Abstract
Background and aims:
Despite mechanistic data implicating unresolving inflammation in stroke pathogenesis, data regarding circulating immune cell phenotypes – key determinants of inflammation propagation versus resolution - and incident stroke are lacking. Therefore, we aimed to comprehensively define associations of circulating immune phenotypes and activation profiles with incident stroke.
Methods:
We investigated circulating leukocyte phenotypes and activation profiles with incident adjudicated stroke in 2104 diverse adults from the Multi-Ethnic Study of Atherosclerosis (MESA) followed over a median of 16.6 years. Cryopreserved cells from the MESA baseline examination were thawed and myeloid and lymphoid lineage cell subsets were measured using polychromatic flow cytometry and intracellular cytokine activation staining. We analyzed multivariable-adjusted associations of cell phenotypes, as a proportion of parent cell subsets, with incident stroke (overall) and ischemic stroke using Cox regression models.
Results:
We observed associations of intermediate monocytes, early-activated CD4+ T cells, and both CD4+ and CD8+ T cells producing interleukin-4 after cytokine stimulation (Th2 and Tc2, respectively) with higher risk for incident stroke; effect sizes ranged from 35% to 62% relative increases in risk for stroke. Meanwhile, differentiated and memory T cell phenotypes were associated with lower risk for incident stroke. In sex-stratified analyses, positive and negative associations were especially strong among men but null among women.
Conclusions:
Circulating IL-4 producing T cells and intermediate monocytes were significantly associated with incident stroke over nearly two decades of follow-up. These associations were stronger among men and not among women. Further translational studies are warranted to define more precise targets for prognosis and intervention.
Keywords: Inflammation, Immune cells, Stroke, Epidemiology, Biomarkers
Graphical Abstract
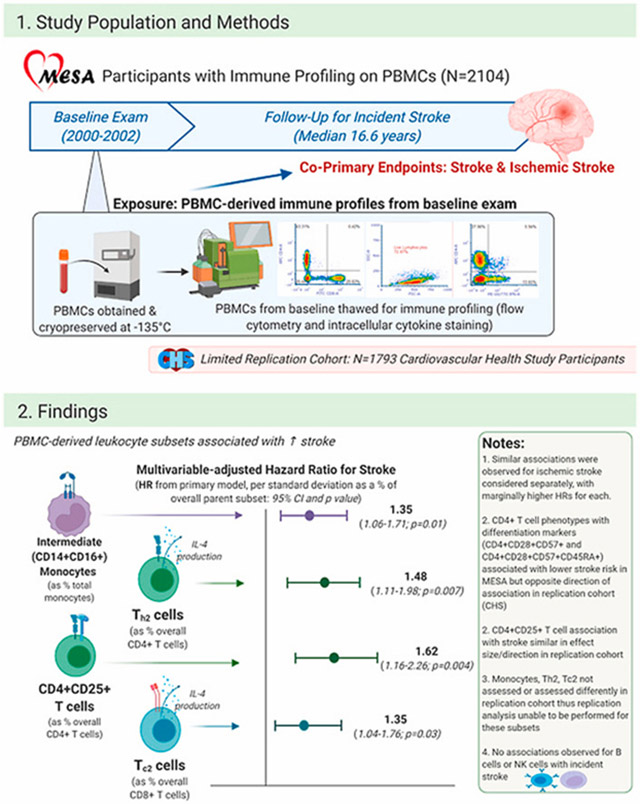
1. Introduction
Observational studies and experimental models suggest an important role of immune response and regulation in ischemic stroke pathogenesis. In cohort studies, circulating markers of inflammation and thrombosis such as C-reactive protein, interleukin-6, and fibrinogen are associated with incident stroke [1-7]. Complementing these are (1) observational findings that inflammatory leukocytes from excised plaque in carotid endarterectomy patients are strongly associated with subsequent ischemic stroke [8] and (2) experimental data that implicate activated leukocytes in athero-thrombotic events by mechanisms including endothelial apoptosis and plaque erosion/rupture [9-11]. In studies of peri- and post-stroke prognosis, experimental data demonstrate a clear role of innate-adaptive immune interplay in determining extent of injury following cerebral ischemia/ischemia-reperfusion [12]. These findings, taken together, highlight the likely importance of pro-inflammatory immune responses in stroke pathophysiology. However, few studies have prospectively investigated the relationship between specific peripheral blood leukocyte subpopulations and incident stroke. The two largest studies to our knowledge investigated associations of B cell subsets and CD4+ T cell subsets with stroke in a Swedish cohort of individuals aged 68–73 years (N = 700) [13,14], and observed varied significant associations of certain B cell subsets, but not T regulatory cells (Tregs), with stroke. However, outside of this ethnically homogenous Swedish population, sparse data exist regarding prospective associations of circulating leukocyte subsets with incident stroke. Additional unresolved questions include (1) associations of circulating monocyte subsets – which we have observed as associated with carotid intima-media thickness progression [15] – and CD8+ T cell subsets with incident stroke, and (2) associations of circulating leukocyte subsets with ischemic stroke as well as all stroke (combining ischemic and hemorrhagic as previously done [13,14]).
2. Patients and methods
In this study, we analyzed prospective associations between 28 pre-specified leukocyte subsets and incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis (MESA), a multi-center prospective cohort study based at 6 sites in the United States [16]. Of 6814 participants in MESA, 6793 were free from stroke at baseline (Exam 1; 2000–2002); 2193 of these participants had polychromatic flow cytometry and intracellular cytokine staining performed on cryopreserved peripheral blood mononuclear cells (PBMCs; see Online Methods for details) [15,17].
2.1. Immune profiling
Our methods for immune profiling of cryopreserved PBMCs have been described previously [15,17], including gating strategies; details regarding sample processing and storage are included in the Supplementary Methods. Briefly, cryopreserved (−145 °C) cells from the MESA baseline examination were thawed and myeloid and lymphoid lineage cell subsets were measured using polychromatic flow cytometry and intracellular cytokine activation staining. Supplementary Table 1 displays specific subsets measured, their corresponding cellular markers, and frequencies within parent cell subsets, and Supplementary Fig. 1 displays correlations of cell subsets with one another.
2.2. Statistical analyses
We analyzed associations of each of the 28 leukocyte subsets measured with incident stroke, both all stroke and ischemic stroke separately as co-primary endpoints. Due to multiple comparisons, we defined significant as associations with a p value of 0.0018 (reflecting a Bonferroni correction of 0.05/28) whereas associations with a p value between 0.0018 and 0.05 were considered borderline significant. Our base model (Model 0) adjusted for age (years), sex, race/ethnicity, and MESA field center. Our primary model (Model 1) adjusted for age (years), sex, race/ethnicity, MESA field center, educational attainment, smoking status, alcohol use, systolic blood pressure, body-mass index, and diabetes mellitus. Given our prior findings of significant associations of circulating monocyte subsets with carotid intima-media thickness progression in men but not women [15], we also performed pre-specified sex-stratified analyses using Model 1 covariates for adjustment to evaluate associations of baseline immune cell subsets and incident stroke; we focused on stroke (all) only for these sex-stratified analyses due to limited sample size and related power after stratification.
For secondary analyses, we added four models. Model 2 adjusted for Model 1 variables in addition to the analytical batch in which cells were measured and the seasons in which blood draws occurred. Model 3 adjusted for Model 1 variables in addition to batch, season, and baseline low- and high-density lipoprotein cholesterol (LDL, HDL), and Model 4 adjusted for Model 1 variables in addition to batch, season, LDL, HDL, and cytomegalovirus (CMV) viral load given prior findings in MESA that CMV viral load is associated with T cell biasing, which is in turn associated with atherosclerosis [18]. Finally, Model 5 adjusted for baseline antihypertensive and statin medication use in addition to Model 3 variables. The purpose of our approach to these models was to minimize the potential for overadjustment bias [19] in the primary model, while also accounting for variables of interest that could be potential meaningful confounders in not only the primary model but subsequent models used in secondary analyses.
2.3. Sample weighting
The participants’ empirical weights were computed using multiple imputation and a logistic regression model. Specifically, 100 imputations were done using the mice package in R, with predicted probabilities calculated for each imputation, then averaged across the imputations. Covariates in the sampling weights model included age, gender, race, CAC, HDL, cholesterol, triglycerides, BMI, systolic BP, diabetes, statin use, antihypertensive drugs use, stroke, angina, MI and HF.
Several nested Cox regression models using the weighted sample and robust standard errors were used to address the association between stroke or ischemic stroke with individual immune cell subsets. To account for multiple testing, Bonferroni correction of 0.05/18 was used. Analysis was performed in R: (Ref: R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
2.4. Replication analyses in the Cardiovascular Health Study (CHS)
Twenty-five leukocyte subsets were measured in CHS participants with PBMCs cryopreserved from Exam 11 (1998–1999) in the same manner as they were measured in MESA participants in the primary analyses. A total of N = 1793 participants free from stroke at Exam 11 had these 25 leukocyte subsets measured; given the composition of CHS as an older cohort, the age at Exam 11 of CHS participants analyzed (79.6 ± 4.4 years old, vs. 62.2 in MESA) was substantially older. Cell phenotypes were measured at the same central laboratory and using the same methods for the CHS analyses as for MESA, but with the difference that several subsets measured in MESA were not measured in CHS [20]. The leukocyte subsets measured in CHS that were in common with those measured in MESA included the following subsets: natural killer cells (CD3 = CD16 + CD56+), gamma-delta T cells (CD3+ gamma-delta T cell receptor+), pan CD4+, Tregs (CD4+CD25+CD127−), CD4+CD25+, CD4+CD45RA+, CD4+CD28−, CD4+CD57+, CD4+CD28−CD57+, CD4+CD28−CD57+CD45RA+, CD8+, CD8+CD45RA+, CD8+CD28−, CD8+CD57+, CD8+CD28−CD57+, and CD19+ B cells. Of the N = 1793 participants with these leukocyte subsets measured, N = 293 had incident stroke events over a median follow-up of 9.9 years (maximum 17 years) and N = 226 of these were ischemic strokes. Incidence rates were relatively similar in men and women for all stroke (1.56 per 100 person-years for men and 1.86 per 100 person-years for women) and ischemic stroke (1.28 and 1.40, respectively). All available CHS participants were included, so sampling weights were not needed. The central replication analysis was a meta-analysis of the associations of each of these fifteen leukocyte subsets (measured in parallel fashion for MESA and CHS) with incident stroke.
2.5. Stroke adjudication
Incident stroke events were adjudicated and subtyped in MESA and CHS using parallel methods, described previously [21,22], in which independent adjudicators filled out answers to detailed questions related to stroke subtypes based on their review of comprehensive clinical and imaging data. Possible events were determined by at least yearly contacts via phone with cohort participants as well as in person follow-up examinations, with medical records, death certificates, and autopsy reports subsequently reviewed for suspected events. For out-of-hospital events, participants were interviewed and outpatient clinical summaries and testing were reviewed; for out-of-hospital deaths, physicians and family members were interviewed. All records were anonymized and then made available for adjudicators. Adjudicators reviewed all events thought possibly related to transient ischemic attack (TIA) or stroke (or neither) and determined these to be events based on symptoms, signs, and imaging results. Strokes were subtyped into hemorrhagic, ischemic, other, or undetermined. Any disagreements were resolved by consensus between reviewers.
2.6. Data availability
The datasets generated during and/or analyzed during the current study are available from the MESA and CHS coordinating centers on reasonable request.
3. Results
Of 2193 participants in MESA with immune cell profiling performed, 2104 had complete baseline data. These 2104 individuals comprise the sample for this study and empirical sampling weights were constructed to ensure this population (a combination of two case-cohorts, neither of which focused on stroke) was representative of the overall MESA population (Table 2). Follow up for stroke events (ischemic stroke and all stroke), adjudicated in standard fashion [21], occurred from Exam 1 until 12/31/18 or death, with a maximum follow-up of 18.5 years. In our study sample (N = 2104), a total of 103 individuals had incident ischemic strokes and 124 had incident stroke of any type during follow-up. Incidence rates for all stroke were 0.45 per 100 person-years for men and 0.29 per 100 person-years for women; for ischemic stroke, these numbers were 0.39 and 0.22, respectively. A limited replication analysis was performed using data from the Cardiovascular Health Study (CHS), an older population-based cohort in which leukocyte subsets were measured in N = 1793 individuals using similar immune profiling methods in the same laboratory.
Table 2.
Associations of circulating immune cell subsets with incident stroke (all) and ischemic stroke in MESA (N = 2104).
| Stroke (all) |
Ischemic stroke |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Model 0 |
Model 1 (Primary) |
Model 0 |
Model 1 (Primary) |
||||||||
| Cell subset | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value |
| Classical monocytes | 0.84 | (0.70, 1.01) | 0.06 | 0.83 | (0.64, 1.08) | 0.16 | 0.83 | (0.68, 1) | 0.05 | 0.76 | (0.56,1.04) | 0.08 |
| Intermediate monocytes | 1.33 | (1.14, 1.56) | <0.001 | 1.35 | (1.06, 1.71) | 0.01 | 1.31 | (1.10, 1.56) | 0.003 | 1.41 | (0.99, 2.00) | 0.055 |
| Nonclassical monocytes | 0.89 | (0.72, 1.11) | 0.3 | 0.86 | (0.61, 1.21) | 0.38 | 0.95 | (0.76, 1.18) | 0.63 | 0.99 | (0.71, 1.38) | 0.96 |
| Natural killer cells | 1.03 | (0.87, 1.22) | 0.74 | 0.96 | (0.75, 1.23) | 0.76 | 1.03 | (0.86, 1.23) | 0.75 | 0.93 | (0.71, 1.22) | 0.61 |
| Gamma-delta T cells | 0.92 | (0.71, 1.18) | 0.51 | 0.89 | (0.59, 1.35) | 0.60 | 0.95 | (0.74, 1.23) | 0.69 | 0.98 | (0.66, 1.45) | 0.92 |
| Pan CD4+ T cells | 1.06 | (0.82, 1.36) | 0.68 | 1.14 | (0.79, 1.65) | 0.49 | 1.09 | (0.82, 1.44) | 0.56 | 1.15 | (0.73,1.81) | 0.54 |
| Th1 | 1.02 | (0.75, 1.37) | 0.93 | 0.88 | (0.64, 1.20) | 0.41 | 1.06 | (0.78, 1.45) | 0.71 | 0.95 | (0.68, 1.34) | 0.77 |
| Th2 | 1.33 | (1.09, 1.61) | 0.004 | 1.48 | (1.11, 1.98) | 0.007 | 1.35 | (1.09, 1.67) | 0.007 | 1.51 | (1.09, 2.08) | 0.013 |
| Th17 | 0.89 | (0.67, 1.18) | 0.41 | 0.79 | (0.49, 1.27) | 0.33 | 0.95 | (0.74, 1.24) | 0.72 | 0.92 | (0.62, 1.38) | 0.70 |
| Treg (CD4+CD25+ CD127−) | 1.09 | (0.90, 1.32) | 0.41 | 1.20 | (0.90, 1.61) | 0.21 | 1.08 | (0.89, 1.32) | 0.44 | 1.17 | (0.86, 1.57) | 0.32 |
| CD4+CD45RA+ | 0.84 | (0.68, 1.03) | 0.10 | 0.98 | (0.73, 1.31) | 0.88 | 0.82 | (0.64, 1.04) | 0.097 | 0.95 | (0.68, 1.34) | 0.78 |
| CD4+CD45RO+ | 1.04 | (0.85, 1.27) | 0.68 | 0.97 | (0.73, 1.27) | 0.80 | 1.05 | (0.83, 1.33) | 0.68 | 1.00 | (0.71, 1.41) | 0.99 |
| CD4+CD25+ | 1.44 | (1.17, 1.78) | <0.001 | 1.62 | (1.16, 2.26) | 0.004 | 1.52 | (1.23, 1.88) | <0.001 | 1.74 | (1.23, 2.45) | 0.002 |
| CD4+CD28− | 0.81 | (0.64, 1.01) | 0.06 | 0.72 | (0.54, 0.97) | 0.03 | 0.86 | (0.68, 1.10) | 0.24 | 0.77 | (0.55, 1.07) | 0.12 |
| CD4+CD57− | 0.91 | (0.71, 1.16) | 0.42 | 0.93 | (0.69, 1.26) | 0.64 | 0.91 | (0.70, 1.18) | 0.46 | 0.88 | (0.62, 1.26) | 0.49 |
| CD4+CD28−CD57+ | 0.73 | (0.56, 0.95) | 0.02 | 0.61 | (0.43, 0.85) | 0.003 | 0.78 | (0.59, 1.04) | 0.09 | 0.64 | (0.44, 0.93) | 0.02 |
| CD4+CD28−CD57+CD45RA+ | 0.79 | (0.64, 0.97) | 0.03 | 0.77 | (0.59, 0.99) | 0.047 | 0.82 | (0.66, 1.02) | 0.08 | 0.78 | (0.58, 1.05) | 0.1 |
| Pan CD8+ T cells | 0.9 | (0.72, 1.13) | 0.36 | 0.81 | (0.60, 1.08) | 0.15 | 0.92 | (0.72, 1.19) | 0.54 | 0.82 | (0.57, 1.18) | 0.29 |
| Tc1 | 1.19 | (0.92, 1.54) | 0.19 | 1.28 | (0.89, 1.83) | 0.18 | 1.30 | (0.99, 1.72) | 0.06 | 1.51 | (1.02, 2.23) | 0.04 |
| Tc2 | 1.27 | (1.01, 1.58) | 0.04 | 1.35 | (1.04, 1.76) | 0.03 | 1.32 | (1.04, 1.67) | 0.02 | 1.42 | (1.07, 1.88) | 0.02 |
| Tc17 | 1.07 | (0.89, 1.30) | 0.47 | 1.08 | (0.85, 1.36) | 0.54 | 1.1 | (0.90, 1.34) | 0.35 | 1.09 | (0.84, 1.41) | 0.51 |
| CD8+CD45RA+ | 0.99 | (0.77, 1.27) | 0.91 | 1.18 | (0.84, 1.64) | 0.34 | 1.09 | (0.84, 1.42) | 0.54 | 1.24 | (0.87, 1.77) | 0.24 |
| CD8+CD45RO+ | 0.94 | (0.73, 1.21) | 0.61 | 0.85 | (0.62, 1.17) | 0.32 | 0.79 | (0.61, 1.04) | 0.09 | 0.77 | (0.54, 1.10) | 0.15 |
| CD8+CD28− | 0.85 | (0.67, 1.08) | 0.19 | 0.90 | (0.65, 1.23) | 0.50 | 0.89 | (0.69, 1.15) | 0.36 | 0.98 | (0.69, 1.39) | 0.90 |
| CD8+CD57+ | 0.87 | (0.67, 1.12) | 0.28 | 1.05 | (0.76, 1.45) | 0.79 | 0.86 | (0.66, 1.12) | 0.26 | 0.99 | (0.70, 1.42) | 0.99 |
| CD8+CD28−CD57+ | 0.85 | (0.66, 1.08) | 0.18 | 0.98 | (0.70, 1.35) | 0.89 | 0.85 | (0.66, 1.10) | 0.22 | 1.003 | (0.70, 1.44) | 0.99 |
| CD8+CD28−CD57+CD45RA+ | 0.91 | (0.71, 1.17) | 0.46 | 1.11 | (0.81, 1.53) | 0.51 | 0.97 | (0.75, 1.26) | 0.82 | 1.17 | (0.83, 1.65) | 0.37 |
| CD19+ | 1.03 | (0.85, 1.25) | 0.78 | 1.01 | (0.76, 1.35) | 0.94 | 0.94 | (0.74, 1.19) | 0.60 | 0.97 | (0.65, 1.44) | 0.88 |
HR, Hazard Ratio; CI, Confidence Interval.
Red indicates association of subset with increased risk for stroke (all or ischemic) and blue indicates association with decreased risk for stroke; dense red or blue indicates significance after Bonferroni correction (p < 0.0018) and lighter red or blue indicates borderline significance (p < 0.05 but > 0.0018).
Model 0: Adjusted for age (years), gender, race, MESA site.
Primary Model (Model 1): Adjusted for age (years), sex, race/ethnicity, MESA site, educational attainment, smoking status, alcohol, systolic blood pressure, body-mass index, and diabetes mellitus (DM).
Table 1 displays multivariable-adjusted associations of leukocyte subsets with incident stroke (all stroke) and ischemic stroke in MESA. Hazard ratios (HRs) are displayed per standard-deviation (SD) difference in cell subtype as a proportion of parent immune cell subset. In the base model (Model 0), adjusted for age, sex, race, ethnicity, and MESA field center, higher proportions of intermediate monocytes (CD14+CD16+, as % of total monocyte population) were associated with significantly higher risk for all stroke [HR 1.33, 95% confidence interval (CI) 1.14–1.56), p < 0.001] and borderline significantly higher risk for ischemic stroke (HR 1.31, 95% CI 1.10–1.56, p=0.003). In the pre-specified primary model (Model 1), which was adjusted additionally for educational attainment, smoking status, alcohol use, systolic blood pressure, body-mass index, and diabetes mellitus (Table 2), patterns were similar, with higher effect sizes but attenuated significance. Likewise, in sensitivity analyses adding adjustment for season, lipid parameters, and CMV (Models 2–4; see Supplementary Data), patterns and effect sizes of the association of CD14+CD16+ cells with all stroke (HRs ranging from 1.29 to 1.35) and ischemic stroke (HRs ranging from 1.34 to 1.46) were similar but with significance attenuated. These results demonstrate a consistent association in which each SD increase in intermediate monocytes (as a proportion of total monocytes) was associated with a 30–40% higher risk for stroke (all and ischemic). The other monocyte subsets, CD14++CD16− and CD14+CD16++, were not associated with stroke endpoints.
Table 1.
Baseline characteristics of weighted study sample (N = 2104) and overall MESA cohort free from stroke at baseline (N = 6783).
| Study sample (N = 2104) | Overall MESA (N = 6783) | |
|---|---|---|
| Mean (± Standard Deviation) | Mean (± Standard Deviation) | |
| Age (years) | 62.2 ± 10.5 | 62.2 ± 10.2 |
| Male sex (%) | 47.0 | 47.0 |
| Race/Ethnicity (%) | ||
| Black | 27.9 | 27.7 |
| Chinese | 12.1 | 11.8 |
| Hispanic | 22.4 | 22.0 |
| White | 37.6 | 38.5 |
| Education ≥ high school diploma (or equivalent) | 81.7 | 82.0 |
| Systolic blood pressure (mmHg) | 126.8 ± 21.2 | 126.6 ± 21.5 |
| Diastolic blood pressure (mmHg) | 72.1 ± 10.2 | 71.9 ± 10.3 |
| Body-Mass Index (kg/m2) | 28.3 ± 5.5 | 28.3 ± 5.5 |
| Total cholesterol (mg/dL) | 193.7 ± 34.7 | 194.1 ± 35.7 |
| Low density lipoprotein (LDL) cholesterol (mg/dL) | 116.5 ± 29.9 | 117.2 ± 31.5 |
| High density lipoprotein (HDL) cholesterol (mg/dL) | 51.1 ± 14.8 | 51.0 ± 14.8 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 81.4 ± 18.9 | 81.2 ± 18.5 |
| Diagnosis of hypertension (%) | 46.0 | 45.0 |
| diagnosis of diabetes mellitus (%) | 13.0 | 13.0 |
| Cigarette smoking status (%) | ||
| current | 12.2 | 13.1 |
| former | 37.0 | 36.5 |
| never | 50.8 | 50.4 |
| Alcohol intake (%) | ||
| 0 drinks/week | 41.5 | 40.9 |
| 1-7 drinks/week | 44.7 | 43.7 |
| >7 drinks/week | 13.8 | 15.4 |
Several interesting patterns were observed related to T cell subsets and ischemic stroke (Table 2). In the primary model, each 1-SD increase in CD4+CD25+ (early activated) CD4+ T cells was associated with 1.62 (95% CI 1.16–2.23, p=0.004) and 1.74 (95% CI 1.23–2.45, p=0.002) times higher risk for all stroke and ischemic stroke, respectively. Of note, the subset of CD4+CD25+ T cells representing T regulatory cells (Tregs, CD4+CD25+CD127−) was not associated with stroke or ischemic stroke (p > 0.2 for all).
Meanwhile, differentiated and memory phenotypes of CD4+ T cells were associated with lower risks for stroke; effect sizes were similar for all stroke and ischemic stroke but the associations were not significant for ischemic stroke. Cell subsets associated with all stroke in the primary model included CD4+CD28− (HR 0.722, 95% CI 0.537–0.972, p=0.032), CD4+CD28−CD57+ (HR 0.61, 95% CI 0.43–0.85, p=0.003), and CD4+CD28−CD57+ CD45RA+ (HR 0.77, 95% CI 0.59–0.997, p=0.047); these represent differentiated, terminally differentiated/senescent, and effector memory RA+ (TEMRA) phenotypes, respectively (Table 2).
Cytokine activation profiling (Table 2) revealed that CD4+ T cells expressing IL-4 (Th2) were associated with higher risk for stroke (HR 1.48, 95% CI 1.11–1.98, p=0.007) and ischemic stroke (1.51, 95% CI 1.88–2.08, p=0.013). Likewise, CD8+ T cells expressing IL-4 (Tc2) were associated with higher risk for stroke (HR 1.35, 95% CI 1.04–1.76, p=0.027) and ischemic stroke (1.42, 95% CI 1.07–1.88, p=0.016).
In secondary analyses (see Supplementary Data), overall similar patterns of association were observed after adjustment adding batch and season (Model 2); LDL and HDL in addition to Model 2 covariates (Model 3); cytomegalovirus (CMV) viral load (log-adjusted) in addition to Model 3 covariates (Model 4); and baseline antihypertensive and statin use to Model 3 covariates (Model 5).
Given our previous findings of significant sex-based interactions and related differences in associations of immune phenotypes with carotid intima-media thickness (C-IMT) progression, we performed pre-specified sex-stratified analyses (Table 3). As in our previous analyses, several associations were significant for men but not women, with clear and substantial differences in effect sizes. Among men, intermediate monocytes were associated with incident stroke with a substantially larger effect size than in the overall population (HR 1.64, 95% CI 1.01–2.67), whereas the association was null for women (HR 1.06, 95% CI 0.66–1.69). Likewise, Th2 cells (HR 1.79, 95% CI 1.23–2.62), CD4+CD25+T cells (HR 1.83, 95% CI 1.20–2.81), and Tc2 cells (HR 1.64, 95% CI 1.14–2.35) were associated with significantly higher incident stroke risk for men but not women. Interestingly, Tc1 cells, which are CD8+ T cells producing interferon-gamma on intracellular cytokine profiling, were associated strongly with stroke risk for men (HR 1.89, 95% CI 1.19–3.01) whereas the direction of association clearly differed among women (HR 0.87, 95% CI 0.57–1.34). Men were also observed to have significant associations of CD4+CD28− (HR 0.61, 95% CI 0.38–0.99) and CD4+CD28−CD57+ (HR 0.53, 95% CI 0.31–0.91) cells with lower stroke risk, whereas these associations were null among women.
Table 3.
Sex-stratified associations of circulating immune cell subsets with incident stroke in MESA (N = 2104).
| Women |
Men |
Interaction Term p value | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Classical monocytes | 1.08 | (0.73,1.61) | 0.70 | 0.72 | (0.49,1.05) | 0.09 | 0.039 |
| Intermediate monocytes | 1.06 | (0.66,1.69) | 0.82 | 1.64 | (1.01,2.67) | 0.045 | 0.134 |
| Nonclassical monocytes | 0.80 | (0.44,1.45) | 0.46 | 0.88 | (0.58,1.33) | 0.54 | 0.562 |
| Natural killer cells | 1.02 | (0.61,1.70) | 0.95 | 0.89 | (0.67,1.18) | 0.42 | 0.931 |
| Gamma-delta T cells | 1.04 | (0.71,1.53) | 0.85 | 0.84 | (0.46,1.54) | 0.57 | 0.651 |
| Pan CD4+ T cells | 1.14 | (0.69,1.87) | 0.62 | 1.18 | (0.67,2.08) | 0.57 | 0.948 |
| Th1 | 0.89 | (0.63,1.26) | 0.52 | 0.87 | (0.46,1.64) | 0.67 | 0.979 |
| Th2 | 1.27 | (0.93,1.73) | 0.13 | 1.79 | (1.23,2.62) | 0.003 | 0.46 |
| Th17 | 0.63 | (0.25,1.59) | 0.33 | 0.94 | (0.61,1.45) | 0.79 | 0.364 |
| Treg (CD4+CD25+ CD127−) | 1.29 | (0.84,1.98) | 0.24 | 1.14 | (0.80,1.64) | 0.47 | 0.654 |
| CD4+CD45RA+ | 1.11 | (0.75,1.64) | 0.60 | 0.82 | (0.52,1.3) | 0.40 | 0.153 |
| CD4+CD45RO+ | 0.81 | (0.56,1.17) | 0.26 | 1.16 | (0.77,1.77) | 0.48 | 0.093 |
| CD4+CD25+ | 1.52 | (0.85,2.71) | 0.16 | 1.83 | (1.20,2.81) | 0.005 | 0.569 |
| CD4+CD28− | 0.89 | (0.62,1.29) | 0.54 | 0.61 | (0.38,0.99) | 0.04 | 0.206 |
| CD4+CD57− | 1.21 | (0.78,1.86) | 0.39 | 0.78 | (0.52,1.18) | 0.24 | 0.148 |
| CD4+CD28−CD57+ | 0.73 | (0.47,1.13) | 0.15 | 0.53 | (0.31,0.91) | 0.02 | 0.355 |
| CD4+CD28−CD57+CD45RA+ | 0.95 | (0.70,1.28) | 0.73 | 0.67 | (0.43,1.03) | 0.07 | 0.193 |
| Pan CD8+ T cells | 0.71 | (0.43,1.18) | 0.19 | 0.87 | (0.58,1.31) | 0.51 | 0.584 |
| Tc1 | 0.87 | (0.57,1.34) | 0.53 | 1.89 | (1.19,3.01) | 0.007 | 0.021 |
| Tc2 | 1.26 | (0.99,1.60) | 0.06 | 1.64 | (1.14,2.35) | 0.007 | 0.203 |
| Tc17 | 1.17 | (0.79,1.72) | 0.44 | 1.05 | (0.76,1.47) | 0.75 | 0.886 |
| CD8+CD45RA+ | 1.07 | (0.62,1.87) | 0.8 | 1.18 | (0.79,1.75) | 0.43 | 0.944 |
| CD8+CD45RO+ | 0.86 | (0.48,1.53) | 0.60 | 0.85 | (0.6,1.22) | 0.38 | 0.606 |
| CD8+CD28− | 0.90 | (0.56,1.42) | 0.64 | 0.84 | (0.54,1.30) | 0.43 | 0.961 |
| CD8+CD57+ | 1.19 | (0.76,1.85) | 0.45 | 0.90 | (0.57,1.43) | 0.67 | 0.44 |
| CD8+CD28−CD57+ | 1.04 | (0.66,1.65) | 0.85 | 0.91 | (0.58,1.42) | 0.67 | 0.752 |
| CD8+CD28−CD57+CD45RA+ | 1.09 | (0.69,1.73) | 0.70 | 1.08 | (0.71,1.62) | 0.73 | 0.899 |
| CD19+ | 1.26 | (0.98,1.60) | 0.07 | 0.73 | (0.36,1.50) | 0.39 | 0.123 |
HR, Hazard Ratio; CI, Confidence Interval.
Red indicates association of subset with increased risk for stroke (all or ischemic) and blue indicates association with decreased risk for stroke; dense red or blue indicates significance after Bonferroni correction (p < 0.0018) and lighter red or blue indicates borderline significance (p < 0.05 but > 0.0018).
Primary Model (Model 1): Adjusted for age (years), sex, race/ethnicity, MESA site, educational attainment, smoking status, alcohol, systolic blood pressure, body-mass index, and diabetes mellitus (DM).
Given the long duration of time between baseline and follow up (maximum 18.5 years), and related possibility that this could blunt informative associations, we performed sensitivity analyses of leukocyte phenotypes at baseline and ischemic stroke occurring within 10 years of baseline. Overall, patterns of association were similar to those observed in the primary analyses (see Supplementary Data).
Finally, we performed a limited replication analysis in which we meta-analyzed associations of certain cell phenotypes with incident stroke in MESA and CHS. We analyzed only subsets that were measured in the same laboratory using the same methods in MESA and CHS; monocytes and intracellular cytokine activation profiling were not measured in CHS, nor were certain T cell phenotypes. Age at PBMC measurement differed considerably for MESA (median = 62.2 years) and CHS (median = 79.6 years). The associations of CD4+CD28−CD57+ and CD4+CD28−CD57+CD45RA+T cells with lower stroke risk in MESA, and CD4+CD25+ T cells with higher stroke risk in MESA, were not replicated in CHS (Table 4).
Table 4.
Meta-analysis of leukocyte subsets and incident stroke in MESA (N = 2104) and CHS (N = 1793).
| MESA (N = 2104) |
CHS(N = 1793) |
Meta-Analysis (N = 3897) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| CD19+ | 1.002 | (0.96,1.045) | 0.941 | 1.002 | (0.993,1.011) | 0.627 | 1.002 | (0.993,1.011) | 0.623 |
| Gamma-delta T cells | 0.981 | (0.912,1.054) | 0.597 | 1.007 | (0.979,1.035) | 0.648 | 1.003 | (0.977,1.03) | 0.814 |
| Natural killer cells | 0.993 | (0.953,1.036) | 0.755 | 0.957 | (0.924,0.992) | 0.016 | 0.972 | (0.946,0.999) | 0.041 |
| Pan CD4+ T cells | 1.012 | (0.979,1.046) | 0.488 | 1 | (0.991,1.009) | 0.995 | 1.001 | (0.992,1.01) | 0.849 |
| CD4+CD25+ | 1.043 | (1.013,1.074) | 0.004 | 1.005 | (0.996,1.015) | 0.288 | 1.009 | (1,1.018) | 0.057 |
| CD4+CD25+CD127− | 1.087 | (0.954,1.238) | 0.21 | 0.997 | (0.966,1.029) | 0.864 | 1.002 | (0.972,1.033) | 0.897 |
| CD4+CD28− | 0.968 | (0.939,0.997) | 0.032 | 1.004 | (0.995,1.014) | 0.384 | 1.001 | (0.991,1.01) | 0.874 |
| CD4+CD28−CD57+ | 0.941 | (0.904,0.98) | 0.003 | 1.009 | (0.995,1.023) | 0.213 | 1.002 | (0.989,1.015) | 0.817 |
| CD4+CD28−CD57+CD45RA+ | 0.945 | (0.894,0.999) | 0.047 | 1 | (0.978,1.022) | 0.982 | 0.992 | (0.971,1.013) | 0.444 |
| CD4+CD45RA+ | 0.998 | (0.975,1.021) | 0.877 | 0.997 | (0.986,1.007) | 0.529 | 0.997 | (0.987,1.006) | 0.524 |
| CD4+CD45+RO+ | 0.998 | (0.979,1.017) | 0.802 | 0.996 | (0.988,1.005) | 0.398 | 0.997 | (0.989,1.004) | 0.383 |
| CD4+CD57+ | 0.994 | (0.971,1.018) | 0.641 | 1.009 | (0.999,1.018) | 0.066 | 1.007 | (0.998,1.016) | 0.125 |
| Pan CD8+ T cells | 0.977 | (0.947,1.009) | 0.153 | 1.002 | (0.988,1.015) | 0.819 | 0.998 | (0.985,1.01) | 0.716 |
| CD8+CD28− | 0.993 | (0.973,1.013) | 0.499 | 0.998 | (0.991,1.005) | 0.531 | 0.997 | (0.99,1.004) | 0.414 |
| CD8+CD28−CD57+ | 0.999 | (0.978,1.019) | 0.886 | 0.997 | (0.989,1.005) | 0.416 | 0.997 | (0.99,1.004) | 0.417 |
| CD8+CD28−CD57+CD45RA+ | 1.008 | (0.986,1.03) | 0.506 | 0.997 | (0.988,1.006) | 0.501 | 0.998 | (0.99,1.007) | 0.716 |
| CD8+CD45RA+ | 1.011 | (0.989,1.034) | 0.34 | 1.001 | (0.993,1.009) | 0.766 | 1.002 | (0.995,1.01) | 0.548 |
| CD8+CD45+RO+ | 0.986 | (0.959,1.014) | 0.319 | 0.999 | (0.989,1.008) | 0.794 | 0.997 | (0.989,1.006) | 0.569 |
| CD8+CD57+ | 1.003 | (0.983.1.023) | 0.785 | 1.001 | (0.993,1.008) | 0.877 | 1.001 | 0.994,1.008) | 0.809 |
Model: Adjusted for age (years), sex, race/ethnicity, MESA site, educational attainment, smoking status, alcohol, systolic blood pressure, body-mass index, and diabetes mellitus (DM). Hazard ratios are expressed per unit (1%) increase in exposure.
4. Discussion
This study uses a large cohort to investigate the relationships between specific leukocyte subsets and incident stroke. In a cohort of 2104 individuals followed for nearly two decades, we observed several novel prospective associations of monocyte and T cell phenotypes with incident stroke. These findings not only add granularity to existing data examining broad inflammatory biomarkers and stroke, but also several precise immune phenotypes that may be implicated in the inflammation-stroke relationship.
Our findings related to T cell subsets and stroke revealed several consistent patterns. T cell subsets expressing IL-4 after activation (Th2 and Tc2) were associated with higher stroke risk. Activated CD4+ T cells (CD4+CD25+) were likewise associated with higher stroke risk in both longer term (up to 18.5 years) and shorter term (up to 10 years) follow up analyses; notably, the T regulatory subset of CD4+CD25+ cells (Tregs: CD4+CD25+CD127−) was not associated with stroke in any analyses. To provide context related to effect size, each one standard deviation increase in Th2 cells (as a proportion of all CD4+ T cells) was associated with an approximately 1.5-fold (50% increase) higher multivariable-adjusted hazard of stroke. In other words, a person in the 84th percentile of Th2 cells (one standard deviation higher than someone in the 50th percentile) would have an approximately 50% higher risk for incident stroke. To provide context, each year older in age was associated with a hazard ratio of 1.05 (approximately 5% relative increase per year in stroke risk), with 8 years older age corresponding an approximately 1.5-fold increase (1.05^8 = 1.48; a 48% relative increase) in stroke risk. Accordingly, each standard deviation increase in Th2 cell proportion was equivalent to slightly more than 8 years of older age with respect to risk for stroke.
These relatively consistent findings underscore the potentially important role of activated T cell phenotypes in atherosclerosis pathogenesis and suggest that the controversial role of IL-4-producing T cell phenotypes in development of vs. protection from Refs. [23-25] warrants further investigation. Classically, IL-4 and related Th2-type immune responses were thought to oppose pro-atherogenic Th1 activities, and indeed IL-4 has been demonstrated to aid in tissue recovery/damage resolution following ischemic stroke [26]. Yet, the simplified paradigm of Th2 and IL-4 as purely athero-protective, inflammation-resolving has been challenged by other mechanistic and clinical data implicating these allergic-type responses (e.g. Th2-driven mast cell activation and degranulation) in atherosclerosis and thrombosis [27]. In experimental models, mast cell activation and related immunoglobin E/Toll-like receptor 4-driven inflammatory macrophage activation lead to inflammatory plaque development [28]. Similarly, in humans, carotid intra-plaque mast cells are associated prospectively with increased stroke risk and autopsy findings of excess degranulated mast cells have been observed in ruptured coronary plaque and thrombi from patients with acute coronary syndromes [8]. Further supporting these findings, IL-6 – which is causally implicated in ischemic stroke [29] – plays an essential role in Th cell differentiation, promoting differentiation to Th2-type cells preferentially and inhibiting Th1 polarization [30].
Given the role of atherosclerosis and thrombosis in ischemic stroke and blood-brain barrier permeability to inflammatory cell infiltration [31,32], it is plausible that Th2-type responses are implicated in diverse etiologies of ischemic stroke; however, it was beyond the scope of the present study to analyze of associations of immune cell populations with further subtyped ischemic stroke events. The clinical and immunotherapeutic implications of investigating propensities to allergic-type inflammation in stroke – and more broadly, athero-thrombosis – pathogenesis are substantial and warrant further mechanistic investigation.
We also observed that differentiated CD4+ phenotypes tended to be associated with lower risk of stroke in MESA, although these findings were not replicated in the older CHS cohort. The lack of replication in CHS is not necessarily surprising, and could result from differences in study populations – with CHS representing an older cohort (subject to related survival bias) with a less diverse race/ethnicity make-up than MESA – and/or true lack of a replicable effect (e.g. false positive in MESA). Meanwhile, naïve and early-differentiated CD4+ T cells were associated with higher stroke risk in both cohorts (with a stronger and more statistically significant signal in MESA). Potential causes for this include more potent inflammatory responses induced by naïve and early-differentiated CD4+ T cells. It is also possible that certain T cell subsets are simply markers of existing atherosclerotic lesions (which in turn, drive stroke risk); in this case, the harmful association of memory CD4+T cells and protective association of naïve CD4+T cells with ischemic stroke may reflect antigen-experienced memory in the setting of existing atherosclerosis (or a lack thereof), and therefore a (not surprising) association of immune markers of this atherosclerosis with ischemic stroke. However, the long duration of time between cell measurement and events makes this perhaps less likely.
Interestingly, we observed strong associations of immune cell subsets with incident stroke for men but not women. These findings, including an association of intermediate monocytes with increased stroke risk, provide an interesting corollary to – and perhaps more clinically relevant extension of – our group’s recent findings in the same cohort that nonclassical monocytes were associated with carotid intima-media thickness (C-IMT) progression whereas classical monocytes were associated with C-IMT regression [15]. In this analysis, we also observed particularly robust associations of IL-4 producing T cell subsets (Th2 and Tc2) with stroke for men but not women. In light of our findings and known sex dimorphism in acquired immune responses [33] and incident stroke [34], future studies on sex-dimorphic immune factors implicated in vulnerability vs. resilience to athero-thrombotic events are warranted.
Several limitations warrant discussion. PBMCs were obtained at a single time point and not serially between baseline and follow-up for events, which limited our ability to probe dynamic changes in immune response leading up to events. Our immune profiling consisted of phenotypic assessments and cytokine stimulation experiments, which provide important surface marker and functional data but do not offer the granularity of single-cell or bulk sequencing. Another potential limitation relates to use of cryopreserved PBMCs instead of freshly obtained whole blood, although studies have demonstrated that cryopreserved cells show similar results as fresh whole blood [35]. However, using cryopreserved PBMCs enabled us to analyze cells from multiple MESA sites, collected over a two-year period (the baseline MESA examination), in a standardized way and minimize potential batch effects. Another potential limitation is the use of percentages rather than absolute cell counts, which were not available due to the absence of a complete blood cell count with differential performed at the time of blood draw. While the effect sizes observed are unlikely to inform clinically relevant risk prediction models, the purpose of these analyses was not to generate a new stroke risk prediction model, but rather to highlight potential relationships between circulating immune cell profiles of interest and incident stroke. An additional limitation was our limited power to evaluate associations of immune cell subsets with further subtypes of stroke beyond ischemic stroke only vs. overall (including hemorrhagic) stroke. This is potentially important given the proportion of ischemic strokes (25–30% in CHS and MESA [21,22]) that are cardio-embolic in etiology. We were not powered to evaluate further stroke subtypes beyond ischemic only vs. overall stroke as outcomes for this analysis, but future investigations should ideally further distinguish among ischemic stroke subtypes. Overall, despite these limitations, the size, representativeness, and follow-up of the cohort, coupled with the systematic and large-scale immune profiling, provided a unique opportunity to investigate our hypotheses that would not readily be investigable in other cohorts. One exception to the latter point is the available data from CHS we used in our limited replication analyses; however, the subsets most strongly and consistently associated with stroke in our analysis of MESA were not measured in CHS, precluding replication of our central findings.
In conclusion, in a diverse study of middle-aged and older adults, we observed significant associations of circulating IL-4 producing T cells and intermediate monocytes with incident stroke over nearly two decades of follow-up. Future translational investigations investigating tissue-level mediators of the observed immune profile-stroke associations are needed to enhance depth of insights into related diagnostic and therapeutic targets.
Supplementary Material
Financial support
The research was also supported by grant funding from the American Heart Association (Fellow-to-Faculty Award 16FTF312000010) and National Institutes of Health (R01-HL-156792, R01HL144483, and R01HL120854).
Multi-Ethnic Study of Atherosclerosis: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). This publication was also developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S. Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Cardiovascular Health Study: This CHS research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006; and NHLBI grants U01HL080295, R01HL087652, R01HL103612, R01HL120393, R01HL144483, and R01HL120854 and U01HL130114 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at.
Footnotes
CRediT authorship contribution statement
Matthew Feinstein: study design, data analysis, interpretation, manuscript writing, and critical revision; Petra Buzkova: data analysis and interpretation; Nels Olson: study design, interpretation, and critical revision; Margaret Doyle: study design, immune profiling analysis, interpretation, and critical revision; Russell Tracy: study design, immune profiling analysis, interpretation, and critical revision; William Longstreth: study design, data acquisition and analysis, interpretation, and critical revision; Bruce Psaty and Joseph Delaney: study design, immune profiling analysis, interpretation, and critical revision; Colleen Sitlani, Alison Fohner, Sally Huber, James Floyd, Arjun Sinha, Edward Thorp, Alan Landay, Matthew Freiberg: data interpretation and critical revision..
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2022.05.007.
References
- [1].Elkind MS, Tai W, Coates K, Paik MC, Sacco RL, High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke, Arch. Intern. Med 166 (19) (Oct 23 2006) 2073–2080, 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- [2].Whiteley W, Jackson C, Lewis S, et al. , Association of circulating inflammatory markers with recurrent vascular events after stroke: a prospective cohort study, Stroke. 42 (1) (2011) 10–16, 10.1161/STROKEAHA.110.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Di Napoli M, Papa F, Bocola V, C-reactive protein in ischemic stroke: an independent prognostic factor, Stroke. 32 (4) (2001) 917–924, 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- [4].Patterson CC, Smith AE, Yarnell JW, Rumley A, Ben-Shlomo Y, Lowe GD, The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: the Caerphilly Study, Atherosclerosis. 209 (2) (2010) 551–557, 10.1016/j.atherosclerosis.2009.09.030. [DOI] [PubMed] [Google Scholar]
- [5].Liu Y, Wang J, Zhang L, et al. , Relationship between C-reactive protein and stroke: a large prospective community based study, PLoS One 9 (9) (2014), e107017, 10.1371/journal.pone.0107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fibrinogen Studies C, Danesh J, Lewington S, et al. , Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis, JAMA : J. Am. Med. Assoc 294 (14) (Oct 12 2005) 1799–1809, 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- [7].Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, et al. , C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis, Lancet. 375 (9709) (September 2010) 132–140, 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Willems S, Vink A, Bot I, et al. , Mast cells in human carotid atherosclerotic plaques are associated with intraplaque microvessel density and the occurrence of future cardiovascular events, Eur. Heart J 34 (48) (Dec 2013) 3699–3706, 10.1093/eurheartj/eht186. [DOI] [PubMed] [Google Scholar]
- [9].Heikkila HM, Latti S, Leskinen MJ, Hakala JK, Kovanen PT, Lindstedt KA, Activated mast cells induce endothelial cell apoptosis by a combined action of chymase and tumor necrosis factor-alpha, Arteriosclerosis, thrombosis, and vascular biology 28 (2) (2008) 309–314, 10.1161/ATVBAHA.107.151340. [DOI] [PubMed] [Google Scholar]
- [10].Kovanen PT, Mast cells: multipotent local effector cells in atherothrombosis, Immunol Rev. 217 (2007) 105–122, 10.1111/j.1600-065X.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- [11].Libby P, The changing landscape of atherosclerosis, Nature. 592 (7855) (2021) 524–533, 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- [12].Iadecola C, Anrather J, The immunology of stroke: from mechanisms to translation, Nat. Med 17 (7) (Jul 7 2011) 796–808, 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mantani PT, Ljungcrantz I, Andersson L, et al. , Circulating CD40+ and CD86+ B cell subsets demonstrate opposing associations with risk of stroke, Arteriosclerosis, thrombosis, and vascular biology 34 (1) (2014) 211–218, 10.1161/ATVBAHA.113.302667. [DOI] [PubMed] [Google Scholar]
- [14].Wigren M, Bjorkbacka H, Andersson L, et al. , Low levels of circulating CD4+ FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke, Arterioscler. Thromb. Vasc. Biol 32 (8) (Aug 2012) 2000–2004, 10.1161/ATVBAHA.112.251579. [DOI] [PubMed] [Google Scholar]
- [15].Feinstein MJ, Doyle MF, Stein JH, et al. , Nonclassical monocytes (CD14dimCD16+) are associated with carotid intima-media thickness progression for men but not women: the multi-ethnic study of atherosclerosis-brief report, Arterioscler. Thromb. Vasc. Biol 41 (5) (May 5 2021) 1810–1817, 10.1161/ATVBAHA.120.315886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bild DE, Bluemke DA, Burke GL, et al. , Multi-ethnic study of atherosclerosis: objectives and design, Am. J. Epidemiol 156 (9) (Nov 1 2002) 871–881, 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- [17].Olson NC, Doyle MF, Jenny NS, et al. , Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis, PLoS One 8 (8) (2013), e71498, 10.1371/journal.pone.0071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tracy RP, Doyle MF, Olson NC, et al. , T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis, J. Am. Heart Assoc 2 (3) (May 20 2013), e000117, 10.1161/JAHA.113.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schisterman EF, Cole SR, Platt RW, Overadjustment bias and unnecessary adjustment in epidemiologic studies, Epidemiology. 20 (4) (2009) 488–495, 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Olson NC, Sitlani CM, Doyle MF, et al. , Innate and adaptive immune cell subsets as risk factors for coronary heart disease in two population-based cohorts, Atherosclerosis 300 (May 2020) 47–53, 10.1016/j.atherosclerosis.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Longstreth WT Jr., Gasca NC, Gottesman RF, Pearce JB, Sacco RL, Adjudication of transient ischemic attack and stroke in the multi-ethnic study of atherosclerosis, Neuroepidemiology 50 (1–2) (2018) 23–28, 10.1159/000486174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Longstreth WT Jr., Bernick C, Fitzpatrick A, et al. , Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study, Neurology 56 (3) (Feb 13 2001) 368–375, 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- [23].Bartlett B, Ludewick HP, Misra A, Lee S, Dwivedi G, Macrophages and T cells in atherosclerosis: a translational perspective, Am. J. Physiol. Heart Circ. Physiol 317 (2) (Aug 1 2019) H375–H386, 10.1152/ajpheart.00206.2019. [DOI] [PubMed] [Google Scholar]
- [24].Mallat Z, Taleb S, Ait-Oufella H, Tedgui A, The role of adaptive T cell immunity in atherosclerosis, J Lipid Res. 50 (Suppl) (2009) S364–S369, 10.1194/jlr.R800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bot I, Bot M, van Heiningen SH, et al. , Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in ApoE−/− mice, Cardiovasc Res. 89 (1) (2011) 244–252, 10.1093/cvr/cvq260. [DOI] [PubMed] [Google Scholar]
- [26].Zhu H, Hu S, Li Y, et al. , Interleukins and ischemic stroke, Front. Immunol 13 (2022) 828447, 10.3389/fimmu.2022.828447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Potaczek DP, Links between allergy and cardiovascular or hemostatic system, Int. J. Cardiol 170 (3) (Jan 1 2014) 278–285, 10.1016/j.ijcard.2013.11.029. [DOI] [PubMed] [Google Scholar]
- [28].Weber C, Zernecke A, Libby P, The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models, Nat. Rev. Immunol 8 (10) (Oct 2008) 802–815, 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- [29].Georgakis MK, Malik R, Gill D, et al. , Interleukin-6 signaling effects on ischemic stroke and other cardiovascular outcomes: a mendelian randomization study, Circ Genom Precis Med. 13 (3) (2020), e002872, 10.1161/CIRCGEN.119.002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diehl S, Rincon M, The two faces of IL-6 on Th1/Th2 differentiation, Mol. Immunol 39 (9) (Dec 2002) 531–536, 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- [31].Ribatti D, The crucial role of mast cells in blood-brain barrier alterations, Exp. Cell Res 338 (1) (Oct 15 2015) 119–125, 10.1016/j.yexcr.2015.05.013. [DOI] [PubMed] [Google Scholar]
- [32].Silverman AJ, Sutherland AK, Wilhelm M, Silver R, Mast cells migrate from blood to brain, J Neurosci. 20 (1) (2000) 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moulton VR, Sex hormones in acquired immunity and autoimmune disease, Front. Immunol 9 (2018) 2279, 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Appelros P, Stegmayr B, Terent A, Sex differences in stroke epidemiology: a systematic review, Stroke. Apr 40 (4) (2009) 1082–1090, 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- [35].Thyagarajan B, Barcelo H, Crimmins E, et al. , Effect of delayed cell processing and cryopreservation on immunophenotyping in multicenter population studies, J Immunol Methods. 463 (2018) 61–70, 10.1016/j.jim.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the MESA and CHS coordinating centers on reasonable request.


