Abstract
We have examined the localization of inducible nitric oxide synthase (iNOS) and nitrotyrosine (the product of nitration of tyrosine by peroxynitrite, a highly reactive derivative of nitric oxide [NO]) in demyelinating lesions from (i) two young adult patients with acute multiple sclerosis (MS), (ii) a child with MS (consistent with diffuse sclerosis), and (iii) five adult patients with chronic MS. Previous reports have suggested a possible correlation between iNOS, peroxynitrite, related nitrogen-derived oxidants, and the demyelinating processes in MS. We have demonstrated iNOS-immunoreactive cells in both acute-MS and diffuse-sclerosis-type lesions. In acute-MS lesions, iNOS was localized in both monocytes/macrophages and reactive astrocytes. However, foamy (myelin-laden) macrophages and the majority of reactive astrocytes were iNOS negative. In specimens from the childhood MS patient, iNOS protein was present only in a subpopulation of reactive or hypertrophic astrocytes. In contrast, no iNOS staining was detected in chronic-MS lesions. Immunohistochemical staining of acute-MS lesions with an antibody to nitrotyrosine revealed codistribution of iNOS- and nitrotyrosine-positive cells, although nitrotyrosine staining was more widespread in cells of the monocyte/macrophage lineage. In diffuse-sclerosis-type lesions, nitrotyrosine staining was present in hypertrophic astrocytes, whereas it was absent in chronic-MS lesions. These results suggest that NO and nitrogen-derived oxidants may play a role in the initiation of demyelination in acute-MS lesions but not in the later phase of the disease.
Nitric oxide (NO) is a radical molecule, synthesized by nitric oxide synthase (NOS) from l-arginine by nitrogen oxidation of guanidino nitrogen to form l-citrulline (43, 44, 50). There are two constitutive isoforms of NOS (type I or brain or neuronal NOS and type III or endothelial NOS) and one inducible form (iNOS or type II) (9, 15, 16, 43, 51). NO produced by constitutively expressed NOS (types I and III) plays a major role as regulator and mediator of numerous processes, including muscle relaxation, vasodilation, and neurotransmission (43, 44, 50, 51). NO produced by type II NOS (iNOS) is generated in chronic and acute conditions of inflammation (9, 15, 16, 19, 26, 30, 34, 48, 52, 64). Type II NOS is produced by many different cell types in response to endotoxins and cytokines, such as gamma interferon, interleukin 1, and tumor necrosis factor alpha (9, 15, 16, 19, 26, 30, 48). Type II NOS has been detected in several inflammatory diseases of the central nervous system (CNS), including experimental allergic encephalomyelitis (EAE) (27) and encephalitis induced by coronavirus, rhabdovirus, flavivirus, rabies virus, Borna virus, herpesvirus, Sindbis virus, and Theiler’s murine encephalomyelitis virus (15, 16, 19, 25–27, 30, 34, 37, 42, 48, 52, 56, 61, 62, 64). Experiments using specific inhibitors of iNOS revealed that NO may exhibit a protective role in viral encephalitis by inhibition of viral replication or it may contribute to the pathogenesis of the disease (7, 17, 37, 42).
It has been reported that iNOS inhibitors may ameliorate EAE in mice (12, 18, 69). NO produced by microglia could be a potent neurotoxin and can mediate tumor necrosis factor alpha toxicity towards oligodendrocytes (20, 47, 49). Therefore, NO produced by iNOS may be both friend and foe. NO and its degradation products are reactive molecules and have been implicated in blocking mitochondrial respiration by forming iron-NO complexes with respiratory enzymes and enzymes playing a role in DNA replication and repair (40, 66, 67). These results suggest that NO may participate in demyelinating diseases such as multiple sclerosis (MS), in myelin damage, or in damage of myelin-producing cells. Dysfunction of mitochondria may also be the result of formation of peroxynitrite, a reaction product of NO and superoxide (4, 11, 31, 41, 59). Peroxidation of membranes as well as swollen oligodendrocyte cell bodies have been found in the brains of MS patients (29). Peroxynitrite may react with tyrosine in proteins to form nitrotyrosine by adding a nitro group to the 3-position adjacent to the hydroxyl group of tyrosine (5). Nitrosylation of tyrosine has been observed in cells derived from patients with several acute inflammatory or neurodegenerative diseases, including acute lung injury, arteriosclerosis, and Alzheimer’s disease (5, 24, 35). With one exception, iNOS expression has been examined only in brain lesions of chronic-MS patients, and iNOS has been found in active demyelinating lesions but not in chronic inactive lesions (3, 8, 13, 21, 28). However, there are discrepancies regarding the cell types that express iNOS. In one study, macrophage/microglial cells have been reported to be the major source of iNOS (3, 21, 28), while in another, astrocytes have been identified as the NO-producing cells (8, 13). However, NADPH diaphorase staining, which does not permit the distinction between type I and type II NOS, has been used to identify iNOS-positive cells in these studies (8, 13).
In this report, we compared the expression of iNOS in the brain in two cases of acute MS in young adults, one case of diffuse sclerosis in a child, and five cases of chronic MS. Acute MS represents a distinct variant of MS and differs both clinically and pathologically from the much more common classic chronic MS. Acute MS occurs more frequently in a relatively younger group of patients and is characterized by rapid and extensive neurological deficit (2, 33, 46, 58). These patients have multiple, extensive white matter lesions of uniform age and lack the healed lesions found in chronic-MS patients (2, 33, 46, 58). Diffuse sclerosis represents a rather subacute form of demyelinating disease which occurs in children and has a relentlessly progressive clinical course (58). We report here differential expression of iNOS and nitrotyrosine in brain lesions of patients with acute and chronic MS.
MATERIALS AND METHODS
Patients.
The patients described in Table 1 were the source of the paraffin-embedded specimens used in these studies.
TABLE 1.
Surgical biopsy and autopsy data
| Disease and patient no. | Patient age (yr) | Sex | Tissuea | Postmortem interval (h) |
|---|---|---|---|---|
| Acute MSb | ||||
| 1 | 19 | F | B | |
| 2 | 26 | F | B | |
| Diffuse sclerosisc | ||||
| 3 | 11 | M | A | NAd |
| Chronic MS | ||||
| 4 (CPe) | 66 | M | A | 3 |
| 5 (RRf) | 40 | F | A | 2 |
| 6 (CP) | 42 | M | A | 4 |
| 7 (RR) | 82 | F | A | 2.5 |
| 8 (CP) | 54 | F | A | NA |
A, autopsy specimen; B, biopsy specimen.
The acute-MS patients were diagnosed based on the following findings: acute monophasic lesion(s) involving predominantly cerebral white matter; increased T2-weighted signal pattern on magnetic resonance imaging; exhibition in both patients of a mass effect with internal shift; on patient followup, abnormal visual evoked potential; CSF IgGs showing oligoclonal banding; detection in biopsy specimens of acute demyelinating lesions (of the same age) accompanied by variably prominent perivascular and parenchymal mononuclear infiltrates and reactive astrocytosis; preservation of axons; lack of gray matter involvement.
Rapidly progressive, essentially unremitting demyelinating disease was diagnosed in an 11-year-old African-American male. Clinically, there was predominant cerebral hemispheric white matter involvement. Magnetic resonance imaging exhibited increased signal on T2-weighted images. Optic neuritis was evident. The patient died 18 months after the onset of illness. Postmortem neuropathology included extensive, confluent-to-diffuse demyelinating lesions involving the cerebral hemispheric white matter, bilaterally, and virtually all funiculi of the spinal cord. There was no evidence of dysmyelinating disorder in the context of leukodystrophy. The adrenal glands, visceral organs, peripheral nervous system, and musculoskeletal system were unremarkable, and there was no evidence of mitochondrial cytopathy.
NA, not available.
CP, chronic progressive.
RR, remitting relapsing.
(i) Specimens from acute-MS patients.
Paraffin-embedded brain biopsy specimens from two patients with acute MS were provided by the Department of Pathology and Laboratory Medicine, The University of Texas Health Science Center Medical School of Houston, Tex., and Baylor College of Medicine, Houston, Tex.
(ii) Specimens from diffuse-cerebral-sclerosis patient.
Paraffin-embedded brain and spinal cord tissues collected at the time of postmortem from an 11-year-old male with diffuse sclerosis, who died 18 months after diagnosis of the disease, were obtained from St. Christopher’s Hospital for Children, Philadelphia, Pa.
(iii) Specimens from chronic-MS patients.
Paraffin-embedded autopsy brain tissue was obtained from Rocky Mountain Multiple Sclerosis Center, Englewood, Colo. (four patients) and Graduate Hospital, Philadelphia, Pa. (one patient).
Antibodies.
The antibodies employed in these studies were affinity-purified rabbit anti-iNOS antibody (Transduction Laboratories, Lexington, Ky.), rabbit anti-glial fibrillary acidic protein (anti-GFAP) antibody (DAKO, Carpinteria, Calif.), HAM-56 immunoglobulin M (IgM) monoclonal antibody (MAb) (DAKO), and affinity-purified antinitrotyrosine antibody (Upstate Biotechnology, Lake Placid, N.Y.). The HAM-56 MAb recognizes an antigen expressed on macrophages, monocytes, and certain microglia (1). We have previously determined (56) that (i) immunohistochemical staining with the affinity-purified rabbit anti-iNOS antibody employed in these studies closely correlates with the presence of iNOS transcripts and (ii) this affinity-purified rabbit anti-iNOS antibody does not cross-react with NOS.
Immunohistochemistry.
Five-millimeter-thick paraffin sections were deparaffinized and rehydrated in xylene and alcohols and immersed in phosphate-buffered saline (PBS) for 5 to 10 min. Endogenous peroxidase was blocked by incubation for 30 min in 1.2% hydrogen peroxide in cold methanol. To eliminate nonspecific staining, sections were incubated for 1 h with goat serum. iNOS, nitrotyrosine, and GFAP were detected by an immunoperoxidase technique (54) using an ABC-ELITE Kit (Vector Laboratories, Burlingame, Calif.). Sections were incubated with primary antibody, either anti-iNOS (1.25 μg/ml), anti-GFAP antibody (diluted 1:1,000), or antityrosine (1:200), for 1 h. Antigen-antibody complexes were detected with an biotinylated anti-rabbit–horseradish peroxidase complex and visualized with 3,3′-diaminobenzidine as the substrate in accordance with the manufacturer’s specifications. Sections were counterstained with Mayer’s hematoxylin (Sigma Chemical Co., St. Louis, Mo.). Controls included sections incubated with PBS instead of primary antibody or sections incubated with normal rabbit IgG (1.25 μg/ml), which was used instead of a specific rabbit anti-iNOS, anti-GFAP, or antinitrotyrosine primary antibody.
To confirm the specificity of antinitrotyrosine staining, antinitrotyrosine antibody was preincubated for 1 h with 10 mM nitrotyrosine in potassium phosphate buffer (pH 7.4) and used to stain tissue sections. Such treatment totally abolished staining of tissue, which was otherwise positive for nitrotyrosine.
HAM-56-positive cells were detected by using a Universal DAKO LSAB Kit in accordance with the manufacturer’s instruction. Deparaffinized and rehydrated tissue sections were digested with 0.025% protease type XXIV (Sigma) for 6 min at room temperature. Tissue sections were washed for 5 min in PBS and blocked for 5 min in blocking buffer before incubation with HAM-56 MAb. Antigen-antibody complexes were detected by sequential incubation of tissue sections with biotinylated anti-mouse immunoglobulin and streptavidin conjugated to horseradish peroxidase. The reaction was visualized by using 3,3′-diaminobenzidine as the substrate.
RESULTS
Expression of iNOS and nitrotyrosine in acute monophasic lesions in the brains of two patients with acute MS.
For both patients with acute MS, tissue was procured from surgical biopsy material at the time of initial clinical presentation. The pathological changes were essentially similar in both patients and were consistent with a diagnosis of acute MS. Histologic parameters of these specimens are shown in Table 2. All lesions were of the same age, suggestive of a monophasic process. There was no evidence of shadow plaques in multiple tissue sections examined. Brisk, predominantly perivascular mononuclear cell infiltrates and HAM-56-immunoreactive monocytes/macrophages, including foamy macrophages (laden with myelin debris), were admixed with GFAP-positive reactive astrocytes. There was overt myelin loss but preservation of axons.
TABLE 2.
Histologic parameters of MS and its variants
| Disease | Cellular contributionsa of:
|
||
|---|---|---|---|
| Mononuclear infiltratesb | Foamy macrophagesc | Astrocytes | |
| Acute MS | +++ | ++ | ++d |
| Diffuse sclerosis (Schilder type) | ++ | +++ | +++d |
| Chronic MS | +F | +F | +++e |
Cellular contributions are rated as follows: +, scant; ++, moderate or intermediate; +++, pronounced. The topographic distribution of all the cell types is diffuse unless indicated otherwise by the superscript letter F, for focal. With focal distribution, infiltrates involve the walls and perivascular spaces of scattered intralesional vessels.
Comprised predominantly of lymphocytes and monocytes and to a lesser extent of immunoblasts and plasma cells.
Myelin-laden macrophages, which are present in the Virchow-Robin (perivascular) spaces and also throughout the white matter substance.
Principally hypertrophic (large cell bodies).
Principally fibrous (fibrillary).
Scattered iNOS-labeled cells of the monocyte/macrophage series (Fig. 1a and b) and reactive astrocytes (Fig. 1b) were present within the lesions. The former were also demonstrated in perivascular locations (Fig. 1b). The identity of the two iNOS-positive cell types was confirmed on subserial sequential sections immunostained with anti-HAM-56 (Fig. 1c) or anti-GFAP (Fig. 1d) antibodies, respectively. It should be noted that iNOS localization was detected in only a minority (<10%) of reactive astrocytes, as well as of cells of the monocyte/macrophage lineage. Moreover, virtually all foamy macrophages were distinctly iNOS negative (Fig. 1a).
FIG. 1.
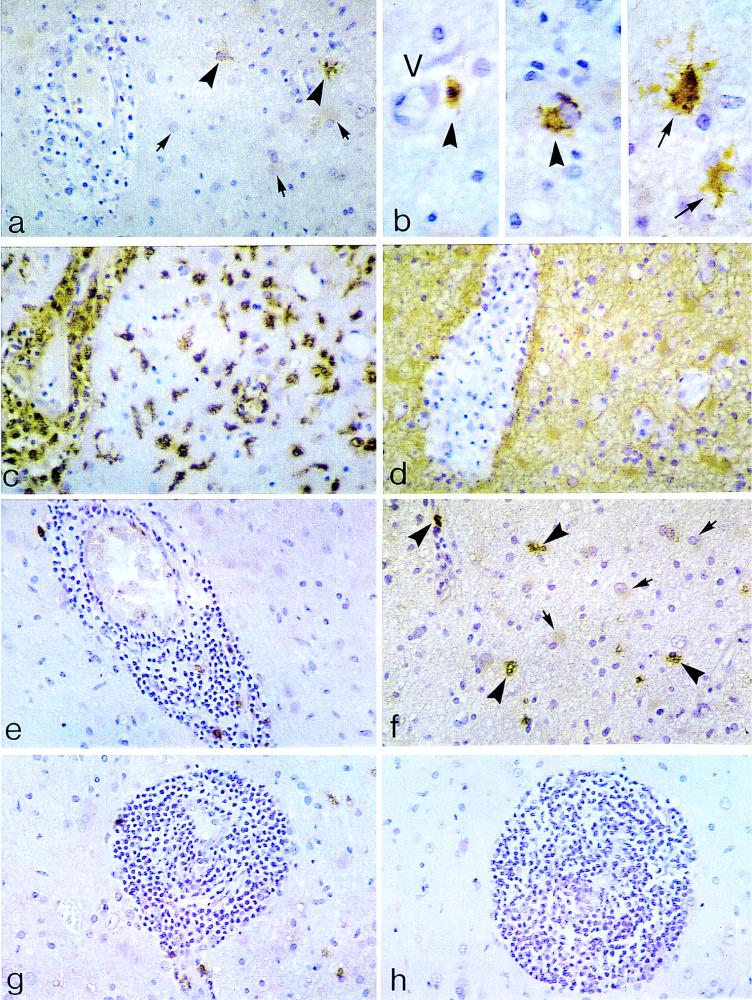
Immunocytochemical detection of iNOS protein (a and b), HAM-56 (c), GFAP (d), and nitrotyrosine (e, f, and g) in active, monophasic, demyelinating lesions consistent with acute MS. (a) Evidence of florid demyelinating process characterized by perivascular inflammatory infiltrates (lymphocytes and monocytes/macrophages), myelin pallor, and a mixture of mononuclear cells and reactive astrocytes in the white matter. iNOS localization is present in scattered cells of the monocyte/macrophage lineage (large arrowheads). In this field, there is lack of iNOS staining in reactive astrocytes (small arrows) and in perivascular inflammatory cell cuffs. Original magnification, ×400. (b) Higher magnification (under oil-immersion lens) of a field adjoining that of panel a. iNOS-positive cells of the monocyte/macrophage series (large arrowheads) and somewhat larger, multipolar cells consistent with reactive astrocytes (small arrows) are visible. It should be noted that only a minority (<10%) of mononuclear cells and reactive astrocytes combined are iNOS positive in these acute lesions. Original magnification, ×1,000. (c) Deeper, sequential tissue section relative to that of panel a. Widespread HAM-56 staining of monocytes/macrophages is visible in the perivascular space and in the white matter. Note morphologic variation among these cells, including round (monocyte-like), plump (macrophage-like), rod-shaped, and ameboid (microglia-like) forms. Original magnification, ×400. (d) Homologous microscopic field in a subserial section relative to sections shown in panels a and c. There is diffuse GFAP staining in reactive astrocytes and their fibrillary processes, surrounding a Virchow-Robin (perivascular) space packed with GFAP-negative foamy (myelin-laden) macrophages. Due to the abundance of macrophages, the vascular lumen is obliterated. Original magnification, ×400. (e) Perivascular mononuclear cell cuffing in a white matter blood vessel. Note scattered monocytes/macrophages within the inflammatory cuff, showing nitrotyrosine-like immunoreactivity. Original magnification, ×400. (f) Nearby field relative to panel e field. Nitrotyrosine-like staining is visible in cells of the monocyte/macrophage lineage (large arrowheads). In this field, reactive astrocytes (small arrows) are nitrotyrosine negative. During the acute phase of demyelination, the majority of nitrotyrosine-labeled cells are monocytes/macrophages; only rare reactive astrocytes are nitrotyrosine positive (not shown). Original magnification, ×400. (g) Dense perivascular mononuclear cell infiltrates. As in panel f, nitrotyrosine-like immunoreactivity is present in scattered monocytes/macrophages. Also, note nitrotyrosine-positive monocytic elements in the contiguous white matter (upper right corner). Original magnification, ×400. (h) Deeper tissue section relative to panel g section. A lack of staining is apparent in perivascular mononuclear cell infiltrates after the use of nonspecific IgG in lieu of the primary antibody. Original magnification, ×400.
In acute-MS lesions, variable numbers of overt mononuclear cells in the Virchow-Robin (perivascular) spaces (Fig. 1e and g) and white matter (Fig. 1f) exhibited nitrotyrosine-positive staining. It appears that at this phase, there is a preponderance of nitrotyrosine immunoreactivity in cells of the mononuclear phagocytic series and a paucity of staining in reactive astrocytes (Fig. 1f). However, rare nitrotyrosine-positive glial cells are also present (data not shown). As compared to the cellular localization of iNOS, the distribution of nitrotyrosine immunoreactivity in acute-MS lesions is notably more widespread. The immunohistochemical findings for these patients are summarized in Table 3.
TABLE 3.
Immunohistochemistry of MS and its variants
| Cell type | Immunoreactivitya
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute-MS monophasic lesionsb
|
Diffuse-sclerosis-type lesionsc
|
Chronic lesions, classic MS
|
||||||||||
| iNOS | HAM-56 | GFAP | NTyr | iNOS | HAM-56 | GFAP | NTyr | iNOS | HAM-56 | GFAP | NTyr | |
| Monocytes/macrophagesd | + | + | − | +/++e | − | + | − | − | − | − | − | − |
| Foamy macrophagesf | − | +++ | − | − | − | +++ | − | − | − | +++ | − | − |
| Astrocytes | + | − | +++ | + | + | − | ++ | +++ | − | − | +++ | − |
Immunoreactivities to anti-iNOS, HAM-56, anti-GFAP, and antinitrotyrosine (NTyr) were rated as follows: +, focal (<10% of cells); ++, intermediate; +++, prominent (>50% of cells); −, absent.
Lesions from acute rampant monophasic demyelinating illness as observed in biopsy specimen at time of initial clinical presentation; clinicopathologically consistent with diagnosis of acute MS.
Lesions from an 11-year-old terminal-stage MS patient, with rapidly progressive, unremitting features resembling acute MS, as seen at time of autopsy.
Includes cells in the Virchow-Robin (perivascular) spaces, as well as cells infiltrating the white matter. Other mononuclear cells, such as lymphocytes, do not show immunoreactivity with any one of the antibodies employed in this study.
Intralesional staining varies among microscopic fields of a single patient specimen. The relative count of immunoreactive cells is influenced by the lymphocyte-to-monocyte/macrophage ratio that may be increased in the hyperacute phase of acute MS.
Myelin-laden phagocytes in areas of overt demyelination.
Expression of iNOS and nitrotyrosine in specimens from a patient with diffuse sclerosis.
Brain and spinal cord tissues were obtained at the time of autopsy of an 11-year-old patient, who died as a result of a rapidly progressive, unremitting white matter disease resembling acute MS, with diffuse cerebral and spinal cord involvement, consistent with so-called diffuse sclerosis (Schilder type). Sections from multiple gross lesions throughout the neuraxis confirmed a diffuse demyelinating process. All lesions were remarkably of the same age and exhibited signs of an overt demyelinating process. Extensive multifocal and confluent lesions characterized by massive accumulations of HAM-56-positive foamy macrophages (data not shown) imperceptibly merged with GFAP-positive hypertrophic astrocytes (Fig. 2a and b). Focal and relatively sparse mononuclear infiltrates were present, but in contrast to findings in acute-MS lesions, they did not dominate the pathological lesions. Also, as compared to findings in acute-MS lesions, the demyelinating lesions in this case contained an increased number of myelin-laden macrophages, often presenting as extensive tightly packed accumulations of foamy phagocytic cells (Fig. 2a to c). The histologic parameters of these specimens are shown in Table 2. Immunohistochemical staining for iNOS was detected in only a minority (<10%) of GFAP-positive cells. On the whole, these cells were reactive or hypertrophic astrocytes (Fig. 2a and c) on the basis of their morphology and immunoreactivity for GFAP in adjacent sections (Fig. 2b). In addition, robust nitrotyrosine immunoreactivity was present in these hypertrophic astrocytes (Fig. 2d) but not in foamy macrophages (Fig. 2d, inset). As with iNOS-positive astrocytes (Fig. 2c, inset), a predilection for a perivascular location for nitrotyrosine-positive astrocytes (Fig. 2d) was observed. These immunohistological findings are summarized in Table 3.
FIG. 2.
Immunocytochemical detection of iNOS protein (a and c), GFAP (b), and nitrotyrosine (d) in active, confluent demyelinating lesions consistent with diffuse sclerosis (Schilder type). (a) Extensive, confluent demyelinating lesions involving cerebral white matter. Scattered iNOS-positive reactive or hypertrophic astrocytes are visible amidst heavy accumulations of iNOS-negative foamy macrophages. Original magnification, ×100. (b) Subserial section relative to that of panel a. Accumulations of GFAP-positive hypertrophic astrocytes intermingled with GFAP-negative foamy macrophages are visible. Original magnification, ×100. (c) Higher magnification of the microscopic field depicted in panel a. There is distinctive iNOS immunoreactivity in large astrocytic cell bodies; however, some degree of staining variability among morphologically identical hypertrophic astrocytes is visible. The inset shows discrete iNOS localization in hypertrophic astrocytes and its processes encroaching on the adventitia of a parenchymal blood vessel. Original magnification, ×400. (d) Robust nitrotyrosine-like staining in hypertrophic astrocytes surrounding a white matter blood vessel. Also, linear-type staining in the luminal endothelial surface, as well as lack of immunoreactivity in foamy macrophages (inset), is visible. Original magnification, ×400. (e to h) Immunocytochemical profile of HAM-56 (e), GFAP (f), iNOS (g), and nitrotyrosine (h) in subserial sections from a chronic-MS plaque. (e) Perivascular aggregates of HAM-56-positive monocytes/macrophages surrounding a sclerotic vessel within a chronic-MS plaque. Original magnification, ×400. (f) GFAP staining highlights fibrous gliosis surrounding an intralesional blood vessel. GFAP-negative mononuclear cells are visible in the perivascular space. Original magnification, ×400. (g) Lack of iNOS staining in perivascular mononuclear cells and in fibrous astrocytes. Original magnification, ×400. (h) Lack of nitrotyrosine-like staining in perivascular mononuclear cells and in fibrous astrocytes mirroring the profile of iNOS. Original magnification, ×400.
Expression of iNOS and nitrotyrosine in chronic lesions of classic MS.
The tissue samples from patients with chronic MS revealed classical chronic MS plaques involving cerebral hemispheric, particularly periventricular, white matter. Areas of myelin loss were accompanied by prominent fibrous astrogliosis, as confirmed by widespread GFAP staining (Fig. 2f). Focal accumulations of HAM-56-positive foamy macrophages, exhibiting a predilection for a perivascular location (Fig. 2e), were present in half of the patients. Scant, perivascular and/or transmural lymphocytic and monocyte/macrophage infiltrates were demonstrable in a focal distribution within chronic plaques in three patients (Fig. 2e and h). The vascular walls, of presumptive veins, were thickened and hyalinized, but the vessel lumens were patent (Fig. 2e and h). Intralesional axons were unaffected. Neither iNOS (Fig. 2g)- nor nitrotyrosine (Fig. 2h)-positive staining was detected in either astrocytes or macrophages. Histologic and immunohistochemical findings for these patients are summarized in Tables 2 and 3, respectively.
DISCUSSION
NO and NO-dependent oxidants, such as peroxynitrite, are believed to play a role in the pathogenesis of inflammatory diseases of the CNS, such as viral and maltose-binding protein-induced encephalitis and demyelinating diseases, including MS (6, 7, 12, 17, 18, 25, 27, 28, 32, 36, 42, 53, 54, 56, 61, 62, 69). High levels of nitrate, nitrite, and neopterin, a precursor of tetrahydrobiopterin (a cofactor for NOS), have been found in the CNS of MS patients (6, 23, 32, 65).
Several mechanisms have been proposed for NO toxicity, including deamination of DNA, direct inhibition of mitochondrial respiration, and depletion of NAD by activation of poly(ADP-ribose) synthetase and formation of peroxynitrite (4, 11, 31, 40, 41, 59, 66, 67). Peroxynitrite is formed by a diffusion-limited reaction of NO with superoxide, and it is a powerful oxidant. Membrane lipid peroxidation of oligodendrocytes has been demonstrated in the brains of patients with MS (29). Nitration on the ortho position of tyrosine is the major product of peroxynitrite attack on proteins. NO does not directly nitrate tyrosine residues. One of the known effects of tyrosine nitration on protein function is inhibition of phosphorylation, which is critical in controlling cellular regulation and signal transduction (45). The presence of nitrotyrosine in the brains of MS patients suggests the action of peroxynitrite or other toxic products of NO, such as nitrogen dioxide, in these brains.
Expression of iNOS-derived NO in the brains of MS patients has been examined by several investigators. However, the cell types that express iNOS are still a matter of controversy. Bagasra et al. (3) and De Groot et al. (21) reported that in active MS lesions, iNOS immunoreactivity was found exclusively in perivascular and parenchymal macrophages, within the region of active demyelination. Additionally, De Groot et al. (21) demonstrated that macrophages isolated from active MS lesions produced NO, were stained with antibody specific to iNOS, and constitutively expressed type I NOS. In contrast, Brosnan et al. (13) and Bö et al. (8) reported that reactive astrocytes were the major cell type that expressed iNOS. In the latter study, NADPH diaphorase was used as a marker for NOS activity, which does not distinguish type I from type II NOS, suggesting that the NOS detected in astrocytes in these studies (8, 13) may include type I and/or type II NOS. However, Brosnan et al. (13) and Bo et al. (8) did not detect any NADPH diaphorase activity in cells of monocyte/macrophage lineage. These discrepancies regarding the cell types that express iNOS in the brains of MS patients prompted us to examine whether iNOS expression and its cellular localization in the brains of MS patients is associated with the type of the disease, namely, acute MS versus chronic MS. We have examined iNOS expression in the brains of two patients with acute MS, one patient with diffuse sclerosis, and five patients with classical chronic MS. In the brains of patients with acute MS, immunoreactivity for iNOS was found mainly in perivascular and parenchymal macrophages and in scattered reactive astrocytes within the demyelinating lesions. In contrast, in the brains of patients with diffuse sclerosis, which represents a rather subacute, albeit progressive form of MS in young patients, staining for iNOS was detected solely in reactive astrocytes. iNOS-positive staining was not detected in demyelinating lesions of patients with classical chronic MS. Our results suggest that iNOS expression in acute-MS lesions is different from iNOS expression in the active demyelinating lesions in patients with chronic MS described by Bagasra et al. (3), De Groot et al. (21), and Hooper et al. (28). These investigators reported (3, 28) iNOS localization exclusively in cells of the monocyte/macrophage lineage. They did not observe any iNOS-positive staining of reactive astrocytes. In contrast, in acute-MS lesions there is iNOS expression in cells of monocyte/macrophages lineage and in reactive astrocytes. We did not observe iNOS expression in five patients with classical chronic MS. Our results are in agreement with those of De Groot et al. (3), who also did not find any significant iNOS immunoreactivity in demyelinating lesions in patients with chronic MS. However, diffuse sclerosis might represent a distinctive form of acute-like MS, since iNOS protein was detected solely in reactive astrocytes, not in cells of monocyte/macrophage lineage.
It is of interest that nitrotyrosine-positive staining colocalized with iNOS immunoreactivity in the brains of two patients with acute MS and the brain of one patient with diffuse sclerosis, although it was more widespread than iNOS-positive staining. Tyrosine nitration or iNOS protein was not detected in patients with chronic MS. Although it has been suggested that nitration of protein will affect protein stability, the half-life of nitrotyrosine is unknown. In EAE, expression of iNOS has been correlated with production of nitrotyrosine in astrocytes in the spinal cord of mice and reached its maximum at the peak of disease (53). However, nitrotyrosine-positive cells were eliminated or degraded 4 to 6 days after the disease was resolved (68). A similar mechanism may be responsible for the lack of nitrotyrosine-positive cells in chronic-MS lesions.
The discrepancy between our results and those of De Groot et al. (21) and Hooper et al. (28) regarding cell types which express iNOS perhaps may be attributed to the stage of the disease. Thus, brain tissue examined by Hooper et al. (28) was obtained at the time of postmortem examination from patients who had disease for at least 10 years. The age of the patients was not provided, but these patients had in all probability chronic MS. Among three brain tissue specimens studied by De Groot et al. (21), two were from elderly patients (81 years old) and one was from a 46-year-old patient who also suffered from long-standing disease. Although the lesions examined by De Groot et al. (21) and Hooper et al. (28) were characterized as the result of active demyelinating disease, they are definitely different from the lesions of the brains of two patients with acute MS examined in this study. Surgical biopsy specimens from these two patients were obtained at the time of clinical diagnosis, and they revealed not only acute but also monophasic lesions, with no evidence of the secondary progressive demyelinating lesions observed in patients with chronic MS as reported by De Groot et al. (21) and Hooper et al. (28). It has been suggested that progression of demyelinating disease could be the result of epitope spreading, and lesions at the end stage of the disease may be the result of a different pathological mechanism than the one that initiated the disease (39, 63, 68).
It remains to be determined whether expression of iNOS in astrocytes and monocytes/macrophages in brains of patients with acute MS (this report) versus expression of iNOS in monocytes/macrophages only in active lesions of patients with chronic MS (3, 21, 28) can be attributed to different stages of the same disease or to the involvement of different pathogenic mechanisms responsible for the initiation of acute MS. It is well documented, at least in vitro, that both monocytes/macrophages and astrocytes can produce NO (10, 13, 14, 22, 38, 57, 65).
Our results support the hypothesis that NO and reactive derivatives of NO, produced by monocytes/macrophages (both perivascular and parenchymal) and reactive astrocytes, may participate in the demyelination process in acute MS and diffuse sclerosis. These results are in agreement with the profile of iNOS production in the CNS of mice infected with Theiler’s murine encephalomyelitis virus (56). Infection of mice with this neurotropic virus results in encephalitis and, later, in a demyelinating disease which resembles MS (reviewed in references 55 and 60). We have found high levels of iNOS transcripts and protein in astrocytes and monocytes/macrophages in Theiler’s murine encephalomyelitis virus-infected mice during the encephalitic stage of the disease and the early stage of the demyelinating phase (56). However, iNOS transcripts and protein were absent during progression of demyelinating disease (56), which suggests that NO plays a role in encephalitis and possibly in initiation of myelin damage but is not involved in progression of the demyelinating disease.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the N. Eleanor Naylor Dana Charitable Trust to E.L.O. The Rocky Mountain Multiple Sclerosis Center is a National Multiple Sclerosis funded project.
We thank N. Karmazin and J. P. de Chadarevian, Department of Anatomic Pathology, St. Christopher’s Hospital for Children, Philadelphia, Pa., for providing the CNS specimen from the patient with diffuse sclerosis.
REFERENCES
- 1.Adams C W, Poston R N. Macrophage histology in paraffin-embedded multiple sclerosis plaques is demonstrated by the monoclonal pan-macrophage marker HAM-56: correlation with chronicity of the lesion. Acta Neuropathol. 1990;80:208–211. doi: 10.1007/BF00308926. [DOI] [PubMed] [Google Scholar]
- 2.Adams C W M, Poston R N, Buk S J. Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J Neurol Sci. 1989;92:291–306. doi: 10.1016/0022-510x(89)90144-5. [DOI] [PubMed] [Google Scholar]
- 3.Bagasra O, Michaels F H, Zheng Y M, Bobroski L E, Spitsin S V, Fu Z F, Tawadros R, Koprowski H. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci USA. 1997;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman J S, Crow J P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 5.Beckman J S, Ye Y Z, Anderson P G, Chen J, Accavitti M A, Tarpey M M, White C R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 6.Benveniste E N. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 7.Bi Z, Reiss C S. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bö L, Dawson T M, Wesselingh S, Mörk S, Choi S, Kong P A, Hanley D, Trapp B. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- 9.Bogdan C, Vodovotz Y, Paik J, Xie Q-W, Nathan C. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J Leukocyte Biol. 1994;55:227–233. doi: 10.1002/jlb.55.2.227. [DOI] [PubMed] [Google Scholar]
- 10.Boje K M, Arora P K. Microglia-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 11.Bolanos J P, Heales S J R, Land J M, Clark J B. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 12.Brenner T, Brocke S, Szafer F, Sobel R A, Parkinson J F, Perex D H, Steinman L. Inhibition of nitric oxide synthase for treatment of experimental autoimmune encephalomyelitis. J Immunol. 1997;158:2940–2946. [PubMed] [Google Scholar]
- 13.Brosnan C F, Battistini L, Raine C S, Dickson D W, Casadevall A, Lee S C. Reactive nitrogen intermediates in human neuropathology: an overview. Dev Neurosci. 1994;16:152–161. doi: 10.1159/000112102. [DOI] [PubMed] [Google Scholar]
- 14.Chao C C, Hu S, Molitor T W, Shaskan E G, Peterson P K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 15.Chesrown S E, Monier J, Visner G, Nick H S. Regulation of inducible nitric oxide synthase mRNA levels by LPS, IFN-γ, TGF-β, and IL-10 in murine macrophage cell lines and rat peritoneal macrophages. Biochem Biophys Res Commun. 1994;200:126–134. doi: 10.1006/bbrc.1994.1424. [DOI] [PubMed] [Google Scholar]
- 16.Corradin S B, Fasel N, Buchmüller-Rouiller Y, Ransijn A, Smith J, Manuel J. Induction of macrophage nitric oxide production by interferon-γ and tumor necrosis factor-α is enhanced by interleukin-10. Eur J Immunol. 1993;23:2045–2048. doi: 10.1002/eji.1830230851. [DOI] [PubMed] [Google Scholar]
- 17.Croen K D. Evidence for an antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross A H, Misko T P, Lin R F, Hickey W F, Trotter J L, Tilton R G. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994;93:2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha F Q, Moncada S, Liew F Y. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-γ in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–1159. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 20.Dawson V L, Dawson T M, London E D, Bredt D S, Snyder S H. Nitric oxide mediates glutamate neurotoxicity in primary cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeGroot C J A, Ruuls S R, Theeuwes J W M, Dukstra C D, Van der Valk P. Immunocytochemical characterization of the expression of inducible and constitutive isoforms on nitric oxide synthase in demyelinating multiple sclerosis lesions. J Neuropathol Exp Neurol. 1997;56:10–20. doi: 10.1097/00005072-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Galea E, Reis D J, Feinstein D L. Cloning and expression of inducible nitric oxide synthase from rat astrocytes. J Neurosci Res. 1994;37:406–414. doi: 10.1002/jnr.490370313. [DOI] [PubMed] [Google Scholar]
- 23.Giovannoni G, Heales S J R, Silver N C, O’Riordan J, Miller R F, Land J M, Clark J B, Thompson E J. Raised serum nitrate and nitrite levels in patients with multiple sclerosis. J Neurol Sci. 1997;145:77–81. doi: 10.1016/s0022-510x(96)00246-8. [DOI] [PubMed] [Google Scholar]
- 24.Good P F, Werner P, Hsu A, Olanow C W, Perl D P. Evidence for neuronal oxidative damage in Alzheimer’s disease. Am J Pathol. 1966;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 25.Grzybicki D M, Kwack K B, Perlman S, Murphy S P. Nitric oxide synthase type II expression by different cell types in MHV-JHM encephalitis suggests distinct roles for nitric oxide in acute versus persistent virus infection. J Neuroimmunol. 1997;73:15–27. doi: 10.1016/S0165-5728(96)00159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewett S J, Corbett J A, McDaniel M L, Choi D W. Interferon-γ and interleukin-1β induce nitric oxide formation from primary mouse astrocytes. Neurosci Lett. 1993;164:229–232. doi: 10.1016/0304-3940(93)90898-u. [DOI] [PubMed] [Google Scholar]
- 27.Hooper D C, Ohnishi S T, Kean R, Numagami Y, Dietzschold B, Koprowski H. Local nitric oxide production in viral and autoimmune diseases of the CNS. Proc Natl Acad Sci USA. 1995;92:5312–5316. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper D C, Bagasra O, Marini J C, Sborek A, Ohnishi S T, Kean R, Champion J M, Sarker A B, Bobroski L, Farber J L, Akaike T, Maeda H, Koprowski H. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA. 1997;94:2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter M I S, Niemadim B C, Davidson D L W. Lipid peroxidation production and antioxidant proteins in plasma and cerebrospinal fluid from multiple sclerosis. J Clin Immunol. 1989;10:1645–1652. doi: 10.1007/BF00988606. [DOI] [PubMed] [Google Scholar]
- 30.Ialenti A, Ianaro A, Moncada S, Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992;211:177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- 31.Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- 32.Johnson A W, Land J M, Thompson E J, Bolanos J P, Clark J B, Heales S R. Evidence for increased nitric oxide production in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995;58:107. doi: 10.1136/jnnp.58.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kepes J J. Large focal tumor-like demyelinating lesions of the brain; intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]
- 34.Kolb H, Kolb-Bachofen V. Nitric oxide: a pathogenic factor in autoimmunity. Immunol Today. 1992;13:157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- 35.Kooy N W, Royall J A, Ye Y Z, Kelly D R, Beckman J S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995;151:1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- 36.Koprowski H, Zhen Y M, Heber-Katz E, Fraser N, Rorke L, Fu Z F, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreil T R, Eibl M M. Nitric oxide and viral infection: no antiviral activity against a flavivirus in vitro, and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- 38.Lee S C, Dickson D W, Liu W, Brosnan C F. Induction of nitric oxide synthase activity in human astrocytes by IL-1β and IFN-γ. J Neuroimmunol. 1993;46:19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann P V, Forsthuber T, Miller A, Sercarz E E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 40.Lepoivre M, Fieschi F, Coves J, Thelander L, Fontecave M. Inactivation of ribonucleotide reductase by nitric oxide. Biochem Biophys Res Commun. 1991;179:442–448. doi: 10.1016/0006-291x(91)91390-x. [DOI] [PubMed] [Google Scholar]
- 41.Lipton S A, Choi Y B, Pan Z H, Lei S Z, Chen H S V, Sucher N J, Loscalzo J, Singel D J, Stamier J S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitrosocompounds. Nature. 1993;364:626–631. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 42.Lowenstein C J, Hill S L, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose N R, Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marlena M A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268:12231–12234. [PubMed] [Google Scholar]
- 44.Marletta M A, Yocr P S, Lyengar R, Leaf C D, Wishnok J S. Macrophage oxidation of l-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988;27:8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- 45.Martin B L, Wu D, Jakes S, Graves D J. Chemical influences on the specificity of tyrosine phosphorylation. J Biol Chem. 1990;265:7108–7111. [PubMed] [Google Scholar]
- 46.Mendez M F, Pogacar S. Malignant monophasic multiple sclerosis or “Marburg’s disease.”. Neurology. 1988;38:1153–1155. doi: 10.1212/wnl.38.7.1153. [DOI] [PubMed] [Google Scholar]
- 47.Merrill J E, Ignarro L J, Sherman M P, Melinek J, Lane T E. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 48.Minc-Golomb D, Tsarfaty I, Schwartz J P. Expression of inducible nitric oxide synthase by neurones following exposure to endotoxin and cytokine. Br J Pharmacol. 1994;112:720–722. doi: 10.1111/j.1476-5381.1994.tb13136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitrovic B, Ignarro L J, Montestruque S, Smoll A, Merrill J E. Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience. 1994;61:575–585. doi: 10.1016/0306-4522(94)90435-9. [DOI] [PubMed] [Google Scholar]
- 50.Nathan C, Xie Q-W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 51.Nathan C, Xie Q-W. Nitric oxide synthases: roles, tolls and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 52.Nussler A K, Billiar T R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukocyte Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- 53.Okuda Y, Sakoda S, Fujimura H, Yanagihara T. Nitric oxide via an inducible isoform of nitric oxide synthase is a possible factor to eliminate inflammatory cells from the central nervous system of mice with experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:107–116. doi: 10.1016/s0165-5728(96)00194-4. [DOI] [PubMed] [Google Scholar]
- 54.Oleszak E L, Murdoch G, Manuelidis L, Manuelidis E E. Growth factor production by Creutzfeldt-Jacob disease cell lines. J Virol. 1988;62:3103–3108. doi: 10.1128/jvi.62.9.3103-3108.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oleszak E L, Kuzmak J, Good R A, Platsoucas C D. Immunobiology of TMEV infection. Immunology of Theiler’s murine encephalomyelitis virus infection. Immunol Res. 1995;14:13–33. doi: 10.1007/BF02918495. [DOI] [PubMed] [Google Scholar]
- 56.Oleszak E L, Katsetos C D, Kuzmak J, Varadhachary A. Inducible nitric oxide synthase in Theiler’s murine encephalomyelitis virus infection. J Virol. 1997;71:3228–3235. doi: 10.1128/jvi.71.4.3228-3235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parkinson J F, Mirtovic B, Merrill J E. The role of nitric oxide in multiple sclerosis. J Mol Med. 1997;75:174–186. doi: 10.1007/s001090050102. [DOI] [PubMed] [Google Scholar]
- 58.Prineas, J. W., and W. I. McDonald. Demyelinating diseases, p. 813–896. In D. I. Graham and P. L. Lantos (ed.), Greenfield’s neuropathology, 6th ed., chapter 13. Arnold, London, United Kingdom.
- 59.Radi R, Beckman J S, Bush K M, Freeman B A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 60.Rodriguez M, Oleszak E, Leibowitz J. Theiler’s murine encephalomyelitis: a model of demyelination and persistence of virus. CRC Crit Rev Immunol. 1987;7:325–365. [PubMed] [Google Scholar]
- 61.Tucker P C, Griffin D E, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Dam A-M, Bauer J, Man-A-Hing W K H, Marquette C, Tilders F J H, Berkenbosch F. Appearance of inducible nitric oxide synthase in the rat central nervous system after rabies virus infection and during experimental allergic encephalomyelitis but not after peripheral administration of endotoxin. J Neurosci Res. 1995;40:251–260. doi: 10.1002/jnr.490400214. [DOI] [PubMed] [Google Scholar]
- 63.Voskuhl R R, Farris II R W, Nagasato K, McFarland H F, Dalcq M D. Epitope spreading occurs in active but not passive EAE induced by myelin basic protein. J Neuroimmunol. 1996;70:103–111. doi: 10.1016/s0165-5728(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 64.Wei X, Charles I G, Smith A, Ure J, Feng G, Huang F, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 65.Weinberg J B, Misukonis M A, Shami P J, Mason S N, Sauls D L, Dittman W A, Wood E R, Smith G K, McDonald B, Bachus K E, Haney A F, Granger D L. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production of blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 66.Wink D A, Kasprzak K S, Maragos C M, Elespuru R K, Misra M, Dunams T M, Cebula T A, Koch W H, Andrews A W, Allen J S, Keefer L K. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 67.Wink D A, Laval J. The Fpg protein, a DNA repair enzyme, is inhibited by the biomediator nitric oxide in vitro and in vivo. Carcinogenesis. 1994;15:2125–2129. doi: 10.1093/carcin/15.10.2125. [DOI] [PubMed] [Google Scholar]
- 68.Yu M, Johnson J M, Tuohy V K. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis for peptide-specific therapy after onset of clinical disease. J Exp Med. 1996;183:1777–1788. doi: 10.1084/jem.183.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zielasek J, Jung S, Gold R, Liew F Y, Toyka K V, Hartung H P. Administration of nitric oxide synthase inhibitors in experimental autoimmune neuritis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 1995;58:81–88. doi: 10.1016/0165-5728(94)00192-q. [DOI] [PubMed] [Google Scholar]