Abstract
Acute pancreatitis (AP), resulting from inflammation of the pancreas, accounts for over 300,000 US hospital discharges per year. Although glucose intolerance has been known as a complication of severe AP, this effect was thought to be transient. Recently, cohort studies and meta-analysis of 24 published studies of 1100 patients who survived one or more episodes of AP revealed that 30–40% of patients developed diabetes or impaired glucose tolerance within 3–4 years of even a single episode of AP. The National Institute of Diabetes and Digestive and Kidney Diseases funded the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC) to undertake a prospective observational study of the occurrence of diabetes during an AP episode or subsequently, with emphasis on type 1 diabetes (T1D). Key factors for funding T1DAPC are the increasing incidence and prevalence of AP, its association with the development of T1D and other forms of diabetes after AP, its complications, and associated health care cost. The T1DAPC structure, governance and research objectives are described in this article. The DREAM (Diabetes RElated to Acute Pancreatitis and its Mechanisms) study to be undertaken by the T1DAPC is described in other articles in this journal’s issue.
Keywords: acute pancreatitis, type 1 diabetes mellitus, diabetes post pancreatitis, Consortium for the Study of T1 Diabetes in Acute Pancreatitis, DREAM study
INTRODUCTION
The spectrum of pancreatic diseases, including acute, recurrent and chronic pancreatitis and the diabetes associated with these pancreatic diseases, represent some of the most challenging medical disorders of our time. Based on input from patient advocacy groups, diabetes and gastroenterological professional societies and from the National Diabetes and Digestive and Kidney Diseases Advisory Council, the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC) was established by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) as a unique multidisciplinary research program designed to accelerate progress in understanding the relationship between these complex disorders.
WHAT LED TO THE CREATION OF T1DAPC?
Although the term ‘pancreatogenic diabetes’ suggests a single entity, it can arise from a variety of pathophysiological etiologies depending on the underlying pancreatic disease, as previously described for chronic pancreatitis (CP), pancreatic cancer, and cystic fibrosis.1 New onset diabetes that occurs within several years following acute pancreatitis (AP) can be diagnosed as type 1 diabetes (T1D), type 2 diabetes (T2D), or a form tied to exocrine pancreatic dysfunction and pancreas destruction, type 3c diabetes (T3cD). Acute pancreatitis is an inflammatory disease arising from the exocrine pancreas that accounts for more than 300,000 hospitalizations, with health care costs exceeding $2 billion annually in the United States.2,3 Acute pancreatitis may be due to gallstones, duct obstruction, trauma, a genetic (hereditary) predisposition to inappropriate activation of intra-pancreatic proteases, or the toxic effects of alcohol, drugs, infectious agents, or metabolites. Approximately 80% of patients have a mild clinical course with hospitalization lasting less than a week, although those with more severe AP experience pancreatic necrosis and/or organ failure, a protracted hospital course, and a mortality rate of 20–30%.4 Long-term sequelae include exocrine pancreatic insufficiency, complications from walled-off pancreatic necrosis, and recurrent episodes of AP in up to 20% of patients.5–7 Pancreatogenic diabetes is likely the most common complication after AP, with a cumulative incidence ranging from 23% to 40%.8–10 The temporal relationship between endocrine dysfunction and AP is highly variable and poorly described.11–13 Thus, the development of dysregulated glucose metabolism is common in patients with AP but may have a reversible component such that hyperglycemia experienced during or shortly after AP is resolved over time.
While prior studies have provided key observations regarding diabetes secondary to AP, their conclusions are limited by the source of the data (clinical diagnosis or diagnostic coding for diabetes case ascertainment via chart reviews or administrative databases), and the heterogeneity in study designs.7,9 Therefore, definitive studies on the incidence rate and risk factors for diabetes secondary to AP require prospective, longitudinal follow-up with serial assessments of glycemic status and evaluation of potential patient and disease related factors. Such studies have not yet been undertaken, likely due to the substantial costs and other resources required.
The conceptual origin of the T1DAPC represents the combined efforts of NIDDK’s Divisions of Diabetes, Endocrinology, and Metabolic Diseases and Digestive Diseases and Nutrition as well as patient-based non-profit and professional organizations and many dedicated clinicians and scientists. This led to the issue of Request For Applications (RFA)-DK-19-022 to fund clinical sites, and RFA-DK-19-023 to fund a coordinating center. The objectives of these RFAs were to form multi-disciplinary teams composed of pancreatologists, endocrinologists, immunologists, and radiologists to undertake a prospective longitudinal observational clinical study to investigate the incidence, etiology and pathophysiology of diabetes following AP with a particular emphasis on the auto-immune processes that result in T1D. This includes monitoring parameters such as the temporal relationship between pancreatitis and diabetes, relationship to pancreatitis severity, the roles of pancreatitis etiology, genetic and genomic risk factors, environmental and biological factors, and potential biomarkers for the development of T1D. In addition, a major collaborative effort within the Consortium will be the establishment of an annotated repository of biospecimens (blood, peripheral blood mononuclear cells, RNA, DNA, stool) to provide the resources and collaborative opportunities necessary to identify the interrelationship between the exocrine and the endocrine pancreas in the development of post-AP diabetes. Through the acquisition of a cohort of well-characterized patients and associated biospecimens, the proposed clinical research network will address many of the research objectives identified in the strategic plans of the NIDDK (goals 1, 2 and 3, Fig. 1) (https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/niddk-strategic-plan-for-research).
FIGURE 1.

NIDDK Strategic Plan for Research
From applications received in response to RFA-DK-19-02214 and RFA-DK-19-023,15 and based on Study Section review, NIDDK program staff evaluations, and with the concurrence of NIDDK Advisory Council, a Consortium for the study of Type 1 Diabetes in Acute Pancreatitis was created with the investigators and institutions listed in Table 1. These investigators represent the largest combined assembly of pancreatic investigative expertise in both exocrine and endocrine disease in the US, and represent top academic clinical centers distributed across the US, providing an impressive “catchment” area encompassing a diverse population (Fig. 2 and Table 1).
TABLE 1.
T1DAPC Clinical Centers and Data Coordination Center
| Institution* | Principal Investigator(s) |
|---|---|
| T1DAPC Clinical Center | |
| Benaroya Research Institute at Virginia Mason | Carla Greenbaum and Richard Kozarek |
| Cedars-Sinai Medical Center | Mark Goodarzi and Stephen J. Pandol |
| Indiana University and Purdue University at Indianapolis | Evan Fogel and Carmella Evans-Molina |
| Johns Hopkins University | Vikesh Singh and Zhaoli Sun |
| Ohio State University | Georgios I. Papachristou, Darwin L. Conwell, and Phil A. Hart |
| University of Florida | Christopher E. Forsmark, Steven J. Hughes, and Richard E. Pratley |
| University of Illinois at Chicago | Cemal Yazici and Brian T. Layden |
| University of Minnesota, Minneapolis | Melena D. Bellin† |
| University of Pittsburgh | Dhiraj Yadav* and Frederico G.S. Toledo |
| Stanford University | Walter G. Park, Marina Basina, and Aida Habtezion |
| T1DAPC Data Coordinating Center | |
| Pennsylvania State University Hershey Medical Center | Vernon M. Chinchilli |
In alphabetical order
T1DAPC co-chair
FIGURE 2.
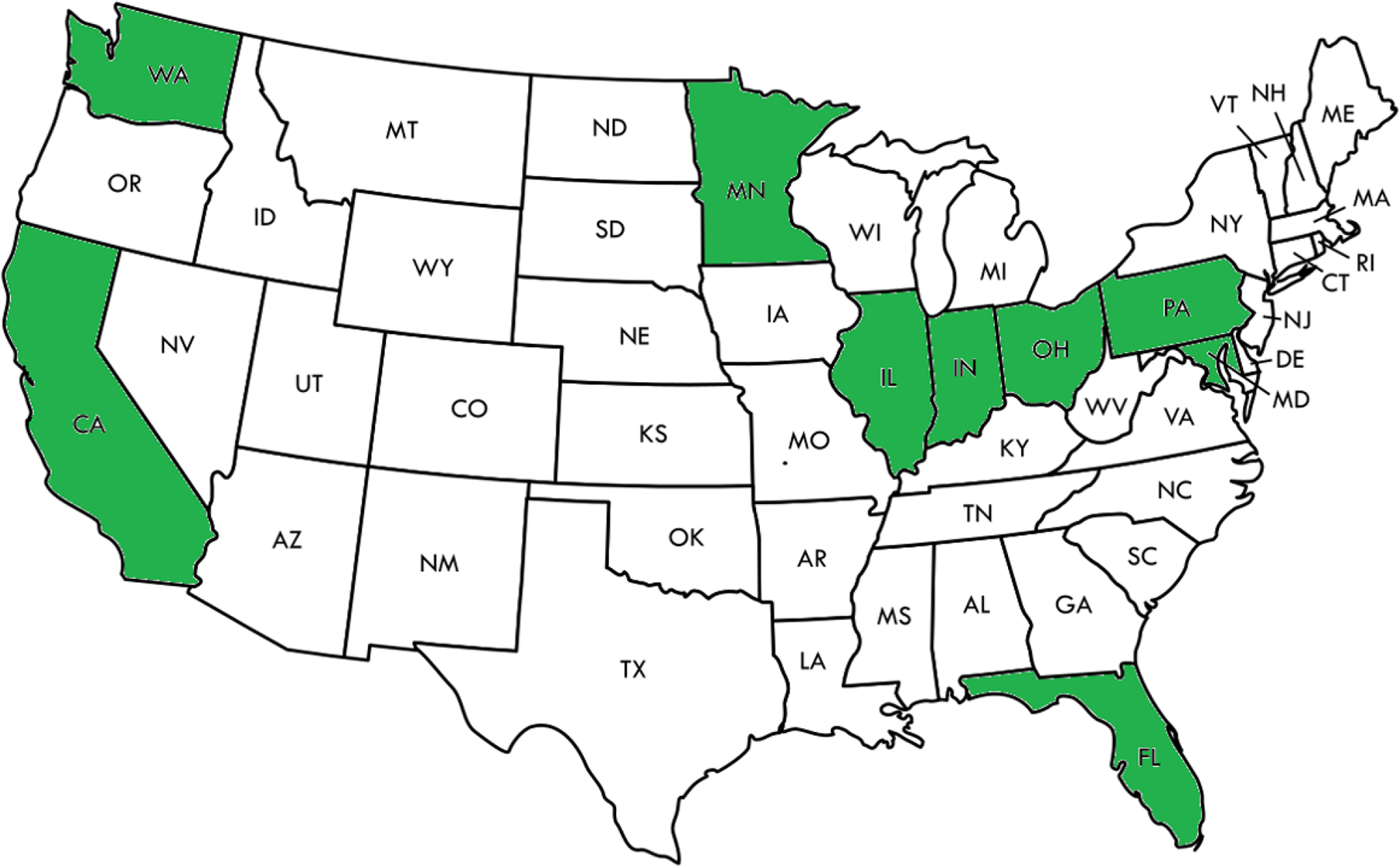
Geographical location of the T1DAPC members.
ORGANIZATION OF THE T1DAPC CONSORTIUM
The T1DAPC consists of representatives from NIDDK, ten Clinical Centers (CC), and a Data Coordinating Center (DCC). The structure is comprised of an Executive Committee, a Steering Committee and its subcommittees, and a Data and Safety Monitoring Board (DSMB) (Fig. 3).
FIGURE 3.
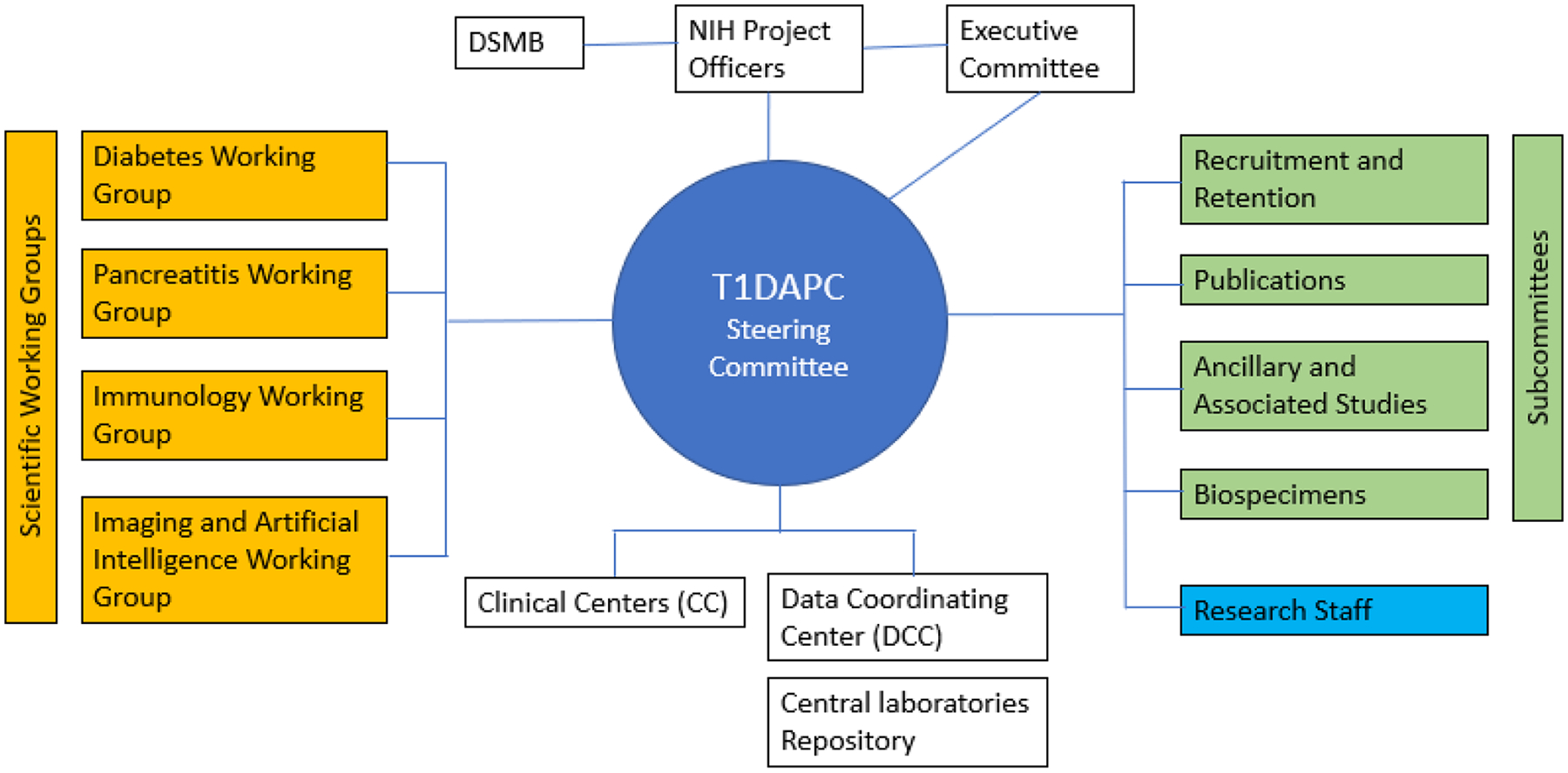
Organization and governance of the T1DAPC.
The NIDDK Project Scientists provide programmatic oversight, monitor study progress and adherence to NIDDK policies. Clinical Centers are comprised of outstanding clinicians, scientists, coordinators, and technicians, and are responsible for all aspects of planning and executing the study, which entails recruiting and collecting data on participants for the duration of the study. All interactions with participants take place at the CCs. Collaboration amongst the CCs, and the creativeand diligence of the consortium members, are all notable characteristics of the T1DAPC.
The DCC is central to the success of the T1DAPC. It provides administrative and logistic support for the consortium, including developing and maintaining public and secured consortium websites, supporting conference calls and future in-person meetings, and establishing and managing subawards for the CCs, central laboratories and biorepositories. The DCC also provides coordination for protocol development and its execution including regulatory compliance and coordination with the NIDDK, DSMB, and single institutional review board. Central to its function is data management including collection, storage, and quality control. The DCC also provides statistical support for all consortium collaborative studies, such as for study design, data analysis, and data interpretation. It develops new statistical methodology as necessary to meet study needs.
T1DAPC STUDY GOVERNANCE
Steering Committee and Subcommittees
The Steering Committee (SC) is composed of the Principal Investigators (PI) of each CC and the DCC, and the NIDDK Project Scientists (Fig. 3). It is the main governing body of the T1DAPC. The SC has primary responsibility for the activities of the Consortium. During the first year of the consortium, working groups established under the SC designed the DREAM study and created necessary documents including the protocol, consent forms and the manual of operations. These working groups are described below. Working through its many subcommittees, the SC is responsible for the design, conduct and monitoring of studies and reporting study results, and approving ancillary studies and publications. The SC oversees many subcommittees, including Recruitment and Retention; Publications, Ancillary and Associated Studies; Biospecimens; Coordinators; and discipline specific working groups.
Executive Committee
The Executive Committee (EC) is comprised of the T1DAPC Study Co-Chairs, the PI of the DCC, and the NIDDK Project Scientists. It convenes to effect management decisions as required for the function of the consortium.
Data and Safety Monitoring Board
An independent Data and Safety Monitoring Board (DSMB) has been established by the NIDDK to review protocols, study materials and the Data and Safety Monitoring Plan, and to monitor patient safety and study performance. As a part of its responsibilities, the DSMB submits recommendations to the NIDDK regarding the continuation of each study. All protocols or changes to protocols are approved by a single Institutional Review Board and the local IRBs at each CC institution, the SC, the DSMB, and the NIDDK before implementation.
Scientific Working Groups
The investigators organized themselves into four scientific working groups, designed around the main research objectives: These include the Diabetes Working Group, the Pancreatitis Working Group, the Immunology Working Group, and the Imaging and Artificial Intelligence Working Group. Working group members provided the subsequent papers in the current series describing the DREAM study.
With significant cross-fertilization across the four working groups, the consortium’s investigators have developed a prospective longitudinal observational clinical study (DREAM study) to investigate the incidence, etiology, and pathophysiology of diabetes mellitus following acute pancreatitis, pursuing three aims:
AIM 1
Determine the cumulative incidence and clinical characteristics associated with the development of DM after one or more episodes of AP.
AIM 2
Comprehensively characterize beta cell function and endocrine alterations after AP and their relationship with the development of DM after AP.
AIM 3
Determine the immunologic mechanisms of DM after AP, including the contribution of β-cell autoimmunity.
The primary study design will be a longitudinal study of adults (age 18 years and older) who have experienced one or more episodes of acute pancreatitis in whom periodic glycemic parameter testing identifies subjects who develop impaired glucose tolerance (IGT) or diabetes after the onset of the acute pancreatitis over a period of at least three years. The type of diabetes which occurs after acute pancreatitis will be characterized using biomarkers of T1DM (eg, Glutamic acid decarboxylase (GAD-65), insulin antibody (IA-2) and measures of insulin secretion), Type 2 DM (T2DM) (eg, measures of insulin sensitivity), and Type 3c diabetes (T3cDM) (eg, basal and post-test meal levels of pancreatic polypeptide and other biomarkers of T3cDM) to determine the prevalence of all disease types among subjects who develop diabetes after or as a consequence of AP.
The Consortium also provides an environment that fosters internal and external collaborations through ancillary studies which in the future will provide new information on the epidemiology, pathogenesis and treatment of post-pancreatitis diabetes.
In conclusion the Type 1 Diabetes in Acute Pancreatitis Consortium represents a significant joint effort of two NIDDK Divisions to support a comprehensive research program to determine the incidence of diabetes after AP for clinical, epidemiological, and biological characterization of patients who develop diabetes following AP, to gain insight into the pathophysiology and relationships between these pancreatic diseases; and to develop better methods (and biomarkers) for diagnosis, prevention, monitoring, early detection, and therapy.
The results will identify strategies for more effective care and targeted interventions for patients with these increasing conditions.
ACKNOWLEDGMENTS
The authors would like to acknowledge all the Investigators, Co-investigators and Collaborators participating in the Consortium for the study of Type 1 Diabetes in Acute Pancreatitis (T1DAPC):
Benaroya Research Institute At Virginia Mason : Carla Greenbaum, Richard Kozarek, Cate Speake, Sandra Lord, Shayan Irani, Peter Linsley, William Kwok, Adam Lacy-Hulbert, Jessica Hamerman, Henry Bahnson, Brooke Grubb, Cassandra Williams, Sarah Ackermann, Kimberly Varner
Cedars-Sinai Medical Center: Mark O. Goodarzi, Stephen J. Pandol, James Buxbaum, Marcio Diniz, Marc Goodman, O. Joe Hines, Christie Jeon, Murray Korc, Debiao Li, Maxim Petrov, Joseph Pisegna, Jennifer Van Eyk, Santhi Swaroop Vege
Indiana University: Evan Fogel, Carmella Evans-Molina, Temel Tirkes, Stuart Sherman, Jeffrey Easler, Zed Saeed, Emily Sims, Richard Oram, Michael Weedon
Johns Hopkins University: Vikesh Singh, Zhaoli Sun, Elham Afghani, Rita Kalyani, Michael Jacobs, Atif Zaheer, Heng Zhu, Venkata S. Akshintala
The Ohio State University: Georgios I. Papachristou, Phil A. Hart, Darwin L. Conwell, David Bradley, Kathleen Dungan, Zarine Shah, Zoe Krebs, and Samantha Hazelett.
University of Florida: Christopher E. Forsmark, Anna Casu (Advent Health), Steven Hughes, Richard E. Pratley (Advent Health), Martha Campbell Thompson, Clive Wasserfall, Joseph Grajo
University of Illinois At Chicago: Cemal Yazici, Brian Layden, Kirstie Danielson, Brian Boulay, Edward Villa, Ece Mutlu, Yinglin Xia, Stefan Green, Lisa Tussing-Humphreys, Bellur Prabhakar, Ron Gaba, Kiira Ratia, Martha Daviglus, Mario Spaggiari, Paul J. Grippo, Veronica Setiawan, Stephen J. Pandol. Northwestern University: Rajesh Keswani, Srinadh Komanduri, Aziz Aadam.
University of Minnesota: Melena Bellin, Guru Trikudanathan, Brian Fife, Martin Freeman, Tasma Harindhanavudhi, Antoinette Moran, Benjamin Spilseth, James Hodges
University of Pittsburgh: Dhiraj Yadav, Frederico G. S. Toledo, Anil Dasyam, Anna E. Phillips, Chae-Ryon Kang, Vijay Yechoor, Jami Saloman, Dorothy Becker, Shari Reynolds, Kristin Hall, Kim Stello, Melanie Mays, Melissa Saul.
Stanford University: Walter G. Park, Marina Basina, Aida Habtezion , Peter Poullos, and Everett Meyer
Pennsylvania State University Hershey Medical Center: Vernon M. Chinchilli, Lan Kong, Dajiang Liu, Jennifer Maranki, Jennifer McCormick, Ariana Pichardo-Lowden, Ayesha Siddiqui, Nazia Raja-Khan, Ming Wang, Xiang Zhan, Rita Bottino, Georgia Faulkner, Kendall Thomas Baab, Anne-Marie Dyer, Jennifer Guzman, Beth Holmes, Rose Baron, Aimee Merchlinski, Paula Valencia-Moulton, James Broach, David Bradley
The T1DAP Consortium is funded the National Institute of Diabetes and Digestive and Kidney Diseases: grants U01DK127384; U01DK127367; U01DK127377; U01DK127392; U01DK127382; U01DK127403; U01DK127404; U01DK127388; U01DK127395; U01DK127378 and U01DK127400. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest/disclosures:
M.D.B. receives research support from Viacyte and Dexcom and is on the advisory board for Insulet. The remaining authors do not have any potential conflicts to disclose.
REFERENCES
- 1.Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019;156:254–272.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagenholz PJ, Fernandez-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35:302–307. [DOI] [PubMed] [Google Scholar]
- 4.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nature reviews Gastroenterol Hepatol. 2019;16:479–496. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–1103. [DOI] [PubMed] [Google Scholar]
- 6.Yadav D, Lee E, Papachristou GI,. A population-based evaluation of readmissions after first hospitalization for acute pancreatitis. Pancreas. 2014;43:630–637. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738–746. [DOI] [PubMed] [Google Scholar]
- 8.Woodmansey C, McGovern AP, McCullough KA, et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (Type 3c): A retrospective cohort study. Diabetes Care. 2017;40:1486–1493. [DOI] [PubMed] [Google Scholar]
- 9.Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818–831. [DOI] [PubMed] [Google Scholar]
- 10.Zhi M, Zhu X, Lugea A, et al. Incidence of new onset diabetes mellitus secondary to acute pancreatitis: A systematic review and meta-analysis. Frontiers Physiol. 2019;10:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibars EP, Sanchez de Rojas EA, Quereda LA, et al. Pancreatic function after acute biliary pancreatitis: does it change? World J Surg. 2002;26:479–486. [DOI] [PubMed] [Google Scholar]
- 12.Angelini G, Cavallini G, Pederzoli P, et al. Long-term outcome of acute pancreatitis: a prospective study with 118 patients. Digestion. 1993;54:143–147. [DOI] [PubMed] [Google Scholar]
- 13.Shen HN, Yang CC, Chang YH, et al. Risk of diabetes mellitus after first-attack acute pancreatitis: A national population-based study. Am J Gastroenterol. 2015;110:1698–1706. [DOI] [PubMed] [Google Scholar]
- 14.Type 1 Diabetes in Acute Pancreatitis Consortium - Clinical Centers (T1DAPC-CCs) (U01 Clinical Trial Optional). Updated August 23, 2019. Available at: https://grants.nih.gov/grants/guide/rfa-files/RFA-DK-19-022.html. Accessed December 3, 2021
- 15.Type 1 Diabetes in Acute Pancreatitis Consortium - Data Coordinating Center (T1DAPCDCC) (U01 Clinical Trial Optional). Updated August 23, 2019. Available at: https://grants.nih.gov/grants/guide/rfa-files/RFA-DK-19-022.html. Accessed December 3, 2021


