Highlights
-
•
An overview of systematic reviews on the application of AI including 129 studies.
-
•
AI use is prominent in Universal Health Coverage, featuring image analysis in neoplasms.
-
•
Half of the reviews did not evaluate validation procedures nor reporting guidelines.
-
•
Risk of bias was only included un a third of the reviews.
-
•
There is not sufficient evidence to transfer AI to actual healthcare delivery.
Keywords: Machine learning, Universal health coverage, Health emergencies, Health and well-being, European region
Abbreviations: AI, Artificial intelligence; BHW, Better Health and Well-being; DL, Deep Learning; EPW, European Programme of Work; HEP, Health Emergencies Protection; ML, Machine Learning; NL, Neural Learning; RL, Reinforcement Learning; SVM, Support Vector Machines; GPW13, The Thirteenth General Programme of Work; UHC, Universal Health coverage; WHO, World Health Organization
Abstract
Background
Artificial intelligence is fueling a new revolution in medicine and in the healthcare sector. Despite the growing evidence on the benefits of artificial intelligence there are several aspects that limit the measure of its impact in people’s health. It is necessary to assess the current status on the application of AI towards the improvement of people’s health in the domains defined by WHO’s Thirteenth General Programme of Work (GPW13) and the European Programme of Work (EPW), to inform about trends, gaps, opportunities, and challenges.
Objective
To perform a systematic overview of systematic reviews on the application of artificial intelligence in the people’s health domains as defined in the GPW13 and provide a comprehensive and updated map on the application specialties of artificial intelligence in terms of methodologies, algorithms, data sources, outcomes, predictors, performance, and methodological quality.
Methods
A systematic search in MEDLINE, EMBASE, Cochrane and IEEEXplore was conducted between January 2015 and June 2021 to collect systematic reviews using a combination of keywords related to the domains of universal health coverage, health emergencies protection, and better health and wellbeing as defined by the WHO’s PGW13 and EPW. Eligibility criteria was based on methodological quality and the inclusion of practical implementation of artificial intelligence. Records were classified and labeled using ICD-11 categories into the domains of the GPW13. Descriptors related to the area of implementation, type of modeling, data entities, outcomes and implementation on care delivery were extracted using a structured form and methodological aspects of the included reviews studies was assessed using the AMSTAR checklist.
Results
The search strategy resulted in the screening of 815 systematic reviews from which 203 were assessed for eligibility and 129 were included in the review. The most predominant domain for artificial intelligence applications was Universal Health Coverage (N = 98) followed by Health Emergencies (N = 16) and Better Health and Wellbeing (N = 15). Neoplasms area on Universal Health Coverage was the disease area featuring most of the applications (21.7 %, N = 28). The reviews featured analytics primarily over both public and private data sources (67.44 %, N = 87). The most used type of data was medical imaging (31.8 %, N = 41) and predictors based on regions of interest and clinical data. The most prominent subdomain of Artificial Intelligence was Machine Learning (43.4 %, N = 56), in which Support Vector Machine method was predominant (20.9 %, N = 27). Regarding the purpose, the application of Artificial Intelligence I is focused on the prediction of the diseases (36.4 %, N = 47). With respect to the validation, more than a half of the reviews (54.3 %, N = 70) did not report a validation procedure and, whenever available, the main performance indicator was the accuracy (28.7 %, N = 37). According to the methodological quality assessment, a third of the reviews (34.9 %, N = 45) implemented methods for analysis the risk of bias and the overall AMSTAR score below was 5 (4.01 ± 1.93) on all the included systematic reviews.
Conclusion
Artificial intelligence is being used for disease modelling, diagnose, classification and prediction in the three domains of GPW13. However, the evidence is often limited to laboratory and the level of adoption is largely unbalanced between ICD-11 categoriesand diseases. Data availability is a determinant factor on the developmental stage of artificial intelligence applications. Most of the reviewed studies show a poor methodological quality and are at high risk of bias, which limits the reproducibility of the results and the reliability of translating these applications to real clinical scenarios. The analyzed papers show results only in laboratory and testing scenarios and not in clinical trials nor case studies, limiting the supporting evidence to transfer artificial intelligence to actual care delivery.
1. Introduction
The Thirteenth General Programme of Work (GPW 13) defines the World Health Organization’s (WHO) strategy for the period between 2019 and 2023 which focuses on measurable impacts on people’s health [1]. Based on the GPW 13, the core priorities and the roadmap for the 53 countries of the WHO European region are described in the European Programme of Work 2020–2025 (EPW) [2]. The GPW 13 and the EPW aim to transform public health, focusing on measurable impacts on people’s health at the national level with three core features: enhanced Universal Health coverage (UHC), Health Emergencies Protection (HEP), and Better Health and Well-being (BHW).
The UHC domain involves primary care, community care and person-centered health systems [3]. Health promotion and disease prevention are the key principles in which UHC should be constructed and maintained. Furthermore, primary care services should also ensure access to curative, rehabilitative and palliative health services regardless the geographical location and the financial status [4]. The HEP domain is focused on preparing health care systems to better react when a health emergency is declared, this is to provide adequate and timely services that range from disease prevention to life-saving interventions. The BHW domain is focused on improving the general health and wellbeing of people, involving the prevention of noncommunicable disease, promoting mental health, minimizing and eradicating high impact communicable disease and addressing the health effects of climate change.
Artificial intelligence (AI) is a discipline which seeks to reproduce human-like ways of perceiving, reasoning, learning, and solving problems and is an area of interest in clinical applications [5]. AI expands traditional statistical techniques, allowing to extract information to support decision-making and research. These methods have been deployed in many clinical research areas and technological domains [6], [7], [8].
AI has been implemented in healthcare over a wide typology of clinical applications, for example, from molecular and genetic testing to medical images of different modalities, diagnostic codes and social media [9]. The ultimate goal of AI is to learn and identify associations between data and outcomes of interest [10]. AI needs data generated from healthcare activities such as diagnosis, treatments and follow-up to develop, test and validate algorithms. Digitalized data in healthcare is available in a wide range of formats, including structured and non-structured schemas [11].
The landscape of AI methods can be divided in four main categories: regression and probabilistic methods, machine learning, deep learning and reinforcement learning [12], [13]. One of the most prominent areas of AI is Machine Learning (ML), in which models can adapt to improve their performance according to the changes in the data and the experiences [14]. ML algorithms combine the strengths of computer science and data science to find the optimal fit between theoretical approaches and data-driven solutions, enabling the development of tools that can solve problems human cannot do in reasonable timespans. The basic principle relies in the ability of the model to predict the output whenever new input data is given and thus inform about possible scenarios to understand the information. There are two major approaches in ML, the supervised learning to solve problems of classification and regression based on sets of labelled input data and the unsupervised learning, which seeks to find patterns in sets of unexplained and unlabeled data.
Linear and logistic regression, Support Vector Machines (SVM) and decision trees are relevant techniques in the supervised approach. An extended use of unsupervised learning focuses on finding associations of data in clusters and the identification of principal components that explain multidimensional data [15]. These associations do not consider the outcome information but the nature of the input data, providing categories of patients based on their similarities. Typical algorithms for clustering are k-means and hierarchical clustering. The regular pipeline involves using unsupervised learning to pre-process data and select features that explain the nature of data, to thereafter apply supervised learning to provide clinically relevant results. These modelling techniques pursue to minimize the classification (or misclassification) error (e.g.: the quadratic loss function).
The evolution of ML is known as Neural Learning (NL) and has the ability of generating multiple non-linear combinations of data for creating exhaustive models. This approach combines artificial neural networks that replicate the structure and behavior of a human brain in the way it connects several processing units in multiple layers to adjust their configuration based on the data. Artificial neurons are grouped into layers driving signals from the input to the output. The combination of both ML and NL has led to the definition of Deep Learning (DL), which consists in a hierarchical combination of processing units grouped and connected into layers to transform and extract information [16]. DL approach conducted to the definition of Reinforcement Learning (RL) in which the algorithms learn actions based on the maximization of a predefined reward [17]. The basic principle of RL is based on the interaction of an agent that makes decisions and its environment with the goal of reaching preferable states. After every interaction the agent receives feedback, which can be positive (a reinforcement) or negative, and then RL model will prosecute the actions that maximizes the number of positive rewards.
AI involves a wide variety of methods that expand traditional statistical techniques and can find patterns that support the process of decision making as well as the formulation of hypotheses in the domains of UCH, EHP and BHW. AI can provide powerful tools to automate tasks and to support and inform clinicians, epidemiologists and policy makers on what are the most efficient strategies to promote health at a population and individual level. But, due to the broad range of applications of AI in healthcare is necessary to assess the current status on the application of AI and in which way they can improve people’s health. This overview of systematic reviews has the objective of providing a comprehensive landscape on the most recent evidence on the application of AI to in the three domains defined by the GPW 13 and the EPW. The overview includes regression algorithms, machine learning, deep learning and reinforcement learning approaches, and their application in any medical and clinical specialty domain. The selection criteria are defined as real AI applications on health and care services that can be transferred to real clinical scenarios. The ultimate goal is to provide a comprehensive and updated map on the fields of application of AI to improve people’s health and reveal the medical prominent specialties, the modelling techniques, what type of data is used and, importantly, the methodological quality of the recent scientific literature.
2. Methods
2.1. Search strategy
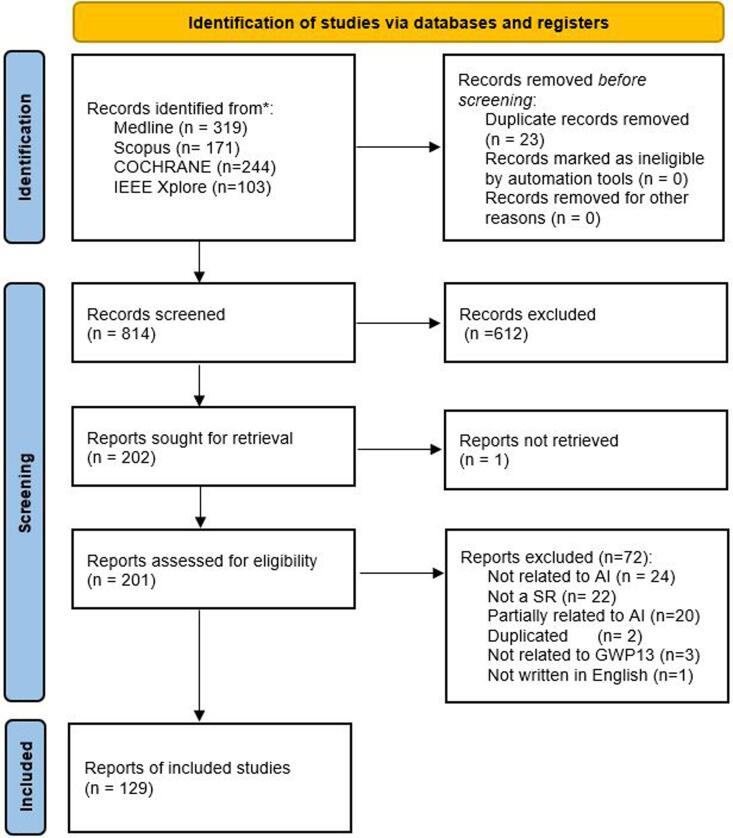
A systematic literature search on systematic reviews featuring qualitative and/or meta-analysis was conducted using four electronic databases: MEDLINE, IEEE Xplore, EMBASE and COCHRANE. The search string included a combination of keywords (artificial intelligence OR machine learning OR deep learning) AND (universal health coverage OR emergency care OR public health) and the search queries listed in Appendix I. The Preferred Reporting Items for Systematic reviews and meta-analyses (PRISMA 2020 statement) [18] was used to funnel the article selection process (Fig. 1). From the total articles (n = 837), duplicated entries were removed (n = 23). No additional studies were excluded using automation tools or because of other reasons. Afterwards, the hits were screened based on the title and abstract to identify relevant studies matching three basic criteria: 1) Being a Systematic Review; 2) Analysis of Artificial Intelligence modelling; 3) Related to one domain of the GPW 13 and the EPW. Once the relevant articles were identified (n = 203), a full-text review was performed to assess their eligibility. Four articles were excluded because of the following reasons: not in English (n = 1), duplicated (n = 2) and not retrieved – it was not possible to find the full publication available – (n = 1). A total of 72 articles were excluded after the in-depth assessment because the entry was not related or partially related to AI (n = 24) (the paper is not focused on AI development or validation), it was not a systematic review (n = 21), they were partially related to AI (n = 20), duplicated studies (n = 2), not related to the GPW 13 and EPW domains (n = 3) and not written in English (n = 1). Two authors participated in the abstract screening and full- text review. When authors did not reach a consensus a third author evaluated the possible exclusion to break the deadlock.
Fig. 1.
Systematic Reviews selection process.
2.2. Eligibility criteria
Systematic reviews were included in the overview if they reported on the implementation and evaluation of artificial intelligence in a health or disease area related to universal health coverage, emergencies and better health and wellbeing. Systematic reviews covering a specific medical technology (e.g.: radiology) were included in the analysis. Studies on data-driven models, natural language processing and image processing were included only if they explicitly designated a relationship with a disease or a health management area. Editorials, protocols and reports were excluded. Also, studies not following the methodology of a systematic review were excluded.
2.3. Data extraction
The following data were extracted systematically for all the studies: authors, title, year, journal, GPW 13 and EPW domain, relevant type of disease according to the International Code of Disease, version eleven (ICD-11), number of included studies, type of data source (public/private), data types used by the models, predictors used by the models (input data categories), outcome of the model (screening, diagnose, classification, treatment), type of modelling (regression based, machine learning, deep learning or reinforcement learning), predominant modelling technique, validation methodology (clinical trial, case study or statistical appraisal), performance indicators and risk of bias assessment. Two authors participated in the data extraction and classification of the articles. When authors did not reach a consensus a third author evaluated the entry and assigned the correspondent label.
2.4. Quality assessment
Included studies were evaluated with the Assessment of Multiple Systematic Reviews checklist (AMSTAR) [19] to assess the methodological quality of the systematic review. Every item of the AMSTAR checklist was scored with 1 point if it was successfully reported and 0 points otherwise.
3. Results
The analysis of the systematic reviews was divided into the three domains of the GPW 13 and the EPW priorities, that is 98 in universal health coverage, 16 in health emergencies protection and 15 in better health and wellbeing. Appendix II contains the complete list of the systematic reviews included in the overview, their domain and related ICD-11 chapter and the number of included studies (qualitative and meta-analysis). Appendix III presents a descriptive analysis of the systematic review with respect to the health/disease area, the type of data and modelling technique and the highlight challenges and opportunities. Appendix IV depicts the descriptive analysis for each GPW13- EPW domain of the extracted data (quantitative and qualitative). Appendix V contains the AMSTAR evaluation scores and the individual quality of each review included in the analysis.
With respect to the domain of universal health coverage, neoplasms are the predominant disease application (N = 28), followed by mental and behavioral disorders (N = 17), diseases of the circulatory system (N = 9) and the musculoskeletal system (N = 8). In the domain of emergencies, the predominant disease category is infectious or parasitic diseases (N = 13) and in the domain of better health and wellbeing all fall into the category of factors influencing health status or contact with health services (N = 14). The studies included heterogeneous designs, focusing on different approaches to the health promotion and the disease management, sources of data, target population, predictors and assessment of the outcomes. The following subsections describe the collated results of the review for each of the GPW 13 and the EPW domains.
3.1. Artificial intelligence for areas related to universal health coverage
The application of AI in UHC domain is mainly focused on neoplasms (N = 28) [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47] and secondary on mental health (N = 17) [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64] as depicted in Fig. 2. There is an intermediate group including diseases of the circulatory system (N = 9) [65], [66], [67], [68], [69], [70], [71], [72], [73], diseases of the musculoskeletal system (N = 8) [74], [75], [76], [77], [78], [79], [80], [81], diseases of the digestive system (N = 7) [82], [83], [84], [85], [86], [87], [88] and the nervous system (N = 7) [89], [90], [91], [92], [93], [94], [95]. There are a few reviews focused on other categories such as diseases of respiratory system (N = 4) [96], [97], [98], [99], visual system (N = 3) [100], [101], [102] and the other chapters include only one or two systematic reviews [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116]. The number of studies included in the reviews is 41.29 ± 41.9 (mean ± standard deviation) with an IQR = [16–47]. In the predominant categories, the number of reviews is sparse with 34.23 ± 31.78 for neoplasms, 43.00 ± 36.97 for factors influencing health and 57.93 ± 73.29 for mental health. Only the 20 of these reviews included a meta-analysis, 5 of them belonged to the disease area of neoplasms while the other categories included only one or two meta-analysis.
Fig. 2.
Dashboard and descriptive analytics on the use of AI in areas related to Universal Health Coverage.
The studies included in the reviews featured analytics primarily over public and private data sources (68.37 %) and less on public data (28.57 %). Only a 3.06 % did not disclose the sources of data. Medical imaging (38.78 %) is the most used type of data, followed by clinical data from laboratory tests and examinations (15.31 %), registries from clinical studies (11.22 %) and electronic health records (10.20 %). Some reviews used mixed types of data, but these account for the 8.16 % of them. Other reviews were focused on social media data (4.08 %) and genomic data (3.06 %). With respect to the predictors (the specific type of variables used develop the models), the majority is based on Regions of Interest (39.8 %) and mixed predictors such as imaging features, clinical assessment and notes (15.3 %). The reviews analyzed similarly clinical and demographic variables (6.12 %) and signal features and vital signs (6.12 %). A few reviews were focused on the analysis of notes (5.1 %) and histological data (4.1 %). Importantly, less than a quarter of the reviews did not disclose the type of predictors used by the models (23.5 %).
In the domain of UHC the application of AI is focused on the prediction of the diseases (32.65 %) and the detection (24.49 %). Some reviews focused on the classification of diseases degrees and severity scales (18.37 %). A few reviews focused on segmentation (4.08 %) and other unspecific outcomes (6.12 %). Machine learning techniques accounted for the 44.9 % of the types of AI, and their combination with regression methods (23.47 %) and deep learning techniques (16.33 %). Deep learning was used in the 13.27 % of the reviews. The predominant modelling technique was Support Vector Machines (24.49 %) and Convolutional Neural Networks (20.41 %).
With respect to the validation, slightly more than a half (52.04 %) did not report any validation procedure. Almost a third of the reviews reported internal validation (32.65 %), only 5.1 % reported external validation, and 12.24 % reported both internal and external validation. When described, the main performance indicator was de performance (31.63 %), followed by a compendium of indicators of C statistic (AUC), sensitivity and specificity (24.49). The AUC was reported as the single indicator in the 20.41 % of the reviews. and the sensitivity and specificity on the 12.24 %,
Regarding the quality assessment, the 61.22 % of the reviews did not implement any method for analyzing the risk of bias, and for the remaining 38.78 % of reviews performing it, QUADAS-2 was the most used method (17.35 %). From the 71 qualitative analysis, 19 (26.7 %) performed a quality assessment, and from the 27 meta-analysis (some studies included a qualitative and a meta-analysis), 20 (74.1 %) performed a quality assessment. The overall AMSTAR score was low (4.05 ± 1.99), with a few reviews yielding scores over 5 points. Table 1 in the Appendix III contains the complete descriptive analysis of the systematic reviews classified into areas related to Universal Health Coverage domain.
3.2. Artificial intelligence for areas related to health emergencies protection
The application of AI in HEP is mainly focused on infectious or parasitic diseases (N = 13) [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128] and secondary on factors influencing health (N = 2) [129], [130] and mental health (N = 1) [131], as depicted in Fig. 3. SARS-CoV-2 was the main topic on 10 of these reviews. The number of studies included in the reviews is 46.75 ± 44.66 with an IQR = [16–65.5]. In the predominant category, the number of reviews is 52.46 ± 47.98. Only the 12.5 % of these reviews include meta-analysis, one of them belonged to infectious diseases and the other according to factors influencing health.
Fig. 3.
Dashboard and descriptive analytics on the use of AI in areas related to Health Emergencies Protection.
The studies included in the reviews featured primarily over public and private data sources (75 %) and less on public data (25 %). In this case, the most used typed of data is clinical data (25 %), followed by medical imaging (18.75 %), social media data (12.5 %), laboratory test (6.25 %) and empirical data (6.25 %). Besides, some reviews used mixed types of data (18.75 %). With respect to the predictors, the majority is based on regions of interest (25 %), followed by analysis of notes (12.5 %), clinical or demographic variables (12.5 %), genome sequences (6.25 %) and mixed predictors (6.25 %). Nevertheless, 37.5 % of the reviews did not disclose the type of predictors used by the models.
On the other hand, the application of AI in the domain of Health Emergencies Protection is focused on the prediction of the diseases (50 %), followed by the detection (25 %) and classification of diseases degrees and severity scales (25 %). Machine learning and regression techniques accounted for the 43.75 % of the types of AI, 25 % of machine learning and deep learning methods and 18.75 % of only machine learning. The predominant modelling technique was Convolutional Neural Networks (18.75 %) and Logistic regression (12.5 %).
With respect to the validation, more than a half (56.25 %) did not report any validation procedure. A quarter of the reviews (25 %) reported both internal and external validation, and 18.75 % reported only internal validation. No external validation was reported. In addition, the main performance indicator was the accuracy (25 %) and AUC (25 %), followed by a compendium of indicators (12.5 %) such as sensitivity and specificity, C statistic and R2.
Regarding the quality assessment, the 75 % of the reviews did not implement any method for analyzing the risk of bias. From the 14 qualitative analysis, 3 (21.4 %) performed a quality assessment, and from the 2 meta-analysis, only one (50 %) performed a quality assessment. The PROBAST tool was the most used method (17.35 %). The overall AMSTAR score was low (4.06 ± 1.98), with a few reviews yielding scores over 5 points. Table 2 in the Appendix III contains the complete descriptive analysis of the systematic reviews classified into health emergencies protection.
3.3. Artificial intelligence for areas related to a better health and Well-being
The application of AI in BHW domain is mainly focused on factors influencing health (N = 14) [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145] and secondary on mental health (N = 1) [146], as depicted in Fig. 4. The number of studies included in the reviews is 62 ± 66.36 with an IQR = [18–81.25]. In the predominant category, the number of reviews is 65.21 ± 67.65. No review included meta-analysis.
Fig. 4.
Dashboard and descriptive analytics on the use of AI in areas related to a Better Health and Wellbeing.
The studies included in the reviews featured analytics primarily over public and private data sources (53.3), followed by public data (40 %) and less on private data (6.7 %). Electronic health record (33.3) is the most used typed of data, followed by clinical data (20 %) and environmental data (13.3 %). Other reviews were focused on social media data (6.7 %) and mixed types of data (6.7 %). According to predictors, the majority is based on analysis of notes (40 %), followed by clinical variables (6.7 %) and mixed predictors (6.7 %). Importantly, almost the half (46.7 %) of the reviews did not disclose the type of predictors used by the models.
In the domain of Better health and Wellbeing, the application of AI is focused on the prediction of the diseases (46.7 %), secondary on detection (33.3 %) and classification of diseases degrees and severity scales (13.3 %). Machine learning techniques accounted the 60 % of the types of AI and their combination with deep learning (26.7 %). The predominant modelling technique was Natural Language Processing (NLP) (33.3 %), followed by Support Vector Machine (13.3 %) and Neural Networks (13.3 %).
Regarding the validation techniques, more than a half (66.7 %) did not report any validation procedure. A quarter of the reviews (26.7 %) reported internal validation and only 6.7 % reported both internal and external validation. The main performance indicator was a compendium of indicators (20 %) such as AUC, sensitivity and specificity, followed by the accuracy (13.3 %).
When it comes to the quality assessment, the 80 % of the reviews did not implement any method for analysis the risk of bias. None of the included studies in BHW performed a meta-analysis. Cochrane’s tool was the most used method (13.3 %). The overall AMSTAR score was low (3.67 ± 1.54), with a few reviews yielding scores over 5 points. Table 1 in the Appendix III contains the complete descriptive analysis of the systematic reviews classified into BHW.
4. Discussion
Despite the recent increase in the AI literature and the publication of systematic reviews about AI applications in healthcare, the research in this field is focused on certain diseases. The type of data which algorithms are implemented with and the availability in public repositories could be a possible cause. Neoplasms is one of the most prominent areas due to the use of different medical image modalities and the recent advances in image processing techniques with DL approaches, moreover, because an accurate and on-time diagnose is crucial to determine the treatment and minimize the causes that lead to the death in neoplasms. One of the most recurrent applications of AI is the early diagnosis (predictions) and classification of disease severity, which can be improved by using other data sources such as Electronic Medical Reports. The impact of AI in care delivery in the chosen domains cannot be measured as all the reviewed studies featured results in laboratory settings and did not include clinical evaluation.
The effective management of public health systems is a multifactorial responsibility with a wide range of actors and effects. Like any other medical act, the provision of care services at a populational level involves the screening and diagnosis of certain conditions, the treatment and the follow-up of those conditions [71], [95], [115]. In this context, the use of AI has demonstrated that it can provide powerful tools to support and inform decisions and even automate tasks to aid clinicians, epidemiologists and policy makers on the most efficient strategies to promote health at a population level [137], including the current COVID-19 pandemic [125]. However, despite the great advances and the high level of maturity of AI in certain clinical domains, the review of systematic reviews leads to conclude that the use of AI is still scarce in clinical practice and depends strongly upon the clinical application domain [58], [72]. Published evidence mainly consists of tests in laboratory settings and early-phase validation of ML and DL models.
This overview is a broad summary on the development and application status of AI in healthcare but also stands as an assessment on the quality of the systematic reviews in the use of AI. The use of reporting guidelines on the methodology related to the elaboration and selection of papers for the systematic review is a good practice implemented in the vast majority of the reviews. However, the quality assessment of individual studies is only implemented in a minority of them. Quality assessment tools and risk of bias methods are an utmost important tool to understand the level of evidence reached by the authors and the real impact on health outcomes. Recently, there have been important updates on standard reporting guidelines such as the CONSORT-AI for clinical trial reports involving AI [147], the SPIRIT-AI for clinical trial protocols involving AI [148], the MI-CLAIM checklist on minimum information about clinical AI modelling [149] and the PROBAST tool to assess the risk of bias and applicability of prediction model studies [150]. These updates and recommendations address important issues related to the development, implementation and evaluation of clinical interventions based on AI in a broad set of factors identified in this overview. Individual studies and systematic reviews should describe the intended workflow in the use of the AI intervention, with a specific statement on the disease context, intended users and the purpose of the intervention.
Despite the rapid growth and generalization of AI in several fields of medicine, this overview shows that there is a limited level of maturity on its use in clinical practice. None of the analyzed reviews reported studies on the real impact of these tools in real clinical scenarios. This outcome shows an important gap between the development of the models in laboratory settings and the implementation of this models under real conditions. However, in some specific clinical areas, the level of maturity of the models is unarguably high and the current body of literature shows consistent results in specific indicators, methodologies, and comparison metrics. These situations should ease the transference of these models to improve the current techniques and protocols used for the assessment of disease conditions.
The type of data used to implement AI models is heterogeneous and frequently not consistent across the same type of clinical domains and applications [151]. Diagnostic medical imaging is the principal data source for Neoplasms, Infections and Surgery among others and constitutes the main trend of research in the application of AI. The analysis of time series and natural language processing from social networks and medical records are emerging fields of research and an interesting field for the development of AI.
4.1. Challenges
One of the common pitfalls identified in this overview is the lack of standardization protocol designs on the interventions of AI, including the approaches to perform the statistical analysis of the outcomes. The high level of heterogeneity found in the approaches to select observable variables, outcomes and the performance analysis makes impossible to compare disease-specific cut-off points. The challenge of standardization is not new in clinical research and should be taken under deep consideration when referring to the use of AI in medicine [40]. It is common to find studies which are biased by imbalanced classes, specifically when the study includes limited sample sizes and outcomes that are difficult to measure (e.g.: mental health, suicide, etc.). This affects the interpretation of the results because it does not show the ability of the models to discriminate positive and negative cases. Another common challenge found in the systematic reviews is the heterogeneity of software infrastructures used to collect, store, and analyze personal and clinical data.
One of the most recurrent issues in the analyzed reviews is the scarcity of data and the risk of model overfitting, especially in models developed for medical imaging processing. Penalization techniques are recommended to address overfitting in models, but they should be applied carefully depending on the data size. Penalization techniques could be an unreliable choice when the sample size is small [152].
The use of performance metrics to compare and evaluate AI models is common in the revised systematic reviews. Study design limitations in the statistical analysis can lead to wrong conclusions and under/over-estimations in the accuracy and the classification ability of the models, however, the quality of this models rely on the data used to train and validate them. Beyond issues of data heterogeneity and availability, understanding the data biases applied in the model development is crucial.
Another challenge of AI in clinical practice is that it must show acceptable and reproductible results. Limitations in the reporting of AI models are a challenge to homogenize the use of AI in research and how to adapt the specific models to the particular needs of clinical units and populations in terms of healthcare service characteristics [101], [111]. One of the modelling approaches most frequently used are the decision trees because of its performance and its ability to illustrate in human understandable way how a decision was made. In opposite, other modelling techniques have no ability to indicate how the decision was made without falling into a (frequently) complex mathematical formulation [110].
4.2. Opportunities
The overview has also spotted many opportunities in the field of AI and medicine. Future studies should include deeper considerations on the pathophysiology of the specific disease when designing the protocol for the intervention. The incorporation of a control group and using cross-validation will increase the evidence and the credibility on these types of studies [39]. Despite not having found real applications in clinical contexts in which AI drives a decision. Many reviews conclude that ML will play an important role helping clinicians to identify specific indicators and this will lead to a better diagnose, treatment and outcomes.
Open databases and basic principles of data sharing will be paramount to develop and implement AI models, allowing to reproduce results, compare the accuracy of different methods and approaches and confirming scientific findings [153]. The main barrier to modelling lies between laboratory conditions and free-living clinical and practical environments, but still the incorporation of contextual factors will ease the transference of these models to real life scenarios. Digital data interventions have the opportunity to enhance population’s health and wellbeing, health coverage and protection from emergencies, but they should be boosted by the application of ML algorithms in population-based clinical decision making with the use of Big Data and new communication technologies.
The degree of interdisciplinarity of the research teams has demonstrated to be an influential factor in the quality of the research, as demonstrated in the clinical case of thoracic cancer [154]. Studies driven by a clinical meaningful need, supported by an adequate design and focused on a clinical practice target will generate more transferable results. The implementation of sustained educational training programs, such as diplomas, Masters and PhD’s focused at healthcare professionals in collaboration with stakeholders from the engineering fields will allow to build new capacities and teams to spread the theories and findings of AI based tools in medicine [155]. However, their effectiveness and trustworthiness will be only demonstrated through the implementation of well-designed clinical trials, and herein collaborative partnerships can play a significant role for sharing resources, knowledge and scientific expertise between countries to optimize training and research opportunities. An increment in the interdisciplinarity of teams will lead to a more mature and clinical practice-oriented research.
5. Conclusion
Artificial intelligence applications in the three domains of GPW13 have proved to increase our insights for disease modelling, diagnose, classification and prediction in a wide range of clinical domains and different scenarios. However, this evidence is often limited to laboratory and testing scenarios. Cross-sectional and longitudinal data from public repositories, clinical registries, clinical trials and other datasets is continuously being used to develop and validate AI models showing excellent results in the context of their respective study designs. However, there is a huge need of improving the methodological reporting of these studies and to improve the robustness of these models to consider variabilities from different sources. Explainable AI is a growing field of research that will respond to the needs of understanding how models are inferred from clinical and health data. The combination of AI modelling and explainable strategies will have a better clinical value in the diagnose and treatment of diseases, allowing healthcare systems to improve the quality of universal healthcare coverage, the responses to emergencies and to support healthier populations.
Authors’ contributions
AMM, VT, NAM, and DNO designed the study. AMM, ASS, and VT performed first- and second-stage screening, and extracted the presented data. VT solved any disagreements. AMM, ASS and VT carried out the quality assessment. AMM, ASS, RTC, VT, and DNO drafted the manuscript and its final version. All authors contributed to the article and approved the submitted version.
Disclaimer
DN-O and NA-M are staff members of the WHO. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the WHO.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijmedinf.2022.104855.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.13th General Programme of Work (GPW13). WHO Impact Framework, no. January, 2019.
- 2.WHO Regional Office for Europe, European Programme of Work 2020 - 2025, vol. 2023, no. September, pp. 1–25, 2020.
- 3.Moreno-Serra R., Smith P.C. Does progress towards universal health coverage improve population health? The Lancet. 2012;380(9845):917–923. doi: 10.1016/S0140-6736(12)61039-3. [DOI] [PubMed] [Google Scholar]
- 4.W.H. Organization, Delivering Quality Health Services: A Global Imperative, OECD Publishing, 2018. [DOI] [PMC free article] [PubMed]
- 5.Reddy S., Fox J., Purohit M.P. Artificial intelligence-enabled healthcare delivery. J. R. Soc. Med. 2019;112(1):22–28. doi: 10.1177/0141076818815510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynn L.A. Artificial intelligence systems for complex decision-making in acute care medicine: a review. Patient Saf. Surg. 2019;13(1):6. doi: 10.1186/s13037-019-0188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.N.R. Tadapaneni, Artificial Intelligence in Finance and Investments, Int. J. Innov. Res. Sci. Eng. Technol., 2020.
- 8.Jha K., Doshi A., Patel P., Shah M. A comprehensive review on automation in agriculture using artificial intelligence. Artificial Intell. Agric. 2019;2:1–12. [Google Scholar]
- 9.He J., Baxter S.L., Xu J., Xu J., Zhou X., Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019;25(1):30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzinger A., Langs G., Denk H., Zatloukal K., Müller H. Causability and explainability of artificial intelligence in medicine. Wiley Interdisciplinary Rev.: Data Min. Knowledge Discovery. 2019;9(4) doi: 10.1002/widm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.I.J. do Nascimento, et al., Impact of Big Data Analytics on People’s Health: Overview of Systematic Reviews and Recommendations for Future Studies, J. Med. Internet Res. 23(4) (2021) p. e27275, doi: 10.2196/27275. [DOI] [PMC free article] [PubMed]
- 12.Y. Lecun, Y. Bengio, G. Hinton, Deep learning, 2015, doi: 10.1038/nature14539. [DOI] [PubMed]
- 13.Yu K.-H., Beam A.L., Kohane I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018;2(10):719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- 14.Rajkomar A., Dean J., Kohane I. Machine learning in medicine. N. Engl. J. Med. 2019;380(14):1347–1358. doi: 10.1056/NEJMra1814259. [DOI] [PubMed] [Google Scholar]
- 15.C. Orphanidou, D. Wong, Machine learning models for multidimensional clinical data, in: Handbook of Large-Scale Distributed Computing in Smart Healthcare, Springer, 2017, pp. 177–216.
- 16.Wang F., Casalino L.P., Khullar D. Deep learning in medicine—promise, progress, and challenges. JAMA Intern Med. 2019;179(3):293–294. doi: 10.1001/jamainternmed.2018.7117. [DOI] [PubMed] [Google Scholar]
- 17.Petersen B.K., Yang J., Grathwohl W.S., Cockrell C., Santiago C., An G., Faissol D.M. Deep reinforcement learning and simulation as a path toward precision medicine. J. Comput. Biol. 2019;26(6):597–604. doi: 10.1089/cmb.2018.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.M.J. Page, et al., The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, Int. J. Surg. 88 (2021) p. 105906, doi: 10.1016/J.IJSU.2021.105906. [DOI] [PubMed]
- 19.B.J. Shea, et al., AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both, BMJ (Online) 358 (2017) pp. 1–9, doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed]
- 20.Z. Salod, Y. Singh, A five-year (2015 to 2019) analysis of studies focused on breast cancer prediction using machine learning: A systematic review and bibliometric analysis, J. Public Health Res. 9(1) (2020), doi: 10.4081/jphr.2020.1772. [DOI] [PMC free article] [PubMed]
- 21.Kothari G., Korte J., Lehrer E.J., Zaorsky N.G., Lazarakis S., Kron T., Hardcastle N., Siva S. A systematic review and meta-analysis of the prognostic value of radiomics based models in non-small cell lung cancer treated with curative radiotherapy. Radiother. Oncol. 2021;155:188–203. doi: 10.1016/j.radonc.2020.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Al Husaini M.A.S., Habaebi M.H., Hameed S.A., Islam M.R., Gunawan T.S. A Systematic Review of Breast Cancer Detection Using Thermography and Neural Networks. IEEE Access. 2020;8:208922–208937. doi: 10.1109/access.2020.3038817. [DOI] [Google Scholar]
- 23.Zhong J., Hu Y., Si L., Jia G., Xing Y., Zhang H., Yao W. A systematic review of radiomics in osteosarcoma: utilizing radiomics quality score as a tool promoting clinical translation. Eur. Radiol. 2021;31(3):1526–1535. doi: 10.1007/s00330-020-07221-w. [DOI] [PubMed] [Google Scholar]
- 24.Grothen A.E., Tennant B., Wang C., Torres A., Bloodgood Sheppard B., Abastillas G., Matatova M., Warner J.L., Rivera D.R. Application of Artificial Intelligence Methods to Pharmacy Data for Cancer Surveillance and Epidemiology Research: A Systematic Review. JCO Clin. Cancer Inform. 2020;(4):1051–1058. doi: 10.1200/CCI.20.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charalambides M., Singh S. Artificial intelligence and melanoma detection: friend or foe of dermatologists? Br. J. Hosp. Med. 2020;81(1):1–5. doi: 10.12968/hmed.2019.0322. [DOI] [PubMed] [Google Scholar]
- 26.Barua I., Vinsard D.G., Jodal H.C., Løberg M., Kalager M., Holme Ø., Misawa M., Bretthauer M., Mori Y. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53(03):277–284. doi: 10.1055/a-1201-7165. [DOI] [PubMed] [Google Scholar]
- 27.Jin P., Ji X., Kang W., Li Y., Liu H., Ma F., Ma S., Hu H., Li W., Tian Y. Artificial intelligence in gastric cancer: a systematic review. J. Cancer Res. Clin. Oncol. 2020;146(9):2339–2350. doi: 10.1007/s00432-020-03304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocak B., Kaya O.K., Erdim C., Kus E.A., Kilickesmez O. Artificial Intelligence in Renal Mass Characterization: A Systematic Review of Methodologic Items Related to Modeling, Performance Evaluation, Clinical Utility, and Transparency. Am. J. Roentgenol. Nov. 2020;215(5):1113–1122. doi: 10.2214/ajr.20.22847. [DOI] [PubMed] [Google Scholar]
- 29.O.T. Jones, et al., Artificial Intelligence Techniques That May Be Applied to Primary Care Data to Facilitate Earlier Diagnosis of Cancer: Systematic Review, J. Med. Internet Res. 23(3) (2021) p. e23483, doi: 10.2196/23483. [DOI] [PMC free article] [PubMed]
- 30.Marka A., Carter J.B., Toto E., Hassanpour S. Automated detection of nonmelanoma skin cancer using digital images: a systematic review. BMC Med. Imaging. 2019;19(1) doi: 10.1186/s12880-019-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bang C.S., Lee J.J., Baik G.H. Computer-aided diagnosis of esophageal cancer and neoplasms in endoscopic images: a systematic review and meta-analysis of diagnostic test accuracy. Gastrointest. Endosc. May 2021;93(5):1006–1015.e13. doi: 10.1016/j.gie.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Ferrante di Ruffano L., Takwoingi Y., Dinnes J., Chuchu N., Bayliss S.E., Davenport C., Matin R.N., Godfrey K., O'Sullivan C., Gulati A., Chan S.A., Durack A., O'Connell S., Gardiner M.D., Bamber J., Deeks J.J., Williams H.C. Computer-assisted diagnosis techniques (dermoscopy and spectroscopy-based) for diagnosing skin cancer in adults. Cochrane Database of System. Rev. 2018;2018(12) doi: 10.1002/14651858.cd013186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R.D. Nindrea, T. Aryandono, L. Lazuardi, I. Dwiprahasto, Diagnostic Accuracy of Different Machine Learning Algorithms for Breast Cancer Risk Calculation: a Meta-Analysis, Asian Pacific J. Cancer Prevention 19(7) (Jul. 2018), doi: 10.22034/APJCP.2018.19.7.1747. [DOI] [PMC free article] [PubMed]
- 34.Mehta T.I., Heiberger C., Kazi S., Brown M., Weissman S., Hong K., Mehta M., Yim D. Effectiveness of Radiofrequency Ablation in the Treatment of Painful Osseous Metastases: A Correlation Meta-Analysis with Machine Learning Cluster Identification. J. Vasc. Interv. Radiol. 2020;31(11):1753–1762. doi: 10.1016/j.jvir.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Sugano D., Sanford D., Abreu A., Duddalwar V., Gill I., Cacciamani G.E. Impact of radiomics on prostate cancer detection: a systematic review of clinical applications. Curr. Opin. Urol. Sep. 2020;30(6):754–781. doi: 10.1097/mou.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 36.Patil S., et al. Machine learning and its potential applications to the genomic study of head and neck cancer{ extemdash}A systematic review. J. Oral Pathol. Med. 2019;48(9):773–779. doi: 10.1111/jop.12854. [DOI] [PubMed] [Google Scholar]
- 37.Xie C.-Y., Pang C.-L., Chan B., Wong E.-Y.-Y., Dou Q., Vardhanabhuti V. Machine Learning and Radiomics Applications in Esophageal Cancers Using Non-Invasive Imaging Methods{\textemdash}A Critical Review of Literature. Cancers (Basel) May 2021;13(10):2469. doi: 10.3390/cancers13102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.T.B. Lacerda, A. Medeiros, R.B. Perez, A.P.C. Furtado, Machine Learning Applied to survival prediction of elderly cancer patients: Systematic Review, Jun. 2020. doi: 10.23919/cisti49556.2020.9140861.
- 39.Mekki A., Dercle L., Lichtenstein P., Nasser G., Marabelle A., Champiat S., Chouzenoux E., Balleyguier C., Ammari S. Machine learning defined diagnostic criteria for differentiating pituitary metastasis from autoimmune hypophysitis in patients undergoing immune checkpoint blockade therapy. Eur. J. Cancer. 2019;119:44–56. doi: 10.1016/j.ejca.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Alabi R.O., et al. Machine learning in oral squamous cell carcinoma: Current status, clinical concerns and prospects for future{\textemdash}A systematic review. Artif. Intell. Med. May 2021;115 doi: 10.1016/j.artmed.2021.102060. [DOI] [PubMed] [Google Scholar]
- 41.Yassin N.I.R., Omran S., El Houby E.M.F., Allam H. Machine learning techniques for breast cancer computer aided diagnosis using different image modalities: A systematic review. Comput. Methods Programs Biomed. Mar. 2018;156:25–45. doi: 10.1016/j.cmpb.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Bradley A., van der Meer R., McKay C. Personalized Pancreatic Cancer Management. Pancreas. May 2019;48(5):598–604. doi: 10.1097/mpa.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 43.Lai Q., Spoletini G., Mennini G., Laureiro Z.L., Tsilimigras D.I., Pawlik T.M., Rossi M. Prognostic role of artificial intelligence among patients with hepatocellular cancer: A systematic review. World J. Gastroenterol. 2020;26(42):6679–6688. doi: 10.3748/wjg.v26.i42.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh D., Singh A.K. Role of image thermography in early breast cancer detection- Past, present and future. Comput. Methods Programs Biomed. 2020;183 doi: 10.1016/j.cmpb.2019.105074. [DOI] [PubMed] [Google Scholar]
- 45.T.J. Brinker, et al., Skin Cancer Classification Using Convolutional Neural Networks: Systematic Review, J. Med. Internet Res. 20(10) (2018), p. e11936, doi: 10.2196/11936. [DOI] [PMC free article] [PubMed]
- 46.M.O. Khairandish, M. Sharma, K. Kusrini, The Performance of Brain Tumor Diagnosis Based on Machine Learning Techniques Evaluation - A Systematic Review, Nov. 2020. doi: 10.1109/icoiact50329.2020.9332131.
- 47.Li J., Sang T., Yu W.-H., Jiang M., Hunag S.-Y., Cao C.-L., Chen M., Cao Y.-W., Cui X.-W., Dietrich C.F. The value of S-Detect for the differential diagnosis of breast masses on ultrasound: a systematic review and pooled meta-analysis. Med. Ultrasonography. May 2020;22(2):211. doi: 10.11152/mu-2402. [DOI] [PubMed] [Google Scholar]
- 48.N.F. Zulkifli, Z.C. Cob, A.A. Latif, S.M. Drus, A Systematic Review of Machine Learning in Substance Addiction, 2020. doi: 10.1109/icimu49871.2020.9243581.
- 49.Rahman R.A., Omar K., Noah S.A.M., Danuri M.S.N.M., Al-Garadi M.A. Application of Machine Learning Methods in Mental Health Detection: A Systematic Review. IEEE Access. 2020;8:183952–183964. doi: 10.1109/access.2020.3029154. [DOI] [Google Scholar]
- 50.Lee Y., Ragguett R.-M., Mansur R.B., Boutilier J.J., Rosenblat J.D., Trevizol A., Brietzke E., Lin K., Pan Z., Subramaniapillai M., Chan T.C.Y., Fus D., Park C., Musial N., Zuckerman H., Chen V.-H., Ho R., Rong C., McIntyre R.S. Applications of machine learning algorithms to predict therapeutic outcomes in depression: A meta-analysis and systematic review. J. Affect. Disord. 2018;241:519–532. doi: 10.1016/j.jad.2018.08.073. [DOI] [PubMed] [Google Scholar]
- 51.Bernert R.A., Hilberg A.M., Melia R., Kim J.P., Shah N.H., Abnousi F. Artificial Intelligence and Suicide Prevention: A Systematic Review of Machine Learning Investigations. Int. J. Environ. Res. Public Health. 2020;17(16):5929. doi: 10.3390/ijerph17165929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.X. Geng, X. Kang, P.C.M. Wong, Autism spectrum disorder risk prediction: A systematic review of behavioral and neural investigations, in: Progress in Molecular Biology and Translational Science, Elsevier, 2020, pp. 91–137. doi: 10.1016/bs.pmbts.2020.04.015. [DOI] [PubMed]
- 53.Ebrahimighahnavieh M.A., Luo S., Chiong R. Deep learning to detect Alzheimer{\textquotesingle}s disease from neuroimaging: A systematic literature review. Comput. Methods Programs Biomed. 2020;187 doi: 10.1016/j.cmpb.2019.105242. [DOI] [PubMed] [Google Scholar]
- 54.Bruin W., Denys D., van Wingen G. Diagnostic neuroimaging markers of obsessive-compulsive disorder: Initial evidence from structural and functional MRI studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;91:49–59. doi: 10.1016/j.pnpbp.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Sanfelici R., Dwyer D.B., Antonucci L.A., Koutsouleris N. Individualized Diagnostic and Prognostic Models for Patients With Psychosis Risk Syndromes: A Meta-analytic View on the State of the Art. Biol. Psychiatry. 2020;88(4):349–360. doi: 10.1016/j.biopsych.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 56.A.L. Dallora, S. Eivazzadeh, E. Mendes, J. Berglund, P. Anderberg, Machine learning and microsimulation techniques on the prognosis of dementia: A systematic literature review, {PLOS} {ONE} 12(6) (2017) p. e0179804, doi: 10.1371/journal.pone.0179804. [DOI] [PMC free article] [PubMed]
- 57.A. Le Glaz, et al., Machine Learning and Natural Language Processing in Mental Health: Systematic Review, J. Med. Internet Res. 23(5) (2021), p. e15708, doi: 10.2196/15708. [DOI] [PMC free article] [PubMed]
- 58.Bracher-Smith M., Crawford K., Escott-Price V. Machine learning for genetic prediction of psychiatric disorders: a systematic review. Mol. Psychiatry. Jun. 2020;26(1):70–79. doi: 10.1038/s41380-020-0825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shatte A.B.R., Hutchinson D.M., Teague S.J. Machine learning in mental health: a scoping review of methods and applications. Psychol. Med. Feb. 2019;49(09):1426–1448. doi: 10.1017/s0033291719000151. [DOI] [PubMed] [Google Scholar]
- 60.Levman J., Takahashi E. Multivariate analyses applied to fetal, neonatal and pediatric MRI of neurodevelopmental disorders. {NeuroImage}: Clinical. 2015;9:532–544. doi: 10.1016/j.nicl.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wongkoblap A., Vadillo M.A., Curcin V. Researching Mental Health Disorders in the Era of Social Media: Systematic Review. J. Med. Internet Res. Jun. 2017;19(6) doi: 10.2196/jmir.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.A.Z. Antosik-Wójcińska, et al., Smartphone as a monitoring tool for bipolar disorder: a systematic review including data analysis, machine learning algorithms and predictive modelling, Int. J. Med. Inform. 138 (2020), p. 104131, doi: 10.1016/j.ijmedinf.2020.104131. [DOI] [PubMed]
- 63.Rashidan M.A., Sidek S.N., Yusof H.M., Khalid M., Dzulkarnain A.A.A., Ghazali A.S., Zabidi S.A.M., Sidique F.A.A. Technology-Assisted Emotion Recognition for Autism Spectrum Disorder ({ASD}) Children: A Systematic Literature Review. IEEE Access. 2021;9:33638–33653. [Google Scholar]
- 64.Burke T.A., Ammerman B.A., Jacobucci R. The use of machine learning in the study of suicidal and non-suicidal self-injurious thoughts and behaviors: A systematic review. J. Affect. Disord. Feb. 2019;245:869–884. doi: 10.1016/j.jad.2018.11.073. [DOI] [PubMed] [Google Scholar]
- 65.Dwivedi A.K., Imtiaz S.A., Rodriguez-Villegas E. Algorithms for Automatic Analysis and Classification of Heart Sounds{\textendash}A Systematic Review. IEEE Access. 2019;7:8316–8345. doi: 10.1109/access.2018.2889437. [DOI] [Google Scholar]
- 66.Millán C.A., Girón N.A., Lopez D.M. Analysis of Relevant Features from Photoplethysmographic Signals for Atrial Fibrillation Classification. Int. J. Environ. Res. Public Health. 2020;17(2):498. doi: 10.3390/ijerph17020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raffort J., Adam C., Carrier M., Ballaith A., Coscas R., Jean-Baptiste E., Hassen-Khodja R., Chakfé N., Lareyre F. Artificial intelligence in abdominal aortic aneurysm. J. Vasc. Surg. 2020;72(1):321–333.e1. doi: 10.1016/j.jvs.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 68.Burlacu A., Iftene A., Popa I.V., Crisan-Dabija R., Brinza C., Covic A. Computational Models Used to Predict Cardiovascular Complications in Chronic Kidney Disease Patients: A Systematic Review. Medicina (B Aires) May 2021;57(6):538. doi: 10.3390/medicina57060538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.R. Fernandes, J. Paredes, J. Salinet, Detection and Classification of Cardiac Arrhythmias by Machine Learning: a Systematic Review, 2020. doi: 10.22489/cinc.2020.333.
- 70.V.S. de Siqueira, et al., Machine Learning Applied to Support Medical Decision in Transthoracic Echocardiogram Exams: A Systematic Review, Jul. 2020. doi: 10.1109/compsac48688.2020.0-215.
- 71.Rjoob K., Bond R., Finlay D., McGilligan V., Leslie S.J., Rababah A., Guldenring D., Iftikhar A., Knoery C., McShane A., Peace A. Machine learning techniques for detecting electrode misplacement and interchanges when recording {ECGs}: A systematic review and meta-analysis. J. Electrocardiol. 2020;62:116–123. doi: 10.1016/j.jelectrocard.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 72.Mahajan S.M., Heidenreich P., Abbott B., Newton A., Ward D. Predictive models for identifying risk of readmission after index hospitalization for heart failure: A systematic review. Eur. J. Cardiovasc. Nursing. Sep. 2018;17(8):675–689. doi: 10.1177/1474515118799059. [DOI] [PubMed] [Google Scholar]
- 73.Javan S.L., Sepehri M.M., Aghajani H. Toward analyzing and synthesizing previous research in early prediction of cardiac arrest using machine learning based on a multi-layered integrative framework. J. Biomed. Inform. 2018;88:70–89. doi: 10.1016/j.jbi.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Hassanipour S., Ghaem H., Arab-Zozani M., Seif M., Fararouei M., Abdzadeh E., Sabetian G., Paydar S. Comparison of artificial neural network and logistic regression models for prediction of outcomes in trauma patients: A systematic review and meta-analysis. Injury. 2019;50(2):244–250. doi: 10.1016/j.injury.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 75.J. Kedra, et al., Current status of use of big data and artificial intelligence in {RMDs}: a systematic literature review informing {EULAR} recommendations, {RMD} Open 5(2) (2019), p. e001004, doi: 10.1136/rmdopen-2019-001004. [DOI] [PMC free article] [PubMed]
- 76.Anteby R., Klang E., Horesh N., Nachmany I., Shimon O., Barash Y., Kopylov U., Soffer S. Deep learning for noninvasive liver fibrosis classification: A systematic review. Liver Int. 2021;41(10):2269–2278. doi: 10.1111/liv.14966. [DOI] [PubMed] [Google Scholar]
- 77.M. Prados-Privado, J.G. Villalón, C.H. Mart\’\inez-Mart\’\inez, C. Ivorra, J.C. Prados-Frutos, Dental Caries Diagnosis and Detection Using Neural Networks: A Systematic Review, J. Clin. Med. 9(11) (2020), p. 3579, doi: 10.3390/jcm9113579. [DOI] [PMC free article] [PubMed]
- 78.Groot O.Q., Bongers M.E.R., Ogink P.T., Senders J.T., Karhade A.V., Bramer J.A.M., Verlaan J.-J., Schwab J.H. Does Artificial Intelligence Outperform Natural Intelligence in Interpreting Musculoskeletal Radiological Studies? A Systematic Review. Clin. Orthop. Relat. Res. 2020;478(12):2751–2764. doi: 10.1097/CORR.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith A., López-Solà M., McMahon K., Pedler A., Sterling M. Multivariate pattern analysis utilizing structural or functional {MRI}{\textemdash}In individuals with musculoskeletal pain and healthy controls: A systematic review. Semin. Arthritis Rheum. 2017;47(3):418–431. doi: 10.1016/j.semarthrit.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 80.Hung K., Montalvao C., Tanaka R., Kawai T., Bornstein M.M. The use and performance of artificial intelligence applications in dental and maxillofacial radiology: A systematic review. Dentomaxillofacial Radiol. 2020;49(1):20190107. doi: 10.1259/dmfr.20190107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langerhuizen D.W.G., Janssen S.J., Mallee W.H., van den Bekerom M.P.J., Ring D., Kerkhoffs G.M.M.J., Jaarsma R.L., Doornberg J.N. What Are the Applications and Limitations of Artificial Intelligence for Fracture Detection and Classification in Orthopaedic Trauma Imaging? A Systematic Review. Clin. Orthop. Relat. Res. 2019;477(11):2482–2491. doi: 10.1097/CORR.0000000000000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lui T.K.L., Tsui V.W.M., Leung W.K. Accuracy of artificial intelligence{\textendash}assisted detection of upper GI lesions: a systematic review and meta-analysis. Gastrointest. Endosc. Oct. 2020;92(4):821–830.e9. doi: 10.1016/j.gie.2020.06.034. [DOI] [PubMed] [Google Scholar]
- 83.Decharatanachart P., Chaiteerakij R., Tiyarattanachai T., Treeprasertsuk S. Application of artificial intelligence in chronic liver diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21(1) doi: 10.1186/s12876-020-01585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar H., DeSouza S.V., Petrov M.S. Automated pancreas segmentation from computed tomography and magnetic resonance images: A systematic review. Comput. Methods Programs Biomed. Sep. 2019;178:319–328. doi: 10.1016/j.cmpb.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Soffer S., Klang E., Shimon O., Nachmias N., Eliakim R., Ben-Horin S., Kopylov U., Barash Y. Deep learning for wireless capsule endoscopy: a systematic review and meta-analysis. Gastrointest. Endosc. 2020;92(4):831–839.e8. doi: 10.1016/j.gie.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 86.Shung D., Simonov M., Gentry M., Au B., Laine L. Machine Learning to Predict Outcomes in Patients with Acute Gastrointestinal Bleeding: A Systematic Review. Dig. Dis. Sci. May 2019;64(8):2078–2087. doi: 10.1007/s10620-019-05645-z. [DOI] [PubMed] [Google Scholar]
- 87.Aziz M., Fatima R., Dong C., Lee-Smith W., Nawras A. The impact of deep convolutional neural network-based artificial intelligence on colonoscopy outcomes: A systematic review with meta-analysis. J. Gastroenterol. Hepatol. 2020;35(10):1676–1683. doi: 10.1111/jgh.15070. [DOI] [PubMed] [Google Scholar]
- 88.Wingfield L.R., Ceresa C., Thorogood S., Fleuriot J., Knight S. Using Artificial Intelligence for Predicting Survival of Individual Grafts in Liver Transplantation: A Systematic Review. Liver Transpl. Jun. 2020;26(7):922–934. doi: 10.1002/lt.25772. [DOI] [PubMed] [Google Scholar]
- 89.W. Wang, et al., A systematic review of machine learning models for predicting outcomes of stroke with structured data, {PLOS} {ONE} 15(6) (2020) p. e0234722, doi: 10.1371/journal.pone.0234722. [DOI] [PMC free article] [PubMed]
- 90.Murray N.M., Unberath M., Hager G.D., Hui F.K. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: a systematic review. J. NeuroInterventional Surg. Oct. 2019;12(2):156–164. doi: 10.1136/neurintsurg-2019-015135. [DOI] [PubMed] [Google Scholar]
- 91.Sarmento R.M., Vasconcelos F.F.X., Filho P.P.R., Wu W., de Albuquerque V.H.C. Automatic Neuroimage Processing and Analysis in Stroke{\textemdash}A Systematic Review. IEEE Rev. Biomed. Eng. 2020;13:130–155. doi: 10.1109/rbme.2019.2934500. [DOI] [PubMed] [Google Scholar]
- 92.R. Balakrishnan, M. del C. Valdés Hernández, A.J. Farrall, Automatic segmentation of white matter hyperintensities from brain magnetic resonance images in the era of deep learning and big data {\textendash} A systematic review, Computerized Med. Imaging Graphics 88 (2021) p. 101867, doi: 10.1016/j.compmedimag.2021.101867. [DOI] [PubMed]
- 93.Senders J.T., Staples P.C., Karhade A.V., Zaki M.M., Gormley W.B., Broekman M.L.D., Smith T.R., Arnaout O. Machine Learning and Neurosurgical Outcome Prediction: A Systematic Review. World Neurosurg. 2018;109:476–486.e1. doi: 10.1016/j.wneu.2017.09.149. [DOI] [PubMed] [Google Scholar]
- 94.R. Gautam, M. Sharma, Prevalence and diagnosis of neurological disorders using different deep learning techniques: A∼Meta-Analysis, J. Med. Syst. 44(2) (2020), doi: 10.1007/s10916-019-1519-7. [DOI] [PubMed]
- 95.Xu L.u., He B., Zhang Y., Chen L.u., Fan D., Zhan S., Wang S. Prognostic models for amyotrophic lateral sclerosis: a systematic review. J. Neurol. 2021;268(9):3361–3370. doi: 10.1007/s00415-021-10508-7. [DOI] [PubMed] [Google Scholar]
- 96.M. Harris, et al., A systematic review of the diagnostic accuracy of artificial intelligence-based computer programs to analyze chest X-rays for pulmonary tuberculosis, {PLOS} {ONE} 14(9) (2019), p. e0221339, doi: 10.1371/journal.pone.0221339. [DOI] [PMC free article] [PubMed]
- 97.Valente I.R.S., Cortez P.C., Neto E.C., Soares J.M., de Albuquerque V.H.C., Tavares J.M.R.S. Automatic 3D pulmonary nodule detection in CT images: A survey. Comput. Methods Programs Biomed. Feb. 2016;124:91–107. doi: 10.1016/j.cmpb.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Nikolaou V., Massaro S., Fakhimi M., Stergioulas L., Price D. {COPD} phenotypes and machine learning cluster analysis: A systematic review and future research agenda. Respir. Med. Sep. 2020;171 doi: 10.1016/j.rmed.2020.106093. [DOI] [PubMed] [Google Scholar]
- 99.Khan W., Zaki N., Ali L. Intelligent pneumonia identification from chest X-rays: A systematic literature review. IEEE Access. 2021;9:51747–51771. doi: 10.1109/access.2021.3069937. [DOI] [Google Scholar]
- 100.Islam M.M., Yang H.-C., Poly T.N., Jian W.-S., (Jack) Li Y.-C. Deep learning algorithms for detection of diabetic retinopathy in retinal fundus photographs: A systematic review and meta-analysis. Comput. Methods Programs Biomed. 2020;191:105320. doi: 10.1016/j.cmpb.2020.105320. [DOI] [PubMed] [Google Scholar]
- 101.Nielsen K.B., Lautrup M.L., Andersen J.K.H., Savarimuthu T.R., Grauslund J. Deep Learning{\textendash}Based Algorithms in Screening of Diabetic Retinopathy: A Systematic Review of Diagnostic Performance. Ophthalmol. Retina. 2019;3(4):294–304. doi: 10.1016/j.oret.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 102.S. Wang, et al., Performance of deep neural network-based artificial intelligence method in diabetic retinopathy screening: a systematic review and meta-analysis of diagnostic test accuracy, Eur. J. Endocrinol. 183(1) (2020), pp. 41–49, doi: 10.1530/eje-19-0968. [DOI] [PubMed]
- 103.Fleuren L.M., Klausch T.L.T., Zwager C.L., Schoonmade L.J., Guo T., Roggeveen L.F., Swart E.L., Girbes A.R.J., Thoral P., Ercole A., Hoogendoorn M., Elbers P.W.G. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020;46(3):383–400. doi: 10.1007/s00134-019-05872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gupta V., Braun T.M., Chowdhury M., Tewari M., Choi S.W. A Systematic Review of Machine Learning Techniques in Hematopoietic Stem Cell Transplantation ({HSCT}) Sensors. Oct. 2020;20(21):6100. doi: 10.3390/s20216100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gregório T., Pipa S., Cavaleiro P., Atanásio G., Albuquerque I., Chaves P.C., Azevedo L. Prognostic models for intracerebral hemorrhage: systematic review and meta-analysis. BMC Med. Res. Method. 2018;18(1) doi: 10.1186/s12874-018-0613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tulloch J., Zamani R., Akrami M. Machine Learning in the Prevention, Diagnosis and Management of Diabetic Foot Ulcers: A Systematic Review. IEEE Access. 2020;8:198977–199000. doi: 10.1109/access.2020.3035327. [DOI] [Google Scholar]
- 107.De Silva K., Lee W.K., Forbes A., Demmer R.T., Barton C., Enticott J. Use and performance of machine learning models for type 2 diabetes prediction in community settings: A systematic review and meta-analysis. Int. J. Med. Inf. Nov. 2020;143 doi: 10.1016/j.ijmedinf.2020.104268. [DOI] [PubMed] [Google Scholar]
- 108.Liu N.T., Salinas J. Machine learning in burn care and research: A systematic review of the literature. Burns. 2015;41(8):1636–1641. doi: 10.1016/j.burns.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Senanayake S., White N., Graves N., Healy H., Baboolal K., Kularatna S. Machine learning in predicting graft failure following kidney transplantation: A systematic review of published predictive models. Int. J. Med. Inf. Oct. 2019;130 doi: 10.1016/j.ijmedinf.2019.103957. [DOI] [PubMed] [Google Scholar]
- 110.H. Sufriyana, et al., Comparison of Multivariable Logistic Regression and Other Machine Learning Algorithms for Prognostic Prediction Studies in Pregnancy Care: Systematic Review and Meta-Analysis, {JMIR} Med. Inform. 8(11) (2020), p. e16503, doi: 10.2196/16503. [DOI] [PMC free article] [PubMed]
- 111.A.L. Dallora, P. Anderberg, O. Kvist, E. Mendes, S.D. Ruiz, J.S. Berglund, Bone age assessment with various machine learning techniques: A systematic literature review and meta-analysis, {PLOS} {ONE} 14(7) (2019), p. e0220242, doi: 10.1371/journal.pone.0220242. [DOI] [PMC free article] [PubMed]
- 112.Muralitharan S., et al. Machine Learning{\textendash}Based Early Warning Systems for Clinical Deterioration: Systematic Scoping Review. J. Med. Internet Res. Feb. 2021;23(2) doi: 10.2196/25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vallmuur K. Machine learning approaches to analysing textual injury surveillance data: A systematic review. Accid. Anal. Prev. 2015;79:41–49. doi: 10.1016/j.aap.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 114.Young I.J.B., Luz S., Lone N. A systematic review of natural language processing for classification tasks in the field of incident reporting and adverse event analysis. Int. J. Med. Inf. 2019;132 doi: 10.1016/j.ijmedinf.2019.103971. [DOI] [PubMed] [Google Scholar]
- 115.Medic G., Kosaner Kließ M., Atallah L., Weichert J., Panda S., Postma M., EL-Kerdi A. Evidence-based Clinical Decision Support Systems for the prediction and detection of three disease states in critical care: A systematic literature review. F1000Res. 2019;8:1728. doi: 10.12688/f1000research.20498.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arji G., Safdari R., Rezaeizadeh H., Abbassian A., Mokhtaran M., Ayati M.H. A systematic literature review and classification of knowledge discovery in traditional medicine. Comput. Methods Programs Biomed. 2019;168:39–57. doi: 10.1016/j.cmpb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 117.Islam M.N., Inan T.T., Rafi S., Akter S.S., Sarker I.H., Islam A.K.M.N. A Systematic Review on the Use of AI and ML for Fighting the COVID}-19 Pandemic. IEEE Trans. Artif. Intell. 2020;1(3):258–270. doi: 10.1109/tai.2021.3062771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Musulin J., Baressi Šegota S., Štifanić D., Lorencin I., Anđelić N., Šušteršič T., Blagojević A., Filipović N., Ćabov T., Markova-Car E. Application of Artificial Intelligence-Based Regression Methods in the Problem of {COVID}-19 Spread Prediction: A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18(8):4287. doi: 10.3390/ijerph18084287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chee M.L., Ong M.E.H., Siddiqui F.J., Zhang Z., Lim S.L., Ho A.F.W., Liu N. Artificial Intelligence Applications for {COVID}-19 in Intensive Care and Emergency Settings: A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18(9):4749. doi: 10.3390/ijerph18094749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adamidi E.S., Mitsis K., Nikita K.S. Artificial intelligence in clinical care amidst {COVID}-19 pandemic: A systematic review. Comput. Struct. Biotechnol. J. 2021;19:2833–2850. doi: 10.1016/j.csbj.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.A. Abd-Alrazaq, et al., Artificial Intelligence in the Fight Against {COVID}-19: Scoping Review, J. Med. Internet Res. 22(12) (2020), p. e20756, doi: 10.2196/20756. [DOI] [PMC free article] [PubMed]
- 122.Scardoni A., Balzarini F., Signorelli C., Cabitza F., Odone A. Artificial intelligence-based tools to control healthcare associated infections: A systematic review of the literature. J. Infect. Public Health. 2020;13(8):1061–1077. doi: 10.1016/j.jiph.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 123.Ghaderzadeh M., Asadi F., Maietta S. Deep Learning in the Detection and Diagnosis of {COVID}-19 Using Radiology Modalities: A Systematic Review. J. Healthcare Eng. 2021;2021:1–10. doi: 10.1155/2021/6677314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baldominos A., Puello A., Ogul H., Asuroglu T., Colomo-Palacios R. Predicting Infections Using Computational Intelligence {\textendash} A Systematic Review. IEEE Access. 2020;8:31083–31102. doi: 10.1109/access.2020.2973006. [DOI] [Google Scholar]
- 125.Payedimarri A.B., Concina D., Portinale L., Canonico M., Seys D., Vanhaecht K., Panella M. Prediction Models for Public Health Containment Measures on {COVID}-19 Using Artificial Intelligence and Machine Learning: A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18(9):4499. doi: 10.3390/ijerph18094499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Albahri O.S., Zaidan A.A., Albahri A.S., Zaidan B.B., Abdulkareem K.H., Al-qaysi Z.T., Alamoodi A.H., Aleesa A.M., Chyad M.A., Alesa R.M., Kem L.C., Lakulu M.M., Ibrahim A.B., Rashid N.A. Systematic review of artificial intelligence techniques in the detection and classification of {COVID}-19 medical images in terms of evaluation and benchmarking: Taxonomy analysis, challenges, future solutions and methodological aspects. J. Infection Public Health. 2020;13(10):1381–1396. doi: 10.1016/j.jiph.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li W.T., Ma J., Shende N., Castaneda G., Chakladar J., Tsai J.C., Apostol L., Honda C.O., Xu J., Wong L.M., Zhang T., Lee A., Gnanasekar A., Honda T.K., Kuo S.Z., Yu M.A., Chang E.Y., Rajasekaran M.“.R., Ongkeko W.M. Using machine learning of clinical data to diagnose COVID}-19: a systematic review and meta-analysis. BMC Med. Inform. Decision Making. 2020;20(1) doi: 10.1186/s12911-020-01266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Al-garadi M.A., Khan M.S., Varathan K.D., Mujtaba G., Al-Kabsi A.M. Using online social networks to track a pandemic: A systematic review. J. Biomed. Inform. 2016;62:1–11. doi: 10.1016/j.jbi.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 129.Kareemi H., Vaillancourt C., Rosenberg H., Fournier K., Yadav K., Mitchell A.M. Machine Learning Versus Usual Care for Diagnostic and Prognostic Prediction in the Emergency Department: A Systematic Review. Acad. Emerg. Med. 2021;28(2):184–196. doi: 10.1111/acem.14190. [DOI] [PubMed] [Google Scholar]
- 130.Miles J., Turner J., Jacques R., Williams J., Mason S. Using machine-learning risk prediction models to triage the acuity of undifferentiated patients entering the emergency care system: a systematic review. Diagnostic Prognostic Res. 2020;4(1):Oct. doi: 10.1186/s41512-020-00084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karmegam D., Ramamoorthy T., Mappillairajan B. A Systematic Review of Techniques Employed for Determining Mental Health Using Social Media in Psychological Surveillance During Disasters. Disaster Med. Public Health Preparedness. Jul. 2019;14(2):265–272. doi: 10.1017/dmp.2019.40. [DOI] [PubMed] [Google Scholar]
- 132.Shillan D., Sterne J.A.C., Champneys A., Gibbison B. Use of machine learning to analyse routinely collected intensive care unit data: a systematic review. Crit. Care. 2019;23(1) doi: 10.1186/s13054-019-2564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.V. Nunavath, M. Goodwin, The Use of Artificial Intelligence in Disaster Management - A Systematic Literature Review, 2019. doi: 10.1109/ict-dm47966.2019.9032935.
- 134.M. Milne-Ives, et al., The Effectiveness of Artificial Intelligence Conversational Agents in Health Care: Systematic Review, J. Med. Internet Res. 22(10) (2020), p. e20346, doi: 10.2196/20346. [DOI] [PMC free article] [PubMed]
- 135.Kreimeyer K., Foster M., Pandey A., Arya N., Halford G., Jones S.F., Forshee R., Walderhaug M., Botsis T. Natural language processing systems for capturing and standardizing unstructured clinical information: A systematic review. J. Biomed. Inform. 2017;73:14–29. doi: 10.1016/j.jbi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koleck T.A., Dreisbach C., Bourne P.E., Bakken S. Natural language processing of symptoms documented in free-text narratives of electronic health records: a systematic review. J. Am. Med. Inform. Assoc. Feb. 2019;26(4):364–379. doi: 10.1093/jamia/ocy173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oskar S., Stingone J.A. Machine Learning Within Studies of Early-Life Environmental Exposures and Child Health: Review of the Current Literature and Discussion of Next Steps. Curr. Environ. Health Reports. 2020;7(3):170–184. doi: 10.1007/s40572-020-00282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cresswell K., Callaghan M., Khan S., Sheikh Z., Mozaffar H., Sheikh A. Investigating the use of data-driven artificial intelligence in computerised decision support systems for health and social care: A systematic review. Health Inform. J. 2020;26(3):2138–2147. doi: 10.1177/1460458219900452. [DOI] [PubMed] [Google Scholar]
- 139.Ford E., Carroll J.A., Smith H.E., Scott D., Cassell J.A. Extracting information from the text of electronic medical records to improve case detection: a systematic review. J. Am. Med. Inform. Assoc. Feb. 2016;23(5):1007–1015. doi: 10.1093/jamia/ocv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Payrovnaziri S.N., et al. Explainable artificial intelligence models using real-world electronic health∼record data: a systematic scoping review. J. Am. Med. Inform. Assoc. May 2020;27(7):1173–1185. doi: 10.1093/jamia/ocaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.J. Shen, et al., Artificial Intelligence Versus Clinicians in Disease Diagnosis: Systematic Review, {JMIR} Med. Inform. 7(3), p. e10010, 2019, doi: 10.2196/10010. [DOI] [PMC free article] [PubMed]
- 142.Dreisbach C., Koleck T.A., Bourne P.E., Bakken S. A systematic review of natural language processing and text mining of symptoms from electronic patient-authored text data. Int. J. Med. Inf. May 2019;125:37–46. doi: 10.1016/j.ijmedinf.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kearns W.R., Chi N.-C., Choi Y.K., Lin S.-Y., Thompson H., Demiris G. A Systematic Review of Health Dialog Systems. Methods Inf. Med. 2019;58(06):179–193. doi: 10.1055/s-0040-1708807. [DOI] [PubMed] [Google Scholar]
- 144.Bellinger C., Jabbar M.S.M., Zaïane O., Osornio-Vargas A. A systematic review of data mining and machine learning for air pollution epidemiology. BMC Public Health. 2017;17(1):Nov. doi: 10.1186/s12889-017-4914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yuan M., Boston-Fisher N., Luo Y., Verma A., Buckeridge D.L. A systematic review of aberration detection algorithms used in public health surveillance. J. Biomed. Inform. 2019;94 doi: 10.1016/j.jbi.2019.103181. [DOI] [PubMed] [Google Scholar]
- 146.Mak K.K., Lee K., Park C. Applications of machine learning in addiction studies: A systematic review. Psychiatry Res. May 2019;275:53–60. doi: 10.1016/j.psychres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Liu X., Rivera S.C., Moher D., Calvert M.J., Denniston A.K. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI Extension. The BMJ. 2020;370 doi: 10.1136/bmj.m3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rivera S.C., Liu X., Chan A.W., Denniston A.K., Calvert M.J. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI Extension. The BMJ. 2020;370:1–14. doi: 10.1136/bmj.m3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.B. Norgeot, et al., Modeling : the MI-CLAIM checklist, vol. 26, no. 9, pp. 1320–1324, 2020, doi: 10.1038/s41591-020-1041-y.Minimum. [DOI] [PMC free article] [PubMed]
- 150.Wolff R.F., Moons K.G.M., Riley R.D., Whiting P.F., Westwood M., Collins G.S., Reitsma J.B., Kleijnen J., Mallett S. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019;170(1):51. doi: 10.7326/M18-1376. [DOI] [PubMed] [Google Scholar]
- 151.Jiang F., Jiang Y., Zhi H., Dong Y.i., Li H., Ma S., Wang Y., Dong Q., Shen H., Wang Y. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017;2(4):230–243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Riley R.D., et al. Penalisation and shrinkage methods produced unreliable clinical prediction models especially when sample size was small. J. Clin. Epidemiol. 2020;132:88–96. doi: 10.1016/j.jclinepi.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Forero D.A., Curioso W.H., Patrinos G.P. The importance of adherence to international standards for depositing open data in public repositories. BMC Res. Notes. 2021;14(1):1–5. doi: 10.1186/S13104-021-05817-Z/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sollini M., Gelardi F., Matassa G., Delgado Bolton R.C., Chiti A., Kirienko M. Interdisciplinarity: an essential requirement for translation of radiomics research into clinical practice – a systematic review focused on thoracic oncology. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition) 2020;39(3):146–156. doi: 10.1016/j.remnie.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 155.Curioso W.H. Building capacity and training for digital health: challenges and opportunities in Latin America. J. Med. Internet Res. 2019;21(12) doi: 10.2196/16513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.