ABSTRACT
Synbiotics combine probiotics and prebiotics and are being investigated for potential health benefits. In this single-group-design trial, we analyzed changes in the gut microbiome, stool quality, and gastrointestinal well-being in 15 healthy volunteers after a synbiotic intervention comprising Lacticaseibacillus rhamnosus (LGG), Lactobacillus acidophilus (LA-5), Lacticaseibacillus paracasei subsp. paracasei (L. CASEI 431), and Bifidobacterium animalis subsp. lactis BB-12 and 20 g of chicory-derived inulin powder consumed daily for 4 weeks. Fecal samples were collected at baseline and at completion of the intervention, and all participants completed a fecal diary based on the Bristol Stool Scale and recorded their gastrointestinal well-being. No adverse effects were observed after consumption of the synbiotic product, and stool consistency and frequency remained almost unchanged during the trial. Microbiome analysis of the fecal samples was achieved using shotgun sequencing followed by taxonomic profiling. No changes in alpha and beta diversity were seen after the intervention. Greater relative abundances of Bifidobacteriaceae were observed in 12 subjects, with indigenous bifidobacteria species constituting the main increase. All four probiotic organisms increased in abundance, and L. rhamnosus, B. animalis, and L. acidophilus were differentially abundant, compared to baseline. Comparison of the fecal strains to the B. animalis subsp. lactis BB-12 reference genome and the sequenced symbiotic product revealed only a few single-nucleotide polymorphisms differentiating the probiotic B. animalis subsp. lactis BB-12 from the fecal strains identified, indicating that this probiotic strain was detectable after the intervention.
IMPORTANCE The effects of probiotics/synbiotics are seldom investigated in healthy volunteers; therefore, this study is important, especially considering the safety aspects of multiple probiotics together with prebiotic fiber in consumption by humans. The study explores at the potential of a synbiotic intervention with lactobacilli, bifidobacteria, and inulin in healthy volunteers and tracks the ingested probiotic strain B. animalis subsp. lactis.
KEYWORDS: synbiotics, probiotics, prebiotics, inulin, bifidobacteria, lactobacilli, metagenomics, microbiome, shotgun, fecal
INTRODUCTION
In recent years, more knowledge has been gained about the role of the human gut microbiota in host physiology and human health. Gut microbiota alterations have been linked to a wide range of conditions, from inflammatory bowel diseases, irritable bowel syndrome, obesity, and type 2 diabetes mellitus to behavioral disorders, schizophrenia, and autism (1–8). Prebiotics, which are carbohydrates that are metabolized only by gut bacteria, have gained much attention in recent years for their potential health benefits. Apart from being bifidogenic, their effects are also mediated through metabolism by microbes, generating short-chain fatty acids, including acetate, propionate, and butyrate, as well as promotion of ion and trace element absorption and regulation of the immune system (9–11).
The composition of the human gut microbiome is influenced by geographic location, age, diet, and especially treatment with antibiotics (12–14). The term “transient microbiome” has been proposed to describe how the composition of the microbiome is dynamic and variable and is influenced by factors such as diet, exposure to ingested probiotic bacteria, environmental conditions of the gut, and other host-associated factors (15). As well as being composed of commensal residents, the gut microbiota is constantly exposed to exogenous microbes from the diet that transiently become part of this ecosystem (16). The term “colonization resistance” denotes the ability of indigenous bacterial communities to undergo colonization by nonindigenous bacteria. Some strains, such as probiotic strains, can pass safely through the stomach, reach the intestine, and affect the composition and activity of the resident gut communities, as has been shown for the well-documented probiotic Bifidobacterium animalis subsp. lactis BB-12 (17).
A synbiotic is defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (18). Synbiotics have been demonstrated to change the microbiota composition in different patient populations, but little is known regarding the effect of synbiotics on modulation of the gut microbiome in healthy individuals (19, 20). While simple sugars, such as lactose and sucrose, are metabolized in the upper gut by bacteria such as lactobacilli, prebiotics, such as nondigestible oligosaccharides, are metabolized in the lower gut by bifidobacterial species, among others (21). On average, 12% of the bifidobacterial genome is predicted to encode enzymes involved in carbohydrate metabolism (22). Strategies used by bifidobacteria to metabolize carbohydrates has been thoroughly reviewed elsewhere (23).
Recent literature has suggested a beneficial role of a diet rich in inulin both in the gut microbiome and in food-related behavior, such as satiety and reduced desire to eat sweet and salty food (24). A recent systematic review found that inulin supplementation led to a consistent increase in the relative abundance of bifidobacteria and a decrease in the relative abundance of Bacteroides, and it has been implied to give rise to a beneficial microbiota and promote weight loss in obese patients (25, 26).
The aims of our study were to monitor the gastrointestinal function and explore the modulation of the healthy bacterial microbiota through intake of a synbiotic product in a pilot trial of healthy volunteers. This will lead to more insight into the potential role of synbiotics in the gut microbiota and gastrointestinal well-being.
RESULTS
Gastrointestinal well-being and stool quality.
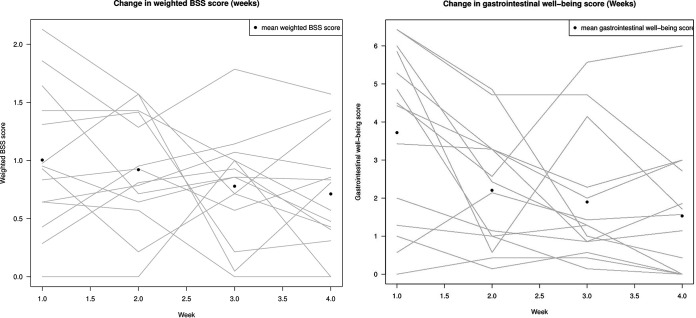
Fifteen healthy participants were enrolled to consume a synbiotic product consisting of Lacticaseibacillus rhamnosus (LGG), Lactobacillus acidophilus (LA-5), Lacticaseibacillus paracasei subsp. paracasei (L. CASEI 431), Bifidobacterium animalis subsp. lactis (BB-12), and inulin (fructo-oligosaccharide). One subject was noncompliant with the protocol and was excluded from further analyses. The synbiotic product was generally well tolerated. Of a total of 24 possible points, the average score among the participants during the 4-week intervention period was 2.4 (range, 0.2 to 4.8 points) (Fig. 1, right). Because the participants were healthy volunteers, the symptom score, which was very low, was considered only in terms of possible adverse effects. Bloating and gases were especially reported by participants during the first 2 weeks of the study. Three (21%) of 14 participants reported worse gastrointestinal function after the intervention, while 5 participants (36%) reported better gastrointestinal function and 6 participants (43%) reported unchanged gastrointestinal function in the self-observed result diary. This finding should be seen in the light of it being a self-observed result, there being no discontinuations, and the average symptom score being very low. There was a trend toward better stool quality in terms of consistency (type 3 and 4) at the end, compared with the start, of the intervention (Fig. 1, left) (nonsignificant). The average stool frequency was 1.7 stools per day during the intervention period (range, 1 to 5 stools per day) in the self-reported result diary; the average frequency before the intervention started (baseline) was 1.55 stools per day (range, 1 to 35 stools per day).
FIG 1.
wBSSS and gastrointestinal well-being scores. (Right) Changes in wBSSS values during the intervention for each participant. The wBSSS was calculated from the BSS by assigning a score between 0 and 3 to each stool type, with 0 representing the closest to normal stool and 3 the greatest divergence from normal stool. Each participant is represented by a line. (Left) Changes in gastrointestinal well-being during the intervention. The symptoms evaluated were nausea, stomach cramps, stomach pain, bloating, rumbling, and gases, graded from 0 (none) to 4 (unbearable) (giving a possible total maximum score of 24). The dots represent the average symptom scores for all participants during the intervention, while the lines show the average symptom scores during the intervention for each participant.
Microbiome of the fecal samples.
Sequencing of the fecal samples resulted in total numbers of reads per sample (forward and reverse) ranging from 12,441,554 to 47,725,002 reads per sample (median, 20,409,274 reads per sample). These numbers were practically unchanged after depletion of human DNA reads, with the median number of reads being 20,232,764. For the positive control, 42,869,146 reads were obtained; for the negative control, only 14,028 reads were obtained.
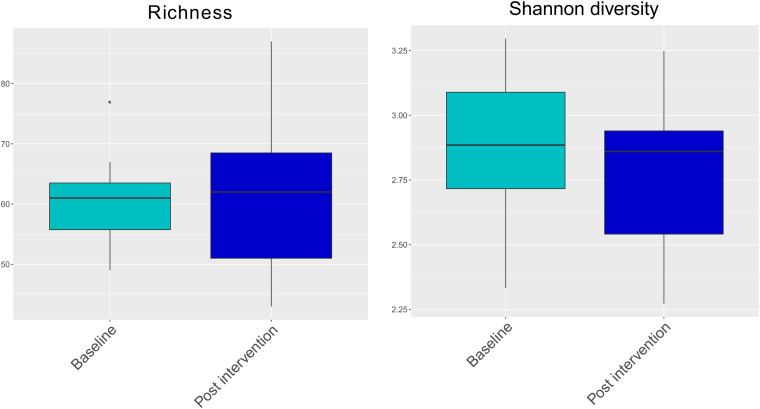
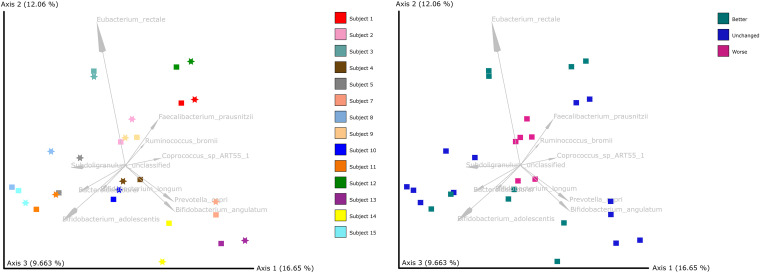
Taxonomic profiling with MetaPhlAn2 detected a total of 205 different bacterial species in the fecal samples (see the supplemental material). After organisms with low abundance were filtered out, 142 different bacterial species remained. No significant changes in species richness and Shannon diversity were seen between baseline and postintervention samples (Fig. 2). Beta diversity analyses showed grouping by subject (P = 0.001, permutational multivariate analysis of variance [PERMANOVA] with 999 permutations) rather than by sampling time (P = 0.222) (Fig. 3, left). Samples from subjects who reported overall gastrointestinal function to be worse after the intervention tended to cluster together (Fig. 3, right).
FIG 2.
Alpha diversity (Shannon index and richness). (Left) Species richness (number of species detected) for the participants at baseline and postintervention (P = 0.8064, Wilcoxon signed-rank test). (Right) Shannon diversity index for the participants at baseline and postintervention (P = 0.391, Wilcoxon signed-rank test). The lines in the boxes represent the medians.
FIG 3.
PCoA plots based on Aitchison distances. (Left) Samples colored by subject, where squares represent baseline samples and stars represent postintervention samples. (Right) Samples colored by gastrointestinal function status at the end of the intervention.
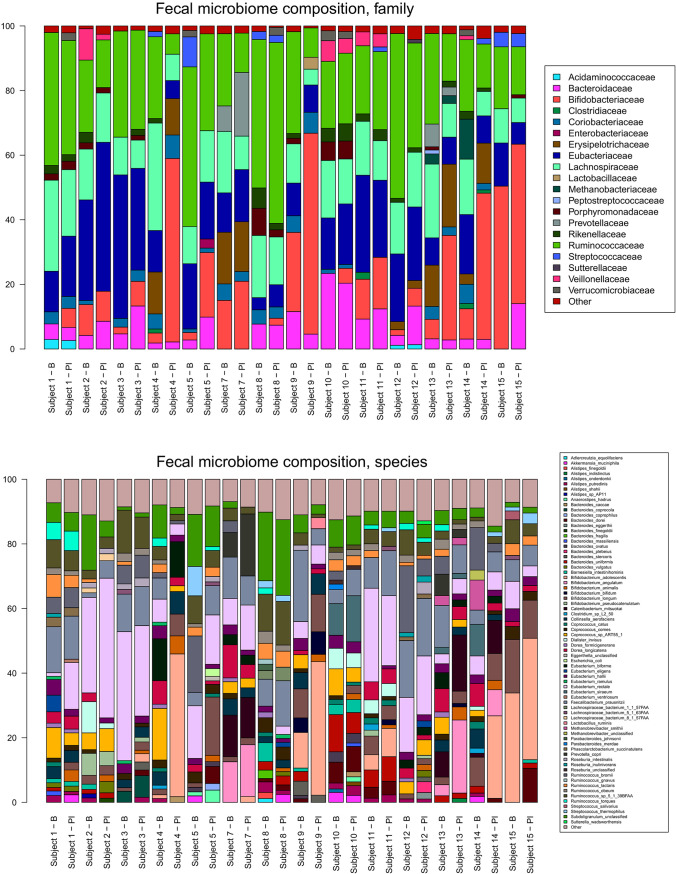
At the species level, Bifidobacterium adolescentis and Bifidobacterium longum were the most prevalent bifidobacterial species at baseline (Fig. 4, bottom). Large increases in the relative abundance of B. adolescentis were observed in subjects 4, 9, and 14, while a large increase in B. longum was seen in subject 5. Bifidobacterium angulatum was found in only 2 of the subjects at baseline and 3 of the subjects postintervention and increased most noticeably in subjects 13 and 14. Bifidobacterium animalis was found in only 2 of the subjects at baseline but in all subjects except subject 15 after the intervention.
FIG 4.
Relative abundances of families (top) and species (bottom) detected in the metagenomic data. Species or families occurring at relative abundances below 1% are grouped as “Other.” B, baseline; PI, postintervention.
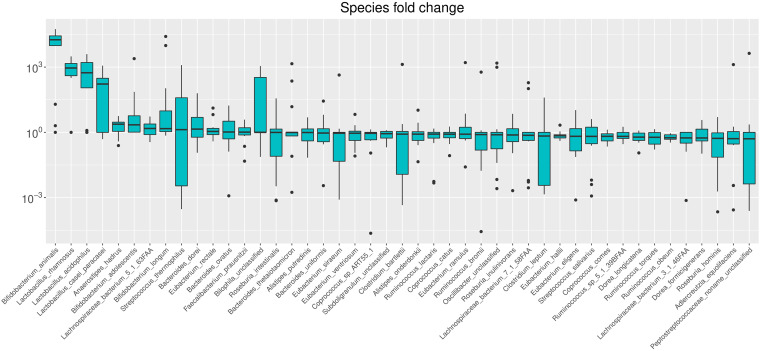
To obtain an overview of which species in general increased or decreased during the intervention, we estimated the ratio between baseline and postintervention relative abundances (Fig. 5). The four species showing the largest fold increases in relative abundance were the four probiotic strains ingested. Anaerostipes hadrus, Lachnospiraceae bacterium, B. adolescentis, and B. longum also showed general trends toward increasing relative abundances, although the median fold increases were smaller for these species. At the other end of the scale, Dorea formicigenerans, Roseburia hominis, Adlercreutzia equolifaciens, and an unclassified Peptostreptococcaceae species showed general trends toward decreasing relative abundances, although the median fold decreases were small.
FIG 5.
Changes in species relative abundance. The ratios between postintervention and baseline relative abundances are shown. Relative abundances of zero were set to 0.0001% prior to ratio calculation. The species are sorted according to median ratio (highest first and lowest last), and only species with a median ratio different from 1 are included in the figure.
The three administered probiotic Lactobacillus species were each detected in 1 (L. acidophilus and L. paracasei subsp. paracasei) or none (L. rhamnosus) of the subjects at baseline; after the intervention, they were detected in 12 (L. acidophilus), 9 (L. paracasei subsp. paracasei), and 13 (L. rhamnosus) of the subjects (see the supplemental material). The relative abundances of these species were low, however, ranging from 0.01 to 0.39%.
Relative abundances are not always easily interpretable on their own because they are not independent of each other, reflecting the compositional nature of microbiome data (27). Therefore, we used ALDEx2 to identify species that were differentially abundant between baseline and postintervention samples. ALDEx2 identified L. rhamnosus, B. animalis, and L. acidophilus to be differentially abundant between baseline and postintervention samples (Benjamini-Hochberg corrected P value from Welch’s t test of <0.005, with effect size of >1) (see Fig. S1 and Table S1 in the supplemental material). This finding indicates that these three probiotic species were the species showing the most consistent increases across the study subjects, relative to baseline levels.
All bacterial species present in the positive control were detected by MetaPhlan2. No additional species were detected except for a small fraction of sequences belonging to unclassified Listeria species, probably originating from Listeria monocytogenes present in the positive control.
Analysis of the family-level microbiome composition revealed Ruminococcaceae, Eubacteriaceae, and in some subjects Bifidobacteriaceae to be predominant (Fig. 4, top). The relative abundance of Bifidobacteriaceae increased in all subjects except subjects 2 and 15, and particularly large increases were seen for subjects 4, 5, 9, 13, and 14. Bacteroidaceae also increased in several subjects, most markedly subject 15 (species Bacteroides dorei), Eubacteriaceae increased in subjects 1 and 2 (species Eubacterium rectale), and Prevotellaceae (species Prevotella copri) increased in subject 7.
Detection of the probiotic strains in the fecal samples.
Mapping of the metagenomic reads to reference genomes for the four probiotic strains showed that the abundances of the four strains in the fecal samples generally were higher after completion of the intervention (see Table S2). B. animalis subsp. lactis BB-12 was the only probiotic organism identified in large enough amounts to be included in further analyses, and only 6 postintervention samples contained levels above the applied threshold (minimum depth of coverage of 10× and nearly complete genome coverage). Phylogenetic analysis with the sequenced probiotic Bifidobacterium animalis subsp. lactis (BB-12, referred to as the probiotic BB-12 strain) and available B. animalis genomes confirmed that the probiotic BB-12 strain was identical to the BB-12 reference genome (NCBI accession number NC_017214) (see Table S3), and this reference was used for single-nucleotide polymorphism (SNP)-based analysis of the B. animalis subsp. lactis sequences from the fecal samples (referred to as the fecal strains).
We identified between 23 and 171 SNPs between the BB-12 reference and fecal strains (see Table S4). Further inspection of the SNPs revealed that many of them occurred in regions of high mapping depth (see Fig. S2), suggesting unspecific mapping of homologous sequences from other bacterial species. Disregarding SNPs occurring in positions with more than 2× mean mapping depth resulted in 2 to 60 remaining SNPs between the fecal strains and the probiotic BB-12 strain, with 5 of the samples showing only 2 to 15 SNPs. The variant allele frequency (percentage of sequence reads different from the reference) for the SNPs ranged from 50 to 82%; for the 5 samples with the maximum 15 SNPs, it ranged from 50 to 71%. This implies that 29 to 50% of the BB-12 B. animalis subsp. lactis reads in the fecal samples have the same sequence as the probiotic BB-12 strain, suggesting that this probiotic strain is present in the fecal samples.
DISCUSSION
Synbiotics have been shown to possess the ability to shift the predominant bacteria of the fecal microbiota in the human colon and thereby potentially manipulate the microbiota with advantages to human health (28). The average daily consumption of the prebiotic inulin in Europe is estimated to be between 3 and 11 g. A series of clinical studies showed that up to 20 g/day of inulin and/or oligofructose is well tolerated (29). In this study, we aimed for a high dosage of 15 g/day.
Previous studies showed that consumption of inulin stimulates the growth of both Bifidobacterium and Lactobacillus species (30, 31). The ability of prebiotics to alter the fecal microbiota composition, creating a potentially health-promoting community by stimulating benign genera, has raised awareness of prebiotics in recent years (32). There is increasing evidence that lactobacilli and bifidobacteria have the ability to develop antimicrobial activities that participate in the host's gastrointestinal defense system (33, 34). Many previous studies looked at either probiotics, prebiotics, or a combination thereof to transiently alter the microbiome. The alteration is not permanent with the use of only probiotics, but studies using a onetime fecal transplant in patients suffering from Clostridioides difficile infection showed semipermanent alteration of the microbiome for up to 5 years (35).
The present study revealed no significant alteration in alpha diversity between baseline and postintervention samples, which is in alignment with other similar studies (24, 28). The overall microbiome composition (beta diversity) varied by subject rather than time of sampling, also in line with previous findings (13). The most marked alterations in microbiome composition were increases in bifidobacterial species. These species were found to increase in earlier inulin intervention studies, suggesting that inulin is the main driver behind the increase in indigenous bifidobacteria in fecal microbiota (30, 31). In a double-blind, randomized, and placebo-controlled crossover study with 16 healthy individuals 20 to 30 years of age, participants received synbiotics consisting of L. acidophilus NCFM in combination with cellobiose for 3 weeks (19). An increase in the relative abundance of lactobacilli and bifidobacteria was seen, while there was no change in alpha diversity. Self-reported gastrointestinal symptoms did not differ between the groups. In a study with a setup similar to ours, administration of a high-inulin diet (minimum of 9 g of inulin daily) to 26 healthy volunteers did not alter alpha diversity, while beneficial bifidobacteria increased and food-related behavior, in terms of satiety and reduced desire to eat sweet, salty, and fatty food, was improved (24).
All four probiotic organisms were found to increase after the intervention, with three of them being identified as differentially abundant. In five of six fecal samples analyzed, we found evidence suggesting the presence of the ingested probiotic B. animalis subsp. lactis strain BB-12 after the intervention. In the last subject, the high number of SNPs identified, combined with the high level of variant allele frequencies seen, suggests that more than one B. animalis subsp. lactis strain was present. The low levels of the remaining three probiotic organisms identified could indicate that these probiotics did not efficiently colonize the intestine. Larger increases were seen for indigenous bifidobacterial species already present at baseline, indicating that these changes in the microbiome composition are mainly related to the intake of inulin, which upregulated bifidobacterial species in general.
A strength of this study was the robust bioinformatic methodology and analyses. A limitation of the study was the sample size and the fact that the study was not placebo controlled. However, the participants were their own controls in the study, with regard to their baseline microbiomes.
Conclusion.
The 4-week synbiotic intervention with a synbiotic product consisting of Lacticasebacillus rhamnosus (LGG), Lactobacillus acidophilus (LA-5), Lacticaseibacillus paracasei subsp. paracasei (L. CASEI 431), Bifidobacterium animalis subsp. lactis (BB-12), and chicory-derived inulin powder was well tolerated and led to a significant increase in Bifidobacterium species. Alpha diversity and beta diversity were unaltered. This study underlines the ability of synbiotics to beneficially alter the microbiome in healthy people by increasing the relative abundance of the beneficial bifidobacterial species.
MATERIALS AND METHODS
Study design.
A 4-week pilot intervention study was conducted in healthy volunteers from the Copenhagen, Denmark, area. The volunteers were their own controls in the design of the study. The inclusion criteria were individuals 18 to 50 years of age with BMI values of 20 to 25 kg/m². The exclusion criteria were any chronic or intestinal condition, use of probiotics and/or prebiotics (within the previous 2 months), excessive alcohol use, pregnancy or lactation, and use of antibiotics (within the previous 6 months).
The product was provided as a 6-g sachet consisting of 1 g of freeze-dried culture of Lacticasebacillus rhamnosus (LGG) (DSM 33156) (at least 109 CFU/g), Lactobacillus acidophilus (LA-5) (DSM 13241) (at least 109 CFU/g), Lacticaseibacillus paracasei subsp. paracasei (L. CASEI 431) (DSM 33451) (at least 109 CFU/g), and Bifidobacterium animalis subsp. lactis (BB-12) (DSM 15954) (at least 109 CFU/g) and 5 g of chicory-derived inulin powder. The participants consumed 2 sachets twice daily (total of 4 sachets per day) for 4 weeks. Study products were produced at Chr. Hansen A/S.
The clinical study was conducted in accordance with international Good Clinical Practice guidelines and the Declaration of Helsinki. The study was approved by the National Ethics Committee of the Capital Region of Denmark (approval number H-18049898). All study participants signed written informed consent forms.
Diary.
At the end of each test day, participants filled out a diary, recording the stool consistency using the validated Bristol Stool Scale (BSS) (scale of 1 [very hard lumps] to 7 [liquid]), as well as the frequency of their stool (36). In the diary, they also noted whether they had taken their daily synbiotic dosage, as well as recording any gastrointestinal symptoms. Gastrointestinal well-being was based on the following symptoms: nausea, stomach cramps, stomach pain, bloating, rumbling, and gases, graded from 0 (none) to 4 (unbearable) (giving a possible total maximum score of 24). An additional question referring to whether the gastrointestinal function had remained the same, worsened, or improved was also included at the end of the study.
BSS calculations.
A weighted version of the BSS was designed to represent multiple daily stool measurements as a single value. The weighted BSS score (wBSSS) was designed to represent a divergence from a “normal” stool. BSS levels 3 and 4 were weighted 0 to indicate closest to normal stool, level 5 was weighted 1, levels 2 and 6 were weighted 2, and levels 1 and 7 were weighted 3, with 3 thus representing the largest divergence from normal stool on the BSS at both ends of the scale (37). The wBSSS was calculated as the average of all wBSSS values. As an example, four stool measures with BSS levels of 2, 3, 4, and 7 would give a wBSSS of (2 + 0 + 0 + 3)/4 = 1.25. Additionally, days with no stool were considered not normal; therefore, the wBSSS for these days was calculated as the number of cumulative consecutive days with no stool (wBSSS of 1 for day 1, wBSSS of 2 for day 2, and so on). If more than 3 consecutive days persisted with no stool, the score was set to 3 for all subsequent consecutive days with no stool.
Fecal sample collection, library preparation, and sequencing.
Two fecal samples were collected, at baseline and 1 day after the end of the intervention. Fecal samples were immediately stored in the participants’ own freezer before being transported in a cooling transport system to Copenhagen University Hospital (Hvidovre, Denmark), where samples were subsequently stored at −80°C until analysis.
DNA was extracted from 100 mg feces with the DNeasy PowerSoil Pro kit (Qiagen, Germany) according to the manufacturer’s instructions. A negative control consisting of water and a positive control consisting of the ZymoBIOMICS microbial community standard (Zymo Research, USA) were included throughout the process. Libraries were prepared with the Nextera XT DNA sample preparation kit (Illumina). Samples were sequenced on the NextSeq 550 system (Illumina) with 150-bp paired-end reads.
Bioinformatic analysis. (i) Preprocessing of data.
NextSeq bcl files were converted to the fastq format and demultiplexed using bcl2fastq v. 2.19.1 (Illumina) with the default setting of 1 barcode mismatch allowed. Fastq reads were trimmed using Trimmomatic v. 0.38 with trimming of leading and trailing bases with Phred quality scores below 20, trimming using a 15-base sliding window, and cutting when the average quality per base dropped below 20, with exclusion of reads shorter than 50 bases after trimming. FastQC v. 0.11.7 was used to assess the quality of the reads before and after trimming (38, 39). The data were depleted of human sequences by aligning the trimmed reads to the human genome (hg38; University of California, Santa Cruz, Santa Cruz, CA) using Bowtie2 v. 2.3.4.1 with end-to-end alignment (40).
(ii) Microbial profiling.
Clade-based microbial profiling of the human-sequence-depleted reads was performed using MetaPhlAn2 v. 2.7.7 with default settings except addition of the parameters –ignore_viruses, –ignore_eukaryotes, and –t rel_ab_w_read_stats, the latter to obtain an estimate of the number of reads coming from each clade (41). Relative abundance tables for all samples were merged using the MetaPhlAn2 script merge_metaphlan_tables.py (see Data Set S1 in the supplemental material).
(iii) Identification of the probiotic strains.
To assess whether sufficient amounts of sequences for SNP analyses originated from the four probiotic organisms, read pairs (singletons from trimming and human sequence depletion excluded) were aligned to the reference genomes Lactobacillus rhamnosus GG (GenBank accession number NC_013198.1), Lactobacillus acidophilus NCFM (GenBank accession number NC_006814), Lactobacillus paracasei ATCC 334 (GenBank accession number NC_008526.1), and Bifidobacterium animalis subsp. lactis BB-12 (GenBank accession number NC_017214.2) using Bowtie2 with default settings except for the addition of the parameters –no-mixed –no-discordant –X 2000. Duplicate reads were removed using the Picard toolkit v. 2.20.2 MarkDuplicates function, and depth and coverage for alignments with mapping quality (MAPQ) scores of ≥20 were calculated using the SAMtools v. 1.9 view function and the BEDTools v. 2.28.0 genomecov function (42, 43). To account for uneven sequencing depth when assessing coverage and depth, mapping was repeated using read files subsampled to 6 million read pairs using seqtk v. 1.3 (https://github.com/lh3/seqtk).
Only B. animalis subsp. lactis BB-12 was included in further analyses. To confirm whether the public reference genome for Bifidobacterium animalis subsp. lactis BB-12 (GenBank accession number NC_017214.2) was the genome most closely related to the probiotic Bifidobacterium animalis subsp. lactis BB-12 (DSM 15954), the variant-calling pipeline NASP was run with default parameters using GenBank accession number NC_017214.2 as the reference, the raw reads from sequencing of the synbiotic product, and available B. animalis genomes as input (44). SNP distances were calculated from the resulting SNP alignment.
Human-DNA-depleted metagenomic read pairs from samples with mapping depth of ≥10× and almost complete (99%) genome coverage of B. animalis subsp. lactis BB-12 were aligned to GenBank accession number NC_017214.2 using Bowtie2 as detailed above. Duplicate reads were removed using the Picard toolkit, and variants were called using SAMtools mpileup with alignments with MAPQ scores of ≥20, followed by VarScan v. 2.4.3 with the parameters –mpileup2cns –min-var-freq 0.5 –min-coverage 10 (45). Depth and coverage for alignments with MAPQ scores of ≥20 were calculated using SAMtools view and BEDTools genomecov, and coverage plots were generated using R (https://www.rstudio.com). The variants were also filtered based on a maximum depth of 2 times the mean depth for a given sample. Command-line analysis jobs were executed in parallel using GNU Parallel v. 20181222 (46).
Taxonomic and diversity analyses.
The taxonomic data were processed in R v. 3.6.1, and ggplot2 was used for certain visualizations (47, 48). Rare species with sample-level relative abundances of <0.01% were filtered out, and species found in <2 samples were removed. For presentation in bar plots, species or families present at relative abundances of <1% were grouped as “other.” To visualize general changes in species relative abundance between the two samplings, the ratios between baseline and postintervention samples were calculated. Because some relative abundances were 0%, the zeros were replaced by 0.0001% (just below the smallest relative abundance in the data set) for estimation of the ratios. Only species for which the median of the postintervention/baseline ratio was different from 1 (no change) were included in the analysis.
(i) Diversity analysis.
Species richness and Shannon diversity were calculated based on the filtered relative abundance data using the vegan package, and differences were assessed using the paired Wilcoxon signed-rank test (49). Beta diversity analysis was performed using QIIME2 v. 2021.2 with the species-level taxonomic abundance table containing the estimated read counts generated by MetaPhlAn2 (50). Read count values representing <0.01% relative abundance and species present in <2 samples were filtered out using the QIIME2 feature-table plugin. Aitchison distance was chosen as the beta diversity metric to account for the compositionality of the data (27, 51). Aitchison distances were computed by using the QIIME2 diversity plugin and adding a pseudocount of 1. Differences in beta diversity between baseline and postintervention samples and between subjects were assessed based on the Aitchison distance matrix with PERMANOVA. Principal-coordinate analysis (PCoA) was performed using the diversity plugin and visualized using the emperor plugin (52).
(ii) Differential abundance analysis.
Differential abundance analysis was performed using the R package ALDEx2 (53–55). The data were transformed using the aldex.clr function, which generates random instances of the centered log ratio-transformed values, using mc.samples=1000 (number of Monte-Carlo instances) and denom=“all” (using all features as the denominator for the geometric mean calculation). The aldex.ttest function was used to perform the Welch t test and Wilcoxon signed-rank test comparing baseline and postintervention samples using paired.test=TRUE. The aldex.effect function was then applied to estimate effect size and the within- and between-condition values for baseline versus postintervention; finally, the aldex.plot function was used to make MA (Bland-Altman style) and MW (effect) plots using test=“welch” and default settings otherwise (Benjamini-Hochberg false-discovery rate [FDR] cutoff default, 0.1; effect size cutoff default, 1).
Ethics approval and consent to participate.
The trial has been approved by the Scientific Ethics Committee for Copenhagen Regional Hospitals (permission number H-18049798). Because the synbiotic product is considered a dietary supplement and not a pharmaceutical, no authorization by the Danish Medical Agency was required. The study was performed in accordance with the requirements of the Revised Declaration of Helsinki, and all participants signed informed consent forms.
Data availability.
Raw sequencing reads depleted of human reads as described have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA759733.
ACKNOWLEDGMENTS
The synbiotic product was provided by Chr. Hansen A/S. We thank Thomas Kallemose for his help with the statistical analysis. We also thank Cathrine Melsaether for her help with the protocol writing and for providing information about the synbiotic product.
C.B. and A.B. were (at the time of the study) employed or financially supported by Chr. Hansen A/S, which produces and markets the synbiotic product. I.M.C.R. received an unrestricted grant from Chr. Hansen A/S. The remaining authors report no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Ingrid Maria Cecilia Rubin, Email: irub0009@regionh.dk.
Johanna Björkroth, University of Helsinki.
REFERENCES
- 1.Matsuoka K, Kanai T. 2015. The gut microbiota and inflammatory bowel disease. Semin Immunopathol 37:47–55. 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. 2018. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 11:1–10. 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 3.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, Moayyedi P. 2019. Gut microbiota in patients with irritable bowel syndrome: a systematic review. Gastroenterology 157:97–108. 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Ley RE. 2010. Obesity and the human microbiome. Curr Opin Gastroenterol 26:5–11. 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ. 2017. Microbes and diet-induced obesity: fast, cheap, and out of control. Cell Host Microbe 21:278–281. 10.1016/j.chom.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto J-M, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. 2012. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60. 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 7.Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, Smeekens SP, Netea MG, Buitelaar JK, Franke B, van Hijum SAFT, Arias Vasquez A. 2017. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One 12:e0183509. 10.1371/journal.pone.0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. 2011. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108:3047–3052. 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosscher D, Van Loo J, Franck A. 2006. Inulin and oligofructose as prebiotics in the prevention of intestinal infections and diseases. Nutr Res Rev 19:216–226. 10.1017/S0954422407249686. [DOI] [PubMed] [Google Scholar]
- 10.Roberfroid MB. 2005. Introducing inulin-type fructans. Br J Nutr 93(Suppl 1):S13–S25. 10.1079/BJN20041350. [DOI] [PubMed] [Google Scholar]
- 11.Cerdó T, García-Santos JA, Bermúdez MG, Campoy C. 2019. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 11:635. 10.3390/nu11030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, Wang J, Imhann F, Brandsma E, Jankipersadsing SA, Joossens M, Cenit MC, Deelen P, Swertz MA, Weersma RK, Feskens EJM, Netea MG, Gevers D, Jonkers D, Franke L, Aulchenko YS, Huttenhower C, Raes J, Hofker MH, Xavier RJ, Wijmenga C, Fu J, LifeLines Cohort Study . 2016. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352:565–569. 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Personalized Microbiome Class Students, Walter J, Menon R, Koecher K, Knights D. 2019. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 25:789–802.e5. 10.1016/j.chom.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J. 2016. Population-level analysis of gut microbiome variation. Science 352:560–564. 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 15.Derrien M, van Hylckama Vlieg JET. 2015. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol 23:354–366. 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Derrien M, Levenez F, Brazeilles R, Ballal SA, Kim J, Degivry M-C, Quéré G, Garault P, van Hylckama Vlieg JET, Garrett WS, Doré J, Veiga P. 2016. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J 10:2235–2245. 10.1038/ismej.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ba Z, Lee Y, Meng H, Kris-Etherton PM, Rogers CJ, Lewis ZT, Mills DA, Furumoto EJ, Rolon ML, Fleming JA, Roberts RF. 2021. Matrix effects on the delivery efficacy of Bifidobacterium animalis subsp. lactis BB-12 on fecal microbiota, gut transit time, and short-chain fatty acids in healthy young adults. mSphere 6:e00084-21. 10.1128/mSphere.00084-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME. 2020. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17:687–701. 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Zanten GC, Krych L, Röytiö H, Forssten S, Lahtinen SJ, Al-Soud WA, Sørensen S, Svensson B, Jespersen L, Jakobsen M. 2014. Synbiotic Lactobacillus acidophilus NCFM and cellobiose does not affect human gut bacterial diversity but increases abundance of lactobacilli, bifidobacteria and branched-chain fatty acids: a randomized, double-blinded cross-over trial. FEMS Microbiol Ecol 90:225–236. 10.1111/1574-6941.12397. [DOI] [PubMed] [Google Scholar]
- 20.Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT. 2013. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther 38:804–816. 10.1111/apt.12453. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan EE, Heilig HGHJ, Ben-Amor K, De Vos WM. 2005. Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. FEMS Microbiol Rev 29:477–490. 10.1016/j.fmrre.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Milani C, Lugli GA, Duranti S, Turroni F, Bottacini F, Mangifesta M, Sanchez B, Viappiani A, Mancabelli L, Taminiau B, Delcenserie V, Barrangou R, Margolles A, van Sinderen D, Ventura M. 2014. Genomic encyclopedia of type strains of the genus Bifidobacterium. Appl Environ Microbiol 80:6290–6302. 10.1128/AEM.02308-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Callaghan A, van Sinderen D. 2016. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 7:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiel S, Bindels LB, Pachikian BD, Kalala G, Broers V, Zamariola G, Chang BPI, Kambashi B, Rodriguez J, Cani PD, Neyrinck AM, Thissen J-P, Luminet O, Bindelle J, Delzenne NM. 2019. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr 109:1683–1695. 10.1093/ajcn/nqz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bastard Q, Chapelet G, Javaudin F, Lepelletier D, Batard E, Montassier E. 2020. The effects of inulin on gut microbial composition: a systematic review of evidence from human studies. Eur J Clin Microbiol Infect Dis 39:403–413. 10.1007/s10096-019-03721-w. [DOI] [PubMed] [Google Scholar]
- 26.Hiel S, Gianfrancesco MA, Rodriguez J, Portheault D, Leyrolle Q, Bindels LB, Gomes da Silveira Cauduro C, Mulders MDGH, Zamariola G, Azzi AS, Kalala G, Pachikian BD, Amadieu C, Neyrinck AM, Loumaye A, Cani PD, Lanthier N, Trefois P, Klein O, Luminet O, Bindelle J, Paquot N, Cnop M, Thissen JP, Delzenne NM. 2020. Link between gut microbiota and health outcomes in inulin-treated obese patients: lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr 39:3618–3628. 10.1016/j.clnu.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. 2017. Microbiome datasets are compositional: and this is not optional. Front Microbiol 8:2224. 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Zanten GC, Knudsen A, Röytiö H, Forssten S, Lawther M, Blennow A, Lahtinen SJ, Jakobsen M, Svensson B, Jespersen L. 2012. The effect of selected synbiotics on microbial composition and short-chain fatty acid production in a model system of the human colon. PLoS One 7:e47212. 10.1371/journal.pone.0047212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carabin IG, Flamm WG. 1999. Evaluation of safety of inulin and oligofructose as dietary fiber. Regul Toxicol Pharmacol 30:268–282. 10.1006/rtph.1999.1349. [DOI] [PubMed] [Google Scholar]
- 30.Gibson GR, Beatty ER, Wang X, Cummings JH. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975–982. 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 31.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. 2004. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut 53:1610–1616. 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolida S, Tuohy K, Gibson GR. 2002. Prebiotic effects of inulin and oligofructose. Br J Nutr 87(Suppl 2):S193–S197. 10.1079/BJN/2002537. [DOI] [PubMed] [Google Scholar]
- 33.Servin AL. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440. 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Gorbach S, Doron S, Magro F. 2017. Lactobacillus rhamnosus GG, p 79–88. In Floch MH, Ringel Y, Walker WA (ed), The microbiota in gastrointestinal pathophysiology: implications for human health, prebiotics, probiotics, and dysbiosis. Academic Press, Cambridge, MA. 10.1016/B978-0-12-804024-9.00007-0. [DOI] [Google Scholar]
- 35.Aggarwala V, Mogno I, Li Z, Yang C, Britton GJ, Chen-Liaw A, Mitcham J, Bongers G, Gevers D, Clemente JC, Colombel J-F, Grinspan A, Faith J. 2021. Precise quantification of bacterial strains after fecal microbiota transplantation delineates long-term engraftment and explains outcomes. Nat Microbiol 6:1309–1318. 10.1038/s41564-021-00966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell LJ, Virjee J, Heaton KW. 1990. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 300:439–440. 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis SJ, Heaton KW. 1997. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32:920–924. 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 38.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 40.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, Tett A, Huttenhower C, Segata N. 2016. Erratum: MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods 13:101. 10.1038/nmeth0116-101b. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahl JW, Lemmer D, Travis J. 2016. NASP: an accurate, rapid method for the identification of SNPs in WGS datasets that supports flexible input and output formats. Microb Genom 2:e000074. 10.1099/mgen.0.000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koboldt DC, Chen K, Wylie T, Larson DE, McLellan MD, Mardis ER, Weinstock GM, Wilson RK, Ding L. 2009. VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 25:2283–2285. 10.1093/bioinformatics/btp373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tange O. 2011. GNU Parallel: the command-line power tool. USENIX Mag 36:42–47. [Google Scholar]
- 47.R Foundation for Statistical Computing. 2015. R: a language and environment for statistical computing. https://www.r-project.org.
- 48.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, NY. https://ggplot2.tidyverse.org. [Google Scholar]
- 49.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2015. Package vegan: community ecology package. https://cran.r-project.org/web/packages/vegan.
- 50.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aitchison J, Barceló-Vidal C, Martín-Fernández JA, Pawlowsky-Glahn V. 2000. Logratio analysis and compositional distance. Math Geol 32:271–275. 10.1023/A:1007529726302. [DOI] [Google Scholar]
- 52.Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R. 2013. EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience 2:16. 10.1186/2047-217X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. 2013. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS One 8:e67019. 10.1371/journal.pone.0067019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandes AD, Reid JNS, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. 2014. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2:15. 10.1186/2049-2618-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gloor GB, Macklaim JM, Fernandes AD. 2016. Displaying variation in large datasets: plotting a visual summary of effect sizes. J Comput Graph Stat 25:971–979. 10.1080/10618600.2015.1131161. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2 and Tables S2 and S4. Download aem.01087-22-s0001.pdf, PDF file, 1.0 MB (1MB, pdf)
Table S1. Download aem.01087-22-s0002.xlsx, XLSX file, 0.03 MB (35.5KB, xlsx)
Table S3. Download aem.01087-22-s0003.xlsx, XLSX file, 0.02 MB (19.2KB, xlsx)
Data Set S1. Download aem.01087-22-s0004.xlsx, XLSX file, 0.2 MB (202.5KB, xlsx)
Data Availability Statement
Raw sequencing reads depleted of human reads as described have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA759733.