Abstract
Oral antivirals can potentially reduce the burden of COVID-19. However, low SARS-CoV-2 clinical testing rates in many low- and middle-income countries (LMICs) (mean <10 tests/100,000 people/day, July 2022) makes the development of effective test-and-treat programs challenging. Here, we used an agent-based model to investigate how testing rates and strategies could affect development of test-and-treat programs in three representative LMICs. We find that at <10 tests/100,000 people/day, test-and-treat programs are unlikely to have any impact on the public health burden of COVID-19. At low effective transmission rates (Rt ≤1.2), increasing to 100 tests/100,000 people/day and allowing uncapped distribution of antivirals to LMICs (estimate = 26,000,000–90,000,000 courses/year for all LMICs), could avert up to 65% of severe cases, particularly in countries with older populations. For higher Rt, significant reductions in severe cases are only possible by substantially increasing testing rates or restricting clinical testing to those with higher risk of severe disease.
Introduction
Antiviral therapies such as anti-SARS-CoV-2 monoclonal antibodies, replication inhibitors, protease inhibitors, and host-directed therapies can be used to treat COVID-19, reducing the probability of severe disease to varying degrees.1 Direct-acting antiviral drugs, such as molnupiravir2 and nirmatrelvir–ritonavir (Paxlovid),3 have the potential to substantially lower disease burden given their efficacy and convenience of oral dosing. Nirmatrelvir–ritonavir, in particular, can reduce incidence of adverse events in high-risk individuals (i.e. ≥60 years of age (over-60y) or an adult ≥18 years with a relevant comorbidity) by 46–89%.3,4 Given their ability to lower viral load,3 these drugs could also potentially be used to control SARS-CoV-2 transmission.5 To achieve maximum impact, these drugs must typically be administered within a few days of symptom onset. Given the current limited availability and relatively high cost of these drugs,6 along with the need to administer drugs quickly after symptom onset,2,3 diagnostic testing remains an essential first step for identifying suitable drug recipients.
Just like in high-income countries, oral antivirals (the term “antivirals” refers only to oral direct antivirals for the rest of this article) have the potential to reduce the disease burden of COVID-19 outbreaks in low- and middle-income countries (LMICs). However, there have been substantial gaps in COVID-19 testing equity across country income groups throughout the pandemic. Between January 2020 and March 2022, LMICs were only testing at an average of 27 tests/100,000 people/day (tests/100K/day) as compared to >800 tests/100K/day in high-income countries (HICs).7 In the post-crisis phase of the pandemic, testing rates dwindled down to just 10 tests/100K/day and 500 tests/100K/day on average for LMICs and HICs respectively (as of June 2022).7 Persistently low testing rates severely underestimate COVID-19 cases in LMICs,8 which not only complicate antiviral demand forecasts but create additional barriers to the effective use of antivirals if and when they become widely available in LMICs.
Here, we used the Propelling Action for Testing And Treating (PATAT) agent-based model9,10 to demonstrate how testing rates and testing strategies affect the use and impact of antivirals, particularly in LMICs. In the model, we focused on antigen rapid diagnostic tests (Ag-RDTs) which can easily be performed at point of care or be used as self-tests with short turnaround time needed to quickly identify high-risk infected individuals.11 We computed the potential impact of test-and-treat programs on infections, severe cases, and deaths averted in three LMICs with distinct demographic structures – Brazil, Georgia, and Zambia – as well as the Netherlands as a HIC example, all under varying levels of vaccination coverage. Our findings highlight the limits and expected outcomes of COVID-19 oral antiviral treatment programs under realistic testing and vaccination landscapes.
Results
Impact of oral antivirals in low- and middle-income countries
We first simulated Omicron BA.1-like epidemic waves in three different LMICs (Brazil, Georgia, and Zambia) with distinct population demographics (i.e. age distribution and contact networks; Extended Data Fig. 1) under different levels of vaccine coverage, epidemic intensity (Rt), and test availability. We assumed that only symptomatic individuals seek clinical testing, and that only test-positive, high-risk (i.e. ≥60 years of age (over-60y) or an adult ≥18 years with a relevant comorbidity) individuals receive a course of antivirals.
At the mean LMIC testing rate of 10 tests/100K/day, test-and-treat programs are unlikely to have any population-level impact on disease transmission in any setting (Extended Data Fig. 2). At higher testing rates (≥100 tests/100K/day) and lower Rt (≤1.5) there were modest differences between simulated countries. We found that current antivirals have only limited impact on total infections averted (Extended Data Fig. 2), in large part because 58–67% of all transmission events are attributed to asymptomatic and pre-symptomatic individuals (Extended Data Fig. 3A). In Georgia, where >30% of the population are over-60y and highrisk individuals transmitted almost half of all infections (Extended Data Fig. 3B), increasing testing rates to 100 (500) tests/100K/day, accompanied by uncapped distribution of antivirals, could reduce total infections by 12% (22%). On the other hand, regardless of testing rates, infections averted diminished to <12% and <4% in Brazil and Zambia respectively, both of which have small over-60y populations (i.e. Brazil: 15%; Zambia: 6% of population; Extended Data Fig. 3A) and where most infections are transmitted by low-risk individuals (Extended Data Fig. 3B). Across all settings and testing rates, increasing vaccination coverage did not change the impact of antiviral distribution on infections averted substantially.
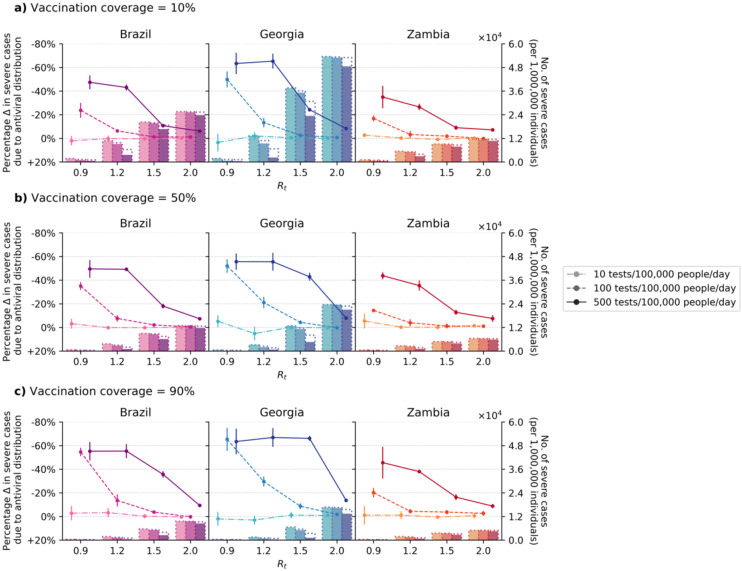
If testing rates could be increased to 500 tests/100K/day, the proportion of severe cases averted due to antivirals depends on the proportion of over-60y in the population, with Georgia, Brazil, and Zambia maximally reducing up to an average of 67%, 55%, and 46% of severe cases respectively through test-and-treat strategies (Fig. 1). Linking antiviral treatment to testing programs at a rate of 10 tests/100K/day does not generate any impact under any scenario, including when 90% of the population are vaccinated. Raising testing rates to 100 tests/100K/day – a widely publicized global target – and treating all high-risk, test-positive patients with antivirals substantially increased the proportion of severe cases averted at lower Rt (i.e. proportion of severe cases averted at Rt = 0.9 (1.2) with 10–90% vaccination coverage: Brazil, 24–55% (6–14%); Zambia, 17–20% (3–4%); and Georgia 50–65% (13–30%) (Fig. 1); the impact was greatest in Georgia given its substantial >60y population. As Rt increases (≥1.5), the likely population demand for tests also increases, and correspondingly >100 tests/100K/day is needed to ensure that high-risk individuals can be identified to initiate treatment (i.e. proportion of severe cases averted at Rt = 1.5 (2.0) with 10–90% vaccination coverage at 100 tests/100K/day: Brazil, 1–4% (0–1%); Zambia, 2–4% (0–3%); Georgia, 3–9% (1–2%); At 500 tests/100K/day: Brazil, 11–36% (6–9%); Zambia, 9–16% (7–9%); Georgia, 2466% (8–14%); Fig. 1).
Fig. 1: Impact of oral antiviral therapy on severe cases in low- and middle-income countries.
No restrictions on access to symptomatic testing at clinics (i.e. all symptomatic individuals who sought testing at clinics would receive one if in stock) and high-risk household contacts of test-positive individuals are not tested. All eligible high-risk individuals (i.e. ≥60 years of age or an adult ≥18 years with a relevant comorbidity) who tested positive were given a course of oral antivirals. Line plots (left y-axis) show the percentage change in severe cases relative to no distribution of antivirals under different levels of mean test availability (different shades of color) after a 90-day Omicron BA.1-like epidemic wave in a population of 1,000,000 individuals with (a) 10%, (b) 50%, and (c) 90% vaccination coverage for different epidemic intensities (measured by the initial instantaneous reproduction number (Rt); x-axis). Bar plots (right y-axis) show the number of severe cases in each corresponding scenario. The dotted outline of each bar shows the number of severe cases of each scenario when no antivirals were distributed.
While no degree of vaccination coverage enables an effective antiviral treatment program at low testing rates, when testing levels are adequate, increasing vaccination coverage will augment the benefit of antivirals in reducing severe cases (Table 1). The greatest benefit increase of antivirals through wider vaccination coverage is at levels of Rt where testing rates would have otherwise been insufficient to satisfy symptomatic testing demand at lower vaccine coverage. For instance, at Rt = 1.5 and 100 tests/100K/day, there is a 3.0-fold increase in the proportion of severe cases averted by boosting vaccination coverage from 10% to 90% in Brazil, and a 2.0-fold increase in Zambia; in Georgia with its larger over-60y population, boosting vaccination coverage to 90% results in a 3.4-fold increase in severe cases averted. Although we did not model the impact of antivirals in reducing the likelihood of death, developing severe disease precedes dying from COVID-19 in our model (see Methods), the number of deaths averted thus follow similar trends as severe cases averted (Extended Data Fig. 4).
Table 1. Fold increase in proportion of severe cases averted due to distribution of oral antivirals when increasing vaccination coverage from 10% to 90%.
No restrictions on access to symptomatic testing at clinics (i.e. all symptomatic individuals who sought testing at clinics would receive one if in stock) and high-risk household contacts of test-positive individuals are not tested.
| Country | Testing rate (tests/100,000 people/day) | R t | Fold increase |
|---|---|---|---|
| Brazil | 100 | 0.9 | 2.30 |
| 1.2 | 2.13 | ||
| 1.5 | 3.00 | ||
| 2.0 | No further increase | ||
| 500 | 0.9 | 1.17 | |
| 1.2 | 1.28 | ||
| 1.5 | 3.33 | ||
| 2.0 | 1.53 | ||
| Georgia | 100 | 0.9 | 1.31 |
| 1.2 | 2.21 | ||
| 1.5 | 3.40 | ||
| 2.0 | No further increase | ||
| 500 | 0.9 | 1.00 | |
| 1.2 | 1.02 | ||
| 1.5 | 2.72 | ||
| 2.0 | 1.63 | ||
| Zambia | 100 | 0.9 | 1.19 |
| 1.2 | 1.36 | ||
| 1.5 | 1.96 | ||
| 2.0 | No further increase | ||
| 500 | 0.9 | 1.30 | |
| 1.2 | 1.43 | ||
| 1.5 | 1.81 | ||
| 2.0 | 1.23 |
Distribution of oral antivirals to high-risk household contacts
As antivirals must be administered quickly after symptom onset, one way to promptly identify and treat infected high-risk individuals is to secondarily distribute self-tests to highrisk household contacts who were exposed to the test-positive individuals. We repeated our simulations with high-risk household contacts receiving Ag-RDTs to self-test over the ensuing three days, initiating antiviral treatment upon a positive diagnosis. In this scenario, however, there is little reduction in total infections due to antivirals (Extended Data Fig. 5). In fact, when Rt is low (≤1.2) and at 100 tests/100K/day, self-testing high-risk household contacts diverted test stocks away from test-seeking symptomatic individuals that would otherwise might have been diagnosed and changed their behavior to lower transmissions. In other words, secondary self-testing and treatment approach resulted in more infections than if antivirals were not distributed at all.
At 100 tests/100K/day across all Rt values, or at 500 tests/100K/day and higher Rt, the proportion of severe cases and in turn, deaths averted diminished substantially by a factor of two- to ten-fold relative to no secondary distribution of Ag-RDTs (Extended Data Figs. 6–7). Even when there were ample tests for both symptomatic individuals and high-risk household contacts (i.e. 500 tests/100K/day and Rt = 0.9), there was no substantial reduction in severe cases and deaths. Crucially, 100 tests/100K/day remains inadequate to meet the testing demand of symptomatic individuals and high-risk household contacts that the beneficial effects on severe case reduction under higher vaccination coverage was only observed at 500 tests/100K/day (i.e. at Rt = 1.5 with 500 tests/100K/day, fold increase in proportion of severe cases averted by boosting vaccination coverage from 10% to 90%: Brazil, 3.2-fold; Zambia, 2.2-fold; Georgia, 4.3-fold).
Restricting symptomatic testing to high-risk individuals
Given the limited impact of current antivirals in reducing transmissions, testing could be targeted to high-risk individuals only in order to distribute antivirals to as many infected high-risk individuals as possible. This strategy can be effective when Ag-RDT availability is inadequate to test all symptomatic individuals who seek testing, which has been a common scenario in LMICs throughout the pandemic. Otherwise, if most individuals only isolate themselves after a positive test, the testing restriction would lead to excess tests available that are not effectively used to alter the behaviour of low-risk infected individuals that curb onward transmissions.
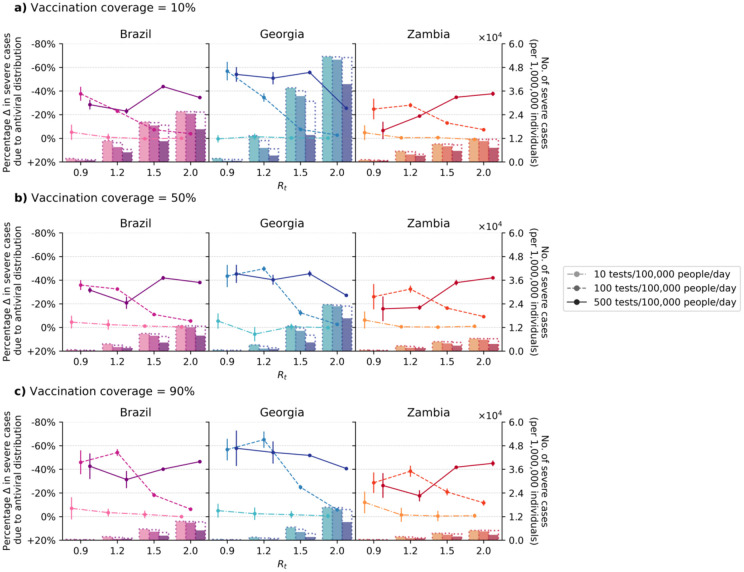
In our model, restricting testing to high-risk groups when there are sufficient amounts of test to diagnose all symptomatic individuals resulted in more transmissions (up to 56% more infections particularly when Rt ≤1.5, 500 tests/100K/day and/or higher vaccination coverage; Extended Data Fig. 8) and a higher number of severe cases (Fig. 2; e.g. 52% (66%) reduction in severe cases in Georgia at Rt = 1.5, 500 tests/100K/day and 90% vaccination coverage with (without; Fig. 1) symptomatic testing restrictions). On the other hand, when operating under limited test availability relative to Rt, restricting symptomatic testing to maximally test-and-treat high-risk individuals could be an effective strategy to further reduce severe cases (i.e. Fold increase in proportion of severe cases averted than no symptomatic testing restrictions when Rt ≥1.5, across all vaccination coverage and LMICs simulated: 100 tests/100K/day, median 4.9-fold (interquartile range (IQR) = 3.3–6.4); 500 tests/100K/day, median 3.2-fold (IQR = 2.4–5.1)) and in turn, deaths as well (Extended Data Fig. 9).
Fig. 2: Impact of oral antiviral therapy on severe cases when restricting symptomatic testing at clinics to high-risk individuals only.
High-risk household contacts of test-positive individuals are not tested. All eligible high-risk individuals (i.e. ≥60 years of age or an adult ≥18 years with a relevant comorbidity) who tested positive were given a course of oral antivirals. Line plots (left y-axis) show the percentage change in severe cases relative to no distribution of antivirals under different levels of mean test availability (different shades of color) after a 90-day Omicron BA.1-like epidemic wave in a population of 1,000,000 individuals with (a) 10%, (b) 50%, and (c) 90% vaccination coverage for different epidemic intensities (measured by the initial instantaneous reproduction number (Rt); x-axis). Bar plots (right y-axis) show the number of severe cases in each corresponding scenario. The dotted outline of each bar shows the number of severe cases of each scenario when no antivirals were distributed.
Impact of oral antivirals in high-income countries
We also simulated Omicron BA.1-like epidemic in the Netherlands as a HIC archetype. We assumed that 80% of the population have been fully vaccinated and that over-the-counter Ag-RDTs for self-testing are widely available, such that only a small proportion (10%) of symptomatic individuals seek clinic-provided testing directly. Most individuals who did not seek clinic-provided testing (80%) would instead perform a self-test using over-the-counter Ag-RDTs. All high-risk individuals who tested positive using self-tests would then seek reflexive testing at clinics on the same day to be administered antivirals (see Methods).
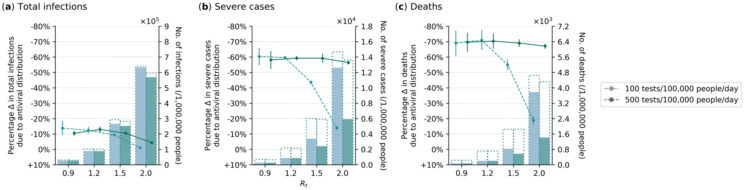
Under these assumptions, we found that in combination with the current mean HIC clinic-provided testing rate of 500 tests/100K/day, distribution of antivirals could avert 56–59% of severe cases and 67–70% of deaths on average, regardless of the epidemic intensity (Fig. 3). Given that the age distribution of the Netherlands is broadly similar to that of Georgia, there was a modest reduction in total infections due to antivirals, but it did not amount to more than an average of 13%. However, if mean clinic-provided testing rates were to fall to 100 tests/100K/day, the mean proportion of severe cases and deaths averted would also drop precipitously to as low as 14% and 19% respectively when Rt ≥ 1.5. Since antivirals must be administered promptly upon a positive diagnosis, we also computed the proportion of high-risk, symptomatic individuals that would miss the treatment window if they had sought reflexive testing late. Regardless of clinical testing rate and Rt, for ≥90% of high-risk symptomatic individuals who were able to avert severe disease outcomes through the antiviral to be treated with the drug, they must not seek reflexive testing at clinics (if reflexive testing is required) later than two days after being tested positive with over-the-counter self-tests (Extended Data Fig. 11).
Fig. 3: Impact of oral antiviral therapy in a high-income country (Netherlands).
No restrictions on access to symptomatic testing at clinics (i.e. all symptomatic individuals who sought testing at clinics would receive one if in stock) and high-risk household contacts of test-positive individuals are not tested. Over-the-counter antigen rapid diagnostic tests (Ag-RDTs) are assumed to be widely available. As such, we assumed that only 10% of symptomatic individuals would seek clinical testing directly while 80% of those who opted not to seek clinic-provided testing would perform self-testing using over-the-counter Ag-RDTs. All high-risk individuals who tested positive through self-testing would seek reflexive testing at clinics on the same day. All eligible high-risk individuals (i.e. ≥60 years of age or an adult ≥18 years with a relevant comorbidity) who tested positive at clinics, either directly or through reflexive testing, were given a course of oral antivirals. Line plots (left y-axis) show the percentage change in (a) total infections, (b) severe cases and (c) deaths relative to no distribution of antivirals under different clinical testing rates (different shades of color) after a 90-day Omicron BA.1-like epidemic wave in a population of 1,000,000 individuals 80% vaccination coverage for different epidemic intensities (measured by the initial instantaneous reproduction number (Rt); x-axis). Bar plots (right y-axis) show the number of severe cases in each corresponding scenario. The dotted outline of each bar shows the number of severe cases of each scenario when no antivirals were distributed.
Oral antiviral need
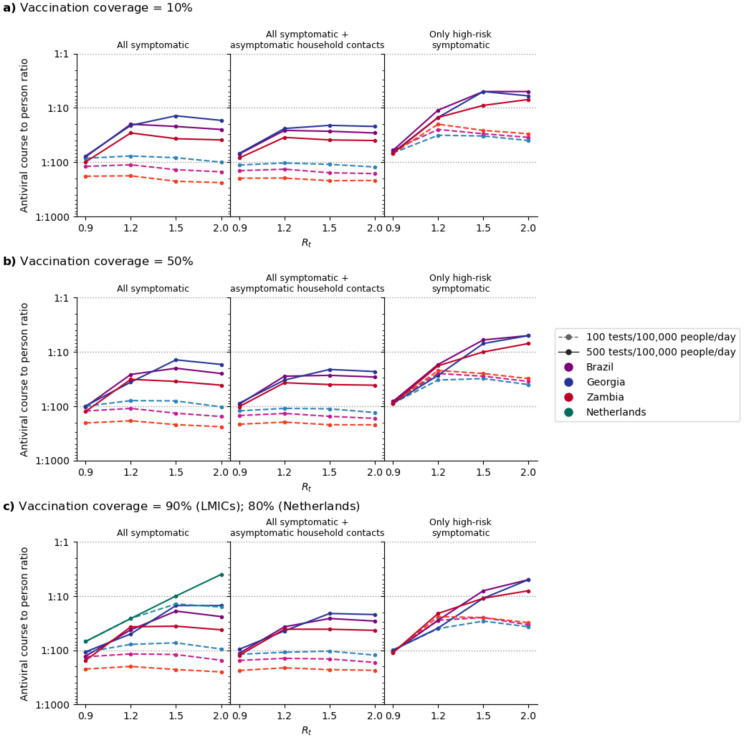
By assuming that all test-positive, high-risk individuals received an antiviral course, we estimated the amount of antiviral needed in each simulated scenario (Fig. 4). We assumed that vaccine protection against infection was low (30%) and that antivirals were distributed regardless of vaccination status. As such, increasing vaccination coverage did not lower antiviral need substantially (median 0.93-fold change (IQR = 0.70–1.00) when vaccination coverage increased from 10% to 90%). Conversely, the amount of antivirals distributed depends on Rt (median 2.60-fold change (IQR = 0.97–4.35) when Rt increases from 0.9 to 2.0), country demographics (median 1.72-fold change (IQR=1.02–2.04) when distributing antivirals in Georgia relative to Zambia), testing rates (median 4.31-fold change (IQR = 1.49–5.77) when increasing from 100 to 500 tests/100K/day), and how tests were targeted (median 2.57-fold change (IQR = 1.52–4.55) when testing only high-risk as opposed to all symptomatic individuals).
Fig. 4: Estimated need of oral antivirals.
Line plots show the ratio of estimated oral antiviral courses needed to number of people per year (expressed as 1 oral antiviral course per n number of individuals; assuming two epidemic waves a year) in various countries (color) under different simulated scenarios (i.e. testing rate at 100 or 500 tests/100,000 people/day (shading and linestyle) and distribution modality (left plot panel: test all symptomatic individuals who sought testing at clinics; middle plot panel: test all symptomatic individuals who sought testing as well as distributing clinic-provided self-tests to high-risk asymptomatic household contacts of test-positive individuals; right plot panel: test only high-risk symptomatic individuals who sought testing at clinics). All test-positive eligible high-risk individuals from clinic-provided testing would receive a course of oral antivirals. For the Netherlands, over-the-counter antigen rapid diagnostic tests (Ag-RDTs) are assumed to be widely available that most high-risk individuals would perform a self-test first and only seek reflexive testing at clinics if their over-the-counter tests were positive. (a) 10%, (b) 50% and (c) 90% (Low and middle-income countries; LMICs); 80% (Netherlands) vaccination coverage assumed for the simulated population.
In the Netherlands, even though only 10% of symptomatic individuals sought clinic-provided testing directly in the model, the availability and assumed wide uptake (80%) of over-the-counter self-tests, coupled with the possibility to perform a reflex test promptly to qualify for antiviral administration (≤2 days since a positive over-the-counter test), ensured that high-risk individuals can be identified promptly, and yielded the highest average antiviral need at one course for every 4–69 individuals per year (assuming testing rate of 500 tests/100K/day and two 90-day epidemic waves per year; Fig. 4C). For the three LMICs simulated, one antiviral course was distributed for every 73–251 (14–154) persons on average if testing rate was 100 (500) tests/100K/day.
Discussion
The current mean LMIC testing rate of 10 tests/100K/day is inadequate to facilitate a test-and-treat program aimed at reducing population-level disease burden. Assuming that antiviral needs can be fully met, increasing test availability to at least 100 tests/100K/day, without imposing any restrictions in access to clinic-provided testing, could avert severe cases by up to 65% in LMICs experiencing an epidemic wave that initialized at Rt ≤1.2. Populations that have an older, high-risk population would avert a larger proportion of severe cases. Crucially, if testing rates are high enough to facilitate a test-and-treat program, the expected reduction in severe cases and deaths due to antivirals improves with the higher vaccination coverage (i.e. between 2.0 and 3.4-fold increase in severe cases averted by antivirals as vaccination coverage increases from 10% to 90%. This emphasizes the importance of linking expanding vaccination coverage in both LMICs and HICs to adequate testing, on top of distributing antivirals.
If Rt ≥1.5, 100 tests/100K/day is likely insufficient to fully meet testing demand for symptomatic, infected persons who seek clinic-based testing, impeding the identification of high-risk individuals for antiviral treatment. Given that antivirals are unlikely to have an impact of population-level transmission5, if the main objective of testing is to maximize the distribution of antivirals to infected high-risk individuals, restricting clinic-based testing to only high-risk symptomatic individuals at testing rates of 100 tests/100K/day could lead to 3.3–6.4-fold increase in proportions of severe cases averted relative to the default scenario where no restrictions to clinic-provided testing was imposed. It is also possible to require asymptomatic, high-risk household contacts of test-positive symptomatic individuals to perform self-tests in order to initiate as many high-risk infected individuals to early antiviral treatment as possible. However, setting aside tests for asymptomatic screening when already facing test availability constraints at 100 tests/100K/day would likely diminish the utility of those tests. The proportion of severe cases and deaths averted due to antiviral distribution would also decrease by a relative factor of two to ten-fold under this strategy.
On the other hand, the availability of over-the-counter self-testing and high testing rates in HICs like the Netherlands is further evidence that high testing volume and the wide accessibility to testing, especially self-testing, are key to the success of antiviral test-and-treat programs. Among the countries simulated, only the Netherlands averted high proportions of severe cases (56–59%) and deaths (67–70%) when Rt ≥ 1.5 without the need to impose testing restrictions. These results, however, are only possible if clinic-provided testing is maintained at the mean HIC rate of 500 tests/100K/day. If clinical testing volumes were to drop further to 100 tests/100K/day, the expected reduction in severe cases and deaths attributable to antivirals would fall to only 14% and 19% respectively in an epidemic wave initializing at Rt = 2.0.
There have been other modelling efforts estimating the impact of antivirals on epidemic outcomes. First, Leung et al.12 estimated that distributing antivirals to 50% of all symptomatic infected individuals would only reduce hospitalizations by 10–13% in a population with high vaccination coverage (70–90%).12 For the Netherlands, we also simulated a population with 80% vaccination coverage and adequate testing availability (including both clinic-based and over-the-counter self-tests) such that at least 50% of all symptomatic individuals were diagnosed. We estimated that 56–59% of severe cases count be averted if only high-risk symptomatic individuals were administered antivirals. When we reconfigured our simulations to now distribute antivirals to 50% of symptomatic infected individuals, the proportion of severe cases averted lower to only 18% which is more in line with Leung et al. A second modelling study found that initiating 20% of infected individuals that were >65 years of age on antivirals daily could avert 32–43% of deaths in an Omicron-like wave (Rt ≥ 2) for an unvaccinated population in LMICs such as Kenya and Mexico.5 We had estimated that 31–62% of deaths could be averted at Rt = 2 at low (10%) vaccination coverage in LMICs but only if test availability was at the current average HIC mean of 500 tests/100K/day and clinic-provided symptomatic testing were restricted to high-risk individuals, in which we would then initiate a daily average of 19–20% of high-risk infected individuals on treatment each day. If there are no restrictions on access to clinic-provided tests, testing rate must be at least 750 tests/100K/day to initiate 20% of infected >65-years on antivirals daily with >95% probability, which is 50% more than the current mean HIC testing rate indicating the previous results for Kenya and Mexico were predicated on very high testing rates.
There are a few limitations to our work. First, our simulations were based on the estimated effectiveness of nirmatrelvir–ritonavir. We did not consider the clinical benefits of other oral antivirals as nirmatrelvir–ritonavir is the most efficacious antiviral available during the development of this work. Second, we also assumed that vaccine effectiveness against infection is low (29%) based on the average reported protection estimates against Omicron BA.1.13–15 Others have shown that with greater vaccine effectiveness against infection (60%), a high vaccination coverage (~70–80%) coupled with antivirals that have an effect in lowering transmissions could synergistically reduce infections in the population.5 However, for only ~20% of infections to be averted in an Omicron-like wave, the antiviral must be able to block onward transmission completely after initiating treatment and 30% of symptomatic infected adults must be administered antivirals daily.5 Even if an antiviral that is 100% effective in truncating transmissions be developed, testing rate must at least be 764 tests/100K/day to initiate 30% of symptomatic infected individuals to treatment daily with >95% probability based on our estimates. Finally, we only simulated scenarios where the only public health interventions against COVID-19 are testing, vaccination and distribution of antivirals. We also did not factor in changes to individual immunity levels due to previous infections or waning. As a simplification, we assumed that the consolidatory effects from other public health measures and varying immunity landscape have been implicitly captured by various initial Rt values when the epidemic wave started.
As of July 2022, Global Fund and UNICEF are procuring up to 10 million courses of nirmatrelvir–ritonavir for LMICs in 2022/2023.16,17 In other words, there would only be one treatment course for every 660 people in LMICs in the coming year (given that the total population size in LMICs stands at ~6.6 billion people18). In contrast, the United States have procured one course for every 16 persons so far,6 well within the range of estimated antiviral need with the expectation of two epidemic waves over the next year (one course per 4–69 individuals) in the Netherlands as a HIC archetype. Strikingly, the current 10 million courses of nirmatrelvir–ritonavir set aside for LMICs cannot even fully satisfy the antiviral need averaged across the three LMICs simulated at 100 tests/100K/day for one epidemic wave that begins with at Rt = 0.9, meeting only 39–47% of potential need. Realistically, having at least two epidemic waves ranging between Rt = 1.2 – 1.5 over the next year and aiming to maximally satisfy all antiviral need of LMICs, would mean that the 10 million courses only amount to 4–7% of potential total need. We estimated that LMICs would likely need between 26 and 90 million courses in a year if testing rates can be boosted to 100 tests/100K/day. Although Pfizer has agreed to grant sublicenses to manufacture generic versions of nirmatrelvir–ritonavir, it will still take at least one year before they enter the market. Furthermore, middle-income countries are prohibited from procuring generics, thus leaving them to compete with HICs for the remaining 90 million courses Pfizer plans to produce in the second-half of 2022.6 Given that unequal access to vaccines and testing have loomed over LMICs over the last two years of the pandemic,19,20 the global distribution of oral antiviral therapeutics is likely to only further inequity.
Online Methods
The Propelling Action for Testing And Treating simulation model
Briefly, PATAT creates an age-structured population of individuals within contact networks of multi-generational households, schools, workplaces, regular mass gatherings (e.g. religious gatherings) and random community settings with country-specific demographic data. All simulations begin with 1% of the population infected with SARS-CoV-2 and compute transmissions between individuals across different contact networks each day. Disease progression of infected individuals follows an SEIRD epidemic model, further distinguished by symptom presentation (i.e. asymptomatic, pre-symptomatic, mild or severe disease). For each infected individual, PATAT randomly draws a within-host viral load trajectory, which impacts the sensitivity of Ag-RDTs21, based on known distributions for Omicron BA.122 using previously developed methods.23 Similar viral load trajectories were drawn for both asymptomatic and symptomatic infected individuals.24
Simulation variables
We simulated 90-day epidemic waves caused by an BA.1-like virus in a community of 1,000,000 individuals using demographic data collected from three LMICs (i.e. Brazil, Georgia, Zambia) and the Netherlands as a HIC counterpart. For LMICs, we simulated different vaccination coverage (10%, 50% and 90%) while 80% of the population were assumed to be vaccinated in the Netherlands based on estimates on July 2022,25 which is largely comparable to other HICs.26 We randomly assigned vaccination status across the simulated population but assumed that vaccination was age-tiered such that the older individuals were vaccinated first. Based on estimated vaccine effectiveness against BA.1 averaged across different vaccines, we assumed that protection rates against infection and severe disease were 29% and 70% respectively.13–15
We did not model varying levels of population immunity due to difficulties in parameterizing the proportion and protection conferred to individuals with infections by single or multiple variants-of-concern in the past. However, we simulated a range of epidemic intensities, measured by the average instantaneous reproduction number (i.e. Rt = 0.9, 1.2, 1.5, and 2.0) during the first week of each simulation for different vaccination landscapes without test-and-treat programs. As such, the different Rt values can be viewed as the collective outcome of population immunity, intrinsic transmissibility of the transmitted virus as well as effects of existing any public health interventions.
Besides an age-structured probability of developing severe disease (Extended Data Table 1), we randomly assigned 20% of the population to have a 40% increase in relative risk to developing severe disease due to pre-existing comorbidities (e.g. people living with HIV, obesity, diabetes etc.).27,28 As a simplification, we assumed that the prevalence of comorbidities was independent of age.
Diagnostic testing
In the model, individuals with symptomatic COVID-19 have a probability of seeking testing at a healthcare facility based on their ability to access a facility (see Supplementary Information). We also estimated symptomatic testing demand from individuals without COVID-19 who sought clinic-provided testing (e.g. individuals who present with similar respiratory symptoms) by assuming a 10% test positivity rate at the start as well as end of an epidemic wave and a 20% test positivity rate at the peak, linearly interpolating the demand for periods between these time points.9,10
We also simulated scenarios where household contacts of clinic-provided positively-tested individuals were given Ag-RDTs for self-testing for three consecutive days following the positive clinical test of the latter. Adherence (likelihood) to testing by asymptomatic household contacts was assumed to decrease linearly to 50% by the third day. We also simulated an alternative test distribution strategy where we restricted clinic-provided symptomatic testing to high-risk individuals only.
For LMICs, we modelled three levels of average test availability at healthcare clinics: 10 (mean LMIC testing rate as of Q2/2022),7 100 and 500 (mean HIC testing rate as of Q2/2022)7 tests/100K/day. For the Netherlands, we performed simulations at clinic-provided testing rates of 100 and 500 tests/100K/day only.
Based on surveys of pre-COVID-19 pandemic health-seeking behaviour, we assumed that on average 65% of mild symptomatic individuals would seek clinic-provided testing for LMICs29 (and were only tested if there were available test stocks).
For the Netherlands, however, we assumed that Ag-RDTs are widely available over-the-counter, with no cap on availability. We also assumed that only 10% of mild symptomatic individuals in the Netherlands would seek clinic-provided testing upon symptom onset based on average daily testing rates reported by all Dutch municipal health services in 2021Q1/2022 (i.e. approximately up to the end of the Omicron BA.1 wave; 7551 tests/100K/day) and Q2/2022 (post Omicron BA.1 wave; 641 tests/100K/day).30 We assumed that 80% of individuals who opted not to seek clinic-provided testing would perform a self-test using an over-the-counter Ag-RDT. We assumed that all high-risk individuals who tested positive would then seek reflexive testing at clinics to be disbursed an antiviral course.
Oral antivirals
Regardless of their vaccination status (per WHO guidance31), all high-risk individuals who tested positive within five days after symptom onset were eligible for a course of antiviral therapy.3,4 We did not impose any caps on antiviral availability as we wanted to estimate the potential number of antiviral courses needed and thus their maximum achievable impact on epidemic outcomes in different scenarios. For all countries, we assumed that antivirals were only administered if high-risk individuals tested positive at clinics (e.g. a self-reported self-test would be insufficient to access antivirals). Although a phase 2/3 trial of nirmatrelvir–ritonavir reported 89% relative risk reduction among unvaccinated high-risk patients infected by the Delta variant-of-concern,3 we assumed that an antiviral course conferred a 46% risk reduction for infected high-risk individuals to severe disease outcomes based on a separate cohort study on the effectiveness of nirmatrelvir–ritonavir among high-risk patients infected by Omicron BA.1 independent of their vaccination status.4 We did not factor any risk reduction in transmissions and deaths given the lack and low certainty of evidence of the impact of oral antivirals on protection against infection and mortality respectively.31 However, in our model, individuals could only die from COVID-19 if they had progressed to severe disease.
We performed five independent simulations for each combination of parameters described above. All key parameters are tabulated in Extended Data Table 1. Full details of PATAT are described in Han et al.9,10 and the Supplementary Information. The PATAT model source code is available at https://github.com/AMC-LAEB/PATAT-sim.
Supplementary Material
Acknowledgements
The authors are pleased to acknowledge that all computational work reported in this paper was performed on the Shared Computing Cluster which is administered by Boston University’s Research Computing Services (www.bu.edu/tech/support/research/).
Funding
This work was supported by the European Research Council [NaviFlu 818353 to A.X.H. and C.A.R.], the National Institutes of Health [5R01AI132362-04 to C.A.R.] and the Dutch Research Council (Nederlandse Organisatie voor Wetenschappelijk Onderzoek) [Vici 09150182010027 to C.A.R.]. This work was supported by the Rockefeller Foundation, and the Governments of Germany, Canada, UK, Australia, Norway, Saudi Arabia, Kuwait, Netherlands and Portugal [all authors].
Footnotes
Potential conflicts of interest
The authors declare that they have no competing interests.
Data availability
All data relevant to the study are included in the Article, the Supplementary Information and the GitHub repository (https://github.com/AMC-LAEB/PATAT-sim). The PATAT model source code can also be found in the GitHub repository (https://github.com/AMC-LAEB/PATAT-sim).
References
- 1.Singh M. & de Wit E. Antiviral agents for the treatment of COVID-19: Progress and challenges. Cell Rep Med 3, 100549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayk Bernal A. et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. New England Journal of Medicine 386, 509–520 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond J. et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. New England Journal of Medicine 386, 1397–1408 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najjar-Debbiny R. et al. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clinical Infectious Diseases (2022) doi: 10.1093/CID/CIAC443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matrajt L., Brown E. R., Cohen M. S., Dimitrov D. & Janes H. Could widespread use of antiviral treatment curb the COVID-19 pandemic? A modeling study. BMC Infect Dis 22, 1–16 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usher A. D. The global COVID-19 treatment divide. The Lancet 399, 779–782 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FIND. Test tracker - FIND. https://www.finddx.org/covid-19/test-tracker/ (2022).
- 8.Gill C. J. et al. Sustained high prevalence of COVID-19 deaths from a systematic post-mortem study in Lusaka, Zambia: one year later. medRxiv 2022.03.08.22272087 (2022) doi: 10.1101/2022.03.08.22272087. [DOI] [Google Scholar]
- 9.Han A. X. et al. Strategies for using antigen rapid diagnostic tests to reduce transmission of SARS-CoV-2 in low- and middle-income countries: a mathematical modelling study applied to Zambia. medRxiv 2022.06.16.22276516 (2022) doi: 10.1101/2022.06.16.22276516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han A. X. et al. SARS-CoV-2 diagnostic testing rates determine the sensitivity of genomic surveillance programs. medRxiv 2022.05.20.22275319 (2022) doi: 10.1101/2022.05.20.22275319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wroe E. B., Seung K. J., Baker B. K. & Farmer P. E. Test and treat: a missing link in the global fight against COVID-19. Lancet Glob Health 10, e181–e182 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung K., Jit M., Leung G. M. & Wu J. T. The allocation of COVID-19 vaccines and antivirals against emerging SARS-CoV-2 variants of concern in East Asia and Pacific region: A modelling study. Lancet Reg Health West Pac 21, 100389 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews N. et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. New England Journal of Medicine 386, 1532–1546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchan S. A. et al. Effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. medRxiv 2021.12.30.21268565 (2022) doi: 10.1101/2021.12.30.21268565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng H. F. et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nature Medicine 2022 28:5 28, 1063–1071 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UNICEF. UNICEF signs supply agreement with Pfizer for oral COVID-19 treatment. https://www.unicef.org/press-releases/unicef-signs-supply-agreement-pfizer-oral-covid-19-treatment (2022).
- 17.Global Fund. Global Fund Signs Letter of Intent with Pfizer for Oral COVID-19 Treatment. https://www.theglobalfund.org/en/news/2022/2022-05-20-global-fund-signs-letter-of-intent-with-pfizer-for-oral-covid-19-treatment/ (2022).
- 18.United Nations. World Population Prospects - Population Division. https://population.un.org/wpp/Download/SpecialAggregates/EconomicTrading/ (2022).
- 19.Batista C. et al. The silent and dangerous inequity around access to COVID-19 testing: A call to action. EClinicalMedicine 43, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Y. et al. Equitable access to COVID-19 vaccines makes a life-saving difference to all countries. Nature Human Behaviour 2022 1–10 (2022) doi: 10.1038/s41562-02201289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brümmer L. E. et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med 18, e1003735- (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay J. A. et al. The impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound. medRxiv 2022.01.13.22269257 (2022) doi: 10.1101/2022.01.13.22269257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quilty B. J. et al. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. Lancet Public Health 6, e175–e183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyton R. J. & Altmann D. M. The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? Nature Reviews Immunology 2021 21:12 21, 762–768 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute For Public Health and The Environment (RIVM). Figures on the COVID-19 vaccination programme. https://www.rivm.nl/en/covid-19-vaccination/figures-vaccination-programme (2022).
- 26.Ritchie H. et al. Coronavirus Pandemic (COVID-19). Our World in Data (2020). [Google Scholar]
- 27.Rawshani A. et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: A nationwide retrospective cohort study. The Lancet Regional Health - Europe 4, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertagnolio S. et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV 9, e486–e495 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dovel K. et al. Frequency of visits to health facilities and HIV services offered to men, Malawi. Bull World Health Organ 99, 618–626 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute for Public Health and the Environment (RIVM). COVID-19 dataset. https://data.rivm.nl/covid-19/ (2022).
- 31.World Health Organization. Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3 (2022). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the Article, the Supplementary Information and the GitHub repository (https://github.com/AMC-LAEB/PATAT-sim). The PATAT model source code can also be found in the GitHub repository (https://github.com/AMC-LAEB/PATAT-sim).