Abstract
Introduction
Lung cancer is the deadliest cancer worldwide and in Brazil. Despite strong evidence, lung cancer screening by low-dose computed tomography (LDCT) in high-risk individuals is far from a reality in many countries, particularly in Brazil. Brazil has a universal public health system marked with important inequalities. One affordable strategy to increase the coverage of resources is to use mobile units.
Objectives
To describe the implementation and results of an innovative lung cancer prevention program that integrates tobacco cessation and lung cancer screening using a mobile CT unit.
Methodology
From May 2019 to Dec 2020, health professionals from 18 public primary health care units in Barretos, Brazil, were trained to offer smoking cessation counseling and treatment. Eligible high-risk participants of this program were also invited to perform lung cancer screening in a mobile LDCT unit that was specially conceived to be dispatched to the community. A detailed epidemiological questionnaire was administered to the LDCT participants.
Results
Among the 233 screened participants, the majority were women (54.9%), and the average age was 62 years old. A total of 52.8% of participants showed high or very high nicotine dependence. After 1 year, 27.8% of participants who were involved in smoking cessation groups had quit smoking. The first LDCT round revealed that the majority of participants (83.7%) exhibited lung-Rads 1 or 2; 7.3% exhibited lung-Rads 3; 7.7% exhibited lung-Rads 4a; and 3% exhibited lung-Rads 4b or 4x. The three participants with lung-Rads 4b were further confirmed, and their surgery led to the diagnosis of early-stage cancer (1 case of adenocarcinoma and two cases of squamous cell carcinoma), leading to a cancer diagnosis rate of 12.8/1000.
Conclusion
Our results indicate promising outcomes for an onsite integrative program enrolling high-risk individuals in a middle-income country. Evidence barriers and challenges remain to be overcome.
Keywords: tobacco smoking, smoking prevention, lung cancer, multidetector computed tomography, mobile health units, Brazil
Introduction
Lung cancer is the most lethal cancer, and its mortality rates are higher than the combined mortality rates of breast, prostate, and colon cancers.1 This dismal prognosis is mainly due to its silent development, which leads to late diagnosis.2 In Brazil, the National Cancer Institute estimated 17,760 new lung cancer cases in men and 12,440 in women between 2020 and 2022, corresponding to an estimated risk of 16.99 new cases for every 100 thousand men and 11.56 for every 100 thousand women.3 The mortality rate of lung cancer increased between 1979 and 2004 in Brazil, from 10.6-13.1 deaths/100 thousand men and from 3.0-5.4 deaths/100 thousand women.4
Cigarette smoking is the main etiological factor for lung cancer5,6 and constitutes the main criterion for defining high-risk individuals for lung cancer in the Western world. In Brazil, the implementation of public health policies in recent decades7 has had a significant impact on reducing tobacco consumption, leading to a decrease of approximately 50% in the prevalence of smoking and related deaths.7 Nevertheless, it was estimated that by 2020, the proportion of lung cancer cases attributable to smoking in Brazil would be 83.3% among men and 64.8% among women.8
Since tobacco-exposed individuals have a significantly increased cancer risk, an early diagnosis method for these high-risk individuals has been researched for decades, and favorable results were obtained after the development of low-dose computed tomography (LDCT).9 LDCT screening of high-risk subjects showed a reduction in lung cancer mortality,10,11 so it has been progressively applied to lung cancer screening worldwide ever since. Several studies reported that LDCT screening could be more effective when offered with smoking cessation programs.12-17 However, lung LDCT screening in Brazil is still in its early steps; it is composed of sparse initiatives and is not widely available in the public setting.18
The Brazilian health care system is divided into a public and unified health system (Sistema Único de Saúde [SUS]) and a private health care system, which covers approximately 26% of the population, mostly higher-income individuals.19 It is a complex structure, with chronic underfunding; concentration and inequity of resources; high-cost technology; and breaches in articulation between the primary health care and the other levels of complexity.20-22 Therefore, the health care network is inadequately equipped for the early diagnosis of lung cancer.23,24
Considering the substantial role of LDCT screening in diminishing lung cancer mortality, any program designed to significantly decrease lung cancer deaths must include LDCT screening as a secondary prevention tool allied to primary prevention.13 Importantly, there is evidence of an inverse relationship between the availability of health apparatuses and access, recruitment, and engagement of the target population in secondary prevention, especially among the most vulnerable,25,26 making CT device availability a critical factor in planning a lung cancer screening program. A structural barrier to offering lung cancer screening nationwide in Brazil is posed, as only 15.5% of all Brazilian cities have CT devices, considering the combining equipment from both public and private units, a proportion that falls below 5% in parts of the Brazilian northeast.27,28
Given the impossibility of overcoming the profound structural limitations in access to lung cancer screening in Brazil, a rational and creative solution could integrate current resources to include mobile units, thereby increasing effective coverage. Our hospital has a long and great expertise in such a context, employing mobile units for assistance or secondary prevention among underserved populations. The onsite strategy employing mobile units has demonstrated favorable recruitment and follow-up rates in remote areas and vulnerable populations.29-31 The use of mobile CT units for lung cancer screening has been recently explored,32,33 with promising preliminary results related to the recruitment of underserved, high-risk populations.32
The present study aimed to describe the implementation process of an integrated program for lung cancer prevention that combines smoking cessation and screening employing a mobile LDCT unit for high-risk individuals. Herein, we present its initial results and discuss some operationalization challenges.
Materials and Methods
The current integrated lung cancer screening program was implemented in the city of Barretos, located in the northeast of São Paulo state, Brazil. Barretos is a typical mid-range city in Sao Paulo state, with 112,000 inhabitants and a territorial area measuring 1566 km2 in total.28 Crossing demographic survey data with data from national health surveys34-36 and using high-risk criteria for lung cancer defined similarly as in the National Lung Screening Trial10 (ie, age 55-74, a 30 + pack year smoking history and current smoking status or having quit in the last 15 years), we estimated a required sample size of 1765 high-risk men and 1611 high-risk women in Barretos, for whom LDCT screening would be indicated.
The program is part of our institution’s prevention actions, including breast,29,37,38 cervical,30,39-41 skin,31,42 oral, and colorectal43 cancer programs, and now lung cancer.
Health Services for Implementation
As a representative of the Brazilian health system, in Barretos, there are public and private health care facilities, with primary care practices predominantly carried out by public coverage and performed in community-based primary care units. In contrast, secondary and tertiary care are performed both by public and private units. Particularly in Barretos, a private institution manages a large proportion of the city’s health system, including our private cancer hospital, the public primary health care facilities, the public secondary health care facility (ambulatory clinics), and another private general hospital, allowing a favorable environment to integrate health care practices across different levels and administrations.
CT Scan Mobile Unit
A mobile unit was specifically conceived for the lung cancer screening program by our hospital mobile unit factory. The assembled mobile CT unit consists of an air-conditioned truck trailer adapted to rustic traffic conditions, containing an operational Optima 540 (GE Healthcare, USA) CT device, control room, and working facilities, with entry access by latter or hydraulic elevator (Figure 1).
Figure 1.
The Mobile CT Unit: (A) inside view of the operational CT room; (B) Mobile CT unit in displacement; (C) external lateral view, with the CT room already expanded on the back, the hydraulic elevator, and the entrance latter on its right, and (D) inside view of the CT room from the command room.
Implementation Steps of Lung Cancer Prevention
The program was designed to reinforce primary prevention in public domain primary health care units while using its potential to reach the community and recruit high-risk individuals for screening in the mobile unit or hospital (Supplementary Fig.).
Human Resource Preparation
A training phase was first conducted to introduce health professionals to the strategy designed, underlining the role of primary and secondary prevention, the strength of the association of approaches, the high-risk group concept, and the screening flow. This training phase was planned to be held twice a year to gather information from ongoing practice to overcome eventual barriers and limitations.
Eighteen smoking cessation teams were trained within the scope of the program in twelve primary health care units throughout the city, offering tobacco cessation counseling and treatment to the community in an opt-in system. Accordingly, doctors, nurses, dentists, pharmacists, and other related professionals of public primary care units were invited to enroll in a 16-hour training course taught by the Reference Center for Alcohol Tobacco and Other Drugs (CRATOD) of the state of São Paulo, which addressed issues of smokers approach, support groups, prescription and dispensing of medicines, according to the professional’s area of expertise.
The treatment for smoking cessation followed the Brazilian Clinical protocol and therapeutic guidelines for nicotine addiction.44 The treatment is preceded by a clinical consultation with and assessment of the patient’s motivation to quit smoking; the degree of nicotine dependence, accessed through the Fagerstrom scale, a survey of clinical history and existence of psychological comorbidities; and whether there is indication or contraindication to the use of medication. The intensive approach to smokers consists of individual sessions or in a support group of 10 to 15 participants, coordinated by 1 to two higher-level health professionals, with four initial weekly sessions, followed by two biweekly sessions and then a monthly open session to prevent relapse, until completing 1 year. The drug treatment consisted of nicotine replacement therapy through a transdermal patch (with the release of 7 mg, 14 mg, or 21 mg in 24 h) and bupropion hydrochloride (tablets 150 mg),44 if clinically indicated.
Screening Development for the Target Population
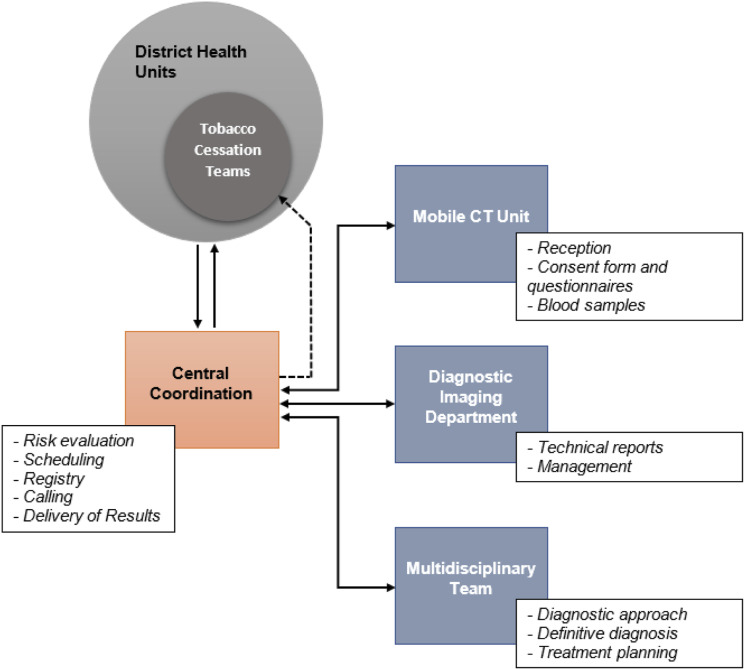
The selection and recruitment for LDCT screening were initially carried out by previously trained professionals from the primary health care units, mainly doctors, nurses, and dentists who constituted the smoking cessation support groups. Potentially eligible high-risk individuals matching NLST criteria were identified and assessed, and LDCT screening was offered. At medical discretion, some cases beyond the NLST criteria were included, subject to the participants' agreement and informed consent. For high-risk individuals, a first-round LDCT screening examination was scheduled in the mobile CT unit placed in the community or in our hospital. A central coordination department managed the scheduling, including ancillary and follow-up examinations, and was responsible for navigating the participant through the screening process and delivering results to general practitioners from the referring primary health care (Figure 2).
Figure 2.
Schematic flow. Central coordination departments is the main interface between municipal health teams and the HCB that also coordinates and track the events following the initial LDCT examination.
Direct access to the screening flow was planned in parallel by direct appointment with the central coordination department or onsite opportunistic presentation to the mobile unit. In those cases, the reference primary health care unit of the individual’s residence was identified to where the results were sent, allowing the same assessment opportunity as the regular flow. Regardless of the entrance, the participant received a brief assessment for smoking cessation by the navigator staff. The LDCT screening was designed to be offered annually, as long as they satisfy the high-risk selection criteria or until they are diagnosed with lung cancer.
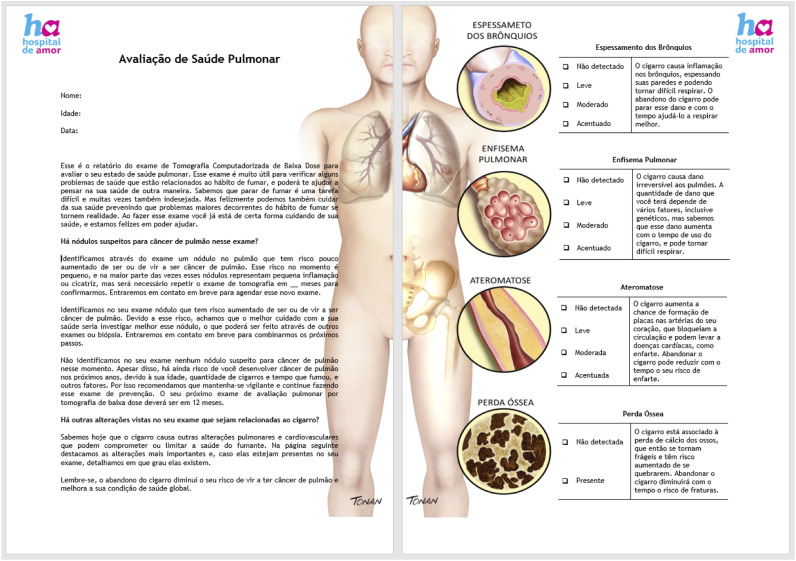
Thoracic radiologists assessed LDCT examinations and filled in structured reports. Two reports were delivered for each examination, 1 in technical and precise information, serving as a guide to clinical management and meant to be filed in medical records. An additional report was illustrated and adapted with informal language to translate the most critical screening results and raise awareness (Figure 3). Both reports were sent to the referring health care unit and delivered to the participant’s assistant doctor. In the return appointment, the participants could clarify their doubts and may once again be assessed regarding tobacco cessation susceptibility. The illustrated report would also serve as a persuasion tool in the reassessment of tobacco cessation susceptibility, thus taking advantage of a potential teachable moment.
Figure 3.
Illustrated report sample (in the Portuguese language). Adapted language to explain screening rationale and individual results, illustrating physiopathology effects of tobacco smoking, aimed to maintain awareness.
The central coordination department called the cases that required reassessment, carried out in our hospital; cases that were considered to need invasive procedures were previously discussed in a multidisciplinary board of medical specialists in our hospital, individualizing the medical management. All necessary diagnostic procedures for cancer suspicion after the initial examination were performed in the hospital. Interventional radiologists performed needed biopsies, and the treatment, when applicable, was managed by the Departments of Thoracic Surgery and Clinical Oncology (Thoracic and Head and Neck division) from the hospital.
Complementarily, blood and sputum were collected from all participants and stored at the biobank of the hospital for future biomarker analysis. Data from all the endings were entered into a secure online database (REDCap©)45,46 and made available to every user according to each credential’s allowance level. Informed consent was obtained from all the participants undergoing LDCT screening and before collecting biological samples or undergoing medical procedures.
This study was approved by the Barretos Cancer Hospital Ethics Committee (n° 2.907.024), and all participants signed informed consent forms for study participation, including questionnaire answering, LDTC screening and blood and sputum collection.
Quality Control of Cancer Prevention
As a quality control intervention, the central coordination department double-checks the screening eligibility of appointments, as does the mobile unit team onsite. Every involved physician is board certified. Expert thoracic radiologists assessed the LDCT, and the structured report was made under the LungRads 1.147 categorization. The multidisciplinary team has substantial experience in thoracic oncology. The involved interventional radiologists have 10 years or more of experience in thoracic percutaneous biopsy. The database results are periodically reviewed to check for discrepant tendencies, and sample analysis from the issued reports is performed in peer review. The mobile CT unit and the regular CT units undertake weekly quality control tests by a physicist.
Radiometric surveys and quality control tests were performed on the mobile CT unit before its usage to satisfy security requirements and reliability. The mobile unit’s displacement was restricted to the city boundaries, thus allowing necessary adjustments or unpredicted malfunctions correction to be promptly addressed.
Measurements and Outcomes
One year after starting the combined smoking cessation and LDCT screening intervention, participants were contacted by telephone call by the research team and asked about their current smoking status. Participant characteristics, such as age, sex, self-reported ethnicity, and years of education, were pooled together with smoking-related data, such as current smoking status, pack-years, and degree of nicotine dependence by the Fagerstrom score scale. Eligibility criteria and source entry into the program were also included.
For those who underwent LDCT screening, the lung-RADS category of the first exam, tomographic findings related to smoking, medical procedures, diagnoses and clinical staging were recorded.
As a secondary objective in this study, the effectiveness of mobile LDCT tomographic screening and participation in smoking cessation groups were investigated. The association between quit smoking and participation in a smoking cessation group was analyzed by the chi-square test, which was performing using SPSS version 22. The level of significance for the statistical tests was 5%.
Results
The program officially began on May 31st, 2019, the World No Tobacco Day, when the mobile CT unit was dispatched to downtown Barretos and stayed for two days in a promotional and educational event with the municipal health teams (Figure 4).
Figure 4.
Displacement of the mobile CT unit to Barretos downtown on “World No Tobacco Day” for awareness and assessment of high-risk individuals. Indicated LDCT screening examinations were performed immediately, and the results were posteriorly sent to the municipal health care unit of the screenee’s neighborhood.
Overall, 233 high-risk individuals were included in the program, 177 were current smokers, and 55 were former smokers. One year after the beginning of the program, a telephone survey obtained information from 107 respondents (60.5%). Among smokers, 54 (30.5%) participants were involved in smoking cessation groups, of which all received nicotine substitutes and medication, and 15 (27.8%) quit smoking. In total, 23 (13,0%) high-risk participants quit smoking, and the majority quit smoking over 1 year (83.4%) (Table 1). Participation in a smoking cessation group increased the odds of quitting smoking by 2-fold – OR 2.16 (CI 95%: .83-5.64), yet this increase was not statistically significant (P value = .158) (Table 1).
Table 1.
Status of 177 Smokers 1 Year after LDCT Intervention.
| Initially smokers (n = 177) | ||||
|---|---|---|---|---|
| Status one year after LDCT intervention | ||||
| Current smoking | Quit smoking | No information | ||
| Variable | Parameters | 84 (47.4%) | 23 (13.0%) | 72 (40.6%) |
| Smoking cessation group | Yes = 54 | 39 (46.4%) | 15 (65.2%) | |
| No = 53 | 45 (53.6%) | 8 (34.8%) | ||
| Abstinence time | <12 months | — | 3 (13,3%) | |
| ≥12 months | — | 19 (83,4%) | ||
| No info | — | 1 (3.3%) | ||
Association between quit smoking and participation in a smoking cessation group: OR 2.16 (CI 95%: .83 – 5.64); Chi-square P-value = .158.
Among the screened subjects, 128 (54.9%) were women and 105 (45.1%) were men. The average age was 62 years old, 123 (52.8%) had high or very high nicotine dependence on the Fagerstrom score scale, and 114 (48.9%) had over 45 pack-years of history. Seventy-two (30.9%) participants were referred from primary care general practitioners, and 130 (55.8%) were included without a prior referral in the abovementioned and on-site opportunistic screening campaign on the “World No Tobacco Day” (Table 2). We found that 77.3% of participants reported at least 1 prior tobacco cessation attempt, and 87.5% accepted professional guidance to quit. Moreover, 74.3% of these subjects assumed that stopping smoking could moderately decrease the risk of developing lung cancer, but 25.6% reported never having a concern about the possibility of developing lung cancer.
Table 2.
Summary of LDCT Screening Participants Features.
| Variables | Parameters | n/measure | (%) |
|---|---|---|---|
| Age, years | Mean (range) | 62 (38-81) | — |
| Sex | Female | 128 | (54.9) |
| Male | 105 | (45.1) | |
| Self-reported color of the skin/etnicitya | White | 129 | (55.4) |
| Black | 17 | (7.3) | |
| Brown | 80 | (34.3) | |
| Asian | 5 | (2.1) | |
| Missing | 2 | (.9) | |
| Escolarity | Illiterate | 4 | (1.7) |
| ≤4 years of education | 68 | (29.2) | |
| >4 and ≤8 years of education | 53 | (22.7) | |
| >8 and ≤12 years of education | 61 | (26.2) | |
| >12 years of education | 41 | (17.6) | |
| Missing | 6 | (2.6) | |
| Smoking status | Smoker | 177 | (76.0) |
| Former smoker | 56 | (24.0) | |
| Pack-years | Mean (range) | 56.9 (10-302.5) | — |
| Median | 45 | — | |
| Nicotine dependence grade | Low or medium | 104 | (44.6) |
| High or very high | 123 | (52.8) | |
| Missing | 6 | (2.6) | |
| NLST eligible | NLST criteria | 183 | (78.5) |
| PLCOm2012 riskb | ≥1.5 | 164 | (70.4) |
| ≥1.61 | 159 | (68.2) | |
| ≥2.0 | 136 | (58.4) | |
| Provenience | Smoking cessation groups | 103 | (44.2) |
| On-site opportunistic | 130 | (55.8) |
aSelf-reported color of the skin/ethnicity according to Brazilian Institute of Geography and Statistics (IBGE).
bestimated 6-year risk of lung cancer using PLCOM2012 international model, retrospectively applied to the sample.
The first round LDCT screening revealed 195 (83.7%) of category Lung-Rads 1 or 2, 17 (7.3%) Lung-Rads 3, 18 (7.7%) Lung-Rads 4a and 3 (1.3%) Lung-Rads 4b or 4x, revealing adequate concordance to ACR prevalence estimates47 despite the small sample of these preliminary results (Table 3). Individuals with baseline scans classified as Lung-Rads categories 3 or 4a were reassessed with a supplemental LDCT scan at 6 months or 3 months, strictly according to the Lung-Rads protocol. In this sample, all 35 Lung-Rads Category 3 or 4a nodules were reclassified as category Lung-Rads 2 in the control examination due to stability or regression of findings. Only three (1.3%) of the screened patients needed a biopsy, all of whom were diagnosed with lung cancer.
Table 3.
Summary of LDCT Screening Results.
| Variables | Parameters | n | (%) |
|---|---|---|---|
| Lung-rads categories | Category 1 or 2 | 195 | (83.7) |
| Category 3 | 17 | (7.3) | |
| Category 4a | 18 | (7.7) | |
| Category 4b ou 4x | 3 | (1.3) | |
| Category modifier S | 10 | (4.3) | |
| Tobacco-related findingsa | Pulmonary emphysema | 128 | (54.9) |
| Bronchial wall thickening | 133 | (57.1) | |
| Coronary arteries calcification | 146 | (62.7) | |
| Invasive diagnosis | Transthoracic percutaneous needle biopsy | 3 | (100) |
| Histology | Adenocarcinoma | 1 | (33.3) |
| Squamous cell carcinoma | 2 | (66.6) | |
| Disease staging | Stage IA | 2 | (66.6) |
| Stage IB | 1 | (33.3) |
asimplified qualitative or semiquantitative classification by thoracic radiologists.
Three cases of lung cancer were diagnosed among the 233 participants, leading to a diagnosis rate of 12.8/1000. The lung cancer cases were clinical stage I, including two adenocarcinomas (clinical stages IA and IB), and 1 squamous cell carcinoma (clinical stage IA). These numbers contrast with the proportion of early-stage clinical cases attended in our hospital (9.2% clinical stage I in 2017) or reported nationwide (8% clinical stage I). Additionally, a nonsuspected breast cancer, clinical stage I, and 1 ascending aortic aneurysm elected for surgical intervention were diagnosed.
Discussion
The present study described the implementation of integrated lung cancer prevention and screening with the first Latin American low-dose CT mobile unit for lung cancer screening. In the program’s scope, 18 smoking cessation groups were trained in the public primary health care units, serving hundreds of smokers. In parallel, 233 high-risk participants underwent LDCT screening in the mobile unit, leading to the detection of three early-stage and curable lung cancers. Quality control ensured that only high-risk individuals underwent LDCT screening. A similar proportion of Lung-RADS categories were obtained compared to ACR estimates, and all invasive diagnoses indicated and performed proved underlying malignancy.
This program broadens and enhances primary health care duties, offering a resource that is not yet available in the current public health context in a public–private partnership model to reduce lung cancer mortality in the long run. However, some ancillary benefits already achieved by the initiative may be highlighted, as the instrumentation of the primary care to leverage the smoker assessment, the better illustration of the impact of other health conditions related to tobacco usage, the renewal of smoker self-care, and the potential strengthening of the bond between the individual and primary health care professionals.
A great challenge in our design setting was the effective recruitment of the target population, which should be worked out creatively. In the program’s first year, less than 10% of the total high-risk population estimates were recruited. A higher proportion of women and more educated individuals was observed than internal data from lung cancer individuals treated in our hospital, indicating the need to expand or better orientate the actions on recruitment to reach those that are more socially and economically vulnerable. We still need more data to better understand possible cultural and functional barriers. A potential limitation of the present study is the bias and representation of the population included, and further studies with a larger sample also focusing on public primary health care hospitals and clinics and on smoking cessation outcomes are warranted to fully ascertain the efficacy of the present approach.
It comes to attention that 55.8% of the individuals were recruited in the two days in which the mobile CT unit was dispatched to the community, suggesting that recruitment from the primary health care units is still incipient but also that there is excellent potential for opportunistic recruitment in the community by the mobile unit itself. Interestingly, the idea to use mobile LDCT units for lung cancer screening has been explored in recent studies,32,33 with evidence indicating that it can improve outreach among distant populations and among those that are reluctant to be screened.33 Moreover, the mobile unit may also be an advertising and educational tool,33 promoting awareness of lung cancer risks and the benefits of screenings.
Although the mobile CT unit was designed to displace, even on secondary roads, the equipment’s dimensions and sensibility pose limits to extend the usage in any context, so remote areas with bad traffic conditions could be problematic, and a proper logistic arrangement should be set.
Although there is confidence in the role of LDCT screening in reducing lung cancer mortality, the high-risk group’s proper definition remains unclear. The concept of a high-risk group is evolving to a more individualized estimate, explored by some authors in mathematical models.48,49 The more accurate the high-risk definition is, the higher the pretest probability and the higher the proportion of lung cancer diagnosis, thereby improving the whole screening process’s cost-effectiveness.50 Moreover, bimolecular research and the identification of serum or sputum biomarkers could be useful in selecting high-risk individuals for screening. As part of this evolution, in 2020, we incorporated the PLCOM2012 international risk model51 as a tool for the complementary selection of high-risk individuals for LDCT screening. Whether there are genotypic-phenotypic differences in the highly miscegenated Brazilian population that impact the high-risk selection criteria concerning other better-known populations has yet to be studied. Nevertheless, our group has shown the association of genetic ancestry with important genetic alterations of lung cancer.52,53
Of note, health-care financing is a great limiting factor26 to implement a lung cancer screening program in Brazil, as experienced in this study. The Brazilian governmental regulatory office does not yet recognize LDCT as a secondary lung cancer prevention tool, and there was no payment set up for this application. The lack of provision precludes the private sector’s engagement, the owner of a significant proportion of the available CT devices. A national guideline could provide conditions to direct payment for the procedure and establish quality metrics to be met, accreditation criteria, and surveillance protocols. Due to the lack of national guidelines and government support, lung cancer screening in Brazil is still incipient and almost restricted to the private initiative. The very little available evidence49,54,55,56 points to favorable preliminary results and potential adequacy of international high-risk selection criteria and recommendations, although the representativeness of those findings to the general population still needs to be investigated.
Additionally, since it was built, our mobile CT unit has also been used in actions outside this program’s scope due to its great versatility and new urgent demands, such as coronavirus pandemics. The mobility of a high-technology tool with high diagnostic sensibility and limited availability is certainly a resource to be explored in different scenarios, the more diversified as its scarcity increases.
Conclusions
The Brazilian health system is complex, with insufficient and poorly distributed resources. Here, we present an innovative lung cancer prevention and screening program using a mobile CT unit that operates in the national public health model based in the community, enhancing it. This creative solution can reach high-risk individuals, with promising preliminary results that may constitute a reference for future national models. New strategies are necessary to improve recruitment. On the other hand, new perspectives are opened to better characterize the population, highlighting pretest risk prospection and cancer biomarker research.
Supplemental Material
Supplemental Material for Implementation of an Integrated Lung Cancer Prevention and Screening Program Using a Mobile Computed Tomography (CT) Unit in Brazil by Rodrigo Sampaio Chiarantano, Fabiana Lima Vazquez, Alexander Franco, Larissa Cristina Ferreira, Maraísa Cristina da Costa, Thais Talarico, Ângela Neves Oliveira, José Elias Miziara, Edmundo Carvalho Mauad, Eduardo Caetano da Silva, Luis Marcelo Ventur, Raphael Haikel Junior, Letícia Ferro Leal, and Rui Manuel Reis in Cancer Control
Acknowledgments
The authors would like to thank Dr. Martin C. Tammemägi for the use of the PLCOM2012 international risk model, all members of the GTOP group (Translational Group of Pulmonary Oncology – Barretos Cancer Hospital, Brazil) for scientific discussion and suggestions, and Dr. Ricardo Sales dos Santos for the preliminary discussion of the lung cancer program.
Appendix.
Abbreviations
- CT
computed tomography
- LDCT
low-dose computed tomography
Author’s Contribution: Rodrigo Sampaio Chiarantano: conceptualization, methodology, investigation, writing – original draft, visualization; Fabiana Lima Vazquez: methodology, writing – review and editing; Alexander Franco: resources; Larissa Cristina Ferreira: data collection and curation; Maraisa Cristina da Costa: data collection and curation; Ângela Neves Oliveira: data collection and curation; Thais Talarico: data collection and curation; José Elias Miziara: conceptualization, data collection; Eduardo Caetano da Silva: conceptualization, data collection; Luis Marcelo Ventura: conceptualization, data collection; Raphael Haikel Junior: conceptualization, data collection; Leticia Ferro Leal: conceptualization, data collection, writing – review and editing; Edmundo Carvalho Mauad: conceptualization, data collection; Rui Manuel Reis: conceptualization, resources, writing – review and editing, supervision, project administration.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research: This work was supported by Barretos Cancer Hospital and the Public Ministry of Labor Campinas (Research, Prevention and Education of Occupational Cancer). FV, LCL, MCF, and LFL are supported by the Public Ministry of Labor Campinas (Research, Prevention, and Education of Occupational Cancer) in Campinas, Brazil. RMR is a recipient of a CNPq Productivity (Brazil) fellowship.
Ethical Approval: This study was approved by the Barretos Cancer Hospital Ethics Committee (n° 2.907.024), and all participants signed informed consent forms for study participation, including questionnaire answering, LDTC screening and blood and sputum collection.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Ângela Neves Oliveira https://orcid.org/0000-0002-4775-6417
Rui Manuel Reis https://orcid.org/0000-0002-9639-7940
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Read C, Janes S, George J, Spiro S. Early lung cancer: Screening and detection. Prim Care Respir J. 2006;15(6):332-336. doi: 10.1016/j.pcrj.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.INCA . Estimate/2020 - Cancer Incidence in Brazil. 2019. http://www.inca.gov.br [Google Scholar]
- 4.Boing AF, Rossi TF. Tendência temporal e distribuição espacial da mortalidadepor câncer de pulmão no Brasil entre 1979 e 2004: Magnitude, padrões regionais e diferenças entre sexos. J Bras Pneumol. 2007;33(5):544-551. https://www.jornaldepneumologia.com.br/how-to-cite/250/en-US [DOI] [PubMed] [Google Scholar]
- 5.Dela Cruz C, Tanoue L, Matthay R. Lung cancer: Epidemiology, etiology, and prevention. Clin Chest MedLung. 2011;32(4):1-61. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridge C, McErlean A, Ginsberg M. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(02):093-098. doi: 10.1055/s-0033-1342949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy D, de Almeida LM, Szklo A. The Brazil simSmoke policy simulation model: The effect of strong tobacco control policies on smoking prevalence and smoking-attributable deaths in a middle income nation. PLoS Med. 2012;9(11):e1001336. doi: 10.1371/journal.pmed.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azevedo E, Silva G, De Moura L, et al. The fraction of cancer attributable to ways of life, infections, occupation, and environmental agents in Brazil in 2020. PLoS One. 2016;11(2):1-13. doi: 10.1371/journal.pone.0148761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midthun DE. Screening for lung cancer. Clin Chest Med. 2011;32(4):659-668. doi: 10.1016/j.ccm.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 10.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Koning HJ, Van Der Aalst CM, De Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 12.Wood DE, Kazerooni EA, Baum SL, et al. Lung cancer screening, version 3.2018. JNCCN J Natl Compr Cancer Netw. 2018;16(4):412-441. doi: 10.6004/jnccn.2018.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren GW, Ostroff JS, Goffin JR. Lung cancer screening, cancer treatment, and addressing the continuum of health risks caused by tobacco. Am Soc Clin Oncol Educ B. 2016;36:223-229. doi: 10.14694/edbk_158704. [DOI] [PubMed] [Google Scholar]
- 14.Goffin JR, Flanagan WM, Miller AB, et al. Cost-effectiveness of lung cancer screening in Canada. JAMA Oncol. 2015;1(6):807-813. doi: 10.1001/jamaoncol.2015.2472. [DOI] [PubMed] [Google Scholar]
- 15.Cao P, Jeon J, Levy DT, et al. Potential impact of cessation interventions at the point of lung cancer screening on lung cancer and overall mortality in the United States. J Thorac Oncol. 2020;15(7):1160-1169. doi: 10.1016/j.jtho.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen JH, Tønnesen P, Ashraf H. Smoking cessation and lung cancer screening. Ann Transl Med. 2016;4(8):157. Published online. doi: 10.21037/atm.2016.03.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steliga MA, Yang P. Integration of smoking cessation and lung cancer screening. Transl Lung Cancer Res. 2019;8(suppl 1):S88-S94. doi: 10.21037/tlcr.2019.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochhegger B, Camargo S, da Silva Teles GB, et al. Challenges of implementing lung cancer screening in a developing country: Results of the second Brazilian early lung cancer screening trial (BRELT2). JCO Glob Oncol. 2022;8:1-6. doi: 10.1200/GO.21.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paim J, Travassos C, Almeida C, Bahia L, MacInko J. The Brazilian health system: History, advances, and challenges. Lancet. 2011;377(9779):1778-1797. doi: 10.1016/S0140-6736(11)60054-8. [DOI] [PubMed] [Google Scholar]
- 20.Macinko J, Harris MJ. Brazil’s family health strategy — delivering community-based primary care in a universal health system. N Engl J Med. 2015;372(23):2177-2181. doi: 10.1056/NEJMp1501140. [DOI] [PubMed] [Google Scholar]
- 21.da Silva SF. Organização de redes regionalizadas e integradas de atenção à saúde: Desafios do sistema ÚNico de saúde (Brasil). Cienc e Saude Coletiva. 2011;16(6):2753-2762. doi: 10.1590/S1413-81232011000600014. [DOI] [PubMed] [Google Scholar]
- 22.Castro MC, Massuda A, Almeida G, et al. Brazil’s unified health system: The first 30 years and prospects for the future. Lancet. 2019;394(10195):345-356. doi: 10.1016/S0140-6736(19)31243-7. [DOI] [PubMed] [Google Scholar]
- 23.Lista M, Bes F, Pereira J, Ikari F, Nikaedo S. Excessiva demora no diagnóstico clínico do câncer de pulmão. Depende do médico, do paciente ou do sistema. Arq Med Hosp Fac Cienc Med St Casa São Paulo. 2008;53(1):6-9. [Google Scholar]
- 24.Araujo LH, Baldotto C, Castro G, Jr, et al. Lung cancer in Brazil. J Bras Pneumol. 2018;44(1):55-64. doi: 10.1590/s1806-37562017000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bingham A, Bishop A, Coffey P, et al. Factors affecting utilization of cervical cancer prevention services in low-resource settings. Salud Publica Mex. 2003;45(5):408-416. doi: 10.1590/S0036-36342003000900015. [DOI] [PubMed] [Google Scholar]
- 26.Barrios CH, Werutsky G, Mohar A, et al. Cancer control in latin America and the caribbean: recent advances and opportunities to move forward. Lancet Oncol. 2021;22(11):e474-e487. doi: 10.1016/S1470-2045(21)00492-7. [DOI] [PubMed] [Google Scholar]
- 27.DATASUS . Brasil. Ministério da saúde. Informações de saúde (TABNET). Rede Assistencial. 2021. http://www2.datasus.gov.br/DATASUS/index.php?area=0204&id=11671&VObj=http://tabnet.datasus.gov.br/cgi/deftohtm.exe?cnes/cnv/equipo [Google Scholar]
- 28.(IBGE) (Brasil. Instituto Brasileiro de Geografia e Estatística. Brasil | Cidades e Estados | IBGE. 2021. https://www.ibge.gov.br/cidades-e-estados
- 29.Haikel RL, Mauad EC, Silva TB, et al. Mammography-based screening program: Preliminary results from a first 2-year round in a Brazilian region using mobile and fixed units. BMC Womens Health. 2012;12:32. doi: 10.1186/1472-6874-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauad EC, Nicolau SM, Gomes UA, et al. Can mobile units improve the strategies for cervical cancer prevention? Diagn Cytopathol. 2009;36(4):727-730. doi: 10.1002/dc.21287. [DOI] [PubMed] [Google Scholar]
- 31.Mauad EC, Silva TB, Latorre MRDO, et al. Opportunistic screening for skin cancer using a mobile unit in Brazil. BMC Dermatol. 2011;11:7-12. doi: 10.1186/1471-5945-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crosbie PA, Balata H, Evison M, et al. Implementing lung cancer screening: Baseline results from a community-based “Lung health check” pilot in deprived areas of Manchester. Thorax; 2018. [DOI] [PubMed] [Google Scholar]
- 33.Headrick JR, Morin O, Miller AD, Hill L, Smith J. Mobile lung screening: Should we all get on the bus? Ann Thorac Surg. 2020;110(4):1147-1152. doi: 10.1016/j.athoracsur.2020.03.093. [DOI] [PubMed] [Google Scholar]
- 34.Instituto Brasileiro de Geografia e Estatística (IBGE) . Censo. 2010. http://www.censo2010.ibge.gov [Google Scholar]
- 35.Instituto Brasileiro de Geografia e Estatística (IBGE) . Pesquisa nacional de saúde;2013. http://www.ibge.gov.br [Google Scholar]
- 36.Brasileiro de Georgafia e Estatística, Instituto Nacional de Câncer José Alencar Gomes da Silva . Pesquisa Especial de Tabagismo - PETab. Relatório Brasil;2009. [Google Scholar]
- 37.De Castro Mattos JS, Mauad EC, Syrjänen K, et al. The impact of breast cancer screening among younger women in the barretos region, Brazil. Anticancer Res. 2013;33(6):2651-2656. [PubMed] [Google Scholar]
- 38.Greenwald ZR, Fregnani JH, Longatto-Filho A, et al. The performance of mobile screening units in a breast cancer screening program in Brazil. Cancer Causes Control. 2018;29(2):233-241. doi: 10.1007/s10552-017-0995-7. [DOI] [PubMed] [Google Scholar]
- 39.Mauad EC, Gomes UA, Nogueira JL, Melani AGF, Lemos DL, Hidalgo GS. Prevention of cervical cancer in a poor population in Brazil. Fam Pract. 2002;19(2):189-192. doi: 10.1093/fampra/19.2.189. [DOI] [PubMed] [Google Scholar]
- 40.Hunt B, Fregnani JHTG, Schwarz RA, et al. Diagnosing cervical neoplasia in rural Brazil using a mobile van equipped with in vivo microscopy: A cluster-randomized community trial. Cancer Prev Res. 2018;11(6):359-370. doi: 10.1158/1940-6207.CAPR-17-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Possati-Resende JC, Vazquez FDL, Biot ST, et al. Organized cervical cancer screening program in barretos, Brazil: Experience in 18 municipalities of são paulo state. Acta Cytol. 2018;62(1):19-27. doi: 10.1159/000480446. [DOI] [PubMed] [Google Scholar]
- 42.Silveira C, Mauad E. Analysis of a decade of skin cancer prevention using a mobile unit in Brazil. Rural Remote Health. 2019;19(3):4599. doi: 10.22605/RRH4599. [DOI] [PubMed] [Google Scholar]
- 43.Guimarães DP, Mantuan LA, de Oliveira MA, et al. The performance of colorectal cancer screening in Brazil: The first two years of the implementation program in Barretos Cancer Hospital. Cancer Prev Res. 2020;1:0179. canprevres. doi: 10.1158/1940-6207.CAPR-20-0179. [DOI] [PubMed] [Google Scholar]
- 44.INCA . Protocolo Clínico e Diretrizes Terapêuticas do Tabagismo. Published. 2020. https://www.cevs.rs.gov.br/upload/arquivos/201704/25092135-protocolo-clinico-e-diretrizes-terapeuticas-dependencia-a-nicotina-inca-2014.pdf [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lung-RADS®RC on ACof . Lung-RADS Version 1.1. 2019. https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf
- 48.Gray EP, Teare MD, Stevens J, Archer R. Risk prediction models for lung cancer: A systematic review. Clin Lung Cancer. 2016;17(2):95-106. doi: 10.1016/j.cllc.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Miranda-Filho A, Charvat H, Bray F, et al. A modeling analysis to compare eligibility strategies for lung cancer screening in Brazil. eClinicalMedicine. 2021;42:101176. doi: 10.1016/j.eclinm.2021.101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cressman S, Peacock SJ, Tammemägi MC, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol. 2017;12(8):1210-1222. doi: 10.1016/j.jtho.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: Screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11(12):e1001764. doi: 10.1371/journal.pmed.1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leal LF, Laus AC, Cavagna R, et al. EGF+61 A’G polymorphism does not predict response to first-generation EGFR tyrosine kinase inhibitors in lung cancer patients. Thorac Cancer. 2020;11(10):2987-2992. doi: 10.1111/1759-7714.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leal LF, de Paula FE, De Marchi P, et al. Mutational profile of Brazilian lung adenocarcinoma unveils association of EGFR mutations with high Asian ancestry and independent prognostic role of KRAS mutations. Sci Rep. 2019;9(1):1-10. doi: 10.1038/s41598-019-39965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos RSdos, Franceschini JP, Chate RC, et al. Do current lung cancer screening guidelines apply for populations with high prevalence of granulomatous disease? results from the first brazilian lung cancer screening trial (BRELT1). Ann Thorac Surg. Published online. 2015;101(2):1-8. doi: 10.1016/j.athoracsur.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Teles GBDS, Macedo ACS, Chate RC, Valente VAT, Funari MBDG, Szarf G. LDCT lung cancer screening in populations at different risk for lung cancer. BMJ Open Respir Res. 2020;7(1):1-6. doi: 10.1136/bmjresp-2019-000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hochhegger B, Camargo S, da Silva Teles GB, et al. Challenges of implementing lung cancer screening in a developing Country: Results of the Second Brazilian Early Lung Cancer Screening Trial (BRELT2). JCO Glob Oncol. 2022;8:e2100257. doi:10.1200/GO.21.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Implementation of an Integrated Lung Cancer Prevention and Screening Program Using a Mobile Computed Tomography (CT) Unit in Brazil by Rodrigo Sampaio Chiarantano, Fabiana Lima Vazquez, Alexander Franco, Larissa Cristina Ferreira, Maraísa Cristina da Costa, Thais Talarico, Ângela Neves Oliveira, José Elias Miziara, Edmundo Carvalho Mauad, Eduardo Caetano da Silva, Luis Marcelo Ventur, Raphael Haikel Junior, Letícia Ferro Leal, and Rui Manuel Reis in Cancer Control