Abstract
Purpose
To assess the repeatability of peripapillary OCT angiography (OCTA) in those with Alzheimer disease (AD), mild cognitive impairment (MCI), Parkinson disease (PD), or normal cognition.
Design
Cross-sectional.
Participants
Patients with a clinical diagnosis of AD, MCI, PD, or normal cognition were imaged. Those with glaucoma, diabetes mellitus, vitreoretinal pathology, and poor-quality images were excluded.
Methods
Each eligible eye of each participant underwent 2 OCTA 4.5 × 4.5-mm peripapillary scans in a single session using a Zeiss Cirrus HD-OCT 5000 with AngioPlex (Carl Zeiss Meditec). The Zeiss software (v11.0.0.29946) quantified measures of perfusion in the radial peripapillary capillary (RPC) plexus in 4 sectors (superior, nasal, inferior, temporal). The average of these sectors was calculated and reported.
Main Outcome Measures
Radial peripapillary capillary plexus perfusion was quantified using 2 parameters: capillary perfusion density (CPD) and capillary flux index (CFI). Intraclass correlation coefficients (ICCs) were used to quantify repeatability. For subjects who had both eyes included, the average values of each scan pair were used to assess interocular symmetry of CPD and CFI.
Results
Of 374 eyes, 46 were from participants who had AD, 85 were from participants who had MCI, 87 were from participants who had PD, and 156 were from participants who had normal cognition. Capillary perfusion density ICC in AD = 0.88 (95% confidence interval [CI], 0.79–0.93), MCI = 0.95 (0.92–0.96), PD = 0.91 (0.87–0.94), and controls = 0.90 (0.87–0.93). Capillary flux index ICC in AD = 0.82 (0.70–0.90), MCI = 0.87 (0.80–0.91), PD = 0.91 (0.87–0.94) and controls = 0.85 (0.79–0.89). There were no significant differences in interocular variation in average CPD and CFI in AD, MCI, or PD (all P > 0.05). Isolated interocular sectoral CPD differences were noted in AD (nasal, P = 0.049; temporal, P = 0.024), PD (nasal, P = 0.036), and controls (nasal, P = 0.016). Interocular differences in CFI in the superior sector in MCI (P = 0.028) and in average CFI for controls (P = 0.035) were observed.
Conclusions
Peripapillary OCTA repeatability in AD, MCI, and PD is good-excellent and similar to those with normal cognition. Insignificant interocular asymmetry in peripapillary OCTA suggests neurodegeneration may proceed uniformly; future studies may reveal the appropriateness of single-eye imaging.
Keywords: Alzheimer disease, Mild cognitive impairment, Neurodegeneration, OCT angiography, Parkinson disease, Peripapillary, Repeatability, Retina
Abbreviations and Acronyms: AD, Alzheimer disease; CI, confidence interval; CFI, capillary flux index; CPD, capillary perfusion density; ICC, intraclass correlation coefficient; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; OCTA, OCT angiography; PD, Parkinson disease; RPC, radial peripapillary capillary; SD, standard deviation
The optic nerve connects the retina to the brain and may be affected in numerous medical and ocular conditions.1, 2, 3 OCT and OCT angiography (OCTA) provide a targeted means of assessing the structure and vascularity of the peripapillary retina.4 The radial peripapillary capillary (RPC) plexus is a network of capillaries that runs in parallel with retinal nerve fiber layer axon bundles and is instrumental in nourishing the axons of retinal ganglion cells in the peripapillary neurosensory retina.5, 6, 7 Peripapillary vessel density decreases with normal aging and in ocular pathologies such as glaucoma.8, 9, 10
Because pathologic changes in the central nervous system may be reflected in the retinal tissue, as an extension of the brain, the peripapillary vasculature has also been studied in the context of neurodegenerative disease.11,12 The development of novel ocular imaging biomarkers for these conditions using OCT and OCTA technology is a growing area of research.13,14 A study of Alzheimer disease (AD) and mild cognitive impairment (MCI) found no difference in peripapillary vessel density when compared with those with normal cognition.15 In Parkinson disease (PD), our group recently demonstrated increased perfusion in the RPC plexus compared with those with normal cognition and without PD.16
Previous studies in healthy eyes of cognitively normal individuals have described good-excellent repeatability of macular OCTA measures, and our recent investigation of peripapillary OCTA repeatability in a smaller cohort of healthy older adults found moderate-good correlation.9,17 Although there is a growing body of literature on microvascular changes in neurodegenerative disease, the differential repeatability of these measures among the various diagnoses has yet to be studied. Patients with neurodegenerative disease may have impaired executive function (in AD and MCI) or impaired motor function and head tremor (in PD). Because image acquisition requires eye fixation and OCTA is susceptible to motion artifact, individuals with these conditions may have less repeatable scans than those with normal cognition. As OCTA technology becomes a more readily available, facile, and noninvasive means of studying the retina to better understand cerebral disease, establishing its repeatability in research protocols is a meaningful undertaking.
Previous studies have described varying correlation of ocular parameters measured between 2 eyes of cognitively normal adults ranging from moderate to high correlation in retinal thickness,18 foveal avascular zone area, superficial vessel density,19 ganglion cell complex measures,20 capillary plexus measures,21 and fractal dimension.17 Our prior work assessing interocular symmetry of peripapillary OCTA measures found significant asymmetry in temporal capillary flux index (CFI) between the eyes of cognitively normal older adults; however, other parameters were similar between eyes of the same patient.9 The inclusion of both eyes of a subject in ocular studies of systemic disease has been controversial, because it is possible that neurodegenerative disease affects each eye differently. Demonstrating that interocular symmetry of OCTA parameters exists in individuals with neurodegenerative disease may support the inclusion of only 1 eye of a patient with neurodegenerative disease, which would facilitate shorter image acquisition times and potentially allow for larger studies.
In this study, we investigate the repeatability and interocular symmetry of peripapillary OCTA imaging parameters in AD, MCI, and PD compared with normal cognition.
Methods
Subjects were recruited from the Duke Alzheimer’s Disease Prevention Registry and the Duke Neurological and Movement Disorders Clinics. This included persons with a clinical diagnosis of AD, MCI, or PD confirmed by an expert neurologist, and cognitively normal participants without a neurologic diagnosis. Patients with potentially confounding medical or ocular conditions including diabetes mellitus, glaucoma, uncontrolled hypertension, other neurodegenerative disease, and vitreoretinal pathology were excluded.
Each subject underwent 2 consecutive 4.5 × 4.5-mm optic disc-centered OCTA scans in at least 1 eye. Imaging was performed by an experienced technician using a Zeiss Cirrus HD-OCT 5000 with AngioPlex (Carl Zeiss Meditec, Version 11.0.0.29946). The image set was manually reviewed by the investigators (J.P.M., C.B.R.) for images of insufficient signal strength (<7/10), segmentation artifact, or motion artifact. Scan pairs in which at least 1 scan was of poor quality were excluded from analysis. Image quality reviewers were masked to diagnosis and masked to the quantitative data between repeated scans. All imaged patients had neurocognitive status assessed with a Mini Mental State Examination (MMSE) on the day of imaging, and PD patients had disease severity scored by an expert neurologist (B.L.S.) using the Modified Hoehn and Yahr scale within 7 days of imaging.22,23
The main measurements in this study were that of peripapillary vessel perfusion quantified by 2 parameters: capillary perfusion density (CPD) and CFI. These were measured in the RPC plexus, isolated as a tissue slab including the retinal layers between the internal limiting membrane and the outer boundary of the retinal nerve fiber layer. The Zeiss AngioPlex software converted the en face image into a binary map, in which each pixel was assigned as either vessel or not-vessel. The resultant binary map was further skeletonized to a vessel network 1 pixel in width. To ensure quantification of perfusion exclusively at the capillary level, vessels with a pre-skeletonized width > 32 μm were excluded, because this width exceeds capillary size and would represent larger retinal vessels (arterioles/venules).
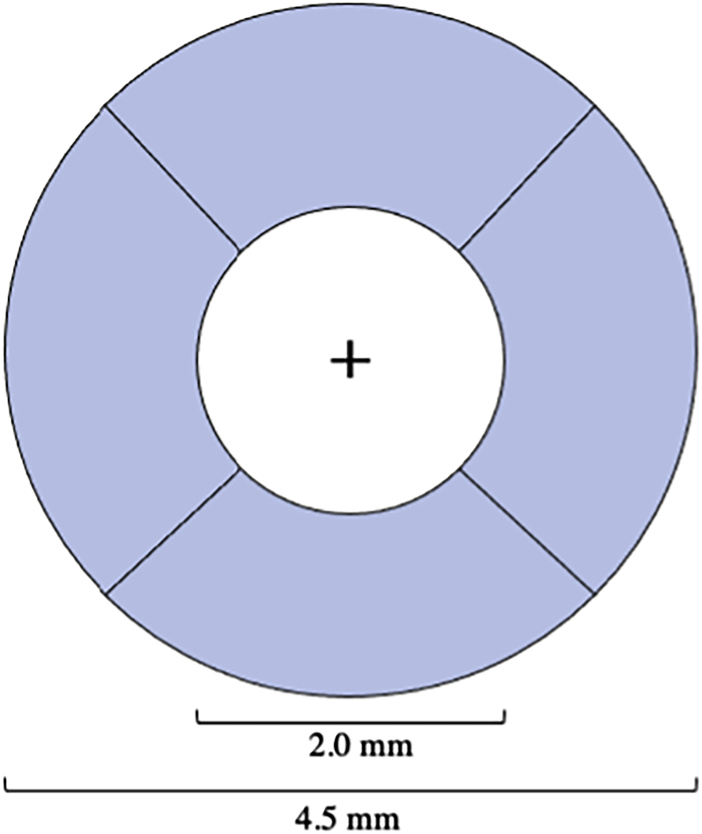
The CPD and CFI values were extracted from the binary map and vessel skeleton by the software exclusively within the peripapillary region, defined as a ring-shaped region of interest bounded by optic disc-centered circles of 4.5-mm diameter exteriorly and 2.0-mm diameter interiorly (Figure 1). Measurements were reported by sector (superior, nasal, temporal, inferior) and as an average of these areas. The CPD was calculated as the percentage of pixels in the binary map that were also in the vessel skeleton. The CFI was calculated as a unitless ratio representing the perfused vascular area per unit area, weighted by the flow within the vessel, extracted from brightness of constituent pixels of the vessels in the en face image.24,25
Figure 1.
Annular peripapillary angiography sectors (superior, nasal, temporal, inferior) formed by circles of 2 mm and 4.5 mm in diameter about the center of optic disc (+). Capillary perfusion density (CPD) and capillary flux index (CFI) were measured in each sector and as an average of all 4 sectors.
Statistical analysis was performed by an experienced statistician (S.S.S.). The MMSE and Modified Hoehn and Yahr scale scores were compared between included and excluded patients within each diagnosis using the Wilcoxon rank-sum test for difference between medians. The repeatability of average and sectoral CPD and CFI was quantified using intraclass correlation coefficients (ICCs), which describe the overall correlation between individual measures, and Bland–Altman limit of agreement analysis. We used benchmarks for qualitative interpretation of ICCs provided by Koo and Li:26 poor (ICC = 0.00–0.50), moderate (0.5–0.75), good (0.75–0.90), and excellent repeatability (> 0.90).27, 28, 29, 30
We also assessed interocular symmetry in AD, MCI, and PD using the averages of repeat scans for a more robust measure of each parameter in each subject. Only patients with both eyes imaged were included in this sub-analysis. The average of the 2 repeated measures in each of a given pair of patient’s eyes were compared using the Wilcoxon signed-rank test. To assess potential association of repeatability with age, patients in each group were stratified into 2 age brackets (<70 years of age and ≥70 years of age), and ICCs were calculated in each stratum.
This study was sanctioned by the Duke University Institutional Review Board (Pro00082598) in compliance with the Health Insurance Portability and Accountability Act and the Declaration of Helsinki. Informed consent was obtained for all participants. Clinical trial registration is available at clinicaltrials.gov with the identifier NCT03233646.
Results
A total of 67 AD eyes, 108 MCI eyes, 125 PD eyes, and 195 eyes of cognitively normal subjects were imaged. After diagnosis-masked image quality exclusion, 46 eyes with AD (68.7% yield of initial eyes, 35 subjects), 85 eyes with MCI (78.7% yield, 55 subjects), 87 eyes with PD (69.6% yield 53 subjects), and 156 normal cognition eyes (80.0% yield, 92 subjects) were analyzed. Chi-square testing indicated no significant differences among or between diagnostic groups in the proportion of images excluded because of poor quality (P > 0.05).
Demographic comparisons of average age and sex distribution in each group are reported in Table 1. The average age of the AD group was 74.5 years with a standard deviation (SD) of 7.1 years, MCI = 72.5 years (SD = 6.7), PD = 69.0 years (SD = 9.2), and normal cognition = 68.6 years (SD = 6.7). F-test and analysis of variance testing revealed that the mean ages between these groups were unequal (P < 0.001). Chi-square testing showed significant differences in sex distribution between groups (P < 0.001).
Table 1.
Age and Sex Demographic Distributions
| Statistic | Alzheimer Disease | Mild Cognitive Impairment | Parkinson Disease | Normal Cognition | P | |
|---|---|---|---|---|---|---|
| Age (yrs) | N (Subjects) | 35 | 55 | 53 | 92 | <0.001∗ |
| Mean (SD) | 74.5 (7.1) | 72.5 (6.7) | 69.0 (9.2) | 68.6 (6.7) | ||
| Min, Median, Max | 59.3, 74.3, 87.3 | 53.5, 73.9, 89.5 | 44.7, 69.5, 85.3 | 50.6, 69.4, 80.6 | ||
| Sex, male (%) | N (%) | 8 (23) | 26 (47) | 31 (58) | 24 (26) | <0.001† |
SD = standard deviation.
P value for age-based on F-test from analysis of variance.
P value for sex based on chi-square test.
Table 2 illustrates the degree of neurocognitive and neurodegenerative impairment between the patients who were included in the study compared with those excluded in each group with poor image quality, which could be partly due to such impairment. As quantified by the MMSE, there was no difference in cognitive impairment among the AD, MCI, PD, or cognitively normal groups when included subjects were compared with excluded subjects. However, there was a statistically significant difference in degree of motor symptoms as described by the Modified Hoehn and Yahr scale score for PD staging between included and excluded participants, with excluded participants demonstrating more impairment on the 4-point scale (mean excluded = 2.19; mean included = 1.88; P = 0.002).
Table 2.
Cognitive Impairment of Included Subjects versus Excluded Subjects
| Diagnosis | Statistic | Included Subjects† | Excluded Subjects† | P∗ |
|---|---|---|---|---|
| Alzheimer disease | N (subjects,∗ eyes) | 35, 47 | 15, 20 | |
| Mean MMSE (SD) | 22.0 (4.8) | 22.9 (4.7) | 0.519 | |
| Mild cognitive impairment | N (subjects,∗ eyes) | 55, 85 | 21, 23 | |
| Mean MMSE (SD) | 26.9 (3.3) | 26.6 (3.3) | 0.684 | |
| Parkinson disease | N (subjects,∗ eyes) | 53, 87 | 20, 38 | |
| Mean MMSE (SD) | 28.8 (1.6) | 27.8 (2.4) | 0.043 | |
| Mean Modified Hoehn and Yahr scale (SD) | 2.04 (0.58) | 2.15 (0.56) | 0.053 | |
| Normal cognition | N (subjects,∗ eyes) | 92, 156 | 28, 39 | |
| Mean MMSE (SD) | 29.4 (1.3) | 29.3 (1.3) | 0.767 |
MMSE = Mini Mental State Examination (range, 0–30); SD = standard deviation. Boldface indicates statistical significance.
Modified Hoehn & Yahr Scale, describing the severity of Parkinson disease motor symptoms (range: 1 least involved to 4 most involved).
P value based on a Mann–Whitney U test for unequal means for MMSE and Modified Hoehn Yahr Scale.
The sum of included subjects and excluded subjects, N, may not match the total number of subjects reported per diagnosis in Table 1, due to some subjects having 1 eye included and 1 eye excluded.
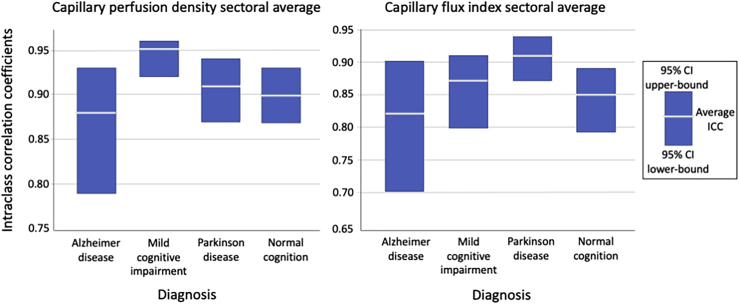
The ICCs for all sectors and averages are reported in Table 3. The ICCs were similar for measures of CPD and CFI across all diagnoses, with the average of sectoral measures having ICCs with overlapping 95% confidence intervals (CIs) for both parameters between all diagnoses (Figure 2).
Table 3.
Capillary Perfusion Density and Capillary Flux Index Repeatability
| Alzheimer Disease | Mild Cognitive Impairment | Parkinson Disease | Normal Cognition | |||||
|---|---|---|---|---|---|---|---|---|
| N (eyes) | 46 | 85 | 87 | 156 | ||||
| Capillary Perfusion Density Repeatability | ||||||||
| ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | |
| Average | 0.88 | 0.79–0.93 | 0.95 | 0.92–0.96 | 0.91 | 0.87–0.94 | 0.90 | 0.87–0.93 |
| Superior | 0.77 | 0.62–0.87 | 0.89 | 0.83–0.92 | 0.95 | 0.92–0.97 | 0.87 | 0.82–0.90 |
| Nasal | 0.68 | 0.48–0.81 | 0.86 | 0.79–0.91 | 0.79 | 0.69–0.86 | 0.66 | 0.56–0.74 |
| Inferior | 0.86 | 0.77–0.92 | 0.89 | 0.83–0.93 | 0.87 | 0.80–0.91 | 0.89 | 0.86–0.92 |
| Temporal | 0.75 | 0.59–0.85 | 0.82 | 0.73–0.88 | 0.76 | 0.66–0.84 | 0.76 | 0.68–0.82 |
| Capillary Flux Index Repeatability | ||||||||
| ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | |
| Average | 0.82 | 0.70–0.90 | 0.87 | 0.80–0.91 | 0.91 | 0.87–0.94 | 0.85 | 0.79–0.89 |
| Superior | 0.80 | 0.67–0.88 | 0.80 | 0.71–0.87 | 0.90 | 0.84–0.93 | 0.75 | 0.68–0.82 |
| Nasal | 0.71 | 0.53–0.83 | 0.74 | 0.63–0.82 | 0.90 | 0.84–0.93 | 0.84 | 0.79–0.88 |
| Inferior | 0.80 | 0.66–0.88 | 0.84 | 0.76–0.89 | 0.84 | 0.76–0.89 | 0.83 | 0.78–0.88 |
| Temporal | 0.77 | 0.63–0.87 | 0.81 | 0.72–0.87 | 0.86 | 0.80–0.91 | 0.75 | 0.67–0.81 |
CI = confidence interval (lower bound-upper bound); ICC = intraclass correlation coefficient.
Figure 2.
The intraclass correlation coefficient (ICC) for each diagnosis with upper and lower bounds of the 95% confidence interval (CI) for capillary perfusion density (CPD) (left) and capillary flux index (CFI) (right).
The CPD sectoral average had excellent repeatability (ICC > 0.90) in MCI, PD, and normal cognition; AD had good repeatability (ICC = 0.75–0.90) using Koo and Li’s26 guidelines for interpretation of ICC ranges. For measures of CFI, there was good repeatability in the AD, MCI, and normal cognition groups, and excellent repeatability in the PD group. Individual sectoral measures were largely in the good or excellent (ICC > 0.75) range for both CPD and CFI; however, several instances of moderate repeatability (ICC = 0.50–0.75) were observed. Nasal sector measures of CPD exhibited only moderate repeatability in the AD group (ICC = 0.68) and the normal cognition group (ICC = 0.66). For CFI measurements, moderate repeatability was observed in the MCI group (ICC = 0.74) and again in the AD group (ICC = 0.71). The AD group exhibited the lowest average ICC for both CPD and CFI; however, this was not statistically different from other diagnostic groups.
Given that the average age between groups differed, we calculated ICCs stratified by age (< 70 and ≥ 70 years). The sectoral average is reported by age per diagnosis in Table 4. The 95% CIs of CPD measures were all overlapping between age strata in all neurologic diagnoses. For measures of CFI, there were some nonoverlapping 95% CIs, with younger PD subjects having worse average CFI ICC, and younger individuals with normal cognition having better CFI ICC on average.
Table 4.
Capillary Perfusion Density and Capillary Flux Index Sectoral Average Repeatability by Age
| Diagnosis | Age < 70 Yrs |
Age ≥ 70 Yrs |
||||
|---|---|---|---|---|---|---|
| N (Eyes) | ICC | 95% CI | N (Eyes) | ICC | 95% CI | |
| Capillary Perfusion Density | ||||||
| Alzheimer disease | 12 | 0.58 | 0.05–0.85 | 34 | 0.91 | 0.83–0.96 |
| Mild cognitive impairment | 25 | 0.93 | 0.86–0.91 | 60 | 0.81 | 0.71–0.88 |
| Parkinson disease | 42 | 0.86 | 0.76–0.92 | 45 | 0.93 | 0.88–0.96 |
| Normal cognition | 87 | 0.90 | 0.86–0.94 | 69 | 0.89 | 0.82–0.93 |
| Capillary Flux Index | ||||||
| Alzheimer disease | 12 | 0.55 | 0.02–0.84 | 34 | 0.87 | 0.75–0.93 |
| Mild cognitive impairment | 25 | 0.87 | 0.73–0.94 | 60 | 0.84 | 0.75–0.90 |
| Parkinson disease | 42 | 0.77 | 0.63–0.87 | 45 | 0.93 | 0.88–0.96 |
| Normal cognition | 87 | 0.90 | 0.85–0.93 | 69 | 0.74 | 0.61–0.83 |
CI = confidence interval (lower bound-upper bound); ICC = intraclass correlation coefficient.
In addition to ICC analysis, the numerical differences between parameter values in each pair of repeated scans were compared using the Kruskal–Wallis test of difference among medians across all groups and within each group difference using the Wilcoxon signed-rank test of median difference from zero. There were no significant differences in between-group or within-group analysis of CPD or CFI in any group (all P > 0.05). In general, the average percent difference between repeated scans was at most 1.26% in CPD (Table S1, available at www.ophthalmologyscience.org) and 0.92% in CFI (Table S2, available at www.ophthalmologyscience.org).
These differences between scan pairs are presented visually using Bland–Altman plots, which examine 2 values of a given parameter between scan pairs and plot the average value of the parameter against the difference between the 2 individual scan measurements. The limits of agreement, the +1.96σ and −1.96σ values of difference, are plotted about the mean difference. Bland–Altman analysis showed that the mean intrasession difference was generally small, near 0 in most cases, with few measurement pairs outlying the limits of agreement, and a generally symmetric appearance to each plot (Figs S1 and S2, available at www.ophthalmologyscience.org).
We observed some isolated sectoral differences in the symmetry of parameter values between the right and left eyes of subjects in all groups, with findings reported in Table 5. Notably, in measures of CPD, the AD and PD groups and individuals with normal cognition showed significant differences in the nasal sector (all P > 0.05). Additionally, the AD group showed significant asymmetry in the temporal sector (P = 0.024). For measures of CFI, isolated sectoral asymmetry was observed in the MCI group (superior sector, P = 0.028; temporal sector, P = 0.040). In the control group, there was a significant degree of asymmetry of CFI in the temporal sector (P = 0.035), but also in the CFI area average (P = 0.035). The equivalent percent differences of these measures were generally less than 2.5%, with no exceptional value exceeding 5.0% difference.
Table 5.
Interocular Symmetry of Capillary Perfusion Density and Capillary Flux Index
| Diagnosis |
Alzheimer Disease |
Mild Cognitive Impairment |
Parkinson Disease |
Normal Cognition |
||||
|---|---|---|---|---|---|---|---|---|
| N (eye pairs) | 11 |
30 |
34 |
60 |
||||
| Mean Δ∗ (SD, %Δ) | P† | Mean Δ∗ (SD, %Δ) | P† | Mean Δ∗ (SD, %Δ) | P† | Mean Δ∗ (SD, %Δ) | P† | |
| Capillary Perfusion Density | ||||||||
| Average | 0.47 (2.17, 4.09%) | 0.206 | 0.13 (0.97, 0.33%) | 0.725 | 0.20 (1.70, 0.57%) | 0.927 | 0.14 (1.17, 0.31%) | 0.222 |
| Superior | 0.27 (1.72, 1.32%) | 0.765 | 0.39 (2.07, 0.91%) | 0.307 | 0.20 (3.61, 0.27%) | 0.055 | 0.22 (2.17, 0.55%) | 0.575 |
| Nasal | 1.33 (1.96, 3.14% | 0.049 | 0.53 (2.29, 1.29%) | 0.260 | 0.77 (2.09, 1.94%) | 0.036 | 0.71 (2.20, 1.70%) | 0.016 |
| Inferior | 1.15 (2.57, 2.77%) | 0.102 | 0.30 (2.54, 0.69%) | 0.312 | 0.54 (2.09, 1.35%) | 0.227 | 0.21 (1.83, 0.46%) | 0.205 |
| Temporal | 1.79 (2.17, 4.09%) | 0.024 | 0.57 (2.01, 1.26%) | 0.130 | 0.31 (2.26, 0.65%) | 0.329 | 0.22 (1.94, 0.50%) | 0.536 |
| Capillary Flux Index | ||||||||
| Average | 0.007 (0.018, 1.80%) | 0.413 | 0.008 (0.023, 2.06%) | 0.107 | 0.006 (0.022, 1.42%) | 0.226 | 0.005 (0.021, 1.12%) | 0.035 |
| Superior | 0.008 (0.018, 2.07%) | 0.249 | 0.009 (0.020, 2.25%) | 0.028 | 0.004 (0.023, 1.16%) | 0.580 | 0.004 (0.022, 0.85%) | 0.061 |
| Nasal | 0.010 (0.018, 2.49%) | 0.086 | 0.007 (0.024, 1.81%) | 0.130 | 0.005 (0.028, 1.36%) | 0.598 | 0.005 (0.026, 1.26%) | 0.218 |
| Inferior | 0.008 (0.020, 2.15%) | 0.288 | 0.004 (0.024, 1.04%) | 0.450 | 0.004 (0.020, 1.05%) | 0.481 | 0.003 (0.019, 0.85%) | 0.149 |
| Temporal | 0.003 (0.018, 1.80%) | 0.770 | 0.012 (0.033, 3.07%) | 0.040 | 0.010 (0.030, 2.29%) | 0.054 | 0.006 (0.029, 1.48%) | 0.035 |
SD = standard deviation. Boldface indicates statistical significance.
Mean difference calculated by subtracting maximum of the mean parameter value (right or left eye) from the minimum value (corresponding left or right eye), with the corresponding percent difference calculated as this difference divided by the minimum mean parameter value.
P value calculated using the Wilcoxon signed-rank test using the mean difference between eyes.
Discussion
Repeatability of peripapillary OCTA measures of CPD and CFI was good (ICC = 0.75–0.90) or excellent (ICC > 0.90) for nearly all measures for all groups: AD, MCI, PD, and normal cognition. The AD group had the widest 95% CI bounds for repeatability in measures of CPD and CFI, suggesting more variability in the imaging of these subjects. The repeatability of CPD and CFI in our expanded control cohort is consistent with our previous investigation showing good-excellent sectoral average repeatability in those with normal cognition, with some individual sectors with ICCs in the moderate quality range.9 Similar to our prior observations, average sectoral CPD was generally better than that of CFI measures.9 A small early study of peripapillary OCTA found very high ICC (0.966) in 10 healthy volunteers using the same device (Zeiss Cirrus HD-OCT 5000 with AngioPlex) that we used in this investigation, which exceeds the value of any ICC in any sectoral or average measurement in our study.31 Pappelis and Jansonius,32 using peripapillary OCTA obtained from a Canon OCT HS-100 (Canon), found somewhat lower repeatability values in the good range (ICC = 0.76–0.88).
The average ICC was generally higher than that of any individual sector. As the average scan area exceeds that of any single sector, the average CPD and CFI are mathematically, and likely anatomically, a more robust measure and may be more resistant to factors that would affect repeatability. The temporal and nasal sectors had lower ICCs in comparison with the superior and inferior sectors in all diagnoses as well as controls with normal cognition for CPD. It is possible that measurements of CPD are qualitatively less reproducible in the temporal and nasal peripapillary sectors; however, a similar trend was not observed with CFI, suggesting that this phenomenon is likely not due to an anatomic difference or a systematic flaw in patient positioning or scan tilt. As the 95% CIs for ICC overlapped across each cohort, these slight differences are likely within the normal variation of sectoral repeatability.
While we interpret the overlapping ICC 95% CIs as an indicator of general similarity of the repeatability of peripapillary OCTA between groups, it is notable that the normal cognition control group did not have the highest sectoral average ICC in CPD or CFI. The cognitively normal controls in our study lacked the executive and physical symptoms of AD, MCI, and PD participants, facilitating image acquisition with more precision and accuracy, thereby contributing to higher repeatability. It is possible that images from participants with cognitive or motor symptoms due to AD, MCI, and PD would be more likely to be excluded from analysis due to poor image quality, causing disproportionate image yields between groups. However, although the AD and PD groups had the lower image yields, our analysis found that this difference in patient yield across all groups, including the cognitively normal control cohort, was not significant.
Given significant differences in age across groups, we performed sub-analyses stratifying patients by age (< 70 years or ≥ 70 years). In both AD and PD, the two groups hypothesized to have worse repeatability with age, older patients demonstrated similar if not better repeatability compared with the younger strata. However, the 95% CIs were overlapping, suggesting that repeatability is comparable between groups for these diagnoses; the sample size for younger AD patients was lower, decreasing the robustness of ICC calculation for that subgroup. For cognitively normal controls, however, the ICC in the older group was less (moderate ICC quality) than that of the younger group (excellent ICC quality) and outside of the 95% CI, indicating the hypothesized trend in decreasing repeatability with age. That our quantitative analysis of parameter differences between scan pairs, both between groups and within groups, found no significant differences in CPD or CFI for any study group further supported the robust repeatability of peripapillary OCTA in the research environment, even for subjects with neurodegenerative conditions.
Our findings of only small differences between the right and left eye measures for any given parameter in the AD, MCI, and PD groups suggest that there is only a minor degree of asymmetric peripapillary microvascular impairment in these conditions. Although asymmetric findings on brain imaging or symptomology have been observed in neurodegenerative conditions such as AD and PD, and unilateral central pathology may correspond with unilateral signs and symptoms, it is unclear how common asymmetric progression may manifest at the population level or what the etiology and clinical significance of these phenomena are specific to the eye.33, 34, 35 It is possible that the eye may be less overtly affected by asymmetric progression of disease centrally or that our sample size was insufficient to capture many such patients with asymmetric disease; larger studies may consider performing a sub-analysis on patients who demonstrate more asymmetric retinal findings in the contexts of repeatability but also in the primary pathophysiology of disease. By convention, subjects with only 1 eye included (if both eyes are eligible) tended to have the right eye imaged (we routinely image the right eye first, followed by the left eye); however, our findings of symmetry between eyes indicates that this is unlikely to have introduced appreciable bias in our results. Notably, it was the control group that demonstrated a significant difference in symmetry in average CFI. Although significant, the magnitude of the difference was small, and the literature has suggested that such a minor degree of asymmetry may be an expected finding in the context of normal aging.30 Although this component was a sub-analysis, our findings indicate that AD, MCI, and PD do not demonstrate appreciable asymmetry in peripapillary OCTA, thereby providing some initial support for the inclusion of single eyes in research studies of neurodegeneration that are studying the peripapillary vasculature; however, more study with longitudinal follow-up is needed.
A limitation of this study lies in the potential bias due to the poor-quality images from the analysis and their effect on aggregate repeatability.36 The quality of OCTA metrics is sensitive to patient movement during image acquisition, inability to fixate on the target, and signal loss due to media opacity or miosis.37,38 These challenges can create artifacts of projection, shadowing, poor saturation, displacement, segmentation, and errors in flow rate measurement, which could confound the true measurement of various imaging parameters. As discussed, subjects with neurodegenerative conditions exhibit varying degrees of executive and movement-related symptoms and may be more likely to produce poor-quality images; thus, inadequate quality control could have led to an underestimation of ICC because the underlying cause of both poor image quality and poor image repeatability are the same. Although the proportion of patients’ images excluded for quality was higher than 30% in AD and PD (compared with 20% in those with normal cognition), perhaps reflecting the fact that rigorous quality control would be expected to exclude images of patients with neurodegeneration-related impairment more frequently, this difference in proportion of subjects excluded across groups ultimately was not statistically significantly. Additionally, the reviewers in the image exclusion process were blinded to diagnosis. As such, although our findings may not apply to all subjects in the population, many of whom are likely unable to provide research-quality images, our measurements of effective repeatability are representative of the high-quality data upon which research and clinical practice using these images would need to rely upon.
The lack of difference in neurocognitive testing (by MMSE) in the AD, MCI, PD, and cognitively normal groups suggests that our included sub-sample of subjects with good image quality are likely a good representative population across the spectrum of neurocognitive impairment. However, we did find more motor impairment in the excluded PD subjects in comparison with those included. The average Modified Hoehn and Yahr score of the excluded patients of 2.19 reflects the lower-bound of a Stage 2 score, which indicates bilateral and axial motor symptoms without balance impairment.22,23 The included patients had an average Modified Hoehn and Yahr score of 1.88, reflecting a lower-bound of a Stage 1.5 score, which indicates only unilateral and axial motor symptoms without balance impairment.22,23 The key difference between these clinical stages is bilateral motor involvement in the excluded group compared with unilateral motor involvement in the included PD subjects. Although the Modified Hoehn and Yahr score does not directly account for head motion, in terms of retinal imaging, any motor involvement, particularly head movements, may affect image quality. These findings suggest that in PD, advanced Parkinsonism may have a greater impact on image quality than cognitive status alone. We also recognize that excluding such PD subjects may overestimate the repeatability of peripapillary OCTA imaging in this group and that these findings may not be generalizable to those with advanced Parkinsonism. These findings reiterate the critical importance of scan quality in analyzing OCTA metrics in neurodegeneration. With one of the key goals in development of retinal imaging in neurodegeneration being earlier detection along the clinical continuum of the disease, it is reassuring that in those patients able to provide good-quality images, the metrics have good-excellent repeatability. This is even more important when performing longitudinal studies as change over time analysis requires high-quality baseline data.
Our use of uniformly high-quality images enables a more consistent comparison between groups. Notably, the exclusion rate of approximately 20% in even individuals with normal cognition highlights the need for future studies of neurodegeneration and retinal imaging to account for image loss in sample size calculations, because quality control is critical in producing accurate data. Furthermore, our study cohorts of AD and PD in particular were relatively small; however, this is among the larger studies of its kind to consider subjects with neurodegeneration. The effect of small study populations was likely compounded in our sub-analysis by age in which even smaller groups were created with stratification. The significant differences in study cohort demographic composition also limits the generalizability of these results. Notably, because the PD group was slightly younger on average than the other groups, it is possible that older patients with more severe motor symptoms resulting in poor-quality images were systemically removed from the study. Challenges in technical interoperability among various OCTA machines, analysis software versions, and platform-specific metrics may similarly limit the broad application of our findings. These challenges are not unique to our study and reiterate the need to establish consensus guidelines for retinal imaging in neurodegeneration.
In conclusion, our results suggest that with good-quality images, peripapillary OCTA has high repeatability and deserves further study as a potential clinical biomarker for disease in neurodegenerative conditions. These findings offer support for conclusions drawn by studies of neurodegeneration in the eye that use peripapillary OCTA. Because OCTA is particularly susceptible to artifact issues, more so than structural OCT, assessing small differences in scan quality–sensitive metrics, such as CPD and CFI, requires reliance on good-quality images. Continuing to evaluate the differential imaging repeatability in various neurodegenerative diagnoses is important to collect meta-data in this field of study. Future studies of imaging modality performance should include larger populations of subjects with neurodegenerative conditions. Consideration of the effect of poor-quality images and how they should be interpreted in context of large data sets will also be important to understand the true imaging characteristics of these subjects and limitations of OCTA imaging.
Manuscript no. D-21-00147.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
This study was funded in part by the Alzheimer’s Drug Discovery Foundation.
Presented as a poster at: the Duke Ophthalmology Scientific Sessions; A Presentation of Trainee Scientific Discoveries Conference, June 4, 2021.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at the Duke University approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Ma, Robbins, Stinnett, Grewal, Fekrat
Data collection: Ma, Robbins, Stinnett, Johnson, Scott
Analysis and interpretation: Ma, Robbins, Stinnett, Grewal, Fekrat
Obtained funding: Fekrat; Study was performed as part of regular employment duties at Duke University School of Medicine No additional funding was provided.
Overall responsibility: Ma, Robbins, Stinnett, Johnson, Scott, Grewal, Fekrat
Supplementary Data
References
- 1.Cao D., Yang D., Yu H., et al. Optic nerve head perfusion changes preceding peripapillary retinal nerve fibre layer thinning in preclinical diabetic retinopathy. Clin Exp Ophthalmol. 2019;47:219–225. doi: 10.1111/ceo.13390. [DOI] [PubMed] [Google Scholar]
- 2.Hoorbakht H., Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65–72. doi: 10.2174/1874364101206010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreb R.N., Aung T., Medeiros F.A. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akil H., Falavarjani K.G., Sadda S.R., Sadun A.A. Optical coherence tomography angiography of the optic disc; an overview. J Ophthalmic Vis Res. 2017;12:98–105. doi: 10.4103/2008-322X.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alterman M., Henkind P. Radial peripapillary capillaries of the retina. II. Possible role in Bjerrum scotoma. Br J Ophthalmol. 1968;52:26. doi: 10.1136/bjo.52.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henkind P. Radial peripapillary capillaries of the retina. I. Anatomy: human and comparative. Br J Ophthalmol. 1967;51:115–123. doi: 10.1136/bjo.51.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Y., Wei E., Wang X., et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121:1322–1332. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo Y.H., Sung K.R., Shin J.W. Effects of age on peripapillary and macular vessel density determined using optical coherence tomography angiography in healthy eyes. Invest Ophthalmol Vis Sci. 2019;60:3492–3498. doi: 10.1167/iovs.19-26848. [DOI] [PubMed] [Google Scholar]
- 9.Robbins C.B., Grewal D.S., Thompson A.C., et al. Repeatability of peripapillary optical coherence tomography angiography parameters in older adults. J Vitreoretin Dis. 2020 doi: 10.1177/2474126420953968. 2474126420953968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lévêque P.M., Zéboulon P., Brasnu E., et al. Optic disc vascularization in glaucoma: value of spectral-domain optical coherence tomography angiography. J Ophthalmol. 2016;2016:6956717. doi: 10.1155/2016/6956717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London A., Benhar I., Schwartz M. The retina as a window to the brain—from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera DeBuc D., Somfai G.M., Koller A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am J Physiol Heart Circ Physiol. 2017;312:H201–H212. doi: 10.1152/ajpheart.00201.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon S.P., Grewal D.S., Thompson A.C., et al. Retinal microvascular and neurodegenerative changes in Alzheimer's disease and mild cognitive impairment compared with control participants. Ophthalmol Retina. 2019;3:489–499. doi: 10.1016/j.oret.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabel P., Kaluzny J.J., Wilkosc-Debczynska M., et al. Comparison of retinal microvasculature in patients with Alzheimer's Disease and primary open-angle glaucoma by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60:3447–3455. doi: 10.1167/iovs.19-27028. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Zhao Q., Tao R., et al. Decreased retinal vascular density in Alzheimer’s Disease (AD) and mild cognitive impairment (MCI): an optical coherence tomography angiography (OCTA) study. Front Aging Neurosci. 2021;12:572484. doi: 10.3389/fnagi.2020.572484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins C.B., Grewal D.S., Thompson A.C., et al. Identifying peripapillary radial capillary plexus alterations in Parkinson's disease using optical coherence tomography angiography. Ophthalmol Retina. 2021;S2468-6530(21):00091–00099. doi: 10.1016/j.oret.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Fang D., Tang F.Y., Huang H., et al. Repeatability, interocular correlation and agreement of quantitative swept-source optical coherence tomography angiography macular metrics in healthy subjects. Br J Ophthalmol. 2019;103:415–420. doi: 10.1136/bjophthalmol-2018-311874. [DOI] [PubMed] [Google Scholar]
- 18.Bayraktar Z., Pehlivanoglu S., Bayraktar S., et al. Inter-ocular symmetry of vascular density and retinal thickness in unilateral anisometropic amblyopia. Clin Ophthalmol. 2020;14:1261–1267. doi: 10.2147/OPTH.S234294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F.K., Menghini M., Hansen A., et al. Intrasession repeatability and interocular symmetry of foveal avascular zone and retinal vessel density in OCT angiography. Transl Vis Sci Technol. 2018;7 doi: 10.1167/tvst.7.1.6. 6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Gallego A., De la Cruz J., Rodríguez-Salgado M., et al. Interobserver reproducibility and interocular symmetry of the macular ganglion cell complex: assessment in healthy children using optical coherence tomography. BMC Ophthalmol. 2020;20:197. doi: 10.1186/s12886-020-01379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G., Keyal K., Wang F. Interocular symmetry of vascular density and association with central macular thickness of healthy adults by optical coherence tomography angiography. Sci Rep. 2017;7:16297. doi: 10.1038/s41598-017-16675-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 23.Goetz C.G., Poewe W., Rascol O., et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 24.Chang R., Chu Z., Burkemper B., et al. Effect of scan size on glaucoma diagnostic performance using OCT angiography en face images of the radial peripapillary capillaries. J Glaucoma. 2019;28:465–472. doi: 10.1097/IJG.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durbin M.K., An L., Shemonski N.D., et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135:370–376. doi: 10.1001/jamaophthalmol.2017.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo T.K., Li M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarica A., Vasta R., Novellino F., et al. MRI asymmetry index of hippocampal subfields increases through the continuum from the mild cognitive impairment to the Alzheimer's Disease. Front Neurosci. 2018;12:576. doi: 10.3389/fnins.2018.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C., Zhong S., Zhou X., Wei L., et al. The abnormality of topological asymmetry between hemispheric brain white matter networks in Alzheimer's disease and mild cognitive impairment. Front Aging Neurosci. 2017;9 doi: 10.3389/fnagi.2017.00261. 261-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claassen D.O., McDonell K.E., Donahue M., et al. Cortical asymmetry in Parkinson's disease: early susceptibility of the left hemisphere. Brain Behav. 2016;6 doi: 10.1002/brb3.573. e00573-e00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minkova L., Habich A., Peter J., et al. Gray matter asymmetries in aging and neurodegeneration: a review and meta-analysis. Hum Brain Mapp. 2017;38:5890–5904. doi: 10.1002/hbm.23772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C.L., Bojikian K.D., Xin C., et al. Repeatability and reproducibility of optic nerve head perfusion measurements using optical coherence tomography angiography. J Biomed Opt. 2016;21:65002. doi: 10.1117/1.JBO.21.6.065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pappelis K., Jansonius N.M. Quantification and repeatability of vessel density and flux as assessed by optical coherence tomography angiography. Transl Vis Sci Technol. 2019;8 doi: 10.1167/tvst.8.3.3. 3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marinus J., van Hilten J.J. The significance of motor (a)symmetry in Parkinson's disease. Mov Disord. 2015;30:379–385. doi: 10.1002/mds.26107. [DOI] [PubMed] [Google Scholar]
- 34.Roe J.M., Vidal-Piñeiro D., Sørensen Ø., et al. Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer’s disease. Nat Commun. 2021;12:721. doi: 10.1038/s41467-021-21057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weintraub S., Wicklund A.H., Salmon D.P. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spaide R.F., Fujimoto J.G., Waheed N.K. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czakó C., István L., Ecsedy M., et al. The effect of image quality on the reliability of OCT angiography measurements in patients with diabetes. Int J Retina Vitreous. 2019;5:46. doi: 10.1186/s40942-019-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmen I.C., Konda S.M., Pak J.W., et al. Prevalence and severity of artifacts in optical coherence tomographic angiograms. JAMA Ophthalmol. 2020;138:119–126. doi: 10.1001/jamaophthalmol.2019.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.