Abstract
Introduction
Non-alcoholic fatty liver disease (NAFLD) is highly prevalent (~75%) in people with type 2 diabetes (T2D). Since exercise and weight loss (WL) are recommended for the management of both NAFLD and T2D, this study examined whether progressive resistance training (PRT) plus WL could lead to greater improvements in the fatty liver index (FLI), an indicator of NAFLD, compared with WL alone in older adults with T2D.
Research design and methods
This study represents a secondary analysis of a 12-month, two-arm randomised controlled trial including 36 overweight and obese adults (60–80 years) with T2D randomly allocated to supervised PRT plus WL (hypocaloric diet) (n=19) or WL plus sham (stretching) (n=17) for 6 months (phase I), followed by 6-months home-based training with ad libitum diet (phase II). FLI, which is an algorithm based on waist circumference, body mass index, triglycerides and gamma-glutamyl transferase, was assessed at baseline and every 3 months. Linear mixed models were used to analyse between-group differences over time, adjusting for baseline values.
Results
At baseline, the mean±SD FLI was 76.6±18.5 and the likelihood of NAFLD (FLI >60) in all participants was 86%. Following phase I, both groups had similar statistically significant improvements in FLI (mean change (95% CI): PRT+WL, −12 (−20 to –4); WL, −9 (−15 to –4)), with no significant between-group difference. After the subsequent 6-month home-based phase, the improvements in FLI tended to persist in both groups (PRT+WL, −7 (−11 to –2); WL, −4 (−10 to 1)), with no between-group differences.
Conclusions
In older overweight adults with T2D, PRT did not enhance the benefits of WL on FLI, a predictor of NAFLD.
Trial registration number
ACTRN12622000640707.
Keywords: diabetes mellitus, type 2; non-alcoholic fatty liver disease; exercise; weight loss
What is already known on this topic
Lifestyle strategies such as weight loss through hypocaloric diets and exercise, including progressive resistance training (PRT), are cornerstone to prevention and management of both type 2 diabetes (T2D) and non-alcoholic liver disease (NAFLD).
However, whether PRT plus weight loss is more effective for improving NAFLD than weight loss alone is not known.
What this study adds
In this 12-month randomized controlled clinical trial including 36 older overweight and obese sedentary adults with T2D, weight loss with or without PRT was associated with similar significant reductions in the fatty liver index (FLI), an indicator of NAFLD.
This was likely due to the favourable changes (losses) in both weight and adiposity which were similar between the groups, despite PRT providing some additional benefits to muscle (lean) mass.
How this study might affect research, practice or policy
While this study indicates that weight loss is the key factor driving improvements in the FLI in older adults with T2D, further research is needed to explore the potential synergistic effects of exercise combined weight loss on other liver outcomes (eg, hepatic fat) in a large sample of older adults with T2D and NAFLD to help shape future clinical guidelines.
Introduction
Type 2 diabetes (T2D) is a serious global public health issue, estimated to affect 462 million people globally.1 Individuals with T2D are also at significantly higher risk of multiple other chronic diseases, including non-alcoholic fatty liver disease (NAFLD).2 In fact, the prevalence of NAFLD in people with T2D has been identified as high as 60%–75%,3 with the rapidly rising burden of both diseases heightened by poor dietary habits, physical inactivity and sedentary behaviours.4 5 Both conditions also share several common metabolic risk factors and pathophysiological and inflammatory pathways,6 with a growing body of evidence indicating a bidirectional relationship between T2D and NAFLD.4 7
For both T2D and NAFLD, lifestyle strategies including weight loss (WL) through dietary modification and exercise are the cornerstone for prevention and management.8 9 Available evidence indicates calorie restriction used to induce WL (7%–10% total body weight) is effective for improving glycemic control and reducing liver fat in those with T2D and NAFLD.10 11 However, WL is often associated with a concurrent loss in muscle (lean) mass,12 which is important as there is growing evidence that low muscle mass is independently associated with poor glycemic control13 and increased risk of T2D,14 including in those with NAFLD.15 16 A meta-analysis of 18 cross-sectional studies involving 48 709 adults also found that low muscle mass was associated with a 1.3-fold and 2.4-fold increased risk and severity of NAFLD, respectively.17 This suggest that optimising muscle (lean) mass may represent an important approach to prevention and management of both T2D and NAFLD.
Progressive resistance training (PRT) is one method that has been shown to improve body composition, particularly muscle (lean) mass, as well as glycemic control and blood lipids in people with T2D.18 Resistance training has also been shown to reduce liver fat (10%–25%), liver enzymes and the FLI, as well as improve muscle (lean) mass in people with NAFLD.19 20 In older adults with obesity and those with T2D, we and others have shown that PRT can prevent the concurrent loss of muscle (lean) mass associated with WL while resulting in similar reductions in fat mass and total body weight, as achieved by WL alone.21–23 Furthermore, in older overweight adults with T2D we have shown that high-intensity PRT (75%–85% of one repetition maximum strength) in combination with moderate WL (−2.5 to −3.1 kg) was more effective at improving glycemic control (glycated hemoglobin (HbA1c)) and lean mass compared with WL alone, despite similar losses in fat mass.22 23 However, whether PRT combined with WL is more effective at improving NAFLD outcomes, compared with WL, has not been examined. Therefore, the aim of this study, which is a secondary analysis of our previous 12-month RCT,22 23 was to investigate whether 6 months of high-intensity PRT combined with WL can reduce the risk of NAFLD (improve FLI) in older overweight and obese adults with T2D compared with WL alone. A secondary aim was to evaluate whether any improvement in FLI following the supervised and structured training can be maintained following 6 months of home-based exercise training without any further instruction to lose weight.
Methods
Study design
This is a secondary analysis of a prior two-arm, 12-month RCT consisting of two phases involving 36 older overweight and obese adults with T2D.22 23 Phase I incorporated 6 months of supervised and structured gym-based PRT with WL and phase II involved a further 6 months of home-based PRT with ad libitum diet. Participants were randomly assigned (via a computer-generated random number table in Excel) to either of the two groups by an independent researcher. Recruitment to the intervention occurred over a 2-year period (February 1999 to January 2001). Repeated measures were conducted at 3, 6, 9 and 12 months for all outcomes, other than body composition (6 and 12 months). As previously reported,22 23 participants were initially randomized to either PRT+WL (n=19) or sham (flexibility) training+WL (n=17) for the first 6 months. All assessments and training were performed at Deakin University, Melbourne, Australia. The study was retrospectively registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12622000640707).
Participants
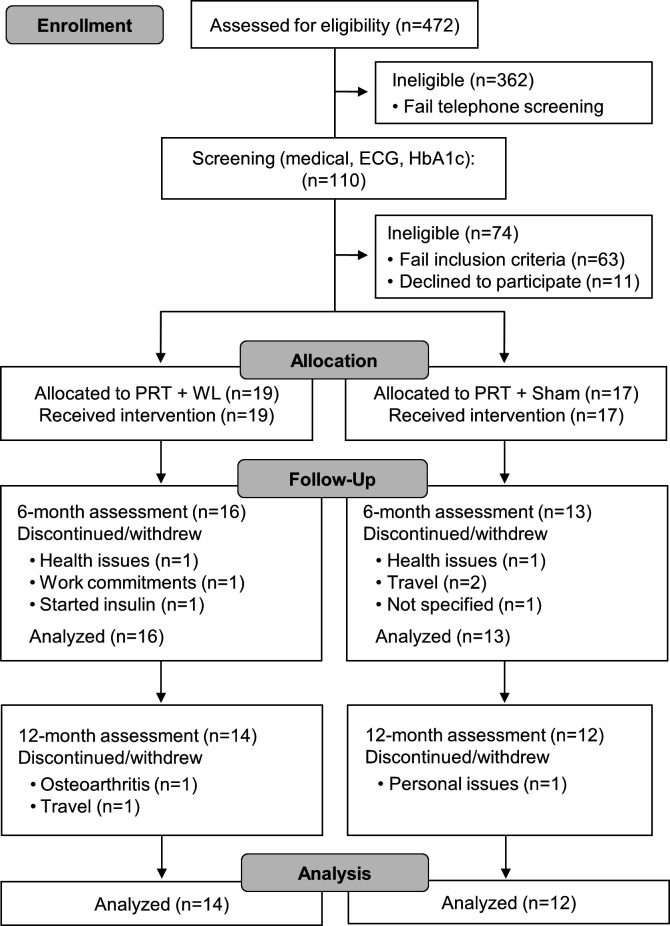
As previously reported,22 23 36 overweight and obese adults aged 60–80 years with T2D were recruited from the International Diabetes Institute Clinics. Participants were initially screened by telephone, with eligible participants (n=110) required to undertake further assessments to determine eligibility (HbA1c, resting blood pressure, ECG, medical history). Participants were included based on the following criteria: established T2D (>6 months), being treated with diet or a oral hypoglycemic agent (excluding insulin), HbA1c range 7%–10%, overweight or obese (body mass index (BMI) >27 kg/m2 and ≤40 kg/m2), not participating in regular PRT and engaging in <150 min moderate or <60 min vigorous exercise/week (preceding 6 months), non-smoker and consuming <2 alcoholic drinks/day. Exclusion criteria were: history/evidence of ischemic heart disease, systemic diseases, hypertension (>160/90 mm Hg), advanced diabetic neuropathy and/or retinopathy and conditions (severe orthopedic, cardiovascular or respiratory) that prevent participation and those with absolute exercise contraindications according to the American College of Sports Medicine guidelines.24 A total of 47 participants (24 men, 23 women) were deemed eligible, of which 36 agreed to participate. As reported previously,22 23 in the first 8 weeks six participants (PRT+WL group, n=2, sham+WL group, n=4) withdrew due to non-related health problems or commitments and one participant was excluded due to starting insulin. In total, 81% of participants (PRT+WL, n=16, 84%; sham+WL, n=13, 76%) completed phase I. An additional three participants (PRT+WL, n=2; sham+WL, n=1) withdrew during the first 2 weeks of phase II (home-based training) due to travel, osteoarthritis knee pain and unrelated personal issues. Thus, 26 participants (PRT+WL, n=14, 74%; sham+WL, n=12, 71%) completed the 12-month intervention (figure 1).
Figure 1.
Study flow chart. HbA1c, glycated hemoglobin; PRT, progressive resistance training; WL, weight loss.
Intervention
Phase I: supervised gym-based intervention
A detailed description of the exercise intervention has been previously reported.22 23 Briefly, for the first 6-month gym-based intervention, all participants attended the exercise laboratory at Deakin University three non-consecutive days per week. Those randomized to PRT performed an individually prescribed 45–60 min, high-intensity (75%–85% of their one repetition maximum strength) program consisting of free weights and weights machines (three sets of 8–10 repetitions, nine exercises). To ensure correct technique and progression, all sessions were fully supervised. The sham flexibility group sessions consisted of 5 min of stationary cycling (no workload) followed by a sequence of static stretching exercises (~30 min) designed to provide participation and improve flexibility but not to elicit changes in muscle strength or fitness.
Phase II: home-based training
Following the 6-month supervised gym-based intervention, participants were prescribed a home-based exercise program in which they were provided with individualised instructions and equipment (dumbbells and ankle weights for PRT group and flexibility chart for flexibility group). Participants were asked to train 3 days/week at home and/or at a community or commercial leisure centre. To facilitate transition, participants in the PRT+WL group performed the home-based PRT program within the structured and supervised gym setting for the final month of phase I. The home-based exercises replaced weight machines with dumbbells and ankle weights and participants were requested to complete nine exercises (three sets of 8–10 repetitions) with the aim to exercise at a moderate intensity (at least 60% of maximum). Participants attended the gymnasium monthly to monitor technique and progression and completed weekly exercise diaries to monitor adherence. In addition, weekly phone calls (first month) and subsequent fortnightly calls monitored adherence and enabled participants to ask questions and receive feedback. A single home visit was conducted early during the home program to ensure safety and provide additional weights to facilitate progression. Participants in the control flexibility group were requested to maintain the flexibility program at home.
Weight loss intervention
Four weeks prior to the commencement of phase I, all participants were placed on a healthy eating plan supplying ≤30% total energy from total fat (≤10% saturated fat) with protein and carbohydrate being distributed for remaining energy. Individually prescribed by a dietitian, the plan was designed to induce moderate WL (~0.25 kg/week) throughout phase I. Interviews every 2 weeks by the dietitian and completion of a weekly checklist were used to assess adherence. Changes in nutrient intake were assessed via a 3-day food record conducted at 3 and 6 months. Nutrition information was analyzed using Foodworks nutrient analysis software (Xyris, Brisbane, Queensland, Australia). Following the gym-based intervention (phase I), participants were not required to adhere to the healthy eating (WL) plan and did not receive further dietary recommendations.
Measurements
Health and medical history
Information on participants health and medical history were assessed via an interviewer questionnaire conducted at baseline. Information collected included: duration of diabetes (years), age of diabetes onset, oral hypoglycemic medication use, lipid-lowering medication use, history of several diseases (eg, hypertension, retinopathy, neuropathy and arthritis/osteoarthritis) and supplement usage.
Anthropometry
Height (cm) was measured using a Holtain stadiometer (Holtain, Croswell, Wales) and weight (kg) using SECA electronic scales, assessed to the nearest 0.1 kg. Waist circumference (WC) was measured at the midpoint between the iliac crest and lower edge of rib cage using a non-elastic measuring tape.
Biochemical measures and the fatty liver index
Morning venous blood samples were collected at baseline, 3, 6, 9 and 12 months with all samples obtained after an overnight fast and at least 48 hours post exercise. The biomarkers (gamma-glutamyl transferase (GGT) and triglycerides (TG)) were analyzed using standard laboratory procedures. The FLI, a well-validated and simple algorithm, was defined by the following formula25:
FLI=(e0.953×LN (TG)+0.139×BMI+0.718×LN (GGT)+0.053×WC–15.745)/(1+e0.953×LN (TG)+0.139×BMI+0.718×LN (GGT)+0.053×WC–15.745)×100
Scores for the FLI range from 0 to 100, with a score ≥60 being used to consider the likely presence of NAFLD and <30 to rule out the presence of liver steatosis.25 Blood measures were available for the following number of participants: baseline, 3 and 6 months (PRT+WL, n=16; sham+WL, n=13), 9 and 12 months (PRT+WL, n=14; sham+WL, n=12).
Habitual physical activity
An interview-administered validated 7-day physical activity recall questionnaire was used to estimate habitual physical activity (energy expenditure, kcal/day), excluding the exercise intervention.26
Statistical analysis
Statistical analysis was conducted using Stata SE Statistical software. The data were analyzed using a modified intention-to-treat approach, including all randomized participants with at least one follow-up measurement. A linear mixed model was used to analyze the data, with random intercepts for participants, time as a repeated measure and an interaction between group and time. For all models, normality and homogeneity of variance of the residuals were checked using quantile-quantile plots and scatter plots, respectively. No data imputation was undertaken. The results were analyzed adjusted for baseline values (model 1), and model 1 plus baseline total lean body mass (model 2), and model 1 plus baseline physical activity (model 3). A mixed effects logistic regression model was used to analyze group differences for the changes in proportion of participants with an FLI ≥60. Statistical significance was set at p<0.05.
Results
Participant characteristics
Baseline characteristics are shown in table 1. The mean±SD age and BMI of the participants was 67.3±5.1 years and 31.9±3.5 kg/m2, respectively, with 69% of the participants classified as obese (BMI >30). The mean duration of diabetes was 8.1±6.5 years and 86.2% of participants were taking oral hypoglycemic medication (other than insulin) and 34.5% of participants were taking lipid-lowering medications. During phase I, four participants (PRT+WL n=3, sham+WL n=1) decreased their oral hypoglycemic medication dosage while four participants (PRT+WL n=2, sham+WL n=2) increased medication. During phase II, one participant increased and one decreased hypoglycemic medication dosage (both PRT+WL). Regarding lipid-lowering medication, one participant commenced lipid-lowering medication (sham+WL) during phase I, while during phase II, one participant (PRT+WL) commenced and one ceased (sham+WL) medication. At baseline, 86.2% of participants had a likely presence of NAFLD (FLI >60), while 3.5% indicated an absence of NAFLD (FLI <30) (table 1).
Table 1.
Baseline characteristics of participants in the progressive resistance training plus moderate weight loss (PRT+WL) and moderate weight loss group (sham+WL)
| Characteristic | PRT+WL (n=16) | Sham+WL (n=13) |
| Male/Female, n | 10/6 | 6/7 |
| Age (years) | 67.6±5.2 | 66.9±5.3 |
| Height (cm) | 167.8±8.7 | 166.0±9.2 |
| Weight, kg | 88.7±10.9 | 89.5±12.1 |
| BMI, kg/m2 | 31.5±10.9 | 32.5±3.8 |
| Waist circumference (cm) | 105.3±7.5 | 103.3±11.4 |
| Age at diagnosis (years) | 60.1±5.0 | 58.1±8.6 |
| Duration diabetes (years) | 7.6±5.4 | 8.8±7.9 |
| Oral hypoglycemic medication use, n (%) | 15 (94%) | 10 (78%) |
| Lipid-lowering medication use, n (%) | 5 (31%) | 5 (38%) |
| Estimated physical activity (kJ/day) | 3022±413 | 3110±428 |
| FLI <30, n (%) | 0 (0%) | 1 (8%) |
| 30–59, n (%) | 2 (13%) | 1 (8%) |
| >60, n (%) | 14 (87%) | 11 (85%) |
Values presented are mean±SDs unless otherwise indicated.
BMI, body mass index; FLI, fatty liver index.
Adherence to intervention
As reported previously,22 23 average adherence to the exercise program was 88% and 85% (PRT+WL and sham+WL, respectively) during phase I. During the home-based training (phase II), mean adherence was 73% in PRT+WL and 78% in sham+WL.
Anthropometry and biochemical measures
There was a comparable statistically significant reduction in body weight in both the PRT+WL and sham+WL groups after 3 months (mean±SD change: −1.8±2.0 kg vs −2.0±1.5 kg, both p<0.01) and 6 months (−2.5±2.9 kg vs −3.1±2.1 kg, both p<0.01) relative to baseline. At completion of 12 months, both groups experienced similar increases in weight (mean change relative to baseline: PRT+WL, -1.7±1.9 kg; sham+WL, -1.6±2.0 kg, both p<0.05), however weight did remain significantly lower than baseline values for both groups.
The absolute within-group changes and net between-group differences for the change in BMI, WC, GGT, TG and FLI relative to baseline are shown in table 2. After phase I, comparable statistically significant reductions in BMI and WC were observed within each group. Neither group experienced a significant change in TG levels after the initial 6 months. PRT+WL had a significant 7.4 and 9.1 U/L (both p<0.05) decrease in GGT after 3 and 6 months, respectively, but these changes did not differ significantly from sham+WL after 3 months (p=0.06) or 6 months (p=0.09). At completion of phase II, BMI and WC remained significantly lower than baseline values for both groups with no significant between-group differences (table 2). TG levels remained relatively unchanged (both groups) and there were no significant within-group changes relative to baseline nor group differences in GGT after 9 or 12 months.
Table 2.
Baseline values and absolute within-group changes after the supervised, gym-based training (phase I, 3 and 6 months) and the home-based training (phase II, 9 and 12 months) in PRT+WL and sham+WL for BMI, waist circumference, triglycerides, gamma-glutamyl transferase and FLI and the net between-group differences for change relative to baseline
| Mean (95% CI) absolute change from baseline | |||||
| Baseline | ∆ 3 months | ∆ 6 months | ∆ 9 months | ∆ 12 months | |
| BMI, kg/m2 | |||||
| PRT+WL | 31.5±3.4 | −0.6 (−1.0 to –0.3)** | −0.9 (−1.4 to –0.3)** | −0.8 (−1.3 to –0.3)** | −0.6 (−1.0 to –0.2)** |
| Sham+WL | 32.5±3.8 | −0.7 (−1.0 to –0.4)*** | −1.1 (−1.5 to –0.7)*** | −1.1 (−1.7 to –0.6)** | −0.6 (−1.0 to –0.1)* |
| Net difference (95% CI) | 0.1 (−0.4 to 0.6) | 0.2 (−0.5 to 0.9) | 0.3 (−0.4 to 1.0) | 0.0 (−0.5 to 0.5) | |
| P value† | 0.76 | 0.62 | 0.32 | 0.79 | |
| Waist circumference, cm | |||||
| PRT+WL | 105.3±7.5 | −3.8 (−5.6 to –1.9)*** | −6.9 (−10.0 to –3.9)*** | −6.8 (−10.5 to –3.0)** | −3.7 (−6.5 to –1.0)* |
| Sham+WL | 103.3±11.4 | −3.1 (−5.2 to –1.0)** | −6.7 (−10.4 to –3.0)** | −6.4 (−10.0 to –2.7)** | −2.3 (−4.3 to –0.2)* |
| Net difference (95% CI) | −0.7 (−3.3 to 2.0) | −0.3 (−4.8 to 4.2) | −0.4 (−5.4 to 4.6) | −1.4 (−4.8 to 1.9) | |
| P value† | 0.74 | 0.88 | 0.75 | 0.32 | |
| Triglycerides, mmol/L | |||||
| PRT+WL | 1.83±0.75 | −0.23 (−0.60 to 0.15) | −0.24 (−0.63 to 0.15) | −0.39 (−0.80 to 0.01) | −0.09 (−0.44 to 0.26) |
| Sham+WL | 1.85±0.78 | −0.05 (−0.58 to 0.47) | −0.08 (−0.45 to 0.28) | 0.12 (−0.56 to 0.79) | 0.28 (−0.49 to 1.04) |
| Net difference (95% CI) | −0.17 (−0.77 to 0.43) | −0.16 (−0.68 to 0.36) | −0.51 (−1.23 to 0.21) | −0.37 (−1.13 to 0.39) | |
| P value† | 0.52 | 0.48 | 0.39 | 0.60 | |
| Gamma-glutamyl transferase, U/L | |||||
| PRT+WL | 45.9±47.6 | −7.4 (−13.7 to –1.0)* | −9.1 (−16.1 to –2.1)* | −4.8 (−14.4 to 4.8) | −2.1 (−11.5 to 7.3) |
| Sham+WL | 34.2±28.5 | 3.2 (−6.9 to 13.4) | 7.0 (−15.3 to 29.3) | 5.2 (−9.0 to 8.6) | 0.9 (−6.0 to 7.8) |
| Net difference (95% CI) | −10.6 (−21.5 to 0.3) | −16.1 (−36.4 to 4.2) | −10.0 (−27.5 to 7.6) | −3.0 (−14.4 to 8.4) | |
| P value† | 0.06 | 0.09 | 0.07 | 0.10 | |
| FLI | |||||
| PRT+WL | 77.2±20.0 | −7.0 (−12.0 to –2.0)** | −12.3 (−20.3 to –4.2)** | −11.7 (−17.6 to -5.9)*** | −6.5 (−10.8 to –2.2)** |
| Sham+WL | 75.9±19.8 | −10.8 (−23.6 to 2.1) | −9.3 (−14.8 to –3.8)** | −10.1 (−19.1 to –1.0)* | −4.3 (−9.9 to 1.4) |
| Net difference (95% CI) | 3.8 (−8.3 to 15.9) | −2.9 (−12.7 to 6.9) | −1.6 (−11.5 to 8.2) | −2.3 (−8.9 to 4.3) | |
| P value† | 0.50 | 0.56 | 0.95 | 0.75 | |
All baseline values are unadjusted means±SDs. All change values are unadjusted means (95% CI) and expressed as absolute changes from baseline. Mean net differences (95% CI) were calculated by subtracting within-group changes for PRT+WL from within-group changes from WL.
*P<0.05, **p<0.01, ***p<0.001 within-group change from baseline.
†P values represent group-by-time interaction from linear mixed models adjusted for baseline values.
BMI, body mass index; FLI, fatty liver index; PRT, progressive resistance training; WL, weight loss.
Fatty liver index
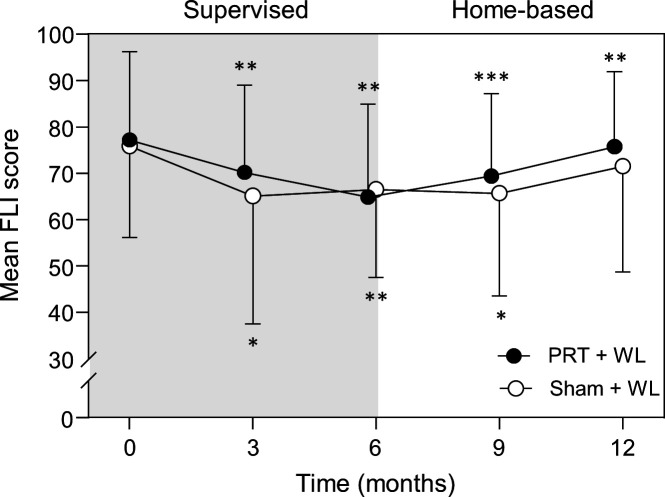
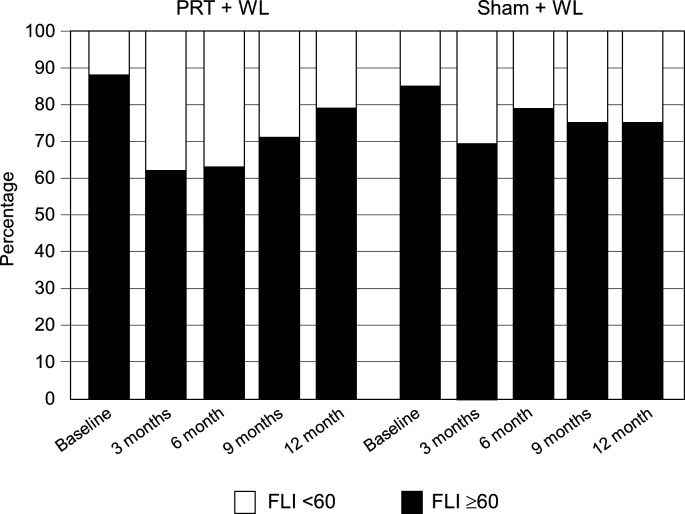
At completion of 6 months, both groups experienced a statistically significant reduction in FLI, with no significant between-group differences for the change after 3 months (interaction, p=0.50) or 6 months (interaction, p=0.56) (table 2 and figure 2). All results for FLI remained unchanged after further adjusting for baseline total lean body mass and baseline physical activity. After 12 months (phase II), the significant improvement in FLI persisted in PRT+WL relative to baseline but not sham+WL, however between-group differences for the change over time were not significant (interaction, p=0.75). Regarding NAFLD risk, the proportion of participants with an FLI score ≥60 after 3 months and 6 months decreased from baseline by 26% and 25% in the PRT+WL and 16% and 8% in the sham+WL, respectively (figure 3). At completion of 12 months, there was a 9% and 10% reduction from baseline in the PRT+WL and sham+WL for the proportion of participants with a FLI ≥60. There was no statistically significant difference between groups for the change in proportion of participants with a FLI ≥60 at 3 months (p=0.93), 6 months (p=0.37), 9 months (p=0.48) or 12 months (p=0.73).
Figure 2.
Mean (±SD) fatty liver index (FLI) scores in the progressive resistance training plus weight loss (● PRT+WL) and weight loss (○ sham+WL) group at baseline, 3, 6, 9 and 12 months. *P<0.05, **p<0.01, ***p<0.001 vs baseline. The grey shaded region represents the supervised, gym-based training phase and when both groups were prescribed a moderate weight loss diet and the white region represents the home-based training phase with the ad libitum diet.
Figure 3.
Proportion of participants in the progressive resistance training plus weight loss (PRT+WL) and weight loss (sham+WL) groups that were classified as likely to have non-alcoholic fatty liver disease (fatty liver index (FLI) ≥60) at baseline, 3, 6, 9 and 12 months. The first 6 months represent the supervised, gym-based training and the second 6 months the home-based training.
Discussion
The main finding from this RCT in older overweight and obese adults with T2D was that 6 months of moderate WL, with or without supervised high-intensity PRT, was associated with similar significant improvements (reductions) in FLI. A second key finding was that FLI tended to increase in both groups in parallel with weight regain during the second 6 months following the ad libitum diet, but remained below baseline levels in those undertaking the home-based PRT. While this indicates that home-based PRT may help to attenuate some of the weight-related regains in FLI, collectively, findings from this study suggest that the primary factors driving changes in FLI are alterations in body weight and adiposity and that PRT provided no or little additional benefits in older overweight and obese adults with T2D. However, the findings related to PRT should be interpreted with caution considering the modest sample size which likely limited our ability to detect any additive benefits of PRT over WL on FLI in this study involving secondary data analysis.
Since we have previously reported significantly greater improvements in glycemic control (HbA1c −1.2±1.0% vs −0.4±0.8%) and total body lean mass (0.5±1.1 kg vs −0.4±1.0 kg), and greater reductions in inflammatory markers interleukin-10 and tumor necrosis factor-α, compared with sham+WL group,22 23 we hypothesized that there would be a greater improvement in FLI in those undertaking PRT. This hypothesis was also informed by previous research demonstrating PRT may benefit NAFLD through enhanced insulin sensitivity,27 reduced inflammation28 and/or increased muscle mass.29 30 However, few studies have assessed the effect of PRT on FLI directly, and studies assessing other liver outcomes (eg, liver fat, liver enzymes), are limited and have reported mixed findings.19 27 31 For example, previous research has indicated 8–12 weeks of PRT at moderate intensity (50%–70% of one repetition maximum strength), independent of WL, can achieve modest reductions in liver fat (10%–13%) in those with NAFLD.30 32 More notable decreases in liver fat (26%) and improvements in the liver enzyme ALT have been demonstrated after 4 months of high-intensity PRT (70%–80% of one repetition maximum strength) combined with dietary advice in T2D participants with NAFLD.19 Together, these findings suggest that high-intensity PRT may result in more favorable NAFLD outcomes. Therefore, the lack of any additional benefits of PRT in our study were somewhat unexpected as participants were prescribed a high-intensity (75%–85% of one repetition maximum strength) PRT program (phase I) in which adherence was excellent (mean 88%) and the program resulted in multiple other benefits over WL alone as indicated above. Furthermore, in our cohort of older overweight and obese adults with T2D, 86% of participants were identified to be at high risk of NAFLD (FLI >60). One possible explanation is that the FLI includes only selected measurements of body fat (WC) and liver function (eg, GGT), and thus may not be sensitive to changes in liver fat in response to the PRT above WL alone, or the difference was not substantial enough to be detected by the small sample size of our trial. Although FLI is well validated in population studies,25 it is conceivable there may have been benefits to other key liver outcomes (eg, liver fat, liver enzymes) that we did not assess and are not reflected in the FLI or conversely a longer duration of high-intensity PRT may be required to elicit improvements in FLI. However, there is some evidence supporting the benefits of 10–24 weeks of PRT for improving FLI (13%–18%) in older menopausal women with obesity33 and those with T2D,34 but these studies included PRT in conjunction with aerobic training (AT). Aerobic training is often associated with greater reductions in visceral fat relative to PRT and changes in adipocytokines,35 which may explain the observed improvements in FLI in these studies.
There is emerging evidence indicating that lean (muscle) mass may play an important role in NAFLD,36 with research highlighting an inverse association between measures of muscle mass and NAFLD risk and severity.17 For instance, a population-based study involving 10 534 community-dwelling adults (2631 with NAFLD) aged 51.4 (SD 8.3) years found those in the highest tertile for body weight-adjusted appendicular skeletal muscle mass gain after 1 year, exhibited a significant reduction in liver fat, and resolution of baseline NAFLD after 7 years.37 Therefore, it is possible that PRT-related improvement in muscle mass may play a role in improving NAFLD outcomes. This is likely mediated by improved insulin resistance and muscle-liver crosstalk via modification in myokines.38 There is consistent evidence from clinical trials highlighting the effectiveness of PRT in healthy people and those with chronic conditions as a strategy to improve lean (muscle) mass,39 40 including people with NAFLD.29 30 However, whether PRT-related changes in muscle (lean) mass are associated with improvements in liver outcomes in people with NAFLD remains uncertain. A 12-week RCT in sedentary obese men with NAFLD reported a ~14% reduction in liver fat following a high-intensity PRT program, without any accompanying WL,30 in which the men experienced a mean 1.2 kg gain in muscle mass. As we have reported elsewhere,22 23 the PRT+WL group in our study experienced a significant mean 0.5 kg increase in total body lean mass after 6 months while the WL group had a mean 0.4 kg reduction, with both groups experiencing similar losses in fat mass. It is plausible that the lean mass gain of 0.5 kg in our study was not substantial enough to translate into any benefits to FLI. Whether there is an optimal gain in muscle (lean) mass that may elicit improvements in FLI is not known, but further prospective studies and intervention trials are needed to evaluate whether a given exercise-induced change in lean (muscle) mass may be associated with improvements in liver-specific outcomes in people with NAFLD, independent of changes in weight or fat mass.
The finding that there were similar significant reductions in FLI in both the PRT+WL and sham+WL groups in our study is likely attributed to the modest WL experienced by both groups. Furthermore, both groups achieved comparable improvements in measures of adiposity, including BMI, WC and fat mass (mean change after 6 months: −2.1 to −2.4 kg).22 23 Given that WL is recommended as one of the key strategies to reduce liver fat, it is possible the effects of PRT in our study were masked by the effects of changes in body weight and fat mass. There is compelling evidence that hypocaloric diets resulting in WL of 7%–10% of total body weight are associated with reductions in liver fat (~40%–50%) and liver markers in those with NAFLD.41–45 In terms of FLI, a 32%–38% reduction was reported following WL of ~9%–11% via hypocaloric diets in 98 overweight and obese adults with NAFLD.46 In our study of older overweight and obese adults with T2D, a significant although more modest (~13%–16%) reduction in FLI was observed after 6 months. The smaller changes in FLI observed in our study are likely related to the smaller reductions in body weight (mean change 2.8% PRT+WL and 3.5% sham+WL). In agreement with our findings, another study conducted in 716 participants with NAFLD reported that a 3.4% reduction in total body weight was associated with a 12% reduction in FLI, after 6 months of a lifestyle intervention (diet and habitual physical activity).47 Collectively, these findings support previous research highlighting a strong relationship between the magnitude of change (loss) in weight (adiposity) and subsequent changes in FLI.48
Another key outcome from our study was that the WL-related improvements in FLI during phase I in both groups tended to increase (return to baseline values) during phase II with the ad libitum diet and concomitant gains in weight and fat mass. In agreement with these findings, previous research conducted in 98 overweight and obese adults with NAFLD demonstrated that WL (diet induced) reductions in FLI were reversed ensuing subsequent weight regain.48 However, it is worth noting that FLI did remain significantly below baseline values in the PRT+WL group after phase II in our study. Nevertheless, this was not significantly different from the sham+WL group which limits our ability to make claims about the potential benefits of PRT alone as a modality to maintain WL-induced improvements in FLI. A potential reason for why the home-based PRT training did not result in greater benefits to FLI relative to the sham exercise may relate in part to a reduction in training adherence (88%–73%) and total training volume (~52%) in phase II, as machine weights were replaced by free weights (dumbbells, ankle weights) which limited (and reduced related to phase I) the total training load prescribed.23
The strengths of this study are that it is the first to examine the effect of PRT+WL versus WL alone in NAFLD using FLI in older overweight and obese adults with T2D. The RCT design with a long-term follow-up (12 months), and high adherence to the high-intensity PRT are also noteworthy strengths. However, there are several limitations. First, this study represents a secondary analysis of a previous RCT with a small sample size that was not designed to detect any potential between-group differences in FLI. Second, liver enzymes and direct measures of liver fat were not available, and FLI was used as a surrogate determinant of NAFLD risk. Third, both BMI and WC are parameters in the FLI equation, and therefore changes in FLI are primarily mediated by reductions in body weight, and thus does not capture the potential beneficial effects of changes in lean mass on liver fat. Therefore, it is possible that PRT may have induced additive improvements in liver outcomes unable to be captured in the present study. Given most previous studies report liver fat as the primary outcome, capacity for more meaningful comparison between other PRT-related studies was also limited. Future studies may also consider a more comprehensive assessment of body composition, including muscle adiposity, as there is evidence that individuals with high muscle fat are more likely to have higher liver fat.49
Conclusion
In sedentary, older overweight and obese adults with T2D, 6 months of moderate WL was associated with improvements in FLI, but high-intensity PRT did not provide any added benefits. While these findings support the role for improving (reducing) weight and adiposity as a key strategy for the management of NAFLD in overweight and obese adults with T2D, the lack of any significant added benefits of PRT must be considered given the modest sample size. This likely limited our ability to detect any additive effects from this trial which represents secondary data analysis. Further large-scale and appropriately powered RCTs assessing liver-specific outcomes and other forms of exercise (eg, PRT combined with AT) are required to provide greater insight into the potential synergistic effects of exercise with WL in this cohort.
bmjdrc-2022-002950supp001.pdf (157.9KB, pdf)
Footnotes
Contributors: DWD: study design. RMD, ESG and S-YT: research concept. RMD and DWD conducted the research. CLF, GA and RMD: analyzed the data. CLF wrote the manuscript. ESG, S-YT, GA, DWD and RMD: reviewed and edited the manuscript. RMD is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of data analysis.
Funding: This study was financially supported by a grant (grant number: 98-0146) from the Victorian Health Promotion Foundation (VicHealth). The Rotary Club of Kew, Victoria, Australia and Soroptimist International, Brighton Division provided funds for the purchase of exercise equipment.
Competing interests: All authors have completed the ICMJE uniform disclosure form and declare: no support from any organization for the submitted work, DWD has received research grants from the National Health and Medical Research Council, no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the International Diabetes Institute and Deakin University Human Research Ethics Committees (EC 26-99). All participants provided written informed consent prior to participation.
References
- 1.Khan MAB, Hashim MJ, King JK. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J Epidemiol Glob Health 2020;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baena-Díez JM, Peñafiel J, Subirana I. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care 2016;39:1987–95. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Golabi P, de Avila L. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793–801. [DOI] [PubMed] [Google Scholar]
- 4.Targher G, Marchesini G, Byrne CD. Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: causal association or epiphenomenon? Diabetes Metab 2016;42:142–56. [DOI] [PubMed] [Google Scholar]
- 5.Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol Clin North Am 2016;45:639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomah S, Alkhouri N, OJCd H. Nonalcoholic fatty liver disease and type 2 diabetes: where do Diabetologists stand? Clin Diabetes Endocrinol 2020;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muzica CM, Sfarti C, Trifan A. Nonalcoholic fatty liver disease and type 2 diabetes mellitus: a bidirectional relationship. Can J Gastroenterol 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed IA, Mikail MA, Mustafa MR. Lifestyle interventions for non-alcoholic fatty liver disease. Saudi J Biol Sci 2019;26:1519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perumpail BJ, Cholankeril R, Yoo ER, et al. An overview of dietary interventions and strategies to optimize the management of non-alcoholic fatty liver disease. Diseases 2017;5. 10.3390/diseases5040023. [Epub ahead of print: 22 10 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plauth M, Bernal W, Dasarathy S. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38:485–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parry SA, Hodson L. Managing NAFLD in type 2 diabetes: the effect of lifestyle interventions, a narrative review. Adv Ther 2020;37:1381–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batsis JA, Gill LE, Masutani RK. Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J Am Geriatr Soc 2017;65:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzo A, Massimino E, Riccardi G. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients 2021;13:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anagnostis P, Gkekas NK, Achilla C. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Osaka T, Fukuda T. The relationship between hepatic steatosis and skeletal muscle mass index in men with type 2 diabetes. Endocr J 2016;63:877–84. [DOI] [PubMed] [Google Scholar]
- 16.Kim TN, Park MS, Yang SJ. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes. Diabetes Care 2010;33:1497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai C, Song X, Chen Y. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol Int 2020;14:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Ye W, Chen Q. Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: a systematic review and meta-analysis. Int J Env Res Public Health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacchi E, Negri C, Targher G. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease. Hepatology 2013;58:1287–95. [DOI] [PubMed] [Google Scholar]
- 20.Balducci S, Cardelli P, Pugliese L. Volume-Dependent effect of supervised exercise training on fatty liver and visceral adiposity index in subjects with type 2 diabetes the Italian diabetes exercise study (ides). Diabetes Res Clin Pract 2015;109:355–63. [DOI] [PubMed] [Google Scholar]
- 21.Sardeli AV, Komatsu TR, Mori MA. Resistance training prevents muscle loss induced by caloric restriction in obese elderly individuals: a systematic review and meta-analysis. Nutrients 2018;10:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunstan DW, Daly RM, Owen N. High-Intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 2002;25:1729–36. [DOI] [PubMed] [Google Scholar]
- 23.Dunstan DW, Daly RM, Owen N. Home-Based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care 2005;28:3–9. [DOI] [PubMed] [Google Scholar]
- 24.Medicine ACoS . ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Bedogni G, Bellentani S, Miglioli L. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. J BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallis JF, Haskell WL, Wood PD. Physical activity assessment methodology in the Five-City project. A J Epidemiol 1985;121:91–106. [DOI] [PubMed] [Google Scholar]
- 27.Hallsworth K, Fattakhova G, Hollingsworth KG. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman SL, Neuschwander-Tetri BA, Rinella M. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi A, Imaizumi H, Hayashi M. Simple resistance exercise for 24 weeks decreases alanine aminotransferase levels in patients with non-alcoholic fatty liver disease 2017;1:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh S, So R, Shida T. High-Intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease 2017;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slentz CA, Bateman LA, Willis LH. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab 2011;301:E1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallsworth K, Thoma C, Hollingsworth KG. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial 2015;129:1097–105. [DOI] [PubMed] [Google Scholar]
- 33.Barsalani R, Riesco E, Lavoie JM. Effect of exercise training and isoflavones on hepatic steatosis in overweight postmenopausal women. Climacteric : the Journal of the International Menopause Society 2013;16:88–95. [DOI] [PubMed] [Google Scholar]
- 34.Banitalebi E, Faramarzi M, Nasiri S. Effects of different exercise modalities on novel hepatic steatosis indices in overweight women with type 2 diabetes 2019;25:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson NA, Sachinwalla T, Walton DW. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–12. [DOI] [PubMed] [Google Scholar]
- 36.Moon JS, Yoon JS, Won KC. The role of skeletal muscle in development of nonalcoholic fatty liver disease. Diabetes Metab J 2013;37:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim G, Lee S-E, Lee Y-B. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a 7-year longitudinal study. Hepatology 2018;68:1755–68. [DOI] [PubMed] [Google Scholar]
- 38.Hashida R, Kawaguchi T, Bekki M. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol 2017;66:142–52. [DOI] [PubMed] [Google Scholar]
- 39.Csapo R, Alegre LM. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: a meta-analysis. Scand J Med Sci Sports 2016;26:995–1006. [DOI] [PubMed] [Google Scholar]
- 40.Schoenfeld BJ, Ogborn D, Krieger JW. Dose-Response relationship between Weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J Sports Sci 2017;35:1073–82. [DOI] [PubMed] [Google Scholar]
- 41.Browning JD, Baker JA, Rogers T. Short-Term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. The American Journal of Clinical Nutrition 2011;93:1048–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haufe S, Engeli S, Kast P. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects 2011;53:1504–14. [DOI] [PubMed] [Google Scholar]
- 43.Steven S, Hollingsworth KG, Al-Mrabeh A. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders 2016;39:808–15. [DOI] [PubMed] [Google Scholar]
- 44.Lewis MC, Phillips ML, Slavotinek JP. Change in liver size and fat content after treatment with Optifast® very low calorie diet. Obes Surg 2006;16:697–701. [DOI] [PubMed] [Google Scholar]
- 45.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis 2015;149:367–78. [DOI] [PubMed] [Google Scholar]
- 46.Marin-Alejandre BA, Abete I, Cantero I. The metabolic and hepatic impact of two personalized dietary strategies in subjects with obesity and nonalcoholic fatty liver disease: the fatty liver in obesity (FLiO) randomized controlled trial. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazzotti A, Caletti MT, Brodosi L. An Internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol 2018;69:1155–63. [DOI] [PubMed] [Google Scholar]
- 48.Marin‐Alejandre BA, Cantero I, Perez‐Diaz‐del‐Campo N. Effects of two personalized dietary strategies during a 2‐year intervention in subjects with nonalcoholic fatty liver disease: a randomized trial 2021. [DOI] [PubMed]
- 49.Pasco JA, Sui SX, West EC. Fatty liver index and skeletal muscle density 2022:1–9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2022-002950supp001.pdf (157.9KB, pdf)
Data Availability Statement
Data are available on reasonable request.