Abstract
Purpose
To elucidate the incidence and treatment pattern of active exudative age-related macular degeneration (AMD).
Design
A population-based cohort study conducted using the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), a national claims database managed by the Japanese Ministry of Health, Labour, and Welfare (MHLW).
Participants
The entire Japanese population aged 40 years or older (76 million people).
Methods
With the permission of the MHLW, we accessed the complete NDB dataset and identified patients with newly diagnosed active exudative AMD between 2011 and 2018. The incidence of active exudative AMD was categorized by age and sex per year between 2011 and 2018; moreover, details regarding first-line therapy and number of anti-vascular endothelial growth factor (VEGF) injections per elapsed year since initial treatment were obtained and changes in treatment pattern were investigated.
Main Outcome Measures
Incidence rate of active exudative AMD.
Results
During the specified 8-year period, 246 064 incident cases of active exudative AMD were identified; 61.4% of these patients were men. The overall incidence rate was 40.66 per 100 000 person-years (95% confidence interval [CI], 40.49–40.82) in the general population aged 40 years or older, 53.22 (95% CI, 52.95–53.49) in men, and 29.78 (95% CI, 29.60–29.98) in women. Mean age of onset was lower in men than in women (72.51 ± 10.50 years vs. 73.90 ± 10.46 years). Among patients with newly diagnosed active exudative AMD, 92.9% received anti-VEGF injections for initial treatments, whereas 1.8% underwent combination therapy with photodynamic therapy. The number of anti-VEGF injections in the first year (0–12 months), second year (13–24 months), and third year (25–36 months) after the initial injection was 3.66 ± 2.30, 1.39 ± 2.20, and 1.23 ± 2.19, respectively. Patients who received fewer injections in the first year received fewer injections in subsequent years and vice versa.
Conclusions
This is a relatively large population-based study on the detailed epidemiology and actual treatment patterns of active exudative AMD in clinical practice. Our results can be a fundamental information source to ensure healthy eyes and promote well-being for all at all ages.
Keywords: Age-related macular degeneration, Epidemiology, Health insurance claims database, Incidence rate
Abbreviations and Acronyms: AMD, age-related macular degeneration; CI, confidence interval; ICD-10, International Classification of Diseases, Tenth Revision; MHLW, Ministry of Health, Labour, and Welfare; NDB, National Database of Health Insurance Claims and Specific Health Checkups of Japan; PDT, photodynamic therapy with verteporfin; PPV, pars plana vitrectomy; SDG, sustainable development goal; VEGF, vascular endothelial growth factor
Age-related macular degeneration (AMD) is currently recognized as an important sight-threatening disease and is the leading cause of blindness in developed countries.1 As aging is the major risk factor for developing AMD, the number of patients with AMD, along with the global social burden, is expected to only increase with time in this era of global aging.2 It is estimated that 8.7% of the worldwide population is affected by AMD, and this is expected to increase to approximately 196 million by 2020 and 288 million by 2040.3 In Asia especially, the number of patients with AMD is estimated to increase more significantly in the future compared with that in other regions.3
Age-related macular degeneration is classified into early AMD and late AMD, with the latter being the main cause for vision loss.2 Late AMD can be further classified into 2 types according to the absence or presence of choroidal neovascularization, namely, dry AMD and wet AMD (exudative AMD).2 Although there is no effective treatment for dry AMD so far, anti-vascular endothelial growth factor (VEGF) therapy is the first-line treatment for exudative AMD and generates approximately $8.6 billion in worldwide revenue annually.4 Anti-VEGF therapy is administered to patients intensively at the early stage of AMD treatment, and the frequency of administration generally decreases as disease activity declines. Although most studies on AMD have focused on treatment outcomes, it is also important to consider the health economic aspects of AMD in the context of the sustainable development goals (SDGs), namely, to ensure healthy lives and promote well-being for all at all ages (Goal 3; Good Health and Well-being). The first step in this process would be to clarify the fundamental information regarding AMD treatment—the incidence of active exudative AMD, that is, AMD that requires treatment and can be treated, and its treatment patterns. Among several approaches we have used for epidemiological studies, claims database study would be the most appropriate method for this purpose, especially in the countries of universal health coverage.5, 6, 7, 8 Although a large number of cohort studies and cross-sectional studies have reported the prevalence of late AMD, few studies have reported its incidence, especially with long-term follow-up.9 Furthermore, limited epidemiological information specific to exudative AMD is available, and there is no epidemiological information limited to active exudative AMD.
The purpose of this study was to determine the incidence of exudative AMD that requires treatment and to analyze the acutal clinical practices of AMD treatment. To achieve these objectives, we conducted a nationwide population-based cohort study using the largest claims database in the world. This is a relatively large epidemiological study on AMD to report the prevalence and incidence of active exudative AMD.
Methods
This retrospective, nationwide population-based cohort study was approved by the Institutional Review Board and the Ethics Committee of Kyoto University Hospital and Kyoto University Graduate School of Medicine (No. R2035). All investigations were conducted in accordance with the tenets of the Declaration of Helsinki and its later amendments. The results are reported according to the STrengthening the Reporting of OBservational studies in Epidemiology and REporting of studies Conducted using Observational Routinely collected health Data guidelines.
Database
The National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) is a national claims database managed by the Japanese Ministry of Health, Labour, and Welfare (MHLW).10 As Japan provides universal health coverage, the NDB covers > 95% of all medical care claims for the entire Japanese population of more than 127 million people. The data stored in the NDB are anonymized to protect personal information, but a unique identification is assigned to identify individual patients. The data are made available for academic use through several options and have led to several significant reports.11,12 The features of the offering options are briefly summarized in Table S1 (available at www.ophthalmologyscience.org).
In the current study, the onsite option was used. With the approval of the MHLW, we accessed the database via the NDB Onsite Research Center, Kyoto, which is 1 of only 2 centers with onsite remote access to the complete NDB dataset.10,13 The current study was conducted during the authorized research period between October 11, 2019, and April 10, 2020.
The NDB data includes coded information on drugs and procedures for both outpatients and inpatients as well as on diagnosed diseases, which are coded according to the International Classification of Diseases, Tenth Revision (ICD-10).14 This information is also coded as per the local claim codes of Japan: NDB diagnostic codes, NDB drug codes, and NDB procedure codes. At the time of research initiation, data for more than 14 billion claims covering the entire Japanese population (N ≥ 127 million) generated between 2008 and 2019 were available. Detailed information regarding the NDB is available in other publications.10,12,13
Active Exudative AMD
After we had linked all the claims of individuals aged 40 years and older as previously described,8 we identified the patients (1) with newly diagnosed AMD between April 1, 2009, and December 31, 2018, and (2) who received postdiagnosis treatment for neovascular AMD, that is, vitreous injections of anti-VEGF medication, photodynamic therapy with verteporfin (PDT), or combination therapy. The incident cases of active exudative AMD between April 2009 and December 2010 were excluded from the current study to washout recurrent cases.
A diagnosis of AMD was assigned on the basis of the NDB diagnostic codes, which are the original diagnostic codes of Japan used in the NDB, because these codes are known to be able to identify diseases more specifically than the ICD-10 codes. The correspondence table for the NDB diagnostic codes and the ICD-10 codes is provided in Table S2 (available at www.ophthalmologyscience.org). We validated the NDB diagnostic codes in a previous study.15 Patients were determined to have undergone PDT on the basis of prescriptions for verteporfin. Likewise, they were determined to have received vitreous injections of anti-VEGF drugs based on prescriptions for either pegaptanib, ranibizumab, or aflibercept. Patients who underwent both PDT and anti-VEGF treatment within 1 week were considered to have undergone combination therapy.
Onset and Initial Treatment for Active Exudative AMD
In the current study, we defined the onset date of active exudative AMD as the date of the first treatment after diagnosis of AMD, that is, we did not consider the AMD active unless it was treated.
The number of patients with active exudative AMD, namely, the incidence of active exudative AMD, by age and sex per year from 2011 to 2018 was calculated. Incidence rates stratified by age and sex were determined by dividing the incidence of AMD within each group by the population at risk in the corresponding group. The population estimates for each year between 2011 and 2018 provided by the Japanese Ministry of Internal Affairs and Communications were used to define the entire population and each subgroup population as the population at risk.16 In addition, because the NDB includes information on prefectures, we calculated the incidence rate for each prefecture where the AMD onset of each patient was identified for reference.
The changes in ophthalmologists’ first-choice treatment for new-onset active exudative AMD from 2011 to 2018 were also investigated. With regard to anti-VEGF therapy, we also investigated the use of each of the 3 anti-VEGF drugs.
Consequences after the Initial Treatment
To address the actual anti-VEGF treatment pattern in clinical practice, the number of anti-VEGF injections received by each patient was determined. The average number of anti-VEGF injections received in the accumulative years under observation or by the year from initial treatment was also calculated to assess the trend according to the year of treatment. Furthermore, the associations between the number of anti-VEGF injections in the first year and those after the second year, in the accumulative years, or by the year from initial treatment, were also assessed.
To analyze the local adverse events of anti-VEGF injections, we counted the patients who had endophthalmitis or retinal detachment requiring pars plana vitrectomy (PPV) within 1, 2, or 3 years from the date of first anti-VEGF injection. Both adverse events were defined based on the corresponding NDB diagnostic code and NDB procedure code (Table S2, available at www.ophthalmologyscience.org). The incident proportions of the adverse events were calculated by dividing the number of incident cases by the number of patients who started AMD treatment with anti-VEGF injections (n = 246 064).
Association between Number of Anti-VEGF Injections and Copayment Proportion
Japan has a universal health insurance system in which all citizens are covered by medical insurance, with copayment proportions varying depending on the patient's annual income. Although the basic copayment proportion is 30%, for older patients with relatively lower incomes, namely, those with an annual income of < 3.7 million yen (∼34 000 dollars, considering an exchange rate of 1 dollar = 109 yen), the copayment proportion is reduced—copayment proportion decreases to 10% if they are aged ≥ 75 years and 20% if they are aged 70–74 years. The NDB contains information only on copayment proportion, not on patient annual incomes. Because anti-VEGF treatment is expensive, to assess whether copayment proportion affects the number of anti-VEGF injections administered, we compared the number of anti-VEGF injections between the different copayment proportion groups. As mentioned, the copayment proportion of the patients aged 70–74 years was 30% or 20%, and that of patients aged ≥ 75 years old was 30% or 10%. Thus, we compared the 30% and 20% subgroups in the 70–74 years age group and the 30% and 10% subgroups in the ≥ 75 years age group. The number of anti-VEGF injections administered within the first 12 months and 36 months was evaluated.
Statistical Analyses
All statistical analyses were performed using Oracle R Enterprise 1.4.1 (Oracle Corporation) and R version 3.4.1 (R Foundation for Statistical Computing). All values are presented with 95% confidence intervals (CIs) based on the Poisson distribution. Between-group differences were assessed using the t test or analysis of variance. Two-sided P values < 0.05 were considered statistically significant.
Results
The patient selection process is summarized as a flowchart in Figure S1 (available at www.ophthalmologyscience.org). In total, 246 064 incident cases of active exudative AMD during the 8-year study period and 72 378 prevalent cases in 2015 were identified. The 72 378 prevalent cases of patients with active exudative AMD in 2015 corresponded to a prevalence of 0.10%, calculated by dividing the number of prevalent cases by the number of at-risk individuals aged 40 years and older. Table 1 shows a comparison of the prevalence of exudative or late AMD with data from previous studies in Asian populations and countries.5,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31
Table 1.
Reported Prevalence of Age-Related Macular Degeneration in Asian Populations and Countries
| Study | Country | Data Source | Study Period | Age, yrs | Number at Risk | Detected Cases (Prevalence (%)) |
||
|---|---|---|---|---|---|---|---|---|
| Active Exudative AMD | Exudative AMD | Late AMD | ||||||
| Present Study | Japan | NDB | 2011–2018 | ≥40 | 75 761 015 | 72 378 (0.10) | NA | NA |
| Hisayama Study | Japan | Regional Cohort | 1998 | ≥50 | 1482 | NA | NA | 7 (0.47) |
| Shihpai Eye Study | Taiwan | Regional Cohort | 1999–2000 | ≥65 | 1058 | NA | 14 (1.32) | NA |
| Funagata Study | Japan | Regional Cohort | 2000–2002 | ≥40 | 1556 | NA | 7 (0.45) | 8 (0.51) |
| Kumejima Study | Japan | Regional Cohort | 2005–2006 | ≥40 | 3068 | NA | 4 (0.13) | 4 (0.13) |
| Handan Eye Study | China | Regional Cohort | 2006–2007 | ≥50 | 4049 | NA | NA | 4 (0.10) |
| Hisayama Study | Japan | Regional Cohort | 2007 | ≥50 | 2663 | NA | 32 (1.20) | 33 (1.24) |
| Nagahama Study | Japan | Regional Cohort | 2008–2010 | 74 ≥, ≥ 50 | 5595 | NA | 28 (0.50) | 29 (0.52) |
| KNHANES | Korea | KNHANES | 2008–2012 | ≥40 | 20 419 | NA | 100 (0.49) | NA |
| HIRA | Korea | HIRA | 2008–2012 | ≥40 | 22 376 510 | NA | 81 513 (0.36) | NA |
| Beijing Eye Study | China | Regional Cohort | 2011 | ≥50 | 3467 | NA | 48 (1.38) | NA |
| Tsuruoka Metabolomics Cohort Study | Japan | Regional Cohort | 2012–2013 | 74 ≥, ≥ 35 | 3988 | NA | 2 (0.05) | 2 (0.05) |
| Handan Eye Study | China | Regional Cohort | 2012–2013 | ≥40 | 5048 | NA | 11 (0.22) | NA |
| Yangxi Study | China | Regional Cohort | 2014 | ≥50 | 4881 | NA | NA | 42 (0.86) |
| Hong Kong Eye Study | Hong Kong | Regional Cohort | 2018 | ≥40 | 1565 | NA | 8 (0.51) | NA |
AMD = age-related macular degeneration; HIRA = Health Insurance and Review Assessment; KNHANES = Korean National Health and Nutritional Survey; NA = not available; NDB = National Database of Health Insurance Claims and Specific Health Checkups of Japan.
The first 12 months refer to the 12 months from each initial treatment. Injection numbers are presented as mean ± standard deviation.
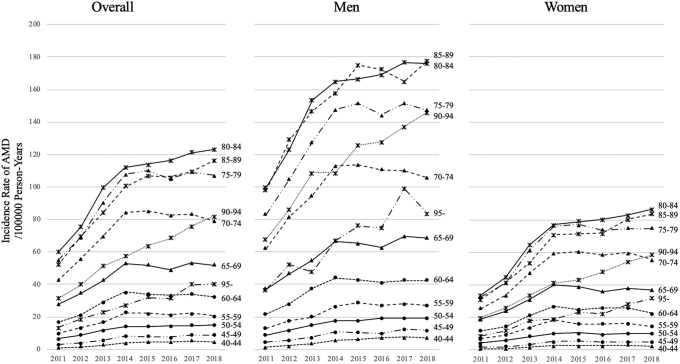
In the study period, 61.4% (151 029/246 064) of patients in the NDB dataset were men. Table 2 shows the number of incident cases, incidence rates per 100 000 person-years, and mean ages of onset of active exudative AMD between 2011 and 2018. The incidence rate in the overall population was 40.66 per 100 000 person-years (95% CI, 40.49–40.82). The overall incidence rate was 53.22 per 100 000 person-years (95% CI, 52.95–53.49) for men and 29.78 per 100 000 person-years (95% CI, 29.60–29.98) for women; both had an increasing trend. Mean age of onset was lower in men than in women (72.51 ± 10.50 years vs. 73.90 ± 10.46 years); both were observed to keep increasing slightly over the years (Table 2). In addition, age- and sex-stratified data are shown in Figure 1. The incidence rate of active exudative AMD in men was almost twice as high as that in women in every age group. The highest incidence rate was observed in the 80–84 years group, both in men and women. The changes in the age- and sex-stratified incidence rates of active exudative AMD from 2011 to 2018 are presented in Figure 2. The incidence rate was steadily increasing in almost all age groups over 65 years of age between 2011 and 2018.
Table 2.
Annual Incidence of Active Exudative Age-related Macular Degeneration, 2011–2018
| Year | Incidence |
Incidence Rate (95% CI) |
Mean Age (±SD) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | Men | Women | Total | |
| 2011 | 10 700 | 5711 | 16 411 | 31.22 (30.62–31.81) | 14.75 (14.37–15.14) | 22.49 (22.14–22.83) | 72.46 ± 9.93 | 73.17 ± 10.16 | 72.70 ± 10.01 |
| 2012 | 14 057 | 7507 | 21 564 | 40.58 (39.91–41.25) | 19.21 (18.77–19.64) | 29.25 (28.86–29.64) | 72.53 ± 10.01 | 73.47 ± 10.14 | 72.86 ± 10.07 |
| 2013 | 17 407 | 10 808 | 28 215 | 49.73 (48.99–50.47) | 27.39 (26.87–27.90) | 37.89 (37.45–38.33) | 72.53 ± 10.14 | 73.41 ± 10.23 | 72.87 ± 10.18 |
| 2014 | 20 723 | 13 865 | 34 588 | 58.62 (57.82–59.41) | 34.82 (34.24–35.40) | 46.01 (45.52–46.49) | 72.04 ± 10.40 | 73.25 ± 10.45 | 72.52 ± 10.43 |
| 2015 | 21 464 | 14 014 | 35 478 | 59.96 (59.16–60.76) | 34.90 (34.32–35.47) | 46.71 (46.22–47.19) | 72.37 ± 10.53 | 73.64 ± 10.48 | 72.87 ± 10.53 |
| 2016 | 21 298 | 13 843 | 35 141 | 59.08 (58.28–59.87) | 34.25 (33.68–34.82) | 45.95 (45.47–46.43) | 72.59 ± 10.69 | 74.04 ± 10.51 | 73.16 ± 10.64 |
| 2017 | 22 609 | 14 646 | 37 255 | 62.38 (61.57–63.19) | 36.06 (35.48–36.64) | 48.47 (47.98–48.97) | 72.58 ± 10.79 | 74.39 ± 10.61 | 73.29 ± 10.76 |
| 2018 | 22 771 | 14 641 | 37 412 | 62.56 (61.75–63.38) | 35.91 (35.33–36.49) | 48.48 (47.99–48.97) | 72.92 ± 10.86 | 75.03 ± 10.57 | 73.75 ± 10.80 |
| Total | 151 029 | 95 035 | 246 064 | 53.22 (52.95–53.49) | 29.78 (29.60–29.98) | 40.66 (40.49–40.82) | 72.51 ± 10.50 | 73.90 ± 10.46 | 73.05 ± 10.50 |
CI = confidence interval; SD = standard deviation.
The incidence rate is reported per 100 000 person-years.
Figure 1.
Annual age- and sex-stratified incidence rate of active exudative age-related macular degeneration (AMD) per 100 000 person-years. Men and women had significantly different distributions. The incidence rate of exudative AMD in men was approximately twice that in women in every age group. The highest incidence rate was observed in the 80–84 years group in both men and women.
Figure 2.
Overall age- and sex-stratified incidence rate of active exudative age-related macular degeneration (AMD) per 100 000 person-years between 2011 and 2018. Beyond the age of 65 years, the incidence rate increased steadily in almost all groups between 2011 and 2018.
Data regarding the initial treatment options among 246 064 cases of active exudative AMD during the study period are presented in Table 3. In this period, 228 665 patients (92.9%) underwent anti-VEGF therapy as the initial treatment for AMD, and the proportion of anti-VEGF treatment cases steadily increased over the study period. The proportions of patients undergoing PDT and combination therapy declined until 2016, but since then they increased again slightly. Data regarding anti-VEGF therapy as the initial treatment for AMD are presented in Table 4. The number of patients with active exudative AMD treated with anti-VEGF therapy increased over the 8 years and especially rapidly before 2014. Ranibizumab was the predominantly used anti-VEGF drug until aflibercept was approved in Japan. After 2015, aflibercept has been the primary treatment option for active exudative AMD, with a market share of approximately 70%. Only a small fraction of patients with active exudative AMD (0.8% in the overall study period, with the number steadily decreasing from 2.6% to 0.3%) used pegaptanib.
Table 3.
Initial Treatment Option Data for Age-related Macular Degeneration, 2011–2018
| Year | Treated Cases (%) | Initial Treatment Option (%) |
||
|---|---|---|---|---|
| Anti-VEGF Therapy | PDT | Combination | ||
| 2011 | 16 411 (100) | 13 165 (80.2) | 2304 (14.0) | 942 (5.7) |
| 2012 | 21 564 (100) | 18 495 (85.8) | 2129 (9.9) | 940 (4.4) |
| 2013 | 28 215 (100) | 26 218 (92.9) | 1513 (5.4) | 484 (1.7) |
| 2014 | 34 588 (100) | 32 740 (94.7) | 1468 (4.2) | 380 (1.1) |
| 2015 | 35 478 (100) | 33 741 (95.1) | 1439 (4.1) | 298 (0.8) |
| 2016 | 35 141 (100) | 33 513 (95.4) | 1342 (3.8) | 286 (0.8) |
| 2017 | 37 255 (100) | 35 411 (95.1) | 1444 (3.9) | 400 (1.1) |
| 2018 | 37 412 (100) | 35 382 (94.6) | 1445 (3.9) | 585 (1.6) |
| Total | 246 064 (100) | 228 665 (92.9) | 13 084 (5.3) | 4315 (1.8) |
PDT = photodynamic therapy with verteporfin; VEGF = vascular endothelial growth factor.
Table 4.
Details of Anti-VEGF Therapy for Initial Age-related Macular Degeneration Treatment, 2011–2018
| Year | Cases Treated Using Anti-VEGF Therapy (%) | Initial Anti-VEGF Treatment (%) |
||
|---|---|---|---|---|
| Ranibizumab | Aflibercept | Pegaptanib | ||
| 2011 | 14 107 (100) | 13 743 (97.4) | - | 364 (2.6) |
| 2012 | 19 435 (100) | 18 652 (96.0) | 306 (1.6) | 477 (2.4) |
| 2013 | 26 702 (100) | 15 070 (56.5) | 11 304 (42.3) | 328 (1.2) |
| 2014 | 33 120 (100) | 18 111 (54.7) | 14 792 (44.7) | 217 (0.6) |
| 2015 | 34 039 (100) | 12 873 (37.8) | 20 978 (61.6) | 188 (0.6) |
| 2016 | 33 799 (100) | 9908 (29.3) | 23 763 (70.3) | 128 (0.4) |
| 2017 | 35 811 (100) | 10 944 (30.6) | 24 751 (69.1) | 116 (0.3) |
| 2018 | 35 967 (100) | 10 867 (30.2) | 25 011 (69.5) | 89 (0.3) |
| Total | 232 980 (100) | 110 168 (47.3) | 120 905 (51.9) | 1907 (0.8) |
VEGF = vascular endothelial growth factor.
The number of anti-VEGF injections administered for active exudative AMD, both accumulative and by the year, is shown in Table 5. The number of injections in the first year (0–12 months), second year (13–24 months), and third year (25–36 months) after the initial injection was 3.66 ± 2.30, 1.39 ± 2.20, 1.23 ± 2.19, respectively. The number of injections per year has remained almost unchanged since 2012. The number of anti-VEGF injections administered after the first year, categorized according to the number of anti-VEGF injections in the first year, is presented in Table 6. The most frequently observed number of anti-VEGF injections in the first year was 3 (26.8%), followed by 1 (18.7%). For all groups categorized by the average number of injections in the first year, the number of injections after the second year was lower than that in the first year. Patients who received fewer injections in the first year received fewer injections in subsequent years and vice versa.
Table 5.
Number of Anti-VEGF Injections Administered for Age-related Macular Degeneration
| Year of Initial Treatment | Number of Anti-VEGF Injections within the Specified Period |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accumulative | By the Year | ||||||||||
| 0–12 mos | 0–24 mos | 0–36 mos | 0–48 mos | 0–60 mos | 0–72 mos | 0–84 mos | 0–12 mos | 13–24 mos | 25–36 mos | ||
| 2011 | Mean ± SD | 3.46 ± 1.88 | 4.65 ± 3.41 | 5.82 ± 4.96 | 6.97 ± 6.55 | 8.18 ± 8.26 | 9.42 ± 10.03 | 10.75 ± 11.90 | 3.46 ± 1.88 | 1.20 ± 2.04 | 1.15 ± 2.07 |
| Median (quantile) | 3 (2–4) | 3 (3–6) | 4 (3–8) | 4 (3–9) | 5 (3–11) | 5 (3–13) | 6 (3–14) | 3 (2–4) | 0 (0–2) | 0 (0–2) | |
| 2012 | Mean ± SD | 3.73 ± 2.16 | 5.09 ± 3.84 | 6.34 ± 5.53 | 7.57 ± 7.22 | 8.86 ± 9.00 | 10.26 ± 10.91 | - | 3.73 ± 2.16 | 1.36 ± 2.16 | 1.24 ± 2.20 |
| Median (quantile) | 3 (2–5) | 4 (3–7) | 4 (3–9) | 5 (3–10) | 5 (3–12) | 6 (3–14) | - | 3 (2–5) | 0 (0–2) | 0 (0–2) | |
| 2013 | Mean ± SD | 3.73 ± 2.21 | 5.09 ± 3.89 | 6.33 ± 5.57 | 7.53 ± 7.27 | 8.87 ± 9.11 | - | - | 3.73 ± 2.21 | 1.36 ± 2.15 | 1.21 ± 2.15 |
| Median (quantile) | 3 (2–5) | 4 (3–7) | 4 (3–9) | 5 (3–10) | 5 (3–12) | - | - | 3 (2–5) | 0 (0–2) | 0 (0–2) | |
| 2014 | Mean ± SD | 3.60 ± 2.32 | 5.00 ± 4.08 | 6.24 ± 5.82 | 7.51 ± 7.60 | - | - | - | 3.60 ± 2.32 | 1.38 ± 2.20 | 1.21 ± 2.17 |
| Median (quantile) | 3 (2–5) | 4 (2–7) | 4 (2–9) | 5 (2–10) | - | - | - | 3 (2–5) | 0 (0–2) | 0 (0–2) | |
| 2015 | Mean ± SD | 3.61 ± 2.34 | 5.05 ± 4.17 | 6.41 ± 6.03 | - | - | - | - | 3.61 ± 2.34 | 1.42 ± 2.24 | 1.29 ± 2.28 |
| Median (quantile) | 3 (2–5) | 4 (2–7) | 4 (2–9) | - | - | - | - | 3 (2–5) | 0 (0–2) | 0 (0–2) | |
| 2016 | Mean ± SD | 3.68 ± 2.41 | 5.22 ± 4.31 | - | - | - | - | - | 3.68 ± 2.41 | 1.50 ± 2.32 | - |
| Median (quantile) | 3 (2–5) | 4 (2-7) | - | - | - | - | - | 3 (2–5) | 0 (0–3) | - | |
| 2017 | Mean ± SD | 3.77 ± 2.46 | - | - | - | - | - | - | 3.77 ± 2.46 | - | - |
| Median (quantile) | 3 (2–5) | - | - | - | - | - | - | 3 (2–5) | - | - | |
| Average | 3.66 ± 2.30 | 5.05 ± 4.03 | 6.27 ± 5.69 | 7.44 ± 7.27 | 8.69 ± 8.88 | 9.90 ± 10.55 | 10.75 ± 11.90 | 3.66 ± 2.30 | 1.39 ± 2.20 | 1.23 ± 2.19 | |
SD = standard deviation; VEGF = vascular endothelial growth factor.
Table 6.
Number of Anti-VEGF Injections after the First Year Categorized According to Number of Anti-VEGF Injections in the First Year
| Number of Anti-VEGF in the First 12 Mos | Cases (No./%) | Average Number of Anti-VEGF Injections within the Specified Period (±SD) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Accumulative |
By the Year |
|||||||||
| 0–24 mos | 0–36 mos | 0–48 mos | 0–60 mos | 13–24 mos | 25–36 mos | 37–48 mos | 49–60 mos | |||
| 1 | 35 108 | 18.7 | 1.26 ± 0.85 | 1.55 ± 1.62 | 1.91 ± 2.49 | 2.41 ± 3.60 | 0.26 ± 0.85 | 0.29 ± 0.99 | 0.33 ± 1.09 | 0.38 ± 1.22 |
| 2 | 25 473 | 13.6 | 2.52 ± 1.14 | 3.05 ± 2.14 | 3.62 ± 3.27 | 4.34 ± 4.59 | 0.52 ± 1.14 | 0.52 ± 1.28 | 0.54 ± 1.39 | 0.59 ± 1.55 |
| 3 | 50 136 | 26.8 | 3.65 ± 1.31 | 4.33 ± 2.47 | 5.02 ± 3.66 | 5.76 ± 4.93 | 0.65 ± 1.31 | 0.68 ± 1.50 | 0.69 ± 1.58 | 0.73 ± 1.68 |
| 4 | 23 073 | 12.3 | 5.41 ± 1.76 | 6.67 ± 3.25 | 7.88 ± 4.77 | 9.14 ± 6.37 | 1.41 ± 1.76 | 1.24 ± 1.87 | 1.16 ± 1.95 | 1.19 ± 2.11 |
| 5 | 16 306 | 8.7 | 7.09 ± 2.08 | 8.87 ± 3.88 | 10.56 ± 5.66 | 12.25 ± 7.49 | 2.09 ± 2.08 | 1.77 ± 2.21 | 1.63 ± 2.29 | 1.60 ± 2.41 |
| 6 | 14 665 | 7.8 | 8.53 ± 2.27 | 10.66 ± 4.27 | 12.58 ± 6.30 | 14.48 ± 8.39 | 2.53 ± 2.27 | 2.12 ± 2.46 | 1.95 ± 2.55 | 1.87 ± 2.63 |
| 7 | 10 693 | 5.7 | 10.5 ± 2.47 | 13.4 ± 4.83 | 16.00 ± 7.16 | 18.82 ± 9.63 | 3.50 ± 2.47 | 2.89 ± 2.80 | 2.65 ± 2.85 | 2.57 ± 3.00 |
| 8 | 5730 | 3.1 | 12.3 ± 2.71 | 15.92 ± 5.31 | 18.98 ± 7.89 | 21.87 ± 10.45 | 4.31 ± 2.71 | 3.57 ± 3.07 | 3.10 ± 3.19 | 2.99 ± 3.29 |
| 9 | 2983 | 1.6 | 14.0 ± 3.01 | 18.21 ± 3.04 | 21.35 ± 7.81 | 24.38 ± 11.85 | 5.01 ± 3.01 | 4.14 ± 3.57 | 3.47 ± 3.47 | 3.31 ± 3.65 |
| 10 | 1655 | 0.9 | 15.86 ± 3.25 | 20.41 ± 6.26 | 24.64 ± 9.44 | 28.47 ± 12.45 | 5.86 ± 3.25 | 4.71 ± 3.63 | 4.27 ± 3.89 | 4.00 ± 3.78 |
| 11 | 976 | 0.5 | 17.84 ± 3.51 | 23.29 ± 6.82 | 27.48 ± 10.05 | 31.72 ± 13.92 | 6.84 ± 3.51 | 5.53 ± 3.90 | 4.70 ± 4.15 | 4.22 ± 4.29 |
| 12 | 621 | 0.3 | 19.6 ± 3.80 | 25.22 ± 7.83 | 29.79 ± 11.92 | 33.68 ± 14.28 | 7.58 ± 3.80 | 5.72 ± 4.61 | 4.82 ± 4.70 | 4.09 ± 4.19 |
SD = standard deviation; VEGF = vascular endothelial growth factor.
The first 12 months refer to the 12 months from each initial treatment.
Injection numbers are presented as mean ± standard deviation.
Table 7 presents data regarding local adverse events such as endophthalmitis and retinal detachment after the initial anti-VEGF injection. Endophthalmitis treated by PPV was observed in 132 (0.054%), 170 (0.069%), and 200 (0.081%) cases within 1 year, 2 years, and 3 years of the initial anti-VEGF injection, respectively. Retinal detachment treated by PPV was observed in 468 (0.19%), 658 (0.26%), and 778 (0.32%) cases within 1 year, 2 years, and 3 years of the initial anti-VEGF injection, respectively.
Table 7.
Local Adverse Events After Initial Anti-VEGF Injection for Age-related Macular Degeneration
| Adverse Event | Duration After Initial Treatment | Incident Cases |
Incident Proportion |
||
|---|---|---|---|---|---|
| Men | Women | Total | Total | ||
| Endophthalmitis | In 1 yr | 81 | 51 | 132 | 0.054% |
| In 2 yrs | 105 | 65 | 170 | 0.069% | |
| In 3 yrs | 122 | 78 | 200 | 0.081% | |
| Retinal detachment | In 1 yr | 326 | 142 | 468 | 0.19% |
| In 2 yrs | 460 | 198 | 658 | 0.26% | |
| In 3 yrs | 549 | 229 | 778 | 0.32% | |
VEGF = vascular endothelial growth factor.
Endophthalmitis and retinal detachment were defined on the basis of diagnosis codes and pars plana vitrectomy (PVV).
Incident proportions were calculated by dividing the number of incident cases by the number of patients who received anti-VEGF injections as the initial treatment for AMD (n = 246 064).
Table 8 presents the number of anti-VEGF injections administered in the different copayment proportion groups. Patients aged 75 years or more with copayment proportion 30% received 3.94 ± 2.37 injections in the first year, and those aged 70–74 years received 3.98 ± 2.37 injections. They received a significantly higher number of injections than patients of the same age with a lower copayment proportion (≥ 75 years: 3.74 ± 2.29, P < 0.0001 and 70–74 years: 3.83 ± 2.35, P = 0.0003).
Table 8.
Number of Anti-VEGF Injections in Different Copayment Proportion Groups
| Age |
≥75 Yrs |
70–74 Yrs |
||||
|---|---|---|---|---|---|---|
| Copayment proportion | 30% | 10% | P Value | 30% | 20% | P Value |
| Newly treated AMD patients | 10 463 | 104 717 | - | 4947 | 37 633 | - |
| Anti-VEGF injection numbers in the first 1 yr (mean ± SD) | 3.94 ± 2.37 | 3.74 ± 2.29 | <0.0001 | 3.98 ± 2.37 | 3.83 ± 2.35 | 0.0003 |
| Anti-VEGF Injection Numbers in 3 yrs (mean ± SD) | 6.95 ± 5.95 | 6.37 ± 5.64 | <0.0001 | 7.45 ± 6.40 | 6.94 ± 5.98 | 0.0003 |
AMD = age-related macular degeneration; SD = standard deviation; VEGF = vascular endothelial growth factor.
The basic copayment is 30%. If the annual income is <33 700 000 Japanese Yen, copayment is reduced to 10% and 20% for individuals aged ≥75 and 70–74 years, respectively.
Discussion
Age-related macular degeneration is one of the leading causes of blindness in developed countries, and many treatments, such as anti-VEGF therapy, have been developed and are used for active exudative AMD. In this study, we analyzed the Japanese national administrative database of claims data with the permission of the MHLW to clarify the epidemiology of active exudative AMD and its actual treatment patterns. This is a relatively large epidemiological study that reports the prevalence and incidence of active exudative AMD.
Although there are many reports on the epidemiology of AMD, there have been no reports regarding exudative AMD requiring treatment. It is important to create awareness among the general public regarding the heavy burden, which AMD treatment places on the social insurance system because of the high cost of anti-VEGF therapy, which is the first-line treatment for AMD. In addition, the expensive drugs also often make it difficult for AMD patients to continue effective treatment, which should be avoided, especially in the context of SDGs. To improve the situation, it is extremely important for societies to be aware of the data regarding the epidemiology and actual clinical practices of AMD that requires treatment. Accordingly, we analyzed Japan’s national claims database—Japanese patients with AMD represent a population that is expected to have easy access to anti-VEGF treatment because (1) the patient’s copayment is limited to 10% to 30% depending on age and income under Japan’s universal health coverage system; (2) OCT equipment has been widely disseminated to ophthalmology clinics; and (3) patients have free access to macula-specialized hospitals. Thus, in Japan, most patients who are medically indicated for anti-VEGF treatment will receive the treatment promptly. Considering this situation, it is reasonable to evaluate the epidemiology of treated AMD as a strong surrogate for active exudative AMD. We believe the current study is an important first step toward SDG goals regarding AMD.
Although the current study determined the incidence rate of active exudative AMD, the results were within the reported epidemiological range of exudative AMD (including both active and nonactive cases)—the previously reported incidence of exudative AMD in Asian populations ranges from 20 to 160 per 100 000 person-years in regional cohort studies and from 29 to 30 per 100 000 person-years in claims database studies.18,26,28,32,33 As discussed, most cases of exudative AMD would have been appropriately treated using anti-VEGF drugs in Japan. The trajectory of annual incidence rate (Fig 1) reveals that the current study period can be divided into 2 periods: the rapid-increase period from 2011 to 2014 and the plateau period after 2015. By comparison with the graph of the number of OCT units sold (data provided by the Japan Ophthalmic Instruments Association; Fig S2, available at www.ophthalmologyscience.org), we speculate that the rapid growth period corresponds to the OCT dissemination period in Japan. The widespread use of OCT would have made it easier to detect slight exudative changes, increasing referrals to tertiary hospitals and resulting in the increase in the number of patients with AMD introduced to anti-VEGF drugs. However, the increase in incidence during the plateau phase after the spread of OCT may better reflect the true incidence of active exudative AMD. Even during the plateau period, the incidence of active exudative AMD in both men and women aged 75 years and older increased year by year. One possible reason for this could be that patients with AMD would be able to live longer than before because of advancement in medical care, although they are known to have cardiovascular risk factors such as hyperlipidemia and hypertension.
Tables 4 and 5 present several interesting findings. First, despite the increased market share of aflibercept compared with that of ranibizumab from 2012 to 2018, both the number of anti-VEGF injections per patient per year and the cumulative number during this period did not decrease. Although the differences in duration of effect between aflibercept and ranibizumab have been debated, it is possible that there may not be any significant difference in this duration between them. Second, the greater the number of anti-VEGF injections given in the first year after onset, the greater the number of anti-VEGF injections given in the subsequent years. As this trend was clear even in patients with 1 to 6 anti-VEGF injections in the first year, namely, those who would have received anti-VEGF treatment pro re nata, it is likely that disease activity in the first year is associated with that in subsequent years. Patients who received 3 or fewer injections in the first year continued to receive < 1 additional injection per year thereafter, whereas those who received 4 or more injections in the first year received, on average, > 1 injection per year, even in the fifth year after treatment. These results suggest that careful long-term follow-up is required for patients in whom the disease was not controlled with the loading dose in the first year.
We also examined the local complications associated with the anti-VEGF drug injection. In this study, 0.054% of patients developed endophthalmitis requiring PPV in the initial 1 year of treatment for AMD. Previous reports have shown that endophthalmitis after the use of injections of anti-VEGF drugs occurs in the range of 0.019% to 1.4% per injection unit.34 In the present study, we reported the incidence rate of endophthalmitis on a per-patient basis rather than a per-injection basis. Considering that the average number of injections in the first year was 3.66, the incidence of endophthalmitis in this study was comparable to that reported in previous studies.
Several studies have evaluated the association between medical care–seeking behavior of patients and copayment. However, these studies did not provide consistent evidence, concluding that the impact of copayment on health outcomes varies by disease.35,36 However, Kato and Goto36 reported that the differences in copayment significantly affected patient behavior in low-income groups,36 which supports our finding that lower-income patients received significantly fewer injections. We also found that the reduction of copayment to 10% or 20% for low-income patients may not be enough to encourage appropriate treatment; nevertheless, this aspect needs further study in the future.
Study Limitations
Although this nationwide population-based study has several strengths in terms of the large sample size and its representativeness, it also has certain limitations. First, we defined active exudative AMD on the basis of both diagnosis and treatment; thus, patients with active exudative AMD who did not receive treatment for some reason (e.g., short life expectancy or comorbid illness) would have been missed. Second, we were not able to identify patients who did not visit a hospital, which may have also led to an underestimation of incidence. However, because active exudative AMD causes obvious vision loss, patients are usually aware of their symptoms and visit a hospital promptly. Thus, underestimation due to this reason is less likely to have occurred. Third, although the NDB is a comprehensive administrative database covering most of the healthcare claims for the entire Japanese population, it does not contain data regarding medical care not covered by health insurance, such as that paid for by public assistance and industrial accident compensation insurance; therefore, we must have missed such cases, although they are not common, and the impact is expected to be limited. Lastly, it was impossible to strictly distinguish between unilateral and bilateral AMD using the NDB data. This may have led to an overestimation of the number of injections per eye unit. However, because it is not likely for AMD in both eyes to become active at the same time, we believe that the impact is relatively minimal.
In conclusion, the epidemiology and actual treatment patterns in clinical practice of active exudative AMD were clarified in this study. Because the widespread use of expensive anti-VEGF drugs places a considerable burden on society and individuals, it is important in the context of SDGs to comprehend the actual clinical practice pattern of treatment for active exudative AMD and to use this information as a foundation for treatment optimization and planning. The clarification of the actual clinical situation in Japan would be an important first step in this direction.
Acknowledgments
This work was partly supported by JSPS KAKENHI grant number 21K09740. The authors thank Rei Goto of Graduate School of Business Administration, Keio University, for his advice and comments from the viewpoint of health economics.
Manuscript no. XOPS-D-21-00132
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): S.H.: Grants – JSPS KAKENHI 19K19386.
K.K.: Grants – Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Pfizer Inc., Stella Pharma Corporation, CMIC Co., Ltd., Suntory Beverage & Food Ltd., Mitsubishi Corporation, Real World Data Co., Ltd., Eisai Co., Ltd.; Consulting fees – LEBER Inc., JMDC Inc., Shin Nippon Biomedical Laboratories Ltd., Kaken Pharmaceutical Co., Ltd., Advanced Medical Care Inc.; Honoraria – Mitsubishi Corporation, Mitsubishi Chemical Holdings Corporation, Pharma Business Academy
T.Kuroda.: Contracts – Ministry of Health Labour and Welfare (Japan)
M.M.: Grants – JPSP KAKENHI 21K09740; Contracts – Novartis Pharma; Honoraria for lectures, presentations, speakers bureaus – Novartis Pharma, Bayer Pharma, Santen Pharma
S.O.: Honoraria for lectures, presentations, speakers bureaus – Alcon Japan, Santen Pharma, Senju Pharma, Bayer, Novartis Pharma, Nidek
M.T.: Consulting fees – Eisai Co., Ltd.
H.T.: Grants – Findex, Ministry of Health, Labour and Welfare (Japan), JSPS KAKENHIb; Academic consultant fees – Suntory, ASCA corporation; Payment for lectures – Novartis, Otsuka Pharmaceutical; Support for travel – Novartisb, Bayer Yakuhin
A.Tsujikawa.: Grants – Canon, Findex, Santen Pharmaceutical, Kowa Pharmaceutical, Pfizer, AMO Japan, Senju Pharmaceutical, Wakamoto Pharmaceutical, Alcon Japan, Novartis Pharma, Otsuka Pharmaceutical, Bayer Yakuhin, Nitten Pharmaceutical; Consulting fees – Senju Pharmaceutical, Bayer Yakuhin, Novartis Pharma, HOYA, Ellex, MSD, Allegan Japan, Eisai, Daiich-Sankyo, Chugai Pharmaceutical; Honoraria for lectures – Bayer Yakuhin, Senju Pharmaceutical, Novartis Pharma, Santen Pharmaceutical, Alcon Pharma, Alcon Japan, AbbVie GK, AMO Japan, Kowa Pharmaceutical, Canon, Otsuka Pharmaceutical, Wakamoto Pharmaceutical
Financial Support: JSPS KAKENHI grant no. 21K09740. The funding organization had no role in the design or conduct of this research.
Data Availability: The permission to access NDB expired after the authorized research period, so we can no longer access raw data without obtaining access permission again. The raw data can be accessed only after the permission from the MHLW. Those who want to access the raw data will need to apply to the MHLW. The program codes used during the current study are available from the corresponding author on reasonable request.
HUMAN SUBJECTS: Human subjects were included in this study. This retrospective, nationwide population-based cohort study was approved by the Institutional Review Board and by the Ethics Committee of Kyoto University Hospital and Kyoto University Graduate School of Medicine (No. R2035). All investigations were conducted in accordance with the tenets of the Declaration of Helsinki and its later amendments. The requirement for informed consent was waived because of the retrospective nature of the study.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Kido, Miyake, Tamura, Hiragi
Data collection: Kido, Miyake, Tamura, Hiragi, Kimura, Ohtera
Analysis and interpretation: Kido, Miyake, Tamura, Hiragi, Yoshida, Takeuchi, Takahashi, Ooto, Kawakami, Kuroda, Tsujikawa
Obtained funding: Miyake; Study was performed as part of regular employment duties at Kyoto University. No additional funding was provided.
Overall responsibility: Kido, Miyake, Tamura, Hiragi, Kimura, Yoshida, Takeuchi, Ohtera, Takahashi, Ooto, Kawakami, Kuroda, Tsujikawa
Supplementary Data
References
- 1.Flaxman S.R., Bourne R.R.A., Resnikoff S., et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 3.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.GlobalNewswire. Age-Related Macular Degeneration: Global Drug Forecast and Market Analysis to 2028. Vol. 2020. https://www.globenewswire.com/news-release/2020/04/24/2021766/0/en/Age-Related-Macular-Degeneration-Global-Drug-Forecast-and-Market-Analysis-to-2028.html2020. Accessed May 24, 2020.
- 5.Nakata I., Yamashiro K., Nakanishi H., et al. Prevalence and characteristics of age-related macular degeneration in the Japanese population: the Nagahama study. Am J Ophthalmol. 2013;156:1002–1009.e2. doi: 10.1016/j.ajo.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Rim T.H., Kawasaki R., Tham Y.C., et al. Prevalence and pattern of geographic atrophy in Asia: The Asian Eye Epidemiology Consortium. Ophthalmology. 2020;127:1371–1381. doi: 10.1016/j.ophtha.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Nakao S.Y., Miyake M., Hosoda Y., et al. Myopia prevalence and ocular biometry features in a general Japanese population: The Nagahama Study. Ophthalmology. 2021;128:522–531. doi: 10.1016/j.ophtha.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Kido A, Miyake M, Tamura H, et al. Incidence of central serous chorioretinopathy 2011-2018: a nationwide population-based cohort study of Japan. Br J Ophthalmol. 2021 Jul 14;bjophthalmol-2021-319403. doi: 10.1136/bjophthalmol-2021-319403. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 9.Rudnicka A.R., Kapetanakis V.V., Jarrar Z., et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85–93. doi: 10.1016/j.ajo.2015.04.003. e3. [DOI] [PubMed] [Google Scholar]
- 10.Kato G. History of the secondary use of National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) Trans Jpn Soc Med Biol Eng. 2017;55:143–150. [Google Scholar]
- 11.Sugihara T., Yasunaga H., Matsui H., et al. Regional clinical practice variation in urology: usage example of the Open Data of the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Int J Urol. 2019;26:303–305. doi: 10.1111/iju.13840. [DOI] [PubMed] [Google Scholar]
- 12.Kido A., Tamura H., Ikeda H.O., et al. Nationwide incidence of central retinal artery occlusion in Japan: an exploratory descriptive study using the National Database of Health Insurance Claims (2011-2015) BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirose N., Ishimaru M., Morita K., Yasunaga H. A review of studies using the Japanese National Database of Health Insurance Claims and Specific Health Checkups. Ann Clin Epidemiol. 2020;2:13–26. [Google Scholar]
- 14.Matsuda S., Fujimori K. The claim database in Japan. Asian Pac J Dis Manag. 2014;6:55–59. [Google Scholar]
- 15.Tamiya R., Miyake M., Kido A., et al. Validation study of the claims-based definition for age-related macular degeneration at a single university hospital in Japan. Jpn J Ophthalmol. 2021;65:388–394. doi: 10.1007/s10384-021-00816-w. [DOI] [PubMed] [Google Scholar]
- 16.Ministry-of-Internal-Affairs-and-Communications Result of the Population Estimates. https://www.stat.go.jp/english/data/jinsui/2.html
- 17.Miyazaki M., Nakamura H., Kubo M., et al. Risk factors for age related maculopathy in a Japanese population: the Hisayama study. Br J Ophthalmol. 2003;87:469–472. doi: 10.1136/bjo.87.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda M., Kiyohara Y., Hata Y., et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population the Hisayama study. Ophthalmology. 2009;116:2135–2140. doi: 10.1016/j.ophtha.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara K., Yasuda M., Hata J., et al. Prevalence and risk factors for polypoidal choroidal vasculopathy in a general Japanese population: The Hisayama Study. Semin Ophthalmol. 2018;33:813–819. doi: 10.1080/08820538.2018.1506483. [DOI] [PubMed] [Google Scholar]
- 20.Hsu W.M., Cheng C.Y., Liu J.H., et al. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004;111:62–69. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Chen S.J., Cheng C.Y., Peng K.L., et al. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2008;49:3126–3133. doi: 10.1167/iovs.08-1803. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki R., Wang J.J., Ji G.J., et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: the Funagata study. Ophthalmology. 2008;115:1376–1381. doi: 10.1016/j.ophtha.2007.11.015. 81.e1-2. [DOI] [PubMed] [Google Scholar]
- 23.Obata R., Yanagi Y., Inoue T., et al. Prevalence and factors associated with age-related macular degeneration in a southwestern island population of Japan: the Kumejima Study. Br J Ophthalmol. 2018;102:1047–1053. doi: 10.1136/bjophthalmol-2016-309980. [DOI] [PubMed] [Google Scholar]
- 24.Yang K., Liang Y.B., Gao L.Q., et al. Prevalence of age-related macular degeneration in a rural Chinese population: the Handan Eye Study. Ophthalmology. 2011;118:1395–1401. doi: 10.1016/j.ophtha.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Mao F., Yang X., Yang K., et al. Six-year incidence and risk factors for age-related macular degeneration in a rural Chinese population: The Handan Eye Study. Invest Ophthalmol Vis Sci. 2019;60:4966–4971. doi: 10.1167/iovs.19-27325. [DOI] [PubMed] [Google Scholar]
- 26.Park S.J., Kwon K.E., Choi N.K., et al. Prevalence and incidence of exudative age-related macular degeneration in South Korea: a nationwide population-based study. Ophthalmology. 2015;122:2063–2070.e1. doi: 10.1016/j.ophtha.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Park S.J., Lee J.H., Woo S.J., et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014;121:1756–1765. doi: 10.1016/j.ophtha.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 28.You Q.S., Xu L., Yang H., et al. Five-year incidence of age-related macular degeneration: the Beijing Eye Study. Ophthalmology. 2012;119:2519–2525. doi: 10.1016/j.ophtha.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 29.Jin G., Ding X., Xiao W., et al. Prevalence of age-related macular degeneration in rural southern China: the Yangxi Eye Study. Br J Ophthalmol. 2018;102:625–630. doi: 10.1136/bjophthalmol-2017-310368. [DOI] [PubMed] [Google Scholar]
- 30.You Q.S., Choy B.K.N., Chan J.C.H., et al. Prevalence and causes of visual impairment and blindness among adult Chinese in Hong Kong - The Hong Kong Eye Study. Ophthalmic Epidemiol. 2020;27:354–363. doi: 10.1080/09286586.2020.1755444. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki M., Harada S., Kawasaki Y., et al. Gender-specific association of early age-related macular degeneration with systemic and genetic factors in a Japanese population. Sci Rep. 2018;8:785. doi: 10.1038/s41598-017-18487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rim T.H., Yoo T.K., Kim S.H., et al. Incidence of exudative age-related macular degeneration and treatment load under the Korean National Health Insurance System in 2010-2015. Br J Ophthalmol. 2019;103:1361–1366. doi: 10.1136/bjophthalmol-2018-312693. [DOI] [PubMed] [Google Scholar]
- 33.Cheung C.M.G., Ong P.G., Neelam K., et al. Six-year incidence of age-related macular degeneration in Asian Malays: The Singapore Malay Eye Study. Ophthalmology. 2017;124:1305–1313. doi: 10.1016/j.ophtha.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 34.Cheung C.S., Wong A.W., Lui A., et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119:1609–1614. doi: 10.1016/j.ophtha.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Baicker K., Taubman S.L., Allen H.L., et al. The Oregon experiment--effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368:1713–1722. doi: 10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato H., Goto R. Effect of reducing cost sharing for outpatient care on children's inpatient services in Japan. Health Econ Rev. 2017;7:28. doi: 10.1186/s13561-017-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.