Abstract
Huntington’s disease (HD) is a genetic neurodegenerative disease caused by an expanded CAG repeat in the Huntingtin (HTT) gene that codes for an expanded polyglutamine (polyQ) repeat in exon-1 of the human mutant huntingtin (mHTT) protein. The presence of this polyQ repeat results in neuronal degeneration for which there is no cure nor treatment that modifies disease progression. In previous studies we have shown that small molecules that bind selectively to σ2R/TMEM97 can have significant neuroprotective effects in models of Alzheimer’s disease, traumatic brain injury and several other neurodegenerative diseases. In the present work we extend these investigations and show that certain σ2R/TMEM97-selective ligands decrease mHTT induced neuronal toxicity. We first synthesized a set of compounds designed to bind to σ2R/TMEM97 and determined their binding profiles (Ki values) for σ2R/TMEM97 and other proteins in the central nervous system. Modulators with high affinity and selectivity for σ2R/TMEM97 were then tested in our HD cell model. Primary cortical neurons were cultured in vitro for seven days and then co-transfected with either a normal HTT construct (Htt N-586–22Q/GFP) or the mHTT construct Htt-N586–82Q/GFP. Transfected neurons were treated with either σ2R/TMEM97 or σ1R modulators for 48 h. After treatment, neurons were fixed and stained with Hoechst, and condensed nuclei were quantified to assess cell death in the transfected neurons. Significantly, σ2R/TMEM97 modulators reduce the neuronal toxicity induced by mHTT, and their neuroprotective effects are not blocked by NE-100, a selective σ1R antagonist known to block neuroprotection by σ1R ligands. These results indicate for the first time that σ2R/TMEM97 modulators can protect neurons from mHTT-induced neuronal toxicity, suggesting that targeting σ2R/TMEM97 may lead to a novel therapeutic approach to treat patients with HD.
Keywords: Huntington’s disease, σ2R/TMEM97, neuroprotection, neuronal survival, nucleus condensation
Graphical Abstract
INTRODUCTION
Huntington’s disease (HD) is an autosomal-dominant neurodegenerative disease for which there is neither a cure nor an approved treatment that slows or reverses its progression. HD patients typically develop symptoms at mid-adulthood, and the associated disabilities worsen over time ending in death within 10–20 years following the onset of symptoms.1 This devastating disease is caused by an abnormal expansion of CAG repeats in exon-1 of the human Huntingtin gene (HTT) coding for a mutant huntingtin protein (mHTT) with an elongated polyglutamine (polyQ) sequence.2 This mHTT preferentially affects the striatum and deep cortical pyramidal neurons of HD patients, and the disease is manifested as progressive movement disorders including chorea, cognitive decline, and emotional alterations.3 Although some symptomatic treatments are available, there is no disease modifying treatment for HD, so there is an urgent need for neuroprotective drugs or other therapies.4, 5
Small molecules have a rich history in drug discovery because of their ability to selectively target and inhibit or activate proteins involved in pathogenic pathways. In this context, compounds that bind to sigma receptors (σRs) are gaining prominence.6, 7 The sigma 1 receptor (σ1R), which shows no homology with any other mammalian protein, is located in the endoplasmic reticulum (ER) where it is enriched in the mitochondria-associated membrane subregion and is involved in calcium modulation.8, 9 Small molecules that bind to the σ1R have been shown to exhibit promising attributes in neurodegenerative and neurological disorders,10–13 and several σ1R ligands have neuroprotective effects in animal models of HD,14–16 including pridopidine that is in human clinical trials.17–20
The sigma 2 receptor (σ2R), which is biochemically distinct from σ1R, was initially associated with cancer diagnosis and therapy,21–24 but it has more recently been implicated in neurological disorders.25, 26 Compounds that bind to σ2R have been shown to affect intracellular Ca2+ levels and signaling.27, 28 The molecular identity of σ2R was an enigma from its discovery until several years ago, when it was cloned and identified as the ER-resident transmembrane protein 97 (TMEM97),29 herein referred to as σ2R/TMEM97. Although the biological function of σ2R/TMEM97 is not well characterized, it is known to play a role in cholesterol trafficking and homeostasis,30, 31 and 20(S)-hydroxycholesterol has recently been identified as an endogenous ligand.32 σ2R/TMEM97 appears to be a partner of the lysosomal cholesterol transporter NPC1,33 a mutation in which results in Niemann-Pick disease type C, and several other proteins including progesterone membrane component 1 (PGRMC1)31, 34–36 and the low-density lipoprotein receptor (LDLR).31, 36 Small molecules that modulate σ2R/TMEM97-mediated pathways show beneficial effects in different disease contexts, including cancer,37, 38 neuropathic pain,39 traumatic brain injury (TBI),40 alcohol use disorder,35, 41 and Alzheimer’s disease (AD).34, 36, 42, 43 Moreover, a putative σ2R/TMEM97 antagonist is in Phase II clinical trials for treating AD.44
The findings that modulating σ2R/TMEM97 exhibits neuroprotection in several models of neurodegenerative disease prompted us to query whether compounds that bind to σ2R/TMEM97 might provide beneficial effects in an HD model. Toward testing this hypothesis, we evaluated a small panel of compounds with differing affinities and selectivities for σ2R/TMEM97 and σ1R in a primary neuron model of HD. These compounds include racemic AMA-1127, DKR-1051, DKR-1677, UKH-1114, JJS-1678, BJM-1679, EES-1686, BEA-1687, MPC-1154, and HLJ-1560 (Figure 1), some of which have been previously tested in other disease models.34, 39, 40 In this study, we used an HD cell model to assess the effects of these compounds upon mHTT-induced neuronal toxicity. Briefly, primary neurons were co-transfected with plasmid expression of a 586 N-terminal Htt polypeptide with either normal Q (Htt-N586–22Q) or expanded Q (Htt-N586–82Q) repeats and green fluorescent protein (GFP). Compounds that are selective for σ2R/TMEM97 showed strong protective effects on mHTT-induced neuronal cell death as did several different compounds having σ1R selectivity. To exclude the possible involvement of σ1R modulation as a possible mechanism of action for the σ2R/TMEM97 ligands, the σ1R-selective antagonist, NE-100, was used. Notably, the protective effect of σ2R/TMEM97-selective compounds is not blocked by NE-100, clearly demonstrating that neuroprotection by these small molecules is mediated by their interaction with σ2R/TMEM97, not σ1R. These studies are the first to demonstrate that compounds that bind selectively to σ2R/TMEM97 are neuroprotective in an HD model, and they support further mechanistic studies of the function of σ2R/TMEM97 in mHTT protection as a possible new approach to treat HD patients.
Figure 1. Structure of σ2R/TMEM97-selective modulators and their binding affinities.
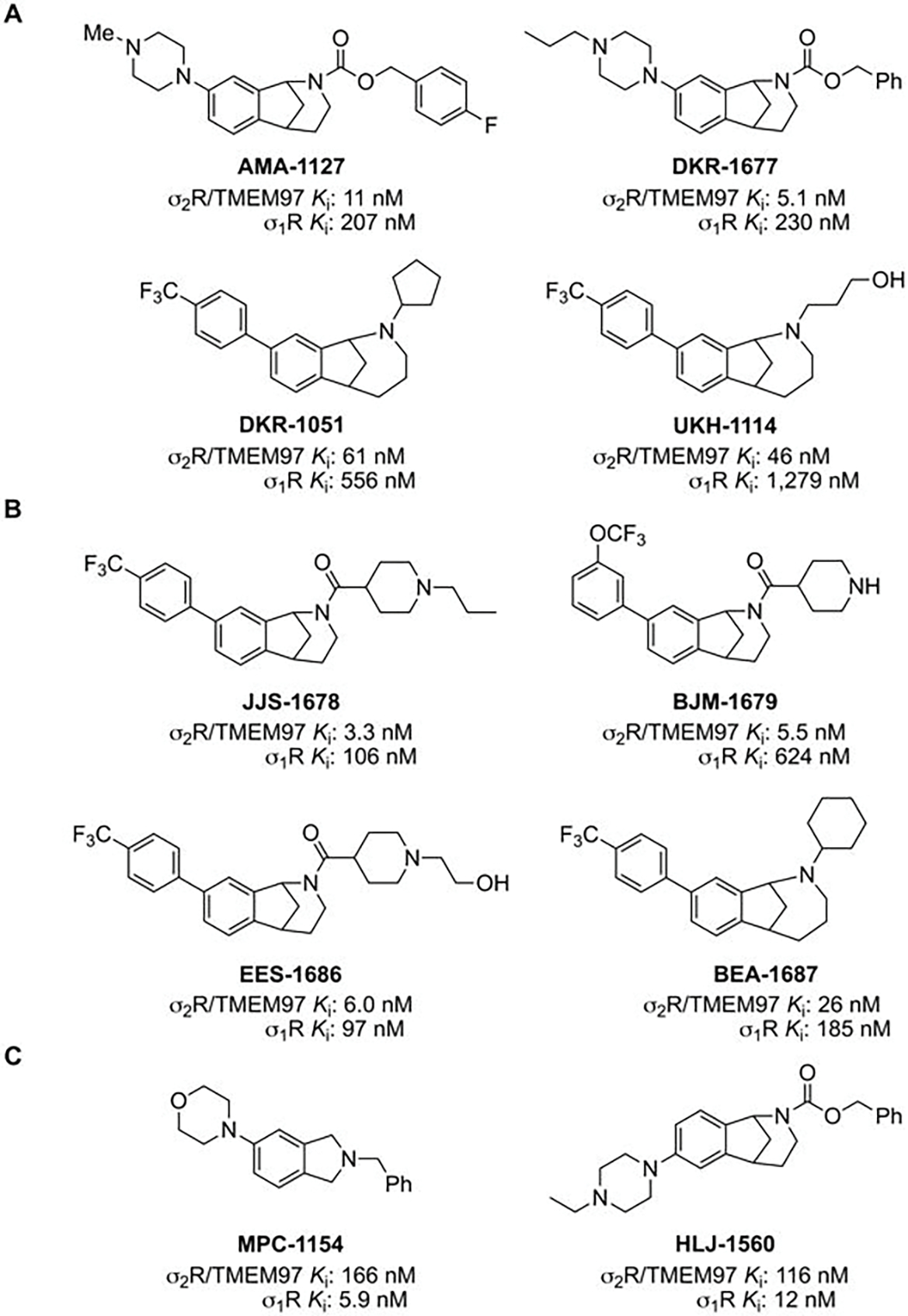
A. Structures of racemic B-norbenzomorphans (AMA-1127 and DKR-1677) and methanobenzazocines (DKR-1051 and UKH-1114) that are selective modulators of σ2R/TMEM97 and their binding affinities. Ki, for σ2R/TMEM97 (rat) and σ1R (guinea pig). B. Structures of racemic B-norbenzomorphans (JJS-1678, BJM-1679, and EES-1686) and methanobenzazocines (BEA-1687) that are selective modulators of σ2R/TMEM97 and their binding affinities. Ki, for σ2R/TMEM97 (human) and σ1R (human). C. Structures of σ1R-selective modulators MPC-1154 and racemic HLJ-1560 and their binding affinities, Ki, for σ2R/TMEM97 (rat) and σ1R (guinea pig).
MATERIALS AND METHODS
Chemical Synthesis and Characterization.
Acetonitrile was dried by filtration through two columns of activated molecular sieves, and toluene was dried by filtration through one column of activated, neutral alumina followed by one column of Q5 reactant. Methylene chloride and diisopropylethylamine (Hünigs base) were distilled from calcium hydride immediately prior to use. Dioxane was distilled from sodium metal and benzophenone prior to use. All solvents were determined to have less than 50 ppm H2O by Karl Fischer coulometric moisture analysis. All reagents were reagent grade and used without purification unless otherwise noted. All reactions involving air or moisture sensitive reagents or intermediates were performed under an inert atmosphere of nitrogen or argon in glassware that was flame or oven dried. Solutions were degassed using three freeze-thaw cycles under vacuum. Reaction temperatures refer to the temperature of the cooling/heating bath. Volatile solvents were removed under reduced pressure using a Büchi rotary evaporator at 25–30 °C. Thin layer chromatography was performed using run on pre-coated plates of silica gel with a 0.25 mm thickness containing 60F-254 indicator (Merck). Chromatography was performed using forced flow (flash chromatography) and the indicated solvent system on 230–400 mesh silica gel (E. Merck reagent silica gel 60). Radial Preparative Liquid Chromatography (radial plc) was performed on a Chromatotron® using glass plates coated with Merck, TLC grade 7749 silica gel with gypsum binder and fluorescent indicator. All compounds submitted for in vivo testing were >95% purity as determined by LC via AUC at 214- and 254 nm. Proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR) spectra were obtained at the indicated field as solutions in CDCl3 unless otherwise indicated. Chemical shifts are referenced to the deuterated solvent and are reported in parts per million (ppm, δ) downfield from tetramethylsilane (TMS, δ = 0.00 ppm). Coupling constants (J) are reported in Hz and the splitting abbreviations used are: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; comp, overlapping multiplets of magnetically nonequivalent protons; br, broad; app, apparent. Racemic intermediates 1,45 2,46 5,47 9,39 and 10,48 as well as (±)-MPC-115449 were prepared as previously described. New compounds were prepared according to the reactions summarized in Schemes 1–5.
Scheme 1.
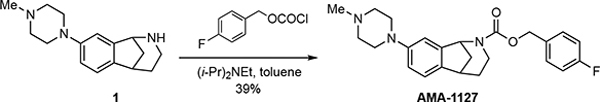
Synthesis of AMA-1127
Scheme 5.
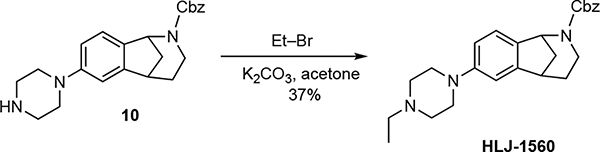
Synthesis of HLJ-1560.
(±)-4-Fluorobenzyl-8-(4-methylpiperazin-1-yl)-1,3,4,5-tetrahydro-2H-1,5-methanobenzo[c]azepine-2-carboxylate (AMA-1127).
4-Fluorobenzyl chloroformate was prepared by slowly adding a solution of phosgene (111 μL of 15 wt % in toluene, 0.155 mmol) to a stirred solution of 4-fluorobenzyl alcohol (21 mg, 0.163 mmol) and diisopropylethylamine (30 mg, 41 μL, 0.233 mmol) in toluene (1 mL) at 0 °C. A solution of amine 1 (19 mg, 0.075 mmol) in toluene (0.5 mL) was then added with stirring, the cooling bath was removed, and the solution was stirred for 1 h. The mixture was diluted with aqueous NaOH (1 M, 10 mL), and the aqueous mixture was extracted with EtOAc (3×10 mL). The combined organic extracts were washed with brine (1×10 mL), dried (Na2SO4), and concentrated under reduced pressure. The residue was purified via flash column chromatography (SiO2), eluting with MeOH/CH2Cl2 (2% v/v), to afford 12 mg (39%) of AMA-1127 as a pale yellow oil. 1H NMR (500 MHz) (rotamers) δ 7.46–7.28 (comp, 2 H), 7.11 (d, J = 8.1 Hz, 1 H), 7.10–7.00 (comp, 2 H), 6.97 (brs, 0.5 H), 6.81 (dd, J = 8.1, 2.0 Hz, 1 H), 6.77 (brs, 0.5 H), 5.45 (dd, J = 2.9 Hz, 0.5 H), 5.31 (dd, J = 2.9 Hz, 0.5 H), 5.23–5.02 (comp, 2 H), 3.85–3.70 (m, 1 H), 3.25–3.10 (comp, 5 H), 2.60 (t, J = 4.9 Hz, 4 H), 2.52–2.38 (m, 1 H), 2.37 (s, 3 H), 2.25–2.12 (m, 1 H), 2.03–1.89 (m, 1 H), 1.87–1.80 (m, 1 H), 1.62–1.50 (m, 1 H). 13C NMR (125 MHz) (rotamers) δ 155.1, 154.8, 151.2, 142.3, 141.9, 137.7, 133.2, 132.9, 130.1, 130.1, 130.0, 129.9, 123.3, 123.2, 116.3, 115.9, 115.6, 115.4, 112.6, 112.2, 66.4, 58.2, 57.9, 55.3, 49.8, 46.2, 43.9, 39.1, 38.8, 30.6; HRMS (ESI) m/z calcd for C24H28N3O2F (M+H)+, 410.2238; found 410.2242.
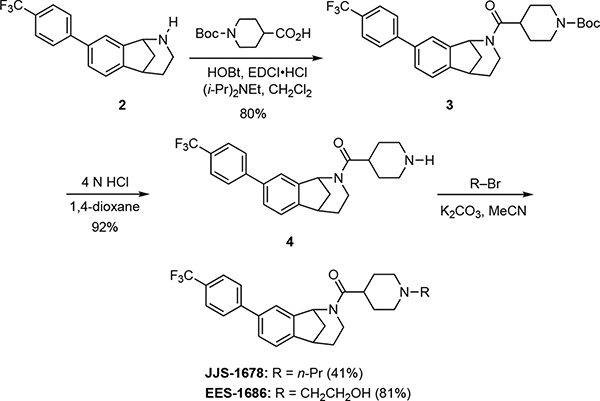
(±)-tert-Butyl 4-(8-(4-(trifluoromethyl)phenyl)-2,3,4,5-tetrahydro-1H-1,5-methanobenzo[c]azepine-2-carbonyl)piperidine-1-carboxylate (3).
EDCI•HCl (52 mg, 0.27 mmol) and hydroxybenzotriazole (42 mg, 0.27 mmol) were added to a solution of 1-(tert-butoxycarbonyl)piperidine-4-carboxylic acid (69 mg, 0.30 mmol) in CH2Cl2 (3.0 mL). A solution of the amine 2 (75 mg, 0.24 mmol) and Hünig’s base (74 mg, 100 μL, 0.54 mmol) in CH2Cl2 (0.95 mL) was then added, and the solution was stirred for 12 h. The mixture was concentrated under reduced pressure, and the crude mixture product was purified via radial preparative layer chromatography, eluting with hexanes → hexanes/EtOAc (9/1 → 1/3 → 1/1) to provide 102 mg (80%) of the carbamate 3 as a colorless oil. 1H NMR (400 MHz) δ 7.51 (d, J = 8.0 Hz, 1 H), 7.49–7.42 (comp, 3 H), 7.42–7.37 (m, 1 H), 7.37–7.31 (m, 1 H), 7.22–7.16 (m, 1 H), 6.01 (d, J = 4.0 Hz, 0.6 H), 5.20–5.17 (m, 0.4 H), 4.32 (dd, J = 14.0, 7.0 Hz, 0.4 H), 4.28–4.02 (comp, 2 H), 3.60 (dd, J = 14.0, 7.0 Hz, 0.6 H), 3.43–3.36 (m, 1 H), 2.94–2.80 (m, 1 H), 2.72 (td, J = 12.0, 5.3 Hz, 2 H), 2.56–2.46 (m, 1 H), 2.38–2.19 (m, 1 H), 2.07–1.97 (m, 1 H), 1.86–1.77 (comp, 2 H), 1.75–1.63 (comp, 2 H), 1.63–1.52 (comp, 2 H), 1.51–1.40 (comp, 9 H); 13C NMR (100 MHz) δ 172.4, 154.7, 149.7, 146.5, 144.4, 142.0, 139.2, 129.3 (q, JC–F = 25.5 Hz), 127.5, 127.4, 127.2, 125.7 (q, JC–F = 3.0 Hz), 123.4, 123.3, 123.1, 122.8, 122.3, 79.5, 54.6, 44.0, 43.2, 39.8, 38.9, 31.1, 29.7, 28.6, 28.4. HRMS (ESI) m/z calcd for C29H33F3N2NaO3 (M+Na)+, 537.2335; found 537.2339.
(±)-Piperidin-4-yl(8-(4-(trifluoromethyl)phenyl)-1,3,4,5-tetrahydro-2H-1,5-methanobenzo[c]azepin-2-yl)methanone (4).
A solution of 4 N HCl in 1,4-dioxane (3.5 mL) was added to a solution of carbamate 3 (88 mg, 0.17 mmol) in 3.0 mL 1,4-dioxane at room temperature and stirring was continued for 24 h. The solution was concentrated under reduced pressure at room temperature, and the residue was dissolved in CH2Cl2 (5 mL). The mixture was made basic by the addition of aqueous NaOH (1 M, 3.0 mL), the organic layer was separated, and the aqueous mixture was extracted with CH2Cl2 (3 × 5 mL). The combined organic extracts were dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was dissolved in Et2O, filtered, and concentrated under reduced pressure to give 65 mg (92%) of amine 4 that was of sufficient purity to be used in subsequent reactions. 1H NMR (400 MHz, CD3OD) δ 7.78–7.72 (comp, 2 H), 7.71–7.66 (comp, 2 H), 7.61–7.59 (m, 1 H), 7.59–7.57 (m, 0.5 H), 7.55–7.53 (m, 0.5 H), 7.40–7.36 (m, 1 H), 5.87 (d, J = 4.0 Hz, 0.6 H), 5.42 (d, J = 4.0 Hz, 0.4 H), 4.19 (dd, J = 12.0, 6.0 Hz, 0.4 H), 3.78–3.59 (comp, 1.6 H), 3.38–3.33 (m, 1 H), 3.29 (pent, J = 2.0 Hz, 0.6 H), 3.16–3.04 (comp, 1.4 H), 3.04–2.94 (m, 1 H), 2.79–2.49 (comp, 2.6 H), 2.35–2.28 (m, 0.4 H), 2.26–2.12 (m, 1 H), 2.09–1.95 (m, 1 H), 1.92 (d, J = 12.0 Hz, 0.4 H), 1.85–1.79 (m, 1 H), 1.81 (d, J = 12.0 Hz, 0.6 H), 1.75–1.62 (comp, 3 H), 1.62–1.46 (m, 1 H); 13C NMR (100 MHz) δ 171.1, 170.9, 146.6, 146.4, 144.3, 141.7, 140.7, 139.4, 139.3, 128.3, 127.8, 127.4, 127.3, 125.7 (q, JC–F = 3.0 Hz), 123.4, 123.2, 122.8, 122.4, 59.1, 44.4, 43.8, 40.2, 39.7, 39.6, 36.7, 35.1, 31.1, 30.4. HRMS (ESI) m/z calcd for C24H26F3N2O (M+H)+, 415.1992; found 415.2004.
(±)-(1-Propylpiperidin-4-yl)(8-(4-(trifluoromethyl)phenyl)-1,3,4,5-tetrahydro-2H-1,5-methanobenzo[c]azepin-2-yl)methanone (JJS-1678).
1-Bromopropane (22 mg, 0.18 mmol) was added to a mixture of 4 (25 mg, 0.060 mmol) and K2CO3 (34 mg, 0.24 mmol) in CH3CN (800 μL). The mixture was heated at 45 °C for 20 h, cooled to room temperature, and concentrated under reduced pressure. The crude residue was purified via flash column chromatography (SiO2), eluting with EtOAc → MeOH/EtOAc (1/19) to give 11 mg (41%) of JJS-1678 as an off white foam. 1H NMR (400 MHz) δ 7.71–7.62 (comp, 4 H), 7.57–7.54 (m, 0.6 H), 7.51 (dd, J = 8.0, 2.0 Hz, 1 H), 7.43–7.40 (m, 0.4 H), 7.35 (t, J = 4.0 Hz, 1 H), 6.02 (J = 4.0 Hz, 0.6 H), 5.18 (J = 4.0 Hz, 0.4 H), 4.32 (dd, J = 12.0, 4.0 Hz, 0.4 H), 3.59 (dd, J = 12.0, 4.0 Hz, 0.6 H), 3.42–3.35 (m, 1 H), 3.13–2.95 (comp, 2 H), 2.71 (td, J = 26.0, 4.0 Hz, 1 H), 2.48–2.11 (comp, 5 H), 2.08–1.77 (comp, 6 H), 1.73–1.64 (m, 1 H), 1.62–1.48 (comp, 2 H), 0.95–0.89 (m, 1 H), 0.89 (t, J = 12.0 Hz, 3 H); 13C NMR (100 MHz) δ 172.2, 171.9, 146.6, 146.4, 144.4, 141.9, 140.9, 139.3, 139.2, 128.2, 127.7, 127.4, 127.3, 125.7 (q, JC–F = 3.0 Hz), 123.4, 123.2, 122.7, 122.3, 59.5, 59.4, 44.4, 43.8, 40.1, 39.8, 39.7, 36.4, 31.1, 30.4, 29.67, 18.4, 18.3, 11.6, 11.5. HRMS (ESI) m/z calcd for C27H32F3N2O (M+H)+, 457.2461; found 457.2465.
(±)-(1-(2-Hydroxyethyl)piperidin-4-yl)(8-(4-(trifluoromethyl)phenyl)-1,3,4,5-tetrahydro-2H-1,5-methanobenzo[c]azepin-2-yl)methanone (EES-1686).
2-Bromoethanol (15 mg, 0.12 mmol) was added to a mixture of 4 (25 mg, 0.060 mmol) and K2CO3 (34 mg, 0.24 mmol) in CH3CN (800 μL), and then the mixture was heated at 50 °C for 20 h. The mixture was cooled to room temperature and concentrated under reduced pressure, and the crude residue was purified via flash column chromatography (SiO2), eluting with hexanes → hexanes/EtOAc (1/1) → EtOAc → CH2Cl2 → MeOH/CH2Cl2 (1:9) to give 22 mg (81%) of a EES-1686 as a light yellow oil. 1H NMR (400 MHz) δ 7.71–7.62 (comp, 4 H), 7.55 (br s, 0.6 H), 7.52 (dd, J = 8.0, 2.0 Hz, 1 H), 7.42 (br s, 0.4 H), 7.35 (t, J = 8.0 Hz, 1 H), 6.02 (d, J = 4.0 Hz, 0.6 H), 5.18 (d, J = 4.0 Hz, 0.4 H), 4.31 (dd, J = 12.0, 8.0 Hz, 0.4 H), 3.71–3.61 (comp, 2 H), 3.58 (dd, J = 12.0, 8.0 Hz, 0.6 H), 3.43–3.36 (m, 1 H), 3.16–2.98 (comp, 2 H), 2.87–2.78 (m, 1 H), 2.73 (td, J = 26.0, 4.0 Hz, 1 H), 2.66 (t, J = 4.0 Hz, 0.7 H), 2.61 (t, J = 4.0 Hz, 1.3 H), 2.53–2.19 (comp, 4 H), 2.08–1.80 (comp, 5 H), 1.78–1.64 (comp, 2 H), 0.92–0.78 (m, 1 H); 13C NMR (100 MHz) δ 171.8, 171.5, 146.6, 146.3, 144.3, 141.7, 140.7, 139.4, 139.3, 128.3, 127.8, 127.4, 127.3, 125.7 (q, JC–F = 3.0 Hz), 123.5, 123.3, 122.7, 122.3, 60.7, 59.1, 56.4, 51.6, 44.4, 43.8, 40.3, 39.7, 39.6, 36.5, 31.1, 29.7. HRMS (ESI) m/z calcd for C26H30F3N2O2 (M+H)+, 459.2263; found 459.2254.
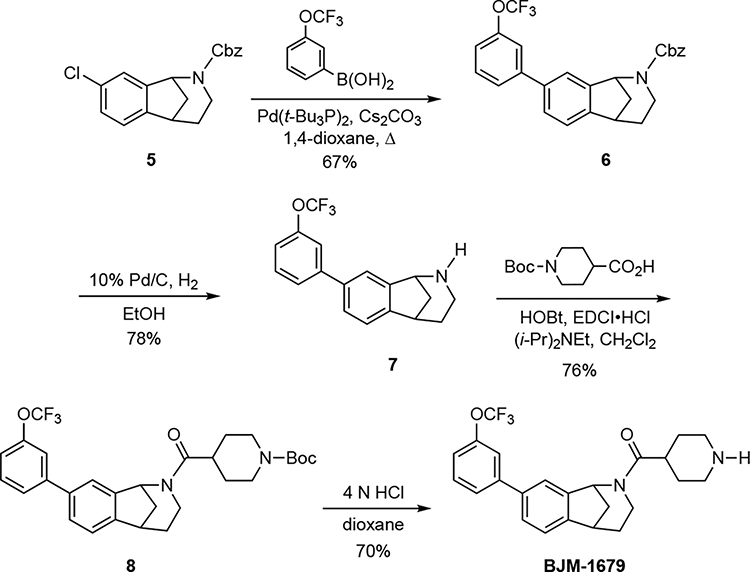
(±)-Benzyl-8-(3-(trifluoromethoxy)phenyl)-1,3,4,5-tetrahydro-2H-1,5-methanobenzo[c]azepine-2-carboxylate (6).
A solution of carbamate 5 (148 mg, 0.45 mmol), 3-trifluroromethoxyphenylboronic acid (186 mg, 0.90 mmol), Cs2CO3 (294 mg, 0.90 mmol), Pd[(t-butyl)3P]2 (12.0 mg, 0.02 mmol) in degassed 1,4-dioxane (2.0 mL) was stirred for 24 h at 100 °C. The reaction was cooled to room temperature, and EtOAc (3 mL) was added. The mixture was filtered through a pad of Celite® and the filter cake rinsed with EtOAc (10 mL), and the combined filtrate and washings were concentrated under reduced pressure to provide the crude product, which was purified via flash column chromatography (SiO2) eluting with hexanes → EtOAc/hexanes (1:4) to give 137 mg (67%) of 6 as a colorless oil. 1H NMR (400 MHz) δ 7.60–7.23 (comp, 11 H), 7.23–7.16 (m, 1 H), 5.55 (br d, J = 48.0 Hz, 1 H), 5.34–5.06 (comp, 2 H), 3.96–3.77 (m, 1 H), 3.37–3.31 (m, 0.9 H), 3.30–3.24 (m, 0.1 H), 2.60–2.38 (m, 1 H), 2.35–2.15 (m, 1 H), 2.13–1.97 (m, 1 H), 1.94 (d, J = 12.0 Hz, 0.9 H), 1.88 (d, J = 12.0 Hz, 0.1 H), 1.74–1.52 (m, 1 H); 13C NMR (100 MHz) δ 154.9, 149.6, 146.2, 143.2, 141.9, 139.0, 136.9, 130.0, 128.5, 128.0, 127.9, 127.4, 125.4, 123.1, 122.5, 121.5, 119.6, 119.4, 67.0, 57.4, 43.6, 39.5, 38.6, 30.21. HRMS (ESI) m/z calcd for C26H22F3NNaO3 (M+Na)+, 476.1444; found 476.1449.
(±)-8-(3-(Trifluoromethoxy)phenyl)-2,3,4,5-tetrahydro-1H-1,5-methanobenzo[c]azepine (7).
A solution of 6 carbamate (137 mg, 0.30 mmol) in ethanol (5 mL) was purged with argon (× 2), whereupon 10% Pd/C (100 mg) was added, and the flask was purged and re-filled with H2 gas (× 3). The mixture was stirred for 2 h under an atmosphere of H2 gas (balloon). The catalyst was removed by filtration through a pad of Celite®, and the filter cake was rinsed with EtOH (3 mL). The combined filtrate and washings were concentrated under reduced pressure, and the crude product was purified via flash column chromatography (SiO2), eluting with hexanes → EtOAc → MeOH/EtOAc (1:19) to give 75 mg (78%) of 7 as a light yellow oil. 1H NMR (400 MHz, CD3OD) δ 7.63–7.61 (m, 1 H), 7.56–7.55 (m, 1 H), 7.53–7.49 (comp, 3 H), 7.31 (d, J = 8.0 Hz, 1 H), 7.25–7.21 (m, 1 H), 4.22 (d, J = 4.0 Hz, 1 H), 3.26–3.22 (m, 1 H), 2.66 (dd, J = 12.0, 4.0 Hz, 1 H), 2.29 (td, d, J = 12.0, 4.0 Hz, 1 H), 2.23–2.16 (m, 1 H), 2.04–1.95 (m, 1 H), 1.98 (d, J = 8.0 Hz, 1 H), 1.63–1.53 (m, 1 H); 13C NMR (100 MHz) δ 149.6, 146.2, 143.4, 142.9, 138.8, 130.0, 127.0, 125.4, 122.8, 122.0, 119.6, 119.3, 58.67, 44.8, 39.8, 38.9, 30.6; HRMS (ESI) m/z calcd for C18H17F3NO (M+H)+, 320.1257; found 320.1266.
(±)-tert-Butyl 4-(8-(3-(trifluoromethoxy)phenyl)-2,3,4,5-tetrahydro-1H-1,5-methanobenzo[c]azepine-2-carbonyl)piperidine-1-carboxylate (8).
EDCI•HCl (23 mg, 0.15 mmol) and hydroxybenzotriazole (23 mg, 0.15 mmol) were added to a solution of 1-(tert-butoxycarbonyl)piperidine-4-carboxylic acid (37 mg, 0.16 mmol) in CH2Cl2 (3.0 mL) at room temperature. A solution of the secondary amine 7 (43 mg, 0.13 mmol) and Hünig’s base (38 mg, 52 μL, 0.30 mmol) in CH2Cl2 (800 μL) was added, and stirring was continued for 12 h. The mixture was concentrated under reduced pressure, and the crude product was purified via radial plc, eluting with hexanes → hexanes/EtOAc (9/1 → 1/3 → 1/1) to provide 54 mg (76%) of carbamate 8 as a colorless oil. 1H NMR (400 MHz) δ 7.56–7.30 (comp, 6 H), 7.19 (d, J = 6.4 Hz, 1 H), 6.02 (br s, 0.5 H), 4.26–4.02 (comp, 2 H), 3.62 (br s, 0.5 H), 3.39 (s, 1 H), 2.95–2.17 (comp, 5 H), 2.07–1.96 (m, 1 H), 1.92–1.52 (comp, 7 H), 1.46 (s, 9 H);13C NMR (100 MHz) δ 172.4, 154.7, 149.7 (q, JC–F = 1.5 Hz), 146.5, 143.1, 139.1, 130.1, 127.5, 127.4, 125.4, 123.6, 123.1, 122.6, 121.5, 119.6, 119.5, 119.4, 79.5, 54.7, 44.0, 43.3, 39.8, 38.9, 31.1, 29.7, 28.7, 28.4. HRMS (ESI) m/z calcd for C29H33F3N2NaO4 (M+Na)+, 553.2285; found 553.2288.
(±)-Piperidin-4-yl(8-(3-(trifluoromethoxy)phenyl)-1,3,4,5-tetrahydro-2H-1,5-methanobenzo[c]azepin-2-yl)methanone (BJM-1679).
Prepared from carbamate 8 (54 mg, 0.10 mmol) according to the procedure described above for the preparation of 4 to give 30 mg (70%) of BJM-1679 as a white foam. 1H NMR (400 MHz) δ 7.53–7.36 (comp, 5 H), 7.31 (t, J = 8.0 Hz, 1 H), 7.21–7.14 (m, 1 H), 5.99 (d, J = 4.0 Hz, 0.6 H), 5.18 (d, J = 4.0 Hz, 0.4 H), 4.30 (dd, J = 12.0, 8.0 Hz, 0.4 H), 3.59 (dd, J = 12.0, 8.0 Hz, 0.6 H), 3.40–3.32 (m, 1 H), 3.27–3.07 (comp, 2 H), 2.92–2.46 (comp, 3 H), 2.38 (br s, 1.4 H), 2.35–2.16 (comp, 1.6 H), 2.05–1.94 (m, 1 H), 1.89 (d, J = 8.0 Hz, 0.4 H), 1.86–1.76 (m, 1 H), 1.81 (d, J = 8.0 Hz, 0.6 H), 1.77–1.53 (comp, 3 H), 0.91–0.77 (m, 1 H); 13C NMR (100 MHz) δ 172.7, 172.4, 149.7, 146.6, 146.4, 143.2, 143.1, 142.1, 141.0, 139.1, 139.0, 130.1, 130.0, 128.0, 127.4, 125.5, 125.3, 123.3, 123.0, 122.7, 122.2, 121.5, 119.7, 119.6, 119.5, 119.4, 58.7, 44.4, 43.8, 39.85, 39.82, 39.7, 38.6, 36.4, 31.2, 30.5. HRMS (ESI) m/z calcd for C24H26F3N2O2 (M+H)+, 431.1941; found 431.1949.
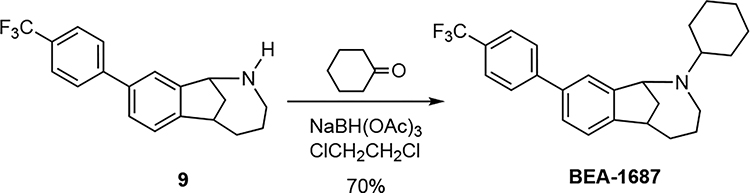
(±)-2-Cyclohexyl-9-(4-(trifluoromethyl)phenyl)-1,2,3,4,5,6-hexahydro-1,6-methanobenzo[c]azocine (BEA-1687).
Cyclohexanone (22 mg, 23 μL, 0.22 mmol) was added to a solution of 9 (23 mg, 0.072 mmol) in 1,2-dichloroethane at room temperature, and the solution was stirred for 30 min. Sodium triacetoxyborohydride (47 mg, 0.22 mmol) and acetic acid (100 μL) were added sequentially, and the mixture was stirred 12 h at room temperature. Aqueous saturated NaHCO3 solution (2 mL) was then added, and the mixture was stirred for 10 min. The layers were separated, and the aqueous layer was extracted with CH2Cl2 (3 × 15 mL). The combined organic layers were washed with brine (3 mL), dried (MgSO4), filtered and concentrated under reduced pressure. The crude residue was purified via radial preparative layer chromatography, eluting with hexanes hexanes/EtOAc (100/0 → 95/5) to provide 20 mg (70%) of BEA-1687 as a light yellow oil. 1H NMR (400 MHz, CD3OD) δ 7.80 (d, J = 12.0 Hz, 2 H), 7.71 (d, J = 12.0 Hz, 2 H), 7.56–7.54 (comp, 1.5 H), 7.53 (d, J = 4.0 Hz, 0.5 H), 7.27 (d, J = 8.0 Hz, 1 H), 4.43–4.39 (m, 1 H), 3.39–3.34 (m, 1 H), 3.13 (t, J = 12.0 Hz, 1 H), 2.65 (m, 1 H), 2.60–2.52 (m, 1 H), 2.20–2.10 (comp, 3 H), 2.00 (br d, J = 12.0 Hz, 1 H), 1.93–1.85 (comp, 2 H), 1.82 (pent, J = 4.0 Hz, 2 H), 1.71.–1.64 (m, 1 H), 1.64–1.52 (m, 1 H), 1.47–1.12 (comp, 5 H), 1.07–0.94 (m, 1 H).13C NMR (100 MHz) δ 146.6, 143.3, 139.5, 129.7, 129.4, 129.1, 127.9, 125.7 (q, JC–F = 3.0 Hz), 125.4, 123.8, 123.2, 66.3, 62.3, 50.5, 42.7, 34.9, 33.8, 29.7, 25.4, 25.3, 25.2. HRMS (ESI) m/z calcd for C25H29F3N (M+H)+, 400.2247; found 400.2253.
(±)-Benzyl-7-(4-ethylpiperazin-1-yl)-1,3,4,5-tetrahydro-2H-1,5-methanobenzo-[c]azepine-2-carboxylate (HLJ-1560).
A mixture of K2CO3 (15 mg, 0.106 mmol), 10 (20 mg, 0.053 mmol), and ethyl bromide (7 mg, 4.7 μL, 0.064 mmol) in acetone (1 mL) was stirred at room temperature for 24 h. The mixture was filtered, and the filtrate was concentrated under reduced pressure. The residue was purified via flash column chromatography (SiO2), eluting with MeOH/CH2Cl2 (2% v/v) to afford to afford 8 mg (37%) of HLJ-1560 as a clear oil. 1H NMR (400 MHz) (rotamers) δ 7.48–7.26 (comp, 5 H), 7.21 (d, J = 7.8 Hz, 0.5 H), 7.10 (d, J = 7.5 Hz, 0.5 H), 6.84 (d, J = 2.2 Hz, 1 H), 6.78–6.71 (m, 1 H), 5.44 (brs, 0.5 H), 5.33 (brs, 0.5 H, 5.23–5.05 (comp, 2 H), 3.87–3.73 (m, 1 H), 3.27–3.17 (comp, 5 H), 2.62 (t, J = 4.9 Hz, 4 H), 2.49 (q, J = 7.1 Hz, 2 H), 2.51–2.38 (m, 1 H), 2.24–2.12 (m, 1 H), 2.02–1.80 (comp, 2 H), 1.64–1.49 (m, 1 H), 1.13 (t, J = 7.1 Hz, 3 H). 13C NMR (125 MHz) (rotamers) δ 155.0, 154.9, 152.1, 147.8, 137.3, 137.1, 132.4, 132.2, 128.6, 128.0, 127.9, 124.5, 124.3, 114.6, 111.0, 67.0, 57.3, 57.1, 53.0, 52.5, 49.6, 44.1, 40.4, 38.7, 30.5, 12.1; IR (neat) 2937, 1695, 1418, 1237, 1096 cm−1; HRMS (ESI) m/z calcd for C25H31N3O2 (M+H)+, 406.2489; found 406.2503.
Preparation of solutions of σ2R/TMEM97 modulators.
Stock solutions of σ2R/TMEM97 modulators were prepared by dissolving the compound in DMSO to a concentration of 10 mM. For the in vitro assays, the stock solution was diluted with culture medium (1:1000) to a working concentration of 10 μM of σ2R/TMEM97 modulator. Serial dilutions were then performed using culture medium to prepare other concentrations of the modulator. The final DMSO concentration is less than 0.1%. The vehicle group was performed using 0.1% DMSO in culture medium.
Receptor binding assays.
Receptor binding assays for compounds determined by LC-MS to be >95% pure were performed by the Psychoactive Drug Screening Program (PDSP) at Chapel Hill, North Carolina.50 Briefly, binding affinities, Ki, for σ2R/TMEM97 (rat PC12 cells) were determined through competition binding assays using the radioligand [3H]-ditolylguanidine in the presence of (+)-pentazocine to block σ1R binding sites, whereas binding affinities, Ki, for σ1R (guinea pig brain) were determined through competition binding assays with [3H]-(+)-pentazocine. Binding affinities, Ki, for σ2R/TMEM97 (human clone transiently expressed in HEK293 cells) were determined through competition binding assays using the radioligand [3H]-ditolylguanidine in the presence of (+)-pentazocine to block σ1R binding sites, and binding affinities, Ki, for σ1R (human clone transiently expressed in HEK293 cells) were determined through competition binding assays with [3H]-(+)-pentazocine. Ki values are calculated from best-fit IC50 determinations performed in triplicate. The Ki values for other proteins in the central nervous system (CNS) of neuroprotective σ2R/TMEM97 modulators were also determined by the PDSP, and these binding profiles may be found in the Supporting Information. Detailed experimental protocols are available on the NIMH PDSP website at https://pdspdb.unc.edu/pdspWeb.
Primary cortical neuron preparation.
Primary neurons were isolated from E17 embryos of CD1 mice in accord with published procedures.51, 52 Animals used in this study were cared for following the National Institutes of Health guidelines for the use of experimental animals. All experimental protocols involving animals were approved by Johns Hopkins Institutional Animal Care and Use Committee. Briefly, E17 embryonic mouse brain were removed, and the neocortex was dissected under stereomicroscope by removing midbrain and hippocampus and striatum. Cortex tissue was digested using trypsin for 15 min and then digested with DNase for 2 minutes. Cortical tissue was dissociated by pipetting, and single cell suspension was achieved filtering digested tissue through cell strainer. Cortical neurons were plated at 106 cells/mm2 in 24-well plates coated with poly -D-Lysine and laminin. Neurons were maintained at 37°C / 5% CO2 in Neurobasal medium containing 2% B27, 2 mM Glutamax and 1 % Pen/strep. All cell culture supplies were obtained from Corning, and all media were from ThermoFisher Scientific.
Co-transfection of primary neurons.
Neurons were co-transfected using Lipofectamine 2000 (ThermoFisher Scientific) at day in vitro (DIV) 6 according to our previously published protocol.51, 52 Cells were co-transfected with GFP and a plasmid expressing 586 N-terminal amino acids of human huntingtin with either 22 or 82 polyglutamines in exon 1. Neurons were treated with or without test compounds at transfection. At 48 h, neurons were fixed using 4% PFA. Nuclei were stained with Hoechst.
Nuclear condensation assay.
Cell toxicity experiments in primary cortical neurons were conducted according to the established protocol.51, 52 After fixing with PFA, nuclei were stained using Hoechst (0.2 μg/mL in PBS for 5 min). Automated picture acquisition was performed using a Zeiss Axiovert 200 inverted microscope with a 20x objective, and mosaic images were obtained. Automatic quantification of the nuclear intensity of transfected cells was performed using Volocity. Cells were considered dead when their nuclear intensity was higher than the average intensity by two standard deviations. Each condition was performed in quadruplicate within each experiment, and each experiment was repeated in at least six independent neuronal preparations unless specifically indicated. Data were represented as mean ± SEM.
Statistical analysis.
Statistical analysis was conducted using GraphPad Prism software version 8 (GraphPad, San Diego, CA, USA). Two-way ANOVA was used to analyze data. Results were considered significant if the p value was <0.05. Error bars indicate SEM in all figures.
RESULTS
Receptor binding profiles
The binding affinities (Ki) of all synthetic compounds for σ2R/TMEM97 and σ1R were determined at the Psychoactive Drug Screening Program. Prior to the identification of σ2R as TMEM97, Ki values were measured using σ2R sourced from rat PC12 cells and σ1R sourced from guinea pig brain, but subsequently σ2R/TMEM97 and σ1R binding isotherms were determined using human protein obtained by transfection in HEK293T cells. Examination of the Ki values for AMA-1127, DKR-1051, DKR-1677 and UKH-1114, which were obtained using rat σ2R/TMEM97 and guinea pig σ1R proteins, show that each of these compounds has high affinity and good selectivity for σ2R/TMEM97 versus σ1R (Figure 1A). Similarly, the Ki values for JJS-1678, BJM-1679, EES-1686 and BEA-1687, which were obtained using human σ2R/TMEM97 and σ1R proteins, also display high affinity and good selectivity for σ2R/TMEM97 vs σ1R (Figure 1B). The structures of new σ2R/TMEM97-selective compounds comprise the pharmacophoric elements of a basic amino group and a hydrophobic group that is characteristic of structural classes and similar compounds we have previously reported.45, 46, 48 Although the two molecular scaffolds differ by the presence of an extra methylene group in the methanobenzazocines DKR-1051 and UKH-1114, two distinct chemotypes are also represented. Namely, AMA-1127 and DKR-1677 have a basic piperazine group on the aromatic ring of the B-norbenzomorphan core, whereas all of the other compounds have an aryl substituent at this position on the parent molecular framework. We have also identified compounds that are selective for σ1R (guinea pig) relative to σ2R/TMEM97 (rat), and we used two of these, MPC-1154 and HLJ-1560 (Figure 1C), as controls. MPC-1154 represents a completely different class of compounds selective for σ1R,49 whereas HLJ-1560 differs from the σ2R/TMEM97-selective ligands AMA-1127 and DKR-1677 by the orientation of the piperazine ring on the B-norbenzomorphan core.45, 46, 48
Compounds binding selectively to σ2R/TMEM97 protect cortical primary neurons from mHTT-induced toxicity.
To evaluate the extent to which selective σ2R/TMEM97 modulators exhibit neuroprotective effects in neurodegenerative processes associated with HD, we used a HD neuronal model as previously described.51 Briefly, primary cortical neurons were isolated from embryonic day 17 mouse brains and cultured in neurobasal medium for seven days. The neurons were then co-transfected with plasmids expressing HTT and GFP. Plasmids expressing the 586 N-terminal amino acids of the human huntingtin gene with either 22 polyglutamine (Htt-N586–22Q) or 82 polyglutamine (Htt-N586–82Q) repeats within exon 1 were used. Parallel experiments were performed in which neurons were simultaneously treated with σ2R/TMEM97 or σ1R modulators, and neurons treated with vehicle were used as controls. Forty-eight hours after transfection, neurons were fixed, and the effects of the various sigma receptor modulators on neuronal cell death were evaluated. Representative images of GFP+ neurons transfected with 22Q, 82Q and 82Q with σ2R/TMEM97 modulators show that mHTT (82Q) transfected neurons had condensed nuclei, whereas the mHTT (82Q) transfected neurons that were treated with σ2R/TMEM97 ligands AMA-1127, DKR-1051, UKH-1114, EES-1686 and BEA-1687 had relatively normal nuclei (Figure 2A and B). Similarly, mHTT (82Q) transfected neurons treated with the selective σ1R ligand HLJ-1560 also had relatively normal nuclei (Figure 2B).
Figure 2. Neuroprotective effect of σ2R/TMEM97-selective modulators on mHTT induced toxicity.
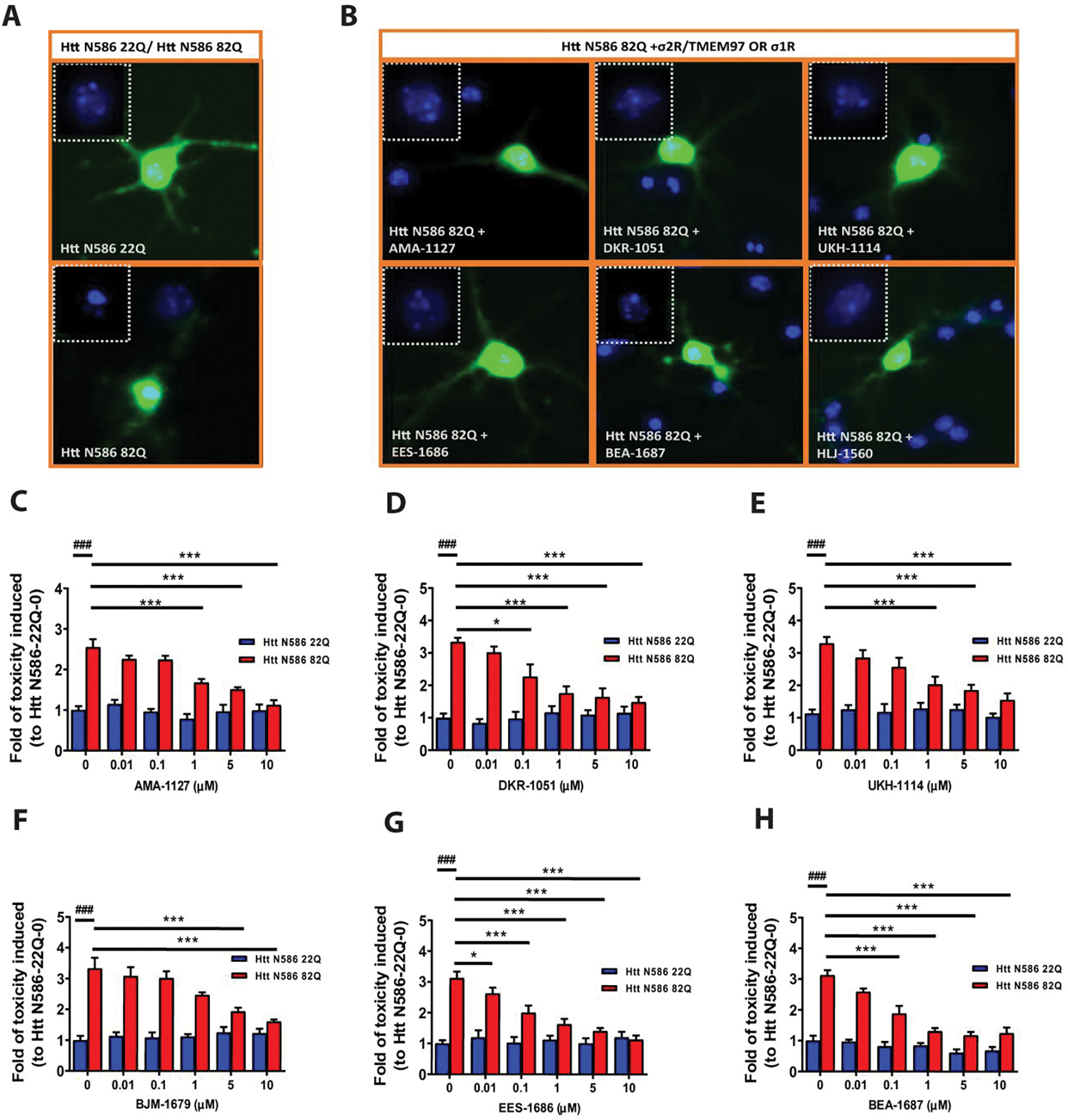
Primary cortical neurons were co-transfected with either Htt-N586–22Q or Htt-N586–82Q and GFP. Four hours after transfection, neurons were treated with either σ1R or σ2R/TMEM97 modulators for 48 h, whereupon neurons were fixed, and nuclei were stained with Hoechst. Cells with condensed nuclei were counted as dead cells. Only neurons transfected with plasmid were counted. A. Representative pictures for neurons transfected with Htt-N586–22Q or Htt-N586–82Q and GFP. B. Representative pictures for neurons transfected with Htt-N586–82Q/GFP and treated with σ2R/TMEM97 or σ1R modulators. Neurons were treated with 1 μM of the indicated modulators. Insert boxes indicated viable cells with normal nucleic morphology. C-H. σ2R/TMEM97-selective modulators tested in HD cell model showing neuroprotection. These compounds are AMA-1127 (C), DKR-1051 (D), UKH-1114 (E), BJM-1679 (F), EES-1686 (G), and BEA-1687 (H). ### p<0.0001 vs Htt N586–22Q with 0. * p<0.05, *** p<0.0001 vs Htt N586–82Q with 0. n=6–8 independent experiments.
The effects of selective σ2R/TMEM97 modulators on mHTT-induced neuronal toxicity were then assessed using a nuclear condensation assay (Figure 2C–H).51 Neurons were treated with σ2R/TMEM97 modulators at concentrations varying from 0.01–10 μM, and those compounds having protective effects are AMA-1127 (Figure 2C), DKR-1051 (Figure 2D), UKH-1114 (Figure 2E), BJM-1679 (Figure 2F), EES-1686 (Figure 2G) and BEA-1687 (Figure 2H). These compounds provide significant neuroprotection at concentrations that range from a low of 10 nM for EES-1686 (Figure 2G) to a high of 5 μM for BJM-1679 (Figure 2F). Importantly, the observed neuroprotective effects were dose dependent with higher concentrations of the σ2R/TMEM97 modulator having greater protective effects (Figure 2C–H). Because these compounds have no effect on neurons transfected with Htt-N586–22Q, none appear to exhibit any intrinsic toxicity. Of the σ2R/TMEM97 modulators tested in these experiments, only DKR-1677 (Figure S1A) and JJS-1678 (Figure S1B) had no notable protective effect on neurons transfected with HTT-N586–82Q.
We also examined the effects of several selective σ1R modulators on neuron survival using the same assays, and both MPC-1154 (Figure 3A) and HLJ-1560 (Figure 3B) protected neurons from mHTT-induced cell toxicity at levels of 1 μM. The neuroprotective effects of these compounds are also dose dependent (Figure 3A, B).
Figure 3. Neuroprotection effect of σ1R-selective modulators on mHTT induced toxicity.
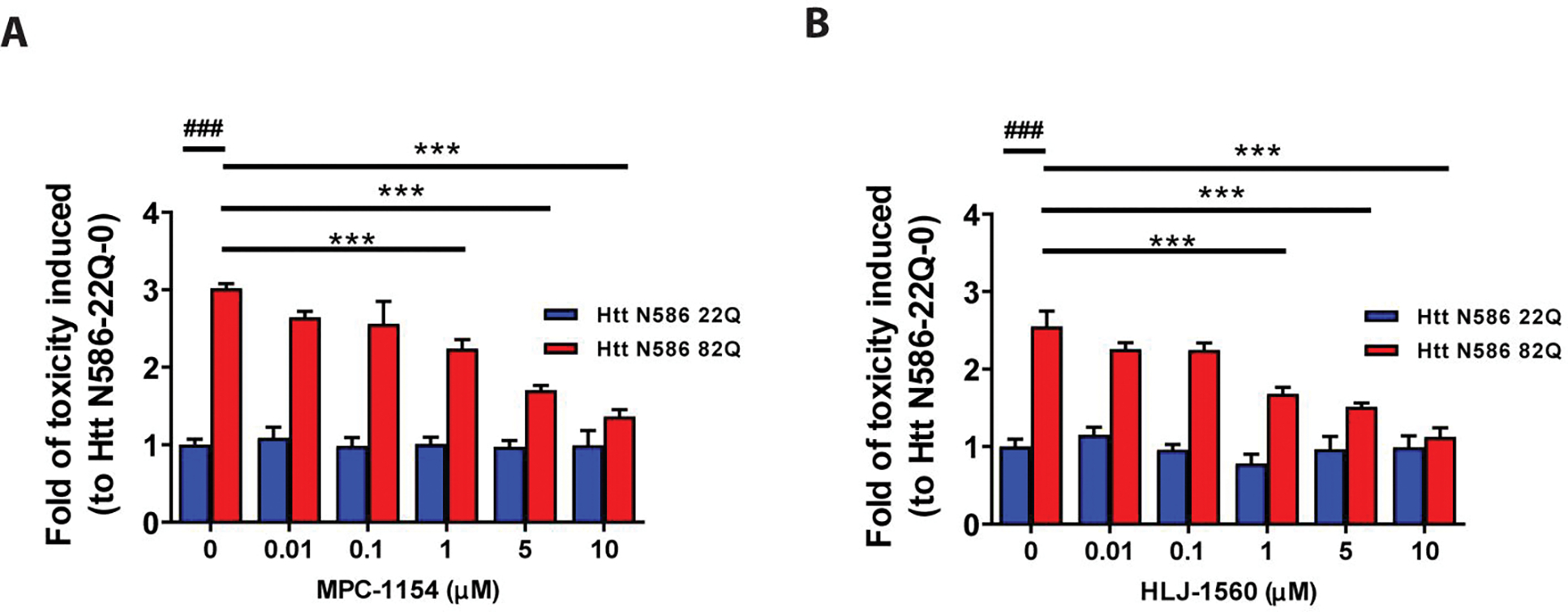
Primary cortical neurons were co-transfected with either Htt-N586–22Q or Htt-N586–82Q and GFP. Four hours after transfection, neurons were treated with or without σ1R modulators at different concentrations. σ1R-selective modulators tested in HD cell model were as follows: MPC-1154 (A) or HLJ-1560 (B). ### p<0.0001 vs Htt N586–22Q with 0. *** p<0.0001 vs Htt N586–82Qwith 0. n=6–8 independent experiments.
NE-100 did not block the neuroprotective effect of σ2R/TMEM97 ligands on mHTT-induced toxicity.
The binding profiles of AMA-1127, DKR-1051, UKH-1114, BJM-1679, EES-1686, and BEA-1687 show that they are all selective for σ2R/TMEM97 relative to σ1R and other CNS proteins (see Supporting Information), suggesting that their protective effect arises from modulating a pathway involving σ2R/TMEM97. However, the function of σ2R/TMEM97 is not well understood, and because there are no ligands that are confirmed σ2R/TMEM97 antagonists, we employed an established σ1R antagonist, NE-100, to block any σ1R pathway that might be operative.53, 54 NE-100 had no effect on the protective attributes of the σ2R/TMEM97-selective modulators AMA-1127, DKR-1051, UKH-1114, EES-1686 and BEA-1687, whereas NE-100 treatment abolished the neuroprotective effects of the selective σ1R modulators HLJ-1560 (Figure 4) and MCP-1154 (Figure S2). These results support the hypothesis that compounds that selectively bind to σ2R/TMEM97 mitigate mHTT-induced neuronal toxicity by a pathway that is distinct from interacting with σ1R.
Figure 4. Specificity of σ2R/TMEM97 modulators.
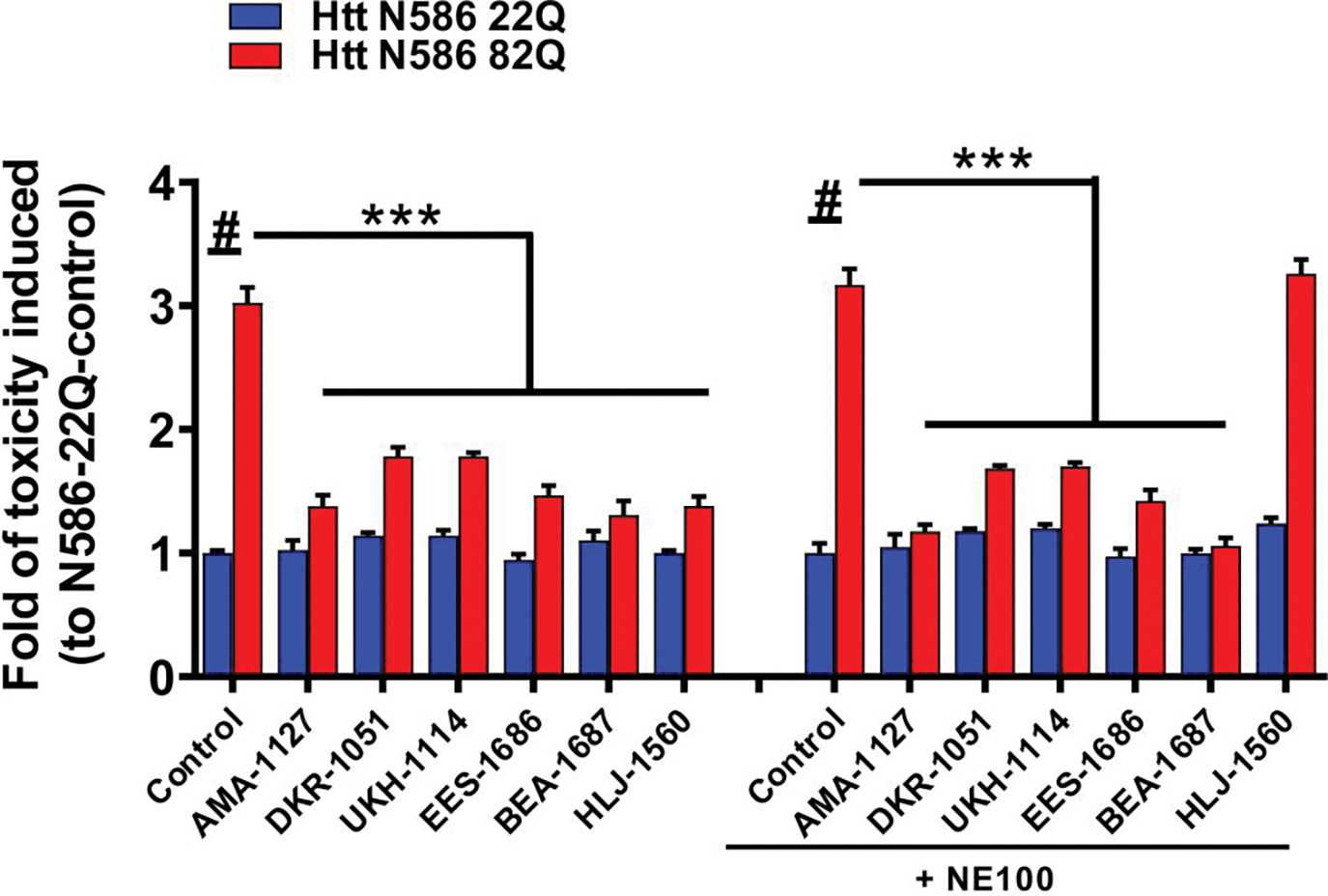
To further explore the specificity of σ2R/TMEM97 modulators, selective σ1R antagonist, NE-100 was used in the primary cortical neurons treated σ1R or σ2R/TMEM97 modulators (10 μM). Primary cortical neurons were co-transfected with either Htt-N586–22Q or Htt-N586–82Q and GFP. Four hours after transfection, neurons were treated with modulators with or without a pretreatment with 10 μM of NE-100. After 48 h, neurons were fixed and nuclei were stained. We included one σ1R-selective modulator, HLJ-1560, as a positive control, which its effect will be blocked by NE-100. Cell death were quantified using a nuclei condensation assay. NE-100 abolished the protective effect of the σ1R modulator, HLJ-1560, but it did not influence the effects of σ2R/TMEM97 modulators. *** p<0,0001 vs Htt N586–82Q, # p<0,001 vs Htt N586–22Q. n=4–6 independent experiments.
DISCUSSION
Research using cell and animal models has resulted in significant progress toward understanding the etiology and pathology of HD, but treatments that slow disease progression have been elusive. mHTT is specifically toxic to striatal medium spiny neurons causing neuronal death in the striatum.1, 55, 56 The mechanism of neuronal death includes mHTT-related transcriptional dysregulation, neurotrophic factor deficit, abnormal mitochondrial function, energy and cholesterol metabolic abnormalities, and impaired protein degradation.57 Although numerous attempts to discover drugs that reduce or reverse mHTT-induced toxicity have been unsuccessful, genetic modification of mHTT expression has emerged as a promising strategy, albeit one limited by the need for CNS delivery of large molecules and the accompanying toxicity or the toxicity of small molecule splicing modifiers.58, 59
The present findings are significant because they are the first to demonstrate that small molecules that bind selectively to σ2R/TMEM97 are neuroprotective in an HD model. In particular, each of the σ2R/TMEM97 ligands AMA-1127, DKR-1051, UKH-1114, EES-1686 and BEA-1687 protects neurons from mHTT-induced toxicity in a dose-dependent manner at concentrations as low as 10 nM. We also show that the σ1R-selective ligands MPC-1154 and HLJ-1560 are neuroprotective, but this is not surprising because σ1R agonists have been shown to be neuroprotective in HD cell and animal models.14–16 Indeed, the σ1R agonist pridopidine is in clinical trials for treating patients with HD.17–19
Compounds AMA-1127, DKR-1051, UKH-1114, EES-1686 and BEA-1687 have high affinity for σ2R/TMEM97, and each has excellent selectivity for σ2R/TMEM97 relative to approximately 45 receptors, transmembrane proteins, and neurotransmitter transporters in the CNS (see Supporting Information). We thus surmise that modulation of σ2R/TMEM97 is responsible for their ability to protect neurons from mHTT-induced toxicity. Unfortunately, the standard pharmacological technique of blocking their activity with other σ2R/TMEM97 ligands is not a meaningful approach to demonstrate target engagement because little is known about the function of σ2R/TMEM97, and there is no reliable method to assign agonist or antagonist activity. Accordingly, we turned to an alternative approach to gather evidence supporting the neuroprotective role of σ2R/TMEM97. Because each of these ligands has some affinity for σ1R, we wanted to exclude the possibility that σ1R binding was involved in reducing mHTT-induced toxicity. Toward this end, co-transfected neurons were treated with AMA-1127, DKR-1051, UKH-1114, EES-1686 and BEA-1687 together with the known σ1R antagonist NE-100;53, 54 the σ1R ligand HLJ-1560 served as a positive σ1R control. NE-100 blocked the protective effect of the σ1R ligand HLJ-1560, but it did not block the effects of AMA-1127, DKR-1051, UKH-1114, EES-1686 or BEA-1687. These results exclude the possibility that the observed neuroprotective effects of these compounds arise from binding to σ1R.
It is notable that DKR-1051 and UKH-1114, which are neuroprotective in this HD model, also relieve mechanical hypersensitivity in an animal model of neuropathic pain,39 but neither mitigates behavioral deficits in a model of alcohol withdrawal.35 This is not the first time we have observed that σ2R/TMEM97 modulators that are active in one bioassay or disease model may be inactive in another. For example, DKR-1677 reduces axonal degeneration in a blast model of TBI and improves survival of cortical neurons and oligodendrocytes in the controlled cortical impact injury model of TBI;40 however, it has no neuroprotective activity in this model of HD. These observations suggest that biological outcomes arising from interactions of σ2R/TMEM97 with structurally distinct ligands can vary depending upon the pathology of the neurological condition. Understanding the effects of modulating σ2R/TMEM97 with small molecules is further complicated by findings that the benefits of a bioactive σ2R/TMEM97 ligand in one disease model can be blocked by another compound that binds to σ2R/TMEM97 and is active in a different model. For example, DKR-1051 induces a rapid Ca2+ transient in human SK-N-SH neuroblastoma cells that is blocked by pretreating the cells with SAS-0132, the norfluoro analog of AMA-1127. SAS-0132 does not induce significant Ca2+ release at similar concentrations, but it does have significant neuroprotective effects in AD models.34 We have also shown that SAS-0132, which has no antinociceptive effects, blocks the antinociceptive properties of UKH-1114.39 Collectively, these results demonstrate that the functional activities of bioactive σ2R/TMEM97 ligands do not fall into well-defined categories such as agonist or antagonist.
There is accumulating evidence that modulating σ2R/TMEM97 with small molecules may induce pleiotropic effects, and changes, sometimes relatively minor, in the structure of the ligand can have a profound influence upon biological outcomes, which appear to be dependent upon the etiology of the disease or condition. Based upon what little is known about the function of σ2R/TMEM97 in cells, regulating cholesterol and/or Ca2+ levels in some way may be an essential component of its mechanism of action.27, 28, 30, 31 It also appears that interactions of σ2R/TMEM97 with other membrane proteins including PGRMC1, NPC1, and the LDLR are important,31, 33–36 but details of the roles of such protein-protein interactions are lacking. Similarly, the role of any endogenous ligands such as (20S)-hydroxycholesterol must be clarified.32 Before one can understand the downstream effects of small molecule binding to σ2R/TMEM97, the function of σ2R/TMEM97 in stressed cells must be elucidated.
Work toward developing a better understanding of the mechanism and function of σ2R/TMEM97 are ongoing, but the results presented herein are significant because they show for the first time that compounds modulating σ2R/TMEM97 are neuroprotective in a HD cell model. EES-1686, the most potent compound studied, is a promising lead compound for advancing to in vivo experiments. Although further studies are needed, these investigations suggest that targeting σ2R/TMEM97 may be a novel therapeutic strategy for developing HD treatments.
Supplementary Material
Scheme 2.
Syntheses of JJS-1678 and EES-1686
Scheme 3.
Synthesis of BJM-1679
Scheme 4.
Synthesis of BEA-1687.
Acknowledgments.
This work was supported by NIH R01 NS086452 (C.A.R.), the Robert A. Welch Foundation (F-0652) (S.F.M), and the Dell Medical School’s Texas Health Catalyst program (S.F.M. and J.J.S.). The Ki determinations were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271–2018-00023-C (NIMH PDSP), which is directed by Bryan L. Roth at the University of North Carolina at Chapel Hill and Jamie Driscoll, project officer at NIMH, Bethesda MD. For more details on the PDSP and experimental details for binding assays, see the PDSP website at https://pdspdb.unc.edu/pdspWeb/.
Footnotes
Competing Interests Statement. S.F.M., J.J.S., K.T.L., and T.R.H. report being co-inventors on patents and pending patent applications related to work described in this article, and S.F.M. and J.J.S. report being co-founders of NuvoNuro, Inc.
Supporting Information. Tables of σ2R/TMEM97 and σ1R binding affinities, binding profiles at non-σR sites, supplementary figures, copies of 1H and 13C NMR spectra of new compounds.
References
- 1.Tabrizi SJ, Flower MD, Ross CA, and Wild EJ (2020) Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities, Nat Rev Neurol 16, 529–546. [DOI] [PubMed] [Google Scholar]
- 2.(1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group, Cell 72, 971–983. [DOI] [PubMed] [Google Scholar]
- 3.Walker FO (2007) Huntington’s Disease, Semin Neurol 27, 143–150. [DOI] [PubMed] [Google Scholar]
- 4.Adam OR, and Jankovic J (2008) Symptomatic treatment of Huntington disease, Neurotherapeutics 5, 181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dash D, and Mestre TA (2020) Therapeutic Update on Huntington’s Disease: Symptomatic Treatments and Emerging Disease-Modifying Therapies, Neurotherapeutics 17, 1645–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt HR, and Kruse AC (2019) The Molecular Function of sigma Receptors: Past, Present, and Future, Trends Pharmacol Sci 40, 636–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SBS, T (2017) Sigma Receptors: Their Role in Disease and as Therapeutic Agents, Springer, New York. [DOI] [PubMed] [Google Scholar]
- 8.Pontisso I, and Combettes L (2021) Role of Sigma-1 Receptor in Calcium Modulation: Possible Involvement in Cancer, Genes (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi T, and Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival, Cell 131, 596–610. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T (2015) Sigma-1 receptor: the novel intracellular target of neuropsychotherapeutic drugs, J Pharmacol Sci 127, 2–5. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen L, Lucke-Wold BP, Mookerjee SA, Cavendish JZ, Robson MJ, Scandinaro AL, and Matsumoto RR (2015) Role of sigma-1 receptors in neurodegenerative diseases, J Pharmacol Sci 127, 17–29. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen L, Lucke-Wold BP, Mookerjee S, Kaushal N, and Matsumoto RR (2017) Sigma-1 Receptors and Neurodegenerative Diseases: Towards a Hypothesis of Sigma-1 Receptors as Amplifiers of Neurodegeneration and Neuroprotection, Adv Exp Med Biol 964, 133–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryskamp DA, Korban S, Zhemkov V, Kraskovskaya N, and Bezprozvanny I (2019) Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases, Front Neurosci 13, 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyrskyluoto A, Pulli I, Tornqvist K, Ho TH, Korhonen L, and Lindholm D (2013) Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-kappaB pathway, Cell Death Dis 4, e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Yebenes JG, Landwehrmeyer B, Squitieri F, Reilmann R, Rosser A, Barker RA, Saft C, Magnet MK, Sword A, Rembratt A, Tedroff J, and Mermai H. D. s. i. (2011) Pridopidine for the treatment of motor function in patients with Huntington’s disease (MermaiHD): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Neurol 10, 1049–1057. [DOI] [PubMed] [Google Scholar]
- 16.Ryskamp D, Wu J, Geva M, Kusko R, Grossman I, Hayden M, and Bezprozvanny I (2017) The sigma-1 receptor mediates the beneficial effects of pridopidine in a mouse model of Huntington disease, Neurobiol Dis 97, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squitieri F, Di Pardo A, Favellato M, Amico E, Maglione V, and Frati L (2015) Pridopidine, a dopamine stabilizer, improves motor performance and shows neuroprotective effects in Huntington disease R6/2 mouse model, J Cell Mol Med 19, 2540–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geva M, Kusko R, Soares H, Fowler KD, Birnberg T, Barash S, Wagner AM, Fine T, Lysaght A, Weiner B, Cha Y, Kolitz S, Towfic F, Orbach A, Laufer R, Zeskind B, Grossman I, and Hayden MR (2016) Pridopidine activates neuroprotective pathways impaired in Huntington Disease, Hum Mol Genet 25, 3975–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahlholm K, Valle-Leon M, Taura J, Fernandez-Duenas V, and Ciruela F (2018) Effects of the Dopamine Stabilizer, Pridopidine, on Basal and Phencyclidine-Induced Locomotion: Role of Dopamine D2 and Sigma-1 Receptors, CNS Neurol Disord Drug Targets 17, 522–527. [DOI] [PubMed] [Google Scholar]
- 20.Eddings CR, Arbez N, Akimov S, Geva M, Hayden MR, and Ross CA (2019) Pridopidine protects neurons from mutant-huntingtin toxicity via the sigma-1 receptor, Neurobiol Dis 129, 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mach RH, Zeng C, and Hawkins WG (2013) The sigma2 receptor: a novel protein for the imaging and treatment of cancer, J Med Chem 56, 7137–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YS, Lu HL, Zhang LJ, and Wu Z (2014) Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy, Med Res Rev 34, 532–566. [DOI] [PubMed] [Google Scholar]
- 23.Qiu G, Sun W, Zou Y, Cai Z, Wang P, Lin X, Huang J, Jiang L, Ding X, and Hu G (2015) RNA interference against TMEM97 inhibits cell proliferation, migration, and invasion in glioma cells, Tumour Biol 36, 8231–8238. [DOI] [PubMed] [Google Scholar]
- 24.Zeng C, and Mach RH (2017) The Evolution of the Sigma-2 (sigma2) Receptor from Obscure Binding Site to Bona Fide Therapeutic Target, Adv Exp Med Biol 964, 49–61. [DOI] [PubMed] [Google Scholar]
- 25.Izzo NJ, Staniszewski A, To L, Fa M, Teich AF, Saeed F, Wostein H, Walko T 3rd, Vaswani A, Wardius M, Syed Z, Ravenscroft J, Mozzoni K, Silky C, Rehak C, Yurko R, Finn P, Look G, Rishton G, Safferstein H, Miller M, Johanson C, Stopa E, Windisch M, Hutter-Paier B, Shamloo M, Arancio O, LeVine H 3rd, and Catalano SM (2014) Alzheimer’s therapeutics targeting amyloid beta 1–42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits, PLoS One 9, e111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L, and Zhen X (2015) Sigma-2 receptor ligands: neurobiological effects, Curr Med Chem 22, 989–1003. [DOI] [PubMed] [Google Scholar]
- 27.Vilner BJ, and Bowen WD (2000) Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells, J Pharmacol Exp Ther 292, 900–911. [PubMed] [Google Scholar]
- 28.Cassano G, Gasparre G, Niso M, Contino M, Scalera V, and Colabufo NA (2009) F281, synthetic agonist of the sigma-2 receptor, induces Ca2+ efflux from the endoplasmic reticulum and mitochondria in SK-N-SH cells, Cell Calcium 45, 340–345. [DOI] [PubMed] [Google Scholar]
- 29.Alon A, Schmidt HR, Wood MD, Sahn JJ, Martin SF, and Kruse AC (2017) Identification of the gene that codes for the sigma2 receptor, Proc Natl Acad Sci U S A 114, 7160–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartz F, Kern L, Erz D, Zhu M, Gilbert D, Meinhof T, Wirkner U, Erfle H, Muckenthaler M, Pepperkok R, and Runz H (2009) Identification of cholesterol-regulating genes by targeted RNAi screening, Cell Metab 10, 63–75. [DOI] [PubMed] [Google Scholar]
- 31.Riad A, Zeng C, Weng CC, Winters H, Xu K, Makvandi M, Metz T, Carlin S, and Mach RH (2018) Sigma-2 Receptor/TMEM97 and PGRMC-1 Increase the Rate of Internalization of LDL by LDL Receptor through the Formation of a Ternary Complex, Sci Rep 8, 16845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng YS, Zhang T, Ma X, Pratuangtham S, Zhang GC, Ondrus AA, Mafi A, Lomenick B, Jones JJ, and Ondrus AE (2021) A proteome-wide map of 20(S)-hydroxycholesterol interactors in cell membranes, Nat Chem Biol 17, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahimi-Fakhari D, Wahlster L, Bartz F, Werenbeck-Ueding J, Praggastis M, Zhang J, Joggerst-Thomalla B, Theiss S, Grimm D, Ory DS, and Runz H (2016) Reduction of TMEM97 increases NPC1 protein levels and restores cholesterol trafficking in Niemann-pick type C1 disease cells, Hum Mol Genet 25, 3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi B, Sahn JJ, Ardestani PM, Evans AK, Scott LL, Chan JZ, Iyer S, Crisp A, Zuniga G, Pierce JT, Martin SF, and Shamloo M (2017) Small molecule modulator of sigma 2 receptor is neuroprotective and reduces cognitive deficits and neuroinflammation in experimental models of Alzheimer’s disease, J Neurochem 140, 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott LL, Sahn JJ, Ferragud A, Yen RC, Satarasinghe PN, Wood MD, Hodges TR, Shi T, Prakash BA, Friese KM, Shen A, Sabino V, Pierce JT, and Martin SF (2018) Small molecule modulators of sigma2R/Tmem97 reduce alcohol withdrawal-induced behaviors, Neuropsychopharmacology 43, 1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riad A, Lengyel-Zhand Z, Zeng C, Weng CC, Lee VM, Trojanowski JQ, and Mach RH (2020) The Sigma-2 Receptor/TMEM97, PGRMC1, and LDL Receptor Complex Are Responsible for the Cellular Uptake of Abeta42 and Its Protein Aggregates, Mol Neurobiol 57, 3803–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu CC, Yu CF, Wang SC, Li HY, Lin CM, Wang HH, Abate C, and Chiang CS (2019) Sigma-2 receptor/TMEM97 agonist PB221 as an alternative drug for brain tumor, BMC Cancer 19, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang K, Zeng C, Wang C, Sun M, Yin D, and Sun T (2020) Sigma-2 Receptor-A Potential Target for Cancer/Alzheimer’s Disease Treatment via Its Regulation of Cholesterol Homeostasis, Molecules 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahn JJ, Mejia GL, Ray PR, Martin SF, and Price TJ (2017) Sigma 2 Receptor/Tmem97 Agonists Produce Long Lasting Antineuropathic Pain Effects in Mice, ACS Chem Neurosci 8, 1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Rosa E, Watson MR, Sahn JJ, Hodges TR, Schroeder RE, Cintron-Perez CJ, Shin MK, Yin TC, Emery JL, Martin SF, Liebl DJ, and Pieper AA (2019) Neuroprotective Efficacy of a Sigma 2 Receptor/TMEM97 Modulator (DKR-1677) after Traumatic Brain Injury, ACS Chem Neurosci 10, 1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quadir SG, Tanino SM, Rohl CD, Sahn JJ, Yao EJ, Cruz LDR, Cottone P, Martin SF, and Sabino V (2021) The Sigma-2 receptor / transmembrane protein 97 (sigma2R/TMEM97) modulator JVW-1034 reduces heavy alcohol drinking and associated pain states in male mice, Neuropharmacology 184, 108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mondal S, Hegarty E, Sahn JJ, Scott LL, Gokce SK, Martin C, Ghorashian N, Satarasinghe PN, Iyer S, Sae-Lee W, Hodges TR, Pierce JT, Martin SF, and Ben-Yakar A (2018) High-Content Microfluidic Screening Platform Used To Identify sigma2R/Tmem97 Binding Ligands that Reduce Age-Dependent Neurodegeneration in C. elegans SC_APP Model, ACS Chem Neurosci 9, 1014–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izzo NJ, Xu J, Zeng C, Kirk MJ, Mozzoni K, Silky C, Rehak C, Yurko R, Look G, Rishton G, Safferstein H, Cruchaga C, Goate A, Cahill MA, Arancio O, Mach RH, Craven R, Head E, LeVine H 3rd, Spires-Jones TL, and Catalano SM (2014) Alzheimer’s therapeutics targeting amyloid beta 1–42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity, PLoS One 9, e111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundman M, Morgan R, Lickliter JD, Schneider LS, DeKosky S, Izzo NJ, Guttendorf R, Higgin M, Pribyl J, Mozzoni K, Safferstein H, and Catalano SM (2019) A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease, Alzheimers Dement (N Y) 5, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahn JJ, Hodges TR, Chan JZ, and Martin SF (2016) Norbenzomorphan Framework as a Novel Scaffold for Generating Sigma 2 Receptor/PGRMC1 Subtype-Selective Ligands, ChemMedChem 11, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahn JJ, and Martin SF (2012) Expedient synthesis of norbenzomorphan library via multicomponent assembly process coupled with ring-closing reactions, ACS Comb Sci 14, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sunderhaus JD, Dockendorff C, and Martin SF (2009) Synthesis of Diverse Heterocyclic Scaffolds via Tandem Additions to Imine Derivatives and Ring-Forming Reactions, Tetrahedron 65, 6454–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahn JJ, Hodges TR, Chan JZ, and Martin SF (2017) Norbenzomorphan Scaffold: Chemical Tool for Modulating Sigma Receptor-Subtype Selectivity, ACS Med Chem Lett 8, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linkens K, Schmidt HR, Sahn JJ, Kruse AC, and Martin SF (2018) Investigating isoindoline, tetrahydroisoquinoline, and tetrahydrobenzazepine scaffolds for their sigma receptor binding properties, Eur J Med Chem 151, 557–567. [DOI] [PubMed] [Google Scholar]
- 50.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, and Hopkins AL (2012) Automated design of ligands to polypharmacological profiles, Nature 492, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arbez N, Ratovitski T, Roby E, Chighladze E, Stewart JC, Ren M, Wang X, Lavery DJ, and Ross CA (2017) Post-translational modifications clustering within proteolytic domains decrease mutant huntingtin toxicity, J Biol Chem 292, 19238–19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hegde RN, Chiki A, Petricca L, Martufi P, Arbez N, Mouchiroud L, Auwerx J, Landles C, Bates GP, Singh-Bains MK, Dragunow M, Curtis MA, Faull RL, Ross CA, Caricasole A, and Lashuel HA (2020) TBK1 phosphorylates mutant Huntingtin and suppresses its aggregation and toxicity in Huntington’s disease models, EMBO J 39, e104671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogawa S, Okuyama S, Araki H, and Otomo S (1994) Effect of NE-100, a novel sigma receptor ligand, on phencyclidine-induced cognitive dysfunction, Eur J Pharmacol 263, 9–15. [DOI] [PubMed] [Google Scholar]
- 54.Narita M, Yoshizawa K, Aoki K, Takagi M, Miyatake M, and Suzuki T (2001) A putative sigma1 receptor antagonist NE-100 attenuates the discriminative stimulus effects of ketamine in rats, Addict Biol 6, 373–376. [DOI] [PubMed] [Google Scholar]
- 55.Ross CA (2004) Huntington’s disease: new paths to pathogenesis, Cell 118, 4–7. [DOI] [PubMed] [Google Scholar]
- 56.Ross CA, and Tabrizi SJ (2011) Huntington’s disease: from molecular pathogenesis to clinical treatment, Lancet Neurol 10, 83–98. [DOI] [PubMed] [Google Scholar]
- 57.Saudou F, and Humbert S (2016) The Biology of Huntingtin, Neuron 89, 910–926. [DOI] [PubMed] [Google Scholar]
- 58.Barker RA, Fujimaki M, Rogers P, and Rubinsztein DC (2020) Huntingtin-lowering strategies for Huntington’s disease, Expert Opin Investig Drugs 29, 1125–1132. [DOI] [PubMed] [Google Scholar]
- 59.Marxreiter F, Stemick J, and Kohl Z (2020) Huntingtin Lowering Strategies, Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.