Abstract
Background
There is no clear consensus regarding the safety and efficacy of immune checkpoint inhibitors (ICIs) in patients with advanced non-small cell lung cancer (NSCLC) and pre-existing interstitial lung disease (ILD). We aimed to elucidate the impact of ICIs on pre-existing ILD.
Methods
We systematically queried PubMed-MEDLINE, Embase-Scopus, and ISI Web of Science databases up to January 10, 2022. The pooled any-grade and grade 3–5 ICI-associated pneumonitis (ICIP) rate and objective response rate (ORR) in patients with pre-existing ILD were mainly evaluated. The relative risk (RR) was also evaluated for pre-existing ILD and usual interstitial pneumonia (UIP) patterns. Sensitivity and subgroup analyses were performed to assess the heterogeneity.
Results
In total, 17 studies involving 5,529 patients were included in the meta-analysis. The pooled ICIP rate was 30% [95% confidence interval (CI): 24–36%]; it was found to be significantly higher in patients with pre-existing ILD relative to those without (RR =3.05, 95% CI: 2.53–3.69; I2=0.0%). The pooled grade 3–5 ICIP rate was 12% (95% CI: 9–15%); this was also significantly higher in patients with pre-existing ILD (RR =3.19, 95% CI: 2.32–4.38; I2=0.0%). According to subgroup analysis, these ICIP rates were not significantly different among the treatment lines (first, ≥ second, and mixed) (P=0.33) whereas the pooled ORR was 36% (95% CI: 24–48%; I2=53.7%) with a significant difference among the treatment lines (P=0.027). The pooled ICIP rate was independent of the UIP pattern (RR =1.06, 95% CI: 0.86–1.32; I2=0.0%).
Conclusions
Overall, ICIs should be administered cautiously in patients with pre-existing ILD, regardless of the treatment line. Moreover, the risks of ICIP may outweigh ICI benefits, especially in second-or later-line treatment. These results need to be further confirmed by meta-analyses including more observational cohort studies in clinical setting.
Keywords: Immune checkpoint inhibitors (ICIs), non-small cell lung cancer (NSCLC), pre-existing interstitial lung disease, pneumonitis
Introduction
Globally, non-small cell lung cancer (NSCLC) is a common cause of cancer-related mortality (1). Most patients with NSCLC are at an advanced stage on first diagnosis (2).
In recent years, the continued development of immune checkpoint inhibitors (ICIs), a breakthrough treatment strategy for advanced NSCLC, have enabled durable survival for several years (3,4). A favorable response to ICIs results in longer survival for patients. However, a small number of patients achieve such benefits; some patients show immune-related adverse events (irAEs), including cutaneous lesions, nephritis, hepatitis, colitis, endocrinopathies, neuropathies, and pneumonitis (5). These irAEs can potentially become serious and fatal; in NSCLC particularly, pneumonitis is reported to be the most serious and fatal AE (6).
Interstitial lung disease (ILD) is one of the most common complications at the diagnosis of lung cancer (7). A previous study reports that approximately 10–20% of patients with ILD have combined LC conditions in the real-world setting (8). Despite such a staggering number, patients with pre-existing ILD are almost always excluded from clinical trials of NSCLC owing to the concerns regarding treatment-associated pneumonitis due to chemotherapies and radiotherapy. Therefore, there are relatively fewer studies in this group of patients (9,10). ICIs are drugs that also cause treatment-associated pneumonitis, and pre-existing ILD is a risk factor associated with ICI-associated pneumonitis (ICIP) in patients with different cancer types (11).
Although the safety and efficacy have been evaluated in a few prospective observational studies with a small number of cases and multiple retrospective observational studies (12-28), there is no consensus regarding the use of ICIs in NSCLC patients with pre-existing ILD. Thus, we performed a meta-analysis using the available reports to elucidate the risks and benefits of ICIs for advanced NSCLC patients with pre-existing ILD. Our systematic review and meta-analysis were conducted following the PRISMA reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-162/rc).
Methods
Search strategy
Our systematic review and meta-analysis were conducted following the PRISMA guidelines. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD 42022302783). We systematically queried the PubMed-MEDLINE, Embase-Scopus, and Web of Science databases to screen eligible studies published before January 10, 2022. The following search terms were used for the literature screen: “Non-Small Cell Lung Cancer” OR “NSCLC” AND “immune checkpoint inhibitor” OR “ICI” OR “immunotherapy” OR “nivolumab” OR “pembrolizumab” OR “atezolizumab” OR “durvalumab” OR “programmed death-1” OR “PD-1” OR “programmed death ligand-1” OR “PD-L1” AND “interstitial pneumonia” OR “interstitial pneumonitis” OR “interstitial lung”.
Selection criteria
The inclusion criteria were as follows: (I) cohort studies that evaluated advanced NSCLC patients who had undergone ICI-based treatment; (II) non-comparative or comparative studies including patients with pre-existing ILD; (III) studies with safety data on any-grade and grade 3–5 ICIP rate, and (IV) articles written in English. Reviews, case reports, animal studies, and duplicated publications were excluded.
Data extraction and quality assessment
Two investigators (KM and TS) independently extracted the following data from the included studies: the first name of the authors, year of publication, type of study, sample size, age, treatment lines, treatment regimen, any-grade ICIP rate, grade 3–5 ICIP rate, objective response rate (ORR), disease control rate (DCR), and median progression-free survival (PFS). The main outcomes were pooled any-grade ICIP rate, grade 3–5 ICIP rate, and ORR. Additionally, we evaluated the pooled DCR, and the relative risk (RR) for pre-existing ILD (yes vs. no) and radiological patterns (UIP vs. non-UIP). The Newcastle-Ottawa scale (NOS) standard was used for research quality assessment of observational studies (29), wherein low quality referred to studies with scores ≤4. The two authors evaluated the quality of the extracted studies. Any differences in opinions regarding the studies between the two authors were resolved with the help of a third investigator (TK).
Statistical analysis
The Stata (version 17.0; Stata Corporation, College Station, TX) tool was used to calculate the pooled any-grade ICIP rate, grade 3–5 ICIP rate, ORR, DCR, and the RR for pre-existing ILD and radiological patterns.
Heterogeneity between studies was assessed using the I2 statistics (30). According to the heterogeneity test using I2 statistics, the fixed-effects model was preferred over the random-effects model in the absence of statistically moderate or high heterogeneity (I2>50%) between the studies. An integrated analysis was performed to calculate the 95% confidence interval (CI); subsequently, the forest plots were plotted for the pooled effect sizes and RR.
The potential effects in presence of a significant heterogeneity (I2>50%) in the pooled effect size (31) were assessed. Subgroup analyses were performed for the treatment lines (first, ≥ second, and mixed). Differences between subgroups were evaluated using the I2 statistics. Additionally, sensitivity analyses were performed using leave-one-out method and the heterogeneity was re-evaluated after excluding the study that most affected the pooled outcomes and had the lowest sample size.
Potential publication biases were further validated using Funnel plots, Begg’s and Egger’s tests and trim and fill analysis (32). All statistical analyses were two-sided. Statistical significance was set at P<0.05.
Results
Search results
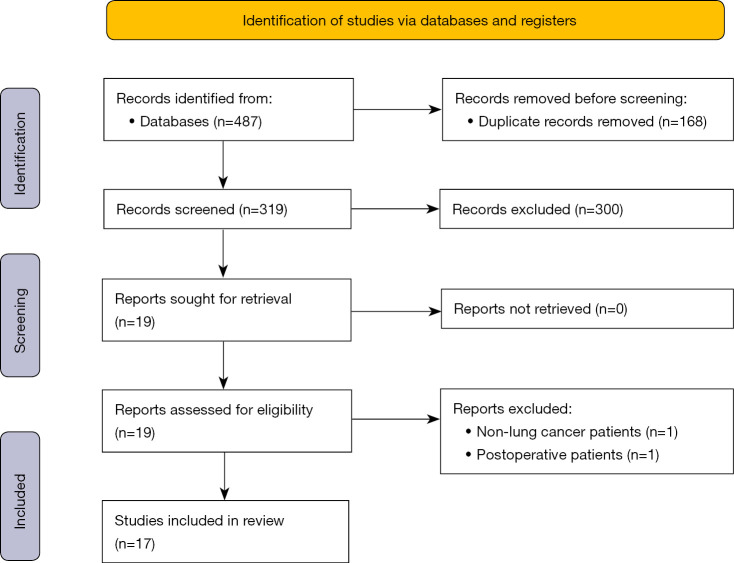
The PRISMA flowchart for this meta-analysis is shown in Figure 1. Duplicate and irrelevant studies were removed after construing a total of 487 titles. In total, 319 records were screened and 300 articles were excluded based on their titles and abstracts. All investigators fully assessed the remaining 19 articles and agreed to include 17 eligible studies comprising 5,529 patients in the subsequent meta-analysis (12-28).
Figure 1.
Flow diagram for search and selection of studies for meta-analysis.
Study characteristics
The extracted data are listed in Table 1. Three prospective and 14 retrospective cohort studies were included. The eligible studies were published between 2017 and 2021 and the sample sizes ranged between 5 and 221; a total of 543 patients with pre-existing ILD and 4,986 patients without ILD were analyzed. In most studies, the median age was around 70 years (range, 63–78 years) and the treatment lines and regimen were described. Two studies described the first-line treatment of patients with pembrolizumab alone (18,25), while six studies described only the second-line treatment (12-14,19,23,26). Of the six studies, only nivolumab was administered in five studies (12-14,23,26), and atezolizumab in one study (19). In the remaining nine studies, ICIs such as nivolumab, pembrolizumab, atezolizumab, and durvalumab were administered in various treatment lines (15-17,20-22,24,27,28).
Table 1. Characteristics of cohort studies.
| Author, year | Type of study | Pre-existing ILD group | Non-ILD group | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age, years | Treatment line | Regimen | ORR (%) | DCR (%) | mPFS (months) | Any grade ICIP rate (%) | Grade≥3 ICIP rate (%) | UIP pattern, n | ICIP rate (%) (UIP pattern) | Non-UIP pattern, n | ICIP rate (%) (non-UIP pattern) | n | Age, years | Treatment line | Regimen | Any grade ICIP rate (%) | Grade ≥3 ICIP rate (%) | |||
| Fujimoto, 2017 (12) | Prospective | 6 | 72 [64–81] | ≥2nd line: 6 | Nivolumab: 6 | 50.0 (3/6) | 100 (6/6) | 5.2 (NA) | 0 (0/6) | 0 (0/6) | 0 | 0 | 6 | 0 (0/6) | NA | NA | NA | NA | NA | NA | |
| Kanai, 2018 (13) | Retrospective | 26 | 71 [55–85] | ≥2nd line: 26 | Nivolumab: 26 | 26.9 (7/26) | 57.7 (15/26) | 2.7 [1.7–5.3] | 30.8 (8/26) | 19.2 (5/26) | 12 | 25.0 (3/12) | 14 | 35.7 (5/14) | 190 | 69 [30–89] | ≥2nd line: 190 | Nivolumab: 190 | 11.6 (22/190) | 5.3 (10/190) | |
| Fujimoto, 2019 (14) | Prospective | 18 | 71.5 [68.5–76.3] | ≥2nd line: 18 | Nivolumab: 18 | 38.9 (7/18) | 72.2 (13/18) | 7.4 [1.8–16.8] | 11.1 (2/18) | 0 (0/18) | 0 | 0 | 18 | 11.1 (2/18) | NA | NA | NA | NA | NA | NA | |
| Shibaki, 2019 (15) | Retrospective | 14 | 63 [33–83] | 1st line: 4; ≥2nd line: 10 | Nivolumab: 9; Pembrolizumab: 5 | 21.4 (3/14) | 57.1 (8/14) | 4.3 [1.1–19] | 28.6 (4/14) | 7.1 (1/14) | NA | NA | NA | NA | 196 | 61 [30–83] | 1st line: 35; ≥2nd line: 161 | Nivolumab: 118; Pembrolizumab: 78 | 11.2 (22/196) | 4.1 (8/196) | |
| Byeon, 2020 (17) | Retrospective | 6 | 63 [59–72] | 1st line: NA; ≥2nd line: NA | Nivolumab: NA; Pembrolizumab: NA | 16.7 (1/6) | 50.0 (3/6) | 1.4 (NA) | 0 (0/6) | 0 (0/6) | 5 | 0 (0/5) | 1 | 0 (0/1) | 231 | NA | 1st line: NA; ≥2nd line: NA | Nivolumab: NA; Pembrolizumab: NA | 3.9 (9/231) | 1.7 (4/231) | |
| Nakanishi, 2019 (16) | Retrospective | 13 | NA | 1st line: NA; ≥2nd line: NA | Nivolumab: NA; Pembrolizumab: NA | NA | NA | NA | 46.2 (6/13) | 15.4 (2/13) | 3 | 66.7 (2/3) | 10 | 40.0 (4/10) | 70 | NA | 1st line: NA; ≥2nd line: NA | Nivolumab: NA; Pembrolizumab: NA | 11.4 (8/70) | 8.6 (6/70) | |
| Fujita, 2020 (18) | Retrospective | 5 | 78 [75–81] | 1st line: 5 | Pembrolizumab: 5 | 60.0 (3/5) | 80 (4/5) | NA | 80.0 (4/5) | 40.0 (2/5) | 1 | 100 (1/1) | 4 | 75.0 (3/4) | NA | NA | NA | NA | NA | NA | |
| Ikeda, 2020 (19) | Prospective | 17 | 70 [66–73] | ≥2nd line: 17 | Atezolizumab: 17 | 6.3 (1/16) | 62.5 (10/16) | 3.4 [0.8–5.9] | 29.4 (5/17) | 23.5 (4/17) | 7 | 57.1 (4/7) | 11 | 9.1 (1/11) | NA | NA | NA | NA | NA | NA | |
| Nishiyama, 2020 (20) | Retrospective | 48 | 70 [52–83] | 1st line: 13; ≥2nd line: 35 | Nivolumab: 21; Pembrolizumab: 25; Atezolizumab: 2 | 45.8 (22/48) | 68.8 (33/48) | 4.7 (NA) | 14.6 (7/48) | 10.4 (5/48) | 9 | 11.1 (1/9) | 39 | 15.4 (6/39) | NA | NA | NA | NA | NA | NA | |
| Shibaki, 2020 (21) | Retrospective | 17 | 66 [33–83] | 1st line: 5; ≥2nd line: 12 | Nivolumab: 12; Pembrolizumab: 5 | NA | NA | NA | 29.4 (5/17) | 11.8 (2/17) | NA | NA | NA | NA | 314 | 62 [30–84] | 1st line: 36; ≥2nd line: 278 | Nivolumab: 236; Pembrolizumab: 78 | 9.9 (31/314) | 3.8 (12/314) | |
| Ichimura, 2022 (28) | Retrospective | 33 | NA | 1st line: 8; ≥2nd line: 25 | Nivolumab: 18; Pembrolizumab: 12; Atezolizumab: 3 | NA | NA | NA | 33.3 (11/33) | 21.2 (7/33) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Takahara, 2021 (22) | Retrospective | 14 | NA | 1st line: NA; ≥2nd line: NA | Nivolumab: NA; Pembrolizumab: NA; Durvalumab: NA | NA | NA | NA | 57.1 (8/14) | 28.6 (4/14) | 3 | 66.7 (2/3) | 11 | 54.5 (6/11) | NA | NA | NA | NA | NA | NA | |
| Yamamoto, 2021 (23) | Retrospective | 221 | NA | ≥2nd line: 221 | Nivolumab: 221 | NA | NA | NA | 25.3 (56/221) | 10.9 (24/221) | NA | NA | NA | NA | 3380 | NA | ≥2nd line: 3,380 | Nivolumab: 3,380 | 8.5 (288/3,380) | 3.5 (117/3,380) | |
| Tasaka, 2021 (24) | Retrospective | 49 | 71 [57–83] | 1st line: 14; ≥2nd line: 35 | Nivolumab: 22; Pembrolizumab: 27 | 49.0 (24/49) | 69.4 (34/49) | 5.9 (NA) | 30.6 (15/49) | 16.3 (8/49) | 8 | NA | 41 | NA | 412 | 69 [34–88] | 1st line: 97; ≥2nd line: 315 | Nivolumab: 247; Pembrolizumab: 165 | 9.5 (39/412) | 3.6 (15/412) | |
| Yamaguchi, 2021 (25) | Retrospective | 10 | NA | 1st line: 10 | Pembrolizumab: 10 | 70.0 (7/10) | 90.0 (9/10) | 8.6 (NA) | 20.0 (2/10) | 10.0 (1/10) | 1 | 0 (0/1) | 9 | 22.2 (2/9) | 62 | NA | 1st line: 62 | Pembrolizumab: 62 | 22.6 (14/62) | 11.3 (7/62) | |
| Yamaguchi, 2021 (26) | Retrospective | 26 | NA | ≥2nd line: 26 | Nivolumab: 26 | NA | NA | NA | 38.5 (10/26) | 7.7 (2/26) | 9 | 44.4 (4/9) | 17 | 35.3 (6/17) | 70 | NA | ≥2nd line: 70 | Nivolumab: 70 | 5.7 (4/70) | 0 (0/70) | |
| Isono, 2021 (27) | Retrospective | 20 | NA | 1st line: NA; ≥2nd line: NA | Nivolumab: 12; Pembrolizumab: 8 | 35.0 (7/20) | NA | NA | 35.0 (7/20) | NA | 3 | NA | 17 | NA | 61 | NA | NA | NA | 6.6 (4/61) | NA | |
ILD, interstitial lung disease; ORR, objective response rate; DCR, disease control rate; PFS, progression-free survival; ICIP, immune checkpoint inhibitor-associated pneumonitis; UIP, usual interstitial pneumonia; NA, not applicable.
Most observational studies (14 of 17) were scored 6 to 8 points for the NOS assessment without inclusion of the low-quality studies. The results of the quality assessment of the included studies are presented in Table S1.
The pooled ICIP rate in NSCLC patients with pre-existing ILD
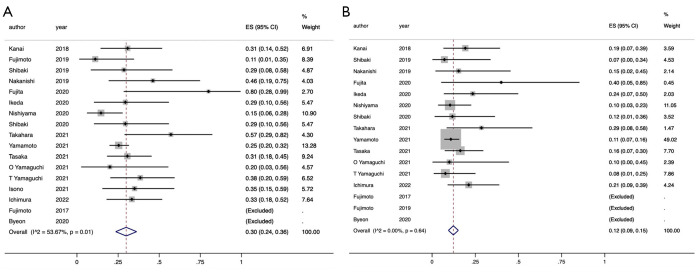
The pooled any-grade and grade 3–5 ICIP rates were obtained from 17 and 16 eligible studies, respectively (12-28). The pooled any-grade ICIP rate was 30% [95% confidence interval (CI): 24–36%] (12-28) and statistically significant heterogeneity was obtained through analysis (I2=53.7%, P=0.01) (Figure 2A). Therefore, a subgroup analysis was performed based on the treatment line to examine the cause of heterogeneity. The treatment lines could not explain the heterogeneity between the studies because the subgroup interactions did not show any statistical significance (P=0.33) (Figure S1A). Moreover, we performed a sensitivity analysis using leave-one-out method and excluding the study by Fujita et al. (18) (Figure S1B). The pooled any-grade ICIP rate was almost unchanged (28%; 95% CI: 22–33%), which suggested the robustness of the outcome whereas significant heterogeneity disappeared (I2=38.8%, P=0.07) (Figure S1C).
Figure 2.
Forest plot of ICIP rates in advanced NSCLC patients with pre-existing ILD. The point estimate of ICIP rate for each study is represented by the filled diamond, and the horizontal line crossing the diamond represents the 95% CI. The open diamond represents the pooled ES. (A) Any grade ICIP rates; (B) grade 3–5 ICIP rates. ICIP, immune checkpoint inhibitor-associated pneumonitis; NSCLC, non-small cell lung cancer; ILD, interstitial lung disease; CI confidence interval; ES, effect size.
Second, the pooled grade 3–5 ICIP rate was 12% (95% CI: 9–15%) (12-26, 28). No statistically significant heterogeneity was obtained (I2=0.0%, P=0.64) and there was little variation among the studies (Figure 2B).
The funnel plots for the pooled any-grade and grade 3–5 ICIP rates, shown in Figure S2, suggest the presence of publication biases (Begg’s and Egger’s tests, P=0.07 and 0.034, and P=0.009 and 0.022, respectively). The symmetry in the funnel plots may have been shown if the studies by Fujimoto et al. and Byeon et al. were not excluded in these meta-analyses (12,14,17). Additionally, a trim and fill method revealed that the re-evaluated pooled any-grade and grade 3–5 ICIP rates with imputed studies were similar to the observed outcomes (28%; 95% CI: 20–35% and 10%; 95% CI: 8–13%, respectively), indicating the stability against publication biases.
The pooled ORR and DCR of ICIs in NSCLC patients with pre-existing ILD
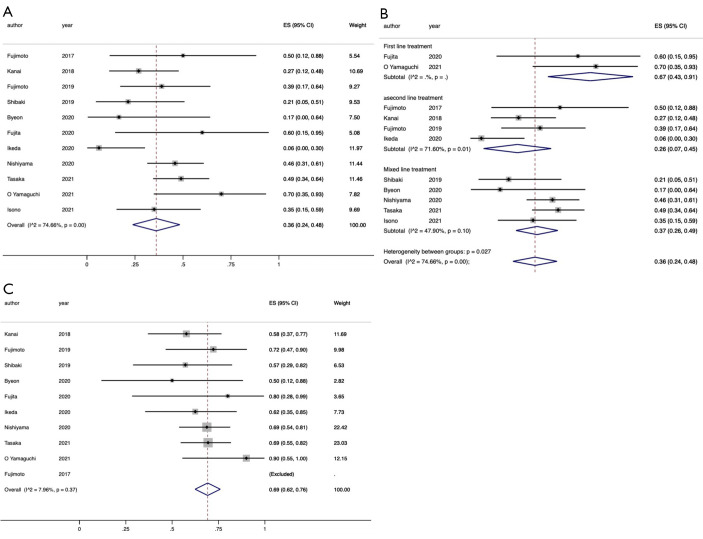
The ORR data were obtained from a total of 11 eligible studies (12-15,17-20,24,25,27). The pooled ORR was 36% (95% CI: 24–48%), indicating high heterogeneity (I2=74.7%, P<0.001) (Figure 3A). A subgroup analysis based on the treatment line was performed, and the results suggested that the treatment line could explain the heterogeneity among the studies since the subgroup interactions were statistically significant (P=0.027) (Figure 3B). When the ICIs were used in the first line and ≥second line, the pooled ORR were 67% (95% CI: 43–91%) and 26% (95% CI: 7–45%), respectively.
Figure 3.
Forest plot of ORR and DCR in advanced NSCLC patients with pre-existing ILD. The point estimates of ORR and DCR for each study are represented by the filled diamond, and the horizontal line crossing the diamond represents the 95% CI. The open diamond represents the pooled ES. (A) ORR; (B) subgroup analysis of ORR based on treatment line (first line, ≥ second line, and mixed line); (C) DCR. ORR, objective response rate; DCR, disease control rate; NSCLC, non-small cell lung cancer; ILD, interstitial lung disease; CI, confidence interval; ES, effect size.
The DCR data were obtained from ten eligible studies (12-15, 17-20, 24, 25). The pooled DCR was 69% (95% CI: 62–76%), suggesting little heterogeneity (I2=8.0%, P=0.37) (Figure 3C).
Funnel plots for the pooled ORR and DCR were largely symmetrical (Figure S3), which indicated no publication biases (Begg’s and Egger’s tests, P=0.64 and 0.26, and P=0.47 and 0.62, respectively).
The association between the ICIP and pre-existing ILD
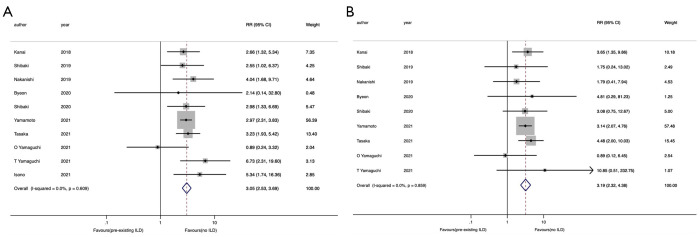
The any-grade and grade 3–5 ICIP rates were compared between patients with and without pre-existing ILD. The data were obtained from ten (13,15-17,21,23-27) and nine (13,15-17,21,23-26) eligible studies, respectively. The any-grade ICIP rate was significantly higher in patients with pre-existing ILD relative to those without (RR =3.05, 95% CI: 2.53–3.69). Moreover, no heterogeneity was observed in this analysis (I2=0.0%, P=0.61) (Figure 4A).
Figure 4.
Forest plots of RR of ICIP rates in advanced NSCLC patients with pre-existing ILD compared to patients without ILD. The point estimate of RR for each study is represented by the filled diamond, and the horizontal line crossing the diamond represents the 95% CI. The open diamond represents the pooled RR. (A) Any grade ICIP rates; (B) grade 3–5 ICIP rates. RR, relative risk; ICIP, immune checkpoint inhibitor-associated pneumonitis; NSCLC, non-small cell lung cancer; ILD, interstitial lung disease; CI, confidence interval.
The grade 3–5 ICIP rate was also significantly higher in patients with pre-existing ILD relative to those without (RR =3.19, 95% CI: 2.32–4.38); no heterogeneity was observed in this analysis (I2=0.0%, P=0.86) (Figure 4B).
Funnel plots showed the absence of publication biases (Begg’s and Egger’s tests, P=0.59 and 0.49, and P=0.92 and 0.69, respectively) (Figure S4).
The association between the ICIP and radiological patterns
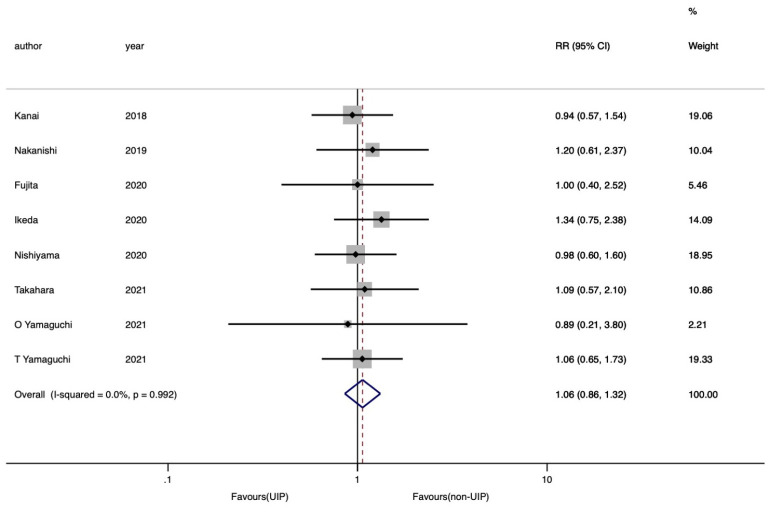
Finally, the any-grade ICIP rate was compared between the patients with UIP and non-UIP patterns, and the data were obtained from eight eligible studies (13,16,18-20,22,25,26). The any-grade ICIP rate was almost equivalent between the two groups of patients (RR =1.06, 95% CI: 0.86–1.32). Moreover, no heterogeneity was observed in this analysis (I2=0.0%, P=0.99) (Figure 5).
Figure 5.
Forest plots of RR of any grade ICIP rates in advanced NSCLC patients with UIP pattern compared to patients with non-UIP patterns. The point estimates of RR for each study are represented by the filled diamond, and the horizontal line crossing the diamond represents the 95% CI. The open diamond represents the pooled RR. RR, relative risk; ICIP, immune checkpoint inhibitor-associated pneumonitis; NSCLC, non-small cell lung cancer; UIP, usual interstitial pneumonia; CI, confidence interval.
Funnel plots showed the absence of any publication biases (Begg’s and Egger’s tests, P=0.90 and 0.97) (Figure S5).
Discussion
This systematic review and meta-analysis demonstrated that pre-existing ILD was associated with the incidence of pneumonitis caused by ICIs in patients with advanced NSCLC. The ORR and DCR were slightly higher in patients with pre-existing ILD than reported previously such as Checkmate 017 and 057 (3,4); however, these may be dependent on the treatment line. Any-grade and grade 3–5 ICIP rates were markedly higher in patients with pre-existing ILD (3,4,33). Additionally, the findings elucidated that the UIP pattern did not significantly increase the ICIP rate.
Thus, our findings provide the greatest evidence of the clinical safety and efficacy of immunotherapies for NSCLC patients with pre-existing ILD and may also have a large impact on combination therapy with ICIs (34).
Previously, several chemotherapies have been reported to increase the risk of pneumonitis in NSCLC patients with pre-existing ILD. Some retrospective cohort studies demonstrate that 14% and 12% of patients with ILD who received docetaxel and pemetrexed monotherapy, respectively, developed drug-associated pneumonitis (35,36). Many mechanisms, including cytotoxic and immune disorders, are involved in drug-associated pneumonitis. These mechanisms, sometimes independently or in combination, may be implicated in different forms of lung injury (37).
The mechanisms underlying ICIP remain poorly understood in patients with pre-existing ILD. However, a study showed that Th2 inflammation caused by the blockade of PD-1/PD-L2 interaction is a possible underlying mechanism (38). Moreover, others demonstrate that interleukin-6 (IL-6) plays an important role in ICIP, and anti-IL-6 antibodies are effective against ICIP (39,40). Increased IL-6 levels are detected in patients with acute exacerbation of idiopathic pulmonary fibrosis (IPF); moreover, IL-6 is a poor prognostic prediction factor in patients with ILD (41,42). Thus, ICIs may induce Th2 inflammation, thereby enhancing levels of IL-6 in pre-existing ILD, which may induce pneumonitis and allergy-like immune responses.
Recently, Zhang et al. first reported a meta-analysis regarding the clinical outcomes of ICIs in patients with advanced NSCLC and pre-existing ILD (43). They interpreted that the ICIs had favorable efficacy in patients with pre-existing ILD and ICIP was often mild and easily manageable. According to a report by Fujita et al. in the meta-analysis, three out of four patients with ICIP used steroid pulse therapy and managed to recover; however, two of them finally needed home oxygen therapy (18). Hence, the interpretation that ICIP is often mild and easily manageable may be risky. Moreover, we added seven more studies in addition to the studies reported by Zhang et al., including approximately three times as many patients with pre-existing ILD (543 patients vs. 179 patients). We further performed a subgroup analysis by treatment lines thorough the study protocol, and newly revealed that the pooled ORR may be dependent on the treatment lines and was 26% (95% CI: 7–45%) in the second-or later-line treatment, which was equivalent to the real-world data with ICI monotherapies in pretreated patients with NSCLC (44). Another real-world study reported the incidence of any-grade and grade 3–5 ICIP rates, which were approximately 10% and 4%, in the NSCLC patients treated with nivolumab (45). Therefore, considering the present ICIP rates, which were 30% in any-grade and 12% in grade 3–5, the risks of ICI monotherapy may outweigh its benefits, especially in second-or later-line treatment.
In this study, the ICIP rate was not significantly different between the UIP and non-UIP groups. IPF generally indicates a poorer prognosis relative to other idiopathic interstitial pneumonias and the incidence of acute exacerbation increases according to the UIP pattern (46,47). The reason for such difference remains unclear, and therefore, this warrants further investigation.
Finally, ICIs currently are used as key drugs for the treatment of patients with advanced NSCLC, along with various agents such as anti-angiogenic, cytotoxic, and molecularly targeted compounds (34,48). Such combination therapies are more effective than previous treatments; however, they also exert greater toxic effects. A previous meta-analysis of ICIs plus chemotherapy demonstrated that the RR was 2.92 (95% CI: 1.95–4.37) for the pooled ICIP compared to chemotherapy alone (49). The present meta-analysis included few patients with ICIs plus chemotherapy; therefore, ICIP may develop at a higher rate when ICIs plus chemotherapy are administered to patients with pre-existing ILD. Thus, treatments including ICIs should be cautiously administered for patients with pre-existing ILD.
This study had certain limitations. First, our meta-analysis included a small number of studies and sample sizes, which may have led to statistical insignificance of the results. Second, most of our sample included retrospective observational studies; therefore, there may have been several causes of inherent bias owing to the study design and unaccounted confounding factors. Third, due to the lack of detailed data, such as age, tumor proportion score, and respiratory function values, we could not perform appropriate subgroup and sensitivity analyses. Forth, most of the selected studies were of relatively poor-to-mild quality because the follow-up period as well as the final follow-up results were not stated. Therefore, all studies were selected to avoid systematic selection bias, which may have led to overestimation. Fifth, all the studies included only Asians, who may be more likely to develop ICIP compared to non-Asians, thereby, limiting the external validity of our findings (50). Sixth, we evaluated single arm of ICI monotherapies, and it is desirable to compare with a control arm in patients with pre-existing ILD. Finally, we mainly aimed to evaluate the safety and we did not assess the efficacy using indicators, such as PFS and OS. In clinical practice, a treatment should be decided on the basis of both efficacy and safety. Our findings are beneficial for generating hypotheses for future studies.
In summary, based on the findings of this meta-analysis, pre-existing ILD may be a risk factor for the incidence of ICIP in patients with NSCLC. Both any and severe grade ICIP rates were much higher in patients with pre-existing ILD than those of the previous reports whereas the ORR was comparable to that of previous reports on second-or later-line treatment. Therefore, ICIs should be administered cautiously in patients with pre-existing ILD, regardless of the treatment line. Moreover, the risk of ICIP may outweigh ICI benefits, especially in second-or later-line treatment. These results need to be further confirmed by meta-analyses, including more observational cohort studies in clinical setting.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported in part by the Center of Innovation program (COISTREAM) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (to AK); the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP18H05282 to AK); the Japan Agency for Medical Research and Development (AMED) (J210705582, J200705023, J200705710, J200705049, JP18cm016335 and JP18cm059042 to AK); and grants from the Kansai Economic Federation (KANKEIREN); Mitsubish Zaidan1 (to AK). The research was designed, conducted, analyzed, and interpreted by the authors, independent of the funding sources.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-162/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-162/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-162/coif). The authors have no conflicts of interest to declare.
References
- 1.Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013;24:20-30. Correction appears in Ann Oncol 2013;24:1133. [DOI] [PubMed]
- 2.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Govindan R, Anders RA, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer 2018;6:75. 10.1186/s40425-018-0382-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Zhao H, Zhao J. Correction to: Serious adverse events and fatal adverse events associated with nivolumab treatment in cancer patients. J Immunother Cancer 2019;7:9. 10.1186/s40425-018-0487-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829-44. 10.21037/jtd.2018.05.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawahara T, Sakashita H, Suzuki T, et al. Real world data of combined lung cancer and interstitial lung disease. J Thorac Dis 2019;11:4144-51. 10.21037/jtd.2019.10.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011;6:1242-6. 10.1097/JTO.0b013e318216ee6b [DOI] [PubMed] [Google Scholar]
- 10.Ozawa Y, Abe T, Omae M, et al. Impact of Preexisting Interstitial Lung Disease on Acute, Extensive Radiation Pneumonitis: Retrospective Analysis of Patients with Lung Cancer. PLoS One 2015;10:e0140437. 10.1371/journal.pone.0140437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimoji K, Masuda T, Yamaguchi K, et al. Association of Preexisting Interstitial Lung Abnormalities With Immune Checkpoint Inhibitor-Induced Interstitial Lung Disease Among Patients With Nonlung Cancers. JAMA Netw Open 2020;3:e2022906. 10.1001/jamanetworkopen.2020.22906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto D, Morimoto T, Ito J, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017;111:1-5. 10.1016/j.lungcan.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018;9:847-55. 10.1111/1759-7714.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto D, Yomota M, Sekine A, et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: A multicenter, open-label single-arm phase II trial. Lung Cancer 2019;134:274-8. 10.1016/j.lungcan.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 15.Shibaki R, Murakami S, Matsumoto Y, et al. Tumor expression and usefulness as a biomarker of programmed death ligand 1 in advanced non-small cell lung cancer patients with preexisting interstitial lung disease. Med Oncol 2019;36:49. 10.1007/s12032-019-1274-0 [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi Y, Masuda T, Yamaguchi K, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Investig 2019;57:451-9. 10.1016/j.resinv.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Byeon S, Cho JH, Jung HA, et al. PD-1 inhibitors for non-small cell lung cancer patients with special issues: Real-world evidence. Cancer Med 2020;9:2352-62. 10.1002/cam4.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita T, Kuroki T, Hayama N, et al. Pembrolizumab for Previously Untreated Patients with Advanced Non-small-cell Lung Cancer and Preexisting Interstitial Lung Disease. Intern Med 2020;59:1939-45. 10.2169/internalmedicine.4552-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda S, Kato T, Kenmotsu H, et al. A Phase 2 Study of Atezolizumab for Pretreated NSCLC With Idiopathic Interstitial Pneumonitis. J Thorac Oncol 2020;15:1935-42. 10.1016/j.jtho.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama N, Honda T, Sema M, et al. The utility of ground-glass attenuation score for anticancer treatment-related acute exacerbation of interstitial lung disease among lung cancer patients with interstitial lung disease. Int J Clin Oncol 2020;25:282-91. 10.1007/s10147-019-01576-x [DOI] [PubMed] [Google Scholar]
- 21.Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother 2020;69:15-22. 10.1007/s00262-019-02431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahara Y, Tanaka T, Ishige Y, et al. Risk factors for acute exacerbation in lung cancer complicated by interstitial lung disease with slight reticular shadows. Thorac Cancer 2021;12:2758-66. 10.1111/1759-7714.14121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto N, Nakanishi Y, Gemma A, et al. Real-world safety of nivolumab in patients with non-small-cell lung cancer in Japan: Postmarketing surveillance. Cancer Sci 2021;112:4692-701. 10.1111/cas.15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasaka Y, Honda T, Nishiyama N, et al. Non-inferior clinical outcomes of immune checkpoint inhibitors in non-small cell lung cancer patients with interstitial lung disease. Lung Cancer 2021;155:120-6. 10.1016/j.lungcan.2021.03.014 [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi O, Kaira K, Shinomiya S, et al. Pre-existing interstitial lung disease does not affect prognosis in non-small cell lung cancer patients with PD-L1 expression ≥50% on first-line pembrolizumab. Thorac Cancer 2021;12:304-13. 10.1111/1759-7714.13725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing interstitial lung disease is associated with onset of nivolumab-induced pneumonitis in patients with solid tumors: a retrospective analysis. BMC Cancer 2021;21:924. 10.1186/s12885-021-08661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isono T, Kagiyama N, Takano K, et al. Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer 2021;12:153-64. 10.1111/1759-7714.13736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichimura T, Hinata M, Ichikura D, et al. Safety of immune checkpoint inhibitors in non-small-cell lung cancer patients with idiopathic interstitial pneumonia: a matched case-control study. Cancer Chemother Pharmacol 2022;89:21-30. 10.1007/s00280-021-04362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018;74:785-94. 10.1111/biom.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 34.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe N, Niho S, Kirita K, et al. Second-line docetaxel for patients with platinum-refractory advanced non-small cell lung cancer and interstitial pneumonia. Cancer Chemother Pharmacol 2015;76:69-74. 10.1007/s00280-015-2775-y [DOI] [PubMed] [Google Scholar]
- 36.Kato M, Shukuya T, Takahashi F, et al. Pemetrexed for advanced non-small cell lung cancer patients with interstitial lung disease. BMC Cancer 2014;14:508. 10.1186/1471-2407-14-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietra GG. Pathologic mechanisms of drug-induced lung disorders. J Thorac Imaging 1991;6:1-7. 10.1097/00005382-199101000-00003 [DOI] [PubMed] [Google Scholar]
- 38.Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019;25:551-7. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 39.Ke W, Zhang L, Dai Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs). Thorac Cancer 2020;11:835-9. 10.1111/1759-7714.13341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Addeo A, Obeid M, Friedlaender A. COVID-19 and lung cancer: risks, mechanisms and treatment interactions. J Immunother Cancer 2020;8:e000892. 10.1136/jitc-2020-000892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papiris SA, Tomos IP, Karakatsani A, et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine 2018;102:168-72. 10.1016/j.cyto.2017.08.019 [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Jang JH, Park JH, et al. The role of interleukin-6 as a prognostic biomarker for predicting acute exacerbation in interstitial lung diseases. PLoS One 2021;16:e0255365. 10.1371/journal.pone.0255365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Fan Y, Nie L, et al. Clinical Outcomes of Immune Checkpoint Inhibitor Therapy in Patients With Advanced Non-small Cell Lung Cancer and Preexisting Interstitial Lung Diseases: A Systematic Review and Meta-analysis. Chest 2022;161:1675-86. 10.1016/j.chest.2021.12.656 [DOI] [PubMed] [Google Scholar]
- 44.Pasello G, Pavan A, Attili I, et al. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev 2020;87:102031. 10.1016/j.ctrv.2020.102031 [DOI] [PubMed] [Google Scholar]
- 45.Morita R, Okishio K, Shimizu J, et al. Real-world effectiveness and safety of nivolumab for advanced non-small-cell lung cancer: a retrospective observational study in Japan. Lung Cancer. 2020;140:8-18. 10.1016/j.lungcan.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 46.Antoniou KM, Wells AU. Acute exacerbations of idiopathic pulmonary fibrosis. Respiration 2013;86:265-74. 10.1159/000355485 [DOI] [PubMed] [Google Scholar]
- 47.Miyashita K, Kono M, Saito G, et al. Prognosis after acute exacerbation in patients with interstitial lung disease other than idiopathic pulmonary fibrosis. Clin Respir J 2021;15:336-44. 10.1111/crj.13304 [DOI] [PubMed] [Google Scholar]
- 48.Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. 10.1016/S1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 49.Tun AM, Thein KZ, Thein WL, et al. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future Sci OA 2019;5:FSO421. 10.2144/fsoa-2019-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol 2018;13:1930-9. 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as