Abstract
A major component of aging is chronic, low-grade inflammation, attributable in part by impaired gut barrier function. We previously reported that deletion of ghrelin, a peptidergic hormone released mainly from the gut, exacerbates experimental muscle atrophy in aged mice. In addition, ghrelin has been shown to ameliorate colitis in experimental models of inflammatory bowel disease (IBD), although the role of endogenous ghrelin in host–microbe interactions is less clear. Here, we showed that 22-month-old global ghrelin knockout (Ghrl−/−) mice exhibited significantly increased depressive-like behaviors, while anxiety levels and working memory were similar to littermate wild-type (WT) mice. Furthermore, old Ghrl−/− mice showed significantly increased intestinal permeability to fluorescein isothiocyanate (FITC)-dextran, significantly higher colonic interleukin (IL-1β) levels, and trends for higher colonic IL-6 and tumor necrosis factor-α (TNF-α) compared to WT mice. Interestingly, young Ghrl−/− and WT mice showed comparable depressive-like behavior and gut permeability, suggesting age-dependent exacerbation in gut barrier dysfunction in Ghrl−/− mice. While fecal short-chain fatty acids levels were comparable between old Ghrl−/− and WT mice, serum metabolome revealed alterations in metabolic cascades including tryptophan metabolism. Specifically, tryptophan and its microbial derivatives indole-3-acetic acid and indole-3-lactic acid were significantly reduced in old Ghrl−/−mice. Furthermore, in an experimental model of dextran sulfate sodium (DSS)-induced colitis, Ghrl−/− mice showed exacerbated disease symptoms, and higher levels of chemoattractant and pro-inflammatory cytokines in the colon. Overall, these data demonstrated that ghrelin deficiency is associated with gut barrier dysfunction, alterations in microbially derived tryptophan metabolites, and increased susceptibility to colitis. These data suggested that endogenous ghrelin contributes to maintaining a healthy host–microbe environment, ultimately impacting on brain function.
Keywords: Ghrelin, tryptophan metabolism, indoles, gut barrier, aging
Impact Statement
The colonic tissue microenvironment helps to maintain a symbiotic microbial community. This study aimed to evaluate ghrelin’s role in chronic and acute pro-inflammatory conditions, and demonstrated that endogenous ghrelin contributes to host–microbe interactions. The study showed that ghrelin deficiency leads to gut dysbiosis, with reduced microbial tryptophan metabolism and thereby reduced production of indoles and related catabolites, which were associated with increased susceptibility to acute inflammation, and pro-inflammatory pathologies including intestinal permeability and depression in aging. The results contributed to further insights to host–microbe interactions in health and disease.
Introduction
A major component of aging is chronic, low-grade inflammation, which is an important risk factor for morbidity and mortality in the older adults, since inflammatory pathogenesis underlies most age-associated diseases.1,2 Accumulating evidence implicate bacterial contributions to chronic inflammation via imbalance in the gut microbial communities (gut dysbiosis).3,4 Gut dysbiosis and the decrease in barrier function, indicated by augmented mucosal permeability and translocation of bacterial toxins to circulation, have been linked to systemic inflammation, metabolic dysfunction, neuroinflammation, stress-related psychiatric disorders, and cognitive decline in aging.5 –9 While the symbiotic relationships between host and microbes are now considered as critical determinants of health and disease, the molecular mechanisms regulating the host–microbe interactions during aging remain largely undetermined.
Ghrelin, a hormone consisting of 28 amino acids, is produced and secreted by X/A-like cells of the stomach,10,11 and functions in inducing growth hormone release, increasing feeding, and promoting adiposity.12 –15 On the contrary, through loss-of-function studies, we and others have shown that body weight and feeding were not altered in ghrelin-deficient mice compared to wild-type (WT) mice on either high-fat diet or regular diet.16,17 Interestingly, pharmacological administration of ghrelin attenuates sepsis-induced gut barrier impairment 18 and experimental colitis, 19 suggesting that ghrelin may exert anti-inflammatory effects in preclinical models of acute inflammation. However, little is known regarding the role of endogenous ghrelin in gut barrier function. Clinical data showed decreased circulating ghrelin levels in older subjects.20,21 Furthermore, we found that with a high fructose corn syrup supplement, ghrelin-null (Ghrl−/−) mice exhibited exacerbated adiposity and inflammation of the adipose tissue, suggesting increased susceptibility to pro-inflammatory stimulus. 22 In addition, we recently reported that old Ghrl−/− mice (18–20 months) exhibited predisposition to fasting-induced muscle atrophy, and administration of exogenous ghrelin exerted protective effects. 23 Notably, microbiome analysis showed an increase in Firmicutes and a decrease in Bacteroidetes in the gut microbiota composition in Ghrl−/− mice at young age (6 months), prior to manifestation of age-associated increase in adiposity. 23 Bacteroidetes and Firmicutes have been associated with healthy lean metabolic state and obese inflammatory state, respectively.24,25 Taken together, these data suggested that ghrelin deficiency may predispose the host to pro-inflammatory pathologies. Whether microbiota dysbiosis in ghrelin-deficient mice contributes to aging-associated physiological changes remains to be determined.
Here, we first studied whether old Ghrl−/− mice show impaired intestinal permeability and cognitive functions. Given that we previously showed age-associated alterations in serum metabolome and microbial tryptophan metabolism, 26 we next assessed serum metabolome in old Ghrl−/− and WT mice. Finally, to further investigate the role of ghrelin in gut barrier function, we subjected Ghrl−/− and WT mice to dextran sulfate sodium (DSS)-induced experimental model of ulcerative colitis. Our data demonstrated that ghrelin-deficient mice are more vulnerable to pro-inflammatory pathologies associated with aging and colitis, suggesting that endogenous ghrelin contributes to intestinal homeostasis.
Materials and methods
Animals
Global Ghrelin knockout (Ghrl−/−) mice were generated by Sun et al. 16 Mice were housed in the animal facility of Texas A&M University (College station, TX, USA), maintained on 12 h light/dark cycles (lights on at 6:00) at 22°C ± 2°C. Mice were given ad libitum access to water and chow diet (TD. 2018, Envigo, Madison, WI, USA, with 18%, 58%, and 24% calories from fat, carbohydrates, and protein, respectively). All experimental procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M University, and all methods were performed in accordance with the relevant guideline and regulations.
Experimental design
Three independent cohorts of WT and Ghrl−/− mice were used in the study. In Cohort 1, male WT and Ghrl−/− mice from 22 months (22M) of age were subjected to fecal collection for short-chain fatty acids (SCFA) measurements, behavior testing, intestinal permeability test, and euthanized at 28 months (28M) and serum collected for metabolome analysis. In Cohort 2, male WT and Ghrl−/− mice at 6–12 months (6–12M) were subjected forced swim test (FST) and intestinal permeability test. In Cohort 3, male WT and Ghrl−/− mice at 18 months (18M) were subjected to DSS-induced colitis (n = 4, 4, and 5 for WT, DSS–WT, and DSS–Ghrl−/− groups, respectively). For feces collection, mice were transferred to individual autoclaved cages in the morning between 8:00 and 9:00, fecal pellets produced were collected, flash frozen and stored at −80°C until targeted metabolome analysis of SCFAs. Mice were euthanized and serum samples collected as we described before. 26 Colons were dissected, flushed with phosphate-buffered saline (PBS), opened longitudinally, flash frozen, and stored at −80°C until cytokine analysis.
Serum metabolome analysis
Serum metabolites of male 28M WT and Ghrl−/− mice, at fed state, were assessed using untargeted liquid chromatography–high-resolution accurate mass spectrometry (LC-HRMS) analysis as we described before. 26 For data analysis with the web-based tool MetaboAnalyst 5.0, 27 the peak intensity table (Supplementary Table 1, containing 10 [samples] by 1820 [peaks[mz/rt] data matrix]) was uploaded to the website, and data were then normalized by the sum followed by auto-scaling (boxplots of data after normalization are shown in Supplementary Figure 1). Data were then analyzed by the principal component analysis (PCA) and univariate analysis, and the output table of significantly altered metabolites was then used in pathway analysis (Supplementary Table 2).
Fecal SCFA measurement
Feces were processed for quantitation of SCFAs with a gas chromatography triple quadrupole mass spectrometer (TSQ EVO 8000, Thermo Scientific, Waltham, MA, USA) as we described. 28 Briefly, 50 mg of lyophilized feces were extracted in ethyl acetate, spiked with heptadeuterated butyric acid to normalize for extraction efficiency; the absolute levels in μM were normalized to mg dried fecal weights. TraceFinder 3.3 (Thermo Scientific) was used in sample acquisition and analysis.
Behavior testing
Mice were acclimatized to the procedure room for 30 min before each testing session. Behavioral testing was performed between 10:00 and 16:00.
Open field test
Open field test was performed as we described, 29 with slight modification, in the Rodent Preclinical Phenotyping Core. Each animal was put in the center of a clear Plexiglas chamber (25 cm × 25 cm × 38 cm) with photo beams to record horizontal activities (TRU SCAN Activity Monitoring System, Coulbourn Instruments, Holliston, MA, USA). Testing was performed with overhead lights (∼700 lux of illumination). Activities were monitored over a 30-min period; physical activity (total distance traveled) and anxiety level (time spent in center zone) were analyzed.
Novel object recognition test
Novel object recognition test (NORT) was carried out as described, with slight modification.30,31 On the first day, each mouse was subjected to a 5-min trial to acclimatize to the arena (40 cm × 40 cm × 30 cm) without objects. Twenty-four hours after acclimatization session, each mouse was given 5 min to explore (defined as sniffing or touching) identical objects (F1 and F2), placed in fixed adjacent corners. Two hours after training trial, F2 was switched to a novel object N, and interactions with F1 or N were recorded for 5 min to test for short-term memory test. Activities during all testing trials were recorded with a camera. A scorer blinded to the genotype of the mice scored the recorded videos for time spent interacting with the familiar or novel object.
FST
FST was performed as we previously described. 29
Intestinal permeability
Mice were fasted for 4 h prior to oral gavage of fluorescein isothiocyanate (FITC)-labeled dextran (3–5 kDa; Sigma-Aldrich, St. Louis, MO, USA). 200 and 150 μL of 80 mg/mL FITC-dextran were administered to old and young mice, respectively. After gavage, mice were returned to home cage and left undisturbed for 4 h, without access to food or water. Blood was collected from tail vein 4 h after gavage into ethylenediaminetetraacetic acid (EDTA)-coated microvette 100 capillary blood collection tubes (Sarstedt, Newton, NC, USA), centrifuged at 5000g for 5 min and plasma transferred to fresh tubes. Fluorescence intensity was measured in black-welled plates with microplate reader CLARIOstar Plus (BMG Labtech, Ortenberg, Germany).
Induction of colitis
Animals received normal drinking water (control group) or DSS (2%) (MP Biomedicals = 36–50 kDa) in drinking water for seven days; water was refreshed every two to three days. All mice were assessed for body weight, fecal consistency, and macroscopic fecal blood scores as described. 32 Animals were terminated on study Day 7 after final scoring, and colon samples collected as described above.
Cytokine analysis
Colons were weighed and homogenized at 10:1 ratio (10 mL per 1 g of tissue) in T-PER and quantitated as described. 32 25 μL of each sample was used in cytokine analysis using the Mouse Cytokine/Chemokine Milliplex Map Kit (Millipore, Burlington, MA, USA), according to the manufacturer’s instructions. The plate was analyzed on a BioPlex 200 (Bio-Rad, Hercules, CA, USA). Data were normalized to per mg protein.
Statistical analysis
Results were expressed as mean value ± standard error (SE) at a significant level of P < 0.05. Student’s t-tests were used to compare genotype effect. For the comparison between more than two groups, one-way analysis of variance (ANOVA) was used if there was a single independent variable, or two-way ANOVA for repeated measures (treatment × time). To further test pairwise differences between treatment groups, Tukey’s post hoc tests were used. Statistical analyses were carried out using GraphPad PRISM 9.2.0 (GraphPad, San Diego, CA, USA).
Results
Aged ghrelin-deficient mice exhibited increased depressive-like behaviors
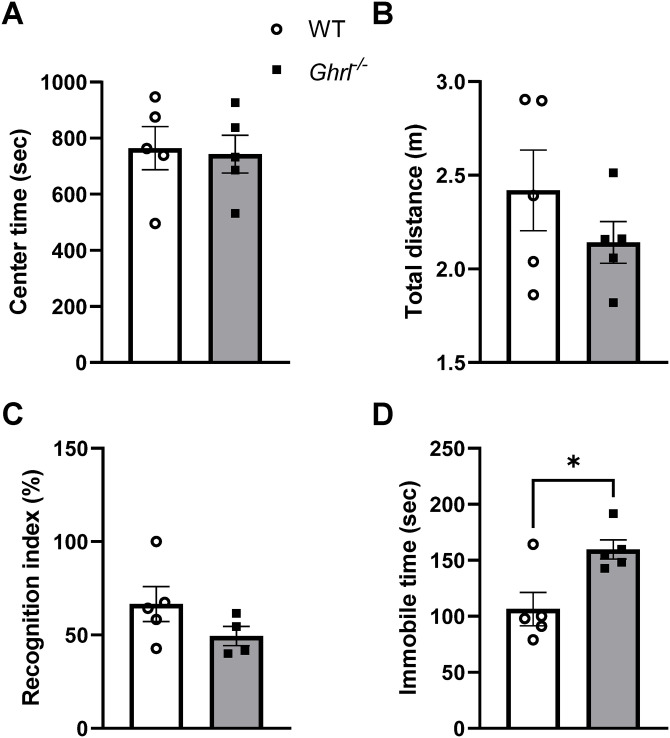
Previously, we reported gut dysbiosis in Ghrl−/− mice at young age. 23 To test the functional consequences of chronic gut dysbiosis in aging, we subjected 22-month-old male Ghrl−/− and littermate control WT mice to behavior tests assessing anxiety, working memory, and depressive-like behavior. Open field test showed that there was no significant difference in the time spent in the center zone of an open arena between old Ghrl−/− and WT mice, suggesting similar anxiety levels (Figure 1(A)). In addition, old Ghrl−/− and WT mice traveled similar distances in the 30-min test period, suggesting comparable locomotor activity levels in a novel environment (Figure 1(B)). In the NORT, WT mice spent more time interacting with the novel objects than familiar objects (recognition index >50%) as expected, while there was a trend for reduced recognition index in old Ghrl−/− mice (Figure 1(C)). In the FST which models behavioral despair, old Ghrl−/− mice showed significantly increased immobile time, suggesting increased depressive-like behavior compared to WT mice (Figure 1(D)).
Figure 1.
Old Ghrl−/− mice showed increased depressive-like behavior. Anxiety levels and locomotor activity were measured in the open field test (A and B). (A) Time spent in the center zone. (B) Total distance traveled in the open field arena. (C) Learning and memory function was tested in novel object recognition test, indicated by recognition index. (D) Depressive-like behaviors were measured in the forced swim test, indicated by immobile time.
*P < 0.05, WT versus Ghrl−/− mice.
Aged ghrelin-deficient mice exhibited increased gut permeability and inflammation
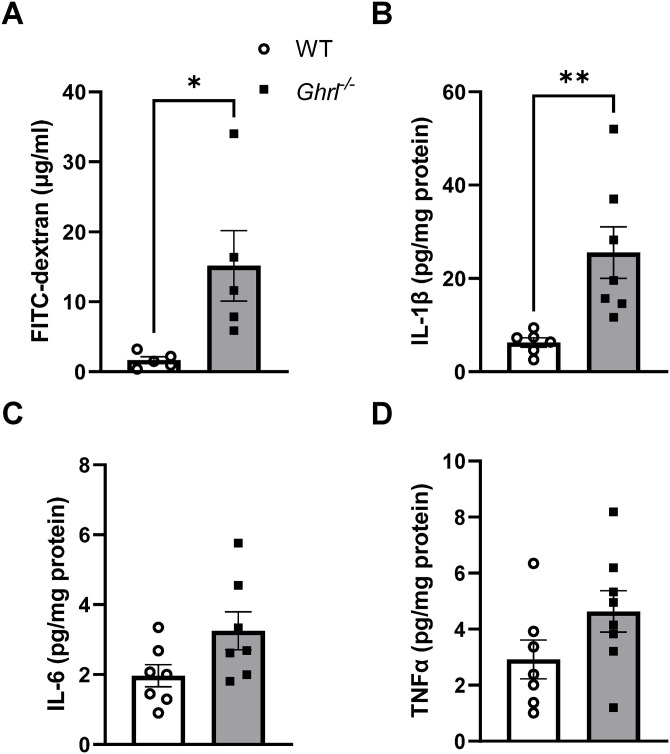
Given that depression has been linked to impaired gut barrier function, 7 and aging is associated with increased leakiness of the gut, 33 next we assessed gut barrier function in these mice using an in vivo intestinal permeability test. We found that at 23 months of age, there was a significant increase in plasma FITC-dextran levels in old Ghrl−/− mice compared to their WT littermates, suggesting that intestinal permeability was significantly impacted by ghrelin deficiency (Figure 2(A)). Measurements of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) showed that old Ghrl−/− mice exhibited significantly increased IL-1β, and a trend for increased IL-6 and TNF-α in colons (Figure 2(C) and (D)).
Figure 2.
Old Ghrl−/− mice showed increased gut permeability and inflammation. (A) Plasma level of fluorescence emitted from FITC-dextran translocated from gut. (B and D) Colon samples from 22M to 28M mice were processed and used for cytokine measurements: (B) IL-1β, (C) IL-6, and (D) TNF-α.
*P < 0.05, WT versus Ghrl−/− mice.
Younger ghrelin-deficient mice showed normal depressive-like behavior and gut permeability
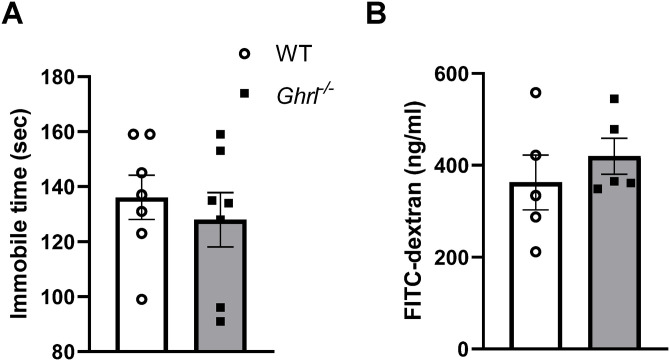
To test whether the increased depressive-like behavior and gut permeability in Ghrl−/− mice is age-dependent, next we assessed these parameters in a cohort of younger mice. Ghrl−/− mice at 6–12M of age showed similar immobile time compared to littermate WT mice (Figure 3(A)). In addition, there was no significant difference between Ghrl−/− and WT mice in plasma FITC-dextran levels (Figure 3(B)). Taken together, these in vivo phenotyping data suggested that Ghrl−/− mice exhibited increased depressive-like behavior and gut permeability in an age-dependent manner.
Figure 3.
Younger Ghrl−/− mice showed normal depressive-like behaviors and gut permeability comparable to age-matched WT mice. (A) Depressive-like behaviors and (B) plasma level of fluorescence emitted from FITC-dextran translocated from gut.
Fecal SCFA levels were not altered in old Ghrl−/− mice
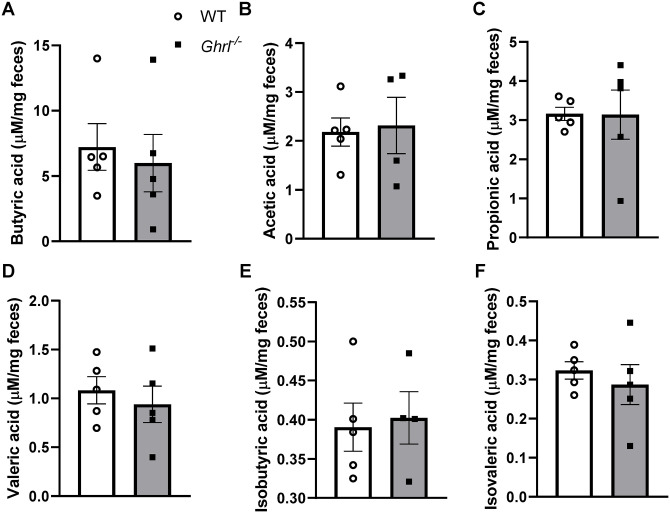
Previously, we identified reduced populations of Roseburia and Clostridium XIVb, Gram-positive anaerobic bacteria that produce the SCFA butyrate, in young Ghrl−/− mice. 23 To test whether SCFA levels are altered in old Ghrl−/− mice, we performed targeted gas chromatography–mass spectrometry (GC-MS) on feces collected from old mice in Cohort 1. Levels of SCFAs (propionate and butyrate) and branched chain fatty acids (BCFAs: isovalerate and valerate) in feces were measured; there was no significant difference between old Ghrl−/− mice and WT mice in the SCFAs and BCFAs assessed (Figure 4).
Figure 4.
Old Ghrl−/− mice showed fecal short-chain fatty acid levels comparable to WT mice. Fecal samples collected from old Ghrl−/− and WT mice were processed for SCFA measurements. (A) butyric acid, (B) acetic acid, (C) propionic acid, (D) valeric acid, (E) isobutyric acid, and (F) isovaleric acid.
Old ghrelin-deficient mice exhibited altered serum metabolome
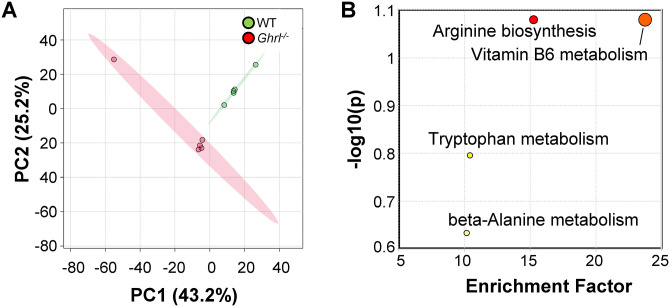
Next, we screened serum metabolites in old Ghrl−/− and WT mice, using untargeted LC-MS as we previously described. 26 We obtained 1820 metabolite features annotated with Compound Discoverer and performed a series of analyses using the web-based tool MetaboAnalyst 5.0. 27 PCA of the metabolites showed distinct separation of the two clusters of Ghrl−/− and WT mice, suggesting that chronic ghrelin deficiency exerted a strong impact on serum metabolome (Figure 5(A)). We calculated the mean fold change and the statistically significant difference between the two groups using t-tests, adjusting for false discovery rate (FDR). We identified 211 significant metabolites, which were then subjected to the Mummichog pathway analysis through the MetaboAnalyst. We found four pathways that were significantly altered by chronic ghrelin deficiency: arginine biosynthesis, vitamin B6 metabolism, tryptophan metabolism, and beta-alanine metabolism (Figure 5(B)). Given the role of tryptophan metabolism in gut barrier function, we pulled out the relative abundance of metabolites in the tryptophan pathway, to assess the direction of change.
Figure 5.
Fed-state serum metabolome and enriched pathways were altered in old Ghrl−/− mice. Serum samples from old male Ghrl−/− and WT mice were analyzed (n = 5 and 5, respectively). (A) PCA analysis showed distinct clusters separated by genotype. (B) Mummichog pathway analysis plot, generated by peaks-to-pathway analysis module in MetaboAnalyst 5.0. Each circle represents a pathway: the color corresponds to its P value, while the size represents the enrichment factor, defined as the ratio between the number of significant pathway hits and the expected number of compound hits within the pathway. (A color version of this figure is available in the online journal.)
We found that there were significant decreases in relative abundance of tryptophan as well as l-kynurenine, a metabolite in the tryptophan catabolism pathway in old Ghrl−/− mice (Figure 6). Notably, the microbially derived indole-3-acetic acid, indole-3-lactic acid, and 5-hydroxyindole-3-acetic acid showed significant decreases in old Ghrl−/− mice compared to WT mice (Figure 6). Taken together, the serum metabolome data suggested that chronic ghrelin deficiency is associated with decreased tryptophan metabolism in the host as well as in the microbial communities residing in old Ghrl−/− mice.
Figure 6.
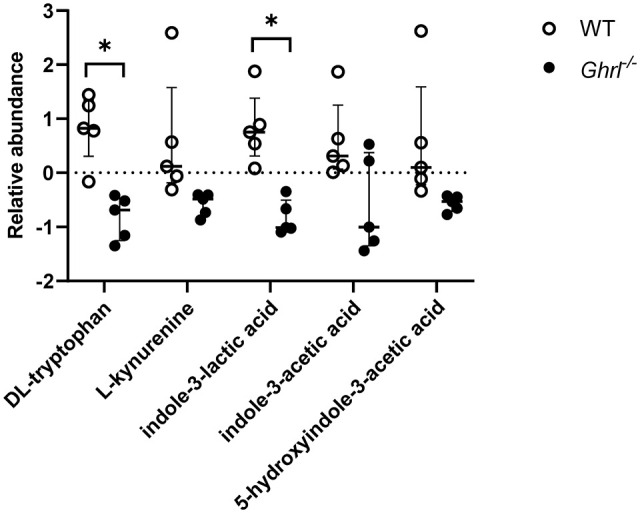
Bacterial tryptophan metabolism was decreased in old Ghrl−/− mice. Serum levels of tryptophan and indole-3-lactic acid, metabolite from tryptophan catabolism by gut bacteria, were significantly decreased in old Ghrl−/− mice. Serum levels of l-kynurenine, indole-3-acetic acid, and 5-hydrozyindole-3-acetic acid were also decreased in old Ghrl−/− mice, albeit did not reach significance level, after correcting for multiple t-test comparisons using the false discovery rate (FDR, set at 1%).
DSS-induced colitis was aggravated in old ghrelin-deficient mice
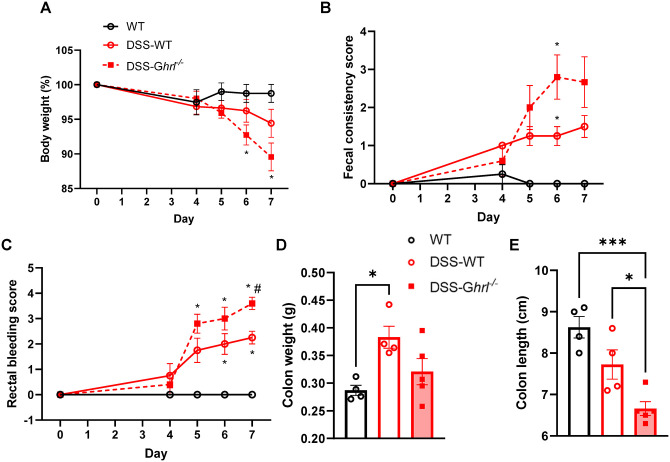
To investigate further the role of endogenous ghrelin in gut barrier function, we used an experimental model of ulcerative colitis, where DSS induces epithelial cell damage and inflammatory processes similar to those observed in human ulcerative colitis patients. 34 We subjected another cohort of male Ghrl−/− and littermate WT mice at 18M of age to 2% DSS for seven days, and assessed daily the disease progression, manifested as body weight loss, fecal consistency (from pellets to diarrhea), and rectal bleeding (presence of visible blood in feces) (Figure 7). DSS-induced colitis was exacerbated in DSS–Ghrl−/− mice compared to DSS–WT mice, as indicated by increased body weight loss, increased fecal consistency score, and rectal bleeding score (Figure 7(A) to (C)). In addition, while colon weights were significantly increased in DSS–WT mice and not significantly changed in DSS–Ghrl−/− mice, colon lengths were significantly shortened in DSS–Ghrl−/− mice compared to DSS–WT mice (Figure 7(D) and (E)). The decrease in colon lengths suggested increased tissue inflammation, consistent with the increased disease activities in DSS–Ghrl−/− mice.
Figure 7.
DSS-induced colitis was exacerbated in 18-month-old Ghrl−/− mice. Normal or 2% DSS in drinking water was given to mice for seven days, and water was changed every two days. Disease activities were monitored, including body weight (A), stool consistency (B), and rectal bleeding (C). Mice were euthanized on Day 7 after assessing disease activities, and colon dissected, weight (D) and length (E) measured. For (A to C), data were analyzed with two-way ANOVA (treatment group × time) followed by Tukey’s post hoc tests. For (D and E), data were analyzed with one-way ANOVA (treatment) followed by Tukey’s post hoc tests. (A color version of this figure is available in the online journal.)
*P < 0.05; ***P < 0.001, WT versus Ghrl−/− mice; #P < 0.05, DSS-WT versus DSS-Ghrl−/−.
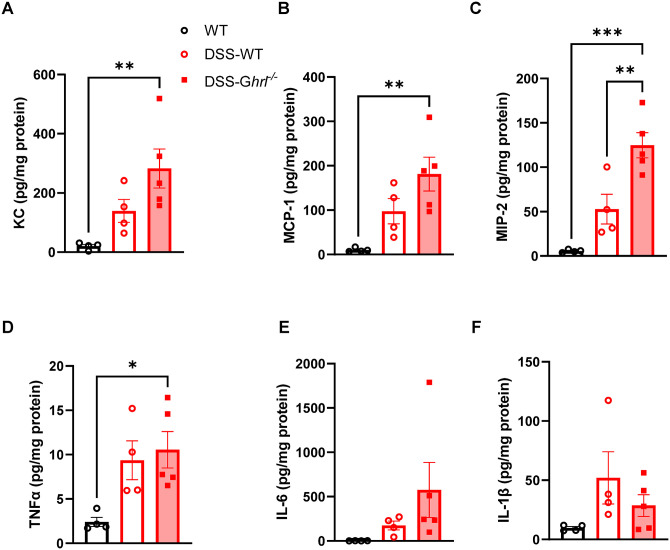
Next, we assessed cytokines levels in the colon tissue. Levels of the chemoattractant KC (also known as CXCL1), monocyte chemoattractant protein (MCP)-1, and macrophage inflammatory protein (MIP)-2 were increased by DSS treatment; however, the extent of increase was exaggerated in DSS–Ghrl−/− mice compared to control DSS–WT mice (Figure 8(A) to (C)). Similarly, the inflammatory cytokine TNF-α was increased by DSS treatment, and the extent of increase was exaggerated in DSS–Ghrl−/− mice (Figure 8(D)). There was a trend for increased tissue IL-6 levels in DSS–Ghrl−/− mice (Figure 8(E)), while IL-1β was increased in both DSS–WT and DSS–Ghrl−/− mice (Figure 8(F)). These results showed that DSS-induced release of chemoattractant and inflammatory cytokines in colon tissues were exacerbated in old Ghrl−/− mice compared to old WT mice. In summary, these data demonstrated that ghrelin deficiency led to exaggerated inflammatory response and disease manifestation in DSS-induced colitis, underscoring a role for endogenous ghrelin in intestinal homeostasis.
Figure 8.
Immune response associated with DSS-induced colitis was exacerbated in 18-month-old Ghrl−/− mice. Colon samples were processed and used for cytokine measurements. (A) KC (CXCL1), (B) MCP-1, (C) MIP-2, (D) TNF-α, (E) IL-6, and (F) IL-1β. Data were analyzed with one-way ANOVA (treatment) followed by Tukey’s post hoc tests. (A color version of this figure is available in the online journal.)
*P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Gut dysbiosis and gut barrier dysfunction have been associated with augmentation of systemic inflammation in chronic pro-inflammatory conditions including type II diabetes, obesity, and aging. In addition, growing evidence suggest that gut dysbiosis also contributes to the pathophysiology of depression.35 –38 Consistent with gut dysbiosis in young Ghrl−/− mice, here we showed that chronic ghrelin deficiency was associated with increased gut permeability and depressive-like behavior. Furthermore, aged Ghrl−/− mice showed exacerbated DSS-induced ulcerative colitis as well as increased chemoattractant levels in colon tissue, suggesting a role for endogenous ghrelin in intestinal epithelial cell function and subsequent immune response. This was further supported by serum metabolome data showing that the microbially derived indole metabolites were significantly decreased in old Ghrl−/− mice; this suggested dysregulated microbial metabolism and impaired gut barrier in old Ghrl−/− mice. Of note, fecal SCFA levels were not significantly impacted by ghrelin deficiency, even though we previously identified that butyrate-producing Roseburia was significantly reduced in young male Ghrl−/− mice. 23 Nevertheless, our data demonstrated that chronic ghrelin deficiency is associated with exacerbated loss of gut barrier function and depression in aged mice.
Depression is a leading cause of disability; however, despite its global prevalence, the pathophysiology remains undefined. 39 Several concepts have been used to elucidate the pathophysiology of depression, including neurotransmission deficit, neurotrophic changes, neuroanatomical abnormalities, impaired responses to stress with dysregulated hypothalamic–pituitary–adrenal (HPA) axis, endocrine-immune system dysfunction, and inflammation.40 –44 Interestingly, depressive disorders are prevalent in patients with IBD. 45 The current understanding of underlying mechanisms common to both IBD and depression has revealed many humoral and neural pathways of gut–brain communication, 46 and gut microbiota have emerged as a novel link in this system.35,47,48 Gut dysbiosis has been reported in depressed patients,49,50 as well as in animal models of depression. 51 Furthermore, clinical studies have also shown that ingestion of probiotics decreases depression. 52 Interestingly, Zheng et al. 50 demonstrated the causality of “depression microbiota,” showing that transplantation of germ-free mice with “depression microbiota” derived from patients with depression resulted in depressive-like behaviors. In this study, we showed that chronic ghrelin deficiency was associated with increased depressive-like behaviors and intestinal permeability in aged mice. The observation that these phenomena were pronounced in aged mice but not in young mice suggested that the increase of depressive-like behaviors in Ghrl-/- mice may be due to accumulative gut barrier dysfunction and gut microbiota dysbiosis developed during the aging process, and less likely due to direct effect of lack of ghrelin signaling in the brain.
Aging is associated with augmented intestinal permeability and systemic inflammation. 33 In addition, we previously reported age-associated microbiota dysbiosis and decrease in microbial tryptophan metabolism; notably, there were significant negative correlations between age and serum levels of indoles and indole-3-lactic acids. 26 Here, we showed that serum levels of the indole derivatives indole-3-lactic acids and indole-3-acetic acids were significantly decreased in old Ghrl−/− mice compared to littermate WT mice. Indole and its derivatives, which are tryptophan catabolites generated by the microbiota, have been shown to regulate microbiota–host crosstalk in health and disease.53,54 Indoles provide protective effects for the gut barrier through increasing the mucus production of endothelial cells and restoring tight junction proteins in the epithelial cell barrier, thus reducing pathogenic invasion. 55 Moreover, administration of indole-containing capsules to germ-free mice increased expression of junctional proteins and resistance to DSS-induced epithelial injury. 56 Indoles and its derivatives can act as activators of aryl hydrocarbon receptor, which consequently regulates local IL-22 production and thereby modulates immune response.57 –59 Taken together, our data suggested that the decreased indole-related metabolites associated with ghrelin deficiency may be one of the molecular mechanisms contributing to the decline in gut barrier function in old Ghrl−/− mice. Whether the increased depressive-like behavior observed in old Ghrl−/− mice was due to altered tryptophan metabolism or due to gut barrier dysfunction and subsequent increase in systemic inflammation is not clear. Further work is required to determine causal links between these bacterial tryptophan catabolites and depression. 8
In line with a role of ghrelin in gut barrier function during aging, our data also showed that ghrelin-deficient mice were more vulnerable to DSS-induced colitis. This is consistent with previous studies showing that pharmacological treatment with ghrelin provided protective effects in several models of experimental colitis.19,60 Matuszyk et al. 60 showed that ghrelin treatment promoted faster regeneration of the colonic wall from acetic acid-induced colitis, and decreased colonic levels of pro-inflammatory cytokines IL-1β and TNF-α, and myeloperoxidase (a marker for neutrophil infiltration). Given the important roles of microbially derived indoles and its derivatives in gut health, the significant decrease in these metabolites could be an underlying mechanism for the exacerbation of colitis in old Ghrl−/− mice. As discussed above, indole and its derivatives act as agonists for the ligand-dependent aryl hydrocarbon receptor, which has been shown to modulate intestinal homeostasis by acting on epithelial regeneration, barrier function, and immune cells.53,58 Interestingly, a study on caspase recruitment domain family member 9 (CARD9), a susceptibility gene for IBD, demonstrated that microbial tryptophan metabolism is decreased in Card9−/− mice, resulting in reduced aryl hydrocarbon receptor activation and impaired restitution from DSS-induced colitis. 61 This study also showed that patients with IBD have reduced aryl hydrocarbon receptor activity and tryptophan metabolites indoles and indole-3-acetic acids. Thus, this study and our data suggest a causal link between impaired tryptophan metabolism by the microbiota and hyper-susceptibility to colitis.
Consistent with a role for endogenous ghrelin in intestinal homeostasis, we recently showed that deletion of its receptor growth hormone secretagogue receptor (GHS-R) led to gut dysbiosis in an age-dependent manner, and also showed increased susceptibility to DSS-induced colitis. 62 At the phylum level, the ratio of Firmicutes and Bacteroidetes (F/B ratio) was similar in young but increased in older GHS-R KO mice. At the family level, young GHS-R KO mice showed lower Lachnospiraceae and Prevotellaceae, but higher Erysipelotrichaceae. This gut dysbiosis pattern persisted in older GHS-R KO mice, and the proportion of Erysipelotrichaceae was further increased in older GHS-R KO mice. The bacterial family Erysipelotrichaceae belongs to the Firmicutes phylum and increased abundance of Erysipelotrichaceae have been associated with intestinal inflammation in colorectal cancer, IBD, and metabolic diseases. 63 In addition, DSS-induced colitis was exacerbated in both young and older GHS-R KO mice, with older GHS-R KO showing more severe disease activity than young GHS-R KO mice. 62 Collectively, these data suggested that endogenous ghrelin signaling has an important role in maintaining intestinal homeostasis.
The symbiotic relationships between host and microbes are now emerging as critical determinants of health and disease. Dysregulated mucosal microbial communities or mucosal barrier may induce or aggravate intestinal inflammation. 64 It has been suggested that metabolism in colonic epithelial cells may function as a control switch, mediating shifts between homeostatic and dysbiotic microbial communities.65,66 Interestingly, ghrelin levels have been found to be increased in active IBD patients compared to those in remission.67,68 Whether this is a protective host response to contain inflammation and/or promote tissue repair is unknown. Future studies examining the potential effect of ghrelin on the colonic metabolism, and its impact on the microbial communities such as indole-producing bacteria versus pathobionts such as Erysipelotrichaceae, will provide further insights into the causal relationship between dysregulated host metabolism and dysbiotic microbial communities in the progression of inflammatory pathologies in aging and in colitis.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221110647 for Deletion of ghrelin alters tryptophan metabolism and exacerbates experimental ulcerative colitis in aged mice by Ellie Tuchaai, Valerie Endres, Brock Jones, Smriti Shankar, Cory Klemashevich, Yuxiang Sun and Chia-Shan Wu in Experimental Biology and Medicine
Acknowledgments
The authors acknowledge Jennifer DeLuca and Clinton Allred for technical advice on the experimental model of DSS-induced colitis. The authors acknowledge Jiahua Yang for technical assistance in performing and scoring FSTs. The authors also acknowledge the expertise and support of the Rodent Preclinical Phenotyping Core, Integrated Metabolomics Analysis, and Molecular Genomics cores supported in part by P30 ES029067.
Footnotes
Authors’ Contributions: Y.S. and C.-S.W. contributed to the conceptualization. E.T., V.E., B.J., S.S., C.K., and C.-S.W. contributed to the formal analysis. Y.S. and C.-S.W. contributed to the funding acquisition. E.T., S.S., C.K., and C.-S.W. contributed to the investigation. C.-S.W. contributed to the project administration. V.E. and C.-S.W. contributed to the writing – original draft. Y.S. and C.-S.W. contributed to the writing – review and editing. All authors have read and agreed to the published version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging (R21AG061726) and the US Army Medical Research and Development Command (W81XWH-20-1-0127) to C.-S.W., and the National Institute on Aging (R01AG064869) to Y.S.
ORCID iD: Chia-Shan Wu  https://orcid.org/0000-0002-6034-939X
https://orcid.org/0000-0002-6034-939X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014;69:S4–9 [DOI] [PubMed] [Google Scholar]
- 2. Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and “Garb-aging.” Trends Endocrinol Metab 2017;28:199–212 [DOI] [PubMed] [Google Scholar]
- 3. Agusti A, Garcia-Pardo MP, Lopez-Almela I, Campillo I, Maes M, Romani-Perez M, Sanz Y. Interplay between the gut-brain axis, obesity and cognitive function. Front Neurosci 2018;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 2020;28:180–9 [DOI] [PubMed] [Google Scholar]
- 5. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–72 [DOI] [PubMed] [Google Scholar]
- 6. Kohler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctot KL, Carvalho AF. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr Pharm Des 2016;22:6152–66 [DOI] [PubMed] [Google Scholar]
- 7. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merchak A, Gaultier A. Microbial metabolites and immune regulation: new targets for major depressive disorder. Brain Behav Immun Health 2020;9:100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020;11:135–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000;141:4255–61 [DOI] [PubMed] [Google Scholar]
- 11. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60 [DOI] [PubMed] [Google Scholar]
- 12. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–13 [DOI] [PubMed] [Google Scholar]
- 13. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 2004;101:4679–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu CS, Bongmba OYN, Yue J, Lee JH, Lin L, Saito K, Pradhan G, Li DP, Pan HL, Xu A, Guo S, Xu Y, Sun Y. Suppression of GHS-R in AgRP neurons mitigates diet-induced obesity by activating thermogenesis. Int J Mol Sci 2017;18:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta D, Patterson AM, Osborne-Lawrence S, Bookout AL, Varshney S, Shankar K, Singh O, Metzger NP, Richard CP, Wyler SC, Elmquist JK, Zigman JM. Disrupting the ghrelin-growth hormone axis limits ghrelin’s orexigenic but not glucoregulatory actions. Mol Metab 2021; 53:101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 2003;23:7973–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 2004;101:8227–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu R, Dong W, Qiang X, Wang H, Blau SA, Ravikumar TS, Wang P. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit Care Med 2009;37:2421–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006;130:1707–20 [DOI] [PubMed] [Google Scholar]
- 20. Nass R, Farhy LS, Liu J, Pezzoli SS, Johnson ML, Gaylinn BD, Thorner MO. Age-dependent decline in acyl-ghrelin concentrations and reduced association of acyl-ghrelin and growth hormone in healthy older adults. J Clin Endocrinol Metab 2014;99:602–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rigamonti AE, Pincelli AI, Corra B, Viarengo R, Bonomo SM, Galimberti D, Scacchi M, Scarpini E, Cavagnini F, Muller EE. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol 2002;175:R1–5 [DOI] [PubMed] [Google Scholar]
- 22. Ma X, Lin L, Yue J, Wu CS, Guo CA, Wang R, Yu KJ, Devaraj S, Murano P, Chen Z, Sun Y. Suppression of ghrelin exacerbates HFCS-induced adiposity and insulin resistance. Int J Mol Sci 2017;18:1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu CS, Wei Q, Wang H, Kim DM, Balderas M, Wu G, Lawler J, Safe S, Guo S, Devaraj S, Chen Z, Sun Y. Protective effects of ghrelin on fasting-induced muscle atrophy in aging mice. J Gerontol A Biol Sci Med Sci 2020;75:621–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009;15:1546–58 [DOI] [PubMed] [Google Scholar]
- 25. Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 2016;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu CS, Muthyala SDV, Klemashevich C, Ufondu AU, Menon R, Chen Z, Devaraj S, Jayaraman A, Sun Y. Age-dependent remodeling of gut microbiome and host serum metabolome in mice. Aging 2021; 13:6330–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinformatics 2019;68:e86 [DOI] [PubMed] [Google Scholar]
- 28. Muthyala SDV, Shankar S, Klemashevich C, Blazier JC, Hillhouse A, Wu CS. Differential effects of the soluble fiber inulin in reducing adiposity and altering gut microbiome in aging mice. J Nutr Biochem 2022;105:108999. [DOI] [PubMed] [Google Scholar]
- 29. Wu CS, Morgan D, Jew CP, Haskins C, Andrews MJ, Leishman E, Spencer CM, Czyzyk T, Bradshaw H, Mackie K, Lu HC. Long-term consequences of perinatal fatty acid amino hydrolase inhibition. Br J Pharmacol 2014;171:1420–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp 2017:55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang HY, Wu M, Diao JL, Li JB, Sun YX, Xiao XQ. Huperzine A ameliorates obesity-related cognitive performance impairments involving neuronal insulin signaling pathway in mice. Acta Pharmacol Sin 2020; 41:145–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeLuca JA, Allred KF, Menon R, Riordan R, Weeks BR, Jayaraman A, Allred CD. Bisphenol-A alters microbiota metabolites derived from aromatic amino acids and worsens disease activity during colitis. Exp Biol Med 2018;243:864–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larche MJ, Davidson DJ, Verdu EF, Surette MG, Bowdish DME. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017;21:455e4–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Czarnewski P, Parigi SM, Sorini C, Diaz OE, Das S, Gagliani N, Villablanca EJ. Conserved transcriptomic profile between mouse and human colitis allows unsupervised patient stratification. Nature Communications 2019;10:2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013;36:305–12 [DOI] [PubMed] [Google Scholar]
- 36. Zheng P, Wu J, Zhang H, Perry SW, Yin B, Tan X, Chai T, Liang W, Huang Y, Li Y, Duan J, Wong ML, Licinio J, Xie P. The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol Psychiatry 2021;26:2380–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu M, Tian T, Mao Q, Zou T, Zhou CJ, Xie J, Chen JJ. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry 2020; 10:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chevalier G, Siopi E, Guenin-Mace L, Pascal M, Laval T, Rifflet A, Boneca IG, Demangel C, Colsch B, Pruvost A, Chu-Van E, Messager A, Leulier F, Lepousez G, Eberl G, Lledo PM. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nature Communications 2020;11:6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 2012;379:1045–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011;16:383–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 2012;17:1130–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry 2007;12:988–1000 [DOI] [PubMed] [Google Scholar]
- 43. Schlosser RG, Wagner G, Koch K, Dahnke R, Reichenbach JR, Sauer H. Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage 2008;43:645–55 [DOI] [PubMed] [Google Scholar]
- 44. Smith RS. The macrophage theory of depression. Med Hypotheses 1991; 35:298–306 [DOI] [PubMed] [Google Scholar]
- 45. Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res 2016;87:70–80 [DOI] [PubMed] [Google Scholar]
- 46. Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr 2016;21:184–98 [DOI] [PubMed] [Google Scholar]
- 47. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12 [DOI] [PubMed] [Google Scholar]
- 48. Flowers SA, Ellingrod VL. The microbiome in mental health: potential contribution of gut microbiota in disease and pharmacotherapy management. Pharmacotherapy 2015;35:910–6 [DOI] [PubMed] [Google Scholar]
- 49. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015;48:186–94 [DOI] [PubMed] [Google Scholar]
- 50. Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–96 [DOI] [PubMed] [Google Scholar]
- 51. Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil 2013;25:733–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression. Neurogastroenterol Motil 2013;25:713–9 [DOI] [PubMed] [Google Scholar]
- 53. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23:716–24 [DOI] [PubMed] [Google Scholar]
- 54. Levy M, Blacher E, Elinav E. Microbiome, metabolites and host immunity. Curr Opin Microbiol 2017;35:8–15 [DOI] [PubMed] [Google Scholar]
- 55. Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA 2010; 107:228–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS ONE 2013;8:e80604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013;39:372–85 [DOI] [PubMed] [Google Scholar]
- 58. Safe S, Jayaraman A, Chapkin RS. Ah receptor ligands and their impacts on gut resilience: structure–activity effects. Crit Rev Toxicol 2020;50:463–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Keir M, Yi Y, Lu T, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med 2020;217:e20192195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matuszyk A, Ceranowicz P, Warzecha Z, Cieszkowski J, Ceranowicz D, Galazka K, Bonior J, Jaworek J, Bartus K, Gil K, Olszanecki R, Dembinski A. Exogenous ghrelin accelerates the healing of acetic acid-induced colitis in rats. Int J Mol Sci 2016;17:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 2016;22:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Noh JY, Wu CS, DeLuca JAA, Devaraj S, Jayaraman A, Alaniz RC, Tan XD, Allred CD, Sun Y. Novel role of ghrelin receptor in gut dysbiosis and experimental colitis in aging. Int J Mol Sci 2022;23:2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaakoush NO. Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol 2015;5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjovall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014;63:281–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine – a review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol 2015;309:C350–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Litvak Y, Byndloss MX, Baumler AJ. Colonocyte metabolism shapes the gut microbiota. Science 2018;362:eaat9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peracchi M, Bardella MT, Caprioli F, Massironi S, Conte D, Valenti L, Ronchi C, Beck-Peccoz P, Arosio M, Piodi L. Circulating ghrelin levels in patients with inflammatory bowel disease. Gut 2006;55:432–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ates Y, Degertekin B, Erdil A, Yaman H, Dagalp K. Serum ghrelin levels in inflammatory bowel disease with relation to disease activity and nutritional status. Dig Dis Sci 2008;53:2215–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221110647 for Deletion of ghrelin alters tryptophan metabolism and exacerbates experimental ulcerative colitis in aged mice by Ellie Tuchaai, Valerie Endres, Brock Jones, Smriti Shankar, Cory Klemashevich, Yuxiang Sun and Chia-Shan Wu in Experimental Biology and Medicine