Abstract
Backgrond and Objective
Crohn’s disease (CD) is a chronic inflammatory bowel disease that affects a wide age range. Hence, CD patients receive a variety of drugs over their life beyond those used for CD itself. The changes to the integrity of the intestine and its drug metabolising enzymes and transporters (DMETs) can alter the oral bioavailability of drugs. However, there are other changes in systems parameters determining the fate of drugs in CD, and understanding these is essential for dose adjustment in patients with CD.
Methods
The current analysis gathered all the available clinical data on the kinetics of drugs in CD (by March 2021), focusing on orally administered small molecule drugs. A meta-analysis of the systems parameters affecting oral drug pharmacokinetics was conducted. The systems information gathered on intestine, liver and blood proteins and other physiological parameters was incorporated into a physiologically based pharmacokinetic (PBPK) platform to create a virtual population of CD patients, with a view for guiding dose adjustment in the absence of clinical data in CD.
Results
There were no uniform trends in the reported changes in reported oral bioavailability. The nature of the drug as well as the formulation affected the direction and magnitude of variation in kinetics in CD patients relative to healthy volunteers. Even for the same drug, the reported changes in exposure varied, possibly due to a lack of distinction between the activity states of CD. The highest alteration was seen with S-verapamil and midazolam, 8.7- and 5.3-fold greater exposure, respectively, in active CD patients relative to healthy volunteers. Only one report was available on liver DMETs in CD, and indicated reduced CYP3A4 activity. In a number of reports, mRNA expression of DMETs in the ileum and colon of CD patients was measured, focussing on P-glycoprotein (p-gp) transporter and CYP3A4 enzyme, and showed contradictory results. No data were available on protein expression in duodenum and jejunum despite their dominant role in oral drug absorption.
Conclusion
There are currently inadequate dedicated clinical or quantitative proteomic studies in CD to enable predictive PBPK models with high confidence and adequate verification. The PBPK models for CD with the available systems parameters were able to capture the major physiological influencers and the gaps to be filled by future research. Quantification of DMETs in the intestine and the liver in CD is warranted, alongside well-defined clinical drug disposition studies with a number of index drugs as biomarkers of changes in DMETs in these patients, to avoid large-scale dedicated studies for every drug to determine the effects of disease on the drug’s metabolism and disposition and the consequential safety and therapeutic concerns.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-022-01169-4.
Key Points
| There was no uniform and simple trend linking the changes in physiology/biology in Crohn’s disease patients to changes in drug bioavailability and disposition. |
| Inadequate clinical and proteomics data were identified as hurdles to incorporate relevant information into physiologically based pharmacokinetic models for creation of reliable predictive virtual populations of Crohn’s disease patients. Initial assessment of the models identified the major physiological influencers and the knowledge gaps for prudent prediction of altered pharmacokinetics in Crohn’s disease. |
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that can affect any part of the gastrointestinal (GI) system. The mucosal layers of the intestine, ileum and colon segments are predominantly affected [1, 2]. Although duodenum and jejunum involvement is not as common, these segments play a major role in drug pharmacokinetics (PK), as they represent the largest surface area and have an abundance of metabolising enzymes and transporters [3, 4]. Most CD patients are diagnosed before the age of 30, and patients are susceptible to cancer and arthritis, as well as cardiovascular, respiratory, kidney and liver diseases [5, 6]. Hence, over a lifetime, these patients receive many different drugs beyond those aimed to control CD itself. It is estimated that the prevalence of CD is up to 322 per 100,000 persons in Europe, with corresponding numbers in Asia and North America being 67.9 and 319, respectively [7]. Despite the high prevalence of CD, unlike other organ impairments such as those related to renal or hepatic function [8, 9], there are no mandatory requirements by regulatory agencies for clinical studies to be conducted on drugs entering market, to assess variable exposure to drugs in these patients. It might be assumed that changes to the fate of drugs in CD, based on the current knowledge on drug studies in CD, indicates minimal variations from healthy volunteers, which has led to a lack of legislation. However, this is not the case, and the more likely reason is associated with practical issues in adding further dedicated studies to those already in place for renal and hepatic impairment. Indeed, many other comorbidities may be excluded from clinical trials during drug development, for example, a typical, similar case would be patients undergoing bariatric surgery [10]. The issue of a lack of diversity in patient populations during drug development has been addressed in recent guidance by the US Food and Drug Administration (FDA), which encouraged the sponsors to broaden the inclusion criteria for clinical studies [11].
Following several position papers on special populations [12, 13], the possibility of using physiologically based pharmacokinetic (PBPK) models to fill in the gaps in guiding informed dosage adjustment (when necessary) in a sub-group of patients, as an alternative to leaving the drug labels silent, seems a prudent approach until dedicated studies become available. Indeed, the recent FDA guidance for the conduct of renal impairment studies during drug development [9] has mentioned PBPK as a potential approach for informing prescribers regarding any actions until adequate data are gathered from dedicated clinical studies or real-world data analysis.
The major aims of the current report are twofold:
Gather evidence regarding changes to the fate of drugs in CD, with a view to indicating any trend that can be discerned.
Extract relevant systems information for PBPK in CD and create a repository that helps with the creation of CD population files in PBPK modelling.
We also conducted a preliminary application of PBPK for CD for two case examples (the benzodiazepine midazolam and the corticosteroid budesonide) for verification and testing the adequacy of the model versus existing major gaps. The two drugs are both substrates of cytochrome P450 3A4 (CYP3A4). However, as would be expected from their different uses, there are subtle differences between midazolam and budesonide that may be used to identify the influence of different systems parameters. Midazolam is a well-known CYP3A4 probe, which allows investigation of the influence of CD on the liver and intestine separately by comparing relative PK in CD patients versus healthy volunteers after an intravenous (IV) and oral dose. The investigated dosage form is given as a solution, allowing for assessment of the effects on the upper intestine. On the other hand, budesonide is a substrate for several enzymes (CYP3A4, CYP2C9 and CYP1A2) and P-glycoprotein (p-gp), which allows us to investigate the influence of other factors beyond CYP3A4. Budesonide is a very high extraction ratio drug (Eh = 0.9) [14]; hence, it allows the assessment of the effects from changes to mesenteric blood flow (cardiac output) in CD. Budesonide is given in a controlled-release formulation, where its release is triggered by entering a basic environment (pH > 5.5); it is expected to be sensitive to changes that occur in the lower intestine. The two drugs are highly bound to albumin (80–90%). Both drugs have shown significant alteration in oral bioavailability in CD patients, based on clinical studies. Moreover, they are clinically relevant to CD patients, as IV midazolam is used before endoscopy procedures as an anxiolytic, while oral budesonide is used for treatment of CD itself.
Methods
Data Gathering and Analysis for Kinetics of Orally Administered Drugs in the Crohn’s Disease (CD) Population
A systematic review of the reported PK in the CD population was conducted with the specific aim of assessing any exposure (bioavailability/clearance) changes compared to what was known for the same drug in healthy volunteers. A literature search concluded by March 2021 was run in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) using the terms (“Crohn’s disease” and “inflammatory bowel diseases”) in combination with (“pharmacokinetic”, “bioavailability”) for oral and IV drug formulations (Fig. 1). The search was not limited to particular time periods. Only original research articles concerning small molecule agents were included. An initial screening of titles and abstracts was carried out to identify the inclusion of a PK analysis of the drug in CD adult patients. This was followed by extended screening of the results to identify the presence of the required PK data to allow the drug exposure/clearance analysis. Restriction of the search to reports where PK were assessed in both CD and healthy volunteers within the same study could severely reduce the number of cases for analysing the trends. The same was true in narrowing the studies only to those conducted in the fasting state or excluding the studies due to small sample size. Whenever a drug lacked the information in healthy volunteers in the same study, a literature search was done by the name of the drug and the terms “pharmacokinetic” and “bioavailability”. Hence, the only exclusion was dis-similarity of the trial methodology (dosage regimens, fed or fasted state, formulation and route of administration) between the studies conducted in CD patients versus those in healthy volunteers, particularly when a large dose normalisation was required.
Fig. 1.

An illustration of the process of literature search in relation reports on the systemic exposure of drugs in adults Crohn’s disease relative to healthy volunteers (carried out using PubMed; March 2021). PK pharmacokinetics
The area under the curve (AUC) of the concentration–time profile for each of the drugs both in CD patients and healthy volunteers was the basis for the calculation of the relative drug exposure following dose normalisation (when needed) and the relative clearance (CL) when accounting for IV drug in each group, respectively. Since the values of the two populations could arise from different studies, we had to ensure the nature of the AUC for comparison was the same (e.g. extrapolated AUC0–∞, steady-state AUCss or truncated AUC0–t). All drugs without information on AUC (for oral) and CL (for IV) from healthy volunteers in studies reasonably similar in design to those performed in the CD population were excluded, since no reliable relative bioavailability or clearance could be inferred.
The calculation of relative exposure and clearance between the CD and healthy population (CD/healthy), , and its 95% confidence interval (CI), applied Fieller’s Theorem [15–17], as per Eq. 1:
| 1 |
where a is the mean AUC for the CD population and b is the mean AUC for the healthy volunteer population, is the inverse of the cumulative t distribution (two tailed) for a significance level of α (0.05), and degrees of freedom (df) is equal to na + nb − 2, where na and nb are the number of participants in the study for the estimation of a and b, respectively, and is the combined standard error for the ratio , and it is calculated from Eq. 2:
| 2 |
where the quantity g is given by Eq. 3, and SEa and SEb are the standard error of the means a and b, respectively:
| 3 |
Demography of the CD Population
In general, CD is more common in the Caucasian population than in people of African and Hispanic ethnicities in North America. Various prevalence values are reported for Europe, North America, Oceania, Africa, South America and Asia in the literature [7, 18, 19].
For adults, the peak incidence of CD is between the ages of 20 and 40, with a less common second peak around the age of 60–70, making the demography of CD bimodal [20–22]. The incidence of CD is marginally higher in females compared with males, as suggested by several studies [23–28].
In our study, the demography used mimics the demography of the corresponding clinical studies that we were simulating for the model verification.
Systems Parameters Defining Physiologically Based Pharmacokinetics (PBPK) in CD Patients
A meta-analysis of the literature data was conducted to identify the relevant sources of changes in the bioavailability of oral drugs, and to extract data related to the physiological and anatomical changes of the intestine and whole body in CD patients. The literature search was run in PubMed (October 2021), using the following terms (“small intestine pH”, “large intestine pH”, “gastric emptying time”, “intestine transit time”, “serum albumin”, “alpha acid glycoprotein”, “liver blood flow”, “portal vein flow”, “superior mesenteric artery blood flow”, “cardiac output”, “ABC transporter”, “solute carrier transporter”, “metabolising enzymes”, “CYP” and “proteomics”) in combination with “Crohn’s disease”. Screening of titles and abstracts was carried out to identify the studies with a clear indication of the required information in CD patients. No time frame of the research was applied.
Inclusion of data was restricted to original articles, studies that provided information where determination of the mean, SD and CI is possible, studies that reported the level of the parameter at the baseline (before the treatment intervention), and studies including both CD patients and healthy volunteers. Only the adult population and English publications were considered. Studies that did not report the values of the CD group separately from other diseases were excluded. Some studies included non-IBD patients as the control group instead of healthy volunteers. When such studies are included in the analysis (owing to the low number of the available studies), it is indicated in the results section. For all parameters, differentiation of the results based on active or inactive CD phase is considered. If it was not clearly indicated in the study whether the patients were active or inactive, they were considered mixed (active and inactive).
The activity of CD disease is defined by a subjective scoring index based on the clinical manifestation of the patient in most of the included studies. The most common scoring systems used are the Harvey-Bradshaw Index (HBI) [29] and Crohn’s Disease Activity Index (CDAI) [30]. In the case of reporting the activity based on an objective index where biomarkers, endoscopy or other markers are assessed, this is reported in the results section if applicable. Clinical remission does not always reflect histological/endoscopic remission, hence the reported activity according to the symptom-based scores and the objective markers [31, 32].
For drug metabolising enzymes and transporters (DMET) expression, all studies are included if a fold change of CD value to control is reported or can be determined without distinguishing between inflamed or non-inflamed tissue. If both values from inflamed and non-inflamed tissues are separately available, only the values of inflamed tissues are included. Only studies or values that reported the expression level in CD patients and not in a mixed set of IBD (ulcerative colitis [UC] and CD) patients are included. Several reports did not provide the raw values; thus, the values used here are based on a digitally extracted estimate from the figures. As only a few reports are available, the weighted ratio was calculated from all the included studies regardless of the nature of the assay used to determine the expression.
The identified physiological aspects that can alter oral drug bioavailability depending on the nature of the drug and formulation are summarised in Fig. 2. Previous review [33] and analysis [34] covered some of the aspects considered in our analysis in CD patients, which was checked to assure inclusion of the collected available data. The collected information was stratified based on different segments of the intestine, differentiation between active and inactive CD, sex and fasting state whenever possible. Figure 3 shows a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of the flow for inclusion and exclusion of studies in the meta-analysis. Inadequate or incomplete information is highlighted, as such information can compromise the accuracy of the PBPK model projections.
Fig. 2.
Illustration of physiological parameters that were the subject of the systematic literature review and subsequent meta-analysis in relation to CD. The arrows represent the general trend seen in active (black arrow), inactive (grey arrow) and mixed (undistinguished active and inactive) [stripe arrow] CD patients. The question marks represent the parameters that are not incorporated in the model due to unavailability of data in the targeted population, inconsistencies in the methods or unclear utility in Simcyp® simulator. (Figure is adapted using a public domain image by Mikael Häggström, CC0, via Wikimedia Commons: https://commons.wikimedia.org/wiki/File:Adult_male_with_organs.png). AGP acid glycoprotein, CD Crohn’s disease, DMET drug metabolising enzymes and transporters, GI gastrointestinal
Fig. 3.
PRISMA flowchart of studies systemically reviewed to be included in the meta-analysis of the physiological parameters related to CD. CD Crohn’s disease, CI confidence interval, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
The collected physiological parameters were analysed based on the weighted mean and overall standard deviation using the reported mean and SD as previously reported [35]. Similarly, we followed the previously reported procedure for converting median values and ranges to mean and SD when not provided in the publications [36].
PBPK Application for Obtaining Systems Parameters and Its Applications for Verification of the CD Model
Reverse translation Two CYP3A substrates, midazolam and budesonide, were investigated by means of PBPK modelling, utilising the Simcyp® Simulator M-ADAM absorption model. The details of model assumptions and infrastructure for a multilayer gut wall [37] and the general attributes of the advanced dissolution, absorption and metabolism (ADAM) model are described elsewhere [38]. However, to build a CD population with all relevant known physiological differences in intestine and other organs, we had to incorporate relative changes in each parameter into the population library files within the simulator, based on our analysis of the systems parameters described in the previous section. Additionally, a reverse translation approach was used based on a midazolam IV clinical study in CD [39] to determine the value of the liver CYP3A4. Hence, the value of hepatic clearance (CLH) was used to estimate the intrinsic unbound metabolic clearance (CLu-int), knowing the hepatic blood flow (QH) and fraction unbound in blood (fuB) according to Eq. 4:
| 4 |
The relative reduction in intrinsic clearance (CLint) in CD patients compared to healthy volunteers [39, 40] was considered as a reflection of reduced hepatic CYP3A4 expression. These changes were incorporated in the CD population file that was used in modelling other cases. The input parameters used to create the inactive and active CD population framework are summarised in Table 1.
Table 1.
Summary of the Input system parameters in Simcyp® simulator of the active and inactive CD population model verification, based on their relative alteration from healthy population as per the data analysis carried out in this study
| Physiological parameter | Inactive CD (fed) | Active CD (fasted) | Source of alteration in CD | |||
|---|---|---|---|---|---|---|
| GIT alterations | ||||||
| pH proximal SI | Fed | Fasted | Fed | Fasted | Reference | |
| Unaltered from normal valuesa |
Duodenum = 6.3 Jejunum I and II = 6.4 and 6.5 |
Unaltered from normal valuesa |
Duodenum = 6.7 Jejunum I and II = 6.8 and 6.9 |
Relative change based on our meta-analysis | ||
| pH distal SI | Fed | Fasted | Fed | Fasted | ||
| Unaltered from normal valuesa | Ileum (I–IV) = 7, 7.2, 7.3 and 7.5 | Unaltered from normal valuesa | Ileum (I–IV) = 7.1, 7.2, 7.3 and 7.4 | |||
| pH large intestine | Fed | Fasted | Fed | Fasted | ||
| Unaltered from normal valuesa | Colon = 7.6 | Unaltered from normal valuesa | Colon = 6.5 | |||
| GIT alterations, segregated transit time model | ||||||
| Gastric MRT (h) | Fed | Fasted | Fed | Fasted | Reference | |
| 1.46 | 0.3 | 1.8 | 0.26 | Relative change based on our analysis | ||
| SITT (h) | Fed | Fasted | Fed | Fasted | ||
| 3.8 | 3.2 | 3.8 | 4.2 | |||
| Ascending colon transit time (h) | Fed | Fasted | Fed | Fasted | ||
|
Male = 9.9 Female = 12.1 |
Unaltered from normal valuesa |
Male = 3.7 Female = 4.5 |
Unaltered from normal valuesa | |||
| Intestine CYP3A4 | SI (nmol/SI) | Colon (nmol/colon) | SI (nmol/SI) | Colon (nmol/colon) | ||
| 52.3 | 2.4 | 52.3 | 2.4 | |||
| Intestine CYP2C9b | SI (nmol/SI) | Colon (nmol/colon) | SI (nmol/SI) | Colon (nmol/colon) | ||
| 13.75 | 0.1 | 13.75 | 0.1 | |||
| Intestine p-gpb | SI (pmol/mg) | Colon (pmol/mg) | SI (pmol/mg) | Colon (pmol/mg) | ||
| Ileum I–IV = 1.2 | 0.17 | Ileum I–IV = 1.2 | 0.55 | |||
| Systemic circulation alterations | ||||||
| SI blood flow (% of CO) | Male | Female | Male | Female | Reference | |
| 11.1 | 12.2 | 15.3 | 16.8 | Relative change based on our meta-analysis | ||
| QH (% of CO) | Male | Female | Male | Female | Reference | |
| 21 | 23.8 | 25.73 | 27.39 | Proportional to the increase in the intestine flow ratec | ||
| HSA (g/L) | Scenario 1 | |||||
| Male | Female | Male | Female | |||
| 44.8 | 43.9 | 30.13 | 25.2 | |||
| Reference | Inactive based on the relative change based on our analysis (no differentiation between active and inactive CD) | Reduction of HSA was derived from a study that reported the albumin level of male and female separately in active CD [41]. The HSA reduced by 49% in female and 40% in male relative to healthy reference level | ||||
| Scenario 2 | ||||||
| Unaltered from normal values in one simulation. (Two active CD simulations were carried out once with reduced HSA and once with normal HSA levels) | ||||||
| Reference | The reduction of HSA was not assumed to be the definite state of CD patients, as only around 20% of CD population suffer from HSA drop based on the collected literature data | |||||
| Liver alterations | ||||||
| Liver CYP3A4 (pmol/mg protein) | Unaltered from normal valuesa | Male | Female | Reference | ||
| 55.4 | 80.5 | Reverse translation based on FH value [31] | ||||
CD Crohn’s disease, CO cardiac output, GIT gastrointestinal track, HSA human serum albumin, MRT mean residence time, QH hepatic blood flow, p-gp P-glycoprotein, SI small intestine, SITT small intestine transit time, FH the fraction of drug escaping first-pass hepatic metabolism and biliary secretion
aNo available information that supports alteration from normal values
bUsed with budesonide only
cNo available literature data on the liver blood flow
The study design taken from each clinical case was used for building and verifying the drug PBPK models in healthy volunteers (drug PBPK models in healthy volunteers: budesonide IV [41], budesonide oral as Entocort® [42], midazolam oral [40]) in the Simcyp® Simulator V19 and using the default healthy volunteer population. The study design included the information on age range, female-to-male ratio, fasting status and dosage regimen. The clinical studies for budesonide in the CD population were conducted under different conditions [39, 42–44]. Our focus was on the study that showed a significant difference [42] in budesonide bioavailability in CD patients compared to healthy volunteers. Midazolam was studied in only one study for assessing bioavailability difference in CD [39]. The drug-specific parameters of budesonide were mainly taken from the report by Effinger et al. [45], since there is no budesonide default library in the library set of compounds in the Simcyp® simulator. For midazolam, the available Simcyp® drug model from the compound library was used as a starting point; this is verified and published in several reports and frequently used in regulatory submissions [46, 47]. The key modifications that we had to make, for the purpose of later applications in CD, were the selection of the M-ADAM absorption model option (in preference to the default ADAM) and the selection of full-PBPK (as opposed to the default of minimal-PBPK). Input parameters used in the Simcyp® simulator for building budesonide and midazolam drug profiles are summarised in Tables S1 and S2, respectively (see the Electronic Supplementary Material).
Application The observed concentration–time profiles of budesonide and midazolam were derived using GetData Graph Digitizer 2.26. We considered females and males as having different CYP3A4 abundance in all simulations (one of the options in the Simcyp® simulator), as this is more consistent with the gender difference reported for CYP3A4 substrate clearance values [48]. All trial sizes were based on 100 participants (ten participants in ten trials). Details of the trial design for clinical studies of budesonide IV [43], budesonide controlled-release formulation [42] and midazolam oral solution [39] are in Table S3 (see the electronic supplementary material). The simulated concentration–time profiles of budesonide and midazolam were visually inspected by comparing the mean and 95% prediction interval against the observed clinical data. The similarity of the predicted relative mean values of maximum drug concentration (Cmax), AUC0–∞ and time to reach Cmax (Tmax) to the observed oral budesonide and midazolam values were assessed.
Global Sensitivity Analysis (GSA)
The impact of the identified systems parameters in Table 1 on the PK properties of the oral drugs of interest is investigated utilising the Global Sensitivity Analysis (GSA) function within Simcyp®. GSA is important as the physiological alterations encountered by CD patients vary in their effects on different oral drugs based on several disease and drug factors. The determination of the impact spectrum and identification of the key parameters were performed based on physicochemical and PK attributes of budesonide and midazolam and the integrated systems data in the CD population framework. The frequency distributions of the investigated systems parameter values, within the selected range for each parameter, were considered to be uniform. The applied range of the lower bound and upper bound was based on the collected literature systems parameters in healthy volunteers and the active CD population. The Morris method for determining GSA was selected, where it defines the parameters’ impact based on their influence on the output variable. It also accounts partly for the interaction between the investigated parameters [49].
The influence of the different systems parameters on altered bioavailability is reflected in the simulated PK properties Cmax, AUC and Tmax of budesonide and midazolam oral formulations based on their trial specifications. The influential parameters determined by the GSA were compared with the extracted available literature data, to identify the gaps in the key parameters and to guide the decision for future research needed to be carried out to fill these gaps, to allow for the reliable prediction of active and inactive CD PBPK population models.
Results
Altered Kinetics of Orally Administered Drugs in the CD Population
The systematic data analysis on the exposure of orally administered drugs in the CD population relative to healthy volunteers, based on the criteria set in Sect. 2, is summarised in Fig. 4. Knowing there are several modified physiological parameters in the GI tract of CD patients, the number of cases that passed the rigor of the inclusion criteria was not sufficiently high to indicate a consistent trend. For instance, the exposure of CYP3A substrates did not show the same magnitude of change [39, 42, 43, 50–56] in the same phase of the disease (active/inactive). Also, when S- and R-verapamil [50] and midazolam and budesonide [39] were given to the same CD patients, the exposure behaviour was not similar. The results from different studies on the same drug were also inconsistent, as seen with budesonide and mesalamine [39, 42, 43, 57, 58]. It should be noted that the type of formulation and other attributes of the drugs could play a role in these observations. Although S-verapamil showed the highest level of increased relative exposure (8.7-fold, with 9.6-fold higher Cmax), since there was no comparative IV data in the CD population, these could not be assigned solely to changes in bioavailability. The relative fold changes in exposure for midazolam and budesonide were 5.3 and 1.9 higher, respectively. There were comparative IV data for CL in CD and healthy populations to discern the source of variation [39, 43].
Fig. 4.

The exposure to given oral doses of drugs given to Crohn’s disease (CD) patients relative to healthy volunteers. The lower panel represents controlled-release formulations, and the upper panel shows the values in the case of immediate-release/solution formulations. The markers represent the mean of relative exposure and the bars represent the 95% confidence intervals. The black, grey and patterned fill of markers indicates active, inactive and a mixture of CD patients, respectively. All studies were done under fasted conditions unless indicated as ‘fed’
A number of studies showed comparable exposure in CD patients and healthy volunteers [43, 51, 53, 54]. p-gp substrates (propranolol, fexofenadine and budesonide) did not show the same direction of change [39, 42, 59, 60]. Of the three drugs, fexofenadine is a p-gp probe, while propranolol and budesonide are metabolised by CYP enzymes. The activity of the CD patients in the propranolol study was determined based on the erythrocyte sedimentation rate (ESR). The details of the relative exposure of orally administered drugs in CD patients are in Table S4 (see the Electronic Supplementary Material).
Some of the included drugs were studied under specific conditions that might have contributed to the outcome. One of the two prednisolone studies included had inactive CD patients as the control group, and the diseased group was a mix of active UC and CD patients, where the activity was determined by clinical, biochemical, endoscopic and biopsy criteria [53]. Yet, the exposure result was similar to the other study, where the control group was healthy volunteers and only CD patients were in the test group [52]. For mesalamine, one of the two included studies had CD patients in the remission state or with low activity only [57]. In the other mesalamine study, six out of the nine CD patients had undergone resection of the terminal ileum, where they have reported lower AUC values [58]. A similar observation was reported with cyclosporine [54] and metronidazole [51] in patients with a history of bowel resection, indicating a lower absorption profile compared with other CD patients with an intact intestine.
The reports on CL values for various drugs following IV administration to CD patients showed no substantial difference compared to healthy volunteers except in the case of midazolam, which was 3.7-fold lower (Fig. 5). Since all the other drugs included in the comparison were also CYP3A4 substrates, midazolam being distinct from the other drugs cannot be related to the route of metabolism alone. The details of the relative CL for drugs administered via the IV route in CD patients are in Table S5 (see the Electronic Supplementary Material).
Fig. 5.

The clearance of drugs in Crohn’s disease (CD) patients relative to corresponding values in healthy volunteers following intravenous (IV) dose. The markers represent the mean of relative clearance and the bars represent the 95% confidence intervals. The black and patterned fill of markers indicates active or a mixture of active/inactive CD patients, respectively. All drugs are substrates of CYP3A to varying degrees
Demography of the CD Population
Although the simulation of a large group of CD patients requires implementation of the general demography for the CD population, the objective in our study was not to simulate such a large CD cohort, but was to focus on replicating the observed clinical studies on the kinetics of midazolam and budesonide. Hence, we only applied the demography of the patients reported in each study, rather than the overall demography of CD patients in the general population.
Major Systems Parameters Defining PBPK in CD Patients
Intestinal pH The pH values of the small and large intestines of CD patients were reported in five studies (Fig. 6a) based on the criteria previously described in Sect. 2 [61–65]. The CD activity in one study was based on the level of serum proteins [65], but it did not show difference in the alteration of the pH compared to the other studies where the CDAI was used to indicate the activity of CD. All the studies were relatively small (≤ 15 CD patients). From our meta-analysis (Fig. 6b), only the pH of the large intestine in inactive CD patients showed a statistically significant increase relative to healthy volunteers. The reports were not consistent, but the reported pH of the proximal small intestine showed a general trend towards higher values, particularly in the active stage. The trends for the distal small intestine and large intestine were slightly more divergent, showing pH values to be higher, similar or lower than those for healthy volunteers (Fig. 6a). Only two studies reported the pH values for the active and inactive groups separately. No pH data in CD patients were reported in the fed state, although pH values are reported in a mix of IBD (UC and CD) patients in the fed state. Details of the reported values in the literature are given in Table S6 (see the Electronic Supplementary Material).
Fig. 6.
The pH of the proximal and distal small intestine (SI) and the large intestine in active and inactive Crohn’s disease (CD) populations compared to healthy volunteers in the fasted state. Part a shows the reported pH values. Part b shows the weighted mean pH from all the studies and the associated 95% confidence intervals. The white, black, grey and patterned fill of markers indicates healthy, active, inactive and a mixture of CD, respectively
Gastric emptying time Ten studies reported gastric emptying time in CD patients alongside the control groups, under fed [42, 66–70] and fasted conditions [71–74]. Under fed conditions, a general trend of a higher gastric emptying time in CD patients was observed, while a similar value was seen under fasted conditions (Fig. 7a). Two studies had non-IBD patients as the control group [73, 74] and one had a mix of healthy and non-IBD patients [72], where gastric emptying time was measured under fasted conditions. Our analysis showed statistical significance of the gastric emptying time in both active and inactive CD patients compared to healthy volunteers only under fed conditions (Fig. 7b). Five of the included studies reported mixed active and inactive CD patients without distinguishing between the two states of the disease [42, 67, 70–72]. The sizes of the studies were between six and 96 CD patients. Details of reports on gastric emptying time in CD patients are provided in Table S7 (see the Electronic Supplementary Material).
Fig. 7.
The gastric emptying time (GET) in Crohn’s disease (CD) patients compared to healthy volunteers in fasted and fed states. Part a shows the values of GET in each study. Part b shows the weighted value of GET from all studies associated 95% confidence intervals. The white, black, grey and patterned fill of markers indicates healthy, active, inactive and mixture of CD, respectively
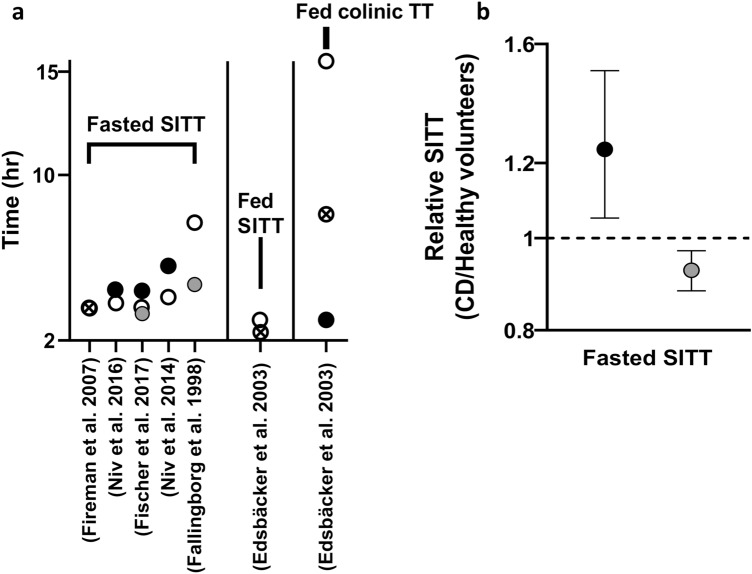
Intestinal motility and transit time Three studies reported the small intestine transit time in CD patients alongside the control healthy groups [42, 62, 71], and three studies had non-IBD patients as the control group [73–75]. The observations from these studies are summarised in Fig. 8a. One study reported the transit time in the fed state and in the colon [42], which does not allow for comparative analysis of the changes between the diseased and healthy states. The highest reduction in small intestine transit time was seen in a study in which patients with inactive CD who had undergone ileocecal resection [62] were compared with controls. A statically significant prolonged small intestine transit time in active CD and shortened time in inactive CD compared to healthy volunteers are indicated from our analysis (Fig. 8b). The sizes of the studies were between nine and 96 CD patients. Details of all studies on intestinal transit time in CD patients are included in Table S8 (see the Electronic Supplementary Material).
Fig. 8.
The small intestine transit time (SITT) and the colonic transit time (TT) in Crohn’s disease (CD) patients compared to healthy volunteers. Part a shows the values of TT in each individual study. Part b shows the weighted values for SITT from all studies and the associated 95% confidence intervals. The white, black, grey and patterned fill of markers indicates healthy, active, inactive and mixture of CD, respectively
Vascularity and haemodynamics of blood flow The intestine morphology and vascularity differ in the active stage of the disease and in the stage where the inflammation is controlled. The key identified differences were linked to gut blood flow and, hence, oral drug PK; our focus here is on the change of the superior mesenteric artery (SMA) blood flow rate, as it represents an important aspect for oral drugs with high extraction. Ten studies reported the SMA blood flow in CD patients alongside that of healthy volunteers (Fig. 9a) [76–85] based on our inclusion criteria. The meta-analysis showed a statistically significant increase in the SMA flow in both active and inactive CD patients relative to healthy volunteers (Fig. 9b), with a higher increase recorded in the active CD phase. One study used X-ray alongside clinical signs to indicate the disease activity [77]; the alteration in the SMA of both active and inactive CD patients was very similar to the observed alteration in other studies where the CDAI was used. The sizes of the studies were between nine and 74 CD patients. Details of all studies on intestinal transit time in CD patients are included in Table S9 (see the Electronic Supplementary Material).
Fig. 9.
The superior mesenteric artery (SMA) blood flow (mL/min) in Crohn’s disease (CD) patients compared to healthy volunteers. Part a shows the SMA blood flow from each study. Part b shows the weighted value of SMA blood flow from all the studies and the associated 95% confidence intervals. The white, black, grey and patterned fill of markers indicates healthy, active, inactive and mixture of CD, respectively
There are few studies measuring the blood flow of the portal vein. An increase is seen in the portal vein blood flow [83, 86], which is ~ 50% higher in active and inactive CD patients than in healthy volunteers [87].
Another aspect is the intestine regional blood flow, which was shown to differ between intestinal regions based on the activity of the disease, where the colonic blood flow was significantly increased in the active stage, while the ileum blood flow was significantly decreased in the inactive stage compared with healthy levels [88]. Other changes encountered by the intestine due to inflammation in CD that might affect the PK of oral drugs are reported. These include dilation of the blood vessels [78, 89], change of the intestine vascularity [90–92], change of intestine blood perfusion [90–94], increase in the thickness of the intestine wall (active CD > 4 mm) [78, 80, 81, 83, 89, 93], submucosal fibrosis and muscularisation [91] and wrapping of the intestinal wall in fat wrap [95], but they are beyond the focus of this analysis.
Expression of DMETs in the intestine The effect of CD inflammation on the expression of DMETs has not been extensively studied. In total, we found 13 publications that measured DMETs from CD patients in inflamed and non-inflamed intestinal tissues. Seven reported drug metabolising enzymes (DMEs) (mainly CYPs) [96–102], seven reported ABC transporters [96–101, 103, 104] and six reported solute carrier (SLC) and/or solute carrier organic anion (SLOC) expression [98, 101, 105–108]. In addition, a case report study on a duodenum biopsy reported an increase in p-gp expression of greater than twofold in a CD patient, as compared with healthy specimens [109].
Four studies were excluded from our analysis. Two had combined UC and CD patients, considered gene expression values from rectum inflamed tissue [96] and non-inflamed tissue from upper and lower segments of the intestine, and had no control group to extract fold change values for use in our analysis [101]. One study reported the expression value of DMEs from the ileum and colon combined, which did not allow for differential analysis of the expression based on the segment [102]. The last study had the values reported in a previous study compared to healthy instead to non-inflamed tissue [104].
The available abundance data are of two kinds: relative abundances of proteins measured at the protein level by immunoblotting techniques, and reports of mRNA levels being used as proxies for the protein abundances. The gold standard for measuring absolute abundances of proteins in complex mixtures is now generally considered to be tandem mass spectrometry (LC/MS-MS) of protein digests, and in no case has such an analysis been reported. Six of the included publications had healthy volunteers as the control group [97, 98, 100, 103, 106, 107], while the rest had non-IBD patients as the control group [99, 105, 108]. DMET abundances in the ileum, which is known to have a more dominant role in oral drugs than the colon, were reported in six studies. There are no reports of expression of DMETs in the upper intestine segments (duodenum, jejunum) in CD, but these are rarely affected by inflammation and are, therefore, less relevant.
The lack of a clear distinction between active and inactive CD in the available studies makes it hard to determine the extent of inflammation-caused changes in DMET expression in the different phases. Only one study compared expression levels in active and inactive CD patients [98]. In both disease phases, the investigated DMETs were altered compared with control, but, unsurprisingly, alterations were more pronounced in active CD patients. The expression of absorption, distribution, metabolism and excretion (ADME) proteins was reported in samples that were not differentiated for being in an active or inactive state of the disease [100, 105], with an indication that the active state does not affect the expression of CYP3A4 and p-gp [100]. The degree of change of expression from normal was shown to be proportional to the tissue inflammation [103, 105, 106].
The reported expression data show high variability in the changes (CD/control) of the ileum (Fig. 10a) and colon (Fig. 10b) DMETs of CD patients. From our analysis, OATP2B1 showed the highest increase in its expression in the ileum and colon, seven- and eightfold, respectively. Another protein, ASBT, showed the highest reduction in its expression in CD (~3.5- and 35-fold in the ileum and colon, respectively). In general, limited numbers of DMET isoforms were studied and very few were reported in more than one publication. UGTs are strikingly absent from these reports, with only UGT1A3 reported. In addition, there is no in vitro activity data on the human CD population. Details of the studied DMET abundances are summarised in Table S10 and S11 (see the electronic supplementary material).
Fig. 10.
The drug metabolising enzymes and transporters (DMETs) expression in Crohn’s disease (CD) patients relative to healthy volunteers. Part a shows the expression in ileum based on individual studies, and part b shows corresponding values in the colon. The bars represent the 95% confidence intervals (no bar means there was only a single measure)
Expression of drug metabolising enzymes and transporters in the liver There is no available direct information on the abundance or activity of DMETs in the livers of CD patients. One study reported a reduction of the hepatic extraction ratio (EH) in eight active CD patients using midazolam as a CYP3A4 probe [39]. From this study, we calculated the fraction of drug entering the liver and escaping first pass hepatic metabolism and biliary secretion (FH) in CD patients as 0.89 and in healthy volunteers as 0.57 [40]. This shows that the liver clearance (CYP3A4 activity) is decreased in CD and midazolam absorption has increased by ~ 56% compared with samples from healthy volunteers. We used an FH relative change (CD patients/healthy volunteers) of 1.5 as a reflection of the fold reduction in CYP3A4 abundance. Although, the FH value is not linearly related to the change in abundance or activity of CYP3A4, and a back-calculation of intrinsic clearance through Eq. 4 would be needed.
We assessed the suppression of CYP3A4 by back-calculation from the observed midazolam clearance after IV administration and accounted for the changes to blood flow and protein binding. An alternative approach would be to estimate the level of suppression based on the inflammatory proteins circulating in blood, particularly interleukin-6 (IL-6) [110–115]. The suppressive level of hepatic IL-6 is not known in CD patients. However, considering relative higher levels of IL-6 in the blood stream of CD patients [116, 117], some inferences on the lower level of CYP3A4 could be made. A recent simulation study has used such an approach [118]. Broadening the cases related to 3A4 activity in gut and liver as well as proteomics analysis of tissue samples from CD patients will give more confidence on the quantitative level of the changes that occur in these patients.
Blood proteins (albumin and α1-AGP) Human serum albumin (HSA) and α1-acid glycoprotein (α1-AGP) levels show dysregulation during CD. Dysregulation of drug–blood protein binding levels would be expected to affect the bioavailability of drugs, depending on the extent of drug–protein binding.
A literature search for HSA in CD patients and healthy adults identified a total of 27 studies (Fig. 11a). In 26 studies, participants with active and inactive CD had a lower albumin level compared with controls [52, 55, 57, 119–142], where one study used serum sialic acid as the activity indicator [134], which did not show a difference in the albumin changes compared to other studies that used the CDAI and HBI, and one study showed a slightly higher albumin level in inactive CD patients, where all participants had undergone an ileocecal resection [62]. Our analysis showed a statistically significant decrease in HSA in both CD phases, with active CD exhibiting a higher drop relative to healthy volunteers (Fig. 11b). The sizes of the studies were between nine and 247 CD patients. Details of HSA level studies in CD patients are summarised in Table S12 (see the Electronic Supplementary Material).
Fig. 11.
The human serum albumin (HSA) level (g/L) in Crohn’s disease (CD) patients compared to healthy volunteers. Part a shows the HSA level reported in individual studies. Part b shows the weighted HSA level relative to healthy volunteers from all the studies and the associated 95% confidence intervals. The white, black, grey and patterned fill of markers indicates healthy, active, inactive and mixture of CD, respectively
Among active CD patients, females showed a greater depletion in HSA than males, with both being significantly lower than healthy values [143]. The HSA level was significantly lower when the same set of CD patients was examined in active state compared with after remission [144]. The results of this study are in agreement with other publications where active CD patients showed a significant drop in albumin compared with inactive CD patients [57, 62, 145].
Importantly, the albumin level is not always low in CD patients, as illustrated by two large-scale publications of 117 [146] and 6082 [147] CD patients. The percentage of the patients who encountered a severe drop in their albumin (< 30 g/L) during active inflammation did not exceed 22%.
A total of eight studies were identified reporting α1-AGP levels in CD patients based on our research criteria [119, 120, 135, 142, 148–151]. The number of CD patients was between two and 51. The reported values of α1-AGP in active and inactive CD patients compared to healthy volunteers (Table S13; see the Electronic Supplementary Material) showed that only active CD patients had a significantly higher level compared to healthy volunteers, with the weighted mean determined to be 1.59 g/L (95% CI 0.75–2.97) compared with 0.85 g/L (95% CI 0.37–1.7) in healthy volunteers [135, 142, 148–150]. The α1-AGP of the inactive CD patients was in close proximity to that of healthy volunteers, with weighted means (95% CIs) of 1.15 g/L (0.7–1.8) and 0.86 g/L (0.34–1.8), respectively.
Verification Status PBPK Model with Current Systems Data
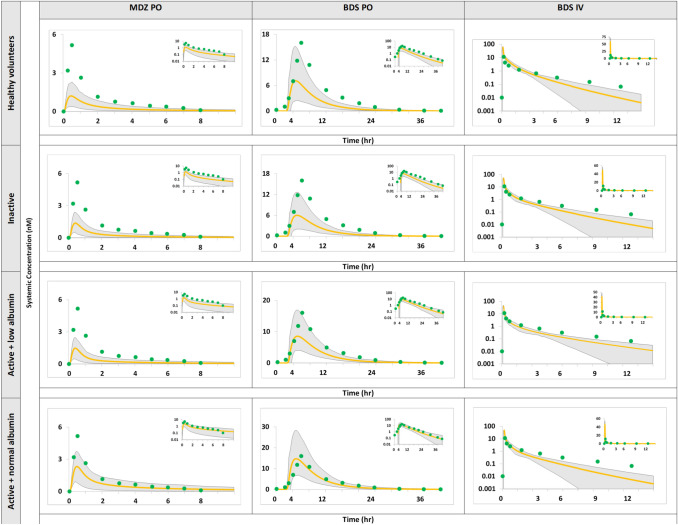
We created two separate files for the CD patients within the Simcyp® simulator for the active and non-active stage, respectively, based on all available data gathered in the report. The created CD populations were alternately applied to budesonide and midazolam (IV and oral administration). The simulations of budesonide and midazolam in Fig. 12 show the overlay of plasma concentration–time profiles of Simcyp® healthy volunteers and active and inactive CD population models on the observed clinical data in CD.
Fig. 12.
Simulation of concentration–time profile of midazolam (MDZ) following oral (PO) administration of 0.1 mg solution in the fasted state [39] and budesonide (BDS) after administration of an 18-mg controlled-release PO dose in the fed state and a 0.5-mg intravenous (IV) dose [42], 43. Observed data (green circles) are compared with the 95% prediction interval (grey region) and the central tendency of profile (yellow line) generated from physiologically based pharmacokinetic models of active and inactive Crohn’s disease patients and healthy volunteers
As seen from the overlay of the created populations and the PK parameters predicted/observed ratio (Table 2), the inactive population did not capture the observed clinical outcome. Both the budesonide and midazolam simulations were greater than twofold relative to the observed.
Table 2.
Comparison of the predicted and observed PK parameters of oral midazolam and budesonide in inactive CD populations
| Parameter | Midazolam oral solution, fasted | Parameter | Budesonide controlled release, fed | ||||
|---|---|---|---|---|---|---|---|
| Predicted | Observed Mean ± SD |
Predicted/Observed | Predicted | Observed Mean (95% CI) |
Predicted/Observed | ||
| AUC0–∞ (nM*h) | 2.1 | 14 ± 6.38 | 0.15 | AUC0–∞ (nM*h) | 46.7 | 114 (81.4–159.5) | 0.41 |
| Cmax (nM) | 1.3 | 8.4 ± 5.13 | 0.16 | Cmax (nM) | 6.22 | 14.3 (6–13.7) | 0.43 |
| Tmax (h) | 0.39 | 0.53 ± 1.3 | 0.5 | Tmax (h) | 4.8 | 6 (3–8) | 0.8 |
| F % | 22 | 31 ± 22 | 0.8 | F % | 10 | 20.5 (8.8–15) | 0.5 |
AUC area under the curve, CD Crohn’s disease, CI confidence interval, Cmax maximum drug concentration, PK pharmacokinetics, Tmax time to reach Cmax, F oral bioavailability
The simulated PK of oral budesonide was compared with the clinical observations using two different models, one assuming normal levels of albumin and another assuming lower albumin than healthy individuals. Although both predictions were within twofold of the observed data, the predictions assuming normal levels of albumin were closer to the observed values. PK parameters were all within twofold of the observed data as well (Tables 3 and 4). The reason the active CD model (with normal albumin levels) was considered superior to the alternative model with low albumin levels was the fact that the latter was crossing the observed plasma concentration on the borders of the 95% prediction interval. The 95% prediction interval of the simulated midazolam shows that none of the active CD models were able to capture its in vivo plasma concentration (Tables 3 and 4). Thus, these models cannot be relied on as reproducible PBPK models for the CD population. A global sensitivity analysis was, therefore, carried out to identify the literature gaps that primarily determine the observed alteration of the bioavailability in CD patients.
Table 3.
Comparison of the predicted and observed PK parameters of oral midazolam and budesonide based on in active CD population with reduced HSA
| Parameter | Midazolam oral solution, fasted | Parameter | Budesonide controlled-release, fed | ||||
|---|---|---|---|---|---|---|---|
| Predicted | Observed Mean ± SD |
Predicted/observed | Predicted | Observed Mean (95% CI) |
Predicted/observed | ||
| AUC0–∞ (nM*h) | 2.4 | 14 ± 6.38 | 0.2 | AUC0–∞ (nmol*h/L) | 61.6 | 114 (81.4–159.5) | 0.54 |
| Cmax (nM) | 1.45 | 8.4 ± 5.13 | 0.2 | Cmax (nM) | 7.8 | 14.3 (6–13.7) | 0.55 |
| Tmax (h) | 0.37 | 0.53 ± 1.3 | 0.7 | Tmax (h) | 4.84 | 6 (3–8) | 0.81 |
| F % | 27 | 31 ± 22 | 0.9 | F % | 14 | 20.5 (8.8–15) | 0.7 |
AUC area under the curve, CD Crohn’s disease, CI confidence interval, Cmax maximum drug concentration, HSA human serum albumin, PK pharmacokinetics, Tmax time to reach Cmax, F oral bioavailability
Table 4.
Comparison of the predicted and observed PK parameters of oral midazolam and budesonide in active CD populations with normal HSA
| Parameter | Midazolam oral solution, fasted | Parameter | Budesonide controlled release, fed | ||||
|---|---|---|---|---|---|---|---|
| Predicted | Observed Mean ± SD |
Predicted/Observed | Predicted | Observed Mean (95% CI) |
Predicted/Observed | ||
| AUC0–∞ (nM*h) | 5 | 14 ± 6.38 | 0.36 | AUC0–∞ (nM*h) | 104.6 | 114 (81.4–159.5) | 0.92 |
| Cmax (nM) | 2.31 | 8.4 ± 5.13 | 0.3 | Cmax (nM) | 14.1 | 14.3 (6–13.7) | 0.99 |
| Tmax (h) | 0.45 | 0.53 ± 1.3 | 0.85 | Tmax (h) | 4.75 | 6 (3–8) | 0.8 |
| F % | 34 | 31 ± 22 | 1.1 | F % | 21 | 20.5 (8.8–15) | 1.02 |
AUC area under the curve, CD Crohn’s disease, CI confidence interval, Cmax maximum drug concentration, HSA human serum albumin, PK pharmacokinetics, Tmax time to reach Cmax, F oral bioavailability
GSA
All the identified systems parameters that are altered during the course of CD were included in the GSA, but only the parameters that showed the highest influence on AUC, Cmax and Tmax are shown in Fig. 13a–c. The other parameters with low impact are excluded from the presented output data.
Fig. 13.
Relative sensitivity of budesonide (BDS) and midazolam (MDZ) kinetics to the systems parameters from global sensitivity analysis based on physiologically based pharmacokinetics in Crohn’s disease: a area under the curve (AUC), b maximum drug concentration (Cmax) and c time to reach Cmax (Tmax). GET gastric emptying time, SITT small intestine transit time
Cardiac output is found to have the highest influence on AUC, Cmax and Tmax of the budesonide PK parameters investigated. HSA comes second for AUC and Cmax and third for Tmax. Gastric emptying time is one of the highest contributors in Cmax and Tmax observations. When it comes to CYP influence, liver CYP3A4 is the most impactful on all three investigated parameters, while small intestine CYP3A4 abundance has its highest impact on Tmax.
Liver CYP3A4 abundance was found to be the major influencer of midazolam AUC, while small intestine CYP3A4 abundance is the major influencer of Cmax. Gastric emptying time has an important influence on Tmax. HSA comes second for AUC and Cmax and third for Tmax, and liver CYP3A4 abundance comes second for Tmax and third for Cmax. Cardiac output, upper intestine pH and small intestine transit time have varying influences on the three investigated PK parameters.
Discussion
The systematic data analysis for the kinetics of drugs in CD patients demonstrated clearly that the internal exposure to given doses of drugs can be significantly different from that of healthy volunteers. However, there is no consistent and uniform effect for different drugs or formulations to enable a “one size fits all” recommendation for alteration of a dosage regimen or, indeed, of keeping it the same as for other patients. Unlike in the case of hepatic and renal impairment, where there are regulatory requirements to assess the potential changes in the kinetics, there are no such requirements. Moreover, whilst the changes, if there are any, in the cases of renal and hepatic impairment are in one direction (reduced clearance), the changes in the case of CD patients seem to happen in both directions; exposure may be increased or decreased compared with healthy volunteers.
The classification of CD can be addressed differently when considering the clinical representation of the patient or the objective disease biomarkers. The most commonly used classification of CD in the literature is the symptoms-based system, classifying CD into active and inactive. This is simple, but not very accurate, due to patient variations and the apparent lack of use of more reliable objective markers to define disease activity. For example, magnetic resonance enterography (MRE) can reliably evaluate small bowel CD activity [152], which was not reported in any of the studies assessing the CD activity of this segment. A new classification of CD was introduced based on the localisation (ileal and colonic) of the disease and its relevant clinical presentation and biomarkers profile [153]. If this classification were to be considered, several PBPK population models of CD would be distinct according to the different clinical phenotypes.
Fistula and stricture are common complications of CD that can present alongside the initial diagnosis of CD or occur later [154]. Epithelial to mesenchymal transition (EMT) and inflammatory biomarkers are associated with their formation. Such complications do not heal with the control of inflammation, and no preventive treatment is in place. Developing an appropriate PBPK model for such CD patients should be done independently from the current models, as their presence alters CD from an inflammatory disease phenotype to a strictured or fistulated phenotype. Currently, this is hard to apply, as various drawbacks prevent accurate assessment of CD activity. The literature does not usually distinguish between CD types based on the location of the disease; rather, it distinguishes the disease, based on the clinical profile of the patient, as active and inactive CD.
When examining the systemic exposure of CYP3A oral substrates, which represent the majority of drugs with available clinical data, a trend of higher exposure is more common, but cannot be generalised, as the available data on active and inactive CD are clouded by uncertainties, which hinders obtaining a definite conclusion. The same applies for the clearance of IV drugs. For the non-CYP3A substrates, no clear trend could be concluded. A higher variability is seen, but is inferred from a small number of studies. The changes are multi-factorial, and the interplay of the drug/formulation with the changes in CD can help in postulating the likely effects. This necessitates further clinical data to establish the disease impact and the magnitude of its activity.
Hence, application of PBPK might be the best chance for rationalising the dosage changes (when needed) in CD patients, as suggested in the case of bariatric surgery patients, who also do not get dedicated studies for various drugs coming to the market [10].
To develop the CD PBPK population, the current systems data defining active and inactive CD patients are captured in this article, whether obtained from independent studies or from a reverse translation approach. The data are not abundant, and some parameters lack definition. More dramatic alteration is seen with active disease patients relative to patients in remission. The predominant alterations are seen with the increase of SMA flow, small intestine transit time and reduction of albumin, where the dysregulation severity in HSA level, SMA and the intestine flow were reported to correlate with the active disease severity [88, 94, 136, 155–157]. The increase of gastric emptying time and small intestine transit time will affect the absorption rate and solubility of oral drugs.
Although an increase of gut wall thickness can affect gut fluid dynamics, a meta-analysis was not carried out for this parameter even though it showed an approximately twofold increase in active CD patients compared to healthy volunteers in the few studies encountered [78, 80, 81, 93, 158]. This is because the utility of the intestine loop morphology is not clear in the Simcyp® M-ADAM model; instead, the villous morphology is established in Simcyp®. For CD patients, reduction of the duodenum and ileum villous length was reported in paediatric patients [159]. From the carried GSA in this study, the villous morphological changes had little impact on midazolam and budesonide AUC and Cmax, and only a very limited impact was seen on midazolam Tmax; thus, this was not incorporated in our model.
Enterocyte turnover rate is another aspect not included in our model. Turnover of the epithelial cells in CD patients was reported to be faster than in healthy volunteers, but the measurement varies from one study to another. Moreover, some of these reports are on preclinical models of CD, rather than real patients [160]. There are also reports on faster production of cytokines, cadherin or other proteins [161–164] and the excretion of bile acids [165] as an indicator of the different cell turnover. Therefore, currently, it is difficult to reach a consensus regarding a specific value for enterocyte turnover in CD patients, despite general agreement that it would be faster than in healthy individuals.
The intestine microbiota is an important aspect to include when creating a CD virtual population. Unfortunately, the topic suffers from the same problem, as it is not clearly defined for the different clinical phenotypes of the disease, and the diversity of the addressed bacteria and their role hinders its accurate representation in the current, developed models. CD patients suffer from change in the intestine microbiota, which might alter the intestine environment and homeostasis [166–168], affecting oral drug bioavailability, although, the evidence for such effects is lacking and most oral drug absorption takes place before the drug comes into contact with the microbiota. In future work, focus on this topic should be prioritised, especially if the studied drugs are activated/metabolised by the bacterial enzymes of the intestine [169, 170]. Dysregulation of DMETs in the liver has not been addressed except for the reduction of CYP3A4 activity in active CD patients [39]. This reduction might be a major contributor in the significant difference of the systemic exposure seen with CYP3A substrates midazolam, verapamil and budesonide in the CD population. In patients with primary sclerosing cholangitis (PSC), a comorbidity associated with CD [171], investigators reported an insignificant reduction of liver CYP3A4 expression and a notable increase of OSTβ in participants with diseases other than CD [172, 173]. This supports the possible deterioration of liver metabolism in CD and promotes investigation into the abundance and activity of liver DMETs in the CD population, with and without relation to comorbidities. Liver DMETs alteration will cause a considerable change in drug clearance, as the liver is the major xenobiotic-metabolising organ [174].
CYP3A4 expression has shown different results in the ileum and colon [97, 99, 100], but overall, a reduction of its expression is seen. Similar observations are seen with p-gp expression [97–100, 103]. The intestine CYP3A4 reduction influence might seem less pronounced, but the simulation of midazolam and budesonide based on the liver CYP3A4 alteration did not capture the clinical data and the intestine involvement is greater than anticipated based on our GSA results. Other non-CYP DMEs abundance/activity should be addressed in the liver and the intestine, as they metabolise about 30% of approved drugs [175]. Undesired drug–disease interactions can occur, which might result in several complications and delays in disease control.
Intestinal permeability is another major player in the observed changes. It is altered in response to the elevation of inflammatory biomarkers changing the drug transporter expression during the course of CD [176–178]. The available data on the intestine transporters in CD are mainly based on gene expression levels. The gene expression analysis does not have a high correlation with direct protein expression measurements for all proteins [179–190]. Establishing a relationship that would reflect the correlation between protein mRNA expression and direct concentration is challenging, as it was found that in around 60% of proteins, mRNA levels do not highly correlate with abundance [191].
Propranolol, a CYP (2C9, 1A2, 2D6) and p-gp substrate, showed a significant difference between its exposure in CD patients and that in healthy volunteers [59, 192]. More severe inflammation indicated by higher ESR was associated with higher propranolol concentration. When ESR was low, the concentration of propranolol in rheumatoid arthritis patients was similar to that in the healthy group, but this was not the case in the CD group, as the concentration remained significantly higher [59]. This might be attributed to localisation of the inflammation to the intestine in CD causing alteration of the DMET protein abundances. Therefore, systemic exposure of oral drugs in CD cannot be explained by the liver factors alone or intestine factors alone.
In vitro data for DMETs are lacking in the CD population; these data would allow identification of the intestinal intrinsic clearance and the microsomal fraction unbound to enable reproducible and more accurate prediction of oral drug PK, and differentiation between the involvement of the main metabolising organs.
In inflammatory conditions, albumin synthesis by the liver can be shifted [193, 194]. Albumin reduction will affect the drug fraction unbound in the plasma (fuP). This will affect drug disposition and lead to an increase in free drug plasma concentration. The volume of distribution (Vss) and clearance are sensitive to fuP values [195, 196]. Underprediction of fuP can lead to underprediction of clearance and hinder the prediction accuracy for drugs with a high protein binding affinity [197, 198]. Correlation between albumin elevation and IL-6 reduction was reported as an indicator of medication effectiveness and progression to a remission state in CD patients [144] and also to the severity of the disease of IBD patients (CD and UC) [199]. The displacement of the high extraction ratio drugs like budesonide from plasma proteins will be of similar magnitude to the displacement of low extraction ratio drugs.
Patients who underwent resection surgery would suffer from consequences that might alter the physiological aspects that affect oral drugs absorption. Short bowel syndrome (SBS) is a disorder where patients suffer from malabsorption due to bowel resection [200]. SBS complications, alteration in the gut microbiota, reduction of gastric emptying time and small intestine transit time, higher intestine pH, reduction of the absorption area and altered transporters profile [62, 201–207], can affect the absorption of a wide range of drugs [208]. Further to these complications, the bile acid composition is altered as a result of its malabsorption [209, 210], especially when the surgical procedure included removal of the upper intestine parts (jejunum and ileum), where the bile acids get absorbed [211, 212]. Surgeries targeting the ileum could lead to loss of ASBT transporter, a major contributor in the absorption of bile acid [213]. Loss of the bile acid would affect the absorption and solubilisation of lipophilic drugs such as cyclosporine [214], lovastatin [215] and ampicillin [216], impairing their bioavailability. Along with the size of the resection, the location of the surgery influences the extent of the impact on absorption following resection. Colostomy’s influence is expected to be more restrained, affecting special formulation drugs directed to be released in the lower segments of the intestine. Ileostomy, on the other hand, could lead to more drastic absorption alteration, since it represents a larger surface area and higher abundance of DMETs, where most conventional oral formulations are absorbed [54, 217]. Therefore, caution should be taken when dealing with patients with prior bowel surgery, as their physiological profile can be significantly different from that of other CD patients. Thus, rectification of the created PBPK model is necessary to deliver a more accurate insight into the appropriate dose adjustment for these patients.
The case of oral budesonide requires further investigation as the different studies reporting its bioavailability in CD did not show significant variation from healthy volunteers [39, 43, 44] except for one [42]. The different studies followed different dose regimens under fed and fasted conditions, but this was reported to carry no significant effect on the drug PK. The male-to-female proportion and the inclusion of active and inactive study participants varies among the studies and could contribute to the disparities noticed between studies. Budesonide is a high extraction ratio drug that is highly affected by the blood flow, which is subject to individual variability. The fed state results in increasing the splanchnic blood flow, which leads to increased absorption [218]. This indicates that when an oral high extraction drug is under investigation, many different factors contribute to its PK behaviour, and these factors are changeable, specifically in a disease situation, where the change is caused by a combination of organ dysfunction and alterations in physiological factors all together at the same time.
The budesonide immediate-release and controlled-release formulations encountered varying changes in systemic exposure, which might be linked to the additive relationship of CYP3A4 and p-gp, as it is a substrate of both. The function of CYP3A4 and p-gp as a barrier for drug absorption [219–221] has been supported by in silico modelling [222–225] and in vitro experiments [226, 227]. The difference between immediate-release and controlled-release formulations for combined CYP3A4 and p-gp substrates was examined, and no significant differences in the AUC between the two formulations were reported [17].
The active CD model only captured the budesonide [42] plasma concentration–time profile in CD patients, but not the midazolam profile [39]. A previously reported CD population PBPK model was capable of capturing budesonide based on modifying several parameters in CD [45]. The patients included in the budesonide study were both active and inactive CD patients, while in the corresponding midazolam study, there were only active CD patients. Therefore, the models applied to the selected drugs fully characterised in CD patients following IV and oral dose (budesonide and midazolam) are not sufficient to produce robust PBPK models that can predict the likely outcome of PK studies.
To provide a map for future studies, we conducted a global sensitivity analysis showing variations in the intensities of the effects of the most influential physiological parameters on midazolam and budesonide PK. The observed variations are attributed to different factors related to the drug’s nature and formulation, in addition to the patient activity state, where the budesonide study included a mixture of active and inactive patients and the midazolam study included only active state participants.
New oral molecules have been investigated and introduced for the treatment of CD. Filgotinib and upadacitinib are Janus kinase (JAK) inhibitors that have successfully passed phase II of the clinical trials, with promising efficacy and safety profiles. Other JAK agents are still under development [228]. Sphingosine 1-phosphate (S1P) modulators such as ozanimod, etrasimod and amiselimod are another class of oral drugs that has shown promising clinical results for CD [229]. Other oral anti-trafficking therapies and PDE4 inhibitors did not pass clinical trials for CD, but showed efficacy for UC [230]. The use of a reliable PBPK model would aid in the drug development process and give insight into what to expect from the drug in such a special population.
Therefore, going forward, differentiation between active and inactive CD is essential. This is identified from the available literature data collected for the systems parameters as well as limited clinical observations and PBPK simulations. However, the definition of the activity state of the disease in all of the included studies was hard to unify. The activity state was mainly based on subjective clinical patient data, with or without objective biomarkers participating in this variability. Due to the multifactorial physiological alterations of active and inactive CD, attempts to explain observed changes to oral bioavailability in the CD population using single attributes of drugs or simplified modelling is not successful.
Conclusion
The most influential parameters determining the changes to bioavailability of the two investigated drugs in the CD population were the expression/activity of liver and intestine enzymes. Both of these parameters currently lack a reliable value in CD patients. This gap stands between the aspiration to apply PBPK for predicting the alterations of drug fate in CD populations to guide dosing, and the practical ability to do such predictions. Therefore, we urge all researchers in this field to boost efforts to generate such information, which should be provided separately for the active and inactive status of CD patients when possible.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The PBPK models were provided by Certara. Amin Rostami-Hodjegan is an employee of Certara UK Limited and holds shares in Certara. The rest of the authors have no conflicts of interest to declare.
Funding
This work was supported by the Saudi Ministry of Education, King Saud University, KSA (to SA) and The Centre for Applied Pharmacokinetic Research (CAPKR), University of Manchester, UK.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SA and ARH. The first draft of the manuscript was written by SA, and all authors have commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable. Code availability Not applicable.
Code availability
Not applicable.
Availability of data and material
The datasets generated or analysed during the current study are included in this article and in its supplementary material files, which can be found in the online version of this article.
References
- 1.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 2.Hart AL, Ng SC. Crohn’s disease. Medicine (Baltim) 2011;39:229–236. doi: 10.1016/j.mpmed.2011.01.004. [DOI] [Google Scholar]
- 3.Doherty MM, Pang KS. First-pass effect: significance of the intestine for absorption and metabolism. Drug Chem Toxicol. 1997;20:329–344. doi: 10.3109/01480549709003891. [DOI] [PubMed] [Google Scholar]
- 4.Berggren S, Gall C, Wollnitz N, Ekelund M, Karlbom U, Hoogstraate J, et al. Gene and protein expression of P-glycoprotein, MRP1, MRP2, and CYP3A4 in the small and large human intestine. Mol Pharm. 2007;4:252–257. doi: 10.1021/mp0600687. [DOI] [PubMed] [Google Scholar]
- 5.Xu F, Dahlhamer JM, Zammitti EP, Wheaton AG, Croft JB. Health-risk behaviors and chronic conditions among adults with inflammatory bowel disease—United States, 2015 and 2016. MMWR Morb Mortal Wkly Rep. 2018;67:190–195. doi: 10.15585/mmwr.mm6706a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92:1088–1103. doi: 10.1016/j.mayocp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 8.FDA. Pharmacokinetics in Patients with Impaired Hepatic Function : Study Design , Data Analysis , and Impact on Dosing and Labeling. FDA Guid [Internet]. 2003 [cited 2021 Jun 12]; Available from: https://www.fda.gov/media/71311/download.
- 9.FDA. Guidance for industry pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing. FDA Publication. 2020 [cited 2021 Jun 12]; Available from: https://www.fda.gov/media/78573/download.
- 10.Darwich AS, Henderson K, Burgin A, Ward N, Whittam J, Ammori BJ, et al. Trends in oral drug bioavailability following bariatric surgery: examining the variable extent of impact on exposure of different drug classes. Br J Clin Pharmacol. 2012;74:774–787. doi: 10.1111/j.1365-2125.2012.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs guidance for industry. 2020 [cited 2021 Jun 20]; Available from: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugsand/or.
- 12.Jadhav PR, Cook J, Sinha V, Zhao P, Rostami-Hodjegan A, Sahasrabudhe V, et al. A proposal for scientific framework enabling specific population drug dosing recommendations. J Clin Pharmacol. 2015;55:1073–1078. doi: 10.1002/jcph.579. [DOI] [PubMed] [Google Scholar]
- 13.Younis IR, Robert Powell J, Rostami-Hodjegan A, Corrigan B, Stockbridge N, Sinha V, et al. Utility of model-based approaches for informing dosing recommendations in specific populations: report from the public AAPS Workshop. J Clin Pharmacol. 2017;57:105–109. doi: 10.1002/jcph.787. [DOI] [PubMed] [Google Scholar]
- 14.Astrazeneca. FDA clinical pharmacology review: Entocort EC (budesonide capsules). 2009 [cited 2021 Oct 13]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021324s008lbl.pdf.
- 15.Fieller EC. Some problems in interval estimation. J R Stat Soc Ser B. 1954;16:175–185. [Google Scholar]
- 16.Motulsky H. Intuitive biostatistics: a nonmathematical guide to statistical thinking. Oxford: Oxford University Press; 2010. [Google Scholar]
- 17.Olivares-Morales A, Kamiyama Y, Darwich AS, Aarons L, Rostami-Hodjegan A. Analysis of the impact of controlled release formulations on oral drug absorption, gut wall metabolism and relative bioavailability of CYP3A substrates using a physiologically-based pharmacokinetic model. Eur J Pharm Sci. 2015;67:32–44. doi: 10.1016/j.ejps.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Mahid SS, Mulhall AM, Gholson RD, Eichenberger RM, Galandiuk S. Inflammatory bowel disease and African Americans: a systematic review. Inflamm Bowel Dis. 2008;14:960–967. doi: 10.1002/ibd.20389. [DOI] [PubMed] [Google Scholar]
- 19.Reddy SI, Burakoff R. Inflammatory bowel disease in African Americans. Inflamm Bowel Dis. 2003;9:380–385. doi: 10.1097/00054725-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794.e4. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 21.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 22.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Ramadas AV, Gunesh S, Thomas GAO, Williams GT, Hawthorne AB. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–1206. doi: 10.1136/gut.2009.202101. [DOI] [PubMed] [Google Scholar]
- 24.Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjær M, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003–2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–1282. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 25.Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/S0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 26.Moum B, Vatn MH, Ekbom A, Aadland E, Fausa O, Lygren I, et al. Incidence of Crohn’s disease in four counties in southeastern Norway, 1990–93 a prospective population-based study. Scand J Gastroenterol. 1996;31:355–361. doi: 10.3109/00365529609006410. [DOI] [PubMed] [Google Scholar]
- 27.Jussila A, Virta LJ, Kautiainen H, Rekiaro M, Nieminen U, Färkkilä MA. Increasing incidence of inflammatory bowel diseases between 2000 and 2007: a nationwide register study in Finland. Inflamm Bowel Dis. 2012;18:555–561. doi: 10.1002/ibd.21695. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian Province: a population-based study. Am J Epidemiol. 1999;149:916–924. doi: 10.1093/oxfordjournals.aje.a009735. [DOI] [PubMed] [Google Scholar]
- 29.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/S0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 30.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. Gastroenterology. 1976;70:439–444. doi: 10.1016/S0016-5085(76)80163-1. [DOI] [PubMed] [Google Scholar]
- 31.Zittan E, Kabakchiev B, Kelly OB, Milgrom R, Nguyen GC, Croitoru K, et al. Development of the Harvey-Bradshaw Index-pro (HBI-PRO) score to assess endoscopic disease activity in Crohn’s disease. J Crohns Colitis. 2016;11:jjw200. doi: 10.1093/ecco-jcc/jjw200. [DOI] [PubMed] [Google Scholar]
- 32.Freeman HJ. Use of the Crohn’s disease activity index in clinical trials of biological agents. World J Gastroenterol. 2008;14:4127. doi: 10.3748/wjg.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Effinger A, O’Driscoll CM, McAllister M, Fotaki N. Impact of gastrointestinal disease states on oral drug absorption—implications for formulation design—a PEARRL review. J Pharm Pharmacol. 2019;71:674–698. doi: 10.1111/jphp.12928. [DOI] [PubMed] [Google Scholar]
- 34.McNamara PJ, Meiman D. Predicting drug binding to human serum albumin and alpha one acid glycoprotein in diseased and age patient populations. J Pharm Sci. 2019;108:2737–2747. doi: 10.1016/j.xphs.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Darwich AS, Aslam U, Ashcroft DM, Rostami-Hodjegan A. Meta-analysis of the turnover of intestinal epithelia in preclinical animal species and humans. Drug Metab Dispos. 2014;42:2016–2022. doi: 10.1124/dmd.114.058404. [DOI] [PubMed] [Google Scholar]
- 36.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Certara (Simcyp). Simcyp Simulator Version 19 Help File. 2019. Certara UK Ltd., Sheffield, UK. Available from: https://members.simcyp.com/
- 38.Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11:225–237. doi: 10.1208/s12248-009-9099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson A, Tirona RG, Kim RB. CYP3A4 activity is markedly lower in patients with Crohnʼs disease. Inflamm Bowel Dis. 2017;23:804–813. doi: 10.1097/MIB.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 40.Hohmann N, Kocheise F, Carls A, Burhenne J, Haefeli WE, Mikus G. Midazolam microdose to determine systemic and pre-systemic metabolic CYP3A activity in humans. Br J Clin Pharmacol. 2015;79:278–285. doi: 10.1111/bcp.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorsson L, Edsbäcker S, Conradson T-B. Lung deposition of budesonide from Turbuhaler® is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7:1839–1844. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 42.Edsbäcker S, Bengtsson B, Larsson P, Lundin P, Nilsson Å, Ulmius J, et al. A pharmacoscintigraphic evaluation of oral budesonide given as controlled-release (Entocort) capsules. Aliment Pharmacol Ther. 2003;17:525–536. doi: 10.1046/j.1365-2036.2003.01426.x. [DOI] [PubMed] [Google Scholar]
- 43.Lundin P, Naber T, Nilsson M, Edsbacker S. Effect of food on the pharmacokinetics of budesonide controlled ileal release capsules in patients with active Crohn’s disease. Aliment Pharmacol Ther. 2001;15:45–51. doi: 10.1046/j.1365-2036.2001.00910.x. [DOI] [PubMed] [Google Scholar]
- 44.Lundin PDP, Edsbäcker S, Bergstrand M, Ejderhamn J, Linander H, Högberg L, et al. Pharmacokinetics of budesonide controlled ileal release capsules in children and adults with active Crohn’s disease. Aliment Pharmacol Ther. 2003;17:85–92. doi: 10.1046/j.1365-2036.2003.01386.x. [DOI] [PubMed] [Google Scholar]
- 45.Effinger A, O’Driscoll CM, McAllister M, Fotaki N. Predicting budesonide performance in healthy subjects and patients with Crohn’s disease using biorelevant in vitro dissolution testing and PBPK modeling. Eur J Pharm Sci. 2021;157:105617. [DOI] [PubMed]
- 46.Han B, Mao J, Chien JY, Hall SD. Optimization of drug–drug interaction study design: comparison of minimal physiologically based pharmacokinetic models on prediction of CYP3A inhibition by ketoconazole. Drug Metab Dispos. 2013;41:1329–1338. doi: 10.1124/dmd.112.050732. [DOI] [PubMed] [Google Scholar]
- 47.Wang H-Y, Chen X, Jiang J, Shi J, Hu P. Evaluating a physiologically based pharmacokinetic model for predicting the pharmacokinetics of midazolam in Chinese after oral administration. Acta Pharmacol Sin. 2016;37:276–284. doi: 10.1038/aps.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chetty M, Mattison D, Rostami-Hodjegan A. Sex differences in the clearance of CYP3A4 substrates: exploring possible reasons for the substrate dependency and lack of consensus. Curr Drug Metab. 2012;13:778–786. doi: 10.2174/138920012800840464. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Li L, Rostami-Hodjegan A, Bois FY, Jamei M. Considerations and caveats when applying global sensitivity analysis methods to physiologically based pharmacokinetic models. AAPS J. 2020;22:93. doi: 10.1208/s12248-020-00480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanaee F, Clements JD, Waugh AWG, Fedorak RN, Lewanczuk R, Jamali F. Drug−disease interaction: Crohn’s disease elevates verapamil plasma concentrations but reduces response to the drug proportional to disease activity. Br J Clin Pharmacol. 2011;72:787–797. doi: 10.1111/j.1365-2125.2011.04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melander A, Kahlmeter G, Kamme C, Ursing B. Bioavailability of metronidazole in fasting and non-fasting healthy subjects and in patients with Crohn’s disease. Eur J Clin Pharmacol. 1977;12:69–72. doi: 10.1007/BF00561408. [DOI] [PubMed] [Google Scholar]
- 52.Tanner AR, Halliday JW, Powell LW. Serum prednisolone levels in Crohn’s disease and coeliac disease following oral prednisolone administration. Digestion. 1981;21:310–315. doi: 10.1159/000198583. [DOI] [PubMed] [Google Scholar]
- 53.Milsap RL, George DE, Szefler SJ, Murray KA, Lebenthal E, Jusko WJ. Effect of inflammatory bowel disease on absorption and disposition of prednisolone. Dig Dis Sci. 1983;28:161–168. doi: 10.1007/BF01315146. [DOI] [PubMed] [Google Scholar]
- 54.Flückiger SS, Schmidt C, Meyer A, Kallay Z, Johnston A, Kutz K. Pharmacokinetics of orally administered cyclosporine in patients with Crohn’s disease. J Clin Pharmacol. 1995;35:681–687. doi: 10.1002/j.1552-4604.1995.tb04108.x. [DOI] [PubMed] [Google Scholar]
- 55.Nader A, Stodtmann S, Friedel A, Mohamed MF, Othman AA. Pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis, Crohn’s disease, ulcerative colitis, or atopic dermatitis: population analyses of phase 1 and 2 clinical trials. J Clin Pharmacol. 2020;60:528–539. doi: 10.1002/jcph.1550. [DOI] [PubMed] [Google Scholar]
- 56.Jiang F, Peng X, Cai D, Wen D, Liu Y, Zhi M, et al. A validated LC-MS/MS method for the simultaneous determination of thalidomide and its two metabolites in human plasma: application to a pharmacokinetic assay. Biomed Chromatogr. 2018;32:e4240. doi: 10.1002/bmc.4240. [DOI] [PubMed] [Google Scholar]
- 57.Norlander B, Gotthard R, Strom M. Pharmacokinetics of a 5-aminosalicylic acid enteric-coated tablet in patients with Crohn’s disease or ulcerative colitis and in healthy volunteers. Aliment Pharmacol Ther. 1990;4:497–505. doi: 10.1111/j.1365-2036.1990.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 58.Gionchetti P, Campieri M, Belluzzi A, Boschi S, Brignola C, Miglioli M, et al. Bioavailability of single and multiple doses of a new oral formulation of 5-ASA in patients with inflammatory bowel disease and healthy volunteers. Aliment Pharmacol Ther. 1994;8:535–540. doi: 10.1111/j.1365-2036.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 59.Schneider R, Bishop H, Hawkins C. Plasma propranolol concentrations and the erythrocyte sedimentation rate. Br J Clin Pharmacol. 1979;8:43–47. doi: 10.1111/j.1365-2125.1979.tb05907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwab M, Klotz U. Pharmacokinetic considerations in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2001;40:723–751. doi: 10.2165/00003088-200140100-00003. [DOI] [PubMed] [Google Scholar]
- 61.Press AG, Hauptmann IA, Hauptmann L, Fuchs B, Fuchs M, Ewe K, et al. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 1998;12:673–678. doi: 10.1046/j.1365-2036.1998.00358.x. [DOI] [PubMed] [Google Scholar]
- 62.Fallingborg J, Pedersen P, Jacobsen BA. Small intestinal transit time and intraluminal pH in ileocecal resected patients with Crohn’s disease. Dig Dis Sci. 1998;43:702–705. doi: 10.1023/A:1018893409596. [DOI] [PubMed] [Google Scholar]
- 63.Ewe K, Schwartz S, Petersen S, Press AG. Inflammation does not decrease intraluminal pH in chronic inflammatory bowel disease. Dig Dis Sci. 1999;44:1434–1439. doi: 10.1023/A:1026664105112. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki Y, Hada R, Nakajima H, Fukuda S, Munakata A. Improved localizing method of radiopill in measurement of entire gastrointestinal pH profiles: colonic luminal pH in normal subjects and patients with Crohn’s disease. Am J Gastroenterol. 1997;92:114–118. [PubMed] [Google Scholar]
- 65.Lucas ML, Cooper BT, Lei FH, Johnson IT, Holmes GK, Blair JA, et al. Acid microclimate in coeliac and Crohn’s disease: a model for folate malabsorption. Gut. 1978;19:735–742. doi: 10.1136/gut.19.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nóbrega ACM, Ferreira BRS, Oliveira GJ, Sales KMO, Santos AA, Nobre e Souza MÂ, et al. Dyspeptic symptoms and delayed gastric emptying of solids in patients with inactive Crohn’s disease. BMC Gastroenterol. 2012;12:175. doi: 10.1186/1471-230X-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keller J, Beglinger C, Holst JJ, Andresen V, Layer P. Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am J Physiol Liver Physiol. 2009;297:G861–G868. doi: 10.1152/ajpgi.00145.2009. [DOI] [PubMed] [Google Scholar]
- 68.Annese V, Bassotti G, Napolitano G, Frusciante V, Bruno M, Conoscitore P, et al. Gastric emptying of solids in patients with nonobstructive Crohnʼs disease is sometimes delayed. J Clin Gastroenterol. 1995;21:279–282. doi: 10.1097/00004836-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Keller J, Binnewies U, Rösch M, Juul Holst J, Beglinger C, Andresen V, et al. Gastric emptying and disease activity in inflammatory bowel disease. Eur J Clin Investig. 2015;45:1234–1242. doi: 10.1111/eci.12542. [DOI] [PubMed] [Google Scholar]
- 70.Damião AO, Sipahi AM, Vezozzo DP, Gonçalves PL, Laudanna AA. Measurement of gastric emptying time by real-time ultrasonography in patients with Crohn’s disease. Rev Hosp Clin Fac Med Sao Paulo. 1996;51:154–156. [PubMed] [Google Scholar]
- 71.Fireman Z, Kopelman Y, Friedman S, Ephrath H, Choman E, Debby H, et al. Age and indication for referral to capsule endoscopy significantly affect small bowel transit times: the given database. Dig Dis Sci. 2007;52:2884–2887. doi: 10.1007/s10620-007-9789-1. [DOI] [PubMed] [Google Scholar]
- 72.Holt S, Heading RC, Clements JA, Tothill P, Prescott LF. Acetaminophen absorption and metabolism in celiac disease and Crohn’s disease. Clin Pharmacol Ther. 1981;30:232–238. doi: 10.1038/clpt.1981.153. [DOI] [PubMed] [Google Scholar]
- 73.Niv E, Pinchasovich H, Yanai H. Impact of demographic and clinical parameters on video capsule transit time. Eur J Gastroenterol Hepatol. 2016;28:1161–1165. doi: 10.1097/MEG.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 74.Niv E, Fishman S, Kachman H, Arnon R, Dotan I. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn’s disease. J Crohns Colitis. 2014;8:1616–1623. doi: 10.1016/j.crohns.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Fischer M, Siva S, Wo JM, Fadda HM. Assessment of small intestinal transit times in ulcerative colitis and Crohn’s disease patients with different disease activity using video capsule endoscopy. AAPS PharmSciTech. 2017;18:404–409. doi: 10.1208/s12249-016-0521-3. [DOI] [PubMed] [Google Scholar]
- 76.van Oostayen JA, Wasser MNJM, van Hogezand RA, Griffioen G, de Roos A. Activity of Crohn disease assessed by measurement of superior mesenteric artery flow with Doppler US. Radiology. 1994;193:551–554. doi: 10.1148/radiology.193.2.7972778. [DOI] [PubMed] [Google Scholar]
- 77.van Oostayen J. Diagnosis of Crohn’s ileitis and monitoring of disease activity: value of Doppler ultrasound of superior mesenteric artery flow. Am J Gastroenterol. 1998;93:88–91. doi: 10.1111/j.1572-0241.1998.088_c.x. [DOI] [PubMed] [Google Scholar]
- 78.Yekeler E, Danalioglu A, Movasseghi B, Yilmaz S, Karaca C, Kaymakoglu S, et al. Crohn disease activity evaluated by Doppler ultrasonography of the superior mesenteric artery and the affected small-bowel segments. J Ultrasound Med. 2005;24:59–65. doi: 10.7863/jum.2005.24.1.59. [DOI] [PubMed] [Google Scholar]
- 79.Bonnin P, Coelho J, Pocard M, Levy BI, Marteau P. Anti-TNFα therapy early improves hemodynamics in local intestinal and extraintestinal circulations in active Crohn’s disease. J Crohns Colitis. 2013;7:451–459. doi: 10.1016/j.crohns.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Na Y, Zhou J, Xiao X-M, Zhang W-X, Gao H-L, Wang Y, et al. Assessment of Crohn’s disease activity by Doppler sonography. Saudi Med J. 2017;38:391–395. doi: 10.15537/smj.2017.4.17855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sjekavica I, Barbarić-Babić V, Krznarić Ž, Molnar M, Čuković-Čavka S, Štern-Padovan R. Assessment of Crohn’s disease activity by Doppler ultrasound of superior mesenteric artery and mural arteries in thickened bowel wall: cross-sectional study. Croat Med J. 2007;48:822–830. doi: 10.3325/cmj.2007.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Britton I, Maguire C, Adams C, Russell RI, Leen E. Assessment of the role and reliability of sonographic post-prandial flow response in grading Crohn’s disease activity. Clin Radiol. 1998;53:599–603. doi: 10.1016/S0009-9260(98)80153-0. [DOI] [PubMed] [Google Scholar]
- 83.Maconi G, Parente F, Bollani S, Imbesi V, Ardizzone S, Russo A, et al. Factors affecting splanchnic haemodynamics in Crohn’s disease: a prospective controlled study using Doppler ultrasound. Gut. 1998;43:645–650. doi: 10.1136/gut.43.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karoui S, Nouira K, Serghini M, Ben Mustapha N, Boubaker J, Menif E, et al. Assessment of activity of Crohn’s disease by Doppler sonography of superior mesenteric artery flow. J Crohns Colitis. 2010;4:334–340. doi: 10.1016/j.crohns.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Byrne MF, Farrell MA, Abass S, Fitzgerald A, Varghese JC, Thornton F, et al. Assessment of Crohn’s disease activity by Doppler sonography of the superior mesenteric artery, clinical evaluation and the Crohn’s disease activity index: a prospective study. Clin Radiol. 2001;56:973–978. doi: 10.1053/crad.2001.0794. [DOI] [PubMed] [Google Scholar]
- 86.Maconi G, Asthana AK, Bolzacchini E, Dell’Era A, Furfaro F, Bezzio C, et al. Splanchnic hemodynamics and intestinal vascularity in Crohn’s disease: an in vivo evaluation using Doppler and contrast-enhanced ultrasound and biochemical parameters. Ultrasound Med Biol. 2016;42:150–158. doi: 10.1016/j.ultrasmedbio.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 87.Maconi G, Imbesi V, Porro GB. Doppler ultrasound measurement of intestinal blood flow in inflammatory bowel disease. Scand J Gastroenterol. 1996;31:590–593. doi: 10.3109/00365529609009132. [DOI] [PubMed] [Google Scholar]
- 88.Hultén L, Lindhagen J, Lundgren O, Fasth S, Ahrén C. Regional intestinal blood flow in ulcerative colitis and Crohn’s disease. Gastroenterology. 1977;72:388–396. doi: 10.1016/S0016-5085(77)80245-X. [DOI] [PubMed] [Google Scholar]
- 89.Lunderquist A, Knutsson H. Angiography in Crohn’s disease of the small bowel and colon. Am J Roentgenol Radium Ther Nucl Med. 1967;101:338–344. doi: 10.2214/ajr.101.2.338. [DOI] [PubMed] [Google Scholar]
- 90.Bacaner MB. Quantitative measurement of regional colon blood flow in the normal and pathological human bowel. Gastroenterology. 1966;51:764–777. doi: 10.1016/S0016-5085(19)34333-1. [DOI] [PubMed] [Google Scholar]
- 91.Angerson WJ, Allison MC, Baxter JN, Russell RI. Neoterminal ileal blood flow after ileocolonic resection for Crohn’s disease. Gut. 1993;34:1531–1534. doi: 10.1136/gut.34.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brahme F. Mesenteric angiography in regional enterocolitis. Radiology. 1966;87:1037–1042. doi: 10.1148/87.6.1037. [DOI] [PubMed] [Google Scholar]
- 93.Di Sabatino A, Fulle I, Ciccocioppo R, Ricevuti L, Tinozzi FP, Tinozzi S, et al. Doppler enhancement after intravenous Levovist injection in Crohn’s disease. Inflamm Bowel Dis. 2002;8:251–257. doi: 10.1097/00054725-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 94.Su KC, Leung FW, Guth PH. Assessment of mucosal hemodynamics in normal human colon and patients with inflammatory bowel disease. Gastrointest Endosc. 1989;35:22–27. doi: 10.1016/S0016-5107(89)72680-8. [DOI] [PubMed] [Google Scholar]
- 95.Sheehan AL, Warren BF, Gear MWL, Shepherd NA. Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–958. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- 96.Thörn M, Finnström N, Lundgren S, Rane A, Lööf L. Expression of cytochrome P450 and MDR1 in patients with proctitis. Ups J Med Sci. 2007;112:303–312. doi: 10.3109/2000-1967-203. [DOI] [PubMed] [Google Scholar]
- 97.Plewka D, Plewka A, Szczepanik T, Morek M, Bogunia E, Wittek P, et al. Expression of selected cytochrome P450 isoforms and of cooperating enzymes in colorectal tissues in selected pathological conditions. Pathol Res Pract. 2014;210:242–249. doi: 10.1016/j.prp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 98.Jahnel J, Fickert P, Hauer AC, Högenauer C, Avian A, Trauner M. Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug Metab Dispos. 2014;42:1423–1431. doi: 10.1124/dmd.114.058065. [DOI] [PubMed] [Google Scholar]
- 99.Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 100.Wilson A, Urquhart BL, Ponich T, Chande N, Gregor JC, Beaton M, et al. Crohn’s disease is associated with decreased CYP3A4 and P-glycoprotein protein expression. Mol Pharm. 2019;16:4059–4064. doi: 10.1021/acs.molpharmaceut.9b00459. [DOI] [PubMed] [Google Scholar]
- 101.Takayama K, Ito K, Matsui A, Yamashita T, Kawakami K, Hirayama D, et al. In vivo gene expression profile of human intestinal epithelial cells: from the viewpoint of drug metabolism and pharmacokinetics. Drug Metab Dispos. 2021;49:221–232. doi: 10.1124/dmd.120.000283. [DOI] [PubMed] [Google Scholar]
- 102.Klotz U, Hoensch H, Schütz T, Beaune P, Zanger U, Bode JC, et al. Expression of intestinal drug-metabolizing enzymes in patients with chronic inflammatory bowel disease. Curr Ther Res. 1998;59:556–563. doi: 10.1016/S0011-393X(98)85095-9. [DOI] [Google Scholar]
- 103.Blokzijl H, Borght SV, Bok LIH, Libbrecht L, Geuken M, van den Heuvel FAJ, et al. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflamm Bowel Dis. 2007;13:710–720. doi: 10.1002/ibd.20088. [DOI] [PubMed] [Google Scholar]
- 104.Blokzijl H, van Steenpaal A, Borght SV, Bok LIH, Libbrecht L, Tamminga M, et al. Up-regulation and cytoprotective role of epithelial multidrug resistance-associated protein 1 in inflammatory bowel disease. J Biol Chem. 2008;283:35630–35637. doi: 10.1074/jbc.M804374200. [DOI] [PubMed] [Google Scholar]
- 105.Wojtal KA, Eloranta JJ, Hruz P, Gutmann H, Drewe J, Staumann A, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871–1877. doi: 10.1124/dmd.109.027367. [DOI] [PubMed] [Google Scholar]
- 106.Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, et al. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133:1916–1927. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 107.Jung D. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53:78–84. doi: 10.1136/gut.53.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Girardin M, Dionne S, Goyette P, Rioux J, Bitton A, Elimrani I, et al. Expression and functional analysis of intestinal organic cation/l-carnitine transporter (OCTN) in Crohn’s disease. J Crohns Colitis. 2012;6:189–197. doi: 10.1016/j.crohns.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 109.Buchman AL, Paine MF, Wallin A, Ludington SS. A higher dose requirement of tacrolimus in active Crohn’s disease may be related to a high intestinal P-glycoprotein content. Dig Dis Sci. 2005;50:2312–2315. doi: 10.1007/s10620-005-3053-3. [DOI] [PubMed] [Google Scholar]
- 110.Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease–drug–drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89:735–740. doi: 10.1038/clpt.2011.35. [DOI] [PubMed] [Google Scholar]
- 111.Frye RF, Schneider VM, Frye CS, Feldman AM. Plasma levels of TNF-α and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail. 2002;8:315–319. doi: 10.1054/jcaf.2002.127773. [DOI] [PubMed] [Google Scholar]
- 112.Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br J Cancer. 2002;87:277–280. doi: 10.1038/sj.bjc.6600448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sunman JA, Hawke RL, LeCluyse EL, Kashuba ADM. Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab Dispos. 2004;32:359–363. doi: 10.1124/dmd.32.3.359. [DOI] [PubMed] [Google Scholar]
- 114.Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–1693. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–1422. doi: 10.1124/dmd.111.038679. [DOI] [PubMed] [Google Scholar]
- 116.Korolkova OY, Myers JN, Pellom ST, Wang L, M’koma AE. Characterization of serum cytokine profile in predominantly colonic inflammatory bowel disease to delineate ulcerative and Crohn’s colitides. Clin Med Insights Gastroenterol. 2015;8:CGast.S20612. [DOI] [PMC free article] [PubMed]
- 117.Gombošová L, Lazúrová I, Zakuciová M, Čurová K, Kmeťová M, Petrášová D, et al. Genes of intestinal Escherichia coli and their relation to the inflammatory activity in patients with ulcerative colitis and Crohn’s disease. Folia Microbiol (Praha) 2011;56:367–372. doi: 10.1007/s12223-011-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang L, Chen Y, Zhou W, Miao X, Zhou H. Utilization of physiologically-based pharmacokinetic model to assess disease-mediated therapeutic protein-disease-drug interaction in immune-mediated inflammatory diseases. Clin Transl Sci. 2022;15:464–476. doi: 10.1111/cts.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baruzzi A, Contin M, Perucca E, Albani F, Riva R. Altered serum protein binding of carbamazepine in disease states associated with an increased? 1-acid glycoprotein concentration. Eur J Clin Pharmacol. 1986;31:85–89. doi: 10.1007/BF00870992. [DOI] [PubMed] [Google Scholar]
- 120.Weeke B, Jarnum S. Serum concentration of 19 serum proteins in Crohn’s disease and ulcerative colitis. Gut. 1971;12:297–302. doi: 10.1136/gut.12.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou F-S, Gao N, Sun X, Jiang X-Y, Chen J-J, Mao Q-Q, et al. C-reactive protein/abumin ratio is a useful biomarker for predicting the mucosal healing in the Crohn disease. Medicine (Baltim) 2021;100:e24925. doi: 10.1097/MD.0000000000024925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szczeklik K, Krzyściak W, Cibor D, Kozioł K, Pocztar H, Pytko-Polończyk J, et al. Evaluation of plasma concentrations of selected antioxidant parameters in patients with active Crohn’s disease. Folia Med Crac. 2018;58:119–130. doi: 10.24425/fmc.2018.124663. [DOI] [PubMed] [Google Scholar]
- 123.Saadoun MM, Nosair NA el-A, Abdel-Azeez HA-H, Sharaf SM, Ahmed MH. Serum visfatin as a diagnostic marker of active inflammatory bowel disease. J Gastrointest Liver Dis. 2021;30:339–45. [DOI] [PubMed]
- 124.Sahin A, Calhan T, Cengiz M, Kahraman R, Aydin K, Ozdil K, et al. Serum interleukin 17 levels in patients with Crohn’s disease: real life data. Dis Markers. 2014;2014:1–6. doi: 10.1155/2014/690853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagy J, Lakner L, Poór VS, Pandur E, Mózsik G, Miseta A, et al. Serum prohepcidin levels in chronic inflammatory bowel diseases. J Crohns Colitis. 2010;4:649–653. doi: 10.1016/j.crohns.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 126.Li C, Shoji S, Beebe J. Pharmacokinetics and C-reactive protein modelling of anti-interleukin-6 antibody (PF-04236921) in healthy volunteers and patients with autoimmune disease. Br J Clin Pharmacol. 2018;84:2059–2074. doi: 10.1111/bcp.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iwamoto J, Honda A, Miyamoto Y, Miyazaki T, Murakami M, Saito Y, et al. Serum carnitine as an independent biomarker of malnutrition in patients with impaired oral intake. J Clin Biochem Nutr. 2014;55:221–227. doi: 10.3164/jcbn.14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mijač DD, Janković GLJ, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315–319. doi: 10.1016/j.ejim.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 129.Karbach U, Ewe K, Dehos H. Antiinflammatory treatment and intestinal? 1-antitrypsin clearance in active Crohn’s disease. Dig Dis Sci. 1985;30:229–235. doi: 10.1007/BF01347889. [DOI] [PubMed] [Google Scholar]
- 130.Ahrenstedt Ö, Hällgren R, Knutson L. Jejunal release of prostaglandin E 2 in Crohn’s disease: relation to disease activity and first-degree relatives. J Gastroenterol Hepatol. 1994;9:539–543. doi: 10.1111/j.1440-1746.1994.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 131.Cibor D, Szczeklik K, Brzozowski B, Mach T, Owczarek D. Serum galectin 3, galectin 9 and galectin 3-binding proteins in patients with active and inactive inflammatory bowel disease. J Physiol Pharmacol. 2019;70:95–104. [DOI] [PubMed]
- 132.Garg M, Burrell LM, Velkoska E, Griggs K, Angus PW, Gibson PR, et al. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: a pilot study. J Renin Angiotensin Aldosterone Syst. 2015;16:559–569. doi: 10.1177/1470320314521086. [DOI] [PubMed] [Google Scholar]
- 133.Engel T, Ben-Horin S, Beer-Gabel M. Autonomic dysfunction correlates with clinical and inflammatory activity in patients with Crohnʼs disease. Inflamm Bowel Dis. 2015;21:1. doi: 10.1097/MIB.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 134.Baba R, Yashiro K, Nagasako K, Obata H. Significance of serum sialic acid in patients with Crohn’s disease. Gastroenterol Jpn. 1992;27:604–610. doi: 10.1007/BF02774974. [DOI] [PubMed] [Google Scholar]
- 135.Eivindson M, Grønbæk H, Skogstrand K, Thorsen P, Frystyk J, Flyvbjerg A, et al. The insulin-like growth factor (IGF) system and its relation to infliximab treatment in adult patients with Crohn’s disease. Scand J Gastroenterol. 2007;42:464–470. doi: 10.1080/00365520601010115. [DOI] [PubMed] [Google Scholar]
- 136.Su Q, Li X, Mo W, Yang Z. Low serum bilirubin, albumin, and uric acid levels in patients with Crohn’s disease. Medicine (Baltim) 2019;98:e15664. doi: 10.1097/MD.0000000000015664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qin G, Tu J, Liu L, Luo L, Wu J, Tao L, et al. Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s disease activity. Med Sci Monit. 2016;22:4393–4400. doi: 10.12659/MSM.897460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaplan M, Yuksel M, Ates I, Kilic ZMY, Kilic H, Kuzu UB, et al. Is ischemia modified albumin a disease activity marker for inflammatory bowel diseases? J Gastroenterol Hepatol. 2016;31:1120–1125. doi: 10.1111/jgh.13254. [DOI] [PubMed] [Google Scholar]
- 139.Bourgonje AR, von Martels JZH, Bulthuis MLC, van Londen M, Faber KN, Dijkstra G, et al. Crohn’s disease in clinical remission is marked by systemic oxidative stress. Front Physiol. 2019;10:499. doi: 10.3389/fphys.2019.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cibor D, Szczeklik K, Kozioł K, Pocztar H, Mach T, Owczarek D. Serum concentration of selected biochemical markers of endothelial dysfunction and inflammation in patients with varying activities of inflammatory bowel disease. Pol Arch Intern Med. 2020;130:598–606. doi: 10.20452/pamw.15463. [DOI] [PubMed] [Google Scholar]
- 141.Krzystek-Korpacka M, Neubauer K, Berdowska I, Boehm D, Zielinski B, Petryszyn P, et al. Enhanced formation of advanced oxidation protein products in IBD. Inflamm Bowel Dis. 2008;14:794–802. doi: 10.1002/ibd.20383. [DOI] [PubMed] [Google Scholar]
- 142.Reimund J-M, Arondel Y, Escalin G, Finck G, Baumann R, Duclos B. Immune activation and nutritional status in adult Crohn’s disease patients. Dig Liver Dis. 2005;37:424–431. doi: 10.1016/j.dld.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 143.Jarnum S, Jensen KB. Fecal radioiodide excretion following intravenous injection of 131I-albumin and 125I-immunoglobulin G in chronic inflammatory bowel disease. Gastroenterology. 1975;68:1433–1444. doi: 10.1016/S0016-5085(75)80129-6. [DOI] [PubMed] [Google Scholar]
- 144.Suzuki Y, Matsui T, Ito H, Ashida T, Nakamura S, Motoya S, et al. Circulating interleukin 6 and albumin, and infliximab levels are good predictors of recovering efficacy after dose escalation infliximab therapy in patients with loss of response to treatment for Crohnʼs disease. Inflamm Bowel Dis. 2015;21:2114–2122. doi: 10.1097/MIB.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 145.Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. J Parenter Enter Nutr. 2007;31:311–319. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 146.Liu X, Wu X, Zhou C, Hu T, Ke J, Chen Y, et al. Preoperative hypoalbuminemia is associated with an increased risk for intra-abdominal septic complications after primary anastomosis for Crohn’s disease. Gastroenterol Rep. 2017;5:298–304. doi: 10.1093/gastro/gox002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nguyen GC, Du L, Chong RY, Jackson TD. Hypoalbuminaemia and postoperative outcomes in inflammatory bowel disease: the NSQIP surgical cohort. J Crohns Colitis. 2019;13:1433–1438. doi: 10.1093/ecco-jcc/jjz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ricci G, Ambrosi A, Resca D, Masotti M, Alvisi V. Comparison of serum total sialic acid, C-reactive protein, α1-acid glycoprotein and β2-microglobulin in patients with non-malignant bowel diseases. Biomed Pharmacother. 1995;49:259–262. doi: 10.1016/0753-3322(96)82632-1. [DOI] [PubMed] [Google Scholar]
- 149.Gesink-van der Veer BJ, Burm AG, Hennis PJ, Bovill JG. Alfentanil requirement in Crohn’s disease. Increased alfentanil dose requirement in patients with Crohn’s disease. Anaesthesia. 1989;44:209–11. [DOI] [PubMed]
- 150.Easton JA, Hardwicke J, Whitehead PH. The estimation of two alpha1 glycoproteins (orosomucoid and another alpha1 acid glycoprotein) in health and disease. J Clin Pathol. 1962;15:585–590. doi: 10.1136/jcp.15.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Darroch CJ, Barnes RMR, Dawson J. Circulating antibodies to Saccharomyces cerevisiae (bakers’/brewers’ yeast) in gastrointestinal disease. J Clin Pathol. 1999;52:47–53. doi: 10.1136/jcp.52.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Griffin N, Grant LA, Anderson S, Irving P, Sanderson J. Small bowel MR enterography: problem solving in Crohn’s disease. Insights Imaging. 2012;3:251–263. doi: 10.1007/s13244-012-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dulai PS, Singh S, Vande Casteele N, Boland BS, Rivera-Nieves J, Ernst PB, et al. Should we divide Crohn’s disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol. 2019;17:2634–2643. doi: 10.1016/j.cgh.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scharl M, Bruckner RS, Rogler G. The two sides of the coin: similarities and differences in the pathomechanisms of fistulas and stricture formations in irritable bowel disease. United Eur Gastroenterol J. 2016;4:506–514. doi: 10.1177/2050640616635957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hodges P, Gee M, Grace M, Sherbaniuk RW, Wensel RH, Thomson ABR. Protein-energy intake and malnutrition in Crohn’s disease. J Am Diet Assoc. 1984;84:1460–1464. doi: 10.1016/S0002-8223(21)08378-4. [DOI] [PubMed] [Google Scholar]
- 156.Tarján Z, Tóth G, Györke T, Mester Á, Karlinger K, Makó EK. Ultrasound in Crohn’s disease of the small bowel. Eur J Radiol. 2000;35:176–182. doi: 10.1016/S0720-048X(00)00240-0. [DOI] [PubMed] [Google Scholar]
- 157.Ludwig D, Wiener S, Brüning A, Schwarting K, Jantschek G, Stange EF. Mesenteric blood flow is related to disease activity and risk of relapse in Crohn’s disease: a prospective follow-up study. Am J Gastroenterol. 1999;94:2942–2950. doi: 10.1111/j.1572-0241.1999.01442.x. [DOI] [PubMed] [Google Scholar]
- 158.Baker ME, Walter J, Obuchowski NA, Achkar J-P, Einstein D, Veniero JC, et al. Mural attenuation in normal small bowel and active inflammatory Crohn’s disease on CT enterography: location, absolute attenuation, relative attenuation, and the effect of wall thickness. Am J Roentgenol. 2009;192:417–423. doi: 10.2214/AJR.08.1267. [DOI] [PubMed] [Google Scholar]
- 159.Vyhlidal CA, Chapron BD, Ahmed A, Singh V, Casini R, Shakhnovich V. Effect of Crohn’s disease on villous length and CYP3A4 expression in the pediatric small intestine. Clin Transl Sci. 2021;14:729–736. doi: 10.1111/cts.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 161.Sipos F, Molnár B, Zágoni T, Berczi L, Tulassay Z. Growth in epithelial cell proliferation and apoptosis correlates specifically to the inflammation activity of inflammatory bowel diseases: ulcerative colitis shows specific p53- and EGFR expression alterations. Dis Colon Rectum. 2005;48:775–786. doi: 10.1007/s10350-004-0831-5. [DOI] [PubMed] [Google Scholar]
- 162.Zbar AP, Simopoulos C, Karayiannakis AJ. Cadherins: an integral role in inflammatory bowel disease and mucosal restitution. J Gastroenterol. 2004;39:413–421. doi: 10.1007/s00535-004-1335-8. [DOI] [PubMed] [Google Scholar]
- 163.Liapis G, Boletis J, Skalioti C, Bamias G, Tsimaratou K, Patsouris E, et al. Histological spectrum of mycophenolate mofetil-related colitis: association with apoptosis. Histopathology. 2013;63:649–658. doi: 10.1111/his.12222. [DOI] [PubMed] [Google Scholar]
- 164.Mäkitalo L, Sipponen T, Kärkkäinen P, Kolho K-L, Saarialho-Kere U. Changes in matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) expression profile in Crohn’s disease after immunosuppressive treatment correlate with histological score and calprotectin values. Int J Colorectal Dis. 2009;24:1157–1167. doi: 10.1007/s00384-009-0756-5. [DOI] [PubMed] [Google Scholar]
- 165.Tougaard L, Giese B, Pedersen BH, Binder V. Bile acid metabolism in patients with Crohn’s disease in terminal ileum. Scand J Gastroenterol. 1986;21:627–633. doi: 10.3109/00365528609003110. [DOI] [PubMed] [Google Scholar]
- 166.Eckburg PB, Relman DA. The role of microbes in Crohn’s disease. Clin Infect Dis. 2007;44:256–262. doi: 10.1086/510385. [DOI] [PubMed] [Google Scholar]
- 167.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152:327–339.e4. doi: 10.1053/j.gastro.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Swanson HI. Drug metabolism by the host and gut microbiota: a partnership or rivalry? Drug Metab Dispos. 2015;43:1499–1504. doi: 10.1124/dmd.115.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Enright EF, Gahan CGM, Joyce SA, Griffin BT. The impact of the gut microbiota on drug metabolism and clinical outcome. Yale J Biol Med. 2016;89:375–382. [PMC free article] [PubMed] [Google Scholar]
- 171.Mertz A. Primary sclerosing cholangitis and inflammatory bowel disease comorbidity: an update of the evidence. Ann Gastroenterol. 2019;32:124–133. doi: 10.20524/aog.2019.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cieślak A, Kelly I, Trottier J, Verreault M, Wunsch E, Milkiewicz P, et al. Selective and sensitive quantification of the cytochrome P450 3A4 protein in human liver homogenates through multiple reaction monitoring mass spectrometry. Proteomics. 2016;16:2827–2837. doi: 10.1002/pmic.201500386. [DOI] [PubMed] [Google Scholar]
- 173.Milkiewicz M, Klak M, Kempinska-Podhorodecka A, Wiechowska-Kozlowska A, Urasinska E, Blatkiewicz M, et al. Impaired hepatic adaptation to chronic cholestasis induced by primary sclerosing cholangitis. Sci Rep. 2016;6:39573. doi: 10.1038/srep39573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Couto N, Al-Majdoub ZM, Achour B, Wright PC, Rostami-Hodjegan A, Barber J. Quantification of proteins involved in drug metabolism and disposition in the human liver using label-free global proteomics. Mol Pharm. 2019;16:632–647. doi: 10.1021/acs.molpharmaceut.8b00941. [DOI] [PubMed] [Google Scholar]
- 175.Cerny MA. Prevalence of non-cytochrome P450-mediated metabolism in food and drug administration-approved oral and intravenous drugs: 2006–2015. Drug Metab Dispos. 2016;44:1246–1252. doi: 10.1124/dmd.116.070763. [DOI] [PubMed] [Google Scholar]
- 176.Ekblad E, Bauer AJ. Role of vasoactive intestinal peptide and inflammatory mediators in enteric neuronal plasticity. Neurogastroenterol Motil. 2004;16:123–128. doi: 10.1111/j.1743-3150.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 177.Abad C, Juarranz Y, Martinez C, Arranz A, Rosignoli F, García-Gómez M, et al. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm Bowel Dis. 2005;11:674–684. doi: 10.1097/01.MIB.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- 178.Soufflet F, Biraud M, Rolli-Derkinderen M, Lardeux B, Trang C, Coron E, et al. Modulation of VIPergic phenotype of enteric neurons by colonic biopsy supernatants from patients with inflammatory bowel diseases: involvement of IL-6 in Crohn’s disease. Neurogastroenterol Motil. 2018;30:e13198. doi: 10.1111/nmo.13198. [DOI] [PubMed] [Google Scholar]
- 179.Picotti P, Bodenmiller B, Mueller LN, Domon B, Aebersold R. Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell. 2009;138:795–806. doi: 10.1016/j.cell.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ohtsuki S, Uchida Y, Kubo Y, Terasaki T. Quantitative targeted absolute proteomics-based ADME research as a new path to drug discovery and development: methodology, advantages, strategy, and prospects. J Pharm Sci. 2011;100:3547–3559. doi: 10.1002/jps.22612. [DOI] [PubMed] [Google Scholar]
- 181.Harwood MD, Neuhoff S, Carlson GL, Warhurst G, Rostami-Hodjegan A. Absolute abundance and function of intestinal drug transporters: a prerequisite for fully mechanistic in vitro-in vivo extrapolation of oral drug absorption. Biopharm Drug Dispos. 2013;34:2–28. doi: 10.1002/bdd.1810. [DOI] [PubMed] [Google Scholar]
- 182.Drozdzik M, Busch D, Lapczuk J, Müller J, Ostrowski M, Kurzawski M, et al. Protein abundance of clinically relevant drug-metabolizing enzymes in the human liver and intestine: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther. 2019;105:1204–1212. doi: 10.1002/cpt.1301. [DOI] [PubMed] [Google Scholar]
- 183.Mouly S, Paine MF. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res. 2003;20:1595–1599. doi: 10.1023/A:1026183200740. [DOI] [PubMed] [Google Scholar]
- 184.Bruyère A, Declèves X, Bouzom F, Ball K, Marques C, Treton X, et al. Effect of variations in the amounts of P-glycoprotein (ABCB1), BCRP (ABCG2) and CYP3A4 along the human small intestine on PBPK models for predicting intestinal first pass. Mol Pharm. 2010;7:1596–1607. doi: 10.1021/mp100015x. [DOI] [PubMed] [Google Scholar]
- 185.Thorn M, Finnstrom N, Lundgren S, Rane A, Loof L. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. Br J Clin Pharmacol. 2005;60:54–60. doi: 10.1111/j.1365-2125.2005.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nakamura T, Sakaeda T, Ohmoto N, Tamura T, Aoyama N, Shirakawa T, et al. Real-time quantitative polymerase chain reaction for MDR1, MRP1, MRP2, and CYP3A-mRNA levels in Caco-2 cell lines, human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Drug Metab Dispos. 2002;30:4–6. doi: 10.1124/dmd.30.1.4. [DOI] [PubMed] [Google Scholar]
- 187.Zimmermann C, Gutmann H, Hruz P, Gutzwiller J-P, Beglinger C, Drewe J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos. 2005;33:219–224. doi: 10.1124/dmd.104.001354. [DOI] [PubMed] [Google Scholar]
- 188.Englund G, Rorsman F, Rönnblom A, Karlbom U, Lazorova L, Gråsjö J, et al. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci. 2006;29:269–277. doi: 10.1016/j.ejps.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 189.Seithel A, Karlsson J, Hilgendorf C, Björquist A, Ungell A-L. Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: comparison between human segments and Caco-2 cells. Eur J Pharm Sci. 2006;28:291–299. doi: 10.1016/j.ejps.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 190.Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell A-L, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333–1340. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 191.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schneider RE, Babb J, Bishop H, Mitchard M, Hoare AM. Plasma levels of propranolol in treated patients with coeliac disease and patients with Crohn’s disease. BMJ. 1976;2:794–795. doi: 10.1136/bmj.2.6039.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J Parenter Enter Nutr. 2019;43:181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Powell-Tuck J. Protein metabolism in inflammatory bowel disease. Gut. 1986;27:67–71. doi: 10.1136/gut.27.Suppl_1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ye M, Nagar S, Korzekwa K. A physiologically based pharmacokinetic model to predict the pharmacokinetics of highly protein-bound drugs and the impact of errors in plasma protein binding. Biopharm Drug Dispos. 2016;37:123–141. doi: 10.1002/bdd.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wilkinson GR, Shand DG. Commentary: a physio-logical approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]
- 197.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–1359. [PubMed] [Google Scholar]
- 198.Baker M, Parton T. Kinetic determinants of hepatic clearance: plasma protein binding and hepatic uptake. Xenobiotica. 2007;37:1110–1134. doi: 10.1080/00498250701658296. [DOI] [PubMed] [Google Scholar]
- 199.Takac B, Mihaljević S, Stefanić M, Glavas-Obrovac L, Kibel A, Samardzija M. Importance of interleukin 6 in pathogenesis of inflammatory bowel disease. Coll Antropol. 2014;38:659–664. [PubMed] [Google Scholar]
- 200.Carroll RE, Benedetti E, Schowalter JP, Buchman AL. Management and complications of short bowel syndrome: an updated review. Curr Gastroenterol Rep. 2016;18:40. doi: 10.1007/s11894-016-0511-3. [DOI] [PubMed] [Google Scholar]
- 201.Boccia S, Torre I, Santarpia L, Iervolino C, Del Piano C, Puggina A, et al. Intestinal microbiota in adult patients with Short Bowel Syndrome: preliminary results from a pilot study. Clin Nutr. 2017;36:1707–1709. doi: 10.1016/j.clnu.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 202.Ziegler TR, Fernández-Estívariz C, Gu LH, Bazargan N, Umeakunne K, Wallace TM, et al. Distribution of the H+/peptide transporter PepT1 in human intestine: up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am J Clin Nutr. 2002;75:922–930. doi: 10.1093/ajcn/75.5.922. [DOI] [PubMed] [Google Scholar]
- 203.Joly F, Mayeur C, Messing B, Lavergne-Slove A, Cazals-Hatem D, Noordine M-L, et al. Morphological adaptation with preserved proliferation/transporter content in the colon of patients with short bowel syndrome. Am J Physiol Liver Physiol. 2009;297:G116–G123. doi: 10.1152/ajpgi.90657.2008. [DOI] [PubMed] [Google Scholar]
- 204.Compher C, Rubesin S, Kinosian B, Madaras J, Metz D. Noninvasive measurement of transit time in short bowel syndrome. J Parenter Enter Nutr. 2007;31:240–245. doi: 10.1177/0148607107031003240. [DOI] [PubMed] [Google Scholar]
- 205.Fitzpatrick WJF, Zentler-Munro PL, Northfield TC. Ileal resection: effect of cimetidine and taurine on intrajejunal bile acid precipitation and lipid solubilisation. Gut. 1986;27:66–72. doi: 10.1136/gut.27.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Van Deest BW, Fordtran JS, Morawski SG, Wilson JD. Bile salt and micellar fat concentration in proximal small bowel contents of ileectomy patients. J Clin Investig. 1968;47:1314–1324. doi: 10.1172/JCI105823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tappenden KA. Intestinal adaptation following resection. J Parenter Enter Nutr. 2014;38:23S–31S. doi: 10.1177/0148607114525210. [DOI] [PubMed] [Google Scholar]
- 208.Severijnen R, Bayat N, Bakker H, Tolboom J, Bongaerts G. Enteral drug absorption in patients with short small bowel a review. Clin Pharmacokinet. 2004;43:951–962. doi: 10.2165/00003088-200443140-00001. [DOI] [PubMed] [Google Scholar]
- 209.Kumpf VJ. Pharmacologic management of diarrhea in patients with short bowel syndrome. J Parenter Enter Nutr. 2014;38:38S–44S. doi: 10.1177/0148607113520618. [DOI] [PubMed] [Google Scholar]
- 210.Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. Gastroenterology. 1972;62:918–934. doi: 10.1016/S0016-5085(72)80109-4. [DOI] [PubMed] [Google Scholar]
- 211.Krag E, Phillips SF. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Investig. 1974;53:1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. Intestinal absorption of bile acids in health and disease. Compr Physiol. 2020;10:21–56. doi: 10.1002/cphy.c190007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803–1823. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- 214.Mehta M, Venkataramanan R, Burckart G, Ptachcinski R, Delamos B, Stachak S, et al. Effect of bile on cyclosporin absorption in liver transplant patients. Br J Clin Pharmacol. 1988;25:579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim K, Yoon I, Chun I, Lee N, Kim T, Gwak HS. Effects of bile salts on the lovastatin pharmacokinetics following oral administration to rats. Drug Deliv. 2011;18:79–83. doi: 10.3109/10717544.2010.512024. [DOI] [PubMed] [Google Scholar]
- 216.Stojančević M, Pavlović N, Goločorbin-Kon S, Mikov M. Application of bile acids in drug formulation and delivery. Front Life Sci. 2013;7:112–122. doi: 10.1080/21553769.2013.879925. [DOI] [Google Scholar]
- 217.Zanni GR, Wick JY. Ostomy care and the consultant pharmacist. Consult Pharm. 2006;21:262–274. doi: 10.4140/TCP.n.2006.262. [DOI] [PubMed] [Google Scholar]
- 218.Rose RH, Turner DB, Neuhoff S, Jamei M. Incorporation of the time-varying postprandial increase in splanchnic blood flow into a PBPK model to predict the effect of food on the pharmacokinetics of orally administered high-extraction drugs. AAPS J. 2017;19:1205–1217. doi: 10.1208/s12248-017-0099-z. [DOI] [PubMed] [Google Scholar]
- 219.Watkins P. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997;27:161–170. doi: 10.1016/S0169-409X(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 220.Benet LZ, Wu C-Y, Hebert MF, Wacher VJ. Intestinal drug metabolism and antitransport processes: a potential paradigm shift in oral drug delivery. J Control Release. 1996;39:139–143. doi: 10.1016/0168-3659(95)00147-6. [DOI] [Google Scholar]
- 221.Benet LZ. The drug transporter−metabolism alliance: uncovering and defining the interplay. Mol Pharm. 2009;6:1631–1643. doi: 10.1021/mp900253n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Darwich AS, Neuhoff S, Jamei M, Rostami-Hodjegan A. Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr Drug Metab. 2010;11:716–729. doi: 10.2174/138920010794328913. [DOI] [PubMed] [Google Scholar]
- 223.Badhan R, Penny J, Galetin A, Houston JB. Methodology for development of a physiological model incorporating CYP3A and P-glycoprotein for the prediction of intestinal drug absorption. J Pharm Sci. 2009;98:2180–2197. doi: 10.1002/jps.21572. [DOI] [PubMed] [Google Scholar]
- 224.Ito K, Kusuhara H, Sugiyama Y. Effects of intestinal CYP3A4 and P-glycoprotein on oral drug absorption–theoretical approach. Pharm Res. 1999;16:225–231. doi: 10.1023/A:1018872207437. [DOI] [PubMed] [Google Scholar]
- 225.Watanabe T, Maeda K, Nakai C, Sugiyama Y. Investigation of the effect of the uneven distribution of CYP3A4 and P-glycoprotein in the intestine on the barrier function against xenobiotics: a simulation study. J Pharm Sci. 2013;102:3196–3204. doi: 10.1002/jps.23623. [DOI] [PubMed] [Google Scholar]
- 226.Cummins CL, Jacobsen W, Benet LZ. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;300:1036–1045. doi: 10.1124/jpet.300.3.1036. [DOI] [PubMed] [Google Scholar]
- 227.Cummins CL, Jacobsen W, Christians U, Benet LZ. CYP3A4-Transfected Caco-2 cells as a tool for understanding biochemical absorption barriers: studies with sirolimus and midazolam. J Pharmacol Exp Ther. 2004;308:143–155. doi: 10.1124/jpet.103.058065. [DOI] [PubMed] [Google Scholar]
- 228.Rogler G. Efficacy of JAK inhibitors in Crohn’s disease. J Crohns Colitis. 2020;14:S746–S754. doi: 10.1093/ecco-jcc/jjz186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Verstockt B, Vetrano S, Salas A, Nayeri S, Duijvestein M, Vande Casteele N, et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2022;19:351–366. doi: 10.1038/s41575-021-00574-7. [DOI] [PubMed] [Google Scholar]
- 230.Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12:569. doi: 10.3389/fphar.2021.651415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.