Abstract
The human gut possesses millions of microbes that define a complex microbial community. The gut microbiota has been characterized as a vital organ forming its multidirectional connecting axis with other organs. This gut microbiota axis is responsible for host-microbe interactions and works by communicating with the neural, endocrinal, humoral, immunological, and metabolic pathways. The human gut microorganisms (mostly non-pathogenic) have symbiotic host relationships and are usually associated with the host’s immunity to defend against pathogenic invasion. The dysbiosis of the gut microbiota is therefore linked to various human diseases, such as anxiety, depression, hypertension, cardiovascular diseases, obesity, diabetes, inflammatory bowel disease, and cancer. The mechanism leading to the disease development has a crucial correlation with gut microbiota, metabolic products, and host immune response in humans. The understanding of mechanisms over gut microbiota exerts its positive or harmful impacts remains largely undefined. However, many recent clinical studies conducted worldwide are demonstrating the relation of specific microbial species and eubiosis in health and disease. A comprehensive understanding of gut microbiota interactions, its role in health and disease, and recent updates on the subject are the striking topics of the current review. We have also addressed the daunting challenges that must be brought under control to maintain health and treat diseases.
Keywords: human gut microbiota, health, disease, eubiosis, dysbiosis, pathogenic
Introduction
The association of human health with the intestine has been long acknowledged as Hippocrates said, “Death sits in the bowls” in 400 B.C. Many studies worldwide have focused on the significant impact of intestinal microbiota on human health and disease (AboNahas et al., 2022). The human body is colonized by a diversity of bacteria, viruses, archaea, and unicellular eukaryotes. Microbes inhabit all human body surfaces, but a significant number of microbes live in the gastrointestinal tract/gut. The human gut possesses approximately more than one thousand microbial species that form a complex ecological community called gut microbiota (Lagier et al., 2016). The human gut microbiota is carrying about 150 times more genes compared to the entire human genome. It is widely accepted that approximately a hundred trillion microbes live on and inside the human body having a key role in various biological processes including health and disease (Wang et al., 2017). They are the primary mediators of body homeostasis, impacting various physiological activities, such as metabolism, barrier homeostasis, inflammation, and hematopoiesis through both intestinal and extra-intestinal actions. The gut microbiota has recently been classified as a “vital organ” because of its multidirectional and communicational connection or axis with other organs through neural, endocrine, humoral, immunological, and metabolic pathways. Any change in the microbial community not only causes gut-related issues but also influences other organs related diseases, though the actual interaction mechanism between the gut and the organs has yet to be fully understood (Ahlawat and Sharma, 2021).
The interaction between host and microbes plays a pivotal role in both health and disease. Gut microbiota diversity is greatly dependent on various host factors including diet, human lifestyle, age, and environmental factors. However, diet is currently considered one of the major factors (modifiers) in modulating the gut microbiota (Simões et al., 2022). Human microbiota has promising potential in altering appetite, increasing nutrient harvest, and exerting energy from various food components. Microbes have also a fundamental role in xenobiotic metabolism. In xenobiotic metabolism, various gut microbes alter the chemical structures of various diet components, drugs, pollutants, and many pesticides (Nakov and Velikova, 2020).
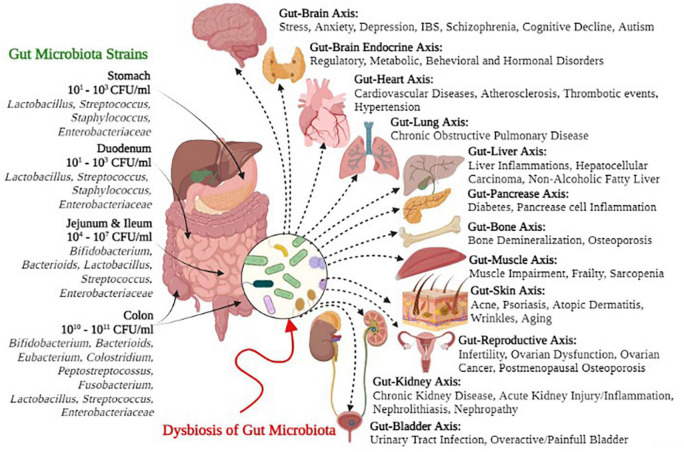
Many research studies have supported the concept that gut microbiota plays a key role in modulating immunity, weight gain or loss, energy homeostasis, and obesity-related disorders (Piccioni et al., 2022). Likewise, gut microbiota and their metabolites are associated with various non-alcoholic fatty liver diseases (NAFLDs), inflammatory bowels diseases (IBDs), hepatocellular carcinoma, cardiovascular diseases (CVDs), alcoholic liver disease (ALD), chronic kidney diseases (CKDs), and cirrhosis (Hsu et al., 2020; Jansen et al., 2021; Ryma et al., 2021; Wang et al., 2021; Zhou et al., 2021; Philips et al., 2022). Figure 1 depicts several symbiotic gut microbial strains and the possible negative health consequences of dysbiosis on the gut-organ axis. Hence, the comprehensive understanding of recent gut microbiota interactions, their eubiotic role in health and disease, and other recent updates on this subject are compiled in this review, with a major focus on controlling the challenges to maintain health and treat various diseases.
FIGURE 1.
Gut microbial strains and negative health outcomes of gut microbial dysbiosis.
Significance of human gut microbiota eubiosis
Comprehensive clinical studies are available on microbiota and involvement in their balance, i.e., eubiosis and related pathophysiological aspects. The compositional difference in gut microbiota has been observed in health and disease conditions. Eubiosis conditions are effective in controlling various diseases caused by microbes. Proper intake of a healthy diet and the development of eubiosis acts in favor of human health. The high intake of antibiotics causes an imbalance in the gut microbiota and favors systemic diseases (Santacroce et al., 2021).
Several population-based studies have revealed the highly beneficial role of human gut microbiota in healthy people, as well as the importance of well-understanding its structure and the factors that influence its composition, such as food, age, geography, systemic disorders, and drugs (Wang et al., 2017; Rowland et al., 2018). Phyla Firmicutes, Bacteroides, Actinobacteria, Proteobacteria, and Verrucomicrobia contribute to the significant resident bacterial populations in the gut microbiome (Fava et al., 2019). The first step in identifying the symbiotic interactions between intestinal microbes and their hosts is to describe the balanced composition of gut microbiota and disease-related variations. The microbes reside in a mutual association with the host in a healthy state, affecting the host’s health by controlling nutrient metabolism, defending against pathogens, and delivering signals to immune cells to promote host physiology and immunity (Ribaldone et al., 2022). An initial underestimation of the total number of microbial species in the intestine has been described through several vivo and ex vivo studies due to complications in culturing certain microorganisms (Lagier et al., 2015).
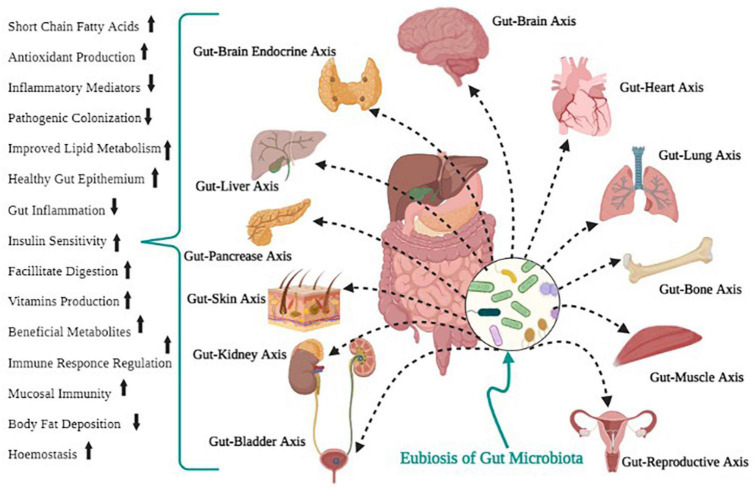
Bacteria and proteobacteria contribute to carbohydrate digestion, gut microbiota, regulation of the immune system, and defense against pathogen colonization (Rosser and Mauri, 2016; Fan and Pedersen, 2021). For survival, microbes in the intestine tract mainly depend on dietary substrates undigested in the upper digestive tract. Saccharolytic bacterial fermentation typically creates advantageous metabolites, while bacteria switch to an alternative energy source if there are insufficient carbohydrates, leading to the development of other metabolites that could be more disadvantageous to human health (Rowland et al., 2018). Methanobrevibacter smithii is the human-associated Archaea that plays a vital function in the synthesis of methane from H2 processed by bacterial metabolism. It is a prominent and essential Archean in the gut microbiota (Hoffmann et al., 2013; Berry, 2016). Some of the beneficial functions of gut microbiota for human health are shown in Figure 2.
FIGURE 2.
Positive health outcomes of gut microbial eubiosis.
It is considered that diet is a significant factor associated with health and disease control, but some recent studies concluded that diet is pivotal for shaping the gut microbial structure and influencing the metabolism of the host. The gut environment, sequentially, can help reproduce, grow, and survive the microbial community (Browne et al., 2016). Carbohydrates are an essential and significant energy source; also, intestinal microbiota has provided a fermentation stage to deliver vital biomolecules to the host (Conlon and Bird, 2015).
A normal balance between the host and gut flora is essential for human health, while disruption is linked with various human diseases, like hypertension, obesity, cardiovascular disorders, diabetes, and IBD (Von Martels et al., 2017; Kho and Lal, 2018; Szablewski, 2018). However, the human microbiome analysis is still at its initial phase in filling the knowledge gap in the microbiome-host relationship and its role in disease pathogenesis and therapeutical importance. Therefore, further in-depth research is needed to unravel this fascinating yet enigmatic area of study.
Gut microbiota and human metabolism
The diverse human microbiome has substantial metabolic activities essential for the functioning of mammalian enzymes in the gut mucosa and liver and the host metabolism. Gut microbiota influence host health by shaping the biochemical profile of the diet. The significant role of gut microbiota in human immunity has promoted research to investigate the contributions of particular microbes in metabolic pathways, especially in dietary components’ metabolism (Cardona and Roman, 2022). Recent studies have found that gut microbiota can metabolize phytochemicals, especially polyphenols, by well-defined paths (Rowland et al., 2018). The human gut microbiota reacts efficiently to major dietary changes. The presence of these fast, diet-induced patterns is confirmed by evidence from individuals switching between plant and meat-based diets, adding to their diet more than 30 g of specific dietary fibers a day or adapting either a high-fiber-low fat diet or a low-fiber-high-fat diet for ten days; in all cases, the structure and composition of microbiome changed over 1–2 days (Wu et al., 2011; David et al., 2014). This flexibility may be an advantageous feature of enlisting microbes as part of the digestive structure, particularly when considering the potential day-to-day variability in food available to foragers. It may also be an inescapable consequence of dealing with such a microbial community that is diverse and competitive and undergoes rapid turnover. Human gut microbiota is associated with the degradation of dietary fibers, proteins, and peptides by fermentation and anaerobic degradation (Yadav et al., 2018).
Carbohydrates and simple sugars are the main components of food metabolized by gut microbiota. Bacterial species, especially the phyla Bacteroidetes and Firmicutes, can ferment fibers (the indigestible carbohydrates) to produce branched-chain and short-chain fatty acids (SCFAs), lactate, ethanol, hydrogen, and carbon dioxide; these products are further used by the host or excreted (Patrascu et al., 2017). Acetate, propionate, and butyrate are the main short-chain fatty acids (SCFAs) distinguished in human feces, usually found in 3:1:1 to 10:2:1 molar ratio; this ratio is consistent with the values reported in the intestine in early sudden deaths (Rowland et al., 2018). These are the main SCFAs that perform several essential functions in the human body (Rauf et al., 2022). Butyrate is perhaps the essential SCFA for human health, as it is the primary source of energy for human colonocytes (Wang et al., 2019). Butyrate has the potential to act as an anti-carcinogen as it persuades apoptosis of colon cancer cells and regulates gene expression by inhibiting histone deacetylase (Havenaar, 2011; Steliou et al., 2012). Propionate is also an essential energy source for the epithelial cells in the liver; it plays a vital role in gluconeogenesis (Cani, 2018). Acetate helps in the growth of other bacteria as an essential co-factor; for example, Faecalibacterium prausnitzii will not grow in pure culture in the absence of acetate (Rowland et al., 2018).
Human gut microbiota can also synthesize essential vitamins, including biotin, folate, and vitamin K, and neutralize carcinogenic compounds, such as pyro lysates (Selber-Hnatiw et al., 2017). Various indications specify that the host metabolism is mainly affected by multiple microbial metabolites that bind to specific host membranes or nuclear receptors (Bhutia et al., 2017). Some of the most important metabolites produced by gut microbiota are described in Table 1. The majority of essential functions for host physiology and maintenance are associated with gut microbiota, e.g., the nervous system’s development, intestinal development, appetite regulation, etc.
TABLE 1.
Metabolites produced by gut microbiota and their functions.
| Metabolites | Functions | References |
| Bile acid metabolites; including deoxycholic acid (DCA) and lithocholic acid (LCA) | Regulate bile acid, cholesterol, lipid, glucose, and energy metabolism, show antimicrobial effects, and activate host nuclear receptors and cell signaling pathways. | Ramírez-Macías et al., 2022 |
| Short-chain fatty acids (SCFAs) metabolites such as propionate and butyrate | Regulate food intake and insulin secretion, also aid in maintaining body weight. | Psichas et al., 2015; Larraufie et al., 2018 |
| Branched-chain fatty acids (BCFA) including isobutyrate, isovalerate | Histone deacetylase (HDAC) inhibition, increased histone acetylation. | Mischke and Plösch, 2016 |
| Indole derivatives including indoxyl sulfate and indole-3-propionic acid (IPA) | IPA exhibits neuroprotective effects, acts as a powerful antioxidant, and regulates intestinal barrier function. Indoxyl sulfate is a uremic toxin that accumulates in the blood of individuals with impaired excretion systems. | Hendrikx and Schnabl, 2019 |
| Lipopolysaccharide (LPS), peptidoglycan (PGN), lipoteichoic acid (LTA) | Epigenetic regulation of genes in colorectal cancer, modulation of chromatin structure and transcriptional activity. | Lightfoot et al., 2013; Mischke and Plösch, 2016 |
| Phenolic derivatives include 4-OH phenylacetic acid, urolithins, enterodiol, and 9-prenylnaringenin | Exhibit antimicrobial effects, maintain intestinal health, and protect against oxidative stress. | Larrosa et al., 2010 |
| Choline metabolites include choline, trimethylamine N-oxide (TMAO), and betaine | Regulating lipid metabolism, and glucose synthesis contribute to the development of cardiovascular disease. | Smallwood et al., 2016 |
| Polyamines include putrescine, spermidine, and spermine | Sustaining the high proliferation rate of intestinal epithelial cells enhances intestinal barrier integrity and enhances the systematic adaptive immune system. | Rooks and Garrett, 2016; Tofalo et al., 2019 |
| Vitamins including thiamine (B1), riboflavin (B2), niacin (B3), pyridoxine (B6), pantothenic acid (B5), biotin (B7), folate (B11-B9), cobalamin (B12), and menaquinone (K2) | Help in red blood cell formation, DNA replication, and repair, work as an enzymatic co-factor, and enhance immune functioning. | Nicholson et al., 2012; Forster et al., 2017 |
| Ethanol | Protein fermentation metabolites may be involved in NAFLD progression. | Yao et al., 2016; Wu et al., 2021 |
| Hydrogen sulfide (H2s) | Reduction/neutralization of reactive oxygen species. | Afanas’ev, 2014; Mischke and Plösch, 2016 |
Gut microbiota in immune homeostasis
The contribution of the human gut microbiota to various aspects of human health, especially the immune system, is crucial for providing the host with several essential benefits. Recent studies have found that early development of the gut microbiota is crucial in preventing autoimmune disorders and proper immune functioning (Lazar et al., 2018; Spencer et al., 2019; Elmassry et al., 2020; Schluter et al., 2020). The intestinal microbiome is essential for the maturation of the immune system, which includes adaptive and innate immune responses. Innate immunity deals with the physical barrier of the epithelia, specialized cells, and circulating chemicals to immediately identify a wide assortment of foreign antigens and eradicate them (Thaiss et al., 2016). The mucosal immune system, in particular, mechanisms are primarily independent of the systemic immune system, and after bacterial colonization of the intestinal tract, it undergoes significant changes. For the immune system’s growth and development, commensal microorganisms are necessary to distinguish between commensal and pathogenic bacteria. Recent studies have demonstrated that gastrointestinal tract microbiota modulates the movement and role of neutrophils and influences the division of populations of T cells into various forms of T helper cells (Th), respectively: Th1, Th2, and Th17 or into regulatory T cells (Francino, 2014; Owaga et al., 2015; Tomkovich and Jobin, 2016). Th17 cells are a subset of TCD4+ cells that secrete several cytokines, affecting immune homeostasis and inflammation (Rossi and Bot, 2013). Gut microbiota contributes to the stimulation and maturation of the immune system in response to pathogens, and it induces and sustains tolerance (Pickard et al., 2017).
Development of the immune system begins at birth, with the introduction of the microbiota, and can only become fully mature in the presence of commensal microflora. Proper immune system maturation is needed to prevent aberrant immune responses, which can cause chronic inflammation and illness (Tibbs et al., 2019). Various strategies, including the germ-free (GF) model, have been taken to demonstrate the importance of gut flora for forming both innate and adaptive immune systems (Uzbay, 2019). In comparison, gut microbiota modulation with antibiotic treatment also demonstrated its importance for immune homeostasis (Hill et al., 2010; Ubeda and Pamer, 2012). Antigen-presenting cells (APCs), having co-evolved with gut microbiota, a key advantage of intestinal APCs is their potential to defend the body from infection while retaining the immune tolerance to the normal gut microbiota (Wu and Wu, 2012). Gut microbiota plays a significant role in controlling the production of APCs. Gut microbiota is also involved in various intestinal and extraintestinal autoimmune diseases, as demonstrated by multiple studies (Andréasson et al., 2016; Rinninella et al., 2019).
Gut microbiota in malnutrition and fasting
Diets and food supplements have a significant influence on the gut’s microbial composition and its variability over time. A high-fat diet is a risk factor for diseases like obesity, metabolic syndrome, and diabetes, all of which are linked to significant gut microbiota composition changes. Disruption of the circadian physiological rhythm increases the probability of intestinal dysbiosis, potentially leading to the pathogenesis of a variety of metabolic and inflammatory disorders, like diabetes, intestinal inflammatory diseases, and even cancer (Reynolds et al., 2017). Studies have also found that gut microbiota responds to malnutrition and fasting (Flint et al., 2015). The impacts of malnourishment on the gut microbiota were only studied under controlled conditions in lab animals due to ethical reasons. In a study, several weeks of nutrient deficiency showed increased microbiome diversity in fish, mice, and toads; geckos showed a decrease while no change was detected in quails (Kohl et al., 2014). Due to these variations, it is challenging to investigate the influence of human nutrient deficiency, which can only be experienced in particular undernourished people. One of the leading causes of child mortality is malnutrition; nutrient-rich therapeutic foods are used to treat severe malnutrition. Also, children cannot completely recover from body mass improvements, probably due to their immature microbiomes. In children, the early development of the intestinal microbiome is particularly significant because microbiome composition keeps changing as they grow and continue changing their diet (Derrien et al., 2019).
Weight loss is promoted by intermittent fasting (IF) regimens, which contribute to enhanced metabolic health. Through metabolic activities, IF participates in the modulation of the gut flora, allowing ongoing interaction with nutrients to be digested and shaping intestinal immune responses during the development of coronary heart disease, blood pressure, and diabetes mellitus (Matías-Pérez et al., 2022). Microbiota reshaping by antibiotic therapy has extended the survival of children with acute malnutrition; even so, severe malnutrition reappeared when the microbiome remained immature, implying that microbiota maturity would anticipate the long-term therapeutic efficacy of the food (Subramanian et al., 2014). Furthermore, a study found that gut microbiota contributes a beneficial impact to the start of severe malnutrition, which can be regenerated by microbiota transplantation into gnotobiotic mice (Smith et al., 2013). Dietary and lifestyle activity such as fasting, and time-restricted eating influences the makeup of the intestinal microbiota. Various microbial products such as SCFAs, trimethylamine N-oxide, tryptophan, and tyrosine derivatives can significantly change with significant microbiota composition changes. However, there are several promising observational studies on human malnutrition, holding out the hope that therapeutic renovation of the gut microbiota will support eradicating mortality linked to malnutrition.
Gut microbiota in major human diseases
From the findings of recent epidemiological, physiological and omics-based studies, supported by cellular and animal experiments, it is demonstrated that intestinal microbiota plays a significant role in both health and disease (Ding et al., 2019). Although this research area is still at a very initial stage, with less understanding of the functional characteristics of the complex gut microbiota, some promising studies have been reported and indicated an enormous potential for revolutionizing the pathogenesis of diseases and therapeutic approaches (Ding et al., 2019; Yin et al., 2019; Rajoka et al., 2020; Bangar et al., 2022). Several major human diseases are associated with an altered gastrointestinal microbiota, for example, obesity, diabetes, cardiovascular disorders, cancer, hypertension, and IBDs (Ding et al., 2019; Nie et al., 2019; Xu et al., 2019) have been discussed individually later in this review. A state called “dysbiosis” is the variation in gut microbiota composition, which is described in many diseases, as shown in Table 2. It is a common problem in the current era because of bacterial infections, diet shifts, and antibiotics (Lindell et al., 2022). It has been challenging to define an appropriate healthy microbiome composition because of inter-individual variation (Lloyd-Price et al., 2016). A well-balanced gut microbial community is essential for the host and the microbiome to co-exist in a mutually beneficial relationship.
TABLE 2.
Diseases associated with gut microbiota abnormalities.
| Disease | Features | References |
| Irritable bowel syndrome | An abundance of Firmicutes and a decrease in Bacteroidetes. | Kennedy et al., 2014 |
| Type 1 diabetes | In genetically predisposed individuals, autoimmune against pancreatic b-cells. Deficient development or alteration of the microbiota may contribute to dysfunctional immunity with the devastation of autoimmune b-cells and increased leakiness of the intestinal epithelial barrier. Variability of microbiomes reduced. | Dunne et al., 2014 |
| Asthma | Outbreaks of Chlamydophila pneumonia during bronchitis and pneumonia development affect the airway microbiome. Gut microbiota is influenced by the introduction of microbiota to the environment, particularly in early life, which helps immune function growth and the development of defending against allergic sensitization. | Huang and Boushey, 2015 |
| Food-borne pathogens and food poisoning | Opportunistic pathogens (Campylobacter, Salmonella, Escherichia coli, Shigella, etc.) disturb the microbiome’s balance leading to dysbiosis. | Josephs-Spaulding et al., 2016 |
| Malnutrition | Decrease or missing species that either process food categories efficiently or produce vitamins may reduce the absorption of nutrients. An overabundance of Enterobacteriaceae can lead to epithelial damage, diarrhea, and limited absorption of nutrients. | Kane et al., 2015 |
| Depression | In physiological systems, Bifidobacterium infantis, generally found in infants’ gastrointestinal tract and administered probiotic drugs, can have antidepressant effects. | Evrensel and Ceylan, 2015 |
| Anxiety | Oral administration of Campylobacter jejuni subclinical doses in murine models induced anxiety-like behavior without stimulating immunity. In a marine model, the Lactobacillus and Bifidobacterium may act as an anxiolytic influencer. | Schnorr and Bachner, 2016 |
Obesity
The global prevalence of obesity has exceeded nearly 650 million people in the last four decades, a total that is six times more than what was reported in the 1990s (Sørensen et al., 2022). That can only be justified by increasing caloric intake and decreasing physical activity (Pascale et al., 2019). Several other diseases, such as diabetes mellitus, coronary heart disease, and cancers, are linked to obesity (Amin et al., 2019; Sun et al., 2019). Thus, weight management and reduction have gained more interest and attention from researchers. The involvement of gut microbiota in obesity is becoming a broad research topic and potentially useful for obesity treatment. Remarkably, the effect of diet on intestinal microbiota composition has become a specific subject of research. In this regard, recent evidence from various studies of humans and mice has demonstrated that changes in gut microbiota composition may play a vital role in the development of obesity (Davis, 2016; Bouter et al., 2017; Stephens et al., 2018; Socol et al., 2022). Several gut microbiota species, called the obesogenic gut microbiota, can significantly contribute to obesity, such as Firmicutes, Bacteroidetes, Rhizobium, Lactococcus, and Clostridium (Cao et al., 2019). In particular, obesogenic gut microbiota could facilitate obesity by producing SCFAs such as butyrate, providing the host with extra energy, and inducing low-grade inflammation caused by intestinal microbiota metabolites (Cao et al., 2019). Genetic aspects and epigenetic variations also play a significant role in the correlation between the composition of the gut microbiota and its contribution to obesity and the production of metabolites.
Some mechanisms have been proposed to define the role of gut microbiota in the development of obesity. Gut microbiota can reduce fatty acid oxidation by suppressing adenosine monophosphate kinase (AMPk) (López, 2017). This enzyme is present in muscle fibers and the liver and serves as a cellular energy indicator. AMPk suppression leads to reduced oxidation of fatty acids and, as a result, increased fat accumulation. By inducing systematic inflammation, intestinal microbiota can also lead to metabolic disturbance observed in obesity (Pindjakova et al., 2017). Another proposed mechanism is the energy regulation and microbes’ potential to ferment dietary polysaccharides that are not digested by humans (Khan et al., 2016). The fermentation of dietary fiber produces SCFAs. SCFA can stimulate lipogenesis after being absorbed and boost triglyceride storage via molecular pathways. Also, SCFA has the potential to suppress the fasting-induced adipocyte factor (FIAF), which inhibits lipoprotein lipase (LPL), causing the accumulation of triglycerides in the host adipocytes (Khan et al., 2016). To acknowledge, how intestinal microbiota promotes the development of obesity, more prospective and interventional studies are needed.
Hypertension
Hypertension is becoming a significant threat to public health and an important risk factor for cardiac, stroke, and kidney diseases (Shah et al., 2019). By 2025, it is estimated that the total number of patients with hypertension will rise to 1.56 billion worldwide (Xu et al., 2020). Studies have shown that various genetic and environmental factors, including dietary salt intake, lack of exercise, and alcohol consumption, also contribute to hypertension progression (Booth et al., 2012; Rust and Ekmekcioglu, 2016). Previous research on animal models and human subjects has shown that hypertension progression is also linked to gut microbiota dysbiosis (Jose and Raj, 2015; Miremadi et al., 2016). Moreover, alterations in the composition of the intestinal microbiota can result in the development of novel antihypertensive therapies. The various mechanisms underlying the relation between gut microbiota and hypertension have been proposed, although there is no definite understanding. The ratio of Bacteroidetes and Firmicutes within intestinal microbiota has been significantly associated with hypertension (Yang et al., 2015). Hypertensive animals and seven hypertensive patients reported an abundance of Bacteroidetes and Firmicutes in their gut microbiota as sequenced by 16S ribosomal RNA (Moghadamrad et al., 2015). Studies using angiotensin II-infused GF mice have shown that gut microbiota is involved in vascular dysfunction and hypertension induced by angiotensin II (Karbach et al., 2016).
Short-chain fatty acids play a crucial role in maintaining gut microbiome homeostasis and host immunity. Recent studies have found that SCFAs produced by gut microbiota is involved in modulating blood pressure (Kang and Cai, 2018). SCFAs have the potential to stimulate host G-protein-coupled receptor (GPR) pathways that affect the secretion of renin and blood pressure (Pluznick et al., 2013). In another study to investigate the correlation between serum metabolites and hypertension, it was found that lyxose levels (a by-product of intestinal microbial fermentation) were higher in patients with newly diagnosed hypertension compared to healthy controls (Hao et al., 2016). However, these findings are preliminary; it is essential to validate other environmental factors like the diet that might affect the gut microbiota.
Furthermore, a beneficial role of Lactobacillus in the regulation of blood pressure has been reported (Gómez-Guzmán et al., 2015). Recent studies and clinical trials demonstrate a close but complex inter-relationship between gut microbiota and hypertension. However, more studies involving human participants are needed to elaborate on the critical role of gut microbiota in hypertension and to demonstrate promising therapeutical approaches.
Cardiovascular diseases
Even with the existing approaches in atherothrombosis prevention and treatment, heart disease is still a significant cause of death globally. It will constantly rise due to increased incidence in low and middle-income countries (Odutayo et al., 2016). In the pathophysiology and progression of CVDs, the intestine has also been involved, primarily due to decreased perfusion of the intestines leading to intestinal barrier dysfunction. The intestinal endothelial barrier is regulated by many mechanisms of a well well-balanced intestinal microbiota (Sabatino et al., 2015). Recently, due to accumulating evidence, intestinal microbiota has been studied as a contributing factor to heart disease and stroke (Tang et al., 2017; Leustean et al., 2018; Jayachandran et al., 2020). Emerging evidence has shown that gut dysbiosis was correlated with the production of many metabolites from intestinal microbiota and also fostered disruption of the function of the gut endothelial barrier.
Furthermore, an essential correlation between the amount of fecal gut microbiota and the intensity of intestinal permeability was identified in patients with CVDs (Pasini et al., 2016). In contrast, patients who had bacterial DNA in the peripheral blood had considerably high plasma levels of inflammatory markers, particularly highly sensitive C-reactive protein and interleukin-6 levels, compared to those who did not have bacterial DNA in their peripheral blood (Wang et al., 2012). Moreover, an increased abundance of Streptococcus and Enterobacteriaceae is linked with coronary artery disease (Jie et al., 2017). Patients with coronary artery disease have altered populations of the most prevalent bacterial species that make up the gut microbiota, with a decrease in Bacteroidetes and an increase in Firmicutes. Trimethylamine-N-oxide is a metabolite that plays an important role in atherosclerosis and can help predict cardiovascular risk (Ramírez-Macías et al., 2022).
Various mechanisms have been proposed to understand the crucial role of gut microbiota in the development and prevention of CVDs. Copies of bacterial genes coding for trimethylamine (TMA) lyase and atherosclerotic CVDs have also been found to be associated (Barrington and Lusis, 2017). TMA lyase contributes to the generation of trimethylamine-N-oxide (TMAO), a metabolite derived from the gut microbiota (Witkowski et al., 2022). TMAO has been shown to contribute to the development of cardiovascular atherosclerotic disease in animal studies and seems to be significantly linked in human studies, identifying the primary function that TMAO may perform in developing atherosclerotic CVD (Tang and Hazen, 2014; Jonsson and Bäckhed, 2017). Thus, a rapid increase in cardiovascular and metabolic disorders has concentrated on gut microbiota regulation as an effective treatment option.
Diabetes mellitus
Diabetes mellitus causes a significant adverse effect on the health condition of human populations worldwide. Diabetes-related risk factors include aspects like a family history of diabetes, poor eating habits, and being overweight. Regarding the continuous rise of urbanization, shifts in diet, and the emergence of more unhealthy lifestyles, the growing incidence of diabetes is a global crisis. According to a report, about 463 million people globally reported diabetes in 2019, and future estimates predict that by 2045, the number of diabetic patients will exceed 700 million (Saeedi et al., 2019). Recent studies have demonstrated that the progression of diabetes is closely correlated to the alterations in the composition of intestinal microbiota (Sender et al., 2016; Gurung et al., 2020). Diet is among the key determinants of the composition of the intestinal microbiota and a significant causal factor in the development of diabetes (Meijnikman et al., 2018).
Given that the development and formation of the gut microbiota depend on the availability of nutrients, it is vitally important to demonstrate that metabolite production depends on food consumption. It has been found that, in response to a shift from a low-fat, plant polysaccharide-rich diet to a high-fat, high-sugar diet, the microbiome composition changed rapidly (Turnbaugh et al., 2009). Human eating patterns have evolved over the past few decades, with fats preferred over fibers; in response to recent eating habits, intestinal microbiota has also changed. Therefore, it was suggested that diabetes could be linked to the intestinal microbiota’s systematic alterations (Sircana et al., 2018).
It was observed in the diabetes prevention and prediction (DIPP) study that new-onset type-1 diabetes subjects had a distinct composition of gut microbiota compared to controls (Brown et al., 2011). It was found that mucin formation was caused by lactate and butyrate-producing bacteria in the control group to sustain gut integrity. In contrast, mucin synthesis was inhibited by non-butyrate producing lactate-utilizing bacteria contributing to autoimmunity of β-cells and type 1 diabetes (Brown et al., 2011). Also, an increase in the occurrence of Akkermansia muciniphila has been observed to be inversely related to the probability of developing type 1 diabetes (Hansen et al., 2012; Navab-Moghadam et al., 2017). A. muciniphila may is a potential probiotic in the treatment of type 1 diabetes. Many other studies have reported the variations in the composition of gut microbiota between type 1 diabetes and their matched health controls, illustrating the need for a better understanding of the function that these bacteria can play in the development of diabetes (Murri et al., 2013; Gülden et al., 2015).
It has been indicated that the influence of microbiota on type 2 diabetes can be mediated through mechanisms involving changes in the butyrate and incretins secretions (Nøhr et al., 2013; Baothman et al., 2016). In patients with type 2 diabetes, a study showed a moderate degree of intestinal microbial dysbiosis, a decrease in bacteria-producing universal butyrate, and an increase in opportunistic pathogens (Baothman et al., 2016). Other studies have also shown the significant influence of gut microbiota on type 2 diabetes pathways, including insulin signaling, inflammation, and glucose homeostasis (Baothman et al., 2016; Cani, 2018). However, more studies are needed to deeply understand the mechanisms and influential role of gut microbiota in the development of diabetes.
Cancer
Cancer is the second most common cause of death globally (Fitzmaurice et al., 2017). Many factors significantly influence cancer risks, such as exposure to pathogens, UV radiation and toxic substances, diet, and lifestyle. However, the risk mainly depends on the dosage, the period, and the combination of such factors, along with the genetic background of the patient (Vivarelli et al., 2019). There is a growing interest in the characterization and functionality of intestinal microbiota due to its complicated relationship with the host (Tao et al., 2020). Different studies have indicated that abrogation or alteration of gut microbiota significantly contributes to developing colorectal carcinoma in genetic and carcinogenic tumorigenesis models (Arthur et al., 2012; Vivarelli et al., 2019). Metabolomics and metagenomics studies have demonstrated the dual role of gut microbiota in cancer risk reduction and tumor growth, and anti-cancer therapies (Bultman, 2014).
A greater abundance of Bacteroides massiliensis was found in patients with prostate cancer, while Eubacterium rectale and F. prausnitzii have been identified in comparatively less abundance, indicating the potential contribution of these specific microorganisms in the pathogenesis of prostate cancer (Chung et al., 2018). It has also been found that the gut microbiota is linked with the development of colorectal cancer, with Fusobacterium nucleatum, Bacteroides fragilis, and Peptostreptococcus anaerobic being identified in its development as important players (Hsieh et al., 2018). Gut bacteria, especially F. nucleatum and Clostridium colicanis, were proposed as indicative markers in gastric cancer’s carcinogenesis (Mehta et al., 2017). Recent studies have indicated that F. nucleatum can suppress the host’s immune response and upgrade cellular proliferation. In contrast, a diet rich in whole grains and dietary fiber have a lower risk of F. nucleatum positive cancer, indicating that the gut microbiome may be a significant mediator between dietary and colorectal cancer interactions (Hall et al., 2017). Various preclinical studies using GF mice have proposed the mechanism and considerable impact of gut microbiota on genesis and cancer progression (Arthur et al., 2012). A deeper understanding of the influential role of gut microbiota in the development of cancer has increased the interest in research for microbiome-based therapeutics in cancer treatment. However, more studies involving human participants are required to deeply understand the mechanism of gut microbiota in the development of cancer and its anti-carcinogenic characteristics.
Inflammatory bowel diseases
Inflammatory bowel disease is a significant disease with the highest prevalence in western countries; its incidence has risen rapidly in newly industrialized countries in Asia, the Middle East, Africa, and South America (Kaplan and Ng, 2017). It is also imperative to examine the exact etiology and pathogenesis of IBD. Notable advancements have been achieved in identifying the development of IBD in the last few years. The most significant and clinically beneficial aspect of this advancement was the identification of gut microbiota as a crucial multifunctional inflammatory factor. Recently, the role of intestinal microbiota in the pathogenesis of IBD has been emphasized. Several lines of evidence indicate the essential part of the gut microbiota in intestinal inflammation. Most studies have demonstrated decreased intestinal microbiota diversity in patients with IBD (Willing et al., 2010; Matsuoka and Kanai, 2015). Significant decreases in Firmicutes and proteobacteria are the most important observations of altered composition of gut microbiota in patients with IBD. The decreased diversity of intestinal microbiota found in patients with IBD was primarily due to the reduction of Firmicutes. A decline in the Clostridium leptum groups, particularly F. prausnitzii, has been observed among Firmicutes (Wang et al., 2014). In biologically susceptible hosts, alterations of the gut microbiota have been associated with aberrant mucosal immune responses that result in a variety of intestinal and extraintestinal disorders, including IBD. As a result, restoring immunological homeostasis by modifying the gut microbiota is currently considered to be a potential therapeutic strategy to treat IBD patients (Facciotti, 2022).
The majority of discovered human pathogenic bacteria belong to the phylum Proteobacteria, which play an increasingly important role in IBD (Mukhopadhya et al., 2012). Analysis of microbial diversity shows a rise in the number of bacterial species belonging to this phylum, implying an active role in initiating chronic inflammation in patients with IBD (Hold et al., 2014). The abundance of Ruminococcus gnavus is also found to be higher in IBD (Örtqvist et al., 2019). Although more clinical studies are required to examine and deeply understand the mechanism through which gut microbiota contribute to IBD progression.
Eubiosis and food
Dietary effects and influences on our gut microbiome are not new subjects of research. Food causes transient changes in the gut microbiota composition, which are primarily due to fish, meat, and fiber, which have long-term effects (Bajinka et al., 2020). More than two macronutrients can be found in one diet, which alters the gut microbiota while also altering metabolic output (Qiu et al., 2020). The positive benefits of dietary fiber on human metabolism have been explored and found to be significant. Dietary fiber has been shown to alter the microbiota and produce beneficial metabolites like butyrate (Silva et al., 2020). While a balanced nutritional diet is important for overall health, a diet high in fiber is particularly essential to maintain the diversity of the intestinal microbiota (Zhang et al., 2013).
Microbiota ferment complex undigested carbohydrates, also known as microbiota-accessible carbohydrates (MAC), leads to an increase in SCFA levels and, as a result, a positive health effect (Seo et al., 2020). These complex carbohydrates, which include resistant starch, oligosaccharides, and dietary fiber, can positively modulate a variety of gut microbes that are beneficial to health (Yang et al., 2020). Unsaturated plant-based fats in the diet reduce detrimental bacteria while increasing the abundance of Bifidobacterium and butyrate-producing bacteria (Roseburia and Faecalibacterium), all of which have been associated with positive health effects (Muralidharan et al., 2019). Micronutrients, in addition to macronutrients, may play a key role in gut reshaping, according to various studies (Ramos and Martín, 2021). All of these findings point to the importance of dietary factors as modulators of the microbial community, which can therefore have an impact on human physiology and disease processes.
Conclusion
The crucial role of probiotics in health, disease, and nutrition has increased their scientific and marketing significance across the globe. The attention has been shifted from prospective studies to clinical trials to have a better understanding of how microbiota can interplay in human health and disease. Eubiosis is important in exerting the health endorsing benefits of probiotics. An unhealthy diet intake, such low intakes of fruits and vegetables intakes and overuse of antibiotics can result in dysbiosis. In nutshell, probiotics aid in the treatment of various infectious diseases, dysfunctions of the GI tract, and inflammatory disorders as well as in controlling obesity and diabetes. The advances in gut microbiota modeling and analysis will enhance our knowledge of how they influence health and disease, allowing us to adapt current and forthcoming therapeutic and preventive strategies. Understanding the specific roles played by the gut microbiome in our growth and development, as well as how it functions in health and disease, holds the potential to improve many parts of our daily lives, from improving the formula for infants to offering new approaches in fighting obesity and cancer, among others. As gut microbiota is a complex topic, future research should focus on multidisciplinary approaches, taking into consideration recent innovations in various scientific fields.
Author contributions
MA and RA: conceptualization and writing—original draft preparation. MA, FS, YS, MH, RR, AH, AR, MP, JL, and CS: writing—review and editing. RA: supervision. All authors read and agreed to the final version of the manuscript.
Acknowledgments
We are thankful for all the support from Government College University Faisalabad, Pakistan. We also address thanks to the University of Oradea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- AboNahas H. H., Darwish A. M., Abd El-kareem H. F., AboNahas Y. H., Mansour S. A., Korra Y. H., et al. (2022). “Trust Your Gut: The Human Gut Microbiome in Health and Disease,” in Microbiome-Gut-Brain Axis, eds Sayyed R. Z., Khan M. (Singapore: Springer; ), 53–96. 10.1007/978-981-16-1626-6_3 [DOI] [Google Scholar]
- Afanas’ev I. (2014). New nucleophilic mechanisms of ros-dependent epigenetic modifications: Comparison of aging and cancer. Aging Dis. 5:52. 10.14336/ad.2014.050052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlawat S., Sharma K. K. (2021). Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 72 636–668. 10.1111/lam.13333 [DOI] [PubMed] [Google Scholar]
- Amin M. N., Hussain M. S., Sarwar M. S., Moghal M. M. R., Das A., Hossain M. Z., et al. (2019). How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab. Syndr. 13 1213–1224. 10.1016/j.dsx.2019.01.041 [DOI] [PubMed] [Google Scholar]
- Andréasson K., Alrawi Z., Persson A., Jönsson G., Marsal J. (2016). Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease. Arthrit. Res. Ther. 18:278. 10.1186/s13075-016-1182-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. C., Perez-Chanona E., Mühlbauer M., Tomkovich S., Uronis J. M., Fan T. J., et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338 120–123. 10.1126/science.1224820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajinka O., Tan Y., Abdelhalim K. A., Özdemir G., Qiu X. (2020). Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express. 10:130. 10.1186/s13568-020-01066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangar P. S., Singh Sandhu K., Trif M., Rusu A., Pop I. D., Kumar M. (2022). Enrichment in Different Health Components of Barley Flour Using Twin-Screw Extrusion Technology to Support Nutritionally Balanced Diets. Front. Nutr. 8:823148. 10.3389/fnut.2021.823148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baothman O. A., Zamzami M. A., Taher I., Abubaker J., Abu-Farha M. (2016). The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis. 15:108. 10.1186/s12944-016-0278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington W. T., Lusis A. J. (2017). Association between the gut microbiome and atherosclerosis. Nat. Rev. Cardiol. 14 699–700. 10.1038/nrcardio.2017.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. (2016). The emerging view of F irmicutes as key fibre degraders in the human gut. Environ. Microbiol. 18 2081–2083. 10.1111/1462-2920.13225 [DOI] [PubMed] [Google Scholar]
- Bhutia Y. D., Ogura J., Sivaprakasam S., Ganapathy V. (2017). Gut microbiome and colon cancer: Role of bacterial metabolites and their molecular targets in the host. Curr. Colorectal Cancer Rep. 13 111–118. 10.1007/s11888-017-0362-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth F. W., Roberts C. K., Laye M. J. (2012). Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2:1143. 10.1002/cphy.c110025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouter K. E., Van Raalte D. H., Groen A. K., Nieuwdorp M. (2017). Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology 152 1671–1678. 10.1053/j.gastro.2016.12.048 [DOI] [PubMed] [Google Scholar]
- Brown C. T., Davis-Richardson A. G., Giongo A., Gano K. A., Crabb D. B., Mukherjee N., et al. (2011). Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One 6:e25792. 10.1371/journal.pone.0025792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne H. P., Forster S. C., Anonye B. O., Kumar N., Neville B. A., Stares M. D., et al. (2016). Culturing of ‘unculturable’human microbiota reveals novel taxa and extensive sporulation. Nature 533 543–546. 10.1038/nature17645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman S. J. (2014). Emerging roles of the microbiome in cancer. Carcinogenesis 35 249–255. 10.1093/carcin/bgt392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P. D. (2018). Human gut microbiome: Hopes, threats and promises. Gut 67 1716–1725. 10.1136/gutjnl-2018-316723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S. Y., Zhao C. N., Xu X. Y., Tang G. Y., Corke H., Gan R. Y., et al. (2019). Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 92 194–204. 10.1016/j.tifs.2019.08.004 [DOI] [Google Scholar]
- Cardona D., Roman P. (2022). New Perspectives in Health: Gut Microbiota. Int. J. Environ. Res. Public Health 19:5828. 10.3390/ijerph19105828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L., Orberg E. T., Geis A. L., Chan J. L., Fu K., Shields C. E. D., et al. (2018). Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe 23 203–214.e205. 10.1016/j.chom.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon M. A., Bird A. R. (2015). The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7 17–44. 10.3390/nu7010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L. A., Maurice C. F., Carmody R. N., Gootenberg D. B., Button J. E., Wolfe B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. D. (2016). The gut microbiome and its role in obesity. Nutr. Today 51:167. 10.1097/NT.0000000000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M., Alvarez A. S., De Vos W. M. (2019). The gut microbiota in the first decade of life. Trends Microbiol. 27 997–1010. 10.1016/j.tim.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Ding R. X., Goh W. R., Wu R. N., Yue X. Q., Luo X., Khine W. W. T., et al. (2019). Revisit gut microbiota and its impact on human health and disease. J. Food Drug Anal. 27 623–631. 10.1016/j.jfda.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne J. L., Triplett E. W., Gevers D., Xavier R., Insel R., Danska J. (2014). The intestinal microbiome in type 1 diabetes. Clin. Exp. Immunol. 177 30–37. 10.1111/cei.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmassry M. M., Zayed A., Farag M. A. (2020). Gut homeostasis and microbiota under attack: Impact of the different types of food contaminants on gut health. Crit. Rev. Food Sci. Nutr. 62 738–763. 10.1080/10408398.2020.1828263 [DOI] [PubMed] [Google Scholar]
- Evrensel A., Ceylan M. E. (2015). The gut-brain axis: The missing link in depression. Clin. Psychopharmacol. Neurosci. 13:239. 10.9758/cpn.2015.13.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciotti F. (2022). Modulation of intestinal immune cell responses by eubiotic or dysbiotic microbiota in inflammatory bowel diseases. Pharmanutrition 21:100303. 10.1016/j.phanu.2022.100303 [DOI] [Google Scholar]
- Fan Y., Pedersen O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19 55–71. 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- Fava F., Rizzetto L., Tuohy K. (2019). Gut microbiota and health: Connecting actors across the metabolic system. Proc. Nutr. Soc. 78 177–188. 10.1017/S0029665118002719 [DOI] [PubMed] [Google Scholar]
- Fitzmaurice C., Allen C., Barber R. M., Barregard L., Bhutta Z. A., Brenner H., et al. (2017). Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 3 524–548. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H. J., Duncan S. H., Scott K. P., Louis P. (2015). Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 74 13–22. 10.1017/S0029665114001463 [DOI] [PubMed] [Google Scholar]
- Forster V. J., McDonnell A., Theobald R., McKay J. A. (2017). Effect of methotrexate/vitamin B12 on DNA methylation as a potential factor in leukemia treatment-related neurotoxicity. Epigenomics 9 1205–1218. 10.2217/epi-2016-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francino M. P. (2014). Early development of the gut microbiota and immune health. Pathogens 3 769–790. 10.3390/pathogens3030769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Guzmán M., Toral M., Romero M., Jiménez R., Galindo P., Sánchez M., et al. (2015). Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol. Nutr. Food Res. 59 2326–2336. 10.1002/mnfr.201500290 [DOI] [PubMed] [Google Scholar]
- Gülden E., Wong F. S., Wen L. (2015). The gut microbiota and type 1 diabetes. Clin. Immunol. 159 143–153. 10.1016/j.clim.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung M., Li Z., You H., Rodrigues R., Jump D. B., Morgun A., et al. (2020). Role of gut microbiota in type 2 diabetes pathophysiology. Ebio Med. 51:102590. 10.1016/j.ebiom.2019.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. B., Yassour M., Sauk J., Garner A., Jiang X., Arthur T., et al. (2017). A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 9:103. 10.1186/s13073-017-0490-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C., Krych L., Nielsen D., Vogensen F., Hansen L., Sørensen S., et al. (2012). Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 55 2285–2294. 10.1007/s00125-012-2564-7 [DOI] [PubMed] [Google Scholar]
- Hao Y., Wang Y., Xi L., Li G., Zhao F., Qi Y., et al. (2016). A nested case-control study of association between metabolome and hypertension risk. Biomed. Res. Int. 2016:7646979. 10.1155/2016/7646979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenaar R. (2011). Intestinal health functions of colonic microbial metabolites: A review. Benef. Microbes 2 103–114. 10.3920/BM2011.0003 [DOI] [PubMed] [Google Scholar]
- Hendrikx T., Schnabl B. (2019). Indoles: Metabolites produced by intestinal bacteria capable of controlling liver disease manifestation. J. Intern. Med. 286 32–40. 10.1111/joim.12892 [DOI] [PubMed] [Google Scholar]
- Hill D. A., Hoffmann C., Abt M. C., Du Y., Kobuley D., Kirn T. J., et al. (2010). Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 3 148–158. 10.1038/mi.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C., Dollive S., Grunberg S., Chen J., Li H., Wu G. D., et al. (2013). Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PLoS One 8:e66019. 10.1371/journal.pone.0066019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hold G. L., Smith M., Grange C., Watt E. R., El-Omar E. M., Mukhopadhya I. (2014). Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years?. World J. Gastroenterol. 20:1192. 10.3748/wjg.v20.i5.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. N., Hou C. Y., Chang-Chien G. P., Lin S., Yang H. W., Tain Y. L. (2020). Perinatal resveratrol therapy prevents hypertension programmed by maternal chronic kidney disease in adult male offspring: Implications of the gut microbiome and their metabolites. Biomedicines 8:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y. Y., Tung S. Y., Pan H. Y., Yen C. W., Xu H. W., Lin Y. J., et al. (2018). Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci. Rep. 8:158. 10.1038/s41598-017-18596-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. J., Boushey H. A. (2015). The microbiome in asthma. J. Allergy Clin. Immunol. 135 25–30. 10.1016/j.jaci.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen V. L., Gerdes V. E., Middeldorp S., Van Mens T. E. (2021). Gut microbiota and their metabolites in cardiovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 35:101492. 10.1016/j.beem.2021.101492 [DOI] [PubMed] [Google Scholar]
- Jayachandran M., Chung S. S. M., Xu B. (2020). A critical review on diet-induced microbiota changes and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 60 2914–2925. 10.1080/10408398.2019.1666792 [DOI] [PubMed] [Google Scholar]
- Jie Z., Xia H., Zhong S. L., Feng Q., Li S., Liang S., et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8:845. 10.1038/s41467-017-00900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson A. L., Bäckhed F. (2017). Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 14 79–87. 10.1038/nrcardio.2016.183 [DOI] [PubMed] [Google Scholar]
- Jose P. A., Raj D. (2015). Gut microbiota in hypertension. Curr. Opin. Nephrol. Hypertens. 24:403. 10.1097/MNH.0000000000000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs-Spaulding J., Beeler E., Singh O. V. (2016). Human microbiome versus food-borne pathogens: Friend or foe. Appl. Microbiol. Biotechnol. 100 4845–4863. 10.1007/s00253-016-7523-7 [DOI] [PubMed] [Google Scholar]
- Kane A. V., Dinh D. M., Ward H. D. (2015). Childhood malnutrition and the intestinal microbiome. Pediatr. Res. 77 256–262. 10.1038/pr.2014.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Cai Y. (2018). Gut microbiota and hypertension: From pathogenesis to new therapeutic strategies. Clin. Res. Hepatol. Gastroenterol. 42 110–117. 10.1016/j.clinre.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Kaplan G. G., Ng S. C. (2017). Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 152 313–321.e312. 10.1053/j.gastro.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Karbach S. H., Schönfelder T., Brandão I., Wilms E., Hörmann N., Jäckel S., et al. (2016). Gut microbiota promote angiotensin II–induced arterial hypertension and vascular dysfunction. J. Am. Heart Assoc. 5:e003698. 10.1161/JAHA.116.003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P. J., Cryan J. F., Dinan T. G., Clarke G. (2014). Irritable bowel syndrome: A microbiome-gut-brain axis disorder?. World J. Gastroenterol. 20:14105. 10.3748/wjg.v20.i39.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. J., Gerasimidis K., Edwards C. A., Shaikh M. G. (2016). Role of gut microbiota in the aetiology of obesity: Proposed mechanisms and review of the literature. J. Obes. 2016:7353642. 10.1155/2016/7353642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho Z. Y., Lal S. K. (2018). The human gut microbiome–a potential controller of wellness and disease. Front. Microbiol. 9:1835. 10.3389/fmicb.2018.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl K. D., Amaya J., Passement C. A., Dearing M. D., Mccue M. D. (2014). Unique and shared responses of the gut microbiota to prolonged fasting: A comparative study across five classes of vertebrate hosts. FEMS Microbiol. Ecol. 90 883–894. 10.1111/1574-6941.12442 [DOI] [PubMed] [Google Scholar]
- Lagier J. C., Edouard S., Pagnier I., Mediannikov O., Drancourt M., Raoult D. (2015). Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 28 208–236. 10.1128/CMR.00110-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier J. C., Khelaifia S., Alou M. T., Ndongo S., Dione N., Hugon P., et al. (2016). Culture of previously uncultured members of the human gut microbiota by culturomics. Nat. Microbiol. 1:16203. 10.1038/nmicrobiol.2016.203 [DOI] [PubMed] [Google Scholar]
- Larraufie P., Martin-Gallausiaux C., Lapaque N., Dore J., Gribble F. M., Reimann F., et al. (2018). SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 8:74. 10.1038/s41598-017-18259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrosa M., González-Sarrías A., Yáñez-Gascón M. J., Selma M. V., Azorín-Ortuño M., Toti S., et al. (2010). Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 21 717–725. 10.1016/j.jnutbio.2009.04.012 [DOI] [PubMed] [Google Scholar]
- Lazar V., Ditu L. M., Pircalabioru G. G., Gheorghe I., Curutiu C., Holban A. M., et al. (2018). Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front. Immunol. 9:1830. 10.3389/fimmu.2018.01830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustean A. M., Ciocoiu M., Sava A., Costea C. F., Floria M., Tarniceriu C. C., et al. (2018). Implications of the intestinal microbiota in diagnosing the progression of diabetes and the presence of cardiovascular complications. J. Diabetes Res. 2018:5205126. 10.1155/2018/5205126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot Y. L., Yang T., Sahay B., Mohamadzadeh M. (2013). Targeting aberrant colon cancer-specific DNA methylation with lipoteichoic acid-deficient Lactobacillus acidophilus. Gut Microbes 4 84–88. 10.4161/gmic.22822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell A. E., Zimmermann-Kogadeeva M., Patil K. R. (2022). Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 20 431–443. 10.1038/s41579-022-00681-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J., Abu-Ali G., Huttenhower C. (2016). The healthy human microbiome. Genome Med. 8:51. 10.1186/s13073-016-0307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M. (2017). EJE PRIZE 2017: Hypothalamic AMPK: A golden target against obesity? Eur. J. Endocrinol. 176 R235–R246. 10.1530/EJE-16-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matías-Pérez D., Hernández-Bautista E., García-Montalvo I. A. (2022). Intermittent fasting may optimize intestinal microbiota, adipocyte status and metabolic health. Asia Pac. J. Clin. Nutr. 31 16–23. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Kanai T. (2015). The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 37 47–55. 10.1007/s00281-014-0454-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. S., Nishihara R., Cao Y., Song M., Mima K., Qian Z. R., et al. (2017). Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 3 921–927. 10.1001/jamaoncol.2016.6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijnikman A. S., Gerdes V. E., Nieuwdorp M., Herrema H. (2018). Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr. Rev. 39 133–153. 10.1210/er.2017-00192 [DOI] [PubMed] [Google Scholar]
- Miremadi F., Sherkat F., Stojanovska L. (2016). Hypocholesterolaemic effect and anti-hypertensive properties of probiotics and prebiotics: A review. J. Funct. Foods 25 497–510. 10.1016/j.jff.2016.06.016 [DOI] [Google Scholar]
- Mischke M., Plösch T. (2016). The gut microbiota and their metabolites: Potential implications for the host epigenome. Microb. Hum. Body 902 33–44. 10.1007/978-3-319-31248-4_3 [DOI] [PubMed] [Google Scholar]
- Moghadamrad S., Mccoy K. D., Geuking M. B., Sägesser H., Kirundi J., Macpherson A. J., et al. (2015). Attenuated portal hypertension in germ-free mice: Function of bacterial flora on the development of mesenteric lymphatic and blood vessels. Hepatology 61 1685–1695. 10.1002/hep.27698 [DOI] [PubMed] [Google Scholar]
- Mukhopadhya I., Hansen R., El-Omar E. M., Hold G. L. (2012). IBD—what role do Proteobacteria play?. Nat. Rev. Gastroenterol. Hepatol. 9 219–230. 10.1038/nrgastro.2012.14 [DOI] [PubMed] [Google Scholar]
- Muralidharan J., Galiè S., Hernández-Alonso P., Bulló M., Salas-Salvadó J. (2019). Plant-based fat, dietary patterns rich in vegetable fat and gut microbiota modulation. Front. Nutr. 6:157. 10.3389/fnut.2019.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murri M., Leiva I., Gomez-Zumaquero J. M., Tinahones F. J., Cardona F., Soriguer F., et al. (2013). Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 11:46. 10.1186/1741-7015-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakov R., Velikova T. (2020). Chemical metabolism of xenobiotics by gut microbiota. Curr. Drug Metab. 21 260–269. 10.2174/1389200221666200303113830 [DOI] [PubMed] [Google Scholar]
- Navab-Moghadam F., Sedighi M., Khamseh M. E., Alaei-Shahmiri F., Talebi M., Razavi S., et al. (2017). The association of type II diabetes with gut microbiota composition. Microb. Pathog. 110 630–636. 10.1016/j.micpath.2017.07.034 [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W. (2012). Host-gut microbiota metabolic interactions. Science 336 1262–1267. 10.1126/science.1223813 [DOI] [PubMed] [Google Scholar]
- Nie P., Li Z., Wang Y., Zhang Y., Zhao M., Luo J., et al. (2019). Gut microbiome interventions in human health and diseases. Med. Res. Rev. 39 2286–2313. 10.1002/med.21584 [DOI] [PubMed] [Google Scholar]
- Nøhr M. K., Pedersen M. H., Gille A., Egerod K. L., Engelstoft M. S., Husted A. S., et al. (2013). GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154 3552–3564. 10.1210/en.2013-1142 [DOI] [PubMed] [Google Scholar]
- Odutayo A., Wong C. X., Hsiao A. J., Hopewell S., Altman D. G., Emdin C. A. (2016). Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 354:i4482. 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- Örtqvist A. K., Lundholm C., Halfvarson J., Ludvigsson J. F., Almqvist C. (2019). Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: A population-based study. Gut 68 218–225. 10.1136/gutjnl-2017-314352 [DOI] [PubMed] [Google Scholar]
- Owaga E., Hsieh R. H., Mugendi B., Masuku S., Shih C. K., Chang J. S. (2015). Th17 cells as potential probiotic therapeutic targets in inflammatory bowel diseases. Int. J. Mol. Sci. 16 20841–20858. 10.3390/ijms160920841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A., Marchesi N., Govoni S., Coppola A., Gazzaruso C. (2019). The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: New insights into old diseases. Curr. Opin. Pharmacol. 49 1–5. 10.1016/j.coph.2019.03.011 [DOI] [PubMed] [Google Scholar]
- Pasini E., Aquilani R., Testa C., Baiardi P., Angioletti S., Boschi F., et al. (2016). Pathogenic gut flora in patients with chronic heart failure. JACC 4 220–227. 10.1016/j.jchf.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Patrascu O., Béguet-Crespel F., Marinelli L., Le Chatelier E., Abraham A. L., Leclerc M., et al. (2017). A fibrolytic potential in the human ileum mucosal microbiota revealed by functional metagenomic. Sci. Rep. 7:40248. 10.1038/srep40248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips C. A., Augustine P., Ganesan K., Ranade S., Chopra V., Patil K., et al. (2022). The role of gut microbiota in clinical complications, disease severity, and treatment response in severe alcoholic hepatitis. Indian J. Gastroenterol. 41 37–51. 10.1007/s12664-021-01157-9 [DOI] [PubMed] [Google Scholar]
- Piccioni A., Cicchinelli S., Valletta F., De Luca G., Longhitano Y., Candelli M., et al. (2022). Gut Microbiota and Autoimmune Diseases: A Charming Real World Together With Probiotics. Curr. Med. Chem. 29 3147–3159. 10.2174/0929867328666210922161913 [DOI] [PubMed] [Google Scholar]
- Pickard J. M., Zeng M. Y., Caruso R., Núñez G. (2017). Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 279 70–89. 10.1111/imr.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindjakova J., Sartini C., Lo Re O., Rappa F., Coupe B., Lelouvier B., et al. (2017). Gut dysbiosis and adaptive immune response in diet-induced obesity vs. systemic inflammation. Front. Microbiol. 8:1157. 10.3389/fmicb.2017.01157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznick J. L., Protzko R. J., Gevorgyan H., Peterlin Z., Sipos A., Han J., et al. (2013). Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. U. S. A. 110 4410–4415. 10.1073/pnas.1215927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psichas A., Sleeth M. L., Murphy K. G., Brooks L., Bewick G. A., Hanyaloglu A. C., et al. (2015). The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39 424–429. 10.1038/ijo.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Ye Q., Sun M., Wang L., Tan Y., Wu G. (2020). Saturated hydrogen improves lipid metabolism disorders and dysbacteriosis induced by a high-fat diet. Exp. Biol. Med. 245 512–521. 10.1177/1535370219898407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajoka M. S. R., Mehwish H. M., Xiong Y., Song X., Hussain N., Zhu Q., et al. (2020). Gut microbiota targeted nanomedicine for cancer therapy: Challenges and future considerations. Trends Food Sci. Technol. 107 240–251. 10.1016/j.tifs.2020.10.036 [DOI] [Google Scholar]
- Ramírez-Macías I., Orenes-Piñero E., Camelo-Castillo A., Rivera-Caravaca J. M., López-García C., Marín F. (2022). Novel insights in the relationship of gut microbiota and coronary artery diseases. Crit. Rev. Food Sci. Nutr. 62 3738–3750. 10.1080/10408398.2020.1868397 [DOI] [PubMed] [Google Scholar]
- Ramos S., Martín M. Á. (2021). Impact of diet on gut microbiota. Curr. Opin. Food Sci. 37 83–90. 10.1016/j.cofs.2020.09.006 [DOI] [Google Scholar]
- Rauf A., Khalil A. A., Rahman U. U., Khalid A., Naz S., Shariati M. A., et al. (2022). Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 62, 6034–6054. [DOI] [PubMed] [Google Scholar]
- Reynolds A. C., Paterson J. L., Ferguson S. A., Stanley D., Wright K. P., Jr., Dawson D. (2017). The shift work and health research agenda: Considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med. Rev. 34 3–9. 10.1016/j.smrv.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Ribaldone D. G., Pellicano R., Fagoonee S., Actis G. C. (2022). Modulation of the gut microbiota: Opportunities and regulatory aspects. Minerva Gastroenterol. 10.23736/S2724-5985.22.03152-7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G. A. D., Gasbarrini A., et al. (2019). What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7:14. 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks M. G., Garrett W. S. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16 341–352. 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser E. C., Mauri C. (2016). A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun. 74 85–93. 10.1016/j.jaut.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Rossi M., Bot A. (2013). The Th17 cell population and the immune homeostasis of the gastrointestinal tract. Int. Rev. Immunol. 32, 471–474. [DOI] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., et al. (2018). Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 57 1–24. 10.1007/s00394-017-1445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust P., Ekmekcioglu C. (2016). “Impact of salt intake on the pathogenesis and treatment of hypertension,” in Hypertension: From basic research to clinical practice, ed. Islam S. (Cham: Spinger; ), 61–84. 10.1007/5584_2016_147 [DOI] [PubMed] [Google Scholar]
- Ryma T., Samer A., Soufli I., Rafa H., Touil-Boukoffa C. (2021). Role of probiotics and their metabolites in inflammatory bowel diseases (IBDs). Gastroenterol. Insights 12 56–66. 10.3390/gastroent12010006 [DOI] [Google Scholar]
- Sabatino A., Regolisti G., Brusasco I., Cabassi A., Morabito S., Fiaccadori E. (2015). Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol. Dial. Transplant. 30 924–933. 10.1093/ndt/gfu287 [DOI] [PubMed] [Google Scholar]
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- Santacroce L., Man A., Charitos I. A., Haxhirexha K., Topi S. (2021). Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 26 135–148. 10.52586/4930 [DOI] [PubMed] [Google Scholar]
- Schluter J., Peled J. U., Taylor B. P., Markey K. A., Smith M., Taur Y., et al. (2020). The gut microbiota is associated with immune cell dynamics in humans. Nature 588 303–307. 10.1038/s41586-020-2971-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr S. L., Bachner H. A. (2016). Focus: Microbiome: Integrative therapies in anxiety treatment with special emphasis on the gut microbiome. Yale J. Biol. Med. 89:397. [PMC free article] [PubMed] [Google Scholar]
- Selber-Hnatiw S., Rukundo B., Ahmadi M., Akoubi H., Al-Bizri H., Aliu A. F., et al. (2017). Human gut microbiota: Toward an ecology of disease. Front. Microbiol. 8:1265. 10.3389/fmicb.2017.01265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R., Fuchs S., Milo R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14:e1002533. 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y. S., Lee H. B., Kim Y., Park H. Y. (2020). Dietary carbohydrate constituents related to gut dysbiosis and health. Microorganisms 8:427. 10.3390/microorganisms8030427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah T. G., Sutaria J. M., Vyas M. V. (2019). The association between pulmonary hypertension and stroke: A systematic review and meta-analysis. Int. J. Cardiol. 295 21–24. 10.1016/j.ijcard.2019.07.085 [DOI] [PubMed] [Google Scholar]
- Silva F. M. D. C. E., Oliveira E. E. D., Ambrósio M. G. E., Ayupe M. C., Souza V. P. D., Gameiro J., et al. (2020). High-fat diet-induced obesity worsens TH2 immune response and immunopathologic characteristics in murine model of eosinophilic oesophagitis. Clin. Exp. Allergy 50 244–255. 10.1111/cea.13533 [DOI] [PubMed] [Google Scholar]
- Simões C. D., Maganinho M., Sousa A. S. (2022). FODMAPs, inflammatory bowel disease and gut microbiota: Updated overview on the current evidence. Eur. J. Nutr. 61 1187–1198. 10.1007/s00394-021-02755-1 [DOI] [PubMed] [Google Scholar]
- Sircana A., Framarin L., Leone N., Berrutti M., Castellino F., Parente R., et al. (2018). Altered gut microbiota in type 2 diabetes: Just a coincidence?. Curr. Diabetes Rep. 18:98. 10.1007/s11892-018-1057-6 [DOI] [PubMed] [Google Scholar]
- Smallwood T., Allayee H., Bennett B. J. (2016). Choline metabolites: Gene by diet interactions. Curr. Opin. Lipidol. 27:33. 10.1097/MOL.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. I., Yatsunenko T., Manary M. J., Trehan I., Mkakosya R., Cheng J., et al. (2013). Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339 548–554. 10.1126/science.1229000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socol C. T., Chira A., Martinez-Sanchez M. A., Nuñez-Sanchez M. A., Maerescu C. M., Mierlita D., et al. (2022). Leptin Signaling in Obesity and Colorectal Cancer. Int. J. Mol. Sci. 23:4713. 10.3390/ijms23094713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen T. I., Martinez A. R., Jørgensen T. S. H. (2022). “Epidemiology of Obesity,” in Handbook of Experimental Pharmacology, eds Eckel J., Clément K. (Heidelberg: Springer; ), 3–27. 10.1007/164_2022_581 [DOI] [PubMed] [Google Scholar]
- Spencer S. P., Fragiadakis G. K., Sonnenburg J. L. (2019). Pursuing human-relevant gut microbiota-immune interactions. Immunity 51 225–239. 10.1016/j.immuni.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steliou K., Boosalis M. S., Perrine S. P., Sangerman J., Faller D. V. (2012). Butyrate histone deacetylase inhibitors. Biores. Open Access 1 192–198. 10.1089/biores.2012.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. W., Arhire L., Covasa M. (2018). Gut microbiota: From microorganisms to metabolic organ influencing obesity. Obesity 26 801–809. 10.1002/oby.22179 [DOI] [PubMed] [Google Scholar]
- Subramanian S., Huq S., Yatsunenko T., Haque R., Mahfuz M., Alam M. A., et al. (2014). Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510 417–421. 10.1038/nature13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Tan Y., Rexiati M., Dong M., Guo W. (2019). Obesity is a common soil for premature cardiac aging and heart diseases-role of autophagy. Biochim. Biophys. Acta Mol. Basis Dis. 1865 1898–1904. 10.1016/j.bbadis.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Szablewski L. (2018). Human gut microbiota in health and Alzheimer’s disease. J. Alzheimers Dis. 62 549–560. 10.3233/JAD-170908 [DOI] [PubMed] [Google Scholar]
- Tang W. W., Hazen S. L. (2014). The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 124 4204–4211. 10.1172/JCI72331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. W., Kitai T., Hazen S. L. (2017). Gut microbiota in cardiovascular health and disease. Circ. Res. 120 1183–1196. 10.1161/CIRCRESAHA.117.309715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Li S., Gan R. Y., Zhao C. N., Meng X., Li H. B. (2020). Targeting gut microbiota with dietary components on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 60 1025–1037. 10.1080/10408398.2018.1555789 [DOI] [PubMed] [Google Scholar]
- Thaiss C. A., Zmora N., Levy M., Elinav E. (2016). The microbiome and innate immunity. Nature 535 65–74. 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- Tibbs T. N., Lopez L. R., Arthur J. C. (2019). The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microb. Cell 6:324. 10.15698/mic2019.08.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofalo R., Cocchi S., Suzzi G. (2019). Polyamines and gut microbiota. Front. Nutr. 6:16. 10.3389/fnut.2019.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkovich S., Jobin C. (2016). Microbiota and host immune responses: A love–hate relationship. Immunology 147 1–10. 10.1111/imm.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P. J., Ridaura V. K., Faith J. J., Rey F. E., Knight R., Gordon J. I. (2009). The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1:6ra14. 10.1126/scitranslmed.3000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C., Pamer E. G. (2012). Antibiotics, microbiota, and immune defense. Trends Immunol. 33 459–466. 10.1016/j.it.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbay T. (2019). Germ-free animal experiments in the gut microbiota studies. Curr. Opin. Pharmacol. 49 6–10. 10.1016/j.coph.2019.03.016 [DOI] [PubMed] [Google Scholar]
- Vivarelli S., Salemi R., Candido S., Falzone L., Santagati M., Stefani S., et al. (2019). Gut microbiota and cancer: From pathogenesis to therapy. Cancers 11:38. 10.3390/cancers11010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Martels J. Z., Sadabad M. S., Bourgonje A. R., Blokzijl T., Dijkstra G., Faber K. N., et al. (2017). The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe 44 3–12. 10.1016/j.anaerobe.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Wang B., Yao M., Lv L., Ling Z., Li L. (2017). The human microbiota in health and disease. Engineering 3 71–82. 10.1016/J.ENG.2017.01.008 [DOI] [Google Scholar]
- Wang F., Jiang H., Shi K., Ren Y., Zhang P., Cheng S. (2012). Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 17 733–738. 10.1111/j.1440-1797.2012.01647.x [DOI] [PubMed] [Google Scholar]
- Wang M., Wichienchot S., He X., Fu X., Huang Q., Zhang B. (2019). In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 88 1–9. 10.1016/j.tifs.2019.03.005 [DOI] [Google Scholar]
- Wang R., Tang R., Li B., Ma X., Schnabl B., Tilg H. (2021). Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 18 4–17. 10.1038/s41423-020-00592-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Chen L., Zhou R., Wang X., Song L., Huang S., et al. (2014). Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 52 398–406. 10.1128/JCM.01500-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing B. P., Dicksved J., Halfvarson J., Andersson A. F., Lucio M., Zheng Z., et al. (2010). A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139 1844–1854.e1841. 10.1053/j.gastro.2010.08.049 [DOI] [PubMed] [Google Scholar]
- Witkowski M., Witkowski M., Friebel J., Buffa J. A., Li X. S., Wang Z., et al. (2022). Vascular endothelial Tissue Factor contributes to trimethylamine N-oxide-enhanced arterial thrombosis. Cardiovasc. Res. 118 2367–2384. 10.1093/cvr/cvab263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. D., Chen J., Hoffmann C., Bittinger K., Chen Y. Y., Keilbaugh S. A., et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334 105–108. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. J., Wu E. (2012). The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3 4–14. 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Wang K., Wang X., Pang Y., Jiang C. (2021). The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 12 360–373. 10.1007/s13238-020-00814-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Liu M., Cao J., Li X., Fan D., Xia Y., et al. (2019). The dynamic interplay between the gut microbiota and autoimmune diseases. J. Immunol. Res. 2019:7546047. 10.1155/2019/7546047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., White A. J., Niehoff N. M., O’brien K. M., Sandler D. P. (2020). Airborne metals exposure and risk of hypertension in the Sister Study. Environ. Res. 191:110144. 10.1016/j.envres.2020.110144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M., Verma M. K., Chauhan N. S. (2018). A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 200 203–217. 10.1007/s00203-017-1459-x [DOI] [PubMed] [Google Scholar]
- Yang C., Fei Y., Qin Y., Luo D., Yang S., Kou X., et al. (2015). Bacterial flora changes in conjunctiva of rats with streptozotocin-induced type I diabetes. PLoS One 10:e0133021. 10.1371/journal.pone.0133021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Liang Q., Balakrishnan B., Belobrajdic D. P., Feng Q. J., Zhang W. (2020). Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 12:381. 10.3390/nu12020381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C. K., Muir J. G., Gibson P. R. (2016). Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 43 181–196. 10.1111/apt.13456 [DOI] [PubMed] [Google Scholar]
- Yin R., Kuo H. C., Hudlikar R., Sargsyan D., Li S., Wang L., et al. (2019). Gut microbiota, dietary phytochemicals, and benefits to human health. Curr. Pharmacol. Rep. 5 332–344. 10.1007/s40495-019-00196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Li S., Yang L., Huang P., Li W., Wang S., et al. (2013). Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat. Commun. 4:2163. 10.1038/ncomms3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Tripathi M., Sinha R. A., Singh B. K., Yen P. M. (2021). Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 7:11. 10.20517/2394-5079.2020.134 [DOI] [PMC free article] [PubMed] [Google Scholar]