Abstract
Objective: Multivariate logistic regression analysis of preeclampsia in patients with pregnancy induced hypertension and the risk predictive value of monitoring platelet, coagulation function and thyroid hormone in pregnant women. Methods: The data of 120 pregnant women who delivered their babies at Xinyu Maternal and Child Health Hospital from January 2019 to January 2022 were analyzed retrospectively. Among the subjects studied, 60 were patients with preeclampsia as a study group and 60 healthy pregnant women were assigned to a control group. The clinical data of pregnant women were recorded, including age, weight gain during pregnancy, nationality, education level, times of antenatal examinations, times of pregnancy and parturition, discovery of gestational weeks, multiple pregnancies, amniotic fluid volume, neonatal weight, history of in vitro fertilization combined with embryo transfer, history of diabetes, kidney disorders or preeclampsia, family background of high blood pressure, anemia and so on. The clinical test data, such as platelet count and volume, coagulation function and thyroid hormone, were collected in two groups of pregnant women. Multivariate logistic regression analysis was performed on preeclampsia. The predictive value of platelet, coagulation function and thyroid hormone on preeclampsia was explored. Results: We compared the general hematological parameters. Univariate Logistic analysis found that age, history of diabetes, nephropathy or preeclampsia, family background of elevated blood pressure, weight gain during pregnancy, frequency of pregnancy and multiple pregnancies were all risk factors for preeclampsia. Multivariate Logistic regression analysis screened out that age, history of diabetes, kidney disorders or preeclampsia, family background of hypertension were independent risk factors for preeclampsia. The white blood cell count and platelet count of the study group were lower than those of the control group. Moreover, observed patients displayed a larger average platelet volume (P<0.05). Significant differences were found in glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, lactate dehydrogenase, albumin, serum creatinine and uric acid, as well as in thrombin time and activated partial thromboplastin time between the two groups (P<0.05). In terms of thyroid function, obvious differences were found in serum thyrotropin and free thyroxine between the two groups (P<0.05). Conclusion: Age, history of diabetes, kidney disorders or preeclampsia, family background of highly blood pressure are independent risk factors for preeclampsia. Platelet, coagulation function and thyroid hormone levels can have a certain risk predictive value.
Keywords: Preeclampsia, hypertension, platelet, coagulation function, thyroid hormone
Introduction
Hypertensive disorder complicating pregnancy (HDCP), which consists of chronic hypertension with preeclampsia, chronic hypertension, preeclampsia, pregnancy-induced hypertension, is the pregnancy-specific disease with a global incidence of 5%-8% [1,2]. The onset of HDCP is from 20 weeks of pregnancy to 12 weeks postpartum. The main clinical manifestations are elevated blood pressure (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg), edema, proteinuria, accompanied by damage or failure of important organs. In severe cases, convulsions, coma, cardiorenal failure, placental abruption, diffuse intravascular coagulation (DIC) and cerebral hemorrhage, even maternal and fetal death may occur. HDCP has become a serious obstetrical complication affecting maternal and infant health and is an important cause of poor perinatal prognosis and death [3-5]. HDCP has always been a hot but challenging topic in pathological obstetrical research. It is of significance to improve the treatment of HDCP [6].
Preeclampsia is a kind of HDCP. The basic pathophysiological changes are systemic small vasospasm, decreased perfusion of various organ systems, and irreversible damage in severe cases. It is one of the important cause of maternal and perinatal mortality. Also, it can increase the risk of long-term maternal and fetal complications [7]. Preeclampsia is a difficult research focus in perinatal medicine at present. The etiology of this disease has not been fully elucidated, and there is a lack of simple and accurate prediction methods clinically. Most preeclampsia can only be found after the occurrence of symptoms. Interventions were carried out when complications occurred, with severe organ and fetal damage, as well as poor pregnancy outcome [8,9].
At present, the pathogenesis theory recognized by scholars is the “two-stage theory”. In stage 1, patients develop uterine spiral artery trophoblast recasting disorder, leading to placental ischemia and hypoxia, releasing a variety of placental factors. In stage 2, placental factors enter the blood, promote the activation of systemic inflammatory response and vascular endothelial damage, leading to the disease [10-12]. There are many high-risk factors for preeclampsia: age, gravidity and parity, history of preeclampsia, family history of hypertension, multiple pregnancies, low socio-economic status, malnutrition, assisted reproductive technology, medical complications (such as diabetes, hypertension, kidney disease, thrombosis, urinary infection, periodontitis and autoimmune diseases) [13,14]. Through long-term and continuous practice, clinicians continue to explore and gradually summarize corresponding prevention methods, which can play a predictable protective effect on both pregnant women and fetuses [15]. Among them, the change of blood coagulation mechanism may become an effective starting point [16]. Coagulation system activity may increase in patients with preeclampsia at the initial stage. Many studies have indicated that plasma D-dimer levels in pregnant women with preeclampsia are much higher than those without preeclampsia [17]. The purpose of this study was to assess the implications of multiple factors on preeclampsia and to find the relationship between the indexes of routine prenatal examination and preeclampsia.
Materials and methods
General information
The data of 120 pregnant women delivered their babies in Xinyu Maternal and Child Health Hospital from January 2019 to January 2022 were analyzed retrospectively, including 60 patients with preeclampsia as a study group and 60 healthy pregnant women as a control group. The control cases were 23-37 years old (27.43±3.46), and the cases in the study group were 23-38 years old (28.25±3.52). There was no significant difference in age between the two groups (P>0.05). Ethical approval was obtained from our clinic’s Health Policy Affiliation (20220032).
Inclusion criteria of the study group: 1) pregnant women with systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg after 20 weeks of pregnancy with any of the subsequent followings: urinary protein ≥ 0.3 g/24 h, urinary protein/creatinine ratio ≥ 0.3 or arbitrarily urinary protein ≥ (+); 2) pregnant women with no previous history of cognitive impairment and mental illness; 3) pregnant women who signed informed consent.
Inclusion criteria of the control group: pregnant women without HDCP in the same period.
Exclusion criteria of the two group: 1) patients with benign or malignant tumors, inflammatory diseases, hepatitis B, acquired immune deficiency syndrome (AIDS) or other infectious diseases; 2) patients with blood system diseases, endocrine system diseases, immune diseases or other organ system basic diseases; 3) patients with other obstetrical complications.
Treatment methods
The clinical data of pregnant women were recorded, including age, weight gain during pregnancy, nationality, education level, times of antenatal examination, times of pregnancy and parturition, discovery of gestational weeks, multiple pregnancies, amniotic fluid volume, neonatal weight, history of in vitro fertilization combined with embryo transfer, history of diabetes, kidney disorders or preeclampsia, family background of high blood pressure, anemia and so on. The clinical test data, such as platelet, coagulation function, thyroid hormone, were collected in two groups of pregnant women.
Observation index
Univariate Logistic analysis
Univariate Logistic analysis was carried out to identify the risk factors associated with preeclampsia by univariate Logistic analysis of 14 variables, such as age, body weight during pregnancy, times of pregnancy and parturition, multiple pregnancies and history of preeclampsia.
Comparison of blood routine indexes
After EDTA anticoagulation treatment during fasting state in the morning, the red blood cell (RBC) count, hemoglobin (Hb), white blood cell (WBC) count, platelet count (PLT) and mean platelet volume (MPV) were measured and compared.
Comparison of liver function indexes
After EDTA anticoagulation treatment during fasting state in the morning, the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), albumin (ALB) and globulin (GLB) were measured and analyzed.
Comparison of renal function indexes
After EDTA anticoagulation treatment during fasting state in the morning, serum creatinine (Cr), urea nitrogen (BUN) and uric acid (UA) were measured and analyzed.
Comparison of blood coagulation function indexes
After EDTA anticoagulation treatment during fasting state in the morning, prothrombin time (PT), thrombin time (TT), activated partial thromboplastin time (APTT) and fibrinogen (FIB) were recorded and analyzed.
Comparison of thyroid function indexes
After EDTA anticoagulation treatment during fasting state in the morning, serum thyrotropin (TSH) and free thyroxine (FT4) were measured in pregnant women.
Statistical analysis
SPSS 24.0 software was applied to analyze the data. For measurement data which were presented by (x̅±s), independent samples t-test was adopted for the inter-group comparison, and paired t-test was adopted for the comparison between before and after treatment. The counting data were presented by n (%), and χ2 test was adopted for the comparison. The risk factors were assessed by Logistic regression analysis, advanced univariate Logistic analysis, and then the factors with statistically significant differences were calculated using multivariate Logistic Regression analysis. When P value was less than 0.05, the difference was statistically significant.
Results
Comparison of the general data between the two groups
We conducted comparison of the general data between the two groups, including age, history of diabetes, nephropathy or preeclampsia, family background of high blood pressure, weight gain during pregnancy, frequency of pregnancy and multiple pregnancies, and all the differences were statistically significant (P<0.05). All the results are shown in Table 1.
Table 1.
Logistic univariate analysis of risk factors for preeclampsia
| Variable | Regression coefficient | Standard error | Wald Value | P Value | OR Value (95% CI) |
|---|---|---|---|---|---|
| Age (≥ 35 years old) | 2.14 | 1.043 | 4.210 | 0.040 | 8.499 (1.100-65.646) |
| History of diabetes mellitus | 1.264 | 0.276 | 20.974 | 0.000 | 3.540 (2.061-6.080) |
| History of kidney disease | 1.248 | 0.352 | 12.570 | 0.000 | 3.483 (1.747-6.944) |
| Family history of hypertension | 1.784 | 0.374 | 22.753 | 0.000 | 5.954 (2.860-12.392) |
| History of preeclampsia | 1.574 | 0.327 | 23.169 | 0.000 | 4.826 (2.542-9.161) |
| Weight gain during pregnancy | 1.582 | 0.293 | 29.153 | 0.000 | 4.865 (2.739-8.639) |
| Number of pregnancy and parturition | 0.873 | 0.353 | 6.116 | 0.013 | 2.394 (1.199-4.782) |
| Multiple pregnancy | 0.845 | 0.318 | 7.061 | 0.008 | 2.328 (1.248-4.342) |
Multivariate Logistic regression analysis
Multivariate Logistic regression analysis screened out that age, history of diabetes, kidney disorders or preeclampsia, family background of hypertension were independent risk factors for preeclampsia. All the results are shown in Table 2.
Table 2.
Logistic multivariate analysis of risk factors for preeclampsia
| Variable | Regression coefficient | Standard error | Wald Value | P Value | OR Value (95% CI) |
|---|---|---|---|---|---|
| Preeclampsia | 19.43 | 0.682 | 320.413 | 0.000 | / |
| Age (≥ 35 years old) | 3.26 | 0.879 | 13.755 | 0.000 | 26.050 (4.651-145.888) |
| Family history of hypertension | 1.347 | 0.531 | 6.435 | 0.011 | 3.846 (1,358-10.889) |
| History of kidney disease | 1.186 | 0.361 | 10.793 | 0.001 | 3.274 (1.614-6.643) |
| History of diabetes mellitus | 1.025 | 0.348 | 8.675 | 0.003 | 2.787 (1.409-5.513) |
Comparison of blood routine indexes
The WBC count and PLC of the study group were lower than those of the control group. Moreover, the observed patients displayed a larger average platelet volume (P<0.05, Table 3).
Table 3.
Comparison of general hematological parameters between the two groups [x̅±s]
| Group | N | RBC (×1012/L) | Hb (g/L) | WBC (%) | PLT (×109/L) | MPV (fl) |
|---|---|---|---|---|---|---|
| Control group | 60 | 4.02±1.24 | 123.64±13.25 | 9.87±1.61 | 198.84±46.37 | 10.26±1.08 |
| Study group | 60 | 4.16±1.21 | 124.21±12.36 | 9.21±1.56 | 178.53±53.13 | 11.67±1.25 |
| t | 0.625 | 0.243 | 2.280 | 2.230 | 6.611 | |
| P | 0.532 | 0.807 | 0.024 | 0.027 | 0.000 |
RBC: red blood cell, Hb: hemoglobin, WBC: white blood cell, PLT: platelet count, MPV: mean platelet volume.
Comparison of liver function indexes
Significant differences were discovered in glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, lactate dehydrogenase and albumin between the two groups (P<0.05, Figure 1).
Figure 1.
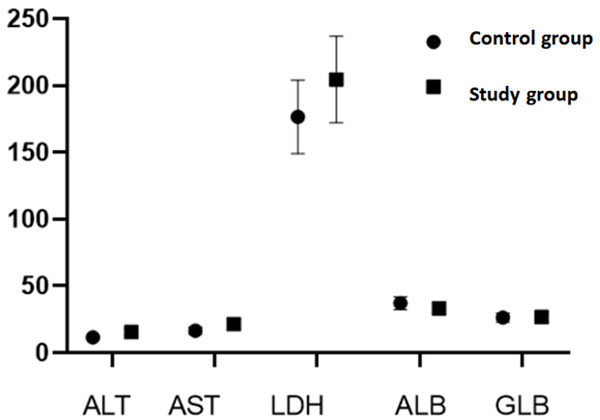
Comparison of liver functional indexes between the two groups.
The renal functional indexes
Great differences were found in serum creatinine and uric acid between the two groups (P<0.05, Figure 2).
Figure 2.
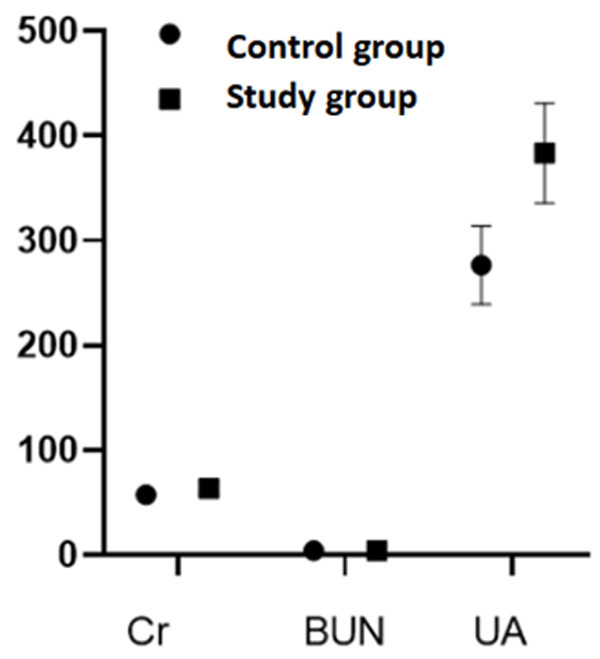
Comparison of renal functional indexes between the two groups.
Blood coagulation function
There were markable differences in thrombin time and activated partial thromboplastin time between the two groups (P<0.05, Table 4).
Table 4.
Comparison of coagulation function indexes between the two groups [x̅±s]
| Group | N | PT (s) | TT (s) | APTT (s) | FIB (g/L) |
|---|---|---|---|---|---|
| Control group | 70 | 57.42±5.37 | 3.93±1.48 | 276.43±37.37 | 7.74±2.14 |
| Study group | 70 | 63.47±5.64 | 4.24±1.67 | 383.12±47.54 | 7.34±2.72 |
| t | 6.017 | 1.076 | 26.476 | 1.119 | |
| P | 0.000 | 0.284 | 0.000 | 0.265 |
PT: prothrombin time, TT: thrombin time, APTT: activated partial thromboplastin time, FIB: fibrinogen.
The thyroid function
In terms of the indexes of thyroid function, obvious differences were discovered in serum thyrotropin and free thyroxine between the two groups (P<0.05, Table 5).
Table 5.
Comparison of thyroid function indexes between the two groups [x̅±s]
| Group | N | TSH (mIU/L) | FT4 (pmol/L) |
|---|---|---|---|
| Control group | 60 | 2.31±0.89 | 12.43±3.24 |
| Study group | 60 | 3.82±1.03 | 10.02±2.68 |
| t | 8.592 | 4.439 | |
| P | 0.000 | 0.000 |
TSH: thyrotropin, FT4: free thyroxine.
Discussion
Pregnancy induced hypertension syndrome is characterized by hypertension, albuminuria and edema in women after 20 to 24 weeks of pregnancy, often accompanied by multiple organ damage, and even coma, convulsions, cerebral hemorrhage, placental abruption and heart failure in severe cases, possibly leading to maternal death [18]. Preeclampsia and eclampsia are the results of the further development of HDCP [19-21]. The main causes of neonatal death are preterm delivery and asphyxia caused by eclampsia. Studies have indicated that patients with preeclampsia have poor maternal uterine and placental blood circulation, which hinder fetal oxygen and nutrient intake, thereby affecting fetal growth and development. The incidence of preterm delivery in patients with early-onset serious preeclampsia is significantly higher than that in patients with other types of preeclampsia. Additionally, the mothers often have to terminate pregnancy early due to the progression of the disease [22,23]. A cohort study in 354676 Swedish pregnant women found that women with a history of preeclampsia had a significantly augmented risk of developing preeclampsia when they were pregnant again, and the risk of stillbirth in a second pregnancy doubles. Researchers also compared the effects of preeclampsia on outcomes and found that the incidence of adverse outcomes such as preterm delivery and low birth weight infants increased by 6-9 times [24-26]. In addition, 67% of pregnant women with severe eclampsia are prone to thrombosis [27]. Related genetic studies have found a significant increase in the mutation rate of placenta and prothrombin gene in preeclampsia [28]. The blood coagulation alters in patients with preeclampsia, especially in the process of early DIC. Embolism of the capillary bed and microcirculation disturbance in the hypercoagulable state can lead to the death of perinatal infants with poor placental circulation [29].
So far, the pathogenesis of preeclampsia is still unclear. The main theories about the pathogenesis of preeclampsia are inflammatory and immune overactivation, uterine spiral arteriole recasting disorder, vascular endothelial cell injury, genetic factors and insulin resistance [30]. A study found that history of diabetes and kidney disease in pregnant women were the main risk factors for preeclampsia. When they were suffering from diabetes and nephropathy at the same time, the incidence of HDCP would be over 50% [31]. When pregnant women suffer from hypertension, diabetes, kidney and other medical diseases, the pathophysiology of placenta will change, which injures systemic endothelial cells and cause systemic arteriole spasm, leading to preeclampsia [32]. This study support the notion that when pregnant women suffer from kidney disease and diabetes, the risk of preeclampsia is significantly increased. Genetic factors have also been confirmed as the causes of HDCP. When the patients’ parent(s) suffered from hypertension, the risk of preeclampsia is significantly augmented in pregnant women. Therefore, family history of hypertension is one of the main risk factors for preeclampsia [33-35]. Age is another important influencing factors of preeclampsia. At present, China recommends that the best childbearing age is from 23 to 30 years old, and women over 35 years old are elderly parturients [36,37]. A case-control study in Bangkok also indicated that pregnant women aged 35 or older had a 1.7-fold risk of developing preeclampsia. Pregnant women over 30 years old should strengthen their awareness of health care and pay attention to their blood pressure. If an abnormal rise in blood pressure was observed, they should communicate with their doctors in time to avoid the development of preeclampsia. The level of thyroid hormone during pregnancy changes with the change of gestational weeks. The levels of TSH and FT4 change throughout the whole gestational period. TSH, FT4 and TPOAb are triple indicators of thyroid function screening during pregnancy. Some prior reports believed women with severe preeclampsia had higher serum TSH level and lower serum FT4 level than healthy pregnant women, indicating that there is a close relationship between severe preeclampsia and thyroid hormone levels. This corresponds to previous studies that hypothyroidism during pregnancy is a high-risk factor for preeclampsia. The reasons may be as follows. (1) Deficiency of free triiodothyronine in hypothyroidism weakens its relaxing effect on aortic endothelial cells and vascular smooth muscle cells, and decreases the synthesis rate of nitric oxide, a vasodilator, in hypothyroidism. It leads to endothelium-dependent vasodilation dysfunction and increases vascular resistance. The decrease of thyroid level can reduce systemic hemodynamics, renal blood flow and glomerular filtration rate, promoting the development of hypertension. (2) During hypothyroidism, the ability of liver to synthesize protein decreased significantly, which decreases plasma colloid osmotic pressure, increases blood volume and easily elevates blood pressure. (3) The deficiency of thyroid hormone can lead to the disorder of fat metabolism and glucose metabolism and increase the risk of preeclampsia. During the development of severe eclampsia, changes in renal structures occur, such as vascular endothelial hyperplasia and increased glomerular permeability, resulting in the excretion of thyroid hormones in the urine, leading to a decrease in various thyroxine levels and the development of low FT4. The decrease of thyroid hormone level counteracts on the kidney tissue and promotes the change of renal tissue structure, which reduces the renal blood flow and glomerular filtration capacity in varying degrees, thus increasing the burden of the kidney and causing a vicious circle of renal function damage. Therefore, severe preeclampsia and hypothyroidism promote and influence each other, thus worsening the prognosis of pregnant women and newborns. Several studies in patients with severe pre-eclampsia with thyroid dysfunction have indicated differences in pregnancy outcomes between those with early onset and those with late onset [38-40]. In this study, univariate Logistic analysis indicated that age, history of diabetes, nephropathy or preeclampsia, family background of hypertension, weight gain during pregnancy, frequency of pregnancy and multiple pregnancies were all risk factors for preeclampsia. In addition, the age, history of diabetes, renal disorders or preeclampsia records, family background of hypertension were independent-risk factors for preeclampsia. We compared the blood routine indexes. The WBC count and PLC of patients were lower than those in healthy subjects. In contract, the observation cases showed a larger average platelet volume. In addition, there were significant differences in glutamic pyruvic transaminase, aspartate aminotransferase, lactate dehydrogenase and albumin between the two groups (P<0.05). In terms of liver function, great differences were discovered in serum creatinine and uric acid between the two groups (P<0.05). Significant differences in thrombin time and activated partial thromboplastin time were found between the two groups (P<0.05). Also, there were significant differences in serum thyrotropin and free thyroxine between the two groups (P<0.05). The analysis indicated that due to the change of physiological function, the fibrinolytic system and blood system of pregnant women were affected accordingly, so that the blood anticoagulant components were decreased rapidly, and the blood coagulation factor II and V were activated continuously. The contents in the blood increased accordingly, which inhibited the activation of the fibrinolytic system, and finally led to hypercoagulable blood. When the blood system of pregnant women changes during pregnancy, the dynamic balance of the original fibrinolysis system and coagulation system is impacted. Then, the activity of coagulation factors becomes stronger, and the fibrinogen in the fibrinolysis system is changed accordingly. Moreover, a new balance system is re-established. Although the hypercoagulable state of maternal blood system during pregnancy contributes to placental abruption and avoids massive bleeding, it also increases the incidence of complications such as thrombotic and obstetrical diseases. The platelets in human body mainly promote the release of endogenous TXA2 and ADP by adhering to subcutaneous collagen to inhibit platelet aggregation and stop bleeding, which has important application value in evaluating vasospasm and severity of injury in pregnant women. Common indicators include PLT, PMV and PDW. PLT reflects the status of platelet production and death, which is an important index to monitor the existence of coagulation dysfunction in pregnant women. MPV is an important index to reflect platelet volume, macrophage regeneration and metabolism. Larger volume indicates stronger metabolism, adhesion and aggregation ability. PDW is an index reflecting the difference of platelet volume and platelet regeneration. As the distribution coefficient of MPV, it can reflect the change of platelet volume to some extent. The same idea can be found in the study put forward by Benschop [40]. They applied new methods and the conclusion reached supported our study. There are some limitations in this study. The sample size of our study is not large, and it is a single-center study, so bias is inevitable. In future research, we will carry out multi-center, large-sample prospective studies to obtain more valuable conclusions.
In summary, age, history of diabetes, kidney disorders or preeclampsia, family background of hypertension are all high-risk factors for PIH at present. It is therefore important to monitor high-risk factors, prevent them in a timely manner and choose appropriate treatments to protect maternal health and reduce mortality from perinatal disability. Age, history of diabetes, kidney disorders r preeclampsia, family background of high blood pressure are independent risk factors for preeclampsia. Platelets, coagulation function and thyroid hormone levels have some risk predictive value.
Acknowledgements
Project of Xinyu Science and Technology Bureau: Clinical application of direct repair of uterine incision diverticulum in scar uterine recesarean section, No.: 20183090827; Project of Xinyu Science and Technology Bureau: Clinical Research on the correlation of anti-mullerian tubular hormone (ANH) and polycystic ovary syndrome; No.: 20173090854; Ji’an Science and Technology project “Study on the effect of Verdeludin pelvic perfusion on obstructive salpingitis in rats”, No. Ji Science and Technology [2014] No. 4.
Disclosure of conflict of interest
None.
References
- 1.Boakye E, Kwapong YA, Obisesan O, Ogunwole SM, Hays AG, Nasir K, Blumenthal RS, Douglas PS, Blaha MJ, Hong X, Creanga AA, Wang X, Sharma G. Nativity-related disparities in preeclampsia and cardiovascular disease risk among a racially diverse cohort of US women. JAMA Netw Open. 2021;4:e2139564. doi: 10.1001/jamanetworkopen.2021.39564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haile TG, Assefa N, Alemayehu T, Mariye T, Geberemeskel GG, Bahrey D, Mebrahtom G, Demisse B, Gebrekidan H, Getachew T. Determinants of preeclampsia among women attending delivery services in public hospitals of central tigray, northern ethiopia: a case-control study. J Pregnancy. 2021;2021:4654828. doi: 10.1155/2021/4654828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panda S, Das R, Sharma N, Das A, Deb P, Singh K. Maternal and perinatal outcomes in hypertensive disorders of pregnancy and factors influencing it: a prospective hospital-based study in Northeast India. Cureus. 2021;13:e13982. doi: 10.7759/cureus.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen R, Rogozinska E, Sivarajasingam P, Khan KS, Thangaratinam S. Effect of diet- and lifestyle-based metabolic risk-modifying interventions on preeclampsia: a meta-analysis. Acta Obstet Gynecol Scand. 2014;93:973–85. doi: 10.1111/aogs.12467. [DOI] [PubMed] [Google Scholar]
- 5.Jacquemyn Y, Zemtsova O. Risk factors and prediction of preeclampsia. Acta Clin Belg. 2010;65:1–12. doi: 10.1179/acb.2010.001. [DOI] [PubMed] [Google Scholar]
- 6.Zwertbroek EF, Groen H, Fontanella F, Maggio L, Marchi L, Bilardo CM. Performance of the FMF first-trimester preeclampsia-screening algorithm in a high-risk population in The Netherlands. Fetal Diagn Ther. 2021;48:103–111. doi: 10.1159/000512335. [DOI] [PubMed] [Google Scholar]
- 7.Mor M, Shmueli A, Krispin E, Bardin R, Sneh-Arbib O, Braun M, Arbib N, Hadar E. Intrahepatic cholestasis of pregnancy as a risk factor for preeclampsia. Arch Gynecol Obstet. 2020;301:655–664. doi: 10.1007/s00404-020-05456-y. [DOI] [PubMed] [Google Scholar]
- 8.Hercus A, Dekker G, Leemaqz S. Primipaternity and birth interval; independent risk factors for preeclampsia. J Matern Fetal Neonatal Med. 2020;33:303–306. doi: 10.1080/14767058.2018.1489794. [DOI] [PubMed] [Google Scholar]
- 9.Nourollahpour Shiadeh M, Behboodi Moghadam Z, Adam I, Saber V, Bagheri M, Rostami A. Human infectious diseases and risk of preeclampsia: an updated review of the literature. Infection. 2017;45:589–600. doi: 10.1007/s15010-017-1031-2. [DOI] [PubMed] [Google Scholar]
- 10.Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci. 2019;20:4246. doi: 10.3390/ijms20174246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckers KF, Sones JL. Maternal microbiome and the hypertensive disorder of pregnancy, preeclampsia. Am J Physiol Heart Circ Physiol. 2020;318:H1–H10. doi: 10.1152/ajpheart.00469.2019. [DOI] [PubMed] [Google Scholar]
- 12.Omani-Samani R, Alizadeh A, Almasi-Hashiani A, Mohammadi M, Maroufizadeh S, Navid B, Khedmati Morasae E, Amini P. Risk of preeclampsia following assisted reproductive technology: systematic review and meta-analysis of 72 cohort studies. J Matern Fetal Neonatal Med. 2020;33:2826–2840. doi: 10.1080/14767058.2018.1560406. [DOI] [PubMed] [Google Scholar]
- 13.Brown MA, Roberts L, Hoffman A, Henry A, Mangos G, O’Sullivan A, Pettit F, Youssef G, Xu L, Davis GK. Recognizing cardiovascular risk after preeclampsia: the P4 study. J Am Heart Assoc. 2020;9:e018604. doi: 10.1161/JAHA.120.018604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JM, Rich-Edwards JW, McElrath TF, Garmire L, Myatt L Global Pregnancy Collaboration. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. 2021;77:1430–1441. doi: 10.1161/HYPERTENSIONAHA.120.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess A, Feliu K. Women’s Knowledge of cardiovascular risk after preeclampsia. Nurs Womens Health. 2019;23:424–432. doi: 10.1016/j.nwh.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Marić I, Mayo JA, Druzin ML, Wong RJ, Winn VD, Stevenson DK, Shaw GM. Maternal height and risk of preeclampsia among race/ethnic groups. Am J Perinatol. 2019;36:864–871. doi: 10.1055/s-0038-1675205. [DOI] [PubMed] [Google Scholar]
- 17.Lisowska M, Pietrucha T, Sakowicz A. Preeclampsia and related cardiovascular risk: common genetic background. Curr Hypertens Rep. 2018;20:71. doi: 10.1007/s11906-018-0869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross-Barnet C, Courtot B, Benatar S, Hill I. Preeclampsia risk and prevention among pregnant medicaid beneficiaries. J Health Care Poor Underserved. 2020;31:1634–1647. doi: 10.1353/hpu.2020.0123. [DOI] [PubMed] [Google Scholar]
- 19.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 20.Guedes-Martins L. Superimposed preeclampsia. Adv Exp Med Biol. 2017;956:409–417. doi: 10.1007/5584_2016_82. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Wan P, Ng K, Singh K, Cheng TH, Velickovic I, Dalloul M, Wlody D. Preeclampsia among African American pregnant women: an update on prevalence, complications, etiology, and biomarkers. Obstet Gynecol Surv. 2020;75:111–120. doi: 10.1097/OGX.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 22.Jenabi E, Afshari M, Khazaei S. The association between preeclampsia and the risk of metabolic syndrome after delivery: a meta-analysis. J Matern Fetal Neonatal Med. 2021;34:3253–3258. doi: 10.1080/14767058.2019.1678138. [DOI] [PubMed] [Google Scholar]
- 23.Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398:341–354. doi: 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 24.Paauw ND, Lely AT. Cardiovascular sequels during and after preeclampsia. Adv Exp Med Biol. 2018;1065:455–470. doi: 10.1007/978-3-319-77932-4_28. [DOI] [PubMed] [Google Scholar]
- 25.Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15:275–289. doi: 10.1038/s41581-019-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alese MO, Moodley J, Naicker T. Preeclampsia and HELLP syndrome, the role of the liver. J Matern Fetal Neonatal Med. 2021;34:117–123. doi: 10.1080/14767058.2019.1572737. [DOI] [PubMed] [Google Scholar]
- 27.Dhariwal NK, Lynde GC. Update in the management of patients with preeclampsia. Anesthesiol Clin. 2017;35:95–106. doi: 10.1016/j.anclin.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Chourdakis E, Oikonomou N, Fouzas S, Hahalis G, Karatza AA. Preeclampsia emerging as a risk factor of cardiovascular disease in women. High Blood Press Cardiovasc Prev. 2021;28:103–114. doi: 10.1007/s40292-020-00425-7. [DOI] [PubMed] [Google Scholar]
- 29.Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am J Physiol Renal Physiol. 2020;318:F1315–F1326. doi: 10.1152/ajprenal.00071.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Le Ray I, Zhu J, Zhang J, Hua J, Reilly M. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw Open. 2021;4:e218401. doi: 10.1001/jamanetworkopen.2021.8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Le Ray I, Zhu J, Zhang J, Hua J, Reilly M. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw Open. 2021;4:e218401. doi: 10.1001/jamanetworkopen.2021.8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 33.Wright D, Wright A, Nicolaides KH. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol. 2020;223:12–23. e7. doi: 10.1016/j.ajog.2019.11.1247. [DOI] [PubMed] [Google Scholar]
- 34.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019:CD004659. doi: 10.1002/14651858.CD004659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010:CD000025. doi: 10.1002/14651858.CD000025.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Döbert M, Varouxaki AN, Mu AC, Syngelaki A, Ciobanu A, Akolekar R, De Paco Matallana C, Cicero S, Greco E, Singh M, Janga D, Del Mar Gil M, Jani JC, Bartha JL, Maclagan K, Wright D, Nicolaides KH. Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation. 2021;144:670–679. doi: 10.1161/CIRCULATIONAHA.121.053963. [DOI] [PubMed] [Google Scholar]
- 37.Gudu W. Prodromal symptoms, health care seeking in response to symptoms and associated factors in eclamptic patients. BMC Pregnancy Childbirth. 2017;17:87. doi: 10.1186/s12884-017-1272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewandowska M, Więckowska B, Sajdak S, Lubiński J. Pre-pregnancy obesity vs. other risk factors in probability models of preeclampsia and gestational hypertension. Nutrients. 2020;12:2681. doi: 10.3390/nu12092681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Le Ray I, Zhu J, Zhang J, Hua J, Reilly M. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw Open. 2021;4:e218401. doi: 10.1001/jamanetworkopen.2021.8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019;105:1273–1278. doi: 10.1136/heartjnl-2018-313453. [DOI] [PMC free article] [PubMed] [Google Scholar]


