Abstract
Background
Anticoagulants are promising regimens for treating coronavirus disease 2019 (COVID-19). However, whether prophylactic or intermediate-to-therapeutic dosage is optimal remains under active discussion.
Methods
We comprehensively searched PubMed, Embase, Scopus, Web of Science, Cochrane Library, ClinicalTrials, and MedRxiv databases on April 26, 2022. Two independent researchers conducted literature selection and data extraction separately according to predetermined criteria. Notably, this is the first meta-analysis on COVID-19, taking serious consideration regarding the dosage overlap between the 2 comparison groups of prophylactic anticoagulation (PA) and intermediate-to-therapeutic anticoagulation (I-TA).
Results
We included 11 randomized controlled trials (RCTs) and 36 cohort studies with 27,051 COVID-19 patients. By analyzing all the RCTs, there was no significant difference in mortality between the PA and I-TA groups, which was further confirmed by trial sequential analysis (TSA) (odds ratio [OR]: 0.93; 95% confidence interval [CI]: 0.71–1.22; P = 0.61; TSA adjusted CI: 0.71–1.26). The rate of major bleeding was remarkably higher in the I-TA group than in the PA group, despite adjusting for TSA (OR: 1.73; 95% CI: 1.15–2.60; P = 0.009; TSA adjusted CI: 1.09–2.58). RCTs have supported the beneficial effect of I-TA in reducing thrombotic events. After including all studies, mortality in the I-TA group was significantly higher than in the PA group (OR: 1.38; 95% CI: 1.15–1.66; P = 0.0005). The rate of major bleeding was similar to the analysis from RCTs (OR: 2.24; 95% CI: 1.86–2.69; P < 0.00001). There was no distinct difference in the rate of thrombotic events between the 2 regimen groups. In addition, in both critical and noncritical subgroups, I-TA failed to reduce mortality but increased major bleeding rate compared with PA, as shown in meta-analysis of all studies, as well as RCTs only. Meta-regression of all studies suggested that there was no relationship between the treatment effect and the overall risk of mortality or major bleeding (P = 0.14, P = 0.09, respectively).
Conclusion
I-TA is not superior to PA for treating COVID-19 because it fails to lower the mortality rate but increases the major bleeding rate in both critical and noncritical patients.
Keywords: Anticoagulation, COVID-19, Major bleeding, Mortality, Prophylactic, Therapeutic
Introduction
Coronavirus disease 2019 (COVID-19), an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a global pandemic. Although many studies have been conducted, effective treatment of patients with COVID-19 is still needed.[1,2] According to a report by the World Health Organization (WHO), as of May 2, 2022, there were more than 511 million confirmed COVID-19 cases, with approximately 6 million deaths worldwide.[3]
As the understanding of the mechanisms of COVID-19 continues to grow, microthrombi subsequent to the hypercoagulable state have been widely recognized as a key factor in organ failure and death.[4–11] SARS-CoV-2 enters target cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor, activating the renin-angiotensin system and the immune system, and triggering the release of angiotensin II and excessive inflammatory factors. This subsequently causes endothelial injury, thus leading to hypercoagulability. As the disease progresses, disseminated viral replication leads to widespread endotheliopathy, aggravating the prothrombotic state.[5,6] Several studies have found that patients with COVID-19 have a hypercoagulable state, with altered parameters including d-dimer, prothrombin time, activated partial thromboplastin time, fibrinogen, fibrin degradation product, and platelet count.[7,12,13] The hypercoagulable state provides the necessary conditions for extensive formation of microthrombus. [9–11] Parra-Medina et al.[10] identified in 151 autopsies of patients with COVID-19 that 60% had microthrombi in the lungs, heart, kidneys, and liver. In an observational study, diffuse intravascular coagulation was reported in 71.4% of mortalities and 0.6% of surviving patients with COVID-19 during hospitalization.[8] Hypercoagulability and thrombosis in COVID-19 are possible causes of increased mortality.[9] A meta-analysis revealed that the mortality of patients with COVID-19 who took anticoagulants was significantly lower than that of patients who did not use anticoagulants.[14] Therefore, anticoagulants are a promising treatment option for patients with COVID-19, because of their thromboprophylactic effect.
However, the optimal anticoagulant dosage remains controversial. Although the latest versions of the guidelines issued by the WHO and the United States all recommend prophylactic dosage,[15,16] many studies found that despite using prophylactic anticoagulants, the incidence of thrombotic events is still high rather than intermediate or therapeutic dosage in hospitalized patients with COVID-19 without evidence of thromboembolism.[17–19] Therefore, administering a higher anticoagulant dose (intermediate or therapeutic) has been proposed and studied. However, it was also observed that intermediate or therapeutic dosage was not more effective than prophylactic dosage in reducing mortality in patients with COVID-19.[17,20] Consequently, anticoagulant dosage for treating COVID-19 remains debatable.
Therefore, we conducted a meta-analysis, trial sequential analysis (TSA), and meta-regression to determine the optimal anticoagulant dosage, that is, intermediate-to-therapeutic (including intermediate and therapeutic) or prophylactic, for treating COVID-19.
Materials and methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,[21] with the PRISMA checklist provided in Supplementary Table 1, http://links.lww.com/ECCM/A31.
Literature search
A strict and comprehensive literature search of eligible studies was performed on April 26, 2022, in PubMed, Embase, Scopus, Web of Science, Cochrane Library, ClinicalTrials, and MedRxiv. The following terms were used in our search strategy: (COVID-19 OR novel coronavirus 2019 OR SARS-CoV-2 OR 2019-nCoV OR SARS-CoV-19 OR coronavirus disease 2019) AND (anticoagulant OR heparin OR Enoxaparin OR Dalteparin OR Fondaparinux OR warfarin OR rivaroxaban OR Dabigatran OR apixaban OR edoxaban OR thrombin inhibitors; Supplementary Table 2, http://links.lww.com/ECCM/A32). This meta-analysis aimed to assess the efficacy and safety of prophylactic anticoagulation (PA) versus intermediate-to-therapeutic (I-TA) therapy in patients with COVID-19. There were no restrictions on the language used, publication status, or publication date.
Inclusion and exclusion criteria
Qualification was inspected carefully according to a predefined selection criteria by reviewing the titles, abstracts, full manuscripts, and supplementary materials. We included studies that (1) enrolled adult inpatients with confirmed SARS-CoV-2 infections; (2) compared PA versus I-TA; (3) contained at least one of the following endpoints or outcomes: mortality, major bleeding, thrombotic events, pulmonary embolism, stroke, myocardial infarction, or venous thromboembolism; and (4) were eligible controlled studies. We excluded (1) studies that did not compare PA versus I-TA; (2) studies for which the numerical data of outcomes could not be acquired; (3) studies that applied the dosage of anticoagulants inconsistent with most studies included in our meta-analysis; and (4) studies with unsatisfactory methodological quality. The doses of the 2 anticoagulation regimens used in the included studies are listed in Supplemental Table 3, http://links.lww.com/ECCM/A33. There were few studies in which the specific doses of anticoagulation regimens were not described; we tacitly assumed that the doses used were widely accepted and could be included to avoid selection bias as much as possible.
Study selection
Two reviewers independently screened all the titles and abstracts to identify potentially eligible studies. The full text was then used to determine whether they could be included in our meta-analysis. Any discrepancies were resolved by discussion or if consensus could not be reached by a third investigator.
Quality assessment and data extraction
The methodological quality of randomized controlled trials (RCTs) and cohort studies was evaluated by 2 independent investigators using the Cochrane risk-of-bias tool Newcastle-Ottawa Scale (NOS), respectively.
Two researchers independently extracted relevant data from each eligible study using a standardized data extraction form. Extracted information included the characteristics of the included studies, baseline characteristics of participants, information on interventions, clinical outcomes, and results of comparison. For the characteristics of the included studies, we extracted the study type and setting, sample size, publication information, etc. For the baseline characteristics of participants, information on age, sex, and comorbidities were extracted. Regarding intervention, drugs and detailed dosages of the 2 regimen groups were extracted. For outcomes, we extracted the information on mortality, major bleeding, thrombotic events, pulmonary embolism, myocardial infarction, stroke, and venous thromboembolism.
Outcomes and definitions
The primary efficacy outcome was mortality and the primary safety outcome was the incidence of major bleeding. The secondary outcomes were the rates of thrombotic events, pulmonary embolism, stroke, myocardial infarction, and venous thromboembolism. Definitions of major bleeding, thrombotic events, and critically ill or noncritically ill patients are reported in the respective studies (Supplemental Table 4, http://links.lww.com/ECCM/A34).
Grading the quality of evidence
Two investigators assessed the quality of each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology. [22] The GRADE Profiler (version 3.6) software was used. Quality was downgraded based on the following evaluations: risk of bias, inconsistency, indirectness, imprecision, and other considerations. Quality was upgraded if the magnitude of the treatment effect was very large, if there was evidence of a dose-response relationship, or if all reasonable biases would reduce but not increase the magnitude of the apparent treatment effect. The overall quality of the evidence was rated as “high,” “moderate,” “low,” or “very low.”
Data synthesis and analysis
Statistical analysis was performed using Review Manager (version 5.3), STATA (version 12.0), and TSA program version 0.9.5.10 (http://www.ctu.dk/tsa). For dichotomous variables, we calculated the risk ratio (RR), odds ratio (OR), and 95% confidence interval (CI) using the Mantel-Haenszel method. When only RCTs were analyzed, RR was selected as the effect value; otherwise, OR was used. Statistical heterogeneity of the included studies was quantified using I2 values. I2 values more than 50% indicate significant heterogeneity, and a random-effects model was used. Otherwise, a fixed-effects model was used for analysis. Visual inspection and quantitative analysis of publication bias were performed using funnel plots and the Begg’s and Egger’s tests, respectively. No statistical difference was considered if P value greater than 0.05. Meta-regression was performed to investigate the association between the treatment effect and overall risk using the event rate of the experimental group. We performed TSA for RCTs to avoid the positive results of meta-analysis being derived from random errors rather than the real effects of interventions. We quantified the required information size (RIS) and trial sequential monitoring boundaries using the O'Brien-Fleming α-spending function. The cumulative Z curve located in regions of, such as the futility area, crossing the trial sequence monitoring boundaries, or neither, may indicate that the result is true negative, true positive, or uncertain, respectively. RIS was calculated using the relative risk increase of 34.53% (mortality) and 105.02% (major bleeding) with a risk of type I error of 5%, at a power of 80%.
Results
Results of literature selection
Through a database search, we identified 47 studies, including 11 RCTs and 36 cohort studies. A detailed literature selection flowchart is shown in Figure 1. In total, 27,051 inpatients with COVID-19 were enrolled, of whom 16,774 underwent PA and 10,277 underwent I-TA. The baseline characteristics of the included patients are shown in Table 1. Among the studies, 23 were single center and 24 were multicenter. Thirteen studies enrolled critically ill patients only, 8 studies enrolled noncritically ill patients only, and 26 studies were unspecified. The characteristics of the included studies are shown in Table 2.
Figure 1.
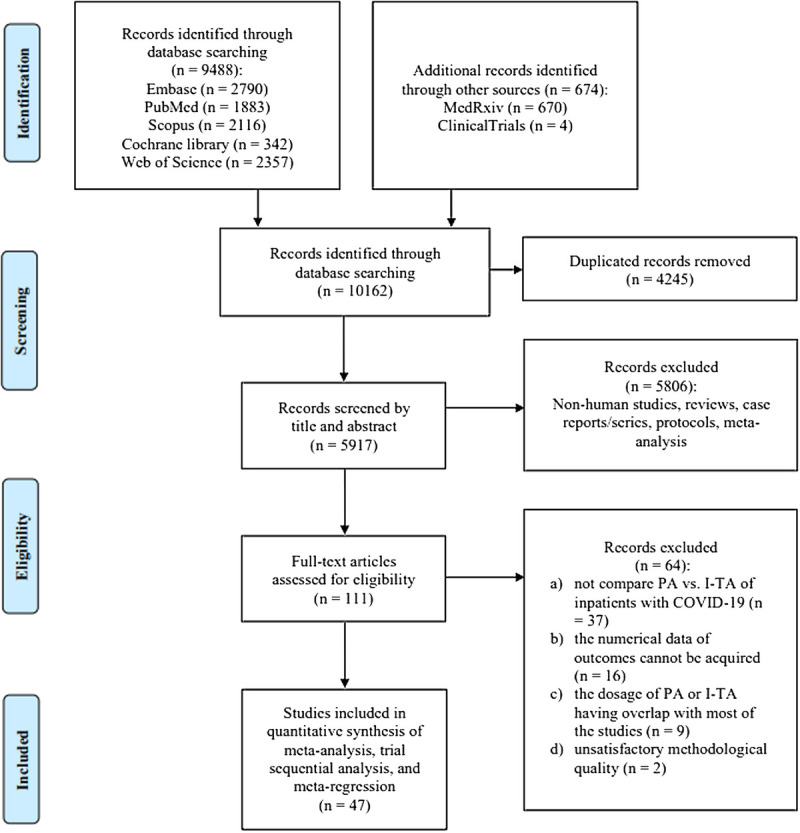
Flowchart of literature selection process. I-TA, intermediate-to-therapeutic anticoagulation; PA, prophylactic anticoagulation.
Table 1.
Baseline Characteristics of Patients With COVID-19 Included in the Meta-analysis
| Baseline Characteristics | I-TA (n = 10,277) | PA (n = 16,774) | P |
|---|---|---|---|
| Age, mean ± SD | 62.84 ± 15.30 | 62.80 ± 15.99 | 0.88 |
| Male, n (%) | 5583/9009 (61.97%) | 8321/14,430 (57.66%) | <0.0001 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 2699/8248 (32.72%) | 3972/13,286 (29.90%) | <0.0001 |
| Cardiovascular disease | 1200/7038 (17.05%) | 1659/10,791 (15.37%) | 0.0029 |
| Hypertension | 3418/6849 (49.91%) | 5322/11,041 (48.20%) | 0.03 |
| Chronic kidney disease | 575/5565 (10.33%) | 1031/9333 (11.05%) | 0.17 |
| Smoker | 862/4508 (19.12%) | 1763/9035 (19.51%) | 0.59 |
| Heart failure | 665/4049 (16.42%) | 643/8270 (7.78%) | <0.0001 |
| Liver disease | 49/3246 (1.51%) | 101/5655 (1.79%) | 0.33 |
| Respiratory disease | 910/7028 (12.95%) | 1011/8109 (12.47%) | 0.38 |
| Cancer | 403/5271 (7.65%) | 764/9696 (7.88%) | 0.61 |
I-TA, intermediate-to-therapeutic anticoagulation; PA, prophylactic anticoagulation; SD, standard deviation.
Table 2.
Characteristics of the Studies Included in the Meta-analysis
| Authors | Study Type | Setting | Severity | Sample Size | Drugs | Dosages | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| I-TA | PA | I-TA | PA | I-TA | PA | |||||
| 1 | Lawler et al[23] | RCT | Multicenter | Noncritical | 1181 | 1050 | Enoxaparin Dalteparin Tinzaparin Heparin |
Enoxaparin Dalteparin Tinzaparin Fondaparinux Heparin |
CrCl ≥ 30 BMI < 40 Enoxaparin, 1 mg/kg sc bid or 1.5 mg/kg sc qd Dalteparin, 200 U/kg sc qd or 100 U/kg sc bid Tinzaparin, 175 U/kg sc qd Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl ≥ 30 BMI < 40 Enoxaparin, 40 mg sc qd Dalteparin, 5000 U sc qd Tinzaparin, 4500 U sc qd Fondaparinux, 2.5 mg sc qd Heparin, 5000 U sc q8–12h |
| CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 1 mg/kg sc bid Dalteparin, 100 U/kg sc bid Tinzaparin, 175 U/kg sc qd Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 40 mg sc bid Dalteparin, 5000 U sc bid Tinzaparin, 9000 U sc qd Heparin, 7500 U sc tid |
|||||||||
| CrCl < 30 Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl < 30 BMI < 40 Heparin, 5000 U sc q8–12h CrCl < 30 BMI ≥ 40 Heparin, 7500 U sc tid |
|||||||||
| 2 | Goligher et al[24] | RCT | Multicenter | Critical | 536 | 567 | Enoxaparin Dalteparin Tinzaparin Heparin |
Enoxaparin Dalteparin Tinzaparin Fondaparinux Heparin |
CrCl ≥ 30 BMI < 40 Enoxaparin, 1 mg/kg sc bid or 1.5 mg/kg sc qd Dalteparin, 200 U/kg sc qd or 100 U/kg sc bid Tinzaparin, 175 U/kg sc qd Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl ≥ 30 BMI < 40 Enoxaparin, 40 mg sc qd Dalteparin, 5000 U sc qd Tinzaparin, 4500 U sc qd Fondaparinux, 2.5 mg sc qd Heparin, 5000 U sc q8–12h |
| CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 1 mg/kg sc bid Dalteparin, 100 U/kg sc bid Tinzaparin, 175 U/kg sc qd Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 40 mg sc bid Dalteparin, 5000 U sc bid Tinzaparin, 9000 U sc qd Heparin, 7500 U sc tid |
|||||||||
| CrCl < 30 Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl < 30 BMI < 40 Heparin, 5000 U sc q8–12h CrCl < 30 BMI ≥ 40 Heparin, 7500 U sc tid |
|||||||||
| 3 | Lopes et al[19] | RCT | Multicenter | Noncritical | 311 | 304 | Rivaroxaban Enoxaparin UFH |
Enoxaparin Fondaparinux UFH |
Stable patients: 30 ≤ CrCl < 49 Rivaroxaban, 15 mg qd Unstable patients CrCl ≥ 30 BMI < 40 Enoxaparin, 1 mg/kg sc bid or 1.5 mg/kg sc qd In patients ≥ 75 y: Enoxaparin, 0.75 mg/kg sc 12/12 h UFH, 60 unit/kg iv bolus, then 12 U/kg/h and titrate for anti-Xa 0.3–0.7 IU/mL or corresponding target value of aPTT |
CrCl ≥ 30 BMI < 40 Enoxaparin, 40 mg sc qd Fondaparinux, 2.5 mg sc qd UFH, 5000 U sc q8–12h |
| Unstable patients CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 1 mg/kg sc bid UFH, 60 U/kg iv bolus, then 12 U/kg/h and titrate for anti-Xa 0.3–0.7 IU/mL or corresponding target value of aPTT |
CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 60 mg sc qd UFH, 7500 U sc q8–12h |
|||||||||
| Stable patients CrCl < 30 Rivaroxaban, 20 mg qd Unstable patients CrCl < 30 BMI < 40 Enoxaparin, 1 mg/kg sc qd UFH, 60 U/kg iv bolus, then 12 U/kg/h and titrate for anti-Xa 0.3–0.7 IU/mL or corresponding target value of aPTT |
CrCl < 30 BMI < 40 UFH, 5000 U sc q8–12h CrCl < 30 BMI ≥ 40 UFH, 7500 U sc q8–12h |
|||||||||
| 4 | Sadegh-ipour et al[25] | RCT | Multicenter | Critical | 276 | 286 | Enoxaparin | Enoxaparin | Intermediate dose 1 mg/kg qd |
40 mg qd |
| 5 | Sholzberg et al[26] | RCT | Multicenter | Moderate | 228 | 237 | Enoxaparin Dalteparin Tinzaparin Heparin |
Enoxaparin Dalteparin Tinzaparin Fondaparinux Heparin UFH |
CrCl ≥ 30 BMI < 40 Enoxaparin, 1 mg/kg sc bid or 1.5 mg/kg sc qd Dalteparin, 200 U/kg sc qd or 100 IU/kg sc bid Tinzaparin, 175 U/kg sc qd Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl ≥ 30 BMI < 40 Enoxaparin, 40 mg sc qd Dalteparin, 5000 U sc qd Tinzaparin, 4500 U sc qd Fondaparinux, 2.5 mg sc qd Heparin, 5000 U sc q8–12h |
| CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 1 mg/kg sc bid Dalteparin, 100 U/kg sc bid Tinzaparin, 175 IU/kg sc qd Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl ≥ 30 BMI ≥ 40 Enoxaparin, 40 mg sc bid Dalteparin, 5000 U sc bid Tinzaparin, 9000 U sc qd UFH, 7500 U sc tid |
|||||||||
| CrCl < 30 Heparin, iv bolus with continuous infusion to titrate to anti-Xa 0.3–0.7 IU/mL or corresponding aPTT values |
CrCl < 30 BMI < 40 Heparin, 5000 U sc q8–12h CrCl < 30 BMI ≥ 40 Heparin, 7500 U sc tid |
|||||||||
| 6 | Morici et al[27] | RCT | Multicenter | All | 91 | 92 | Enoxaparin | Enoxaparin | 40 mg sc bid | 40 mg sc qd |
| 7 | Perepu et al[20] | RCT | Multicenter | Critical | 87 | 86 | Enoxaparin | Enoxaparin | Intermediate dose BMI was < 30 1 mg/kg sc qd BMI was ≥ 30 0.5 mg/kg sc bid |
BMI < 30 40 mg sc qd BMI ≥ 30 30 or 40 mg sc bid |
| 8 | Oliynyk et al[28] | RCT | Single center | Critical | 84 | 42 | LMWH-Enoxaparin | LMWH-Enoxaparin | 80 or 100 anti-Xa IU/kg sc qd | 50 anti-Xa IU/kg sc qd |
| 9 | Varona et al[29] | RCT | Multicenter | Noncritical | 38 | 38 | Bemiparin | Bemiparin | 115 IU/kg sc qd | 3500 IU sc qd |
| 10 | Marcos-Jubilar et al[30] | RCT | Multicenter | Noncritical | 32 | 33 | Bemiparin | Bemiparin | 115 IU/kg sc qd Weight 50–70 kg 7500 IU sc qd Weight 70–100 kg 10,000 IU sc qd Weight > 100 kg 12,500 IU sc qd |
3500 IU sc qd |
| 11 | Lemos et al[31] | RCT | Single center | All | 10 | 10 | UFH Enoxaparin |
UFH Enoxaparin |
CrCl > 50 Enoxaparin, 1 mg/kg bid (younger than 75 y) or 0.75 mg/kg bid (older than 75 y) 30 < CrCl ≤ 50 Enoxaparin, 0.75 mg/kg bid (younger than 75 y) or 1 mg/kg qd (older than 75 y) |
Weight < 120 kg UFH, 5000 IU sc tid Enoxaparin, 40 mg qd |
| 10 ≤ CrCl < 30 Enoxaparin, 1 mg/kg qd (younger than 75 y) or 0.75 mg/kg qd (older than 75 y) CrCl < 10 UFH, sc qd after the last dose of enoxaparin, which was adjusted according to the aPTT aiming at a ratio between 1.5 and 2.0 |
Weight > 120 kg UFH, 7500 IU sc tid Enoxaparin, 40 mg bid |
|||||||||
| 12 | Ionescu et al[32] | Cohort study | Multicenter | All | 998 | 2121 | UFH Enoxaparin Fondaparinux Oral anticoagulants |
UFH Enoxaparin, Fondaparinux |
UFH, with at least 1 documented aPTT in the anticoagulation range (≥45 s) Enoxaparin, 1 mg/kg sc bid or 1.5 mg/kg sc qd Fondaparinux, 5–10 mg qd |
UFH, 5000 U sc bid or tid Enoxaparin, 30–40 mg sc qd Fondaparinux, 2.5 mg sc qd |
| 13 | Nadkarni et al[17] | Cohort study | Multicenter | All | 900 | 1959 | Enoxaparin Apixaban Bivalirudin Argatroban UFH Rivaroxaban dabigatran |
UFH Enoxaparin Apixaban |
Enoxaparin, 1 mg/kg iv bid or 1.5 mg/kg iv qd Apixaban, 5 mg iv bid Bivalirudin, argatroban, UFH, rivaroxaban, or dabigatran Patients >75 y Apixaban, 2.5 mg iv bid or 5 mg iv qd |
UFH, Enoxaparin Patients ≤ 75 y Apixaban, 2.5 mg sc bid or 5 mg sc qd |
| 14 | Meizlish et al[33] | Cohort study | Multicenter | All | 760 | 1395 | Enoxaparin UFH Bivalirudin |
Enoxaparin UFH |
Intermediate dose BMI < 40 Enoxaparin, 0.4–0.7 mg/kg sc bid UFH, 7500 U sc at any frequency Therapeutic dose Enoxaparin, ≥0.7 mg/kg sc bid or ≥1.4 mg/kg qd UFH or bivalirudin CrCl < 30 Enoxaparin, ≥0.7 mg/kg sc qd |
Enoxaparin, 30–40 mg sc qd or <0.7 mg/kg sc qd or <0.4 mg/kg sc bid BMI ≥ 40 UFH, 5000 or 7500 U sc tid |
| 15 | Almohareb et al[34] | Cohort study | Multicenter | All | 711 | 711 | Enoxaparin | Enoxaparin | 40 or 60 or 80 or 120 mg sc bid | 40 mg sc qd |
| 16 | Vaughn et al[35] | Cohort study | Multicenter | All | 219 | 970 | NM | LMWH UFH |
NM | LMWH, 30–40 mg sc qd or bid UFH |
| 17 | Smadja et al[36] | Cohort study | Multicenter | All | 261 | 783 | LMWH UFH |
LMWH UFH |
NM | NM |
| 18 | Kaur et al[37] | Cohort study | Multicenter | All | 381 | 652 | LMWH UFH DOAC warfarin |
NM | NM | NM |
| 19 | Albani et al[38] | Cohort study | Single center | All | 312 | 487 | Enoxaparin | Enoxaparin | Enoxaparin, >40 mg qd | Enoxaparin, <40 mg qd |
| 20 | Kuno et al[39] | Cohort study | Single center | All | 383 | 383 | NM | NM | NM | NM |
| 21 | Lavinio et al[40] | Cohort study | Multicenter | Critical | 274 | 435 | Enoxaparin | NM | Enoxaparin, 100–200 IU/kg sc qd | NM |
| 22 | Kodama et al[41] | Cohort study | Single center | Noncritical | 82 | 498 | NM | NM | NM | NM |
| 23 | Gonzalez-Porras et al[42] | Cohort study | Single center | All | 120 | 410 | Bemiparin Enoxaparin |
Bemiparin Enoxaparin |
Bemiparin, 115 anti-Xa IU/kg sc qd Enoxaparin, 1 mg/kg sc bid |
Bemiparin, 3500 IU sc qd Enoxaparin, 40 mg sc qd |
| 24 | Hsu et al[43] | Cohort study | Single center | All | 64 | 377 | LMWH UFH Apixaban Rivaroxaban |
LMWH UFH Apixaban |
LMWH, 40 mg sc bid or 1 mg/kg sc bid UFH, 7500 U sc tid Apixaban, 5 mg sc bid Rivaroxaban, 20 mg sc qd |
LMWH, 40 mg sc qd UFH, 5000 U sc tid Apixaban, 2.5 mg sc bid |
| 25 | Millet et al[44] | Cohort study | Single center | All | 225 | 215 | NM | NM | NM | NM |
| 26 | Mennuni et al[45] | Cohort study | Multicenter | All | 149 | 287 | Enoxaparin | Enoxaparin | >4000 IU qd | 4000 IU qd |
| 27 | Lynn et al[46] | Cohort study | Single center | All | 152 | 250 | Enoxaparin | NM | 1 mg/kg sc bid or 1.5 mg/kg sc qd | NM |
| 28 | Motta et al[47] | Cohort study | Multicenter | All | 75 | 299 | Enoxaparin Heparin |
Enoxaparin Heparin |
Enoxaparin, 1 mg/kg sc bid or 1.5 mg/kg sc qd or based on renal function, or higher doses titrated to antifactor Xa range of 0.6–1 IU/mL bid and 1–2 IU/mL qd Heparin, titrated to an aPTT between 70 and 110 s |
Enoxaparin, 30 or 40 mg sc qd Heparin, 5000 U sc tid |
| 29 | Aljuhani et al[48] | Cohort study | Multicenter | Critical | 176 | 176 | UFH Enoxaparin |
UFH Enoxaparin |
Enoxaparin, 1 mg/kg sc bid or 1.5 mg/kg sc qd | Enoxaparin, 40 mg sc qd UFH, 5000 U sc tid |
| 30 | Yu et al[49] | Cohort study | Single center | Moderate, severe, critical |
133 | 215 | Enoxaparin Apixaban Fondaparinux UFH |
NM | Enoxaparin, 1 mg/kg bid Apixaban, ≥5 mg bid Fondaparinux, ≥5 mg qd |
NM |
| 31 | Pesavento et al[50] | Cohort study | Single center | Noncritical | 84 | 240 | NM | Enoxaparin Fondaparinux UFH |
NM | Enoxaparin, 4000 U sc qd Fondaparinux, 2.5 mg sc qd UFH, 15,000 U sc qd |
| 32 | Musoke et al[51] | Cohort study | Single center | All | 102 | 178 | LMWH | LMWH Heparin |
1 mg/kg bid | Heparin, 5000 U sc bid tid LMWH, 30–40 mg qd |
| 33 | Martinelli et al[52] | Cohort study | Single center | All | 127 | 151 | Enoxaparin | Enoxaparin | ICU patients 1 mg/kg bid High intensity of care wards patients 0.7 mg/kg bid Low intensity of care wards patients 1 mg/kg qd |
40 mg qd |
| 34 | Gabara et al[53] | Cohort study | Single center | Critical | 123 | 78 | Enoxaparin Dalteparin Tinzaparin Bemiparin |
Enoxaparin Dalteparin Tinzaparin Bemiparin |
CrCl > 30 Intermediate dose Enoxaparin, if > 80 kg: 60 mg sc qd; other condition: 1 mg/kg sc qd Fondaparinux, 5 mg sc qd Tinzaparin, if > 90 kg: 50 IU/kg sc qd; other condition: 75 IU/kg sc qd Bemiparin, 5000 IU sc qd Therapeutic dose Enoxaparin, 1.5 mg/kg sc qd or 1 mg/kg sc bid Fondaparinux, <50 kg: 5 mg sc qd; 51–100 kg: 7.5 mg sc qd; >100 kg: 10 mg sc qd Tinzaparin, 175 IU/kg sc qd Bemiparin, 115 IU/kg sc qd |
CrCl > 30 Enoxaparin, 40 mg sc qd Fondaparinux, 2.5 mg sc qd Tinzaparin, 4500 IU sc qd Bemiparin, 3500 IU sc qd |
| CrCl < 30 Intermediate dose Enoxaparin, if >80 kg: 40 mg sc qd; other condition: 0.5 mg/kg sc qd Fondaparinux, 2.5 mg sc qd Tinzaparin, if >90 kg: 50 IU/kg sc qd; other condition: 75 IU/kg sc qd Bemiparin, 3500 IU sc qd Therapeutic dose Enoxaparin, 1 mg/kg sc qd Tinzaparin, 175 IU/kg sc qd Bemiparin, 85 IU/kg sc qd |
CrCl < 30 Enoxaparin, 20 mg sc qd Fondaparinux, 1.5 mg sc qd Tinzaparin, 4500 IU sc qd Bemiparin, 2500 IU sc qd |
|||||||||
| 35 | Helms et al[54] | Cohort study | Multicenter | Critical | 71 | 108 | LMWH UFH |
LMWH UFH |
LMWH, 100 IU/kg sc bid CrCl < 30 mL/min LMWH, without exceeding 10,000 IU bid UFH, 500 IU/kg qd |
Obese patients LMWH, up to 6000 IU sc bid CrCl < 30 mL/min UFH, 200 IU/kg qd |
| 36 | Shah et al[55] | Cohort study | Multicenter | Critical | 27 | 151 | LMWH UFH |
LMWH UFH |
NM | NM |
| 37 | Jonmarker et al[56] | Cohort study | Single center | Critical | 85 | 67 | Dalteparin Tinzaparin |
Dalteparin Tinzaparin |
Tinzaparin, >4500 IU qd Dalteparin, >5000 IU qd | Tinzaparin, 2500–4500 IU qd Dalteparin, 2500–5000 IU qd |
| 38 | Daughety et al[57] | Cohort study | Single center | All | 27 | 99 | Enoxaparin Heparin |
Enoxaparin UFH |
Enoxaparin, 1 mg/kg bid Heparin, infusion titrated to antifactor Xa levels 0.5–0.7 IU/mL in patients with renal failure |
Weight < 100 kg Enoxaparin, 40 mg qd Weight > 100 kg Enoxaparin, 60 mg qd Patients with renal failure UFH, 5000 IU tid |
| 39 | Copur et al[58] | Cohort study | Single center | All | 46 | 69 | LMWH | LMWH | 1 mg/kg sc bid | 40 mg sc qd |
| 40 | Moll et al[59] | Cohort study | Multicenter | Critical | 47 | 47 | Enoxaparin UFH |
Enoxaparin UFH |
Intermediate dose Enoxaparin, 40 mg bid Extremes of weight Enoxaparin, 0.5 mg/kg bid UFH, 7500 IU tid |
Enoxaparin, 40 mg qd UFH, 5000 IU bid tid |
| 41 | Voicu et al[60] | Cohort study | Single center | Critical | 43 | 50 | Enoxaparin UFH |
Enoxaparin UFH |
Enoxaparin, 40 mg sc bid or 1 mg/kg sc bid | Enoxaparin, 40 mg sc qd CrCl < 15 UFH, 15,000 IU sc qd |
| 42 | Matli et al[61] | Cohort study | Single center | All | 31 | 51 | LMWH UFH Fondaparinux DOAC |
LMWH UFH Fondaparinux DOAC |
NM | NM |
| 43 | Poulakou et al[62] | Cohort study | Multicenter | All | 54 | 26 | LMWH | LMWH | NM | NM |
| 44 | Longhitano et al[63] | Cohort study | Single center | All | 47 | 27 | Enoxaparin UFH |
Enoxaparin UFH |
Enoxaparin, 80 U/kg sc qd to 100 U/kg sc bid UFH, 5000 U sc q8h to 12,500 U sc q8–12h |
Enoxaparin, 80 U/kg sc qd UFH, 5000 U sc q8h |
| 45 | Nadeem et al[64] | Cohort study | Single center | Critical | 40 | 34 | NM | NM | NM | NM |
| 46 | Elmelhat et al[65] | Cohort study | Single center | Noncritical | 39 | 20 | Enoxaparin | Enoxaparin | 1 mg/kg bid | 40 mg qd |
| 47 | Zalivansky et al[66] | Cohort study | Single center | All | 35 | 10 | Enoxaparin | Enoxaparin | 0.5 mg/kg sc bid or 1 mg/kg sc qd/bid or 1.5 mg/kg qd | 40 mg sc qd |
aPTT, activated partial thermoplastic time; bid, twice a day; BMI, body mass index; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; ICU, intensive care unit; I-TA, intermediate-to-therapeutic anticoagulation; IU, international unit; IV, intravenous; LMWH, low molecular weight heparin; NM, not mentioned; PA, prophylactic anticoagulation; qd, once a day; RCT, randomized controlled trial; sc, subcutaneous; tid, 3 times a day; UFH, counteraction heparin.
Mortality
Mortality was reported in 43 studies with 15,523 patients and 9562 patients treated with PA and I-TA, respectively. Meta-analysis of all 43 studies showed that patients with COVID-19 who received I-TA exhibited significantly higher mortality than patients who received PA (24.82% vs 18.45%; OR: 1.38; 95% CI: 1.15–1.66, P = 0.0005; Fig. 2A).
Figure 2.
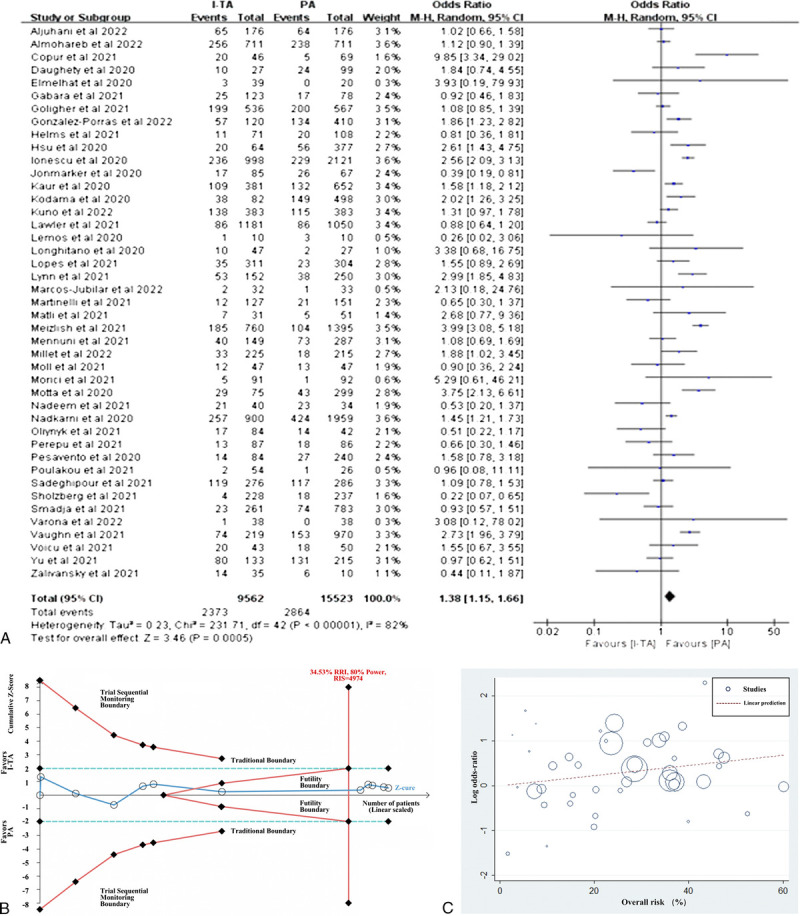
Efficacy of intermediate-to-therapeutic versus prophylactic anticoagulation on mortality in patients with COVID-19. (A) Pooled OR and forest plot of mortality. Forty-three studies were included in the statistical analysis, with 9562 patients in the I-TA and 15,523 in the PA groups. The results showed that the mortality significantly decreased in the PA group compared with I-TA group (OR: 1.38; 95% CI: 1.15–1.66; P = 0.0005). (B) Trial sequential analysis of mortality. The X-axis represents sample size, and the Y-axis represents Z score. The uppermost and lowermost red curves represent trial sequential monitoring boundary lines for positive conclusion. The horizontal blue lines represent the conventional boundaries for statistical significance. The red triangular lines represent the futility boundary. The dark blue line is the Z curve representing the cumulative Z scores of included studies, arranged according to publication date. The RIS of 4974 was calculated using α = 0.05 (2-sided), β = 0.20 (power 80%), and the relative risk of mortality increase was 34.53%. The results showed that the cumulative Z curve exceeded the RIS line, the final cumulative Z score located in the zone between futility boundaries, and the TSA adjusted the 95% CI to be 0.71 to 1.26. (C) Meta-regression of the association between log OR for mortality and overall risk (%). CI, confidence interval; I-TA, intermediate-to-therapeutic anticoagulation; OR, odds ratio; PA, prophylactic anticoagulation; RIS, required information size; TSA, trial sequential analysis.
To investigate RCTs and real-world studies separately, we performed a subgroup analysis. When only RCTs were included, the results showed that mortality was comparable between the I-TA and PA groups (16.77% vs 17.52%; OR: 0.93; 95% CI: 0.71–1.22; P = 0.61; Fig. 4). TSA was performed for 11 RCTs to adjust the results. During TSA, 1 study was excluded because of the small sample size. The cumulative Z curve exceeded the RIS line and was situated within the region of futility boundaries, confirming the negative result from the meta-analysis (TSA adjusted CI, 0.71–1.26), as shown in Figure 2B. However, in real-world studies, pooled mortality was significantly lower in patients receiving I-TA than in those treated with PA (Fig. 4). We assume that in real-world practice, physicians might be prone to prescribe I-TA to patients with more serious conditions.
Figure 4.
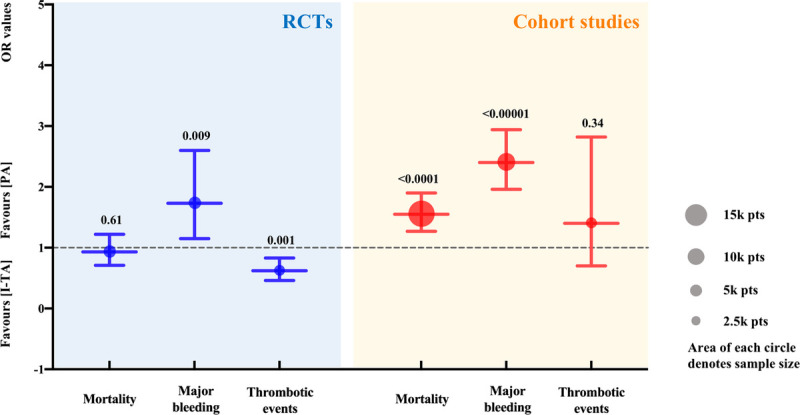
Subgroup analysis according to RCTs or cohort studies. The dashed line represents the null line (OR: 1). The blue and red lines and circles exhibit the results of RCTs and cohort studies, respectively. The size (area) of each circle denotes sample size. The lower and upper limits of the lines correspond to the 95% CI, the nodes in the middle represent pooled OR values, and the numbers on the top of the upper limits represent P values. CI, confidence interval; OR, odds ratio; pts, patients; RCTs, randomized controlled trials.
Regarding disease severity, we conducted a subgroup analysis of critically ill and noncritically ill patients. The mortality was similar between I-TA and PA groups in both critically ill and noncritically ill patients (Fig. 5A). When only RCTs were selected, there was no significant difference in mortality between the 2 treatment regimens in both critically ill and noncritically ill patients (Fig. 5B). This finding was supported by meta-regression of all studies, which suggested that there was no relationship between the treatment effect and overall risk of mortality (P = 0.14; Fig. 2C).
Figure 5.
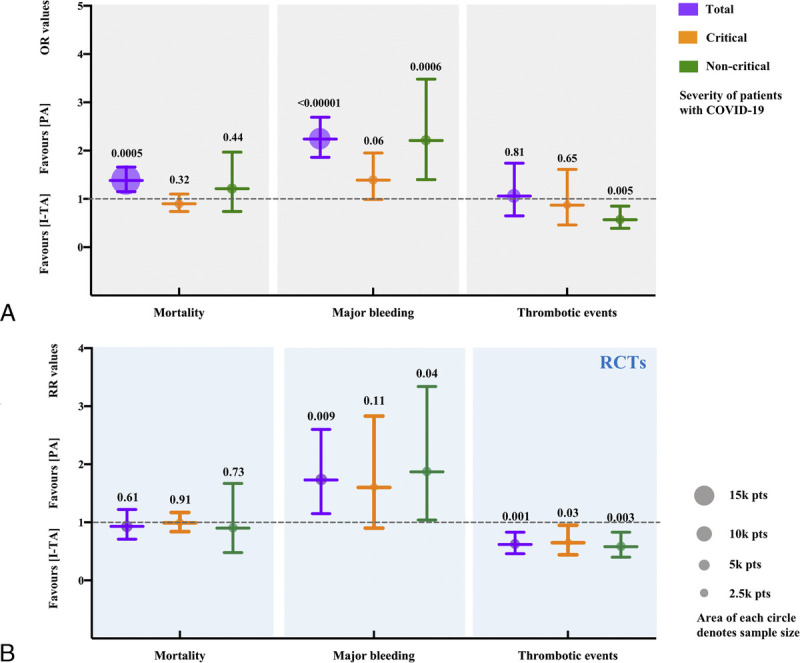
Subgroup analysis according to severity of patients. (A) Results in all types of studies. (B) Results in RCTs only. The dashed line represents the null line (OR/RR: 1). The purple, orange, and green lines as well as circles exhibit the results of total, critical, and noncritical groups, respectively. The area of circles denotes sample sizes. The lower and upper limits of the lines correspond to the 95% CI, the nodes in the middle represent pooled OR or RR values, and the numbers on top represent P values. CI, confidence interval; OR, odds ratio; pts, patients; RCTs, randomized controlled trials; RR, risk ratio.
Major bleeding
Pooled results from 29 studies documenting major bleeding illustrated that I-TA significantly increased the rate of major bleeding compared with PA in patients with COVID-19 (4.49% vs 2.19%; OR: 2.24; 95% CI: 1.86–2.69; P < 0.00001). Further meta-analysis of RCTs confirmed this conclusion, after adjusting for TSA (2.29% vs 1.37%; OR: 1.73; 95% CI: 1.15–2.60; P = 0.009; TSA adjusted CI, 1.09–2.58). TSA showed that the cumulative Z curve exceeded the RIS line and the trial sequential monitoring boundary, confirming that I-TA has a disadvantage due to the increased major bleeding rate (Figs. 3A, B).
Figure 3.
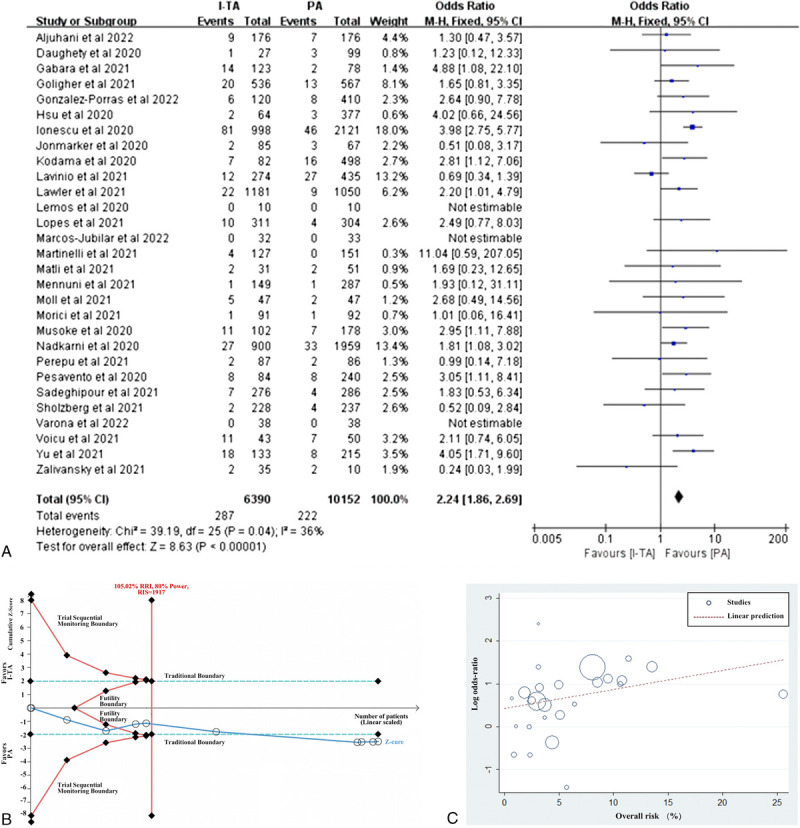
Effect of intermediate-to-therapeutic versus prophylactic anticoagulation on risk of major bleeding in patients with COVID-19. (A) Pooled OR and forest plot of major bleeding. Twenty-nine studies that reported major bleeding were included, with 6390 patients in the I-TA group and 10,152 in the PA group. The results showed that I-TA significantly increased the incidence of major bleeding compared with PA (OR: 2.24; 95% CI: 1.86–2.69; P < 0.00001). (B) Trial sequential analysis of major bleeding. The uppermost and lowermost red curves represent trial sequential monitoring boundary lines for positive conclusion, the red triangle zone represents futility. The vertical red line represents the RIS of 1917, which was calculated using α = 0.05 (2-sided), β = 0.20 (power 80%), and 105.02% of the relative risk of major bleeding increase. The horizontal blue lines represent the traditional boundaries for statistical significance. The cumulative Z curve represents the data of included studies, which were arranged based on publication date. The cumulative Z curve exceeded the line RIS and the conventional boundary. The TSA adjusted the traditional 95% CI to be 1.09 to 2.58. (C) Meta-regression of the association between log OR for major bleeding and overall risk (%). CI, confidence interval; COVID-19, coronavirus disease 2019; I-TA, intermediate-to-therapeutic anticoagulation; OR, odds ratio; PA, prophylactic anticoagulation; RIS, required information size; TSA, trial sequential analysis.
In subgroup analysis based on the type of study or the severity of patients, major bleeding rate showed the same trend, which was also supported by meta-regression (P = 0.09; Fig. 3C). In a small subgroup of critically ill patients in all studies or RCTs only, I-TA tended to increase the rate of major bleeding, but it did not reach statistical significance, compared with PA.
Thrombotic events
Sixteen studies that reported thrombotic events were included, with 3546 patients in the I-TA and 4623 in the PA groups, respectively. The results showed that there were no significant differences in the rates of thrombotic events, pulmonary embolism, myocardial infarction, stroke, or venous thromboembolism between the I-TA and PA groups (Fig. 6). The results from the RCT subgroup supported the idea that I-TA could reduce the incidence of thrombotic events (Fig. 4). Regarding disease severity, noncritically ill patients might benefit from I-TA with a reduced rate of thrombotic events compared with PA (Fig. 5A). RCTs supported the beneficial effect of I-TA in decreasing thrombotic events in both the critically ill and noncritically ill groups (Fig. 5B).
Figure 6.
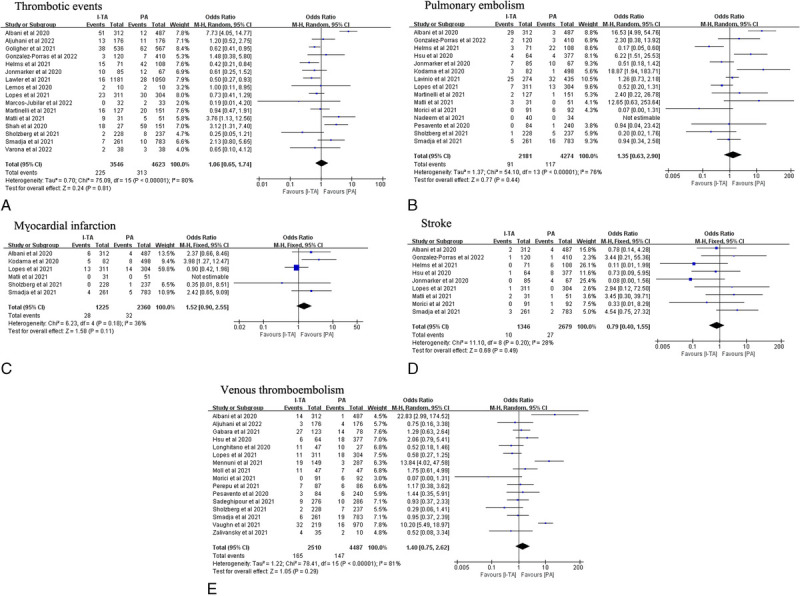
Pooled OR and forest plot of thrombotic events, pulmonary embolism, myocardial infarction, stroke, and venous thromboembolism between intermediate-to-therapeutic versus prophylactic anticoagulation among patients with COVID-19. (A–E) No significant differences on the incidence of thrombotic events (OR: 1.06; 95% CI: 0.65–1.74; P = 0.81), pulmonary embolism (OR: 1.35; 95% CI: 0.63–2.90; P = 0.44), myocardial infarction (OR: 1.52; 95% CI: 0.90–2.55; P = 0.11), stroke (OR: 0.79; 95% CI: 0.40–1.55; P = 0.49), or venous thromboembolism (OR: 1.40; 95% CI: 0.75–2.62; P = 0.29) was found between the 2 groups. CI, confidence interval; OR, odds ratio.
Quality assessment
We used the Cochrane risk-of-bias tool and NOS to assess the quality of RCTs and cohort studies, respectively (Fig. 7). The quality of the controlled studies included in the meta-analysis was satisfactory.
Figure 7.
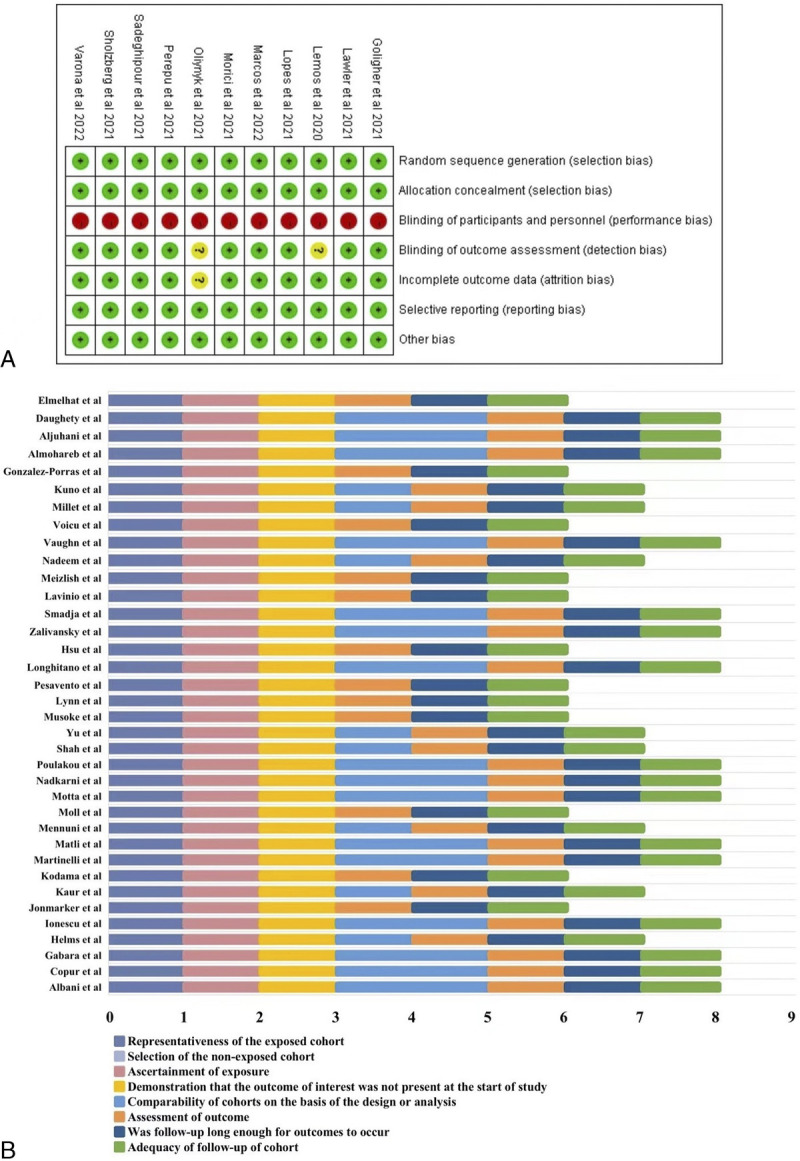
The quality assessment of included studies. (A) The quality assessment of randomized controlled trials by Cochrane risk-of-bias tool. (B) The quality assessment of cohort studies by Newcastle-Ottawa Scale.
Publication bias
Funnel plots were used to analyze the publication bias. Intuitively, the studies were distributed almost symmetrically on both sides (Fig. 8). Furthermore, the absence of publication bias was demonstrated by Begg’s and Egger’s tests (Begg’s test, P = 0.87; Egger’s test, P = 0.29).
Figure 8.
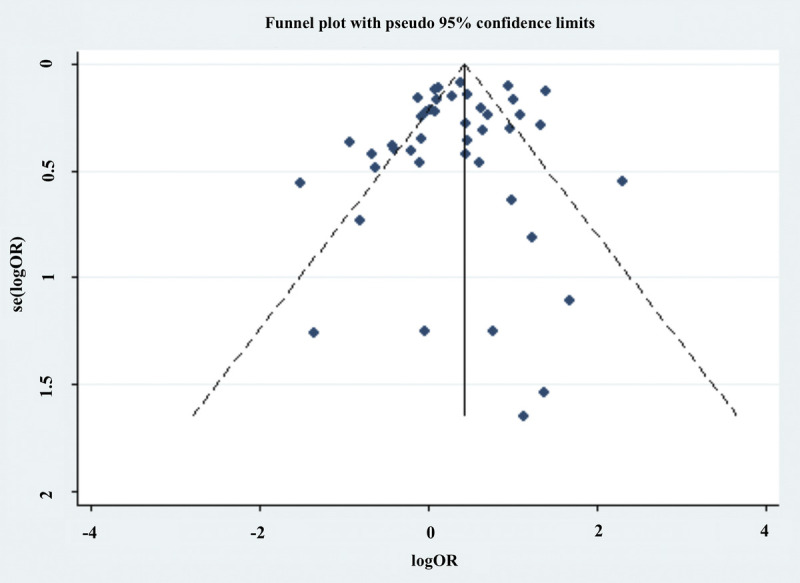
Funnel plot analysis of publication bias.
Grade recommendation
The overall evidence for each outcome of the RCTs and cohort studies was qualified using the GRADE framework. It showed that the certainties of evidence for the outcomes of mortality, major bleeding, and thrombotic events of the RCTs were “moderate.” In detail, we adjudicated the risk of bias as “serious” mainly because all RCTs were open labeled. We found no significant downgrade points for inconsistency, indirectness, imprecision, or publication bias. For cohort studies, the certainties of evidence for the outcomes of mortality, major bleeding, and thrombotic events were “low,” “moderate,” and “very low,” respectively. Specifically, the quality of evidence for major bleeding escalated because of the large effect value. Thrombotic events were downgraded because of “serious” imprecision with a large 95% CI. The GRADE tables are described in detail in Supplementary Table 5, http://links.lww.com/ECCM/A35.
Discussion
This meta-analysis included 47 clinical studies involving 27,051 patients with COVID-19. The results revealed that compared with patients with COVID-19 who received PA, the mortality of patients receiving I-TA was slightly higher. The major bleeding rate was remarkably higher in patients receiving I-TA than in those receiving PA. No statistical difference was found in the rates of thrombotic events, pulmonary embolism, myocardial infarction, stroke, or venous thromboembolism between the 2 treatment groups. These results indicate that PA is a better choice for patients with COVID-19. Subgroup analysis of RCTs showed that there was no significant difference in mortality between the 2 treatment groups, as proven by TSA, and the major bleeding rate was remarkably higher, which was also confirmed by TSA. Meanwhile, the incidence of thrombotic events was markedly lower in the I-TA group than in the PA group. In both critically ill and noncritically ill patients, I-TA failed to reduce mortality but increased the major bleeding rate compared with PA, which was also supported by meta-regression.
Similar to SARS[67] and H1N1,[68] thrombosis is a pathological feature of SARS-CoV-2 infection.[69] Several studies have demonstrated increased levels of coagulation biomarkers in patients with COVID-19,[70,71] the degree of which was positively correlated with disease severity and poor prognosis.[7,70–72] Anticoagulant administration in patients with COVID-19 has been confirmed to decrease mortality.[73] In clinical practice, commonly used anticoagulants include heparinoids (eg, unfractionated heparin and low molecular weight heparin [LMWH]), factor Xa inhibitors (eg, fondaparinux, rivaroxaban, and apixaban), direct thrombin inhibitors (eg, dabigatran and bivalirudin), and vitamin K antagonists (eg, warfarin). Heparin binds to antithrombin, causing a conformational change that accelerates the inactivation of IIa, IXa, Xa, XIa, and XIIa factors, thereby blocking the coagulation cascade and exerting an anticoagulant effect.[74] Compared with heparin, LMWH has more precise target inhibition, but less ability to inhibit other coagulation factors and lower anticoagulation speed.[75] Strikingly, LMWH not only exhibits an anticoagulant effect but also interferes with the binding of SARS-CoV-2 to the ACE2 receptor, thereby limiting viral infectivity and reducing mortality.[76] Oral anticoagulants had no antiviral effect, but patients with COVID-19 using oral anticoagulants also had a reduced risk of mortality compared with those without.[77] Anticoagulation might be an effective way to reduce thrombosis and subsequent organ damage in patients with COVID-19; however, the optimal dosage of anticoagulants remains debatable.[17,19,23,78]
Mortality was the primary outcome of this meta-analysis. The meta-analysis demonstrated significantly lower mortality in PA than I-TA group; however, the result was not supported by meta-analysis nor TSA of RCTs. Cohort studies inevitably have confounding factors, but we believe that including real-world cohort studies can provide more comprehensive information. To clarify whether the severity of disease contributes to the efficacy of I-TA and PA regimens, subgroup analysis and meta-regression were performed. This showed that in both critically ill and noncritically ill patients with COVID-19, the 2 anticoagulant regimens did not affect mortality. Subgroup analysis of RCTs showed a consistent result. Hence, increasing the dose of anticoagulants did not reduce mortality.
The major bleeding rate, as the safety outcome, agreed with common sense. Compared with PA, I-TA significantly increased the rate of major bleeding, which was also confirmed by TSA. Moreover, in the subgroup analysis of RCTs and cohort studies or of critically ill and noncritically ill patients, the same result was observed. However, among the critically ill patients in the subgroup of all studies or RCTs only, the major bleeding rate between groups was similar but was not statistically significant, which may be due to the small sample size.
For thrombotic events, the overall analysis did not find a distinct difference between the 2 regimens; however, subgroup analysis of the RCTs and noncritically ill patients supported the beneficial effect of I-TA in lowering the risk of thrombotic events. The delicate balance between anticoagulation strategies, bleeding, and thrombotic complications should be carefully considered. Critically ill patients with COVID-19, who are characterized by long-term immobilization, systemic inflammation, platelet activation, and endothelial dysfunction, are more likely to develop thromboembolism.[79,80] A retrospective analysis of 400 patients with COVID-19 showed that the incidence of thrombotic events was 4.7% (95% CI, 2.4–8.0) and 18.1% (95% CI, 12.1–25.3) in noncritically ill and critically ill patients, respectively. [81] However, our overall findings did not show that I-TA reduced thrombotic events compared with PA in critically ill patients. In addition to considering possible confounding factors in cohort studies, the overwhelming inflammatory response and concomitant thrombosis were too pronounced in critically ill patients to recover. Meanwhile, in noncritically ill patients, I-TA might sustain an appropriate balance, which may explain the above result.[82] In brief, this study suggests that I-TA is superior to PA in terms of reducing the rate of thrombotic events.
Our study had several limitations. One limitation is that cohort studies have a lower level of evidence than RCTs do. Although cohort studies inevitably have bias, they provide wider insights into real-world practice, especially during the COVID-19 pandemic. The other limitation is that we tried to perform more subgroup analyses, such as types of anticoagulants, but the related data were difficult to extract from studies.
This study has several strengths compared with similar studies. First, all eligible studies until April 26, 2022, were enrolled to yield the latest evidence on this topic. Second, this is the first meta-analysis to focus on the heterogeneity of the definitions of PA and I-TA among studies. To solve this problem, we checked the doses of anticoagulants in each study and excluded 9 studies to avoid dosage overlap between the 2 comparison groups. Third, to achieve a robust conclusion, we performed TSA and meta-regression, which are important for fully understanding the results. This is also the first study to use the TSA approach for this topic. Finally, we used the GRADE framework in addition to other assessment tools to evaluate the quality of the evidence. We believe that this meta-analysis, TSA, and meta-regression will provide valuable information for clinical practice and further research.
Conclusion
I-TA was not superior to PA in terms of reducing mortality but increased the risk of major bleeding. For patients with a high risk of thrombosis and low risk of bleeding, I-TA is appropriate. Further larger-scale RCTs are still needed.
Conflict of interest statement
Yuguo Chen is the Editor-in-Chief of Emergency and Critical Care Medicine, and Feng Xu is an Editorial Board member of Emergency and Critical Care Medicine. The article was subject to the journal's standard procedures, with peer review handled independently of the Editor-in-Chief, this Editorial Board member, and their research groups. The authors declare no conflict of interest.
Author contributions
Guo M and Xing J contributed to the literature research and data extraction. Guo M and Han Q contributed to quality evaluation. Cao S and Xue L helped with the literature search. De Y and Wang X helped with the data extraction. Hao P, Li C, Wang J, and Xu F provided valuable advice on these methods. Yuan Q, Pan C, Wang H, and Bian Y provided valuable advice for the manuscript writing. Guo M, Han Q, and Shan Z wrote the first version of the manuscript. Pang J and Chen Y contributed to study design and manuscript revision.
Funding
This study was supported by the National Key R&D Program of China (2020YFC0846600, 2020YFC1512700, 2020YFC1512705, 2020YFC1512703), National S&T Fundamental Resources Investigation Project (2018FY100600, 2018FY100602), Taishan Pandeng Scholar Program of Shandong Province (tspd20181220), Taishan Young Scholar Program of Shandong Province (tsqn20161065, tsqn201812129), Youth Top-Talent Project of National Ten Thousand Talents Plan, and Qilu Young Scholar Program.
Ethical approval of studies and informed consent
Not applicable.
Acknowledgements
None.
Footnotes
How to cite this article: Guo M, Han Q, Xing J, et al. The optimal anticoagulation strategy for COVID-19, prophylactic or therapeutic?: a metaanalysis, trial sequential analysis, and meta-regression of more than 27,000 participants. Emerg Crit Care Med. 2022;2(3):148–166. doi: 10.1097/EC9.0000000000000059
MG, QH, and JX contributed equally to this article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online: 16 September 2022
Contributor Information
Mingyue Guo, Email: mingyueguo@mail.sdu.edu.cn.
Qi Han, Email: hanqi2019@mail.sdu.edu.cn.
Jiaxuan Xing, Email: 202135960@mail.sdu.edu.cn.
Feng Xu, Email: xufengsdu@126.com.
Jiali Wang, Email: wangjiali_2000@126.com.
Chuanbao Li, Email: bao2460@126.com.
Zechen Shan, Email: 202135941@mail.sdu.edu.cn.
Yuan Bian, Email: bianyuan@sdu.edu.cn.
Hao Wang, Email: wanghao34@126.com.
Li Xue, Email: dream2005xl@126.com.
Qiuhuan Yuan, Email: xiaoqiu0707@aliyun.com.
Chang Pan, Email: panchang0517@163.com.
Yanshan De, Email: deysjy@163.com.
Xingfang Wang, Email: 202120825@mail.sdu.edu.cn.
Panpan Hao, Email: panda.how@126.com.
Shengchuan Cao, Email: caoshengchuan@126.com.
Jiaojiao Pang, Email: jiaojiaopang@email.sdu.edu.cn.
References
- 1.Pang J Xu F Aondio G, et al. Efficacy and tolerability of bevacizumab in patients with severe COVID-19. Nat Commun. 2021;12(1):814. doi: 10.1038/s41467-021-21085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han Q Guo M Zheng Y, et al. Current evidence of interleukin-6 signaling inhibitors in patients with COVID-19: a systematic review and meta-analysis. Front Pharmacol. 2020;11:615972. doi: 10.3389/fphar.2020.615972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Coronavirus disease (COVID-19) outbreak. Accessed November 15, 2021. https://covid19.who.int
- 4.Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10(9):200160. doi: 10.1098/rsob.200160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheblawi M Wang K Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–7144. doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan N, Eikelboom J. Hypercoagulability and thrombosis in COVID-19: a modifiable cause for mortality? Eur Heart J. 2021;42(33):3143–3145. doi: 10.1093/eurheartj/ehab417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F Yu T Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arachchillage DRJ, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(5):1233–1234. doi: 10.1111/jth.14820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miró Ò Jiménez S Mebazaa A, et al. Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur Heart J. 2021;42(33):3127–3142. doi: 10.1093/eurheartj/ehab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parra-Medina R, Herrera S, Mejia J. Systematic review of microthrombi in COVID-19 autopsies. Acta Haematol. 2021;144(5):476–483. doi: 10.1159/000515104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao XH Luo T Shi Y, et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31(8):836–846. doi: 10.1038/s41422-021-00523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C Wang Y Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giossi R Menichelli D Pani A, et al. A systematic review and a meta-analysis comparing prophylactic and therapeutic low molecular weight heparins for mortality reduction in 32,688 COVID-19 patients. Front Pharmacol. 2021;12:698008. doi: 10.3389/fphar.2021.698008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Living guidance for clinical management of COVID-19. Accessed November 23, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 [PubMed]
- 16.National Institutes of Health . Coronavirus disease 2019 (COVID-19) treatment guidelines. Accessed January 22, 2022. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 17.Nadkarni GN Lala A Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klok FA Kruip MJHA van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes RD de Barros E Silva PGM Furtado RHM, et al. ACTION Coalition COVID-19 Brazil IV Investigators . Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated d-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253–2263. doi: 10.1016/S0140-6736(21)01203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perepu US Chambers I Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021;19(9):2225–2234. doi: 10.1111/jth.15450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ McKenzie JE Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt GH Oxman AD Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawler PR Goligher EC Berger JS, et al. ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators . Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goligher EC Bradbury CA Mcverry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeghipour P Talasaz AH Rashidi F, et al. INSPIRATION Investigators . Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION Randomized Clinical Trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sholzberg M Tang GH Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morici N Podda G Birocchi S, et al. Enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients: the X-COVID-19 randomized trial. Eur J Clin Invest. 2022;52(5):e13735. doi: 10.1111/eci.13735 [DOI] [PubMed] [Google Scholar]
- 28.Oliynyk O Barg W Slifirczyk A, et al. Comparison of the effect of unfractionated heparin and enoxaparin sodium at different doses on the course of COVID-19–associated coagulopathy. Life (Basel). 2021;11(10):1032. doi: 10.3390/life11101032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varona JF, Núñez E, Fernández Félix BM, Castellano Vázquez JM, Cubillo A. Efficacy and safety of therapeutic vs. prophylactic bemiparin in noncritically ill patients with COVID-19 pneumonia. Eur J Intern Med. 2022;99:106–108. doi: 10.1016/j.ejim.2022.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcos-Jubilar M Carmona-Torre F Vidal R, et al. Therapeutic versus prophylactic bemiparin in hospitalized patients with nonsevere COVID-19 pneumonia (BEMICOP study): an open-label, multicenter, randomized, controlled trial. Thromb Haemost. 2022;122(2):295–299. doi: 10.1055/a-1667-7534 [DOI] [PubMed] [Google Scholar]
- 31.Lemos ACB do Espírito Santo DA Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ionescu F Jaiyesimi I Petrescu I, et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol. 2021;106(2):165–174. doi: 10.1111/ejh.13533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meizlish ML Goshua G Liu Y, et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am J Hematol. 2021;96(4):471–479. doi: 10.1002/ajh.26102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almohareb SN, Al Yami MS, Assiri AM, Almohammed OA. Impact of thromboprophylaxis intensity on patients' mortality among hospitalized patients with COVID-19: a propensity-score matched study. Clin Epidemiol. 2022;14:361–368. doi: 10.2147/CLEP.S359132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaughn VM Yost M Abshire C, et al. Trends in venous thromboembolism anticoagulation in patients hospitalized with COVID-19. JAMA Netw Open. 2021;4(6):e2111788. doi: 10.1001/jamanetworkopen.2021.11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smadja DM Bonnet G Gendron N, et al. Intermediate- vs. standard-dose prophylactic anticoagulation in patients with COVID-19 admitted in medical ward: a propensity score-matched cohort study. Front Med (Lausanne). 2021;8:747527. doi: 10.3389/fmed.2021.747527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur J, Sule AA, Koehler T, Krishnamoorthy G, Delongpre J. Anticoagulation management and outcomes in COVID-19 patients: a multi-center petrospective cohort study. Blood. 2020;136(suppl 1):34. doi: 10.1182/blood-2020-140997 [DOI] [Google Scholar]
- 38.Albani F Sepe L Fusina F, et al. Thromboprophylaxis with enoxaparin is associated with a lower death rate in patients hospitalized with SARS-CoV-2 infection. A cohort study. EClinicalMedicine. 2020;27:100562. doi: 10.1016/j.eclinm.2020.100562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuno T, So M, Takahashi M, Egorova NN. Prophylactic versus therapeutic anticoagulation for survival of patients with COVID-19 on steroid. J Thromb Thrombolysis. 2022;53(2):352–358. doi: 10.1007/s11239-021-02569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavinio A Ercole A Battaglini D, et al. Safety profile of enhanced thromboprophylaxis strategies for critically ill COVID-19 patients during the first wave of the pandemic: observational report from 28 European intensive care units. Crit Care. 2021;25(1):155. doi: 10.1186/s13054-021-03543-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodama R Kalsi A Singh A, et al. Outcomes in COVID-19 patients on treatment dose anti-coagulation compared to prophylactic dose anti-coagulation. Blood. 2020;136(suppl 1):40–41. doi: 10.1182/blood-2020-142552 [DOI] [Google Scholar]
- 42.Gonzalez-Porras JR Belhassen-Garcia M Lopez-Bernus A, et al. Low molecular weight heparin is useful in adult COVID-19 inpatients. Experience during the first Spanish wave: observational study. Sao Paulo Med J. 2022;140(1):123–133. doi: 10.1590/1516-3180.2021.0098.R1.08062021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu A, Liu Y, Zayac AS, Olszewski AJ, Reagan JL. Intensity of anticoagulation and survival in patients hospitalized with COVID-19 pneumonia. Thromb Res. 2020;196:375–378. doi: 10.1016/j.thromres.2020.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millet C, Narvaneni S, Shafeek F, Roman S, Mechineni A, Manickam R. Use of systemic anticoagulation in COVID-19: delving beyond theoretical hypothesis. Cureus. 2022;14(2):e22061. doi: 10.7759/cureus.22061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mennuni MG Renda G Grisafi L, et al. Clinical outcome with different doses of low-molecular-weight heparin in patients hospitalized for COVID-19. J Thromb Thrombolysis. 2021;52(3):782–790. doi: 10.1007/s11239-021-02401-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynn L Reyes JA Hawkins K, et al. The effect of anticoagulation on clinical outcomes in novel coronavirus (COVID-19) pneumonia in a U.S. cohort. Thromb Res. 2021;197:65–68. doi: 10.1016/j.thromres.2020.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motta JK Ogunnaike RO Shah R, et al. Clinical outcomes with the use of prophylactic versus therapeutic anticoagulation in coronavirus disease 2019. Crit Care Explor. 2020;2(12):e0309. doi: 10.1097/CCE.0000000000000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aljuhani O Al Sulaiman K Hafiz A, et al. Comparison between standard vs. escalated dose venous thromboembolism (VTE) prophylaxis in critically ill patients with COVID-19: a two centers, observational study. Saudi Pharm J. 2022;30(4):398–406. doi: 10.1016/j.jsps.2022.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu B Gutierrez VP Carlos A, et al. Empiric use of anticoagulation in hospitalized patients with COVID-19: a propensity score-matched study of risks and benefits. Biomark Res. 2021;9(1):29. doi: 10.1186/s40364-021-00283-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pesavento R Ceccato D Pasquetto G, et al. The hazard of (sub)therapeutic doses of anticoagulants in non-critically ill patients with COVID-19: the Padua province experience. J Thromb Haemost. 2020;18(10):2629–2635. doi: 10.1111/jth.15022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musoke N Lo KB Albano J, et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. 2020;196:227–230. doi: 10.1016/j.thromres.2020.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinelli I Ciavarella A Abbattista M, et al. Increasing dosages of low-molecular-weight heparin in hospitalized patients with COVID-19. Intern Emerg Med. 2021;16(5):1223–1229. doi: 10.1007/s11739-020-02585-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gabara C Solarat B Castro P, et al. Anticoagulation strategies and risk of bleeding events in critically ill COVID-19 patients. Med Intensiva (Engl Ed). 2021;S0210–5691(21):00178–9. doi: 10.1016/j.medin.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 54.Helms J Severac F Merdji H, et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: bi-center cohort study. Ann Intensive Care. 2021;11(1):14. doi: 10.1186/s13613-021-00809-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah A Donovan K McHugh A, et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Crit Care. 2020;24(1):561. doi: 10.1186/s13054-020-03260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonmarker S Hollenberg J Dahlberg M, et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit Care. 2020;24(1):653. doi: 10.1186/s13054-020-03375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daughety MM Morgan A Frost E, et al. COVID-19 associated coagulopathy: thrombosis, hemorrhage and mortality rates with an escalated-dose thromboprophylaxis strategy. Thromb Res. 2020;196:483–485. doi: 10.1016/j.thromres.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Copur B Surme S Sayili U, et al. Comparison of standard prophylactic and preemptive therapeutic low molecular weight heparin treatments in hospitalized patients with COVID-19. Bratisl Lek Listy. 2021;122(9):626–630. doi: 10.4149/BLL_2021_100 [DOI] [PubMed] [Google Scholar]
- 59.Moll M Zon RL Sylvester KW, et al. Intermediate versus standard dose heparin prophylaxis in COVID-19 ICU patients: a propensity score-matched analysis. Thromb Res. 2021;203:57–60. doi: 10.1016/j.thromres.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voicu S Chousterman BG Bonnin P, et al. Increased anticoagulation reduces proximal deep vein thrombosis in mechanically ventilated COVID-19 patients: venous thrombosis prevention & COVID-19. J Infect. 2021;82(5):186–230. doi: 10.1016/j.jinf.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matli K Chamoun N Fares A, et al. Combined anticoagulant and antiplatelet therapy is associated with an improved outcome in hospitalised patients with COVID-19: a propensity matched cohort study. Open Heart. 2021;8(2):e001785. doi: 10.1136/openhrt-2021-001785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poulakou G Dimakakos E Kollias A, et al. Beneficial effects of intermediate dosage of anticoagulation treatment on the prognosis of hospitalized COVID-19 patients: the ETHRA study. In Vivo. 2021;35(1):653–661. doi: 10.21873/invivo.12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Longhitano Y Racca F Zanza C, et al. Venous thrombo-embolism in hospitalized SARS-CoV-2 patients treated with three different anticoagulation protocols: prospective observational study. Biology (Basel). 2020;9(10):310. doi: 10.3390/biology9100310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nadeem R Thomas SJ Fathima Z, et al. Pattern of anticoagulation prescription for patients with COVID-19 acute respiratory distress syndrome admitted to ICU. Does it impact outcome? Heart Lung. 2021;50(1):1–5. doi: 10.1016/j.hrtlng.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elmelhat A Elbourai E Dewedar H, et al. Comparison between prophylactic versus therapeutic doses of low-molecular-weight heparin in severely ill coronavirus disease 2019 patients in relation to disease progression and outcome. Dubai Med J. 2020;3(4):162–169. doi: 10.1159/000511163 [DOI] [Google Scholar]
- 66.Zalivansky Y, Pereira L, Budovich A. Enoxaparin use in hospitalized SARS-CoV-2-positive patients with elevated d-dimer: a pilot study. J Pharm Pract. 2021;8971900211064184. doi: 10.1177/08971900211064184 [DOI] [PubMed] [Google Scholar]
- 67.Lew TWK Kwek T-K Tai D, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):374–380. doi: 10.1001/jama.290.3.374 [DOI] [PubMed] [Google Scholar]
- 68.Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis. 2011;52(2):e14–e17. doi: 10.1093/cid/ciq125 [DOI] [PubMed] [Google Scholar]
- 69.Lopes RD, Fanaroff AC. Anticoagulation in COVID-19: it is time for high-quality evidence. J Am Coll Cardiol. 2020;76(16):1827–1829. doi: 10.1016/j.jacc.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan WJ Ni ZY Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cotter AH, Yang S-JT, Shafi H, Cotter TM, Palmer-Toy DE. Elevated von Willebrand factor antigen is an early predictor of mortality and prolonged length of stay for coronavirus disease 2019 (COVID-19) inpatients. Arch Pathol Lab Med. 2022;146(1):34–37. doi: 10.5858/arpa.2021-0255-SA [DOI] [PubMed] [Google Scholar]
- 72.Zhao R Su Z Komissarov AA, et al. Associations of d-dimer on admission and clinical features of COVID-19 patients: a systematic review, meta-analysis, and meta-regression. Front Immunol. 2021;12:691249. doi: 10.3389/fimmu.2021.691249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parisi R, Costanzo S, Di Castelnuovo A, de Gaetano G, Donati MB, Iacoviello L. Different anticoagulant regimens, mortality, and bleeding in hospitalized patients with COVID-19: a systematic review and an updated meta-analysis. Semin Thromb Hemost. 2021;47(4):372–391. doi: 10.1055/s-0041-1726034 [DOI] [PubMed] [Google Scholar]
- 74.Warnock LB, Huang D. Heparin. In: StatPearls. Treasure Island, FL: StatPearls Publishing; July 17, 2021. Accessed January 13, https://www.ncbi.nlm.nih.gov/books/NBK538247/. [Google Scholar]
- 75.DeWald TA, Washam JB, Becker RC. Anticoagulants: pharmacokinetics, mechanisms of action, and indications. Neurosurg Clin N Am. 2018;29(4):503–515. doi: 10.1016/j.nec.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 76.Pereyra D Heber S Schrottmaier WC, et al. Low-molecular-weight heparin use in coronavirus disease 2019 is associated with curtailed viral persistence: a retrospective multicentre observational study. Cardiovasc Res. 2021;117(14):2807–2820. doi: 10.1093/cvr/cvab308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rieder M Gauchel N Kaier K, et al. Pre-medication with oral anticoagulants is associated with better outcomes in a large multinational COVID-19 cohort with cardiovascular comorbidities. Clin Res Cardiol. 2022;111(3):322–332. doi: 10.1007/s00392-021-01939-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Talasaz AH Sadeghipour P Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu H, Chen M, Tang S, Yu W. Association of coagulation disturbances with severity of COVID-19: a longitudinal study. Hematology. 2021;26(1):656–662. doi: 10.1080/16078454.2021.1968648 [DOI] [PubMed] [Google Scholar]
- 80.The Lancet Haematology . COVID-19 coagulopathy: an evolving story. Lancet Haematol. 2020;7(6):e425. doi: 10.1016/S2352-3026(20)30151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Samkari H Karp Leaf RS Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leentjens J, van Haaps TF, Wessels PF, Schutgens REG, Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021;8(7):e524–e533. doi: 10.1016/S2352-3026(21)00105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


