Abstract
Cholesterol-enhanced pore formation is one evolutionary means cholesterol-free bacterial cells utilize to specifically target cholesterol-rich eukaryotic cells, thus escaping the toxicity these membrane-lytic pores might have brought onto themselves. Here, we present a class of artificial cholesterol-dependent nanopores, manifesting nanopore formation sensitivity, up-regulated by cholesterol of up to 50 mol% (relative to the lipid molecules). The high modularity in the amphiphilic molecular backbone enables a facile tuning of pore size and consequently channel activity. Possessing a nano-sized cavity of ~ 1.6 nm in diameter, our most active channel Ch-C1 can transport nanometer-sized molecules as large as 5(6)-carboxyfluorescein and display potent anticancer activity (IC50 = 3.8 µM) toward human hepatocellular carcinomas, with high selectivity index values of 12.5 and >130 against normal human liver and kidney cells, respectively.
Subject terms: Self-assembly, Supramolecular polymers, Nanopores
Bacterial cells utilize cholesterol-enhanced pore formation to specifically target eukaryotic cells. Here, the authors present a class of bio-inspired, cholesterol-enhanced nanopores which display anticancer activities in vitro.
Introduction
As an essential structural component of eukaryotic cell membranes1,2, cholesterol is typically absent from bacterial cell membranes3. By leveraging on this difference, bacterial cells manage to evolve cholesterol-dependent cytolysins (CDCs), a family of bacterial protein toxins that form circular membrane-lytic pores only in the presence of cholesterol to specifically target and harm eukaryotic cells, but not themselves4. Other protein channels, which display similarly interesting ion transport activities up-regulated by cholesterol, include nicotinic acetylcholine receptor5, epithelial Na+ channels6, transient receptor potential channels7 and Alzheimer β-amyloid peptides8. Nevertheless, despite a number of examples seen in Nature, within the realm of artificially created membrane transporters9–80, presence of cholesterol is often observed to cause several fold decreases in ion transport activity, and cholesterol-induced enhancement in ion transport activity, to the best of our knowledge, has yet to be demonstrated.
In this work, we report on the examples of cholic acid-derived pore-formers, forming multimeric nanopores that become increasingly active in ion transport when the level of cholesterol increases from 0 to 50 mol% relative to the amount of lipid molecules present in the membrane. Among this series of structurally similar pore-formers, the most active ensemble formed by Ch-C1 encloses a nano-sized cavity of 1.6 nm in diameter, which can be modularly tuned down through side chain modification. Importantly, Ch-C1 further displays potent anticancer activity (IC50 = 3.8 µM) toward human liver cancer cells with low cytotoxicity to normal human liver (IC50 = 47.5 µM) and kidney cells (IC50 > 500 µM).
Results and Discussion
Molecular design of pore-forming Ch-Pns
Attracting wide interests from biomimetic chemists, artificial transmembrane pores can be readily constructed using backbone-rigidified foldamers48,67,78–80. We however envisioned that this type of pores with high structural rigidity unlikely will exhibit cholesterol-enhanced ion transport activities. Instead, those pore-enclosing conformations, which are generated from either a single molecular backbone without a defined conformation9,20,28,39,65 or multiple components assembled via non-covalent forces, are deemed to be more sensitive toward environmental stimuli. In this regard, there exist a wide range of strategies in the literature. Inspiring ones include helical folding via solvophobic forces by Moore9, “rigid rod” β-barrels by Matile13–15, tail-to-tail assembly of half channel molecules16 and pore-forming steroid-modified biscarbamate17 by Kobuke, dendritic19 or linear-shaped steroid derivatives19 by Regen, columnar stacking involving shape-persistent macrocycles by Gong23,27,49, multiblock amphiphiles having alternatingly arranged hydrophilic and hydrophobic units by Muraoka and Kinbara28,39,65, structurally simple pore-forming mono- and tri-peptides71,72 and trimesic amides73 by Zeng, as well as H-bond-assisted formation of pore-containing rosettes derived from mannitol40 or diol-containing 1,3-diethynylbenzene52 by Talukdar, folate by Matile18, G-quartets by Davis22 and Dash44,66 and a fused guanine−cytosine base by Wanunu69.
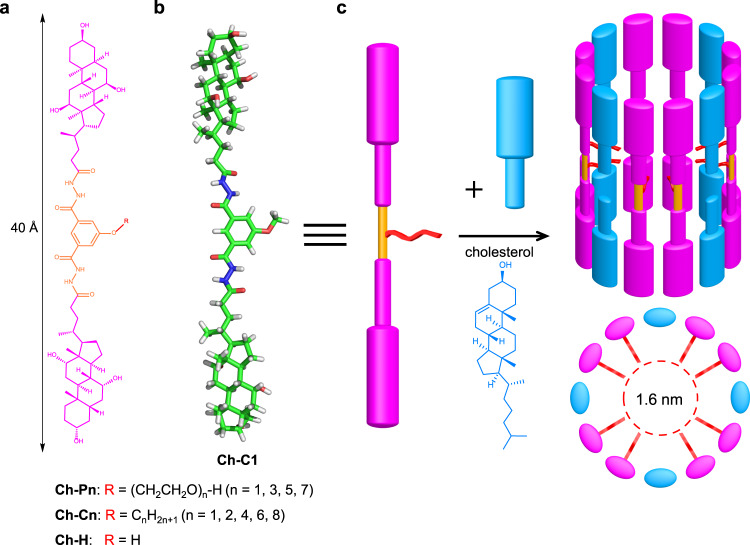
With long-standing interests in developing novel membrane-active artificial transporters71–81, we recently reported molecular machine-inspired molecular swings as a highly efficient K+ transporter82. These molecular swings are constructed by attaching a cation-binding and -transporting crown ether onto the center of a rigid cholic acid-derived linear scaffold via a flexible linker. Possessing two cholic acid units at the ends, this rigid cholic acid-derived scaffold is designed to have a length of 40 Å in for spanning the hydrophobic lipid region (Fig. 1a, b). Inspired by the unusual ability of Amphotericin B (AmB) to form an ergosterol-assisted barrel stave-like transmembrane pore, we wonder correspondingly whether the cholic acid-derived linear scaffold would be able to assemble into a form of circular nanopore in a cholesterol-dependent manner (Fig. 1c). To examine this possibility, we initially tested a series of four structurally similar molecules Ch-Pns (n = 1, 3, 5 and 7, Fig. 1a) that contain one to seven ethylene glycol units, and one control molecule Ch-C1 that has just a methyl group in its side chain (Fig. 1a, b).
Fig. 1. Molecular design and self-assembly of nanopores.
a Chemical structures of cholic acid-derived amphiphilic transmembrane channels Ch-Pn, Ch-Cn and Ch-H. b Computationally optimized structure of Ch-C1 at the level of B3LYP/6-31 G* in the gas phase. c Schematic illustration of one possible means to form a toroidal channel of ~1.6 nm in diameter by Ch-C1, a process that is primarily assisted by cholesterol molecules (Ch in blue), for mediating ion transport across the membrane.
Ch-Pns exhibit cholesterol-enhanced ion transport activities
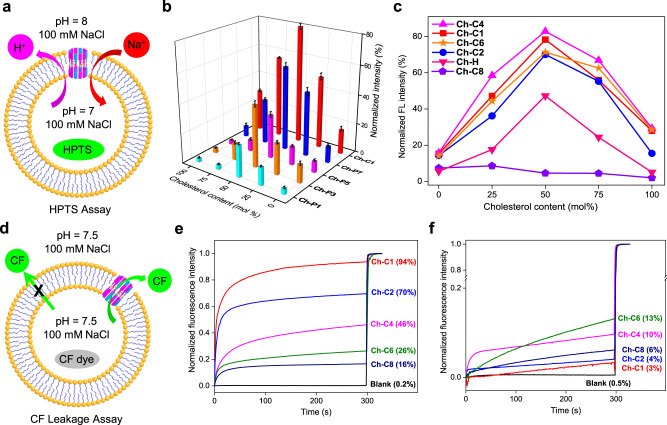
The ability of these five molecules (Ch-Pns and Ch-C1) to self-assemble into a membrane-active ion-transporting pore in the presence of cholesterol (Ch) molecules was assessed using the well-established pH-sensitive fluorescence assay (Fig. 2a), employing large unilamellar vesicles (LUVs) containing EYPC (egg yolk phosphatidylcholine) and Ch at varying molar ratios of 1:0 to 1:1. In typical conditions, pH-sensitive HPTS (8-hydroxy-1,3,6-pyrenetrisulfonate, 0.1 mM) and NaCl (100 mM) were entrapped in EYPC-based LUVs containing 0 mol%, 25 mol%, 50 mol%, 75 mol% and 100 mol% of Ch with respect to lipids in HEPES buffer at pH 7. The LUV stock solution was then diluted to the same buffer at pH 8.0 to create a pH gradient of 1.0 across the membrane. Upon addition of channels at a final concentration of 8 µM (3.7 mol% relative to lipid), the changes in ratiometric value of the fluorescence intensity at 510 nm (I460/I403) of the encapsulated HPTS were recorded for 300 s. After subtracting the background intensity at t = 300 s in the absence of the channel molecules (Supplementary Fig. 2), the signals were further normalized to give fractional ion transport activities summarized in Fig. 2b (For detailed ion transport curves, see: Supplementary Figs. 3, 4). It is clear that, in the absence of Ch, all five channels display weak ion transport activities of ≤16%. Nevertheless, increasing membrane Ch content from 0 mol% to 50 mol% makes these channels increasingly more active, generating 4–6 folds enhancements in ion transport activity at 50 mol% of Ch for all five channels. This interestingly general trend highlights a pivotal role that Ch molecules play in augmenting self-assembling propensities of these molecules. Coincidently, this 50 mol% is also the highest Ch content possible in eukaryotic cells. Further increases in Ch content to 100 mol% result in poorer performances, likely as a result of Ch-mediated over-stiffness of the membrane that attenuates the pore-forming capacity of these molecules83.
Fig. 2. Transmembrane transport activity study of nanopores.
a Schematic illustration of HPTS assay. HPTS = 8-hydroxy-1,3,6-pyrenetrisulfonate. b, c Normalized ion transport activities of channels over a duration of 5 min at 8 μM using LUVs containing different contents of cholesterol (0, 25, 50, 75 and 100 mol % relative to lipid) in the HPTS assay. d Schematic illustration of CF dye leakage assay for qualitatively probing pore size formed by channels Ch-C1, Ch-C2, Ch-C4, Ch-C6 and Ch-C8. CF = 5(6)-carboxyfluorescein. e and f describe changes in fluorescence intensity of self-quenching CF dye (λex = 492 nm, λem = 517 nm) after additions of channel molecules at 8 μM in the e presence or f absence of 50 mol % cholesterol relative to lipid. In b and c, all the relative errors are less than 3% based on a triplicate run. These experiments demonstrate that (i) cholesterol molecules do assist the formation of pores of >1 nm and (ii) side chain elongation decreases the pore size, suggesting side chains to orient toward, not away from the pore interior. Source data are provided as a Source Data file.
Ch-Pns are less active than Ch-Cns (n ≠ 8)
To our surprise, the control molecule Ch-C1, having no ethylene glycol units, consistently exhibits better ion transport activities than Ch-Pns at all Ch contents. Although we are not exactly sure about the reasons behind, this somewhat intriguing finding does suggest oxygen-rich ethylene glycol chains not to be that essential in order to facilitate transmembrane ion flux.
Logically, in the next step, we therefore decided to look into pore-forming capacity of four more molecules Ch-Cns (n = 2, 4, 6 and 8, Fig. 1a), which contain a straight hydrocarbon chain, as well as another control molecule Ch-H, which possesses a hydroxyl group. Similarly, the ion transport activities of these five channels are sensitively and increasingly up-regulated by increasing Ch contents of up to 50 mol% beyond which activities drop (Fig. 2c and Supplementary Fig. 5). Except for Ch-C8, the other four alkyl-containing Ch-Cns (n = 1, 2, 4 and 6) are more active than ethylene glycol-containing Ch-Pns (n = 1, 3, 5 and 7). In more details, at 50 mol% of Ch, Ch-Cns (n = 1, 2, 4 and 6) display fractional ion transport activities of 78%, 70%, 83% and 71%, but Ch-Pns (n = 1, 3, 5 and 7) show much weaker activities of 18%, 40%, 28% and 55%, respectively.
Ch-Cns generate cholesterol-stabilized pores in the cholesterol-rich lipid bilayer
Since these linear-shaped molecules Ch-Cns or Ch-Pns carry no discernable binding groups for binding and transporting ions, we proposed a toroidal model where multiple linear molecules are circularly arranged to produce a multimeric hollow ensemble sensitive to cholesterol molecules and possibly lipid molecules as well (Fig. 1c). The stability, pore size and ion-transporting potential of the resultant nanopores are critically dependent on the Ch content as well as the length and orientation of side chains. In terms of side-chain orientation, they may point toward the channel’s interior as illustrated in Fig. 1c, or away from the interior. The former is believed to be a more likely scenario that is consistent with a negligible ion transport activity of 4.6% exhibited by Ch-C8 (Fig. 2c), especially when compared to high activities of >70% by those within the same series but with side chains shortened by 2–7 carbon atoms (e.g., Ch-Cns, n = 1, 2, 4 and 6). In other words, we hypothesized that it is only when the side chains point toward the interior that a sharp decline in activity from 71% for hexyl-containing Ch-C6 to 4.6% for octyl-containing Ch-C8 may become possible.
To lend some support to this hypothesis, we applied self-quenching 5(6)-carboxyfluorescein (CF) dye leakage assay (Fig. 2d) to qualitatively evaluate the pore sizes and activities of Ch-Cns (n = 1, 2, 4, 6 and 8). Here, CF dye is highly fluorescent at diluted concentrations but self-quenches at high concentration of 50 mM via a self-dimerization process. Having the smallest dimension of 1.0 nm x 1.0 nm (Supplementary Fig. 6), CF molecules trapped inside LUVs can only permeate through a pore of >1 nm to reach the extravesicular region, resulting in increased fluorescence intensity. For this CF leakage assay, we prepared LUVs containing 50 mol% of Ch relative to lipids. As summarized in Fig. 2e, Ch-C1 at 8 µM (3.7 mol% relative to lipid) elicits CF efflux by 94%, which is in sharp contrast to 3% CF efflux obtained using LUVs free of Ch (Fig. 2f). This high activity, which confirms the pore size formed by Ch-C1 to be >1 nm, is followed by Ch-C2 (70%), Ch-C4 (46%), Ch-C6 (26%) and Ch-C8 (16%), indicating increasingly shrunk pore sizes by increasingly lengthened side chains among this series of channels. As summarized in Table 1 and Supplementary Figs. 7, 8, the corresponding EC50 values in the presence of Ch are 5.0, 7.0 and 7.9 µM for Ch-C1, Ch-C2 and Ch-C4, respectively, and greater than 20 µM for both Ch-C6 and Ch-C8. For comparison, the EC50 value for melittin, a pore-forming toxin with estimated pore sizes of 3.5–4.5 nm84, was 0.17 µM (0.08 mol% relative to lipid). In addition, none of these channels is capable of efficient transport of CF dye in the Ch-free membrane environment (Fig. 2f and Supplementary Fig. 9).
Table 1.
EC50 values (µM) determined using CF-leakage assay
| EC50 (µM) | n | |
|---|---|---|
| Ch-C1 | 5.0 ± 0.2 | 4.08 ± 0.60 |
| Ch-C2 | 7.0 ± 0.2 | 6.40 ± 1.02 |
| Ch-C4 | 7.9 ± 0.4 | 3.58 ± 0.62 |
| Ch-C6 | >20 | - |
| Ch-C8 | >20 | - |
| Ch-H | 8.0 ± 0.1 | 6.39 ± 0.37 |
| Melittin | 0.17 ± 0.01 | 1.33 ± 0.07 |
Taken together, the above CF dye leakage data are in great accord with the AmB-inspired hypothetic barrel stave-like model, i.e., Ch molecules may aid the formation of a nanoscale toroidal pore by alternatively interacting with channel molecules whose side chains point toward the pore interior (Fig. 1c). Forming these large pores is also consistent with the ability of Ch-C1 to transport anions (Supplementary Fig. 10), and with the fact that these large pores makes the HPTS assay insignificantly influenced by ions and/or HPTS dye molecules that get transported into the extravesicular region (Supplementary Figs. 11, 12) and thus do not generate a well-defined activity order among Ch-Cns (Fig. 2c).
Ch-Cns generate toroidal pores of <2 nm in diameter
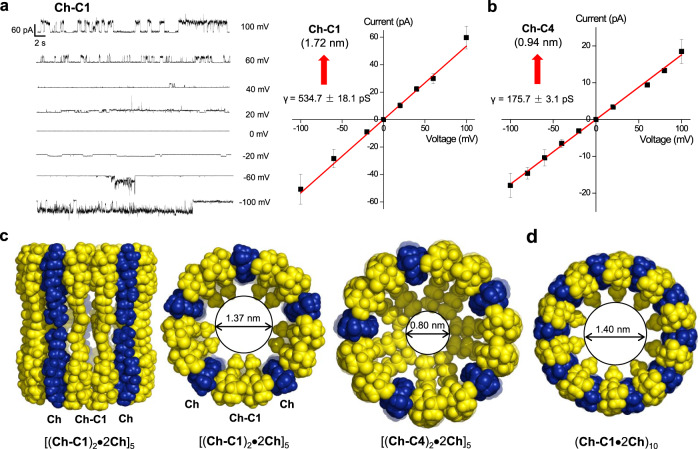
To quantify the pore sizes and also verify the single channel behaviors of the pore-formers, we conducted planar lipid bilayer experiments using the bilayer membrane made up of 1,2-diphytanoyl-sn-glycero-3-phosphocholine and Ch in a 2:1 molar ratio in symmetric (cis chamber = trans chamber = 1 M KCl) baths. The recorded single channel current traces from –100 mV to 100 mV for Ch-C1 undoubtedly confirm its ability to transport ions through a channel mechanism (Fig. 3a). At a lower voltage of −20 mV, channels appear to exist in the form of barrel-shaped ensemble mostly having a single diameter. At other voltages, many multiple level transitions were observed in the current traces, suggesting the existence of toroidal pores of varied diameters85,86. These varied diameters result from dynamic breathing-type interactions of Ch-C1 with Ch and these interactions might be further influences by the lipid molecules that are also in constant movement.
Fig. 3. Single channel study and molecular dynamic simulation of nanopores.
a Single channel current traces of Ch-C1 recorded in symmetric (cis chamber = trans chamber = 1 M KCl) baths, the red lines refer to the mean current values for plotting current-voltage (I-V) curve for obtaining the ion conductance (γ) and pore size (1.72 nm) for Ch-C1. b I-V curve and estimated pore size (0.94 nm) for Ch-C4. Data are represented as mean current values ± half width at half maximum. n = 3 independent experiments. c Molecular dynamics-simulated pore-forming ensembles of different sizes made up of channel (Ch-C1 or Ch-C4 in yellow) and Ch (in blue) molecules in 1:1 molar ratio. d An alternative self-assembling possibility involving Ch-C1 and Ch in 1:2 molar ratio to generate a pore of 1.40 nm.
Accordingly, the histogram of currents at all voltages were plotted to obtain the mean current values and the relative errors (Supplementary Figs. 13–15) for plotting the current-voltage (I-V) curve presented in Fig. 3a. From the linear I-V curve, the single-channel conductance was calculated to be 534.7 ± 18.1 pS, corresponding to a highly efficient transport of 3.4 × 108 ions/s at 100 mV. On the basis of this conductance value, the channel’s pore size was estimated to be 1.67 nm across by using the Hille equation87 (Eq. 4). In contrast, in the single channel current measurements using Ch-free bilayer membrane, we didn’t observe any channel activity even after prolonged recordings, confirming the critical role of Ch molecules in the pore formation. Repeating the single channel current recording twice followed by pore size calculations (Supplementary Figs. 16–20) gives an average conductance value of 496.4 ± 46.5 pS and an average pore size of 1.61 ± 0.09 nm.
The same set of single channel conductance measurement and pore size calculation performed on Ch-C4 in triplicate gives an average conductance value of 188.5 ± 9.6 pS (Supplementary Figs. 21–28) and an average pore size of 0.94 ± 0.03 nm in diameter. A representative I-V curve presented in Fig. 3b gives a conductance value of 175.7 ± 3.1 pS and a pore size of 0.90 nm in diameter.
Pore-formers do associate with cholesterol molecules
The binding between the channel molecules and Ch was initially supported by 1H NMR titration experiments involving titrating 0–100 equivalents of Ch into a THF-d8 solution containing Ch-C2 at 5 mM (Supplementary Fig. 29). Upon addition of up to 100 equivalents of Ch, the signals corresponding to the two amide protons of Ch-C2 show obvious downfield shifts of up to 0.08 and 0.16 ppm, respectively, indicating that not only Ch-C2 can bind to Ch molecules but also such binding likely increases the self-association extent of Ch-C2 molecules. Using UV-vis spectroscopy, we observed (1) red shifts of 2.5 nm and 21 nm for the maximum and minor absorption peaks when increasing the concentration of Ch-C2 from 5 µM to 80 µM in THF at 20 oC (Supplementary Note 1 and Supplementary Fig. 30) and (2) a red shift of 2.5 nm and a 24% decrease in intensity for the maximum absorption peak (Supplementary Fig. 31). These two pieces of data are evidently suggestive of associations among Ch-C2 molecules and between Ch-C2 and Ch molecules. Consistent with the 1H NMR- and UV-vis-based binding data, analysis of the high-resolution mass spectra (HRMS) of the THF solution containing Ch-C2 (10 µM) and Ch (1 mM) reveals dimeric (Ch-C2)2•Na+ and (Ch-C2)2•H+ as well as 4Ch-C2•2Ch•2H+ as the major peaks (Supplementary Figs. 33, 34), followed by much weaker signals, corresponding to the singly charged (Ch-C2)2•H+ that associates with one or two Ch molecules (Supplementary Figs. 35, 36). These HRMS data suggest self-dimerization involving two Ch-C2 molecules to be far much stronger than the mutual-association between Ch-C2 and Ch molecules.
Computational models of the pores formed by Ch-C1 and Ch-C4
In light of (1) the side chain-dependent activity trend deduced from the CF leakage assay, (2) the quantified pore sizes of 1.61 and 0.94 nm for Ch-C1 and Ch-C4, respectively, and (3) the HRMS data that confirm the existence of a dimeric (Ch-C1)2 fragment, we constructed a few Ch-containing toroidal ensembles [(Ch-Cn)2•2Ch)m], with side chains pointing toward the pore interior (Fig. 3c and Supplementary Fig. 37a). This was followed by molecular dynamics simulation (MD) to yield pore sizes of 1.37 and 0.80 nm for [(Ch-C1)2•2Ch)5] and [(Ch-C4)2•2Ch)5], respectively. Moreover, ensembles [(Ch-Cn)2•2Ch)m] with m ≠ 5 generate a pore size that is too small or too large (Supplementary Fig. 37a), and those with half of side chains pointing outward are energetically less stable (Supplementary Fig. 37b). We thus believe pentameric pores containing inward-pointing side chains might be one preferred association mode at least for Ch-C1 and Ch-C4, enabling channel and Ch molecules to act synergistically to produce membrane-active wide pore ensembles. Certainly, there might exist other possible structural models that may also enclose a pore size comparable to the experimentally determined one. Figure 3d illustrates one such ensemble (Ch-C1•2Ch)10, having a pore size of 1.40 nm that closely matches the pore size of ~1.6 nm for Ch-C1.
Ch-C1 mediates potent and specific anticancer effects
Given that disrupting ion homeostasis across the cellular membrane could result in cell death31,52,88–90, it is expected that these hole-punching channels might exert toxicity on cancer cells. In this regard, we applied a standard cell viability assay to evaluate the anticancer activities of Ch-Cns against human hepatocellular carcinomas (HepG2 cell line, Table 2 and Supplementary Fig. 38). We found that channels with different side chain lengths or pore sizes show good anticancer activities. Among them, the most potent Ch-C1 exhibits an IC50 value of as low as 3.8 µM, which is comparable to those of the well-known chemotherapeutic agents doxorubicin (1.5 µM) and paxlitaxel (8.2 µM) and much lower than that of cisplatin (>500 µM) (Table 2 and Supplementary Fig. 38). For human primary glioblastoma cell line (U87-MG) that are refractory to be treated, the above-mentioned three chemotherapeutic agents (cisplatin, doxorubicin and paclitaxel) show no activity (IC50 > 500 µM, Table 2 and Supplementary Fig. 39), whereas Ch-C1 still displays significant therapeutic activity (IC50 = 50.7 µM) (Table 2 and Supplementary Fig. 39). Not very surprisingly, for both cancer cell lines, the anticancer activities of these channels correlate well with the pore sizes (or the side chain lengths), i.e., larger pores (or shorter side chains) show more potent activities.
Table 2.
IC50 values (μM) for Ch-Cn (n = 1 – 8) against HepG2 and U87-MG cancer cells
| IC50 (µM) | ||
|---|---|---|
| HepG2 | U87-MG | |
| Ch-C1 | 3.8 | 50.7 |
| Ch-C2 | 8.4 | 186.7 |
| Ch-C4 | 26.6 | > 500 |
| Ch-C6 | 36.6 | > 500 |
| Ch-C8 | 57.5 | > 500 |
| Cisplatin | > 500 | > 500 |
| Paclitaxel | 8.2 | > 500 |
| Doxorubicin | 1.5 | > 500 |
These good anticancer activities of Ch-C1 promoted us to further evaluate its selectivity and safety towards normal cells by performing the cell viability assay on human normal liver cells (THLE-2) and human renal proximal tubular epithelial cells (HK-2). Desirably, the determined IC50 values of Ch-C1 against THLE-2 cells and HK-2 cells were 47.5 and >500 µM, respectively (Supplementary Fig. 40). Defined as the ratio of IC50 values between normal and cancer cells, the selectivity indexes (SI) determined for Ch-C1 molecules are 12.5 and >130 against THLE-2 and HK-2 cells, respectively. These high SI values are more than one order of magnitude higher than those of doxorubicin and paclitaxel91,92, indicating comparably lower cytotoxicities of Ch-C1 to normal cells than doxorubicin and paclitaxel. We then employed mice blood cells to test the in vitro hemolytic activity of Ch-C1. To our delight, Ch-C1 also showed a low hemolytic activity. The concentration causing 50% hemolysis of red blood cells (HC50) is 127.3 µM (Supplementary Fig. 41), corresponding to a high SI value of 38.5. This high specificity may be caused by the high-level expression of cholic acid receptors on hepatocarcinoma cells that leads to higher uptake of cholic acid-containing channel molecules93,94.
Two types of dye molecules (PI and DAPI) were applied to assess the membrane integrity in the presence of Ch-C1 molecules. While DAPI molecules can enter the cells at high concentrations and become blue upon binding to the AT regions of dsDNA, membrane-impermeable PI enters cells having compromised membranes and binds tightly to the intracellular nucleic acids to emit red fluorescence when excited at 535 nm. After the incubation of HepG2 cells with various concentrations of Ch-C1 (0, 1, 4 and 16 µM) for 36 h, the cells were fixed, stained with PI and DAPI, and analyzed under a laser confocal microscope. As can be seen from Fig. 4, the DAPI-stained cells reveal intact cell membrane, confirming that pore-forming Ch-C1 does not disrupt the cell membranes to a noticeable extent at concentrations of up to 16 µM. And the PI-stained cells do suggest that Ch-C1 make the membrane leaky and more permeable via forming wide pores.
Fig. 4. Nanopores punch holes on cancer cells.
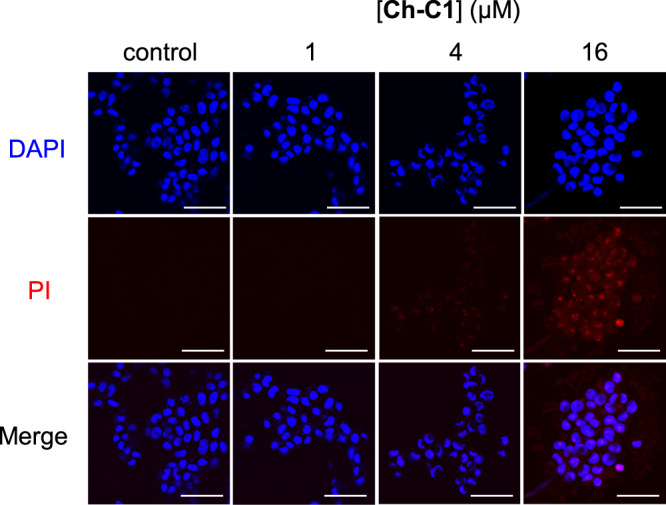
Cell imaging of HepG2 cells treated with Ch-C1 at concentrations of 1 µM, 4 µM and 16 µM for 36 h, followed by staining with blue DAPI and red PI dyes. Blue and red images were merged by ImageJ 1.8.0. Each experiment was repeated twice independently with similar results. Scale bar: 50 μm. DAPI 4’,6-diamidino-2-phenylindole, PI propidium iodide.
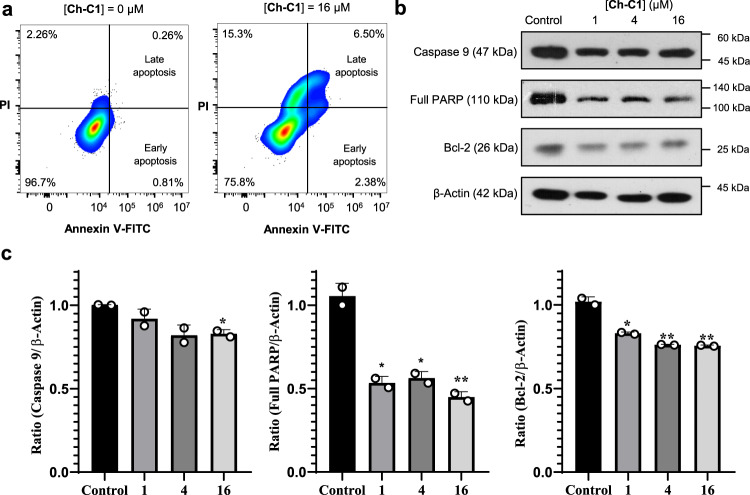
It has been shown that disruption of cellular homeostasis caused by leaky cell membrane leads to cell apoptosis via the caspase signaling pathway95–97. Applying dead cell apoptosis kit, we treated the HepG2 cells with Ch-C1 at 16 µM for 24 h, stained the cells using both green Annexin V- FITC conjugate and red PI dyes and sorted the cells by flow cytometry. Considering that cells stainable by Annexin V-FITC conjugate correspond to early or later apoptotic cells (Fig. 5a), the fact that the percentage of apoptotic cells substantially increases from 1.07% to 8.88% with increased concentrations of Ch-C1 from 0 to 16 μM establishes the capability of Ch-C1 to induce cell apoptosis. Some characteristic proteins involved in apoptosis include (1) apoptosis initiator protein (caspase-9) that initiates cell killing but is cleaved during early apoptosis98,99, (2) poly(ADP-ribose) polymerase (PARP) cleaved by the activated caspase 9 to facilitate apoptosis by preventing DNA repair and (3) anti-apoptotic protein (Bcl-2) that undergoes apoptosis- induced inhibition. Therefore, following an apoptotic stimulus, decreased presences of caspase 9, PARP and Bcl-2, together with the increased presence of cleaved PARP, are expected. And these are indeed what we observed when we treated HepG2 cells with Ch-C1 at 16 µM for 6 h (Fig. 5b, c), prompting us to conclude that Ch-C1-enhanced membrane permeability can induce HepG2 cell apoptosis via the caspase 9 pathway.
Fig. 5. Nanopores induce cancer cell apoptosis.
a Evaluation of HepG2 apoptosis by flow cytometry, with Ch-C1 at 0 μM and 16 μM for 24 h and cells stained using green Annexin V-FITC conjugate and red PI dye. Annexin V = Intracellular protein of the annexin family that recognizes phosphatidylserines; FITC = Fluorescein isothiocyanate. b Immunoblot assay for Caspase 9, PARP and Bcl-2 in HepG2 cells treated with up to 16 µM of Ch-C1 for 24 h. Results were analyzed via ImageJ 1.8.1 and reported as histograms by graphpad prism 8.01 in c. Two-tailed Student’s t-test was used for statistical significance and data are presented as means ± SEM (n = 2 biologically independent samples). P values (Caspase 9) = 0.1827, 0.0524 and 0.0105, respectively. P values (full PARP) = 0.0132, 0.0156 and 0.0093, respectively. P values (Bcl-2) = 0.0117, 0.0060 and 0.0058, respectively. Symbols * and ** stand for significant differences between the control group and other groups, with P < 0.05 and 0.01, respectively. Source data are provided as a Source Data file.
In conclusion, we have conceptualized and demonstrated a class of cholesterol-enhanced nanopores in analogy to the barrel stave-like pores formed by AmB in the presence of ergosterol in the membrane. In assisting the wide pore formation, two cholesterol molecules, which may adopt a bilayer-type structure, alternatively interact with the self-dimerized channel molecules in a 1:1 molar ratio in a concentration-dependent manner, with the most active (likely also the most stable) pores always formed when cholesterol is at 50 mol % with respective to lipid molecules. The pores thus formed further show side chain-dependent activities, and the one with the shortest methyl side chains (e.g., Ch-C1) produces the widest pore of about 1.6 nm across and the highest cross-membrane transport activity. Channel Ch-C1 further displays the best anti-hepatic cancer activity and high specificity to tumor cells, and may serve as a promising candidate for hepatic cancer treatments. This artificial cholesterol-enhanced nanopore system with high structural modularity offers a facile strategy to construct a family of structurally diversified sensitive nanopores, possibly giving rise to a broad range of applications including cholesterol sensors, anticancer agents and controlled drug delivery.
Methods
Ion transport study using the HPTS assay
Egg yolk L-α-phosphatidylcholine (EYPC, 0.6 ml, 25 mg/mL in CHCl3, Avanti Polar Lipids, USA) and cholesterol (0, 1.9, 3.8, 5.7 or 7.6 mg) were dissolved in CHCl3 (10 mL). CHCl3 was removed under reduced pressure at 35 oC. After drying the resulting film under high vacuum overnight at room temperature, the film was hydrated with 1.5 mL 10 mM HEPES buffer solution (100 mM NaCl, pH = 7.0) containing a pH sensitive HPTS dye (0.1 mM) at 37 oC for 2 h to give a milky suspension. The mixture was then subjected to 8 freeze-thaw cycles. The vesicle suspension was extruded through polycarbonate membrane (0.1 μm) to produce a homogeneous suspension of LUVs. The suspension of LUVs was dialyzed for 16 h with gentle stirring (300 r/min, 4 oC) using membrane tube (MWCO = 10,000 Da) against the same HEPES buffer solution (300 mL, without HPTS) for 6 times to remove the unencapsulated HPTS to yield LUVs with lipids at a concentration of 13 mM.
The HPTS-containing LUV suspension (30 μL, 13 mM in 10 mM HEPES buffer containing 100 mM NaCl at pH = 7.0) was added to a HEPES buffer solution (1.75 mL, 10 mM HEPES, 100 mM NaCl at pH = 8.0) to create a pH gradient for ion transport study. A solution of channels in DMSO was then injected into the suspension under gentle stirring. Upon the addition of channels, the emission of HPTS was immediately monitored at 510 nm with excitations at both 460 and 403 nm recorded simultaneously for 300 s using fluorescence spectrophotometer (Hitachi, Model F-7100, Japan). At 300 s, an aqueous solution of Triton X-100 (20 μL, 20% v/v) was immediately added to induce the maximum change in fluorescence emission. The final transport trace was obtained as a ratiometric value of I460/I403 and normalized based on the ratiometric value of I460/I403 after addition of triton using the Eq. (1).
| 1 |
where If = Fractional emission intensity, It = Fluorescence intensity at time t, I1 = Fluorescence intensity after addition of Triton X-100 and I0 = Initial fluorescence intensity.
CF dye leakage assay
Preparation of CF-containing LUVs follows a similar protocol in the HPTS assay. In a typical experiment, the CF-containing LUV suspension (30 μL, 13 mM in 10 mM HEPES buffer containing 100 mM NaCl at pH = 7.5) was diluted to the same HEPES buffer solution (1.75 mL) to create a concentration gradient for CF dye efflux study. A solution of channels or Melittin in DMSO at different concentrations was injected into the suspension under gentle stirring. Upon the addition of channels or melittin, the emission of CF was immediately monitored at 517 nm with excitations at 492 nm for 300 s using fluorescence spectrophotometer (Hitachi, Model F-7100, Japan). At t = 300 s, an aqueous solution of Triton X-100 (20 μL, 20% v/v) was immediately added to achieve the maximum efflux of dye. The final transport trace was obtained by normalizing the fluorescence intensity using Eq. (2).
| 2 |
where, F = Fractional emission intensity, Ft = Fluorescence intensity at time t, F1 = Fluorescence intensity after addition of Triton X-100 and F0 = Initial fluorescence intensity.
The fractional ion transport activity R was calculated for each curve using the normalized fluorescence intensity before the addition of Triton X-100, with the blank as 0 and that of triton set 1. Fitting the fractional transmembrane activity R vs channel concentration using the Hill Eq. (3) gave the Hill coefficient n and EC50 values.
| 3 |
Single channel conductance measurement using planar lipid bilayer system
The chloroform solution containing a mixture of 1,2-diphytanoyl-sn-glycero-3-phosphocholine (diPhyPC, 10 mg/ml, 40 µL) and cholesterol (10 mg/ml, 9.1 μL) was evaporated using nitrogen gas to form a thin film and re-dissolved in n-decane (16 uL). 0.5 μL of this n-decane solution was injected into the aperture of the Delrin® cup (Warner Instruments, Hamden, CT) with the n-decane removed using nitrogen gas. In a typical experiment, both the chamber (cis side) and Delrin cup (trans side) were filled with an aqueous KCl solution (1.0 M, 1.0 mL). Ag-AgCl electrodes were inserted into the two solutions with the cis chamber grounded. Planar lipid bilayer was formed by painting 0.3 μL of the lipid-containing n-decane solution around the n-decane-pretreated aperture. Samples in MeOH (1.0 μL) were added to the cis compartment to reach a final concentration of around 10−6 M and the solution was stirred for a few min until a single current trace appeared. These single channel currents were then measured using a Warner BC-535D bilayer clamp amplifier, collected by PatchMaster (HEKA) with a sample interval at 5 kHz and filtered with an 8-pole Bessel filter at 1 kHz (HEKA). The data were analysed by FitMaster (HEKA) with a digital filter at 100 Hz. Plotting current traces vs voltages yielded ion conductance (γ). The diameter of the channel is calculated from the ion conductance using Hille Eq. (4):
| 4 |
where g = corrected ion conductance (obtained by multiplying measured conductance with Sansom’s correction factor), l = length of channel (40 Å) and ρ = resistivity of 1 M KCl solution (0.0921 Ω•m).
In vitro anticancer study via MTT assay
The cytotoxicity assay of channels relied mainly on ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium) MTT assay in which MTT was used as the detection reagent for cell viability. HepG2, U87-MG, THLE-2 and HK-2 were used as test cells. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco), containing 10% of fetal bovine serum (FBS, Gibco) and 10,000 U/mL of Penicillin-Streptomycin (Gibco) at 37 oC in a humidified atmosphere containing 5% CO2. Active cells were seeded onto 96-well plates with a cell density of 1 × 104 cells/well in 100 µL DMEM medium. Channel molecules at a series of diluted concentrations in DMSO (obtained from Yeasen) were further diluted with the serum-free medium to give final concentration of channel molecules ranging from 0.0625 μM to 512 μM. The cells treated with 0.5% DMSO served as a negative control. After the cell density reached 50–60%, the medium of 96-well plates was replaced with serum-free medium containing channel reagents of various concentrations, and each concentration was repeated four times. After 48 h incubation, the medium containing the channel molecules were replaced with MTT solution (free DMEM as solvent, 500 µg/mL). Then after 4 h incubation, the medium was removed, and 150 µL DMSO were added, followed by uniformly shaking the plates to completely dissolve the purple formazan crystals. The UV-absorption values at 490 nm were obtained by absorbance microplate reader (CMAX PLUS, Molecular Devices, USA). Cell viabilities with the addition of channel molecules at various concentrations were calculated following Eq. (5). Then cell viabilities versus logarithm of channel concentrations were plotted and the IC50 values were calculated using a nonlinear regression curve fit with Graphpad Prism 8.0.1.
| 5 |
Cell membrane integrity assessment
HepG2 cells were seeded onto a 48-well plate with pre-placed circular cell plates at cell density of 2 × 104 cells/well. After incubation at 37 oC in a humidified atmosphere containing 5% CO2 for 12 h, the supernatant was replaced with the medium containing Ch-C1 at 0, 1, 4 and 16 µM, and cell were incubated for another 36 h. The propidium iodide (PI) staining solution (obtained from Yeasen) was diluted 20 times with dye diluent and warmed up to 37 oC in a water bath. The supernatant of the 48-well plate was then replaced with the warm PI staining solution. After incubation for 15 min, the cells were washed twice with phosphate buffered saline (PBS, Solarbio) and fixed at 4 oC with a 4% paraformaldehyde solution for 10 min. The tablets were sealed with 4’,6-diamidino-2-phenylindole (DAPI)-containing cell sealing tablets (obtained from Yeasen) and dried naturally in the dark. The fluorescence images were then photographed using laser confocal microscope (LSM5 EXCITER, Zeiss).
Flow cytometry assay
HepG2 cells were cultured in 6-well plates containing DMEM supplemented with 10% fetal bovine serum at 37 oC in a humidified atmosphere containing 5% CO2 for 12 h. After incubating the cells with Ch-C1 at various concentrations for 24 h, the cells were detached from the plate using 0.25% trypsin (0.5 mL) and resuspended in medium (4 mL) prior to centrifugation (112 × g for 5 min). The obtained cell pellets were washed twice using PBS (2 mL, Solarbio) and stained with Annexin V-FITC (obtained from Yeasen) and propidium iodide (PI) in the dark for 15 min. The percentages of apoptotic cells were determined by flow cytometry using PB450-A (CytoFLEX, Beckman Coulter) for 1 h. Data was analyzed using FlowJo 10.6.2. Example of gating strategy for apoptotic cell analysis is illustrated in Supplementary Fig. 42.
Immunoblot analysis
HepG2 cells were evenly seeded in 6-well plates with cell density of 6 × 105 cells/well and incubated in the medium containing FBS at 37 oC in a humidified atmosphere containing 5% CO2 for 16 h, followed by the replacement of medium containing Ch-C1 at 0, 1, 4 and 16 µM without FBS. Meanwhile, control cell groups were prepared via the same protocol using 0.5% DMSO in the absence of Ch-C1. After incubation with Ch-C1 for 24 h, the cells were washed with PBS once, and lysed in a lysis buffer containing 250 mM NaCl, 20 mM Tris-HCl at pH 7.4, 1.0 mM ethylene diamine tetraacetic acid (EDTA, Solarbio) and 1% Triton X-100 (Adamas) at 4 oC for 30 min. Protein gel electrophoresis was performed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method. The separated proteins were then transferred to polyvinylidene fluoride (PVDF, Millipore) membrane. After blocking with 5% skimmed milk, blots were incubated in primary antibody solution overnight at 4 oC. Antibodies used are sc-8007 (1:1000, Santa) for PARP, 9502 T for Caspase 9 (1:1000, Cell signalling technology), 12324 for BCL2 (1:1000, Abcam) and AC026 for β-Actin (1:5000, Abclonal). After washing with TBST, incubation continued with a peroxidase-conjugated secondary antibody solution (1:5000, Abcam) at 25 oC for 2 h, and the blots were then washed with 1xTBST solution, developed with Pierce™ ECL Western Blotting Substrate (Thermo Scientific) and visualized using Chemiluminescence Imager (ChemiDoc MP, Biorad). Density of each band was analysed with ImageJ 1.8.0 software. Uncropped scans of blots are supplied in the Source Data file.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22001221 to C.R., 81971724 to Y.-L.W. and U1903119 to C.W.), Shenzhen Science and Innovation Committee (JCYJ20210324123411030 to C.R. and 2021Szuvp067 to C.R.), the Fundamental Research Funds for the Central Universities (20720210101 to C.R.) and Fuzhou University.
Source data
Author contributions
J.S. performed the synthesis and ion transport study of channels. Y.G. performed the single channel current measurement. L.K., C.W. and Y.-L. W. performed in vitro anticancer study and hemolytic study. Q.Z. performed flow cytometry study and membrane integrity study. Y.C. and Z.W. performed immunoblot study. Y.M. performed the computational work. Y.L. performed the purification of channels. C.R. and H.Z. conceived and supervised the project and wrote the manuscript. All authors participated in discussion and editing of the manuscript.
Peer review
Peer review information
Nature Communications thanks Ryuji Kawano and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. The datasets that support the finding of this study are available in figshare repository with the identifier(s) 10.6084/m9.figshare.20544585.v1. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Changliang Ren, Email: changliang.ren@xmu.edu.cn.
Huaqiang Zeng, Email: hqzeng@fzu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33639-5.
References
- 1.Yeagle PL. Cholesterol and the cell membrane. Biochim. Biophys. Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 2.Levitan I, Singh DK, Rosenhouse-Dantsker A. Cholesterol binding to ion channels. Front. Physiol. 2014;5:65. doi: 10.3389/fphys.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brender JR, McHenry AJ, Ramamoorthy A. Does cholesterol play a role in the bacterial selectivity of antimicrobial peptides? Front. Immunol. 2012;3:195–195. doi: 10.3389/fimmu.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton CJ, Sani M-A, Parker MW, Separovic F. Cholesterol-dependent cytolysins: membrane and protein structural requirements for pore formation: focus review. Chem. Rev. 2019;119:7721–7736. doi: 10.1021/acs.chemrev.9b00090. [DOI] [PubMed] [Google Scholar]
- 5.Barrantes FJ. Cholesterol effects on nicotinic acetylcholine receptor. J. Neurochem. 2007;103:72–80. doi: 10.1111/j.1471-4159.2007.04719.x. [DOI] [PubMed] [Google Scholar]
- 6.Awayda MS, et al. Acute cholesterol-induced anti-natriuretic effects: role of epithelial Na+ channel activity, protein levels, and processing. J. Biol. Chem. 2011;286:1683–1695. doi: 10.1074/jbc.M110.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales-Lázaro SL, Rosenbaum T. Multiple mechanisms of regulation of transient receptor potential ion channels by cholesterol. Curr. Top. Membr. 2017;80:139–161. doi: 10.1016/bs.ctm.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Di Scala C, et al. Mechanism of cholesterol‐assisted oligomeric channel formation by a short Alzheimer β‐amyloid peptide. J. Neurochem. 2014;128:186–195. doi: 10.1111/jnc.12390. [DOI] [PubMed] [Google Scholar]
- 9.Nelson JC, Saven JG, Moore JS, Wolynes PG. Solvophobically driven folding of nonbiological oligomers. Science. 1997;277:1793–1796. doi: 10.1126/science.277.5333.1793. [DOI] [PubMed] [Google Scholar]
- 10.Bong DT, Clark TD, Granja JR, Ghadiri MR. Self-assembling organic nanotubes. Angew. Chem. Int. Ed. 2001;40:988–1011. doi: 10.1002/1521-3773(20010316)40:6<988::AID-ANIE9880>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Lopez S, et al. Antibacterial agents based on the cyclic D,L-α-peptide architecture. Nature. 2001;412:452–455. doi: 10.1038/35086601. [DOI] [PubMed] [Google Scholar]
- 12.Gokel GW, Mukhopadhyay A. Synthetic models of cation-conducting channels. Chem. Soc. Rev. 2001;30:274–286. doi: 10.1039/b008667n. [DOI] [Google Scholar]
- 13.Das G, Talukdar P, Matile S. Fluorometric detection of enzyme activity with synthetic supramolecular pores. Science. 2002;298:1600–1602. doi: 10.1126/science.1077353. [DOI] [PubMed] [Google Scholar]
- 14.Sakai N, Mareda J, Matile S. Rigid-rod molecules in biomembrane models: from hydrogen-bonded chains to synthetic multifunctional pores. Acc. Chem. Res. 2005;38:79–87. doi: 10.1021/ar0400802. [DOI] [PubMed] [Google Scholar]
- 15.Sakai N, Mareda J, Matile S. Artificial beta-barrels. Acc. Chem. Res. 2008;41:1354–1365. doi: 10.1021/ar700229r. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino N, Satake A, Kobuke Y. An artificial ion channel formed by a macrocyclic resorcin[4]arene with amphiphilic cholic acid ether groups. Angew. Chem. Int. Ed. 2001;40:457–459. doi: 10.1002/1521-3773(20010119)40:2<457::AID-ANIE457>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Goto C, Yamamura M, Satake A, Kobuke Y. Artificial ion channels showing rectified current behavior. J. Am. Chem. Soc. 2001;123:12152–12159. doi: 10.1021/ja010761h. [DOI] [PubMed] [Google Scholar]
- 18.Sakai N, et al. Dendritic Folate Rosettes as Ion Channels in Lipid Bilayers. J. Am. Chem. Soc. 2006;128:2218–2219. doi: 10.1021/ja058157k. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Jing B, Regen SL. Kinetic Evidence for the Existence and Mechanism of Formation of a Barrel Stave Structure from Pore-Forming Dendrimers. J. Am. Chem. Soc. 2003;125:13984–13987. doi: 10.1021/ja036390h. [DOI] [PubMed] [Google Scholar]
- 20.Chen W-H, Shao X-B, Regen SL. Poly(choloyl)-Based Amphiphiles as Pore-Forming Agents: Transport-Active Monomers by Design. J. Am. Chem. Soc. 2005;127:12727–12735. doi: 10.1021/ja053527q. [DOI] [PubMed] [Google Scholar]
- 21.McNally BA, O’Neil EJ, Nguyen A, Smith BD. Membrane Transporters for Anions That Use a Relay Mechanism. J. Am. Chem. Soc. 2008;130:17274–17275. doi: 10.1021/ja8082363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Melegari M, Colombini M, Davis JT. Large and stable transmembrane pores from Guanosine−Bile acid conjugates. J. Am. Chem. Soc. 2008;130:2938–2939. doi: 10.1021/ja7110702. [DOI] [PubMed] [Google Scholar]
- 23.Helsel AJ, et al. Highly conducting transmembrane pores formed by aromatic oligoamide macrocycles. J. Am. Chem. Soc. 2008;130:15784–15785. doi: 10.1021/ja807078y. [DOI] [PubMed] [Google Scholar]
- 24.Yamnitz CR, Negin S, Carasel IA, Winter RK, Gokel GW. Dianilides of dipicolinic acid function as synthetic chloride channels. Chem. Commun. 2010;46:2838–2840. doi: 10.1039/b924812a. [DOI] [PubMed] [Google Scholar]
- 25.Otis F, Racine-Berthiaume C, Voyer N. How far can a sodium ion travel within a lipid bilayer? J. Am. Chem. Soc. 2011;133:6481–6483. doi: 10.1021/ja110336s. [DOI] [PubMed] [Google Scholar]
- 26.Matile S, Jentzsch AV, Montenegro J, Fin A. Recent synthetic transport systems. Chem. Soc. Rev. 2011;40:2453–2474. doi: 10.1039/c0cs00209g. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, et al. Self-assembling subnanometer pores with unusual mass-transport properties. Nat. Commun. 2012;3:949. doi: 10.1038/ncomms1949. [DOI] [PubMed] [Google Scholar]
- 28.Muraoka T, et al. Ion permeation by a folded multiblock amphiphilic oligomer achieved by hierarchical construction of self-assembled nanopores. J. Am. Chem. Soc. 2012;134:19788–19794. doi: 10.1021/ja308342g. [DOI] [PubMed] [Google Scholar]
- 29.Sakai N, Matile S. Synthetic Ion Channels. Langmuir. 2013;29:9031–9040. doi: 10.1021/la400716c. [DOI] [PubMed] [Google Scholar]
- 30.Vargas Jentzsch A, Hennig A, Mareda J, Matile S. Synthetic ion transporters that work with anion−π interactions, halogen bonds, and anion–macrodipole interactions. Acc. Chem. Res. 2013;46:2791–2800. doi: 10.1021/ar400014r. [DOI] [PubMed] [Google Scholar]
- 31.Gokel GW, Negin S. Synthetic ion channels: from pores to biological applications. Acc. Chem. Res. 2013;46:2824–2833. doi: 10.1021/ar400026x. [DOI] [PubMed] [Google Scholar]
- 32.Otis F, Auger M, Voyer N. Exploiting peptide nanostructures to construct functional artificial ion channels. Acc. Chem. Res. 2013;46:2934–2943. doi: 10.1021/ar400044k. [DOI] [PubMed] [Google Scholar]
- 33.Montenegro J, Ghadiri MR, Granja JR. Ion channel models based on self-assembling cyclic peptide nanotubes. Acc. Chem. Res. 2013;46:2955–2965. doi: 10.1021/ar400061d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fyles TM. How do amphiphiles form ion-conducting channels in membranes? Lessons from linear oligoesters. Acc. Chem. Res. 2013;46:2847–2855. doi: 10.1021/ar4000295. [DOI] [PubMed] [Google Scholar]
- 35.Gong B, Shao Z. Self-assembling organic nanotubes with precisely defined, sub-nanometer pores: formation and mass transport characteristics. Acc. Chem. Res. 2013;46:2856–2866. doi: 10.1021/ar400030e. [DOI] [PubMed] [Google Scholar]
- 36.Barboiu M, et al. An artificial primitive mimic of the Gramicidin-A channel. Nat. Commun. 2014;5:4142. doi: 10.1038/ncomms5142. [DOI] [PubMed] [Google Scholar]
- 37.De Riccardis F, Izzo I, Montesarchio D, Tecilla P. Ion transport through lipid bilayers by synthetic ionophores: modulation of activity and selectivity. Acc. Chem. Res. 2013;46:2781–2790. doi: 10.1021/ar4000136. [DOI] [PubMed] [Google Scholar]
- 38.Mosgaard LD, Heimburg T. Lipid ion channels and the role of proteins. Acc. Chem. Res. 2013;46:2966–2976. doi: 10.1021/ar4000604. [DOI] [PubMed] [Google Scholar]
- 39.Muraoka T, et al. Reversible ion transportation switch by a ligand-gated synthetic supramolecular ion channel. J. Am. Chem. Soc. 2014;136:15584–15595. doi: 10.1021/ja5070312. [DOI] [PubMed] [Google Scholar]
- 40.Saha T, et al. Hopping-mediated anion transport through a mannitol-based rosette ion channel. J. Am. Chem. Soc. 2014;136:14128–14135. doi: 10.1021/ja506278z. [DOI] [PubMed] [Google Scholar]
- 41.Kim DS, Sessler JL. Calix[4]pyrroles: versatile molecular containers with ion transport, recognition, and molecular switching functions. Chem. Soc. Rev. 2015;44:532–546. doi: 10.1039/C4CS00157E. [DOI] [PubMed] [Google Scholar]
- 42.Si W, Xin P, Li Z-T, Hou J-L. Tubular unimolecular transmembrane channels: construction strategy and transport activities. Acc. Chem. Res. 2015;48:1612–1619. doi: 10.1021/acs.accounts.5b00143. [DOI] [PubMed] [Google Scholar]
- 43.Sun Z, Barboiu M, Legrand Y-M, Petit E, Rotaru A. Highly selective artificial cholesteryl crown ether K+-channels. Angew. Chem., Int. Ed. 2015;127:14681–14685. doi: 10.1002/ange.201506430. [DOI] [PubMed] [Google Scholar]
- 44.Das RN, Kumar YP, Schütte OM, Steinem C, Dash J. A DNA-Inspired Synthetic Ion Channel Based on G–C Base Pairing. J. Am. Chem. Soc. 2015;137:34–37. doi: 10.1021/ja510470b. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, et al. Nonprotonophoric electrogenic Cl− transport mediated by valinomycin-like carriers. Chem. 2016;1:127–146. doi: 10.1016/j.chempr.2016.04.002. [DOI] [Google Scholar]
- 46.Sun Z, et al. Squalyl crown ether self-assembled conjugates: an example of highly selective artificial K+ channels. Chem. Eur. J. 2016;22:2158–2164. doi: 10.1002/chem.201503979. [DOI] [PubMed] [Google Scholar]
- 47.Huo Y, Zeng H. “Sticky”-ends-guided creation of functional hollow nanopores for guest encapsulation and water transport. Acc. Chem. Res. 2016;49:922–930. doi: 10.1021/acs.accounts.6b00051. [DOI] [PubMed] [Google Scholar]
- 48.Lang C, et al. Biomimetic transmembrane channels with high stability and transporting efficiency from helically folded macromolecules. Angew. Chem., Int. Ed. 2016;55:9723–9727. doi: 10.1002/anie.201604071. [DOI] [PubMed] [Google Scholar]
- 49.Wei X, et al. Persistent organic nanopores amenable to structural and functional tuning. J. Am. Chem. Soc. 2016;138:2749–2754. doi: 10.1021/jacs.5b12698. [DOI] [PubMed] [Google Scholar]
- 50.Benz S, et al. Anion transport with chalcogen bonds. J. Am. Chem. Soc. 2016;138:9093–9096. doi: 10.1021/jacs.6b05779. [DOI] [PubMed] [Google Scholar]
- 51.Choi YR, Lee B, Park J, Namkung W, Jeong K-S. Enzyme-responsive procarriers capable of transporting chloride ions across lipid and cellular membranes. J. Am. Chem. Soc. 2016;138:15319–15322. doi: 10.1021/jacs.6b10592. [DOI] [PubMed] [Google Scholar]
- 52.Saha T, Gautam A, Mukherjee A, Lahiri M, Talukdar P. Chloride transport through supramolecular barrel-rosette ion channels: lipophilic control and apoptosis-inducing activity. J. Am. Chem. Soc. 2016;138:16443–16451. doi: 10.1021/jacs.6b10379. [DOI] [PubMed] [Google Scholar]
- 53.Benke BP, et al. Iodide-selective synthetic ion channels based on shape-persistent organic cages. J. Am. Chem. Soc. 2017;139:7432–7435. doi: 10.1021/jacs.7b02708. [DOI] [PubMed] [Google Scholar]
- 54.Howorka S. Building membrane nanopores. Nat. Nanotechnol. 2017;12:619–630. doi: 10.1038/nnano.2017.99. [DOI] [PubMed] [Google Scholar]
- 55.Lang C, et al. Highly selective artificial potassium ion channels constructed from pore-containing helical oligomers. Angew. Chem. Int. Ed. 2017;129:12842–12845. doi: 10.1002/ange.201705048. [DOI] [PubMed] [Google Scholar]
- 56.Chen J-Y, Hou J-L. Controllable synthetic ion channels. Org. Chem. Front. 2018;5:1728–1736. doi: 10.1039/C8QO00287H. [DOI] [Google Scholar]
- 57.Ren CL, et al. A halogen bond-mediated highly active artificial chloride channel with high anticancer activity. Chem. Sci. 2018;9:4044–4051. doi: 10.1039/C8SC00602D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barboiu M. Encapsulation versus self-aggregation toward highly selective artificial K+ channels. Acc. Chem. Res. 2018;51:2711–2718. doi: 10.1021/acs.accounts.8b00311. [DOI] [PubMed] [Google Scholar]
- 59.Grauwels G, Valkenier H, Davis AP, Jabin I, Bartik K. Repositioning chloride transmembrane transporters: transport of organic ion pairs. Angew. Chem. Int. Ed. 2019;58:6921–6925. doi: 10.1002/anie.201900818. [DOI] [PubMed] [Google Scholar]
- 60.Xin P, et al. Artificial K+ channels formed by pillararene‐cyclodextrin hybrid molecules: tuning cation selectivity and generating membrane potential. Angew. Chem. Int. Ed. 2019;58:2779–2784. doi: 10.1002/anie.201813797. [DOI] [PubMed] [Google Scholar]
- 61.Zhang C, et al. Helical supramolecular polymer nanotubes with wide lumen for glucose transport: towards the development of functional membrane-spanning channels. Chem. Sci. 2019;10:8648–8653. doi: 10.1039/C9SC02336D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howe ENW, Gale PA. Fatty acid fueled transmembrane chloride transport. J. Am. Chem. Soc. 2019;141:10654–10660. doi: 10.1021/jacs.9b02116. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, et al. Voltage-switchable HCl transport enabled by lipid headgroup–transporter interactions. Angew. Chem. Int. Ed. 2019;58:15142–15147. doi: 10.1002/anie.201907466. [DOI] [PubMed] [Google Scholar]
- 64.Zeng LZ, Zhang H, Wang T, Li T. Enhancing K+ transport activity and selectivity of synthetic K+ channels via electron-donating effects. Chem. Commun. 2020;56:1211–1214. doi: 10.1039/C9CC08396K. [DOI] [PubMed] [Google Scholar]
- 65.Muraoka T, et al. A synthetic ion channel with anisotropic ligand response. Nat. Commun. 2020;11:2924. doi: 10.1038/s41467-020-16770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Debnath M, et al. Ionophore constructed from non-covalent assembly of a G-quadruplex and liponucleoside transports K+-ion across biological membranes. Nat. Commun. 2020;11:469. doi: 10.1038/s41467-019-13834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu J, et al. Reversible ligand-gated ion channel via the interconversion between hollow single helix and intertwined double helix. Angew. Chem. Int. Ed. 2020;59:13602. doi: 10.1002/anie.201916755. [DOI] [PubMed] [Google Scholar]
- 68.Huang W-L, Wang X-D, Ao Y-F, Wang Q-Q, Wang D-X. Artificial chloride-selective channel: shape and function mimic of the clc channel selective pore. J. Am. Chem. Soc. 2020;142:13273–13277. doi: 10.1021/jacs.0c02881. [DOI] [PubMed] [Google Scholar]
- 69.Tripathi P, et al. Rosette nanotube porins as ion selective transporters and single-molecule sensors. J. Am. Chem. Soc. 2020;142:1680–1685. doi: 10.1021/jacs.9b10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi S, et al. Foldamer-based potassium channels with high ion selectivity and transport activity. J. Am. Chem. Soc. 2021;143:3284–3288. doi: 10.1021/jacs.0c12128. [DOI] [PubMed] [Google Scholar]
- 71.Ren C, et al. Pore-forming monopeptides as exceptionally active anion channels. J. Am. Chem. Soc. 2018;140:8817–8826. doi: 10.1021/jacs.8b04657. [DOI] [PubMed] [Google Scholar]
- 72.Zeng F, et al. A pore-forming tripeptide as an extraordinarily active anion channel. Org. Lett. 2019;21:4826–4830. doi: 10.1021/acs.orglett.9b01723. [DOI] [PubMed] [Google Scholar]
- 73.Yuan L, Shen J, Ye RJ, Chen F, Zeng HQ. Structurally simple trimesic amides as highly selective anion channels. Chem. Commun. 2019;55:4797–4800. doi: 10.1039/C9CC00248K. [DOI] [PubMed] [Google Scholar]
- 74.Ren C, Shen J, Zeng H. Combinatorial evolution of fast-conducting highly selective K+-channels via modularly tunable directional assembly of crown ethers. J. Am. Chem. Soc. 2017;139:12338–12341. doi: 10.1021/jacs.7b04335. [DOI] [PubMed] [Google Scholar]
- 75.Ye R, et al. Molecular ion fishers as highly active and exceptionally selective K+ transporters. J. Am. Chem. Soc. 2019;141:9788–9792. doi: 10.1021/jacs.9b04096. [DOI] [PubMed] [Google Scholar]
- 76.Shen J, et al. Aquafoldmer-based aquaporin-like synthetic water channel. J. Am. Chem. Soc. 2020;142:10050–10058. doi: 10.1021/jacs.0c02013. [DOI] [PubMed] [Google Scholar]
- 77.Shen J, Ye RJ, Zeng HQ. Crystal packing-guided construction of hetero-oligomeric peptidic ensembles as synthetic 3-in-1 transporters. Angew. Chem. Int. Ed. 2021;60:12924–12930. doi: 10.1002/anie.202101489. [DOI] [PubMed] [Google Scholar]
- 78.Chen F, et al. Pyridine/oxadiazole‐based helical foldamer ion channels with exceptionally high K+/Na+ selectivity. Angew. Chem. Int. Ed. 2020;59:1440–1444. doi: 10.1002/anie.201906341. [DOI] [PubMed] [Google Scholar]
- 79.Roy A, et al. Polyhydrazide-based organic nanotubes as efficient and selective artificial iodide channels. Angew. Chem. Int. Ed. 2020;59:4806–4813. doi: 10.1002/anie.201916287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li N, Shen J, Ang GK, Ye R, Zeng HQ. Molecular tetrahedrons as selective and efficient ion transporters via a two-station swing-relay mechanism. CCS Chem. 2021;3:2269–2279. doi: 10.31635/ccschem.020.202000475. [DOI] [Google Scholar]
- 81.Roy A, et al. Foldamer-based ultrapermeable and highly selective artificial water channels that exclude protons. Nat. Nanotechnol. 2021;16:911–917. doi: 10.1038/s41565-021-00915-2. [DOI] [PubMed] [Google Scholar]
- 82.Ren C, et al. Molecular Swings as Highly Active Ion Transporters. Angew. Chem. Int. Ed. 2019;58:8034–8038. doi: 10.1002/anie.201901833. [DOI] [PubMed] [Google Scholar]
- 83.Lundbaek J, Birn P, Girshman J, Hansen A, Andersen O. Membrane stiffness and channel function. Biochemistry. 1996;35:3825–3830. doi: 10.1021/bi952250b. [DOI] [PubMed] [Google Scholar]
- 84.Van Den Bogaart G, Mika JT, Krasnikov V, Poolman B. The lipid dependence of melittin action investigated by dual-color fluorescence burst analysis. Biophys. J. 2007;93:154–163. doi: 10.1529/biophysj.107.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe H, et al. Analysis of pore formation and protein translocation using large biological nanopores. Anal. Chem. 2017;89:11269–11277. doi: 10.1021/acs.analchem.7b01550. [DOI] [PubMed] [Google Scholar]
- 86.Saigo N, Izumi K, Kawano R. Electrophysiological analysis of antimicrobial peptides in diverse species. ACS Omega. 2019;4:13124–13130. doi: 10.1021/acsomega.9b01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hille, B. Ion Channels of Excitable Membranes, 3rd ed., Sinauer Associates: Sunderland, MA, 2001.
- 88.Malla JA, et al. A glutathione activatable ion channel induces apoptosis in cancer cells by depleting intracellular glutathione levels. Angew. Chem. Int. Ed. 2020;59:7944–7952. doi: 10.1002/anie.202000961. [DOI] [PubMed] [Google Scholar]
- 89.Bai D, et al. Reversible ligand‐gated ion channel via interconversion between hollow single helix and intertwined double helix. Angew. Chem. Int. Ed. 2020;59:13602–13607. doi: 10.1002/anie.201916755. [DOI] [PubMed] [Google Scholar]
- 90.Gill H, et al. Supramolecular pore formation as an antimicrobial strategy. Coord. Chem. Rev. 2020;412:213264. doi: 10.1016/j.ccr.2020.213264. [DOI] [Google Scholar]
- 91.Antoszczak M, et al. Synthesis and antiproliferative activity of silybin conjugates with salinomycin and monensin. Chem. Biol. Drug Des. 2015;86:1378–1386. doi: 10.1111/cbdd.12602. [DOI] [PubMed] [Google Scholar]
- 92.Coghi P, et al. Novel peroxides as promising anticancer agents with unexpected depressed antimalarial activity. ChemMedChem. 2018;13:902–908. doi: 10.1002/cmdc.201700804. [DOI] [PubMed] [Google Scholar]
- 93.Faustino C, Serafim C, Rijo P, Reis CP. Bile acids and bile acid derivatives: use in drug delivery systems and as therapeutic agents. Expert Opin. Drug Deliv. 2016;13:1133–1148. doi: 10.1080/17425247.2016.1178233. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, et al. Bile acid receptors and liver cancer. Curr. Pathobiol. Rep. 2013;1:29–35. doi: 10.1007/s40139-012-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu SP, Canzoniero LM, Choi DW. Ion homeostasis and apoptosis. Curr. Opin. Cell Biol. 2001;13:405–411. doi: 10.1016/S0955-0674(00)00228-3. [DOI] [PubMed] [Google Scholar]
- 96.Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N. Ion channels in the regulation of apoptosis. Biochim. Biophys. Acta. 2015;1848:2532–2546. doi: 10.1016/j.bbamem.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 97.Yu SP, Choi DW. Ions, cell volume, and apoptosis. Proc. Natl Acad. Sci. (U. S. A) 2000;97:9360–9362. doi: 10.1073/pnas.97.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park S-H, et al. Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. J. Environ. Pathol. Toxicol. Oncol. 2014;33:219. doi: 10.1615/JEnvironPatholToxicolOncol.2014010923. [DOI] [PubMed] [Google Scholar]
- 99.Mohan S, et al. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: Its activation coupled with G0/G1 phase cell cycle arrest. J. Ethnopharmacol. 2010;131:592–600. doi: 10.1016/j.jep.2010.07.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. The datasets that support the finding of this study are available in figshare repository with the identifier(s) 10.6084/m9.figshare.20544585.v1. Source data are provided with this paper.