Abstract
It is considered that certain drugs might induce delusional infestation, yet, to date, no studies have been performed to identify the pharmacodynamics associated with these treatments. The aim of this review is to summarize current available knowledge of drug-induced delusional infestation. A literature search was performed for primary studies on suspected drugs reported to induce delusional infestation. Included articles were evaluated systematically using the Naranjo criteria. In addition, drug mechanisms of action were compared. The final selection included 31 studies, in which a total of 26 classes of drugs were identified. Anti-Parkinson drugs were most frequently associated with delusional infestation, followed by antidepressants, antiepileptics, antibiotics, prescription stimulants, and a few other drug groups. The current available literature suggests that the onset of delusional infestation is initiated by drug-induced alterations in neurotransmitter levels, predominantly dopamine, in the central nervous system.
Key words: delusional infestation, drug-induced, secondary delusional infestation, psychodermatology
Psychodermatology, which encompasses the interface of dermatology and psychiatry, is a relatively new subspecialty that is currently gaining momentum. A notable proportion of psychodermatological patients experience delusions of infestation (DI), a rare somatic form of delusional disorder characterized by a false, but immovable, belief that they are infested by living or lifeless organisms and experience cutaneous sensations without any skin disorder or objective evidence to support this (1, 2). A high burden of disease is often demonstrated by patients as a result of extensive engagement with their symptoms (3, 4). Although DI is considered a psychiatric disorder, patients with DI are strongly convinced that their symptoms have a somatic origin and they generally avoid psychiatrists. Therefore, physicians, such as dermatologists and infectious disease physicians, are often in charge of treating the main symptoms of this patient group (5). Identifying risk factors and early diagnosis is essential in this disease in order to develop relevant treatment plans at the outset of care, and to improve prognosis (6).
SIGNIFICANCE
Delusional infestation is a rare psychiatric condition, and drugs may play a significant role in its development. A literature search was performed for studies on drugs reported to induce delusional infestation. Anti-Parkinson drugs were most frequently associated with the condition, followed by antidepressants, antiepileptics, antibiotics, prescription stimulants and a few other drug groups. Discontinuation or dose adjustment of the suspected drug may have a positive influence on the course of delusional infestation. Medication use should therefore be assessed carefully in patients experiencing delusional infestation.
In the current literature DI is subdivided into primary and secondary DI, with primary DI being classified in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as a delusional disorder (somatic type) and in the International Classification of Diseases 10th revision (ICD-10) as a persistent delusion disorder (7, 8). Primary DI can be diagnosed only after secondary causes of delusions have been excluded (Table SI) (2, 3). Management of DI is often a therapeutic challenge; literature on pharmacological treatment strategies is mostly limited to case series and case reports (5). The current recommended therapy for primary DI consists mostly of antipsychotics; however, secondary DI treatment aims to treat underlying causes (where possible). Dopaminergic agents, steroids and incidental antibiotics have been described as predisposing factors of drug-induced DI, although little is known about its pathophysiology (2). To this end, this review sought to provide an overview of cases discussing drug-induced DI and to investigate shared pharmacodynamics properties of these suspected causative drugs.
MATERIALS AND METHODS
Search strategy
A detailed literature search was performed in January 2021 in PubMed. The search strategy was focused on selecting prespecified key words of free text, Medical Subject Headings (MeSH), and used the following terms: “delusional parasitosis”, “acarophobia”, “dermatozoic delusion”, “psychogenic parasitosis”, “Ekbom delusion”, “delusional infestation”, together with “drug therapy”, “aetiology”, “adverse events” and “adverse effects”. This was followed by application of PubMed-specific filters for language (English, German or Dutch) and publication date up to and including 31 December 2020. Inclusion and exclusion criteria were created to focus on studies describing cases of treatment-induced DI (Table I). In addition, the reference lists of all review articles were searched by hand for alternative relevant studies on drug-induced DI.
Table I.
Inclusion and exclusion criteria
| Inclusion criteria |
| Secondary drug-induced delusional infestation |
| Articles with a well-described case of drug-induced delusional infestation |
| Exclusion criteria |
| Primary delusional infestation |
| Secondary delusional infestation caused by other medical, nutritional or psychiatric conditions |
| Conditions often confused with DI, including: psychosis, tactile hallucinations |
Assessment of literature
For each article, the possibly causative drug(s) was/were identified. To objectify a possible causative relationship between individual drugs and symptoms of delusion, the Naranjo criteria (Table II) were used (9). Each of the 10 components can score by answering either yes (+1, +2 or –1) or no (–1, +0, +1 or +2), resulting in a weighted score (adverse drug reaction (ADR) probability scale). A score ≥9 is considered a strong causality, 5–8 a plausible causality, 1–4 possible causality, and <1 unlikely causality. Furthermore, comorbidity and co-medication were identified from the reports. Evaluation of the exact pharmacodynamic mechanism of the suspected drugs was extracted from the corresponding case report.
Table II.
Naranjo criteria (9). A systematic standardized questionnaire that rates the extent of causality between adverse drug reactions (ADRs) and prescribed drugs
| Yes | No | Do not know | Score | |
|---|---|---|---|---|
| 1. Are there previous conclusive reports on this reaction? | + 1 | 0 | 0 | – |
| 2. Did the adverse event appear after the suspected drug was administered? | + 2 | −1 | 0 | – |
| 3. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered (dechallenge)? | +1 | 0 | 0 | – |
| 4. Did the adverse reaction reappear after re-administering the drug (rechallenge)? | +2 | −1 | 0 | – |
| 5. Are there alternative causes (other than the drug) that could on their own have caused the reaction? | −1 | +2 | 0 | – |
| 6. Did the reaction return when a placebo was administered? | −1 | +1 | 0 | – |
| 7. Was the drug detected in the blood (or other bodily fluids) in concentrations known to be toxic? | +1 | 0 | 0 | – |
| 8. Was the reaction more severe when the dose was increased, or less severe when the dose was decreased? | +1 | 0 | 0 | – |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | – |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | – |
| Total score | – | |||
RESULTS
The search strategy identified 323 publications, of which 171 abstracts were eligible for review. After thorough examination, 31 articles met our eligibility criteria and were included in this review (Fig. 1). Clinical characteristics of the patients described in these publications are shown in Tables SII and SIII. In 42 cases with possible drug-induced DI, 26 classes of drugs were associated with DI (Table III): 16 anti-Parkinson medications, 6 antidepressants, 2 antiepileptic drugs, 2 antibiotics, 12 reports of drug abuse, and a few single cases of other drug groups.
Fig. 1.
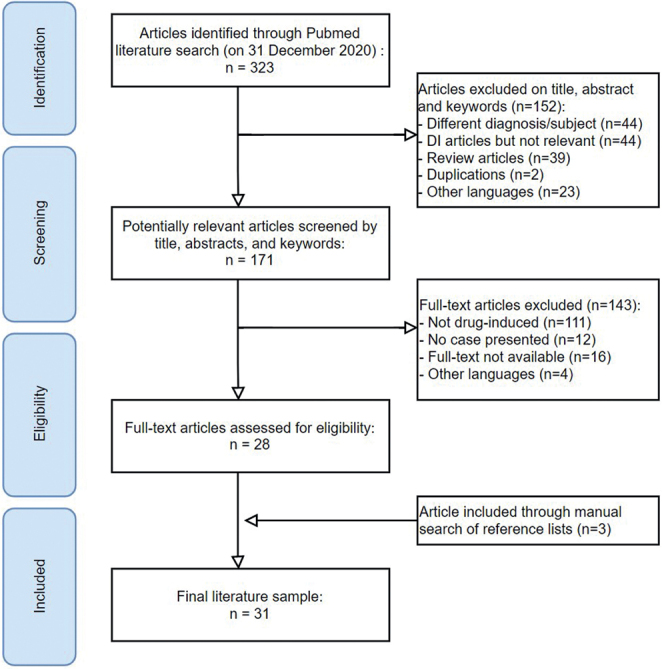
Identification and selection of eligible studies. DI: delusions of infestation.
Table III.
Classes of medication reported to have an association with delusional infestation
| Drug | Neuropharmacological target | Cases, n | Case report (ref) |
|---|---|---|---|
| Parkinsonian drugs | |||
| Ropinirole | 1. D2, D3 and D4 receptor agonist | 6 | 10–12 |
| Amantadine | 1. Matrix protein 2 inhibitor (antiviral effect) | 5 | 12, 13, 15, 16 |
| 2. NMDA 3A receptor antagonist | |||
| 3. D2 receptor agonist | |||
| Piribedil | 1. D2 and D3 receptor agonist | 1 | 17 |
| 2. Alpha-2 adrenergic receptor antagonist | |||
| Cabergoline | 1. D2 receptor agonist | 1 | 11 |
| Levodopa/carbidopa | 1. Conversion to D | 1 | 16 |
| Pramipexole | 1. D2, D3 and D4 receptor agonist | 3 | 13–15 |
| Trihexyphenidyl | 1. Selective M1 muscarinic acetylcholine receptor antagonist | 1 | 12 |
| Entacapone | 1. Selective catechol-o-methyl transferase (COMT) inhibitor | 1 | 12 |
| 2. A increased effect of levodopa | |||
| Drug abuse | |||
| Amphetamine | 1. Blockade of DAT protein | 6 | 10, 27, 31 |
| 2. Blockade of NET protein | |||
| 3. 5-HT1A receptor antagonist | |||
| Cocaine | 1. Blockade of DAT protein | 6 | 32–35 |
| 2. 5-HT3 receptor antagonist | |||
| 3. 5-HT2A and 5-HT2C receptors agonist | |||
| 4. Inhibition of NET and SERT protein | |||
| Cannabis | 1. Stimulation of CB 1 and 2 receptor | 1 | 36 |
| Pemoline | 1. Inhibition of DAT protein | 1 | 37 |
| Antibiotics | |||
| Ciprofloxacin | 1. Blocks bacterial topoisomerase 2 and 4 (DNA gyrase) | 1 | 25 |
| 2. GABA-A receptor antagonist | |||
| Clarithromycin | 1. Blocks bacterial 50S ribosomal subunit | 1 | 26 |
| 2. GABA-A receptor antagonist | |||
| Antiviral drugs | |||
| Interferon alpha-2b | 1. IFNARI/2 receptor activation | 1 | 39 |
| Anti-epileptics | |||
| Topiramate | 1. GABA-A receptor agonist | 1 | 24 |
| 2. AMPA/kainate (glutamate) receptor antagonist | |||
| 3. Na+-channel blocker | |||
| Gabapentin | 1. GABA-A analogue | 1 | 23 |
| 2. Ca2+-channel blocker (P/Q type) | |||
| Antidepressants | |||
| Phenelzine | 1. MAO-A and B inhibitor | 2 | 18, 19 |
| 2. Inhibitor of ALT and GABA-T proteins | |||
| 3. Its metabolite, phenethylamine, has a releasing effect on NE and D | |||
| Doxepin | 1. Alpha-1 adrenergic receptor antagonist | 2 | 20, 21 |
| 2. Muscarinic cholinergic receptor antagonist | |||
| 3. H1 and H2 receptor antagonist | |||
| 4. Inhibition of NET and SERT protein | |||
| Sertraline | 1. Inhibition of SERT protein | 1 | 22 |
| Fluoxetine | 1. Inhibition of SERT, DAT and NET protein | 1 | 22 |
| 2. Antagonist 5-HT(2C) | |||
| Other drugs | |||
| Alprazolam | 1. GABA-A receptor agonist | 1 | 22 |
| Armodafinil | 1. Blockade of DAT protein | 1 | 10 |
| Atomoxetine | 1. Inhibition of NET protein | 1 | 38 |
| Cetirizine | 1. H1 receptor antagonist | 1 | 21 |
| Donepezil | 1. Inhibition of AChE | 1 | 40 |
ALT: alanine transaminase; D: dopamine; DAT: dopamine transporter, DI: delusional infestation; GABA: gamma-aminobutyric acid; H: histamine; 5-HT: serotonin; MAOI: monoamine oxidase inhibitor; NE: norepinephrine; NET: norepinephrine transporter; SERT: serotonin transporter; AChE: acetylcholinesterase; CB: cannabinoid; NMDA: N-methyl-D-aspartate; IFNAR: interferon-a/β receptor; AMPA: a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
Anti-Parkinson medication
Dopaminergic drugs were, with 16 out of 42 cases, the most reported class of medications to cause DI. These reports discussed patients with Parkinson’s disease (PD) receiving dopaminergic therapy, including ropinirole, amantadine, piribedil, levodopa/carbidopa, pergolide, cabergoline and pramipexole (Table II) (10–17). Ropinirole, a dopamine agonist, was both the most frequently reported anti-Parkinson drug associated with DI (n = 6) and had the highest reported Naranjo score, of 7 (9). In addition, 4 out of 6 cases of ropinirole (11–13) received a Naranjo score of 3, adding up to reasonable suspicion for causality. A similar dopamine agonist, pramipexole, was reported in 3 cases, with 1 case scoring the second highest Naranjo score of 6 (13–15). Two out of 3 of these cases were a result of a pramipexole monotherapy, whereas, in the lowest scoring case, DI symptoms occurred after addition of pramipexole and amantadine to a pre-existing levodopa/benserazide treatment. Furthermore, amantadine was associated with 5 cases of DI. All amantadine cases scored a Naranjo score of 2 (12, 13, 15) with the exception of 1 case, treated with both amantadine and levodopa/carbidopa, in which a Naranjo score of 5 was given (16). Other dopamine agonists, such as pergolide, piribedil and cabergoline, were reported in singular cases of DI, receiving Naranjo scores of 3 (11, 12, 17).
Antidepressants
Six cases reported drug-induced DI associated with anti-depressants, including the selective serotonin-reuptake inhibitors (SSRIs) sertraline (n = 1) and fluoxetine (n = 1), doxepin, a tricyclic antidepressant (TCA) (n = 2) and, lastly, phenelzine, a mono-amine oxidase (MAO) inhibitor (n = 2). Both phenelzine cases received high Naranjo scores (5 and 4), indicating a high suspicion of causality (18, 19). High scores were achieved by the timely onset of DI after the start of drug intake, positive dechallenges, and in 1 report, even a positive rechallenge (18). Doxepin was discussed in 2 cases of drug-induced DI (20, 21). In 1 case DI-symptoms resolved after stopping doxepin and cetirizine simultaneously, not distinguishing which change of medication might have led to recovery (21). Fluoxetine and sertraline were both suspected as DI inducers in separate case reports by Wenning et al. (22). However, both cases received low Naranjo scores (both 0) due to the involvement of other suspected medications, such as lithium and alprazolam, absence of dechallenges or rechallenges, and a history of psychiatric disorders and drug abuse in both patients (22).
Anti-epileptic medication
There have been 2 reports of DI associated with antiepileptic drugs (Table SII), namely gabapentin (n = 1) and topiramate (n = 1). Both cases were the first-known reports of DI resulting from these GABAergic drugs; however, psychotic adverse effects are well known in adjunctive and monotherapy with topiramate (23, 24). Both cases resolved after discontinuation of the suspected anti-epileptic drug.
Antibiotics
There have been a few single reported cases of DI associated with the antimicrobial agents ciprofloxacin and clarithromycin (Table SII), both of which are known to cause psychiatric adverse effects (25, 26). However, both reports claim to be the first to associate DI with these drugs. Discontinuation of the specific antimicrobial drug led to symptom resolution in both cases.
Prescription stimulants and recreational drug abuse
DI following the repeated use or abuse of psychostimulant drugs has been reported in both children and adults (Table SIII). Most commonly reported cases are patients using cocaine (n = 6) or amphetamines (n = 6). In all 6 cases, amphetamines were prescribed as treatment for attention-deficit hyperactivity disorder (ADHD) (10, 27–31). Drug dose and treatment of DI symptomatology were either not described at all, or only in a very limited way in all reports discussing cocaine abuse (32–35). Abuse of other drugs, such as cannabis and alternative amphetamine-like drugs, e.g. pemoline, atomoxetine and methylphenidate, have also been reported with DI (36–38).
Other medications
A few other anecdotal reports of drug-induced DI are described (Table SII), including interferon alpha-2B (an antiviral agent) (39), alprazolam (benzodiazepine) (22), cetirizine (antihistamine) (21), trihexyphenidyl (anticholinergic) (12) and donepezil (cholinesterase inhibitor) (40). Reasonable causality was found in 1 case report, discussing interferon alpha-2b in a used to treat chronic hepatitis C infection, where a positive dechallenge and rechallenge of interferon led to a Naranjo score of 4 (39). Other cases received relatively low Naranjo scores.
DISCUSSION
This review focusses on secondary DI and provides an overview of published evidence regarding drug-induced DI. The study identified 26 drugs associated with DI, the vast majority of which are known to influence neurotransmitter systems in the central nervous system (CNS). These include dopaminergic, serotonergic and adrenergic systems, as well as the histamine and gamma-aminobutyric acid (GABA) pathways. Most drugs for which an association with DI has been reported have a dopaminergic mechanism of action, either as a major mechanism or as a “side” mechanism (see Table SIII). This is consistent with well-known theories discussing the level of dopamine in the brain as a major factor in the manifestation of DI and other delusional disorders (2, 12, 41). The decreased functioning of the striatal dopamine transporter (DAT), a key regulator of the dopamine-reup-take system, is the main aetiological foundation for this hypothesis (41). Higher levels of dopamine in the striatum can lead to hyperactivity of dopaminergic neurones (12, 42), resulting in the formation of delusions. As a result, treatment of DI has focused on anti-dopaminergic potency. Both first-generation (i.e. haloperidol, sulpiride or pimozide) and second-generation (i.e. risperidone, amisulpride or olanzapine) antipsychotics block postsynaptic D2 receptors, resulting in a reduction in the effect of extracellular dopamine. The centrality of dopamine can also be found in other DI-associated disorders, such as Parkinson’s disease (PD), Huntington’s disease, depression, and schizophrenia, which have been shown to have diminished DAT functioning, attributing more power to this theory (2, 43, 44).
The pathogenesis of DI is unknown. One theory for the development of somatoform-type disorders is that common, distressing somatic symptoms become amplified and perpetuated following the patient’s new awareness of a known disease through another individual, the media, or publicity by public health agencies (45). It has also been proposed that DI may be related to an excess of extracellular dopamine within the striatum of the brain, due to decreased functioning of the dopamine transporter (46). Furthermore, hyperactivity of serotonin (5HT2A) receptors has been linked to the onset of psychosis (47, 48). Some of the medication discussed in this review acts on 5HT2A receptors besides dopamine receptors.
Initial assessment of patients with DI should therefore address medication (mis)use and misuse of substances. Stimulant drugs, such as cocaine and amphetamines, can cause psychosis, probably through activation of the mesolimbic dopamine pathway (49). Misuse of substances such as amphetamines, cannabis, codeine, cocaine, or opiates was described in a third of patients with DI (50). In sporadic cases substance use withdrawal from heroin or alcohol can induce formication (51). Therefore, toxicological screenings are strongly recommended when assessing patients with DI. Studies of clearly defined groups of medication that cause DI are scarce. The priority of this review is to address this issue and to provide a transparent overview of medications reported to induce DI.
Treatment of DI has proven a therapeutic challenge. In the case of secondary DI, an attempt should be made to treat the underlying problem, which means that medication or substance use should be discontinued where possible to exclude drug-induced DI. If necessary, counselling through an addiction agency can be offered. In primary and secondary DI, oral antipsychotics lead to recovery from DI symptoms in the majority of patients. There is no compelling evidence in the literature for any particular group of antipsychotics (52). The biggest therapeutic challenge is the patient’s conviction of the authenticity of the infestation, which makes him or her reluctant to try medication or accept a referral to a psychiatrist. A strong therapeutic relationship with the patient and a solid alliance between dermatologists (or other doctors patients present to) and psychiatrists are crucial in the management of this disease.
Limitations
This review has a number of limitations. Firstly, the literature summary is derived mostly from case series and single case reports of drug-induced DI. As no clinical trial is included in this review, the established evidence is weak. Many cases are also outdated and may be prone to inaccuracies. Secondly, the Naranjo criteria were adopted to score cases, as it is commonly used to report adverse events in drug use. However, documentation may be incomplete, as significant parts of the Naranjo criteria were not touched upon in most cases. Especially tested toxic blood levels, increase/decrease of drug dose and objective evidence have rarely been described. A positive rechallenge was reported in some cases, which may hint at a likelihood of association between DI and the prescribed drug. Thirdly, the aim of the current study was to identify pharmacological mechanisms responsible for inducing a delusional infestation. Thus, this study focused on reports in which cases were described in order to perform a causality assessment. Observational studies, such as the one by Marshall et al. (53), may therefore have been missed. The same holds true for other prescription medications possibly associated with delusional infestation that could not be stopped to ascertain a causal relationship. Lastly, potential confounding factors, such as patient demographics, are difficult to eliminate. Many patients also had underlying psychiatric disorders, such as depression or ADHD, which raises the question as to whether they are more susceptible to develop drug-induced DI due to pre-existing imbalance in neurotransmitters in the CNS.
Conclusion
A wide variety of drugs is reported to induce DI, especially dopaminergic drugs. Better understanding of the pharmacological pathways that can induce delusional symptoms is essential to better define clear groups of DI-associated medications. Management of suspected drug-induced DI should focus on discontinuation or dose adjustment of the specific drug and, if necessary, the administration of antipsychotics. This review adds to the current knowledge of the role of drugs in secondary DI; however, more studies are required to expand our understanding of the complex pathophysiology of this phenomenon.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Hughes JE, Barraclough BM, Hamblin LG, White JE. Psychiatric symptoms in dermatology patients. Br J Psychiatry 1983; 143: 51–54. [DOI] [PubMed] [Google Scholar]
- 2.Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev 2009; 22: 690–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohorst JJ, Bailey CH, Andersen LK, Pittelkow MR, Davis MDP. Prevalence of delusional infestation – a population-based study. JAMA Dermatol 2018; 154: 615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewley AP, Lepping P, Freudenmann RW, Taylor R. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol 2010; 163:1–2. Erratum in: Br J Dermatol 2010; 163: 899. [DOI] [PubMed] [Google Scholar]
- 5.Vulink NC. Delusional infestation: state of the art. Acta Derm Venereol 2016; 96: 58–63. [DOI] [PubMed] [Google Scholar]
- 6.Lepping P, Aboalkaz S, Squire SB, Romanov DV, Bewley A, Huber M, et al. Later age of onset and longer duration of untreated psychosis are associated with poorer outcome in delusional infestation. Acta Derm Venereol 2020; 100: adv00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- 8.World Health Organization . (1992). The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization. [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 10.Fowler E, Maderal A, Yosipovitch G. Treatment-induced delusions ofinfestation associated with increased brain dopamine levels. Acta Derm Venereol 2019; 99: 327–328. [DOI] [PubMed] [Google Scholar]
- 11.Flann S, Shotbolt J, Kessel B, Vekaria D, Taylor R, Bewley A, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol 2010; 35: 740–742. [DOI] [PubMed] [Google Scholar]
- 12.Davis JL, Kurek JA, Sethi KD, Morgan JC. Delusional infestation in Parkinson’s disease. Mov Disord Clin Pract 2016; 4: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro A, Munhoz RP, Moscovich M, Arruda WO, Teive HA. Delusional misidentification syndrome and other unusual delusions in advanced Parkinson’s disease. Parkinsonism Relat Disord 2013; 19: 751–754. [DOI] [PubMed] [Google Scholar]
- 14.Romero Sandoval K, Festa Neto C, Nico MMS. Delusional infestation caused by pramipexole. Clin Exp Dermatol 2018; 43: 192–193. [DOI] [PubMed] [Google Scholar]
- 15.Ojeda-López C, Aguilar-Venegas LC, Tapia-Orozco M, Cervantes-Arriaga A, Rodríguez-Violante M. Delusional parasitosis as a treatment complication of Parkinson disease. Psychosomatics 2015; 56: 696–699. [DOI] [PubMed] [Google Scholar]
- 16.Swick BL, Walling HW. Drug-induced delusions of parasitosis during treatment of Parkinson’s disease. J Am Acad Dermatol 2005; 53: 1086–1087. [DOI] [PubMed] [Google Scholar]
- 17.Kölle M, Lepping P, Kassubek J, Schönfeldt-Lecuona C, Freudenmann RW. Delusional infestation induced by piribedil add-on in Parkinson’s disease. Pharmacopsychiatry 2010; 43: 240–242. [DOI] [PubMed] [Google Scholar]
- 18.Liebowitz MR, Nuetzel EJ, Bowser AE, Klein DF. Phenelzine and delusions of parasitosis: a case report. Am J Psychiatry 1978; 135: 1565–1566. [DOI] [PubMed] [Google Scholar]
- 19.Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry 1991; 159: 716–717. [DOI] [PubMed] [Google Scholar]
- 20.Damiani JT, Flowers FP, Pierce DK. Pimozide in delusions of parasitosis. J Am Acad Dermatol 1990; 22: 312–313. [DOI] [PubMed] [Google Scholar]
- 21.Reichenberg JS, Magid M, Drage LA. A cure for delusions of parasitosis. J Eur Acad Dermatol Venereol 2007; 21: 1423–1424. [DOI] [PubMed] [Google Scholar]
- 22.Wenning MT, Davy LE, Catalano G, Catalano MC. Atypical antipsychotics in the treatment of delusional parasitosis. Ann Clin Psychiatry 2003; 15: 233–239. [DOI] [PubMed] [Google Scholar]
- 23.Lopez PR, Rachael T, Leicht S, Smalligan RD. Gabapentin-induced delusions of parasitosis. South Med J 2010; 103: 711–712. [DOI] [PubMed] [Google Scholar]
- 24.Fleury V, Wayte J, Kiley M. Topiramate-induced delusional parasitosis. J Clin Neurosci 2008; 15: 597–599. [DOI] [PubMed] [Google Scholar]
- 25.Steinert T, Studemund H. Acute delusional parasitosis under treatment with ciprofloxacin. Pharmacopsychiatry 2006; 39: 159–160. [DOI] [PubMed] [Google Scholar]
- 26.Tse KC, Li FK, Tang S, Lam MF, Chan TM, Lai KN. Delusion of worm infestation associated with clarithromycin in a patient on peritoneal dialysis. Perit Dial Int 2001; 21: 415–416. [PubMed] [Google Scholar]
- 27.Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry 2012; 2012: 624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh TC, Lin YC, Chen LF, Chiang CP, Mao WC, Chang HA, et al. Aripiprazole treatment in a case of amphetamine-induced delusional infestation. Aust N Z J Psychiatry 2014; 48: 681–682. [DOI] [PubMed] [Google Scholar]
- 29.Stanciu CN, Penders TM, Oxentine HN. Delusional infestation following misuse of prescription stimulants. Psychosomatics 2015; 56: 210–212. [DOI] [PubMed] [Google Scholar]
- 30.Beach SR, Kroshinsky D, Kontos N. Case records of the Massachusetts General Hospital. Case 37-2014. A 35-year-old woman with suspected mite infestation. N Engl J Med 2014; 371: 2115–2123. [DOI] [PubMed] [Google Scholar]
- 31.Marschall MA, Dolezal RF, Cohen M, Marschall SF. Chronic wounds and delusions of parasitosis in the drug abuser. Plast Reconstr Surg 1991; 88: 328–230. [DOI] [PubMed] [Google Scholar]
- 32.Elliott A, Mahmood T, Smalligan RD. Cocaine bugs: a case report of cocaine-induced delusions of parasitosis. Am J Addict 2012; 21: 180–181. [DOI] [PubMed] [Google Scholar]
- 33.Jagt YQ, Sutterland AL, Meijer JH, Oudijn MS, Kemperman PM, Vulink NC, et al. Delusional infestation, a therapeutic challenge. Ned Tijdschr Geneeskd 2014; 158: A7548. [PubMed] [Google Scholar]
- 34.Ahmad K, Ramsay B. Delusional parasitosis: lessons learnt. Acta Derm Venereol 2009; 89: 165–168. [DOI] [PubMed] [Google Scholar]
- 35.Brewer JD, Meves A, Bostwick JM, Hamacher KL, Pittelkow MR. Cocaine abuse: dermatologic manifestations and therapeutic approaches. J Am Acad Dermatol 2008; 59: 483–487. [DOI] [PubMed] [Google Scholar]
- 36.Freudenmann RW, Kölle M, Huwe A, Luster M, Reske SN, Huber M, et al. Delusional infestation: neural correlates and antipsychotic therapy investigated by multimodal neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 1215–1222. Erratum in: Prog Neuropsychopharmacol Biol Psychiatry 2012; 36: 324. [DOI] [PubMed] [Google Scholar]
- 37.Krauseneck T, Soyka M. Delusional parasitosis associated with pemoline. Psychopathology 2005; 38: 103–104. [DOI] [PubMed] [Google Scholar]
- 38.Howes CF, Sharp C. Delusional infestation in the treatment of ADHD with atomoxetine. BMJ Case Rep 2018; bcr2018226020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robaeys G, De Bie J, Van Ranst M, Buntinx F. An extremely rare case of delusional parasitosis in a chronic hepatitis C patient during pegylated interferon alpha-2b and ribavirin treatment. World J Gastroenterol 2007; 13: 2379–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenzweig I, Ramachandra P, Freer J, Wong M, Pieters T. Delusional parasitosis associated with donepezil. J Clin Psychopharmacol 2011; 31: 781–782. [DOI] [PubMed] [Google Scholar]
- 41.Huber M, Kirchler E, Karner M, Pycha R. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses 2007; 68: 1351–1358. [DOI] [PubMed] [Google Scholar]
- 42.Huber MK, Schwitzer J, Kirchler E, Lepping P. Delusion and dopamine: neuronal insights in psychotropic drug therapy. NeuroPsychopharmacotherapy Springer, Cham, 2021. [Google Scholar]
- 43.Tost H, Alam T, Meyer-Lindenberg A. Dopamine and psychosis: theory, pathomechanisms and intermediate phenotypes. Neurosci Biobehav Rev 2010; 34: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesby JP, Eyles DW, McGrath JJ, Scott JG. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry 2018; 8: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med 1999; 130: 910–921. [DOI] [PubMed] [Google Scholar]
- 46.Joyce EM. Organic psychosis: the pathobiology and treatment of delusions. CNS Neurosci Ther 2018; 24: 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr 2018; 23: 187–191. [DOI] [PubMed] [Google Scholar]
- 48.Bleich A, Brown SL, Kahn R, van Praag HM. The role of serotonin in schizophrenia. Schizophren Bull 1988; 14: 297–315. [DOI] [PubMed] [Google Scholar]
- 49.Bramness JG, Rognli EB. Psychosis induced by amphetamines. Curr Opin Psychiatry 2016; 29: 236–241. [DOI] [PubMed] [Google Scholar]
- 50.Lepping P, Noorthoorn EO, Kemperman PMJH, Harth W, Reichenberg JS, Squire SB, et al. An international study of the prevalence of substance use in patients with delusional infestation. J Am Acad Dermatol 2017; 77: 778–779. [DOI] [PubMed] [Google Scholar]
- 51.Mowla A, Asadipooya K. Delusional parasitosis following heroin withdrawal: a case report. Am J Addict 2009; 18: 334–345. [DOI] [PubMed] [Google Scholar]
- 52.Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb) 2019; 9: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall CL, Ellis C, Williams V, Taylor RE, Bewley AP. Iatrogenic delusional infestation: an observational study. Br J Dermatol 2016; 175: 800–802. [DOI] [PubMed] [Google Scholar]


