Abstract
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is the causative agent of COVID-19 which was detected in late 2019 in Wuhan, China. As of September 2022, there have been over 612 million confirmed cases of COVID-19 with over 6.5 million associated deaths. In many cases, anosmia and dysgeusia have been identified as primary symptoms of COVID-19 infection in patients. While the loss of smell (anosmia) and loss of taste (dysgeusia) due to COVID-19 infection is transient in most patients, many report that these symptoms persist following recovery. Understanding the pathogenesis of these symptoms is paramount to early treatment of the infection. We conducted a literature review of Google Scholar and PubMed to find and analyze studies discussing anosmia and dysgeusia in the context of COVID-19 to understand the progression and management of these symptoms. The mechanism for dysgeusia is largely unknown; however, pathogenesis of anosmia includes inflammation and cytokine release resulting from the infection that alters neuronal signaling, thus inducing the loss of smell that patients experience. Anosmia may be managed and potentially resolved sooner with a combination therapy of olfactory training and budesonide irrigation of the nasal cavity. It is important to note that the variants of SARS-CoV-2 are genetically distinguished from the original virion due to a mutation in their spike proteins, giving them a different symptom profile regarding anosmia and dysgeusia. This variability in symptomatology is an area of study that needs to be further explored.
Keywords: COVID19, Anosmia, Dysgeusia
Introduction
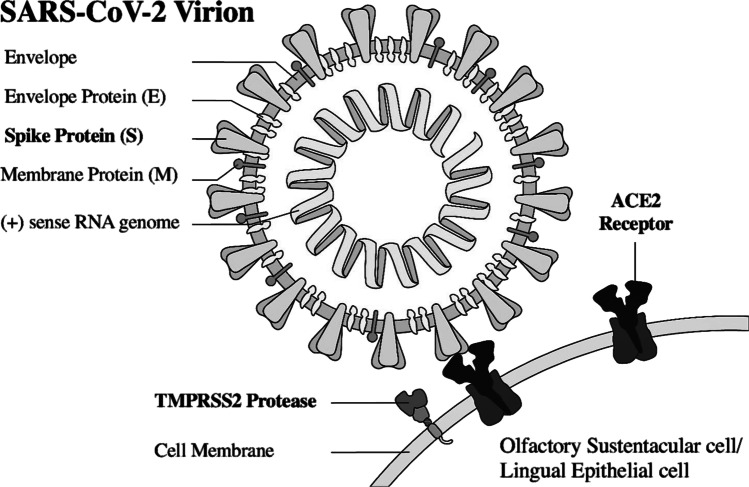
COVID-19, caused by the coronavirus known as SARS-CoV-2, was detected in late 2019 in Wuhan, China [1]. It has since rapidly spread across the country and soon to all other continents, reaching pandemic classification [2]. (p19) 80% similar to SARS-CoV-1 genetically, SARS-CoV-2 is an enveloped virus consisting of many surface glycoproteins, including spike, envelope and membrane proteins [3]. The spike protein mediates binding and entry to the host cell via the host cell’s angiotensin-converting-enzyme-2 (ACE 2) receptor’s peptidase domain [4]. Transmembrane serine protease 2 (TMPRSS2), a target cell protease, also aids in viral entry into host cell as it cleaves part of the spike protein and facilitates a stronger connection between the spike protein and ACE2 receptor [5]. Thus, relative to other coronaviruses, SARS-CoV-2 has structural differences that enable a greater affinity for ACE2 receptors leading to stronger bonding to upper respiratory tract tissue [3].
As of September 2022, there have been over 612 million confirmed cases of COVID-19 globally, with over 6.5 million associated deaths [6]. Typically presenting with flu-like symptoms, COVID-19 poses varying degrees of health hazard and is highly dependent on patients’ comorbidities and risk profiles [7]. Patients can range from asymptomatic to having mild-moderate with potential need for hospitalization to potentially fatal acute respiratory distress syndrome (ARDS), for which many require constant mechanical ventilation [8]. Aside from the common and notable respiratory manifestations in symptomatic patients, COVID-19 has also been found to commonly engender neurologic symptoms [9]. Some transient cardinal neurologic symptoms noted for varying severities of COVID-19 manifestations are anosmia, absent or diminished sense of smell, and dysgeusia, absent or diminished sense of taste [10–12]. In many cases, anosmia and dysgeusia have been identified as primary symptoms of COVID-19 infection in patients. [13]
Sense of taste and smell are typically regained within days to weeks. This sudden onset and recovery period is the distinguishing factor between the COVID-19 presentation of these symptoms when compared to similar viral illnesses [8]. The prevalence of these symptoms, while universally common, has shown great regional variation. While East Asian countries had about a 22.4% prevalence of anosmia in COVID-19 patients, Western countries showed a higher prevalence of 48.4%. A similar discrepancy was found in dysgeusia, as East Asian countries had a 16.2% prevalence in patients with COVID-19 and Western countries held a much higher prevalence of 50.3%. Patients with a mix of both smell and taste deficits showed a 54.7% prevalence in Western and 23.4% prevalence in East Asian COVID-19 patients [14]. These figures, however, are regarded to be underestimated as they depend on patients’ reports of their subjective impressions of sensory deficit. Thus, exact prevalence statistics of these symptoms are difficult to determine.
Methods
A literature review of Google Scholar and PubMed was conducted between March 2020 and September 2022 to find studies related to anosmia and dysgeusia in the setting of COVID-19 infection. Search terms included several combinations of the following keywords: anosmia, dysgeusia, COVID-19, omicron, and delta. Studies were then screened by two separate readers to ensure that they met inclusion criteria. Included studies must have discussed either anosmia or dysgeusia in the setting of COVID-19 in either human or animal models. Studies that specifically discussed mechanisms of pathogenesis of anosmia or dysgeusia in COVID-19 were included. Furthermore, studies that focused on anosmia or dysgeusia in different variants of COVID-19 were also included.
Pathogenesis of anosmia and dysgeusia
Several different pathophysiologic processes can result in anosmia, including head trauma, aging, autoimmunity, and toxic exposures [15]. However, in the setting of infection, loss of smell may arise either through conductive or sensorineural anosmia [16]. Conductive anosmia results from mechanical obstruction and can accompany congestion and rhinitis [5]. Sensorineural anosmia, on the other hand, occurs due to injury to sensory neurons of the olfactory bulbs [17]. Currently, the exact pathogenesis and molecular mechanisms of anosmia in COVID-19 is unknown; however, because the loss of smell often long survives the duration of respiratory symptoms, a sensorineural mechanism is more likely to explain the etiology of a COVID-19-associated anosmia. The pathophysiology of COVID-19-associated dysgeusia is also currently unclear, though many hypotheses exist. In general, the most common etiologies of dysgeusia include head trauma, infections of the upper respiratory tract, exposure to toxic substances, iatrogenic causes, medicines and glossodynia [18]. However, in the setting of a viral infection, the most likely cause of transient dysgeusia is postulated to be associated with the peripheral neurotropism of SARS-CoV-2 or direct toxicity to taste buds or olfactory epithelium [19]. This explains why dysgeusia often presents in conjunction with anosmia in patients with COVID-19.
Recalling the need for ACE2 receptors and TMPRSS2 proteases on the host cell to aid in SARS-CoV-2’s binding and entry via the spike protein, these proteins are not expressed by olfactory sensory neurons nor by the olfactory bulbs. Rather, ACE2 receptors are expressed at very high levels by olfactory mucosal supporting cells, such as microvillar and sustentacular cells [20]. Damage and infection of these supporting cells and resultant inflammation may induce anosmia. SARS-CoV-2 may also infect the ACE 2-expressing vascular pericytes, causing inflammation and hypoperfusion. In addition, it is important to note that in the nasal mucosal microenvironment, ACE2 functions as part of inflammatory response mechanisms and is involved in a variety of respiratory inflammatory conditions [21]. Thus, another hypothesis for the pathogenesis of anosmia includes inflammation and cytokine release resulting from the infection that alters neuronal signaling to cause the anosmia patients experience [22]. This was described in a study by Netland et al., which studied SARS-CoV infection in transgenic mice expressing humane ACE2, as there was a noted upregulation of cytokines, especially IL-6, and chemokines in the brains of infected mice as part of a proinflammatory response [23].
While less is currently understood about the pathogenesis of dysgeusia, ACE2 is significantly expressed in tongue epithelial cells, buccal mucosa, and the oral cavity, as was found in single cell RNA-sequencing studies [14]. The tongue epithelium may thus be a point of infection for SARS-CoV-2 with the buccal mucosa serving as an entry point, though little data are currently available regarding this theory. ACE2 has also been found to regulate taste perception and this effect has been exemplified in chemosensitive side effects of ACE2 inhibitors (ACEI), with dysgeusia being a common adverse effect associated with ACEI [24]. Furthermore, salivary glands have been identified as potential reservoirs for SARS-CoV-2, with localized infection of salivary glands being a potential hypothesis to explain asymptomatic infections [25]. It is likely, still, that either direct or indirect damage of tongue taste receptors through epithelial cell infection and local inflammation plays a role in the pathogenesis of COVID-19 patient dysgeusia [22].
Potential pathogenesis of anosmia and dysgeusia in COVID-19 infections
Symptom profile in COVID-19 variants
Throughout the progression of the pandemic, the SARS-CoV-2 has evolved genetically and thus symptomatically with the development of new variants, including B.1.1.7 (Alpha), B.1.351 (Beta), P1 (Gamma), B.1.617.2 (Delta) [26]. These variants are distinguished from the original SARS-CoV-2 G14 virion and termed G614 viruses, as they harbor the D614G mutation of the spike protein, which has increased their virulence by enhancing viral entry into the host cell [27]. Furthermore, von Bartheld et al. has identified variants with the D614G mutation to be contributors to the prevalence of anosmia and dysgeusia symptoms in COVID-19, as compared to the original D614 virus, G614 variants are significantly more likely to cause symptoms of anosmia and dysgeusia (31.8% vs 5.3% prevalence).
The Omicron variant (B.1.1.529), first detected in December of 2021, carries upward of 50 mutations on the spike protein alone [28]. Since then, this variant has rapidly replaced Delta as the dominant variant of concern globally, further producing descendent lineages that include BA.1, BA.2, BA.3, BA.4, and BA.5 [29, 30]. The symptomatology of this variant also differed drastically from the previous Delta variant, with the three cardinal symptoms of fever, cough, and loss of smell and taste only being reported in 61.5% of Omicron cases compared to 72.2% of Delta cases [31]. Furthermore, when analyzing the percent of cases reporting anosmia and dysgeusia, these symptoms were found to be less common among those with Omicron (13.4%) infections compared to Delta (33.7%) infections (p < 0.001). [31]
The decreased olfactory dysfunction seen in Omicron may be explained by several hypotheses. Butowt et al. proposes that spike protein mutations in Omicron have changed tissue tropism, sparing olfactory and gustatory function [32]. Specifically, Omicron virions prefer an endosomal route of entry as opposed to the TMPRSS2-mediated membrane fusion seen in prior variants. Thus, viral entry into olfactory sustentacular cells that robustly express TMPRESS2 is diminished and potentially explains the decreased frequency of anosmia seen in the new variant [32]. Another proposed mechanism by Rodriguez-Sevilla et al. alludes to the significance of previous immunity among those infected by Omicron, postulating that re-infection in previously exposed populations would lead to a diminished systemic and local inflammatory response, causing less cellular damage in the olfactory epithelium. [33]
Management of anosmia and dysgeusia
According to a 2021 systematic literature review, COVID-19 patients commonly reported onset of dysgeusia and anosmia 4–5 days following the initial onset of symptoms. Whether other symptoms are present, abrupt onset of dysgeusia and anosmia may indicate early signs of possible SARS-CoV-2 infection. The same review found the smell and taste deficits to begin to resolve 1 week following their onset and show significant improvement within 2 weeks. However, these symptoms tended to persist for at least 1 week following the recovery from the COVID-19 infection itself [34]. Furthermore, in a phenomenon known as “Long COVID”, referring to a variety of symptoms affecting different organs reported by people following recovery from an SARS-CoV-2 infection, anosmia and dysgeusia appear to be two of the predominant symptoms that persist [35]. Persistent anosmia after COVID-19 is often associated with extensive damage of olfactory epithelium due to a cascade of effects that result in persistent immune activation [36].
Anosmia may be managed and potentially resolved sooner with a combination therapy of olfactory training and budesonide irrigation of the nasal cavity. This combination therapy helped 43.9% of patients with anosmia improve olfactory ability [34]. Dysgeusia management may be more difficult to achieve due to lack of evidence and literature of its etiology in COVID-19 patients. Despite not identifying an underlying cause, 0.5 to 1 mg of oral clonazepam once a day is encouraged to treat unspecified dysgeusia [37]. Gustatory dysfunction has also been treated with l-carnitine and vitamins. Other treatment modalities for anosmia include saline irrigation, nasal corticosteroids, non-corticoid decongestants, and vitamins. Given that these neurologic symptoms recover spontaneously, supportive and symptomatic treatment for mild sensory deficits may also be appropriate [38].
Conclusion
Anosmia and Dysgeusia symptoms have been highly characteristic of COVID-19 infections thus far in the pandemic. However, the pathogenesis of these symptoms is still unknown. Furthermore, with the development of new variants, the prevalence of these symptoms is subject to change, along with their molecular mechanisms. Further research is required to elucidate the pathogenesis and discrepancies in symptom profiles across variants.
Funding
None.
Declarations
Conflict of interest
The authors do not declare any conflict of interest regarding the publication of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu YC, Kuo RL, Shih SR. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020 doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 4.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Las Casas Lima MH, Cavalcante ALB, Leão SC. Pathophysiological relationship between COVID-19 and olfactory dysfunction: a systematic review. Braz J Otorhinolaryngol. 2021 doi: 10.1016/j.bjorl.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 26 Sep 2022
- 7.Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paludan SR, Mogensen TH. Innate immunological pathways in COVID-19 pathogenesis. Sci Immunol. 2022;7(67):eabm5505. doi: 10.1126/sciimmunol.abm5505. [DOI] [PubMed] [Google Scholar]
- 9.Amanat M, Rezaei N, Roozbeh M, et al. Neurological manifestations as the predictors of severity and mortality in hospitalized individuals with COVID-19: a multicenter prospective clinical study. BMC Neurol. 2021;21(1):116. doi: 10.1186/s12883-021-02152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boesveldt S, Postma EM, Boak D, et al. Anosmia—a clinical review. Chem Senses. 2017;42(7):513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787–1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maheswaran T, Abikshyeet P, Sitra G, Gokulanathan S, Vaithiyanadane V, Jeelani S. Gustatory dysfunction. J Pharm Bioallied Sci. 2014;6(Suppl 1):S30–33. doi: 10.4103/0975-7406.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haldrup M, Johansen MI, Fjaeldstad AW. Anosmia and ageusia as primary symptoms of COVID-19. Ugeskr Laeger. 2020;182(18):V04200205. [PubMed] [Google Scholar]
- 14.Butowt R, von Bartheld CS. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2021;27(6):582–603. doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaines AD. Anosmia and hyposmia. Allergy Asthma Proc. 2010;31(3):185–189. doi: 10.2500/aap.2010.31.3357. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves S, Goldstein BJ. Pathophysiology of olfactory disorders and potential treatment strategies. Curr Otorhinolaryngol Rep. 2016;4(2):115–121. doi: 10.1007/s40136-016-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blioskas S. Anosmia: sensorineural. In: Stavrakas M, Khalil HS, editors. Rhinology and anterior skull base surgery. New York: Springer International Publishing; 2021. pp. 271–274. [Google Scholar]
- 18.Hummel T, Landis BN, Hüttenbrink KB. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011;10:Doc04. doi: 10.3205/cto000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoud MM, Abuohashish HM, Khairy DA, Bugshan AS, Khan AM, Moothedath MM. Pathogenesis of dysgeusia in COVID-19 patients: a scoping review. Eur Rev Med Pharmacol Sci. 2021;25(2):1114–1134. doi: 10.26355/eurrev_202101_24683. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Shen W, Rowan NR, et al. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020;56(3):2001948. doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkubo K, Lee CH, Baraniuk JN, Merida M, Hausfeld JN, Kaliner MA. Angiotensin-converting enzyme in the human nasal mucosa. Am J Respir Cell Mol Biol. 1994;11(2):173–180. doi: 10.1165/ajrcmb.11.2.8049077. [DOI] [PubMed] [Google Scholar]
- 22.Mastrangelo A, Bonato M, Cinque P. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett. 2021;748:135694. doi: 10.1016/j.neulet.2021.135694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020;10(9):1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariz BALA, Brandão TB, Ribeiro ACP, Lopes MA, Santos-Silva AR. New Insights for the Pathogenesis of COVID-19-Related Dysgeusia. J Dent Res. 2020;99(10):1206–1206. doi: 10.1177/0022034520936638. [DOI] [PubMed] [Google Scholar]
- 26.Raman R, Patel KJ, Ranjan K. COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules. 2021;11(7):993. doi: 10.3390/biom11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Bartheld CS, Hagen MM, Butowt R. The D614G virus mutation enhances anosmia in COVID-19 patients: evidence from a systematic review and meta-analysis of studies from South Asia. ACS Chem Neurosci. 2021;12(19):3535–3549. doi: 10.1021/acschemneuro.1c00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poudel S, Ishak A, Perez-Fernandez J, et al. Highly mutated SARS-CoV-2 Omicron variant sparks significant concern among global experts—what is known so far? Travel Med Infect Dis. 2022;45:102234. doi: 10.1016/j.tmaid.2021.102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Wei GW (2022) Omicron BA.2 (B.1.1.529.2): high potential to becoming the next dominating variant. ArXiv. arXiv:2202.05031v1.
- 30.Tracking SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants. Accessed 27 Sep 2022
- 31.Ekroth AKE, Patrzylas P, Turner C, Hughes GJ, Anderson C. Comparative symptomatology of infection with SARS-CoV-2 variants Omicron (B.1.1.529) and Delta (B.1.617.2) from routine contact tracing data in England. Epidemiol Infect. 2022;150:e162. doi: 10.1017/S0950268822001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butowt R, Bilińska K, von Bartheld C. Why does the omicron variant largely spare olfactory function? Implications for the pathogenesis of anosmia in coronavirus disease 2019. J Infect Dis. 2022 doi: 10.1093/infdis/jiac113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Sevilla JJ, Güerri-Fernádez R, Bertran RB. Is there less alteration of smell sensation in patients with omicron SARS-CoV-2 variant infection? Front Med. 2022;9:852998. doi: 10.3389/fmed.2022.852998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos REA, da Silva MG, do Monte Silva MCB, et al (2021) Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: a systematic review. Am J Otolaryngol 42(2):102889. 10.1016/j.amjoto.2020.102889 [DOI] [PMC free article] [PubMed]
- 35.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. Kretzschmar MEE, ed. PLOS Med. 2021;18(9):e1003773. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallée A. Dysautonomia and implications for anosmia in long COVID-19 disease. J Clin Med. 2021;10(23):5514. doi: 10.3390/jcm10235514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman HJ, Rawal S, Li CM, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016;17(2):221–240. doi: 10.1007/s11154-016-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]