Abstract
Plant growth ultimately depends on fixed carbon, thus the available light for photosynthesis. Due to canopy light absorption properties, vegetative shade combines low blue (LB) light and a low red to far-red ratio (LRFR). In shade-avoiding plants, these two conditions independently trigger growth adaptations to enhance light access. However, how these conditions, differing in light quality and quantity, similarly promote hypocotyl growth remains unknown. Using RNA sequencing we show that these two features of shade trigger different transcriptional reprogramming. LB induces starvation responses, suggesting a switch to a catabolic state. Accordingly, LB promotes autophagy. In contrast, LRFR induced anabolism including expression of sterol biosynthesis genes in hypocotyls in a manner dependent on PHYTOCHROME-INTERACTING FACTORs (PIFs). Genetic analyses show that the combination of sterol biosynthesis and autophagy is essential for hypocotyl growth promotion in vegetative shade. We propose that vegetative shade enhances hypocotyl growth by combining autophagy-mediated recycling and promotion of specific lipid biosynthetic processes.
Subject terms: Light responses, Plant physiology, Plant development
Plants subject to vegetative shade receive a low quantity of blue light (LB) and a low ratio of red to far-red light (LFLR). Here the authors show that while LB induces autophagy, LFLR leads to changes in lipid metabolism, and propose that these processes may contribute to shade avoidance responses.
Introduction
Plants use a portion of the electromagnetic spectrum for photosynthesis that is called photosynthetically active radiation (PAR, 400–700 nm) and composed of blue (B, 400–500 nm), green (G, 500–600 nm) and red (R, 600–700 nm) light1. Leaves absorb more than 90% of B and R radiation, whereas they transmit and/or reflect most of the far-red light (FR, 700–760 nm)2. Therefore, plants under vegetative shade receive light combining low B (LB) and a low R/FR ratio (LRFR)3. In shade-avoiding plants, LB and LRFR independently trigger a suite of similar adaptive responses, including the growth of stem-like structures including hypocotyls and petioles to enhance light access4–7. Plants in dense communities also receive LRFR due to FR reflection from neighboring leaves before actual shading. This is perceived as a neighbor proximity/shade threat signal and triggers similar growth adaptation as vegetative shade prior to declining light resources3,4. Plant growth depends on fixed carbon8, which depends on PAR, including B and R light2,9. However, how LB and LRFR with contrasting carbon resource availability promote similar growth adaptations remains unclear10.
While molecular mechanisms underlying hypocotyl growth promotion are relatively well-understood in LRFR, they remain unclear in LB5–7. LRFR inactivates phytochrome B (phyB), leading to de-repression of phytochrome-interacting factors (PIFs) transcription factors (TFs)5,6,11,12. PIF7, with substantial contributions of PIF4 and PIF5, enhances auxin production in cotyledons through induction of YUCCAs (YUC2, YUC5, YUC8, and YUC9) coding for auxin biosynthesis enzymes13–18. Auxin is rapidly transported to the hypocotyl, where it locally induces elongation, presumably through a combined auxin and PIF transcriptional response14,19–21. Additional hormones, notably brassinosteroids (BR) are also important for LRFR-induced hypocotyl growth14,22,23. Indeed, the BR signaling factor BZR1, the auxin response factor ARF6 and PIF4 collectively regulate target gene expression21. PIFs are also important for LB-induced hypocotyl elongation, where PIF4 is the primary one with contributions of PIF5 and PIF724–26. The LB response is controlled by cryptochromes (cry), but how they regulate PIFs remains unclear. Cry1 inhibits PIF4 transcriptional activity following BL-induced interaction26,27. In LB cry2 interacts with PIF4/PIF5, but the functional importance of this complex remains unclear26,27. Despite auxin and BR being indispensable for hypocotyl elongation in LB, this is not apparent from the transcriptional response, which contrasts with LRFR conditions24–26,28,29. Despite these differences, several growth-related pathways are transcriptionally activated in LB and LRFR14,26,28. Nevertheless, the lack of spatial resolution limits our current understanding of LB vs LRFR growth-promoting mechanisms and the role of PIFs in hypocotyls.
Hypocotyl growth occurs by cell elongation where plasma membrane (PM) extension is essential30–32. Although the PM lipid bilayer can transit between tighter or looser packing depending on several parameters, the PM is fairly rigid with limited expansion or contraction ability33. Furthermore, PM curvature is low, also limiting its extension potential34. In rapidly elongating plant cells (e.g., pollen tubes and root hair cells), the PM grows with the deposition of new lipid material through the fusion of Golgi-derived vesicles carrying new cell wall material31,32. We previously reported that LRFR increases carbon allocation to lipids in B. rapa hypocotyls35. Furthermore, LRFR induces sterol biosynthesis gene expression in hypocotyls14. Sterols compose up to 30% of PM lipids33. They influence PM permeability and fluidity33,36. Together with sphingolipids, sterols are enriched in PM lipid microdomains that serve as anchoring platforms for signaling and transporting proteins37,38. Their major structural and functional roles at the PM suggest an important function of sterols in shade-induced hypocotyl elongation.
Production of new material required for cell elongation depends on carbon availability8,39. LRFR does not decrease carbon fixation in B. rapa seedlings, as PAR remains unchanged35. However, reducing PAR either by decreasing B, G, or R light decreases carbon fixation2,9. Thus, carbon fixation is expected to decrease in LB, limiting the availability of newly fixed carbon to sustain hypocotyl growth promotion. Carbon starvation triggered by transferring plants into darkness for several days induces autophagy that recycles unessential cytoplasmic materials by vacuolar hydrolases40–42. The fact that LB and LRFR differ in PAR suggests the deployment of different mechanisms to enable cell elongation in these distinct conditions.
Analyzing light-regulated gene expression in dissected hypocotyls was informative to understand hypocotyl growth regulation during de-etiolation and in LRFR14,43, but we lack equivalent data for LB. Thus, we performed organ-specific gene expression to compare and contrast hypocotyl growth promotion in LB vs LRFR. We show that in LRFR PIFs induce expression of many anabolic processes in the hypocotyl, including sterol biosynthesis. In contrast, LB induces expression of catabolic processes and promotes autophagy, which is important for hypocotyl growth enhancement.
Results
LB and LRFR induce distinct transcriptional changes in elongating hypocotyls
Consistent with previous studies24,26, LB and LRFR treatments independently induced hypocotyl elongation in a PIF and YUC-dependent manner (Fig. 1a). We hypothesized a convergence of LB and LRFR transcriptome in hypocotyls where both light conditions trigger cell elongation. Thus, we analyzed transcriptomes from dissected cotyledons and hypocotyls of Col-0 (wild type—WT) in white light (WL), LB, and LRFR to characterize the organ-specific LB and LRFR responses. pif457 and yuc2589 seedlings were used to determine the role of PIFs and YUC-mediated auxin biosynthesis (Fig. 1b).
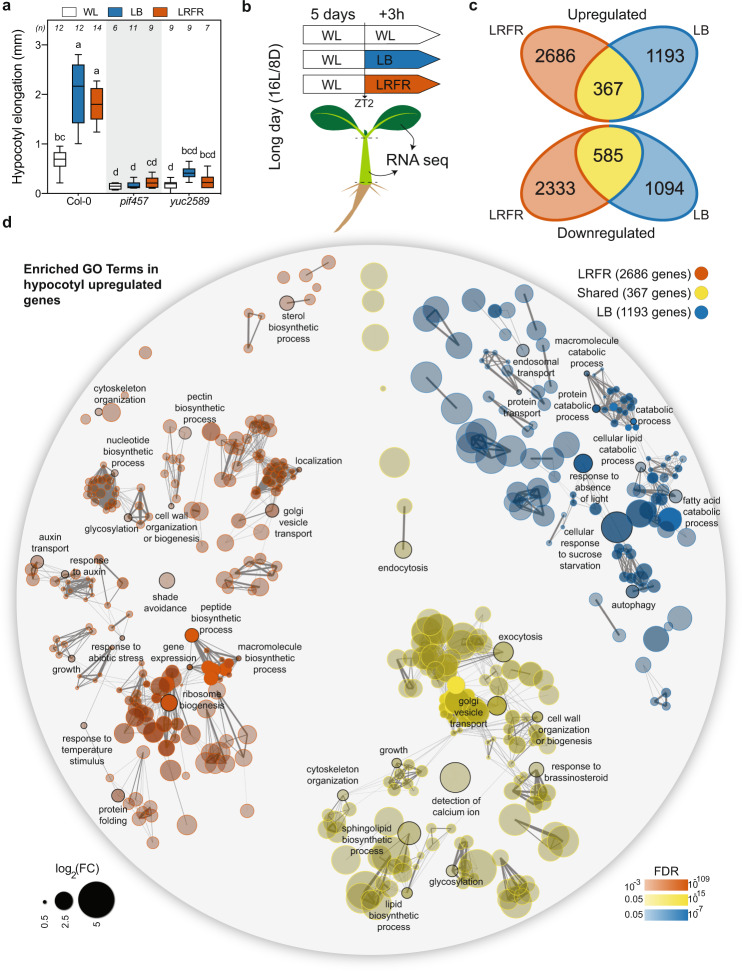
Fig. 1. LB and LRFR induce distinct transcriptional changes in elongating hypocotyls.
a Hypocotyl elongation of the indicated genotypes. The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. Different letters indicate significant differences (P < 0.05, two-way ANOVA with Tukey’s HSD test; the exact P values are available in the Source Data). Sample size (n) that is given on top indicates biologically independent seedlings examined over one experiment. The experiment was repeated two times with similar results. b Schematic summary of the experimental setup used for transcriptome analysis. c Number of up- and downregulated genes in Col-0 hypocotyls in the indicated light conditions (FDR < 0.05, T test with BH correction). d GO term enrichment analysis in Col-0 hypocotyl upregulated gene lists. Each node indicates a significantly enriched GO term (FDR < 0.05). Two terms (nodes) are connected if they share 20% or more genes. The line thickness increases with the increasing number of shared genes between two terms. Only selected GO terms (black outlines) are annotated. The full list of enriched GO terms is in Supplementary Data 2, the interactive version of (d) is available at https://figshare.com/s/864fba30bbd3919d0745. See also Supplementary Fig. 1.
Principal component analysis (PCA) showed that for biological replicates of each genotype, organ, and condition clustered closely (Supplementary Fig. 1a). In WT hypocotyls, LRFR induced more transcriptome changes than LB; while in cotyledons it was the opposite (Fig. 1c, Supplementary Fig. 1b, and Supplementary Data 1). The number of common up- and downregulated genes were higher in hypocotyls than in cotyledons (Fig. 1c and Supplementary Fig. 1b), suggesting a convergence of LB and LRFR transcriptome in elongating hypocotyls. This was confirmed by GO term enrichment analyses for LB-specific, LRFR-specific and shared LB and LRFR-upregulated genes (Fig. 1d, Supplementary Fig. 1c, and Supplementary Data 2). We highlighted selected terms for each organ and light condition that we could easily relate to growth regulation (Fig. 1d, Supplementary Fig. 1c, and full lists in Supplementary Data 2). Genes upregulated by both treatments in hypocotyls were enriched in terms related to cellular elongation including “growth”, “cell wall organization or biogenesis”, “exocytosis”, “endocytosis”, “cytoskeleton organization”, “lipid biosynthetic process”, and “response to brassinosteroid” (Fig. 1d). Genes specifically upregulated by LB in both organs were enriched in GO terms related to starvation (e.g., “cellular response to sucrose starvation”) and catabolic events “protein catabolic process”, “cellular lipid catabolic process” and “autophagy” (Fig. 1d and Supplementary Fig. 1c). In contrast, LRFR-specific genes in hypocotyls were enriched in biosynthetic processes including “ribosome biogenesis”, “peptide biosynthetic process”, “sterol biosynthetic process”, and “cell wall organization and biogenesis” (Fig. 1d). In line with previous reports14, LRFR induced many hormone related responses in cotyledons and “response to auxin” in hypocotyls (Fig. 1d and Supplementary Fig. 1c). Altogether, our GO term enrichment analyses indicate that LB and LRFR transcriptome responses converge in elongating hypocotyls, on the induction of several growth-related mechanisms. However, we observed a striking difference between these treatments with LB upregulating numerous catabolic processes and LRFR inducing many anabolic processes.
Most LRFR-induced genes in hypocotyls require both PIFs and YUCs
In accordance with the established role of PIFs and YUCs for LRFR responses13–18,44, gene expression in pif457 and yuc2589 was largely unresponsive to LRFR (Supplementary Fig. 1a). PCA showed that hypocotyls of LRFR-treated pif457 and yuc2589 grouped with WL samples but yuc2589 cotyledons grouped closer to WT LRFR samples (Supplementary Fig. 1a). We also evaluated the individual roles of PIFs and YUCs by analyzing the interactions between genotypes and light treatments. These comparisons revealed four groups among LRFR-upregulated genes: PIF and YUC dependent, only PIF dependent, only YUC dependent, and dependent on neither (Fig. 2a, Supplementary Fig. 2a, and Supplementary Data 3). In hypocotyls, most LRFR-induced genes required PIFs and YUCs, whereas the largest fraction depended on only PIFs in cotyledons (Fig. 2a and Supplementary Fig. 2a). The extent of LRFR regulation was reduced in pif457 and yuc2589 also in the PIF- and/or YUC-independent categories (Fig. 2b and Supplementary Fig. 2b), indicating that our classification underestimates the importance of PIFs and YUCs. We conclude that LRFR-regulated gene expression largely depends on PIF4, PIF5 and/or PIF7. Moreover, while in cotyledons the regulation of many genes depends on PIFs alone, in the hypocotyl their regulation depends on PIFs and YUCs.
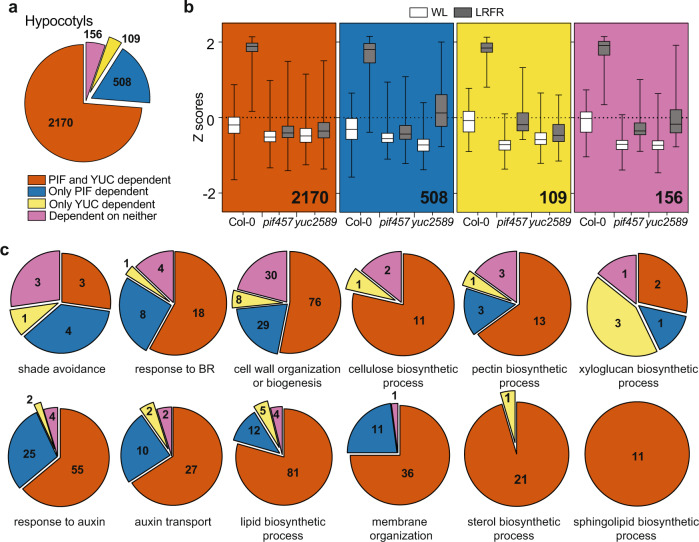
Fig. 2. Most LRFR-induced genes in hypocotyls require both PIFs and YUCs.
a The distribution of hypocotyl-induced genes in LRFR according to the dependence on PIFs and YUCs using the comparison of Col-0, pif457, and yuc2589 transcriptomes (FDR < 0.05, F test with post hoc test). b Distributions of Z-scores computed from replicate averages for categories shown in (a). The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. c The distribution of hypocotyl-induced genes according to the dependence on PIFs and YUCs in each of the selected significantly enriched GO terms. Numbers indicate significantly regulated genes in the given categories and/or GO terms. The full list of misregulated genes and enriched GO terms are given in Supplementary Data 3. See also Supplementary Fig. 2.
We performed GO enrichment analyses to characterize the processes depending on PIFs and YUCs (Supplementary Data 3). In cotyledons terms related to the biosynthesis of multiple hormones required PIFs but not YUCs (Supplementary Fig. 2c). In contrast, in hypocotyls, terms such as “cell wall organization and biogenesis”, “response to brassinosteroids”, “response to auxin”, and “auxin transport” heavily depended on PIFs and YUCs with a particularly strong dependency for terms related to lipid biosynthesis (Fig. 2c). Given that LRFR YUC-dependent auxin production mostly occurs in cotyledons, this suggests that in hypocotyls LRFR gene induction largely depends on auxin transported from the cotyledons with a potential local action of PIFs.
The majority of LB-induced genes do not depend on PIFs or YUCs
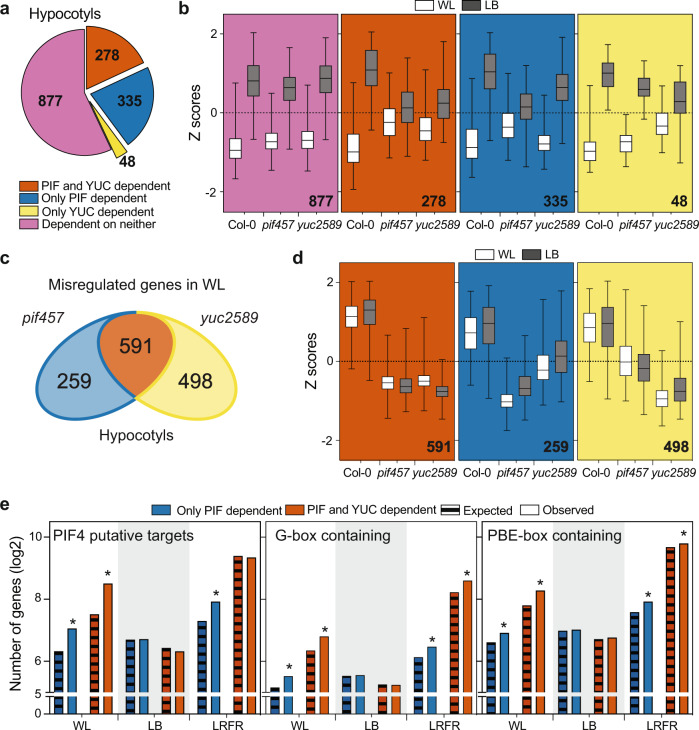
Based on PCA, pif457 and yuc2589 displayed a robust transcriptional response to LB, contrasting with LRFR (particularly in hypocotyls) and the phenotypes of these mutants (Supplementary Fig. 1a and Figs. 1a, 2a, and 3a). Nevertheless, part of the LB-upregulated genes, including some related to protein catabolism and secretion/organelle transport processes, depended on PIFs and/or YUCs (Fig. 3a, b and Supplementary Fig. 3a, gene and GO term list in Supplementary Data 4). However, the biggest fraction of LB-induced genes did not depend on PIFs and/or YUCs (Fig. 3a, b and Supplementary Fig. 3a). One possibility is that LB-induced hypocotyl elongation requires optimal expression of genes in WL (baseline conditions). Indeed, we found numerous genes with lower expression in pif457 and/or yuc2589 compared to WT in WL (Fig. 3c and Supplementary Data 5). We note that most WL-misregulated genes in hypocotyls required both PIFs and YUCs as observed in the hypocotyls of LRFR-treated seedlings (Fig. 3c and Supplementary Fig. 3b). In contrast, in cotyledons more required PIFs but not YUC (Fig. 3c and Supplementary Fig. 3b). Although in the WT LB did not strongly induce these genes, their expression levels in the mutants were also lower in LB (Fig. 3d). Many GO terms related to growth, hormones and cell wall as well as “response to blue light” and “response to starvation” were enriched in the PIF- and YUC-dependent genes in hypocotyls (Supplementary Fig. 3c and Supplementary Data 5). We conclude that in contrast to LRFR, LB-gene induction was less dependent on PIFs and YUCs.
Fig. 3. PIFs & YUCs are required for basal expression of many growth- and hormone-associated genes in hypocotyls of WL-grown seedlings.
a The distribution of LB-induced genes in hypocotyls according to the dependence on PIFs and YUCs using the comparison of Col-0, pif457, and yuc2589 transcriptomes (FDR < 0.05, F test with post hoc test). b Distributions of Z-scores computed from replicate averages for categories shown in (a). c Number of misregulated genes in pif457 and yuc2589 hypocotyls compared to Col-0 in WL (FDR < 0.05, T test with BH correction). d Distributions of Z-scores computed from replicates averages for genes that are grouped as in (c). e Comparison of PIF-dependent genes in hypocotyls with putative PIF4 targets (as listed in ref. 26), promoters (1 kb upstream) containing G-box (CACGTG) or PBE-box (CATGTG). Asterisks (*) indicate the statistically significant overrepresentation compared to expected (P < 0.05, Binomial distribution, one-tailed; the exact P values are available in the Source Data). b, d The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. Numbers indicate significantly regulated genes in the given categories and/or GO terms. The full list of misregulated genes, enriched GO terms, and enriched motifs are given in Supplementary Data 4, 5, and 6. See also Supplementary Fig. 3.
Finally, we compared each set of PIF-dependent genes in WL, LB, and LRFR with putative PIF4 targets26 (Supplementary Data 6). PIF-dependent genes in WL and LRFR (Figs. 2 and 3c and Supplementary Fig. 3b) were significantly enriched in PIF4 targets for both organs (Fig. 3e and Supplementary Fig. 3d). One exception was PIF and YUC-dependent genes in LRFR in hypocotyls, which may indicate that these genes are induced by auxin produced downstream of PIFs (Fig. 3e). Similarly, for both organs, promoter motif enrichment analysis showed that PIF-bound sequences (G-box and PBE-box) were overrepresented among PIF-dependent genes in WL and LRFR but not in LB (Fig. 3e, Supplementary Fig. 3d, and Supplementary Data 6). These results suggest that PIFs directly regulate numerous genes in WL conditions, which may contribute to impaired hypocotyl elongation of pif457 mutants in LB.
In LRFR PIFs selectively induce SMT2 expression in the hypocotyl
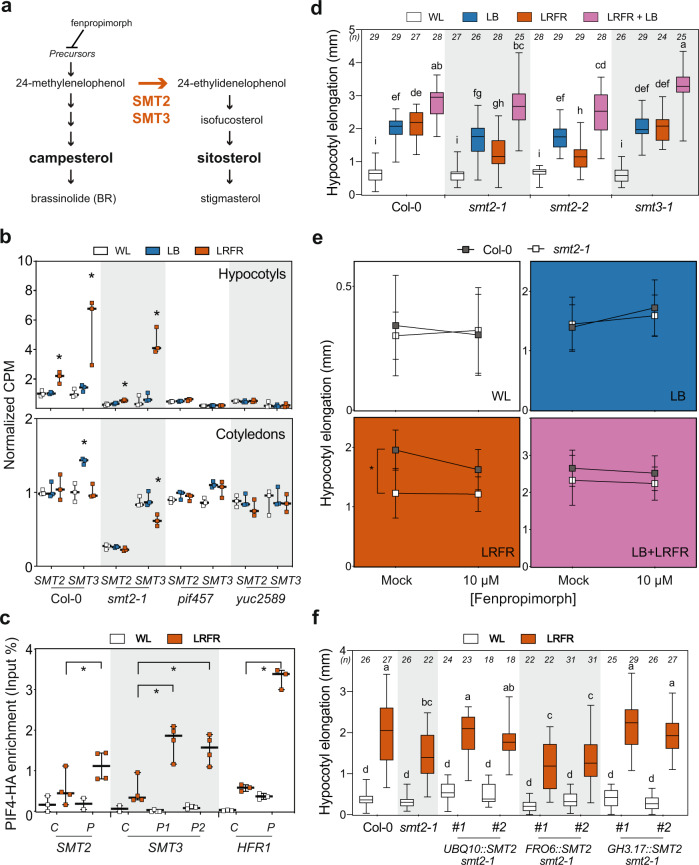
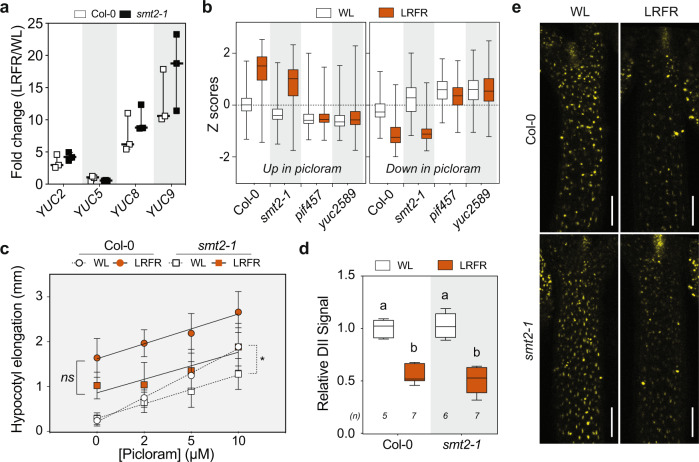
We confirmed that in hypocotyls LRFR induced numerous genes in diverse anabolic processes (Fig. 1d)14, predominantly downstream of PIFs and YUCs (Fig. 2c and Supplementary Data 3). The dependency on PIFs could be direct or indirect as PIFs induce YUC-mediated auxin production in cotyledons13–18. Previously, we reported that more cotyledon-fixed carbon was allocated into the lipid fraction of elongating hypocotyls in LRFR-treated B. rapa35. Lipid biosynthesis is required for membrane expansion in rapidly elongating cells31,32 and was a prominent example of LRFR and PIF-regulated anabolic processes in hypocotyls (Fig. 2c). We focused on sterols because of the global upregulation of the pathway, including several potential direct PIF targets (Figs. 1d and 2c and Supplementary Fig. 4a)26,45. Sterols are indispensable constituents of the PM and precursors of BR growth hormones and several biosynthesis mutants are either embryo lethal or show major growth defects36. C-24 sterol methyltransferases (SMT), encoded by two paralogs SMT2 and SMT3, act at a branch point leading to the synthesis of the predominant PM sterol: sitosterol (Fig. 4a)33,36,46–48. Sitosterol levels decrease dramatically in smt2 and marginally in smt3 mutants but these mutants still contain high levels of other sterols (e.g., campesterol) and BR and they do not have serious growth defects46–48. This enabled us to conduct physiological and molecular experiments using these mutants.
Fig. 4. SMT2 is required locally for LRFR-induced hypocotyl elongation.
a A simplified representation of sterol biosynthesis pathway in Arabidopsis46. b Normalized CPM (counts per million, normalized to Col-0 average in WL) of SMT2 and SMT3 in the indicated genotypes and conditions (data from RNA-seq). c PIF4-HA binding to the promoter of the indicated genes. PIF4-HA enrichment is quantified by qPCR and presented as IP/Input. Control—C, Peak—P. d–f Hypocotyl elongation of the indicated genotypes. d, f The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. b, c Each data point indicates biologically (b) or technically (c) independent samples, the horizontal bar represents the median, whiskers extend to show the data range. e Data are means ± SD with a regression line. Different letters (d, f, two-way ANOVA with Tukey’s HSD test) and asterisks (*) (b, c, Student’s T test, one-tailed) (e, two-way ANOVA) indicate a significant difference (P < 0.05) compared to WL (b) or control (c), and between genotypes in given light condition (e). The exact P values are available in the Source Data. Sample size (n) that is given on top (d, f); e for Col-0; n (WL-Mock) = 21, n (WL-10 µM) = 21, n (LB-Mock) = 26, n (LB-10 µM) = 26; n (LRFR-Mock) = 24, n (LRFR-10 µM) = 24, n (LB + LRFR-Mock) = 25, n (LB + LRFR-10 µM) = 25; for smt2-1; n (WL-Mock) = 12, n (WL-10 µM) = 14, n (LB-Mock) = 20, n (LB-10 µM) = 21, n (LRFR-Mock) = 16, n (LRFR-10 µM) = 18, n (LB + LRFR-Mock) = 24, n (LB + LRFR-10 µM) = 18) indicates biologically independent seedlings examined over one experiment. The experiments were repeated two (e) and three (c, d, f) times with similar results. See also Supplementary Fig. 4.
Induction of SMT2 and SMT3 expression specifically occurred in LRFR, selectively in hypocotyls, and in a PIF and YUC-dependent manner (Fig. 4b). Furthermore, LRFR led to enhanced PIF4-HA binding to the promoter of SMT2 and SMT3 (Fig. 4c) in PIF4p:PIF4-HA (pif4-101) seedlings49. We also detected a significant PIF7-HA enrichment on the SMT3 but not the SMT2 promoter (Supplementary Fig. 4b) in PIF7p:PIF7-HA (pif7-2)50. PIF7-HA binding to SMT3 and HFR1, the latter being used as a positive control, was much lower than PIF4-HA binding (Fig. 4c and Supplementary Fig. 4b). Taken together, our data show that PIFs induce SMT2 and SMT3 expression specifically in hypocotyls and this is accompanied by enhanced PIF binding to SMT promoters in LRFR.
We used smt2 and smt3 mutants46–48 to test the importance of these genes in hypocotyl growth. Hypocotyl elongation was significantly reduced in two independent smt2 alleles and smt2smt3 double mutants, whereas the response to LRFR was unaffected in smt3 (Fig. 4d and Supplementary Fig. 4c). Similarly, when applied simultaneously with light treatments, fenpropimorph, which inhibits sterol biosynthesis upstream of SMTs33,51, reduced hypocotyl elongation in LRFR (Fig. 4a, e). Furthermore, increased drug concentration resulted in a steeper reduction in hypocotyl elongation of WT compared to smt2-1 in LRFR (Supplementary Fig. 4d). Interestingly, hypocotyl elongation of smt2 and smt3 mutants was as in the WT in WL and LB (Fig. 4d). These phenotypes highlight the stronger requirement for SMT2/SMT3 in LRFR than in other light conditions (Fig. 4b, d). Remarkably, LB + LRFR combination mimicking vegetative shade rescued the reduced hypocotyl elongation of smt2-1 in LRFR (Fig. 4d). Similarly, inhibition of sterol biosynthesis during LB and LB + LRFR treatments did not reduce hypocotyl elongation (Fig. 4e). This result contrasts with the hypocotyl phenotype of smt2smt3 in LB and LB + LRFR (Supplementary Fig. 4c). The smt2smt3 double mutant contains only trace amounts of sitosterol, whereas sitosterol biosynthesis is partially impaired in smt2 and upon fenpropimorph treatment (at the tested concentration)46,48,51. Thus, these results indicate that in the smt2 mutant, which contains low levels sitosterol, hypocotyl elongation is primarily affected in LRFR while in the smt2smt3 double mutant, which contains trace amounts of sitosterol, hypocotyl elongation is impaired more broadly.
To determine whether SMT2 is required locally for LRFR-induced hypocotyl growth, we phenotyped smt2-1 transformed with the SMT2 coding sequence controlled by a ubiquitous (UBQ10), cotyledons specific (FRO6)52 or hypocotyls-specific promoter (GH3.17)53 (Supplementary Fig. 4e). UBQ10- and GH3.17-driven SMT2 rescued the smt2-1 phenotype in LRFR in two independent insertion lines for each construct, whereas FRO6 did not (Fig. 4f). Taken together our data shows that PIF selectively regulate SMT2/SMT3 expression in the hypocotyl (Fig. 4b, c) and that SMT2 expression in the hypocotyl is functionally important (Fig. 4f).
One characteristic phenotype of the smt2 and smt2smt3 mutants is an impaired cotyledon vasculature pattern (cvp) (Supplementary Fig. 5a)46–48. Auxin transport from cotyledons to hypocotyls is required for LRFR-induced elongation19,20,28. Thus, we determined hypocotyl growth of other severe cvp mutants, cvp2 and cvp2cvl1 (Supplementary Fig. 5a) that do not interfere with sterol biosynthesis54. Both cvp mutants showed normal hypocotyl elongation in LRFR (Supplementary Fig. 5b), suggesting that the cotyledon vasculature problem of smt2 alone does not explain its hypocotyl growth defect in LRFR. To further analyze the smt2 phenotype, we used RNA sequencing, which showed that the transcriptome of smt2-1 was similar to Col-0 in LB and LRFR in both organs (Supplementary Fig. 5c, d and Supplementary Data 7). LRFR-induced expression of the major genes coding for auxin biosynthesis was similar in Col-0 and smt2-1 (Fig. 5a). Furthermore, comparison of our LRFR transcriptome and 2 h picloram (synthetic auxin)-regulated genes in hypocotyls55 showed a high correlation between these treatments for Col-0 and smt2-1 (Fig. 5b and Supplementary Data 8), but not in pif457 and yuc2589 which are impaired in auxin biosynthesis in LRFR14,18,56. The picloram dose response of WT and smt2-1 hypocotyls was similar in LRFR (Fig. 5c), indicating a similar auxin response. Finally, DII-VENUS, an auxin-input reporter57, similarly decreased in hypocotyls of LRFR-treated seedlings of both genotypes, indicating increased auxin levels (Fig. 5d, e). Altogether, our data suggest that reduced smt2 hypocotyl elongation in LRFR unlikely results from major alterations in auxin biosynthesis, transport or response.
Fig. 5. Auxin biosynthesis, response, and transport are normal in smt2-1 in LRFR.
a LRFR-induction of auxin biosynthetic genes in the indicated genotypes (data from RNA-seq). Each data point indicates biologically independent samples, the horizontal bar represents the median, whiskers extend to show the data range. b Distributions of Z-scores computed from replicates averages for synthetic auxin picloram up- and downregulated genes in hypocotyls of the indicated genotypes (as listed in ref. 55). c Hypocotyl elongation of indicated genotypes with the indicated doses of picloram. Data are means ± SD with a regression line. d, e Quantification (d) and the representative images (e) of the DII-VENUS signal intensity (normalized to mean value of Col-0 in WL) in hypocotyls of the indicated genotypes either kept at WL or transferred to LRFR for 1 h. White bars equal to 100 µm. b, d The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. Different letters (d, two-way ANOVA with Tukey’s HSD test) and asterisks (*) (c, two-way ANOVA) indicate a significant difference (P < 0.05) between genotypes in given light condition (c) and compared to WL (d). The exact P values are available in the Source Data. Sample size (n) that is given on top (d); ((c) For Col-0: n (WL) = 17, 22, 21, 20; n (LRFR) = 19, 22, 17, 22. For smt2-1: n (WL) = 12, 15, 19, 17; n (LRFR) = 13, 18, 17, 17 with an order of Mock, 2 µM, 5 µM, 10 µM respectively) indicates biologically independent seedlings examined over one experiment. The experiments were repeated two (c) and three (d) times with similar results. The full gene list of LRFR-picloram transcriptome comparison is given in Supplementary Data 8. See also Supplementary Fig. 5.
Regulation of the lipid composition in the hypocotyls of LRFR-treated seedlings
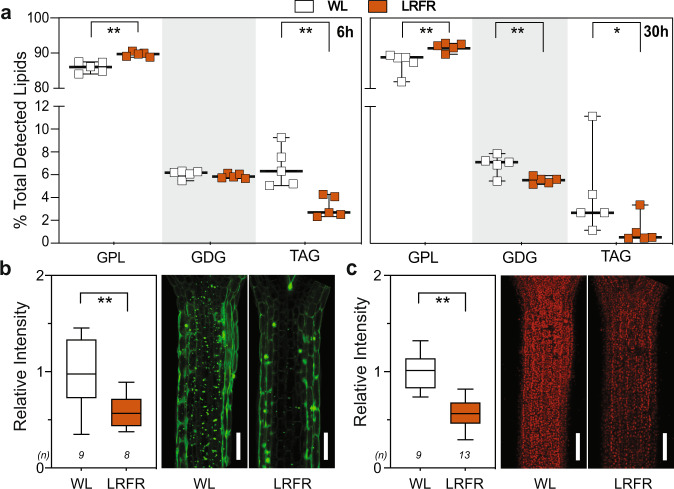
We next analyzed the total lipid content in LRFR using untargeted lipidomics58 in B. rapa hypocotyls, which respond to LRFR similarly to Arabidopsis and allocate more newly fixed carbon to lipids35. This choice was also dictated by technical limitations rendering this experiment impossible with dissected Arabidopsis hypocotyls. The percentage of major PM lipids (glycerophospholipids—GPL) increased, whereas the storage lipids (triacylglycerols—TAG) and the major constituents of thylakoid membranes (glycosyldiacylglycerols—GDG)33 decreased significantly in LRFR in the total lipid pool (Fig. 6a). We then tested whether similar adjustment of lipid profiles occurred in Arabidopsis hypocotyls. We used BODIPY™ 493/503 that stains neutral lipids59 to detect the level of lipid droplets (LD) that mainly contain TAG60. Fluorescence intensity of LDs decreased significantly after 30 h of LRFR treatment in Arabidopsis hypocotyls (Fig. 6b). Furthermore, our transcriptome data showed that the “thylakoid membrane organization” GO term was enriched among hypocotyl downregulated genes in LRFR (Supplementary Data 3). In line with this data and previous reports on tomato stems61, the fluorescence intensity of chloroplasts decreased in Arabidopsis hypocotyls in LRFR (Fig. 6c). Our data indicate that LRFR alters the lipid composition in the hypocotyls of B. rapa and Arabidopsis.
Fig. 6. LRFR changes the lipid composition in hypocotyls.
a Lipid class abundance at the indicated time points is represented as percentage of total detected lipids in B. rapa hypocotyls. Each data point indicates biologically independent samples, horizontal bar represents the median, whiskers extend to show the data range. GPL glycerophospholipids, GDG glycosyldiacylglycerols, TAG triacylglycerols. b, c Quantification (left) and representative images (right) of (b) LDs using BODIPY™ 493/503 and (c) chloroplasts in Col-0 hypocotyls either kept at WL or transferred to LRFR for 30 h. Data is normalized to WL average. White bars are scale bars of 100 µm. b, c The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. a–c Asterisks indicate P values (*<0.1, **<0.05, Student’s T test, one-tailed). The exact P values are available in the Source Data. b, c Sample size (n) that is given on top indicates biologically independent seedlings examined over one experiment. The experiments were repeated three times with similar results. The full list of detected lipid species is given in Supplementary Data 9. See also Supplementary Fig. 6.
Although we focused on SMT2 and SMT3, LRFR led to a coordinated induction of sterol biosynthesis genes in Arabidopsis hypocotyls (Supplementary Fig. 4a)14. This suggests that LRFR induces a general increase in sterols. Thus, we determined the sterol composition in B. rapa hypocotyls where LRFR-expression profiles of BrSMTs were similar to their orthologs in Arabidopsis (Supplementary Fig. 6a, BrIAA29 being a control)20. Campesterol and sitosterol, both major sterols in the PM36, did not change in LRFR during the timeframe of our experiments (Supplementary Fig. 6b). Yet, the percentage of ergosta-5,7-dienol, a precursor for BRs downstream of campesterol, decreased after 3 h of LRFR (Supplementary Fig. 6b). This is in line with a report showing a decreased level of another BR precursor in LRFR62. These results suggest that LRFR induces a total increase in sterols rather than a major change in their composition. We also analyzed plant Sphingolipids using a dedicated LC-MS2 protocol as the methods used for untargeted lipid analysis are poorly suited to study this important class of PM lipids63. As expected, this analysis showed that GIPC (Glycosyl Inositol Phospho Ceramides) were the most abundant class of Sphingolipids in B. rapa hypocotyls and did not reveal any LRFR-induced change within this lipid class (Supplementary Fig. 6c).
LB induces autophagy
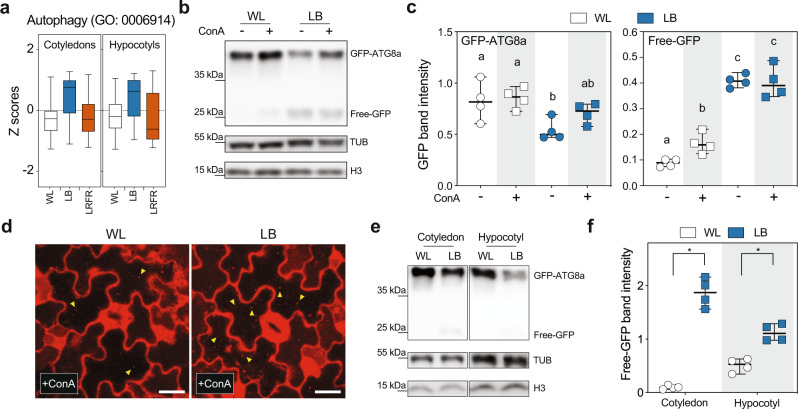
In our LB treatment, PAR was reduced to 66% of WL levels while it remained unchanged in LRFR (Supplementary Fig. 7a). Such a decrease in PAR results in around 50% reduction in the net CO2 assimilation, regardless of the light color used for illumination (B, G, R, or their combinations)2. In contrast, carbon fixation remained unchanged in B. rapa seedlings in LRFR35. Consistently, the “carbon fixation” GO term was enriched only among LB downregulated genes, while terms related to carbon starvation were enriched in LB-induced genes (Fig. 1d, Supplementary Fig. 8a, and Supplementary Data 3, 4). These data suggest that LB induced a switch to a catabolic state to enable growth with declining carbon availability. Accordingly, we observed the selective enrichment of catabolism terms and “Autophagy” in LB-induced genes (Figs. 1d and 7a). To test whether LB induces autophagy, we used a ubiquitously expressed GFP-ATG8a line to quantify autophagic flux64–67. As free GFP is more resistant to vacuolar degradation than GFP-ATG8a, bulk autophagy results in the accumulation of free GFP in the vacuole68. We showed that free GFP intensity increased in LB, whereas the full-length GFP-ATG8a decreased in the absence of a vacuolar-type v-ATPase inhibitor, concanamycin A (ConA) (Fig. 7b, c), indicating LB-induced autophagic flux. Furthermore, GFP-ATG8a levels did not decrease significantly upon ConA application, indicating that LB triggered vacuolar degradation of autophagic bodies. As controls, we showed that GFP levels remained unchanged in a 35 S:GFP line in LB and that in atg7 GFP-ATG8a LB did not lead to an increase in free GFP (Supplementary Fig. 8b, c). Since ATG8 is found in autophagosomal membranes69, we used a ubiquitously expressed mCherry-ATG8e line70 to monitor autophagic activity microscopically using an independent assay. The number of autophagic bodies increased in LB-treated cotyledons in the presence of ConA (Fig. 7d and Supplementary Fig. 8d). We did not detect autophagic bodies in the atg5-1 autophagy-deficient mutant64,67, in LB confirming the nature of these structures (Supplementary Fig. 8e). To test whether LB induces autophagy in hypocotyls and cotyledons, we dissected seedlings and used the GFP-ATG8a western blot assays, which showed that LB led to a significant increase in free GFP in both organs (Fig. 7e, f). The microscopic assay in hypocotyls was not possible presumably due to poor penetration of ConA in this tissue. Altogether, our data indicate that LB promotes autophagy in hypocotyls and cotyledons transcriptionally and by using autophagy reporters.
Fig. 7. LB induces autophagy.
a Distributions of Z-scores computed from replicates averages for genes listed in autophagy GO term in Col-0 seedlings. The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. The full list of genes in the Autophagy GO term is given in Supplementary Data 10. b Representative image of autophagic flux assay using 35 S:GFP-ATG8a (WT), seedlings were grown in the indicated light conditions with or without ConA (0.5 µM). GFP-ATG8a and free GFP levels were detected in total protein extracts with an anti-GFP antibody. c Quantification of GFP-ATG8a and free-GFP bands in autophagic flux assays. Different letters indicate a significant difference (P < 0.05, two-way ANOVA with Tukey’s HSD test; the exact P values are available in the Source Data). d Representative image of cotyledon pavement cells expressing UBQ10:mCherry-ATG8e in the WT background either kept in WL or treated with LB for 8 h in the presence of concanamycin A (ConA, 5 µM). Yellow arrowheads indicate autophagic bodies. White bars equal to 20 µm. The experiment was repeated two times with similar results using 5 to 7 biologically individual seedlings for each condition and the experiment. e Western blot analysis of GFP-ATG8a and free-GFP levels in cotyledons and hypocotyls of dissected WT seedlings treated with 8 h of LB without ConA. f Quantification of free-GFP bands in (e). Asterisks indicate P values (*<0.001, Student’s T test, one-tailed; the exact P values are available in the Source Data). b, e H3 and TUB were used as loading control. The experiment was repeated four times with similar results. c, f Each data point indicates a biologically independent sample (average of two technical replicates for each data point), horizontal bar represents the median, and whiskers extend to show the data range. See also Supplementary Fig. 8.
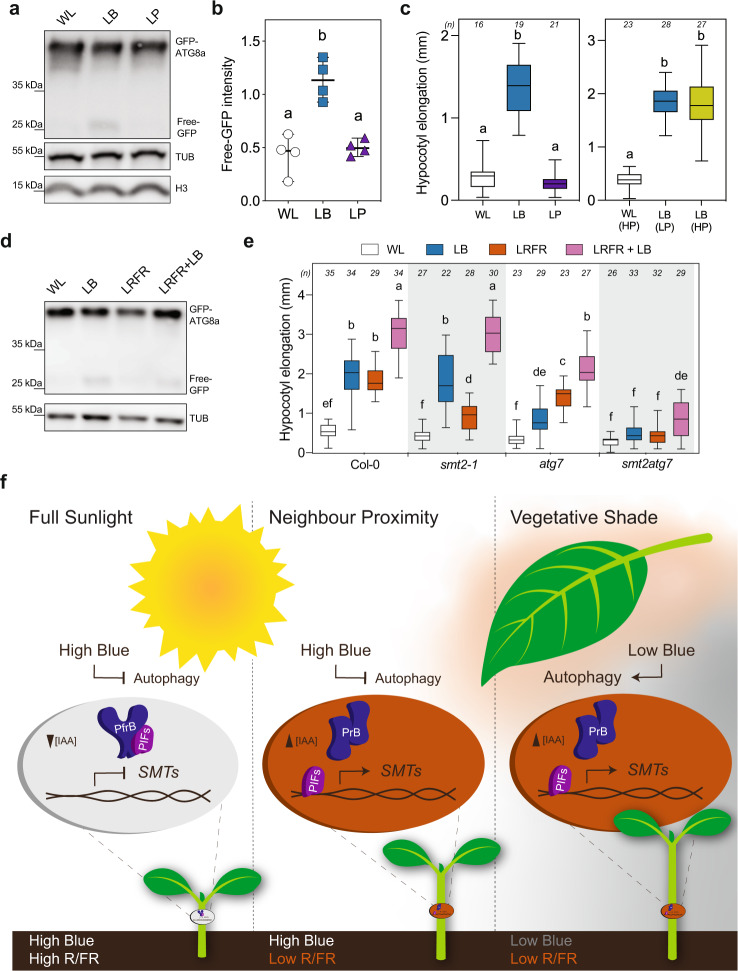
To determine what light feature of LB triggers autophagy, we used the GFP-ATG8a western blot assays to compare WL with LB and WL with low PAR (LP) corresponding to PAR in the LB treatment (66% of WL). This experiment showed that LB but not LP led to an increase in free GFP (Fig. 8a, b). Moreover, we determined how these treatments affect hypocotyl elongation also including a LB treatment with increased PAR corresponding to PAR in WL. This experiment showed that LB (high or low PAR) but not LP induced hypocotyl elongation (Fig. 8c). We next determined whether autophagy is required for LB-induced hypocotyl elongation using the autophagy mutants atg7-2 and atg5-164,71 (Fig. 8e and Supplementary Fig. 9a). Both atg7-2 and atg5-1 hypocotyls elongated less than the WT in all tested light conditions except in WL, with a stronger reduction in LB than LRFR (Fig. 8e and Supplementary Fig. 9a). Remarkably, the atg7 hypocotyl phenotype contrasts with smt2-1, which had the strongest phenotype in LRFR (Fig. 8e). Moreover, LB + LRFR significantly enhanced hypocotyl elongation in atg5, atg7 and smt2-1 single mutants suggesting a degree of compensation between anabolic and catabolic processes mainly promoted by LRFR and LB, respectively (Fig. 8e). Supporting this idea, smt2atg7 hypocotyls elongated neither in LB nor in LRFR (Fig. 8e). Importantly, smt2atg7 hypocotyl elongated marginally in LB + LRFR (simulated vegetative shade), a light condition that induced autophagy similarly to LB (Fig. 8d, e). We further confirmed that these phenotypes are specific to LB, showing that LP did not induce hypocotyl elongation in any of these mutants (Supplementary Fig. 9b, c) Finally, we tested whether the LB hypocotyl growth phenotype of atg7 could be rescued by applying exogenous sucrose and found that atg7 hypocotyl growth was significantly induced by sucrose compared to sorbitol that is used as an osmotic control (Supplementary Fig. 9d). Thus, we conclude that LB and not an equivalent reduction in PAR (without affecting the light spectrum) induces both autophagy and hypocotyl elongation. Moreover, autophagy is important to promote hypocotyl elongation in a vegetative shade that combines LB and LRFR (Fig. 8e, f).
Fig. 8. Hypocotyl elongation phenotypes correlate well with induction of autophagy in response to changing light environments.
a Western blot analysis of GFP-ATG8a and free GFP in WT seedlings treated for 8 h with LB or LP without ConA. b Quantification of free-GFP bands in (a). Each data point indicates a biologically independent sample (average of two technical replicates for each data point), horizontal bar represents the median, and whiskers extend to show the data range. c Hypocotyl elongation of WT seedlings treated with LB, LP, or LB with increased PAR (High PAR, HP) compared to WL. d GFP-ATG8a and free-GFP levels are detected in WT seedlings treated with 8 h of LB, LRFR, or LRFR + LB without ConA. e Hypocotyl elongation of seedlings with the indicated genotypes treated with the indicated light conditions. The sample size (n) that is given on top indicates biologically independent seedlings examined over one experiment. The experiments were repeated four (a, d) and three (e) times with similar results. H3 (a, d) and TUB (d) were used as a loading control. c, e The horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. b, c, e Different letters indicate significant difference (P < 0.05, two-way ANOVA with Tukey’s HSD test, the exact P values are available in the Source Data). f Model for hypocotyl elongation in full sunlight, neighbor proximity, and vegetative shade conditions. In full sunlight (left panel) and neighbor proximity (middle panel), plants receive high-intensity blue light (high blue, shown by the color white) and autophagy remains at basal levels. Vegetative shade (right panel) decreases the blue light intensity (low blue, shown by the dark-gray color) and promotes autophagy-mediated recycling. The reduced R/FR ratio in neighbor proximity and vegetative shade inactivates phyB thereby promoting PIF activity.
Discussion
In young seedlings, cotyledons are the major organs sensing LRFR, while growth promotion occurs in hypocotyls14,20,72. In the cotyledons, the PIF-YUC regulon controls the production of auxin that promotes elongation upon transport to the hypocotyl. However, how PIFs control hypocotyl elongation locally (in the growing organ) and how PIF activity may be induced in hypocotyls is less clear. Modulation of auxin sensitivity in hypocotyls is one identified mechanism10,13,14,73 (Fig. 2c). Here, we show organ-specific transcriptional control of SMT2 and SMT3 by PIFs (Fig. 4b). ChIP data indicate that PIF4 and PIF7 directly control expression of those genes with enhanced binding to their promoter in LRFR (Figs. 4c and 8 and Supplementary Fig. 4b). LRFR-induced SMT2 and SMT3 expression also depends on YUC-mediated auxin production (Fig. 4b), yet auxin alone does not induce their expression in hypocotyls55. This suggests a combined function of PIFs and auxin for LRFR-induced expression of SMT2 and SMT3 (Fig. 8f). Similarly, most LRFR-induced genes in the hypocotyl belonging to GO categories related to growth-promoting processes depend on both PIFs and YUCs (Fig. 2 and Supplementary Data 3). This regulatory pattern suggests that in hypocotyls, increased auxin levels may promote PIF-mediated gene expression. This hypothesis is consistent with coordinated regulation of growth-regulatory genes by ARF6, BZR1, and PIF421. Furthermore, several PIFs are putative targets for ARF6 and BZR121. BZR1 is also known to induce PIF4 expression during thermomorphogenesis74 that induces hypocotyl elongation similarly to LRFR downstream of PIF4 and PIF745,75,76. In contrast to hypocotyls, a substantial fraction of LRFR-induced genes in cotyledons depend on PIFs but not YUCs (Supplementary Fig. 2a). This is consistent with earlier studies identifying several LRFR-induced genes that do not depend on de novo auxin production77. A change in light conditions in cotyledons leads to direct PIF activation potentially explaining these findings. Collectively, our data reveal organ-specific patterns of PIF-mediated gene induction in hypocotyls versus cotyledons and identify SMT2 as an example of a gene that is selectively induced in the hypocotyl and is particularly important during LRFR-induced hypocotyl elongation.
LRFR leads to enhanced reallocation of newly fixed carbon to the lipid fraction of B. rapa hypocotyls35. The expression of many lipid-biosynthetic genes, including sterols is induced in the hypocotyl of LRFR-treated Arabidopsis seedlings (Fig. 1). In addition to sterols, LRFR upregulates the biosynthetic genes for other major components of PM lipids (e.g., sphingolipids) (Fig. 2c)33. PM extension depends on the deposition of lipids that occurs during the delivery of membranes via exocytosis31–34. Of note, endocytosis and exocytosis are also GO terms enriched among upregulated genes in LRFR (Fig. 1d). On the other hand, terms related to chloroplast lipids (GDG) are found among downregulated genes in LRFR (Supplementary Data 3). Consistent with the transcriptional data in Arabidopsis, GPL increases while TAG decreases in B. rapa hypocotyls (Fig. 6a). A similar decrease in chloroplasts was previously observed in tomato stems in LRFR61. Moreover, we show that in Arabidopsis hypocotyls both chloroplasts and storage lipids decrease in LRFR (Fig. 6b, c). Interestingly, during de-etiolation phytochromes also control storage lipid utilization78. Overall, our data indicate that LRFR leads to enhanced production of sterols with a reduction of non-PM lipid classes (Fig. 6a). SMT2 and SMT3 act at a branch point of sterol biosynthesis (Fig. 4a). Their induction by LRFR may therefore lead to changes in sterol composition which is potentially important given their role in PM fluidity and microdomain organization33,36. We cannot rule out this possibility, however our sterol and sphingolipid measurements in LRFR-treated B. rapa do not provide evidence for such a change (Supplementary Fig. 6). We also note that coordinated transcriptional upregulation of the sterol pathway (Supplementary Fig. 4a) is consistent with enhanced overall sterol demand rather than indicative of changes in sterol composition.
PIFs and auxin production are also functionally important for LB-induced hypocotyl elongation24–26,28 (Fig. 1a). However, robust LB-regulated gene expression still occurred in pif457 (Supplementary Fig. 1a). Furthermore, many PIF-dependent genes in LB are probably not direct PIF targets (Fig. 3e, Supplementary Fig. 3d, and Supplementary Data 6). Interestingly, many auxin, BR, and GA response and other growth-related genes show reduced basal (WL) expression in pif457, and this difference persists in LB (Fig. 3 and Supplementary Data 5). Importantly, mutant analysis and pharmacological treatments show an indispensable role for auxin, BR, and GA during LB-induced hypocotyl elongation (Fig. 1a)25,26,28,29. We thus conclude that a more general gene expression defect (already present in WL) may contribute to the hypocotyl growth defect of pif457 mutants in LB. This is consistent with a report showing that PIF4 and PIF5 are dose-dependent inducers of hypocotyl elongation in WL44. However, hypocotyl elongation and PIF4 and PIF5 accumulation occurs more slowly in LB than LRFR26,44,73,79. Hence, our analysis at 3 h may have missed some of the PIF-regulated transcriptional events. Therefore, the link between PIF-regulated gene expression and hypocotyl growth control in LB requires further investigation.
While LRFR leads to transcriptional changes in the hypocotyl, possibly indicative of enhanced production of building blocks required for growth, LB leads to the induction of many catabolic processes and autophagy-related genes (Fig. 1d and Supplementary Fig. 1c). The LRFR gene expression pattern suggests that when light resources remain available (Supplementary Fig. 7a), the Target of Rapamycin (TOR) pathway is on and promotes e.g., the biogenesis of ribosomes and nucleotides80. The rapid and concomitant rise in auxin and many anabolic processes in hypocotyls (Fig. 1d and Supplementary Fig. 1c)14 suggest a potential link between these processes. Consistently, a recent report shows that sugar-dependent TOR activity requires auxin signaling81. Unlike LRFR, LB leads to PAR reduction (Supplementary Fig. 7a). LB presumably induces metabolic adjustments including alternative pathways for respiration (e.g., protein catabolism) to sustain growth, which is consistent with the situation in other carbon limiting conditions82–84. LB transcriptionally promotes autophagy, leads to enhanced production of autophagic bodies (visualized with mCherry-ATG8e) and autophagic processing of GFP-ATG8a in hypocotyls and cotyledons, while LRFR alone does not (Figs. 7 and 8). Importantly, the reduction in PAR alone (maintaining the same light spectrum) to the level in our LB treatment neither induces hypocotyl elongation nor autophagy (Fig. 8 and Supplementary Fig. 9). This indicates that LB rather than the reduction in PAR induces autophagy and hypocotyl elongation. Consistently, atg7 has a hypocotyl growth defect in LB but not WL or LP (Fig. 8 and Supplementary Fig. 9). Interestingly, the combined LB + LRFR treatments, which mimic vegetative shade, largely rescues the phenotypes of atg7 and smt2 single mutants, suggesting a degree of compensation between catabolic and anabolic processes. Consistently, smt2 atg7 double mutants did not elongate in LB or LRFR and had a modest growth response in combined treatments (Fig. 8e). Importantly, SMT2 and SMT3 expression is induced in the hypocotyl of shade-treated seedlings (combined LB and LRFR)85. Altogether, our work indicates a particularly important requirement for enhanced de novo synthesis in LRFR and autophagy in LB, while the combination of both processes contributes to growth enhancement of the hypocotyl in vegetative shade.
In conclusion, our work shows that the mechanisms underlying hypocotyl growth promotion differ during neighbor proximity (LRFR) and in vegetational shade that comprises both LB and LRFR. These differences are illustrated in a model (Fig. 8f). We note that thermomorphogenesis and neighbor proximity both lead to similar growth adaptation using related signaling pathways76,86. Within a temperature range that does not significantly decrease photosynthetic efficiency, it is likely that for thermomorphogenesis as well the anabolic processes described here are relevant.
Methods
Plant material and growth conditions
We used the Arabidopsis thaliana genotypes (cv Columbia-0). yuc2yuc5yuc8yuc9 was recrossed using all yuc alleles that are described in18 except yuc5-1 (SAIL_116_C0). We used the strain R-o-18 for B. rapa experiments. Oligonucleotides used for genotyping are listed in Supplementary Table 1. All materials used in the study are listed in Supplementary Table 2.
Seeds were size-selected and surface-sterilized using 70% (v/v) ethanol and 0.05% (v/v) Triton X-100 for 3 min followed by 10-min incubation in 100% (v/v) ethanol. Seeds were sowed on ½ Murashige and Skoog medium (½ MS) containing 0.8% (w/v) phytoagar and subsequently stratified at 4 °C for 3 days in darkness. For hypocotyl elongation and RNA-seq experiments where seedlings were grown on vertical plates the phytoagar concentration was raised to 1.6% (w/v)87. For all experiments, seedlings were grown in 16 h/8 h, light/dark photoperiod (LD) at 21 °C in a Percival Scientific Model AR-22L (Perry, IA, USA) incubator. WL was emitted from white fluorescence tubes (Lumilux cool white 18 W/840) at a fluence rate of ~120 μmol m−2 s−1 and LRFR was achieved by supplementing WL with ~35 μmol m−2 s−1 FR light (LEDs λmax 740 nm) lowering the R (640–700 nm)/FR (700–760 nm) from 1.4 to 0.2, as measured by Ocean Optics USB2000 + spectrometer. A double layer of yellow filter (010 medium yellow, LEE Filters), lowering blue light from 27 μmol m−2s−1 (WL) to 2.5 μmol m−2 s−1 (LB), was used to cover up the seedlings for LB treatments. The light spectra are shown in Supplementary Fig. 7.
For hypocotyl elongation and microscopy experiments, seedlings were grown for 4 days in WL (except Fig. 1a where seedlings were grown for 5 days) and subsequently kept in WL or transferred to light treatment (at ZT2) for additional 3 days. Fenpropimorph, picloram, sucrose, and sorbitol treatments were done on vertically grown seedlings on nylon meshes which were transferred to new plates containing the drug or the mock and put for 3 additional days into WL or light treatment (at ZT2). Fenpropimorph, picloram, and Concanamycin A were dissolved in DMSO (dimethylsulfoxide) applied at the indicated concentrations in Figure legends (DMSO for mock). For sucrose treatment (1%), the molar equivalent in sorbitol was used as an osmotic control.
For RNA-seq and western blot experiments, seedlings were grown for 5 days in WL and subsequently kept in WL or transferred to light treatment (at ZT2) for 3 h (RNA-seq) or 8 h (western blots) before harvesting. For concanamycin A treatment, seedlings were transferred to liquid ½ MS with shaking (75 rpm).
For ChIP-qPCR experiments, seedlings were grown in WL for 5 days and subsequently kept in WL or transferred to LRFR (at ZT2) for additional 5 days before harvesting.
For RT-qPCR, complex lipid and sterol measurement analysis, B. rapa seedlings were grown for 5 days in WL and subsequently kept in WL or transferred to LRFR (at ZT2) for the indicated time in legends before harvesting.
Seedlings imaging and measurement were done according to standard lab procedures and were described in detail87.
Constructs cloning
PCR amplifications were performed using Phusion® High-Fidelity DNA Polymerase. All cloning was done using In Fusion® HD Cloning kit. First, GUSPlus:tOCS was cloned in pFP100 plasmid carrying pAt2S3:GFP selection marker88 and the new plasmid was named as pYI001. pFR06 and pGH3.17 were cloned into pYI001 in order to obtain pFRO6:GUSPlus:tOCS and pGH3.17:GUSPlus:tOCS, respectively. pUBQ10:SMT2-Flag:tOCS was cloned into pFP100, while pFRO6:SMT2-Flag, and pGH3.17:SMT2-Flag were cloned into pYI001. The primers are listed in Supplementary Table 1. These constructs were transformed into smt2-1 plants using Agrobacterium tumefaciens GV3101 strain by floral dip89.
RNA isolation, quantitative RT-PCR, and RNA sequencing
For RNA isolation, 5 days-old seedlings were harvested in liquid nitrogen and kept at −70 °C for overnight. Next day, seedlings were covered with −70 °C cold RNAlater™-ICE and transferred to −20 °C overnight. Cotyledons and hypocotyls were dissected using sharp needles on top of an ice block under a binocular microscope (Nikon, SMZ1500) and RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) reactions were performed as previously described14. In short, equal amounts of RNA were reverse transcribed into cDNA with Superscript II Reverse Transcriptase (Invitrogen). RT-qPCR was performed in three technical and three biological replicates (nine samples in total). Oligonucleotides are listed in Supplementary Table 1.
For RNA sequencing, RNA quality was assessed on a Fragment Analyzer (Agilent Technologies). From 40 ng total RNA, mRNA was isolated with the NEBNext Poly(A) mRNA Magnetic Isolation Module. RNA-seq libraries were then prepared from the mRNA using the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina. Libraries were quantified by a fluorimetric method, and their quality assessed on a Fragment Analyzer (Agilent Technologies). Cluster generation was performed with the resulting libraries using Illumina HiSeq 3000/4000 SR Cluster Kit reagents. Libraries were sequenced on the Illumina HiSeq 4000 with HiSeq 3000/4000 SBS Kit reagents for 150 cycles. Sequencing data were demultiplexed with the bcl2fastq Conversion Software (v. 2.20, Illumina; San Diego, California, USA).
ChIP-qPCR
10d-old seedlings PIF4p:PIF4-HA in pif4-10149 and PIF7p:PIF7-HA in pif7-2 seedlings50 grown in WL for five days and then either kept in WL or transferred to LRFR for another five days were harvested in liquid nitrogen. Chromatin extraction was performed using standard procedures except that samples were cross-linked only with formaldehyde90. Immunoprecipitation was performed using an anti-HA antibody using standard procedures91. The qPCR was done in triplicates or quadruplicate on input and immunoprecipitated DNA. Peaks (P) were defined using a genome-wide ChIP study from etiolated seedlings that identified PIF4-peaks92. Controls (C) are on coding regions of each gene. Oligonucleotides are listed in Supplementary Table 1.
Western blot analysis
Total protein extracts from seedlings were obtained as previously described50. In short, seedlings were ground in SDS-PAGE FSB (final sample buffer), heated at 95 °C for 5 min, centrifuges and the supernatant loaded on protein gels. Protein samples were separated on 10% Mini-Protean TGX gels and blotted on nitrocellulose membrane using Turbo transfer system. Membranes were blocked with 5% milk overnight at 4 °C or 1 h at room temperature for Anti-GFP JL-8 (1:4000), polyclonal H3 (1:2000), Anti-TUB (1:2000) antibodies before probing with horseradish peroxidase (HRP)-conjugated anti-rabbit (for H3) or anti-mouse (for anti-GFP and anti-TUB) as the secondary antibody (1:5000). Chemiluminescence signal were obtained with Immobilon Western Chemiluminescent HRP Substrate on an ImageQuant LAS 4000 mini (GE Healthcare, Buckinghamshire, UK). All western blot assays were done from four bioreps. Images were processed with ImageJ software.
Microscopy and GUS staining
For DII-Venus microscopy and image quantification14, seedlings were transferred to LRFR or kept in WL for 1 h before imaging. We used an inverted Zeiss confocal microscope (LSM 710, ×20 objective, 0.8 DIC). VENUS signal was detected using an Argon laser (excitation at 514 nm and bandpass emission between 520 and 560 nm). Image stacks (5–6/seedling) were acquired for every hypocotyl until the VENUS signal was lost. The pinhole was opened to collect the maximal signal intensity together with the minimal stack number (5.42 airy units, 20.2-μm section, 10.08-μm interval). We quantified the VENUS signal (ImageJ) via the SUM slices projection of four slices from the stack, excluding the first layer with the stomata.
For the lipid droplet (LD) quantification, seedlings were treated with BODIPY ™ 493/503 (2 µg/mL in H2O) for 20 min at room temperature and imaged on inverted Zeiss confocal microscope (LSM 710, ×20 objective, 0.8 DIC) 30 h after the beginning of light treatment. BODIPY™ 493/503 signal was detected using an Argon laser (excitation at 488 nm and bandpass emission between 500 to 540 nm). Image stacks (6/seedling) were acquired for every hypocotyl. The pinhole was opened to 3.15 Airy Units (11.4 µm section, 10.00 µm interval). We quantified the signal (ImageJ) from a region of interest (ROI) on the upper half of the hypocotyls and analyzed the fluorescence intensity of particles (size = 0–75 circularity = 0.50–1.00) in each image stack with a threshold (>3000) and summed the total intensity using the “Analyze particles” tool.
For chloroplast quantification, we used the same protocol as the LD quantification with the following changes. We used a HeNe633 laser (excitation at 633 nm and bandpass emission between 640 and 685 nm to have an equal contribution from ChlA and ChlB). The pinhole was opened to 3.15 Airy Units (11.4-µm section, 10.00-µm interval). The threshold was adjusted to >10,000; size and circularity were kept as default (0-infinity and 0–100, respectively) in ImageJ.
For visualization of autophagic bodies in UBQ10:mCherry-ATG8e lines67,70, seedlings were transferred to LB or kept in WL for 8 h before imaging in the presence (5 µM) or absence (DMSO) of ConA. We used DPSS 561-10 laser (excitation at 561 nm and bandpass emission between 570 to 635 nm) (LSM 710, ×63 objective, 1.3 oil DIC). The pinhole was opened to 5.32 Airy Units (4.3-µm section, 4.21-µm interval). Two stacks are combined for each image.
The protocol for GUS staining reactions is described in ref. 50. In short, samples were fixed with ice-cold 90% acetone for 30 min, followed by washing twice with 50 mM NaPO4 buffer (pH 7.2). Samples were incubated with staining solution (50 mM NaPO4, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 0.1% triton X-100, and 2 mM X-gluc) overnight at 37 °C in the dark. De-staining was performed with ethanol series at room temperature until samples cleared. Cotyledons were prepared for cotyledon vasculature imaging as described in ref. 47. In short, seedlings were fixed in ethanol:acetic acid [3:1] and then rinsed in 70% and incubated in 100% ethanol at 4 °C overnight. For further clearing, seedlings were treated by 1 h of incubation in 10% NaOH at 42 °C and mounted in 50% glycerol. GUS staining and cotyledon vasculature were imaged using a dissecting microscope (Nikon SMZ1500).
Sterol measurements
Four hypocotyls from 5 days-old B. rapa seedlings per sample were pooled and frozen in liquid nitrogen immediately after fresh weights were recorded. Samples were heated for 1 h in EtOH with 1% H2SO4 at 85 °C. Sterols were extracted in hexane. Free hydroxyl groups were derivatized at 110 °C for 30 min, surplus BSTFA-trimethylchlorosilane was evaporated, and samples were dissolved in hexane for analysis using GC-MS (Agilent 7890, A coupled to a mass spectrometer, MSD 5975, Agilent EI) under the conditions as described93. In short, An HP-5MS capillary column (5% phenyl-methyl-siloxane, 30-m, 250-mm, and 0.25-mm film thickness; Agilent) was used with helium carrier gas at 2 mL/min. The oven temperature was held at 200 °C for 1 min, then programmed with a 10 °C/min ramp to 305 °C (2.5-min hold) and a 15 °C/min ramp to 320 °C. Injection (1 µl) was done in splitless mode; injector and mass spectrometry detector temperatures were set to 250 °C. The ion source in a EI set at 70 eV, the mass range is 40–700 m/z Raw data files were processed using the vendor-specific MassHunter (version B. 07.00, Agilent). Quantification of sterols was based on peak areas, which were derived from total ion current and using cholestanol as the internal standard. Each sterol was normalized to the total amount of detected sterols and presented as a percentage of the total.
Sphingolipid measurements
Samples were collected as described in sterol measurements. For the analysis of sphingolipids by LC-MS/MS, lipids were extracted with Toledo solvent, dried, and then incubated 1 h at 50 °C in 2 mL of methylamine solution (7 mL methylamine 33% (w/v) in EtOH combined with 3 mL of methylamine 40% (w/v) in water (Sigma Aldrich) in order to remove phospholipids. After incubation, methylamine solutions dried at 40 °C under a stream of air. Finally, samples were resuspended into 100 μL of THF/MeOH/H2O (40:20:40, v/v) with 0.1% formic acid containing synthetic internal lipid standards (Cer d18:1/C17:0 and GluCer d18:1/C12:0), thoroughly vortexed, incubated at 60 °C for 20 min, sonicated 5 min and transferred into LC vials.
LC-MS/MS (multiple reaction monitoring mode) analyses were performed with a model QTRAP 6500 (ABSciex) mass spectrometer coupled to a liquid chromatography system (1290 Infinity II, Agilent). Analyses were performed in the positive mode. Nitrogen was used for the curtain gas (set to 30), gas 1 (set to 30), and gas 2 (set to 10). Needle voltage was at +5500 V with needle heating at 400 °C; the declustering potential was adjusted between +10 and +40 V. The collision gas was also nitrogen; collision energy varied from +15 to +60 eV on a compound-dependent basis.
Reverse-phase separations were performed at 40 °C on a Supercolsil ABZ+, 100 × 2.1 mm column, and 5 µm particles (Supelco). The Eluent A was THF/ACN/5 mM Ammonium formate (3/2/5 v/v/v) with 0.1% formic acid, and eluent B was THF/ACN/5 mM Ammonium formate (7/2/1 v/v/v) with 0.1% formic acid. The gradient elution program for Cer and GluCer quantification was as follows: 0 to 1 min, 1% eluent B; 40 min, 80% eluent B; and 40 to 42, 80% eluent B. The gradient elution program for GIPC quantification was as follows: 0 to 1 min, 15% eluent B; 31 min, 45% eluent B; 47.5 min, 70% eluent B; and 47.5 to 49, 70% eluent B. The flow rate was set at 0.2 mL/min, and 5 mL sample volumes were injected.
The areas of LC peaks were determined using MultiQuant software (version 3.0; ABSciex) for sphingolipid quantification.
Untargeted lipidomics mass spectrometry analysis
Four hypocotyls from 5 days-old B. rapa seedlings per sample were pooled, and preheated isopropanol (at 75 °C) was added immediately after fresh weights were recorded. Each sample with isopropanol was incubated at 75 °C for 15 min to inhibit phospholipase activity and cooled down to room temperature. Samples were kept at 4 °C overnight, and isopropanol was then evaporated to dryness using Nitrogen steam. Dry extracts were then reconstituted in 200 μL of IPA spiked with the internal standard mixture (SPLASH® LIPIDOMIX® Mass Spec Standard (92/8; v/v)). This solution was further homogenized in the Cryolys Precellys 24 sample Homogenizer (2 × 20 s at 10,000 rpm, Bertin Technologies, Rockville, MD, USA) with ceramic beads. The bead beater was air-cooled down at a flow rate of 110 L/min at 6 bar. Homogenized extracts were centrifuged for 15 min at 21,000×g at 4 °C (Hermle, Gosheim, Germany) and the resulting supernatant was collected and transferred to an LC-MS vial.
Extracted samples were analyzed by reversed-phase liquid chromatography coupled to a high-resolution mass spectrometry (RPLC-HRMS) instrument (Agilent 6550 IonFunnel QTOF). In both, positive and negative ionization mode, the chromatographic separation was carried out on a Zorbax Eclipse Plus C18. Mobile phase was composed of A = 60:40 (v/v) Acetonitrile:water with 10 mM ammonium acetate and 0.1% acetic acid and B = 88:10:2 Isopropanol:acetonitrile:water with 10 mM ammonium acetate and 0.1% acetic acid. The linear gradient elution from 15 to 30% B was applied for 2 min, then from 30% to 48% B for 0.5 min, from 48 to 72% B, and the last gradient step from 72 to 99% B followed by 0.5 min isocratic conditions and a 3 min re-equilibration to the initial chromatographic conditions. The flow rate was 600 μL/min, column temperature 60 °C, and sample injection volume 2 μl.
ESI source conditions were set as follows: dry gas temperature 200 °C, nebulizer 35 psi and flow 14 L/min, sheath gas temperature 300 °C and flow 11 L/min, nozzle voltage 1000 V, and capillary voltage +/−3500 V. Full scan acquisition mode in the mass range of 100–1700 m/z was applied for MS1 data acquisition while MS/MS data were acquired in the iterative data-dependent acquisition mode to facilitate lipid identification and annotation.
Pooled QC samples (representative of the entire sample set) were analyzed periodically (every six samples) throughout the overall analytical run in order to assess the quality of the data, correct the signal intensity drift and remove the non-reproducible signals (CV >25%)94. In addition, a series of diluted quality controls (dQC) were prepared by dilution with isopropanol: 100% QC, 50% QC, 25% QC, 12.5% QC, and 6.25% QC, and analyzed at the beginning and at the end of the sample batch. This QC dilution series served as a linearity filter to remove the features that do not respond linearly (correlation with dilution factor is <0.65)95.
Data processing
Raw LC-HRMS and HR(MS/MS) data were processed using MS-Dial software96. Relative quantification of lipids was based on EIC (Extracted Ion Chromatogram) areas for the monitored precursor ions at the MS1 level. Peak areas were normalized considering the sample amount (mg) (full lists of lipid species are given in Supplementary Data 9). The obtained data (containing peak areas of detected and identified lipids by MS and MS/MS, and using MS only across all samples) were exported to “R” software http://cran.r-project.org/ where the signal intensity drift correction was done within the LOWESS/Spline normalization program96 followed by noise filtering (CV (QC features) >30%) and visual inspection of linear response.
The abundance of each MS/MS-detected lipid species was normalized to the total amount of MS/MS-detected lipids and presented as a percentage of the total.
RNA-seq initial data analysis
Sequence reads from FASTQ files were processed as follows: Reads that did not pass Illumina’s filtering were removed. Adapters and low-quality 3’ ends were trimmed from the reads using Cutadapt (v1.8)97. Reads matching to ribosomal RNA sequences were removed with fastq_screen (v. 0.11.1). Remaining reads were further filtered for low complexity with reaper (v. 15-065)98. More than 30 million uniquely mapped reads were obtained per library, and reads were aligned against the Arabidopsis thaliana.TAIR10.39 genome using STAR (v. 2.5.3a)99. The number of read counts per gene locus was summarized with htseq-count (v. 0.9.1)100 using the Arabidopsis thaliana.TAIR10.39 gene annotation. The quality of the RNA-seq data alignment was assessed using RSeQC (v. 2.3.7)101.
Statistical analysis was performed for genes independently in R (R version 4.0.2). All steps described here were performed separately for the samples from hypocotyls and cotyledons (except for the initial clustering of all samples together). Genes with low counts were filtered out according to the rule of 1 count(s) per million (cpm) in at least one sample. The number of genes retained in the analyses based on this filtering is different for hypocotyls and cotyledons. Library sizes were scaled using TMM normalization. Subsequently, the normalized counts were transformed to cpm values and a log2 transformation was applied by means of the function cpm with the parameter setting prior.counts = 1 (edgeR)102.
Differential expression was computed with the R Bioconductor package “limma”103 by fitting data to a linear model. The approach limma-trend was used. Fold changes were computed and a moderated t test was applied. P values were adjusted using the Benjamini–Hochberg (BH) method, which controls for the false discovery rate (FDR), globally across several comparisons of experimental conditions. The adjustment was performed in a few different ways customized to different parts of the data analysis. For Supplementary Data 1, Fig. 1, and Supplementary Fig. 1, P values were adjusted globally across pairs of comparisons, LB vs. WL and LRFR vs. WL in each genotype separately. For Supplementary Data 5, Fig. 3, and Supplementary Fig. 3, P values were adjusted globally across three comparisons between different genotypes while keeping the light condition (WL) unchanged: pif457 vs Col-0, yuc2589 vs Col-0 and smt2-1 vs Col-0. For Supplementary Data 3 and 4; Figs. 2 and 3; and Supplementary Fig. 2 and 3, F tests were used that yielded a single P value for three comparisons and post hoc testing as implemented; the R package limma was then applied to identify significantly regulated genes per comparison. For Supplementary Fig. 5, the same F test is applied for two comparisons.
Gene set enrichment analysis for gene ontology
Gene set enrichment analyses were conducted with ShinyGO v0.61:Gene Ontology Enrichment Analysis + more (http://bioinformatics.sdstate.edu/go/)104 in Arabidopsis thaliana using a P value cutoff (FDR) of 0.05 and 500 most significant terms to show. The networks of enriched GO categories were visualized with R software (https://www.r-project.org/) using “visNetwork” and “igraph” libraries. Two terms (nodes) were connected if they share 20% or more genes. The size of the nodes indicates fold change for each term. We highlighted selected terms for each organ and light condition that we could easily relate to growth regulation (Fig. 1d and Supplementary Fig. 1c). The highlighted terms are not necessarily the most significant ones (full lists are available in Supplementary Data 2 and as interactive versions).
Statistical motif analysis in promoter or upstream gene sequences
Motif enrichment analyses were conducted with TAIR’s Motif Analysis tool (https://www.arabidopsis.org/tools/bulk/motiffinder/index.jsp) using 1-kb upstream sequences.
Other statistical analyses and data representation
For all the phenotypic analyses of hypocotyl elongation and the quantification of DII-signal, we performed two-way analysis of variance (ANOVA) (aov) and computed Tukey’s Honest Significance Differences (HSD) test (“agricolae” package) with default parameters using R software. For phenotypic analysis of treatments with fenpropimorph and picloram, we performed two-way analysis of variance (ANOVA) (aov) and represented the significance as genotype*drug interaction in the given light conditions. For the comparisons including qPCR, ChIP-qPCR, sterol measurements, lipidomics analysis and autophagic flux assays, we performed Student’s T test. For lipidomics analysis, we further applied a BH correction for P values. We used binomial distribution for the PIF4 target enrichment, promoter motif, and to determine the significance of PIFs and/or YUCs dependence of enriched GO terms in given light conditions (Supplementary Data 3 and Supplementary Figs. 4 and 5).
In boxplots, the horizontal bar represents the median; boxes extend from the 25th to the 75th percentile, whiskers extend to show the data range. In data point plots, points are given as individual values from biological replicates, the horizontal lines indicate median, and error bars extend to show data range. For the fenpropimorph and picloram treatments, the data show the means ± SD with a simple linear regression line connecting data points for the given light condition and the genotype. Asterisks (*) and different letters in the graphs indicate significant differences as defined by the statistical methods described above (P < 0.05).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Martina Legris, Laure Allenbach Petrolati, Olivier Michaud, Mieke de Wit, Anupama Goyal, Ana Lopez Vazquez, Ganesh Mahadeo Nawkar, and Maud Lagier for providing resources, technical support and/or comments on the manuscript; René Dreos for advice on statistical methods; Niko Geldner, Ersin Gül (ETH Zurich), Sinem Celebioven (University Zurich), Soner Yildiz (Icahn School of Medicine) and Yasin Dagdas (GMI, Vienna) for suggestions and comments on the manuscript; Christian Hardtke, Teva Vernoux (ENS Lyon), Richard Vierstra (Washington University, St Louis), Dany Geelen (University Gent) and Yasin Dagdas for providing resources. We are grateful to the CIF (https://cif.unil.ch/) for help with microscopy, the GTF (https://wp.unil.ch/gtf/) for RNA-seq. experiments and the Metabolomics Unit (https://www.unil.ch/metabolomics/en/home.html) for the lipidomic analysis and the Bordeaux-Metabolome platform for sterol analysis (https://www.biomemb.cnrs.fr/en/lipidomic-plateform/). Work in the Fankhauser lab is supported by the University of Lausanne and the Swiss National Science Foundation (310030B_179558) and the Velux Foundation (Project 1455). The Bordeaux Metabolome-Lipidome Facility-MetaboHUB by a grant from ANR (no. ANR–11–INBS–0010). Manon Genva thanks the National Fund for Scientific Research (FNRS, Belgium, through grant 2022/V 3/5/053-40010130-JG/JN-2724) and the University of Liège for mobility grants.
Source data
Author contributions
Y.C.I.: conceptualization, formal analysis, data curation, methodology, investigation, resources, validation, visualization, and writing—original draft. J.K.: conceptualization, investigation, formal analysis, and validation. A.S.F., M.T., and V.C.G.: investigation, resources, and validation. S.P. and L.W.: formal analysis, data curation, and methodology. S.M.: conceptualization, data curation, validation, investigation, and methodology. L.F., M.G., and P.V.D.: investigation. J.I. and H.G.A.: investigation, data curation, and validation. C.F.: conceptualization, data curation, methodology, project administration, resources, funding acquisition, supervision, validation, and writing—original draft. All authors read and approved the manuscript.
Peer review
Peer review information
Nature Communications thanks Taijoon Chung and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The RNA-seq data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus105 and are accessible through GEO Series accession number GSE174655. The MS data for lipid measurements were deposited in Metabolights (https://www.ebi.ac.uk/metabolights/). Untargeted lipidomic analysis with the unique identifier MTBLS2796, MTBLS5753 for the sphingolipidomics, and MTBLS5766 for sterols. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33384-9.
References
- 1.McCree KJ. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971;9:191–216. doi: 10.1016/0002-1571(71)90022-7. [DOI] [Google Scholar]
- 2.Liu J, van Iersel MW. Photosynthetic physiology of blue, green, and red light: light intensity effects and underlying mechanisms. Front. Plant Sci. 2021;12:619987. doi: 10.3389/fpls.2021.619987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casal JJ. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013;64:403–427. doi: 10.1146/annurev-arplant-050312-120221. [DOI] [PubMed] [Google Scholar]
- 4.Fiorucci AS, Fankhauser C. Plant strategies for enhancing access to sunlight. Curr. Biol. 2017;27:R931–R940. doi: 10.1016/j.cub.2017.05.085. [DOI] [PubMed] [Google Scholar]
- 5.Buti S, Hayes S, Pierik R. The bHLH network underlying plant shade-avoidance. Physiol. Plant. 2020;169:312–324. doi: 10.1111/ppl.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Gao X, Liu Y, Fan S, Ma Q. Progress of research on the regulatory pathway of the plant shade-avoidance syndrome. Front Plant Sci. 2020;11:439. doi: 10.3389/fpls.2020.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballare CL, Pierik R. The shade-avoidance syndrome: multiple signals and ecological consequences. Plant Cell Environ. 2017;40:2530–2543. doi: 10.1111/pce.12914. [DOI] [PubMed] [Google Scholar]
- 8.Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007;30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 9.Moraes TA, et al. Response of the circadian clock and diel starch turnover to one day of low light or low CO2. Plant Physiol. 2019;179:1457–1478. doi: 10.1104/pp.18.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersch M, et al. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl Acad. Sci. USA. 2014;111:6515–6520. doi: 10.1073/pnas.1320355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legris M, Ince YC, Fankhauser C. Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat. Commun. 2019;10:5219. doi: 10.1038/s41467-019-13045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Milmanda GL, Ballare CL. Shade avoidance: expanding the color and hormone palette. Trends Plant Sci. 2021;26:509–523. doi: 10.1016/j.tplants.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Hornitschek P, et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012;71:699–711. doi: 10.1111/j.1365-313X.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 14.Kohnen MV, et al. Neighbor detection induces organ-specific transcriptomes, revealing patterns underlying hypocotyl-specific growth. Plant Cell. 2016;28:2889–2904. doi: 10.1105/tpc.16.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, et al. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012;26:785–790. doi: 10.1101/gad.187849.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller-Moule P, et al. YUCCA auxin biosynthetic genes are required for Arabidopsis shade avoidance. PeerJ. 2016;4:e2574. doi: 10.7717/peerj.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nito K, et al. Spatial Regulation of the gene expression response to shade in Arabidopsis seedlings. Plant Cell Physiol. 2015;56:1306–1319. doi: 10.1093/pcp/pcv057. [DOI] [PubMed] [Google Scholar]
- 18.Nozue K, et al. Shade avoidance components and pathways in adult plants revealed by phenotypic profiling. PLoS Genet. 2015;11:e1004953. doi: 10.1371/journal.pgen.1004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keuskamp DH, Pollmann S, Voesenek LA, Peeters AJ, Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl Acad. Sci. USA. 2010;107:22740–22744. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Procko C, Crenshaw CM, Ljung K, Noel JP, Chory J. Cotyledon-generated auxin is required for shade-induced hypocotyl growth in Brassica rapa. Plant Physiol. 2014;165:1285–1301. doi: 10.1104/pp.114.241844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh, E. et al. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife3, 10.7554/eLife.03031 (2014). [DOI] [PMC free article] [PubMed]
- 22.Cifuentes-Esquivel N, et al. The bHLH proteins BEE and BIM positively modulate the shade avoidance syndrome in Arabidopsis seedlings. Plant J. 2013;75:989–1002. doi: 10.1111/tpj.12264. [DOI] [PubMed] [Google Scholar]
- 23.Kozuka T, et al. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 2010;153:1608–1618. doi: 10.1104/pp.110.156802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wit M, et al. Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Curr. Biol. 2016;26:3320–3326. doi: 10.1016/j.cub.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Keller MM, et al. Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 2011;67:195–207. doi: 10.1111/j.1365-313X.2011.04598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedmale UV, et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell. 2016;164:233–245. doi: 10.1016/j.cell.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma D, et al. Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl Acad. Sci. USA. 2016;113:224–229. doi: 10.1073/pnas.1511437113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keuskamp DH, et al. Blue-light-mediated shade avoidance requires combined auxin and brassinosteroid action in Arabidopsis seedlings. Plant J. 2011;67:208–217. doi: 10.1111/j.1365-313X.2011.04597.x. [DOI] [PubMed] [Google Scholar]
- 29.Pierik R, Djakovic-Petrovic T, Keuskamp DH, de Wit M, Voesenek LA. Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and Della proteins in Arabidopsis. Plant Physiol. 2009;149:1701–1712. doi: 10.1104/pp.108.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gendreau E, et al. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hepler PK, Rounds CM, Winship LJ. Control of cell wall extensibility during pollen tube growth. Mol. Plant. 2013;6:998–1017. doi: 10.1093/mp/sst103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steer MW, Steer JM. Pollen tube tip growth. N. Phytologist. 1989;111:323–358. doi: 10.1111/j.1469-8137.1989.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 33.Mamode Cassim A, et al. Plant lipids: key players of plasma membrane organization and function. Prog. Lipid Res. 2019;73:1–27. doi: 10.1016/j.plipres.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Boutté, Y. & Jaillais, Y. Metabolic cellular communications: feedback mechanisms between membrane lipid homeostasis and plant development. Dev. Cell10.1016/j.devcel.2020.05.005 (2020). [DOI] [PubMed]
- 35.de Wit M, et al. Changes in resource partitioning between and within organs support growth adjustment to neighbor proximity in Brassicaceae seedlings. Proc. Natl Acad. Sci. USA. 2018;115:E9953–E9961. doi: 10.1073/pnas.1806084115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valitova JN, Sulkarnayeva AG, Minibayeva FV. Plant sterols: diversity, biosynthesis, and physiological functions. Biochemistry. 2016;81:819–834. doi: 10.1134/S0006297916080046. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Cui Y, Zhang X, Li R, Lin J. Organization and dynamics of functional plant membrane microdomains. Cell Mol. Life Sci. 2020;77:275–287. doi: 10.1007/s00018-019-03270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gronnier J, et al. Mechanisms governing subcompartmentalization of biological membranes. Curr. Opin. Plant Biol. 2019;52:114–123. doi: 10.1016/j.pbi.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Verbancic J, Lunn JE, Stitt M, Persson S. Carbon supply and the regulation of cell wall synthesis. Mol. Plant. 2018;11:75–94. doi: 10.1016/j.molp.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Chen, Q. et al. Autophagy and nutrients management in plants. Cells8, 10.3390/cells8111426 (2019). [DOI] [PMC free article] [PubMed]
- 41.Li F, Vierstra RD. Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 2012;17:526–537. doi: 10.1016/j.tplants.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang P, Mugume Y, Bassham DC. New advances in autophagy in plants: regulation, selectivity and function. Semin Cell Dev. Biol. 2018;80:113–122. doi: 10.1016/j.semcdb.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun N, et al. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl Acad. Sci. USA. 2016;113:6071–6076. doi: 10.1073/pnas.1604782113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 45.Chung, B. Y. W. et al. An RNA thermoswitch regulates daytime growth in Arabidopsis. Nat. Plants10.1038/s41477-020-0633-3 (2020). [DOI] [PMC free article] [PubMed]
- 46.Carland F, Fujioka S, Nelson T. The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products. Plant Physiol. 2010;153:741–756. doi: 10.1104/pp.109.152587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carland FM. The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell Online. 2002;14:2045–2058. doi: 10.1105/tpc.003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hase Y, et al. Ectopic endoreduplication caused by sterol alteration results in serrated petals in Arabidopsis. J. Exp. Bot. 2005;56:1263–1268. doi: 10.1093/jxb/eri122. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, B. et al. BLADE-ON-PETIOLE proteins act in an E3 ubiquitin ligase complex to regulate PHYTOCHROME INTERACTING FACTOR 4 abundance. eLife610.7554/eLife.26759 (2017). [DOI] [PMC free article] [PubMed]
- 50.Galvao VC, et al. PIF transcription factors link a neighbor threat cue to accelerated reproduction in Arabidopsis. Nat. Commun. 2019;10:4005. doi: 10.1038/s41467-019-11882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He JX, et al. Sterols regulate development and gene expression in Arabidopsis. Plant Physiol. 2003;131:1258–1269. doi: 10.1104/pp.014605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng H, et al. Light-regulated, tissue-specific, and cell differentiation-specific expression of the Arabidopsis Fe(III)-chelate reductase gene AtFRO6. Plant Physiol. 2006;140:1345–1354. doi: 10.1104/pp.105.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Z, et al. Local auxin metabolism regulates environment-induced hypocotyl elongation. Nat. Plants. 2016;2:16025. doi: 10.1038/nplants.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carland F, Nelson T. CVP2- and CVL1-mediated phosphoinositide signaling as a regulator of the ARF GAP SFC/VAN3 in establishment of foliar vein patterns. Plant J. 2009;59:895–907. doi: 10.1111/j.1365-313X.2009.03920.x. [DOI] [PubMed] [Google Scholar]
- 55.Chapman EJ, et al. Hypocotyl transcriptome reveals auxin regulation of growth-promoting genes through GA-dependent and -independent pathways. PLoS ONE. 2012;7:e36210. doi: 10.1371/journal.pone.0036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Wit M, Ljung K, Fankhauser C. Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin responsiveness rather than different auxin levels. N. Phytol. 2015;208:198–209. doi: 10.1111/nph.13449. [DOI] [PubMed] [Google Scholar]
- 57.Brunoud G, et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature. 2012;482:103–106. doi: 10.1038/nature10791. [DOI] [PubMed] [Google Scholar]
- 58.Aldana, J., Romero-Otero, A. & Cala, M. P. Exploring the lipidome: current lipid extraction techniques for mass spectrometry analysis. Metabolites10, 10.3390/metabo10060231 (2020). [DOI] [PMC free article] [PubMed]
- 59.Gocze PM, Freeman DA. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry. 1994;17:151–158. doi: 10.1002/cyto.990170207. [DOI] [PubMed] [Google Scholar]
- 60.Graham IA. Seed storage oil mobilization. Annu. Rev. Plant Biol. 2008;59:115–142. doi: 10.1146/annurev.arplant.59.032607.092938. [DOI] [PubMed] [Google Scholar]
- 61.Cagnola JI, Ploschuk E, Benech-Arnold T, Finlayson SA, Casal JJ. Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol. 2012;160:1110–1119. doi: 10.1104/pp.112.201921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bou-Torrent J, et al. Plant proximity perception dynamically modulates hormone levels and sensitivity in Arabidopsis. J. Exp. Bot. 2014;65:2937–2947. doi: 10.1093/jxb/eru083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mamode Cassim A, et al. Biophysical analysis of the plant-specific GIPC sphingolipids reveals multiple modes of membrane regulation. J. Biol. Chem. 2021;296:100602. doi: 10.1016/j.jbc.2021.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD. Autophagic nutrient recycling in arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 2005;138:2097–2110. doi: 10.1104/pp.105.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin KD, Lee HN, Chung T. A revised assay for monitoring autophagic flux in Arabidopsis thaliana reveals involvement of AUTOPHAGY-RELATED9 in autophagy. Mol. Cells. 2014;37:399–405. doi: 10.14348/molcells.2014.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephani, M. et al. A cross-kingdom conserved ER-phagy receptor maintains endoplasmic reticulum homeostasis during stress. eLife9, 10.7554/eLife.58396 (2020). [DOI] [PMC free article] [PubMed]
- 68.Torggler, R., Papinski, D. & Kraft, C. Assays to monitor autophagy in Saccharomyces cerevisiae. Cells6, 10.3390/cells6030023 (2017). [DOI] [PMC free article] [PubMed]
- 69.Yoshimoto K, et al. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell. 2004;16:2967–2983. doi: 10.1105/tpc.104.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu S, Ye H, Cui Y, Jiang L. AtSec62 is critical for plant development and is involved in ER-phagy in Arabidopsis thaliana. J. Integr. Plant Biol. 2020;62:181–200. doi: 10.1111/jipb.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofius D, et al. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell. 2009;137:773–783. doi: 10.1016/j.cell.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 72.Procko C, et al. The epidermis coordinates auxin-induced stem growth in response to shade. Genes Dev. 2016;30:1529–1541. doi: 10.1101/gad.283234.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pucciariello O, et al. Rewiring of auxin signaling under persistent shade. Proc. Natl Acad. Sci. USA. 2018;115:5612–5617. doi: 10.1073/pnas.1721110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ibanez C, et al. Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr. Biol. 2018;28:303–310 e303. doi: 10.1016/j.cub.2017.11.077. [DOI] [PubMed] [Google Scholar]
- 75.Fiorucci AS, et al. PHYTOCHROME INTERACTING FACTOR 7 is important for early responses to elevated temperature in Arabidopsis seedlings. N. Phytol. 2019;226:50–58. doi: 10.1111/nph.16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu Y. Regulation of PIF4-mediated thermosensory growth. Plant Sci. 2020;297:110541. doi: 10.1016/j.plantsci.2020.110541. [DOI] [PubMed] [Google Scholar]
- 77.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kozuka T, et al. Regulation of sugar and storage oil metabolism by phytochrome during de-etiolation. Plant Physiol. 2020;182:1114–1129. doi: 10.1104/pp.19.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boccaccini A, et al. Low blue light enhances phototropism by releasing cryptochrome1-mediated inhibition of PIF4 expression. Plant Physiol. 2020;183:1780–1793. doi: 10.1104/pp.20.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brunkard JO. Exaptive evolution of target of rapamycin signaling in multicellular eukaryotes. Dev. Cell. 2020;54:142–155. doi: 10.1016/j.devcel.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Leene J, et al. Capturing the phosphorylation and protein interaction landscape of the plant TOR kinase. Nat. Plants. 2019;5:316–327. doi: 10.1038/s41477-019-0378-z. [DOI] [PubMed] [Google Scholar]
- 82.Araujo WL, et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell. 2010;22:1549–1563. doi: 10.1105/tpc.110.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Izumi M, Hidema J, Makino A, Ishida H. Autophagy contributes to nighttime energy availability for growth in Arabidopsis. Plant Physiol. 2013;161:1682–1693. doi: 10.1104/pp.113.215632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buchanan-Wollaston V, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 85.Das D, St Onge KR, Voesenek LA, Pierik R, Sasidharan R. Ethylene- and shade-induced hypocotyl elongation share transcriptome patterns and functional regulators. Plant Physiol. 2016;172:718–733. doi: 10.1104/pp.16.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Casal, J. J. & Balasubramanian, S. Thermomorphogenesis. Annu. Rev. Plant Biol.10.1146/annurev-arplant-050718-095919 (2019). [DOI] [PubMed]
- 87.Ince YC, Galvao VC. Analysis of shade-induced hypocotyl elongation in Arabidopsis. Methods Mol. Biol. 2021;2297:21–31. doi: 10.1007/978-1-0716-1370-2_3. [DOI] [PubMed] [Google Scholar]
- 88.Bensmihen S, et al. Analysis of an activated ABI5 allele using a new selection method for transgenic Arabidopsis seeds. FEBS Lett. 2004;561:127–131. doi: 10.1016/S0014-5793(04)00148-6. [DOI] [PubMed] [Google Scholar]
- 89.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 90.Bourbousse C, Vegesna N, Law JA. SOG1 activator and MYB3R repressors regulate a complex DNA damage network in Arabidopsis. Proc. Natl Acad. Sci. USA. 2018;115:E12453–E12462. doi: 10.1073/pnas.1810582115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gendrel AV, Lippman Z, Martienssen R, Colot V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods. 2005;2:213–218. doi: 10.1038/nmeth0305-213. [DOI] [PubMed] [Google Scholar]
- 92.Oh E, Zhu JY, Wang ZY. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012;14:802–809. doi: 10.1038/ncb2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cacas JL, et al. Revisiting plant plasma membrane lipids in tobacco: a focus on sphingolipids. Plant Physiol. 2016;170:367–384. doi: 10.1104/pp.15.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dunn WB, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011;6:1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- 95.Gagnebin Y, et al. Metabolomic analysis of urine samples by UHPLC-QTOF-MS: Impact of normalization strategies. Anal. Chim. Acta. 2017;955:27–35. doi: 10.1016/j.aca.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 96.Tsugawa H, et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods. 2015;12:523–526. doi: 10.1038/nmeth.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal17, 10.14806/ej.17.1.200 (2011).
- 98.Davis MP, van Dongen S, Abreu-Goodger C, Bartonicek N, Enright AJ. Kraken: a set of tools for quality control and analysis of high-throughput sequence data. Methods. 2013;63:41–49. doi: 10.1016/j.ymeth.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 102.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36:2628–2629. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus105 and are accessible through GEO Series accession number GSE174655. The MS data for lipid measurements were deposited in Metabolights (https://www.ebi.ac.uk/metabolights/). Untargeted lipidomic analysis with the unique identifier MTBLS2796, MTBLS5753 for the sphingolipidomics, and MTBLS5766 for sterols. Source data are provided with this paper.