Key Points
Question
Among individuals who had symptomatic SARS-CoV-2 infection in 2020 and 2021, what proportion experienced common self-reported Long COVID symptom clusters 3 months after initial infection?
Findings
This observational analysis involved bayesian meta-regression and pooling of 54 studies and 2 medical record databases with data for 1.2 million individuals (from 22 countries) who had symptomatic SARS-CoV-2 infection. The modeled estimated proportion with at least 1 of the 3 self-reported Long COVID symptom clusters 3 months after symptomatic SARS-CoV-2 infection was 6.2%, including 3.7% for ongoing respiratory problems, 3.2% for persistent fatigue with bodily pain or mood swings, and 2.2% for cognitive problems after adjusting for health status before COVID-19.
Meaning
This study presents modeled estimates of the proportion of individuals with at least 1 of the 3 self-reported Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) 3 months after symptomatic SARS-CoV-2 infection.
Abstract
Importance
Some individuals experience persistent symptoms after initial symptomatic SARS-CoV-2 infection (often referred to as Long COVID).
Objective
To estimate the proportion of males and females with COVID-19, younger or older than 20 years of age, who had Long COVID symptoms in 2020 and 2021 and their Long COVID symptom duration.
Design, Setting, and Participants
Bayesian meta-regression and pooling of 54 studies and 2 medical record databases with data for 1.2 million individuals (from 22 countries) who had symptomatic SARS-CoV-2 infection. Of the 54 studies, 44 were published and 10 were collaborating cohorts (conducted in Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, Switzerland, and the US). The participant data were derived from the 44 published studies (10 501 hospitalized individuals and 42 891 nonhospitalized individuals), the 10 collaborating cohort studies (10 526 and 1906), and the 2 US electronic medical record databases (250 928 and 846 046). Data collection spanned March 2020 to January 2022.
Exposures
Symptomatic SARS-CoV-2 infection.
Main Outcomes and Measures
Proportion of individuals with at least 1 of the 3 self-reported Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) 3 months after SARS-CoV-2 infection in 2020 and 2021, estimated separately for hospitalized and nonhospitalized individuals aged 20 years or older by sex and for both sexes of nonhospitalized individuals younger than 20 years of age.
Results
A total of 1.2 million individuals who had symptomatic SARS-CoV-2 infection were included (mean age, 4-66 years; males, 26%-88%). In the modeled estimates, 6.2% (95% uncertainty interval [UI], 2.4%-13.3%) of individuals who had symptomatic SARS-CoV-2 infection experienced at least 1 of the 3 Long COVID symptom clusters in 2020 and 2021, including 3.2% (95% UI, 0.6%-10.0%) for persistent fatigue with bodily pain or mood swings, 3.7% (95% UI, 0.9%-9.6%) for ongoing respiratory problems, and 2.2% (95% UI, 0.3%-7.6%) for cognitive problems after adjusting for health status before COVID-19, comprising an estimated 51.0% (95% UI, 16.9%-92.4%), 60.4% (95% UI, 18.9%-89.1%), and 35.4% (95% UI, 9.4%-75.1%), respectively, of Long COVID cases. The Long COVID symptom clusters were more common in women aged 20 years or older (10.6% [95% UI, 4.3%-22.2%]) 3 months after symptomatic SARS-CoV-2 infection than in men aged 20 years or older (5.4% [95% UI, 2.2%-11.7%]). Both sexes younger than 20 years of age were estimated to be affected in 2.8% (95% UI, 0.9%-7.0%) of symptomatic SARS-CoV-2 infections. The estimated mean Long COVID symptom cluster duration was 9.0 months (95% UI, 7.0-12.0 months) among hospitalized individuals and 4.0 months (95% UI, 3.6-4.6 months) among nonhospitalized individuals. Among individuals with Long COVID symptoms 3 months after symptomatic SARS-CoV-2 infection, an estimated 15.1% (95% UI, 10.3%-21.1%) continued to experience symptoms at 12 months.
Conclusions and Relevance
This study presents modeled estimates of the proportion of individuals with at least 1 of 3 self-reported Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) 3 months after symptomatic SARS-CoV-2 infection.
This study estimates the proportion of males and females, younger or older than 20 years of age, affected by at least 1 of the 3 Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) after SARS-CoV-2 infection in 2020 and 2021 and their symptom severity and expected duration of Long COVID.
Introduction
Much of the attention on disease surveillance during the COVID-19 pandemic has concentrated on the number of SARS-CoV-2 infections, hospital admissions, and deaths. Less attention has been given to quantifying the risk for experiencing symptoms after the acute stage of SARS-CoV-2 infection. In October 2021, the World Health Organization (WHO) released a clinical case definition for the post–COVID-19 condition as symptoms that are present 3 months after SARS-CoV-2 infection with a minimum duration of 2 months and cannot be explained by an alternative diagnosis.1 This is often referred to as Long COVID.
Postinfection fatigue syndromes have been described for other viruses and bacteria, including Ebola virus, Epstein-Barr virus, and cytomegalovirus.2,3 Ongoing low-grade inflammation has been postulated to cause these symptoms, but the pathology remains largely unknown and treatments are primarily based on symptom relief.4 The consequences for affected individuals are substantial, and specialized clinics for individuals with Long COVID have arisen to respond to an increasing need for supportive and rehabilitative care.5,6
A systematic review7 of 45 follow-up studies of individuals with COVID-19, of which only 3 had follow-up longer than 3 months, found 84 long-term symptoms with shortness of breath, fatigue, and sleep disorders or insomnia as the most common. Studies have reported most frequently on individual symptoms or counts of symptoms and have reported less frequently on symptom severity, overlapping symptoms, and symptom duration.8,9,10,11
This study collated information on 3 common clusters of Long COVID symptoms largely based on detailed data from ongoing COVID-19 follow-up studies conducted in 10 countries (Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, Switzerland, and the US), supplemented by published data from 44 studies and data from 2 medical record databases. From this pooled information on the occurrence of 3 Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems), estimates were made of the proportion of individuals who had symptomatic SARS-CoV-2 infection and at least 1 of the 3 symptom clusters 3 months after infection, and the duration of these symptom clusters was derived for 2020 and 2021.
Methods
This research was undertaken as part of the Global Burden of Diseases, Injuries, and Risk Factors Study and used deidentified data. A waiver of informed consent was reviewed and approved by the University of Washington institutional review board.
Overview of the Analysis
The analysis comprised 5 components (Figure 1 and eFigure 1 in Supplement 1). First, the proportion of symptomatic survivors with 1 or more of the 3 symptom clusters of Long COVID (persistent fatigue with bodily pain or mood swings, cognitive problems, or ongoing respiratory problems) and the key symptoms of fatigue, cognitive problems, and shortness of breath were extracted from 54 international cohort studies and 2 US medical record databases. Of the 10 collaborating cohort studies with individual case records available, 4 did not report on (1) excess risk of Long COVID symptom clusters compared with controls or (2) self-reported health status prior to COVID-19; therefore, these cohorts were adjusted by the ratio of excess risk of Long COVID symptoms to total symptoms from the 6 that reported both.
Figure 1. Analytical Flowchart of Estimation Process for Long COVID Symptom Clusters.
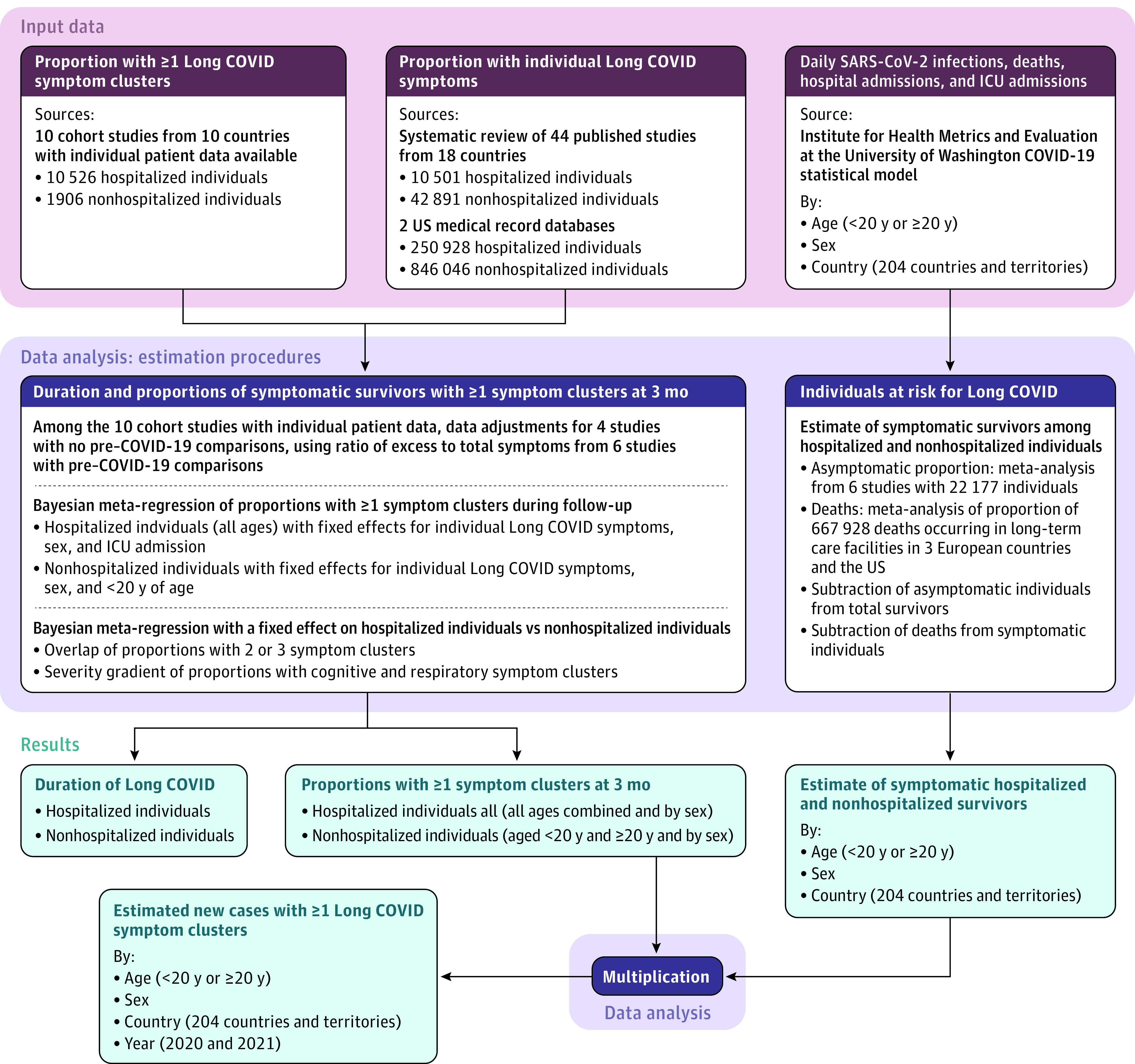
ICU indicates intensive care unit. The 3 Long COVID symptom clusters were persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems and were self-reported 3 months after SARS-CoV-2 infection in 2020 and 2021.
Second, the proportion of individuals with Long COVID symptom clusters after acute SARS-CoV-2 infection were estimated using a bayesian meta-regression tool separately for hospitalized and nonhospitalized individuals. Third, estimates from the studies providing distributions of symptom cluster overlap and severity gradients of cognitive and respiratory problems were pooled.
Fourth, estimates of daily SARS-CoV-2 infections, hospital admissions, intensive care unit (ICU) admissions, and deaths due to SARS-CoV-2 infection were taken from the Institute for Health Metrics and Evaluation at the University of Washington COVID-19 statistical model.12,13 The number of SARS-CoV-2 infections was multiplied by the pooled estimate of the proportion of infections without symptoms, and then deaths were subtracted from the estimate of symptomatic cases to get the estimates by age, sex, and country for symptomatic survivors of SARS-CoV-2 infection. Fifth, the global estimates of symptomatic COVID-19 survivors were multiplied by the proportion of individuals experiencing at least 1 of the 3 Long COVID symptom clusters 3 months after SARS-CoV-2 infection.
Study Population
There were data from 54 studies (44 published studies and 10 collaborating cohort studies) and 2 medical record databases for individuals who had symptomatic SARS-CoV-2 infection. Data from the study populations ranged from a full account of all cases of SARS-CoV-2 infection in the Faroe Islands to cases identified at health facilities, volunteers reporting symptoms in an app, and individuals enrolled in medical insurance. Individuals with Long COVID had new-onset or persisting symptoms 3 months after onset of symptomatic SARS-CoV-2 infection and COVID-19 that were not preexisting. This description aligns with the WHO clinical case definition of the post–COVID-19 condition, which is their preferred term for Long COVID.1
Long COVID Symptom Clusters
The symptom clusters were selected based on reporting frequency in published studies and the ability to characterize them using health state descriptions from the Global Burden of Disease Study. The 3 Long COVID symptom clusters selected were (1) persistent fatigue with bodily pain (myalgia) or mood swings; (2) cognitive problems (forgetfulness or difficulty concentrating, commonly referred to as brain fog); and (3) ongoing respiratory problems (shortness of breath and persistent cough as the main symptoms). In addition, 2 severity levels for cognitive problems were selected as well as 3 severity levels for ongoing respiratory symptoms. The health states and corresponding symptom descriptions used for the 3 Long COVID symptom clusters appear in Table 1.
Table 1. Health States, Symptom Descriptions, and Disability Weights Used for the 3 Long COVID Symptom Clusters.
| Symptom cluster | Health state | Symptom description | Disability weight (95% UI)a |
|---|---|---|---|
| Ongoing respiratory problems | |||
| Mild symptoms | Mild chronic respiratory problems | Cough and shortness of breath after heavy physical activity, but able to walk long distances and climb stairs | 0.02 (0.01-0.04) |
| Moderate symptoms | Moderate chronic respiratory problems | Cough, wheezing, and shortness of breath even after light physical activity; feel tired and can only walk short distances or climb a few stairs | 0.23 (0.15-0.31) |
| Severe symptoms | Severe chronic respiratory problems | Cough, wheezing, and shortness of breath all the time; great difficulty walking even short distances or climbing any stairs, feel tired when at rest, and have anxiety | 0.41 (0.27-0.56) |
| Cognitive problems | |||
| Mild symptomsb | Mild cognitive problems | Some trouble remembering recent events and find it hard to concentrate and make decisions and plans | 0.07 (0.05-0.10) |
| Severe symptomsb | Moderate cognitive problems | Memory problems and confusion, feel disoriented, hear voices sometimes that are not real, and need help with some daily activities | 0.38 (0.25-0.51) |
| Persistent fatigue with bodily pain or mood swings | Postacute consequences of an infectious disease | Always tired and easily upset; feel pain all over the body and have depression | 0.22 (0.15-0.31) |
Abbreviation: UI, uncertainty interval.
Quantifies health loss as a fraction of time lived within a health state (0 indicates full health; 1 indicates complete loss of health).
Also used in the Global Burden of Disease Study for mild and moderate dementia. Additional details appear in eSection 1 in Supplement 1.
Systematic Review and Data Extraction
A systematic review was conducted of the 44 published studies on the long-term symptoms after COVID-19 (eTable 1 and eFigures 2-3 in Supplement 1 and Supplement 2). The published studies were supplemented with more detailed individual-level data from the 10 collaborating cohort studies (eTable 1 and eFigure 4 in Supplement 1) and data from 2 US medical record databases (eTables 1-2 in Supplement 1).
For the 10 collaborating cohort studies, algorithms were developed and applied to extract the 3 Long COVID symptom clusters by symptom severity level to most closely match the symptom descriptions in Table 1 (additional information appears in eSection 1 in Supplement 1). Data from 4 of the collaborating cohort studies that did not report pre–COVID-19 health status were adjusted downward based on the ratio of excess risk of Long COVID symptoms to total symptoms reported from the 6 collaborating cohort studies with available individual-level data on pre–COVID-19 health status (eTable 3 in Supplement 1).14,15,16,17,18,19,20 Respondents with insufficient follow-up data to apply the algorithms were excluded. All extracted data used in the analyses appear in Supplement 3. Data also were extracted from the 44 published follow-up studies reporting on the key defining symptoms of the 3 Long COVID symptom clusters: fatigue, shortness of breath, and cognitive dysfunction.
Long COVID Outcomes
The main outcome was the proportion of individuals with at least 1 of the 3 Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) 3 months after symptomatic SARS-CoV-2 infection and 12 months after COVID-19 illness. Additional outcomes included the duration and relative severity of the Long COVID symptom clusters.
Statistical Analysis
Bayesian meta-regression of the data was performed using the Meta-Regression Tool (MRTool) version 0.0.1 (Institute for Health Metrics and Evaluation at the University of Washington) and R package MR-BRT 002 (R Foundation for Statistical Computing) with tabulated data from each study on the proportion of individuals who experienced at least 1 of the 3 Long COVID symptom clusters during follow-up.21 Indicator variables for male and female sex and study-level random effects were added. Separate models were run for hospitalized and nonhospitalized individuals with an indicator variable for those who were admitted to the ICU in the hospitalized model and for individuals younger than 20 years of age in the nonhospitalized model (eSection 2, eTables 4-9, and eFigures 5-10 in Supplement 1). The statistical differences between the proportion of individuals by sex and age (<20 years or ≥20 years) were determined by estimating the difference at each of 1000 draws of the posterior and presented as means with 95% uncertainty intervals (UIs) and deemed statistically significant if the full range of the 95% UI was either negative or positive.
The overlap of 2 or 3 Long COVID symptom clusters and the severity gradients of the cognitive and respiratory clusters were pooled using the MRTool with indicator variables for individuals who were hospitalized and study-level random effects (eSection 3, eTables 10-12, and eFigures 11-13 in Supplement 1).
The Long COVID symptom cluster duration values for hospitalized and nonhospitalized individuals were derived from the final proportion models having at least 1 symptom cluster (eSection 2 in Supplement 1).
The estimates of SARS-CoV-2 infection were taken from the Institute for Health Metrics and Evaluation at the University of Washington COVID-19 statistical model, which is a statistical susceptible, exposed, infected, and removed compartmental model used to fit data on the daily reported deaths, hospitalizations, and SARS-CoV-2 infections; seroprevalence; and excess mortality data.12,13 The Institute for Health Metrics and Evaluation at the University of Washington COVID-19 statistical model used an ensemble modeling strategy selecting predictive covariate combinations that best accounted for input data variance.
For this analysis, Long COVID was assumed to occur only in those with symptomatic SARS-CoV-2 infection (eSection 4 in Supplement 1). Studies were selected from a published review22 that estimated the proportion of asymptomatic SARS-CoV-2 infections in representative samples screened with antibody testing (Supplement 3). The logit-transformed proportion of individuals with asymptomatic SARS-CoV-2 infection was pooled from 6 studies in a random-effects meta-analysis (eFigure 14 and eTable 13 in Supplement 1), and 1 minus the pooled proportion was multiplied by the number of SARS-CoV-2 infections to estimate the incidence of symptomatic SARS-CoV-2 infections (eFigure 15 in Supplement 1). Deaths were then subtracted from the number of symptomatic SARS-CoV-2 infections to obtain the number of individuals who survived and had symptomatic infection separately for those needing care in a general hospital ward or in the ICU and those not needing such care (eFigures 16-17 in Supplement 1).
The individuals who survived were multiplied by the estimated proportion of individuals who were either hospitalized or not hospitalized with each Long COVID symptom cluster at 3 months (eSection 5 in Supplement 1). Uncertainty was propagated through 1000 posterior draws of every stage of the analysis. The 95% UIs are presented for all estimates based on the 25th and 975th values of the ordered 1000 draws of the final posterior distributions.
To quantify what proportion of cases with Long COVID symptoms would be missed by concentrating on the 3 large symptom clusters, the most detailed and largest cohort with individual records available from Russia was further scrutinized.20 Reported symptoms were tabulated among cases who reported not having recovered from COVID-19 and who reported worse overall health status on the 5-dimension EuroQol 5L index measure compared with their rating using the same measure before having SARS-CoV-2 infection. A sensitivity analysis was conducted to estimate symptom duration using all available data rather than limiting the symptom duration model input data to studies with multiple follow-up points.
This analysis complies with the Guidelines for Accurate and Transparent Health Estimates Reporting23 (Supplement 4).
Results
This observational analysis involved bayesian meta-regression and pooling of 54 studies and 2 medical record databases with data for 1.2 million individuals (from 22 countries) who had symptomatic SARS-CoV-2 infection (mean age range among the data sources, 4-66 years; range for proportion of males, 26%-88%). The participant data were derived from 44 published studies (10 501 hospitalized individuals and 42 891 nonhospitalized individuals), 10 collaborating cohort studies (10 526 hospitalized individuals and 1906 nonhospitalized individuals with COVID-19), and 2 US electronic medical record databases (250 928 hospitalized individuals and 846 046 nonhospitalized individuals) (Table 2,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74 eTable 1 in Supplement 1, and Supplement 3).
Table 2. Summary Characteristics of the Data Sourcesa.
| Studies with access to individual-level data | Studies without access to individual-level data | Medical claims databasesc: matched COVID-19–negative controls | |||
|---|---|---|---|---|---|
| Data on health status before COVID-19 | No data on health status before COVID-19b | COVID-19–negative control group | No control group | ||
| Age, mean (SD), y | 53.7 (20.6) | 48.6 (18.6) | 35.8 (12.8) | 47.2 (14.9) | 52.6 (21.7) |
| Sex, % | |||||
| Male | 50.0 | 53.8 | 45.8 | 48.0 | 44.7 |
| Female | 50.0 | 46.2 | 54.2 | 52.0 | 55.3 |
| Countries with input datad | Austria,19 Iran,15 Italy,18 the Netherlands,16 Russia,17,20 Switzerland14 | Faroe Islands,24 Germany,25,26 Sweden,27,28 US29 | China,30 Denmark,31 Norway,32 UK,10,33,34 US35 | Australia,36 Belgium,37 China,38 France,39,40,41,42 India,43,44 Iran,45 Israel,46,47 Italy,48,49,50,51,52,53 the Netherlands,54 Norway,55 Saudi Arabia,56 South Africa,57 Spain,58,59,60,61,62 Switzerland,63 Turkey,64 UK,11,65,66,67,68,69 US9,70,71,72 | US73,74 |
| Hospitalizede | 10 198 | 328 | 8516 | 9915 | 250 928 |
| Not hospitalized | 1355 | 551 | 34 375 | 586 | 846 046 |
Stratified by method of controlling for non–COVID-attributable symptoms. Details of each study appear in eTable 1 in Supplement 1.
Data were adjusted by the ratio of excess risk of Long COVID symptoms to total symptoms from the 6 collaborating cohort studies that reported these types of data.
Based on a range of demographic and comorbid conditions, 2 US administrative databases were used to match controls to cases with a positive polymerase chain reaction test for COVID-19. The difference between the cases and controls was used as the proportion of symptoms attributable to COVID-19.
All extracted data used in the analyses appear in Supplement 3.
Received care in general hospital ward or intensive care unit.
For data extraction in the 2 US electronic medical record databases, International Classification of Diseases, 10th Revision, codes were used for cognitive symptoms, fatigue, and respiratory symptoms (eTable 2 in Supplement 1). Of the 10 collaborating cohort studies, 3 included individuals who were younger than 20 years of age. Of the 12 432 participants in these collaborating cohort studies, 203 did not have responses required by the Long COVID symptom cluster algorithms and were excluded.
An estimated 6.2% (95% UI, 2.4%-13.3%) of individuals with symptomatic SARS-CoV-2 infection who survived the acute episode experienced at least 1 of the 3 Long COVID symptom clusters (Table 3). The estimated proportion of individuals with at least 1 of the 3 Long COVID symptom clusters was greater in those who were admitted to ICUs (43.1% [95% UI, 22.6%-65.2%]) and in those who were admitted to general hospital wards (27.5% [95% UI, 12.1%-47.8%]) than in those who were not hospitalized (5.7% [95% UI, 1.9%-13.1%]), with higher proportions among females than males (Table 3 and eTable 14 in Supplement 1).
Table 3. Global Proportion of Individuals With at Least 1 of the 3 Long COVID Symptom Clusters.
| Proportion with Long COVID symptom clusters among survivors, % (95% UI)a | ||
|---|---|---|
| 3 mo after symptom onset | 12 mo after symptom onset | |
| All individuals | 6.2 (2.4-13.3) | 0.9 (0.3-2.0)b |
| Both sexes aged <20 yc | 2.8 (0.9-7.0) | 0.3 (0.1-0.8) |
| Women aged ≥20 y | 10.6 (4.3-22.2) | 1.7 (0.7-3.6) |
| Men aged ≥20 y | 5.4 (2.2-11.7) | 0.8 (0.3-1.8) |
| Hospitalized | ||
| Needed care in a general hospital ward | 27.5 (12.1-47.8) | 11.1 (4.7-19.7) |
| Females | 34.8 (16.5-57.3) | 15.1 (5.8-29.7) |
| Males | 21.6 (8.9-40.3) | 8.2 (2.9-17.7) |
| Needed care in an ICU | 43.1 (22.6-65.2) | 20.5 (9.8-32.9) |
| Females | 51.9 (29.7-73.6) | 26.6 (11.5-47.8) |
| Males | 35.8 (17.1-58.1) | 15.7 (6.0-31.9) |
| Not hospitalized | ||
| All individuals | 5.7 (1.9-13.1) | 0.7 (0.2-1.5) |
| Both sexes aged <20 yc | 2.7 (0.8-6.7) | 0.3 (0-0.8) |
| Women aged ≥20 y | 9.9 (3.4-21.2) | 1.3 (0.3-3.4) |
| Men aged ≥20 y | 4.8 (1.5-11.3) | 0.6 (0.1-1.5) |
Abbreviations: ICU, intensive care unit; UI, uncertainty interval.
Long COVID was defined as having at least 1 of the 3 symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) 3 months after symptomatic SARS-CoV-2 infection. Additional details appear in eTables 14-16 in Supplement 1.
Among individuals with Long COVID symptoms 3 months after symptomatic SARS-CoV-2 infection, 15.1% (95% UI, 10.3%-21.2%) continued to experience symptoms at 12 months.
Data were insufficient to stratify proportion estimates by sex.
Among individuals who were hospitalized, the estimated mean Long COVID symptom duration was 9.0 months (95% UI, 7.0-12.0 months) based on data from 6 studies (conducted in 5 high-income countries and in 1 upper-middle-income country) with 8660 respondents with symptomatic SARS-CoV-2 infection (eFigure 5 in Supplement 1). Among individuals who were not hospitalized, the estimated mean Long COVID symptom duration was 4.0 months (95% UI, 3.6-4.6 months) based data from 4 studies (conducted in 4 high-income countries) with 4918 participants with symptomatic SARS-CoV-2 infection.
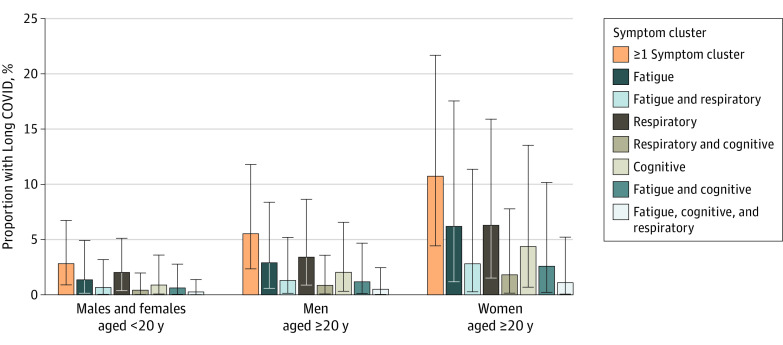
Of individuals with symptomatic SARS-CoV-2 infection, an estimated 3.2% (95% UI, 0.6%-10.0%) had persistent fatigue with bodily pain or mood swings, 3.7% (95% UI, 0.9%-9.6%) had ongoing respiratory problems, and 2.2% (95% UI, 0.3%-7.6%) had cognitive problems after adjusting for health status before COVID-19, comprising an estimated 51.0% (95% UI, 16.9%-92.4%), 60.4% (95% UI, 18.9%-89.1%), and 35.4% (95% UI, 9.4%-75.1%), respectively, of Long COVID cases. In an estimated 38.4% (95% UI, 7.94%-96.0%) of Long COVID cases, 2 or all 3 of the symptom clusters overlapped (Figure 2 and eTable 15 in Supplement 1).
Figure 2. Proportion of Individuals Who Survived Symptomatic SARS-CoV-2 Infection and Who Experienced at Least 1 of the 3 Long COVID Symptom Clusters in 2020 and 2021.
The proportion estimates with the 95% uncertainty intervals appear in eTables 14-15 in Supplement 1.
Globally, an estimated 63.2% (95% UI, 59.7%-66.3%) of individuals with Long COVID were female. The estimated risk of Long COVID at 3 months was lower in individuals with symptomatic SARS-CoV-2 infection who were not hospitalized and were younger than 20 years of age (2.7% [95% UI, 0.8%-6.7%]) than in those aged 20 years or older for both men (4.8% [95% UI, 1.5%-11.3%]) and women (9.9% [95% UI, 3.4%-21.2%]) (Table 3 and eTable 14 in Supplement 1). The difference in the estimated risk of Long COVID between individuals who were younger than 20 years of age and men aged 20 years or older was 2.0% (95% UI, 0.7%-4.6%), the difference between those younger than 20 years of age and women aged 20 years or older was 7.2% (95% UI, 2.6%-15.1%), and the difference between men and women aged 20 years or older was 5.1% (95% UI, 1.8-10.9), which were statistically significant differences.
Among COVID-19 survivors who developed Long COVID in 2020 and 2021 and had symptoms 3 months after SARS-CoV-2 infection, an estimated 15.1% (95% UI, 10.3%-21.1%) continued to have persistent symptoms at 12 months (Table 3). The global new cases with Long COVID symptom clusters by sex and severity of SARS-CoV-2 infection appear in eTable 16 in Supplement 1. The global counts of symptomatic SARS-CoV-2 infection and cases of Long COVID by country appear in eTable 17 in Supplement 1.
The detailed analysis of the Russian cohort found that the 3 Long COVID symptom clusters were present in 136 of 198 individuals reporting not having recovered and having worse general health status than before COVID-19 (eTable 18 in Supplement 1). The majority (48 of 62 persons) of those in the Russian cohort not covered by the 3 Long COVID symptom clusters defined in this article reported fatigue, respiratory, and cognitive symptoms but did not get included by the study’s algorithm because they reported no worsening in their ability to carry out usual activities.
In the sensitivity analysis with all data incorporated into the duration models rather than only studies with follow-up at multiple time points, the length of Long COVID increased slightly from 9.0 months (95% UI, 7.0-12.0 months) to 9.1 months (95% UI, 6.9-12.1 months) for individuals who were hospitalized and increased slightly from 4.0 months (95% UI, 3.6-4.6 months) to 4.7 months (95% UI, 4.0-5.4 months) for individuals who were not hospitalized and the proportion of Long COVID symptom clusters remained stable (eTable 19 in Supplement 1).
Discussion
This modeling study estimated that among patients with symptomatic SARS-CoV-2 infections who survived the acute phase in 2020 and 2021, 6.2% experienced at least 1 of the 3 Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) 3 months after acute infection onset. The risk of Long COVID was greater in females and in those who needed hospitalization for the initial SARS-CoV-2 infection, particularly among those needing ICU care.
The pattern of Long COVID symptoms by sex is distinct from that of severe acute SARS-CoV-2 infection, which tends to affect more males (eFigure 15 in Supplement 1).13 This difference suggests that the underlying mechanism of Long COVID may be different from that of the severity of acute SARS-CoV-2 infection. In general, women respond to viral infections with less severe disease and mount higher antibody responses but also have higher rates of adverse reactions to vaccinations and antiviral drugs; X chromosome–linked genes are thought to influence susceptibility to viral infections as well as autoimmune diseases, lending support to autoimmune processes playing a role in the development of Long COVID.75
A prolonged state of low-grade infection with a hyperimmune response, coagulation or vasculopathy, endocrine and autonomic dysregulation, and a maladaptation of the angiotensin-converting enzyme 2 pathway have been postulated as the underlying pathophysiology of Long COVID.76 Deconditioning due to prolonged immobilization during hospitalization may compound these problems.77
The analyses in this study are based on the WHO case definition that stipulates a minimum period of 3 months after SARS-CoV-2 infection before referring to ongoing symptoms as Long COVID or post–COVID-19 condition. Others have suggested a threshold of 3 weeks to define a case of Long COVID, arguing that no competent virus has been replicated beyond 3 weeks of infection, but periods of up to 12 weeks have been suggested to define the start of Long COVID.76,78,79 This analysis accounts for symptomatic SARS-CoV-2 infections through the end of 2021 and therefore does not cover the Omicron variant wave. Based on data from the UK COVID Symptom Study,80 a reduced odds of Long COVID symptoms between 0.24 and 0.50, depending on time since the last vaccination, was found for the Omicron variants compared with the Delta variants.
The estimated decline in reporting for any of the 3 Long COVID symptom clusters during follow-up among individuals not hospitalized suggests that the majority of Long COVID cases resolve. It is not yet clear if there is a smaller proportion of individuals, especially among those hospitalized for the acute episode of SARS-CoV-2 infection, who develop a more chronic course of Long COVID. Given that the longest follow-up among the included studies was 12 months, the true long-term pattern of symptom persistence for Long COVID will only be revealed as studies conduct longer follow-up. The time-limited course of Long COVID in most people has led to some recommendations to provide rehabilitative support in the community, with specialist rehabilitation services required only for those with protracted and more severe problems, particularly when compounded by postintensive care syndrome.78,81
Quantifying the number of individuals with Long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life. The large number of individuals with Long COVID may provide insights into phenotypical and genotypical characteristics, potentially leading to treatments and predictors of postacute disease syndromes, including those known to occur after other infectious diseases and intensive care for other critical illnesses. Postinfection fatigue syndrome has been previously reported for the Influenza A (H1N1) pandemic in 1918 and SARS-CoV-1 in 2003 and after the Ebola epidemic in West Africa in 2014. Similar symptoms have been reported after other viral infections including the Epstein-Barr virus, mononucleosis, and dengue as well as after nonviral infections such as Q fever, Lyme disease, and giardiasis.82
The collaborative structure of this study helped to provide consistent approaches in dealing with the diverse study methods and instruments used. It led to a definition of Long COVID symptom clusters and quantifying overlap among the symptom clusters. A key step was to correct for overreporting from studies that did not have a comparison with previous health status, leveraging information from the cohort studies that explicitly asked respondents to recall their pre–COVID-19 health status or existence of symptoms. In addition, the large US health insurance databases enabled identification of controls matched on demographic and disease characteristics and thus correct for the occurrence of these symptoms unrelated to SARS-CoV-2 infection. This may in part explain why these estimates of Long COVID are lower than the estimates often reported in the literature. Direct comparisons are unavailable because the Long COVID symptom clusters defined for this study have not been reported by others.
Limitations
This study has several limitations. First, the 95% UIs around the estimates are wide, reflecting limited and heterogeneous data.
Second, separate algorithms had to be formulated for each contributing study to achieve consistency in the case definitions of the 3 chosen Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings, cognitive problems, and ongoing respiratory problems). Efforts to achieve standardization of questions and instruments for studies of Long COVID are underway.1,83 This would make pooling estimates among studies less prone to measurement bias.
Third, it was assumed that Long COVID follows a similar course in all countries and territories. Data were used from western European countries, Australia, China, India, Iran, Israel, Russia, Saudi Arabia, South Africa, Turkey, and the US. Additional reports from Brazil and Bangladesh suggest that Long COVID similarly affects people in other parts of the world.84,85 As more information becomes available, any geographical variation in the occurrence or severity of Long COVID could be explored. The duration estimates for Long COVID relied on studies from high-income countries only. With repeated follow-up being planned in many of the studies, and with new studies being conducted, it should become clearer whether the findings related to the duration of Long COVID are generalizable.
Fourth, apart from the symptoms and Long COVID symptom clusters, new diseases and events have been reported to occur more frequently in patients after COVID-19 diagnosis, including cardiovascular complications like myocarditis, acute myocardial infarction, and thromboembolic events as well as kidney, liver, gastrointestinal, endocrine, and skin disorders.86,87,88 The data sources to quantify these COVID-19–related changes may not yet be sufficient due to lags in the reporting of clinical informatics data, disease registries, and surveys that form the basis of estimation for such diseases.
Fifth, it was assumed that Long COVID only affects those with a symptomatic course of the initial SARS-CoV-2 infection. The participating cohorts included few people with asymptomatic SARS-CoV-2 infection. The study from the Faroe Islands observed 22 individuals with fully asymptomatic SARS-CoV-2 infection, the study from Italy included 53, the study from Switzerland included 182, and the study from the US included 9.14,18,24,29 Long COVID was not identified among any individuals who were followed up in the Italy and US cohorts. In the Faroe Islands and Swiss cohorts, 3 individuals and 5 individuals, respectively, developed at least 1 of the 3 Long COVID symptom clusters during follow-up. The total number of individuals with asymptomatic SARS-CoV-2 infection followed up in these studies was low and, to be cautious, these individuals were excluded from calculations in this study. If Long COVID symptoms do occur in those who have an asymptomatic SARS-CoV-2 infection, the estimates would be higher.
Sixth, the analyses are based on 3 commonly reported Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings, cognitive problems, or ongoing respiratory problems) but not for other common symptoms reported as Long COVID. The main symptoms of the 3 Long COVID symptom clusters are those that reached the highest degree of consensus in the Delphi process that the WHO used to create a clinical case definition for the post–COVID-19 condition.1 The detailed analysis of the most complete cohort from Russia suggested that two-thirds of individuals who were reported as not having recovered or being worse off than before COVID-19 were captured by the 3 Long COVID symptom clusters included in this analysis, whereas most of the remaining one-third of individuals were reported as having the same symptoms but at a less severe level by which the symptoms did not interfere with the ability to perform usual activities (eSection 4 and eTable 18 in Supplement 1).20 The estimates, therefore, do not reflect the burden of the full range of Long COVID outcomes.
Conclusions
This study presents modeled estimates of the proportion of individuals with at least 1 of the 3 self-reported Long COVID symptom clusters (persistent fatigue with bodily pain or mood swings; cognitive problems; or ongoing respiratory problems) 3 months after symptomatic SARS-CoV-2 infection.
eSection 1. Extract long COVID symptom cluster input data
eSection 2. Estimate symptom cluster duration and proportions
eSection 3. Estimate symptom cluster overlap and severity distributions
eSection 4. Estimate symptomatic COVID cases that survive acute episode
eSection 5. Estimate symptom cluster incidence
List of abbreviations
eFigure 1. Flowchart of data, analytical processes, and long COVID symptom cluster outcomes
eFigure 2. PRISMA flow diagram of systematic literature review for long COVID
eFigure 3. Geographic distribution of published input data sources without access to individual-level data, with shading corresponding to total sample size per country
eFigure 4. Geographic distribution of cohort studies with access to individual-level data, with shading corresponding to total sample size per country
eFigure 5. Logit-linear model results of symptom cluster data with multiple follow-up points, used to calculate duration among non-hospitalized COVID cases and hospitalized COVID cases
eFigure 6. Age pattern of the proportion of surviving, symptomatic COVID-19 cases with at least one symptom cluster among the three largest cohort studies with individual record data, by sex and hospitalization status. Error bars represent 95% uncertainty intervals
eFigure 7. Model results: Overall long COVID
eFigure 8. Individual symptom clusters model results: fatigue
eFigure 9. Individual symptom clusters model results: respiratory
eFigure 10. Individual symptom clusters model results: cognitive
eFigure 11. Model results: Overlap of symptom clusters among long COVID patients
eFigure 12. Model results: Respiratory severity distributions
eFigure 13. Model results: Cognitive severity distributions
eFigure 14. Pooled estimate of proportion asymptomatic among SARS-CoV-2 infections
eFigure 15. Age distribution of asymptomatic SARS-CoV-2 infections, non-hospitalized cases, cases needing hospitalization, and cases needing ICU care, by sex
eFigure 16. Pooled estimate of proportion of COVID-19 deaths that occurred in long-term care facilities
eFigure 17. Case fatality ratios among hospitalized and ICU COVID-19 patients by age
eTable 1. Follow-up studies of long COVID, age and sex distributions, their inclusion of community and/or hospitalized cases, sample sizes, follow-up period, comparison method, and reported symptoms or symptom clusters
eTable 2. ICD-10-CM codes used to extract administrative data for cognitive symptoms, fatigue, and respiratory symptoms
eTable 3. Model coefficients for adjustment to account for underlying rates of symptom clusters
eTable 4. Model parameters for non-hospitalized long COVID duration
eTable 5. Model parameters for hospital/ICU long COVID duration
eTable 6. Model parameters for non-hospitalized overall long COVID
eTable 7. Model parameters for hospital/ICU overall long COVID
eTable 8. Model parameters for each symptom cluster model among non-hospitalized cases. Sources of the priors are the same as in the overall long COVID models
eTable 9. Model parameters for each symptom cluster model among hospital/ICU cases. Sources of the priors are the same as in the overall long COVID models
eTable 10. Model parameters for each overlap of symptom clusters model among long COVID cases
eTable 11. Model parameters for severity-specific cognitive symptom models
eTable 12. Model parameters for severity-specific respiratory symptom models
eTable 13. Input data of proportion asymptomatic among COVID infections
eTable 14. Estimated risk of long COVID among symptomatic community, hospitalized, and ICU COVID-19 cases by symptom cluster, sex and age group 3 months after symptom onset
eTable 15. Distribution of symptom clusters and their overlap among long COVID cases at 3 months after symptom onset (proportions are mutually exclusive)
eTable 16. Global new cases of long COVID symptom clusters by sex and severity of initial infection in 2020-2021, in millions
eTable 17. Symptomatic infections and new cases of long COVID by country, 2020 and 2021
eTable 18. Symptoms reported by respondents of the StopCOVID ISARIC Cohort in Russia who did not qualify for any of our long COVID symptoms clusters but reported not having recovered and worse health status than before COVID-19
eTable 19. Sensitivity analysis comparing current method with an alternative method which uses all available data to estimate the duration
eReferences
PRISMA compliance
Input data
GATHER checklist
Data sharing statement
References
- 1.World Health Organization . A clinical case definition of post COVID-19 condition by a Delphi consensus. October 6, 2021. Accessed November 5, 2021. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- 2.Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome: a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickie I, Davenport T, Wakefield D, et al. ; Dubbo Infection Outcomes Study Group . Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. doi: 10.1136/bmj.38933.585764.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374(1648):n1648. doi: 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan P. NICE guideline on Long COVID. Lancet Respir Med. 2021;9(2):129. doi: 10.1016/S2213-2600(21)00031-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanichkachorn G, Newcomb R, Cowl CT, et al. Post–COVID-19 Syndrome (Long Haul Syndrome): description of a multidisciplinary clinic at Mayo Clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96(7):1782-1791. doi: 10.1016/j.mayocp.2021.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417. doi: 10.1001/jamanetworkopen.2021.11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health . NIH launches new initiative to study “Long COVID.” February 23, 2021. Accessed June 7, 2021. https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid
- 9.Cirulli ET, Barrett KMS, Riffle S, et al. Long-term COVID-19 symptoms in a large unselected population. medRxiv. Published online December 1, 2020. doi: 10.1101/2020.10.07.20208702 [DOI]
- 10.Office for National Statistics . UK Coronavirus Infection Survey: updated estimates of the prevalence of Long COVID symptoms. January 21, 2021. Accessed February 2, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/adhocs/12788updatedestimatesoftheprevalenceoflongcovidsymptoms
- 11.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of Long COVID. Nat Med. 2021;27(4):626-631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber RM, Sorensen RJD, Pigott DM, et al. Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis. Lancet. Published online April 8, 2022. doi: 10.1016/S0140-6736(22)00484-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 Forecasting Team . Variation in the COVID-19 infection-fatality ratio by age, time, and geography during the pre-vaccine era: a systematic analysis. Published correction appears in Lancet. 2022;399(10334):1468. Lancet. 2022;399(10334):1469-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ISRCTN Registry . Zurich Coronavirus Cohort: an observational study to determine long-term clinical outcomes and immune responses after coronavirus infection (COVID-19), assess the influence of virus genetics, and examine the spread of the coronavirus in the population of the Canton of Zurich, Switzerland. Accessed September 26, 2022. https://www.isrctn.com/ISRCTN14990068
- 15.Sarrafzadegan N, Mohammadifard N, Haghjooy Javanmard S, et al. Isfahan COVID cohort study: rationale, methodology, and initial results. J Res Med Sci. 2022;27:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bek LM, Berentschot JC, Hellemons ME, et al. ; CO-FLOW Collaboration Group . CO-FLOW: COvid-19 Follow-up care paths and long-term outcomes within the Dutch health care system: study protocol of a multicenter prospective cohort study following patients 2 years after hospital discharge. BMC Health Serv Res. 2021;21(1):847. doi: 10.1186/s12913-021-06813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. medRxiv. Preprint posted online April 26, 2021. doi: 10.1101/2021.04.26.21256110 [DOI] [PMC free article] [PubMed]
- 18.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on Long COVID in children. Acta Paediatr. 2021;110(7):2208-2211. doi: 10.1111/apa.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rass V, Beer R, Schiefecker AJ, et al. Neurological outcome and quality of life 3 months after COVID-19: a prospective observational cohort study. Eur J Neurol. 2021;28(10):3348-3359. doi: 10.1111/ene.14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munblit D, Bobkova P, Spiridonova E, et al. ; Sechenov StopCOVID Research Team . Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021;51(9):1107-1120. doi: 10.1111/cea.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng P, Barber R, Sorensen RJD, Murray CJL, Aravkin AY. Trimmed constrained mixed effects models: formulations and algorithms. ArXiv. Preprint posted online October 27, 2020. https://arxiv.org/abs/1909.10700
- 22.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med. 2020;173(5):362-367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens GA, Alkema L, Black RE, et al. ; GATHER Working Group . Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. PLoS Med. 2016;13(6):e1002056. doi: 10.1371/journal.pmed.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2021;73(11):e4058-e4063. doi: 10.1093/cid/ciaa1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurth F, Roennefarth M, Thibeault C, et al. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID-19 patient cohort (Pa-COVID-19). Infection. 2020;48(4):619-626. doi: 10.1007/s15010-020-01464-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thibeault C, Mühlemann B, Helbig ET, et al. ; Pa-COVID Study Group . Clinical and virological characteristics of hospitalised COVID-19 patients in a German tertiary care centre during the first wave of the SARS-CoV-2 pandemic: a prospective observational study. Infection. 2021;49(4):703-714. doi: 10.1007/s15010-021-01594-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov . Follow-up of Critical COVID-19 Patients (FUP-COVID). Accessed September 26, 2022. https://clinicaltrials.gov/ct2/show/NCT04474249
- 28.Ekbom E, Frithiof R, Emilsson Ö, et al. Impaired diffusing capacity for carbon monoxide is common in critically ill Covid-19 patients at four months post-discharge. Respir Med. 2021;182:106394. doi: 10.1016/j.rmed.2021.106394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. doi: 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89-95. doi: 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikkenborg Berg S, Dam Nielsen S, Nygaard U, et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022;6(4):240-248. doi: 10.1016/S2352-4642(22)00004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Søraas A, Kalleberg KT, Dahl JA, et al. Persisting symptoms three to eight months after non-hospitalized COVID-19: a prospective cohort study. PLoS One. 2021;16(8):e0256142. doi: 10.1371/journal.pone.0256142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephenson T, Shafran R, De Stavola B, et al. ; CLoCk Consortium members . Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study). BMJ Open. 2021;11(8):e052838. doi: 10.1136/bmjopen-2021-052838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayoubkhani D, Pawelek P, Bosworth M; Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. August 5, 2021. Accessed August 24, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/5august2021
- 35.Wanga V, Chevinsky JR, Dimitrov LV, et al. Long-term symptoms among adults tested for SARS-CoV-2—United States, January 2020-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1235-1241. doi: 10.15585/mmwr.mm7036a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5(6):e22-e23. doi: 10.1016/S2352-4642(21)00124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darcis G, Bouquegneau A, Maes N, et al. Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: a monocentric prospective observational cohort study. Int J Infect Dis. 2021;109:209-216. doi: 10.1016/j.ijid.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zayet S, Zahra H, Royer PY, et al. Post-COVID-19 syndrome: nine months after SARS-CoV-2 infection in a cohort of 354 patients: data from the first wave of COVID-19 in Nord Franche-Comté Hospital, France. Microorganisms. 2021;9(8):1719. doi: 10.3390/microorganisms9081719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258-263. doi: 10.1016/j.cmi.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morin L, Savale L, Pham T, et al. ; Writing Committee for the COMEBAC Study Group . Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525-1534. doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4-e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chopra N, Chowdhury M, Singh AK, et al. Clinical predictors of Long COVID-19 and phenotypes of mild COVID-19 at a tertiary care centre in India. Drug Discov Ther. 2021;15(3):156-161. doi: 10.5582/ddt.2021.01014 [DOI] [PubMed] [Google Scholar]
- 44.Naik S, Haldar SN, Soneja M, et al. Post COVID-19 sequelae: a prospective observational study from northern India. Drug Discov Ther. 2021;15(5):254-260. doi: 10.5582/ddt.2021.01093 [DOI] [PubMed] [Google Scholar]
- 45.Asadi-Pooya AA, Nemati H, Shahisavandi M, et al. Long COVID in children and adolescents. World J Pediatr. 2021;17:495-499. doi: 10.1007/s12519-021-00457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkan M, Dvir A, Zaidenstein R, et al. Patient-reported outcome measures after hospitalization during the COVID-19 pandemic: a survey among COVID-19 and non-COVID-19 patients. Int J Gen Med. 2021;14:4829-4836. doi: 10.2147/IJGM.S323316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect. 2021;27(5)769-774. doi: 10.1016/j.cmi.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anastasio F, Barbuto S, Scarnecchia E, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;58(3):2004015. doi: 10.1183/13993003.04015-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. doi: 10.1001/jamanetworkopen.2020.36142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lombardo MDM, Foppiani A, Peretti GM, et al. Long-term coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open Forum Infect Dis. 2021;8(8):ofab384. doi: 10.1093/ofid/ofab384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10):1507-1513. doi: 10.1016/j.cmi.2021.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149:e32. doi: 10.1017/S0950268821000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327(6):559-565. doi: 10.1001/jama.2022.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4):2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tleyjeh IM, Saddik B, AlSwaidan N, et al. Prevalence and predictors of post-acute COVID-19 syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS One. 2021;16(12):e0260568. doi: 10.1371/journal.pone.0260568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dryden M, Mudara C, Vika C, et al. Long COVID in South Africa: findings from a longitudinal cohort of patients at one month after hospitalisation with SARS-CoV-2, using an ISARIC multi-country protocol. Accessed September 26, 2022. https://www.nicd.ac.za/wp-content/uploads/2021/08/COVID-19-Special-Public-Health-Surveillance-Bulletin-August-2nd-edition-2021.pdf
- 58.García-Abellán J, Padilla S, Fernández-González M, et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41:1490-1501. doi: 10.1007/s10875-021-01083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. ; COVID-19-ALC research group . Post-acute COVID-19 syndrome: incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378-383. doi: 10.1016/j.jinf.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sibila O, Albacar N, Perea L, et al. Lung function sequelae in COVID-19 patients 3 months after hospital discharge. Arch Bronconeumol. 2021;57:59-61. doi: 10.1016/j.arbres.2021.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suárez-Robles M, Iguaran-Bermúdez MDR, García-Klepizg JL, et al. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call check list. Pan Afr Med J. 2020;37:289. doi: 10.11604/pamj.2020.37.289.27110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taboada M, Cariñena A, Moreno E, et al. Post-COVID-19 functional status six-months after hospitalization. J Infect. 2021;82(4):e31-e33. doi: 10.1016/j.jinf.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becker C, Beck K, Zumbrunn S, et al. Long COVID 1 year after hospitalisation for COVID-19: a prospective bicentric cohort study. Swiss Med Wkly. 2021;151:w30091. doi: 10.4414/smw.2021.w30091 [DOI] [PubMed] [Google Scholar]
- 64.Kayaaslan B, Eser F, Kalem AK, et al. Post-COVID syndrome: a single-center questionnaire study on 1007 participants recovered from COVID-19. J Med Virol. 2021;93(12):6566-6574. doi: 10.1002/jmv.27198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76(4):399-401. doi: 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013-1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 67.Mandal S, Barnett J, Brill SE, et al. ; ARC Study Group . “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396-398. doi: 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sigfrid L, Drake TM, Pauley E, et al. ; ISARIC4C investigators . Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5(10):708-718. doi: 10.1016/S2352-4642(21)00198-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi: 10.7326/M20-5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horwitz LI, Garry K, Prete AM, et al. Six-month outcomes in patients hospitalized with severe COVID-19. J Gen Intern Med. 2021;36(12):3772-3777. doi: 10.1007/s11606-021-07032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021;73(3):e826-e829. doi: 10.1093/cid/ciab103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.PRA Health Sciences . A global healthcare intelligence partner. Accessed June 15, 2021. https://www.iconplc.com/
- 74.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259-264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 75.Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34(12):1050-1059. doi: 10.1002/bies.201200099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Candan SA, Elibol N, Abdullahi A. Consideration of prevention and management of long-term consequences of post-acute respiratory distress syndrome in patients with COVID-19. Physiother Theory Pract. 2020;36(6):663-668. doi: 10.1080/09593985.2020.1766181 [DOI] [PubMed] [Google Scholar]
- 78.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 79.Huang Y, Pinto MD, Borelli JL, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: looking for clarity in the haze of the pandemic. MedRxiv. Published online March 5, 2021. doi: 10.1101/2021.03.03.21252086 [DOI] [PMC free article] [PubMed]
- 80.Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of Long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263-2264. doi: 10.1016/S0140-6736(22)00941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502-509. doi: 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 82.Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue. 2020;8(2):61-69. doi: 10.1080/21641846.2020.1778227 [DOI] [Google Scholar]
- 83.COMET Initiative . Core outcome measures for post-Covid condition/Long Covid. Accessed August 24, 2021. https://www.comet-initiative.org/Studies/Details/1847
- 84.Barreto APA, Duarte LC, Cerqueira-Silva T, et al. Post-acute COVID syndrome, the aftermath of mild to severe COVID-19 in Brazilian patients. medRxiv. Published online June 9, 2021. doi: 10.1101/2021.06.07.21258520 [DOI]
- 85.Mahmud R, Rahman MM, Rassel MA, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS One. 2021;16(4):e0249644. doi: 10.1371/journal.pone.0249644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504-1507. doi: 10.1016/j.ajem.2020.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017-1032. doi: 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 88.Hultström M, Lipcsey M, Wallin E, Larsson IM, Larsson A, Frithiof R. Severe acute kidney injury associated with progression of chronic kidney disease after critical COVID-19. Crit Care. 2021;25(1):37. doi: 10.1186/s13054-021-03461-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eSection 1. Extract long COVID symptom cluster input data
eSection 2. Estimate symptom cluster duration and proportions
eSection 3. Estimate symptom cluster overlap and severity distributions
eSection 4. Estimate symptomatic COVID cases that survive acute episode
eSection 5. Estimate symptom cluster incidence
List of abbreviations
eFigure 1. Flowchart of data, analytical processes, and long COVID symptom cluster outcomes
eFigure 2. PRISMA flow diagram of systematic literature review for long COVID
eFigure 3. Geographic distribution of published input data sources without access to individual-level data, with shading corresponding to total sample size per country
eFigure 4. Geographic distribution of cohort studies with access to individual-level data, with shading corresponding to total sample size per country
eFigure 5. Logit-linear model results of symptom cluster data with multiple follow-up points, used to calculate duration among non-hospitalized COVID cases and hospitalized COVID cases
eFigure 6. Age pattern of the proportion of surviving, symptomatic COVID-19 cases with at least one symptom cluster among the three largest cohort studies with individual record data, by sex and hospitalization status. Error bars represent 95% uncertainty intervals
eFigure 7. Model results: Overall long COVID
eFigure 8. Individual symptom clusters model results: fatigue
eFigure 9. Individual symptom clusters model results: respiratory
eFigure 10. Individual symptom clusters model results: cognitive
eFigure 11. Model results: Overlap of symptom clusters among long COVID patients
eFigure 12. Model results: Respiratory severity distributions
eFigure 13. Model results: Cognitive severity distributions
eFigure 14. Pooled estimate of proportion asymptomatic among SARS-CoV-2 infections
eFigure 15. Age distribution of asymptomatic SARS-CoV-2 infections, non-hospitalized cases, cases needing hospitalization, and cases needing ICU care, by sex
eFigure 16. Pooled estimate of proportion of COVID-19 deaths that occurred in long-term care facilities
eFigure 17. Case fatality ratios among hospitalized and ICU COVID-19 patients by age
eTable 1. Follow-up studies of long COVID, age and sex distributions, their inclusion of community and/or hospitalized cases, sample sizes, follow-up period, comparison method, and reported symptoms or symptom clusters
eTable 2. ICD-10-CM codes used to extract administrative data for cognitive symptoms, fatigue, and respiratory symptoms
eTable 3. Model coefficients for adjustment to account for underlying rates of symptom clusters
eTable 4. Model parameters for non-hospitalized long COVID duration
eTable 5. Model parameters for hospital/ICU long COVID duration
eTable 6. Model parameters for non-hospitalized overall long COVID
eTable 7. Model parameters for hospital/ICU overall long COVID
eTable 8. Model parameters for each symptom cluster model among non-hospitalized cases. Sources of the priors are the same as in the overall long COVID models
eTable 9. Model parameters for each symptom cluster model among hospital/ICU cases. Sources of the priors are the same as in the overall long COVID models
eTable 10. Model parameters for each overlap of symptom clusters model among long COVID cases
eTable 11. Model parameters for severity-specific cognitive symptom models
eTable 12. Model parameters for severity-specific respiratory symptom models
eTable 13. Input data of proportion asymptomatic among COVID infections
eTable 14. Estimated risk of long COVID among symptomatic community, hospitalized, and ICU COVID-19 cases by symptom cluster, sex and age group 3 months after symptom onset
eTable 15. Distribution of symptom clusters and their overlap among long COVID cases at 3 months after symptom onset (proportions are mutually exclusive)
eTable 16. Global new cases of long COVID symptom clusters by sex and severity of initial infection in 2020-2021, in millions
eTable 17. Symptomatic infections and new cases of long COVID by country, 2020 and 2021
eTable 18. Symptoms reported by respondents of the StopCOVID ISARIC Cohort in Russia who did not qualify for any of our long COVID symptoms clusters but reported not having recovered and worse health status than before COVID-19
eTable 19. Sensitivity analysis comparing current method with an alternative method which uses all available data to estimate the duration
eReferences
PRISMA compliance
Input data
GATHER checklist
Data sharing statement