Abstract
Major storms can alter coastal ecosystems in several direct and indirect ways including habitat destruction, stormwater-related water quality degradation, and organism mortality. From 2010–2020, ten tropical cyclones impacted coastal North Carolina, providing an opportunity to explore ecosystem responses across multiple storms. Using monthly trawl and contemporaneous seagrass surveys conducted in Back Sound, NC, we evaluated how cyclones may affect the nursery role of shallow-water biogenic habitats by examining seagrass-associated fish responses within a temperate-subtropical estuary. We employed a general before-after-control-impact approach using trawls conducted prior (before) and subsequent (after) to storm arrival and years either without (control) or with (impact) storms. We examined whether effects were apparent over short (within ~three weeks of impact) and seasonal (May-October) timescales, as well as if the magnitude of storm-related shifts varied as a function of storm intensity. Our findings suggest that the ability of these shallow-water habitats to support juvenile fishes was not dramatically altered by hurricanes. The resilience exhibited by fishes was likely underpinned by the relative persistence of the seagrass habitat, which appeared principally undamaged by storms based upon review of available–albeit limited seagrass surveys. Increasing cyclone intensity, however, was correlated with greater declines in catch and may potentially underlie the emigration and return rate of fish after cyclones. Whether estuarine fishes will continue to be resilient to acute storm impacts despite chronic environmental degradation and predicted increases major tropical cyclone frequency and intensity remains a pressing question.
Introduction
Pulse disturbances are defined as strong, short-term perturbations that cause a sudden change in ecosystem structure from which the system can recover when stress has subsided. In contrast, press disturbances are typified as a continuous perturbation or stressor that causes a permanent change in ecosystem structure [1, 2]. Tropical cyclones (e.g., hurricanes and typhoons) are typically characterized as extreme pulse disturbances, and current forecasts predict an increase in major tropical cyclone frequency, intensity, and geographical scope as global climate change continues [3–6]. Combined, these ramping forces have the potential to de-stabilize coastal ecosystems and lead to long-term changes in community structure [7]. Thus, understanding the capacity for resistance and resilience within estuarine ecosystems, particularly seagrass meadows that serve as fish nursery habitats, will be critical for forecasting if and how coastal systems shift under these changing climate scenarios.
Estuarine ecosystems are strongly impacted by a variety of interlinked biotic, geophysical, and socioecological processes from both terrestrial and marine sources to form a highly dynamic environment [8]. Water temperature and salinity are spatially and temporally heterogeneous within an estuary and can change rapidly depending upon depth, tides, weather, and other factors that further affect dissolved oxygen content, nutrient fluxes, and water quality [9]. Seasonal patterns of river discharge and warming add an additional layer of biogeochemical complexity and can dictate productivity across trophic levels [10]. Because of these, estuarine flora and fauna are often adapted to withstand high variability in physical conditions [11, 12]. Still, extreme environmental conditions can stress and degrade estuarine foundation species such as seagrasses, salt marshes, and oysters along with the organisms that rely upon them [13–16]. Physical stress from strong winds, waves, and storm surge associated with hurricanes can scour or erode coastal habitats and flood up-estuary mesohaline areas with sea water, while inland rains can inundate coastal estuaries with high volumes of stormwater runoff laden with terrestrial exports that shift physiochemical regimes over hourly-to-monthly scales [17–19]. Combined, large influxes of either fresh- or saltwater can displace organisms from preferred estuarine habitats and cause localized mortality and increased morbidity that shifts community composition towards more freshwater or marine communities, depending on location within the watershed [20–23].
While some fishes can detect environmental cues associated with approaching storms and respond by temporarily seeking refugia [23–27], cyclone-induced biogeochemical shifts can have disproportionate impacts on juvenile fishes by displacing prey, decreasing body condition, and increasing disease prevalence and morbidity [28, 29]. Studies that have examined the impacts of hurricanes on fish communities in shallow coastal ecosystems including estuaries, coral reefs, seagrasses, mangroves, and other nearshore ocean habitats have typically compared conditions before and after singular storm events (Table 1). These studies have found disparate responses: 27% documented abundance decreases, 18% found abundance increases, 14% recorded shifts in species composition but no change in overall abundance, and 41% found no detectable effect within 6 months of hurricane strike (6, 4, 3, and 9 studies, respectively). Over longer time frames (> 6 months after storm), only 3 of 15 studies observed changes in fish abundance, with two documenting post-storm increases [30] and one finding post-storm declines [28]. Notably, the majority were case studies focused on a single hurricane event (16/22) and only seven examined seagrass-associated fishes.
Table 1. Summary table of studies (n = 22) examining change in fish abundance before and after cyclone.
| Study | Location | Habitat | Year | Saff Simp* | < 6 mo | > 6 mo |
|---|---|---|---|---|---|---|
| Adams 2001 | USVI (Carib. Oce) | Coral Reef | 1995 | 2 | No effect | |
| Adams & Ebersole 2004 | USVI (Carib. Oce) | Coral Reef, Seagrass | 1999 | 4 | No effect | No effect |
| Anton et al. 2009 | Alabama (USA) | Seagrass | 2005 | 4 | No effect | No effect |
| Bortone 1976 | Florida (USA) | Jetty, Estuary | 1975 | 3 | Decline | |
| Bouchon et al. 1994 | Guadeloupe (Carib. Oce.) | Mangrove, Coral Reef, Seagrass | 1989 | 4 | Decline | |
| Burkholder et al. 2004 | North Carolina (USA) | Estuary | 1996, 1999 | 3,TS*, 2, 1 | Decline | No effect |
| Cheal et al. 2002 | Queensland (AUS) | Coral Reef | 1999 | 2 | No effect | No effect |
| Davis & Laird 1976 | Virginia (USA) | Estuary, Seagrass | 1972 | TS | No effect | |
| Dolloff et al. 1994 | North Carolina (USA) | Streams and rivers | 1989 | 4 | No effect | No effect |
| Fenner 1991 | Quintana Roo (MEX) | Coral Reef | 1988 | 5 | No effect | No effect |
| Fitzsimmons & Nishimoto 1995 | Hawaii (Pac. Oce) | Streams and rivers | 1992 | 4 | Decline | No effect |
| Greenwood et al. 2006 | Florida (USA) | Seagrass | 2004 | 4, 2, 3, 3 | No effect | No effect |
| Lassig 1983 | Queensland (AUS) | Coral Reef | 1981 | TS | Assemblage change | |
| Letourneur et al. 1993 | Reunion Island (Ind. Oce.) | Coral Reef | 1989 | 2 | Increase | Increase |
| Locascio & Mann, 2005 | Florida (USA) | Estuary, Seagrass | 2004 | 4 | Increase | No effect |
| Paerl et al. 2001 | North Carolina (USA) | Estuary, River | 1999 | TS, 2, 1 | Decline | Decline |
| Paperno et al. 2006 | Florida (USA) | Seagrass | 2004 | 2, 3 | Decline | No effect |
| Springer & McErlean 1962 | Florida (USA) | Coral Reef | 1960 | 4 | No effect | |
| Stevens et al. 2006 | Florida (USA) | Estuary, Seagrass | 2004 | 4 | Assemblage change | No effect |
| Switzer et al. 2006 | Florida (USA) | Estuary | 2004 | 2,3 | Assemblage change | No effect |
| Yu et al. 2013 | South China Sea (CHN) | Marginal Sea | 2009, 2009 | TS, 1 | Increase | Increase |
| Yu et al 2014 | South China Sea (CHN) | Marginal Sea | 2010, 2012, 2012 | 1, 4, 1 | Increase | Increase |
Literature review of studies that examine fish abundances before and after hurricanes in coral reef, seagrass, estuary, and rocky bottom habitats. Effects were separated into responses that were observed within 6 months of storm passage and those observed after 6 months.
*Saff Simp = Saffir Simpson wind scale category, TS = tropical storm. Citations listed in S1 Appendix.
Estuarine fish communities in the temperate-subtropical transition zone of the western Atlantic (~30-35°N latitude) are comprised in significant part by species that ingress as larvae and spend much of their juvenile life stages in estuaries before migrating offshore as adults [31]. Seagrass meadows in this region are recognized as nursery habitats for their ability to enhance juvenile fish growth and survival [32]. It is hypothesized that seagrasses may be somewhat protected from tropical cyclone wave scour due to storm surge submergence, but increased turbidity, phytoplankton blooms, and sediment deposition post-storm can cause light limitation and subsequent mortality [33]. Studies of hurricane impacts on seagrasses have documented both no effect [34–38] as well as declines in productivity and extent [33, 39–44]. Meadows may recover more quickly if dominated by weedy, fast-growing seagrass species [43, 45], but the amount of time between disturbances may play the deciding role in overall ecosystem trajectory [46]. Long-term data on seagrass meadow cover, extent, and health prior to and post-hurricanes can help to elucidate changes in seagrass incurred from hurricane damage from covariates such as seasonal and interannual variation in productivity and how this relates to nursery habitat provisioning.
Our objective was to determine whether hurricanes affected seagrass and seagrass associated fishes and whether changes were correlated with tropical cyclone intensity. Specifically, we employed before-after-control-impact (BACI) frameworks to determine whether seagrass habitat, seagrass-associated fish catch, species richness, and community structure were impacted by hurricanes (winds ≥64 knots), with separate analyses to examine the effect of cyclones that made landfall during early (June-July), peak (August-September), and late (October-November) Atlantic basin hurricane season. We then examined whether increasing cyclone intensity was correlated with greater change in catch and richness for all tropical storms (winds ≥34 knots) and hurricanes to impact our area. Finally, to examine the potential longevity of responses, we examined whether storm impacts were evident over short (~three weeks) and/or longer (seasonal) timescales. We hypothesized that habitat persistence would underlie fish community dynamics [47–49]; thus, if local seagrass meadows were largely unaffected by tropical cyclones, fish communities would also be stable or resilient.
Methods
Tropical cyclones and surveys
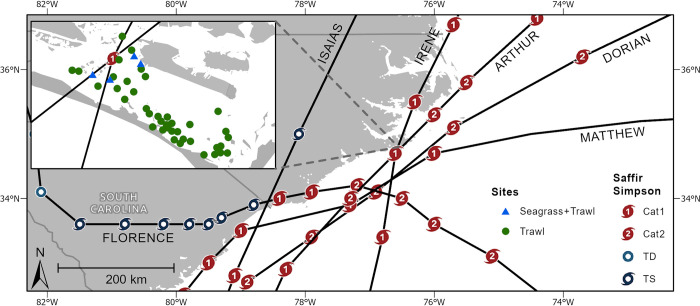
In the Atlantic basin, hurricane season occurs from June 1-Nov 30, with greatest activity occurring between mid-August to mid-October [50]. Our study focuses on the impact of tropical cyclones on seagrass meadows and seagrass-associated fishes within a temperate-subtropical embayment—Back Sound, North Carolina, USA (34° N to 34°39′ N, 76°37′ W to 76°31′ W)—that is among the most frequently cyclone-impacted locations within the continental United States. Back Sound is a shallow (mean depth ~2 m), lagoonal ecosystem enclosed by barrier islands to the south and east (Fig 1). Since 2010, 10 named storms have affected the study area with tropical storm force winds and above (≥34 knots & Table 2). Of these, six made landfall along or struck (came within 65 nautical miles, Fig 1) the study area with at least Saffir Simpson Category 1 (≥64 knot) winds: Hurricanes Irene (8/27/2011), Arthur (7/4/2014), Matthew (10/9/2016), Florence (9/14/2018), Dorian (9/6/2019), and Isaias (8/4/2020). These six hurricanes caused a combined ~30 billion dollars (USD) in storm-related damages to commercial, residential, and agricultural infrastructure within the state [51], with Hurricanes Matthew and Florence also producing 100–1000 year flood events [19]. Rainfall anomalies used in analyses were calculated as the difference between observed year-to-date rainfall and the 30-year (1991–2020) year-to-date normals for NWS Station 315830 [52, 53].
Fig 1. Major hurricane tracks over coastal North Carolina from 2010–2020.
Tracks are based on best track data from HURDAT2 [54]. Cyclone symbology represents hurricane location and Saffir Simpson category at 6-hour intervals (TD = tropical depression, TS = tropical storm). Inset map depicts study area with green circles indicating trawl survey locations and blue triangles indicating seagrass survey locations. Map sources: Esri, HERE, Garmin, SafeGraph, Meti/NASA, USGS, EPA, NPS, USDA.
Table 2. North Carolina tropical cyclone and control year metrics 2010–2020.
| Year | Name/ Treatment | Impact Date | ACE-NC | Winds (kt) | Gusts (kt) | Storm Surge (m) | Rainfall (cm) | Analyses |
|---|---|---|---|---|---|---|---|---|
| 2010 | TS Earl | 3-Sep | 2.59 | 25.27 | 30.71 | 0.17 | 1.25 | Intensity |
| 2011 | Irene | 27-Aug | 5.09 | 33.82 | 46.07 | 0.88 | 22.23 | mBACI, Comm, Intensity |
| 2012 | TS Beryl | 30-May | 1.12 | 26.24 | 31.49 | 0.14 | 7.34 | Reg |
| 2013 | No Storm | 14-Sep | mBACI, Comm | |||||
| 2014 | Arthur | 4-Jul | 5.47 | 46.85 | 60.26 | 0.73 | 5.13 | BACI |
| 2015 | No Storm | 4-Jul, 6-Sep | mBACI, Comm BACI (v. Arthur) | |||||
| 2016 | TS Hermine | 3-Sep | 1.27 | 25.66 | 34.41 | 0.44 | 1.65 | Intensity (short-term) |
| 2016 | Matthew | 9-Oct | 4.23 | 38.88 | 57.15 | 0.64 | 3.33 | BACI |
| 2017 | No Storm | 8-Aug, 9-Oct | mBACI, Comm BACI (v. Matthew) | |||||
| 2018 | Florence | 14-Sep | 12.44 | 51.32 | 71.53 | 1.45 | 29.19 | BACI, Comm, Intensity |
| 2018 | Michael | 11-Oct | 2.63 | 34.76 | 46.51 | 0.27 | 0.91 | Int (short-term) |
| 2019 | Dorian | 6-Sep | 3.79 | -- | 73.86♠ | 0.63 | 14.30 | mBACI, Comm, Intensity |
| 2020 | Isaias | 4-Aug | 1.59 | 42.36 | 52.28 | 0.32 | 3.40 | mBACI, Comm, Intensity |
Characteristics of tropical cyclones and no-storm years used in the listed analyses. Impact date represents date of first arrival of tropical storm force winds. For no storm years/treatments, dummy impact dates were assigned in order to bisect data into time periods and chosen to generally align with observed stormfall dates. ACE-NC = Accumulated Cyclone Energy calculated for the span of time in which the cyclone directly impacted North Carolina. TS = tropical storm as defined by the Saffir Simpson wind scale. Unless otherwise stated, analyses examined both short-term and seasonal time frames. mBACI = multiple Before-After-Control-Impact analysis of Hurricanes Irene, Florence, Dorian, and Isaias, BACI = individual comparison of either Hurricane Matthew or Hurricane Arthur against a single no-storm year, Comm = community structure analyses, Intensity = tobit or linear regression of change versus ACE-NC of all cyclones of tropical storm strength or greater.
Water levels, winds, and gusts were obtained from National Oceanic and Atmospheric Administration (NOAA) Station 8656483: Beaufort, Duke Marine Lab, North Carolina, USA (34°42’58"N 76°40’15"W). This station records 6 min. intervals for water levels & wind speeds; maximum recorded values are reported. Storm surge is calculated as the difference between predicted and measured tide. Rainfall data obtained from NOAA National Weather Service (NWS) Station 315830 in Morehead City, North Carolina, USA (34°42’58"N 76°40’15"W) (NOAA n.d., accessed 3 Feb 2021).
♠ Wind speeds were not available from the Station 8656483 for much of 2019 (i.e., Hurricane Dorian). Value reported obtained from the NOAA Citizen Weather Observer Program in Beaufort, North Carolina, USA.
During 2010–2020, we surveyed fish communities within seagrass meadows in Back Sound, North Carolina (Fig 1) using a 5-m wide otter trawl (5-m head rope, 2-cm mesh size, 0.6-cm cod end mesh) with no tickler chain [55] on a monthly basis from April-November of each year as part of a long-term ecological study. During each monthly sampling interval, at least six seagrass meadows were trawled. Supplemental trawl sampling was conducted from 2010–2016 as part of separate hypothesis-testing studies [56]. At each meadow, two, 2-min tows were completed, with the entire length of each tow conducted within the meadow. Total travel distance for each tow was recorded based on measurements using a Garmin handheld GPS unit (Garmin International, Olathe, Kansas, USA) and averaged 111 m. All tows were conducted within three hours of a diurnal high tide. After each trawl, all fishes were identified to species, enumerated, and weighed. Because the overwhelming majority of fishes caught in trawls are sizes that would constitute juvenile life stages, we assume that catches are synonymous with nursery role metrics [55]. Surface salinity and water temperature were recorded for each tow using handheld refractometers and thermometers, respectively.
Seagrass surveys were not designed with the current study or long-term fish monitoring survey in mind. Therefore, seagrass surveys were not conducted simultaneously with trawls and do not always match trawl sampling frequencies. However, contemporaneous monthly surveys of seagrass percent cover were performed for related studies at four meadows (designated as SG1-SG4, Fig 1, S1 Table) that were also trawled in May-October in 2016 and from April-September in 2019. In addition, seagrass surveys were conducted at SG2 and SG3 in August and October of 2013 and in May and July of 2014. All surveyed seagrass meadows are mixed species (Zostera marina, Halodule wrightii, and occasionally Ruppia maritima); however, SG1 is dominated by Z. marina, and SG2-4 is dominated by H. wrightii. Seagrass surveys were conducted using in situ visual estimates of total seagrass percent cover every 2 or 5 m within a quadrat ranging from 0.0625–0.25 m2 along a 50-m belt transect located entirely within the meadow. Seagrass surveys conducted in 2016 and 2019 occurred over permanent transects; whereas, surveys in 2013 and 2014 were randomly assigned within the meadow. Seagrass species dominance was calculated as the log ratio of the percent cover of Z. marina over the percent cover of H. wrightii + R. maritima.
Animal and field research was conducted under the North Carolina Division of Marine Fisheries permit # 706481 and University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee protocols # 10–114.0, 13–109.0, and 13–130.0.
Statistical approach
Given the multitude of ways that fishes and fish community structure may respond to a perturbation as well as confounding factors associated with timing of storm landfall relative to patterns of fish ingress/egress into estuaries, we employed several analytical approaches, data sub-setting, and data transformations (Table 1, S1 Fig). Short-term responses were limited to trawls conducted within a time span of 23 days before and after impact. This window allowed us to compare fish communities observed most proximate to storm fall while (a) preserving balanced sample sizes across treatments and (b) matching the “days between storm event and fish sampling” in control versus impact years. While this temporal window was selected largely out of logistical necessity rather than a priori hypotheses per se regarding duration of impact, exploring storm impacts within the first ~three weeks post-storm-passage is in line with other studies that have documented short-term responses [28, 57, 58]. Seasonal-scale analyses included all trawls conducted from May-October bisected by storm date, which tended to balance sample sizes between control and impact years given the extended temporal scope and monthly periodicity of trawling.
We presumed that trawls conducted in different years were independent when comparing impact and no impact years, as a new cohort of fishes arrives in the estuary each spring. Because the most abundant fish species, Lagodon rhomboides (pinfish), can comprise the majority of trawl catches in North Carolina seagrass meadows (>80%), we calculated two separate metrics of catch per unit effort (CPUE; fish per 100 m towed): one using the entire trawl catch and the other sans L. rhomboides (hereafter, CPUE and CPUE-Lr, respectively).
mBACI and BACI
To determine if hurricane passage had an effect on seagrass-associated fish catch rates, species richness, and community structure; we employed a de facto multiple before-after-control-impact (mBACI) design following the framework outlined in Hogan et al. (2020), Gericke et al. (2014), Wauchope et al. (2020), and [57, 59, 60]. In this mBACI, we defined “impact” or “storm” years as only those where Saffir Simpson Category 1 or greater windspeeds were observed in the Back Sound study area (Table 2, S1 Fig) during the months of August and September to distinguish hurricanes from ordinary storm events and minimize confounding effects associated with fish ingress/egress. This designated 2011, 2018, 2019, and 2020 as impact/storm years. Years considered as “controls” or “no storm” were defined as those where no tropical storm or cyclone remotely threatened the study area throughout the entire hurricane season (June-November) and included 2013, 2015, and 2017. Years where only tropical storms affected the study area (2010 and 2012) were excluded from mBACI analyses, as environmental impacts of tropical storms are difficult to distinguish from storm events associated with low pressure fronts. Similarly, tropical storms that occurred in the same year as a major hurricane (Hermine in 2016 and Michael in 2018) were not directly examined in the mBACI, as their effects could not be partitioned from the effects of the major hurricane (Matthew in 2016 and Florence in 2018). Within each year, we considered sampling prior to storm events as “before”, and sampling subsequent to storm passage as “after” data. For control years in the mBACI analysis, we applied dummy storm dates of 09/14/2013, 09/06/2015, and 08/08/2017, to designate before- and after periods. These dates were chosen to generally align with observed landfall dates in our dataset (e.g. Florence 09/16/2018, Dorian 09/06/2019, and Isaias 08/04/2020) as well as with peak hurricane season in North Carolina [54].
We conducted two separate BACI analyses to consider the effects of an early- (before August) and a late (after September) storm landing and adjusted our dummy storm dates to match the corresponding hurricane impact date. For these analyses, Hurricane Arthur (7/4/2014) was compared against 2015 (control; dummy storm date: 7/4/2015), and Hurricane Matthew (10/9/2016) was tested against 2017 (control; dummy storm date: 10/9/2017). For both BACIs, we again considered sampling prior to storm arrival as before-impact data, and surveys after storm passage to represent after-impact data.
ANOVA
We used Analysis of Variance (ANOVA) in R (version 4.0.0) [61] to test for the main and interactive effects of time period (before/after) and year type (control/hurricane) on fish CPUEs and species richness for our mBACI and BACI approaches. This approach is based on the expectation that temporal changes (i.e., short-term or seasonal-scale before-after comparisons, separately) during hurricane years could be statistically different from temporal changes that occur during non-storm years [62]. In this context, the ANOVA term of most interest is the potential interaction between ‘time period’ and ‘year’ [63, 64]. Generating these tests for all possible combinations of hurricane contexts (N = 3; multi-storm mBACI, Arthur BACI, and Matthew BACI), response windows (N = 2; short-term and seasonal), and univariate response metrics (N = 3; CPUE, CPUE-Lr, richness) would result in 18 distinct analyses of means (S1 Fig). We note, however, that we could not run short-term comparisons of means for Hurricane Matthew as the closest trawls conducted prior to Hurricane Matthew were conducted 48 days before storm fall and therefore outside of our short-term temporal window.
While ANOVA is largely robust to violations of normality, data transformations were required in several instances to meet parametric assumptions of homoscedasticity (S1 Fig), which was assessed graphically and accepted when group variances were no larger than 1.5x [65]. Given the large sample size within each factor and group (n = 93 to 239 trawls), parametric tests should be robust to unbalanced sample sizes. As part of our exploratory data analysis, we also examined whether surface water temperatures and salinities taken in concert with trawls differed across BACI treatments.
Trend analyses
We also conducted time series analyses on seasonal-scale data to explore differences within the mBACI framework and Arthur-specific BACI [60, 66]. Because many time series exhibit patterns through time independently of perturbations, it is possible for fish communities to respond to hurricanes not only by exhibiting a change in mean but with a shift in overall trend. We did not run temporal models for short-term mBACI or for Hurricane Matthew BACI comparisons as surveys were frequently all conducted within one or a few days within the shortened time frames which did not provide enough samples for accurate trend predictions or account for co-occurring effects of seasonality in fish communities.
For each time period-year type treatment and fish metric for the seasonal time frame mBACI, we ran separate generalized additive models (GAMs) against days since storm as the only predicting variable (mgcv library version 1.3–31 [67, 68]). Models were predicted over the temporal range -119 to 80 days since storm for the mBACI and -46 to 115 days since storm for the Arthur-specific BACI and built using a cubic regression spline with penalized shrinkage, a maximum of three degrees of freedom (knots), negative binomial error distribution with log link function, and restricted maximum likelihood (REML) smoothing parameter (y ~ s(Days since Storm), k = 3). For the Arthur-specific trend analysis, we ran negative binomial generalized linear models (GLMs) due to fewer sampling dates during “before” periods. Pairwise differences between control and hurricane-year trends were calculated from predicted model smooths with a 95% confidence interval. Where difference calculations did not overlap zero, we inferred significant difference between control- and hurricane year trends.
Community structure
We followed the mBACI framework and conducted non-metric multidimensional scaling (NMDS) to examine short-term and seasonal fish communities for Hurricanes Irene, Florence, Dorian, and Isaias combined compared to 2013, 2015, and 2017 combined (Table 2, vegan library version 2.5–6) [69]. We did not compare community structure for pairwise BACI comparisons (i.e., Hurricanes Matthew and Arthur) due to lack of sample size. Community structure analyses were based on a Bray-Curtis extended distance similarity matrix of fish species abundance per trawl sample. Prior to distance calculations, we removed rare species (abundance ≤ 1) [70] and trawls that caught only one fish from the dataset before applying a fourth-root transformation to reduce the influence of extremely abundant species and zero-inflation. A similarity percentages analysis (SIMPER) was used to identify which taxa likely contributed the most to dissimilarity across BACI groups (vegan::simper) [69]. Fish community structure was tested for multivariate homogeneity of group dispersions (PERMDISP) before testing for differences among groups using permutational analysis of variance (PERMANOVA). Water temperature, salinity, days since storm, storm intensity, max gusts, max windspeeds, storm surge, antecedent rainfall, antecedent rainfall anomaly, and storm rainfall were tested for correlation with community ordination and plotted when p < 0.10.
Tropical cyclone intensities and change
We also gauged how tropical cyclone intensity correlated with storm-coincident shifts in catch and species richness. For this analysis, we included most tropical cyclones that impacted the study area as tropical storms or above on the Saffir Simpson wind scale and examined proportional change in catch and raw change in richness at both short-term (n = 9 storms) and seasonal scales (n = 8 storms). Hurricane Hermine (2016) was excluded from short-term change calculations as no trawl surveys were conducted within a month after impact. Tropical storms Hermine and Michael (2016 and 2018, respectively) were not directly examined in seasonal change comparisons as both (a) reached North Carolina over central portions of the state while transitioning from tropical storms into extratropical cyclones, and (b) occurred in years with other more intense local storms that were used to bisect before and after periods (Table 2).
To isolate the effects of the storm on our study area, we calculated Accumulated Cyclone Energy (ACE) using wind speeds and the duration of time in which the cyclone directly impacted North Carolina. We used ACE as our intensity metric as it more accurately captures the effect and relative difference of a slow- versus fast-moving storm [71, 72]. For this analysis, we calculated proportional change in catch (CPUE and CPUE-Lr) and ran tobit regression models censored at -100% decline against ACE (Table 2, VGAM version 1.1–5, VGAM::vglm). We ran linear regressions of raw change in richness (before-storm minus after-storm data) versus ACE, as richness values were low and averages only ranged between six to eight species.
Seagrass temporal patterns
Surveys of seagrass percent cover were not conducted at regular intervals; therefore, they do not fit the BACI framework criteria [73]. Instead, mean seagrass percent cover as a function of time is presented with descriptive rather than inferential statistics to provide available context regarding habitat condition before-and-after storms, as well as potential differences in cover between storm versus non-storm years. For descriptive seagrass statistics, we calculated mean percent cover per meadow for each month and year of observation. Similar to previous fish BACIs, we coded control years as those where no storm threatened the study area (2013) and impact years as those where Saffir Simpson Category 1 winds were observed as hurricane years (Arthur 2014, Matthew 2016, and Dorian 2019) bisected by storm date/dummy storm date (Table 2). Only hurricane-year observations were available for meadows SG1 and SG4 (S1 Table).
Results
Short-term responses in fish metrics in mBACI design
We found no interactive effect of time period and year type on CPUE, CPUE-Lr, or species richness in our mBACI comparisons (ANOVA pperiod*year type = 0.122 & pperiod*year type = 0.109, pperiod*year type = 0.087, respectively, Fig 2A–2C, S2 Table), suggesting that on average, fish catches and richness were not altered by hurricanes. Mean change in CPUE and CPUE-Lr between periods were nearly identical in control and hurricane years (-127 ± 69 & -111 ± 37 CPUE and -4 ± 10 & -22 ± 5 CPUE-Lr, respectively, S3 Table). However, this shift in CPUE-Lr represented a much larger proportional decline in hurricane years (-11% control, -43% impact). Mean richness was higher in hurricane years regardless of period (ANOVA pyear type = 0.001, 8.5 species before and 7.5 species after) compared to control years that demonstrated a slight increase in mean species between periods (6.6 species before and 6.9 species after). Water temperatures and salinities taken in concert with trawls did not differ across mBACI groups (ANOVA pperiod*year type = 0.201, pperiod*year type = 0.792, respectively, S2 Table).
Fig 2. mBACI of short-term fish catches, species richness, and community structure across time periods and year type.
Only means are presented for short-term comparisons of catch per unit effort (A); catch per unit effort calculated sans L. rhomboides (B), and species richness (C). P-values indicate the significance of the ANOVA interaction term. Error bars represent standard error. Non-metric multidimensional scaling (D) of short-term seagrass-associated fish communities based on fourth-root Bray-Curtis extended distances of abundance. Gray coloration and circles indicate control years; blue coloration and triangles indicate impact years. Open symbols indicate before periods and filled symbols indicate after periods. Environmental correlates (p<0.1) are plotted as vectors in the direction of ordination influence. Orange symbols indicate group centroids.
Fish communities observed within the short-term timeframe differed across time periods and year types (PERMANOVA Pperiod*year = 0.002); and the shift in community structure across year type and time periods was not attributable to differences in within group dispersion (PERMDISP F3, 160 = 1.0605, P = 0.3676). However, we found a strong degree of overlap in community ordination across groups using non-metric multi-dimensional scaling (Fig 2D, Stress = 0.22, S2 Fig). Water temperature was the only variable significantly related to ordination and was more strongly correlated with NMDS2 than NMDS1 (p = 0.006, NMDS R2 = 0.208, S5 Table). Changes in L. rhomboides, Orthopristis chrysoptera (pigfish), Gerreidae spp. (mojarra species) abundances consistently contributed the most to community dissimilarities, but all four taxa were common in trawls across years and before-or-after storm contexts (S6 Table).
Seasonal responses in fish metrics in mBACI design
We found that CPUE was not statistically different across time period and year type, but CPUE-Lr and species richness were (CPUE pperiod*year = 0.151, CPUE-Lr pperiod*year = 0.009, Richness pperiod*year = 0.046, Fig 3A and 3B, S7 Table). Despite the statistical significance of these metrics, mean changes between periods suggest small to negligible shifts in raw fish counts and richness. On average, seasonal CPUE and CPUE-Lr declined by comparable amounts between periods in both control and hurricane years (-207 ± 32 and -153 ± 18 CPUE, and -22 ± 4, -33 ± 5 CPUE -Lr, S4 Table). Proportionally, this represented only a 1% greater decline in CPUE and a 14% greater decline in CPUE-Lr during hurricane years than during non-storm years. Mean richness across seasons also did not exhibit a distinct ecological shift and ranged from 6.1 to 6.7 species per tow regardless of time period or year type. At the seasonal timescale, water temperatures and salinities taken in concert with trawls also did not differ across mBACI groups (ANOVA pperiod*year type = 0.201, pperiod*year type = 0.792, respectively, S2 Table).
Fig 3. mBACI of seasonal fish catches, species richness, and community structure across time periods and year type.
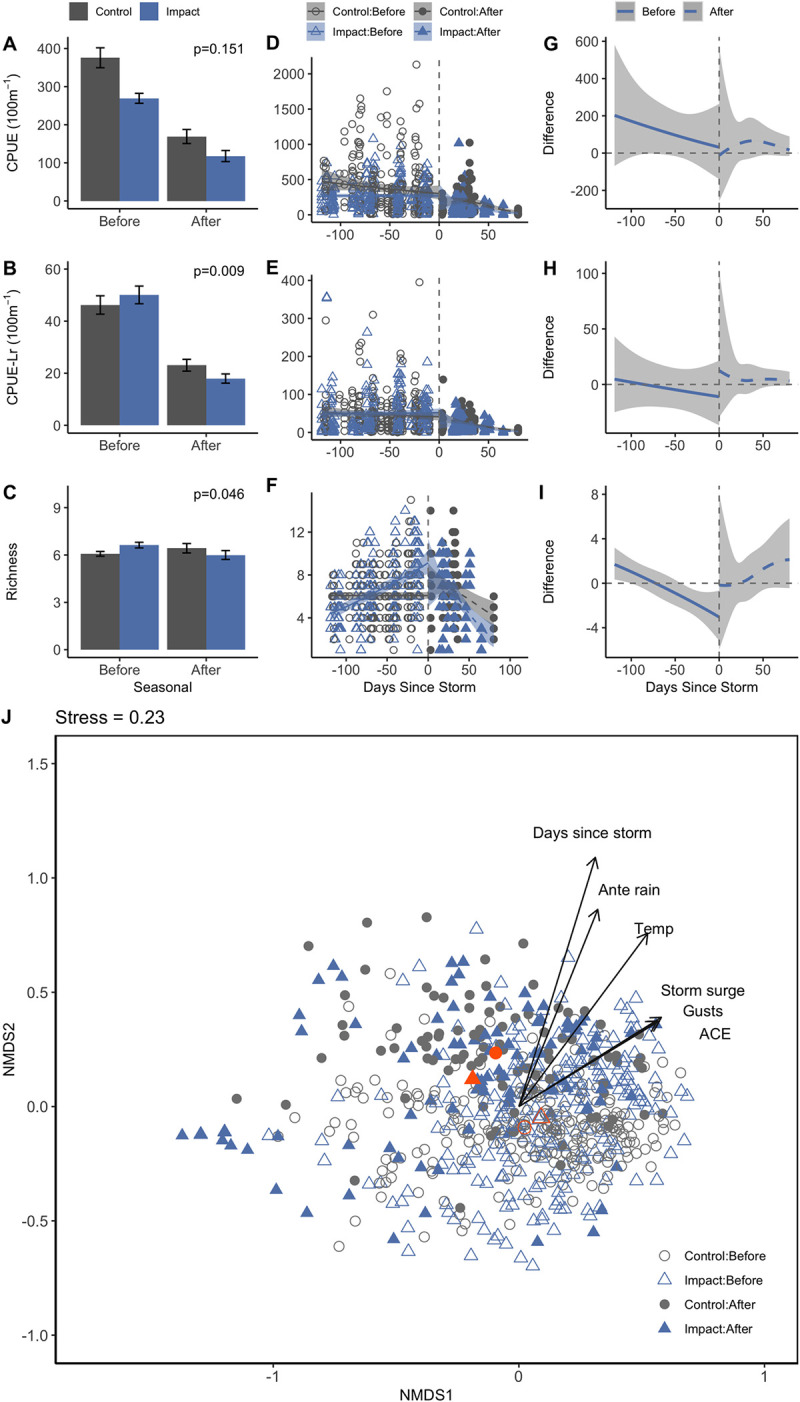
Means (A-C), trend (D-F), and difference between control and impact trends (G-I) are depicted for seasonal comparisons. Confidence intervals and error bars represent 95% confidence and standard errors, respectively. Smoothed lines represent generalized additive models across the seasonal time frame (y ~ s(Days to Storm), k = 3) for both hurricane and storm-free years based on a cubic regression spline with shrinkage. Non-metric multidimensional scaling of seasonal seagrass-associated fish communities (J) are based on fourth-root Bray-Curtis extended distances of abundance. Gray coloration and circles indicate control years; blue coloration and triangles indicate impact years. Open symbols indicate before periods and filled symbols indicate after periods. Environmental correlates (p<0.1) are plotted as vectors in the direction of ordination influence. Orange symbols indicate group centroids.
Based on the GAM analysis, catch trends (CPUE and CPUE-Lr) did not differ across control and hurricane years (Fig 3D and 3E). Notably, CPUE, CPUE-Lr, and richness trends in after periods consistently overlapped and exhibited similar model directions and shapes (p ≤ 0.001 for all “after” period models, Fig 3G–3I). The smoothing term, days since storm, did not significantly explain CPUE or CPUE-Lr trends during “before” periods of hurricane years (p = 0.356, p = 0.426, S8 Table) or CPUE-Lr and species richness trends during “before” periods of control years (p = 0.129, p = 0.466). Species richness trends during “before” periods exhibited dissimilar trends across year types with richness trending steady at roughly six species during control years and increasing from roughly four to nine species during hurricane years (Fig 3F and 3I).
Fish communities observed across the seasonal timeframe differed across time periods and year types (PERMANOVA Pperiod*year = 0.001, Fig 3J); and the shift in community structure across year type and time periods was attributable to differences in within group dispersion (PERMDISP F3, 647 = 7.7587, P < 0.001). However, there was a large degree of overlap across mBACI groups with communities differing primarily across time period observed and not year type (S3 Fig). Days since storm, and antecedent rainfall significantly influenced community ordination along NMDS2 (p = 0.001 & R2 = 0.554, p = 0.001 & R2 = 0.351, respectively, S5 Table). Similar to the short-term analyses, L. rhomboides, O. chrysoptera, Gerreidae spp., and Leiostomus xanthurus (spot), contributed the most to community dissimilarities but all four were common across year types and time periods (S6 Table).
Early- and late-season cyclones in BACI design
When comparing Hurricane Arthur (July 2014) to an analogous control year (2015), we found no interactive effect of time period or year type on any univariate fish metric across the short-term (CPUE pperiod*year = 0.210, CPUE-Lr pperiod*year = 0.271, Richness pperiod*year = 0.797, S3 Table, S4 Fig) or seasonal scale (CPUE pperiod*year = 0.614, CPUE-Lr pperiod*year = 0.068, Richness pperiod*year = 0.519, S7 Table, S4 Fig). Similarly, we found that CPUE, CPUE-Lr, and species richness trends in 2014 did not differ compared to 2015 (S9 Table, S4 Fig).
When comparing Hurricane Matthew (October 2016) to 2017 as the control year, we found an interactive effect of time period and year type on seasonal CPUE but not CPUE-Lr or species richness (CPUE pperiod*year = 0.011, CPUE-Lr pperiod*year = 0.364, Richness pperiod*year = 0.136, S7 Table, S5 Fig). The statistical significance of CPUE across groups was likely driven by the much larger average catch observed before Hurricane Matthew (277 ± 29 fish 100 m-1 S4 Table) compared to the same period in 2017 (94 ± 17 fish 100 m-1), as mean catches during after periods were not substantially different between year types (33 ± 9 fish 100 m-1 after Matthew and 28 ± 5 fish 100 m-1 “after” 2017).
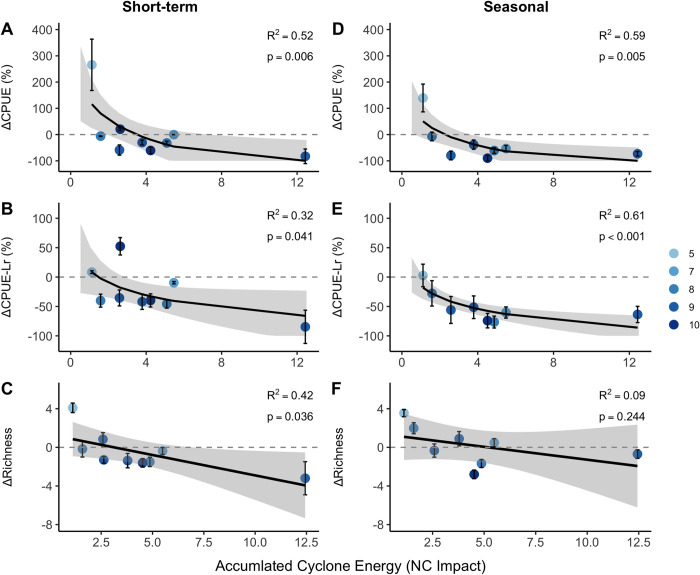
Changes corresponding with cyclone intensity
We found that accumulated cyclone energy was positively correlated with the amount of change in CPUE, CPUE-Lr, and species richness at both the short-term and seasonal time frame, with more intense storms associated with greater declines in each of these metrics (Fig 4). This relationship was particularly pronounced for changes within short-term time frame (R2CPUE = 0.52 & p = 0.006; R2CPUE-Lr = 0.33 & p = 0.041; R2richness = 0.42 & p = 0.036, Fig 4A–4C). CPUE and CPUE-Lr consistently decreased after cyclone impact with two notable exceptions. The only storms where CPUE and CPUE-Lr demonstrated increases were 1) after Tropical Storm Beryl, which was the weakest cyclone and occurred in late spring (5/30/2011), coinciding with seasonal fish ingress into the estuary, and; 2) after Tropical Storm Michael (10/11/18), which occurred less than one month after Hurricane Florence on 9/14/2019. Florence was the strongest storm that impacted Back Sound over the study duration (substantially higher in ACE, rainfall, and storm surge than all other cyclones in our study) and was associated with the greatest short-term decline in CPUE, CPUE-Lr, and species richness, but remained comparable in effect to storms of much lower intensity at the seasonal time frame.
Fig 4. Change in fish metrics as a function of cyclone (tropical storms and hurricanes) intensity.
Short-term changes are depicted column 1, and seasonal-scale changes are represented in column 2. A & D) Proportional change in catch per unit effort. B & E) Proportional change in catch per unit effort sans L. rhomboides, and C & F) change in raw species richness. Models of CPUE and CPUE-Lr represent a tobit regression censored at -100%, and linear regressions are plotted for change in raw species richness. Model shading indicates 95% confidence intervals also censored at -100% for catch metrics. Point coloration (light blue to dark blue) indicates increasing month of impact.
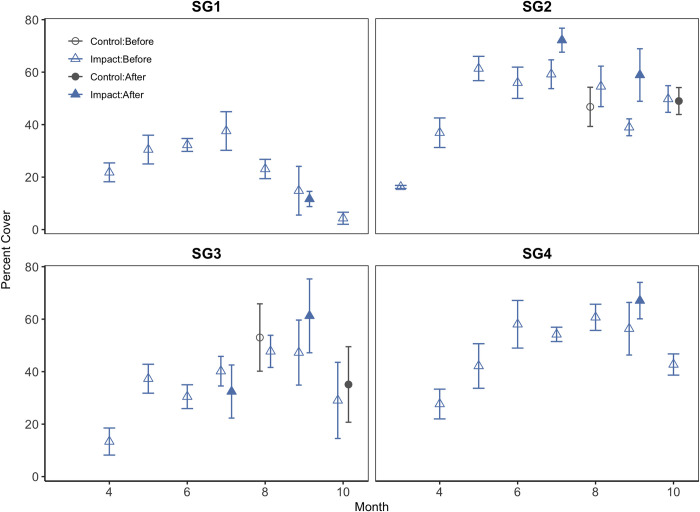
Seagrass trends
Seagrass percent cover displayed consistent seasonal trends associated with productivity patterns of the dominant species within the meadow regardless of year type (Fig 5, S1 Table). Overall, percent cover at surveyed meadows appeared to be stable from 2014–2019. Given the non-standardized sampling frequency, percent cover at SG1 (a meadow that is Z. marina-dominated) after hurricane impact in September 2019 did not differ noticeably from measurements obtained in September 2016 before Hurricane Matthew (before impact). At sites where H. wrightii is the dominant seagrass species (SG2-SG4), percent cover of seagrass during the month of September was often greater after impact (September 2019, after Hurricane Dorian) than that observed prior to impact (September 2016, before Hurricane Matthew). This divergence was greatest at site SG2 where cover averaged 39 ± 3% in September 2016 and 59 ± 10% in September 2019. We also did not detect a consistent effect of hurricanes on seagrass cover during the month of July. Percent cover after Hurricane Arthur in July 2016 was greater at SG2 but slightly less at SG3 compared to average measurements taken in July prior to storms in 2016 and 2019.
Fig 5. Trends in seagrass percent cover by month and BACI treatment for individual meadows.
Gray coloration and circles indicate control year treatments; blue coloration and triangles indicate hurricane years. Open symbols indicate measurements taken before hurricanes, and filled symbols indicate measurements taken after storms.
Discussion
Coastal ecosystems have evolved and adapted to withstand a wide array of environmental conditions and periodic disturbances due to natural climate variability [74]. However, given the likelihood of increasing storm intensity as a result of climate change [75, 76], it is pivotal to consider how these acute pulse disturbances, from which ecosystems can recover, may compound to function more similarly to chronic, press disturbances that push the system towards an alternative state [1, 2]. Hurricanes are predicted to increase in both rainfall and windspeed and potentially impact larger geographical swaths, leading to more devastating impacts across socio-ecological systems [77]. Our study evaluates how seagrass-associated fish communities within a temperate-subtropical estuary along the western Atlantic Ocean have responded after a 10-year period with 10 tropical cyclones. Overall, our findings indicate that fishes were generally resistant to storm impacts across multiple tropical cyclones, with no substantial differences in short-term or seasonal patterns of catch or community structure during storm versus no-storm years. However, increasing storm intensity was correlated with declines in fish catches at the short-term and seasonal scale with greater proportional changes in catches calculated sans L. rhomboides. The overall stability of the fish community was likely underpinned by seagrass meadow persistence, indicating an ability to provide nursery habitat despite multiple notable disturbances. However, if hurricanes increase in intensity, duration, or frequency, it may lead to long-term, large-scale ecosystem shifts that are indicative of press rather than pulse disturbances.
The physical impact of hurricanes can be patchy across space and cause extensive damage in some areas and leave others unaltered [36]. Estuarine seagrass meadows may be more susceptible to negative indirect effects of stormwater runoff and retention, whereas meadows without offshore barrier islands or coral reefs are more likely to be impacted by physical wave scour [78]. Indeed, studies have documented variable impacts on seagrass meadows after tropical cyclones and severe storms including no effect, change in species composition, faster growth, and both long and short-term declines in area [37, 42, 45, 79, 80]. Provided the disturbance does not cause severe habitat degradation, fishes may rapidly repopulate areas [58, 81–83]. We did not detect an obvious decline in seagrass percent cover at four meadows in our system, which may have buffered against drastic shifts in fish community catches and structure. However, we note that this is a limited representation of general seagrass trends across the estuary and long-term surveys are needed to assess habitat trends over time [84]. Seagrass meadows in the temperate-subtropical estuaries of North Carolina are composed of a mix of “weedy” or fast-growing species (i.e. H. wrightii and R. maritima) that can quickly recolonize after disturbance as well as Z. marina that utilizes a mixed-annual reproductive strategy that may enhance seagrass resistance and recovery from physical stressors [85–87]. Given that our trawls were conducted entirely within the seagrass meadow, our surveys may be less sensitive to changes in overall seagrass area. Our results demonstrate that the unit-area function of seagrass persisted [32], but estimates of the overall change in meadow area from 2010–2020 would provide a clearer indication of regional ecosystem resistance and recovery to pulse stressors [88].
In general, estuarine communities are capable of withstanding shifts in salinity [89–91]; however, resilience and ability to adjust to rapidly decreasing or sustained anomalous salinity varies by species [92, 93] and magnitude of perturbation. Antecedent meteorological conditions, such as drought, excessive rainfall, and/or sustained high water temperatures prior to cyclones can affect habitat quality and nursery function prior to hurricane impact. We found that seasonal community structure was most strongly affected by temperature, days since storm, antecedent rainfall, and storm intensity characteristics. However, all environmental covariates were only weakly correlated with community structure, and communities were largely similar irrespective of storm impact. Indeed, previous studies have documented post-hurricane shifts in estuarine nekton species towards both more oligohaline species [22] as well as brackish species [21]. Water temperatures and salinities taken in concert with trawls were not different across impact or time period in our study (S2 Table); however, all trawls conducted post-hurricane were 5 or more days after passage, at which time salinity signals may have dissipated due to our study site proximity to inlets and oceanic influences.
Our findings generally align with the majority of studies (Table 1) that found no distinct effect of hurricane(s) on fish community structure. Fishes that occupy inshore seagrass meadows as juveniles and spend their adult life stages offshore may be buffered from cyclones due to the coinciding timing of migrations to deeper waters offshore with peak hurricane season in North Carolina [27]. We did not find a strong effect of seasonal timing on the overall impact of the storm on fish catch and community structure that was markedly different from control years. However, seasonality may have a different effect in tropical regions where patterns of fish abundance do not fluctuate as substantially. Catches increased in the one-month period between Hurricane Florence (Sept 14) and Tropical Storm Michael, which indicates that despite the effects of Tropical Storm Michael on top of the notable negative impact of Hurricane Florence, fishes were actively repopulating seagrass meadows during peak Atlantic hurricane season. Studies have suggested that storms and other large disturbances may alleviate fishing pressure and potentially promote recovery [94, 95], and in nutrient-poor ecosystems, cyclone-induced upwelling and river discharge can also lead to increases in fish catches [96].
Regardless of disturbance events, there is extensive natural variability in fish population dynamics [97], as evidenced in our study by the wide range of catch in both hurricane and control years during “before” periods. Large-scale climactic processes that influence hurricane activity, local meteorology, and biophysical conditions, such as the Atlantic Multidecadal Oscillation (AMO), can also influence fish populations separately from the direct impact of the storm itself. El Niño Southern Oscillation (ENSO) can heavily influence fisheries in the Pacific Ocean [98], and the AMO can modulate fish abundances in the eastern Atlantic [99]. However, few linkages have been investigated or demonstrated between the AMO and fish populations in estuarine or coastal waters of the western Atlantic [100], and rigorous long-term surveys are needed to examine the interactive effects of climactic oscillations on disturbances and marine ecosystem structure. Our study presents a case study of coastal ecosystem response to cyclones at a unique biogeographic transition zone that may be particularly sensitive to climate-induced shifts in community structure [87, 101] and may serve as a framework for future examinations of tropical cyclones under a global change scenario.
While our data provide reason for optimism regarding seagrass and seagrass-associated fish persistence after multiple cyclones, several climate forecasts and models have indicated that hurricane intensity and associated rainfall will increase in response to rising sea surface temperatures [75, 76, 102, 103], and greater cyclone magnitude was found to correlate strongly with greater declines in fish catch. If return intervals between major tropical cyclones become shorter than those needed for community recovery, the concept of individual storms as acute, pulse disturbances may need to be reframed. Our study and many others examine cyclones as isolated pulse disturbances, but their effects are compounded by and intertwined with escalating press stressors associated with climate change [104], habitat degradation [105], and fishing pressure [106]. Combined, these stressors may lead to an erosion of estuarine ecosystem resistance and resilience on timelines more similar to those of ramp disturbances that increase in intensity over time and/or press disturbances from which the ecosystem may not recover [7]. Studies that provide a single snapshot of before and after a single event can lead to misleading findings if multi-year trends are ignored [107]. Long-term syntheses geared towards understanding how global change will impact disturbance characteristics and collectively push ecosystems towards alternative states are necessary for effective coastal management, especially as society increasingly moves towards building resilient ecosystems [108].
Supporting information
Published studies that examined change in fish abundances before and after hurricanes listed in the literature review.
(PDF)
Meadows surveyed for both fishes and percent cover of seagrass. Seagrass survey dates (month/year) and periodicity listed.
(PDF)
Summary results examining potential correlations between environmental variables, namely surface water temperature and salinity, fish catches and species richness.
(PDF)
ANOVA results for short-term time frame analyses conducted for multi-storm mBACI and Arthur BACI. Post-hoc test results are not presented as no interaction term was significant.
(PDF)
Mean +/- standard error CPUE, CPUE-Lr, and species richness for multi-storm mBACI and BACI comparisons. Percent change is calculated as the decline or increase in catch or richness between before and after periods: . Note that means presented are rounded to whole values for catch and one decimal place for species richness, and percent change presented was calculated using unrounded values.
(PDF)
Summary results of environmental variables tested for correlation with fish community structure for both short-term and seasonal time frames.
(PDF)
Results of Similarity Percentages (SIMPER) analysis indicating the species that contribute the most to dissimilarity across BACI groups based on Bray-Curtis dissimilarities calculated from fourth-root transformed abundance data. Only the top 10 species that contribute the most to between-group dissimilarities are listed.
(PDF)
ANOVA results for seasonal time frame analyses conducted for multi-storm mBACI, Arthur BACI, and Matthew BACI. Post-hoc test results are presented when the interaction term is significant at p<0.05.
(PDF)
All generalized additive models (GAMs) were run for the seasonal time frame against days since storm as the independent variable and built using a cubic regression spline with penalized shrinkage, a maximum of three degrees of freedoms, negative binomial error distribution with log link function, and restricted maximum likelihood smoothing parameter. edf = effective degrees of freedom, logLik = log likelihood, Dev = deviance, df.r = residual degrees of freedom, AIC = Akaike information criterion BIC = Bayesian information criterion.
(PDF)
Negative binomial generalized linear models for seasonal-scale trend analysis of Hurricane Arthur (2014) versus 2015 as the control year. Est = estimate, df.r = residual degrees of freedom, AIC = Akaike information criterion BIC = Bayesian information criterion.
(PDF)
Decision-tree indicating how fish data was subsetted, transformed, and analyzed. If not listed as transformed, raw data was used as the response variable.
(PDF)
This figure demonstrates the potential difference/lack of difference in short-term community dispersion within each mBACI treatment using Principle Coordinates Analysis. Convex hulls are drawn in dashed lines through the outer-must points.
(PDF)
This figure demonstrates the potential difference/lack of difference in seasonal community dispersion within each mBACI treatment using Principle Coordinates Analysis. Convex hulls are drawn in dashed lines through the outer-must points.
(PDF)
Short-term and seasonal fish catches and species richness across time periods (before vs. after) and year type (control vs. impact) for Hurricane Arthur (July 2014) compared to 2015 (control year). Only means are presented for short-term comparisons (column 1); whereas means, trend, and difference between control and impact trends (columns 2–4, respectively) are depicted for seasonal comparisons. Catch per unit effort (CPUE) is presented in row 1 (A, D, H, K); CPUE calculated sans L. rhomboides is presented in row 2 (B, E. I, L), and species richness is row 3 (C, F, J, M). P-values indicate the significance of the interactive ANOVA term. Error bars represent standard error. Smoothed lines represent negative binomial generalized linear models for both hurricane and storm-free years with 95% confidence intervals.
(PDF)
Seasonal fish catch per unit effort (A), catch per unit effort sans Lagodon rhomboides (B) and species richness (C) across time periods (before vs. after) and year type (control vs. impact) for Hurricane Matthew (October 2016) compared to 2017 (control year). Only means are presented for seasonal comparisons (column 1), as the closest trawl samples prior to hurricane Matthew occurred outside of the short-term window and all trawls that occurred after the storm were conducted on the same day. P-values indicate the significance of the interactive ANOVA term. Error bars represent standard error.
(PDF)
Acknowledgments
We thank the numerous technicians, graduate, and undergraduate students who have aided with trawling and seagrass surveys since 2010 with special acknowledgments to Chris Baillie, Danielle Keller, Rebecca van Hoeck, and Amy Yarnall for collecting substantial portions of the data and Abigail Poray for compiling datasets. This manuscript was substantially improved thanks to comments from Janet Nye.
Data Availability
All data, models, and code used for analysis are available on GitHub (https://github.com/yszhang-fish/hurricane_fish). We have also created a DOI and permanent release version for our data on Zenodo. It can be found at: https://zenodo.org/badge/latestdoi/507171785.
Funding Statement
This research was supported by NSF Awards 1926395 and 1906651 to J. Fodrie, NSF Award 1906635 to J. Jarvis and J. Kenworthy, NSF Award 1901746 to R. Gittman, and the North Carolina Division of Marine Fisheries Marine Resources Fund to J. Fodrie. There was no additional external funding received for this study.
References
- 1.Sutherland JP. The fouling community at Beaufort, North Carolina: A study in stability. Am Nat. 1981;118: 499–519. [Google Scholar]
- 2.Bender EA, Case TJ, Gilpin ME. Perturbation experiments in community ecology: theory and practice. Ecology. 1984;65: 1–13. [Google Scholar]
- 3.Webster PJ, Holland GJ, Curry JA, Chang H-R. Changes in tropical cyclone number, duration, and intensity in a warming environment. Science. 2005;309: 1844–1846. doi: 10.1126/science.1116448 [DOI] [PubMed] [Google Scholar]
- 4.Elsner JB, Kossin JP, Jagger TH. The increasing intensity of the strongest tropical cyclones. Nature. 2008;455: 92–95. doi: 10.1038/nature07234 [DOI] [PubMed] [Google Scholar]
- 5.Sobel AH, Camargo SJ, Hall TM, Lee C-Y, Tippett MK, Wing AA. Human influence on tropical cyclone intensity. Science. 2016;353: 242–246. doi: 10.1126/science.aaf6574 [DOI] [PubMed] [Google Scholar]
- 6.Altman J, Ukhvatkina ON, Omelko AM, Macek M, Plener T, Pejcha V, et al. Poleward migration of the destructive effects of tropical cyclones during the 20th century. Proc Natl Acad Sci USA. 2018;115: 11543–11548. doi: 10.1073/pnas.1808979115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nimmo DG, Mac Nally R, Cunningham SC, Haslem A, Bennett AF. Vive la résistance: reviving resistance for 21st century conservation. Trends Ecol Evol. 2015;30: 516–523. doi: 10.1016/j.tree.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 8.Costanza R, Kemp WM, Boynton WR. Predictability, scale, and biodiversity in coastal and estuarine ecosystems: Implications for management. Ambio. 1993; 88–96. [Google Scholar]
- 9.Dyer KR. Estuaries: A Physical Introduction. 1973. [Google Scholar]
- 10.Jia P, Li M. Circulation dynamics and salt balance in a lagoonal estuary. J Geophys Res Oceans. 2012;117. doi: 10.1029/2011JC007124 [DOI] [Google Scholar]
- 11.Gunter G. Some Relations of Faunal Distributions to Salinity in Estuarine Waters. Ecology. 1956;37: 616–619. doi: 10.2307/1930196 [DOI] [Google Scholar]
- 12.Nordlie FG. Fish communities of estuarine salt marshes of eastern North America, and comparisons with temperate estuaries of other continents. Rev Fish Biol Fisher. 2003;13: 281–325. doi: 10.1023/B:RFBF.0000033050.51699.84 [DOI] [Google Scholar]
- 13.Gordon G. Mortality of Oysters and Abundance of Certain Associates as Related to Salinity. Ecology. 1955;36: 601–605. doi: 10.2307/1931298 [DOI] [Google Scholar]
- 14.Short FT, Neckles HA. The effects of global climate change on seagrasses. Aquat Bot. 1999;63: 169–196. doi: 10.1016/S0304-3770(98)00117-X [DOI] [Google Scholar]
- 15.Zieman JC, Fourqurean JW, Frankovich TA. Seagrass die-off in Florida Bay: long-term trends in abundance and growth of turtle grass, Thalassia testudinum. Estuaries. 1999;22: 460–470. [Google Scholar]
- 16.Baggett LP, Powers SP, Brumbaugh RD, Coen LD, DeAngelis BM, Greene JK, et al. Guidelines for evaluating performance of oyster habitat restoration. Restor Ecol. 2015;23: 737–745. [Google Scholar]
- 17.Farris G, Smith G, Crane M, Demas C, Robbins L, Lavoie D. Science and the storms: The USGS response to the hurricanes of 2005. US Geological Survey; 2007. Report No.: 2330–5703. [Google Scholar]
- 18.Paerl HW, Crosswell JR, Van Dam B, Hall NS, Rossignol KL, Osburn CL, et al. Two decades of tropical cyclone impacts on North Carolina’s estuarine carbon, nutrient and phytoplankton dynamics: implications for biogeochemical cycling and water quality in a stormier world. Biogeochemistry. 2018;141: 307–332. doi: 10.1007/s10533-018-0438-x [DOI] [Google Scholar]
- 19.Paerl HW, Hall NS, Hounshell AG, Luettich RA, Rossignol KL, Osburn CL, et al. Recent increase in catastrophic tropical cyclone flooding in coastal North Carolina, USA: Long-term observations suggest a regime shift. Sci Rep. 2019;9: 10620. doi: 10.1038/s41598-019-46928-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Hurricanes and Caribbean coral reefs: impacts, recovery patterns, and role in long‐term decline. Ecology. 2005;86: 174–184. [Google Scholar]
- 21.Paperno R, Tremain D, Adams D, Sebastian A, Sauer J, Dutka-Gianelli J. The disruption and recovery of fish communities in the Indian River Lagoon, Florida, following two hurricanes in 2004. Estuaries Coast. 2006;29: 1004–1010. [Google Scholar]
- 22.Piazza BP, La Peyre MK. The effect of Hurricane Katrina on nekton communities in the tidal freshwater marshes of Breton Sound, Louisiana, USA. Estuar Coast Shelf Sci. 2009;83: 97–104. [Google Scholar]
- 23.Bailey H, Secor DH. Coastal evacuations by fish during extreme weather events. Sci Rep. 2016;6: 30280. doi: 10.1038/srep30280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jury SH, Howell WH, Watson WH. Lobster movements in response to a hurricane. Mar Ecol Prog Ser. 1995;119: 305–310. [Google Scholar]
- 25.Heupel M, Simpfendorfer C, Hueter R. Running before the storm: blacktip sharks respond to falling barometric pressure associated with Tropical Storm Gabrielle. J Fish Biol. 2003;63: 1357–1363. [Google Scholar]
- 26.Sackett DK, Able KW, Grothues TM. Dynamics of summer flounder, Paralichthys dentatus, seasonal migrations based on ultrasonic telemetry. Estuar Coast Shelf Sci. 2007;74: 119–130. [Google Scholar]
- 27.Bacheler NM, Shertzer KW, Cheshire RT, MacMahan JH. Tropical storms influence the movement behavior of a demersal oceanic fish species. Sci Rep. 2019;9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paerl HW, Bales JD, Ausley LW, Buzzelli CP, Crowder LB, Eby LA, et al. Ecosystem impacts of three sequential hurricanes (Dennis, Floyd, and Irene) on the United States’ largest lagoonal estuary, Pamlico Sound, NC. Proc Natl Acad Sci USA. 2001;98: 5655–5660. doi: 10.1073/pnas.101097398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matich P, Moore KB, Plumlee JD. Effects of Hurricane Harvey on the Trophic Status of Juvenile Sport Fishes (Cynoscion nebulosus, Sciaenops ocellatus) in an Estuarine Nursery. Estuaries Coast. 2020;43: 997–1012. doi: 10.1007/s12237-020-00723-2 [DOI] [Google Scholar]
- 30.Letourneur Y, Harmelin-Vivien M, Galzin R. Impact of hurricane Firinga on fish community structure on fringing reefs of Reunion Island, S.W. Indian Ocean. Environ Biol Fishes. 1993;37: 109–120. doi: 10.1007/BF00000586 [DOI] [Google Scholar]
- 31.Deegan LA. Nutrient and energy transport between estuaries and coastal marine ecosystems by fish migration. Can J Fish Aquat Sci. 1993;50: 74–79. [Google Scholar]
- 32.Beck MW, Heck KL, Able KW, Childers DL, Eggleston DB, Gillanders BM, et al. The Identification, Conservation, and Management of Estuarine and Marine Nurseries for Fish and Invertebrates: A better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. BioScience. 2001;51: 633–641. doi: 10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2 [DOI] [Google Scholar]
- 33.Carlson PR, Yarbro LA, Kaufman KA, Mattson RA. Vulnerability and resilience of seagrasses to hurricane and runoff impacts along Florida’s west coast. Hydrobiologia. 2010;649: 39–53. doi: 10.1007/s10750-010-0257-0 [DOI] [Google Scholar]
- 34.Tilmant JT, Curry RW, Jones R, Szmant A, Zieman JC, Flora M, et al. Hurricane Andrew’s effects on marine resources. BioScience. 1994;44: 230–237. [Google Scholar]
- 35.Byron D, Heck KL. Hurricane effects on seagrasses along Alabama’s Gulf Coast. Estuaries Coast. 2006;29: 939–942. [Google Scholar]
- 36.Steward JS, Virnstein RW, Lasi MA, Morris LJ, Miller JD, Hall LM, et al. The impacts of the 2004 hurricanes on hydrology, water quality, and seagrass in the central Indian River Lagoon, Florida. Estuaries Coast. 2006;29: 954–965. [Google Scholar]
- 37.Anton A, Cebrian J, Duarte CM, Heck KL Jr, Goff J. Low impact of Hurricane Katrina on seagrass community structure and functioning in the northern Gulf of Mexico. Bull Mar Sci. 2009;85: 45–59. [Google Scholar]
- 38.Carter GA, Lucas KL, Biber PD, Criss GA, Blossom GA. Historical changes in seagrass coverage on the Mississippi barrier islands, northern Gulf of Mexico, determined from vertical aerial imagery (1940–2007). Geocarto Int. 2011;26: 663–673. doi: 10.1080/10106049.2011.620634 [DOI] [Google Scholar]
- 39.Davis J, Laird B. The effects of tropical storm Agnes on the Chesapeake Bay estuarine system. The Chesapeake Research Consortium; 1976. Report No.: CRC Publication No. 54. [Google Scholar]
- 40.Preen AR, Lee Long WJ, Coles RG. Flood and cyclone related loss, and partial recovery, of more than 1000 km2 of seagrass in Hervey Bay, Queensland, Australia. Aquat Bot. 1995;52: 3–17. doi: 10.1016/0304-3770(95)00491-H [DOI] [Google Scholar]
- 41.Fourqurean JW, Rutten LM. The impact of Hurricane Georges on soft-bottom, back reef communities: site- and species-specific effects in south Florida seagrass beds. Bull Mar Sci. 2004;75: 239–257. [Google Scholar]
- 42.Ridler MS, Dent RC, Arrinton DA. Effects of two hurricanes on Syringodium filiforme, manatee grass, within the Loxahatchee River Estuary, southeast Florida. Estuaries Coast. 2006;29: 1019–1025. [Google Scholar]
- 43.Congdon VM, Bonsell C, Cuddy MR, Dunton KH. In the wake of a major hurricane: Differential effects on early vs. late successional seagrass species. Limnol Oceanogr Lett. 2019;4: 155–163. doi: 10.1002/lol2.10112 [DOI] [Google Scholar]
- 44.Yue S, Zhang X, Xu S, Liu M, Qiao Y, Zhang Y, et al. The super typhoon Lekima (2019) resulted in massive losses in large seagrass (Zostera japonica) meadows, soil organic carbon and nitrogen pools in the intertidal Yellow River Delta, China. Sci Total Environ. 2021;793: 148398. doi: 10.1016/j.scitotenv.2021.148398 [DOI] [PubMed] [Google Scholar]
- 45.Williams SL. Disturbance and recovery of a deep-water Caribbean seagrass bed. Mar Ecol Prog Ser. 1988;42: 63–71. [Google Scholar]
- 46.O’Brien KR, Waycott M, Maxwell P, Kendrick GA, Udy JW, Ferguson AJP, et al. Seagrass ecosystem trajectory depends on the relative timescales of resistance, recovery and disturbance. Mar Pollut Bull. 2018;134: 166–176. doi: 10.1016/j.marpolbul.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 47.Seitz RD, Wennhage H, Bergström U, Lipcius RN, Ysebaert T. Ecological value of coastal habitats for commercially and ecologically important species. ICES J Mar Sci. 2014;71: 648–665. doi: 10.1093/icesjms/fst152 [DOI] [Google Scholar]
- 48.Sundblad G, Bergström U, Sandström A, Eklöv P. Nursery habitat availability limits adult stock sizes of predatory coastal fish. ICES Journal of Marine Science. 2014;71: 672–680. doi: 10.1093/icesjms/fst056 [DOI] [Google Scholar]
- 49.Lipcius RN, Eggleston DB, Fodrie FJ, van der Meer J, Rose KA, Vasconcelos RP, et al. Modeling Quantitative Value of Habitats for Marine and Estuarine Populations. Front Mar Sci. 2019;6. Available: https://www.frontiersin.org/article/10.3389/fmars.2019.00280 [Google Scholar]
- 50.Tropical Cyclone Climatology. [cited 10 May 2022]. Available: https://www.nhc.noaa.gov/climo/ [Google Scholar]
- 51.NOAA. U.S. Billion-dollar Weather and Climate Disasters. National Centers for Environmental Information; 2020. doi: 10.25921/STKW-7W73 [DOI] [Google Scholar]
- 52.Arguez A, Durre I, Applequist S, Vose RS, Squires MF, Yin X, et al. NOAA’s 1981–2010 U.S. Climate Normals: An Overview. Bull Am Meteorol Soc. 2012;93: 1687–1697. doi: 10.1175/BAMS-D-11-00197.1 [DOI] [Google Scholar]
- 53.Wuertz D, Lawrimore J, Korzeniewski B. Cooperative Observer Program (COOP) Hourly Precipitation Data (HPD), Version 2.0. NOAA National Centers for Environmental Information; Available: doi: 10.25921/p7j8-2170 [DOI] [Google Scholar]
- 54.Landsea CW, Franklin JL. Atlantic hurricane database uncertainty and presentation of a new database format. Mon Weather Rev. 2013;141: 3576–3592. [Google Scholar]
- 55.Baillie CJ, Fear JM, Fodrie FJ. Ecotone effects on seagrass and saltmarsh habitat use by juvenile nekton in a temperate estuary. Estuaries Coast. 2015;38: 1414–1430. [Google Scholar]
- 56.Yeager LA, Keller DA, Burns TR, Pool AS, Fodrie FJ. Threshold effects of habitat fragmentation on fish diversity at landscapes scales. Ecology. 2016;97: 2157–2166. doi: 10.1002/ecy.1449 [DOI] [PubMed] [Google Scholar]
- 57.Hogan JA, Feagin RA, Starr G, Ross M, Lin T-C, O’connell C, et al. A Research Framework to Integrate Cross-Ecosystem Responses to Tropical Cyclones. BioScience. 2020;70: 477–489. doi: 10.1093/biosci/biaa034 [DOI] [Google Scholar]
- 58.Patrick CJ, Yeager L, Armitage AR, Carvallo F, Congdon VM, Dunton KH, et al. A System Level Analysis of Coastal Ecosystem Responses to Hurricane Impacts. Estuaries Coast. 2020;43: 943–959. doi: 10.1007/s12237-019-00690-3 [DOI] [Google Scholar]
- 59.Gericke RL, Heck KL, Fodrie FJ. Interactions between Northern-Shifting Tropical Species and Native Species in the Northern Gulf of Mexico. Estuaries Coast. 2014;37: 952–961. doi: 10.1007/s12237-013-9733-x [DOI] [Google Scholar]
- 60.Wauchope HS, Amano T, Geldmann J, Johnston A, Simmons BI, Sutherland WJ, et al. Evaluating impact using time-series data. Trends Ecol Evol. 2020. doi: 10.1016/j.tree.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 61.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 62.Underwood A. On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecol Appl. 1994;4: 3–15. [Google Scholar]
- 63.Underwood AJ. Beyond BACI: the detection of environmental impacts on populations in the real, but variable, world. J Exp Mar Biol Ecol. 1992;161: 145–178. doi: 10.1016/0022-0981(92)90094-Q [DOI] [Google Scholar]
- 64.Keough MJ, Mapstone BD. Designing environmental monitoring for pulp mills in Australia. Water Sci Technol. 1997;35: 397–404. doi: 10.2166/wst.1997.0567 [DOI] [Google Scholar]
- 65.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press; 2002. doi: 10.1017/CBO9780511806384 [DOI] [Google Scholar]
- 66.Chevalier M, Russell JC, Knape J. New measures for evaluation of environmental perturbations using Before-After-Control-Impact analyses. Ecol Appl. 2019;29: e01838. doi: 10.1002/eap.1838 [DOI] [PubMed] [Google Scholar]
- 67.Wood S. The mgcv Package: GAMs with GCV smoothness estimation and GAMMs by REML/PQL. Vienna: R Foundation for Statistical Computing; 2008. p. http://www.R-project.org. [Google Scholar]
- 68.Wood S. mgcv: Mixed GAM computation vehicle with automatic smoothness estimation. 2019. Available: https://CRAN.R-project.org/package=mgcv [Google Scholar]
- 69.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. 2019. Available: https://CRAN.R-project.org/package=vegan [Google Scholar]
- 70.McCune B, Grace JB, Urban DL. Analysis of ecological communities. MjM software design Gleneden Beach, OR; 2002. [Google Scholar]
- 71.Bell GD. Climate assessment for 1999. Bull Amer Meteor Soc. 2000;81: S1–S50. [Google Scholar]
- 72.Bell GD, Chelliah M. Leading tropical modes associated with interannual and multidecadal fluctuations in north Atlantic hurricane activity. J Clim. 2006;19: 590–612. doi: 10.1175/JCLI3659.1 [DOI] [Google Scholar]
- 73.Ludwig DA. Use and misuse of p-values in designed and observational studies: guide for researchers and reviewers. Aviat Space Environ Med. 2005;76: 675–680. [PubMed] [Google Scholar]
- 74.Harley CD, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJ, Thornber CS, et al. The impacts of climate change in coastal marine systems. Ecol Let. 2006;9: 228–241. doi: 10.1111/j.1461-0248.2005.00871.x [DOI] [PubMed] [Google Scholar]
- 75.Knutson T, Camargo SJ, Chan JC, Emanuel K, Ho C-H, Kossin J, et al. Tropical cyclones and climate change assessment: Part II: Projected response to anthropogenic warming. Bull Am Meteorol Soc. 2020;101: E303–E322. [Google Scholar]
- 76.Seneviratne SI, Zhang X, Adnan M, Badi W, Dereczynski C, Luca AD, et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. In Press.; 2021. [Google Scholar]
- 77.Lugo AE. Effects of extreme disturbance events: From ecesis to social–ecological–technological systems. Ecosystems. 2020;23: 1726–1747. doi: 10.1007/s10021-020-00491-x [DOI] [Google Scholar]
- 78.Wilson SS, Furman BT, Hall MO, Fourqurean JW. Assessment of Hurricane Irma impacts on south Florida seagrass communities using long-term monitoring programs. Estuaries Coasts. 2020;43: 1119–1132. doi: 10.1007/s12237-019-00623-0 [DOI] [Google Scholar]
- 79.Fonseca MS, Kenworthy WJ, Whitfield PE. Temporal dynamics of seagrass landscapes: a preliminary comparison of chronic and extreme disturbance events. Biol Mar Mediterr. 2000;7: 373–376. [Google Scholar]
- 80.Marba N, Arias-Ortiz A, Masque P, Kendrick GA, Mazarrasa I, Bastyan GR, et al. Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. J Ecol. 2015;103: 296–302. doi: 10.1111/1365-2745.12370 [DOI] [Google Scholar]
- 81.Fenner DP. Effects of Hurricane Gilbert on coral reefs, fishes and sponges at Cozumel, Mexico. Bull Mar Sci. 1991;48: 719–730. [Google Scholar]
- 82.Syms C, Jones GP. Disturbance, habitat structure, and the dynamics of a coral-reef fish community. Ecology. 2000;81: 2714–2729. [Google Scholar]
- 83.Switzer T, Winner B, Dunham N, Whittington J, Thomas M. Influence of sequential hurricanes on nekton communities in a southeast Florida estuary: short-term effects in the context of historical variations in freshwater inflow. Estuaries Coast. 2006;29: 1011–1018. [Google Scholar]
- 84.Field D, Kenworthy WJ, Carpenter D. Metric Report: Extent of submerged aquatic vegetation, high-salinity estuarine waters (REVISED). Raleigh, NC: Albemarle-Pamlico National Estuary Partnership; 2021. [Google Scholar]
- 85.Thayer GW, Kenworthy WJ, Fonseca MS. Ecology of eelgrass meadows of the Atlantic Coast: a community profile. National Marine Fisheries Service, Beaufort, NC (USA: ). Beaufort Lab.; Virginia Univ., Charlottesville (USA). Dept. of Environmental Sciences; 1984. [Google Scholar]
- 86.Jarvis JC, Moore KA, Kenworthy WJ. Characterization and ecological implication of eelgrass life history strategies near the species’ southern limit in the western North Atlantic. Mar Ecol Prog Ser. 2012;444: 43–56. [Google Scholar]
- 87.Bartenfelder A, Kenworthy WJ, Puckett B, Deaton C, Jarvis JC. The Abundance and Persistence of Temperate and Tropical Seagrasses at Their Edge-of-Range in the Western Atlantic Ocean. Frontiers in Marine Science. 2022;9. doi: 10.3389/fmars.2022.917237 [DOI] [Google Scholar]
- 88.Dahlgren C, Kellison G, Adams A, Gillanders B, Kendall M, Layman C, et al. Marine nurseries and effective juvenile habitats: concepts and applications. Mar Ecol Prog Ser. 2006;312: 291–295. [Google Scholar]
- 89.Gunter G, Hall GE. Biological investigations of the St. Lucie estuary (Florida) in connection with Lake Okeechobee discharges through the St. Lucie canal. Gulf Caribb Res. 1963;1: 189–307. [Google Scholar]
- 90.Haunert DE, Startzman JR. Some seasonal fisheries trends and effects of a 1000 cfs fresh water discharge on the fishes and macroinvertebrates in the St. Lucie estuary, Florida. South Florida Water Management District; 1980. [Google Scholar]
- 91.Haunert D, Startzman J. Short term effects of a freshwater discharge on the biota of St. Lucie Estuary, Florida South Florida Water Management District Technical Publication. 1985; 85–1. [Google Scholar]
- 92.Serafy JE, Lindeman KC, Hopkins TE, Ault JS. Effects of freshwater canal discharge on fish assemblages in a subtropical bay: field and laboratory observations. Mar Ecol Prog Ser. 1997;160: 161–172. [Google Scholar]
- 93.Paperno R, Brodie RB. Effects of environmental variables upon the spatial and temporal structure of a fish community in a small, freshwater tributary of the Indian River Lagoon, Florida. Estuar Coast Shelf Sci. 2004;61: 229–241. [Google Scholar]
- 94.Smee DL, Reustle JW, Belgrad BA, Pettis EL. Storms promote ecosystem resilience by alleviating fishing. Current Biology. 2020;30: R869–R870. doi: 10.1016/j.cub.2020.06.048 [DOI] [PubMed] [Google Scholar]
- 95.Swinea SH, Fodrie FJ. Gulf fisheries supported resilience in the decade following unparalleled oiling. Ecosphere. 2021;12: e03801. doi: 10.1002/ecs2.3801 [DOI] [Google Scholar]
- 96.Yu J, Tang D, Li Y, Huang Z, Chen G. Increase in fish abundance during two typhoons in the South China Sea. Adv Space Res. 2013;51: 1734–1749. doi: 10.1016/j.asr.2012.11.019 [DOI] [Google Scholar]
- 97.Lehodey P, Alheit J, Barange M, Baumgartner T, Beaugrand G, Drinkwater K, et al. Climate Variability, Fish, and Fisheries. J Clim. 2006;19: 5009–5030. doi: 10.1175/JCLI3898.1 [DOI] [Google Scholar]
- 98.Mysak LA. El Niño, interannual variability and fisheries in the northeast Pacific Ocean. Can J Fish Aquat Sci. 1986;43: 464–497. [Google Scholar]
- 99.Alheit J, Licandro P, Coombs S, Garcia A, Giráldez A, Santamaría MTG, et al. Atlantic Multidecadal Oscillation (AMO) modulates dynamics of small pelagic fishes and ecosystem regime shifts in the eastern North and Central Atlantic. J Mar Syst. 2014;131: 21–35. doi: 10.1016/j.jmarsys.2013.11.002 [DOI] [Google Scholar]
- 100.Karnauskas M, Schirripa MJ, Craig JK, Cook GS, Kelble CR, Agar JJ, et al. Evidence of climate-driven ecosystem reorganization in the Gulf of Mexico. Glob Chang Biol. 2015;21: 2554–2568. doi: 10.1111/gcb.12894 [DOI] [PubMed] [Google Scholar]
- 101.Pinsky ML, Selden RL, Kitchel ZJ. Climate-Driven Shifts in Marine Species Ranges: Scaling from Organisms to Communities. Ann Rev Mar Sci. 2020;12: 153–179. doi: 10.1146/annurev-marine-010419-010916 [DOI] [PubMed] [Google Scholar]
- 102.IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: Intergovernmental Panel on Climate Change; 2007. p. 104. [Google Scholar]
- 103.Knutson TR, McBride JL, Chan J, Emanuel K, Holland G, Landsea C, et al. Tropical cyclones and climate change. Nature Geosci. 2010;3: 157–163. [Google Scholar]
- 104.Fodrie FJ, Heck KL Jr, Powers SP, Graham WM, Robinson KL. Climate-related, decadal-scale assemblage changes of seagrass-associated fishes in the northern Gulf of Mexico. Glob Chang Biol. 2010;16: 48–59. doi: 10.1111/j.1365-2486.2009.01889.x [DOI] [Google Scholar]
- 105.Gittman RK, Fodrie FJ, Popowich AM, Keller DA, Bruno JF, Currin CA, et al. Engineering away our natural defenses: an analysis of shoreline hardening in the US. Front Ecol Environ. 2015;13: 301–307. [Google Scholar]
- 106.Powers SP, Fodrie FJ, Scyphers SB, Drymon JM, Shipp RL, Stunz GW. Gulf-wide decreases in the size of large coastal sharks documented by generations of fishermen. Mar Coast Fish. 2013;5: 93–102. [Google Scholar]
- 107.Adams A. Effects of a hurricane on two assemblages of coral reef fishes: Multiple-year analysis reverses a false “snapshot” interpretation. Bull Mar Sci. 2001;69: 341–356. [Google Scholar]
- 108.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413: 591–596. doi: 10.1038/35098000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Published studies that examined change in fish abundances before and after hurricanes listed in the literature review.
(PDF)
Meadows surveyed for both fishes and percent cover of seagrass. Seagrass survey dates (month/year) and periodicity listed.
(PDF)
Summary results examining potential correlations between environmental variables, namely surface water temperature and salinity, fish catches and species richness.
(PDF)
ANOVA results for short-term time frame analyses conducted for multi-storm mBACI and Arthur BACI. Post-hoc test results are not presented as no interaction term was significant.
(PDF)
Mean +/- standard error CPUE, CPUE-Lr, and species richness for multi-storm mBACI and BACI comparisons. Percent change is calculated as the decline or increase in catch or richness between before and after periods: . Note that means presented are rounded to whole values for catch and one decimal place for species richness, and percent change presented was calculated using unrounded values.
(PDF)
Summary results of environmental variables tested for correlation with fish community structure for both short-term and seasonal time frames.
(PDF)
Results of Similarity Percentages (SIMPER) analysis indicating the species that contribute the most to dissimilarity across BACI groups based on Bray-Curtis dissimilarities calculated from fourth-root transformed abundance data. Only the top 10 species that contribute the most to between-group dissimilarities are listed.
(PDF)
ANOVA results for seasonal time frame analyses conducted for multi-storm mBACI, Arthur BACI, and Matthew BACI. Post-hoc test results are presented when the interaction term is significant at p<0.05.
(PDF)
All generalized additive models (GAMs) were run for the seasonal time frame against days since storm as the independent variable and built using a cubic regression spline with penalized shrinkage, a maximum of three degrees of freedoms, negative binomial error distribution with log link function, and restricted maximum likelihood smoothing parameter. edf = effective degrees of freedom, logLik = log likelihood, Dev = deviance, df.r = residual degrees of freedom, AIC = Akaike information criterion BIC = Bayesian information criterion.
(PDF)
Negative binomial generalized linear models for seasonal-scale trend analysis of Hurricane Arthur (2014) versus 2015 as the control year. Est = estimate, df.r = residual degrees of freedom, AIC = Akaike information criterion BIC = Bayesian information criterion.
(PDF)
Decision-tree indicating how fish data was subsetted, transformed, and analyzed. If not listed as transformed, raw data was used as the response variable.
(PDF)
This figure demonstrates the potential difference/lack of difference in short-term community dispersion within each mBACI treatment using Principle Coordinates Analysis. Convex hulls are drawn in dashed lines through the outer-must points.
(PDF)
This figure demonstrates the potential difference/lack of difference in seasonal community dispersion within each mBACI treatment using Principle Coordinates Analysis. Convex hulls are drawn in dashed lines through the outer-must points.
(PDF)
Short-term and seasonal fish catches and species richness across time periods (before vs. after) and year type (control vs. impact) for Hurricane Arthur (July 2014) compared to 2015 (control year). Only means are presented for short-term comparisons (column 1); whereas means, trend, and difference between control and impact trends (columns 2–4, respectively) are depicted for seasonal comparisons. Catch per unit effort (CPUE) is presented in row 1 (A, D, H, K); CPUE calculated sans L. rhomboides is presented in row 2 (B, E. I, L), and species richness is row 3 (C, F, J, M). P-values indicate the significance of the interactive ANOVA term. Error bars represent standard error. Smoothed lines represent negative binomial generalized linear models for both hurricane and storm-free years with 95% confidence intervals.
(PDF)
Seasonal fish catch per unit effort (A), catch per unit effort sans Lagodon rhomboides (B) and species richness (C) across time periods (before vs. after) and year type (control vs. impact) for Hurricane Matthew (October 2016) compared to 2017 (control year). Only means are presented for seasonal comparisons (column 1), as the closest trawl samples prior to hurricane Matthew occurred outside of the short-term window and all trawls that occurred after the storm were conducted on the same day. P-values indicate the significance of the interactive ANOVA term. Error bars represent standard error.
(PDF)
Data Availability Statement
All data, models, and code used for analysis are available on GitHub (https://github.com/yszhang-fish/hurricane_fish). We have also created a DOI and permanent release version for our data on Zenodo. It can be found at: https://zenodo.org/badge/latestdoi/507171785.