Abstract
Introduction
Calcium use during cardiac arrest has conflicting results in terms of efficacy. Therefore, we performed a systematic review evaluating the role of calcium administration in cardiac arrest.
Methods
We searched PubMed, Cochrane, and EMBASE for studies comparing calcium administration versus no calcium administration during cardiac arrest. The study was prospectively registered in PROSPERO (CRD42022316641) adhering to PRISMA guideline recommendations. The primary outcome was return of spontaneous circulation (ROSC) or survival at one hour. The secondary outcomes included survival to discharge or at 30 days, and favorable neurologic outcomes at 30 and 90 days. We planned to perform a random-effects meta-analysis of low risk of bias studies. We evaluated risk of bias with RoB-2 and ROBINS-I.
Results
We identified 1,921 articles and included ten studies with 2509 patients. We were not able to perform a meta-analysis with low-risk of bias studies as only one study was found to be at low-risk of bias. However, for the primary outcome, the three RCTs included showed no benefit with calcium administration during cardiac arrest for ROSC. For the secondary outcomes, based on the most recent study and lower risk of bias, there was a neutral effect for survival to discharge or at 30 days and neurologic outcomes at 30 days. However, there was unfavorable neurologic outcomes at 90 days.
Conclusion
Based on our results, calcium administration in cardiac arrests shows no benefit and can cause harm. Further studies on this matter are likely not advisable.
Keywords: Calcium, Cardiac arrest, Advanced Cardiac Life Support, Resuscitation
Introduction
Calcium has been used in cardiac arrest for more than 70 years. Its first description dates back to 1951 in a paediatric cardiac surgery case series with four successful returns of spontaneous circulation (ROSC) after intracardiac calcium administration.1 Given calcium’s involvement in the contraction of all muscle cell types,2 it was theorised that calcium would increase myocardial contractility and enhance electrical defibrillation. The routine use of calcium in cardiac arrest was then recommended by the American Heart Association (AHA) in 1970.3 However, in 1983, two retrospective studies demonstrated that the role of calcium in cardiac arrest was not as clear as the electrophysiologic rationale4, 5 and showed it could be related to increased mortality.5 Some studies revealed that the cellular ionized calcium removal was impaired during cardiac arrest, and the persistence of such an environment predisposed muscle cells to ischaemia.6 Two blinded randomised controlled trials performed in the early 1980s showed no benefit of calcium in asystole or pulseless electrical activity (PEA) cardiac arrests.7, 8 Further guidelines did not recommend the routine use of calcium, and as a result, a decrease in its administration was noted from 1980s to 1990s.9
Recently, a retrospective study from 2016 revealed that calcium use along with bicarbonate during cardiac arrest in patients with severe hyperkalemia > 6.5 mEq/L had a lesser rate of ROSC (21.1 % in the group of calcium and bicarbonate versus 75 % when neither were administered).10 A more recent double-blinded randomised controlled trial from 2021 found no benefit of calcium administration in out-of-hospital cardiac arrest (OHCA). The aforementioned study was stopped early due to concerns for possible harm.11 The latest American Heart Association (AHA) guidelines from 2020 do not recommend the routine administration of calcium, chiefly based on non-randomised studies of this intervention.12 Nevertheless, despite guideline recommendations, the use of calcium in intra-hospital cardiac arrest (IHCA) has increased since the 2000s.13
Therefore, our objective was to synthesize the evidence on the role of calcium administration in adult patients during OHCA or IHCA in terms of ROSC, survival, and functional outcomes.
Material and methods
The study protocol was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO),14 number PROSPERO 2022 - CRD42022316641, and this report complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15 We used the GRADE (Grading Recommendations, Assessment, Development and Evaluation) tool to evaluate the certainty of evidence of our results.16
Eligibility criteria
We included randomised clinical trials (RCT) and non-randomised studies of interventions (NRSI) with a control group. We decided to include NRSIs because current RCT evidence is scarce and, in this case, the evidence from NRSI could be complementary to allow for more precise estimates. For inclusion, the studies had to meet the following criteria: 1) comparison between calcium and no calcium administration during cardiac arrest in the adult population older than 18 years, including either OHCA or IHCA; 2) reporting outcomes of ROSC, survival to discharge or at 30 days, and/or neurologic outcomes. We excluded publications with no original data, case reports, case series, and animal studies. There were no language restrictions.
Search strategy and data collection
Two independent reviewers (EMHP and BB) performed the literature search in MEDLINE (PubMed), EMBASE, and Cochrane CENTRAL with no language restriction. The terms used were calcium, cardiac arrest, and its respective synonyms. More details regarding keywords and terms used are available in the Supplementary Appendix. We also searched for studies on the reference list of included articles and in the trial registry database Clinicaltrials.gov.
Data collection was performed by two independent reviewers (EMHP and CJD) based on titles, abstracts, and full text articles. The data collected was organized in an electronic spreadsheet. In case of discordance, a third reviewer (RC) was involved for a final decision.
Risk of bias assessment
We evaluated the risk of bias for RCTs using the Cochrane Risk of Bias 2 Tool (RoB 2).17 For NRSI we used the Risk of Bias in Nonrandomised Studies of Interventions (ROBINS-I) tool.18 We considered an ideal feasible randomised clinical trial and compared it with the NRSIs as recommended; more information is available in the Supplementary Appendix. The evaluation of bias was performed by two independent reviewers (EMHP and CJD). In case of discordant results, a third reviewer (RC) made a final decision after discussion with the team.
Outcomes
The primary outcome was to evaluate ROSC or survival at one hour after cardiac arrest. Secondary outcomes included: 1) survival to hospital discharge or at 30 days; 2) neurological outcomes at 30 days; 3) neurological outcomes at 90 days.
Statistical analysis
The analyzed results were all dichotomous outcomes of rare occurrence, where odds ratios approximate risk ratios, so pooling was done on the odds ratio scale with its respective 95 % confidence intervals (CIs) to allow better comparability with NRSI, which report odds ratios. We planned to evaluate statistical heterogeneity with the Cochran Q test and I2 statistics. We planned to perform random-effects meta-analysis throughout the study because we could not assume a single common effect for all studies due to clinical heterogeneity in study design.19 Subgroup and sensitive analyses including IHCA vs OHCA, study design (RCTs vs NRSIs) and study risk of bias (low risk of bias vs serious/critical risk) were planned to be conducted to explore clinical and methodological heterogeneity. When appropriate, we assessed for publication bias using a funnel plot.20 Review Manager 5.4.1 and Stata 17 were used for statistical analysis.
Results
Study selection
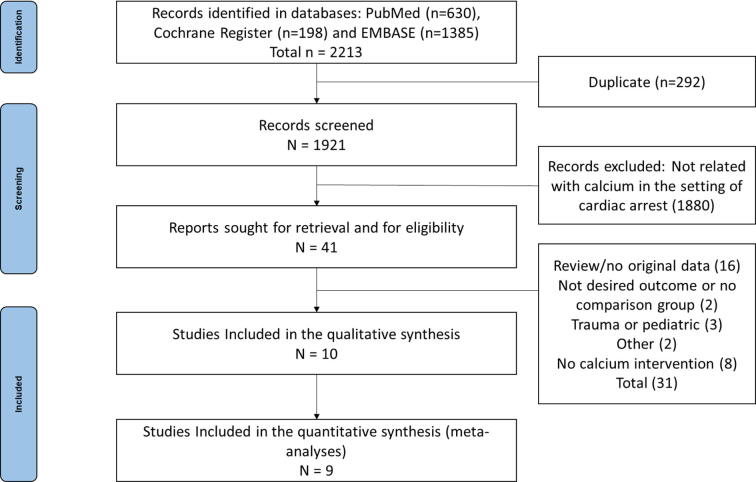
We identified 2213 articles available in the three databases and retrieved 1921 articles after removal of duplicates. Forty-one studies were selected for full text analysis, ten studies were included for qualitative review, and nine were included for meta-analysis. Among them, three RCTs7, 8, 11 and seven NRSI5, 10, 21, 22, 23, 24, 25 were identified. One study included only patients with hyperkalemia measured during the cardiac arrest10 and for this reason, we decided to exclude it from the quantitative analysis. A complete flow diagram is presented in Fig. 1. There were 554 patients randomised and a total of 2509 patients compared either in RCTs or NRSIs.
Fig. 1.
Flow diagram of study selection.
Study characteristics
The three RCTs were blinded and compared calcium chloride with placebo (normal saline).7, 8, 11 Two trials used a dose of 500 mg,7, 8 and the most recent trial used a 735 mg dose.11 Calcium was administered only one time in the trials that used the 500 mg dose,7, 8 however, up to two doses were administered in the most recent trial.11 All of them were undertaken on OHCA patients. All three RCTs included patients with both initial shockable and non-shockable rhythms, however two RCTs administered medications solely when the rhythms were non shockable at the time of drug administration.7, 8 Vallentin et al. used calcium for both shockable and non-shockable rhythms.11
Among the NRSIs, we retrieved four retrospective cohorts,5, 10, 21, 25 one prospective cohort22 and two post-hoc analyses23, 24 of previous cardiopulmonary arrest randomised clinical trials.26, 27 Two retrospective studies compared use of calcium and no calcium treatment in the OHCA scenario.5, 25 The other two retrospective cohorts were IHCA and the researchers compared multiple agents, including the use of calcium.10, 21 The prospective cohort22 was a letter to the editor regarding one of the available retrospective studies, where the authors included their IHCA prospective study findings. They compared the use of calcium with no calcium.22 The last two post-hoc analyses were observational studies that compared the use of multiple drugs during cardiac arrest.23, 24 One of them included both IHCA and OHCA and the data was retrieved from an RCT that compared high dose versus low dose adrenaline (epinephrine).27 The other study included only IHCA and the data was retrieved from an RCT that compared active compression-decompression resuscitation versus standard resuscitation.26 More information regarding the studies is available in Table 1 and Table 2.
Table 1.
Characteristics of the studies included in the analysis.
| Author/Publication | Study Period | Study Design | Inclusion Criteria | Exclusion Criteria | Initial Rhythms | Outcomes Available | Comparison |
|---|---|---|---|---|---|---|---|
| Stueven 19835 | 1980 | Retrospective Observational Cohort | OHCA patients with non-shockable rhythms | Traumatic arrest and poisoning |
Asystole and PEA | ROSC, survival to discharge |
Calcium versus standard care |
| Stueven 1985 (Asystole)8 | 1982–1983 | Blinded Randomized Clinical Trial | OHCA patients in asystole after epinephrine, bicarbonate and atropine | Clinical indications for calcium, traumatic arrest, poisoning and pediatric patients | All | ROSC, survival to discharge |
Calcium versus placebo |
| Stueven 1985 (PEA)7 | 1982–1983 | Blinded Randomized Clinical Trial | OHCA patients in PEA after epinephrine and bicarbonate | Clinical indications for calcium, traumatic arrest, poisoning and pediatric patients | All | ROSC, survival to discharge | Calcium versus placebo |
| Stempien 198625 | 1983–1984 | Retrospective Observational Cohort | OHCA patients only |
No exclusion criteria available | Asystole and PEA | “Survival” (presumed ROSC) | Calcium versus standard care |
| George 198722 | 1985 | Prospective Observational Cohort | IHCA |
No exclusion criteria available | Asystole and PEA | ROSC and survival to discharge | Calcium versus standard care |
| Stiell 199524 | 1989–1992 | Post-hoc analysis of previous Randomized Clinical Trial | IHCA and OHCA patients with time to CPR available | <16-years-old, terminal illness, more than 15 minutes to initiate CPR and trauma | All |
ROSC, survival to discharge |
Calcium and multiple other drugs during cardiac arrest |
| van Walraven 199823 | 1989–1992 | Post-hoc analysis of previous Randomized Clinical Trial | IHCA | < 16-year-old, terminal illness, > 15 min to CPR, trauma, sternotomy, arrest in the OR, delivery or recovery rooms and all OHCA | All Rhythms | ROSC | Calcium and multiple other drugs during cardiac arrest |
| Snipilesky 201621 | 2008–2014 | Retrospective Observational Cohort | Index IHCA | Patients who received uncommon combination of medications during the arrest | All Rhythms | ROSC | Calcium and multiple other drugs during cardiac arrest |
| Wang 201610 | 2006–2012 | Retrospective Observational Cohort | Index IHCA adult patients with hyperkalemia > 6.5 mEq/L collected during cardiac arrest | Traumatic cardiac arrest | All Rhythms | ROSC, survival to discharge, neurologic outcome at discharge | Calcium, sodium bicarbonate and none |
| Vallentin 202111 | 2020–2021 | Double Blinded Randomized Clinical Trial | OHCA, older than 18 years-old who received at least one dose of epinephrine | Traumatic cardiac arrest, pregnancy, clinical indication for calcium, enrollment in other trial, received epinephrine outside of the trial | All Rhythms | ROSC, survival 30 days and 90 days, neurologic outcomes in 30 and 90 days | Calcium versus placebo |
OHCA = out-of-hospital cardiac arrest; PEA = pulseless electrical activity; ROSC = return of spontaneous circulation; IHCA = intra-hospital cardiac arrest; CPR = cardiopulmonary resuscitation; OR = Operation room.
Table 2.
Characteristics of the study population among studies included in the analysis.
| Author/ Year of Publication | N (Ca/No Ca) | Age (YEARS) (MEAN ± SD) | Sex M/F (%) | Location OHCA/IHCA (%) | Rhythm S/NS (%) | Bystander or EMS witnessed (%) | Time to ACLS Minutes (mean ± SD or median, IQR) | Duration of ACLS (min) | Calcium Dose | Mean time to calcium¥ (Minutes, IQR) |
|---|---|---|---|---|---|---|---|---|---|---|
| Stueven 19835 | 42/168 | 66 ± 15 | 65/35 | 100/0 | 0/100 | 47 | NA | 30 | NA | 19 and 23* |
| Stueven 1985 (Asystole)8 | 39/34 | 65 ± 13 | 71/29 | 100/0 | 24.7/75.3 | 44 | Ca 6.2 ± 4.9 Pl 6.6 ± 5.6 |
NA | CaCl2 500 mg | NA |
| Stueven 1985 (PEA)7 | 48/42 | 69 ± 13 | 63/37 | 100/0 | 34.4/64.5 | 61 | Ca 4.3 ± 5.5 Pl 4.6 ± 4.8 |
NA | CaCl2 500 mg | NA |
| Stempien 198625 | 93/17 | NA | NA | 100/0 | 0/100 | NA | NA | NA | CaCl2 1000 mg |
NA |
| George 198722 | 69/61 | NA | NA | 0/100 | NA | NA | NA | Ca 38.8 No Ca 18.4 |
NA | NA |
| Stiell 199524 | 29/500 | 66 ± 13 | 66/34 | 46/54 | 32/68 | 83 | NA | NA | NA | 0.4 to 0.9⁰ |
| van Walraven 199823 | 105/668 | 68.5 ± 14 | 57/43 | 0/100 | 32/68 | 80 | 1.4 | NA | NA | NA |
| Snipilesky 201621 | 34/60 | 65 ± 11 | 53/47 | 0/100 | 8/92 | NA | 0 | Ca 23.1 No Ca 16.2 |
0–6 g | NA |
| Wang 201610 | 61/48 | 64.5 ± 17 | 58/42 | 0/100 | 7/93 | 68 | NA | NA | NA | 16.9 |
| Vallentin 202111 | 193/198 | 68 ± 14 | 71/29 | 100/0 | 25/75 | 59 | Ca 8 (4,12)└ Pl 8 (5,13)└ |
NA | CaCl2 735 mg |
17 (13,23) |
Ca = Calcium; SD = Standard Deviation; EMS = Emergency Medical Service; M = Male; F = Female; OHCA = Out-of-hospital cardiac arrest; IHCA = Intra-hospital cardiac arrest; S = Shockable; NS = Non-shockable; ACLS = Advanced Cardiac Life Support; NA = Not available; Pl = Placebo.
¥Mean time to calcium administration from the cardiac arrest onset.
*19 minutes for asystole and 23 minutes for pulseless electrical activity.
⁰Mean time to calcium after ACLS onset. The study did not provide the mean time to calcium from the cardiac arrest onset.
└Time to ambulance arrival (the study did not provide time to ACLS).
Risk of bias assessment
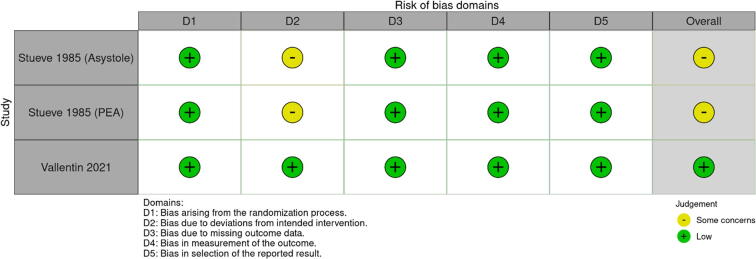
One RCT was considered to be of low risk of bias11 and two RCTs were considered to be of some concern for risk of bias7, 8 due to the exclusion of patients that underwent cross-over or did not follow the protocol (per-protocol analysis). For ROSC and survival at one hour, all NRSI were considered at serious risk of bias and the most common cause was not performing an analysis accounting for resuscitation time bias5, 10, 21, 22, 23 or not performing it appropriately.24 For survival to hospital discharge or at 30 days, the NRSIs were considered at serious risk of bias.5, 22, 24 Only the RCTs and three retrospective studies provided the outcome survival on discharge or at 30 days.5, 7, 8, 11, 22, 24 Fig. 2 provides the risk of bias of the RCTs for ROSC or survival at 1 hour, Figure S1 provides the risk of bias of the RCTs for survival to discharge and at 30 days and Figure S2 provide the risk of bias for NRSI. Only one study provided neurologic outcomes at 30 days and at 90 days, which were considered low risk of bias.11
Fig. 2.
Randomised Clinical Trials Risk of Bias (RoB2).
Outcomes
Return of spontaneous circulation or survival at one hour
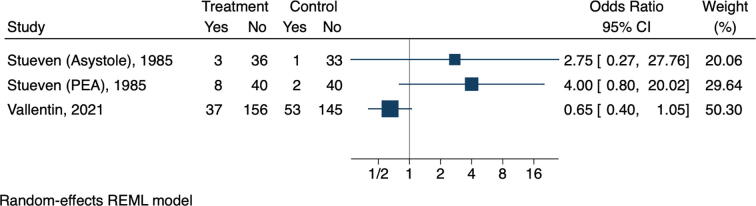
We were unable to perform a meta-analysis with studies with low risk of bias since only one study could be included.11 However, we performed a forest plot (Fig. 3) of the studies that were at no serious risk of bias (some concerns and low risk of bias).7, 8, 11 The last published RCT, and the only with low risk of bias, obtained ROSC in 37 out of 193 patients (19.2 %) in the calcium group versus 53 out of 198 patients (26.7 %) in the placebo group, with an OR of 0.65 (95 % CI 0.40–1.04; p = 0.09).11 The other two RCTs, with some concerns for risk of bias, also had neutral results. The RCT which used intravenous calcium during PEA obtained ROSC in eight out of 48 patients (16.7 %) in the calcium group versus two out of 42 patients in the placebo group (4.8 %), resulting in an OR of 4.00 (95 % CI 0.80–20.02; p = 0.07).7 The RCT which used intravenous calcium during asystole obtained ROSC in three out of 39 patients (7.7 %) in the calcium group versus two out of 42 patients (4.8 %) in the placebo group, resulting in an OR of 2.75 (95 % CI 0.27–27.76; p = 0.37).8 The pooled effect is presented in the Supplementary Appendix (Figure S3). A total of 554 patients were included in the three studies.7, 8, 11 Two sensitivity analyses were performed. Figure S4 shows a post-hoc analysis with the RCTs and the only NRSI that adjusted to resuscitation time bias, although not as recommended.28 There was no change in the neutral effect of calcium. Sensitivity analysis that included all studies showed harm associated with the use of calcium (Figure S5). We also performed a subgroup analysis comparing studies with no serious risk of bias with those with serious risk of bias. Harm was associated with the use of calcium only in the subgroup including studies at serious risk of bias (Figure S5). We initially planned to perform a subgroup analysis of OHCA and IHCA separately, but we could not perform a meta-analysis of IHCA since the studies were at a serious risk of bias. A funnel plot is available in the Supplementary Appendix (Figure S7).
Fig. 3.
Forest plot of return of spontaneous circulation or survival at one hour.
Survival to discharge or at 30 days
We were unable to perform an analysis including studies with low risk of bias. Also, we were unable to perform an analysis with studies with no serious risk of bias since two RCTs had only one event among a total of 163 patients.7, 8 The only RCT with low risk of bias resulted in an OR of 0.55 (95 % CI 0.25–1.22; p = 0.17) for survival to discharge or at 30 days.11 Sensitivity analysis was done and showed harm with calcium administration (Figure S6). A funnel plot is available in the Supplementary Appendix (Figure S8).
Neurologic outcomes
Only one study had available neurologic outcomes11 and a meta-analysis was not performed. The study presented neurologic outcomes at 30 and 90 days. At 30 days, survival with favorable neurologic outcome was seen in seven out of 193 (3.6 %) in the group of calcium and 15 out of 198 (7.6 %) in the control group resulting in an OR of 0.46 (95 % CI 0.18–1.15; p = 0.10). Regarding neurologic outcomes at 90 days, it showed that seven out of 193 (3.6 %) of those who received calcium had a favorable neurologic outcome in 90 days versus 18 of 198 (9.1 %) in the placebo group, resulting in an OR of 0.37 (95 % CI 0.15–0.92; p = 0.03).11
Certainty of evidence
We performed a GRADE assessment16 for the certainty of evidence of our primary and secondary outcomes including the only study with low risk of bias (Fig. 2. ROSC or survival at one hour; survival to hospital discharge or at 30 days; favorable neurologic outcomes in 30 days; and favorable neurologic outcomes in 90 days were all found to have moderate certainty of evidence, mostly due to serious imprecision (Table 3).
Table 3.
GRADE assessment.
|
Certainty assessment |
№ ofstudies |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Calcium | No Calcium | Relative(95 % CI) | Absolute(95 % CI) | ||
| Return of Spontaneous Circulation or Survival at 1 hour | ||||||||||||
| 1 | randomised trial | not serious | not serious | not serious | seriousa | none | 37/193 (19.2 %) | 53/198 (26.8 %) |
OR 0.65 (0.40 to 1.05) |
76 fewer per 1,000 (from 140 fewer to 10 more) |
⊕⊕⊕○ Moderate |
IMPORTANT |
| Survival to discharge or at 30 days | ||||||||||||
| 1 | randomised trial | not serious | not serious | not serious | seriousb | none | 10/193 (5.1 %) | 18/198 (9.1 %) |
OR 0.53 (0.24 to 1.14) |
30 fewer per 1,000 (from 49 fewer to 9 more) |
⊕⊕⊕○ Moderate |
IMPORTANT |
| Neurologic outcomes at 30 days | ||||||||||||
| 1 | randomised trial | not serious | not serious | not serious | seriousc | none | 7/193 (3.6 %) | 15/198 (7.6 %) |
OR 0.46 (0.18 to 1.15) |
47 fewer per 1,000 (from 73 fewer to 12 more) |
⊕⊕⊕○ Moderate |
IMPORTANT |
| Neurologic outcomes at 90 days | ||||||||||||
| 1 | randomised trial | not serious | not serious | not serious | seriousd | none | 7/193 (3.6 %) | 18/198 (9.1 %) |
OR 0.37 (0.15 to 0.92) |
54 fewer per 1,000 (from 76 fewer to 7 fewer) |
⊕⊕⊕○ Moderate |
IMPORTANT |
CI: confidence interval; OR: odds ratio.
Estimated sample (optimal information size) required around 480 patients per group at least, considering 19.2% of ROSC in the calcium group and 26.8% in the placebo group.
Estimated sample (optimal information size) required around 646 patients per group at least, considering 5.1 % of survival to discharge or at 30 days in the calcium group and 9.1 % in the placebo group.
Estimated sample (optimal information size) required around 517 patients per group at least, considering 3.6 % of favorable neurologic outcome at 30 days in the calcium group and 7.6 % in the placebo group.
Estimated sample (optimal information size) required around 307 patients per group at least, considering 3.6 % of favorable neurologic outcome at 90 days in the calcium group and 9.1 % in the placebo group.
Discussion
Our results show that calcium administration during cardiac arrest was associated with no benefit (RR > 1.00) for ROSC or survival at one hour after cardiac arrest. Additionally, we did not observe any benefit for survival to hospital discharge or at 30 days. When evaluating neurologic outcomes, we observed neither benefit nor harm at 30 days, but worse neurologic outcomes or death at 90 days.
These results are mainly due to the most recent and largest randomized clinical trial published. The study was stopped early due to possible harm.11 An OR of 0.65 (95 % CI 0.40–1.04; p = 0.09) was obtained for ROSC. When the authors adjusted the results, an OR of 0.81 (CI CI 95 % 0.47–1.22) was revealed. The Bayesian analysis showed that calcium administration had a probability of benefit of only 4 % for ROSC, 6 % for survival at 30 days and 4 % of the favorable neurological outcomes at 30 days.11
The current data available is limited to three randomised clinical trials in the out-of-hospital setting, which together account for 554 patients.7, 8, 11 No study showed benefit with calcium administration, although there was significant clinical heterogeneity among the studies. Many clinical differences are noticed when comparing both Stueven et al. studies with Vallentin et al.7, 8, 11 Although all three studies included patients in the out-of-hospital setting, they were separated by almost 40 years. Due to the time frame difference, there was improvement of overall post cardiac arrest care occurred and changes in the ACLS protocol. While Vallentin et al. used calcium along with the first and second epinephrine dose despite the rhythm,11 Stueven et al. used calcium later, during asystole after atropine,8 or during PEA.7 Also, the doses between the studies were different. Both Stueven et al. trials7, 8 used 500 mg of calcium gluconate only once while the most recent clinical trial used up to doses two of 735 mg of calcium. A total of 285 out of 391 (73 %) of the patients received two doses.11
We did not include NRSIs in our primary analysis and the analysis to survival to hospital discharge or at 30 days due to serious risk of bias, mostly due to resuscitation time bias.28 The only NRSI24 that had been adjusted to time to drug administration was not adjusted as recommended.28 Nonetheless, the sensitivity analysis for ROSC including Stiell et al.24 and the RCTs showed neutral effect of calcium (Figure S4). The analysis including only RCTs also showed neutral effect of calcium for ROSC (Figure S3). When performing sensitivity analysis, including all studies, for both ROSC and survival to hospital discharge or at 30 days (Figure S5 and Figure S6), calcium administration was associated with harm. This is likely due to resuscitation time bias, which occurs when an exposure, such as calcium administration, is more likely to occur as the cardiac arrest persists. The fact that prolonged cardiac arrest is associated with poor outcomes will likely bias the results towards harm.28, 29, 30
We could not perform a meta-analysis including studies with no serious risk of bias for survival to discharge or at 30 days, and for neurological outcomes at 30 and 90 days. However, the results and the Bayesian analysis from the last RCT11 show that calcium may have a neutral or even harmful effect when administered. Therefore, performing randomized controlled trials may be considered unethical in this context.
We decided not to include Wang et al. in our quantitative analyses, as they studied a specific group of patients with hyperkalemia measured during cardiac arrest. This group of patients have formal indications of intravenous calcium administration during the cardiac arrest,12 although the most recent guidelines from the AHA do not recommend routine point-of-care laboratory work-up during cardiac arrest.12
Although we planned to analyze both OHCA and IHCA separately, we could not perform a meta-analysis of IHCA since studies were at serious risk of bias. However, both types of cardiac arrest have cardiac causes as the primary etiology, and both present 80 % of the time as PEA or asystole as initial rhythm.31 Causes such as hyperkalemia, calcium channel blocker intoxication, and hypocalcemia are rare. In one study evaluating more than 1,000 causes of IHCA, less than 1 % was attributed to hyperkalemia and there was no cardiac arrest due to calcium channel blocker intoxication or hypocalcemia.32
Our current findings show that calcium has no benefit and can instead be harmful. Our results do not endorse the indiscriminate use of calcium in both IHCA and OHCA. Exceptions include specific causes such as hyperkalemia, hypocalcemia, calcium channel blocker intoxication or active participation in a clinical study. Our results are of significance, as current data from a large registry study showed that 20–30 % of patients in IHCA receive calcium during resuscitation, and this trend has been increasing since 2016.13
Limitations
Our systematic review has some limitations. Firstly, we found only three available RCTs despite a comprehensive search strategy and only one was considered to have a low risk of bias. Secondly, we did not perform a search strategy that included grey literature. However, due to the consistency of our findings and the last published RCT,11 we do not believe that any further study would have changed the results. Thirdly, due to paucity of data, we could not perform a meta-analysis with studies with low risk of bias. However, when analyzing the largest RCT, all the Bayesian posterior probability distributions did not show any benefit for neurological outcomes.11 Lastly, we could not analyze IHCA. Nonetheless, as mentioned above, the causes of cardiac arrest that usually require calcium as treatment are less than 1 % of the etiologies in IHCA,32 making it unlikely that the routine use of calcium will have any benefit.
Conclusion
This systematic review does not support the routine use of calcium to improve outcomes in cardiac arrest. Although the available information is not extensive, our study indicates no benefit, but instead may cause possible harm. Based on this data, further studies should likely not be advised.
Role of the funding source
This study was not funded by any source.
Declaration of Interest
The authors declare that they have no conflict of interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2022.100315.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Kaay J.H., Blalock The use of calcium chloride in the treatment of cardiac arrest in patients. Surg Gynecol Obstet. 1951;93:97–102. [PubMed] [Google Scholar]
- 2.Bers D.M. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 3.JAMA. 1974;227:833–868. doi: 10.1001/jama.227.7.833. [DOI] [PubMed] [Google Scholar]
- 4.Harrison E.E., Amey B.D. The use of calcium in cardiac resuscitation. Am J Emerg Med. 1983;1:267–273. doi: 10.1016/0735-6757(83)90103-1. [DOI] [PubMed] [Google Scholar]
- 5.Stueven H., Thompson B.M., Aprahamian C., Darin J.C. Use of calcium in prehospital cardiac arrest. Ann Emerg Med. 1983;12:136–139. doi: 10.1016/s0196-0644(83)80551-4. [DOI] [PubMed] [Google Scholar]
- 6.Fleckenstein A., Kanke J., Döring H.J., Leder O. Key role of Ca in the production of noncoronarogenic myocardial necroses. Recent Adv Stud Cardiac Struct Metab. 1975;6:21–32. [PubMed] [Google Scholar]
- 7.Stueven H.A., Thompson B., Aprahamian C., Tonsfeldt D.J., Kastenson E.H. The effectiveness of calcium chloride in refractory electromechanical dissociation. Ann Emerg Med. 1985;14:626–629. doi: 10.1016/s0196-0644(85)80874-x. [DOI] [PubMed] [Google Scholar]
- 8.Stueven H.A., Thompson B., Aprahamian C., Tonsfeldt D.J., Kastenson E.H. Lack of effectiveness of calcium chloride in refractory asystole. Ann Emerg Med. 1985;14:630–632. doi: 10.1016/s0196-0644(85)80875-1. [DOI] [PubMed] [Google Scholar]
- 9.Levy R.D., Rhoden W.E., Shearer K., Varley E., Brooks N.H. An audit of drug usage for in-hospital cardiopulmonary resuscitation. Eur Heart J. 1992;13:1665–1668. doi: 10.1093/oxfordjournals.eurheartj.a060122. [DOI] [PubMed] [Google Scholar]
- 10.Wang C.H., Huang C.H., Chang W.T., et al. The effects of calcium and sodium bicarbonate on severe hyperkalaemia during cardiopulmonary resuscitation: A retrospective cohort study of adult in-hospital cardiac arrest. Resuscitation. 2016;98:105–111. doi: 10.1016/j.resuscitation.2015.09.384. [DOI] [PubMed] [Google Scholar]
- 11.Vallentin M.F., Granfeldt A., Meilandt C., et al. Effect of Intravenous or Intraosseous Calcium vs Saline on Return of Spontaneous Circulation in Adults With Out-of-Hospital Cardiac Arrest: A Randomised Clinical Trial. JAMA. 2021;326:2268–2276. doi: 10.1001/jama.2021.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panchal A.R., Bartos J.A., Cabañas J.G., et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz A., Ross C.E., Andersen L.W., et al. Trends Over Time in Drug Administration During Adult In-Hospital Cardiac Arrest. Crit Care Med. 2019;47:194–200. doi: 10.1097/CCM.0000000000003506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padrao EMH, Bustos B, Mahesh A, et al: Calcium during cardiopulmonary resuscitation: A systematic review and Meta-analysis. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=316641. Accessed April 19, 2022.
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Published 2021 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serghiou S., Goodman S.N. Random-Effects Meta-analysis: Summarizing Evidence With Caveats. JAMA. 2019;321:301–302. doi: 10.1001/jama.2018.19684. [DOI] [PubMed] [Google Scholar]
- 20.Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
- 21.Snipelisky D., Ray J., Matcha G., et al. Descriptive Analysis of Medication Administration During Inpatient Cardiopulmonary Arrest Resuscitation (from the Mayo Registry for Telemetry Efficacy in Arrest Study) Am J Cardiol. 2016;117:1610–1615. doi: 10.1016/j.amjcard.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 22.George A.L., Jr, Folk B.P., 3rd, Crecelius P.L., Campbell W.B. Calcium and cardiac arrest. Ann Intern Med. 1987;106:472–473. doi: 10.7326/0003-4819-106-3-472_2. [DOI] [PubMed] [Google Scholar]
- 23.van Walraven C., Stiell I.G., Wells G.A., Hébert P.C., Vandemheen K. Do advanced cardiac life support drugs increase resuscitation rates from in-hospital cardiac arrest? The OTAC Study Group. Ann Emerg Med. 1998;32:544–553. doi: 10.1016/s0196-0644(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 24.Stiell I.G., Wells G.A., Hebert P.C., Laupacis A., Weitzman B.N. Association of drug therapy with survival in cardiac arrest: limited role of advanced cardiac life support drugs. Acad Emerg Med. 1995;2:264–273. doi: 10.1111/j.1553-2712.1995.tb03220.x. [DOI] [PubMed] [Google Scholar]
- 25.Stempien A., Katz A.M., Messineo F.C. Calcium and cardiac arrest. Ann Intern Med. 1986;105:603–606. doi: 10.7326/0003-4819-105-4-603. [DOI] [PubMed] [Google Scholar]
- 26.Stiell I.G., Hébert P.C., Wells G.A., et al. The Ontario trial of active compression-decompression cardiopulmonary resuscitation for in-hospital and prehospital cardiac arrest. JAMA. 1996;275:1417–1423. [PubMed] [Google Scholar]
- 27.Stiell I.G., Hebert P.C., Weitzman B.N., et al. High-dose epinephrine in adult cardiac arrest. N Engl J Med. 1992;327:1045–1050. doi: 10.1056/NEJM199210083271502. [DOI] [PubMed] [Google Scholar]
- 28.Andersen L.W., Grossestreuer A.V., Donnino M.W. “Resuscitation time bias”-A unique challenge for observational cardiac arrest research. Resuscitation. 2018;125:79–82. doi: 10.1016/j.resuscitation.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bascom K.E., Dziodzio J., Vasaiwala S., et al. Derivation and Validation of the CREST Model for Very Early Prediction of Circulatory Etiology Death in Patients Without ST-Segment-Elevation Myocardial Infarction After Cardiac Arrest. Circulation. 2018;137:273–282. doi: 10.1161/CIRCULATIONAHA.116.024332. [DOI] [PubMed] [Google Scholar]
- 30.van Walraven C., Forster A.J., Parish D.C., et al. Validation of a clinical decision aid to discontinue in-hospital cardiac arrest resuscitations. JAMA. 2001;285:1602–1606. doi: 10.1001/jama.285.12.1602. [DOI] [PubMed] [Google Scholar]
- 31.Andersen L.W., Holmberg M.J., Berg K.M., Donnino M.W., Granfeldt A. In-Hospital Cardiac Arrest: A Review. JAMA. 2019;321:1200–1210. doi: 10.1001/jama.2019.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallmuller C., Meron G., Kurkciyan I., Schober A., Stratil P., Sterz F. Causes of in-hospital cardiac arrest and influence on outcome. Resuscitation. 2012;83:1206–1211. doi: 10.1016/j.resuscitation.2012.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.