Abstract
Exosomes have a crucial role in intercellular communication and mediate interactions between tumor cells and tumor-associated macrophages (TAMs). Exosome-encapsulated non-coding RNAs (ncRNAs) are involved in various physiological processes. Tumor-derived exosomal ncRNAs induce M2 macrophage polarization through signaling pathway activation, signal transduction, and transcriptional and post-transcriptional regulation. Conversely, TAM-derived exosomal ncRNAs promote tumor proliferation, metastasis, angiogenesis, chemoresistance, and immunosuppression. MicroRNAs induce gene silencing by directly targeting mRNAs, whereas lncRNAs and circRNAs act as miRNA sponges to indirectly regulate protein expressions. The role of ncRNAs in tumor-host interactions is ubiquitous. Current research is increasingly focused on the tumor microenvironment. On the basis of the “cancer-immunity cycle” hypothesis, we discuss the effects of exosomal ncRNAs on immune cells to induce T cell exhaustion, overexpression of programmed cell death ligands, and create a tumor immunosuppressive microenvironment. Furthermore, we discuss potential applications and prospects of exosomal ncRNAs as clinical biomarkers and drug delivery systems.
Keywords: exosome, non-coding RNA, tumor-associated macrophage, tumor microenvironment, immunotherapy
Graphical abstract
Exosomes mediate interactions between tumoral and host immune cells in the tumor environment. Exosome-derived non-coding RNAs (ncRNAs) play multiple roles in physiological and pathological processes, especially cancer. Xu et al. review the current literature on the impacts of exosomes and their ncRNAs in the tumor environment.
Background
Non-coding RNAs (ncRNAs) do not encode proteins but control protein expression and function.1, 2, 3 Several types of ncRNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), affect cell growth, proliferation, and metabolism through multiple mechanisms.3, 4, 5 miRNAs are 20–25 nucleotide ncRNAs that induce gene silencing at the post-transcriptional level by binding to the 3′ untranslated region (3′ UTR) of target mRNAs, thus regulating gene expression and other cellular processes.6,7
lncRNAs are longer than 200 nucleotides that are involved in post-transcriptional regulation in the nucleus and cytoplasm.8,9 lncRNAs modulate the stability, translation, protein stability, and translocation of mRNAs.9,10 lncRNAs increase target mRNA expressions by sponging miRNAs as competitive endogenous RNAs (ceRNAs), and are secreted either alone or bound to proteins.11,12
circRNAs are characterized by a covalently closed-loop structure without a 5′ cap and a poly(A) tail.13,14 These RNAs are implicated in miRNA spongings, protein interactions, and the regulation of nuclear transcription and pre-mRNA splicing.15,16
Exosomes are 40–150 nm extracellular vesicles that regulate multiple physiological and pathological processes by mediating intercellular communication.17,18 The content of exosomes released by donor cells is protected from enzymatic hydrolysis.19, 20, 21 Exosomes have fundamental roles in tumor proliferation, metastasis, and drug resistance.21, 22, 23 Tumoral and immune cells secrete exosomes in the tumor immune microenvironment (TIME).23,24 Tumor-derived exosomes (TEXs) modulate immunological activities, including macrophage polarization, T cell regulation, and inhibition of natural killer (NK) cell activity.25, 26, 27 TEXs also affect tumor malignancy, suggesting their key role in interactions between tumoral and immune cells.28, 29, 30 The roles of exosomal ncRNAs in tumor development and immunosuppression have attracted increasing attention.31, 32, 33 Exosomes can be used in drug delivery because of their high encapsulation efficiency and the ability to transport anti-cancer drugs, natural agents, nucleic acids, and gene-editing systems such as CRISPR-Cas9.34, 35, 36
Macrophages are phagocytic immune cells, and their phenotypes are influenced by cytokines and other factors in the TIME.37 Macrophages can assume a classically activated pro-inflammatory (M1) phenotype and an alternatively activated anti-inflammatory (M2) phenotype.38 Tumor-associated macrophages (TAMs) have an M1 phenotype in the early stages of cancer.37 In the later stage, growth factors and anti-inflammatory mediators, such as IL-4, IL-10, and TGF-β, are expressed in the TIME, inducing M2 polarization.37 M1-M2 polarization is highly dynamic and reversible.
M2 TAMs produce growth factors and inhibit immune activity in the TIME.39 TAM infiltration in solid tumors underscores the role of these cells in tumor progression and immunosuppression.39, 40, 41 M2 macrophages can be divided into M2a, M2b, M2c, and M2d.42 The M2a subgroup is activated by IL-4 and IL-13 and produces CD163, CD206, IL-10, TGF-β, and IL1Ra.42 The M2b subgroup is stimulated by immune complexes and bacterial lipopolysaccharide to produce CD86, IL-10, IL-6, and TNF-α.42 The M2c subgroup is induced by glucocorticoids, IL-10, and TGF-β and produces CD163, CD206, IL-10, and TGF-β; in addition, this subgroup is active against apoptotic cells.42 The M2d subgroup, stimulated by IL-6 and adenosine, secretes anti-inflammatory cytokines (high levels of IL-10 and low levels of IL-12) and vascular endothelial growth factor (VEGF) to promote tumor angiogenesis.42
Some signaling pathways regulate macrophage switch.41,43 Pro-inflammatory cytokines induce malignant behavior, whereas the anti-inflammatory microenvironment promotes tumorigenesis and immune evasion.41,43 Therefore, macrophage activation is fine-tuned by the TIME. Tumor tissues may contain mixed macrophage populations with a spectrum of activation states. However, in this review, we assumed that TAMs have an M2 phenotype, as described in the literature.37
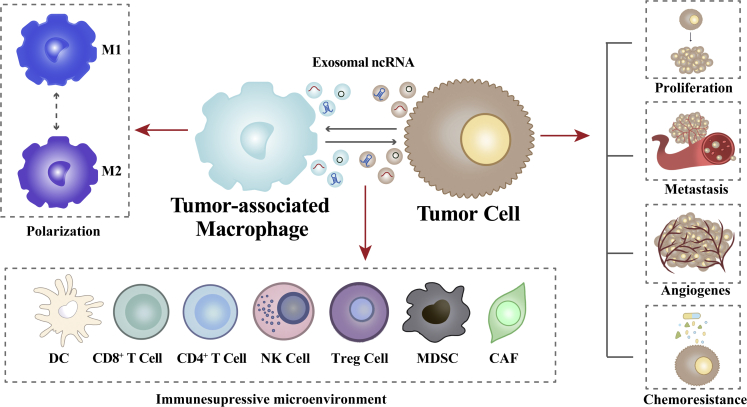
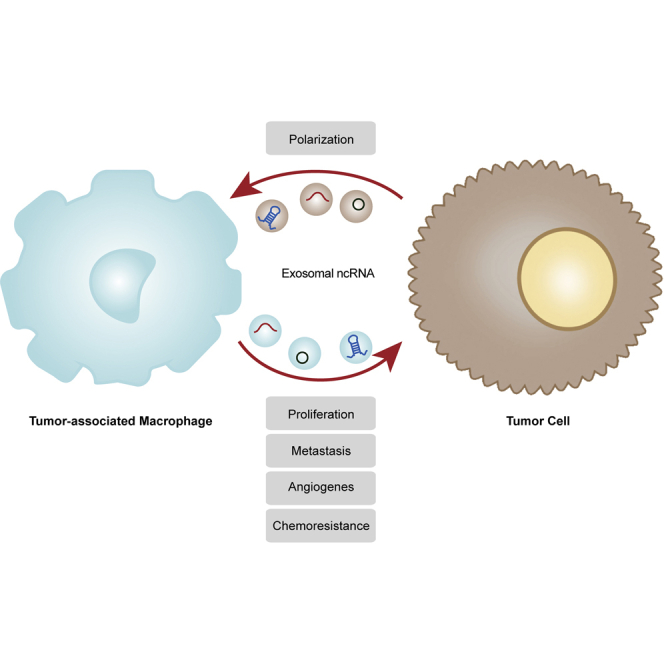
Increased attention has been given to the interactions between tumor cells and TAMs. Tumor cells promote TAM polarization, and polarized TAMs support the malignant phenotype of tumor cells, forming a cycle in which exosomal ncRNAs mediate the communication between tumoral and immune cells (Figure 1). This review discusses the interactions between tumor cells and TAMs during tumor initiation and development, mechanisms controlled by exosomal ncRNAs, the influence of exosomal ncRNAs on the formation of the TIME based on the “cancer-immunity cycle” model, and the potential application of exosomal ncRNAs as diagnostic and prognostic biomarkers and therapeutic targets.
Figure 1.
Interactions between tumor cells and TAMs via exosomal non-coding RNAs
Tumor-derived exosomal ncRNAs induce macrophage M1/M2 polarization. Conversely, TAM-derived exosomal ncRNAs promote tumor proliferation, metastasis, angiogenesis, and chemoresistance. Besides, these ncRNAs contribute to an immunosuppressive microenvironment by regulating immune cells.
Exosomal ncRNAs are important mediators of interactions between tumor cells and TAMs in the TIME
Tumor-derived exosomal ncRNAs promote macrophage polarization
Signaling pathways and macrophage polarization
Macrophage polarization involves multiple signaling pathways and transcriptional and post-transcriptional regulatory networks.38 The phosphatidylinositol 3-kinase (PI3K)/AKT and JAK/STAT pathways and key regulatory factors, including the signal transducer and activator of transcription (STAT) family, peroxisome proliferator-activated receptor-γ (PPARγ), and interferon regulatory factors, are implicated in macrophage polarization.44,45 Tumors can regulate polarization by controlling the function of exosomal ncRNAs and regulatory factors. For instance, in HPV+ head and neck squamous cell carcinoma (HNSCC), miR-9 was enriched in TEXs and was transported to macrophages, inducing M1 polarization by downregulating PPARδ.46 In addition, high levels of miR-451/miR-21 were detected in exosomes from primary human glioblastoma multiforme (GBM) cells and were taken up by TAMs in the brain of mice, decreasing c-Myc mRNA levels. The levels of miR-21 and miR-451 increased in microglia co-cultured with GBM exosomes and heparin reduced this effect.47 In prostate cancer (PCa), miRNAs let-7a-5p and let7-b, -g, -i were enriched in exosomes and downregulated integrin-β3, causing M2 polarization and PCa cell migration.48 Exosomal lncRNA TUC339 was highly expressed in hepatocellular carcinoma (HCC) cells and promoted M2 polarization, leading to reduced pro-inflammatory cytokine production, compromised phagocytosis, and decreased co-stimulatory molecule expression in macrophages.49,50 TUC339 is involved in cytokine receptor signaling pathways and CXCR chemokine receptor binding pathways, which may explain the mechanisms underlying this regulation.50 miR-21-5p was highly enriched in exosomes from colorectal cancer (CRC) cells.51 Exosomes from the CRC cell lines SW480, SW620, and LoVo were injected into nude mice and were significantly enriched in liver macrophages. Furthermore, miR-21 in CRC exosomes promoted M1 polarization via TLR7 to produce IL-6, inducing a pro-inflammatory pre-metastatic niche and CRC cell survival and colonization, ultimately leading to liver metastasis.51
miRNAs also target metabolic enzymes. For instance, melanoma exosomal miR-125b-5p targeted lysosomal acid lipase A in macrophages, leading to phenotypic switching and increasing M2 macrophage survival.52
Several immune factors, including TGF-β, IL-10, and BMP-7, promote M2 polarization via the PI3K/AKT pathway.45,53,54 Phosphatase and tension homolog deleted on chromosome ten (PTEN) inhibits AKT activity by dephosphorylating PIP3.45,55 Exosomal miR-21 from bladder cancer cells regulated PI3K/AKT signaling by inhibiting PTEN activation in macrophages and enhanced STAT3 expression, promoting M2 polarization, leading to cancer cell migration and invasion.56 Exosomal miR-130b-3p, miR-425-5p, and miR-25-3p were transported from CRC cells to macrophages and induced M2 polarization by targeting PTEN and activating the PI3K/AKT pathway. M2 macrophages enhanced epithelial-mesenchymal transition (EMT) and secreted VEGF to promote CRC metastasis.57,58 circFARSA was upregulated in non-small cell lung cancer (NSCLC) tissues and transported to macrophages by exosomes.59 Exosomal circFARSA activated PI3K/AKT signaling in macrophages through ubiquitination and degradation of PTEN, promoting M2 polarization.59 The RNA-binding protein eIF4A3 triggered circFARSA biogenesis and cyclization during M2 polarization, enhancing EMT and metastasis in NSCLC cells.59
The JAK/STAT signaling pathway is implicated in macrophage polarization, and STAT1/5 and STAT 3/6 are involved in M1 and M2 polarization, respectively.60,61 STAT activity is regulated by members of the suppressor of cytokine signaling (SOCS) family.60 Exosomal miR-29a-3p promoted M2 macrophage polarization in oral squamous cell carcinoma by regulating SOCS1/STAT6 signaling.62 In a co-culture system, exosomal miR-223 from cervical squamous cell carcinoma (CSCC) induced IL-6 secretion in M1 macrophages, enhancing STAT3 activity and increasing miR-223 expression in CSCC cells, creating a positive feedback loop.63 Moreover, miR-223 repressed TGFBR3 and HMGCS1 expression in CSCC by targeting their 3′ UTRs, resulting in anchorage-independent growth and tumor growth.63
Factors regulating macrophage polarization
Hypoxia stimulates exosome secretion, and hypoxic exosomes from tumor cells trigger M2 macrophage polarization in a HIF1α- and HIF2α-dependent manner.64,65 Hypoxia induced miR-301a-3p expression in pancreatic cancer (PC) cells and their exosomes and promoted M2 polarization through the PTEN/PI3Kγ signaling pathway.66,67 In addition, hypoxia stimulated hsa_circ_0048117 expression in exosomes from esophageal squamous cell carcinoma (ESCC) cells, and this circRNA promoted M2 polarization by upregulating TLR4 and sponging miR-140.68
The activation or inhibition of the above signaling pathways and regulatory factors is enhanced by hypoxia. For instance, hypoxic tumor exosomes increased oxidative phosphorylation in macrophages via miRNA let-7a, inhibiting the insulin-AKT-mTOR signaling pathway.69 Mammalian target of rapamycin (mTOR) is a downstream molecule of the PI3K-AKT pathway.53 Similarly, hypoxic exosomes induced M2 polarization and enhanced the proliferation, migration, and invasion of glioma in vitro and in vivo.70 MicroRNA-1246 was highly enriched in hypoxic glioma exosomes and mediated M2 polarization by targeting TERF2IP through the STAT3 and NF-κB pathways.70
Different ncRNAs are expressed in tumor exosomes depending on oxygen availability. Under normoxic conditions, miR-222-3p was enriched in macrophage exosomes from epithelial ovarian cancer (EOC) cells and induced M2 polarization via the SOCS3/STAT3 pathway.71 In contrast, miR-940 was expressed in EOC cells and their exosomes and stimulated M2 macrophage polarization under hypoxic conditions.72 In addition, under hypoxia, miR-21-3p, miR-125b-5p, and miR-181d-5p in EOC cell exosomes induced M2 polarization by regulating the SOCS4/5/STAT3 pathway.73 However, the source of exosomes and the mechanisms underlying macrophage polarization by circRNA have not been determined.
EMT is associated with tumor development and chemoresistance.74 Tumor cells control TAMs via exosomal ncRNAs and promote EMT.75,76 EMT is accompanied by a large infiltration of M2 macrophages and exosomes in tumor tissues, including HCC, human head and neck cancer, CRC, and NSCLC.77, 78, 79, 80, 81, 82 Whether EMT leads to M2 polarization or M2 macrophages promote EMT remains controversial; notwithstanding, these processes are complementary. HCC exosomal lncRNA DLX6-AS1 regulated M2 macrophage polarization through competitively binding to miR-15a-5p and regulating the miRNA-15a-5p/CXCL17 axis, thereby promoting HCC migration, invasion, EMT, and pulmonary metastasis.77 The EMT transcription factor Snail activated miR21 transcription and produced miR-21-enriched TEXs. TEXs containing miR-21-, taken up by human monocytes, decreased the expression of M1 markers and increased the expression of M2 markers. In Snail-expressing human head and neck cancer cells, miR-21 knockdown attenuated M2 polarization and inhibited tumor angiogenesis and growth.78 CRC exosomes containing the lncRNA RPPH1 mediated M2 macrophage polarization, promoting cancer cell proliferation and metastasis.79,80 Hsa_circ_0074854 was transferred from HCC tissues and exosomes to macrophages and promoted M2 polarization, and hsa_circ_0074854 knockdown suppressed exosome-mediated polarization and HCC migration and invasion.81 The roles of exosomal lncRNA FGD5-AS1 in NSCLC were similar to those of hsa_circ_0074854 in HCC.82 Nonetheless, the underlying mechanisms remain unclear.
Epigallocatechin gallate exerts antitumor effects by upregulating miR-16 in tumor cells and their exosomes and inhibiting TAM infiltration and M2 polarization.83
The infiltration of M2 macrophages in NSCLC was driven by the Kras mutant and was associated with tumor expansion, Kras-related chemoresistance, and patient survival. Exosome cicHIPK3/PTK2 promoted Kras-driven intratumoral heterogeneity in CD163+ TAMs and lymph node metastasis in mice.84
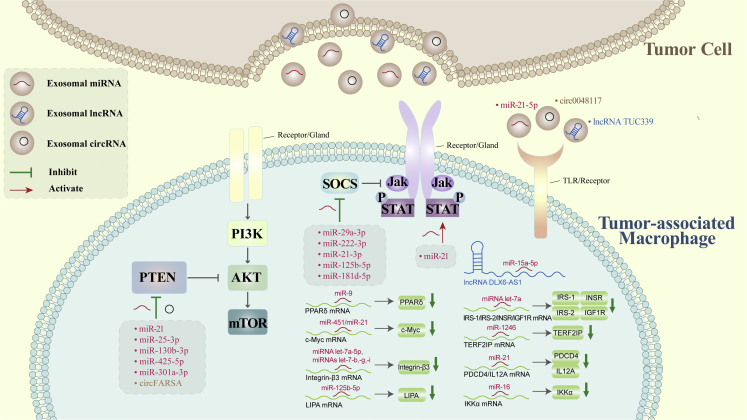
Malignant cells transmit genetic information via exosomal ncRNAs to induce M2 polarization in the TIME (Figure 2; Table 1).
Figure 2.
Tumor-derived exosomal non-coding RNAs promote macrophage polarization
Circ0048117, lncRNA TUC339, and miR-21-5p interfere with TLRs or other receptors on the surface of macrophages to induce M2 polarization. miR-21, miR-25-3p, miR-130b-3p, miR-425-5p, hypoxic miR-301a-3p, and circFARSA inhibit PTEN to activate the PI3K/AKT signaling pathway and induce M2 polarization. miR-29a-3p, miR-222-3p, hypoxic miR-21-3p, hypoxic miR-125b-5p, and hypoxic miR-181d-5p promote M2 polarization by suppressing SOCS signaling. miR-21 activates STAT signaling and promotes M2 polarization. miR-9, miR-451/miR-21, miRNA let-7a-5p/let7-b, -g, -i, miR-125b-5p, miRNA let-7a, miR-1246, miR21, and miR-16 downregulate PPARδ, c-Myc, integrin-β3, LIPA, IRS-1/IRS-2/INSR/IGF1R, TERF2IP, PDCD4/IL12A, and IKKα mRNAs, respectively, to induce M1 or M2 polarization. lncRNA DLX6-AS1 competitively binds to miR-15a-5p to induce M2 polarization.
Table 1.
Tumor-derived exosomal ncRNAs promote macrophage polarization
| Exosomal ncRNAs | Cancer type | Expression levels in tumor cells and their exosomes | Potential direct target gene(s) of ncRNAs in macrophages | Mechanism | Macrophage polarization | Effect | Ref. | |
|---|---|---|---|---|---|---|---|---|
| miRNAs | miR-9 | HPV+ HNSCC | high | PPARδ↓ | downregulate PPARδ mRNA | M1 | increase the radiosensitivity of HPV+ HNSCC | Tong et al.46 |
| miR-451/miR-21 | GBM | high | c-Myc↓ | downregulate c-Myc mRNA | M2 | / | Van der Vos et al.47 | |
| miRNA let-7a-5p miRNAs let7-b, -g, -i | PCa | high | integrin-β3↓ | downregulate integrin-β3 | M2 | tumor migration | Ferguson et al.48 | |
| miR-21-5p | CRC | high | / | bind to TLR7 | M1 | liver metastasis | Shao et al.51 | |
| miR-125b-5p | melanoma | high in exosomes | LIPA↓ | target LIPA | M2 | / | Gerloff et al.52 | |
| miR-21 | bladder cancer | high | PTEN↓ | inhibit PTEN activation and increase STAT3 expression | M2 | cancer migration and invasion | Lin et al.56 | |
| miR-25-3p miR-130b-3p miR-425-5p |
CRC | high | PTEN↓ | downregulate PTEN and activate PI3K/AKT signaling | M2 | tumor metastasis by enhancing EMT and secreting VEGF | Wang and co-workers57,58 | |
| miR-29a-3p | oral squamous cell carcinoma | high | SOCS1↓ | regulate SOCS1/STAT6 signaling activity | M2 | cancer proliferation and invasion | Cai et al.62 | |
| miR-223 | CSCC | high | / | / | M1 | tumor progression | Zhang et al.63 | |
| miR-301a-3p | PC | high under hypoxic conditions | PTEN↓ | activate the PTEN/PI3Kγ signaling pathway | M2 | cancer migration, invasion, and EMT | Wang and co-workers66,67 | |
| miRNA let-7a | / | low in tumor cells and high in exosomes under hypoxic conditions | IRS-1, IRS-2, INSR, and IGF1R↓ | enhance oxidative phosphorylation and inhibit the insulin-Akt-mTOR signaling pathway | M2 | / | Park et al.69 | |
| miR-1246 | glioma | high under hypoxic conditions | TERF2IP↓ | target TERF2IP to activate STAT3 signaling and inhibit NF-κB signaling | M2 | cancer proliferation, migration, and invasion | Qian et al.70 | |
| miR-222-3p | EOC | high | SOCS3↓ | regulate the SOCS3/STAT3 pathway | M2 | cancer proliferation, migration | Ying et al.71 | |
| miR-940 | EOC | high under hypoxic conditions | / | / | M2 | tumor progression | Chen et al.72 | |
| miR-21-3p miR-125b-5p miR-181d-5p |
EOC | high under hypoxic conditions | SOCS4/5↓ | regulate the SOCS4/5/STAT3 pathway | M2 | cancer proliferation and migration | Chen et al.73 | |
| miR21 | head and neck cancer | high | PDCD4 and IL12A↓ | downregulate PDCD4 and IL12A | M2 | tumor growth and angiogenesis | Hsieh et al.78 | |
| miR-16 | BC | high after epigallocatechin gallate treatment | IKKα↓ | downregulate IKKα and accumulate Iκ-B to suppress NF-κB activity | M2→M1 | / | Jang et al.83 | |
| lncRNAs | lncRNA TUC339 | HCC | high | / | regulate cytokine receptor signaling pathways and CXCR chemokine receptor binding pathways | M2 | cancer proliferation and reduce cancer cell adhesion to the extracellular matrix | Kogure and co-workers49,50 |
| lncRNA DLX6-AS1 | HCC | high | miR-15a-5p↓ | regulate the miRNA-15a-5p/CXCL17 axis | M2 | cancer migration, invasion and EMT, including pulmonary metastasis | Wang et al.77 | |
| lncRNA RPPH1 | CRC | high | / | / | M2 | cancer metastasis and proliferation | Liang and co-workers79,80 | |
| lncRNA FGD5-AS1 | NSCLC | high | / | / | M2 | cancer migration and invasion | Lv et al.82 | |
| circRNAs | circFARSA | NSCLC | high | PTEN↓ | increase ubiquitination and degradation of PTEN and activate the PI3K/AKT signaling pathway | M2 | EMT and metastasis | Chen et al.59 |
| circ0048117 | ESCC | high under hypoxic conditions | miR-140↓ | act as a sponge of miR-140 by competing with TLR4 | M2 | tumor invasion and migration | Lu et al.68 | |
| circ0074854 | HCC | high | / | / | M2 | tumor migration and invasion | Wang et al.81 | |
| cicHIPK3 cicPTK2 |
NSCLC | high | / | / | M2 | Kras-associated chemoresistance | Katopodi et al.84 | |
TAM-derived exosomal ncRNAs affect tumor progression
TAM-derived exosomal ncRNAs and tumor cell proliferation
Uncontrolled cell proliferation is an important feature of cancer and is characterized by alterations in the expression and activity of cell-cycle proteins.85 TAM exosomal ncRNAs regulate the transcription, translation, and function of these proteins.
ncRNAs are transferred from TAM exosomes to tumor cells, regulating cancer cell proliferation and apoptosis.86 TAMs promote PCa progression via exosome-mediated miR-95 transfer. In vitro and in vivo experiments showed that miR-95 bound to its target gene, JunB, in PCa cells and further induced tumor proliferation, invasion, and EMT.87 In addition, miR-21 in TAM exosomes enhanced cell proliferation and inhibited apoptosis in gastric cancer (GC) cells by inhibiting PDCD4 expression.88
lncRNAs can reverse miRNA-induced gene silencing. For instance, TAM exosomal lncRNA LIFR-AS1 enhanced osteosarcoma cell proliferation by sponging miR-29a and increasing NFIA protein expression.89
TAM exosomal ncRNAs alter the proliferative capacity of tumor cells via post-transcriptional regulation. Low levels of cyclin-dependent kinase inhibitor 1B (CDKN1B) were associated with EOC progression and poor prognosis. Exosomal miR-221-3p directly targeted and inhibited CDKN1B expression, favoring EOC proliferation and G1/S progression.90 miR-142 and miR-223 were effectively transferred from macrophages to HCC cells via exosomes. These RNAs decreased the expression of reporter proteins and endogenous proteins stathmin-1 and insulin-like growth factor-1 receptor and inhibited cancer cell proliferation.91 miR-125a and miR-125b suppressed HCC cell proliferation by downregulating the cancer stem cell marker CD90.92
TAM-derived exosomal ncRNAs and metastasis
Organ-specific metastasis is complex and dynamic and involves tumor-host intercellular interactions.93,94 TEXs in the circulation can help establish a pre-metastatic niche.95,96 ncRNAs enter the systemic circulation and travel to distant organs to transmit information via cell receptors, allowing these RNAs to regulate tumor cell proliferation.51
Clinical and experimental evidence suggests that TAMs promote cancer cell migration via exosomal signaling.97,98 Moreover, exosomal ncRNAs are involved in different steps of the metastatic cascade. miR-501-3p was highly expressed in pancreatic ductal adenocarcinoma (PDAC) and TAM exosomes.99 This RNA promoted PDAC metastasis by downregulating the tumor-suppressor gene TGFBR3, and miR-501-3p inhibition suppressed tumor formation and metastasis in vivo.99
ncRNAs regulate the expression of proteins involved in invasion or migration. miR-21-5p and miR-155-5p, highly abundant in M2 macrophage exosomes, downregulated the protein expression of BRG1 in CRC cells, promoting cancer cell migration and invasion.100 Decreased Brg-1 expression is implicated in CRC metastases.101 lncRNA SBF2-AS1 acted as ceRNA to inhibit miR-122-5p and upregulate X-linked inhibitor of apoptosis protein.102 The overexpression of exosomal lncRNA SBF2-AS1 promoted PC progression, and the inhibition of this RNA in M2 macrophages attenuated PC tumorigenicity.102 Similarly, lncRNA AFAP1-AS1 downregulated miR-26a and upregulated its direct target ATF2, increasing esophageal cancer cell migration, invasion, and lung metastasis. Moreover, M2 macrophage exosomes showed higher AFAP1-AS1 and ATF2 expression and lower miR-26a expression than M1 macrophage exosomes.103
Androgen receptors (ARs) help regulate HCC initiation and progression.104 Macrophage exosomal miR-92a-2-5p inhibited AR translation by targeting the 3′ UTR and PHLPP/p-AKT/β-catenin signaling, increasing HCC cell invasion. In addition, a preclinical study showed that miR-92a-2-5p inhibitors suppressed HCC progression.104
TAM exosomal miR-223 was upregulated in breast cancer (BC) cells in vitro and promoted BC cell invasion through the Mef2c-β-catenin pathway, and miR-223 inhibition decreased the invasiveness of BC cells.105 Furthermore, macrophage exosomal miR-223 promoted the migration and invasion of GC cells via the PTEN-PI3K/AKT pathway, and RNA silencing reversed this effect. This RNA changed the actin cytoskeleton and upregulated multiple proteins associated with EMT.106 Exosomes from TWEAK-stimulated macrophages were internalized by EOC cells and suppressed metastasis by inhibiting the EGFR/AKT/ERK1/2 pathway. TWEAK increased miR-7 levels—a tumor-suppressive miRNA—in macrophages, macrophage exosomes, and recipient EOC cells.107
Distant metastasis is a complex process in which M1 and M2 facilitate different steps.51,100 It is essential that tumor cells acquire the ability to migrate and distant organs prepare the environment that favors the metastasis of tumor cells during distant organ metastasis.108, 109, 110, 111 As a first step, cancer cells need to become motile and invasive to enter the bloodstream. miR-21-5p and miR-155-5p in exosomal M2 macrophages from primary tumors regulated the expression of cell migration-related proteins and EMT, endowing tumor cells with invasive activity.100 Stephen Paget proposed the “seed and soil” hypothesis, expounding that metastasizing cancer cells “seed” only in certain especially suitable tissues, akin to seeding in “fertile soil.”112 Shao et al. found that exosomes carrying miR-21 traveled from the primary tumor portion to the liver and liver macrophages can phenotype pro-inflammatory M1 by miR-21, which prepared a favorable environment for the metastasis of CRC.51
TAM-derived exosomal ncRNAs and tumor angiogenesis
Tumor angiogenesis involves several processes and cell types.113 In the TIME, exosomes derived from mesenchymal, stromal, and endothelial cells play active roles in this process.114 TAM infiltration in the TIME impacts tumor angiogenesis and epigenetic regulation.115 Exosomes stimulate the formation of tubular structures, the growth of endothelial cells, and the secretion of VEGF. VEGF and other factors secreted by TAMs during tumor progression promote cancer development.116 M2 macrophage exosomes increased the expression of migration and angiogenesis-related proteins in PDAC cells and enhanced metastasis in vivo.99 Exosomal miR-501-3p promoted tumor cell migration, invasion, and tube formation.99 MicroRNA-21 was transferred from glioma to microglia through exosomes. Exogenous miR-21 increased the ability of GBM cells to promote M2 polarization, and miR-21 inhibition reversed this effect.117,118 This miRNA promoted endothelial angiogenesis through VEGFR2 signaling.119 miR-21 was involved in the response to anti-angiogenic therapy, and bevacizumab treatment was associated with increased expression of miR-21 in the serum.120 Moreover, miR-21 silencing increased GBM sensitivity to the anti-angiogenic drug sunitinib.121 Exosomal miR-130b-3p promoted angiogenesis in GC cells.122 Mixed lineage leukemia 3 (MLL3), a poor prognostic factor in GC, increased the expression of the gene grainyhead-like 2 (GRHL2).123 MLL3 inhibited the proliferation, migration, and invasion of GC cells and tube formation in HUEVCs by increasing GRHL2, whereas miR-130b-3p had the opposite effect by inhibiting MLL3 expression.122 In addition, miR-130b-3p downregulation and GRHL2 upregulation inhibited tumor formation and angiogenesis in GC.122
Hypoxia is a crucial regulator of angiogenesis and affects exosome secretion and function and the expression of exosome ncRNAs.124,125 Tissue inhibitor of metalloproteinases-1 upregulated miR-210 and downregulated the mRNA and protein expression of its downstream targets by activating PI3K/AKT/HIF-1 signaling, increasing angiogenesis and tumor growth in vivo.126 Exosome miR-21 activated STAT3, increasing VEGF levels in recipient cells.127
TAM-derived exosomal ncRNAs and tumor chemoresistance
Exosomal ncRNAs increase resistance to antitumor drugs. For instance, M2 macrophage miR-21 increased GC resistance to cisplatin by suppressing apoptosis via PTEN/PI3K/AKT activation.128 lncRNA CRNDE was upregulated in GC tissues and TAM and promoted cisplatin resistance in GC cells via PTEN ubiquitination. CRNDE silencing in M2 macrophage exosomes increased the sensitivity of these cells to cisplatin, and PTEN knockdown reversed this effect.129 MicroRNA-21 stimulated temozolomide resistance in GBM by modulating the STAT3/miR-21/PDCD4 signaling pathway. The STAT3 inhibitor pacritinib overcame temozolomide resistance by decreasing miR-21 levels and the number of miR-21-enriched exosomes.117 The levels of miR-1246 were significantly higher in paclitaxel-resistant ovarian cancer (OC) exosomes than in paclitaxel-sensitive OC exosomes. The Cav1 gene and the multidrug resistance gene were direct targets of miR-1246 and participated in exosome transfer. Cav1 overexpression and miR-1246 mimic treatment sensitized OC cells to paclitaxel.130,131
Hypoxia increases macrophage recruitment and promotes exosome production and release, altering drug sensitivity in tumor cells. For instance, miR-223 was enriched in TAM exosomes under hypoxia and was transferred to EOC cells, inducing drug resistance via the PTEN-PI3K/AKT pathway.132 HIF-1α might be involved in the production of miR-223 in TAM and their exosomes.132 MicroRNA-365 increased PDAC resistance to gemcitabine by upregulating the triphosphate-nucleotide pool and increasing cytidine deaminase expression, whereas miR-365 antagonists reversed this effect.133
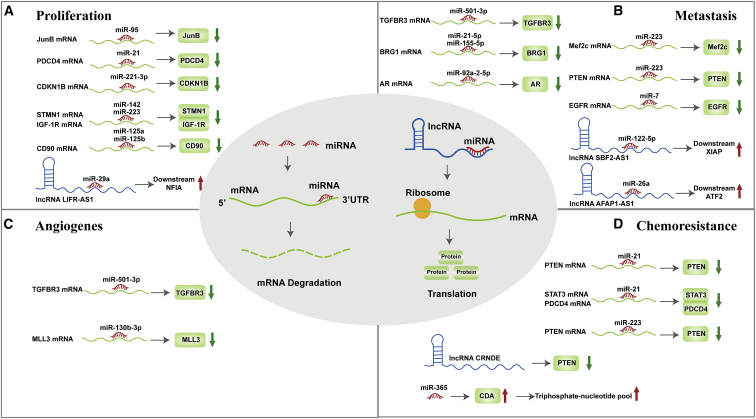
TAM exosomal ncRNAs affect every aspect of tumor progression (Figure 3; Table 2).
Figure 3.
Tumor-associated macrophage-derived exosomal non-coding RNAs affect tumor progression
miRNAs can bind to the 3′ UTR of target mRNAs to induce gene silencing. lncRNAs can sponge miRNAs to increase target mRNA expression. (A) miR-95, miR-21, miR-221-3p, miR-142, miR-223, miR-125a, and miR-125b, promote tumor progression by binding to target mRNAs and downregulating gene expression. lncRNA LIFR-AS1 acts as a miR-29a sponge to upregulate NFIA. (B) miR-501-3p, miR-21-5p, miR-155-5p, miR-92a-2-5p, miR-223, and miR-7 promote tumor cell migration and invasion by targeting the corresponding genes. lncRNA SBF2-AS1 and lncRNA AFAP1-AS1 act as ceRNAs to repress miR-122-5p and miR-26a, respectively, increasing protein expression. (C) miR-501-3p and miR-130b-3p induce tumor angiogenesis by downregulating targeted genes. (D) miR-21, hypoxic miR-223, and lncRNA CRNDE promote chemoresistance by downregulating PTEN. In addition, miR-21 downregulates the STAT3 and PDCD4. miR-365 upregulate cytidine deaminase (CDA) and the triphosphate-nucleotide pool, which is associated with drug resistance in tumors.
Table 2.
Tumor-associated macrophage-derived exosomal ncRNAs affect tumor proliferation, metastasis, angiogenesis, and chemoresistance
| Biological function | Exosomal ncRNAs | Expression levels in macrophages and their exosomes | Potential direct target gene(s) of ncRNAs in tumor cells | Mechanism | Cancer type | Ref. |
|---|---|---|---|---|---|---|
| Tumor cell proliferation | miR-95 | high | JunB↓ | promote tumor progression by binding directly to its downstream target gene JunB to promote PCa cell proliferation, invasion, and EMT | PCa | Guan et al.87 |
| miR-21 | high | PDCD4↓ | promote tumor progression by targeting the PDCD4 gene and inhibiting PDCD4 expression to prevent apoptosis in GC cells | GC | Wang et al.88 | |
| lncRNA LIFR-AS1 | high | miR-29a↓ | act as a miR-29a sponge to upregulate NFIA, promoting tumor cell proliferation but inhibiting tumor cell invasion and apoptosis | osteosarcoma | Zhang et al.89 | |
| miR-221-3p | high | CDKN1B↓ | directly target and inhibit CDKN1B expression to promote EOC cell proliferation and G1/S conversion | EOC | Li and Tang90 | |
| miR-142 miR-223 |
high | STMN1 and IGF-1R↓ | downregulate the expression of reporter proteins and endogenously expressed stathmin-1 and insulin-like growth factor-1 receptor at the post-transcriptional level to inhibit tumor cell proliferation | HCC | Aucher et al.91 | |
| miR-125a miR-125b |
underexpressed in exosomes | CD90↑ | target CD90 to promote HCC cell proliferation | HCC | Wang et al.92 | |
| Tumor metastasis | miR-501-3p | high | TGFBR3↓ | target TGFBR3 to promote PDAC cell metastasis | PDAC | Yin et al.99 |
| miR-21-5p miR-155-5p |
high | BRG1↓ | downregulate BRG1 expression by binding to the BRG1 coding sequence to promote CRC cell migration and invasion | CRC | Lan et al.100 | |
| lncRNA SBF2-AS1 | high | miR-122-5p↓ | act as a ceRNA to repress miR-122-5p and upregulate XIAP to promote PC progression | PC | Yin et al.102 | |
| lncRNA AFAP1-AS1 | high | miR-26a↓ | act as a ceRNA to repress miR-26a and upregulate ATF2 to promote esophageal cancer (EC) cell migration and invasion and lung metastasis | EC | Mi et al.103 | |
| miR-92a-2-5p | high | AR↓ | target the 3′ UTR of AR mRNA and inhibit AR translation to alter PHLPP/p-AKT/β-catenin signaling and promote HCC cell invasion | HCC | Liu et al.104 | |
| miR-223 | high | Mef2c↓ | target the Mef2c-β-catenin pathway to promote BC cell invasion | BC | Yang et al.105 | |
| miR-223 | high | PTEN↓ | target the PTEN-PI3K/AKT pathway, alter the actin cytoskeleton, and upregulate multiple proteins associated with EMT to promote GC cell migration and invasion | GC | Zheng et al.106 | |
| miR-7 | high after TWEAK treatment | EGFR↓ | downregulate the activity of the EGFR/AKT/ERK1/2 pathway to reduce EOC cell metastasis | EOC | Hu et al.107 | |
| Tumor angiogenesis | miR-501-3p | high | TGFBR3↓ | target TGFBR3 to promote tube formation | PDAC | Yin et al.99 |
| miR-130b-3p | high | MLL3↓ | promote GC progression via miR-130b-3p/MLL3/GRHL2 signaling | GC | Zhang et al.122 | |
| Tumor chemoresistance | miR-21 | high | PTEN↓ | activates PI3K/AKT signaling by downregulating PTEN to inhibit GC cell apoptosis and promote DDP resistance | GC | Zheng et al.128 |
| lncRNA CRNDE | high | PTEN↓ | facilitate NEDD4-1-mediated PTEN ubiquitination to increase cisplatin resistance in GC cells | GC | Xin et al.129 | |
| miR-21 | high | STAT3 and PDCD4↓ | regulate the STAT3/miR-21/PDCD4 signaling pathway, which is associated with temozolomide resistance in GBM | GBM | Chuang et al.117 | |
| miR-223 | high | PTEN↓ | activate downstream signaling pathways, including PI3K/AKT, through downregulating PTEN to enhance drug resistance in EOC cells under hypoxia | EOC | Zhu et al.132 | |
| miR-365 | high | CDA↑ | upregulate the triphosphate-nucleotide pool and induce the enzyme cytidine deaminase in cancer cells to induce PDAC resistance to gemcitabine | PDAC | Binebaum et al.133 |
Exosomal ncRNAs and the TIME
The cancer-immunity cycle is the sequence of events that lead to an effective anti-cancer immune response.134 Tumor-specific antigens released from dead tumor cells are recognized by antigen-presenting cells (APCs), especially dendritic cells (DCs), and further processed to form the antigen-peptide-MHC complex. Then, T cell receptors on DCs recognize this complex, whereas B7 molecules on DCs bind to CD28 on T cells, activating these cells. Cytotoxic T lymphocytes (CTLs) infiltrate the tumor and kill cancer cells.
Based on programmed cell death ligand 1 (PD-L1) expression and tumor-infiltrating lymphocytes (TILs), the TIME can be classified as having a “hot” or “cold” phenotype, with distinct pathophysiological characteristics; the former is characterized by the presence of activated lymphocytes and good response to immunotherapy, and the latter is characterized by the lack of T cell infiltrates and poor response to immunotherapy.135 TILs are reflected by CD8A or cytolytic activity. Immunotherapy aims to stimulate the activity of CTLs and initiate and establish effective and long-term anti-cancer immunity.136 However, as the tumor progresses, immune editing mediates immune escape through low tumor antigen expression, high PD-L1 expression, and reduced TILs, and hot tumors may transform into cold tumors, leading to poor immune response.137, 138, 139
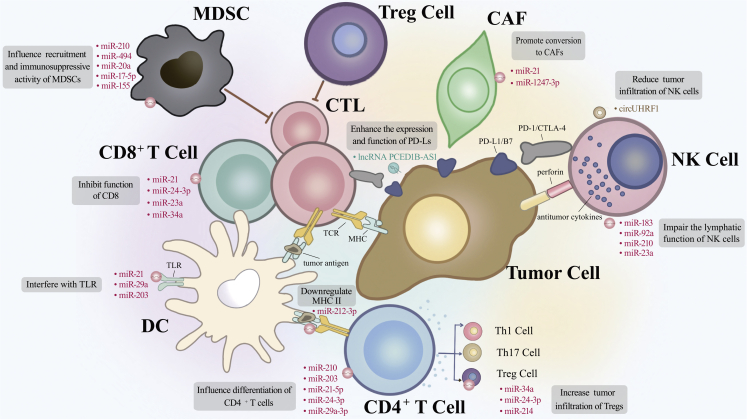
Exosomal ncRNAs regulate the functions, interactions, and infiltration of CTLs—DCs, CTLs, NK cells, TAMs, fibroblasts, myeloid-derived suppressor cells (MDSCs), and Treg cells—in the TIME (Figure 4; Table 3).168, 169, 170
Figure 4.
Exosomal non-coding RNAs of tumor cells or TAMs remodel the tumor immune microenvironment
Dendritic cells (DCs) process tumor antigens and form the antigen-peptide-MHC complex, which activates T lymphocytes. CD4+ T cells can differentiate into Th1, Th17, and Treg cells. Activated CD8+ T cells generate cytotoxic T lymphocytes (CTLs) that kill tumor cells. NK cells kill tumor cells by producing antitumor cytokines, such as IL-4, IFN-γ, FasL, IL-13, and perforin. Myeloid-derived suppressor cells (MDSCs) and Tregs have immunosuppressive activity. miR-21, miR-29a, and miR-203 bind to TLRs on the surface of DCs and inhibit DC maturation. miR-212-3p downregulates MHC II on DCs. miR-21 and miR-24-3p decrease CD8+ T cell proliferation, whereas miR-23a, miR-34a, and miR-21 suppress CD8+ T cell function. miR-29a-3p, miR-21-5p, miR-24-3p, hypoxic miR-210, and miR-203 affect CD4+ T cell differentiation. miR-183, miR-92a, hypoxic miR-210, and hypoxic miR-23a reduce NK cell cytotoxicity. miR-210, miR-494, miR-20a, miR-17-5p, and miR-155 affect the recruitment and function of MDSCs. miR-34a, miR-24-3p, and miR-214 promote Treg recruitment and expansion. lncRNA PCED1B-AS1 suppresses T cell function via enhancing the expression and function of programmed cell death ligands in tumor cells. miR-1247-3p and miR-21 promote conversion from normal fibroblasts to CAFs.
Table 3.
Exosomal ncRNAs of tumor cells or TAMs remodel the tumor immune microenvironment
| Exosomal ncRNAs | Immune cells | Mechanism | Effect | Cancer type | Ref. |
|---|---|---|---|---|---|
| miR-21 miR-29a |
DCs | bind to TLRs to induce primary inflammation | tumor growth and metastasis | NSCLC | Fabbri et al.140 |
| miR-203 | DCs | bind to TLR4 to induce primary inflammation and reduce the expression of cytokines such as TNF-α and IL-12 | inhibit DC maturation and Th1 differentiation to reduce cellular immunity | PC | Zhou et al.141 |
| miR-212-3p | DCs | suppress RFXAP to induce MHC II downregulation | enhance immune tolerance in DCs | PC | Ding et al.142 |
| Exosomes | DCs | / | regulate DC maturation | melanoma | Maus et al.143 |
| Exosomes | DCs | / | inhibit DC differentiation and stimulate MDSC differentiation to promote tumor progression | / | Condamine and Gabrilovich144 |
| miR146a | T cells | / | stimulate M2 polarization and inhibit anti-HCC T cell activity | HCC | Yin et al.39 |
| Exosomes | T cells | / | Decrease the release of antitumor factors such as IFN-γ, IL-2, and IL-17 from CD4+ and CD8+ T cells | nasopharyngeal carcinoma | Ye et al.145 |
| miR-23a | T cells | downregulate the target gene BLIMP-1 | suppress CD8+ T cell function | advanced lung cancer | Lin et al.146 |
| miR-34a | T cells | suppress TGF-β | inhibit CD8+ T cell function and recruit Tregs to the TIME | HCC | Yang et al.147 |
| miR-24-3p | T cells | target FGF11 | stimulate Tregs and inhibit T cell proliferation and Th1 and Th17 differentiation; | nasopharyngeal carcinoma | Ye et al.148 |
| Hypoxia miR-210 | T cells | target HIF1α | promote Th17 differentiation of T cells and promote tumor immune escape | / | Wang et al.149 |
| Exosomes | T cells | uptake by T lymphocytes to activate p38 MAPK | induce ER stress-mediated apoptosis of T lymphocytes and lead to immunosuppression | PC | Shen et al.150 |
| miR-21 (TAM) | T cells | regulate PEG3 | decrease CD8+ T cell proliferation and cytotoxic activity to accelerate immune escape and cancer cell growth, migration, and invasion; inhibit apoptosis to enhance tumor volume decrease | glioma | Yang et al.151 |
| lncRNA PCED1B-AS1 | T cells | sponge hsa-miR-194-5p and enhance the expression and function of PD-Ls in HCC cells | suppress recipient T cells and macrophages to induce immunosuppression in HCC | HCC | Fan et al.152 |
| miR-183 | NK cells | induced by TGF-β, target and inhibit DAP12 | impair the lymphatic function of NK cells and trigger TGF-β-mediated immunosuppression | / | Donatelli et al.153 |
| miR-92a | NK cells | reduce the expression of antitumor cytokines (perforin, FasL, and IFN-γ) by NK cells | induce NK cells to decrease cytotoxic CD8+ T cell activity | glioma | Tang et al.154 |
| circUHRF1 | NK cells | degrade miR-449c-5p and upregulate TIM-3 to inhibit IFN-γ and TNF-α secretion in NK cells | reduce the NK cell ratio and tumor infiltration | HCC | Zhang et al.155 |
| Hypoxic miR-210 | NK cells | control antigen-specific immune responses and tumor hypoxia | reduce NK cell cytotoxicity and function | / | Norman et al.156 |
| Hypoxic miR-23a | NK cells | directly target the expression of CD107a as an immunosuppressive factor | reduce NK cell cytotoxicity and function | / | Berchem et al.157 |
| miR-29a-3p miR-21-5p (TAM) |
Tregs | directly inhibit STAT3 in CD4+ T cells and induce imbalance in the Treg/Th17 ratio to have a synergistic effect on STAT3 dinhibition | increase the Treg/Th17 ratio and create an immunosuppressive microenvironment | EOC | Zhou et al.158 |
| miR-214 | Tregs | decrease PTEN secretion | promote Treg expansion to enhance immunosuppression and tumor growth | / | Yin et al.159 |
| miR-210 miR-494 |
MDSCs | target IL-16 and CXCL12 (miR-210) and PTEN (miR-494), respectively | enhance MDSC recruitment and immunosuppressive activity | / | Liu and co-workers160,161 |
| miR-20a miR-17-5p |
MDSCs | repressed by hypoxia | inhibit MDSC immunosuppressive activity | / | Truettner and co-workers162, 163, 164 |
| miR-155 | MDSCs | repressed by tumor-derived exosomes | enhance MDSC recruitment and immunosuppressive activity | / | Wang et al.165 |
| miR-1247-3p | CAFs | convert HSC to CAFs via activation of PTEN/PDK1/AKT signaling | secret pro-inflammatory cytokines to promote HCC progression | HCC | Fang et al.166 |
| miR-21 | CAFs | convert HSC to CAFs via activation of PTEN/PDK1/AKT signaling | secret angiogenic cytokines to promote HCC progression | HCC | Zhou et al.167 |
Exosomal ncRNAs regulate DC maturation and function
DCs are APCs that express a wide range of TLRs.171,172 Upon TLR stimulation, DCs activate T cells and initiate the immune response by upregulating co-stimulatory molecules and pro-inflammatory cytokines.173 Interference with TLR activation prevents initiating immunity. miR-21 and miR-29a in NSCLC exosomes bound to TLRs, resulting in tumor growth and metastasis.140 In PCs, the overexpression of miR-203 had a similar effect on TLR4.141 In addition, miR-203 reduced the expression of TNF-α and IL-12, which were essential for DC maturation and Th1 differentiation, respectively.141 PC exosomes transferred miR-212-3p to DCs and inhibited the expression of the regulatory factor X-associated protein, decreasing MHC II expression and increasing immune tolerance in DCs.142 Mature DCs stimulated the activation, proliferation, and differentiation of effector T cells, whereas immature DCs promoted T cell tolerance to tumor antigens.174 Melanoma exosomes regulated DC maturation.143 Exosomes blocked DC differentiation from myeloid precursors, producing immature MDSCs that contributed to tumor progression.144 In summary, TEXs in the TIME inhibit the differentiation and maturation of DCs, thus transforming them from beneficial APCs to negative regulators of the immune response.175
Exosomal ncRNAs regulate T cell recruitment, proliferation, and differentiation
T cells are divided into two major groups based on phenotype, surface receptors, and antigen specificity: CD4+ T helper (Th) and CD8+ CLT cells.176,177 Th cells can be further divided into Th1, Th17, and Tregs.177
In addition to inhibiting DC maturation,178 TEXs control the proliferation, differentiation, and function of T lymphocytes and the expression of immune function genes in these cells.179 miR146a in HCC exosomes promoted M2 polarization and impaired T cell function.39 In addition, exosomes decreased the release of antitumor immune factors, such as IFN-γ, IL-2, and IL-17, from CD4+ and/or CD8+ T cells.145 In advanced lung cancer, miR-23a was upregulated in tumor-infiltrating CD8+ T cells and suppressed CD8+ T cell function by downregulating its target gene BLIMP-1.146 In HCC, TGF-β inhibited CD8+ T cell function by suppressing miR-34a, enhancing Treg recruitment to the TIME.147
TEXs are associated with the proliferation and apoptosis of T cells. Exosomal miR-24-3p inhibited T cell proliferation and Th1 and Th17 differentiation and induced Treg differentiation by targeting FGF11 in nasopharyngeal carcinoma.148 Hypoxia increased the level and activity of miR-24-3p.148 Hypoxia-induced miR-210-inhibited Th17 differentiation in T cells by targeting HIF1α.149 PC exosomes induced endoplasmic reticulum-mediated apoptosis of T lymphocytes by activating p38 mitogen-activated protein kinase (MAPK), ultimately leading to immunosuppression.150
TAMs contribute to immune escape and immunosuppression by affecting T lymphocyte infiltration in the TIME. M2 macrophage exosomes promoted immune escape by shuttling miR-21 and decreasing PEG3 mRNA expression in a mouse model of glioma.151 In addition, exosomal miR-21 enhanced tumor volume and reduced the percentage of CD8+ T cells in glioma tissues.151 miR-21 depletion inhibited glioma growth, migration, and invasion, enhanced apoptosis, upregulated IFN-γ levels, and increased CD8+ T proliferation and cytotoxicity.151
ncRNAs mediate the upregulation of immune checkpoints. HCC exosomal lncRNA PCED1B-AS1 enhanced the expression and function of programmed cell death ligands (PD-Ls) in HCC cells via sponging hsa-miR-194-5p and induced immunosuppression by inhibiting recipient T cells and macrophages.152
NK cells are a T cell subpopulation with cytotoxic activity and the ability to produce antitumor cytokines, such as IL-4, IFN-γ, Fas ligand, IL-13, and perforin.180,181 TGF-β inhibits the cytotoxic activity of these cells by upregulating miR-183 and decreasing the protein levels of DNAX-activating protein 12, a signaling adaptor for NK cell function and a key factor in TGF-β-mediated immunosuppression.153 In addition, glioma cell-derived miR-92a significantly decreased the expression of antitumor cytokines in NK cells and increased the ability of NK cells to suppress cytotoxic CD8+ T cell activity.154 HCC-derived circUHRF1 inhibited the secretion of IFN-γ and TNF-α by NK cells and caused NK cell dysfunction by inhibiting miR-449c-5p and upregulating its target gene TIM-3. High levels of plasma exosomal circUHRF1 decreased the NK cell ratio and NK cell infiltration.155 TEXs decreased NK cell cytotoxicity under hypoxic conditions via miR-210 and miR-23a.156,157 As a primary regulator of the hypoxic tumor response, miR-210 controlled antigen-specific immune response,156 whereas miR-23a directly targeted CD107a expression.157 These data demonstrate the vital role of exosomal ncRNAs in mediating T cell exhaustion and creating an immunosuppressive microenvironment.
Exosomal ncRNAs regulate immunosuppressive cell recruitment and function
Other immune cells in the TIME influence the cytotoxicity of CTLs and NK cells.182,183 M1 macrophages enhance the antitumor effects of cytotoxic cells, whereas M2 macrophages, MDSCs, and Treg cells have the opposite effect.182,183 Exosomal ncRNAs stimulate these immunosuppressive cells in the TIME, indirectly influencing the function of CTLs and NK cells.
Treg cells are a major subgroup of immunosuppressive leukocytes. CD25+ CD4+ Tregs produce immunosuppressive cytokines and express co-stimulatory molecules that inhibit tumor-specific CTL function.182 The frequency of Tregs is increased during tumorigenesis and is positively correlated with compromised immune response.182,184,185 The Treg/Th17 ratio was correlated with histological grade and was significantly increased in EOC.158 miR-29a-3p and miR-21-5p were enriched in TAM exosomes.158 These two miRNAs directly inhibited STAT3 in transfected CD4+ T cells but caused an imbalance in the Treg/Th17 ratio and synergistically suppressed STAT3.158 These results indicate that exosomes mediate the crosstalk between TAMs and T cells to create an immunosuppressive microenvironment.158 miR-214 induced Treg expansion in CD4+ T cells by targeting PTEN, causing immunosuppression and tumor growth.159
MDSCs are immature myeloid cells that suppress adaptive and innate immunity in the TIME and are a major driver of tumor immune escape.186 The accumulation of MDSCs and activation of suppressive cells require inflammatory factors regulated by immune-related miRNAs.187 Tumor exosomal miR-210 and miR-494 were upregulated in rodent models.160,161 Targeting CXCL12, PTEN, and IL-16 via miR-210, miR-494, and miR-210/miR-494, respectively, increased the recruitment and suppressive activity of MDSCs.160,161 In addition, miR-20a and miR-17-5p were downregulated in MDSCs under hypoxic conditions, and their overexpression reduced the suppressive activity of MDSCs.162, 163, 164 MicroRNA-155 deficiency increased the recruitment of MDSCs to the TIME and enhanced their immunosuppressive and pro-angiogenic function.165
Exosomal ncRNAs regulate the function of cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs) represent the majority of stromal cells in the TIME.188 In the lung metastatic niche, metastasis-prone HCC cells had advantages in converting normal fibroblasts to CAFs via secreting exosomal miR-1247-3p that targeted B4GALT3 in fibroblasts and activated β1-integrin-NF-κB signaling.166 Similarly, HCC-derived exosomal miR-21 could convert normal hepatocyte stellate cells (HSCs) to CAFs through activating PTEN/PDK1/AKT signaling in HSCs.167 Activated CAFs further promoted HCC progression by secreting angiogenic cytokines, including VEGF, MMP, and TGF-β.167 CAF-derived exosomal ncRNAs are associated with immunosuppression. For example, the levels of miR-92 were higher in CAF exosomes of BC patients than in healthy controls.189 CAF exosomes upregulated PD-L1 in BC cells, decreasing T cell proliferation and increasing apoptosis.189 Hypoxia induced CAFs to release exosomal circEIF3K but inhibited miR-214, which downregulated PD-L1 expression in CRC. As a result, PD-L1 expression was upregulated in CRC under hypoxia.190
In summary, exosomes and their ncRNAs mediate tumor immune escape and the interaction between cancer cells and TAM in the TIME.
Clinical applications of exosomal ncRNAs and exosomes
Exosomes and their ncRNAs are found in virtually all body fluids and thus can be used as non-invasive diagnostic biomarkers in cancer.191 Exosomes are suitable for delivering small interfering RNAs (siRNAs), antitumor agents, and CRISPR-Cas9 systems, decreasing antigenicity and drug toxicity.34,192, 193, 194
Exosomal ncRNAs as promising diagnostic and prognostic biomarkers
Exosomal ncRNAs are highly enriched in biological fluids and can be used for liquid biopsies, improving diagnostic specificity and sensitivity.191 In addition, improving exosome membrane structure can increase miRNA stability.191
circSATB2 was implicated in NSCLC progression and thus can be potentially used as a diagnostic marker for this cancer type.195 Exosomal circ0048117 regulated ESCC progression, and higher serum exosomal circ0048117 was positively and significantly correlated with TNM stage.68 Exosomal circSHKBP1 is a promising biomarker for GC diagnosis and prognosis and a therapeutic target since this RNA was detected in the blood and promoted GC progression.196 CRC exosomal miR-203 promoted the expression of M2 markers in vitro.197 MicroRNA-203-transfected CRC mouse cells developed more liver metastases than the control group.197 Circulating exosomal miR-203 levels were correlated with metastasis, and low miR-203 expression in tumor tissue was a poor prognostic factor in CRC.197 Bioinformatics analysis showed that the high expression of lncRNA GAS5 and miR-221 in tissue, plasma, and exosomes was of diagnostic value in CRC and was a prognostic factor for CRC.198 A prognostic model targeting the STAT3-miR-223-HMGCS1/TGFBR3 axis predicted survival in HCC patients.199 The exosomal lncRNAs RP11-538D16.2 and CTD-2116N20.1 are associated with poor prognosis in CRC.199 The level of exosomal RPPH1 in the plasma was high in treatment-naive CRC patients but low after tumor resection.79,80 Exosomal RPPH1 had a higher diagnostic value than CEA and CA199 in CRC.79,80
Exosomes and ncRNAs as potential therapeutic targets
Exosomes and their ncRNAs play essential roles in physiological and pathological processes. Exosomes are being developed for drug delivery in cancer therapy.34,200 Exosome encapsulation enables the effective transfer of unstable molecules to target cells to participate in antitumor and immunomodulatory processes, increasing drug concentration and decreasing drug toxicity.201,202 Compared with synthetic carriers, such as liposomes and nanoparticles (NPs), exosomes have better biocompatibility, lower immunogenicity, wider distribution, and chemical stability in biological fluids, allowing targeted delivery to the blood-brain barrier and preventing phagocytosis by mononuclear macrophages.200,201 Exosomes enter recipient cells by endocytosis, enhancing drug internalization.200,201 Exosomes have a strong homing property and can target specific tissues and cells, increasing the cytotoxicity of therapeutic agents.200,201 Milk exosomes have been developed for paclitaxel delivery and comply with good manufacturing practices.203 The inhibition of the CD47-SIRPα interaction by engineered exosomes promoted T cell infiltration in syngeneic mouse models of cancer.204 It has been proposed that CAR T cell-derived exosomes are more efficient and less toxic than CAR T therapy.205 Exosomes can potentially be used as cancer vaccines.206 miRNAs and siRNA can be delivered to recipient cells via exosomes to help regulate the expression of relevant genes, particularly oncogenes, which are potential targets for tumor therapy.207,208 Exogenous siRNAs target human monocytes and lymphocytes and have been used to silence the MAPK1 gene.209 Engineered mesenchymal stromal cell exosomes carrying KRASG12D siRNA are currently in phase I clinical trials for patients with metastatic PC and KRASG12D mutations (NCT03608631). Clinical trials on therapeutic exosomes have been well organized previously.200,210,211
Although these advantages make exosomes promising therapeutic targets, some limitations, such as off-targeting, low loading efficiency, fast clearance in vivo, and the lack of standardized production and preparation, need to be resolved before clinical application.
Exosomes are surface-functionalized with ligands to prevent off-targeting and achieve targeted drug delivery.200,212 Common modification methods include chemical ligation of targeted peptides, genetic engineering of progenitor cells and exosomal membranes, magnetic NPs, and electrostatic interactions. Nonetheless, exosome engineering has some limitations.200,212 First, changing the exosome surface structure is challenging. Second, genetically modifying parental cells reduces transfection efficiency and the biological activity of membrane proteins. Third, chemically modified viral proteins may have adverse health effects. Fourth, cationic nanomaterials may cause cytotoxicity and have low loading efficiency when used in electrostatic interactions.
Achieving the efficient, cost-effective, large-scale production of therapeutic exosomes determines whether they can be used in clinical practice. Exosome production is divided into two stages: large-scale cell culture and exosome isolation and processing. In addition, several characterization indexes are needed to assess whether the extracted components are exosomes. Exosomes are characterized based on morphology, size, and protein markers. The most commonly used isolation methods are ultracentrifugation, particle size separation, polymer precipitation, and immunoaffinity capture. However, several methods need to be combined to achieve the desired yield, purity, integrity, price, and other relevant characteristics. Combining different methods can improve purification efficiency and allow cost-effective large-scale production. However, the effectiveness of these strategies has not been determined. Recent advances in therapeutic exosome production are detailed in two reviews.210,212,213
Tumor-suppressive miRNAs are expressed at low levels in tumors and inhibit cancer development. The recovery of these miRNAs using miRNA mimics is a promising therapeutic strategy. A proof-of-concept study evaluated the effectiveness of these mimics as an miRNA-replacement therapy in a preclinical animal model.214,215 Antisense oligonucleotides, miRNA sponges, ribonucleases, small molecules, and the CRISPR-Cas9 system can suppress oncomiRs.216, 217, 218, 219, 220
Despite recent achievements in the study of immune checkpoint inhibitors (ICIs), immunotherapeutic agents have limitations on response rates, toxicity, and resistance.221 Exosomal ncRNAs impact the efficacy of immunotherapy. For instance, endoplasmic reticulum stress promoted the release of exosomes from HCC, and miR-23a-3p-loaded exosomes were phagocytosed by macrophages and activated the PI3K/AKT pathway through inhibiting PTEN. As a result, macrophages were polarized to M2, increasing the expression of PD-L1 and impairing T cell function. The blockade of HCC-macrophage interactions by miR-23a-3p inhibitors may be a novel strategy to treat HCC progression.222 In addition, HCC exosome-educated macrophages suppressed the expression of IFN-γ and TNF-α and upregulated the expression of inhibitory receptors, such as PD-1 and CTLA-4 in T cells.39 HCC exosomal lncRNA PCED1B-AS1 regulated the expression of PD-L1 and PD-2 in HCC, affecting the efficacy of ICIs.152 Serum exosome PCED1B-AS1 correlated with the expression of PD-Ls in HCC and predicted the efficacy of immunotherapy.152 PCED1B-AS1 inhibitors can potentially improve immunotherapy. HCC exosomal circUHRF1 reduced NK infiltration in tumors and inhibited the secretion of IFN-γ and TNF-α by NK cells. In addition, circUHRF1 upregulated TIM-3 expression by inhibiting miR-449c-5p, thereby reducing NK cell function. More importantly, circUHRF1 might be involved in immunosuppression by inducing NK cell dysfunction in HCC, leading to anti-PD1 therapy resistance.155 Blocking circUHRF1 may restore the function of NK cells and improve the efficacy of immunotherapy. Exosomes from drug-resistant BC cells increased the levels of TGF-β1 and the expression of PD-L1, inducing resistance to antitumor agents such as trastuzumab.223 These results suggest that the exosome-mediated transfer of ncRNAs to monocytes contributes to cancer-associated immune escape.224
As an important part of the TIME, macrophages are becoming a new target for antitumor immunity. Current cancer immunotherapies targeting macrophages can inhibit macrophage recruitment, deplete TAMs, reprogram TAMs, and block the CD47-SIRPα pathway.225,226 First, targeting the colony-stimulating factor 1 (CSF-1)/CSF-1R axis, C-C chemokine ligand 5 (CCL5)/CCR5 axis, CCL2/CCR2 axis, or VEGF effectively decreased TAM recruitment in preclinical and clinical studies.227 The delivery of a CCR2 siRNA to monocytes using cationic NPs in peripheral blood, bone marrow, and spleen inhibited monocyte recruitment and reduced TAM infiltration, leading to TIME remodeling.228 Similarly, M2 TAM dual-targeted NPs loaded with anti-CSF-1R siRNA decreased macrophage infiltration in melanoma and tumor size and improved overall survival in mice.229
Hyaluronic acid (HA) NPs loaded miRNA-125b promoted M1 polarization and enhanced antitumor efficacy.230 miRNA mimics are being used in preclinical trials to reprogram TAMs. MicroRNA-125b/wt-p53 plasmids encapsulated in CD44/EGFR-targeted HA NPs triggered M1 polarization and inhibited tumor growth in a mouse model of lung cancer.231 Similarly, the targeted delivery of miR-99b to mice with HCC or subcutaneous Lewis lung cancer induced M1 polarization by targeting κB-Ras2 and mTOR, enhancing immune surveillance and preventing tumor growth.232
CD47 is a transmembrane protein expressed in all cell types, particularly immature red blood cells and cancer cells.233 CD47 overexpression is linked with decreased phagocytosis/apoptosis by TAMs and poor prognosis in tumors.234 Therefore, blocking the CD47-SIRPα pathway can potentially restore the antitumor effect of TAMs. CD47 could be downregulated by antiD47 siRNA.235,236 Exosomes containing SIRPα variants significantly enhanced tumor phagocytosis and induced an effective antitumor T cell response.204 Anti-SIRPα therapy targeting myeloid cells has fewer side effects than anti-CD47 therapy, especially in red blood cells, making it a promising strategy to block the CD47-SIRPα pathway.237 In addition, inhibiting this pathway induces macrophage repolarization.238,239
Discussion
Exosomal ncRNAs usually represent the ncRNA landscape of the mother cell. However, many ncRNAs are differentially expressed between cells and exosomes. In this respect, miR-21 and miR-451 were highly expressed in GBM.47 The relative expression level of miR-21 did not differ significantly between GBM cells and exosomes; however, the level of miR-451 was 1,000–10,000 times higher in exosomes.47 Similarly, 117 ultraconserved RNAs (ucRNAs) were upregulated, 68 miRNAs were downregulated, and 24 ucRNAs were detected exclusively in exosomes.49 The transport of ncRNAs via exosomes is selective, although the underlying mechanisms are unclear. lncRNA LIFR-AS1, a miR-29a sponge, was transferred from macrophages to tumor cells by exosomes. lncRNA LIFR-AS1 was highly expressed and miR-29a was lowly expressed in osteosarcoma tissues. The expression of miR-29a in macrophages was significantly upregulated by lncRNA LIFR-AS1 knockdown, but not in exosomes. This indicated that the two ncRNAs were not co-transported in exosomes, which may be related to selective inclusion of exosomes.89
In addition, the miRNA landscape of exosomes varies depending on the tumor type.48 Viral infection can also affect miRNA expression in tumor cells and exosomes. MicroRNA-9 was highly expressed in HPV+ HNSCC cells but not in HPV− HNSCC cells.46
Hypoxia promoted the secretion of exosomes in tumor cells,66, 67, 68 and increased miRNA levels in TEXs.72 Under hypoxia, the expression of total let-7a miRNA in tumor cells decreased to approximately 30% of that in normal hypoxia controls, while the content of let-7a in exosomes increased to approximately 25 times.69 This reflected the enhanced enrichment ability of exosomes under hypoxia.
The functions of miRNAs in recipient cells depend on the characteristics of exosomes, uptake mechanism, the amount of miRNA transferred to recipient cells in the cytoplasm, and the level of endogenous target mRNAs.47
Prospects and conclusions
Macrophages are one of the most abundant cell types in the TIME and are closely associated with tumor development. The heterogeneity in macrophage activity may have significant diagnostic and therapeutic implications in cancer. Exosomes mediate interactions between tumoral cells and TAMs via ncRNAs.
Immune escape is a hallmark of tumors and disrupts the cancer-immunity cycle, leading to T cell depletion and long-term immunosuppression.
Exosomal ncRNAs are used by cancer cells to evade immune surveillance and can serve as diagnostic markers given their abundance in tumor tissues and peripheral circulation. Nonetheless, their specificity and sensitivity in solid tumors must be further assessed.
Antagonists can be used to reverse the effects of exosomes and their ncRNAs; nonetheless, the development of effective and clinically applicable antagonists is challenging.
Targeting signaling pathways and designing drugs that can reverse macrophage phenotype and tumor drug resistance have tremendous implications in oncotherapy. However, large follow-up studies and clinical validation are needed.
Moreover, miRNAs and siRNAs transferred to recipient cells via exosomes can abrogate the expression of target genes, limiting tumor progression.34 Notwithstanding, establishing standard production practices, improving purity, yield, and targeting, and achieving cost-effective large-scale production are necessary before these nanovesicles advance to clinical trial.
Macrophage-targeting therapies are emerging immunotherapies. More preclinical studies are needed to improve the targeting of these treatments. The exact efficacy and immune-related adverse effects need to be fully evaluated before clinical studies can be conducted.
Acknowledgments
This research was funded by the Jiangsu Provincial Key Research and Development Program (BE2020783(ZE20)) and the National Natural Science Foundation of China (no. 82102981).
Author contributions
L.Z. and Y.S. designed the study. Z.X., Y.C., and L.M. collected the related papers and drafted the manuscript. Z.X., Y.C., and L.M. contributed equally to the manuscript. Y.C., J.L., Y.G., T.Y., and L.Z. revised the manuscript. All authors have read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Lingjun Zhu, Email: zhulingjun@njmu.edu.cn.
Yongqian Shu, Email: shuyongqian1962@162.com.
References
- 1.Hombach S., Kretz M. Non-coding RNAs: classification, biology and functioning. Adv. Exp. Med. Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Chan J.J., Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int. J. Mol. Sci. 2018;19:1310. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guil S., Esteller M. RNA-RNA interactions in gene regulation: the coding and noncoding players. Trends Biochem. Sci. 2015;40:248–256. doi: 10.1016/j.tibs.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Anastasiadou E., Jacob L.S., Slack F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodall G.J., Wickramasinghe V.O. RNA in cancer. Nat. Rev. Cancer. 2021;21:22–36. doi: 10.1038/s41568-020-00306-0. [DOI] [PubMed] [Google Scholar]
- 6.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 7.Dong H., Lei J., Ding L., Wen Y., Ju H., Zhang X. MicroRNA: function, detection, and bioanalysis. Chem. Rev. 2013;113:6207–6233. doi: 10.1021/cr300362f. [DOI] [PubMed] [Google Scholar]
- 8.Chen L.L. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 2016;41:761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 10.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C., Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28:287–301. doi: 10.1016/j.tcb.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C.Y., Kuo H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019;26:29. doi: 10.1186/s12929-019-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 15.Wang R., Zhang S., Chen X., Li N., Li J., Jia R., Pan Y., Liang H. CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res. 2018;78:4812–4825. doi: 10.1158/0008-5472.CAN-18-0532. [DOI] [PubMed] [Google Scholar]
- 16.Qian L., Yu S., Chen Z., Meng Z., Huang S., Wang P. The emerging role of circRNAs and their clinical significance in human cancers. Biochim. Biophys. Acta Rev. Cancer. 2018;1870:247–260. doi: 10.1016/j.bbcan.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 19.Abels E.R., Breakefield X.O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 21.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri R. The biology and function of exosomes in cancer. J. Clin. Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteside T.L. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kok V.C., Yu C.C. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int. J. Nanomed. 2020;15:8019–8036. doi: 10.2147/IJN.S272378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greening D.W., Gopal S.K., Xu R., Simpson R.J., Chen W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Xie F., Zhou X., Fang M., Li H., Su P., Tu Y., Zhang L., Zhou F. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv. Sci. 2019;6:1901779. doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veerman R.E., Güçlüler Akpinar G., Eldh M., Gabrielsson S. Immune cell-derived extracellular vesicles - functions and therapeutic applications. Trends Mol. Med. 2019;25:382–394. doi: 10.1016/j.molmed.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Yan W., Jiang S. Immune cell-derived exosomes in the cancer-immunity cycle. Trends Cancer. 2020;6:506–517. doi: 10.1016/j.trecan.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Sun Z., Shi K., Yang S., Liu J., Zhou Q., Wang G., Song J., Li Z., Zhang Z., Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng J., Meng J., Zhu L., Peng Y. Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications. Mol. Cancer. 2020;19:66. doi: 10.1186/s12943-020-01189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y., Dang W., Zhang S., Yue W., Yang L., Zhai X., Yan Q., Lu J. The role of exosomal noncoding RNAs in cancer. Mol. Cancer. 2019;18:37. doi: 10.1186/s12943-019-0984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pullan J.E., Confeld M.I., Osborn J.K., Kim J., Sarkar K., Mallik S. Exosomes as drug carriers for cancer therapy. Mol. Pharm. 2019;16:1789–1798. doi: 10.1021/acs.molpharmaceut.9b00104. [DOI] [PubMed] [Google Scholar]
- 35.Liu C., Zhang L., Liu H., Cheng K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J. Control. Release. 2017;266:17–26. doi: 10.1016/j.jconrel.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghaemi A., Bagheri E., Abnous K., Taghdisi S.M., Ramezani M., Alibolandi M. CRISPR-cas9 genome editing delivery systems for targeted cancer therapy. Life Sci. 2021;267:118969. doi: 10.1016/j.lfs.2020.118969. [DOI] [PubMed] [Google Scholar]
- 37.Belgiovine C., D'Incalci M., Allavena P., Frapolli R. Tumor-associated macrophages and anti-tumor therapies: complex links. Cell. Mol. Life Sci. 2016;73:2411–2424. doi: 10.1007/s00018-016-2166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orecchioni M., Ghosheh Y., Pramod A.B., Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front. Immunol. 2019;10:1084. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin C., Han Q., Xu D., Zheng B., Zhao X., Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology. 2019;8:1601479. doi: 10.1080/2162402X.2019.1601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruffell B., Coussens L.M. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sica A., Erreni M., Allavena P., Porta C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 44.Czimmerer Z., Daniel B., Horvath A., Rückerl D., Nagy G., Kiss M., Peloquin M., Budai M.M., Cuaranta-Monroy I., Simandi Z., et al. The transcription factor STAT6 mediates direct repression of inflammatory enhancers and limits activation of alternatively polarized macrophages. Immunity. 2018;48:75–90.e76. doi: 10.1016/j.immuni.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 46.Tong F., Mao X., Zhang S., Xie H., Yan B., Wang B., Sun J., Wei L. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020;478:34–44. doi: 10.1016/j.canlet.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 47.van der Vos K.E., Abels E.R., Zhang X., Lai C., Carrizosa E., Oakley D., Prabhakar S., Mardini O., Crommentuijn M.H., Skog J., et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol. 2016;18:58–69. doi: 10.1093/neuonc/nov244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson S., Kim S., Lee C., Deci M., Nguyen J. The phenotypic effects of exosomes secreted from distinct cellular sources: a comparative study based on miRNA composition. AAPS J. 2018;20:67. doi: 10.1208/s12248-018-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kogure T., Yan I.K., Lin W.L., Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4:261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., Lei Y., Wu M., Li N. Regulation of macrophage activation and polarization by HCC-derived exosomal lncRNA TUC339. Int. J. Mol. Sci. 2018;19:2958. doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao Y., Chen T., Zheng X., Yang S., Xu K., Chen X., Xu F., Wang L., Shen Y., Wang T., et al. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39:1368–1379. doi: 10.1093/carcin/bgy115. [DOI] [PubMed] [Google Scholar]
- 52.Gerloff D., Lützkendorf J., Moritz R.K.C., Wersig T., Mäder K., Müller L.P., Sunderkötter C. Melanoma-derived exosomal miR-125b-5p educates tumor associated macrophages (TAMs) by targeting lysosomal acid lipase A (LIPA) Cancers. 2020;12:464. doi: 10.3390/cancers12020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Covarrubias A.J., Aksoylar H.I., Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin. Immunol. 2015;27:286–296. doi: 10.1016/j.smim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao S.J., Kong F.Q., Jie J., Li Q., Liu H., Xu A.D., Yang Y.Q., Jiang B., Wang D.D., Zhou Z.Q., et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics. 2020;10:17–35. doi: 10.7150/thno.36930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu J., Xie L., Liu C., Zhang Q., Sun S. PTEN/PI3k/AKT regulates macrophage polarization in emphysematous mice. Scand. J. Immunol. 2017;85:395–405. doi: 10.1111/sji.12545. [DOI] [PubMed] [Google Scholar]
- 56.Lin F., Yin H.B., Li X.Y., Zhu G.M., He W.Y., Gou X. Bladder cancer cell-secreted exosomal miR-21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int. J. Oncol. 2020;56:151–164. doi: 10.3892/ijo.2019.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D., Wang X., Si M., Yang J., Sun S., Wu H., Cui S., Qu X., Yu X. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020;474:36–52. doi: 10.1016/j.canlet.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Wang D., Wang X., Si M., Yang J., Sun S., Wu H., Cui S., Qu X., Yu X. Corrigendum to “Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages” [Canc. Lett. 474 (2020) 36-52] Cancer Lett. 2022;525:200–202. doi: 10.1016/j.canlet.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Chen T., Liu Y., Li C., Xu C., Ding C., Chen J., Zhao J. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat. Res. Commun. 2021;28:100412. doi: 10.1016/j.ctarc.2021.100412. [DOI] [PubMed] [Google Scholar]
- 60.Zhao M., Bian Y.Y., Yang L.L., Chen Y.Q., Wang Y.J., Ma Y.T., Pei Y.Q., Li W.L., Zeng L. HuoXueTongFu formula alleviates intraperitoneal adhesion by regulating macrophage polarization and the SOCS/JAK2/STAT/PPAR-γ signalling pathway. Mediators Inflamm. 2019;2019:1769374. doi: 10.1155/2019/1769374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu X., Chen J., Wang L., Ivashkiv L.B. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 62.Cai J., Qiao B., Gao N., Lin N., He W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol. Cell Physiol. 2019;316:C731–C740. doi: 10.1152/ajpcell.00366.2018. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Jiang M., Qian L., Lin X., Song W., Gao Y., Zhou Y. The STAT3-miR-223-TGFBR3/HMGCS1 axis modulates the progression of cervical carcinoma. Mol. Oncol. 2020;14:2313–2331. doi: 10.1002/1878-0261.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escribese M.M., Casas M., Corbí A.L. Influence of low oxygen tensions on macrophage polarization. Immunobiology. 2012;217:1233–1240. doi: 10.1016/j.imbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Díaz-Bulnes P., Saiz M.L., López-Larrea C., Rodríguez R.M. Crosstalk between hypoxia and ER stress response: a key regulator of macrophage polarization. Front. Immunol. 2019;10:2951. doi: 10.3389/fimmu.2019.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., Liu B., Su L., Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 67.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., Liu B., Su L., Qiu Z. Correction: hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2020;80:922. doi: 10.1158/0008-5472.CAN-19-3872. [DOI] [PubMed] [Google Scholar]
- 68.Lu Q., Wang X., Zhu J., Fei X., Chen H., Li C. Hypoxic tumor-derived exosomal Circ0048117 facilitates M2 macrophage polarization acting as miR-140 sponge in esophageal squamous cell carcinoma. OncoTargets Ther. 2020;13:11883–11897. doi: 10.2147/OTT.S284192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park J.E., Dutta B., Tse S.W., Gupta N., Tan C.F., Low J.K., Yeoh K.W., Kon O.L., Tam J.P., Sze S.K. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene. 2019;38:5158–5173. doi: 10.1038/s41388-019-0782-x. [DOI] [PubMed] [Google Scholar]
- 70.Qian M., Wang S., Guo X., Wang J., Zhang Z., Qiu W., Gao X., Chen Z., Xu J., Zhao R., et al. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene. 2020;39:428–442. doi: 10.1038/s41388-019-0996-y. [DOI] [PubMed] [Google Scholar]
- 71.Ying X., Wu Q., Wu X., Zhu Q., Wang X., Jiang L., Chen X., Wang X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–43087. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X., Ying X., Wang X., Wu X., Zhu Q., Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol. Rep. 2017;38:522–528. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 73.Chen X., Zhou J., Li X., Wang X., Lin Y., Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91. doi: 10.1016/j.canlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Pastushenko I., Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Sun D., Luo T., Dong P., Zhang N., Chen J., Zhang S., Dong L., Janssen H.L.A., Zhang S. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma. J. Cell. Biochem. 2020;121:2828–2838. doi: 10.1002/jcb.29514. [DOI] [PubMed] [Google Scholar]
- 76.Yao R.R., Li J.H., Zhang R., Chen R.X., Wang Y.H. M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J. Surg. Oncol. 2018;16:9. doi: 10.1186/s12957-018-1312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L.P., Lin J., Ma X.Q., Xu D.Y., Shi C.F., Wang W., Jiang X.J. Exosomal DLX6-AS1 from hepatocellular carcinoma cells induces M2 macrophage polarization to promote migration and invasion in hepatocellular carcinoma through microRNA-15a-5p/CXCL17 axis. J. Exp. Clin. Cancer Res. 2021;40:177. doi: 10.1186/s13046-021-01973-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Hsieh C.H., Tai S.K., Yang M.H. Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering MiR-21-abundant exosomes. Neoplasia. 2018;20:775–788. doi: 10.1016/j.neo.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang Z.X., Liu H.S., Wang F.W., Xiong L., Zhou C., Hu T., He X.W., Wu X.J., Xie D., Wu X.R., Lan P. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019;10:829. doi: 10.1038/s41419-019-2077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang Z.X., Liu H.S., Wang F.W., Xiong L., Zhou C., Hu T., He X.W., Wu X.J., Xie D., Wu X.R., Lan P. Correction: LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2020;11:465. doi: 10.1038/s41419-020-2661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Gao R., Li J., Tang S., Li S., Tong Q., Li S. Downregulation of hsa_circ_0074854 suppresses the migration and invasion in hepatocellular carcinoma via interacting with HuR and via suppressing exosomes-mediated macrophage M2 polarization. Int. J. Nanomed. 2021;16:2803–2818. doi: 10.2147/IJN.S284560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lv J., Li Q., Ma R., Wang Z., Yu Y., Liu H., Miao Y., Jiang S. Long noncoding RNA FGD5-AS1 knockdown decrease viability, migration, and invasion of non-small cell lung cancer (NSCLC) cells by regulating the MicroRNA-944/MACC1 axis. Technol. Cancer Res. Treat. 2021;20 doi: 10.1177/1533033821990090. 1533033821990090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jang J.Y., Lee J.K., Jeon Y.K., Kim C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katopodi T., Petanidis S., Domvri K., Zarogoulidis P., Anestakis D., Charalampidis C., Tsavlis D., Bai C., Huang H., Freitag L., et al. Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit. Sci. Rep. 2021;11:15455. doi: 10.1038/s41598-021-94671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feitelson M.A., Arzumanyan A., Kulathinal R.J., Blain S.W., Holcombe R.F., Mahajna J., Marino M., Martinez-Chantar M.L., Nawroth R., Sanchez-Garcia I., et al. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015;35:S25–S54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maia J., Caja S., Strano Moraes M.C., Couto N., Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guan H., Peng R., Fang F., Mao L., Chen Z., Yang S., Dai C., Wu H., Wang C., Feng N., et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J. Cell. Physiol. 2020;235:9729–9742. doi: 10.1002/jcp.29784. [DOI] [PubMed] [Google Scholar]
- 88.Wang J.J., Wang Z.Y., Chen R., Xiong J., Yao Y.L., Wu J.H., Li G.X. Macrophage-secreted exosomes delivering miRNA-21 inhibitor can regulate BGC-823 cell proliferation. Asian Pac. J. Cancer Prev. 2015;16:4203–4209. doi: 10.7314/apjcp.2015.16.10.4203. [DOI] [PubMed] [Google Scholar]
- 89.Zhang H., Yu Y., Wang J., Han Y., Ren T., Huang Y., Chen C., Huang Q., Wang W., Niu J., et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int. 2021;21:192. doi: 10.1186/s12935-021-01893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X., Tang M. Exosomes released from M2 macrophages transfer miR-221-3p contributed to EOC progression through targeting CDKN1B. Cancer Med. 2020;9:5976–5988. doi: 10.1002/cam4.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aucher A., Rudnicka D., Davis D.M. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 2013;191:6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y., Wang B., Xiao S., Li Y., Chen Q. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J. Cell. Biochem. 2019;120:3046–3055. doi: 10.1002/jcb.27436. [DOI] [PubMed] [Google Scholar]
- 93.Gao Y., Bado I., Wang H., Zhang W., Rosen J.M., Zhang X.H. Metastasis organotropism: redefining the congenial soil. Dev. Cell. 2019;49:375–391. doi: 10.1016/j.devcel.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambert A.W., Pattabiraman D.R., Weinberg R.A. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu J., Gao W., Tang Q., Yu Y., You W., Wu Z., Fan Y., Zhang L., Wu C., Han G., et al. M2 macrophage-derived exosomes facilitate HCC metastasis by transferring α(M) β(2) integrin to tumor cells. Hepatology. 2021;73:1365–1380. doi: 10.1002/hep.31432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Yang C., Dou R., Wei C., Liu K., Shi D., Zhang C., Liu Q., Wang S., Xiong B. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol. Ther. 2021;29:2088–2107. doi: 10.1016/j.ymthe.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yin Z., Ma T., Huang B., Lin L., Zhou Y., Yan J., Zou Y., Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lan J., Sun L., Xu F., Liu L., Hu F., Song D., Hou Z., Wu W., Luo X., Wang J., et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 101.Wang G., Fu Y., Yang X., Luo X., Wang J., Gong J., Hu J. Brg-1 targeting of novel miR550a-5p/RNF43/Wnt signaling axis regulates colorectal cancer metastasis. Oncogene. 2017;36:5915. doi: 10.1038/onc.2017.317. [DOI] [PubMed] [Google Scholar]
- 102.Yin Z., Zhou Y., Ma T., Chen S., Shi N., Zou Y., Hou B., Zhang C. Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. J. Cell. Mol. Med. 2020;24:5028–5038. doi: 10.1111/jcmm.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mi X., Xu R., Hong S., Xu T., Zhang W., Liu M. M2 macrophage-derived exosomal lncRNA AFAP1-AS1 and MicroRNA-26a affect cell migration and metastasis in esophageal cancer. Mol. Ther. Nucleic Acids. 2020;22:779–790. doi: 10.1016/j.omtn.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu G., Ouyang X., Sun Y., Xiao Y., You B., Gao Y., Yeh S., Li Y., Chang C. The miR-92a-2-5p in exosomes from macrophages increases liver cancer cells invasion via altering the AR/PHLPP/p-AKT/β-catenin signaling. Cell Death Differ. 2020;27:3258–3272. doi: 10.1038/s41418-020-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang M., Chen J., Su F., Yu B., Su F., Lin L., Liu Y., Huang J.D., Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng P.M., Gao H.J., Li J.M., Zhang P., Li G. [Effect of exosome-derived miR-223 from macrophages on the metastasis of gastric cancer cells] Zhonghua Yi Xue Za Zhi. 2020;100:1750–1755. doi: 10.3760/cma.j.cn112137-20200425-01309. [DOI] [PubMed] [Google Scholar]
- 107.Hu Y., Li D., Wu A., Qiu X., Di W., Huang L., Qiu L. TWEAK-stimulated macrophages inhibit metastasis of epithelial ovarian cancer via exosomal shuttling of microRNA. Cancer Lett. 2017;393:60–67. doi: 10.1016/j.canlet.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Vanharanta S., Massagué J. Origins of metastatic traits. Cancer Cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bergers G., Fendt S.M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer. 2021;21:162–180. doi: 10.1038/s41568-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., Psaila B., Kaplan R.N., Bromberg J.F., Kang Y., et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 111.Doglioni G., Parik S., Fendt S.M. Interactions in the (Pre)metastatic niche support metastasis formation. Front. Oncol. 2019;9:219. doi: 10.3389/fonc.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 113.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L., Zhang S., Yao J., Lowery F.J., Zhang Q., Huang W.C., Li P., Li M., Wang X., Zhang C., et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Arabey A.A., Denizli M., Kanlikilicer P., Bayraktar R., Ivan C., Rashed M., Kabil N., Ozpolat B., Calin G.A., Salama S.A., et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell. Signal. 2020;68:109539. doi: 10.1016/j.cellsig.2020.109539. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y., Zhao L., Li D., Yin Y., Zhang C.Y., Li J., Zhang Y. Microvesicle-delivery miR-150 promotes tumorigenesis by up-regulating VEGF, and the neutralization of miR-150 attenuate tumor development. Protein Cell. 2013;4:932–941. doi: 10.1007/s13238-013-3092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chuang H.Y., Su Y.K., Liu H.W., Chen C.H., Chiu S.C., Cho D.Y., Lin S.Z., Chen Y.S., Lin C.M. Preclinical evidence of STAT3 inhibitor pacritinib overcoming temozolomide resistance via downregulating miR-21-enriched exosomes from M2 glioblastoma-associated macrophages. J. Clin. Med. 2019;8:959. doi: 10.3390/jcm8070959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abels E.R., Maas S.L.N., Nieland L., Wei Z., Cheah P.S., Tai E., Kolsteeg C.J., Dusoswa S.A., Ting D.T., Hickman S., et al. Glioblastoma-associated microglia reprogramming is mediated by functional transfer of extracellular miR-21. Cell Rep. 2019;28:3105–3119.e7. doi: 10.1016/j.celrep.2019.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun X., Ma X., Wang J., Zhao Y., Wang Y., Bihl J.C., Chen Y., Jiang C. Glioma stem cells-derived exosomes promote the angiogenic ability of endothelial cells through miR-21/VEGF signal. Oncotarget. 2017;8:36137–36148. doi: 10.18632/oncotarget.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siegal T., Charbit H., Paldor I., Zelikovitch B., Canello T., Benis A., Wong M.L., Morokoff A.P., Kaye A.H., Lavon I. Dynamics of circulating hypoxia-mediated miRNAs and tumor response in patients with high-grade glioma treated with bevacizumab. J. Neurosurg. 2016;125:1008–1015. doi: 10.3171/2015.8.JNS15437. [DOI] [PubMed] [Google Scholar]
- 121.Costa P.M., Cardoso A.L., Nóbrega C., Pereira de Almeida L.F., Bruce J.N., Canoll P., Pedroso de Lima M.C. MicroRNA-21 silencing enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma. Hum. Mol. Genet. 2013;22:904–918. doi: 10.1093/hmg/dds496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y., Meng W., Yue P., Li X. M2 macrophage-derived extracellular vesicles promote gastric cancer progression via a microRNA-130b-3p/MLL3/GRHL2 signaling cascade. J. Exp. Clin. Cancer Res. 2020;39:134. doi: 10.1186/s13046-020-01626-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Wang L., Zhao Z., Ozark P.A., Fantini D., Marshall S.A., Rendleman E.J., Cozzolino K.A., Louis N., He X., Morgan M.A., et al. Resetting the epigenetic balance of Polycomb and COMPASS function at enhancers for cancer therapy. Nat. Med. 2018;24:758–769. doi: 10.1038/s41591-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shao C., Yang F., Miao S., Liu W., Wang C., Shu Y., Shen H. Role of hypoxia-induced exosomes in tumor biology. Mol. Cancer. 2018;17:120. doi: 10.1186/s12943-018-0869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang X., Wang J., Deng X., Xiong F., Zhang S., Gong Z., Li X., Cao K., Deng H., He Y., et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020;39:204. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cui H., Seubert B., Stahl E., Dietz H., Reuning U., Moreno-Leon L., Ilie M., Hofman P., Nagase H., Mari B., et al. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene. 2015;34:3640–3650. doi: 10.1038/onc.2014.300. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y., Luo F., Wang B., Li H., Xu Y., Liu X., Shi L., Lu X., Xu W., Lu L., et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370:125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 128.Zheng P., Chen L., Yuan X., Luo Q., Liu Y., Xie G., Ma Y., Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 2017;36:53. doi: 10.1186/s13046-017-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xin L., Zhou L.Q., Liu C., Zeng F., Yuan Y.W., Zhou Q., Li S.H., Wu Y., Wang J.L., Wu D.Z., et al. Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO Rep. 2021;22:e52124. doi: 10.15252/embr.202052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kanlikilicer P., Bayraktar R., Denizli M., Rashed M.H., Ivan C., Aslan B., Mitra R., Karagoz K., Bayraktar E., Zhang X., et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine. 2018;38:100–112. doi: 10.1016/j.ebiom.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kanlikilicer P., Bayraktar R., Denizli M., Rashed M.H., Ivan C., Aslan B., Mitra R., Karagoz K., Bayraktar E., Zhang X., et al. Corrigendum to 'Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer' [EBioMedicine 38 (2018) 100-112] EBioMedicine. 2020;52:102630. doi: 10.1016/j.ebiom.2020.102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu X., Shen H., Yin X., Yang M., Wei H., Chen Q., Feng F., Liu Y., Xu W., Li Y. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J. Exp. Clin. Cancer Res. 2019;38:81. doi: 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Binenbaum Y., Fridman E., Yaari Z., Milman N., Schroeder A., Ben David G., Shlomi T., Gil Z. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 2018;78:5287–5299. doi: 10.1158/0008-5472.CAN-18-0124. [DOI] [PubMed] [Google Scholar]
- 134.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 135.Chen Y.P., Zhang Y., Lv J.W., Li Y.Q., Wang Y.Q., He Q.M., Yang X.J., Sun Y., Mao Y.P., Yun J.P., et al. Genomic analysis of tumor microenvironment immune types across 14 solid cancer types: immunotherapeutic implications. Theranostics. 2017;7:3585–3594. doi: 10.7150/thno.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Borst J., Ahrends T., Bąbała N., Melief C.J.M., Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 137.Ochoa de Olza M., Navarro Rodrigo B., Zimmermann S., Coukos G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol. 2020;21:e419–e430. doi: 10.1016/S1470-2045(20)30234-5. [DOI] [PubMed] [Google Scholar]
- 138.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E., Lichtor T., Decker W.K., Whelan R.L., Kumara H.M.C.S., et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 139.Rosenthal R., Cadieux E.L., Salgado R., Bakir M.A., Moore D.A., Hiley C.T., Lund T., Tanić M., Reading J.L., Joshi K., et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567:479–485. doi: 10.1038/s41586-019-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U S A. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou M., Chen J., Zhou L., Chen W., Ding G., Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell. Immunol. 2014;292:65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Ding G., Zhou L., Qian Y., Fu M., Chen J., Chen J., Xiang J., Wu Z., Jiang G., Cao L. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6:29877–29888. doi: 10.18632/oncotarget.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maus R.L.G., Jakub J.W., Nevala W.K., Christensen T.A., Noble-Orcutt K., Sachs Z., Hieken T.J., Markovic S.N. Human melanoma-derived extracellular vesicles regulate dendritic cell maturation. Front. Immunol. 2017;8:358. doi: 10.3389/fimmu.2017.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Condamine T., Gabrilovich D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ye S.B., Li Z.L., Luo D.H., Huang B.J., Chen Y.S., Zhang X.S., Cui J., Zeng Y.X., Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin R., Chen L., Chen G., Hu C., Jiang S., Sevilla J., Wan Y., Sampson J.H., Zhu B., Li Q.J. Targeting miR-23a in CD8+ cytotoxic T lymphocytes prevents tumor-dependent immunosuppression. J. Clin. Invest. 2014;124:5352–5367. doi: 10.1172/JCI76561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang P., Li Q.J., Feng Y., Zhang Y., Markowitz G.J., Ning S., Deng Y., Zhao J., Jiang S., Yuan Y., et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ye S.B., Zhang H., Cai T.T., Liu Y.N., Ni J.J., He J., Peng J.Y., Chen Q.Y., Mo H.Y., Jun-Cui T., et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016;240:329–340. doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 149.Wang H., Flach H., Onizawa M., Wei L., McManus M.T., Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat. Immunol. 2014;15:393–401. doi: 10.1038/ni.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen T., Huang Z., Shi C., Pu X., Xu X., Wu Z., Ding G., Cao L. Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. FASEB J. 2020;34:8442–8458. doi: 10.1096/fj.201902186R. [DOI] [PubMed] [Google Scholar]
- 151.Yang F., Wang T., Du P., Fan H., Dong X., Guo H. M2 bone marrow-derived macrophage-derived exosomes shuffle microRNA-21 to accelerate immune escape of glioma by modulating PEG3. Cancer Cell Int. 2020;20:93. doi: 10.1186/s12935-020-1163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan F., Chen K., Lu X., Li A., Liu C., Wu B. Dual targeting of PD-L1 and PD-L2 by PCED1B-AS1 via sponging hsa-miR-194-5p induces immunosuppression in hepatocellular carcinoma. Hepatol. Int. 2021;15:444–458. doi: 10.1007/s12072-020-10101-6. [DOI] [PubMed] [Google Scholar]
- 153.Donatelli S.S., Zhou J.M., Gilvary D.L., Eksioglu E.A., Chen X., Cress W.D., Haura E.B., Schabath M.B., Coppola D., Wei S., et al. TGF-β-inducible microRNA-183 silences tumor-associated natural killer cells. Proc. Natl. Acad. Sci. U S A. 2014;111:4203–4208. doi: 10.1073/pnas.1319269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tang B., Wu W., Wei X., Li Y., Ren G., Fan W. Activation of glioma cells generates immune tolerant NKT cells. J. Biol. Chem. 2014;289:34595–34600. doi: 10.1074/jbc.M114.614503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang P.F., Gao C., Huang X.Y., Lu J.C., Guo X.J., Shi G.M., Cai J.B., Ke A.W. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer. 2020;19:110. doi: 10.1186/s12943-020-01222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Noman M.Z., Buart S., Romero P., Ketari S., Janji B., Mari B., Mami-Chouaib F., Chouaib S. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012;72:4629–4641. doi: 10.1158/0008-5472.CAN-12-1383. [DOI] [PubMed] [Google Scholar]
- 157.Berchem G., Noman M.Z., Bosseler M., Paggetti J., Baconnais S., Le Cam E., Nanbakhsh A., Moussay E., Mami-Chouaib F., Janji B., Chouaib S. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology. 2016;5:e1062968. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou J., Li X., Wu X., Zhang T., Zhu Q., Wang X., Wang H., Wang K., Lin Y., Wang X. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol. Res. 2018;6:1578–1592. doi: 10.1158/2326-6066.CIR-17-0479. [DOI] [PubMed] [Google Scholar]
- 159.Yin Y., Cai X., Chen X., Liang H., Zhang Y., Li J., Wang Z., Chen X., Zhang W., Yokoyama S., et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res. 2014;24:1164–1180. doi: 10.1038/cr.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu Y., Lai L., Chen Q., Song Y., Xu S., Ma F., Wang X., Wang J., Yu H., Cao X., Wang Q. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J. Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 161.Noman M.Z., Janji B., Hu S., Wu J.C., Martelli F., Bronte V., Chouaib S. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. 2015;75:3771–3787. doi: 10.1158/0008-5472.CAN-15-0405. [DOI] [PubMed] [Google Scholar]
- 162.Truettner J.S., Katyshev V., Esen-Bilgin N., Dietrich W.D., Dore-Duffy P. Hypoxia alters MicroRNA expression in rat cortical pericytes. MicroRNA. 2013;2:32–44. doi: 10.2174/2211536611302010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eismann J., Hirschfeld M., Erbes T., Rücker G., Jäger M., Ritter A., Weiss D., Gitsch G., Mayer S. Hypoxia- and acidosis-driven aberrations of secreted microRNAs in endometrial cancer in vitro. Oncol. Rep. 2017;38:993–1004. doi: 10.3892/or.2017.5717. [DOI] [PubMed] [Google Scholar]
- 164.Zhang M., Liu Q., Mi S., Liang X., Zhang Z., Su X., Liu J., Chen Y., Wang M., Zhang Y., et al. Both miR-17-5p and miR-20a alleviate suppressive potential of myeloid-derived suppressor cells by modulating STAT3 expression. J. Immunol. 2011;186:4716–4724. doi: 10.4049/jimmunol.1002989. [DOI] [PubMed] [Google Scholar]
- 165.Wang J., Yu F., Jia X., Iwanowycz S., Wang Y., Huang S., Ai W., Fan D. MicroRNA-155 deficiency enhances the recruitment and functions of myeloid-derived suppressor cells in tumor microenvironment and promotes solid tumor growth. Int. J. Cancer. 2015;136:E602–E613. doi: 10.1002/ijc.29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fang T., Lv H., Lv G., Li T., Wang C., Han Q., Yu L., Su B., Guo L., Huang S., et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018;9:191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou Y., Ren H., Dai B., Li J., Shang L., Huang J., Shi X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J. Exp. Clin. Cancer Res. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Radoja S., Rao T.D., Hillman D., Frey A.B. Mice bearing late-stage tumors have normal functional systemic T cell responses in vitro and in vivo. J. Immunol. 2000;164:2619–2628. doi: 10.4049/jimmunol.164.5.2619. [DOI] [PubMed] [Google Scholar]
- 169.Vesely M.D., Kershaw M.H., Schreiber R.D., Smyth M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 170.Que R.S., Lin C., Ding G.P., Wu Z.R., Cao L.P. Increasing the immune activity of exosomes: the effect of miRNA-depleted exosome proteins on activating dendritic cell/cytokine-induced killer cells against pancreatic cancer. J. Zhejiang Univ. Sci. B. 2016;17:352–360. doi: 10.1631/jzus.B1500305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vénéreau E., Ceriotti C., Bianchi M.E. DAMPs from cell death to new life. Front. Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180:1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hammer G.E., Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu. Rev. Immunol. 2013;31:743–791. doi: 10.1146/annurev-immunol-020711-074929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morelli A.E., Thomson A.W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 175.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 176.Montacchiesi G., Pace L. Epigenetics and CD8(+) T cell memory. Immunol. Rev. 2022;305:77–89. doi: 10.1111/imr.13057. [DOI] [PubMed] [Google Scholar]
- 177.Oh D.Y., Fong L. Cytotoxic CD4(+) T cells in cancer: expanding the immune effector toolbox. Immunity. 2021;54:2701–2711. doi: 10.1016/j.immuni.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang C., Kim S.H., Bianco N.R., Robbins P.D. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6:e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muller L., Mitsuhashi M., Simms P., Gooding W.E., Whiteside T.L. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 2016;6:20254. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mandal A., Viswanathan C. Natural killer cells: in health and disease. Hematol. Oncol. Stem Cell Ther. 2015;8:47–55. doi: 10.1016/j.hemonc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 181.Berzins S.P., Ritchie D.S. Natural killer T cells: drivers or passengers in preventing human disease? Nat. Rev. Immunol. 2014;14:640–646. doi: 10.1038/nri3725. [DOI] [PubMed] [Google Scholar]
- 182.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 183.Gabrilovich D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fu J., Xu D., Liu Z., Shi M., Zhao P., Fu B., Zhang Z., Yang H., Zhang H., Zhou C., et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 185.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 186.Geis-Asteggiante L., Belew A.T., Clements V.K., Edwards N.J., Ostrand-Rosenberg S., El-Sayed N.M., Fenselau C. Differential content of proteins, mRNAs, and miRNAs suggests that MDSC and their exosomes may mediate distinct immune suppressive functions. J. Proteome Res. 2018;17:486–498. doi: 10.1021/acs.jproteome.7b00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen S., Zhang Y., Kuzel T.M., Zhang B. Regulating tumor myeloid-derived suppressor cells by MicroRNAs. Cancer Cell Microenviron. 2015;2:e637. doi: 10.14800/ccm.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Biffi G., Tuveson D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021;101:147–176. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dou D., Ren X., Han M., Xu X., Ge X., Gu Y., Wang X. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Front. Immunol. 2020;11:2026. doi: 10.3389/fimmu.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 190.Yang K., Zhang J., Bao C. Exosomal circEIF3K from cancer-associated fibroblast promotes colorectal cancer (CRC) progression via miR-214/PD-L1 axis. BMC Cancer. 2021;21:933. doi: 10.1186/s12885-021-08669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lässer C., Alikhani V.S., Ekström K., Eldh M., Paredes P.T., Bossios A., Sjöstrand M., Gabrielsson S., Lötvall J., Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J. Transl. Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.El-Andaloussi S., Lee Y., Lakhal-Littleton S., Li J., Seow Y., Gardiner C., Alvarez-Erviti L., Sargent I.L., Wood M.J. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 193.Kim S.M., Yang Y., Oh S.J., Hong Y., Seo M., Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release. 2017;266:8–16. doi: 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 194.Saari H., Lázaro-Ibáñez E., Viitala T., Vuorimaa-Laukkanen E., Siljander P., Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 195.Zhang N., Nan A., Chen L., Li X., Jia Y., Qiu M., Dai X., Zhou H., Zhu J., Zhang H., Jiang Y. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol. Cancer. 2020;19:101. doi: 10.1186/s12943-020-01221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xie M., Yu T., Jing X., Ma L., Fan Y., Yang F., Ma P., Jiang H., Wu X., Shu Y., Xu T. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer. 2020;19:112. doi: 10.1186/s12943-020-01208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Takano Y., Masuda T., Iinuma H., Yamaguchi R., Sato K., Tobo T., Hirata H., Kuroda Y., Nambara S., Hayashi N., et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget. 2017;8:78598–78613. doi: 10.18632/oncotarget.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu L., Meng T., Yang X.H., Sayim P., Lei C., Jin B., Ge L., Wang H.J. Prognostic and predictive value of long non-coding RNA GAS5 and mircoRNA-221 in colorectal cancer and their effects on colorectal cancer cell proliferation, migration and invasion. Cancer Biomark. 2018;22:283–299. doi: 10.3233/CBM-171011. [DOI] [PubMed] [Google Scholar]
- 199.Hou Y., Yu Z., Tam N.L., Huang S., Sun C., Wang R., Zhang X., Wang Z., Ma Y., He X., Wu L. Exosome-related lncRNAs as predictors of HCC patient survival: a prognostic model. Am. J. Transl. Res. 2018;10:1648–1662. [PMC free article] [PubMed] [Google Scholar]
- 200.Xu Z., Zeng S., Gong Z., Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol. Cancer. 2020;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shao J., Zaro J., Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int. J. Nanomed. 2020;15:9355–9371. doi: 10.2147/IJN.S281890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van den Boorn J.G., Schlee M., Coch C., Hartmann G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- 203.Agrawal A.K., Aqil F., Jeyabalan J., Spencer W.A., Beck J., Gachuki B.W., Alhakeem S.S., Oben K., Munagala R., Bondada S., Gupta R.C. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13:1627–1636. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 204.Koh E., Lee E.J., Nam G.H., Hong Y., Cho E., Yang Y., Kim I.S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 205.Tang X.J., Sun X.Y., Huang K.M., Zhang L., Yang Z.S., Zou D.D., Wang B., Warnock G.L., Dai L.J., Luo J. Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget. 2015;6:44179–44190. doi: 10.18632/oncotarget.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fu C., Zhou L., Mi Q.S., Jiang A. DC-based vaccines for cancer immunotherapy. Vaccines. 2020;8:706. doi: 10.3390/vaccines8040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wahlgren J., De L.K.T., Brisslert M., Vaziri Sani F., Telemo E., Sunnerhagen P., Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhu L., Sun H.T., Wang S., Huang S.L., Zheng Y., Wang C.Q., Hu B.Y., Qin W., Zou T.T., Fu Y., Shen X.T. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020;13:152. doi: 10.1186/s13045-020-00987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Colao I.L., Corteling R., Bracewell D., Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol. Med. 2018;24:242–256. doi: 10.1016/j.molmed.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 214.Reid G., Kao S.C., Pavlakis N., Brahmbhatt H., MacDiarmid J., Clarke S., Boyer M., van Zandwijk N. Clinical development of TargomiRs, a miRNA mimic-based treatment for patients with recurrent thoracic cancer. Epigenomics. 2016;8:1079–1085. doi: 10.2217/epi-2016-0035. [DOI] [PubMed] [Google Scholar]
- 215.Trang P., Wiggins J.F., Daige C.L., Cho C., Omotola M., Brown D., Weidhaas J.B., Bader A.G., Slack F.J. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lima J.F., Cerqueira L., Figueiredo C., Oliveira C., Azevedo N.F. Anti-miRNA oligonucleotides: a comprehensive guide for design. RNA Biol. 2018;15:338–352. doi: 10.1080/15476286.2018.1445959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ebert M.S., Sharp P.A. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Aquino-Jarquin G. Emerging role of CRISPR/Cas9 technology for MicroRNAs editing in cancer research. Cancer Res. 2017;77:6812–6817. doi: 10.1158/0008-5472.CAN-17-2142. [DOI] [PubMed] [Google Scholar]
- 219.Yang J., Meng X., Pan J., Jiang N., Zhou C., Wu Z., Gong Z. CRISPR/Cas9-mediated noncoding RNA editing in human cancers. RNA Biol. 2018;15:35–43. doi: 10.1080/15476286.2017.1391443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Patutina O.A., Bichenkova E.V., Miroshnichenko S.K., Mironova N.L., Trivoluzzi L.T., Burusco K.K., Bryce R.A., Vlassov V.V., Zenkova M.A. miRNases: novel peptide-oligonucleotide bioconjugates that silence miR-21 in lymphosarcoma cells. Biomaterials. 2017;122:163–178. doi: 10.1016/j.biomaterials.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 221.Jackson C.M., Choi J., Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat. Immunol. 2019;20:1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 222.Liu J., Fan L., Yu H., Zhang J., He Y., Feng D., Wang F., Li X., Liu Q., Li Y., et al. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology. 2019;70:241–258. doi: 10.1002/hep.30607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ning S., Liu H., Gao B., Wei W., Yang A., Li J., Zhang L. miR-155, miR-96 and miR-99a as potential diagnostic and prognostic tools for the clinical management of hepatocellular carcinoma. Oncol. Lett. 2019;18:3381–3387. doi: 10.3892/ol.2019.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Haderk F., Schulz R., Iskar M., Cid L.L., Worst T., Willmund K.V., Schulz A., Warnken U., Seiler J., Benner A., et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci. Immunol. 2017;2:eaah5509. doi: 10.1126/sciimmunol.aah5509. [DOI] [PubMed] [Google Scholar]
- 225.Xia Y., Rao L., Yao H., Wang Z., Ning P., Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 2020;32:e2002054. doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 226.Rao L., Zhao S.K., Wen C., Tian R., Lin L., Cai B., Sun Y., Kang F., Yang Z., He L., et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv. Mater. 2020;32:e2004853. doi: 10.1002/adma.202004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shen S., Zhang Y., Chen K.G., Luo Y.L., Wang J. Cationic polymeric nanoparticle delivering CCR2 siRNA to inflammatory monocytes for tumor microenvironment modification and cancer therapy. Mol. Pharm. 2018;15:3642–3653. doi: 10.1021/acs.molpharmaceut.7b00997. [DOI] [PubMed] [Google Scholar]
- 229.Qian Y., Qiao S., Dai Y., Xu G., Dai B., Lu L., Yu X., Luo Q., Zhang Z. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11:9536–9549. doi: 10.1021/acsnano.7b05465. [DOI] [PubMed] [Google Scholar]
- 230.Parayath N.N., Parikh A., Amiji M.M. Repolarization of tumor-associated macrophages in a genetically engineered nonsmall cell lung cancer model by intraperitoneal administration of hyaluronic acid-based nanoparticles encapsulating MicroRNA-125b. Nano Lett. 2018;18:3571–3579. doi: 10.1021/acs.nanolett.8b00689. [DOI] [PubMed] [Google Scholar]
- 231.Talekar M., Trivedi M., Shah P., Ouyang Q., Oka A., Gandham S., Amiji M.M. Combination wt-p53 and MicroRNA-125b transfection in a genetically engineered lung cancer model using dual CD44/EGFR-targeting nanoparticles. Mol. Ther. 2016;24:759–769. doi: 10.1038/mt.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang L., Hu Y.Y., Zhao J.L., Huang F., Liang S.Q., Dong L., Chen Y., Yu H.C., Bai J., Yang J.M., et al. Targeted delivery of miR-99b reprograms tumor-associated macrophage phenotype leading to tumor regression. J. Immunother. Cancer. 2020;8:e000517. doi: 10.1136/jitc-2019-000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kulkarni A., Chandrasekar V., Natarajan S.K., Ramesh A., Pandey P., Nirgud J., Bhatnagar H., Ashok D., Ajay A.K., Sengupta S. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat. Biomed. Eng. 2018;2:589–599. doi: 10.1038/s41551-018-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Majeti R., Chao M.P., Alizadeh A.A., Pang W.W., Jaiswal S., Gibbs K.D., Jr., van Rooijen N., Weissman I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang Y., Xu Z., Guo S., Zhang L., Sharma A., Robertson G.P., Huang L. Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Mol. Ther. 2013;21:1919–1929. doi: 10.1038/mt.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wu J., Li Z., Yang Z., Guo L., Zhang Y., Deng H., Wang C., Feng M. A glutamine-rich carrier efficiently delivers anti-CD47 siRNA driven by a “glutamine trap” to inhibit lung cancer cell growth. Mol. Pharm. 2018;15:3032–3045. doi: 10.1021/acs.molpharmaceut.8b00076. [DOI] [PubMed] [Google Scholar]
- 237.Voets E., Paradé M., Lutje Hulsik D., Spijkers S., Janssen W., Rens J., Reinieren-Beeren I., van den Tillaart G., van Duijnhoven S., Driessen L., et al. Functional characterization of the selective pan-allele anti-SIRPα antibody ADU-1805 that blocks the SIRPα-CD47 innate immune checkpoint. J. Immunother. Cancer. 2019;7:340. doi: 10.1186/s40425-019-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhang M., Hutter G., Kahn S.A., Azad T.D., Gholamin S., Xu C.Y., Liu J., Achrol A.S., Richard C., Sommerkamp P., et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS One. 2016;11:e0153550. doi: 10.1371/journal.pone.0153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen Q., Wang C., Zhang X., Chen G., Hu Q., Li H., Wang J., Wen D., Zhang Y., Lu Y., et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019;14:89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]