Abstract
Introduction
Traumatic brain injury (TBI) rates in the elderly are increasing worldwide, mainly due to fall accidents. However, TBI's impact on elderly patients' lives has not been thoroughly investigated.
Research question
This systematic review and meta-analysis aims at describing post-TBI incidence of functional decline, dependency, nursing home admission, reduced quality of life and depression in the elderly.
Materials and methods
A systematic literature search was performed in PubMed, EMBASE, Web Of Science, BIOSIS, Current Contents Connect, Data Citation Index, MEDLINE, SciELO, Cochrane library and CINAHL. Study selection was conducted by two independent reviewers. Meta-analysis was performed using a random-effects model.
Results
Twenty-seven studies were included in the qualitative synthesis and twenty-five in a random-effects meta-analysis. The prevalence of unfavorable functional outcomes after TBI was 65.2% (95% CI: 51.1–78.0). Admission to a nursing home had a pooled prevalence of 28.5% (95% CI: 17.1–41.6) and dependency rates ranged between 16.9% and 74.0%. A reduced quality of life was documented throughout follow-up with SF12/36 scores between 35.3 and 52.3/100.2.6–4.8% of the patients with mild TBI reported depressive symptoms. A large heterogeneity was found among studies for functional outcomes and discharge destination.
Discussion and conclusion
In conclusion, elderly patients have a significant risk for functional decline, dependency, nursing home admission and low quality of life following TBI. Moreover, more severe injuries lead to worse outcomes. These findings are important to provide accurate patient and family counseling, set realistic treatment targets and aim at relevant outcome variables in prognostic models for TBI in elderly patients.
Keywords: Traumatic brain injury, Elderly, Dependency, Quality of life, Depression, Systematic review, meta-Analysis
Highlights
-
•
Traumatic Brain Injury in the elderly has a major impact on functional outcomes.
-
•
Traumatic Brain Injury in elderly leads to dependency and nursing home admission.
-
•
Elderly patients have a lower quality of life after Traumatic Brain Injury.
-
•
Older age and injury severity are risk factors for poor functional outcome.
1. Background
Traumatic brain injury (TBI) is defined as a physical and/or functional injury to the brain caused by an external force (Menon et al., 2010) and its incidence amongst elderly patients has been increasing in the last decades (Mosenthal et al., 2002; Steyerberg et al., 2019; Peeters et al., 2015). Most elderly cases of TBI are classified as mild TBI (Styrke et al., 2007), following the Glasgow Coma Scale (GCS) (Teasdale and Jennett, 1974). However, the term “mild TBI” could be a misnomer, as these patients are particularly at risk for injuries with delayed mass effect and secondary deterioration (Hofman et al., 2001; Stiell et al., 2001; Haydel et al., 2000).
Elderly patients, conventionally defined as patients with a chronological age ≥65 years old in most TBI studies (Hawley et al., 2017; Choi et al., 2019; Røe et al., 2015; Susman et al., 2002; Mosenthal et al., 2004; Julien et al., 2017; Deb et al., 1998; Akbik et al., 2019; Erlebach et al., 2017; Haller et al., 2017; Wan et al., 2016; Brazinova et al., 2010), are at risk for a worse recovery after TBI (Mosenthal et al., 2002, 2004; Yu and Richmond, 2005; Thompson et al., 2012; Whitehouse et al., 2016; van Aalst et al., 1991). Poor premorbid condition, neurological sequelae and overall deconditioning lead to an increased risk for psychosocial changes (Rapoport and Feinstein, 2001), disability (Gardner et al., 2018) and secondary medical complications (Thompson et al., 2006).
The primary goal of medical management in TBI is to safeguard the patient's Quality of Life (QoL) (Seibert et al., 2002), which can be affected by TBI long after the initial medical treatment phase (Susman et al., 2002; de Guise et al., 2015). However, the lifelong impact of TBI on elderly patients' wellbeing remains a relatively neglected area in the field of TBI-related research to date (Gaastra et al., 2016).
Better insight into this matter may help (1) clinicians to set realistic treatment targets, (2) patients and families to gain understanding by improved counseling, and (3) researchers to underpin most relevant outcome determinants in the development of prognostic models for this population. We hypothesize that TBI in elderly patients is associated with a high likelihood of significant functional decline, which easily leads to dependency, and in turn is associated with reduced QoL and depression in this age group (Yu and Richmond, 2005; Rapoport and Feinstein, 2001; Gardner et al., 2018; Albrecht et al., 2015).
Therefore, the main objective of the current study is to document risk factors for and incidence of post-TBI functional decline and dependency, nursing home admission, depression and poor QoL in the elderly population.
2. Methods
2.1. Study registration
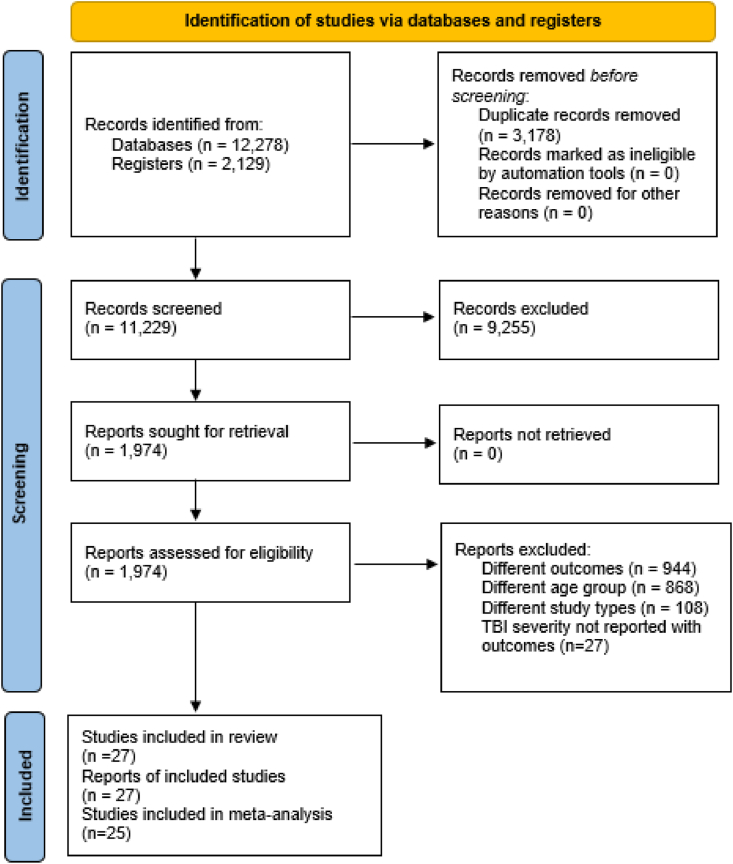
The protocol for this systematic review was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO, registration number [CRD42020212288]) and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) 2020 statement (Page et al., 2021) [Fig. 1] and the Cochrane Handbook for Systematic Reviews of Interventions (Cumpston et al., 2019).
Fig. 1.
Prisma flow diagram.
2.2. Search strategy and study selection
The systematic search was performed in October 2020 in PubMed, EMBASE, Web Of Science (WOS) Core Collection, BIOSIS Citation Index, Current Contents Connect, Data Citation Index, MEDLINE, SciELO Citation Index, the Cochrane Central Register of Controlled Trials and Cumulative Index to Nursing and Allied Health Literature (CINAHL). Language was restricted to English. There was no restriction on publication date. The used MESH (Medical Subject Headings) terms were “Aged”, “Geriatrics'’, ‘‘gerontology’‘, “Nursing Homes”, “Health Services for the Aged”, “Homes for the Aged”, “Housing for the Elderly”, “Brain Injuries, Traumatic”, “qualitative research” and “Case-Control Studies".
Studies’ inclusion was limited to clinical, multi-center, case-control or qualitative studies; performed in hospitals, rehabilitation centers and health care facilities for the elderly; including ≥15 patients who sustained a TBI at ≥65 years (as a subset of a broader cohort or as an independent cohort of elderly patients); using one of the assessment instruments included in Appendix A or reporting nursing home admission rates.
Studies were excluded if they included patients with a different pre-existing neurological condition or previous alcohol/drugs abuse or did not specifically associate outcomes to the TBI severity (given by the GCS).
2.3. Data extraction and analysis
First, references obtained from the systematic search were entered and deduplicated into EndNote X9 (Clarivate Analytics, Philadelphia). Second, titles and abstracts were screened for concordance with the in- and exclusion criteria using Rayyan QCRI software. Full texts of selected studies were then reviewed for final inclusion. The selection process was performed independently by two researchers blinded from each other. Disagreement in results was resolved through mutual discussion. When necessary, a third reviewer was consulted for arbitrage. Data extraction was performed using a standardized data collection form, including the first author's name, publication year, study design, sample size and number of dropouts, TBI injury severity, brain damage location, age of participants, sex of participants, number of months between TBI and assessment, metrics used and outcomes. In case of incomplete or ambiguous data, the corresponding author was contacted via e-mail. All included studies were subjected to descriptive analyses. For studies including a ≥16 years old cohort, only the results of the subset of patients ≥65 years were taken into account.
2.4. Quality assessment
The methodological quality of the studies was assessed using the Downs and Black Scale (Downs and Black, 1998) [Appendix B] and classified as excellent (26–27), good (20–25), fair (15–19) and poor (≤14).
2.5. Statistical analysis
Two random-effects meta-analyses were conducted using tidyverse, meta and metafor packages in R 4.1.0, using the inverse variance weighted average method (IVW) (Lee et al., 2016), in order to calculate combined prevalences. The DerSimonian and Laird method was used to obtain Tau2 (DerSimonian and Laird, 1986) and the Freeman-Turkey double arcsine transformation method to calculate combined prevalences (Freeman and Tukey, 1950). Clopper-Pearson confidence intervals for individual studies were reported (CLOPPER and PEARSON, 1934). Heterogeneity was quantified using I2 and interpreted as low (0%–30%), moderate (30%–50%), substantial (50%–80%) and considerable (80%–100%) (Cochrane Handbook, 2021). Publication bias was assessed using funnel plots of the combined prevalences.
3. Results
The database search retrieved a total of 14,407 citations. 11,229 articles were screened by title and abstract and 1974 full-text articles were assessed for eligibility, of which 27 articles were included in the qualitative synthesis [Fig. 1 and Table 1, Table 2, Table 3, Table 4, Table 5] and 25 were meta-analyzed. 40.7% of the included studies had a poor quality and 59.3% a fair quality [Appendix B].
Table 1.
Summary of studies which assessed functional outcomes using GOS and GOS-E.
| Study (year) | TBI severity (given by GCS) | ISS | Type of study | Study settings | Country | N ≥ 65 years old | LOS (days) | Comorbidities | Pre-injury anticoagulation (N) | Time post-TBI (months) | Outcome |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death (%) | Persistent vegetative state (%) | Severe disability (%) | Moderate disability (%) | Good recovery (%) | |||||||||||
| Hawley et al. (2017) (Hawley et al., 2017) | All | NR | Retrospective | National database | UK | 575 (439 mild TBI and 136 moderate or severe TBI) | 65–74 years old: median (IQR)=11(15) 75–84 years old: median (IQR)=14(25) ≥85 years old: median (IQR)=16(23) | NR | NR | Hospital discharge | 143 (24.9%) patients: 35 patients 65–74 years old, 46 75–84 years old and 62≥85 years old. 80 of them had a moderate or severe TBI. | 29 (5.0%) patients: 11 patients 65–74 years old, 13 75–84 years old and 5 ≥85 yearsold. 14 of them had a moderate or severe TBI | 79 (13.7) patients: 27 patients 65–74 years old, 33 75–84 years old and 19 ≥85 years old. 10 of them had a moderate or severe TBI. | 358 (62.3%) patients: 127 patients 65–74 years old, 130 75–84 years old and 101 ≥85 years old. 32 of them had a moderate or severe TBI and 306 had a mild TBI. | |
| Mohindra et al. (2008) (Mohindra et al., 2008) | All | NR | Retrospective | Hospital | India | 45 ≥70 years old (5 mild TBI, 7 moderate TBI and 33 severe TBI) | NR | NR | NR | 6 | 5 (71.4%) patients with moderate TBI and 24 (72.7%) of the patients with severe TBI | 1 (14.3%) patients with moderate TBI and 8 (24.2%) of the patients with severe TBI | 1 (20.0%) patients with mild TBI | 4(80.0%) patients with mild TBI, 1 (14.3%) of the patients with moderate TBI and 1 (3.0%) patient with severe TBI | |
| Choi et al. (2019) (Choi et al., 2019) | All | 21 patients minor (1–8); 53 moderate (9–15); 56 serious (16–24); 40 severe (≥25) | Retrospective | Republic of Korea | 170 (129 mild TBI, 15 moderate TBI and 26 severe TBI) | NR | NR | NR | 6 | 8 (6.2%) patients with mild TBI, 8 (53.3%) with moderate TBI and 23 (88.5%) with severe TBI | 118 (91.5%) patients with mild TBI, 10 (66.7%) with moderate TBI and 3 (11.5%) with severe TBI | ||||
| Mosenthal et al. (2004) (Mosenthal et al., 2004) | Mild | NR | Retrospective | Multi-center | US | 44 | NR | NR | NR | Hospital discharge | Unknown | Unknown | Unknown | Unknown | 13 (29.5%) |
| Julien et al. (2017) (Julien et al., 2017) | Mild | Median=25 (results only reported for 952 patients) | Retrospective | Hospital | Canada | 982 | Median (IQR)=11 (17) | NR | 439 (44.7%) | Hospital discharge | 162 (16.5%) | 31 (3.2%) | 256 (26.1%) | 508 (51.7%) | 24 (2.4%) |
| Deb et al. (1998) (Deb et al., 1998) | Mild | NR | Retrospective | Hospital | UK | 40 | NR | NR | NR | 12 | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) | 12 (30.0%) | – |
| Akbik et al. (2019) (Akbik et al., 2019) | Moderate and severe | NR | Retrospective | Level I trauma center | US | 62 (31 GCS >9 and 31 GCS≤9) | Median=9 | 37 patients hypertension, 10 atrial fibrillation, 12 diabetes, 14 coronary artery disease, 8 CVA, 8 neoplastic process, 7 dementia | 37 | Hospital discharge | 24 (38.7%) patients with a mean GCS = 7.81 ±4.09 | 4 (6.5%) patients with a mean GCS = 7.81 ±4.09 | 26 (41.9%) patients with a mean GCS=11.16± 3.6 | 6 (9.7%) patients with a mean GCS=9.86±4.34 | 2 (3.2%) patients with a mean GCS=9.86±4.34 |
| Erlebach et al. (2017) (Erlebach et al., 2017) | Moderate and severe | Median (IQR)=20 (11) | Retrospective | Hospital | Switzerland | 50 | 9.2±8.5 (in ICU) | 1 patient stroke, 5 TBI, 1 epilepsy, 10 diabetes, 35 cardiovascular diseases, 4 psychiatric disorder and 8 alcohol/drug abuse | 27 | 6 | 40 (80.0%) | 10 (20.0%) | |||
| Gritti et al. (2019) (Gritti et al., 2019) | Moderate and severe | NR | Retrospective | Hospital | Italy | 38 ≥70 years old | NR | 15 patients diabetes and 27 hypertension | 35 | 12 | 15 (39.5%) | 1(2.6%) | 8 (21.0%) | 7(18.4%) | 7(18.4%) |
| Won et al. (2017) (Won et al., 2017) | Severe | NR | Retrospective | Single center | Germany | 29 ≥80 years old | 9.1±6.2 | 59 patients hypertension, 30 atrial fibrillation, 26 type 2 diabetes, 44 cardiovascular diseases, 20 respiratory insufficiency, 14 renal failure, 8 hematological disease, 51 metabolic disease, 8 previous stroke, 34 pneumonia, 3 sepsis | 51 | Hospital discharge | 23 (79.3%) | 6 (20.7%) | |||
| Tokutomi et al. (2008) (Tokutomi et al., 2008) | Severe | Mean (SD)= 26 (9) | Retrospective | National database | Japan | 189> 70 years old | NR | 52.4% of the cases | NR | 3–6 | 131 (69.3%) | 17 (9.0%) | 22 (11.6%) | 9 (4.8%) | |
| Haller et al. (2017) (Haller et al., 2017) | Severe | Median (IQR)=25 (12) | Prospective observational | Multi-center | Switzerland | 97 | NR | 10 patients psychiatric disorders and 16 alcohol abuse | 26 | 3, 6 and 12 | Mean GOS-E 6 (range 3–8) at 3 and 6 months, and mean GOS-E 7 (range 5–8) at 12 months | Unknown | |||
| Wan et al. (2016) (Wan et al., 2016) | Severe | NR | Retrospective | Hospital | China | 328 | NR | 51 patients | NR | 6 | 60 (18.3%) | 77 (23.5%) | 35 (10.7%) | ||
| Brazinova et al. (2010) (Brazinova et al., 2010) | Severe | Median=20 | Prospective observational | Multi-center | Austria, Bosnia and Herzegovina, Croatia, Macedonia, and Slovakia | 100 | NR | NR | NR | 12 | 89 (89.0%) | 11 (11.0%) | |||
| Røe et al. (2015) (Røe et al., 2015) | Severe | NR | Prospective observational | Multi-center | Norway | 97 (46 patients 65–74 years old and 51 ≥71 years old) | NR | Present in 35 patients 65–74 years old and 45 patients ≥75 years old | 21 patients 65–74 years old and 37 patients ≥75 years old | 12 | 20 (44.0%) the patients 65–74 years old and 44 (86.0%) patients ≥75 years old | 26 (56.0%) patients 65–74 years old and 7 (14 .0%) patients ≥75 years old | |||
N=number; GCS=Glasgow Coma Scale; All=Mild, moderate and severe TBI; ISS=Injury Severity Score; NR=not reported; LOS=length of hospital stay due to TBI; CVA=cerebrovascular accident; Severe disability=GOS of 3 or GOS-E of 3 or 4; Moderate disability= GOS of 4 or GOS-E of 5 or 6; Good recovery= GOS of 5 or GOS-E of 7 or 8; UK=United Kingdom; US=United States of America.
Table 2.
Summary of studies which assessed ADL dependency after TBI using FIM.
| Study (year) | TBI severity (given by GCS) | ISS | Type of study | Study settings | Country | N ≥ 65 years old | LOS (days) | Comorbidities | Pre-injury anticoagulation | Time post-TBI (months) | Outcomes |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FIM motor (mean) | FIM cognition (mean) | FIM total (mean) | |||||||||||
| Mosenthal et al. (2004) (Mosenthal et al., 2004) | Mild | NR | Retrospective | Multi-center | US | 44 | NR | NR | NR | Hospital discharge and 6 | 27 (61.4%) total independence at hospital discharge and at 6 months, 3 patients (6.8%) worse FIM than at discharge, 20 patients (45.5%) unchanged FIM and 9 patients (20.5%) improved FIM | ||
| Susman et al. (2002) (Susman et al., 2002) | Moderate and severe (mean GCS 8.7) | Mean ± SD= 17.4± 8.7 | Retrospective observational study | Database | US | 3244 | NR | NR | NR | Hospital discharge | Abnormal FIM locomotion: 1161 patients (35.8%) | ||
| Abnormal FIM expression: 548 patients (16.9%) | |||||||||||||
| Abnormal FIM feeding: 1090 patients (33.6%) | |||||||||||||
| Lecours et al. (2012) (Lecours et al., 2012) | Severe | NR | Retrospective observational study | Level I and level II trauma center | Canada | 95 (44 patients 65–74 years old, 42 75–84 years old and 9 ≥85 years old) | NR | NR | NR | 24–48 | 42.6 (SD 9.5): 40.5 (SD 11.6) in the 65–74 years old, 41.5 (SD 10.5) in the 65–74 years old and 41.0 (SD 17.0) in the ≥85 years old | 17.8 (SD 5.4) | Unknown |
N=number; GCS=Glasgow Coma Scale; ISS=Injury Severity Score; NR=not reported; LOS=length of hospital stay due to TBI; US=United States of America.
Table 3.
Summary of studies which assessed ADL dependency and integration using CIQ, OARS and non-standardized assessments.
| Study (year) | TBI severity (given by GCS) | ISS | Type of study | Study settings | Country | N ≥ 65 years old | LOS (days) | Comorbidities | Pre-injury anticoagulation | Time post-TBI | Type of assessment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kinsella et al. (2014) (Kinsella et al., 2014) | Mild | NR | Retrospective | Hospital | Australia | 50 | Median (IQR)=4 (5) | NR | NR | 3 months | CIQ | CIQ home mean 5.8 (SD 2.9); CIQ social 8.3 (SD 2.6) |
| Brousseau et al. (2017) (Brousseau et al., 2017) | Mild | NR | Prospective observational | Multi-center | Canada | 344 | NR | 0-1 comorbidities in 51 patients,2–4 in 148 and 5–13 in 143 | NR | 6 months | OARS | 24 patients (7.0%) had functional decline |
| Thompson et al. (2012) (Thompson et al., 2012) | Mild | 65–74 years old: mean (SD)=17.9 (11.7 75–84 years old: mean (SD)= 7.5 (10.9) | Retrospective | National database | US | 309 | NR | In the 65–74 years group: 42.9% of the patients CCI=0, 28.4% CCI=1, 14.5% CCI=2 and 14.2% CCI≥3 In the 75–84 years group: 28.8% CCI=0, 29.6% CCI=1, 17.0% CCI=2, 24.6% ≥3 | NR | 12 months | Interview/non-standardized assessment | In the 65–74 years group patients had by mean 1.3 (SD 2.7) ADL difficulties and in the 75–84 years group a mean of 2.2 (SD 3.2). The number of ADL difficulties pre-injury was 0 in 87% of the 65-74 years-old patients and 71.5% of the 75-84 years-old patients |
| Miller et al. (2017) (Miller et al., 2017) | Mild and moderate | NR | Retrospective | National database | US | 36288 | NR | NR | NR | Hospital discharge | Interview/non-standardized assessment | 76.0% of the 70–79 years old group, 67.0% of the 80–89 years old group and 61.0% of the ≥90 years old group were independent at discharge |
| Lilley et al. (2016) (Lilley et al., 2016) | Severe | Median (IQR)= 5 (10) | Retrospective | National database | US | 90 | Median (IQR)=8 (14) | 41.9% CCI=0, 30.2% CCI=1, 16.3% CCI=2, 11.6 CCI ≥3 | 78.3% of the patients | Hospital discharge | Interview/non-standardized assessment | 61 patients (67.8%) were dependent for one or more ADL |
| Lecours et al. (2012) (Lecours et al., 2012) | Severe | NR | Retrospective | Level I and level II trauma center | Canada | 95 | NR | NR | NR | 24–48 months | Interview/non-standardized assessment | 69 of 136 participants (>55 years old) needed no help to transfer to chair, wheelchair, or bed and 79 of 136 participants (>55 years old) needed minimal help for going up or down the stairs |
N=number; GCS=Glasgow Coma Scale; ISS=Injury Severity Score; NR=not reported; LOS= length of hospital stay due to TBI; CCI=Charlson Comorbidity Index; US=United States of America.
Table 4.
Summary of studies which addressed nursing home admission after TBI.
| Study (year) | TBI severity (given by GCS) | ISS | Type of study | Study settings | Country | N ≥ 65 years old | LOS (days) | Comorbidities | Pre-injury anticoagulation (N) | Time post-TBI (months) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N patients discharged to a nursing home (%) | |||||||||||
| Khan et al. (2017) (Khan et al., 2017) | Mild | Median (IQR)=17 (11) | Retrospective | National database | US | 8750 | NR | NR | NR | Hospital discharge | 2993 (34.2%) |
| Schmidt et al. (2019) (Schmidt et al., 2019) | Mild | NR | Retrospective | Level I trauma center | Switzerland | 344 (141 patients 65–74 years old, 125 75–84 years old and 78 ≥85 years old) | Mean (SD)=2(2) | 9.3% of the patients had diabetes, 22.7% psychiatric diseases and 5.2% musculoskeletal diseases | NR | Hospital discharge | 20 (5.8%): 1 patient 65–74 years old, 6 75–84 years old and 13 ≥85 years old |
| Velez et al. (2020) (Velez et al., 2020) | Mild | Median=11 | Retrospective | National database | US | 19664 | NR | Median CCI=4 | NR | Hospital discharge | 5821(29.6%) |
| Thompson et al. (2012) (Thompson et al., 2012) | Mild | 65–74 years old: mean (SD)=17.9 (11.7) 75–84 years old: mean (SD)= 7.5 (10.9) | Retrospective | National database | US | 309 | NR | In the 65–74 years group: 42.9% of the patients CCI=0, 28.4% CCI=1, 14.5% CCI=2 and 14.2% CCI≥3 In the 75–84 years group: 28.8% CCI=0, 29.6% CCI=1, 17.0% CCI=2, 24.6% ≥3 | NR | 12 | 108 (35.0%), of which 10.4% were 65–74 years old and 24.6 75–84 years old |
| Miller et al. (2017) (Miller et al., 2017) | Mild and moderate | NR | Retrospective | National database | US | 36288 | NR | NR | NR | Hospital discharge | ∗76.0% of the 70–79 years old group, 67.0% of the 80–89 years old group and 61.0% of the ≥90 years old group were discharged to home or prison |
| Bhullar et al. (2010) (Bhullar et al., 2010) | Moderate | 65–80 years old: mean=12 >80 years old: mean=11 | Retrospective | Level II trauma center | US | 328 | NR | NR | NR | Hospital discharge | 27 (8.2%) |
| Gorman et al. (2020) (Gorman et al., 2020) | Moderate and severe | Median (IQR)= 17 (9) | Retrospective | National database | US | 3292 | NR | Median CCI=4 | NR | Hospital discharge | 931 (28.3%) |
| Susman et al. (2002) (Susman et al., 2002) | Moderate and severe (mean GCS=8.7) | Mean ± SD= 17.4± 8.7 | Retrospective | Database | US | 3244 | NR | NR | NR | Hospital discharge | 1752 (54.0%) |
| Lilley et al. (2016) (Lilley et al., 2016) | Severe | Median (IQR)= 5 (16–26) | Retrospective | National database | US | 90 | Median (IQR)=8 (14) | 41.9% CCI=0, 30.2% CCI=1, 16.3% CCI=2, 11.6 CCI ≥3 | 78.3% | Hospital discharge | 61 (67.8%) |
| Haller et al. (2017) (Haller et al., 2017) | Severe | Median (IQR)=25 (12) | Prospective observational | Multi-center | Switzerland | 97 | NR | 10 patients psychiatric disorders and 16 alcohol abuse | 26 | 3 | 10 (10.3%) at 3 months, 14 (14.4%) at 6 months and 16 (16.5%) at 12 months |
| Røe et al. (2015) (Røe et al., 2015) | Severe | NR | Prospective observational | Multi-center | Norway | 97 (46 65–74 years old and 51 ≥75 years old) | NR | 35 patients 65–74 years old and 45 patients ≥75 years old | 21 65–74 years old and 37 p ≥75 years old | 12 | 5 (5.2%): 4 patients 65–74 years old and 1 patient ≥75 years old |
| Lecours et al. (2012) (Lecours et al., 2012) | Severe | NR | Retrospective | Level I and level II trauma center | Canada | 95 | NR | NR | NR | 24–48 | 21 (22.1%) |
N=number; GCS=Glasgow Coma Scale; ISS=Injury Severity Score; NR=not reported; LOS= length of hospital stay due to TBI; CCI=Charlson Comorbidity Index; US=United States of America.
Table 5.
Summary of studies which addressed QoL after TBI.
| Study (year) | TBI severity (given by GCS) | ISS | Type of study | Study settings | Country | N ≥ 65 years old | LOS (days) | Comorbidities | Pre-injury anticoagulation | Time post-TBI (months) | Type of assessment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kinsella et al. (2014) (Kinsella et al., 2014) | Mild | NR | Retrospective | Hospital | Australia | 50 | Median (IQR)=4 (5) | NR | NR | 6 | SF-12 | Mean SF-12 PCS score 36.71 (SD 10.7); mean SF-12 MCS score 53.2 (SD 8.8) |
| Thompson et al. (2012) (Thompson et al., 2012) | Mild | 65–74 years old: mean (SD)=17.9 (11.7 75–84 years old: mean (SD)= 7.5 (10.9) | Retrospective | National database | US | 309 | NR | NR | NR | 12 | SF-36 and SF-6D | Mean SF-36 PCS score: 39.5(SD 17.2) in the 65–74 years old group and 35.3 (SD 17.4) in the 75–84 years old group |
| Mean SF-36 MCS score: 51.7 (SD 16.5) in the 65–74 years old group and 48.2(SD 18.4) in the 75–84 years old group | ||||||||||||
| Mean SF-6D: 0.73 (SD 0.25) in the 65–74 years old group and 0.67 (SD 0.26) in the 75–84 years old group | ||||||||||||
| Haller et al. (2017) (Haller et al., 2017) | Severe | Median (IQR)=25 (12) | Prospective observational | Multi-center study | Switzerland | 97 | NR | 10 patients psychiatric disorders, 16 alcohol abuse | 26 patients | 3, 6 and 12 | SF-12 | Mean SF-12 PCS score 39.2 (range 30.7–50.0); mean SF-12 MCS score 52.3 (range 44.7–55.7) at 3 months, mean SF-12 PCS score 42.3 (34.9–52.8); mean SF-12 MCS score 51.2 (45.3–57.1) at 6 months and mean SF-12 PCS score 44.2 (34.3–52.8); mean SF-12 MCS score 52.3 (45.8–57.2) at 12 months |
N=number; GCS=Glasgow Coma Scale; ISS=Injury Severity Score; NR=not reported; LOS= length of hospital stay due to TBI; US=United States of America.
3.1. Demographic and study characteristics
The included studies were retrospective or prospective observational studies performed in single or multi-center settings, or based on regional or national databases. The number of included patients ranged between 29 and 36,288. Follow-up duration across studies ranged from hospital discharge to 4 years post-TBI [Table 1, Table 2, Table 3, Table 4, Table 5].
3.2. Functional outcome
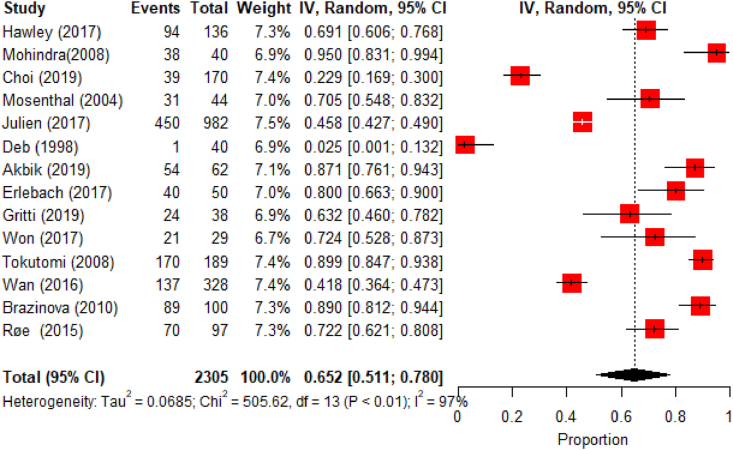
Functional outcome was assessed using the Glasgow Outcome Score (GOS) and the Extended Glasgow Outcome Score (GOS-E) in 15 studies [Table 1], of which 14 could be meta-analyzed [Fig. 2, Fig. 3].
Fig. 2.
Meta-analysis results for unfavorable functional outcomes.
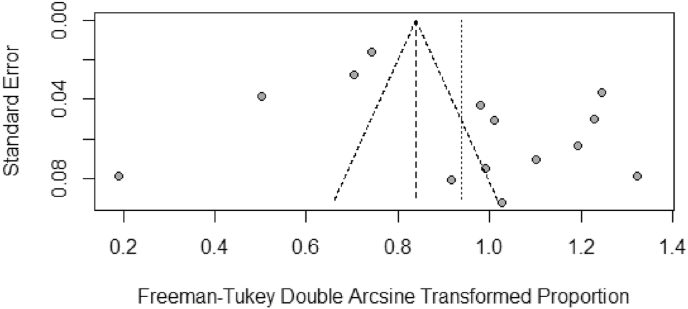
Fig. 3.
Funnel plot for the studies used in the unfavorable functional outcomes meta-analysis.
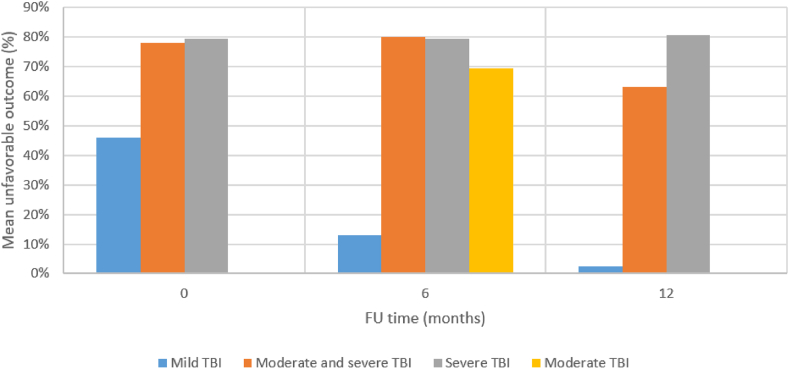
GOS is scored following a scale where 1 corresponds to death, 2 to vegetative state, 3 to severe disability, 4 to moderate disability and 5 to good recovery (Jennett and Bond, 1975). GOS-E is scored as: 1 (death), 2 (vegetative state), 3 (lower severe disability), 4 (upper severe disability), 5 (lower moderate disability), 6 (upper moderate disability), 7 (lower good recovery) to 8 (upper good recovery) (Wilson et al., 1998). GOS 1–3 and GOS-E 1–4 are considered as unfavorable outcomes. Results are visualized in Fig. 4.
Fig. 4.
Mean rates of unfavorable functional outcomes for the different TBI severities at different follow-up time points.
The pooled prevalence of unfavorable outcomes was 65.2% (95% CI: 51.1–78.0) at 12 months post TBI in mild, moderate and severe TBI patients. A significant heterogeneity between studies was found (I2=97%, p<0.01) [Fig. 2, Fig. 3] and the asymmetric funnel plot indicates publication bias [Fig. 3].
In patients with mild TBI, 45.8% had unfavorable outcomes at hospital discharge, including 16.5% who died (Julien et al., 2017). Rates of good recovery at discharge after mild TBI varied between 2.4 and 71.0% (Hawley et al., 2017; Mosenthal et al., 2004; Julien et al., 2017). At 6 months post mild TBI, rates of unfavorable outcomes between 6.2 and 20% were reported (Choi et al., 2019; Mohindra et al., 2008), while these were of 2.5% at 1 year (Deb et al., 1998).
For moderate TBI, at 6 months FU, 53.3–85.7% had unfavorable outcomes (Choi et al., 2019; Mohindra et al., 2008), and for moderate and severe TBI combined the 6 months unfavorable outcome rate reportedly was 80.0% (Erlebach et al., 2017).
For patients who sustained severe TBI, outcomes were unfavorable in 79.3% at hospital discharge (Won et al., 2017), 41.8–89.9% 3–6 months post TBI (Choi et al., 2019; Wan et al., 2016; Tokutomi et al., 2008) and 72.2–89.0% 1 year post-TBI (Røe et al., 2015; Brazinova et al., 2010).
Differences regarding recovery in patients with mild TBI were observed across studies performed in different regions. While Mohindra et al. (2008) and Julien et al. (2017) reported very low or unexisting ‘‘good recovery’’ rates in India and Canada, respectively, higher recovery rates were reported by Hawley et al. (2017), Choi et al. (2019) and Mosenthal et al. (2004) in the United Kingdom (UK), Korea and United States (US).
3.3. Activities of daily living (ADL) dependency and social integration
ADL dependency was assessed in 8 studies using the Functional Independence Measure (FIM), Older Americans' Resources and Services scale (OARS) (Milligan et al., 1988), the Community Integration Questionnaire (CIQ) (Willer et al., 1994) and non-standardized evaluations [Table 2, Table 3].
FIM is an 18-item functional assessment scale containing a motor and cognitive domain. Motor scores range between 13 (lowest) and 91 (highest level of independence), cognitive scores between 5 (lowest) and 35 (highest level of independence) and total score between 18 (lowest) and 126 (highest level of independence) (Linacre et al., 1994).
OARS is a 28-point questionnaire which assesses different ADL activities, scored on a 0–2 scale. Scores range between 0 (complete dependency) and 28 (complete independency) (Milligan et al., 1988).
The CIQ is a 15-item questionnaire which assesses home integration, social integration and productive activity. Total scores range from 0 (low integration) to 29 points (high integration), with a maximum score of 10 points for home integration, 12 points for social interaction and 7 points for productive activity (Willer et al., 1994).
In patients with mild TBI, at hospital discharge, a dependency rate of 38.6% was registered (Mosenthal et al., 2004). At 6 months follow-up, Brousseau et al. (2017) found a functional decline in 7.0% of the patients, defined as a reduction of 3 points on the OARS scale from their emergency department visit to FU, while on the other hand Mosenthal et al. (2004) showed an improvement in FIM scores in 20.5% of the patients at 6 months post mild TBI. At 1 year follow-up, 65-74 year-old patients had an average of 1.3 ADL difficulties and 75-84 year-old patients an average of 2.2 ADL difficulties (Thompson et al., 2012).
Considering patients with mild and moderate TBI, Miller et al. (2017) found that at hospital discharge 24.0% of the patients 70–79 years old, 33.0% of the patients 80–89 years old and 39.0% of the patients ≥90 years old were dependent.
For patients with moderate and severe TBI, at hospital discharge Susman et al. reported dependency rates of 35.8%, 16.9% and 33.6%, for motor function, expression and feeding, respectively (Susman et al., 2002).
For severe TBI cases, Lilley et al. found that 67.8% of the patients were dependent for one or more ADL activities at hospital discharge (Lilley et al., 2016). At 2–4 years FU, 74.0% of the patients were dependent for mobility, of which 49.3% needed help for transfers and 41.9% needed help for climbing stairs, and 73.0% were dependent for self-care (Lecours et al., 2012).
3.4. Discharge destination
Discharge destination from hospital discharge to 4 years post TBI was reported in 12 articles [Table 4].
In patients with mild TBI, 5.8%–34.2% were discharged to a nursing home (Thompson et al., 2012; Schmidt et al., 2019; Velez et al., 2020; Khan et al., 2017) and 35.0% continued residence beyond one year (Thompson et al., 2012).
Considering patients with mild and moderate TBI, Miller et al. (2017) found that 24.0% of the 70-79 years-old, 33.0% of the 80-89 years-old and 39.0% of the ≥90 years-old patients were discharged to an institution (Miller et al., 2017).
Rates of nursing homes discharge ranged between 28.3 and 54.0% in studies considering both moderate and severe TBI (Susman et al., 2002; Gorman et al., 2020). In patients with severe TBI, this rate was 67.8% at hospital discharge (Lilley et al., 2016), 10.3% at 3 months, 14.4% at 6 months (Haller et al., 2017), 5.1–16.5% at 12 months (Røe et al., 2015; Haller et al., 2017) and 22.1% at 24–48 months (Lecours et al., 2012).
Similar rates of nursing home admissions for patients with mild TBI were reported by Khan et al. (2017), Velez et al. (2020) and Thompson et al. (2012) in the US, which were higher than the rate reported by Schmidt et al. (2019) in Switzerland. No similarities were found in studies conducted in the US for patients with moderate and severe TBI (Thompson et al., 2012; Gorman et al., 2020; Bhullar et al., 2010).
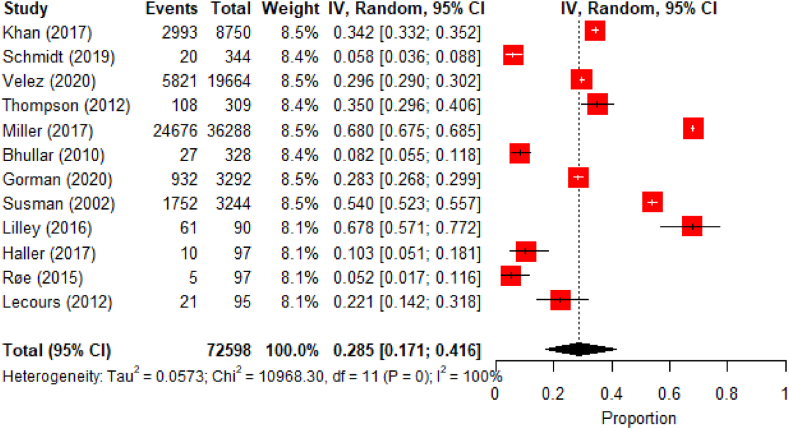
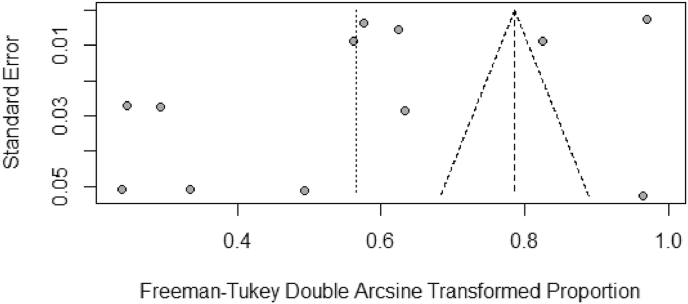
A high heterogeneity between studies was found in the 12 meta-analyzed studies (I2 =100%, p<0.01) [Fig. 5, Fig. 6]. The asymmetric funnel plot indicates publication bias [Fig. 6]. The pooled prevalence of nursing home admission was of 28.5% (95% CI: 17.1–41.6).
Fig. 5.
Meta-analysis results for nursing home admissions.
Fig. 6.
Funnel plot for the studies used in the nursing home admissions meta-analysis.
3.5. QoL
QoL was assessed using the 12-Item Short Form Survey (SF-12) (Ware et al., 1996), 36-Item Short Form Survey (SF-36) (Ware and Sherbourne, 1992) and Six-dimensional health state short form (SF-6D) (Ferreira et al., 2013) in 3 studies [Table 5 and Fig. 7].
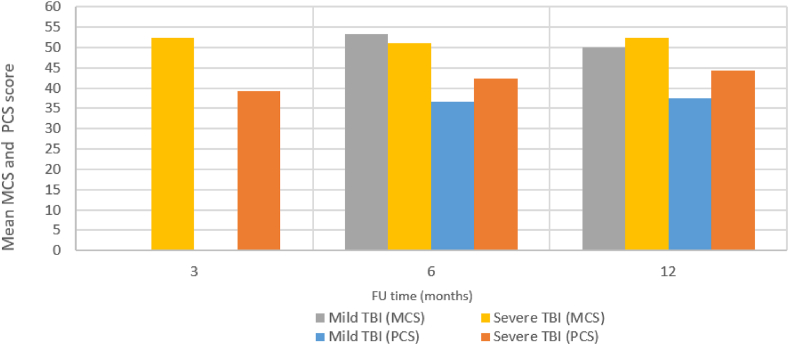
Fig. 7.
Mean MCS and PCS scores for the different TBI severities at different follow-up time point.
SF-12 (Ware et al., 1996) and SF-36 (Ware and Sherbourne, 1992) assess the impact of health on patients’ everyday life on a scale ranging between 0 (low QoL) and 100 (high QoL). Both questionnaires contain a Physical Component Summary (PCS) and a Mental Component Summary (MCS) (Ware et al., 1996; Ware and Sherbourne, 1992). The average reported population score is 50 (Huo et al., 2018; Maglinte et al., 2012). SF-6D evaluates role participation, social functioning, bodily pain, mental health and vitality (Ferreira et al., 2013). Scores range between 0.0 (worst health state) to 1.0 (best health state) (Ferreira et al., 2013).
In patients with mild TBI, Kinsella et al. reported an average PCS score of 36.7 (Kinsella et al., 2014), and average MCS of 52.1 at 6 months follow-up (Kinsella et al., 2014). At 12 months, Thompson et al. reported PCS SF-12 scores of 39.5 for the 65–74 year old patients and 35.3 for the 75–84 year olds (Thompson et al., 2012), while average MCS scores were 51.7 for the 65–74 year old patients and 48.2 for the 75–84 year olds (Thompson et al., 2012).
In severe TBI cases, Haller et al. found a mean SF-12 PCS score of 39.2 and a mean MCS score of 52.3 at 3 months, a mean PCS score of 42.3 and mean MCS score of 51.2 at 6 months, and a mean PCS score of 44.2 and mean MCS score of 52.3 at 12 months (Haller et al., 2017).
3.6. Depression
One study retrospectively studied depression 1 year post mild TBI in 309 patients ≥65 years old from a national database in US (Thompson et al., 2012). Assessment was performed through the self-report question: ‘‘Before your injury, did a doctor ever tell you that you had depression?’’ and through the Center for Epidemiologic Studies Depression Scale-Revised at follow-up, which is a 20-item questionnaire whose scores range from 0 to 60 (Eaton et al., 2004). Higher scores indicate the presence of more depressive symptomatology (Eaton et al., 2004).
One year after mild TBI, depressive symptoms were found in 4.8% of the 65–74 years old patients and 2.6% of the 75–84 years old patients (Thompson et al., 2012).
4. Discussion
This study assesses QoL, functional outcome, dependency, nursing home admission rate and incidence of depression in elderly patients of ≥65 years old who sustained TBI.
The results show a prevalence for unfavorable functional outcomes of 65.2% (95% CI: 51.1–78.0) and a prevalence of nursing home admission of 28.5% (95% CI: 17.1–41.6). Dependency rates range between 16.9% and 74.0%, with outcomes worsening with increasing severity and age and with unclear recovery over time. QoL was found to be particularly decreased as indicated by reported PCS scores ranging between 35.3 and 44.2/100 at 3–12 months post TBI. This is far below the population average scores (50/100 (Huo et al., 2018; Maglinte et al., 2012). In contrast, the MCS scores were similar to the population average scores, ranging between 48.2 and 52.3/100 at 6–12 months follow-up. 2.6–4.8% of patients with mild TBI reported depressive symptoms.
Higher injury severity was associated with poorer functional outcomes. However, this is not the case for QoL, where only subtle differences were observed between patients with mild and severe TBI and, in some cases, the reported QoL scores were lower for patients with mild TBI.
To date, there is a relative scarcity of evidence and lack of detail in studies reporting on elderly TBI outcomes (Steyerberg et al., 2019; Peeters et al., 2015). Particularly, only three studies investigated QoL in elderly patients. While dependency in ADL is a strong predictor of declining QoL (Enkvist et al., 2012; WILHELMSON et al., 2005), the relation between mood changes and function is not straightforward. As the safeguarding of acceptable QoL is the primordial goal of any medical treatment, documented QoL outcomes following TBI in elderly are of major importance for treatment guidance and counseling. Nevertheless, to date, we could find no guidelines on the management of TBI in the elderly.
We hypothesized that TBI in elderly patients often results in significant functional decline and poor QoL, and the obtained results seem to confirm this. Moderate and severe TBI have a significant impact on dependency and QoL in elderly, while mild TBI has a potentially strong impact. Interestingly, Haller et al., Kinsella et al. and Thompson et al. found that health-related QoL was impaired to the same extent in mild as in severe TBI (Haller et al., 2017; Thompson et al., 2012; Kinsella et al., 2014). This might be explained by the definition of ‘‘mild TBI’‘, following the GCS, not being sufficiently reliable in elderly patients. These patients are at a particular risk of deterioration after hospital admission (Hofman et al., 2001; Stiell et al., 2001; Haydel et al., 2000) and, therefore, the impact of mild TBI should not be underestimated. Furthermore, we believe that QoL depends on a large amount of factors about the patients' lives and not only the patients' status after TBI. This could lead, in some cases, to subjective results. Therefore, it could be that in some cases patients with mild TBI potentially perceive their limitations as a serious burden, while patients surviving moderate and severe TBI may be rather positive, outweighing the disabilities by their happiness to be still alive.
A limitation of the current study is the scarcity of published literature documenting outcomes of TBI in elderly, particularly in terms of QoL and depression. Moreover, the review was limited to studies written in English. However, the main limitation is the inter-study variability in outcome scales used, study settings and timings of assessment applied.
Furthermore, the included studies generally contained insufficient detail to correct for patients’ heterogeneity, comorbidities, injury characteristics and clinical management in this population. An attempt to compare studies with similar characteristics has been performed. However, this has not been possible in all the cases due to the variability in outcome scoring, in combination with the impossibility for actual stratification. The establishment and application of an international reporting standard would be very useful.
Finally, if all the included studies would have contained more detail on patient and injury characteristics and management, this could have enabled a meta-analysis for all the included outcomes, which was not possible.
The results from this review illustrate the major burden of TBI in the elderly, primarily for the patients and their families. Second, to society, which is reflected in needs for outpatient supplies and residential care facilities and associated costs. In contrast, research in this field is, to date, rather limited and no clinical guidelines for the management of elderly TBI exist. Better insight into outcomes and risk factors for poor outcome, ideally resulting in the development of prognostic models specific for elderly TBI, can improve counseling of patients and their families, and help caregivers to set realistic treatment targets, particularly in situations of severe TBI where often surgical decisions need to be made fast and treatment withdrawal might be a humane alternative. Further clinical research in this field is therefore urgently needed in order to facilitate clinical guideline development.
5. Conclusion
TBI in the elderly has a major impact on patients’ lives, often leading to functional decline, dependency, nursing home admission and poor QoL. This is particularly true in moderate and severe TBI, but potentially also true in mild TBI. Older age and injury severity are risk factors for poor functional outcome, while poor QoL is seen in all severities of TBI.
6. Authorship confirmation statement
All authors contributed to the study conception and design. The articles screening and selection was performed by Rebeca Alejandra Gavrila Laic and Liedewij Bogaert. Studies' quality assessment and data extraction was performed by Rebeca Alejandra Gavrila Laic. The first draft of the manuscript was written by Rebeca Alejandra Gavrila Laic and Bart Depreitere. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
7. Author(s’) disclosure statement(s)
The authors declare that they have no competing interests.
Funding statement
This work was supported by Fund for Scientific Research – Flanders (FWO) and Helaers foundation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors wish to thank Thomas Vandendriessche, Kristel Paque and Krizia Tuand, the biomedical reference librarians of the KU Leuven Libraries, 2Bergen Learning Center Désiré Collen (Leuven, Belgium), for their help in conducting the systematic literature search. Also, we would like to thank An Van Damme for the language editing.
Appendix A. Assessment techniques included in our study selection inclusion criteria
| Assessment technique | Outcome |
|---|---|
| WHO Disability Assessment Schedule (WHODAS) | Functional outcome |
| Functional Independence Measure (FIM) | Functional outcome |
| Glasgow Outcome Scale (GOS) | Functional outcome |
| GOS-Extended (GOS-E) | Functional outcome |
| Disability Rating Scale (DRS) | Functional outcome |
| Functional Assessment Measure (FAM) | Functional outcome |
| Functional Status Examination (FSE) | Functional outcome |
| Community Integration Questionnaire (CIQ) | Functional outcome |
| Barthel Index | Functional outcome |
| Patient Reported Outcomes Measurement Information System (PROMIS) | Functional outcome |
| Rivermead Postconcussion Symptoms Questionnaire | Functional outcome/depression |
| Trauma outcome profile (TOP) | Functional outcome/depression |
| European Brain Injury Questionnaire (EBIQ) | Functional outcome/QoL |
| General Health Questionnaire-30 (GHQ-30) | Functional outcome/depression |
| Hamilton Depression Rating Scale (HAM-D) | Depression |
| Mini International Neuropsychiatric Interview | Depression |
| Wimbledon Self-Report Scale | Depression |
| Beck's Depression Inventory (BDI) | Depression |
| Patient Health Questionnaire 9-item depression scale (PHQ-9) | Depression |
| Center for Epidemiologic Studies-Depression Scale [CES-D] | Depression |
| Anxiety and depression scales from the Symptom Checklist-90 | Depression and anxiety |
| Hospital anxiety and depression scale (HADS) | Depression and anxiety |
| Beck's anxiety inventory (BAI) | Anxiety |
| 36-Item Short Form Survey (SF-36) | QoL |
| 12-Item Short Form Survey (SF-12) | QoL |
| Short-Form Six-Dimension (SF- 6D) | QoL |
| Quality of Life after Brain Injury (QOLIBRI) | QoL |
| Quality of Life after Brain Injury Overall Scale (QOLIBRI-OS) | QoL |
| Quality of Life Interview (QoLI) | QoL |
| QOLIBRI Proxy version (Q-Pro) | QoL |
| Traumatic Brain Injury Quality of Life (TBI-QOL) | QoL |
| World Health Organization Quality of Life-BREF (WHOQOL-BREF) | QoL |
| World Health Organization Quality of Life 100 (WHOQOL-100) | QoL |
| EuroQol-5D (EQ-5D) | QoL |
| NeuroQol | QoL |
| Sickness Impact Profile (SIP) | QoL |
| Flanagan Quality of Life Scale (FQolS) | QoL |
| Perceived Quality of Life Scale (PQoL) | QoL |
| Satisfaction With Life Scale (SWLS) | QoL |
| Life Satisfaction Questionnaire (LiSat-11) | QoL |
| Life Satisfaction Index I-A (LSI-A) | QoL |
| Freiburg Questionnaire of Coping with Illness (FQCI) | QoL/depression |
| Profile of mood states (POMS) | QoL/depression |
| Qualitative interviews | Functional outcome/depression/dependency/nursing home admissions |
Appendix B. Studies' quality assessment based on the Downs and Blacks scale
| Author (year) | Reporting |
External validity |
Internal validity-bias |
Internal validity - confounding (selection bias) |
Power |
T | Quality | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | |||
| Akbik et al. (2019) (Akbik et al., 2019) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 16 | Fair |
| Bhullar et al. (2010) (Bhullar et al., 2010) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 11 | Poor |
| Brazinova et al. (2010) (Brazinova et al., 2010) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | Poor |
| Brousseau et al. (2017) (Brousseau et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | Poor |
| Choi et al. (2019) (Choi et al., 2019) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 16 | Fair |
| Deb et al. (1998) (Deb et al., 1998) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 17 | Fair |
| Erlebach et al. (2017) (Erlebach et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 17 | Fair |
| Gorman et al. (2020) (Gorman et al., 2020) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | Poor |
| Gritti et al. (2019) (Gritti et al., 2019) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 17 | Fair |
| Haller et al. (2017) (Haller et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 16 | Fair |
| Hawley et al. (2017) (Hawley et al., 2017) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 15 | Fair |
| Julien et al. (2017) (Julien et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 16 | Fair |
| Khan et al. (2017) (Khan et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 16 | Fair |
| Kinsella et al. (2014) (Kinsella et al., 2014) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | Fair |
| Lecours et al. (2012) (Lecours et al., 2012) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 15 | Fair |
| Lilley et al. (2016) (Lilley et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 18 | Fair |
| Miller et al. (2017) (Miller et al., 2017) | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 12 | Poor |
| Mosenthal et al. (2004) (Mosenthal et al., 2004) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 16 | Fair |
| Mohindra et al. (2008) (Mohindra et al., 2008) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 10 | Poor |
| Røe et al. (2015) (Røe et al., 2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 15 | Fair |
| Schmidt et al. (2019) (Schmidt et al., 2019) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 16 | Fair |
| Susman et al. (2002) (Susman et al., 2002) | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | Poor |
| Thompson et al. (2012) (Thompson et al., 2012) | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 13 | Poor |
| Tokutomi et al. (2008) (Tokutomi et al., 2008) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 13 | Poor |
| Velez et al. (2020) (Velez et al., 2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 16 | Fair |
| Wan et al. (2016) (Wan et al., 2016) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | Poor |
| Won et al. (2017) (Won et al., 2017) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 13 | Poor |
1-27 refer to questions 1–27 of the Downs and Blacks scale (Downs and Black, 1998); T=total score.
Downs and Blacks scale's questions (Downs and Black, 1998):
-
1.
Is the hypothesis/aim/objective of the study clearly described?
-
2.
Are the main outcomes to be measured clearly described in the Introduction or Methods section?
-
3.
Are the characteristics of the patients included in the study clearly described ?
-
4.
Are the interventions of interest clearly described?
-
5.
Are the distributions of principal confounders in each group of subjects to be compared clearly described?
-
6.
Are the main findings of the study clearly described?
-
7.
Does the study provide estimates of the random variability in the data for the main outcomes?
-
8.
Have all important adverse events that may be a consequence of the intervention been reported?
-
9.
Have the characteristics of patients lost to follow-up been described?
-
10.
Have actual probability values been reported(e.g. 0.035 rather than <0.05) for the main outcomes except where the probability value is less than 0.001?
-
11.
Were the subjects asked to participate in the study representative of the entire population from which they were recruited?
-
12.
Were those subjects who were prepared to participate representative of the entire population from which they were recruited?
-
13.
Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive?
-
14.
Was an attempt made to blind study subjects to the intervention they have received ?
-
15.
Was an attempt made to blind those measuring the main outcomes of the intervention?
-
16.
If any of the results of the study were based on “data dredging”, was this made clear?
-
17.
In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case-control studies, is the time period between the intervention and outcome the same for cases and controls ?
-
18.
Were the statistical tests used to assess the main outcomes appropriate?
-
19.
Was compliance with the intervention/s reliable?
-
20.
Were the main outcome measures used accurate (valid and reliable)?
-
21.
Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited from the same population?
-
22.
Were study subjects in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited over the same period of time?
-
23.
Were study subjects randomised to intervention groups?
-
24.
Was the randomised intervention assignment concealed from both patients and health care staff until recruitment was complete and irrevocable?
-
25.
Was there adequate adjustment for confounding in the analyses from which the main findings were drawn?
-
26.
Were losses of patients to follow-up taken into account?
-
27.
Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%?
All questions were scored on the following scale: yes=1, unable to determine=0 and no=0.
References
- Akbik O.S., Starling R.V., Gahramanov S., Zhu Y., Lewis J. Mortality and functional outcome in surgically evacuated acute subdural hematoma in elderly patients. World Neurosurg. 2019;126:e1235–e1241. doi: 10.1016/j.wneu.2019.02.234. [DOI] [PubMed] [Google Scholar]
- Albrecht J.S., Kiptanui Z., Tsang Y., et al. Depression among older adults after traumatic brain injury: a national analysis. Am. J. Geriatr. psychiatry. Off. J. Am. Assoc. Geriatr. Psychiatry. 2015;23(6):607–614. doi: 10.1016/j.jagp.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar I.S., Roberts E.E., Brown L., Lipei H. The effect of age on blunt traumatic brain-injured patients. Am. Surg. 2010;76(9):966–968. [PubMed] [Google Scholar]
- Brazinova A., Mauritz W., Leitgeb J., et al. Outcomes of patients with severe traumatic brain injury who have Glasgow Coma Scale scores of 3 or 4 and are over 65 years old. J. Neurotrauma. 2010;27(9):1549–1555. doi: 10.1089/neu.2010.1315. [DOI] [PubMed] [Google Scholar]
- Brousseau A.-A., Émond M., Sirois M.-J., et al. Comparison of functional outcomes in elderly who have sustained a minor trauma with or without head injury: a prospective multicenter cohort study. CJEM. 2017;19(5):329–337. doi: 10.1017/cem.2016.368. [DOI] [PubMed] [Google Scholar]
- Choi M.S., Jeong D., You N., Roh T.H., Kim S.-H. Identification of clinical characteristics and factors predicting favorable treatment outcomes in elderly patients with traumatic brain injury. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019;69:61–66. doi: 10.1016/j.jocn.2019.08.035. [DOI] [PubMed] [Google Scholar]
- Clopper C.J., Pearson E.S. The use OF confidence or fiducial limits illustrated IN the case OF the binomial. Biometrika. 1934;26(4):404–413. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- Cochrane Handbook . 2021. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training.https://training.cochrane.org/handbook [Google Scholar]
- Cumpston M., Li T., Page M.J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guise E., LeBlanc J., Dagher J., et al. Traumatic brain injury in the elderly: a level 1 trauma centre study. Brain Inj. 2015;29(5):558–564. doi: 10.3109/02699052.2014.976593. [DOI] [PubMed] [Google Scholar]
- Deb S., Lyons I., Koutzoukis C. Neuropsychiatric sequelae one year after a minor head injury. J. Neurol. Neurosurg. Psychiatry. 1998;65(6):899–902. doi: 10.1136/jnnp.65.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W.W., Smith C., Ybarra M., Muntaner C., Tien A. Center for epidemiologic studies depression scale: review and revision (CESD and CESD-R) use Psychol. Test Treat. Plan. outcomes Assess. Instruments adults. 2004;3:363–377. third ed. [Google Scholar]
- Enkvist A., Ekström H., Elmståhl S. Life satisfaction (LS) and symptoms among the oldest-old: results from the longitudinal population study called Good Aging in Skåne (GÅS) Arch. Gerontol. Geriatr. 2012;54(1):146–150. doi: 10.1016/j.archger.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Erlebach R., Pagnamenta A., Klinzing S., et al. Age-related outcome of patients after traumatic brain injury: a single-center observation. Minerva Anestesiol. 2017;83(11):1169–1177. doi: 10.23736/S0375-9393.17.11837-7. [DOI] [PubMed] [Google Scholar]
- Ferreira L.N., Ferreira P.L., Pereira L.N., Rowen D., Brazier J.E. Exploring the consistency of the SF-6D. Value Health. 2013;16(6):1023–1031. doi: 10.1016/j.jval.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950;21(4):607–611. doi: 10.1214/aoms/1177729756. [DOI] [Google Scholar]
- Gaastra B., Longworth A., Matta B., et al. The ageing population is neglected in research studies of traumatic brain injury. Br. J. Neurosurg. 2016;30(2):221–226. doi: 10.3109/02688697.2015.1119240. [DOI] [PubMed] [Google Scholar]
- Gardner R.C., Dams-O’Connor K., Morrissey M.R., Manley G.T. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J. Neurotrauma. 2018;35(7):889–906. doi: 10.1089/neu.2017.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman E., Frangos S., DiMaggio C., et al. Is trauma center designation associated with disparities in discharge to rehabilitation centers among elderly patients with Traumatic Brain Injury? Am. J. Surg. 2020;219(4):587–591. doi: 10.1016/j.amjsurg.2020.02.026. [DOI] [PubMed] [Google Scholar]
- Gritti P., Zangari R., Carobbio A., et al. Acute and subacute outcome predictors in moderate and severe traumatic brain injury: a retrospective monocentric study. World Neurosurg. 2019;128:e531–e540. doi: 10.1016/j.wneu.2019.04.190. [DOI] [PubMed] [Google Scholar]
- Haller C.S., Delhumeau C., De Pretto M., et al. Trajectory of disability and quality-of-life in non-geriatric and geriatric survivors after severe traumatic brain injury. Brain Inj. 2017;31(3):319–328. doi: 10.1080/02699052.2016.1255777. [DOI] [PubMed] [Google Scholar]
- Hawley C., Sakr M., Scapinello S., Salvo J., Wrenn P. Traumatic brain injuries in older adults-6 years of data for one UK trauma centre: retrospective analysis of prospectively collected data. Emerg. Med. J. 2017;34(8):509–516. doi: 10.1136/emermed-2016-206506. [DOI] [PubMed] [Google Scholar]
- Haydel M.J., Preston C.A., Mills T.J., Luber S., Blaudeau E., DeBlieux P.M. Indications for computed tomography in patients with minor head injury. N. Engl. J. Med. 2000;343(2):100–105. doi: 10.1056/NEJM200007133430204. [DOI] [PubMed] [Google Scholar]
- Hofman P.A., Stapert S.Z., van Kroonenburgh M.J., Jolles J., de Kruijk J., Wilmink J.T. MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. AJNR Am. J. Neuroradiol. 2001;22(3):441–449. [PMC free article] [PubMed] [Google Scholar]
- Huo T., Guo Y., Shenkman E., Muller K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: a report from the wellness incentive and navigation (WIN) study. Health Qual. Life Outcome. 2018;16(1):34. doi: 10.1186/s12955-018-0858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennett B., Bond M. Assessment of outcome after severe brain damage. Lancet (London, England) 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Julien J., Alsideiri G., Marcoux J., et al. Antithrombotic agents intake prior to injury does not affect outcome after a traumatic brain injury in hospitalized elderly patients. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2017;38:122–125. doi: 10.1016/j.jocn.2016.12.032. [DOI] [PubMed] [Google Scholar]
- Khan M., O'Keeffe T., Jehan F., et al. The impact of Glasgow Coma Scale-age prognosis score on geriatric traumatic brain injury outcomes. J. Surg. Res. 2017;216:109–114. doi: 10.1016/j.jss.2017.04.026. [DOI] [PubMed] [Google Scholar]
- Kinsella G.J., Olver J., Ong B., Gruen R., Hammersley E. Mild traumatic brain injury in older adults: early cognitive outcome. J. Int. Neuropsychol. Soc. 2014;20(6):663–671. doi: 10.1017/S1355617714000447. [DOI] [PubMed] [Google Scholar]
- Lecours A., Sirois M.-J., Ouellet M.-C., Boivin K., Simard J.-F. Long-term functional outcome of older adults after a traumatic brain injury. J. Head Trauma Rehabil. 2012;27(6):379–390. doi: 10.1097/HTR.0b013e31823b2385. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Cook S., Lee J.S., Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of Z-scores. Genomics Inform. 2016;14(4):173–180. doi: 10.5808/GI.2016.14.4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley E.J., Williams K.J., Schneider E.B., et al. Intensity of treatment, end-of-life care, and mortality for older patients with severe traumatic brain injury. J. Trauma. Acute Care Surg. 2016;80(6):998–1004. doi: 10.1097/TA.0000000000001028. [DOI] [PubMed] [Google Scholar]
- Linacre J.M., Heinemann A.W., Wright B.D., Granger C.V., Hamilton B.B. The structure and stability of the functional independence measure. Arch. Phys. Med. Rehabil. 1994;75(2):127–132. [PubMed] [Google Scholar]
- Maglinte G.A., Hays R.D., Kaplan R.M. US general population norms for telephone administration of the SF-36v2. J. Clin. Epidemiol. 2012;65(5):497–502. doi: 10.1016/j.jclinepi.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon D.K., Schwab K., Wright D.W., Maas A.I. Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91(11):1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Miller P.R., Chang M.C., Hoth J.J., et al. Predicting mortality and independence at discharge in the aging traumatic brain injury population using data available at admission. J. Am. Coll. Surg. 2017;224(4):680–685. doi: 10.1016/j.jamcollsurg.2016.12.053. [DOI] [PubMed] [Google Scholar]
- Milligan W.L., Powell D.A., Furchtgott E. The Older Americans Resources and Services interview and the medically disabled elderly. J. Geriatr. Psychiatr. Neurol. 1988;1(2):77–83. doi: 10.1177/089198878800100204. [DOI] [PubMed] [Google Scholar]
- Mohindra S., Mukherjee K.K., Gupta R., Chhabra R. Continuation of poor surgical outcome after elderly brain injury. Surg. Neurol. 2008;69(5):474–477. doi: 10.1016/j.surneu.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Mosenthal A.C., Lavery R.F., Addis M., et al. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J. Trauma. 2002;52(5):907–911. doi: 10.1097/00005373-200205000-00015. [DOI] [PubMed] [Google Scholar]
- Mosenthal A.C., Livingston D.H., Lavery R.F., et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J. Trauma. 2004;56(5):1042–1048. doi: 10.1097/01.ta.0000127767.83267.33. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters W., van den Brande R., Polinder S., et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015;157(10):1683–1696. doi: 10.1007/s00701-015-2512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M.J., Feinstein A. Age and functioning after mild traumatic brain injury: the acute picture. Brain Inj. 2001;15(10):857–864. doi: 10.1080/02699050110065303. [DOI] [PubMed] [Google Scholar]
- Røe C., Skandsen T., Manskow U., Ader T., Anke A. Mortality and one-year functional outcome in elderly and very old patients with severe traumatic brain injuries: observed and predicted. Behav. Neurol. 2015;2015:845491. doi: 10.1155/2015/845491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B.R., Moos R.M., Könü-Leblebicioglu D., et al. Higher age is a major driver of in-hospital adverse events independent of comorbid diseases among patients with isolated mild traumatic brain injury. Eur. J. trauma. Emerg. Surg. Off. Publ. Eur. Trauma. Soc. 2019;45(2):191–198. doi: 10.1007/s00068-018-1029-1. [DOI] [PubMed] [Google Scholar]
- Seibert P.S., Reedy D.P., Hash J., et al. Brain injury: quality of life's greatest challenge. Brain Inj. 2002;16(10):837–848. doi: 10.1080/02699050210131939. [DOI] [PubMed] [Google Scholar]
- Steyerberg E.W., Wiegers E., Sewalt C., et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–934. doi: 10.1016/S1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- Stiell I.G., Wells G.A., Vandemheen K., et al. The Canadian CT Head Rule for patients with minor head injury. Lancet (London, England) 2001;357(9266):1391–1396. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
- Styrke J., Stålnacke B.-M., Sojka P., Björnstig U. Traumatic brain injuries in a well-defined population: epidemiological aspects and severity. J. Neurotrauma. 2007;24(9):1425–1436. doi: 10.1089/neu.2007.0266. [DOI] [PubMed] [Google Scholar]
- Susman M., DiRusso S.M., Sullivan T., et al. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma. 2002;53(2):214–219. doi: 10.1097/00005373-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England) 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thompson H.J., McCormick W.C., Kagan S.H. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 2006;54(10):1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H.J., Weir S., Rivara F.P., et al. Utilization and costs of health care after geriatric traumatic brain injury. J. Neurotrauma. 2012;29(10):1864–1871. doi: 10.1089/neu.2011.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutomi T., Miyagi T., Ogawa T., et al. Age-associated increases in poor outcomes after traumatic brain injury: a report from the Japan Neurotrauma Data Bank. J. Neurotrauma. 2008;25(12):1407–1414. doi: 10.1089/neu.2008.0577. [DOI] [PubMed] [Google Scholar]
- van Aalst J.A., Morris J.A.J., Yates H.K., Miller R.S., Bass S.M. Severely injured geriatric patients return to independent living: a study of factors influencing function and independence. J. Trauma. 1991;31(8):1092–1096. [PubMed] [Google Scholar]
- Velez A.M., Frangos S.G., DiMaggio C.J., Berry C.D., Avraham J.B., Bukur M. Trauma center transfer of elderly patients with mild Traumatic Brain Injury improves outcomes. Am. J. Surg. 2020;219(4):665–669. doi: 10.1016/j.amjsurg.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Wan X., Liu S., Wang S., et al. Elderly patients with severe traumatic brain injury could benefit from surgical treatment. World Neurosurg. 2016;89:147–152. doi: 10.1016/j.wneu.2016.01.084. [DOI] [PubMed] [Google Scholar]
- Ware J.E.J., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Ware J.J., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Whitehouse K.J., Jeyaretna D.S., Enki D.G., Whitfield P.C. Head injury in the elderly: what are the outcomes of neurosurgical care? World Neurosurg. 2016;94:493–500. doi: 10.1016/j.wneu.2016.07.057. [DOI] [PubMed] [Google Scholar]
- Wilhelmson K., Andersson C., Waern M., Allebeck P. Elderly people's perspectives on quality of life. Ageing Soc. 2005;25(4):585–600. doi: 10.1017/S0144686X05003454. [DOI] [Google Scholar]
- Willer B., Ottenbacher K.J., Coad M.L. The community integration questionnaire. A comparative examination. Am. J. Phys. Med. Rehabil. 1994;73(2):103–111. doi: 10.1097/00002060-199404000-00006. [DOI] [PubMed] [Google Scholar]
- Wilson J.T., Pettigrew L.E., Teasdale G.M. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J. Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Won S.-Y., Dubinski D., Brawanski N., et al. Significant increase in acute subdural hematoma in octo- and nonagenarians: surgical treatment, functional outcome, and predictors in this patient cohort. Neurosurg. Focus. 2017;43(5):E10. doi: 10.3171/2017.7.FOCUS17417. [DOI] [PubMed] [Google Scholar]
- Yu F., Richmond T. Factors affecting outpatient rehabilitation outcomes in elders. J. Nurs. Scholarsh. an Off. Publ. Sigma. Theta. Tau. Int. Honor. Soc. Nurs. 2005;37(3):229–236. doi: 10.1111/j.1547-5069.2005.00040.x. [DOI] [PubMed] [Google Scholar]