Abstract
Introduction
We aimed to identify risk factors associated with ICU mortality in critically ill patients with COVID-19 pneumonia treated with Extracorporeal membrane oxygenation (ECMO). We also aimed to assess protocol violations of the local eligibility criteria of ECMO initiation.
Methods
All 31 consecutive adult patients with confirmed COVID-19 pneumonia admitted to ICU and treated with ECMO from March 13th 2020 to 8 December 2021 were enrolled. Eligibility criteria for ECMO initiation were: P/F-ratio<50 mmHg >3 hours, P/F-ratio<80 mmHg >6 hours or pH<7.25 + PaCO2>60 mmHg >6 hours, despite maximal protective invasive ventilation. Primary outcome was ICU mortality. Univariate logistic regression analyses were performed to identify predictors of ICU mortality.
Results
12 out of 31 patients (38.7%) did not survive ECMO treatment in ICU. Half of the non-survivors suffered from acute kidney failure compared to 3 out of 19 survivors (15.79%) (p = .04). Half of the non-survivors required CRRT treatment versus 1 patient in the survivor group (5.3%) (p < .01). Higher age (2.45 (0.97–6.18), p = .05), the development of AKI (5.33 (1.00–28.43), p = .05), need of CRRT during ICU stay (18.00 (1.79–181.31), p = .01) and major bleeding during ECMO therapy (0.51 (0.19–0.89), p < .01) were identified to be predictors of ICU mortality.
Conclusion
Almost 60% of patients could be treated successfully with ECMO with sustained results at 3 months. Predictors for ICU mortality were development of AKI and need of CRRT during ICU stay, higher age category and major bleeding. Inadvertent ECMO allocation was noted in almost one in five patients.
Keywords: COVID-19, extracorporeal membrane oxygenation, intensive care unit, predictor, mortality
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the ongoing pandemic of coronavirus disease (COVID-19). The spectrum of disease severity of patients infected with SARS-CoV-2 is very wide: from an asymptomatic carrier state to severe pneumonia and the development of acute respiratory distress syndrome (ARDS).1
Conventional treatment options established for ARDS include lung-protective mechanical ventilation, neuromuscular blockade, and prone positioning.2 Depending on the availability of resources, International guidelines also recommend the consideration of venovenous (VV) extracorporeal membrane oxygenation (ECMO) in selected patients with COVID-19 who develop severe ARDS and hypoxemia that is refractory to optimal ventilator management and prone positioning.2–4 According to the Rescue Lung Injury in Severe ARDS (EOLIA) trial criteria,5 VV ECMO should be timely considered in selected patients if they meet one of the following three criteria despite maximal conservative therapy: a ratio of partial pressure of arterial oxygen (Pao2) to the fraction of inspired oxygen (Fio2) < 50 mmHg for more than 3 h, PaO2/Fi02 Ratio (P/F Ratio) < 80 mmHg for more than 6 h or pH < 7.25+PaCO2 > 60 mmHg for more than 6h. Veno Arterial (VA) ECMO should be considered in selected patients with coexistence of refractory cardiogenic shock.4
On the downside, ECMO therapy is a resource-demanding procedure with an average cost exceeding 70.000USD6 and carries an increased risk of bleeding and thromboembolic events7 which may counteract its beneficial effects. The first results of ECMO therapy in COVID-19 patients from small Chinese cohorts were discouraging, reporting a very high mortality.8,9 Other studies however show more promising results with a reported cumulative in-hospital mortality rate between 31% and 58.9%.10,11 Finally, ECMO exemplifies a scarce resource that requires thoughtful risk-benefit evaluation and allocation strategy. A national survey demonstrated that the majority of US citizens advocate for the continued use of ECMO to treat COVID patients during periods of resource scarcity but would prioritize those with the highest likelihood of recovery.12
As a result, there is a need for a more in-depth understanding of the risk factors associated with poor outcomes in critically ill patients with COVID-19 pneumonia treated with ECMO. In this respect, the association of medical history, demographic factors, laboratory results, point-of-care echocardiography data, ventilator settings, ventilator-derived parameters, and treatment factors with outcomes in these patients needs to be analyzed. An optimal characterization of these patients with the highest likelihood of recovery can lead to improved ECMO resource utilization. ECMO resource utilization can further be improved by minimizing the risk of inadvertent ECMO allocation to patients not meeting the eligibility criteria for ECMO candidacy.
Hence, the primary aim of this study was to identify predictors of ICU mortality in a cohort of critically ill patients with COVID-19 pneumonia treated with extracorporeal membrane oxygenation.
Materials and methods
This single-center, investigator-initiated, longitudinal, ambispective, cohort study was performed at Jessa Hospital, Hasselt, Belgium. This study is approved by the ethical committee of Jessa Hospital, Hasselt, Belgium on 8 September 2021, and registered on clinicaltrials. gov (NCT05158816). In light of the urgent need to collect data on the ongoing pandemic and the retrospective nature of this study, written informed consent was waived. This study is reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.13
Study population
All adult patients with acute hypoxaemic respiratory failure due to diagnosed COVID-19 pneumonia admitted to ICU and treated with ECMO from 13 March 2020 until 8 December 2021 were included in the study. Following the World Health Organisation (WHO) protocol,14 laboratory confirmation of COVID-19 infection was defined as a positive result on polymerase chain reaction (PCR) assays of nasopharyngeal swab samples or bronchoalveolar lavage. Only laboratory-confirmed patients were included in the analysis. From 13 March 2020 until 8 December 2021, data from 363 consecutive patients admitted to the ICU were prospectively entered into a customized database that included medical history, demographic data, clinical symptoms and signs, laboratory results, ventilator settings, ventilator-derived parameters, echocardiographic parameters, and clinical outcomes. This database was retrospectively reviewed. APACHE II and IV scores were calculated at admission to ICU.15,16 The Sequential Organ Failure Assessment (SOFA) score17 was evaluated daily.
At admission, all patients were classified into 3 categories: 1) full code with ECMO, 2) No ECMO, and 3) Do-not-intubate (DNI). Inclusion criteria for ECMO candidacy during the first wave were: age < 60 years, clinical frailty scale 1 or 2, sustained severe hypoxemia or hypercapnia despite maximum ventilator support, prone ventilation, and neuromuscular blockade. Sustained severe hypoxemia was defined as “a P/F < 50 mmHg for more than 3 h or a PaO2/Fi02 Ratio (P/F Ratio) < 80 mmHg for more than 6 h. Sustained severe hypercapnia was defined as a pH < 7.25+PaCO2 > 60 mmHg for more than 6 h. After the first wave, inclusion criteria were extended to age>70 years (with age between 70 and 80 years a relative contra-indication) and clinical frailty scale < 5. Exclusion criteria for ECMO were severe bleeding, active malignancy, hepatic cirrhosis Child-Pugh B or C, COPD GOLD IV, cardiac arrest, multiple organ failure, severe neurological injury, or cognitive impairment (including CVA or dementia).
Patients were classified into two groups, i.e. the “early ECMO group” and the “late ECMO group”. The early ECMO group included the group of patients who began ECMO within 24h or even before meeting the EOLIA trial criteria.5 The delayed ECMO group referred to the patients that did not begin ECMO until more than 24h after meeting one of the EOLIA trial criteria.
Extracorporeal membrane oxygenation approach
A standard ECMO/ECLS circuit was used for all patients, including a LivaNova Stöckert console with a Revolution centrifugal pump system, a Sechrist gas blender, a Medtronic Biotrend SvO2 meter, and a Hico Variotherm 550 heater/cooler. The disposables consisted of a heparin-coated VA-tubing set with a PMP fiber ECMO oxygenator (LivaNova EOS ECMO or Eurosets A.L. ONE ECMO) and a LivaNova Revolution centrifugal pump head with a line pressure control on 3 places: P1 negative drainage pressure, P2 pre-oxygenator pressure and P3 post-oxygenator pressure. 5000IU of UFH were administered IV before cannulation according to our protocol. Placement of the cannulae was performed by the cardiac surgeon in collaboration with the cardiac anesthesiologist. After disinfection and preparing the groin, a 21Fr. or 25Fr. Medtronic multistage venous cannula was introduced percutaneously through the femoral vein by the Seldinger technique and positioned in the inferior caval vein at the subdiaphragmatic level. After the puncture of the internal jugular vein under echo guidance, the arterial return cannula is positioned in the superior caval vein with the tip at the level of the caval ostium. Attention is taken to avoid recirculation, this is supported by transoesophageal guidance. Simultaneously, the venous return cannula (Edwards Optisite 20Fr. or 22Fr.) was inserted by a cardiac anesthesiologist into the RIJV (right internal jugular vein) with the tip positioned towards the tricuspid valve. ECMO was initiated after an ACT check and ultrasound control of the position of both cannulas. Blood flow was increased with a target of 2,4 LPM CI (cardiac index), taking the limitations of negative venous drainage pressures into account. The weaning strategy for VV-ECMO consists of following parameters: sweep gas flow 0 L/min, ECMO FiO2: 21%, pump flow 2–3 l/min, bloodgas: PaO2> 60 mmHG and SpO2> 90%, PCO2: <55 mmHg, FiO2 ventilator <60%, Pinsp plateau <30 mmHg, RR < 30/min and duration of trial: minimal 1–2 h, maximal 12 h. The magnitude of the pandemic resulted in limited alterations to our conventional weaning strategy. Those alterations can be seen as “more tolerant to both hypoxia and hypercapnia” in comparison to our conventional weaning strategy.
Outcome parameters
The primary outcome was ICU mortality. As a result, the study population was divided into participants who had died during their ICU stay and participants who were discharged from the ICU alive. All participants reached the primary outcome. Secondary outcomes included the incidence of acute kidney injury (AKI) and continuous renal replacement therapy (CRRT), complications during ECMO, length of stay (LOS) in the ICU, hospital LOS and protocol violations of the local eligibility criteria for ECMO initiation. The data set was closed on 1 April 2022.
Point-of-care echocardiography data
All collected data originated from pre-ECMO transthoracic echocardiography (TTE). Five echocardiographic parameters assessing right ventricular (RV) function were included: tricuspid annular plane systolic excursion (TAPSE), right ventricular systolic pressure (RVSP) calculated from continuous wave Doppler by peak velocity over tricuspid regurgitant jet, right ventricle free wall global longitudinal strain (RVfwLS), right ventricular fractional area change (FAC) and right ventricle end-diastolic diameter/left ventricle end-diastolic diameter (RV EDA/LV EDA). RVSP and RV EDA/LV EDA were determined in the apical 4 chamber view whereas FAC TAPSE and RVfwLS were calculated from the apical focused RV view. Cut-off values were TAPSE of 17 mm, RVSP of 35mmHg, RV GLS of −20, RV FAC of 35% and RV EDA/LV EDA of 0,6.18,19
Definitions
Acute kidney failure was diagnosed according to the KDIGO clinical practice guidelines.20 ARDS was diagnosed according to the Berlin Definition.21 Sepsis and septic shock were defined according to the 2016 Third International Consensus Definition for Sepsis and Septic Shock.
COVID-19 waves
The date of ICU admission was categorized according to the COVID-19 wave. Waves 1 and 2 in Belgium were caused by the D614 G variant, wave 3 by the alpha variant, and wave 4 by the delta variant. These virus variants may differ in disease severity and consequently mortality rate.
Statistical analysis
Continuous data are shown as mean±standard deviation (SD) and categorical data are presented as frequencies (%). Comparisons between groups were performed with the Student’s t-tests for normally distributed data and with the Mann Whitney U test for nonnormally distributed data. Categorical variables were analyzed with a Chi-Square test. The following variables were tested for significance: medical factors, demographics, laboratory results, point-of-care echocardiography data, ventilator settings, ventilator-derived parameters, treatment variables, and secondary outcome variables. To predict ICU mortality, univariate logistic regression was performed for the binary variables and univariate linear regression was performed for the continuous variables. A p-value < 0.05 was considered statistically significant. All analyses were performed with SPPS Version 27.
Results
Patient characteristics
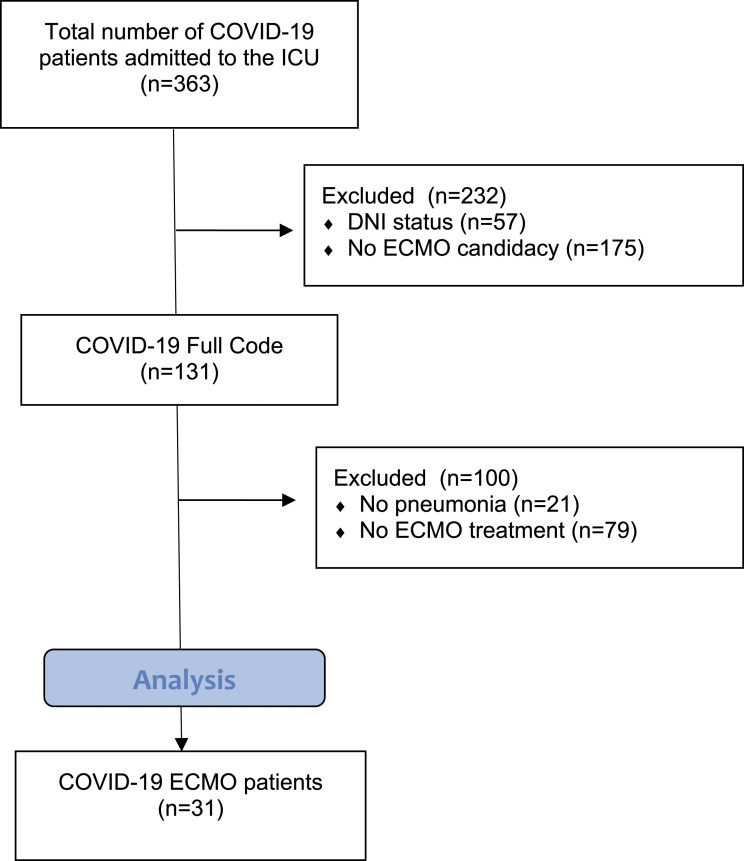
Between 13 March 2020, and 8 December 2021, 363 COVID-19 patients were admitted to the ICU, of which 31 patients were treated with ECMO therapy. A STROBE flowchart depicting the inclusion and exclusion of patients is presented in Figure 1. Detailed baseline characteristics of all COVID-19 patients supported by ECMO therapy, stratified for survival, are presented in Table 1. Patients admitted to the ICU had a mean age of 56.03 ± 10.40 years. Four out of 12 patients in the non-survivor group (33.3%) were active or former smokers, versus none in the survivor group (p = .02).
Figure 1.
STROBE flowchart depicting inclusion and exclusion.
Table 1.
Baseline characteristics.
| COVID-19 ECMO patients (n = 31) | COVID-19 ECMO survivors (n = 19) | COVID-19 ECMO non-survivors (n = 12) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 56.03 ± 10.40 | 53.32 ± 10.82 | 60.33 ± 8.40 | 0.09 |
| Age categories: | ||||
| <50 | 10 (32.3%) | 8 (42.1%) | 2 (16.7%) | 0.23 |
| 51–60 | 9 (29.0%) | 6 (31.6%) | 3 (25.0%) | |
| 61–70 | 11 (35.5%) | 5 (26.3%) | 6 (50.0%) | |
| >71 | 1 (3.2%) | 0 (0.0%) | 1 (8.3%) | |
| Gender (males/females) | 23 (67.6%)/8 (23.5%) | 14 (73.68%)/5 (26.32%) | 9 (75%)/3 (25%) | 0.93 |
| BMI (kg/m2) | 32.43 ± 8.71 | 32.73 ± 9.57 | 31.96 ± 7.53 | 0.98 |
| BMI categoires: | ||||
| Normal and overweight (18.50–29.99) | 17 (54.8%) | 10 (52.6%) | 7 (58.3%) | 0.92 |
| Moderate obesity (30.00–39.99) | 9 (29.0%) | 6 (31.6%) | 3 (25.0%) | |
| Severe obesity (>40) | 5 (16.2%) | 3 (15.8%) | 2 (16.7%) | |
| Date of inclusion | 0.06 | |||
| Wave 1 (March 2020 - August 2020) | 3 (9.7%) | 3 (15.8%) | 0 (0.0%) | 0.92 |
| Wave 2 (September 2020 -December 2020) | 9 (29.0%) | 6 (31.6%) | 3 (25.0%) | |
| Wave 3 (January 2021 - April 2021) | 10 (32.3%) | 3 (15.8%) | 7 (58.3%) | |
| Wave 4 (May 2021 - October 2021) | 9 (29.0%) | 7 (36.8%) | 2 (16.7%) | |
| Scoring systems | ||||
| SOFA score at admission | 4.03 ± 2.67 | 4.06 ± 3.02 | 4.08 ± 2.31 | 0.67 |
| Rockwood clinical frailty index | 2.06 ± 1.12 | 2.00 ± 1.29 | 2.17 ± 0.83 | 0.66 |
| Apache II | 10.23 ± 5.05 | 10.16 ± 5.50 | 10.33 ± 4.48 | 0.93 |
| Apache IV | 37.90 ± 19.20 | 36.68 ± 15.56 | 39.83 ± 17.66 | 0.62 |
| Charlson comorbidity index | 1.77 ± 0.88 | 1.68 ± 0.89 | 1.92 ± 0.90 | 0.48 |
| Admission parameters | ||||
| PaO2 At admission | 65.97 ± 18.10 | 67.42 ± 13.92 | 63.67 ± 23.81 | 0.53 |
| PaCO2 At admission | 40.84 ± 13.78 | 42.95 ± 15.64 | 37.50 ± 9.89 | 0.27 |
| pH at admission | 7.42 ± 0.10 | 7.39 ± 0.12 | 7.45 ± 0.05 | 0.34 |
| SaO2 at admission | 91.19 ± 5.76 | 92.05 ± 4.22 | 89.93 ± 7.26 | 0.73 |
| Lactate at admission | 1.63 ± 0.65 | 1.47 ± 0.53 | 1.87 ± 0.77 | 0.09 |
| P/F ratio at admission | 88.78 ± 55.85 | 85.82 ± 60.67 | 93.49 ± 49.44 | 0.44 |
| Comorbidities | ||||
| Smoking (no/yes/ex) | 27 (87.1%)/1 (3.2%)/3 (9.7%) | 19 (100%)/0 (0%)/0 (0%) | 8 (66.7%)/1 (8.3%)/3 (9.7%) | 0.02 |
| Cardiovascular disease | 4 (12.9%) | 3 (15.8%) | 1 (8.3%) | 0.55 |
| Hypertension | 10 (29.4%) | 4 (21.0%) | 6 (50.0%) | 0.90 |
| Diabetes | 4 (12.9%) | 3 (15.8%) | 1 (8.3%) | 0.55 |
| Respiratory disease | 3 (9.6%) | 3 (15.8%) | 0 (0.0%) | 0.15 |
| Malignancy | 2 (5.9%) | 2 (10.5%) | 0 (0.0%) | 0.25 |
| Chronic kidney disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Chronic liver disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Chronic bowel disease | 1 (3.2%) | 0 (0.0%) | 1 (8.3%) | 0.20 |
| Chronic nervous disease | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Cerebrovascular disease | 5 (16.2%) | 4 (21.0%) | 1 (8.3%) | 0.35 |
| HIV/AIDS | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Hematological disease | 1 (3.2%) | 1 (5.3%) | 0 (0.0%) | 0.42 |
| Rheumatological disease | 3 (9.6%) | 2 (10.5%) | 1 (8.3%) | 0.84 |
| Dementia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Obesity | 14 (45.2%) | 8 (42.1%) | 6 (50.0%) | 0.67 |
Data are expressed as mean ± standard deviation or as frequencies. A p-value <0.05 is considered statistically significant.
Outcome measures, progress, and complications
Details on the progress, complications, and outcomes are shown in Table 2.
Table 2.
Progress, complications and outcomes.
| COVID-19 ECMO patients (n=31) | COVID-19 ECMO survivors (n=19) | COVID-19 ECMO non-survivors (n=12) | p-value | |
|---|---|---|---|---|
| Progress | ||||
| ECMO signals: | 0.04 | |||
| No signal | 6 (19.4%) | 5 (27.8%) | 1 (8.4%) | 0.20 |
| pF ratio <80 mmHg | 11 (35.5%) | 8 (42.1%) | 3 (25.0%) | 0.33 |
| pH <7.25 | 4 (12.9%) | 0 (0.0%) | 4 (33.3%) | <0.01 |
| No information (externally placed) | 10 (32.2%) | 6 (31.2%) | 4 (33.3%) | |
| ECMO (early/late) | 13 (41.9%)/18 (58.1%) | 9 (47.4%)/10 (52.6%) | 4 (33.3%)/8 (66.7%) | 0.44 |
| P/F ratio during ICU stay | 85.82 ± 56.78 | 81.02 ± 61.88 | 93.41 ± 49.23 | 0.21 |
| Invasive mechanical ventilation | 31 (100.0%) | 19 (100%) | 12 (100%) | |
| Number of days | 31.37 ± 25.57 | 34.44 ± 28.61 | 26.75 ± 20.48 | 0.49 |
| Prone ventilation | 31 (100%) | 19 (100%) | 12 (100%) | 1.00 |
| Neuromuscular blocker before ECMO | 26 (100%) (n=26) | 17 (100%) (n=17) | 9 (100%) (n=9) | 1.00 |
| Vasopression | 30 (96.77%) | 18 (94.7%) | 12 (100%) | 0.42 |
| Highest SOFA score | 11.81 ± 2.09 | 11.03 ± 2.30 | 12.67 ± 1.50 | 0.09 |
| Complications | ||||
| CVA/Stroke | 3 (9.7%) | 0 (0.0%) | 3 (25.0%) | 0.02 |
| Major bleeding | 22 (71.0%) | 10 (54.5%) | 12 (100%) | <0.01 |
| Catheter dislocation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| HIT | 2 (6.5%) | 1 (5.3%) | 1 (8.3%) | 0.73 |
| Acute kidney failure | 9 (29.03%) | 3 (15.79%) | 6 (50.00%) | 0.04 |
| CRRT during ECMO | 7 (22.6%) | 1 (5.3%) | 6 (50%) | <0.01 |
| Acute heart failure | 11 (35.18%) | 7 (36.80%) | 4 (33.33%) | 0.84 |
| Sepsis | 29 (93.55%) | 17 (89.50% | 12 (100%) | 0.25 |
| Thromboembolism | 18 (58.06%) | 10 (52.60%) | 8 (66.70%) | 0.48 |
| DVT | 17 (54.84%) | 9 (47.40%) | 8 (66.7%) | |
| Lung | 1 (3.20%) | 1 (100%) | 0 (0.00%) | |
| Outcomes | ||||
| Length of ECMO (days) | 18.43 17.35 | 17.17 ± 14.78 | 20.33 ± 21.19 | 0.92 |
| Duration of ECMO ≥28 days | 8 (25.8%) | 5 (27.8%) | 3 (25.0%) | 0.86 |
| LOS before ICU (days) | 4.65 ± 6.14 | 4.42 ± 7.08 | 5.00 ± 4.53 | 0.28 |
| LOS ICU (days) | 41.03 ± 29.74 | 49.83 ± 31.74 | 30.50 ± 22.67 | 0.09 |
| LOS total (days) | 46.27 ± 31.32 | 60.94 ± 33.7 | 35.50 ± 22.62 | 0.01 |
| ICU mortality | 12 (38.71%) | |||
| 90-days mortality | 13 (41.93%) | |||
Data are expressed as mean ± standard deviation or as frequencies. A p-value <0.05 is considered statistically significant.
In total, 12 patients did not survive ICU admission (38.7%) and one patient did not survive until a 90-day follow-up (41.9%). Six patients (19.4%) did not fulfill the local eligibility criteria for ECMO therapy. Of these patients, only 1 patient did not survive ICU admission (p = .04).
Half of the non-survivors suffered from AKI versus 3 out of 19 patients (15.79%) in the survivor group (p = .04). These non-survivors (50%) required CRRT treatment during the ECMO therapy versus only 1 survivor (5.3%) (p < .01). Three patients in the non-survivor group (25%) suffered from a stroke, compared to none of the survivors (p = .02). Major bleeding (71%) was noted in all 12 non-survivors compared to about half of the survivors (p < .01).
Ventilatory parameters are presented in Table 3.
Table 3.
Ventilatory parameters.
| COVID-19 ECMO survivors (n = 19) | COVID-19 ECMO non-survivors (n = 12) | p-value | |
|---|---|---|---|
| Parameters measured immediately before placement ECMO | |||
| PaO2 | 64.78 ± 17.36 | 65.80 ± 10.13 | 0.52 |
| PaCO2 | 49.72 ± 16.23 | 55.30 ± 14.47 | 0.16 |
| pH | 7.32 ± 0.11 | 7.31 ± 0.08 | 0.58 |
| SaO2 | 89.06 ± 4.69 | 90.10 ± 3.79 | 0.59 |
| Lactate | 1.93 ± 2.80 | 1.60 ± 1.03 | 0.77 |
| P/F ratio | 66.44 ± 17.89 | 70.45 ± 21.92 | 0.44 |
| Platelets | 307.59 ± 121.70 | 246.00 ± 73.34 | 0.09 |
| GFR | 87.94 ± 29.71 | 90.80 ± 28.0 | 0.95 |
| aPTT | 47.56 ± 30.48 | 43.89 ± 16.88 | 0.94 |
| WBC | 13.93 ± 7.52 | 12.30 ± 2.94 | 0.87 |
| Haemoglobin | 11.72 ± 2.39 | 11.44 ± 1.30 | 0.61 |
| Days of prone ventilation | 5.53 ± 5.32 | 6.89 ± 6.37 | 0.49 |
| PEEP | 10.60 ± 1.50 | 11.22 ± 2.68 | 0.63 |
| Tidal Volume | 0.54 ± 0.13 | 0.44 ± 0.18 | 0.09 |
| PIP | 24.87 ± 3.85 | 22.22 ± 5.67 | 0.20 |
| Lung compliance | 42.47 ± 23.43 | 38.57 ± 32.86 | 0.45 |
| Days mechanical ventilation | 5.23 ± 7.27 | 4.89 ± 4.46 | 0.51 |
| Days of ICU | 7.61 ± 7.01 | 7.33 ± 5.79 | 0.92 |
| Parameters measured immediately after placement ECMO | |||
| PaO2 | 86.37 ± 29.02 | 82.18 ± 19.83 | 0.79 |
| PaCO2 | 45.00 ± 13.50 | 42.45 ± 8.87 | 0.76 |
| pH | 7.40 ± 0.07 | 7.43 ± 0.08 | 0.25 |
| SaO2 | 95.42 ± 2.34 | 94.91 ± 4.08 | 0.70 |
| Lactate | 1.70 ± 1.32 | 2.38 ± 1.74 | 0.11 |
| P/F ratio | 145.95 ± 59.35 | 155.73 ± 50.40 | 0.77 |
| PEEP | 8.95 ± 2.12 | 10.00 ± 2.00 | 0.28 |
| Tidal Volume | 0.37 ± 0.15 | 0.26 ± 0.14 | 0.07 |
| PIP | 27.16 ± 8.42 | 23.91 ± 6.86 | 0.33 |
Data are expressed as mean ± standard deviation or as frequencies. A p-value <0.05 is considered statistically significant.
Seven patients (22.6%) needed a second ECMO treatment. Four patients survived both ECMO treatments (p = .79). 30 patients were treated with VV ECMO, while 1 patient received VA ECMO. In order to facilitate mobilization of patients with very long VV-ECMO runtimes, three out of six patients with a VV-ECMO runtime of >1 month were converted to a single dual lumen cannula.
Predictors of ICU mortality
Univariate analysis of risk factors for ICU mortality is presented in Table 4. Higher age (2.45 (0.97–6.18), p = .05), the development of AKI (5.33 (1.00–28.43), p = .05), the need for CRRT during ICU stay (18.00 (1.79–181.31), p = .01) and major bleeding during ECMO therapy (0.51 (0.19–0.89), p < .01) were identified to be predictors of ICU mortality.
Table 4.
Univariate regression analysis.
| OR (95% CI) | p-value | |
|---|---|---|
| Age | 1.08 (0.99–1.17) | 0.07 |
| Age categories | 2.45 (0.97–6.18) | 0.05 |
| BMI | 0.99 (0.91–1.08) | 0.81 |
| BMI categories | 0.92 (0.34–2.42) | 0.86 |
| Rockwood clinical frailty index | 1.14 (0.59–2.12) | 0.68 |
| SOFA at admission | 1.01 (0.77–1.33) | 0.93 |
| Gender | 0.93 (0.18–4.9) | 0.94 |
| Wave | 1.22 (0.57–2.6) | 0.61 |
| LOS before ICU | 1.01 (0.90–1.14) | 0.79 |
| Length of ECMO | 1.01 (0.97–1.06) | 0.62 |
| Early versus Late ECMO | 0.55 (0.12–2.49) | 0.44 |
| Lung compliance before ECMO | 0.99 (0.96–1.03) | 0.72 |
| pH before ECMO | 0.22 (0.00–717.79) | 0.71 |
| Days of mechanical ventilation before ECMO | 0.99 (0.86–1.14) | 0.89 |
| PF < 80 | 0.46 (0.09–2.25) | 0.34 |
| CRRT during ECMO | 18.00 (1.79–181.31) | 0.01 |
| Acute kidney failure | 5.33 (1.00–28.43) | 0.05 |
| Major bleeding | 0.51 (0.19–0.89) | <0.01 |
Data are expressed as OR (95% CI). A p-value <.05 is considered statistically significant.
Point-of-care echocardiography data
Table 5 presents the detailed echocardiographic data from pre-ECMO TTE. No significant differences were found.
Table 5.
Point-of-care echocardiography data.
| COVID-19 ECMO survivors (n = 19) | COVID-19 ECMO non-survivors (n = 12) | p-value | |
|---|---|---|---|
| TAPSE | 1/14 (7.1%) | 1/9 (11.1%) | 0.74 |
| RVSP | 1/8 (12.5%) | 0/5 (0.0%) | 0.41 |
| RV EDA/LV EDA | 10/14 (71.4%) | 6/9 (66.7%) | 0.81 |
| RV FAC | 6/14 (42.9%) | 3/9 (33.3%) | 0.65 |
| RVfwLS | 4/14 (28.6%) | 1/9 (11.1%) | 0.32 |
Data are expressed as numbers (frequencies). A p-value <.05 is considered statistically significant.
Discussion
In this monocentric cohort study involving 31 consecutive critically ill COVID-19 patients requiring ECMO therapy, the ICU mortality was 39.7% and the 90-day mortality was 41.93%.
Patients with a history of smoking were overrepresented in the non-survivor group. All 4 patients were treated with ECMO because of low arterial pH (<7.25) and poor lung compliance and all 3 patients who suffered from stroke didn’t survive ICU admission. Hospital LOS was significantly longer in the survival group. Univariate regression analysis showed the development of AKI and the need for CRRT during ICU stay, higher age category, and major bleeding to be predictors of ICU mortality. Protocol violations of the local eligibility criteria of ECMO initiation were noted in 6 (19.4%) patients.
The association between advanced age and mortality in COVID-19 patients treated with ECMO is well documented in the literature, as five different multicenter cohort studies also observed this association.22–26 This association is not surprising because older age is also known to be a strong independent risk factor for COVID-19 mortality.27
The association between the development of AKI and ICU mortality observed in this study is also supported by literature, as a large multicenter cohort study of 292 patients also concluded that renal dysfunction is an independent predictor of in-hospital mortality.26
The observation in this study that patients with an arterial pH lower than 7.25 before initiation of ECMO are overrepresented in the non-survivor group echoes the finding of Biancari et al. that decreased arterial pH before ECMO is independently associated with increased risk of mortality.23 These findings support the hypothesis that ECMO initiation because of ventilatory failure due to decreased lung compliance is associated with a lower chance of survival compared to ECMO initiation because of oxygenation failure.
In the present study, we couldn’t detect a correlation between BMI and ICU mortality. Two other large multicenter studies also failed to demonstrate a correlation between BMI and ICU mortality in COVID-19 patients treated with ECMO therapy.26,28 In contrast, a large single-center observational cohort study including 76 COVID-19 patients treated with ECMO concluded that obesity is an independent factor associated with improved 90-day survival. Conversely, in-hospital death in a multicenter cohort of 171 COVID-19 patients requiring ECMO in Poland was independently associated with higher BMI. Future research is needed to clarify these inconsistencies.
Dreier et al. showed that patients with COVID-19 induced ARDS might need prolonged (≥28 days) ECMO support and that prolonged ECMO support is not associated with poor outcomes.29 Our study supports these conclusions since 5 out of 8 (62.5%) patients treated with prolonged ECMO support in our cohort survived ICU admission. Conversely, a large meta-analysis found that ECMO duration was associated with increased mortality.30 These contradictory findings might be explained by the so-called self-fulfilling prophecy effect. Beliefs of poor prognosis for critically ill patients with prolonged ECMO support may become self-fulfilling if life-sustaining treatment or resuscitation is subsequently withheld in some centers based on that belief. Prolonged ECMO support might also be associated with an increased risk of complications in a time-dependent manner.
Contrary to Li et al., who found that early initiation of ECMO was associated with decreased mortality, we couldn’t detect a difference in outcome between the “early ECMO group” and the “delayed ECMO group” in the present study.31 The retrospective nature of the former study has the disadvantage that significant biases may affect the selection of controls which may explain these conflicting results.
ICU scoring systems, such as the SOFA, the APACHE II, and IV scores have been validated as prognostic tools to predict mortality in severely ill ICU patients.32,33 The prognostic value of these scoring systems to predict poor outcome seems rather limited in a critically ill COVID-19 population supported with ECMO. A multicenter study reported that the prognostic accuracy for the APACHE II score and SOFA score was low with an area under the receiver operating characteristic (AUC) of 0.572 and 0.602, respectively.34 In the present study, we couldn’t find a significant difference in APACHE II, APACHE IV, or SOFA admission scores between survivors and non-survivors. Daviet et al. were also unable to detect a significant difference in SOFA admission scores between survivors and non-survivors.25 On the contrary, Bergman et al. reported a higher SOFA score before cannulation to be a risk factor for mortality.24
The association between the somatic variable major bleeding and ICU mortality found in the present study seems logical and is in line with the literature, as Bergman et al. reported an increased number of transfusions to be a risk factor for mortality.24 Albeit, the observation that all patients who died in the present study experienced major bleeding during their ICU stay, may suggest that balancing the increased and competing risks of clotting and bleeding in these patients is very difficult and that the applied anticoagulation strategy was too aggressive in individual cases.
In the present study, we couldn’t find an association between five pre-ECMO echocardiographic point-of-care parameters assessing RV function and ECMO survival. This is surprising since Mustafa et al. showed improved survival after VV-ECMO cannulation with the ProTek duo cannula, functionally acting as a right ventricular assist device (RVAD).28 As consequence, determining RV dysfunction on TTE may facilitate tailored management between classic VV-ECMO and RVAD VV-ECMO. Kopanczyk pointed out RV FAC and GLS but not TAPSE to be aberrant on VV-ECMO patients.35 Unfortunately, the author did not examine any association with outcome. In a retrospective study on 35 patients treated with VV-ECMO, Lazzeri et al. found pre-ECMO left ventricular ejection fraction (LVEF), RV EDA/LV EDA, and TAPSE not to be predictors for mortality.36 Importantly, in the latter study, 30% of patients were diagnosed with RV failure based on TAPSE and RV EDA/LV EDA, normalization of these RV values was observed after ECMO initiation, and all survived.36
As consequence, reversibility of RV echocardiographic findings after EMCO initiation may be another predictor for survival. However, in our study, we tried to identify predictors to aid decision-making in the process before ECMO insertion.
This study has several limitations. First, the ambivalent single-center design with relatively low numbers of patients negatively impacts the generalizability of our findings. Second, multivariate prediction analysis is not performed due to the low number of included patients. Third, there is a potential impact of the increasing knowledge of pathophysiology and treatment options in COVID-19 over time, which leads to frequent changes in therapeutic strategies, creating a heterogeneous patient population.
In conclusion, these results suggest that almost 60% percent of patients with need for VV-ECMO due to severe COVID-19 pneumonia can be treated successfully with ECMO with sustained results at 3 months. Predictors for ICU mortality were the development of AKI and the need for CRRT during ICU stay, higher age category, and major bleeding. Inadvertent ECMO allocation was noted in almost one in five patients. These results may help to improve ECMO resource utilization in times of scarcity.
Supplemental Material
Supplemental Material for Predictors of poor outcome in critically ill patients with COVID-19 pneumonia treated with extracorporeal membrane oxygenation by Nick Pans, Jul Vanherf, Jeroen Vandenbrande, Jeroen Lehaen, Alaaddin Yilmaz, Jan Verwerft, Michiel Van Tornout, Laurien Geebelen, Ina Callebaut, Lieven Herbots, Jasperina Dubois and Björn Stessel in Perfusion
Appendix
List of abbreviations
- ARDS
acute respiratory distress syndrome
- MVA
maximum ventilation alone
- DVT
deep vein thrombosis
- VTE
venous thromboembolism
- IMV
invasive mechanical ventilation
- ICU
intensive care unit
- LMWH
low-molecular-weight-heparine
- NMBA
neuromuscular blocking agents
- PEEP
positive end expiratory pressure
- P/F ratio
ratio of arterial oxygen partial pressure to fractional inspired oxygen concentration
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is part of the Limburg Clinical Research Program (LCRP) UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital.
Ethical approval: This study is approved by the ethical committee of JESSA Hospital Hasselt, Belgium.
Consent to participate: Written informed consent was waived in light of the urgent need to collect data in the ongoing pandemic.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Ina Callebaut https://orcid.org/0000-0002-6666-6783
References
- 1.Cornelisssen L, De Muylder G, Lafort Y, et al. FACT SHEET COVID-19 disease (SARS-CoV-2 virus). 15 July 2021. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_fact_sheet_ENG.pdf2021, 84.
- 2.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med 2020; 46(5): 854–887. DOI: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med 2020; 8(5): 518–526. DOI: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shekar K, Badulak J, Peek G, et al. Extracorporeal life support organization Coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J 2020; 66(7): 707–721. DOI: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018; 378(21): 1965–1975. DOI: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 6.Mishra V, Svennevig JL, Bugge JF, et al. Cost of extracorporeal membrane oxygenation: evidence from the Rikshospitalet University Hospital, Oslo, Norway. Eur J Cardiothorac Surg 2010; 37(2): 339–342. DOI: 10.1016/j.ejcts.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 7.Autschbach T, Hatam N, Durak K, et al. Outcomes of extracorporeal membrane oxygenation for acute respiratory distress syndrome in covid-19 patients: a propensity-matched analysis. J Clin Med 2021; 10(12). DOI: 10.3390/jcm10122547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med 2020; 382(18): 1708–1720. DOI: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J Crit Care 2020; 58: 27–28. DOI: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021; 398(10307): 1230–1238. DOI: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M, Hajage D, Lebreton G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med 2020; 8(11): 1121–1131. DOI: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han JJ, Shin M, Patrick WL, et al. How Should ECMO Be Used Under Conditions of Severe Scarcity? A Population Study of Public Perception. J Cardiothorac Vasc Anesth 2021. DOI: 10.1053/j.jvca.2021.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm DGA E., Egger M., et al. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal Clin Epidemiol 2008; 61(4): 344–349. [DOI] [PubMed] [Google Scholar]
- 14.WHO . Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. interim Guidance: 1–7.
- 15.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13(10): 818–829. [PubMed] [Google Scholar]
- 16.Zimmerman JE, Kramer AA, McNair DS, et al. Acute physiology and chronic health evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med 2006; 34(5): 1297–1310. DOI: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22(7): 707–710. DOI: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28(1): 1–39. DOI: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Huston JH, Maron BA, French J, et al. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol 2019; 4(11): 1112–1121. DOI: 10.1001/jamacardio.2019.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Practice 2012; 120(4): c179–c184. DOI: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 21.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307(23): 2526–2533. DOI: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Hall CA, Jacobs JP, Stammers AH, et al. Multi-institutional analysis of 505 patients with Coronavirus disease-2019 supported with extracorporeal membrane oxygenation: predictors of survival. Ann Thorac Surg 2022. DOI: 10.1016/j.athoracsur.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biancari F, Mariscalco G, Dalen M, et al. Six-Month Survival After Extracorporeal Membrane Oxygenation for Severe COVID-19. J Cardiothorac Vasc Anesth 2021; 35(7): 1999–2006. DOI: 10.1053/j.jvca.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman ZR, Wothe JK, Alwan FS, et al. Risk factors of mortality for patients receiving venovenous extracorporeal membrane oxygenation for COVID-19 Acute Respiratory Distress Syndrome. Surg Infect (Larchmt) 2021; 22(10): 1086–1092. DOI: 10.1089/sur.2021.114. [DOI] [PubMed] [Google Scholar]
- 25.Daviet F, Guilloux P, Hraiech S, et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care 2021; 11(1): 157. DOI: 10.1186/s13613-021-00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeed O, Tatooles AJ, Farooq M, et al. Characteristics and outcomes of patients with COVID-19 supported by extracorporeal membrane oxygenation: a retrospective multicenter study. J Thorac Cardiovasc Surg 2021. DOI: 10.1016/j.jtcvs.2021.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho FK, Petermann-Rocha F, Gray SR, et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One 2020; 15(11): e0241824. DOI: 10.1371/journal.pone.0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustafa AK, Joshi DJ, Alexander PJ, et al. Comparative propensity matched outcomes in severe COVID-19 respiratory Failure-Extracorporeal Membrane Oxygenation or Maximum Ventilation Alone. Ann Surg 2021; 274(5): e388–e394. DOI: 10.1097/SLA.0000000000005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreier E, Malfertheiner MV, Dienemann T, et al. ECMO in COVID-19-prolonged therapy needed? A retrospective analysis of outcome and prognostic factors. Perfusion 2021; 36(6): 582–591. DOI: 10.1177/0267659121995997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care 2021; 25(1): 211. DOI: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Hu M, Zheng R, et al. Delayed Initiation of ECMO Is associated with poor outcomes in patients with severe COVID-19: a multicenter retrospective cohort study. Front Med (Lausanne) 2021; 8: 716086. DOI: 10.3389/fmed.2021.716086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoe A, Bakhshi-Raiez F, de Keizer N, et al. Mortality prediction by SOFA score in ICU-patients after cardiac surgery; comparison with traditional prognostic-models. BMC Anesthesiol 2020; 20(1): 65. DOI: 10.1186/s12871-020-00975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286(14): 1754–1758. DOI: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 34.Supady A, DellaVolpe J, Taccone FS, et al. Outcome prediction in patients with severe COVID-19 requiring extracorporeal membrane oxygenation-a retrospective international multicenter study. Membranes (Basel) 2021; 11(3). DOI: 10.3390/membranes11030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopanczyk R, Al-Qudsi OH, Uribe A, et al. Right ventricular dysfunction in patients with Coronavirus Disease 2019 supported with extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 2022; 36(2): 629–631. DOI: 10.1053/j.jvca.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazzeri C, Bonizzoli M, Batacchi S, et al. Persistent right ventricle dilatation in SARS-CoV-2-related acute respiratory distress syndrome on extracorporeal membrane oxygenation support. J Cardiothorac Vasc Anesth 2021. DOI: 10.1053/j.jvca.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Predictors of poor outcome in critically ill patients with COVID-19 pneumonia treated with extracorporeal membrane oxygenation by Nick Pans, Jul Vanherf, Jeroen Vandenbrande, Jeroen Lehaen, Alaaddin Yilmaz, Jan Verwerft, Michiel Van Tornout, Laurien Geebelen, Ina Callebaut, Lieven Herbots, Jasperina Dubois and Björn Stessel in Perfusion