Abstract
Intestinal microbiota plays a key role in regulating the pathogenesis of human disease and maintaining health. Many diseases, mainly induced by bacteria, are on the rise due to the emergence of antibiotic-resistant strains. Intestinal microorganisms include organisms such as bacteria, viruses, and fungi. They play an important role in maintaining human health. Among these microorganisms, phages are the main members of intestinal viromes. In particular, the viral fraction, composed essentially of phages, affects homeostasis by exerting selective pressure on bacterial communities living in the intestinal tract. In recent years, with the widespread use and even abuse of antibacterial drugs, more and more drug-resistant bacteria have been found, and they show a trend of high drug resistance and multidrug resistance. Therefore, it has also become increasingly difficult to treat serious bacterial infections. Phages, a natural antibacterial agent with strong specificity and rapid proliferation, have come back to the field of vision of clinicians and scholars. In this study, the current state of research on intestinal phages was discussed, with an exploration of the impact of phage therapy against infectious diseases, as well as potential application beyond infectious diseases.
1. Background
Intestinal microorganisms are mainly composed of organisms such as bacteria, viruses, fungi, and protozoa. The interaction and homeostasis between various microorganisms are very important to human health. Bacteria and viruses are the two most abundant species in the intestinal microecosystem [1]. Phages are also the main members of intestinal viruses and play an important role in regulating intestinal microbiota. In recent years, with the widespread application of antibiotics, bacterial drug resistance has significantly increased globally. The treatment methods centered on the intestinal microecology have attracted widespread attention. Identifying the composition of intestinal phages and discovering their functions are not only a key point of microecology research but also a new entry point for protecting human health. The total number of bacteria in the human digestive tract exceeds 10^14. The intestinal microbiota of healthy people mainly composed of Firmicutes, Bacteroides, Proteobacteria, and Actinobacteria, accounting for over 90% of the total number of the intestinal bacteria [2]. Phages are the main components of intestinal viromes, containing up to 10^8 billion virus-like particles (VLPs) per mL of fecal filtrate [3]. According to their lytic ability, they are divided into lytic phages (virulent phages) and lysogenic phages (temperate phages). According to the difference in morphology, phages are divided into tailed phages, tailless phages, and filamentous phages and further divided into Siphoviridae, Myoviridae, Podoviridae, and so on. The main steps in the lysis of host bacteria by lytic phages can be summarized as follows: adsorption, invasion, replication, assembly, maturation, and lysis. After the phage is adsorbed on the cell surface of the host bacteria, the enzyme in the tail structure of the phage can penetrate the peptidoglycan layer of the host bacteria and the inner membrane, respectively, to release nucleic acid into the interior of the bacteria. The protein in the phage tail can also inhibit phage nucleic acid injected into the host bacteria from being excreted by them. When the integration of phage nucleic acid with the nucleic acid of host bacteria is completed, it will replicate with the bacterial nucleic acid and can be reassembled with the expressed protein shell to form a new progeny phage with bacteriolytic ability. Under the action of cytolytic enzymes and/or perforin, the infected bacteria are finally lysed, and the progeny phages released after lysis can continue to invade, infect, and lyse surrounding host bacteria in the same way. Since lytic phages can continuously proliferate and lyse bacteria after invading the host bacteria, resulting in the death of the host bacteria, virulent phages are generally used to treat bacterial infection. After the lysogenic phage integrates DNA or RNA into the nucleic acid of the host bacteria, the phage nucleic acid is continuously replicated with the replication of the host bacteria and constantly enters inside host bacteria cells of the next generation with the cell division of the host bacteria, which generally does not cause the lysis of the host bacteria. This phenomenon of simultaneous proliferation with the cellular host without lysing is called lysogenic conversion (lysogenic phenomenon). However, lysogenic conversion is not without lytic properties. Under certain external inducements, phage DNA or RNA integrated into the nucleic acid of the host bacteria can also spontaneously separate from the genomes of the host bacteria, enter the bacteriolytic cycle, and be assembled into a complete progeny phage, thus lysing the host bacteria.
Lysogenic phages are the main components in the human intestine. At present, studies have shown that the most widely distributed intestinal phages across the world are crAss-like phages [4], and healthy people in different regions are taken as the research object to have identified 23 core phages (phages shared by >50% of individuals) and 132 common phages (phages shared by 20∼50% of individuals) from them [5, 6]. Based on the characteristics of genome-wide average nucleotide identity (ANI), the International Committee on Taxonomy of Viruses (ICTVs) proposed a new virological classification system, in which sequence coverage exceeding 85% and ANI exceeding 95% are taken as thresholds, with comprehensive consideration of phylogenetic tree (PT) and gene-sharing networks (GSNs) for classification (Table1) [10]. The relationship between phages and human health can be discovered and their roles in disease diagnosis and treatment can be explored by the use of research into intestinal phageome.
Table 1.
Human intestinal phage classification.
| Reference | Year | Location | Sample size | Object | Phage | Host | Identification method |
|---|---|---|---|---|---|---|---|
| Dutilh et al. [4] | 2014 | United States | 12 | Humans (twins) | crAssphage | Bacteroides | PT |
|
| |||||||
| Devoto et al. [7] | 2019 | United States | 212 | Humans from Bangladesh and Tanzania, two African baboon social groups, and Danish pigs | Lak phage | Prevotella | PT |
|
| |||||||
| Camarillo-Guerrero et al. [8] | 2021 | United Kingdom | 28060 | Humans | Gubaphage | Bacteroides and Parabacteroides | PT |
|
| |||||||
| Benler et al. [9] | 2021 | United States | 5742 | Humans | Quimbyviridae | Prevotella, Bacteroides, and Parabacteroides | PT |
|
| |||||||
| Benler et al. [9] | 2021 | United States | 5742 | Humans | Flandersviridae/Gratiaviridae | Bacteroides and Parabacteroides | PT and GSN |
PT, phylogenetic tree; GSN, gene-sharing networks.
2. The Main Mechanism of Action of Phages Affecting Body Health
2.1. The Regulation of the Composition of Intestinal Microbiota by Phages
2.1.1. Predation
Phages can select target bacteria for “predation” by identifying specific membrane receptors on the bacterial surface. Generally speaking, bacterial infection by lytic phages first requires specific recognition via the structural proteins on the surface of lytic phages and adsorption of them to the surface receptors of host bacteria. This mainly depends on complementarity between protein in the phage tail and the molecular structure on the binding site of the bacterial surface [11]. Tailed phages hydrolyze peptidoglycan of cell walls mainly via lytic enzymes to release progeny phages. Lytic enzymes can lyse bacterial peptidoglycan by inhibiting peptidoglycan synthesis via a single protein or by enzymolysis of peptidoglycan via lysin and perforation-lysozyme systems [12]. There are mainly two kinds of enzymes that destroy bacterial biofilms: exolytic enzymes, which promote the entry of genomes into bacteria in the early stage, and endolytic enzymes, which degrade the host bacteria in the terminal stage to allow the release of progeny phages. After killing intestinal bacteria through this special “predation” effect, intestinal phages will also leave specific CRISPR spacer sequences on the bacterial genomes [13]. Through the identification of these sequences, it was found that bacterial death caused by natural phages in humans and animals is ubiquitous and plays an important role in the stability of intestinal microbiota. This kind of “predation” is mostly specific. For instance, Faecalibacterium prausnitzii phage can selectively infect Faecalibacterium prausnitzii and “turn a blind eye” to other intestinal bacteria [14]. However, macrogenomic analysis of the CRISPR spacer sequences also revealed that some intestinal phages have a wide range of bacterial hosts in human intestines [15].
2.1.2. Lysogenic Conversion
Temperate phages compensate for their own adverse effects on host bacteria by improving the state of host bacteria, which in turn enhances adaptability and also endows the host with a new phenotype. This phenomenon is called “lysogenic conversion.” After entering the host bacteria, the temperate phage carries out gene integration, thereby avoiding recognition and clearance by macrophages and coexisting in the host bacteria for a long time [16]. Taylor et al. [17] and Wahl et al. [18] found that lysogenic conversion can increase the resistance of host bacteria to other phages, adhesion and colonization capability, environmental tolerance, and antibiotic resistance. The lysogenic conversion of phages not only improves the adaptability of phages to the intestine but also cooperates with host bacteria to enhance the evolutionary advantage.
2.1.3. Seesaw Effect
Strains are exposed to the environment of antibacterial drugs and evolve into drug-resistant bacteria under genetic selection. The evolved strains lose the characteristics of phage resistance. Similarly, bacteria exposed to phage conditions lose their antimicrobial resistance after genetic selection [19]. For instance, Ho et al. [20] found that the mutation of bacterial gene EPAR leads to the reduction in adsorption of Enterococcus faecalis by phages; however, changes in this gene lead to an increased bacterial sensitivity of bacteria to daptomycin. When antibacterial drugs induce the phenotypic changes of bacteria, the ability of corresponding phages to prey on bacteria increases. This phenomenon of ebb and flow is also known as the “seesaw effect” [21].
2.1.4. Epithelial Defense
Phages can reduce the colonization of pathogenic bacteria on the surface of the intestinal mucus layer, and they also phagocytize and lyse pathogenic bacteria [22, 23]. Barr et al. [24] found that in the human intestine, some phages adhere to the mucosa and reduce the diffusional movement of the phage itself, thus forming a structure similar to a defensive barrier with the epithelial tissue.
2.2. Phage-Mediated Immune Regulation
Another interesting feature of phages is their regulatory potential for an immune response [25]. Intestinal phages can actively scavenge invasive bacteria, reduce immune as well as inflammatory responses, and also maintain immune homeostasis [26]. Phage-mediated lysis is also involved in the generation of pathogen-associated molecular patterns (PAMPs), and PAMPs may translocate and activate immune response when intestinal permeability increases [27]. Phages can stimulate macrophages to phagocytize bacteria through opsonization to make them more easily enter the immune system [28]. The intestinal mucosa determines the interaction between phages and their hosts. Phage communities establish contacts with mucosal barriers to produce phage-mediated immune responses [29]. In this mode, innate immunity protects commensal microorganisms in the upper layer of mucus through lysis, and acquired immunity kills invasive pathogens in the deepest mucus through lysis [30]. In order to play an effective antibacterial role, adherent phages have to reduce bacterial colonization of mucus. Some phages express the proteins that display C-type lectin folds and immunoglobulin-type domains, interfering with the mucin expression of O-glycosylated MUC2 in the colon [31, 32]. On the other hand, pathogens that disrupt the innate immune response will be handled by the acquired immune system. Ig-type folds of phages were found in antibodies and T cell receptors [28]. Interestingly, the limiting factor of phages in the intestine is considered to be responsible for the production of specific immunoglobulin A (IgA). The results showed that if IgA levels are relatively low, phages will be found in feces; however, if IgA levels are elevated, there are no active phages in the feces, which may also directly explain the interaction between the relative abundance of intestinal phages with IgA-related immunity [28].
In addition, some phages can directly activate the intestinal immune pathway. Gogokhia et al. [31] found that phages can activate intestinal immunity via Toll-like receptor 9-dependent interferon signaling pathway and promote the proliferation of CD4+ and CD8+ T cells in Peyer's patch. Studies [33] also showed that Escherichia coli phages can play an immunosuppressive role and inhibit the expansion of intestinal immune cells, and adhesion proteins expressed by Escherichia coli phages can bind to lipopolysaccharide, which in turn control the lipopolysaccharide-mediated inflammatory response. Through the abovementioned important mechanisms of action, phages can reduce the invasion of foreign pathogens, increase the colonization ability of probiotics, and adjust the structure of intestinal microbiota, with the maintenance of the balance of intestinal microbiota, as well as the maintenance of intestinal homeostasis by regulation of intestinal immunity (Figure 1).
Figure 1.
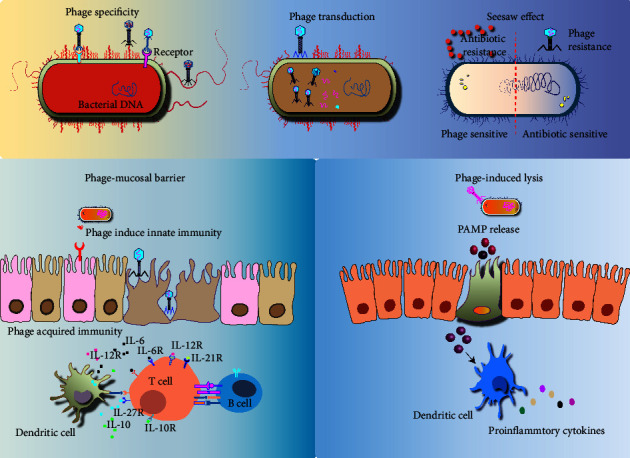
Mechanism of action of the phage therapy.
During the phage lytic infection cycle, phage-encoded binding proteins recognize and attach to receptors on the bacterial surface, such as fimbriae, flagella, porins, or receptor proteins on efflux pumps. The phage attaches and delivers the genomic content to the bacteria, viral replication occurs inside the cytoplasm; after assembly into new phage particles, the process repeats when the bacteria escape by lysing and then infect other susceptible bacteria. Phage therapy kills the target bacteria while at the same time strongly selecting for bacterial virulence or antibiotic resistance when the bacteria mutate to avoid a phage attack. In addition, phage therapy can mediate immune responses in the gut. The intestinal mucosa determines the interaction between phages and their hosts. Phage communities establish contacts with mucosal barriers to produce phage-mediated innate immune responses. Phage-mediated lysis is also involved in the generation of pathogen-associated molecular patterns (PAMPs), and PAMPs may translocate and activate immune response when intestinal permeability increases.
3. Relationship between Phages and Disease and Clinical Application of Phages
3.1. Phages and Infectious Diseases
At the beginning of the 20th century, phages preparations were successively successfully used in the treatment of bacillary dysentery, cholera, and so on [34]. Since then, more and more attempts have been made at phage therapy in various refractory infectious diseases. A research institute in Poland used phage therapy to treat 1307 patients with multidrug-resistant bacteria infections, and 85.9% of them were clinically improved or cured [35, 36]. Fecal filtrate prepared by extraction of phages from healthy human feces can effectively treat refractory Clostridium difficile infection [37]. Additionally, most of the phage preparations entering the clinical trial stage are also aimed at the infection of multidrug-resistant bacteria. The current phage preparations mainly focus on Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, and Staphylococcus aureus [38]. Dedrick et al. [39] reported a patient with cystic fibrosis caused by infection of Pseudomonas aeruginosa and Mycobacterium abscessus, whose symptoms significantly improved after 6 months of treatment with three-phage “cocktails.” Schooley et al. [40] reported a 68-year-old patient with diabetes mellitus infected with multidrug-resistant Acinetobacter baumannii (pancreatic pseudocyst), whose condition continued to worsen despite multiple antibiotics. Nine specific phages were screened out after analysis of the concentrated solution, and they were injected into the abscess cavity in combination with antibiotic therapy. The infection was effectively controlled after combined therapy. Bao et al. [41] reported that a 63-year-old patient with recurrent urinary tract infection caused by sulfamethoxazole-resistant Klebsiella pneumoniae recovered after treatment with phage “cocktail” therapy. Petrovic Fabijan et al. [42] treated 13 patients severely infected with Staphylococcus aureus combined with phage preparation AB-SA01, which not only effectively controlled the infection but also had no adverse reactions. The application of phage therapy in the treatment of infections at different sites is summarized as follows and shown in Table 2.
Table 2.
Phage therapy in different infectious diseases.
| Disease type | Reference | Year | Location | Disease | Object (n) | Study type | Host | Outcome |
|---|---|---|---|---|---|---|---|---|
| Skin and soft tissue infections | Weber Dabrowska et al. [35] | 2000 | Poland | Pyogenic infections of burns | Human (49) | Single arm study | Staphylococcus aureus, Escherichia coli, Klebsiella, Proteus, and Pseudomonas | 86% full recovery while 14% marked improvement. |
| Markoishvili et al. [43] | 2002 | United States | Poorly vascularized and venous stasis ulcers | Human (96) | Single arm study | Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Streptococcus, and Proteus | The wounds/ulcers healed completely in 67 (70%) out of 96 patients. In 22 cases in which microbiologic data were available, healing was associated with the concomitant elimination of, or a reduction in, specific pathogenic bacteria in the ulcers. | |
| Rhoads et al. [44] | 2009 | United States | Venous leg ulcers | Human (42) | RCT | Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli | No adverse events. No significant difference of healing compared to antibiotic control. | |
| Morozova et al. [45] | 2018 | Russian | Infected diabetic foot ulcers | Human (2) | Case report | Staphylococcus, Enterococcus, and Pseudomonas aeruginosa | Wound continues to improve, while MRSA infection is not detected. | |
| Fish et al. [46] | 2018 | United States | Infected diabetic toe ulcers | Human (6) | Case report | Staphylococcus aureus | No adverse effects, tissue breakdown, or recurrence of infection were seen | |
| Kifelew et al. [47] | 2020 | Australia | Diabetic wound infections | Balb/c mice (48) | Animal experiment | Staphylococcus aureus | In phage-treated mice, wound healing was seen as similar to vancomycin treatment. No mortality was recorded associated with infections, and postmortem examinations did not show any evident pathological lesions other than the skin wounds. No adverse effects related to the application of phages were observed. | |
| Kumari et al. [48] | 2011 | India | Burn wound infection | Balb/c mice (30) | Animal experiment | Klebsiella pneumoniae | Significant reduction in mortality and more effective than silver nitrate and gentamicin. | |
| Yin et al. [49] | 2017 | China | Wound infection | Balb/c mice (36) | Animal experiment | Acinetobacter baumannii | Wound sizes in animals receiving locally applied phage were significantly smaller, drier, and cleaner than in mice receiving either systemically administered phage or no treatment. Infected mice receiving no treatment succumbed rapidly. In contrast, all mice treated with phage or polymyxin B survived the entire 7 days of the observation period. | |
| Totte et al. [50] | 2017 | Netherlands | Acne vulgaris and eczema | Human (3) | Case report | Staphylococcus aureus | Reduction and prevention of clinical symptoms and does not interfere with the commensal skin microbes and is also not expected to induce bacterial resistance. | |
|
| ||||||||
| Oral infection | Castillo-Ruiz et al. [51] | 2011 | Chile | Periodontitis | 17 clinical samples were obtained from saliva and wastewater from dental chair drainages (NA) | In vitro | Aggregatibacter actinomycetemcomitans | Kill 99% of the bacteria within a biofilm. |
| Guo et al. [52] | 2015 | United States | Dental caries | 20 bacterial species, including multiple oral Streptococcus (NA) | In vitro | Streptococcus | Potent in killing Streptococcus mutans and Streptococcus salivarius. | |
| Tinoco et al. [53] | 2017 | Brazil | Dentin infection | Enterococcus faecalis V583 (vancomycin resistant strain) or Enterococcus faecalis JH2-2 (fusidic acid and rifampin resistant, vancomycin sensitive strain) (NA) | In vitro | Enterococcus faecalis | The recovered Enterococcus faecalis titer was reduced by 18% for the Enterococcus faecalis JH2-2 infected models and by 99% for the Enterococcus faecalis V583 infected models. | |
| Xu et al. [54] | 2018 | China | Dental caries | Sprague Dawley rats (36) | Animal experiment | Streptococcus | Streptococcus mutans and Streptococcus sobrinus biofilms are significantly decreased after treatment with ClyR for 5 min. Furthermore, continuous administration of ClyR for 40 days significantly reduced the severity of caries in rat models infected with a single or a mixed bacteria of Streptococcus mutans and Streptococcus sobrinus. | |
| Li et al. [55] | 2018 | China | Endodontic infection | Caries-free single-rooted teeth selected from orthodontic extraction (NA) | Ex vivo dental model | Enterococcus faecalis | ClyR degrades Enterococcus faecalis biofilm with high efficacy in a dose-dependent manner. | |
|
| ||||||||
| Gastrointestinal infections | Ott et al. [37] | 2016 | Germany | Diarrhea | Human (5) | Case report | Clostridium difficile | Sufficient to restore normal stool habits and eliminate symptoms. |
| Bruttin and Brussow [56] | 2005 | Switzerland | Healthy volunteers to measure the bioavailability of oral phage for diarrheal diseases | Human (15) | Single arm study | Escherichia coli | Safe | |
| Sarker et al. [57] | 2016 | Bangladesh | Diarrhea | Human (120) | RCT | Escherichia coli | No adverse events. Fecal coliphage was increased in treated over control children, but the titers did not show substantial intestinal phage replication but no amelioration in quantitative diarrhea parameter by phage therapy. | |
| Vahedi et al. [58] | 2018 | Iran | Diarrhea | Balb/c mice (48) | Animal experiment | Enteropathogenic Escherichia coli | Able to control the infection. | |
| Jaiswal et al. [59] | 2013 | India | Diarrhea | New Zealand white rabbits (6) | Animal experiment | Vibrio cholerae | Lowered the shedding of bacteria significantly | |
| Nale et al. [60] | 2016 | United Kingdom | Diarrhea | Hamster (NA) | Animal experiment | Clostridium difficile | Reduced Clostridium difficile colonization at 36 h postinfection. | |
| Galtier et al. [61] | 2016 | France | Uropathogenic Escherichia coli infection | Balb/cYJ mice (5) | Animal experiment | Uropathogenic Escherichia coli | Microbiota diversity was much less affected by phages than by antibiotics and efficiently target uropathogenic Escherichia coli strains residing in the gut. | |
|
| ||||||||
| Respiratory infection | Cao et al. [62] | 2015 | China | Pneumonia | Swiss Webster mice (20) | Animal experiment | Klebsiella pneumoniae | Phage-treated mice exhibited a lower level of Klebsiella pneumoniae burden in the lungs as compared to the untreated control. These mice lost less body weight and exhibited lower levels of inflammatory cytokines in their lungs. |
| Alemayehu et al. [63] | 2012 | Ireland | Pneumonia and cystic fibrosis | Balb/c mice (16) | Animal experiment | Pseudomonas aeruginosa | Effective in killing the pathogen in murine lungs. Pseudomonas was effectively cleared from murine lungs in 6 h. | |
| Oduor et al. [64] | 2016 | Kenya | Haematogenous Staphylococcus aureus pneumonia | Balb/c mice (30) | Animal experiment | Staphylococcus aureus | Histology showed that the mice treated with phage did not develop pneumonia. Phage therapy is effective against haematogenous infection. | |
| Waters et al. [65] | 2017 | United Kingdom | Chronic lung infections | Balb/c mice (60) | Animal experiment | Pseudomonas aeruginosa | Phage therapy was again highly effective against the established 6 d lung infection, completely clearing bacteria from the lungs of 70% of mice and significantly reducing CFU counts in the other 30% compared with controls. | |
| Bao et al. [41] | 2020 | China | Recurrent urinary tract infection | Human (1) | Case report | Klebsiella pneumoniae | The combination of sulfamethoxazole-trimethoprim with the phage cocktail inhibited the emergence of phage resistant mutant in vitro, and the urinary tract infection of the patient was successfully cured by this combination. | |
|
| ||||||||
| Urinary tract infection | Leitner et al. [66] | 2021 | Switzerland | Infection after transurethral resection of the prostate | Human (113) | RCT | Enterococcus spp., Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus spp., and Streptococcus spp. | The efficacy of the phage group was similar to that the of antibiotic group (bacterial titer decreased significantly), but the adverse reactions were less. |
| Kuipers et al. [67] | 2019 | Netherlands | Recurrent urinary tract infection after posttransplant | Human (1) | Case report | Klebsiella pneumoniae | The infection eventually evolved into epididymitis which was successfully treated with meropenem and phages. | |
| Rostkowska et al. [68] | 2021 | Poland | Chronic urinary tract infection after kidney transplantation (caused by polycystic kidney disease) | Human (1) | Case report | Klebsiella pneumoniae | Fully recovered following a nephrectomy of his own left kidney. | |
|
| ||||||||
| Eye infection | Fukuda et al. [69] | 2012 | Japan | Keratitis | C57BL/6 mice (NA) | Animal experiment | Pseudomonas aeruginosa | Significantly improved disease outcome and preserved the structural integrity and transparency of the infected cornea. Suppression of neutrophil infiltration and greatly enhanced bacterial clearance in the infected cornea. |
| Furusawa et al. [70] | 2016 | Japan | Keratitis | C57BL/7 mice (NA) | Animal experiment | Pseudomonas aeruginosa | A great reduction of bacterial proliferation was shown in phage therapy for mouse models of Pseudomonas aeruginosa keratitis (suppressed bacterial multiplication to 0.004%). | |
|
| ||||||||
| Ear infection | Wright et al. [71] | 2009 | United Kingdom | Chronic otitis | Human (24) | RCT | Pseudomonas aeruginosa | No adverse events. Phage-treated group Pseudomonas aeruginosa counts were significantly lower only in the phage-treated group. |
| Hawkins et al. [72] | 2010 | United Kingdom | Otitis | Dogs (13) | Animal experiment | Pseudomonas aeruginosa | 48 h after treatment, the clinical score and Pseudomonas aeruginosa count of all ears had fallen. | |
|
| ||||||||
| Nasal infection | Ooi et al. [73] | 2019 | Australia | Chronic rhinosinusitis | Human (9) | Single arm study | Staphylococcus aureus | Preliminary efficacy results indicated favorable outcomes across all cohorts, with 2 of 9 patients showing clinical and microbiological evidence of eradication of infection. |
|
| ||||||||
| Sepsis/Bacteremia | Schneider et al. [74] | 2018 | Hungary | Sepsis | Balb/c mice (36) | Animal experiment | Escherichia coli | Phage particles administered 10 and 60 min following the bacterial challenge elicited 100% and 95% survival, respectively. But no mice could be rescued if phage administration occurred 3 hours postinfection. |
| Pouillot et al. [75] | 2012 | France | Sepsis and meningitis | Sprague Dawley rat pups (50) | Animal experiment | Escherichia coli | When phages were given at 7 h and 24 h after bacterial injection, the survival rates of rats were 100% and 50%, respectively | |
|
| ||||||||
| Novel coronavirus pneumonia | Li et al. [76] | 2020 | United States | SARS-CoV-2 infection | BALB/c mice (55); Hamster (10) | Animal experiment | SARS-CoV-2 | Potently neutralized mouse-adapted SARS-CoV-2 in wild-type mice at a dose as low as 2 mg/kg and exhibited high prophylactic and therapeutic efficacy in a hamster model of SARS-CoV-2 infection |
Note. NA, not applicable; RCT, randomized controlled trial.
3.1.1. Skin and Soft Tissue Infections
Infection is the main complication of severe burns, which may not only delay wound healing but also induce sepsis and even develop multiple organ dysfunction in severe cases. It is one of the important causes of burn-related death. Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, and Staphylococcus aureus are common bacteria with drug resistance. Phage therapy is also widely applied in the field of burns, and there are many cases with definite curative effects. Weber Dabrowska et al. [35] used phage “cocktail” therapy to treat burn patients by oral administration combined with topical application, and the pathogenic bacteria causing skin infection mainly cover different bacteria such as Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, and Proteus. 85% (42/49) of these patients recovered, and bacteria were no longer detected in the topical skin. Although bacteria were still topically detected in the rest of the patients, the clinical symptoms improved significantly.
A chronic dystrophic ulcer is also prone to coinfections, which is common in diseases such as atherosclerosis and diabetes. Infection is an important influence factor for ulcer healing, and timely removal of infection can promote wound healing. Its pathogenic bacteria are dominated by Staphylococcus aureus and Pseudomonas aeruginosa, and their resistance to antibiotics often occurs, with the poor effect of long-term use of antibiotics [77]. Markoishvili et al. [43] applied a wound dressing impregnated with a variety of phages to treat patients with chronic ulcers, and the results showed that this dressing is not only effective but also safe. In a clinical trial of chronic venous leg ulcers related to bacterial infection conducted by Rhoads et al. [44], the “cocktail” phage WPP-201 was topically applied to leg ulcers. Although the wound healing rate of the phage-treated group was the same as that of the standard antibiotic treatment group, the adverse reactions of the former were significantly reduced compared with that of the latter. Morozova et al. [45] reported that patients with diabetic foot infections receive phage therapy. The previous antibiotic treatment was not effective for them. Their wounds were rinsed with phage preparations, covered with gauze soaked with phages agents for wet compress, with repeated replacement of gauze 4 times a day for 2-3 weeks, and their ulcers improved significantly. Fish et al. [46] successfully cured 6 patients with toe ulcers with commercial Staphylococcus phage SB-1. These patients responded poorly to conventional therapy, with no obvious adverse reaction after phage cure, and the ulcers did not recur. Kifelew et al. [47] found that the AB-SA01 phage cocktail can effectively treat diabetic wound infection caused by multidrug-resistant Staphylococcus aureus and also promote wound healing, whose efficacy is equivalent to vancomycin. Kumari et al. [48] found that in the mouse burn wound model, when the phage is applied topically, its protective efficacy is significantly higher than that of the antibiotics. Yin et al. [49] established a topical wound infection model in mice and confirmed that topical application of phages can effectively promote wound healing and has a better curative effect and higher safety than an injection of phages.
Staphylococcus aureus can not only cause skin and soft tissue infections but is also an important influence factor in many common skin diseases, such as chronic acne, atopic dermatitis, psoriasis, and eczema. Moreover, drug-resistant Staphylococcus aureus is one of the important factors for the protracted course and aggravation of skin diseases in these patients. Brown et al. [78] isolated 10 phages from the human skin and proved that these phages have lytic activity against acne. Totte et al. [50] reported that the patients with eczema, severe acne, and bacterial folliculitis for a long time were treated by local application of endolytic enzymes of phages (Staphefekt SA.100). The clinical symptoms of these patients improved after antibiotic treatment, but they were prone to relapse after drug withdrawal. After topical application of Staphefekt SA.100, Staphylococcus aureus was inhibited for a long time, the clinical symptoms of related skin diseases continued to improve, and the recurrence rate was significantly reduced. More importantly, Staphefekt SA. 100 is effective against both methicillin-susceptible bacteria and methicillin-resistant Staphylococcus aureus and also does not interfere with commensal skin microbiota or induce bacterial resistance [50].
3.1.2. Oral Infection
Studies have shown that phages are one of the causes of oral health problems, especially related to the formation of dental plaque and the occurrence of periodontitis [79]. Naidu et al. [80] isolated phages from dental plaques of healthy people and proved the specificity and potential pathogenicity of phages among different individuals. Zhang et al. [81] proved that the virus and its host bacteria jointly promote the sustainable development of chronic periodontitis by genome analysis of phages isolated from patients with periodontitis. Ly et al. [82] found that there are differences in the composition of oral phages between healthy people and periodontitis patients: in the healthy population, long-tailed phages predominate; under the condition of periodontitis, the relative abundance of myotail phages in the subgingival tissue of patients significantly increases, whose changes may lead to the abnormal relative abundance of pathogenic bacteria in patients' plaques. Pride et al. [83] also found that lysogenic phages in the salivary virus community can store a variety of virulence factor genes, that phages may be a repository of pathogenic genes in the human oral cavity, and that phage imbalance may induce highly virulent pathogens, thus resulting in periodontal diseases. Tronstad et al. [84] found that phages can effectively prevent the formation of dental plaques. Machuca et al. [85] extracted phages of Fusobacterium nucleatum from healthy people, and Fusobacterium nucleatum is the pathogen of periodontitis and its excessive proliferation can mediate inflammation. Castillo-Ruiz et al. [51] extracted the phages of Actinobacillus from the flushing solution of tooth cleaning for the treatment of periodontitis. In recent years, genetically engineered phages have also played an important role in oral diseases. Guo et al. [52] found that the specifically targeted antibacterial peptides produced by genetically engineered phages can efficiently and selectively kill the cariogenic pathogen Streptococcus mutans, thereby reducing the occurrence of dental caries. Smith [86] successfully inserted the genetic information at antibody-antigen binding sites into phage DNA and screened out the phages that can bind highly to antibody specificity, which provided new inspiration for improving the targeting of drugs. Both periodontitis and root canal infection are closely related to Enterococcus faecalis infection. Some phages have been found to specifically attack Enterococcus faecalis, such as phages EF24C [44] and EFDG1 [87]. Tinoco et al. [53] studied the response of phage ϕEf11 after its infection with Enterococcus faecalis. They found that phages can convert prophages into lytic phages by deleting the phage repressor genes and evade the host's inhibitory response by replacing the initiation factor, and the modified phage can effectively destroy bacterial biofilms, thereby improving periodontitis and root canal infection. Xu et al. [54] and Li et al. [55] proved that phage lytic enzyme ClyR can selectively kill bacteria that can cause dental caries and Enterococcus faecalis that can induce periapical dental pulp infection, but it is harmless to common oral commensal bacteria. Also, it has been confirmed by rat dental caries model experiments that phage lytic enzyme ClyR can significantly reduce the degree of dental caries and has a potential therapeutic effect on periodontal disease.
3.1.3. Gastrointestinal Infections
The common pathogenic bacteria of gastrointestinal infections include Escherichia coli, Campylobacter jejuni, and Salmonella shigella, . Some uncommon pathogenic bacteria such as Vibrio cholerae and Clostridium difficile can also cause acute and chronic infectious enteritis. Phage preparation was first successfully used in the treatment of bacillary dysentery and cholera [34]. Subsequently, Ott et al. [37] prepared fecal sterile filtrate from healthy human feces, which can effectively treat refractory Clostridium difficile infection, and it is speculated that the phages in the filtrate play a therapeutic role. Bruttin and Brussow [56] and Sarker et al. [57, 88] used Escherichia coli-T4 phage to treat acute bacterial infectious diarrhea in adults and children. They found that although Escherichia coli-T4 phage is safe, there is still controversy about its therapeutic efficacy. Enteropathogenic Escherichia coli (EPEC) is a drug-resistant Escherichia coli causing severe diarrhea. In recent years, the resistance of EPEC to commonly used antibiotics has been increasing. Vahedi et al. [58] found by comparing the therapeutic effects of phages and ciprofloxacin in the mouse model infected with EPEC that phage therapy can not only reduce the content of EPEC in vivo but also ensure the normal growth of mice. Jaiswal et al. [59] employed the model of rabbit infected with Vibrio cholerae. They found that phage “cocktail” treatment given 6 hours after infection with Vibrio cholerae in rabbits can significantly reduce the proliferation of Vibrio cholerae, but this efficacy of phages may be related to the administration time. If the drug is administered within 6 hours of infection, the number of Vibrio cholerae cannot be reduced. Nale et al. [60] confirmed through the hamster model that phage “cocktail” therapy can significantly reduce the colonization of Clostridium difficile. In addition, Galtier et al. [61] demonstrated that phages can effectively reduce intestinal pathogens, and phages have less impact on the composition of intestinal microbiota than antibiotics.
3.1.4. Respiratory Infection
The respiratory tract is the cavity through which the human body communicates with the outside world, and bacterial infection is also a common cause of respiratory diseases. Similarly, phage therapy also shows a good curative effect on respiratory infection. In the study by Cao et al. [62], multidrug-resistant Klebsiella pneumoniae was inoculated into the lungs of mice, and then, the mice could be protected from fatal pneumonia by nasal inhalation of Klebsiella pneumoniae phage. The research team also used phages to treat a mink model of lung infection, and the result showed that the survival rate of minks after phage treatment is 80%. Alemayehu et al. [63] found that after the lungs of mice are infected with Pseudomonas aeruginosa, the bacterial load in the lungs of mice can be reduced to an undetectable level after nasal inhalation of phage preparations, while Pseudomonas aeruginosa proliferates in large quantities in the lungs of the mice not treated with phage preparation. Oduor et al. [64] found that an intravenous injection of phage preparation can treat Staphylococcus aureus-induced pneumonia in mice and significantly inhibit the bacterial load (from 8 CFU/g to 0.5 CFU/g). In a study of chronic lung infection, Waters et al. [65] found that after the lungs of mice are infected with Pseudomonas aeruginosa, nasal inhalation of phage can effectively reduce the number of bacteria in the lungs of mice, which also suggests the effectiveness of phage therapy in the treatment of chronic respiratory infection.
3.1.5. Urinary Tract Infection
Urinary tract infections are mostly anaerobic bacterial infections in the intestine. Patients with more underlying diseases, critical illnesses, or long-term indwelling urinary catheters are prone to complicated/refractory bacterial infections. Bao et al. [41] reported that a 63-year-old female patient with recurrent urinary tract infections secondary to extensively drug-resistant Klebsiella pneumoniae infection was successfully cured after two rounds of phage “cocktail” therapy combined with antibiotics. Patients with long-term indwelling catheters are prone to urinary tract infection. Garretto et al. reported [89] that after phages are smeared against Pseudomonas aeruginosa, Escherichia coli, and Proteus mirabilis on the indwelling catheter, bacterial biofilm formation on the catheter can be significantly reduced, thus playing an anti-infection role. Leitner et al. [66] conducted a randomized, double-blind, prospective clinical study of intravesical Pyo-phage in patients with postprostatectomy infection. In this study, patients with positive urine culture were selected, the sensitivity of isolated pathogens to phage/antibiotics was first determined, and the subjects with sensitive test results were randomly divided into three groups (phage therapy, antibiotic group, and placebo group); after one week of phage/antibiotic/placebo treatment, it was found that the phage group is better than the antibiotic group, and the effective rate is 66.67% (bacterial titers decrease significantly), with no adverse reactions. In addition, Kuipers et al. [67] and Rostkowska et al. [68] both reported the successful treatment of refractory urinary tract infection with Klebsiella pneumoniae with phages in renal transplant patients after surgery.
3.1.6. Eye Infection
Fukuda et al. [69] topically applied eye drops containing Pseudomonas aeruginosa phages to the cornea of normal mice. The result showed that the eye drops do not cause inflammatory reactions such as corneal opacity or inflammatory cell infiltration. Then, a mouse model of Pseudomonas aeruginosa-induced keratitis was selected, followed by administration of phage-containing eye drops after infection. The result showed that after 5 days, the corneal structure of the mice in the experimental group basically returned to normal, while most of the corneas of the mice in the control group without phage treatment are perforated. Furusawa et al. [70] also administrated a mouse model of corneal infection with Pseudomonas aeruginosa; after 30 minutes and 1, 3, 6, and 12 hours of corneal infection with Pseudomonas aeruginosa in mice, respectively, eye drops containing two kinds of phages were administrated. The results showed that with the administration of phage eye drops within 3 h after corneal infection, the bacterial count of mouse cornea is significantly decreased compared with that in the control group, indicating that phage eye drops have an antibacterial effect.
3.1.7. Ear Infection
Wright et al. [71] conducted a clinical trial evaluating the phage preparation in the treatment of chronic otitis media, 24 patients with chronic otitis media caused by drug-resistant Pseudomonas aeruginosa infection lasting for several years were randomly divided into two groups, and they were treated with phages and placebo, respectively. The results showed that the number of Pseudomonas aeruginosa in the phage treatment group significantly decreases, and no local or systemic side effects are found, while the bacterial count in the placebo group does not change significantly. In an animal experiment administrated by Hawkins et al. [72], a cocktail preparation containing 6 different phages was injected into the ear canal of dogs with chronic otitis media caused by Pseudomonas aeruginosa infection, and the clinical score and bacterial count were found to decrease after 48 h, without the identification of no adverse reactions.
3.1.8. Nasal Infection
Ooi et al. [73] conducted a phase 1b clinical study. Nine patients with chronic sinusitis were treated with AB-SAO1 (a mixture of three natural Staphylococcus aureus phages). The results showed that intranasal phage therapy is safe and well tolerated and has a significant effect on chronic sinusitis caused by Staphylococcus aureus.
3.1.9. Sepsis/Bacteremia
Sepsis refers to the acute systemic infection caused by various pathogenic bacteria invading the blood circulation, growing, multiplying, and producing toxins in blood. If the bacteria invading the bloodstream are removed by the human body's defense function and there are no obvious symptoms of toxemia, it will be called bacteremia. Schneider et al. [74] injected a lethal dose of Escherichia coli into mice, resulting in bacteremia, followed by administration of the same dose of phage 10 min and 60 min after bacterial injection, respectively. It was observed that the survival rates of mice are 100% and 95%, respectively, indicating that the administration of phages within a short time after infection can effectively improve the survival rate, but no mice survive when phages are administrated 3 h after bacterial infection. Another study by Pouillot et al. [75] showed that in a rat model of bacteremia caused by Escherichia coli, the survival rates of mice are 100% and 50% when phages are administrated 7 h and 24 h after bacterial injection, respectively. In addition to mouse models, Jang et al. [90] used drosophila models to evaluate the efficacy of phages in the treatment of bacteremia. The establishment of the bacteremia model is by injection of a lethal dose of Pseudomonas aeruginosa into Drosophila melanogaster, and the model group was fed with phage. The results showed that phages can significantly delay or prevent the death of Drosophila melanogaster.
3.1.10. Novel Coronavirus Pneumonia
Phages are easier to induce the body to produce an immune response due to their immunogenicity and also have advantages such as stability and low cost, and thus, they have been widely studied as a vaccine carrier [91]. Phage display technology (PDT) is to insert the gene sequence of encoding exogenous polypeptide or protein into the coat protein gene of phages to display them on the surface of phage coat protein in the form of the fusion protein, and also, they can maintain good biological activity and original biological function, thereby facilitating the identification and combination of target molecules. This technology has been widely applied in many fields such as immunology, oncology, and pharmacology, with the important value in pathogen detection, disease treatment, vaccine, or drug design [92]. Therefore, the comprehensive use of phage immunogenicity and PDT have a good application prospect in the development of a novel coronavirus vaccine. Staquicini et al. [93] reported the development and research of a novel coronavirus-targeted vaccine based on phages. After analyzing the structure of protein S, the researchers selected 6 antigen epitopes and cloned them into phage vector genomes, respectively. Epitope 4 showed that phages can induce sustained specific humoral immunity in mice without other adverse reactions. After that, the researchers also screened the peptide that can bind to the surface of the pulmonary airway and alveolar inner wall cells through the random peptide library of phages, cloned, and inserted it into phage to construct phage particles. Additionally, they also found that phage particles can induce stronger immune responses [94]. Li et al. [76] identified a phage Ab8 that can bind to antibody V with high affinity by establishing a human antibody V domain phage-displayed library. Through further experiments, they found that the phage can neutralize severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in wild-type mice and shows good preventive and therapeutic effects in a hamster model of SARS-CoV-2 infection.
3.2. Phages and Noninfectious Diseases
Numerous studies have shown that there are significant differences in the diversity and structure of intestinal phageomes between healthy people and patients (especially patients with chronic diseases) [95–97]. Therefore, in-depth analysis of intestinal phageomes can provide new ideas for revealing the pathological mechanism of various diseases and help find new diagnostic and therapeutic markers. As shown in Figure 2, intestinal phages play an important role in the development of inflammatory bowel disease, alcoholic liver disease, diabetes, colorectal cancer, breast cancer, Parkinson's disease, and schizophrenia.
Figure 2.
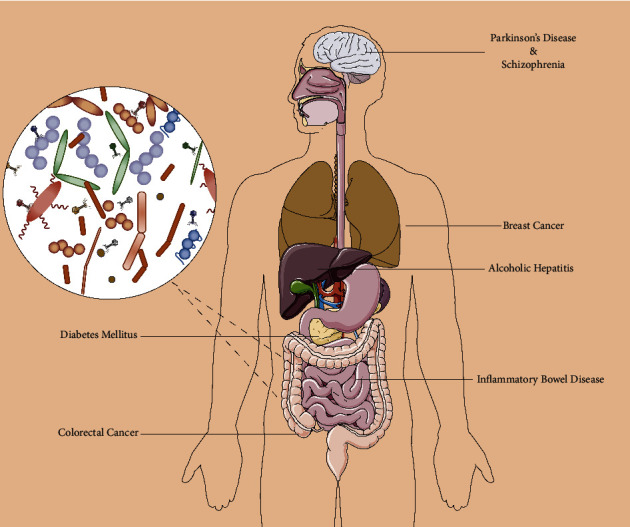
Phages for diagnosis and treatment of diseases beyond infection.
With the application of viromics, the role of phages as biomarkers in disease diagnosis has been gradually discovered. Phage therapy as a targeted bactericidal method plays a considerable role in the treatment of many diseases beyond infection.
3.2.1. Inflammatory Bowel Disease
Inflammatory bowel disease is a chronic intestinal inflammatory disease caused by the interaction between multifactors such as genetics, immunity, microbes, and environmental factors, mainly including ulcerative colitis and Crohn's disease [98]. The long-term recurrent digestive symptoms and other system dysfunction have placed a heavy burden on patients. Studies [6] have confirmed that there are significant differences in the diversity and structure of intestinal viruses between patients with inflammatory bowel disease and healthy members of their families, and they are related to the progression of inflammatory bowel disease. The decreased relative abundance of Faecalibacterium prausnitzii in the intestine of patients with inflammatory bowel disease is an important microbiota feature of inflammatory bowel disease. Further analysis showed that the abundance of a certain phage is related to that of Faecalibacterium prausnitzii, and it is speculated that the increased mortality of Faecalibacterium prausnitzii in the intestine of patients with inflammatory bowel disease is mediated by this phage [14]. Zuo et al. [99] conducted a metagenomic analysis of the samples of domestic patients with ulcerative colitis and found that there is a significant increase in intestinal tailed phages, accompanied by a significant increase in the abundance of Escherichia phages and Enterobacteria phages. Similarly, the inflammatory microenvironment in the intestine may increase the induction of prophages, promote lysis of certain bacterial species, and aggravate microbiota imbalance and intestinal inflammation. The study of Yang et al. [100] suggested that intestinal phages in healthy people can inhibit the occurrence of intestinal inflammation by producing interferon-β mediated by Toll-like receptor 3 and Toll-like receptor 7. Van belleghem et al. [101] also observed that phages can directly interact with innate immune cells, and when peripheral blood monocytes are incubated in vitro, Staphylococcus aureus phages or Pseudomonas aeruginosa phages can induce the transcriptional response of monocytes and significantly increase the transcription of interleukin-1β, interleukin 6, and tumor necrosis factor-α, which in turn aggravate intestinal inflammation.
3.2.2. Alcoholic Hepatitis
Alcoholic hepatitis is a disease associated with a high intake of alcohol. Studies have found that Enterococcus faecalis, an intestinal bacterium, may be involved in the occurrence of alcoholic hepatitis [102]. Duan et al. [103] analyzed the fecal samples from patients with alcoholic hepatitis and healthy people. They found that the level of Enterococcus faecalis in the fecal samples from patients with alcoholic hepatitis is 300 times higher than that of healthy people, and the relative abundance of Enterococcus faecalis significantly increases in the fecal samples of about 80% of alcoholic hepatitis patients. Through further analysis, they found that there is a gene that can encode cytolysins in about 30% of Enterococcus faecalis. The mouse model of alcoholic hepatitis was established by the use of a high alcohol diet. After fecal transplantation containing cytolysin, these alcoholic hepatitis mice show a certain degree of hepatocyte damage and even death, while the mice with alcoholic hepatitis transplanted with cytolysin-deficient fecal samples do not show liver damage. The phage of Enterococcus faecalis can reduce the abundance of cytolysin-containing Enterococcus faecalis and alleviate liver damage in mice with alcoholic hepatitis by targeting, thus playing a protective role.
3.2.3. Diabetes Mellitus
Diabetes mellitus is a group of clinical syndromes characterized by chronic hyperglycemia, with the characteristics such as many complications, hard-to-cure, slow onset, and protracted course. It can cause complications in organs such as kidneys, nerves, and blood vessels, which not only affect the quality of life in patients but also become a public health problem that seriously threatens human health. Ma et al. [104] found that the number of intestinal phages in type 2 diabetes patients is significantly higher than that in healthy individuals, and seven phages are closely related to the occurrence and development of type 2 diabetes. Moreover, the changes in intestinal phages in type 2 diabetes patients are related to changes in the relative abundance and diversity of host bacteria. Zhao et al. [105] analyzed the intestinal microbiota and viral genomes of children at high risk of type 1 diabetes and healthy children. They obtained the following findings: viral genomes of children at high risk for type 1 diabetes change before the onset of disease, and the differences between the two groups increase with age; phage genome sequences are closely related to type 1 diabetes, and before the change in serological parameters of patients with type 1 diabetes, the diversity and relative abundance of intestinal phages significantly decrease, while the intestinal microbiota does not significantly change. This suggested that the change of intestinal microbiota during the development of autoimmune diseases in patients with type 1 diabetes may be caused by the change of phages.
3.2.4. Colorectal Cancer
Colorectal cancer is one of the most malignant tumors with extremely high morbidity and mortality. There are no obvious symptoms in the early stage, and most patients are diagnosed in the middle and late stages. In many studies, it has been reported that the microbiota disturbance in colorectal cancer patients is related to the occurrence and development of colorectal cancer [106]. The diversity of intestinal phage community in colorectal cancer patients significantly increases compared with that of healthy people, and it has been also found that dysregulation of intestinal viromes is related to the progression of colorectal cancer, and there are more than 20 virus genera that differ significantly between colorectal cancer patients and healthy people [107]. Hannigan et al. [108] identified the viruses with significantly altered composition in fecal samples of colorectal cancer patients, mainly the phages from Siphoviridae and Myoviridae. Zheng et al. [109] obtained the following findings: Fusobacterium nucleatum increases in the feces of colorectal cancer patients, while butyrate-producing bacteria such as Clostridium butyricum decrease; Fusobacterium nucleatum is enriched in tumors and can induce autophagy of colorectal cancer cells through Toll-like receptor 4-myeloid differentiation factor 88 pathway to resist chemotherapy, while the butyric acid secreted by Clostridium butyricum can inhibit colorectal cancer cells and further isolate Fusobacterium nucleatum phages from saliva, which can be linked to nanoparticles loaded with colorectal cancer chemotherapy drugs; in animal experiments, the drug was found to be enriched in colorectal tumors of APCmin/+ mice; this significantly prolongs the survival time of colorectal cancer mice, reduces the occurrence of intestinal adenoma and the abundance of Fusobacterium nucleatum, and increases the levels of Clostridium butyricum and colonic butyric acid.
3.2.5. Breast Cancer
Breast cancer often metastasizes to the bone, lung, liver, brain, and so on and causes serious secondary diseases [110]. Currently, new treatment methods for breast cancer mainly include new technologies such as oncolytic virus therapy and virus and phage display immunotherapies. Phage display immunotherapy presents polypeptide or protein antigen by gene fusion with phage coat protein, and phage structure preparation can be used as protective or preventive vaccines against cancer [111]. In addition, filamentous phages can be used as a carrier to combine with anticancer drugs to deliver drugs to cancer cells for targeted therapy [112].
3.2.6. Parkinson's Disease
Parkinson's disease is a common neurodegenerative disease in the elderly, which is related to factors such as heredity, environment, and nervous system aging. Tetz et al. [113] analyzed fecal samples from 32 Parkinson's disease patients and 28 healthy people. After the comparative analysis of bacterial and phage community composition, it was found that Lactobacillus, Streptococcus, and Lactococcus significantly decrease in Parkinson's disease patients. In healthy people, the number of virulent and temperate Lactococcus phages is roughly the same, while virulent phages dominate in Parkinson's disease patients. The increase of lytic c2-like Lactococcus phages and 936-like Lactococcus phages may have a scavenging effect on intestinal Lactococcus, and Lactococcus plays an important regulatory role in the production of dopamine. Therefore, the reduction in phage-mediated Lactococcus may accelerate the development of Parkinson's disease.
3.2.7. Schizophrenia
Schizophrenia is a mental disorder with a complex mechanism. In recent years, with the in-depth study of the brain-gut axis, more and more psychiatric diseases have been found to be related to intestinal microecology. Yolken et al. [114] recruited 41 schizophrenic patients and 33 healthy people and collected DNA samples with throat swabs for metagenomic analysis. Studies showed that Lactobacillus phage phiadh significantly increases in schizophrenic patients. Of the 41 schizophrenic patients included in the study, the sequencing results of 17 patients matched Lactobacillus phage phiadh in one or more places, while there was only one positive match in the healthy control group. After further analysis of drug use in patients, it was found that the abundance of Lactobacillus phage phiadh is also negatively correlated with the use of sodium valproate. 17 patients who did not use sodium valproate tested positive for Lactobacillus phage phiadh.
4. PDT Clinical Application
PDT is a molecular biology technology that uses phage as a carrier to display foreign proteins on the phage surface in the form of fusion expression by integrating exogenous polypeptide genes into phage ones. The PDT clinical application mainly focuses on the following aspects. (1) In antibody production, high-specific monoclonal antibody genotypes can be screened quickly and efficiently through PDT, and corresponding monoclonal antibodies can be obtained after large-scale expression in a prokaryotic system, with the advantages of fast speed and no need for immune animals. In 1993, the human anti-TNF-α antibody D2E7 was produced by PDT [115]. In 2002, D2E7 was officially renamed as adalimumab and FDA-approved for the clinical treatment of rheumatoid arthritis and then approved for the treatment of psoriasis and inflammatory bowel disease. Afterwards, more and more human antibodies were applied in the clinical practice, including anthrax infection—raxibacumab, systemic lupus erythematosus—belimumab, nonsmall-cell lung cancer—necitumumab, gastric, and colorectal cancer—ramucirumab, and so on [116]. (2) In vaccine development, phage-displayed vaccines can display exogenous antigens on the surface of the phage. With the phage proliferation, the expression level of exogenous antigens is also increased so that the body can produce a high immune reaction. Especially in major life-threatening infectious diseases, PDT plays an important role. In addition to the aforementioned COVID-19, Khurana et al. [117] used PDT to develop Ebola virus vaccines; Li et al. [118] adopted PDT to screen single-chain variable fragments targeting influenza virus hemagglutinin subunit 2 (HA2) for influenza vaccine development. (3) In disease diagnosis and treatment, PDT can be utilized to target and identify tumors and organs. The polypeptide can directly target tumor cells or tissues after being coupled with cancer therapeutic drugs by screening the polypeptides specifically bound to tumor cell antigen epitopes. And if fluorescent groups are bound to these specific binding polypeptides, they can also be used for early cancer screening and detection of tumor lesion sites [119]. For example, Shen et al. [120] identified potential ligands of prostate-specific membrane antigen (PSMA) suitable for further development as novel PSMA-targeted peptides by PDT, and the two of the peptide sequences deduced from DNA sequencing of binding phage were SHSFSVGSGDHSPFT and GRFLTGGTGRLLRIS, which may be the basis for further development of peptides for prostate cancer tumor imaging and therapy. Hou et al. [121] adopted PDT to select novel binding peptides for early colon cancer imaging detection and found a peptide termed CBP-DWS, which was demonstrated to be capable of binding to a panel of human colon cancer cell lines and tissues by targeting glypican-3. Han et al. [122] reported a novel 12-mer peptide, GP-5 (IHKDKNAPSLVP), binding to gastric cancer cells specifically and sensitively, providing support for the speculation that the peptide GP-5 is a potential candidate to be developed as a useful molecule fragment for the imaging detection and targeted therapy of gastric cancer.
5. Discussion
As phage therapy has attracted the attention of clinicians and scholars again, its advantages and disadvantages are also known. The advantages of phage therapy are as follows: first, phage specificity is high. General antibiotics are relatively broad-spectrum and the elimination of target pathogens by them also causes destruction of other symbiotic microbiota. Thus, long-term use of antibiotics is not only easy to cause microbiota disturbance but may also lead to opportunistic infection. However, phages can specifically infect host bacteria without affecting other symbiotic microbiota; second, phages have low resistance to bacteria. With the use of antibiotics, the incidence of bacterial resistance to drugs is increasing, while the incidence of bacterial resistance to phages is far below that of antibiotic resistance, and phages can “co-evolve” with bacteria to reduce the incidence of drug resistance [123]; third, the phages, which has the strong bacteriolytic ability, can destroy the bacterial biofilm structure by secreting enzymes such as hydrolase and polysaccharide depolymerases, while antibacterial drugs have limited efficacy against bacterial biofilms [124]; and finally, as a natural antibacterial substance, phages can self-replicate, with a short proliferation cycle. The R&D cycle of phages is shorter than that of general antibacterial drugs. However, phage therapy also has the following common limitations: first, the antibacterial spectrum of phages is narrow. Due to the specific recognition of host bacteria by phages, their curative effect can be significantly reduced in the treatment of multiple infections. Therefore, it is usually necessary to employ the “cocktail therapy” containing multiple phages, which also increases the production cost [125]; second, phages have low resistance to bacterial CRISPR systems. The bacterial CRISPR system can lyse phages and DNA to resist phages. At present, there is no effective way to avoid this defense system [126]; and third, there are also potential risks for phage therapy, such as the potential transfer of virulence or antibiotic resistance genes to infected bacteria, which may lead to such risks as the emergence of highly pathogenic strains [127].
Antibacterial Resistance Leadership Group (ARLG) made recommendations on the potential clinical conditions, laboratory tests, and pharmacokinetics that may need to be considered in phage therapy. Among them, the administration route, dose, frequency, and duration all have indicated that the optimal method has not yet been established. There are currently insufficient data to support a definitive recommendation on the optimal frequency and duration of phage therapy for any given route of administration or a specific type of infection. Extant data suggest that phage needs to be administrated repeatedly to maximize the phage concentration at the infected site, but the ideal frequency and duration of administration are still unclear. The dose and duration required may vary with factors such as phage products, pathogens, disease burden, and infection location [128].
Another aspect that cannot be ignored is that the limitations of phage detection technology also affect our use of similar 16S sequencing methods to make a large-sample analysis of population samples and in turn summarize the phage characteristics of different diseases, which also brings inconvenience to the formulation of phage treatment plans. Temperate phages account for 20–50% of the free phages in the human intestine, and their proportion differs among studies depending on sample sizes and detection methods. For instance, in a study of comparing the virome content of twins, the proportion of temperate phages was estimated according to the copy number of integrase genes, which encodes the enzyme integrating temperate phage genes into bacterial chromosomes [129]. However, since many temperate phages exist as self-replicating free bodies in the host cells and therefore do not express integrase, their copy number is only a rough estimate of the lower limit of the proportion of temperate phages. In addition, the distribution of prophages in the intestinal microbiota is difficult to gauge since most bacterial strains express lysozyme (usually multiple lysozymes), especially Bacteroides, Firmicutes, Actinobacteria, and Proteobacteria [130]. Although metagenomic sequencing has confirmed the presence of prophages in the intestinal microbiota [131], it cannot distinguish between active and defective prophages. Since the latter do not undergo the recovery and dissolution cycle due to mutation, most phages detected by genome sequencing are active phages [132, 133]. Edwards et al. [134] predicted the hosts of different phages by three methods: (1) nucleotide similarity search between phages and bacterial genome, (2) BLAST search, and (3) longest exact nucleotide match between phages and bacterial genomes. However, the predictive accuracies for 820 intact phages are only 37% and 40%. Although CRISPR spacer sequencing can significantly improve the accuracy and efficiency of phage host prediction [15], it can only identify hosts that encode the CRISPR-Cas system. Furthermore, phage infection in these hosts is relatively late, and only 4–13% of the phages can find the target host. Large datasets by Paez Espino et al. [135] and WiSH are used for predicting the hosts of 59% overlapping groups [136]. In a recent study [137], 180 persistent phage clusters were identified by a combination of both methods and 1/3 of which may be related to some bacterial genera (Faecalibacterium and Bacteroides). However, the predictive accuracy and efficiency of either single or combined methods are fuzzy.
In general, the growing problem of antibiotic resistance has brought phages back to the field of vision of clinicians and researchers. In recent years, there have been more and more research studies on phage therapy. According to the clinical trials, there have been 41 clinical studies of phages/phage lytic enzyme therapy (Table 3). From single/cocktail phage therapy, phage lytic enzyme therapy to fecal phage filtrate transplantation, phage therapy is also changing dramatically in form. The advantages of phages, such as high host specificity and low resistance, undoubtedly provide an important breakthrough direction for solving the dilemma of bacterial drug resistance, but phages also have limitations such as potential virulent risks and a narrow antibacterial spectrum. Therefore, the current research is still focused on clinical cases and animal experiments. In these studies, although phage therapy has shown good efficacy and safety, many problems still need to be overcome before it can be officially put into clinical application.
Table 3.
Clinical therapeutic trials involving the use of phage or phage lytic enzymes.
| NCT number | Registry date | Conditions | Interventions | Phases | Locations |
|---|---|---|---|---|---|
| NCT00663091 | 22-April-2008 | Venous leg ulcers | Phages cocktail | Phase 1 | United States |
| NCT00937274 | 10-July-2009 | Diarrhea | Phages cocktail | Not applicable | Bangladesh |
| NCT00945087 | 23-July-2009 | Bacterial infections | Single phage/phages cocktail Phage lytic enzymes |
Not applicable | Poland |
| NCT01617122 | 12-June-2012 | Primary immune deficiency diseases | Single phage | Not applicable | United States |
| NCT01746654 | 11-December-2012 | Infectious disease/bacterial infections | Phage lytic enzymes | Phase 1/phase 2 | Singapore |
| NCT01818206 | 26-March-2013 | Cystic fibrosis | Phages cocktail | Not applicable | France |
| NCT01855048 | 16-May-2013 | Healthy volunteers/antibacterial agents | Phage lytic enzymes | Phase 1 | Korea |
| NCT02116010 | 16-April-2014 | Wound infection | Phages cocktail | Phase 1/phase 2 | Belgium |
| NCT02439359 | 8-May-2015 | Bloodstream infections | Phage lytic enzymes | Phase 1 | United States |
| NCT02664740 | 27-January-2016 | Diabetes/diabetic foot | Phages cocktail | Phase 1/phase 2 | France |
| NCT02757755 | 2-May-2016 | Healthy volunteers | Phages cocktail | Phase 1 | United States |
| NCT02840955 | 21-July-2016 | Atopic dermatitis | Phage lytic enzymes | Not applicable | Netherlands |
| NCT03089697 | 24-March-2017 | Antibacterial agents | Phage lytic enzymes | Phase 2 | Korea |
| NCT03140085 | 4-May-2017 | Urinary tract infections | Phages cocktail | Phase 2/phase 3 | Georgia |
| NCT03163446 | 23-May-2017 | Antibacterial agents | Phage lytic enzymes | Phase 2 | United States |
| NCT03269617 | 1-September-2017 | Gastrointestinal disorder | Phages cocktail | Not applicable | United States |
| NCT03808103 | 17-January-2019 | Crohn disease | Phages cocktail | Phase 1/phase 2 | United States |
| NCT04191148 | 9-December-2019 | Urinary tract infections | Phages cocktail | Phase 1 | United States |
| NCT04287478 | 27-February-2020 | Urinary tract infection, bacterial | Single phage/phages cocktail | Phase 1/phase 2 | United States |
| NCT04289948 | 28-February-2020 | Diabetes/diabetic foot | Phages cocktail | Phase 1/phase 2 | United Kingdom |
| NCT04323475 | 26-March-2020 | Wound infection | Phages cocktail | Phase 1 | Australia |
| NCT04325685 | 27-March-2020 | Trauma injury/brain injuries Abdominal sepsis/pancreatitis Meningitis/encephalitis/seizures Acute respiratory distress syndrome |
Phages cocktail | Not applicable | Russian |
| NCT04596319 | 20-October-2020 | Cystic fibrosis/lung infection | Phages cocktail | Phase 1/phase 2 | United States |
| NCT04650607 | 2-December-2020 | Prosthetic joint infection/bone and joint infection/implant infection | Single phage/phages cocktail | Not applicable | France |
| NCT04682964 | 24-December-2020 | Acute tonsillitis | Single phage | Phase 3 | Uzbekistan |
| NCT04684641 | 24-December-2020 | Cystic fibrosis | Single phage | Phase 1/phase 2 | United States |
| NCT04724603 | 12-January-2021 | Bone and joint infection/prosthetic joint infection | Single phage/phages cocktail | Not applicable | France |
| NCT04737876 | 4-February-2021 | Healthy volunteers | Phages cocktail | Phase 1 | United States |
| NCT04787250 | 8-March-2021 | Prosthetic joint infection | Single phage/phages cocktail | Phase 1/phase 2 | United States |
| NCT04803708 | 18-March-2021 | Diabetic foot ulcer/Pseudomonas aeruginosainfection/Staphylococcus aureus infection/Acinetobacter infection | Phages cocktail | Phase 1/phase 2 | Israel |
| NCT04815798 | 25-March-2021 | Pressure ulcer | Phages cocktail | Phase 1/phase 2 | United Kingdom |
| NCT05010577 | 18-August-2021 | Chronic Pseudomonas aeruginosa infection/cystic fibrosis | Phages cocktail | Phase 1/phase 2 | Israel |
| NCT05177107 | 4-January-2022 | Osteomyelitis/diabetic foot osteomyelitis | Single phage/phages cocktail | Phase 2 | United States |
| NCT05182749 | 10-January-2022 | Shigellosis | Phages cocktail | Phase 1/phase 2 | United States |
| NCT05184764 | 11-January-2022 | Bacteremia/Staphylococcus aureus/Staphylococcusaureus bacteremia/bacteremia due to Staphylococcus aureus | Phages cocktail | Phase 1/phase 2 | United States |
| NCT05240300 | 15-February-2022 | Atopic dermatitis | Single phage/phages cocktail | Phase 1/phase 2 | Israel |
| NCT05269121 | 7-March-2022 | Prosthetic joint infection/bacterial infections | Single phage/phages cocktail | Phase 1/phase 2 | United States |
| NCT05269134 | 7-March-2022 | Prosthetic joint infection | Single phage/phages cocktail | Phase 2/phase 3 | United States |
| NCT05272566 | 9-March-2022 | Feeding patterns/microbial colonization | Fecal phages transfer | Not applicable | Denmark |
| NCT05272579 | 9-March-2022 | Necrotizing enterocolitis/microbial substitution | Fecal phages transfer | Early phase 1 | Denmark |
| NCT05277350 | 14-March-2022 | E. coli infections/bloodstream infection | Phages cocktail | Phase 1 | Denmark |
Data from https://clinicaltrials.gov.
Acknowledgments
The authors are very grateful to Dr. Mei-Juan Luo for her valuable comments in preparing the manuscript and providing literature materials and thank Dr. Yu-Jie Liang for helping design the high-quality figures. In addition, the authors would like to thank KetengEdit for its linguistic assistance. Finally, the authors would like to thank Hao-Xue Xu for giving the first author (H.M.X.) so much accompany and encouragement, during the preparation of this manuscript.
Abbreviations
- VLPs:
Virus-like particles
- ANI:
Average nucleotide identity
- ICTV:
International Committee on Taxonomy of Viruses
- PT:
Phylogenetic tree
- GSNs:
Gene-sharing networks
- PAMPs:
Pathogen-associated molecular patterns
- IgA:
Immunoglobulin A
- EPEC:
Enteropathogenic Escherichia coli
- SARS-CoV-2:
Severe acute respiratory syndrome coronavirus 2
- PDT:
Phage display technology.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Consent
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
H. M. X. and W. M. X. designed the study, summarized literatures, drafted the article, and contributed equally to this article. L. Z. planned and directed the project and interpreted the results. All authors read and approved the final manuscript.
References
- 1.Altves S., Yildiz H. K., Vural H. C. Interaction of the microbiota with the human body in health and diseases. Bioscience of Microbiota, Food and Health . 2020;39(2):23–32. doi: 10.12938/bmfh.19-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimura K. E., Slusher N. A., Cabana M. D., Lynch S. V. Role of the gut microbiota in defining human health. Expert Review of Anti-Infective Therapy . 2010;8(4):435–454. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dion M. B., Oechslin F., Moineau S. Phage diversity, genomics and phylogeny. Nature Reviews Microbiology . 2020;18(3):125–138. doi: 10.1038/s41579-019-0311-5. [DOI] [PubMed] [Google Scholar]
- 4.Dutilh B. E., Cassman N., McNair K., et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nature Communications . 2014;5(1):p. 4498. doi: 10.1038/ncomms5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manrique P., Bolduc B., Walk S. T., van der Oost J., de Vos W. M., Young M. J. Healthy human gut phageome. Proceedings of the National Academy of Sciences of the USA . 2016;113(37):10400–10405. doi: 10.1073/pnas.1601060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman J. M., Handley S. A., Baldridge M. T., et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell . 2015;160(3):447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devoto A. E., Santini J. M., Olm M. R., et al. Megaphages infect prevotella and variants are widespread in gut microbiomes. Nature Microbiology . 2019;4(4):693–700. doi: 10.1038/s41564-018-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camarillo-Guerrero L. F., Almeida A., Rangel-Pineros G., Finn R. D., Lawley T. D. Massive expansion of human gut bacteriophage diversity. Cell . 2021;184(4):1098–1109.e9. doi: 10.1016/j.cell.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benler S., Yutin N., Antipov D., et al. Thousands of previously unknown phages discovered in whole-community human gut metagenomes. Microbiome . 2021;9(1):p. 78. doi: 10.1186/s40168-021-01017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roux S., Adriaenssens E. M., Dutilh B. E., et al. Minimum information about an uncultivated virus genome (MIUViG) Nature Biotechnology . 2019;37(1):29–37. doi: 10.1038/nbt.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee S., Rothenberg E. Interaction of bacteriophage I with its E. coli receptor, LamB. Viruses . 2012;4(11):3162–3178. doi: 10.3390/v4113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermoso J. A., Garcia J. L., Garcia P. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Current Opinion in Microbiology . 2007;10(5):461–472. doi: 10.1016/j.mib.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Safari F., Sharifi M., Farajnia S., et al. The interaction of phages and bacteria: the co-evolutionary arms race. Critical Reviews in Biotechnology . 2020;40(2):119–137. doi: 10.1080/07388551.2019.1674774. [DOI] [PubMed] [Google Scholar]
- 14.Cornuault J. K., Petit M. A., Mariadassou M., et al. Phages infecting faecalibacterium prausnitzii belong to novel viral genera that help to decipher intestinal viromes. Microbiome . 2018;6(1):p. 65. doi: 10.1186/s40168-018-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern A., Mick E., Tirosh I., Sagy O., Sorek R. CRISPR targeting reveals a reservoir of common phages associated with the human gut microbiome. Genome Research . 2012;22(10):1985–1994. doi: 10.1101/gr.138297.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago-Rodriguez T. M., Hollister E. B. Human virome and disease: high-throughput sequencing for virus discovery, identification of phage-bacteria dysbiosis and development of therapeutic approaches with emphasis on the human gut. Viruses . 2019;11(7):p. 656. doi: 10.3390/v11070656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor V. L., Fitzpatrick A. D., Islam Z., Maxwell K. L. The diverse impacts of phage morons on bacterial fitness and virulence. Advances in Virus Research . 2019;103:1–31. doi: 10.1016/bs.aivir.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Wahl A., Battesti A., Ansaldi M. Prophages in Salmonella enterica: a driving force in reshaping the genome and physiology of their bacterial host? Molecular Microbiology . 2019;111(2):303–316. doi: 10.1111/mmi.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber K. E., Ireland C. E., Bukavyn N., Rybak M. J. Observation of seesaw effect with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infectious Disease and Therapy . 2014;3(1):35–43. doi: 10.1007/s40121-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho K., Huo W., Pas S., Dao R., Palmer K. L. Loss-of-Function mutations in epaR confer resistance to ϕNPV1 infection in Enterococcus faecalis OG1RF. Antimicrobial Agents and Chemotherapy . 2018;62(10) doi: 10.1128/aac.00758-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby A. E. Synergistic action of gentamicin and bacteriophage in a continuous culture population of Staphylococcus aureus. PLoS One . 2012;7(11) doi: 10.1371/journal.pone.0051017.e51017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garmaeva S., Sinha T., Kurilshikov A., Fu J., Wijmenga C., Zhernakova A. Studying the gut virome in the metagenomic era: challenges and perspectives. BMC Biology . 2019;17(1):p. 84. doi: 10.1186/s12915-019-0704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo T., Wong S. H., Lam K., et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut . 2018;67(4):634–643. doi: 10.1136/gutjnl-2017-313952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr J. J., Auro R., Furlan M., et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proceedings of the National Academy of Sciences of the USA . 2013;110(26):10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barr J. J. A bacteriophages journey through the human body. Immunological Reviews . 2017;279(1):106–122. doi: 10.1111/imr.12565. [DOI] [PubMed] [Google Scholar]
- 26.Weiss G. A., Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cellular and Molecular Life Sciences . 2017;74(16):2959–2977. doi: 10.1007/s00018-017-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha A., Maurice C. F. Bacteriophages: uncharacterized and dynamic regulators of the immune system. Mediators of Inflammation . 2019;2019:14. doi: 10.1155/2019/3730519.3730519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Belleghem J. D., Dabrowska K., Vaneechoutte M., Barr J. J., Bollyky P. L. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses . 2018;11(1):p. 10. doi: 10.3390/v11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silveira C. B., Rohwer F. L. Piggyback-the-winner in host-associated microbial communities. NPJ Biofilms Microbiomes . 2016;2(1) doi: 10.1038/npjbiofilms.2016.10.16010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr J. J., Youle M., Rohwer F. Innate and acquired bacteriophage-mediated immunity. Bacteriophage . 2013;3(3) doi: 10.4161/bact.25857.e25857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogokhia L., Buhrke K., Bell R., et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host & Microbe . 2019;25(2):285–299.e8. doi: 10.1016/j.chom.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorski A., Dabrowska K., Miedzybrodzki R., et al. Phages and immunomodulation. Future Microbiology . 2017;12(10):905–914. doi: 10.2217/fmb-2017-0049. [DOI] [PubMed] [Google Scholar]
- 33.Gorski A., Miedzybrodzki R., Borysowski J., et al. Phage as a modulator of immune responses: practical implications for phage therapy. Advances in Virus Research . 2012;83:41–71. doi: 10.1016/B978-0-12-394438-2.00002-5. [DOI] [PubMed] [Google Scholar]
- 34.Sabino J., Hirten R. P., Colombel J. F. Review article: bacteriophages in gastroenterology-from biology to clinical applications. Alimentary Pharmacology & Therapeutics . 2020;51(1):53–63. doi: 10.1111/apt.15557. [DOI] [PubMed] [Google Scholar]
- 35.Weber-Dabrowska B., Mulczyk M., Gorski A. Bacteriophage therapy of bacterial infections: an update of our institute’s experience. Archivum Immunologiae et Therapiae Experimentalis . 2000;48(6):547–551. [PubMed] [Google Scholar]
- 36.Zaczek M., Weber-Dabrowska B., Miedzybrodzki R., Lusiak-Szelachowska M., Gorski A. Phage therapy in Poland—a centennial journey to the first ethically approved treatment facility in europe. Frontiers in Microbiology . 2020;11:p. 1056. doi: 10.3389/fmicb.2020.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ott S. J., Waetzig G. H., Rehman A., et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology . 2017;152(4):799–811. doi: 10.1053/j.gastro.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Bhargava K., Nath G., Bhargava A., Aseri G. K., Jain N. Phage therapeutics: from promises to practices and prospectives. Applied Microbiology and Biotechnology . 2021;105(24):9047–9067. doi: 10.1007/s00253-021-11695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dedrick R. M., Guerrero-Bustamante C. A., Garlena R. A., et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nature Medicine . 2019;25(5):730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schooley R. T., Biswas B., Gill J. J., et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant acinetobacter baumannii infection. Antimicrobial Agents and Chemotherapy . 2017;61(10) doi: 10.1128/aac.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao J., Wu N., Zeng Y., et al. Non-active antibiotic and bacteriophage synergism to successfully treat recurrent urinary tract infection caused by extensively drug-resistant Klebsiella pneumoniae. Emerging Microbes & Infections . 2020;9(1):771–774. doi: 10.1080/22221751.2020.1747950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrovic Fabijan A., Lin R. C. Y., Ho J., et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nature Microbiology . 2020;5(3):465–472. doi: 10.1038/s41564-019-0634-z. [DOI] [PubMed] [Google Scholar]
- 43.Markoishvili K., Tsitlanadze G., Katsarava R., Glenn J., Sulakvelidze A. A novel sustained-release matrix based on biodegradable poly (ester amide) s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. International Journal of Dermatology . 2002;41(7):453–458. doi: 10.1046/j.1365-4362.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- 44.Rhoads D. D., Wolcott R. D., Kuskowski M. A., Wolcott B. M., Ward L. S., Sulakvelidze A. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. Journal of Wound Care . 2009;18(6):237–243. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 45.Morozova V. V., Kozlova Y. N., Ganichev D. A., Tikunova N. V. Bacteriophage treatment of infected diabetic foot ulcers. Methods in Molecular Biology . 2018;1693:151–158. doi: 10.1007/978-1-4939-7395-8_13. [DOI] [PubMed] [Google Scholar]
- 46.Fish R., Kutter E., Wheat G., Blasdel B., Kutateladze M., Kuhl S. Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. Methods in Molecular Biology . 2018;1693:159–170. doi: 10.1007/978-1-4939-7395-8_14. [DOI] [PubMed] [Google Scholar]
- 47.Kifelew L. G., Warner M. S., Morales S., et al. Efficacy of phage cocktail AB-SA01 therapy in diabetic mouse wound infections caused by multidrug-resistant Staphylococcus aureus. BMC Microbiology . 2020;20(1):p. 204. doi: 10.1186/s12866-020-01891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumari S., Harjai K., Chhibber S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. Journal of Medical Microbiology . 2011;60(2):205–210. doi: 10.1099/jmm.0.018580-0. [DOI] [PubMed] [Google Scholar]
- 49.Yin S., Huang G., Zhang Y., et al. Phage Abp1 rescues human cells and mice from infection by pan-drug resistant acinetobacter baumannii. Cellular Physiology and Biochemistry . 2017;44(6):2337–2345. doi: 10.1159/000486117. [DOI] [PubMed] [Google Scholar]
- 50.Totte J. E. E., van Doorn M. B., Pasmans S. Successful treatment of chronic Staphylococcus aureus-related dermatoses with the topical endolysin staphefekt SA.100: a report of 3 cases. Case Reports in Dermatology . 2017;9(2):19–25. doi: 10.1159/000473872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castillo-Ruiz M., Vines E. D., Montt C., et al. Isolation of a novel aggregatibacter actinomycetemcomitans serotype b bacteriophage capable of lysing bacteria within a biofilm. Applied and Environmental Microbiology . 2011;77(9):3157–3159. doi: 10.1128/aem.02115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo L., McLean J. S., Yang Y., et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proceedings of the National Academy of Sciences of the USA . 2015;112(24):7569–7574. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tinoco J. M., Liss N., Zhang H., et al. Antibacterial effect of genetically-engineered bacteriophage ϕEf11/ϕFL1C(Δ36)P nisA on dentin infected with antibiotic-resistant Enterococcus faecalis. Archives of Oral Biology . 2017;82:166–170. doi: 10.1016/j.archoralbio.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Xu J., Yang H., Bi Y., Li W., Wei H., Li Y. Activity of the chimeric lysin ClyR against common gram-positive oral microbes and its anticaries efficacy in rat models. Viruses . 2018;10(380):p. 7. doi: 10.3390/v10070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W., Yang H., Gong Y., Wang S., Li Y., Wei H. Effects of a chimeric lysin against planktonic and sessile Enterococcus faecalis hint at potential application in endodontic therapy. Viruses . 2018;10(6):p. 290. doi: 10.3390/v10060290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruttin A., Brussow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrobial Agents and Chemotherapy . 2005;49(7):2874–2878. doi: 10.1128/aac.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarker S. A., Sultana S., Reuteler G., et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine . 2016;4:124–137. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vahedi A., Soltan Dallal M. M., Douraghi M., et al. Isolation and identification of specific bacteriophage against enteropathogenic Escherichia coli (EPEC) and in vitro and in vivo characterization of bacteriophage. FEMS Microbiology Letters . 2018;365(16) doi: 10.1093/femsle/fny136.fny136 [DOI] [PubMed] [Google Scholar]
- 59.Jaiswal A., Koley H., Ghosh A., Palit A., Sarkar B. Efficacy of cocktail phage therapy in treating Vibrio cholerae infection in a rabbit model. Microbes and Infection . 2013;15(2):152–156. doi: 10.1016/j.micinf.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Nale J. Y., Spencer J., Hargreaves K. R., et al. Bacteriophage combinationsz significantly reduce Clostridium difficile growth in vitro and proliferation in vivo. Antimicrobial Agents and Chemotherapy . 2016;60(2):968–981. doi: 10.1128/aac.01774-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galtier M., De Sordi L., Maura D., et al. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environmental Microbiology . 2016;18(7):2237–2245. doi: 10.1111/1462-2920.13284. [DOI] [PubMed] [Google Scholar]
- 62.Cao F., Wang X., Wang L., et al. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. BioMed Research International . 2015;2015:9. doi: 10.1155/2015/752930.752930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alemayehu D., Casey P. G., McAuliffe O., et al. Bacteriophages φMR299-2 and φNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. mBio . 2012;3(2) doi: 10.1128/mBio.00029-12.e00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oduor J. M. O., Onkoba N., Maloba F., Nyachieo A. Experimental phage therapy against haematogenous multi-drug resistant Staphylococcus aureus pneumonia in mice. African Journal of Laboratory Medicine . 2016;5(1):p. 435. doi: 10.4102/ajlm.v5i1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waters E. M., Neill D. R., Kaman B., et al. Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax . 2017;72(7):666–667. doi: 10.1136/thoraxjnl-2016-209265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leitner L., Sybesma W., Chanishvili N., et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urology . 2017;17(1):p. 90. doi: 10.1186/s12894-017-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuipers S., Ruth M. M., Mientjes M., de Sevaux R. G. L., van Ingen J. A dutch case report of successful treatment of chronic relapsing urinary tract infection with bacteriophages in a renal transplant patient. Antimicrobial Agents and Chemotherapy . 2019;64(1) doi: 10.1128/aac.01281-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rostkowska O. M., Miedzybrodzki R., Miszewska-Szyszkowska D., Gorski A., Durlik M. Treatment of recurrent urinary tract infections in a 60-year-old kidney transplant recipient the use of phage therapy. Transplant Infectious Disease . 2021;23(1) doi: 10.1111/tid.13391.e13391 [DOI] [PubMed] [Google Scholar]
- 69.Fukuda K., Ishida W., Uchiyama J., et al. Pseudomonas aeruginosa keratitis in mice: effects of topical bacteriophage KPP12 administration. PLoS One . 2012;7(10) doi: 10.1371/journal.pone.0047742.e47742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Furusawa T., Iwano H., Hiyashimizu Y., et al. Phage therapy is effective in a mouse model of bacterial equine keratitis. Applied and Environmental Microbiology . 2016;82(17):5332–5339. doi: 10.1128/aem.01166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright A., Hawkins C. H., Anggard E. E., Harper D. R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clinical Otolaryngology . 2009;34(4):349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 72.Hawkins C., Harper D., Burch D., Anggard E., Soothill J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: a before/after clinical trial. Veterinary Microbiology . 2010;146(3-4):309–313. doi: 10.1016/j.vetmic.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 73.Ooi M. L., Drilling A. J., Morales S., et al. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to Staphylococcus aureus. JAMA Otolaryngology–Head & Neck Surgery . 2019;145(8):723–729. doi: 10.1001/jamaoto.2019.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider G., Szentes N., Horvath M., et al. Kinetics of targeted phage rescue in a mouse model of systemic Escherichia coli K1. BioMed Research International . 2018;2018:8. doi: 10.1155/2018/7569645.7569645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pouillot F., Chomton M., Blois H., et al. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrobial Agents and Chemotherapy . 2012;56(7):3568–3575. doi: 10.1128/aac.06330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li W., Schafer A., Kulkarni S. S., et al. High potency of a bivalent human VH domain in SARS-CoV-2 animal models. Cell . 2020;183(2):429–441. doi: 10.1016/j.cell.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morozova V. V., Vlassov V. V., Tikunova N. V. Applications of bacteriophages in the treatment of localized infections in humans. Frontiers in Microbiology . 2018;9:p. 1696. doi: 10.3389/fmicb.2018.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown T. L., Petrovski S., Dyson Z. A., Seviour R., Tucci J. The formulation of bacteriophage in a semi solid preparation for control of propionibacterium acnes growth. PLoS One . 2016;11(3) doi: 10.1371/journal.pone.0151184.e0151184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinto G., Silva M. D., Peddey M., Sillankorva S., Azeredo J. The role of bacteriophages in periodontal health and disease. Future Microbiology . 2016;11(10):1359–1369. doi: 10.2217/fmb-2016-0081. [DOI] [PubMed] [Google Scholar]
- 80.Naidu M., Robles-Sikisaka R., Abeles S. R., Boehm T. K., Pride D. T. Characterization of bacteriophage communities and CRISPR profiles from dental plaque. BMC Microbiology . 2014;14(1):p. 175. doi: 10.1186/1471-2180-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Shan T. L., Li F., et al. A novel phage from periodontal pockets associated with chronic periodontitis. Virus Genes . 2019;55(3):381–393. doi: 10.1007/s11262-019-01658-y. [DOI] [PubMed] [Google Scholar]
- 82.Ly M., Abeles S. R., Boehm T. K., et al. Altered oral viral ecology in association with periodontal disease. mBio . 2014;5(3):e01133–e01114. doi: 10.1128/mbio.01133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pride D. T., Salzman J., Haynes M., et al. Evidence of a robust resident bacteriophage population revealed through analysis of the human salivary virome. The ISME Journal . 2012;6(5):915–926. doi: 10.1038/ismej.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tronstad L., Barnett F., Cervone F. Periapical bacterial plaque in teeth refractory to endodontic treatment. Dental Traumatology . 1990;6(2):73–77. doi: 10.1111/j.1600-9657.1990.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 85.Machuca P., Daille L., Vines E., Berrocal L., Bittner M. Isolation of a novel bacteriophage specific for the periodontal pathogen fusobacterium nucleatum. Applied and Environmental Microbiology . 2010;76(21):7243–7250. doi: 10.1128/aem.01135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith G. P. Phage display: simple evolution in a petri dish (nobel lecture) Angewandte Chemie International Edition . 2019;58(41):14428–14437. doi: 10.1002/anie.201908308. [DOI] [PubMed] [Google Scholar]
- 87.Khalifa L., Gelman D., Shlezinger M., et al. Defeating antibiotic- and phage-resistant Enterococcus faecalis using a phage cocktail in vitro and in a clot model. Frontiers in Microbiology . 2018;9:p. 326. doi: 10.3389/fmicb.2018.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sarker S. A., McCallin S., Barretto C., et al. Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology . 2012;434(2):222–232. doi: 10.1016/j.virol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Garretto A., Miller-Ensminger T., Wolfe A. J., Putonti C. Bacteriophages of the lower urinary tract. Nature Reviews Urology . 2019;16(7):422–432. doi: 10.1038/s41585-019-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jang H. J., Bae H. W., Cho Y. H. Exploitation of Drosophila infection models to evaluate antibacterial efficacy of phages. Methods in Molecular Biology . 2019;1898:183–190. doi: 10.1007/978-1-4939-8940-9_15. [DOI] [PubMed] [Google Scholar]
- 91.Palma M. Perspectives on passive antibody therapy and peptide-based vaccines against emerging pathogens like SARS-CoV-2. Germs . 2021;11(2):287–305. doi: 10.18683/germs.2021.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sioud M. Phage display libraries: from binders to targeted drug delivery and human therapeutics. Molecular Biotechnology . 2019;61(4):286–303. doi: 10.1007/s12033-019-00156-8. [DOI] [PubMed] [Google Scholar]
- 93.Staquicini D. I., Tang F. H. F., Markosian C., et al. Design and proof of concept for targeted phage-based COVID-19 vaccination strategies with a streamlined cold-free supply chain. Proceedings of the National Academy of Sciences of the USA . 2021;118(30) doi: 10.1073/pnas.2105739118.e2105739118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staquicini D. I., Barbu E. M., Zemans R. L., et al. Targeted phage display-based pulmonary vaccination in mice and non-human primates. Medical Times . 2021;2(3):321–342.e8. doi: 10.1016/j.medj.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canfield G. S., Duerkop B. A. Molecular mechanisms of enterococcal-bacteriophage interactions and implications for human health. Current Opinion in Microbiology . 2020;56:38–44. doi: 10.1016/j.mib.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segall A. M., Roach D. R., Strathdee S. A. Stronger together? perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Current Opinion in Microbiology . 2019;51:46–50. doi: 10.1016/j.mib.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 97.Seo S. U., Kweon M. N. Virome-host interactions in intestinal health and disease. Current Opinion in Virology . 2019;37:63–71. doi: 10.1016/j.coviro.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 98.Xu H. M., Huang H. L., Xu J., et al. Cross-talk between butyric acid and gut microbiota in ulcerative colitis following fecal microbiota transplantation. Frontiers in Microbiology . 2021;12 doi: 10.3389/fmicb.2021.658292.658292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zuo T., Lu X. J., Zhang Y., et al. Gut mucosal virome alterations in ulcerative colitis. Gut . 2019;68(7):1169–1179. doi: 10.1136/gutjnl-2018-318131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang J. Y., Kim M. S., Kim E., et al. Enteric viruses ameliorate gut inflammation via toll-like receptor 3 and toll-like receptor 7-mediated interferon-beta production. Immunity . 2016;44(4):889–900. doi: 10.1016/j.immuni.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 101.van Belleghem J. D., Clement F., Merabishvili M., Lavigne R., Vaneechoutte M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Scientific Reports . 2017;7(1):p. 8004. doi: 10.1038/s41598-017-08336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clokie M. R. J. Microbial clues to a liver disease. Nature . 2019;575(7783):451–453. doi: 10.1038/d41586-019-03417-3. [DOI] [PubMed] [Google Scholar]
- 103.Duan Y., Llorente C., Lang S., et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature . 2019;575(7783):505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma Y., You X., Mai G., Tokuyasu T., Liu C. A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome . 2018;6(1):p. 24. doi: 10.1186/s40168-018-0410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao G., Vatanen T., Droit L., et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proceedings of the National Academy of Sciences of the USA . 2017;114(30):E6166–E6175. doi: 10.1073/pnas.1706359114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen S., Huo D., Ma C., Jiang S., Zhang J. Expanding the colorectal cancer biomarkers based on the human gut phageome. Microbiology Spectrum . 2021;9(3) doi: 10.1128/spectrum.00090-21.e0009021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakatsu G., Zhou H., Wu W. K. K., et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology . 2018;155(2):529–541. doi: 10.1053/j.gastro.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 108.Hannigan G. D., Duhaime M. B., Ruffin M. T. T., Koumpouras C. C., Schloss P. D. Diagnostic potential and interactive dynamics of the colorectal cancer virome. mBio . 2018;9(6) doi: 10.1128/mbio.02248-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng D. W., Dong X., Pan P., et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nature Biomedical Engineering . 2019;3(9):717–728. doi: 10.1038/s41551-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 110.Hosonaga M., Saya H., Arima Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer and Metastasis Reviews . 2020;39(3):711–720. doi: 10.1007/s10555-020-09881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arab A., Behravan N., Razazn A., et al. The viral approach to breast cancer immunotherapy. Journal of Cellular Physiology . 2019;234(2):1257–1267. doi: 10.1002/jcp.27150. [DOI] [PubMed] [Google Scholar]
- 112.Abbineni G., Modali S., Safiejko-Mroczka B., Petrenko V. A., Mao C. Evolutionary selection of new breast cancer cell-targeting peptides and phages with the cell-targeting peptides fully displayed on the major coat and their effects on actin dynamics during cell internalization. Molecular Pharmaceutics . 2010;7(5):1629–1642. doi: 10.1021/mp100052y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tetz G., Brown S. M., Hao Y., Tetz V. Parkinson’s disease and bacteriophages as its overlooked contributors. Scientific Reports . 2018;8(1) doi: 10.1038/s41598-018-29173-4.10812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yolken R. H., Severance E. G., Sabunciyan S., et al. Metagenomic sequencing indicates that the oropharyngeal phageome of individuals with schizophrenia differs from that of controls. Schizophrenia Bulletin . 2015;41(5):1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kempeni J. Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7. Annals of the Rheumatic Diseases . 1999;58:I70–I72. doi: 10.1136/ard.58.2008.i70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frenzel A., Schirrmann T., Hust M. Phage display-derived human antibodies in clinical development and therapy. mAbs . 2016;8(7):1177–1194. doi: 10.1080/19420862.2016.1212149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khurana S., Fuentes S., Coyle E. M., Ravichandran S., Davey R. T., Jr., Beigel J. H. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nature Medicine . 2016;22(12):1439–1447. doi: 10.1038/nm.4201. [DOI] [PubMed] [Google Scholar]
- 118.Li T. W., Cheng S. F., Tseng Y. T., et al. Development of single-chain variable fragments (scFv) against influenza virus targeting hemagglutinin subunit 2 (HA2) Archives of Virology . 2016;161(1):19–31. doi: 10.1007/s00705-015-2625-6. [DOI] [PubMed] [Google Scholar]
- 119.He B., Chen H., Li N., Huang J. SAROTUP: a suite of tools for finding potential target-unrelated peptides from phage display data. International Journal of Biological Sciences . 2019;15(7):1452–1459. doi: 10.7150/ijbs.31957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen D., Xie F., Edwards W. B. Evaluation of phage display discovered peptides as ligands for prostate-specific membrane antigen (PSMA) PLoS One . 2013;8(7) doi: 10.1371/journal.pone.0068339.e68339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hou L., Zhu D., Liang Y., et al. Identification of a specific peptide binding to colon cancer cells from a phage-displayed peptide library. British Journal of Cancer . 2018;118(1):79–87. doi: 10.1038/bjc.2017.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han J., Gao X., Duan W., et al. The further characterization of the peptide specifically binding to gastric cancer. Molecular and Cellular Probes . 2016;30(3):125–131. doi: 10.1016/j.mcp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Saha D., Mukherjee R. Ameliorating the antimicrobial resistance crisis: phage therapy. IUBMB Life . 2019;71(7):781–790. doi: 10.1002/iub.2010. [DOI] [PubMed] [Google Scholar]
- 124.Kortright K. E., Chan B. K., Koff J. L., Turner P. E. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host & Microbe . 2019;25(2):219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 125.Kaur G., Agarwal R., Sharma R. K. Bacteriophage therapy for critical and high-priority antibiotic-resistant bacteria and phage cocktail-antibiotic formulation perspective. Food and Environmental Virology . 2021;13(4):433–446. doi: 10.1007/s12560-021-09483-z. [DOI] [PubMed] [Google Scholar]
- 126.Azam A. H., Tanji Y. Bacteriophage-host arm race: an update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Applied Microbiology and Biotechnology . 2019;103(5):2121–2131. doi: 10.1007/s00253-019-09629-x. [DOI] [PubMed] [Google Scholar]
- 127.Liu D., van Belleghem J. D., de Vries C. R., et al. The safety and toxicity of phage therapy: a review of animal and clinical studies. Viruses . 2021;13(7):p. 1268. doi: 10.3390/v13071268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suh G. A., Lodise T. P., Tamma P. D., et al. Considerations for the use of phage therapy in clinical practice. Antimicrobial Agents and Chemotherapy . 2022;66(3) doi: 10.1128/aac.02071-21.e0207121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reyes A., Haynes M., Hanson N., et al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature . 2010;466(7304):334–338. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Touchon M., Bernheim A., Rocha E. P. Genetic and life-history traits associated with the distribution of prophages in bacteria. The ISME Journal . 2016;10(11):2744–2754. doi: 10.1038/ismej.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Waller A. S., Yamada T., Kristensen D. M., et al. Classification and quantification of bacteriophage taxa in human gut metagenomes. The ISME Journal . 2014;8(7):1391–1402. doi: 10.1038/ismej.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cornuault J. K., Moncaut E., Loux V., et al. The enemy from within: a prophage of Roseburia intestinalis systematically turns lytic in the mouse gut, driving bacterial adaptation by CRISPR spacer acquisition. The ISME Journal . 2020;14(3):771–787. doi: 10.1038/s41396-019-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oh J. H., Lin X. B., Zhang S., et al. Prophages in Lactobacillus reuteri are associated with fitness trade-offs but can increase competitiveness in the gut ecosystem. Applied and Environmental Microbiology . 2019;86(1) doi: 10.1128/aem.01922-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Edwards R. A., McNair K., Faust K., Raes J., Dutilh B. E. Computational approaches to predict bacteriophage-host relationships. FEMS Microbiology Reviews . 2016;40(2):258–272. doi: 10.1093/femsre/fuv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paez-Espino D., Eloe-Fadrosh E. A., Pavlopoulos G. A., et al. Uncovering earth’s virome. Nature . 2016;536(7617):425–430. doi: 10.1038/nature19094. [DOI] [PubMed] [Google Scholar]
- 136.Galiez C., Siebert M., Enault F., Vincent J., Soding J. WIsH: who is the host? predicting prokaryotic hosts from metagenomic phage contigs. Bioinformatics . 2017;33(19):3113–3114. doi: 10.1093/bioinformatics/btx383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moreno-Gallego J. L., Chou S. P., Di Rienzi S. C., et al. Virome diversity correlates with intestinal microbiome diversity in adult monozygotic twins. Cell Host & Microbe . 2019;25(2):261–272.e5. doi: 10.1016/j.chom.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


