Abstract
Atherosclerosis is the main underlying pathology for many cardiovascular diseases (CVDs), which are the leading cause of death globally and represent a serious health crisis. Atherosclerosis is a chronic condition that can lead to myocardial infarction, ischemic cardiomyopathy, stroke, and peripheral arterial disease. Elevated plasma lipids, hypertension, and high glucose are the major risk factors for developing atherosclerotic plaques. To date, most pharmacological therapies aim to control these risk factors, but they do not target the plaque-causing cells themselves. In patients with acute coronary syndromes, surgical revascularization with percutaneous coronary intervention has greatly reduced mortality rates. However, stent thrombosis and neo-atherosclerosis have emerged as major safety concerns of drug eluting stents due to delayed re-endothelialization. This review summarizes the major milestones, strengths, and limitations of current anti-atherosclerotic therapies. It provides an overview of the recent discoveries and emerging game-changing technologies in the fields of nanomedicine, mRNA therapeutics, and gene editing that have the potential to revolutionize CVD clinical practice by steering it toward precision medicine.
Keywords: atherosclerosis, cardiovascular disease, stroke, coronary artery disease, inflammation, miRNA-switch, mRNA therapeutics, base editing, nanotherapy, nanomedicine, precision medicine
Graphical abstract
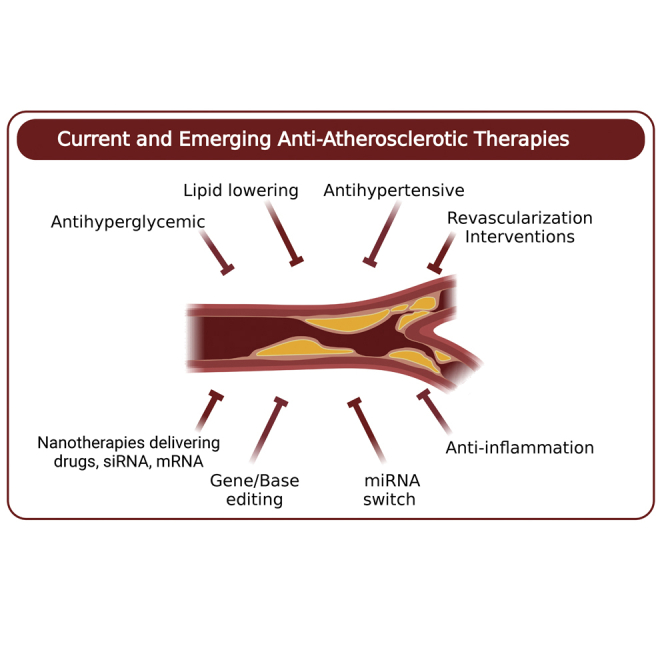
In this review, Hetherington and Totary-Jain describe the major milestones, strengths, and limitations of current anti-atherosclerotic therapies. They additionally provide an overview of emerging game-changing technologies in the fields of nanomedicine, mRNA therapeutics, and gene editing that have the potential to revolutionize CVD clinical practice.
Atherosclerosis
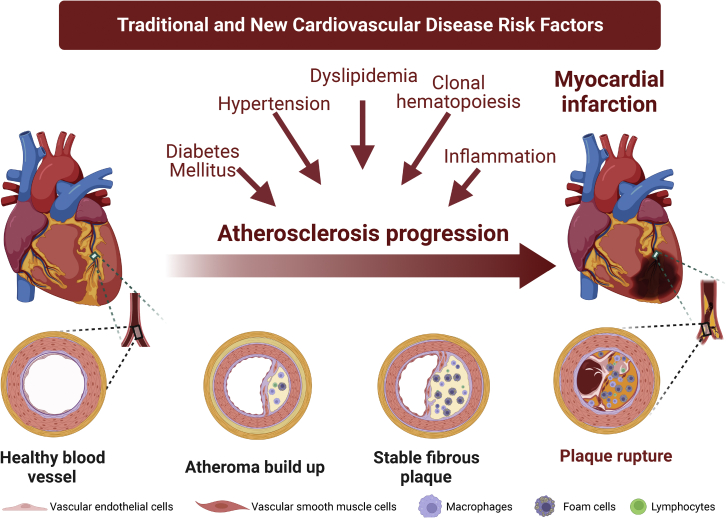
Cardiovascular disease (CVD) is the leading cause of death globally. In 2019, >17 million people died from CVDs; of these, 85% were due to heart attack and stroke,1 which are mainly caused by atherosclerosis of the arteries. Atherosclerosis is a non-resolving chronic inflammatory disease that develops in the medium to large arteries of the arterial tree at branching points with disturbed blood flow.2 Dyslipidemia, hypertension, and diabetes are the major risk factors for atherosclerosis and are implicated in atherosclerotic plaque initiation, progression, and rupture.3 In addition, the role of inflammation4,5 and the recent discovery of clonal hematopoiesis of indeterminate potential (CHIP)6 have expanded CVD risk factors beyond traditional ones (Figure 1).
Figure 1.
Atherosclerosis: Disease progression and risk factors
Atherosclerosis begins with damage to the endothelium lining the arterial intima, initiated by risk factors. This leads to disease progression, lipid retention to the subendothelial space, infiltration of inflammatory cells, and plaque formation. Plaques can form over many decades without manifesting any symptoms but can lead to chronic ischemic heart disease or acute events such as plaque rupture, myocardial infarctions, and strokes. Made with Biorender.com.
Atherosclerosis begins with damage to the vascular endothelial cells (ECs) that line the innermost layer of the vessels, which normally regulate local vascular tone and protect the arteries against inflammation and thrombosis.7,8 Several conditions induce EC dysfunction, including hypercholesterolemia,9 hypertension,10 and diabetes.11,12 EC dysfunction facilitates the deposition of lipids in the subendothelial space, triggering arterial inflammation. Resident patrolling macrophages and infiltrating monocyte-derived macrophages start to proliferate within the arteries and intake oxidized-low-density lipoprotein (LDL), becoming foam cells.13 While resident macrophages promote tissue repair and homeostasis, monocyte-derived macrophages can acquire either pro-inflammatory or pro-resolving phenotypes, which are known as M1/classically activated or M2/alternatively activated phenotypes, respectively.14,15 Macrophages thus play a critical role in not only atherosclerosis progression but also plaque stability and regression by phagocytosis and clearance of apoptotic cells, a process known as efferocytosis, as well as other pro-resolving functions. In addition, in response to the inflammatory cytokines released by macrophages, ECs and vascular smooth muscle cells (VSMCs) contribute to atherosclerotic plaque progression through the endothelial-mesenchymal transition (EMT)16,17 and VSMC de-differentiation into migratory and macrophage-like foam cells.18 The failure to resolve the inflammatory response leads to enhanced inflammatory cell recruitment as well as macrophage and foam cell death. Defective efferocytosis contributes to the formation of the highly inflamed necrotic core of unstable plaques.2,19 Vulnerable plaques with lipid-rich necrotic cores and thin fibrous caps are at a higher risk of rupturing. Plaque rupture has historically accounted for the majority of acute coronary syndromes and is one of the biggest risks associated with myocardial infarctions.20 In contrast, plaque erosion, which is more commonly associated with women and younger individuals, involves the monolayer of ECs lining the arterial intima to become denuded and occurs in 40% of patients with acute coronary syndromes.20, 21, 22, 23
Strengths and limitations of current atherosclerosis treatments
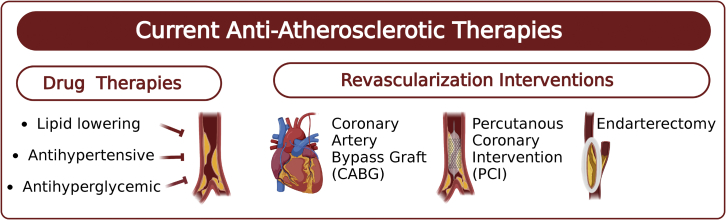
The Framingham Heart Study was the first to identify hyperlipidemia and hypertension as major CVD risk factors.24 Since then, great progress has been made to develop therapies and interventions that have led to better outcomes and lower mortality rates for CVD patients25 (Figure 2). In fact, from 1990 to 2019, the number of people with ischemic heart disease decreased by 4.6% (age-standardized rate per 100,000), and from 2011 to 2017, the age-adjusted mortality for coronary heart disease declined by 2.7% annually.26 Despite these advances, CVD remains the leading cause of death globally, and improvements to current treatments and novel therapeutic strategies are needed.
Figure 2.
Available anti-atherosclerotic drugs and interventions
Made with Biorender.com.
Lipid management therapies
A 30-year follow-up to the Framingham Heart Study showed that for those younger than age 50, cholesterol levels were directly related to 9% of CVD death for each 10 mg/dL.27 Since then, an abundance of clinical, genetic, and epidemiological studies has established that elevated LDL levels are major contributors to the development of atherosclerotic plaques and subsequent CVDs.28, 29, 30 Thus, lowering total and LDL-cholesterol has been a mainstay in the treatment of atherosclerosis, with statin therapy becoming the foundation of lipid management. Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol synthesis.31 Statins also increase LDL clearance from circulation by increasing the expression of hepatic LDL receptors. The 4S clinical trial (Scandinavian Simvastatin Survival Study) was one of the first long-term clinical trials to show that statin therapy increased survival rates and decreased the need for revascularization procedures in patients with coronary heart disease.32 Moreover, statins exert protective pleiotropic effects that are independent of LDL-cholesterol lowering. Several pre-clinical and clinical studies have shown that statins reduce inflammation and slow the progression of atherosclerotic plaques.33 The anti-inflammatory effects of statins stem from their ability to enhance efferocytosis,34 activate transcription factors such as peroxisome proliferator-activated receptors (PPARs) to increased lipid metabolism,35 suppress oxidative stress,36 decrease thrombotic and platelet activity,37 and enhance angiogenesis and EC function.38
Despite these broad benefits, statins may not sufficiently reduce LDL levels in all patients, and many patients do not tolerate statin therapy due to side effects such as myopathy or rare cases of rhabdomyolosis.39 To meet target LDL levels, several agents have been used individually or in combination with statins, including ezetimibe, fibrates, niacin, and bile acid-binding resins, which have shown modest benefits in dyslipidemia management and cardiovascular events.40,41
After the discovery of the proprotein convertase subtilisin/kexin type 9 (PCSK9) gain- and loss-of-function mutations, associated with familial hypercholesterolemia42 and with lower LDL-cholesterol levels,43 respectively, it quickly became one of the most promising targets for the management of LDL-cholesterol levels. PCSK9 is a serine protease that is synthesized in the liver and secreted into the bloodstream and exerts its effects on cholesterol homeostasis by binding LDL receptors both intra- and extracellularly to cause their degradation.44 Thus, PCSK9 inhibition increases hepatic LDL receptors, resulting in a significant reduction in LDL-cholesterol levels and the rate of major adverse cardiovascular events.45,46 In 2015, the US Food and Drug Administration (FDA) approved a new class of lipid-lowering therapies that consisted of humanized monoclonal antibodies (mAb) that inactivate PCSK9, alirocumab, and evolocumab.47 However, PCSK9 mAb treatment requires frequent injections due to their short half-lives and are very expensive, so they are prescribed for high-risk patients who are intolerant to statins or who have failed to reduce LDL-cholesterol levels despite taking the maximum tolerated dose of a statin and ezetimibe. In December 2021, the FDA approved Leqvio (inclisiran), a small interfering RNA (siRNA) therapy that targets PCSK9 mRNA to reduce its synthesis in the liver and decrease LDL-cholesterol levels by ∼50% after subcutaneous administration every 6 months.48,49 Given the need for more affordable and effective lipid-lowering therapies, several other innovative approaches are under development.
Hypertensive management therapies
Hypertension is another significant risk factor for developing atherosclerosis and subsequent CVDs and is a major cause of premature death worldwide.50 Hypertension induces EC dysfunction and exacerbates the formation of atherosclerotic plaques while decreasing their stability.51 Effective management of hypertension has been a major step forward in reducing risks associated with CVDs in patients. A meta-analysis of >48 randomized clinical trials found that a reduction of just 5 mmHg in systolic blood pressure reduced the risk of patients experiencing an adverse cardiac event by 10%.52
Most clinical guidelines for antihypertensive drugs consist of four major classes: β-receptor blockers, calcium channel blockers, renin-angiotensin-aldosterone system (RAAS) blockers, and thiazide-like diuretics. A head-to-head meta-analysis showed that all antihypertensive drugs have a similar effect on major CVD outcomes.53 However, there are limitations to these drugs and patient medical history needs to be carefully examined before prescribing. For example, non-selective β-receptor blockers should not be prescribed to asthmatics as the β2-receptor blockade on the respiratory system can worsen asthma symptoms.54 RAAS blockers may also cause dangerous hyperkalemia in at-risk patients with renal disease and should not be prescribed to those with bilateral renal arterial stenosis, as the renal perfusion of these patients is highly dependent on RAAS.55 In addition, RAAS blockers are contraindicated during pregnancy for their possible teratogenic effects,56 while their effects on newborn infants are largely unknown, so nursing mothers should be educated on the possible risks and alternative antihypertensive treatments. Consequently, methyldopa (α2-receptor blocker), labetalol (β-receptor blocker), and nifedipine (calcium channel blocker) are considered first-line treatments for pregnant women with hypertension.57 A prospective observational cohort study reported that pregnant women treated with methyldopa during the first trimester of pregnancy did not have a significantly increased risk of birth defects, but there was a significant increase in pre-term births.58 Furthermore, a randomized controlled trial evaluated the use of labetalol versus nifedipine in pregnant women, and both were found to be effective in controlling blood pressure to therapeutic targets.59
To achieve contemporary blood pressure targets, combinational therapy is often required. Thus, several single-pill combination therapies have been widely used, and these antihypertensive treatments are among the most remarkable achievements in modern clinical medicine. Even with these accomplishments, resistant hypertension, defined by uncontrolled blood pressure despite the use of a diuretic and ≥2 antihypertensives drugs at the maximum tolerated dose, persists in 12%–14% of treated hypertensive patients.60,61 It has been reported that patients with resistant hypertension experience higher rates of target organ damage compared to those with well-controlled blood pressure.62 Moreover, 3% of these patients who do not achieve blood pressure control even with ≥5 antihypertensive therapies are defined as refractory hypertensive, and those with refractory hypertension were found to have higher prevalence ratios for Black race, diabetes mellitus, and albuminuria, compared to those with resistant hypertension.63, 64, 65 Thus, novel therapeutic strategies are still needed to combat resistant and refractory hypertension.
Diabetes mellitus therapies
Diabetes mellitus (DM) is one of the fastest-growing global health conditions that is a major cause of heart attack, stroke, lower limb amputation, kidney failure, and blindness.66 DM is a chronic metabolic disease characterized by hyperglycemia. Type 1 DM (T1DM) is an autoimmune disorder that affects young people (younger than 30 years of age), which leads to the destruction of the insulin-producing pancreatic beta cells, and patients require lifelong insulin replacement therapy.67 Type 2 DM (T2DM) is a progressive metabolic disease characterized by insulin resistance that progresses into the functional failure of pancreatic beta cells.68 It is vital for patients to control their diabetes diagnosis as they are at higher risk for developing CVD. In fact, a 20-year observation of the Framingham cohort that had prior evidence of diabetes had a 2- to 3-fold increased risk of clinical atherosclerotic disease and subsequent cardiovascular events.69 In addition to insulin replacement therapies for T1DM, there are nearly 60 FDA-approved drugs for T2DM. These include biguanides, sulfonylureas, meglitinides (glinides), α-glucosidase inhibitors, thiazolidinediones, sodium-glucose cotransporter type 2 inhibitors, and incretin-dependent therapies. These therapies have various mechanisms of action such as increasing insulin sensitivity and secretion, decreasing renal glucose reabsorption, inhibiting hepatic glucose production, or inhibiting carbohydrate absorption from the small intestine.70 The recent Cardiovascular Outcomes Trials (CVOTs) and several other studies have shown that over the years, improving T1DM and T2DM management gradually decreased the rates of major cardiovascular events.71, 72, 73 However, despite improved survival, diabetic patients still have a 2.32 hazard ratio for death from CVD compared to the general population, especially among women and African Americans.74, 75, 76
Revascularization intervention
In addition to pharmacological agents to treat risk factors associated with atherosclerosis, surgical interventions such as coronary artery bypass grafting (CABG), endarterectomy, and percutaneous coronary intervention (PCI) have been widely used to treat arterial stenosis. Approximately 371,000 CABG, 480,000 PCI, and 86,000 carotid endarterectomy procedures were performed in the United States in 2014.26 Several randomized trials have shown that in patients with severe coronary artery disease (CAD) and DM, CABG was superior to PCI.77,78 PCI originally started with simple balloon angioplasty to open up occluded vessels and restore blood flow.79 This procedure had a high incidence of acute occlusion caused by the elastic recoil of the arteries, as well as thrombosis and subsequent restenosis.80,81 To overcome acute occlusion, improvements were made to PCI to include the deployment of bare-metal stents (BMSs) and antiplatelet therapies to prevent elastic recoil and thrombosis, respectively. However, in-stent restenosis persisted.82 To overcome in-stent restenosis, drug-eluting stents (DESs) that locally release antiproliferative agents such as sirolimus to the diseased vessel segment were developed. Initial clinical trials comparing DESs to the standard BMSs were highly promising, with those receiving DESs having less frequent neointima hyperplasia and less commonly needing revascularization procedures.83 Nevertheless, the deployment of stents into the arteries inevitably leads to the disruption of the already damaged vascular endothelium. In addition, the compounds released by DESs into vessels are not cell-selective, preventing re-endothelialization of the vasculature.7,81 Thus, stent thrombosis (ST) and neo-atherosclerosis emerged as major safety concerns with the first generation of DESs.84,85 Although ST is uncommon, it is a serious complication of PCI that can cause myocardial infarction in 60%–70% of the cases and increases the risk of mortality by 20%–25%.86 Endarterectomy is another effective revascularization procedure to treat carotid artery stenosis, and long-term studies have shown its efficacy equivalent to that of stenting.87
Newer generations of DESs have been developed to combat their associated risks, consisting of thinner struts and biocompatible polymers.88 However, these DESs still deploy the same non-selective drugs into the arteries and are associated with a similar risk of ST compared to BMSs.89 Therefore, patients must comply with dual antiplatelet therapy when a DES is implanted, despite the increased bleeding risks.90 The lessons learned from PCI is that treatment options for atherosclerosis need to prioritize the protection of the vascular endothelium while also targeting the disease-causing cells that contribute to plaque formation.81 Delivery methods involving site- and cell-selective nanotherapies may provide the answer to this problem.
Emerging anti-atherosclerotic therapies
Despite remarkable advances in systemic anti-atherosclerotic therapies, which manage CVD risk factors and revascularization interventions that restore blood flow after plaque formation, heart disease has remained the leading cause of death globally for the last 20 years.26 This may be due to the increase in the aging population, along with an increased prevalence of obesity and DM. Thus, numerous novel therapeutic targets have been investigated pre-clinically and tested in large-scale clinical trials. Here, we summarize some of the most salient approaches that have the potential to advance the fight against atherosclerosis.
Targeting inflammation
For the last 2 decades, the role of innate and adaptive immunity in atherogenesis has become prominent.4,91 In fact, measuring inflammatory markers, such as C-reactive protein (CRP), is now used clinically to identify high-risk patients and to monitor their treatment.33,92,93 However, targeting inflammation to treat atherosclerosis and decrease adverse cardiac events has only recently been clinically tested.94, 95, 96
Inflammasomes are multimeric protein complexes with key roles in innate immunity and vascular inflammation during atherosclerotic plaque initiation, progression, and rupture.97,98 To date, several stimuli have been shown to activate inflammasomes, including cholesterol crystals,97 oxidized LDL,99 disturbed blood flow,100 hypoxia,101,102 neutrophil extracellular traps,103 and somatic mutations in tet methylcytosine dioxygenase 2 (TET2).104 Activated inflammasomes convert pro-caspase 1 into active caspase-1, which then cleaves pro-interleukin-1β (IL-1β) and pro-interleukin-18 (IL-18) into their active forms. Concurrently, another substrate of active caspase-1, gasdermin D (GSDMD), is cleaved and its N-terminal domain localizes to cell membranes to form pores through which the pro-inflammatory cytokines can escape and further stimulate inflammatory responses.105
IL-1β is a potent pro-inflammatory cytokine that stimulates the production of other cytokines, including tumor necrosis factor (TNF) and interleukin-6 (IL-6) in ECs, VSMCs, macrophages, and hepatocytes.106 Classical IL-6 signaling occurs when the secreted form binds to the IL-6 receptor (IL-6R) on cell membranes. This interaction on its own has no signaling ability, and the IL-6/IL-6R complex requires interaction with the membrane protein gp130 to activate intracellular signaling.107 The IL-6R is mainly found on hepatocytes and leukocytes, but is absent in most other cell types, making them incapable of responding to IL-6 through classical signaling. However, an alternative trans-signaling pathway exists in cells that do not have IL-6R in their membranes, involving a soluble form of the receptor (sIL-6R), which can then bind IL-6. Together they bind membrane bound gp130 to activate an intracellular signal cascade, as gp130 is expressed in all cell types.108 IL-6 has been shown to contribute to atherogenesis by releasing the acute-phase response through the reactants fibrinogen and plasminogen activator inhibitor 1, which are involved in causing blood clots and inhibiting fibrinolysis, respectively, aggravating atherothrombosis.95
Targeting the inflammation component of atherosclerosis started a new era of therapeutic strategies. Numerous clinical trials have been launched to test the efficacy of several anti-inflammatory agents. For example, recent results from the Low-Dose Colchicine 2 (LoDoCo2) trial demonstrated that patients treated daily with 0.5 mg colchicine, an anti-inflammatory drug commonly prescribed to treat gout,109 was effective in reducing the risk of adverse cardiac events in patients, regardless of history of acute coronary syndromes.110 In addition, another study using colchicine evaluated plaques using computed tomographic coronary angiography and showed a decrease in low-attenuation plaque volume and CRP levels in patients at a 1-year follow-up.111
CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study) was the first large-scale, randomized, placebo-controlled, double-blinded clinical trial to show the effectiveness of targeting inflammation to prevent adverse cardiac events.112 This study was specifically designed to test the inflammation hypothesis of atherothrombosis, doing so by testing the efficacy of a mAb targeting IL-1β, canakinumab, at reducing recurrent cardiovascular events in patients with a history of acute myocardial infarctions and residual inflammatory risk despite standard-of-care treatment. Although canakinumab treatment did not reduce lipid levels from baseline, it significantly reduced the plasma marker of inflammation, CRP, and lowered the rate of recurrent cardiovascular events. Outcomes from CANTOS resulted in a paradigm shift in our understanding of the treatment of atherosclerosis, as standard-of-care treatment has focused primarily on lowering LDL-cholesterol levels to reduce the risk of adverse cardiac events.112,113 Despite these favorable results, patients treated with canakinumab also had an increased risk of fatal infections due to the systemic immune suppression, demonstrating the need for more targeted therapy.
After the success of CANTOS, other inflammation targets have been investigated. RESCUE was a recent Phase II clinical trial that aimed to inhibit IL-6 systemically in patients with chronic kidney disease at high atherosclerotic risk.114 The investigators hypothesized that IL-6 may be a more favorable target than IL-1β, as it has been directly implicated in the progression of coronary heart disease.115 Strikingly, treatment with a mAb to neutralize IL-6, ziltivekimab, reduced levels of both pro-inflammatory and thrombotic markers relevant to atherosclerosis.114 Based on these encouraging results, a large-scale cardiovascular outcomes trial is ongoing, and the results are greatly anticipated.
Immunomodulating therapies
In parallel to the promising anti-inflammatory strategies to treat atherosclerosis, several resolution-inducing therapeutic approaches are being tested. Efferocytosis is a process in which macrophages normally recognize and clear apoptotic tissue from plaques. Dysregulation of this process is now recognized as a hallmark of atherosclerosis that increases its pro-inflammatory state.116,117 Plaque cells highly express an antiphagocytosis ligand, Cluster of Differentiation 47 (CD47), on their surfaces, which inhibits them from being cleared by efferocytosis.118 In fact, mice treated with anti-CD47 antibodies restored efferocytosis and prevented atherosclerosis.119 However, these mice also developed anemia due to the antibody’s effects of off-target clearance of red blood cells in the spleen. To overcome off-target toxicity, a single-walled PEGylated carbon nanotubule carrying a small-molecule inhibitor of the CD47 signaling cascade was designed.120 When tested in mouse models, they were preferentially taken up by lesion macrophages and prevented atherosclerosis.
In addition, Fredman et al. used a nanoparticle containing the pro-resolving peptide Ac2-26 that activates the annexin A1 receptor on myeloid cells and stabilized advanced atherosclerotic lesions.121 Another study injected atherosclerotic mice with the potent polarizer IL-13 to induce the M2 state in macrophages and showed reduction in plaque inflammation and atherosclerosis burden.122 In another case, Geng et al. developed an immunomodulatory approach that aimed to reprogram monocytes for atherosclerosis treatment.123 Since oxidized-LDL as well as endotoxemia contribute to the polarization of monocytes toward a non-resolving, constant pro-inflammatory state through TLR4 pathways that involve either Mal/MyD88 or TRIF-related adaptor molecule (TRAM)/TRIF pathways, they showed that the genetic knockout of TRAM in monocytes of ApoE−/− mice had less atherosclerotic plaque development.123 The TRAM-deficient monocytes exhibited a pro-resolving phenotype characterized by both a reduced inflammatory response and a higher expression of anti-inflammatory mediators.123 In addition, several pro-resolving lipid mediators, including omega-3 fatty acids and 12/15-lipoxygenase products, are potent regulators of local inflammatory responses of macrophages and ECs.124,125 These studies show that therapies that induce the pro-resolving macrophage phenotype hold great promise for the treatment of atherosclerosis and prevention of CVDs.
Anti-atherosclerotic nanomedicine
Recent advances in the field of nanotechnology have the potential to revolutionize both diagnostic and therapeutic strategies to treat atherosclerosis.126 There are many advantages to using nanotechnology compared to traditional drug delivery methods. Nanomedicine increases blood circulation time, allowing for lower drug concentrations and less systemic toxicity.127,128 Nanomedicine facilitates the delivery of water-insoluble drugs or the co-delivery of two or more types of combination therapies in a localized manner. More important, nanomedicine facilitates the intracellular delivery of a vast array of small molecules, peptides, proteins, and nucleic acids such as siRNA and messenger RNA (mRNA) therapeutics. A wide range of materials have been used to formulate nanomedicines, including liposomes, polymers, organic, inorganic, and biomimetic materials.129 Of these, liposomes and polymers compose the majority of nanoparticles that are FDA approved or in clinical development.130 Importantly, nanomedicine approaches can be used simultaneously as diagnostics and therapeutics.131
In the last 2 decades, numerous diagnostic nano-sensing materials and anti-atherosclerotic nanotherapeutic approaches have been tested preclinically.129 Some of these strategies leverage the structure and function of high-density lipoprotein (HDL),132, 133, 134, 135 the phagocytic activities of macrophages,13,120,136, 137, 138 and immune cells as carriers of nanotherapeutics to the diseased vascular wall,139 while others targeted thrombosis,140 inflammation,141 defective efferocytosis,120 EC adhesion molecules,142,143 or the extracellular matrix.121 To better protect nanoparticles from innate immune responses, namely the mononuclear phagocyte system (MPS), which can readily opsonize and destroy foreign nanoparticles, biomimetic nanotherapies were designed, in which membranes of red blood cells144 or macrophages145 were used to coat the nanomedicines. These biomimetic nanomedicines offer a favorable strategy to evade the body’s immune response and bypass standard drug elimination.
mRNA therapeutics
mRNA-based therapeutics represent a game-changing technology that has transformed the vaccine field and is rapidly expanding, with the potential to treat chronic diseases, including CVD.146 There are many advantages to mRNA-based therapeutics since they can be produced quickly, cost-effectively, and in a cell-free system.147 Since mRNA is non-replicative, it is considered a very safe biomolecule that allows for transient protein expression in virtually all cell types, including non-dividing cells. Furthermore, coding sequences of any length can be synthetically produced with no nuclear localization, promoter elements, or transcription required, unlike recombinant virus vectors, making the probability of genomic integration nearly nonexistent.148 Importantly, pioneering work by Karikó et al.149 and later by others150,151 showed that replacing uridine residues with the naturally occurring modified nucleoside pseudouridine or N1-methylpseudourdine not only decreases activation of the innate immune pathway but also improves stability and enhances translation levels, making mRNA therapeutics for gene editing, base editing, or protein replacement therapies possible.
Several preclinical studies have shown that synthetic, chemically modified mRNA (modRNA) encoding VEGF-A delivered by direct intracardiac and intramuscular injection without lipid-based carriers resulted in a strong pulse of VEGF-A protein expression that was sufficient to reduce infarct size, enhance vascular regeneration and myocardial perfusion, improving survival after myocardial infarction in mice152 and pigs153 and accelerated wound healing in mice.154 These promising results drove a first-in-human, randomized, double-blind, placebo-controlled Phase I study in men with T2DM, which showed that intradermal delivery of VEGF-A modRNA was well tolerated and produced local functional VEGF-A protein that enhanced transient skin blood flow.155 Based on these positive results, the EPICCURE Phase IIa randomized, double-blind, placebo-controlled clinical trial was designed, which sought to inject VEGF-A modRNA into the myocardium of patients undergoing elective CABG surgery.156,157 The trial was recently completed, and outcomes on the safety, tolerability, and exploratory efficacy are anxiously being awaited.
Other pre-clinical studies have used modRNA encoding insulin-like growth factor-1 (IGF1) as a possible strategy to increase cytoprotection in cardiomyocytes after hypoxia or myocardial infarction,158 or phosphatidylinositol-5-phosphate-4-kinase type 2-gamma (Pip4k2c), which reversed cardiac hypertrophy and fibrosis in a mouse model.159 Research has also been done in primates using lipid nanoparticles (LNPs) to deliver modRNA encoding CRISPR base editors that introduce a precise PCSK9 loss-of-function mutation, with no off-target mutations in genomic DNA. One single infusion of this LNP resulted in nearly a complete knockdown of PCSK9 in the liver, leading to remarkable reductions in the serum levels of both PCSK9 protein and LDL-cholesterol, which lasted for at least 8 months.160,161 Based on these promising results, the first-in-human investigational trial was recently launched testing the safety and efficacy of PCSK9 base editing in patients with familial hypercholesterolemia and CVD.162
Recently, a microRNA (miRNA)-switch strategy was used to achieve cell-selective, antirestenosis therapy.163, 164, 165 This strategy consists of modRNA encoding the cyclin-dependent kinase inhibitor p27Kip1 that contains an EC-specific miRNA target site in its 5′ UTR or 3′ UTR. Thus, exploiting the EC-specific miR-126 allowed the discrimination between proliferating inflammatory cells and VSMCs while sparing ECs to carry on their vital functions. By adding the cationic amphipathic cell-penetrating peptide (p5RHH) to the miRNA switch, it self-assembles into compacted, endonuclease-resistant nanoparticles, which were rapidly taken up by cells and effectively released from the endosomes without inducing cytotoxicity or apoptosis of the transfected cells.163 Systemic administration of modRNA encoding near-infrared fluorescent protein (niRFP) nanoparticles to a wire injury mouse model showed specific expression at endothelial denuded regions, whereas no synthetic mRNA or protein products were detected in other organs, including the liver, spleen, lungs, heart, or kidneys. Strikingly, the repeated administration of nanoparticles containing the p27 Kip1 miR-126 switch reduced neointima formation after wire injury and allowed for vessel re-endothelialization.163 While numerous other therapeutic strategies have aimed to either inhibit inflammation, reduce neointimal hyperplasia, accelerate re-endothelialization, or inhibit thrombosis, the miRNA-switch nanotherapy achieved all of these objectives in a single, comprehensive treatment.163,165 Although the transient nature of the miRNA-switch requires multiple injections to achieve a therapeutic effect, once the EC integrity is restored, the p5RHH nanoparticle will no longer be able to penetrate the EC layer and will be quickly eliminated.
Precision medicine in anti-atherosclerotic therapies
As our knowledge of the human genome advances, precision medicine approaches are becoming more attainable. Precision medicine takes into account not only a patient’s lifestyle, medical history, and environment but also their genetic background and, if known, the molecular defects underlying their disease.166 Precision medicine has been successfully leveraged to treat those with cystic fibrosis, a disease caused by various mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Knowing the exact type of mutation in the CFTR gene has allowed for the targeted treatment of patients based on their specific CFTR mutation, making patient outcomes more favorable.167 Similarly, with the discovery of CHIP and its role in the development of CVD, precision medicine likely has a future in CVD treatment as well. As we age, we accumulate somatic mutations in our tissues, and some of these mutations confer advantages that allow one clonal cell to propagate itself over others.168 This process is common in hematopoietic stem cells, with many of the genes harboring mutations in epigenetic regulators such as TET2. Recent groundbreaking studies have shown that atherosclerosis-prone mice harboring TET2-mutant cells had increased NLRP3 inflammasome-mediated IL-1β secretion and increased atherosclerotic plaque size.104 Importantly, an NLRP3 inhibitor decreased atherosclerotic plaque size in the mice, demonstrating that small-molecule therapeutics may greatly benefit patients with CHIP mutations. Knowing the genetic profile of patients with CVDs and whether they harbor CHIP mutations may allow clinicians to tailor treatment options in a precise and personalized manner.
Conclusions and future perspectives
During the last decade, we have witnessed remarkable advances in understanding the fundamental causes of atherosclerosis to include inflammation, impaired efferocytosis, and CHIP. Moreover, new discoveries and therapeutic targets are regularly being made. In parallel, transformative new technologies such as nanomedicines, mRNA therapeutics, DNA base editing, and miRNA-switches are being developed to combat this global health crisis. Some of these technologies are still in early stages of development while others are being tested in clinical trials. In fact, there are >500 active clinical trials focused on treating atherosclerosis.169 The technological innovations range from coupling diagnostics with therapeutics, delivering mRNA to edit or encode desired genes, or incorporating miRNA recognition sequences for cell-selective expression of therapeutics. To successfully translate these technologies into clinical settings, there are still many limitations that need to be overcome. First, to limit off-target delivery and toxicity, the delivery methods associated with mRNA therapeutics and nanomedicines need to specifically target regions of atherosclerotic plaques. Second, the transient nature of mRNA therapeutic expression in cells, while beneficial in reducing off-target gene editing, may be disadvantageous for the miRNA-switch approach, which requires multiple treatments to achieve therapeutic effects. Finally, to truly have a significant impact on human health and decrease the mortality and morbidity associated with atherosclerosis, these therapies must be made affordable and available to the general population to not increase already prevalent global health disparities.
Acknowledgments
The H.T.-J. laboratory is supported by NIH R01 HL128411 and the Anna D. Valentine and Charles L. Oehler Research Award.
Author contributions
I.H. and H.T.-J. conducted the literature review and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., Tokgözoğlu L., Lewis E.F. Atherosclerosis. Nat. Rev. Dis. Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 3.Kronzon I., Tunick P.A. Aortic atherosclerotic disease and stroke. Circulation. 2006;114:63–75. doi: 10.1161/CIRCULATIONAHA.105.593418. [DOI] [PubMed] [Google Scholar]
- 4.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 5.Campbell I.C., Suever J.D., Timmins L.H., Veneziani A., Vito R.P., Virmani R., Oshinski J.N., Taylor W.R. Biomechanics and inflammation in atherosclerotic plaque erosion and plaque rupture: implications for cardiovascular events in women. PLoS One. 2014;9:e111785. doi: 10.1371/journal.pone.0111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A., et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otsuka F., Finn A.V., Yazdani S.K., Nakano M., Kolodgie F.D., Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat. Rev. Cardiol. 2012;9:439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 8.Gimbrone M.A., Jr., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrault L.P., Mahlberg F., Breugnot C., Bidouard J.P., Villeneuve N., Vilaine J.P., Vanhoutte P.M. Hypercholesterolemia increases coronary endothelial dysfunction, lipid content, and accelerated atherosclerosis after heart transplantation. Arterioscler. Thromb. Vasc. Biol. 2000;20:728–736. doi: 10.1161/01.atv.20.3.728. [DOI] [PubMed] [Google Scholar]
- 10.Higashi Y., Kihara Y., Noma K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. 2012;35:1039–1047. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 11.Mäkimattila S., Virkamäki A., Groop P.H., Cockcroft J., Utriainen T., Fagerudd J., Yki-Järvinen H. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y., Vaziri N.D., Coulson R., Kamanna V.S., Roh D.D. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am. J. Physiol. Endocrinol. Metab. 2000;279:E11–E17. doi: 10.1152/ajpendo.2000.279.1.E11. [DOI] [PubMed] [Google Scholar]
- 13.Dhanasekara C.S., Zhang J., Nie S., Li G., Fan Z., Wang S. Nanoparticles target intimal macrophages in atherosclerotic lesions. Nanomedicine. 2021;32:102346. doi: 10.1016/j.nano.2020.102346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susser L.I., Rayner K.J. Through the layers: how macrophages drive atherosclerosis across the vessel wall. J. Clin. Invest. 2022;132 doi: 10.1172/JCI157011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viola J., Soehnlein O. Atherosclerosis - a matter of unresolved inflammation. Semin. Immunol. 2015;27:184–193. doi: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Chen P.Y., Qin L., Baeyens N., Li G., Afolabi T., Budatha M., Tellides G., Schwartz M.A., Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Invest. 2015;125:4514–4528. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Lui K.O., Zhou B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018;15:445–456. doi: 10.1038/s41569-018-0023-y. [DOI] [PubMed] [Google Scholar]
- 18.Allahverdian S., Chehroudi A.C., McManus B.M., Abraham T., Francis G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 19.Nordestgaard B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ. Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 20.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 21.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto E., Yonetsu T., Kakuta T., Soeda T., Saito Y., Yan B.P., Kurihara O., Takano M., Niccoli G., Higuma T., et al. Clinical and laboratory predictors for plaque erosion in patients with acute coronary syndromes. J. Am. Heart Assoc. 2019;8:e012322. doi: 10.1161/JAHA.119.012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farb A., Burke A.P., Tang A.L., Liang T.Y., Mannan P., Smialek J., Virmani R. Coronary plaque erosion without rupture into a lipid core. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 24.Dawber T.R., Meadors G.F., Moore F.E., Jr. Epidemiological approaches to heart disease: the Framingham study. Am. J. Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons G.H., Seidman C.E., Topol E.J. Conquering atherosclerotic cardiovascular disease - 50 years of progress. N. Engl. J. Med. 2021;384:785–788. doi: 10.1056/NEJMp2033115. [DOI] [PubMed] [Google Scholar]
- 26.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Cheng S., Delling F.N., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 27.Anderson K.M., Castelli W.P., Levy D. Cholesterol and mortality. 30 years of follow-up from the Framingham study. JAMA. 1987;257:2176–2180. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 28.Michos E.D., McEvoy J.W., Blumenthal R.S. Lipid management for the prevention of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2019;381:1557–1567. doi: 10.1056/NEJMra1806939. [DOI] [PubMed] [Google Scholar]
- 29.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramkumar S., Raghunath A., Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol. Sin. 2016;32:631–639. doi: 10.6515/acs20160611a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival study (4S) Lancet. 1994;344:P1383–P1389. doi: 10.1016/S0140-6736(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 33.Ridker P.M., Cannon C.P., Morrow D., Rifai N., Rose L.M., McCabe C.H., Pfeffer M.A., Braunwald E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto K., Janssen W.J., Fessler M.B., McPhillips K.A., Borges V.M., Bowler R.P., Xiao Y.Q., Kench J.A., Henson P.M., Vandivier R.W. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J. Immunol. 2006;176:7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 35.Balakumar P., Mahadevan N. Interplay between statins and PPARs in improving cardiovascular outcomes: a double-edged sword? Br. J. Pharmacol. 2012;165:373–379. doi: 10.1111/j.1476-5381.2011.01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen A.L., Hasselbalch H.C., Nielsen C.H., Poulsen H.E., Ellervik C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox Biol. 2019;21:101088. doi: 10.1016/j.redox.2018.101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Undas A., Brummel-Ziedins K.E., Mann K.G. Statins and blood coagulation. Arterioscler. Thromb. Vasc. Biol. 2005;25:287–294. doi: 10.1161/01.ATV.0000151647.14923.ec. [DOI] [PubMed] [Google Scholar]
- 38.Diamantis E., Kyriakos G., Quiles-Sanchez L.V., Farmaki P., Troupis T. The anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr. Cardiol. Rev. 2017;13:209–216. doi: 10.2174/1573403X13666170426104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mammen A.L. Statin-associated autoimmune myopathy. N. Engl. J. Med. 2016;374:664–669. doi: 10.1056/NEJMra1515161. [DOI] [PubMed] [Google Scholar]
- 40.Cannon C.P., Blazing M.A., Giugliano R.P., McCagg A., White J.A., Theroux P., Darius H., Lewis B.S., Ophuis T.O., Jukema J.W., et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. Overseas. Ed. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 41.Kastelein J.J.P., Bots M.L. Statin therapy with ezetimibe or niacin in high-risk patients. N. Engl. J. Med. 2009;361:2180–2183. doi: 10.1056/NEJMe0908841. [DOI] [PubMed] [Google Scholar]
- 42.Abifadel M., Varret M., Rabès J.P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J.C., Boerwinkle E., Mosley T.H., Jr., Hobbs H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 44.Ferri N. Proprotein convertase subtilisin/kexin type 9: from the discovery to the development of new therapies for cardiovascular diseases. Scientifica. 2012;2012:927352. doi: 10.6064/2012/927352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., Edelberg J.M., Goodman S.G., Hanotin C., Harrington R.A., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 46.Sabatine M.S., Giugliano R.P., Wiviott S.D., Raal F.J., Blom D.J., Robinson J., Ballantyne C.M., Somaratne R., Legg J., Wasserman S.M., et al. Open-Label Study of Long-Term Evaluation against LDLCI. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 47.Kaddoura R., Orabi B., Salam A.M. PCSK9 monoclonal antibodies: an overview. Heart Views. 2020;21:97–103. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_20_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray K.K., Wright R.S., Kallend D., Koenig W., Leiter L.A., Raal F.J., Bisch J.A., Richardson T., Jaros M., Wijngaard P.L.J., Kastelein J.J.P. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 49.Administration USFaD. FDA approves add-on therapy to lower cholesterol among certain high-risk adults 2021. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-lower-cholesterol-among-certain-high-risk-adults
- 50.Collaboration N.C.D.R.F. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chobanian A.V., Alexander R.W. Exacerbation of atherosclerosis by hypertension. Potential mechanisms and clinical implications. Arch. Intern. Med. 1996;156:1952–1956. [PubMed] [Google Scholar]
- 52.Blood Pressure Lowering Treatment Trialists C. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomopoulos C., Parati G., Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J. Hypertens. 2015;33:1321–1341. doi: 10.1097/HJH.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 54.Farzam K., Jan A. StatPearls Publishing; 2021. Beta Blockers.https://www.ncbi.nlm.nih.gov/books/NBK532906/ [PubMed] [Google Scholar]
- 55.Hill R.D., Vaidya P.N. StatPearls Publishing; 2022. Angiotensin II Receptor Blockers (ARB)https://www.ncbi.nlm.nih.gov/books/NBK537027/ [PubMed] [Google Scholar]
- 56.Buawangpong N., Teekachunhatean S., Koonrungsesomboon N. Adverse pregnancy outcomes associated with first-trimester exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: a systematic review and meta-analysis. Pharmacol. Res. Perspect. 2020;8:e00644. doi: 10.1002/prp2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F.P., Saito S., Hall D.R., Warren C.E., Adoyi G., Ishaku S., International Society for the Study of Hypertension in Pregnancy ISSHP The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Hoeltzenbein M., Beck E., Fietz A.K., Wernicke J., Zinke S., Kayser A., Padberg S., Weber-Schoendorfer C., Meister R., Schaefer C. Pregnancy outcome after first trimester use of methyldopa: a prospective cohort study. Hypertension. 2017;70:201–208. doi: 10.1161/HYPERTENSIONAHA.117.09110. [DOI] [PubMed] [Google Scholar]
- 59.Webster L.M., Myers J.E., Nelson-Piercy C., Harding K., Cruickshank J.K., Watt-Coote I., Khalil A., Wiesender C., Seed P.T., Chappell L.C. Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension. 2017;70:915–922. doi: 10.1161/HYPERTENSIONAHA.117.09972. [DOI] [PubMed] [Google Scholar]
- 60.de la Sierra A., Banegas J.R., Oliveras A., Gorostidi M., Segura J., de la Cruz J.J., Armario P., Ruilope L.M. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J. Hypertens. 2012;30:1211–1216. doi: 10.1097/HJH.0b013e328353634e. [DOI] [PubMed] [Google Scholar]
- 61.Daugherty S.L., Powers J.D., Magid D.J., Tavel H.M., Masoudi F.A., Margolis K.L., O'Connor P.J., Selby J.V., Ho P.M. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang Z.R., Gao L.G., Cao J., Cui H., Fan L., Gao D.W. Clinical characteristics, target organ damage and associate risk factors of resistant hypertension determined by ambulatory blood pressure monitoring in patients aged >/= 80 years. J. Geriatr. Cardiol. 2017;14:308–314. doi: 10.11909/j.issn.1671-5411.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calhoun D.A., Booth J.N., 3rd, Oparil S., Irvin M.R., Shimbo D., Lackland D.T., Howard G., Safford M.M., Muntner P. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population-based cohort. Hypertension. 2014;63:451–458. doi: 10.1161/HYPERTENSIONAHA.113.02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dudenbostel T., Siddiqui M., Oparil S., Calhoun D.A. Refractory hypertension: a novel phenotype of antihypertensive treatment failure. Hypertension. 2016;67:1085–1092. doi: 10.1161/HYPERTENSIONAHA.116.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armario P., Calhoun D.A., Oliveras A., Blanch P., Vinyoles E., Banegas J.R., Gorostidi M., Segura J., Ruilope L.M., Dudenbostel T., de la Sierra A. Prevalence and clinical characteristics of refractory hypertension. J. Am. Heart Assoc. 2017;6:e007365. doi: 10.1161/JAHA.117.007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Organization W.H. Diabetes 2021. https://www.who.int/news-room/fact-sheets/detail/diabetes
- 67.Bluestone J.A., Herold K., Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kahn S.E., Cooper M.E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kannel W.B., McGee D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 70.Dahlén A.D., Dashi G., Maslov I., Attwood M.M., Jonsson J., Trukhan V., Schiöth H.B. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front. Pharmacol. 2021;12:807548. doi: 10.3389/fphar.2021.807548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rawshani A., Rawshani A., Franzén S., Eliasson B., Svensson A.M., Miftaraj M., McGuire D.K., Sattar N., Rosengren A., Gudbjörnsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N. Engl. J. Med. 2017;376:1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 72.Gregg E.W., Li Y., Wang J., Burrows N.R., Ali M.K., Rolka D., Williams D.E., Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N. Engl. J. Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt A.M. Diabetes mellitus and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2019;39:558–568. doi: 10.1161/ATVBAHA.119.310961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gregg E.W., Gu Q., Cheng Y.J., Narayan K.M.V., Cowie C.C. Mortality trends in men and women with diabetes, 1971 to 2000. Ann. Intern. Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 75.Fowkes F.G.R., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M., Norman P.E., Sampson U.K.A., Williams L.J., Mensah G.A., Criqui M.H. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 76.Secrest A.M., Becker D.J., Kelsey S.F., LaPorte R.E., Orchard T.J. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny county type 1 diabetes registry. Diabetes Care. 2010;33:2573–2579. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Serruys P.W., Morice M.C., Kappetein A.P., Colombo A., Holmes D.R., Mack M.J., Ståhle E., Feldman T.E., van den Brand M., Bass E.J., et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 78.Farkouh M.E., Domanski M., Sleeper L.A., Siami F.S., Dangas G., Mack M., Yang M., Cohen D.J., Rosenberg Y., Solomon S.D., et al. Strategies for multivessel revascularization in patients with diabetes. N. Engl. J. Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 79.Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1:263. doi: 10.1016/s0140-6736(78)90500-7. [DOI] [PubMed] [Google Scholar]
- 80.Landau C., Lange R.A., Hillis L.D. Percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 1994;330:981–993. doi: 10.1056/NEJM199404073301407. [DOI] [PubMed] [Google Scholar]
- 81.Canfield J., Totary-Jain H. 40 years of percutaneous coronary intervention: history and future directions. J. Pers Med. 2018;8:33. doi: 10.3390/jpm8040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schillinger M., Sabeti S., Loewe C., Dick P., Amighi J., Mlekusch W., Schlager O., Cejna M., Lammer J., Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 83.Moses J.W., Leon M.B., Popma J.J., Fitzgerald P.J., Holmes D.R., O'Shaughnessy C., Caputo R.P., Kereiakes D.J., Williams D.O., Teirstein P.S., et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 84.Lagerqvist B., James S.K., Stenestrand U., Lindbäck J., Nilsson T., Wallentin L., SCAAR Study Group Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 85.Park S.J., Kang S.J., Virmani R., Nakano M., Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J. Am. Coll. Cardiol. 2012;59:2051–2057. doi: 10.1016/j.jacc.2011.10.909. [DOI] [PubMed] [Google Scholar]
- 86.Polimeni A., Sorrentino S., Spaccarotella C., Mongiardo A., Sabatino J., De Rosa S., Gori T., Indolfi C. Stent thrombosis after percutaneous coronary intervention: from bare-metal to the last generation of drug-eluting stents. Cardiol. Clin. 2020;38:639–647. doi: 10.1016/j.ccl.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Brott T.G., Howard G., Roubin G.S., Meschia J.F., Mackey A., Brooks W., Moore W.S., Hill M.D., Mantese V.A., Clark W.M., et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N. Engl. J. Med. 2016;374:1021–1031. doi: 10.1056/NEJMoa1505215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuramitsu S., Sonoda S., Ando K., Otake H., Natsuaki M., Anai R., Honda Y., Kadota K., Kobayashi Y., Kimura T. Drug-eluting stent thrombosis: current and future perspectives. Cardiovasc. Interv. Ther. 2021;36:158–168. doi: 10.1007/s12928-021-00754-x. [DOI] [PubMed] [Google Scholar]
- 89.Tada T., Byrne R.A., Simunovic I., King L.A., Cassese S., Joner M., Fusaro M., Schneider S., Schulz S., Ibrahim T., et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18, 334 patients. JACC. Cardiovasc. Interv. 2013;6:1267–1274. doi: 10.1016/j.jcin.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Chen H., Power D., Giustino G. Optimal duration of dual antiplatelet therapy after PCI: integrating procedural complexity, bleeding risk and the acuteness of clinical presentation. Expert Rev. Cardiovasc. Ther. 2018;16:735–748. doi: 10.1080/14779072.2018.1523718. [DOI] [PubMed] [Google Scholar]
- 91.Hansson G.K., Nilsson J. Introduction: atherosclerosis as inflammation: a controversial concept becomes accepted. J. Intern. Med. 2008;263:462–463. doi: 10.1111/j.1365-2796.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 92.Ridker P.M. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J. Am. Coll. Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 93.Ridker P.M., Rifai N., Clearfield M., Downs J.R., Weis S.E., Miles J.S., Gotto A.M., Jr., Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 94.Soehnlein O., Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat. Rev. Drug Discov. 2021;20:589–610. doi: 10.1038/s41573-021-00198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Libby P., Everett B.M. Novel antiatherosclerotic therapies. Arterioscler. Thromb. Vasc. Biol. 2019;39:538–545. doi: 10.1161/ATVBAHA.118.310958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Libby P., Ridker P.M., Hansson G.K., Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Nuñez G., Schnurr M., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Libby P., Tabas I., Fredman G., Fisher E.A. Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 2014;114:1867–1879. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rhoads J.P., Lukens J.R., Wilhelm A.J., Moore J.L., Mendez-Fernandez Y., Kanneganti T.D., Major A.S. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcgammaR cooperation and is dependent on CARD9. J. Immunol. 2017;198:2105–2114. doi: 10.4049/jimmunol.1601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao H., Lu M., Lin T.Y., Chen Z., Chen G., Wang W.C., Marin T., Shentu T.P., Wen L., Gongol B., et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Folco E.J., Sukhova G.K., Quillard T., Libby P. Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ. Res. 2014;115:875–883. doi: 10.1161/CIRCRESAHA.115.304437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gupta N., Sahu A., Prabhakar A., Chatterjee T., Tyagi T., Kumari B., Khan N., Nair V., Bajaj N., Sharma M., Ashraf M.Z. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc. Natl. Acad. Sci. USA. 2017;114:4763–4768. doi: 10.1073/pnas.1620458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Campos J., Ponomaryov T., De Prendergast A., Whitworth K., Smith C.W., Khan A.O., Kavanagh D., Brill A. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv. 2021;5:2319–2324. doi: 10.1182/bloodadvances.2020003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fuster J.J., MacLauchlan S., Zuriaga M.A., Polackal M.N., Ostriker A.C., Chakraborty R., Wu C.L., Sano S., Muralidharan S., Rius C., et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sborgi L., Ruhl S., Mulvihill E., Pipercevic J., Heilig R., Stahlberg H., Farady C.J., Muller D.J., Broz P., Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin Y., Fu J. Novel insights into the NLRP 3 inflammasome in atherosclerosis. J. Am. Heart Assoc. 2019;8:e012219. doi: 10.1161/JAHA.119.012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ridker P.M., Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021;128:1728–1746. doi: 10.1161/CIRCRESAHA.121.319077. [DOI] [PubMed] [Google Scholar]
- 108.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 109.Dalbeth N., Lauterio T.J., Wolfe H.R. Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 2014;36:1465–1479. doi: 10.1016/j.clinthera.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 110.Opstal T.S.J., Fiolet A.T.L., van Broekhoven A., Mosterd A., Eikelboom J.W., Nidorf S.M., Thompson P.L., Duyvendak M., van Eck J.W.M., van Beek E.A., et al. Colchicine in patients with chronic coronary disease in relation to prior acute coronary syndrome. J. Am. Coll. Cardiol. 2021;78:859–866. doi: 10.1016/j.jacc.2021.06.037. [DOI] [PubMed] [Google Scholar]
- 111.Vaidya K., Arnott C., Martínez G.J., Ng B., McCormack S., Sullivan D.R., Celermajer D.S., Patel S. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: a CT coronary angiography study. JACC. Cardiovasc. Imaging. 2018;11(2 Pt 2):305–316. doi: 10.1016/j.jcmg.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 112.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker JJPK S.D., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl. J. Med. 2017;377 doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 113.Su L., Mittal R., Ramgobin D., Jain R., Jain R. Current management guidelines on hyperlipidemia: the silent killer. J. Lipids. 2021;2021:9883352. doi: 10.1155/2021/9883352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ridker P.M., Devalaraja M., Baeres F.M.M., Engelmann M.D.M., Hovingh G.K., Ivkovic M., Lo L., Kling D., Pergola P., Raj D., et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397:2060–2069. doi: 10.1016/S0140-6736(21)00520-1. [DOI] [PubMed] [Google Scholar]
- 115.Collaboration IRGCERF. Sarwar N., Butterworth A.S., Freitag D.F., Gregson J., Willeit P., Gorman D.N., Gao P., Saleheen D., Rendon A., et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yurdagul A., Jr., Doran A.C., Cai B., Fredman G., Tabas I.A. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovasc. Med. 2017;4:86. doi: 10.3389/fcvm.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kojima Y., Weissman I.L., Leeper N.J. The role of efferocytosis in atherosclerosis. Circulation. 2017;135:476–489. doi: 10.1161/CIRCULATIONAHA.116.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dou M., Chen Y., Hu J., Ma D., Xing Y. Recent advancements in CD47 signal transduction pathways involved in vascular diseases. Biomed. Res. Int. 2020;2020:4749135. doi: 10.1155/2020/4749135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kojima Y., Volkmer J.P., McKenna K., Civelek M., Lusis A.J., Miller C.L., Direnzo D., Nanda V., Ye J., Connolly A.J., et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flores A.M., Hosseini-Nassab N., Jarr K.U., Ye J., Zhu X., Wirka R., Koh A.L., Tsantilas P., Wang Y., Nanda V., et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 2020;15:154–161. doi: 10.1038/s41565-019-0619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fredman G., Kamaly N., Spolitu S., Milton J., Ghorpade D., Chiasson R., Kuriakose G., Perretti M., Farokzhad O., Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 2015;7:275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cardilo-Reis L., Gruber S., Schreier S.M., Drechsler M., Papac-Milicevic N., Weber C., Wagner O., Stangl H., Soehnlein O., Binder C.J. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Geng S., Zhang Y., Yi Z., Lu R., Li L. Resolving monocytes generated through TRAM deletion attenuate atherosclerosis. JCI Insight. 2021;6 doi: 10.1172/jci.insight.149651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li S., Sun Y., Liang C.P., Thorp E.B., Han S., Jehle A.W., Saraswathi V., Pridgen B., Kanter J.E., Li R., et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ. Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flores A.M., Ye J., Jarr K.U., Hosseini-Nassab N., Smith B.R., Leeper N.J. Nanoparticle therapy for vascular diseases. Arterioscler. Thromb. Vasc. Biol. 2019;39:635–646. doi: 10.1161/ATVBAHA.118.311569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu Q., Fang Z., Ge J., Li H. Nanotechnology for cardiovascular diseases. Innovation. 2022;3:100214. doi: 10.1016/j.xinn.2022.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Beltrán-Gracia E., López-Camacho A., Higuera-Ciapara I., Velázquez-Fernández J.B., Vallejo-Cardona A.A. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 2019;10:1–40. doi: 10.1186/s12645-019-0055-y. [DOI] [Google Scholar]
- 131.Chen W., Schilperoort M., Cao Y., Shi J., Tabas I., Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2021 doi: 10.1038/s41569-021-00629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanchez-Gaytan B.L., Fay F., Lobatto M.E., Tang J., Ouimet M., Kim Y., van der Staay S.E.M., van Rijs S.M., Priem B., Zhang L., et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug. Chem. 2015;26:443–451. doi: 10.1021/bc500517k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McMahon K.M., Mutharasan R.K., Tripathy S., Veliceasa D., Bobeica M., Shumaker D.K., Luthi A.J., Helfand B.T., Ardehali H., Mirkin C.A., et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011;11:1208–1214. doi: 10.1021/nl1041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Skajaa T., Cormode D.P., Jarzyna P.A., Delshad A., Blachford C., Barazza A., Fisher E.A., Gordon R.E., Fayad Z.A., Mulder W.J. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials. 2011;32:206–213. doi: 10.1016/j.biomaterials.2010.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cormode D.P., Skajaa T., van Schooneveld M.M., Koole R., Jarzyna P., Lobatto M.E., Calcagno C., Barazza A., Gordon R.E., Zanzonico P., et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tarin C., Carril M., Martin-Ventura J.L., Markuerkiaga I., Padro D., Llamas-Granda P., Moreno J.A., Garcia I., Genicio N., Plaza-Garcia S., et al. Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI. Sci. Rep. 2015;5:17135. doi: 10.1038/srep17135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kircher M.F., Grimm J., Swirski F.K., Libby P., Gerszten R.E., Allport J.R., Weissleder R. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008;117:388–395. doi: 10.1161/CIRCULATIONAHA.107.719765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nahrendorf M., Zhang H., Hembrador S., Panizzi P., Sosnovik D.E., Aikawa E., Libby P., Swirski F.K., Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fish M.B., Banka A.L., Braunreuther M., Fromen C.A., Kelley W.J., Lee J., Adili R., Holinstat M., Eniola-Adefeso O. Deformable microparticles for shuttling nanoparticles to the vascular wall. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su M., Dai Q., Chen C., Zeng Y., Chu C., Liu G. Nano-medicine for thrombosis: a precise diagnosis and treatment strategy. Nanomicro. Lett. 2020;12:96. doi: 10.1007/s40820-020-00434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamaly N., Fredman G., Subramanian M., Gadde S., Pesic A., Cheung L., Fayad Z.A., Langer R., Tabas I., Farokhzad O.C. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc. Natl. Acad. Sci. USA. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serrano D., Bhowmick T., Chadha R., Garnacho C., Muro S. Intercellular adhesion molecule 1 engagement modulates sphingomyelinase and ceramide, supporting uptake of drug carriers by the vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2012;32:1178–1185. doi: 10.1161/ATVBAHA.111.244186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nahrendorf M., Jaffer F.A., Kelly K.A., Sosnovik D.E., Aikawa E., Libby P., Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 144.Wang Y., Zhang K., Qin X., Li T., Qiu J., Yin T., Huang J., McGinty S., Pontrelli G., Ren J., et al. Biomimetic nanotherapies: red blood cell based core-shell structured nanocomplexes for atherosclerosis management. Adv. Sci. 2019;6:1900172. doi: 10.1002/advs.201900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gao C., Huang Q., Liu C., Kwong C.H.T., Yue L., Wan J.B., Lee S.M.Y., Wang R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020;11:2622. doi: 10.1038/s41467-020-16439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chien K.R., Zangi L., Lui K.O. Synthetic chemically modified mRNA (modRNA): toward a new technology platform for cardiovascular biology and medicine. Cold Spring Harb. Perspect. Med. 2014;5:a014035. doi: 10.1101/cshperspect.a014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Damase T.R., Sukhovershin R., Boada C., Taraballi F., Pettigrew R.I., Cooke J.P. The limitless future of RNA therapeutics. Front. Bioeng. Biotechnol. 2021;9:628137. doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schott J.W., Morgan M., Galla M., Schambach A. Viral and synthetic RNA vector technologies and applications. Mol. Ther. 2016;24:1513–1527. doi: 10.1038/mt.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Svitkin Y.V., Cheng Y.M., Chakraborty T., Presnyak V., John M., Sonenberg N. N1-methyl-pseudouridine in mRNA enhances translation through eIF2alpha-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res. 2017;45:6023–6036. doi: 10.1093/nar/gkx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Andries O., Mc Cafferty S., De Smedt S.C., Weiss R., Sanders N.N., Kitada T. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control Release. 2015;217:337–344. doi: 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 152.Zangi L., Lui K.O., von Gise A., Ma Q., Ebina W., Ptaszek L.M., Spater D., Xu H., Tabebordbar M., Gorbatov R., et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carlsson L., Clarke J.C., Yen C., Gregoire F., Albery T., Billger M., Egnell A.C., Gan L.M., Jennbacken K., Johansson E., et al. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 week post-myocardial infarction in swine. Mol. Ther. Methods Clin. Dev. 2018;9:330–346. doi: 10.1016/j.omtm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun N., Ning B., Hansson K.M., Bruce A.C., Seaman S.A., Zhang C., Rikard M., DeRosa C.A., Fraser C.L., Wågberg M., et al. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci. Rep. 2018;8:17509. doi: 10.1038/s41598-018-35570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gan L.M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.C., Rudvik A., Kjaer M., Collén A., Thompson J.D., Joyal J., et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Anttila V., Saraste A., Knuuti J., Jaakkola P., Hedman M., Svedlund S., Lagerstrom-Fermer M., Kjaer M., Jeppsson A., Gan L.M. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol. Ther. Methods Clin. Dev. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Late-breaking science abstracts and featured science abstracts from the American heart association's scientific sessions 2021 and late-breaking abstracts in resuscitation science from the resuscitation science symposium 2021. Circulation. 2021;144:e564–e593. doi: 10.1161/CIR.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 158.Huang C.L., Leblond A.L., Turner E.C., Kumar A.H., Martin K., Whelan D., O'Sullivan D.M., Caplice N.M. Synthetic chemically modified mRNA-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol. Pharm. 2015;12:991–996. doi: 10.1021/mp5006239. [DOI] [PubMed] [Google Scholar]
- 159.Magadum A., Singh N., Kurian A.A., Sharkar M.T.K., Sultana N., Chepurko E., Kaur K., Zak M.M., Hadas Y., Lebeche D., et al. Therapeutic delivery of pip4k2c-modified mRNA attenuates cardiac hypertrophy and fibrosis in the failing heart. Adv. Sci. 2021;8:2004661. doi: 10.1002/advs.202004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Musunuru K., Chadwick A.C., Mizoguchi T., Garcia S.P., DeNizio J.E., Reiss C.W., Wang K., Iyer S., Dutta C., Clendaniel V., et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593:429–434. doi: 10.1038/s41586-021-03534-y. [DOI] [PubMed] [Google Scholar]
- 161.Rothgangl T., Dennis M.K., Lin P.J.C., Oka R., Witzigmann D., Villiger L., Qi W., Hruzova M., Kissling L., Lenggenhager D., et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 2021;39:949–957. doi: 10.1038/s41587-021-00933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Verve Therapeutics, Inc. Verve Therapeutics; 2022. Verve Therapeutics Doses First Human with an Investigational in Vivo Base Editing Medicine, VERVE-101, as a Potential Treatment for Heterozygous Familial Hypercholesterolemia.https://ir.vervetx.com/news-releases/news-release-details/verve-therapeutics-doses-first-human-investigational-vivo-base [Google Scholar]
- 163.Lockhart J.H., VanWye J., Banerjee R., Wickline S.A., Pan H., Totary-Jain H. Self-assembled miRNA-switch nanoparticles target denuded regions and prevent restenosis. Mol. Ther. 2021;29:1744–1757. doi: 10.1016/j.ymthe.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lockhart J., Canfield J., Mong E.F., VanWye J., Totary-Jain H. Nucleotide modification alters MicroRNA-dependent silencing of MicroRNA switches. Mol. Ther. Nucleic Acids. 2019;14:339–350. doi: 10.1016/j.omtn.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Santulli G., Wronska A., Uryu K., Diacovo T.G., Gao M., Marx S.O., Kitajewski J., Chilton J.M., Akat K.M., Tuschl T., et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J. Clin. Invest. 2014;124:4102–4114. doi: 10.1172/JCI76069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ashley E.A. Towards precision medicine. Nat. Rev. Genet. 2016;17:507–522. doi: 10.1038/nrg.2016.86. [DOI] [PubMed] [Google Scholar]
- 167.Manfredi C., Tindall J.M., Hong J.S., Sorscher E.J. Making precision medicine personal for cystic fibrosis. Science. 2019;365:220–221. doi: 10.1126/science.aaw0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jaiswal S., Ebert B.L. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673. doi: 10.1126/science.aan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.U.S. National Library of Medicine ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/results?cond=atherosclerosis&Search=Apply&recrs=a&recrs=d&age_v=&gndr=&type=&rslt=