Abstract
Meditation can exert a profound impact on our mental life, with proficient practitioners often reporting an experience free of boundaries between a separate self and the environment, suggesting an explicit experience of “nondual awareness.” What are the neural correlates of such experiences and how do they relate to the idea of nondual awareness itself? In order to unravel the effects that meditation has on the brain’s spatial topography, we review functional magnetic resonance imaging brain findings from studies specific to an array of meditation types and meditator experience levels. We also review findings from studies that directly probe the interaction between meditation and the experience of the self. The main results are (i) decreased posterior default mode network (DMN) activity, (ii) increased central executive network (CEN) activity, (iii) decreased connectivity within posterior DMN as well as between posterior and anterior DMN, (iv) increased connectivity within the anterior DMN and CEN, and (v) significantly impacted connectivity between the DMN and CEN (likely a nonlinear phenomenon). Together, these suggest a profound organizational shift of the brain’s spatial topography in advanced meditators—we therefore propose a topographic reorganization model of meditation (TRoM). One core component of the TRoM is that the topographic reorganization of DMN and CEN is related to a decrease in the mental-self-processing along with a synchronization with the more nondual layers of self-processing, notably interoceptive and exteroceptive-self-processing. This reorganization of the functionality of both brain and self-processing can result in the explicit experience of nondual awareness. In conclusion, this review provides insight into the profound neural effects of advanced meditation and proposes a result-driven unifying model (TRoM) aimed at identifying the inextricably tied objective (neural) and subjective (experiential) effects of meditation.
Keywords: meditation, self, duality, nonduality, nonduality, DMN, CEN, alignment, neurophenomenological
Introduction
Nondual awareness and the self
What is the experience of one’s seemingly real boundaries of a separate self dissolving into a state of unity with the world, that is, a lack of separation between self and other? One term that aids to describe this phenomenon is “nondual awareness” (Josipovic 2014, 2016, 2019, 2021; Berman and Stevens 2015). A description of nondual awareness has been proposed as “a background awareness that precedes conceptualization and intention and that can contextualize various perceptual, affective, or cognitive contents without fragmenting the field of experience into habitual dualities” (Josipovic 2013). Developing an understanding of the neuro-phenomenal mechanisms (Northoff and Zilio 2022) mediating the explicit experience of nondual awareness (Josipovic 2021) is the goal of this review.
Given that nondual awareness entails a radical change in the experience of the self with respect to others and the environment, we postulate that the self is not homogenous, rather it includes various layers, and that nondual awareness is signified by a radical shift in the relationship among them, both neuronally and experientially. To address this, we consider Qin et al.’s (2020) neural model, which subdivides self-processing into three intimately connected and spatially nested levels with different topographic extensions in the brain: interoceptive-processing, exteroceptive processing, and mental-self-processing.
Box 1.
TRoM’s relationship with the temporo-spatial theory of consciousness (TTC)
| The TTC conceives consciousness as a basic temporo-spatial phenomenon: temporal dynamic and spatial topography are supposed to be shared by both neural and mental/phenomenal levels—the nondual topography on the brain’s neural level surfaces then as nondual experience on the phenomenal or experiential level. The TRoM thus lends further support to the TTC from the viewpoint of an altered state of consciousness, namely proficient meditation. Inextricably tied to the scale-free and nested spatiotemporal structure of the brain is the manner in which the brain can align its spatiotemporal activity to the environment (Northoff and Zilio 2022), something which we posit is fundamental to the explicit experience of nondual awareness. In order to align with the environment, there must both be a sufficient spatiotemporal repertoire (i.e. a sufficient range of functioning intrinsic neural timescales), and these timescales must be functionally aligned (something that is likely impacted during mind-wandering and definitely impacted in states of sedation and anesthesia). By promoting subjects to become increasingly aware of thoughts, emotions, bodily sensations, and environmental activity in a passive manner (depending on the specific meditation type) without yet explicit differentiation between inside/self and outside/other, meditation techniques support the alignment between brain, body, and environment—this allows for the direct experience or perception that all content arises within the shared space of nondual awareness. Nestedness Through increased meditation proficiency, we experience ourself no longer as dual to nonself but as more nested and integrated and thus connected with body and environment, e.g., temporo-spatial nestedness (see temporo-spatial alignment and nestedness as two key mechanisms in the TTC) (Northoff and Huang 2017, Northoff and Zilio 2022). Alignment This alignment (transition from implicit to explicit) of the scale-free spatiotemporally nested layers of self (nondual awareness) is posited as the link between the self and nondual awareness. We must be clear that the self in this case is not merely restricted to cognitive phenomenon and is more accurately viewed as the connection between brain, body, and environment. An effective analogy to nondual awareness proposed by Josipovic (2021) is that of a mirror: which is necessary for all content to be seen, though it is never affected by the contents themselves. |
Box 3.
Relationship of TRoM to predictive coding, active inference, and psychedelics
| How does the TRoM relate to predictive coding? Laukkonen and Slagter (2021) state that during regular thinking (i.e., mind-wandering) one continuously yields top-down predictions (Pezzulo et al. 2021). By increasing the depth of meditative states, the frequency of predictions decreases such that one experiences the incoming inputs and events without prior appraisal. This leads to a recognition of nondual awareness, in which the predictive hierarchy is flattened and “the awareness inherent in experience becomes the foreground” (Laukkonen and Slagter 2021). In Laukkonen and Slagter’s model, they propose that FAM reduces mental or cognitive self-processing and brings the meditator into a more direct and embodied mode of being. Then, going further into the present-moment experience, OMM brings the meditator into a state of nonjudgmental open and bare experience, in a “sensing without appraisal” state (Laukkonen and Slagter 2021). They do not mention NDA meditation itself, which is free of intentional effort, directly aimed at identifying a reflexive awareness, and can be seen as primarily context-oriented, with nondual awareness as the primary context of experience as opposed to attending to the specifics of experience (Josipovic 2012). This discovery of the reflexive and choiceless awareness would be the form of awareness that is freest from predictive modeling since it is most simply the discovery of the space in which the predictions occur, like how a mirror cannot predict what it will reflect, neither can nondual awareness. Empirically, meditation has repeatedly shown evidence of decreased predictive coding. Evidence of the described gradual inhibition of Bayesian predictive modeling has been found in some studies. Valentine and Sweet (1999) found that OMM meditators were less biased by past temporal regularities than FAM meditators. These results may suggest that the OMM meditators develop less strong temporal expectations that biased subsequent perception, potentially because open awareness is further along the progression of awareness. More evidence of the inhibition of predictive modeling that occurs through meditation can be found in Hanley and Garland (2019), in which the authors show that mindfulness training can reduce Pavlovian conditioning. This is very well compatible with our observation of the decrease in the mental self and its underlying regions, DMN. The mental self may no longer yield as strong top-down predictions for especially the lower layer of self, the intero-exteroceptive self. The latter is thus less shaped by the mental self and its duality of self-nonself. Instead, the intero-exterocetpive self may more strongly shaped by bottom-up processing from the relationship of self and environment rather than by top—this allows to focus more on present-moment experience and “sensing without appraisal.” Additionally, the more spontaneous engagement with the world in meditation is also understandable through the perspective of criticality. Systems at a high level of criticality are at a transition point between different states and show scale-free similarity, long-range correlations and critical slowing (Chialvo 2014; Carhart-Harris and Friston 2019). Since meditation experience has been shown to significantly increase the long-range temporal correlations, a measure of criticality (Irrmischer et al. 2018), it is quite probable that meditation, just like psychedelics, reduces the impact of previously formed generative models, allowing for a more spontaneous and free-flowing interaction with the world. The increase in long-range correlations means that more distant regions can be linked to each other and thus synchronize in a positive way to a higher degree—this is not only in tune with the reported findings but also with the reduced degree of the brain’s dual topographic organization including its replacement by nondual topography in proficient meditation. Finally, psychedelics have shown similar neural effects as meditation experience: decreased DMN activity (Muthukumaraswamy et al. 2013; Tagliazucchi et al. 2014; Carhart-Harris et al. 2016), decreased anterior-posterior DMN connectivity (Carhart-Harris et al. 2012a), increased connectivity between the DMN and task-positive networks (Carhart-Harris et al. 2012b), and increased global neural- connectivity (Carhart-Harris et al. 2016; Tagliazucchi et al. 2016). Together with analogous experience of nonduality (Carhart-Harris and Friston 2019), these data provide further evidence of profound topographic reorganization in meditation leading to the experience of nondual awareness (Northoff et al. 2020b). Furthermore, a model for a phenomenon often experienced during psychedelic experiences, which is related to the reduction of mental-self-processing, referred to as ego-loss, has been termed: relaxed beliefs under psychedelics (REBUS) (Carhart-Harris and Friston 2019). In this state, the brain is said to relax the temporally bound top-down processing in exchange for an increased sensitivity to bottom-up processing, hence allowing for a new engagement with the world (Carhart-Harris and Friston 2019; Deane 2020). This again is well compatible with the TRoM. The decrease in DMN–CEN dichotomy and the decrease in mental self (and its underlying regions) strongly suggest decreased top-down predictions and, as a consequence, more relaxed beliefs. Hence, the TRoM and especially the topographic shift from mental to intero-exteroceptive self are well compatible with the REBUS model in psychedelics. Future studies are warranted, though, to better flesh out similarities and differences of meditation and psychedelics. |
What is meditation and how does it relate to nondual awareness?
The term meditation has been referred to as inadequate, considering the extremely wide range of practices that it refers to (Lutz et al. 2007). Nevertheless, there is a common purpose between all practices: to decrease psychological suffering through regulatory (e.g. attentional, emotional, proprioceptive, and interoceptive) processes. Indeed, according to Buddhist traditions, there are a set of correctable defects that are the root cause of human suffering (Gethin 1998; Lutz et al. 2007). Generally speaking, a meditation practice can begin with an emphasis on focusing the mind on an object (such as the breath, a mantra, or an image), followed by an emphasis on the awareness of subjectivity itself, which then at the highest level of practice can progress to a disidentification from any subject or object and thus a realization of the fundamentally changeless and nondual (reflexive) awareness in which all changing contents of experience take place (Lutz et al. 2007).
The array of different meditation types has already been characterized by unique neural correlates, both structurally and functionally (Fox et al. 2016; Laukkonen and Slagter 2021). Despite the neural and psychological differences among the different meditation techniques, there are commonalities between meditation types that are related to the reduction of suffering: (i) monitoring of attention and some aspect of the present moment (Lutz et al. 2008; Lippelt et al. 2014; Delorme and Brandmeyer 2019), (ii) associated with trait increases in mindfulness (Kiken et al. 2015; Lemay et al. 2019; Tang and Tang 2020; Sousa et al. 2021) (i.e. awareness of both mental and environmental content), and (iii) commonly involve decreases in perceived stress (Winzelberg and Luskin 1999; Mohan et al. 2011; Chambers et al. 2016; Lemay et al. 2019). Or put most succinctly, “all meditations in one way or the other try to get you to live in the present” (Osho 2012).
Various brain imaging studies of meditation report changes in attention, memory, and emotional-related regions and networks of the brain (Brefczynski-Lewis et al. 2007; Brewer et al. 2011; Farb et al. 2012; Hasenkamp et al. 2012; Fingelkurts et al. 2015; Tang et al. 2015a; Fox et al. 2016; Travis and Parim 2017), but how this relates to the explicit experience of nondual awareness is a question left unanswered? The capacity to explicitly reach this core state of unity along with other preliminary meditative states is likely a matter of meditation proficiency, which has been correlated with the amount of meditative experience that one has cumulated (van den Hurk et al. 2010; Slagter et al. 2011; Lumma et al. 2015; Tanaka et al. 2015), rather than being strictly tied to the type of meditation that is practiced.
We must be clear that the unique functional and structural effects of distinct meditation types are evident (Fox et al. 2014, 2016; Valk et al. 2017), and we are thus operating under the assumption that all meditation types can lead to or at least contribute in the direction toward an experience of nondual awareness. With repeated evidence of highly proficient long-term meditators from different meditation backgrounds showing the capacity to transcend the self/other duality (Josipovic et al. 2012; Dor-Ziderman et al. 2013; Josipovic 2014, 2019; Ataria et al. 2015; Berman and Stevens 2015; Shiah 2016), we see this as a fair assumption. So, meditation’s orientation toward a present-moment awareness seems to diminish the magnitude of self-referential thinking, hence decreasing the perceived self-other duality (Farb et al. 2007; Lutz et al. 2015; Hasenkamp 2018; Millière et al. 2018; Bauer et al. 2019).
Topographical reorganization model of meditation
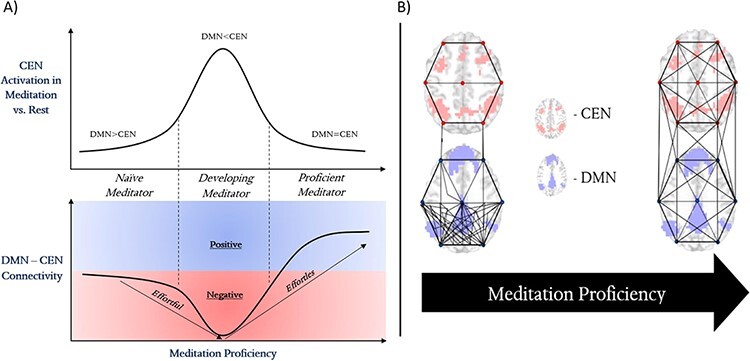
Given the various spatial-topographic and temporal dynamic changes in the brain’s neural activity associated with meditation, we suppose that they translate into more or less corresponding spatiotemporal changes in our experience on the mental level. In other terms, the brain’s topographic reorganization between higher-order self-related regions [default mode network (DMN)] and lower-order nonself-related regions [central executive network (CEN)] translates into a more or less corresponding nondual reorganization of our experience—nonduality is shared by neural and mental levels as their “common currency” (Northoff and Lamme 2020a; Northoff et al. 2020b). We therefore postulate what we describe as “topographic reorganization model of meditation” (TRoM). The TRoM has commonalities to some of the most prominent neuro-phenomenological models of meditation, though it is unique in its own right (see Box 2 for more).
Box 2.
Relationship of TRoM to other neuro-phenomenological models of meditation
|
Fronto-parietal central precuneus network (Josipovic 2021): The fronto-parietal precuneus network model is based on findings that suggest that the central precuneus is capable of functionally connecting with both intrinsically oriented (DMN) and extrinsically oriented (CEN) networks and thus “corresponds to a major function of nondual awareness by increasing the integration of intrinsic self-related and extrinsic environment-related aspects of experience” (Josipovic 2021, see also Josipovic et al. 2012; Josipovic 2014). This hypothesis is well in accordance with the TRoM as stated here. Both emphasize the reorganization of the relationship between the CEN and the DMN. The TRoM extends beyond the fronto-parietal hypothesis by emphasizing the longitudinal transformation between the DMN and CEN and how this relates to meditation proficiency and the layers of self-processing (mental, exteroceptive, and interoceptive). That renders possible to directly link topographic reorganization on the neural level of the brain with the topographic reorganization of the three layers of self, that is, the shift from mental dominant self-processing to an intero-exteroceptive dominant self-processing. The TRoM therefore allows to specify and extend the mechanisms mediating the shift from dual to nondual awareness in our consciousness, not only with measures of connectivity, but more dynamic measures of nestedness and alignment (see Northoff and Zilio 2022). Adaptive coding net/adaptive workspace (Raffone and Srinivasan 2009): The adaptive coding net model postulates that the awareness that meditation (primarily OMM forms) gives access to can be represented by an adaptive coding net (neurons especially located in the anterior PFC), which simultaneously links to multiple brain maps in parallel. This likely yields a large repertoire of potential network patterns available at any time. They further suggest that advanced meditators can elicit increasingly complex firing patterns, thus allowing the brain to more accurately reflect the changing patterns of body and environment. The TRoM is well in accordance with this hypothesis. The relative increase in the intero-exteroceptive self and its underlying neural correlates makes possible an enhanced capacity to stochastically match with the intero-exteroceptive input patterns from body and environment—the coding of these inputs may thus be more adaptive and less disturbed by the top-down predictions of the mental self. Future studies are warranted to add the temporal and thus dynamic dimension like the intrinsic neural timescales (Wolff et al. 2022, Golesorkhi et al. 2021a,b): we assume a broader dynamic range (Irrmischer et al. 2018) to characterize meditation, which may increase the capacity for more adaptive (en)coding of bodily and environmental inputs. Moreover, topographic reorganization goes along with more spatially extended global synchrony as, for instance, the negative relationship of DMN and CEN is replaced by more positive synchrony. Whether such increased synchrony extends to the whole brain thus amounting to a true global synchrony remains to be investigated in the future though. The data discussed here do indeed suggest less difference and more synchrony between unimodal and transmodal regions’ functional connectivity—given that functional connectivity is phase- and thus synchrony-based (Huang et al. 2015), this would be well in line with the assumption of global synchrony. |
|
Minimal phenomenal experience (Metzinger 2020): The model of minimal phenomenal experience (MPE) as posited by Metzinger (2020) states that MPE is an inner representation of tonic alertness, which can co-exist with many other forms of conscious contents. It is further said it is a nonegoic form of self-modeling, which is mostly unnoticed since it functions as the transparent model of an abstract space in which other contents unfold over time. Predictive Bayesian modeling, within the brain, of the functional property of tonic alertness is seen as a primary dimension of MPE. |
| The TRoM agrees and disagrees with model of MPE. It agrees that there is less egoic self-modeling as this corresponds to the decrease in the mental self. At the same time, nonegoic self-modeling increases as manifested in the relative increase of the intero-exteroceptive self-processing. The TRoM thus specifies and further supports the shift from egoic to nonegoic self-modeling as well as the tacit assumption that meditation still involves a self or self-modeling albeit a different sense of self than the one most commonly associated with normal waking state in which nondual awareness is implicit. The TRoM disagrees with a more tacit assumption of the MPE, namely its implicit presupposition that experience is limited and constrained to the head and, at best, the body. This is clearly different in TRoM. Rather than being neuro-cognitive (or neuro-phenomenal), the TRoM is an explicit neuro-ecological model of meditation: it shifts the explicit focus of our experience toward our alignment to the bodily and especially environmental context. Such temporo-spatial alignment is a key component of the TRoM, which distinguishes it from the MPE and, as we would see, also from all other meditation approaches as well as from most consciousness theories (except the TTC; Box 1). Only by taking into view this neuro-ecological component, the nondual nature of experience beyond the self-environment dichotomy can be understood. Nondual awareness shows us in a paradigmatic way that, more generally, consciousness is strongly neuro-ecologically based albeit it commonly remains more tacit or implicit in our experience. |
Our main aim is therefore to review recent studies probing the effect of meditation on the DMN and CEN as well as their relationship with each other. Given that the self and other/nonself are not experienced dichotomously within nondual awareness, we hypothesize that DMN activity is reduced and that the DMN–CEN relationship shifts from a negative to a positive connectivity (already proposed by Josipovic et al. 2012; Josipovic 2014), most likely during meditation itself but quite possibly sustained during the resting state (RS) of highly proficient meditators (see a single case report by Ataria et al. 2015). For that purpose, we review two main lines of imaging [functional magnetic resonance imaging (fMRI)] studies: those specific to the neural effects of meditation and those probing the interaction of meditation and self.
Methods
Search terms
The review focused on meditation-related changes in activation and connectivity from fMRI studies, including the self × meditation interaction. See Fig. 3 for a visual depiction of the search terms used in this review to gather the studies that were implemented.
Figure 3.
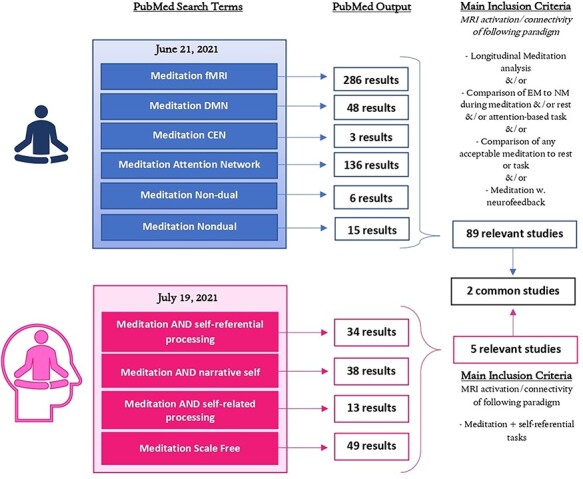
Flow chart of screening process for reviewed studies. Upper blue half represents the screening that was specific to meditation only, and the bottom pink half represents the screening that was specific to the interaction between meditation and the self
Meditation type categorization
It shall be mentioned that there is a large variety of different meditation practices that have been probed in fMRI research. In a recent meta-analysis, Fox et al. (2016) narrowed meditation into four general categories: focused attention meditation (FAM), mantra recitation meditation (MRM), open monitoring meditation (OMM), and loving-kindness and compassion meditation (LKM). In addition, Josipovic et al. (2012) illustrated that a unique form of meditation that has a very precise orientation toward experiencing nondual awareness (nondual awareness meditation) shows a distinct neural signature when compared to FAM and OMM, that is why it has been added here as a distinct form of meditation.
The reason for which this review is very inclusive toward specific meditation practices is based on the viewpoint that the nondual state of awareness is obtainable through all forms of practice (Reddy and Roy 2018, 2019) and that it is consistent across all different meditation types as pointed out especially by Rodriguez-Larios et al. (2020) (see also Berman and Stevens 2015)(Fig. 2). This will serve to create a general framework around meditation and the self; yet, it is understood that this is a simplification that doesn’t fully take all individual meditation types specific to “neural fingerprints” into account. Further investigation into this phenomenon will be necessary to develop a more pedantic framework and see how each type of meditation is directly associated with progression toward the recognition of nondual awareness.
Figure 2.
Practice of any form of meditation practice is seen as contributing toward the development of the capacity to explicitly experience nondual awareness. Meditation proficiency and experience are understood as correlated, although, this relationship is definitely not as straightforward as displayed since different meditation practitioners with the same amount of experience do not necessarily have the same subjective states during rest and meditation
Requirements for inclusion
Meditation section
Inclusion criteria
Our main focus was on the spatial features (mainly fMRI—including activation and connectivity) of meditation. Any study that explicitly studied activation or connectivity measures from fMRI in a relevant comparison or correlational manner was included. Studies that reported on changes in these features either between varying levels of meditation experience (whether it be between different groups or between one group both before and after meditation training), between meditation and RS or a task, or a correlational analysis between meditation type or meditation experience and spatial features of interest were included. Group comparisons of meditation experience (both within- and between-group) were also included for measurements obtained during both RS and meditation.
Borderline inclusion
Studies that analyzed subjects with some form of mild-to-moderate psychological distress (e.g. social anxiety disorder, childhood neglect, and posttraumatic stress disorder) were included in this review, although they were not the primary focus of our attention.
Studies that incorporated some form of neurofeedback during meditation were also included because of their direct capacity to correlate precise regional locations of neural activity with phenomenological experience.
Studies that focused on meditation and its effect on task-based neural activity were only included if the task was attentionally based, as that can provide insight into how meditation experience leads to increased capacity to modulate where attention is placed.
Exclusion criteria
We did not include strictly morphological studies or studies in which their analysis focused on seed-based connectivity with regions not included in our primary focus of the DMN and CEN, event-related potentials, pain perception/modulation, emotional reaction to stimuli, emotional regulation, hypnosis, binaural beats, drug-induced activity, praying, and/or religious chanting (chanting in general was excluded).
Aside from these forms of analysis, the linear activation and connectivity analysis from electroencephalogram (EEG)/Magnetoencephalography (MEG) have been excluded from the main analysis of this review due to their lack of standardization affiliated with the signal processing techniques, which is likely tied to the results of extremely heterogenous nature (Deolindo et al. 2020).
The details of the different studies along with all of their relevant findings can be found in the Supplementary Material. Here, in the main text, we summarize the main and most consistent findings in order to streamline the significant complexity and variety of results and approaches in imaging studies on meditation. We did, although, integrate the meditation type and meditation experience levels of the study subject groups to decipher if these metrics are primary components, which determine or impact the results obtained.
Meditation and self-section
In this section, we focused on studies that included in their experimental paradigm self-processing tasks. We did that in order to see how the different layers of self-processing change in proficient vs naïve meditators.
Inclusion criteria
We looked at studies about self and meditation, which included self-processing tasks in their experimental paradigm, to infer how self-processing changes by gaining experience in meditation.
Also in this section, we focused on the spatial domain (MRI and fMRI—including activation and connectivity). A study that analyzed subjects with a social anxiety disorder was included in this section of the review.
Exclusion criteria
We did not include studies that did not have any kind of self-referential processing task in their experimental paradigm and that focused on self-processing only in the discussion, as an interpretation. We did not include studies that focused on dynamic features (both fMRI and EEG/MEG). Like in the meditation section, here we excluded strictly morphological studies and event-related potentials.
Results
Network activity and connectivity
Regions from within the DMN and CEN [and also the salience network (SN)] proved to show the most promising and relatively replicable results throughout the range of reported findings; hence, their results are selected and cumulated for analysis. To disentangle the various measures used in different studies, we distinguish three main measures: network activation, network intra-connectivity, and network interconnectivity. These parameters are valuable when comparing different meditation techniques to each other as well as to the RS, along with comparing different subject groups with markedly different experience levels. Here, the primary regions that are focused are the posterior cingulate cortex (PCC), precuneus (PCu), and medial prefrontal cortex (mPFC) for the DMN; dorsal lateral prefrontal cortex (dlPFC) for the CEN; and the anterior cingulate cortex (ACC) and insula for the SN.
Core network activity findings
Decreased posterior DMN activation
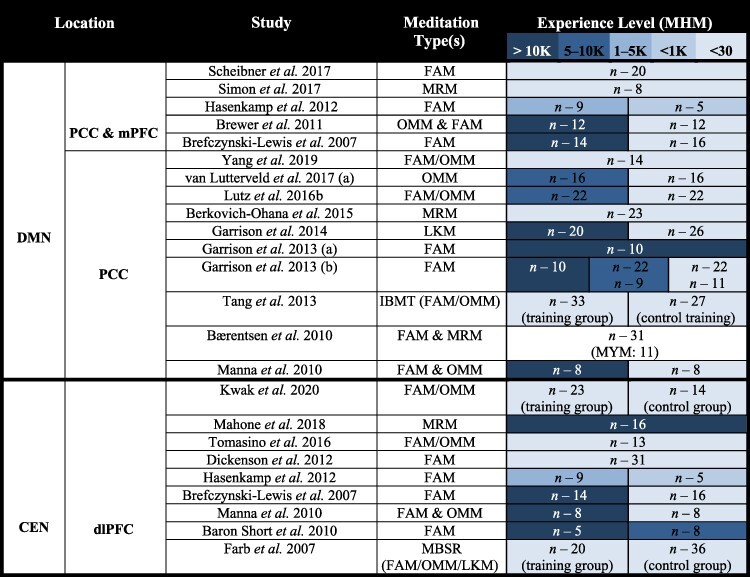
The DMN, and mainly the posterior DMN, consistently deactivates during a range of meditation practices (Table 1 and Fig. 4). Several forms of meditation have directly exhibited a greater DMN deactivation when compared to self-related focus (Farb et al. 2012), self-related tasks (Garrison et al. 2015) as well as other active tasks including a visual task (Ives-Deliperi et al. 2011) and finger tapping (Simon et al. 2017). This reduced DMN activity when comparing meditation to an active task was only seen for experienced meditators but not in naïve controls (Garrison et al. 2015). This signifies the progressive decline of DMN activity during meditation as being associated with meditation experience. Likewise, meditators show a significantly larger decrease of DMN activity than controls, when comparing meditation to the RS (Brewer et al. 2011; Garrison et al. 2015). As evidenced by, deactivation of the PCC was the most replicated finding when it comes to meditation, this shows the robustness of this finding.
Table 1.
Studies that repeatably find decreased DMN activation because of meditation
|
Supporting Information: Text within sections allocated to MHM is to specify either the differentiating factor between groups of similar experience level (e.g. meditation training vs relaxation training) or the level of meditation when mean hours of experience were not provided within the published paper.
Abbreviations: dorsolateral prefrontal cortex (dlPFC); integrative body-mind training (IBMT); mean hours of meditation (MHM); mean years of meditation (MYM); medial prefrontal cortex (mPFC); mindfulness-based stress reduction (MBSR).
Figure 4.
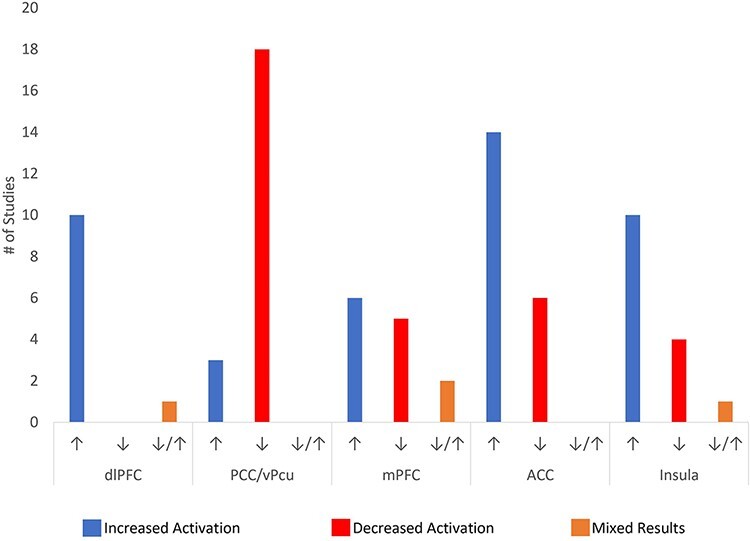
Tally of activations/deactivations of each primary ROI from reviewed articles
See the Supplementary Material for all DMN activity-related findings.
Increased CEN activation
The CEN (primarily the dlPFC) often shows an increase in activation during meditation, with more advanced meditators showing the capacity to keep it more consistently activated (Short et al. 2010). This increase in activation is especially occurrent during focused awareness meditation, yet it is also quite evident in other more open forms of meditation (see Table 1). Yet the trend associated with CEN activation may be nonlinear with respect to meditation proficiency. Significantly, Brefczynski-Lewis et al. (2007) found that the group of meditators with the middle most level of meditation experience elicited the greater dlPFC activity during meditation compared to both naïve meditators as well as the most experienced group of meditators. This is significant considering that the most experienced group of meditators had an average of 44 000 h of experience, while the intermediate meditation group had approximately 19 000 h of experience. Most other studies do not include participants with experience levels greater than 19 000 h (likely because it is a rarity to find subjects with this magnitude of experience), meaning that this study was able to effectively analyze a level of meditation experience commonly overlooked.
This phenomenon alludes to an inverse U-shape trend of CEN activation associated with increasing meditation proficiency, which is in line with previous commentaries on how meditation follows a trajectory from being effortful to effortless (Tang et al. 2015b—see the Discussion for more on this point). That is also in line with the proposition of an evolved default state where open forms of meditation are neuronally very similar to the RS (Manna et al. 2010) and where large-scale networks are increasingly positively connected (Brewer et al. 2011) (see below for network connectivity). It should be noted that this is not yet conclusive evidence, and more research is required to assess if this nonlinear neural phenomenon is robust and how it is related to the subjective transformation of increased meditation proficiency.
See the Supplementary Material for all CEN activity-related findings.
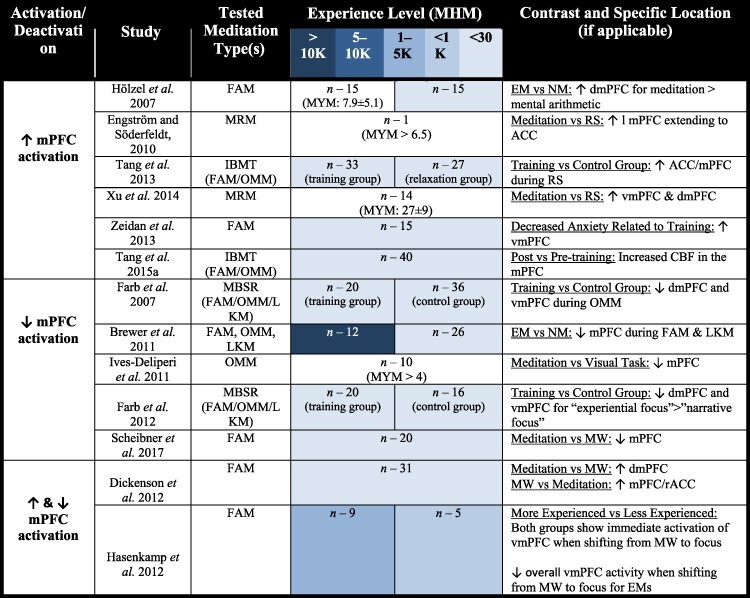
Split Findings for mPFC Activation/deactivation
Meditation’s impact on mPFC activity provides the least consistent finding out of all Region of interests (ROIs) that were assessed in our review (Table 2). We attempted to find any trends regarding mPFC activity level, precise location within the mPFC, meditation type, and experience level, but to no avail. Interestingly, meditation-related increased mPFC activity was found when contrasting highly experienced meditators to naïve meditators, testing longitudinal effects of meditation training, and also when directly comparing neural activity during meditation to rest. Meditation-related decreases of mPFC activation, although, were not found when directly assessing the longitudinal impact of meditation, primarily in the RS. Whether this suggests increased meditation proficiency is not associated with reduced RS mPFC activity is highly uncertain and worthy of investigation. Additionally, deactivations of the mPFC during meditation seemed increasingly relevant to MRM (Engström and Söderfeldt 2010; Xu et al. 2014). The only other study that found an increased activation of the mPFC during meditation utilized a contrast between FAM and mental arithmetic (Hölzel et al. 2007); this is not an ideal contrast for our purposes since mental arithmetic in itself requires focused and deliberate attention, unlike the RS. Meanwhile, mPFC deactivations during meditation were found during FAM (Brewer et al. 2011; Scheibner et al. 2017) and LKM (Brewer et al. 2011) when compared to the RS (Brewer et al. 2011) and mind-wandering (Scheibner et al. 2017). The relationship of meditation-related mPFC activation specificity is still a question that requires further analysis.
Table 2.
Studies that find altered activation in the anterior DMN (mPFC) as a result of meditation
|
Supporting Information: Text within sections allocated to MHM is to specify either the differentiating factor between groups of similar experience level (e.g. meditation training vs relaxation training) or the level of meditation when mean hours of experience were not provided within the published paper.
Abbreviations: anterior cingulate cortex (ACC); dorsomedial prefrontal cortex (dmPFC); experienced meditators (EM); integrative body–mind training (IBMT); mindfulness-based stress reduction (MBSR); medial prefrontal cortex (mPFC); mean hours of meditation (MHM); mean years of meditation (MYM); mind-wandering (MW); naïve meditators (NM); nondual awareness meditation (NDA); ventromedial prefrontal cortex (vmPFC).
Although the meditation-related mPFC activity changes are difficult to make sense of, there have been studies that found a nonlinear mPFC activity phenomenon associated with meditation. Specifically, Hasenkamp and Barsalou (2012) found that there was a nonlinear relationship between activity of the ventromedial prefrontal cortex (vmPFC) and the meditation experience level during a FAM paradigm (Fig. 5). The vmPFC was shown to activate when meditators transitioned from a state of mind-wandering back to the meditation object of focus (in this case the breath). Although, meditation practitioners with a mean of less than 1200 h of meditation showed prolonged vmPFC activation following this shift, whilst meditators with approximately double the meditation experience did not. In fact, the most experienced meditators had an overall negative vmPFC % signal change, where the magnitude of signal decrease was correlated with meditation experience.
Figure 5.
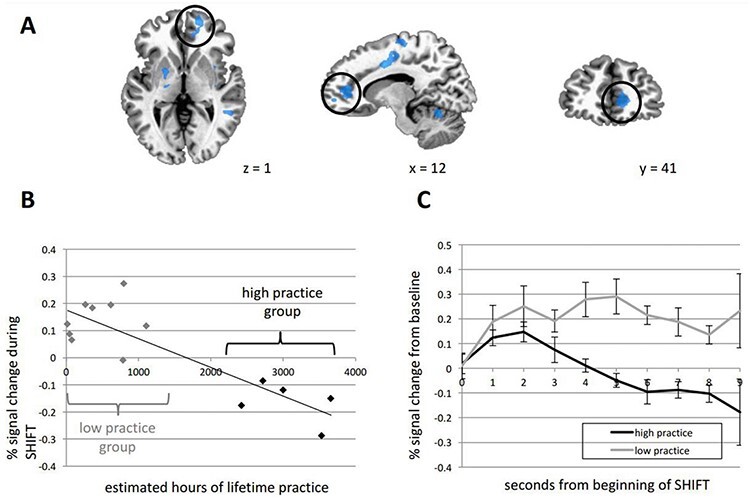
“Practice time effects. (A) Several clusters that were negatively correlated with practice time during the SHIFT phase. The ventromedial PFC cluster that was examined in B and C is circled. (B) Scatter plot of the relationship between practice time and fMRI signal in the ventromedial PFC cluster. Participants with high and low practice time are clearly segregated. (C) Time courses from the ventromedial PFC cluster were extracted, and Hemodynamic response functions (HRFs) were calculated from the onset of the SHIFT phase for each subject. Percent signal change (from mind-wandering, mean ± s.e.m.) over time is plotted for high (n = 5) and low (n = 9) practice participants. The BOLD response is significantly reduced in high practice compared to low practice participants across the modeled time series. *Main effect of group over time by repeated-measures Analysis of variance (ANOVA), p = 0.010.” All sourced directly from Hasenkamp and Barsalou (2012) with slight modification to the caption (changed “MW” to “mind-wandering”). SHIFT represents a shift from mind-wandering to focused attention
Additionally, Dickenson et al. (2012) showed that mind-wandering was associated with increased activity in a cluster sharing the mPFC and rACC, when contrasted with neuronal activity during meditation. Although, meditation, when contrasted with mind-wandering, was associated with increased dmPFC activity, likely an illustration of the multifaceted nature of the mPFC region of the brain.
We further assessed the mPFC-specific findings to see if there is any pattern of coactivation/deactivation with other ROIs (Table 3). Specifically, we were interested to see how the significant change in meditation-related mPFC was associated with the PCC, the CEN, and the SN.
Table 3.
Coactivation/deactivation findings associated with meditation related changes of mPFC activity
| mPFC finding | Coactivation/deactivation ROI | Tested meditation type(s) | Study and findings | |
|---|---|---|---|---|
| ↑ mPFC activation | PCC/vPCu | ↑ Activation | MRM | Xu et al. (2014): increased vmPFC, dmPFC, and PCC activity during nondirective MRM |
| ↓ Activation | IBMT (FAM/OMM) | Tang et al. (2013): following meditation training there was increased ACC/mPFC activity along with decreased PCC activity during RS | ||
| FAM | Zeidan et al. (2013): increased vmPFC along with decreased PCC correlate with decreased anxiety as a result of meditation practice | |||
| CEN | ↑ Activation | IBMT (FAM/OMM) | Tang et al. (2013): following meditation training there was increased ACC/mPFC along with increased IFG/vlPFC activity during RS | |
| MRM | Xu et al. (2014): increased vmPFC, dmPFC, and IPL activity during nondirective MRM | |||
| Insula | ↑ Activation | FAM | Zeidan et al. (2013): increased vmPFC along with increased anterior insula correlate with decreased anxiety as a result of meditation practice | |
| IBMT (FAM/OMM) | Tang et al. (2015a): increased CBF in mPFC and vACC following FAM/OMM training | |||
| ACC | ↑ Activation | FAM | Hölzel et al. (2007): increased dmPFC and rACC activity when comparing Ems to NMs for a meditation vs mental arithmetic contrast | |
| MRM | Engström and Söderfeldt (2010): increased mPFC extending to ACC activity during MRM compared to RS | |||
| FAM | Zeidan et al. (2013): increased vmPFC and ACC activity along with correlate with decreased anxiety as a result of meditation practice | |||
| IBMT (FAM/OMM) | Tang et al. (2015a): increased CBF in mPFC and insula following FAM/OMM training | |||
| ↓ mPFC activation | PCC | ↓ Activation | FAM, OMM, and LKM | Brewer et al. (2011): decreased mPFC and PCC activity during FAM and LKM |
| OMM | Ives-Deliperi et al. (2011): decreased mPFC and PCu activity for OMM vs a visual task | |||
| FAM | Scheibner et al. (2017): decreased mPFC and PCC for FAM vs mind-wandering | |||
| CEN | ↑ Activation | MBSR (FAM/OMM/LKM) | Farb et al. (2007): decreased dmPFC and vmPFC along with increased dPFC and inferolateral PFC activity for EMs vs NMs during OMM | |
| MBSR (FAM/OMM/LKM) | Farb et al. (2012): decreased dmPFC and vmPFC along with increased lPFC activity for EMs vs NMs during “experiential focus” compared to “narrative focus” | |||
| Insula | ↑ Activation | MBSR (FAM/OMM/LKM) | Farb et al. (2007): decreased dmPFC and vmPFC along with increased insula activity for EMs vs NMs during OMM | |
| OMM | Ives-Deliperi et al. (2011): decreased mPFC and insula activity for OMM vs a visual task | |||
| MBSR (FAM/OMM/LKM) | Farb et al. (2012): decreased dmPFC and vmPFC along with increased insula activity for EMs vs NMs during “experiential focus” compared to “narrative focus” | |||
| ACC | ↑ Activation | OMM | Ives-Deliperi et al. (2011): decreased mPFC and vACC activity for OMM vs a visual task | |
| ↑ & ↓ mPFC activation | PCC | ↑ Activation | FAM | Dickenson et al. (2012): increased mPFC/rACC, dmPFC, PCC, and PCu activity during mind-wandering compared to FAM |
| FAM | Hasenkamp et al. (2012): increased mPFC and PCC activity during mind-wandering | |||
| Insula | ↑ Activation | FAM | Dickenson et al. (2012): increased dmPFC and mid insula activity during FAM compared to mind-wandering | |
| ACC | ↑ Activation | FAM | Dickenson et al. (2012): increased dmPFC and dACC activity during FAM compared to mind-wandering | |
Abbreviations: anterior cingulate cortex (ACC); dorsomedial prefrontal cortex (dmPFC); experienced meditators (EM); integrative body–mind training (IBMT); mantra recitation meditation (MRM); mean hours of meditation (MHM); medial prefrontal cortex (mPFC); mind-wandering (MW); mindfulness-based stress reduction (MBSR); naïve meditators (NM); ventromedial prefrontal cortex (vmPFC).
Core network connectivity findings
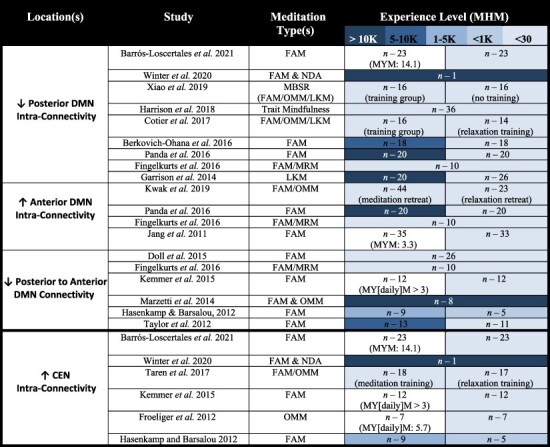
Decreased posterior DMN intra-connectivity, increased anterior DMN intra-connectivity, and decreased posterior-anterior DMN intra-connectivity
A highly replicated result is that of a decoupled intra-network DMN connectivity (Table 4). In this vein, meditation has been shown to exhibit a decrease in operational synchrony within the posterior DMN (Fingelkurts et al. 2015) as well as a reduced nodal connectivity following meditation training (Cotier et al. 2017). Although meditation experience is associated with an increased anterior DMN connectivity (Jang et al. 2011; Fingelkurts et al. 2015; Panda et al. 2016; van Lutterveld et al. 2017a; Kwak et al. 2019). Garrison et al. (2014) indeed found that naïve meditator PCC was increasingly connected to other cortical midline structures of the DMN during LKM, whilst more experienced meditators displayed a PCC with functional connectivity patterns expanded beyond the DMN, especially to the inferior frontal gyrus. Also, since the anterior DMN activation during self-appraisal is significantly larger for experienced as opposed to nonexperienced meditators (Lutz et al. 2016b), this region is likely more affiliated with observing mental phenomenon (Fingelkurts et al. 2015).
Table 4.
Dominantly repeated DMN & CEN intra-connectivity patterns associated with meditation
|
Supporting Information: Text within sections allocated to MHM is to specify either the differentiating factor between groups of similar experience level (e.g. meditation training vs relaxation training) or the level of meditation when mean hours of experience were not provided within the published paper.
Abbreviations: mindfulness-based stress reduction (MBSR); mean hours of meditation (MHM); mean years of meditation (MYM); nondual awareness meditation (NDA).
It should be stated that some outliers from these trends do exist in the literature. Avvenuti et al. (2020) found increased RS functional connectivity between the PCC, precuneus, and superior parietal lobule for a group that trained in transcendental meditation (a form of MRM) for 3 months. Additionally, Bauer et al. (2019) found that experienced meditators had increased functional connectivity between the PCC and the right angular gyrus (another region of the DMN). This being said, the dominant trends of the findings to date are represented in Table 4.
Increased CEN intra-connectivity
The CEN consistently shows to become more intra-connected, which when combined with the decreased interconnectivity of the DMN highlights a global topographic reorganization (see Table 4). This increased CEN intra-connectivity may be a result of the enhanced cognitive/top-down control (Froeliger et al. 2012; Taren et al. 2017; Winter et al. 2020; Barrós-Loscertales et al. 2021), which is granted through meditation experience and is likely tied to the meditators increased capacity to induce activity in the CEN in order to quiet the activity of the DMN (primarily the PCC).
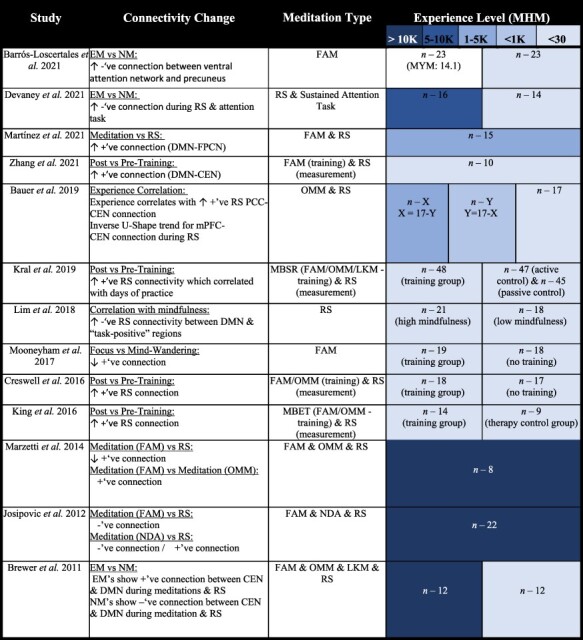
Altered DMN to CEN interconnectivity
The DMN and the CEN have consistently shown to increase in their interconnectivity during both meditation (Brewer et al. 2011; Marzetti et al. 2014; Bauer et al. 2019) and the RS (Brewer et al. 2011; Marzetti et al. 2014; Creswell et al. 2016; Bauer et al. 2019; Kral et al. 2019) (Table 5). Although, it is evident that the relationship between the DMN and CEN is not always constant, with several studies showing that increased meditation proficiency/mindfulness is associated with increased negative connectivity between the two networks. This may be a result of a nonlinear trend (Bauer et al. 2019), much like that found for CEN activation (Brefczynski-Lewis et al. 2007), emphasizing that process of gaining meditation proficiency is not a linear path. Specifically, Bauer et al. (2019) found that meditators with an intermediate amount of experience (with respect to the full sample of participants) showed increasing anticorrelation between the DMN and CEN. Yet more advanced meditators showed a decrease of this anticorrelation—that is referred to being the “ultimate network reconfiguration” since it represents a configuration where the CEN no longer has to interact and interfere with the DMN’s spontaneous activity (Fig. 6). This leads highly experienced meditators brains to exhibit a positive connectivity between the DMN and CEN (Brewer et al. 2011). This coactivation of the DMN and CEN is a key finding in support of nondual awareness (Josipovic et al. 2012). This is not conclusive, although, and it must also be stated that different meditation strategies seem to impact the interaction between these networks in a distinct way (Josipovic et al. 2012). More research is highly encouraged to determine the relationship between meditation type, meditation proficiency, and DMN–CEN connectivity.
Table 5.
Primary DMN–CEN connectivity findings associated with meditation
|
Supporting Information: Text within sections allocated to MHM is to specify either the differentiating factor between groups of similar experience level (e.g. meditation training vs relaxation training) or the level of meditation when mean hours of experience were not provided within the published paper.
Abbreviations: experienced meditators (EM); frontal-parietal control network (FPCN); medial prefrontal cortex (mPFC); mean hours of meditation (MHM); mindfulness-based experiential therapy centre (MBET); mindfulness-based stress reduction (MBSR); naïve meditators (NM); nondual awareness meditation (NDA).
Figure 6.
A visual depiction of the transition from effortful to effortless meditation. (A) The practicing meditator learns to maintain focused attention through CEN activation; although once a certain level of proficiency (a transformed default mode) is reached, there is no longer a need for extensive CEN activation, and there is an extended default mode in which DMN and CEN can be coactive. Coinciding with CEN activity is the connectivity between the DMN and CEN networks of the brain: effort is needed to suppress DMN hyperactivity and activate the CEN (negative connectivity/anticorrelation), which evolves to a coactivation (positive connectivity) of the two networks. This coactivation is affiliated with experience of explicit nondual awareness. (B) The transition from dominant DMN, to dominant CEN, and finally to co-active DMN and CEN is affiliated with global connectivity changes. Primarily, the posterior DMN’s intra-connectivity is decreased, both anterior DMN’s and CEN’s intra-connectivity is increased, and the interconnectivity between the DMN and CEN is increased
SN as a potential mediator between the DMN & CEN
The SN, with anterior insula and dorsal anterior cingulate cortex (dACC) as key regions (besides other cortical and subcortical regions), plays a primary role in several distinct forms of meditation. One study that segregated a meditation session into four different states including “mind-wandering,” “awareness of mind-wandering,” “shifting attention,” and “sustained focus” found that both the anterior insula and the dACC were activated when the meditator became aware that there was no longer sustained attention toward the meditation object, in this case the breath (Hasenkamp and Barsalou 2012; Hasenkamp et al. 2012). This suggests that the SN may very well be a mediator between the DMN and the CEN (Spreng et al. 2010). This finding is in line with another study involving highly advanced Theravada Buddhist Monks that show an anticorrelation between the SN, i.e. dACC, and the CEN, i.e. middle frontal gyrus during focused meditation (Manna et al. 2010). Yet the relationship of the correlation between the SN and the CEN is not fully understood since other impactful studies have shown a coactivation of the SN and the CEN during several different types of meditation (Brewer et al. 2011; Kemmer et al. 2015; Mooneyham et al. 2017).
Regarding the SN’s relationship with the DMN, there is a similar trend of ambiguity in the findings, with some studies suggesting a decreased connectivity during meditation, especially focused meditation (Kemmer et al. 2015). Yet some findings demonstrate a positive connectivity during open forms of meditation (Kilpatrick et al. 2011; Marzetti et al. 2014) and other forms (Manna et al. 2010; Brewer et al. 2011; Hernández et al. 2017). Doll et al.’s (2015) propose that mindfulness is represented by an increase in PCC-insula anticorrelation. Yet other studies show the opposite trend, both during meditation and RS (Brewer et al. 2011; Hernández et al. 2017). Henceforth, the exact mechanism and role of the SN are still uncertain. Although it is possible that the reason behind these contradictory findings is to do with a nonlinear trend of SN activity and connectivity with respect to meditation proficiency. This is supported by the finding of an inverse U-shape trend of anterior insula activity with respect to meditation experience (Brefczynski-Lewis et al. 2007).
Interaction of meditation and self
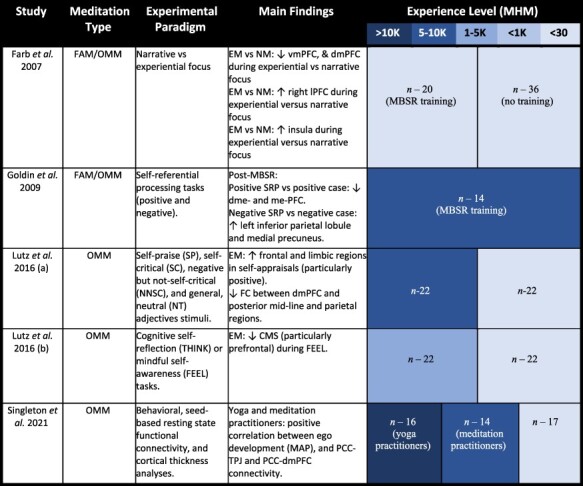
We so far reviewed changes in DMN–CEN relationship as well as three layers of self during proficient meditation compared to beginning meditators. This suggests topographic reorganization of both CEN–DMN relationship and the three layers of self during proficient meditation. Does this mean that the mental self is diminished in the experienced meditators? In order to address this question, one would want to investigate the interaction of meditation × self within one study design. As there are only a few studies on that, we have included a summative table of each study and its respective findings and have included detailed descriptions of each in Table 6
Table 6.
Main findings of studies regarding meditation and self-related processing
|
Supporting Information: Text within sections allocated to MHM is to specify either the differentiating factor between groups of similar experience level (e.g. meditation training vs relaxation training) or the level of meditation when mean hours of experience were not provided within the published paper.
Abbreviations: cortical midline structures (CMS); dorsal medial prefrontal cortex (dmPFC); experienced meditators (EM); focused awareness meditation (FAM); functional connectivity (FC); inferior frontal cortex (IFC); lateral prefrontal cortex (lPFC); naïve meditators (NM); mindfulness-based stress reduction (MBSR); precuneus (Pcu); self-referential processing (SRP); ventral medial prefrontal cortex (vmPFC).
Together, these findings confirm that experienced meditators exhibit a reorganization of the different layers of self-processing, that is instead misaligned in healthy dualistic experiences, as evident from the DMN–CEN anticorrelation. Hence, these findings confirm that experienced meditators show a less pronounced self-bias, which we suggest is linked to the DMN–CEN shift from an anticorrelation to a coactivation.
Discussion
We reviewed various imaging studies that focused on the impact that meditation proficiency, including its interaction with the self, has on spatial topography and connectivity features of the brain. In summary, the most consistent meditation-related findings of the review are (i) decreased posterior DMN (PCC) activity, (ii) increased CEN (dlPFC) activity, (iii) decreased connectivity within posterior DMN as well as between posterior and anterior DMN, (iv) increased connectivity within the anterior DMN and CEN, and (v) significantly impacted connectivity between the DMN and CEN (likely a nonlinear phenomenon) with a final stage of positive connectivity and coactivation. Additionally, the SN has been a common network showing meditation-related alteration in activity and connectivity and may serve as a mediator between the DMN and CEN (Hasenkamp and Barsalou 2012; Hasenkamp et al. 2012).
These results are in accordance with a topographic reorganization of the brain: this, as we postulate, leads to a nondual mode of neural functioning with decreased or nonexisting segregation between the DMN and CEN. We suppose that such nondual topographic organization is manifest on the psychological level in the dissolution of the self-other/environment duality into the experience of nondual awareness (Josipovic et al. 2012; Ataria et al. 2015; Josipovic 2019; Lindahl and Britton 2019). This inextricable tie between the neural topography and subjective phenomena associated with increased meditation proficiency is a primary aspect of the TRoM. The spatial organization or topography of the brain thus provides a link or “common currency” of objective (neural) and subjective (experiential) levels (see Northoff and Lamme 2020a; Northoff et al. 2020b).
We suggest that this topographic reorganization brings to the forefront the unveiling of a most basic, fundamental, experience-based level of self, which becomes explicitly aware of its own nondual nature, e.g. nondual awareness: the nondual organization of our mental life, at its very bottom, is characterized by strong self-body-environment alignment (see Josipovic 2021 for more on the transition from implicit to explicit nondual awareness). Not surprisingly, the region with the most consistent meditation-related finding throughout the reviewed studies (decreased PCC activity) has been reviewed in itself and was found to be associated with “getting caught up in” one’s experience, like getting caught in habits or viewpoints (Brewer et al. 2013). Proficient meditation allows the practitioner to release themselves from identification with and ownership of these habits and viewpoints, thus allowing them to be seen in the context of the overall present moment in which there is a nondual relationship between all phenomena, i.e. nondual awareness. We believe that this transformation, elicited through increased meditation proficiency, is dominantly characterized by a downshift in the dominance of mental self-processing and an increase of exteroceptive and interoceptive self-processing, hence leading to a new default state of the brain (Brewer et al. 2011). This increasingly expansive and integrated default mode has already been shown by Kajimura et al. (2020), where they found that the frontal-parietal network was increasingly merged with the DMN following meditation, leading them to suggest that aside from altered functional connectivity, “meditation leads to reconfiguration of whole-brain network architecture.”
TRoM I: resetting the relationship of DMN and CEN
In subjective states in which nondual awareness is implicit, the duality on the neural level between specific regions/networks like the default-DMN as distinguished from the CEN (Northoff et al. 2006; van der Meer et al. 2010; Andrews-Hanna 2011; Anticevic et al. 2012; Andrews-Hanna et al. 2014; Murray et al. 2014; Davey et al. 2016; Frewen et al. 2020) is mirrored on the subjective duality between of self as distinguished from other (Wolff et al. 2018; Huang et al. 2016; Kolvoort et al. 2020; Northoff et al. 2020b). These findings suggest that the brain’s spatial topography with its duality of DMN and CEN is intimately related to the experienced duality of self and other/world. Accordingly, duality of self-other/world may be shared on neuronal and psychological levels as their “common currency” (Northoff and Huang 2017; Northoff and Lamme 2020a; Northoff et al. 2020b).
This changes during the practice of meditation and especially in the proficient stages. The topographic reorganization of the DMN–CEN relationship changes from primarily a negative to a positive correlation (see Fig. 6). This amounts to a topographic reorganization of the whole brain (see Kajimura et al. 2020 for a direct example of this). In the meditation-related transformed default mode of the brain, the initial DMN likely no longer stands in negative but in positive correlation to CEN, a network of the brain found earlier in the principal gradient system of the brain (Margulies et al. 2016). Due to this transformation, the default mode may now be spatially extended to and include non-DMN regions (see also Bauer et al. 2019 for a somewhat analogous suggestion of the brain’s transformed default mode). This means that, cognitively, both self and other/world-related regions, mediated through meditation experience, may simultaneously function in a new default manner: self- and other-related contents can now be processed and synchronized together, which, in turn, renders superfluous their distinction between the self and other/environment (Brewer et al. 2011; Hasenkamp et al. 2012; Marzetti et al. 2014; Bauer et al. 2019). Hence, we suggest that there is likely a correspondence between the dissolution of neuronal boundaries found between networks like the DMN and CEN and the dissolution of boundaries between the self and other on the psychological level of experience.
Although the state of explicit nondual awareness is one that is inherently effortless, it seems fair to say that effort is required in order to transform the correspondence between the DMN and CEN. Much like the mystic Osho has said in the context of Zen:
“Zen says, achieve it effortlessly; there is no need of effort. Zen is right; there is no need for an effort. But remember, to achieve this point of no-effort you will need a long, long effort.”
Similar to the taming of an elephant that entails binding an elephant to a large post in order to subdue its wild habits, it seems as although the mind must undergo a similar treatment through meditation, an idea already presented in early Buddhist accounts and the modern “Handbook of Mindfulness and Self-Regulation” (Ostafin 2015). The elephant, based on the most replicated fMRI-related finding on meditation, would be the posterior section of the DMN, notably the PCC, while the tamer would likely be that of the CEN, with the dlPFC likely playing a primary role. Through effort, the meditator begins to learn to subdue the hyperactivation of the PCC and cultivate an increasing level of integration between the functional networks of the brain.
It should be noted that the explicit experience of nondual awareness may be increasingly likely during a more open form of attention like OMM, LKM, or NDA, as opposed to FAM. Most, if not all meditation practices, although, have a shared emphasis in their meditation practice on disengaging from mind-wandering and, at the same time, attending to the present moment—this can be observed in both focused or open attentional practices. We emphasize that all forms of meditation are ultimately progressive steps along the continuum of awareness with the ultimate realization being that of nonduality. So, although distinct meditation forms have different neural correlates, we suppose that they all most likely induce a topographic DMN–CEN reorganization, which, in either case, is associated with the experience of nonduality (see Deepeshwar et al. 2019; Schoenberg and Vago 2019 for precise stage-wise progressions). Given that different forms of meditation may all ultimately lead to nonduality, this is very much like the analogy proposed by the 15th-century Zen Buddhist Monk, Ikkyu: “many paths lead from the foot of the mountain, but at the peak we all gaze at the single bright moon.”
TRoM II: A transformed relationship of self-processing layers
Qin et al. (2020) propose a neural model subdividing self-processing into three intimately connected and spatially nested levels with different topographic extensions in the brain: interoceptive-processing, exteroceptive processing, and mental-self-processing, which is similar to previous models (Damasio 1999, 2003a, 2003b, 2010). Interoceptive self-processing refers to the brain’s interaction with the environment at a physiological level, that is, for example, through gastrointestinal and cardiorespiratory sensations (Craig 2009; Seth 2013; Tsakiris and Critchley 2016). At the second layer, the interaction with the environment at a proprioceptive/affective level is processed, that is for example, through what we refer to as bodily and emotional perceptions. At the third layer, it is mental/cognitive self-processing, which consists of representations of self-related nonbodily signals like personal traits and other mental contents that take place.
Recruiting a variety of different imaging findings on both meditation and self, we suppose that the three layers of self (interoceptive, exteroceptive, and mental/cognitive) become more strongly synchronized between each other, allowing a better alignment with the environment (see Fig. 7). This renders more explicit the otherwise more implicit connection of brain, body, and environment, which now becomes the focus of experience (see Glossary of Terms and Northoff and Zilio (2022) for a clarification of terminology).
Figure 7.
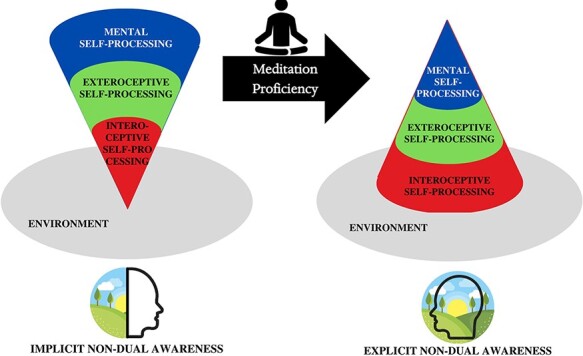
Synchronization between the three different layers of self-related processing due to increased meditation proficiency. The three layers of self-related processing are desynchronized in subjective states of implicit nondual awareness (i.e. duality) and impacted by hyperactive mental-self processing (likely tied with hyperactive PCC activity). Through increased meditation proficiency, the relationship between the layers of self-related processing likely becomes increasingly synchronized and nested within the environment, allowing for a present-moment orientation and a recognition of nondual awareness
It is important for us to underline that the partition of self-processing in the above-described three layers is only a conceptual subdivision, meaning that the mechanism of self-processing is better to be thought of as a continuum. Specifically, presupposing this hierarchical model of self (Qin et al. 2020), we suppose that the upper mental layer is featured by the dichotomy of self and nonself whereas that is not yet present on the more basic layers of self, especially, the self of the interoceptive and exteroceptive layers. As featured by intero-exteroceptive connection, the lowest layer of self is, by default, organized in an increasingly nondual manner. If, through topographic reorganization, the upper mental/cognitive layer of self is synchronized with its intero-exteroceptive layers, the mental/psychological duality of self and nonself is immersed and integrated within the more nondual brain–body–environment connection of the primordial and immediate layers of self (Thompson and Varela 2001; Northoff and Zilio 2022).
In common dualistic experiences, nondual awareness is indeed always implicit (Josipovic 2021) and thus serves as a predisposition or prerequisite for dualistic experiences, which are fundamentally linked to higher-order cognitive processing (see Fig. 1). This is reflected in the hierarchical structure of the three-layered model of self (Qin et al. 2020): the regions of the intero-exteroceptive layers of self are recapitulated within the upper layers of self, that is, in mental/cognitive layers of self (where they are joined by the recruitment of additional regions). The hierarchy among the three layers of self is thus nested such that the lower layer is present within the next upper layer and so forth.
Figure 1.
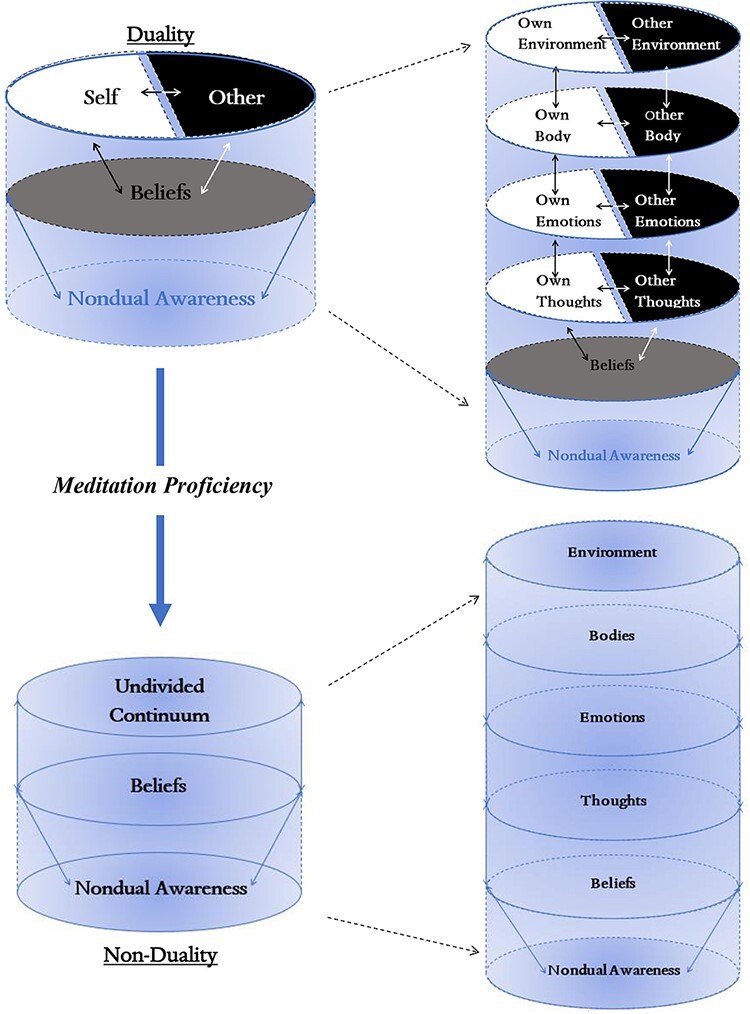
A visual depiction of nondual awareness’s relationship with meditation proficiency. Nondual awareness is necessary for all concepts of “self” and “other” to form, although these conceptualizations that are deeply linked with beliefs (see Box 3: relationship of TRoM to predictive coding, active inference, and psychedelics) are a hindrance to the explicit experience of nondual awareness itself. The text “Nondual Awareness” transition from light to dark to represent the shift from implicit to explicit experience. Though nondual awareness is always present, as represented by the light background/space that is preliminary to all levels and distinction that are fueled by beliefs
Such nested hierarchy carries major implications for the structure of consciousness: even if the dual organization of self and nonself of the mental level is predominant and is thus explicit in our experience, there is still a more implicit experience of nonduality in the background of experience. This seems to lead to an almost paradoxical situation: while the mental/cognitive layer is characterized by strong beliefs, categorization, and projection of a duality between self and other/environment (Josipovic 2021), there remains nevertheless a tacit feeling or implicit experience of nondual connectedness and integration of self and world in the background of our experience (Fig. 1).
Once the upper mental and lower intero-exteroceptive layers of self-processing are synchronized and thus function according to their nested and inter-related nature, then a nonconceptual recognition of nondual awareness by nondual awareness itself is possible: an auto-recognition of reflexive awareness. See, for example, phenomenological reports from one highly proficient meditator in a state of, what we would call, explicit nondual awareness:
“I’m not there basically, just world, so there’s no real location at all (…). It’s very minimal, almost nothing… When there’s no boundary, there’s no personal point of view, it’s the world point of view, it’s like the world looking, not [me] looking, the world is looking.”
“I dissolve into the world and where you have the boundaries and the self as foreground and the world as background or as ground, here there isn’t a ground, there isn’t a foreground, in a way the background is everything, although not identical to the world, I’m not separate from background.”
We suppose that the transition from implicit to explicit nondual awareness takes place when the fundamentally nondual intero-exteroceptive layers shift into the foreground of experience, while the usually more dominating dual mental self at the foreground now becomes nested within these more spatially and temporally expansive layers of self. Hence, we suppose a shift of background and foreground in consciousness: the focus on the mental self shifts to allow the intero-exteroceptive self, the usual more implicit background, to shift into the foreground of our experience and is thereby rendered explicit.
Limitations and recommendations
Some limitations to our review and model need to be considered. First, not all findings of meditative studies form a consensus; thus, these concepts should be taken as approximations that are based on some of the most replicated results. Second, a breadth of meditation types has been included without an in-depth model of how each type is related to one another. This means that each of the study findings is not necessarily in corroboration with each other, rather they may be highlighting specific aspects of the meditation type, which was involved in the experimental procedure. More work is needed to find specific correlates associated with distinct meditation types along distinct stages of meditation proficiency. Finally, a large level of interindividual differences among subjects within the studies along with the studies’ various experimental paradigms (i.e. length of meditation session, specific instructions for meditation, bodily orientation during meditation, and bandwidth of data acquisition) likely play a role in the heterogeneity within the findings and the field would benefit from controlling and directly assessing these variables in later research.
In order to verify and/or modulate the viewpoints developed here, there will need to be further direct testing, with the recommendation of involving experiential questionnaires in order to accurately link neural features to experiential phenomena. Another limitation has been the scarce amount of meditation literature that included in its experimental paradigm the distinction between the different layers of self-processing, i.e. mental-self-processing (or narrative self) and intero-exteroceptive processing (or minimal/experiential/core self). In our opinion, this means that although many studies have focused on the neural correlates of meditative states and traits and on self-dissolution experiences, little attention has been devoted to building studies that focus on how self-processing evolves with increased meditation proficiency and how this correlated with neural measures.
Finally, we encourage future meditation research to incorporate scales of mindfulness such as the Mindfulness Attention Awareness Scale (Brown and Ryan 2003) and nonduality (Hanley et al. 2018) in order to avoid the vagueness of meditation experience levels in terms of hours and years. We deem that this is a limitation of the field since the amount of progression and understanding that one gains through meditation is extremely variable between individuals. This is made evident by the fact that individuals without meditation experience vary in levels of mindfulness. Introducing these scales will allow for more comprehensive correlational analyses to take place, which can more accurately relate the changes in the brain to the proficiency level of the meditator.
Conclusion
Our review illustrates that meditation has a profound neural reorganization on the brain’s spatial topography, particularly in the relation between the DMN and CEN. The shift from naïve to highly proficient meditation likely allows for a reorganization of the brain’s spatial features on the neural level, which leads to a transformed default mode in which the functional networks are increasingly synchronized and thus can operate in an increasingly cooperative manner. Based on preliminary evidence, we suggest that this may manifest on the psychological level as a transition from implicit to explicit nondual experience of self and other/world.
We therefore develop the TRoM that postulates the spatial-topographic (and, in the future, also temporal dynamic) reorganization of the brain from naïve to highly proficient stages of meditation. The key hypothesis of TRoM is that brain and henceforth the psyche (or mind) are organized in a nondual way: this emphasizes the nondual connection of self and nonself/environment on a deeper intero-exteroceptive level over their duality that predominates on the more superficial mental level. On a more general level, this lends further support to the assumption that neural and mental levels share most basic spatial-topographic (and temporal dynamic) features as postulated in the “common currency hypothesis” of brain and mind (Northoff and Lamme 2020a; Northoff et al. 2020b).
Proficient meditation unravels a deeper social and ecological intero-exteroceptive layer of self where it is intimately connected with the environment in a nondual manner. This hints upon a more basic and fundamental notion of self where it is determined by and through its relationship to the other/environment. Reaching beyond the duality of self-other/environment, this deeper relational layer of self is intrinsically neuro-ecological and neuro-social. That connects this deeper layer of self intimately with both other and world in a positive way, that is, integrated and rather than segregated. Proficient meditation can thus provide us with a deeper level in our experience of the own self, a nondual experience where our self is integrated with the other and part of the wider world, as discussed in previous neurophenomenological models of explicit nondual awareness (Josipovic 2019, 2021). This allows to lay bare a deeper ecological and social nondual layer not only of ourself but, correspondingly, also of our brain featured by its deeper most basic and fundamental nondual spatial topography serving as its default or baseline.
Supplementary Material
Contributor Information
Austin Clinton Cooper, Integrated Program of Neuroscience, Room 302, Irving Ludmer Building, 1033 Pine Avenue W., McGill University, Montreal, QC H3A 1A1, Canada.
Bianca Ventura, Mind, Brain Imaging and Neuroethics Research Unit, Institute of Mental Health Research, University of Ottawa, 1145 Carling Avenue, Ottawa, ON K1Z 7K4, Canada.
Georg Northoff, Mind, Brain Imaging and Neuroethics Research Unit, Institute of Mental Health Research, University of Ottawa, 1145 Carling Avenue, Ottawa, ON K1Z 7K4, Canada; Mental Health Center, Zhejiang University School of Medicine, 866 Yuhangtang Road, Hangzhou 310058, China.
Supplementary data
Supplementary data are available at NCONSC online.
Data availability
The technical details and main findings from all reviewed studies may be found in the Supplementary Material.
Funding
This research has received funding from the European Union’s Horizon 2020 Framework Program for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2). G.N. is grateful for funding provided by University Medical Research Fund (UMRF), University of Ottawa Brain and Mind Research Institute (UOBMRI), Canadian Institutes of Health Research (CIHR), and Physicians’ Services Incorporated (PSI) Foundation. We are also grateful to CIHR, NSERC, and SHERRC for supporting our tri-council grant from the Canada-UK Artificial Intelligence Initiative “The self as agent-environment nexus: crossing disciplinary boundaries to help human selves and anticipate artificial selves” (ES/T01279X/1) (together with Karl J. Friston from the UK).
Conflict of interest statement
None declared.
Glossary of Terms
Definitions:
Temporo-spatial nestedness and alignment: this provides the necessary neural conditions of possible consciousness, that is, the neural capacity or neural predisposition and prerequisite of consciousness
Temporo-spatial nestedness: this refers to a particular type of organization of different spatial and temporal scales of
neural activity. Rather than operating in parallel, unconnected, or causally connected but separate ways, the different spatial and temporal scales of neural activity are contained or nested within each other.
Temporo-spatial alignment: this refers to the phenomenon in which the brain encodes the context by adapting (and thereby aligning) its own neural activity to the various inputs/stimuli that shape the context.
Self-related processing:
Interoceptive self: this represents internal sensory signals for cardiorespiratory, gastrointestinal, and urogenital functions. The prerequisite of integration of internal and external environmental signal into the self.
Exteroceptive self: this represents exteroceptive and proprioceptive signals directly relates to one’s own body. An intermediate level that links the internal body and external environment.
Mental-self-processing: this represents self-related nonbodily signals, such as personal traits and other mental contents and finalizes the integration of external environment to the self
Nondual awareness: this is a background awareness that precedes conceptualization and intention and that can contextualize various perceptual, affective, or cognitive contents without fragmenting the field of experience into habitual dualities (Josipovic 2013)
Abbreviations
TRoM: topographic reorganization model of meditationDMN: default mode networkCEN: central executive networkmPFC: medial prefrontal cortexdlPFC: dorsal lateral prefrontal cortexPCC: posterior cingulate cortexACC: anterior cingulate cortexRS: resting state
References
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 2011;18:251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 2014;1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci 2012;16:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataria Y, Dor-Ziderman Y, Berkovich-Ohana A. How does it feel to lack a sense of boundaries? A case study of a long-term mindfulness meditator. Conscious Cogn 2015;37:133–47. [DOI] [PubMed] [Google Scholar]
- Avvenuti G, Leo A, Cecchetti L et al. Reductions in perceived stress following transcendental meditation practice are associated with increased brain regional connectivity at rest. Brain Cogn 2020;139:105517. [DOI] [PubMed] [Google Scholar]
- Bærentsen KB, Stødkilde-Jørgensen H, Sommerlund B et al. An investigation of brain processes supporting meditation. Cogn Process 2009;11:57–84. [DOI] [PubMed] [Google Scholar]
- Baron Short E, Kose S, Mu Q et al. Regional brain activation during meditation shows time and practice effects: an exploratory FMRI study. Evid-based Complement Altern Med 2010;7:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Hernández SE, Xiao Y et al. Resting state functional connectivity associated with Sahaja yoga meditation. Front Hum Neurosci 2021;15:1–11.doi: 10.3389/fnhum.2021.614882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CC, Whitfield-Gabrieli S, Díaz JL et al. From state-to-trait meditation: reconfiguration of central executive and default mode networks. Eneuro 2019;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovich-Ohana A, Glicksohn J, Goldstein A. Studying the default mode and its mindfulness-induced changes using EEG functional connectivity. Soc Cogn Affect Neurosci 2013;9:1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovich-Ohana A, Harel M, Hahamy A et al. Data for default network reduced functional connectivity in meditators, negatively correlated with meditation expertise. Data Brief 2016;8:910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovich-Ohana A, Wilf M, Kahana R et al. Repetitive speech elicits widespread deactivation in the human cortex: the “Mantra” effect? Brain Behav 2015;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AE, Stevens L. EEG manifestations of nondual experiences in meditators. Conscious Cogn 2015;31:1–11. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS et al. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci 2007;104:11483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Front Hum Neurosci 2013;7:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR et al. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci 2011;108:20254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol 2003;84:822–48. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci 2012a;109:2138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Friston KJ. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev 2019;71:316–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Erritzoe D et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 2012b;39:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci 2016;113:4853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J, Phillips B, Burr M et al. Effects of meditation on stress levels of physical therapist students. J Phys Ther Educ 2016;30:33–9. [Google Scholar]
- Chialvo DR Critical brain dynamics at large scale. Criticality in Neural Systems 2014;43–66. 10.1002/9783527651009.ch3. [Google Scholar]
- Cotier FA, Zhang R, Lee TMC. A longitudinal study of the effect of short-term meditation training on functional network organization of the aging brain. Sci Rep 2017;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (Bud) Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Taren AA, Lindsay EK et al. Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: a randomized controlled trial. Biol Psychiatry 2016;80:53–61. [DOI] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotion and the self. Ann N Y Acad Sci 2003a;1001:253–61. [DOI] [PubMed] [Google Scholar]
- Damasio A. Mental self: the person within. Nature 2003b;423:227. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York, NY: Harcourt Inc, 1999. [Google Scholar]
- Damasio AR. When Self Comes to Mind. New York, NY: Pantheon Books, 2010. [Google Scholar]
- Davanger S, Ellingsen Ø, Holen A et al. Meditation-specific prefrontal cortical activation during acem meditation: an FMRI study. Percept Mot Skills 2010;111:291–306. [DOI] [PubMed] [Google Scholar]
- Davey CG, Pujol J, Harrison BJ. Mapping the self in the brain’s default mode network. NeuroImage 2016;132:390–7. [DOI] [PubMed] [Google Scholar]
- Deane G. Dissolving the self: active inference, psychedelics, and ego-dissolution. PhiMiSci 2020;1:2. [Google Scholar]
- Deepeshwar S, Nagendra HR, Rana BB. Evolution from four mental states to the highest state of consciousness: a neurophysiological basis of meditation as defined in yoga texts. Prog Brain Res 2019;244:31–83. [DOI] [PubMed] [Google Scholar]
- Delorme A, Brandmeyer T. When the meditating mind wanders. Curr Opin Psychol 2019;28:133–7. [DOI] [PubMed] [Google Scholar]
- Deolindo CS, Ribeiro MW, Aratanha MA et al. A critical analysis on characterizing the meditation experience through the electroencephalogram. Front Syst Neurosci 2020;14:1–29.doi: 10.3389/fnsys.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney K, Levin E, Tripathi V et al. Attention and default mode network assessments of meditation experience during active cognition and rest. Brain Sci 2021;11:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J, Berkman ET, Arch J et al. Neural correlates of focused attention during a brief mindfulness induction. Soc Cogn Affect Neurosci 2012;8:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodich A, Zollo M, Crespi C et al. Short-term Sahaja yoga meditation training modulates brain structure and spontaneous activity in the executive control network. Brain Behav 2018;9:e01159.doi: 10.1002/brb3.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll A, Hölzel BK, Boucard CC et al. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Front Hum Neurosci 2015;9.doi: 10.3389/fnhum.2015.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor-Ziderman Y, Berkovich-Ohana A, Glicksohn J et al. Mindfulness-induced selflessness: a MEG neurophenomenological study. Front Hum Neurosci 2013;7.doi: 10.3389/fnhum.2013.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen HG, Bernhardt BC, Skottnik L et al. Structural changes in socio-affective networks: multi-modal MRI findings in long-term meditation practitioners. Neuropsychologia 2017;116:26–33. [DOI] [PubMed] [Google Scholar]
- Engström M, Pihlsgård J, Lundberg P et al. Functional magnetic resonance imaging of hippocampal activation during silent mantra meditation. J Altern Complement Med 2010;16:1253–8. [DOI] [PubMed] [Google Scholar]
- Engström M, Söderfeldt B. Brain activation during compassion meditation: a case study. J Altern Complement Med 2010;16:597–9. [DOI] [PubMed] [Google Scholar]
- Escrichs A, Sanjuán A, Atasoy S et al. Characterizing the dynamical complexity underlying meditation. Front Syst Neurosci 2019;131–6.doi: 10.3389/fnsys.2019.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neurosci 2012;8:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci 2007;2:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Kallio-Tamminen T. EEG-guided meditation: a personalized approach. J Physiol Paris 2015;109:180–90. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Kallio-Tamminen T. Long-term meditation training induced changes in the operational synchrony of default mode network modules during a resting state. Cogn Process 2016;17:27–37. [DOI] [PubMed] [Google Scholar]
- Fox KCR, Dixon ML, Nijeboer S et al. Functional neuroanatomy of meditation: a review and meta-analysis of 78 functional neuroimaging investigations. Neurosci Biobehav Rev 2016;65:208–28. [DOI] [PubMed] [Google Scholar]
- Fox KCR, Nijeboer S, Dixon ML et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev 2014;43:48–73. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL et al. From the cover: the human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci 2005;102:9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Schroeter ML, Riva G et al. Neuroimaging the consciousness of self: review, and conceptual-methodological framework. Neurosci Biobehav Rev 2020;112:164–212.doi: 10.1016/j.neubiorev.2020.01.023 [DOI] [PubMed] [Google Scholar]
- Friston K. Am I self-conscious? (Or does self-organization entail self-consciousness?). Front Psychol 2018;9.doi: 10.3389/fpsyg.2018.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, Garland EL, Kozink RV et al. Meditation-state functional connectivity (msFC): strengthening of the dorsal attention network and beyond. Evid-based Complement Altern Med 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino M, Ueda Y, Mizuhara H et al. Open monitoring meditation reduces the involvement of brain regions related to memory function. Sci Rep 2018;8:1–10.doi: 10.1038/s41598-018-28274-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Noggle JJ, Park CL et al. Potential self-regulatory mechanisms of yoga for psychological health. Front Hum Neurosci 2014;8:1–20.doi: 10.3389/fnhum.2014.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Santoyo JF, Davis JH et al. Effortless awareness: using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators’ self-report. Front Hum Neurosci 2013a;7:1–9.doi: 10.3389/fnhum.2013.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Constable RT et al. BOLD signal and functional connectivity associated with loving kindness meditation. Brain Behav 2014;4:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Worhunsky PD et al. Real-time fMRI links subjective experience with brain activity during focused attention. NeuroImage 2013b;81:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Zeffiro TA, Scheinost D et al. Meditation leads to reduced default mode network activity beyond an active task. Cogn Affect Behav Neurosci 2015;15:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gethin R. The Foundations of Buddhism. Oxford University Press, 1998. [Google Scholar]
- Goldin P, Ramel W, Gross J. Mindfulness meditation training and self-referential processing in social anxiety disorder: behavioral and neural effects. J Cogn Psychother 2009;23:242–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golesorkhi M, Gomez-Pilar J, Tumati S et al. Temporal hierarchy of intrinsic neural timescales converges with spatial core-periphery organization. Commun Biol 2021a;4:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golesorkhi M, Gomez-Pilar J, Zilio F et al. The brain and its time: intrinsic neural timescales are key for input processing. Commun Biol 2021b;4:970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley AW, Garland EL. Mindfulness training disrupts Pavlovian conditioning. Physiol Behav 2019;204:151–4. [DOI] [PubMed] [Google Scholar]
- Hanley AW, Nakamura Y, Garland EL. The Nondual Awareness Dimensional Assessment (NADA): new tools to assess nondual traits and states of consciousness occurring within and beyond the context of meditation. Psychol Assess 2018;30:1625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R, Zeidan F, Kitsaras G et al. Trait mindfulness is associated with lower pain reactivity and connectivity of the default mode network. J Pain 2018;20:645–54.doi: 10.1016/j.jpain.2018.10.011 [DOI] [PubMed] [Google Scholar]
- Hasenkamp W. Catching the Wandering Mind. Oxford Handbooks Online. Oxford University Press, 2018.doi: 10.1093/oxfordhb/9780190464745.013.1 [DOI] [Google Scholar]
- Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front Hum Neurosci 2012;6:1–14.doi: 10.3389/fnhum.2012.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E et al. Mind-wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. NeuroImage 2012;59:750–60. [DOI] [PubMed] [Google Scholar]
- Hernández SE, Barros-Loscertales A, Xiao Y et al. Gray matter and functional connectivity in anterior cingulate cortex are associated with the state of mental silence during Sahaja yoga meditation. Neuroscience 2017;371:395–406. [DOI] [PubMed] [Google Scholar]
- Hernández SE, Suero J, Rubia K et al. Monitoring the neural activity of the state of mental silence while practicing Sahaja yoga meditation. J Altern Complement Med 2015;21:175–9. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Hempel H et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett 2007;421:16–21. [DOI] [PubMed] [Google Scholar]
- Huang Z, Obara N, Davis H et al. The temporal structure of resting-state brain activity in the medial prefrontal cortex predicts self-consciousness. Neuropsychologia 2016;82:161–70. [DOI] [PubMed] [Google Scholar]
- Huang Z, Zhang J, Longtin A et al. Is there a nonadditive interaction between spontaneous and evoked activity? Phase-dependence and its relation to the temporal structure of scale-free brain activity. Cereb Cortex 2015;27:bhv288.doi: 10.1093/cercor/bhv288 [DOI] [PubMed] [Google Scholar]
- Irrmischer M, Houtman SJ, Mansvelder HD et al. Controlling the temporal structure of brain oscillations by focused attention meditation. Hum Brain Mapp 2018;39:1825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: an fMRI investigation. Soc Neurosci 2011;6:231–42. [DOI] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang D-H et al. Increased default mode network connectivity associated with meditation. Neurosci Lett 2011;487:358–62. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kim J-H, Yun J-Y et al. Differences in functional connectivity of the insula between brain wave vibration in meditators and non-meditators. Mindfulness 2018;9:1857–66.doi: 10.1007/s12671-018-0928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao T, Li C-W, Vértes PE et al. Large-scale functional brain network reorganization during taoist meditation. Brain Connect 2016;6:9–24. [DOI] [PubMed] [Google Scholar]
- Josipovic Z. Freedom of the mind. Front Psychol 2013;1307:9–18.doi: 10.3389/fpsyg.2013.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josipovic Z. Neural correlates of nondual awareness in meditation. Ann N Y Acad Sci 2014;1307:9–18. [DOI] [PubMed] [Google Scholar]
- Josipovic Z. Love and compassion meditation: a nondual perspective. Ann N Y Acad Sci 2016;1373:65–71. [DOI] [PubMed] [Google Scholar]
- Josipovic Z. Nondual awareness: consciousness-as-such as non-representational reflexivity. In: Srinivasan N (ed.), Progress in Brain Research: Vol. 244. Meditation. Elsevier Academic Press, 2019, 273–98. [DOI] [PubMed] [Google Scholar]
- Josipovic Z. Implicit-explicit gradient of nondual awareness or consciousness as such. Neurosci Conscious 2021;2021:1–13.doi: 10.31231/osf.io/ngupr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josipovic Z, Dinstein I, Weber J et al. Influence of meditation on anti-correlated networks in the brain. Front Hum Neurosci 2012;5.doi: 10.3389/fnhum.2011.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Masuda N, Lau JK et al. Focused attention meditation changes the boundary and configuration of functional networks in the brain. Sci Rep 2020;10:1–11.doi: 10.1038/s41598-020-75396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmer PB, Guo Y, Wang Y et al. Network-based characterization of brain functional connectivity in Zen practitioners. Front Psychol 2015;6:1–14.doi: 10.3389/fpsyg.2015.00603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiken LG, Garland EL, Bluth K et al. From a state to a trait: trajectories of state mindfulness in meditation during intervention predict changes in trait mindfulness. Pers Individ Dif 2015;81:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR et al. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage 2011;56:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK et al. Altered Default Mode Network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress Anxiety 2016;33:289–99. [DOI] [PubMed] [Google Scholar]
- Kolvoort IR, Wainio‐Theberge S, Wolff A et al. Temporal integration as “common currency” of brain and self‐scale‐free activity in resting‐state EEG correlates with temporal delay effects on self‐relatedness. Hum Brain Mapp 2020;41:4355–74.doi: 10.1002/hbm.25129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C, Dentico D, Kral TRA et al. The effect of mindfulness meditation on impulsivity and its neurobiological correlates in healthy adults. Sci Rep 2019;9:1–17.doi: 10.1038/s41598-019-47662-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral TRA, Imhoff-Smith T, Dean DC et al. Mindfulness-based stress reduction-related changes in posterior cingulate resting brain connectivity. Soc Cogn Affect Neurosci 2019;14:777–87.doi: 10.1093/scan/nsz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Sato W, Toichi M et al. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Cogn Brain Res 2001;11:281–7. [DOI] [PubMed] [Google Scholar]
- Kwak S, Kim S-Y, Bae D et al. Enhanced attentional network by short-term intensive meditation. Front Psychol 2020;10:1–12.doi: 10.3389/fpsyg.2019.03073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S, Lee TY, Jung WH et al. The immediate and sustained positive effects of meditation on resilience are mediated by changes in the resting brain. Front Hum Neurosci 2019;13.doi: 10.3389/fnhum.2019.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamblin M, Murawski C, Whittle S et al. Social connectedness, mental health and the adolescent brain. Neurosci Biobehav Rev 2017;80:57–68. [DOI] [PubMed] [Google Scholar]
- Laukkonen RE, Slagter HA. From many to (n)one: meditation and the plasticity of the predictive mind. Neurosci Biobehav Rev 2021;128:199–217. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL et al. Functional brain mapping of the relaxation response and meditation. NeuroReport 2000;11:1581–5. [PubMed] [Google Scholar]
- Lemay V, Hoolahan J, Buchanan A. Impact of a yoga and meditation intervention on students’ stress and anxiety levels. Am J Pharm Educ 2019;83:7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Teng J, Patanaik A et al. Dynamic functional connectivity markers of objective trait mindfulness. NeuroImage 2018;176:193–202. [DOI] [PubMed] [Google Scholar]
- Lindahl J, Britton W. ‘I have this feeling of not really being here’ buddhist meditation and changes in sense of self. J Conscious Stud 2019;26:157–83. [Google Scholar]
- Lippelt DP, Hommel B, Colzato LS. Focused attention, open monitoring and loving kindness meditation: effects on attention, conflict monitoring, and creativity - a review. Front Psychol 2014;5.doi: 10.3389/fpsyg.2014.01083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumma A-L, Kok BE, Singer T. Is meditation always relaxing? Investigating heart rate, heart rate variability, experienced effort and likeability during training of three types of meditation. Int J Psychophysiol 2015;97:38–45. [DOI] [PubMed] [Google Scholar]
- Lutz A, Dunne JD, Davidson RJ. Meditation and the neuroscience of consciousness: an introduction. In: Zelazo PD, Moscovitch M, Thompson E (eds.), The Cambridge Handbook of Consciousness. Cambridge University Press, 2007, 499–551.doi: 10.1017/CBO9780511816789.020 [DOI] [Google Scholar]
- Lutz A, Jha AP, Dunne JD et al. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am Psychol 2015;70:632–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD et al. Attention regulation and monitoring in meditation. Trends Cogn Sci 2008;12:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J, Brühl AB, Doerig N et al. Altered processing of self-related emotional stimuli in mindfulness meditators. NeuroImage 2016a;124:958–67. [DOI] [PubMed] [Google Scholar]
- Lutz J, Brühl AB, Scheerer H et al. Neural correlates of mindful self-awareness in mindfulness meditators and meditation-naïve subjects revisited. Biol Psychol 2016b;119:21–30. [DOI] [PubMed] [Google Scholar]
- Mahone MC, Travis F, Gevirtz R et al. fMRI during transcendental meditation practice. Brain and Cognition 2018;123:30–3. [DOI] [PubMed] [Google Scholar]
- Manna A, Raffone A, Perrucci MG et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res Bull 2010;82:46–56. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences 2016;113:12574–9.doi: 10.1523/eneuro.0341-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez AY, Demertzi A, Bauer CC et al. The time varying networks of the interoceptive attention and rest. Eneuro 2021;8:1–13.doi: 10.1523/eneuro.0341-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Elrahal F, Peters CA et al. Mindfulness and dynamic functional neural connectivity in children and adolescents. Behav Brain Res 2018;336:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti L, Di Lanzo C, Zappasodi F et al. Magnetoencephalographic alpha band connectivity reveals differential default mode network interactions during focused attention and open monitoring meditation. Front Hum Neurosci 2014;8.doi: 10.3389/fnhum.2014.00832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzinger T. Minimal phenomenal experience: meditation, tonic alertness, and the phenomenology of “pure” consciousness. PhiMiSci 2020;1:7. [Google Scholar]
- Millière R, Carhart-Harris RL, Roseman L et al. Psychedelics, meditation, and self-consciousness. Front Psychol 2018;9:1–29.doi: 10.3389/fpsyg.2018.01475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Singh S, Moheb N et al. Changes in functional magnetic resonance imaging with Yogic meditation: a pilot study. AYU 2017;38:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Tanioka K, Yamamoto S et al. Revealing changes in brain functional networks caused by focused-attention meditation using Tucker3 clustering. Front Hum Neurosci 2020;13.doi: 10.3389/fnhum.2019.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Sharma R, Bijlani RL. Effect of meditation on stress-induced changes in cognitive functions. J Altern Complement Med 2011;17:207–12. [DOI] [PubMed] [Google Scholar]
- Mooneyham BW, Mrazek MD, Mrazek AJ et al. States of mind: characterizing the neural bases of focus and mind-wandering through dynamic functional connectivity. J Cogn Neurosci 2017;29:495–506. [DOI] [PubMed] [Google Scholar]
- Murray RJ, Debbané M, Fox PT et al. Functional connectivity mapping of regions associated with self- and other-processing. Hum Brain Mapp 2014;36:1304–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart-Harris RL, Moran RJ et al. Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci 2013;33:15171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg A, Alavi A, Baime M et al. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res: Neuroimaging 2001;106:113–22. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wintering N, Waldman MR et al. Cerebral blood flow differences between long-term meditators and non-meditators. Conscious Cogn 2010;19:899–905. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M et al. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage 2006;31:440–57. [DOI] [PubMed] [Google Scholar]
- Northoff G, Huang Z. How do the brain’s time and space mediate consciousness and its different dimensions? Temporo-spatial theory of consciousness (TTC). Neurosci Biobehav Rev 2017;80:630–45. [DOI] [PubMed] [Google Scholar]
- Northoff G, Lamme V. Neural signs and mechanisms of consciousness: is there a potential convergence of theories of consciousness in sight? Neurosci Biobehav Rev 2020a;118:568–87.doi: 10.1016/j.neubiorev.2020.07.019 [DOI] [PubMed] [Google Scholar]
- Northoff G, Wainio-Theberge S, Evers K. Spatiotemporal Neuroscience – what is it and why we need it. Phys Life Rev 2020b;33:78–87.doi: 10.1016/j.plrev.2020.06.005 [DOI] [PubMed] [Google Scholar]
- Northoff G, Zilio F. Temporo-spatial theory of consciousness (TTC) – bridging the gap of neuronal activity and phenomenal states. Behav Brain Res 2022;424:1–13.doi: 10.1016/j.bbr.2022.113788 [DOI] [PubMed] [Google Scholar]
- Osho . The Book of Secrets 112 Meditations to Discover the Mystery Within. Cork, Ireland: Osho Media International, 2012. [Google Scholar]
- Ostafin BD. Taming the wild elephant: mindfulness and its role in overcoming automatic mental processes. In: Ostafin BD, Robinson MD, Meier BP (eds.), Handbook of Mindfulness and Self-Regulation. New York, NY: Springer, 2015, 47–63.doi: 10.1007/978-1-4939-2263-5_5 [DOI] [Google Scholar]
- Pagnoni G. Dynamical properties of BOLD activity from the ventral posteromedial cortex associated with meditation and attentional skills. J Neurosci 2012;32:5242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda R, Bharath RD, Upadhyay N et al. Temporal dynamics of the default mode network characterize meditation-induced alterations in consciousness. Front Hum Neurosci 2016;10:1–12.doi: 10.3389/fnhum.2016.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Zorzi M, Corbetta M. The secret life of predictive brains: what’s spontaneous activity for? Trends Cogn Sci 2021;25:730–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Wang M, Northoff G. Linking bodily, environmental and mental states in the self – a three-level model based on a meta-analysis. Neurosci Biobehav Rev 2020;115:77–95.doi: 10.1016/j.neubiorev.2020.05.004 [DOI] [PubMed] [Google Scholar]
- Raffone A, Srinivasan N. An adaptive workspace hypothesis about the neural correlates of consciousness: insights from neuroscience and meditation studies. Attention 2009;176:161–80.doi: 10.1016/s0079-6123(09)17620-3 [DOI] [PubMed] [Google Scholar]
- Reddy JSK, Roy S. Commentary: patanjali and neuroscientific research on meditation. Front Psychol 2018;9.doi: 10.3389/fpsyg.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JSK, Roy S. Understanding meditation based on the subjective experience and traditional goal: implications for current meditation research. Front Psychol 2019;10.doi: 10.3389/fpsyg.2019.01827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Larios J, Faber P, Achermann P et al. From thoughtless awareness to effortful cognition: alpha - theta cross-frequency dynamics in experienced meditators during meditation, rest and arithmetic. Sci Rep 2020;10:2020.doi: 10.1038/s41598-020-62392-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Egiziano E, D’Arista S et al. Mindfulness‐based stress reduction training modulates striatal and cerebellar connectivity. J Neurosci Res 2021;99:1236–52. [DOI] [PubMed] [Google Scholar]
- Savostyanov A, Tamozhnikov S, Bocharov A et al. The effect of meditation on comprehension of statements about one-self and others: a pilot ERP and behavioral study. Front Hum Neurosci 2020;13:1–12.doi: 10.3389/fnhum.2019.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibner HJ, Bogler C, Gleich T et al. Internal and external attention and the default mode network. NeuroImage 2017;148:381–9. [DOI] [PubMed] [Google Scholar]
- Schoenberg PL, Vago DR. Mapping meditative states and stages with electrophysiology: concepts, classifications, and methods. Curr Opin Psychol 2019;28:211–7. [DOI] [PubMed] [Google Scholar]
- Schoenberg PLA, Ruf A, Churchill J et al. Mapping complex mind states: EEG neural substrates of meditative unified compassionate awareness. Conscious Cogn 2018;57:41–53. [DOI] [PubMed] [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 2013;17:565–73. [DOI] [PubMed] [Google Scholar]
- Shiah Y-J. From self to nonself: the nonself theory. Front Psychol 2016;7:1–12.doi: 10.3389/fpsyg.2016.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Pihlsgård J, Berglind U et al. Mantra meditation suppression of default mode beyond an active task: a pilot study. J Cognit Enhancement 2017;1:219–27. [Google Scholar]
- Singleton O, Newlon M, Fossas A et al. Brain structure and functional connectivity correlate with psychosocial development in contemplative practitioners and controls. Brain Sci 2021;11:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Davidson RJ, Lutz A. Mental training as a tool in the neuroscientific study of brain and cognitive plasticity. Front Hum Neurosci 2011;5.doi: 10.3389/fnhum.2011.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Allen JW, Haack C et al. The impact of app-delivered mindfulness meditation on functional connectivity and self-reported mindfulness among health profession trainees. Mindfulness 2020;12:92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa GM, Lima-Araújo GL, Araújo DB et al. Brief mindfulness-based training and mindfulness trait attenuate psychological stress in university students: A randomized controlled trial. BMC Psychology 2021;9. doi: 10.1186/s40359-021-00520-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP et al. Default network activity, coupled with the Frontoparietal Control Network, supports goal-directed cognition. NeuroImage 2010;53:303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart-Harris R, Leech R et al. Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp 2014;35:5442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Roseman L, Kaelen M et al. Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol 2016;26:1043–50. [DOI] [PubMed] [Google Scholar]
- Tanaka GK, Maslahati T, Gongora M et al. Effortless attention as a biomarker for experienced mindfulness practitioners. PLoS One 2015;10:e0138561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang -Y-Y, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci 2015b;16:213–25. [DOI] [PubMed] [Google Scholar]
- Tang -Y-Y, Lu Q, Feng H et al. Short-term meditation increases blood flow in anterior cingulate cortex and insula. Front Psychol 2015a;6:1–4.doi: 10.3389/fpsyg.2015.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang -Y-Y, Tang R. Personality and meditation. Neurosci Meditation 2020;15–36.doi: 10.1016/b978-0-12-818266-6.00003-4 [DOI] [Google Scholar]
- Tang -Y-Y, Tang R, Posner MI. Brief meditation training induces smoking reduction. Proc Natl Acad Sci 2013;110:13971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang -Y-Y, Tang Y, Tang R et al. Brief mental training reorganizes large-scale brain networks. Front Syst Neurosci 2017;11.doi: 10.3389/fnsys.2017.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taren AA, Gianaros PJ, Greco CM et al. Mindfulness meditation training and executive control network resting state functional connectivity. Psychosom Med 2017;79:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J et al. Impact of meditation training on the default mode network during a restful state. Soc Cogn Affect Neurosci 2012;8:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telles S, Singh N, Naveen KV et al. A fMRI study of stages of yoga meditation described in traditional texts. J Psychol Psychother 2014;05:1–6.doi: 10.4172/2161-0487.1000185 [DOI] [Google Scholar]
- Thompson E, Varela FJ. Radical embodiment: neural dynamics and consciousness. Trends Cogn Sci 2001;5:418–25. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Fabbro F. Increases in the right dorsolateral prefrontal cortex and decreases the rostral prefrontal cortex activation after-8 weeks of focused attention based mindfulness meditation. Brain Cogn 2016;102:46–54. [DOI] [PubMed] [Google Scholar]
- Travis F, Parim N. Default mode network activation and transcendental meditation practice: focused attention or automatic self-transcending? Brain Cogn 2017;111:86–94. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Critchley H. Interoception beyond homeostasis: affect, cognition and mental health. Philos Trans R Soc B: Biol Sci 2016;371:20160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine ER, Sweet PLG. Meditation and attention: a comparison of the effects of concentrative and mindfulness meditation on sustained attention. Ment Health Relig Cult 1999;2:59–70. [Google Scholar]
- Valk SL, Bernhardt BC, Trautwein FM et al. Structural plasticity of the social brain: Differential change after socio-affective and cognitive mental training. Sci Adv 2017;3:1700489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk PAM, Janssen BH, Giommi F et al. Mindfulness meditation associated with alterations in bottom-up processing: psychophysiological evidence for reduced reactivity. Int J Psychophysiol 2010;78:151–7. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A et al. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev 2010;34:935–46. [DOI] [PubMed] [Google Scholar]
- van Lutterveld R, Houlihan SD, Pal P et al. Source-space EEG neurofeedback links subjective experience with brain activity during effortless awareness meditation. NeuroImage 2017a;151:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lutterveld R, van Dellen E, Pal P et al. Meditation is associated with increased brain network integration. NeuroImage 2017b;158:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter U, LeVan P, Borghardt TL et al. Content-free awareness: EEG-fcMRI correlates of consciousness as such in an expert meditator. Front Psychol 2020;10.doi: 10.3389/fpsyg.2019.03064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzelberg AJ, Luskin FM. The effect of a meditation training in stress levels in secondary school teachers. Stress Med 1999;15:69–77. [Google Scholar]
- Wolff A, Di Giovanni DA, Gómez-Pilar J et al. The temporal signature of self: temporal measures of resting-state EEG predict self-consciousness. Hum Brain Mapp 2018;40:789–803.doi: 10.1002/hbm.24412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A, Berberian N, Golesorkhi M et al. Intrinsic neural timescales: Temporal integration and segregation. Trends in Cognitive Sciences 2022;26:159–73. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Zhao X, Bi G et al. Alterations of regional homogeneity and functional connectivity following short-term mindfulness meditation in healthy volunteers. Front Hum Neurosci 2019;13.doi: 10.3389/fnhum.2019.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Vik A, Groote IR et al. Nondirective meditation activates default mode network and areas associated with memory retrieval and emotional processing. Front Hum Neurosci 2014;8.doi: 10.3389/fnhum.2014.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Tang -Y-Y, Posner M. Short-term meditation increases network efficiency of the anterior cingulate cortex. NeuroReport 2011;22:570–4. [DOI] [PubMed] [Google Scholar]
- Yang -C-C, Barrós-Loscertales A, Li M et al. Alterations in brain structure and amplitude of low-frequency after 8 weeks of mindfulness meditation training in meditation-naïve subjects. Sci Rep 2019;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang -C-C, Barrós-Loscertales A, Pinazo D et al. State and training effects of mindfulness meditation on brain networks reflect neuronal mechanisms of its antidepressant effect. Neural Plast 2016;2016:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA et al. Neural correlates of mindfulness meditation-related anxiety relief. Soc Cogn Affect Neurosci 2013;9:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Luh W, Duan W et al. Longitudinal effects of meditation on brain resting-state functional connectivity. Sci Rep 2021;11:1–14.doi: 10.1038/s41598-021-90729-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The technical details and main findings from all reviewed studies may be found in the Supplementary Material.


