Abstract
The CC or β-chemokines MIP-1α, MIP-1β, and RANTES are the primary components of human immunodeficiency virus type 1 (HIV-1)-suppressive soluble factors in vitro. We studied the relationship between the concentrations of MIP-1α, MIP-1β, and RANTES in plasma and HIV viral load in HIV-infected subjects. The HIV-positive patient group (n = 140) had significantly lower concentrations of all three β-chemokines (MIP-1α, P < 0.0005; MIP-1β, P < 0.005; RANTES, P < 0.0005) than the control group (n = 58 for MIP-1α, n = 27 for MIP-1β, and n = 59 for RANTES). In addition, we divided the patient group into three subgroups (high, moderate, and low) based on the number of HIV-1 RNA copies in the plasma (as measured by quantitative HIV RNA PCR). Again, all three subgroups had significantly lower concentrations of the β-chemokines than the HIV-negative control group. However, there was no significant difference in plasma β-chemokine concentrations among the three subgroups within the patient group (P < 0.3). Although our results demonstrate that HIV-infected individuals had significantly lower concentrations of circulating β-chemokines than healthy uninfected control subjects, we found no correlation between the concentrations of β-chemokines in plasma and HIV-1 viral load in HIV-infected individuals.
The chemokines, a superfamily of factors which possess the properties of both chemoattractants and cytokines, are divided into two subfamilies based on the position of four cysteine residues that form disulfide bonds: CXC, or α-chemokines, and CC, or β-chemokines (3, 18). The α-chemokines primarily activate neutrophils; whereas, the β-chemokines generally activate monocytes, basophils, and eosinophils. Some members of both subfamilies also activate lymphocytes (11, 18).
Chemokines function as modulators of the replication cycle of human immunodeficiency virus type 1 (HIV-1). In particular, the chemokine receptors act as coreceptors with the CD4 molecule for HIV-1 infection (5, 10). Certain members of the β-chemokine subfamily, macrophage inflammatory proteins 1α and 1β (MIP-1α and MIP-1β) and RANTES (for “regulated upon activation, normal T-cell expressed and secreted”), produced by CD8+ T lymphocytes, suppress the replication of macrophage-tropic (M-tropic) HIV-1 isolates in vitro but not T-cell line-adapted viral strains (5). The M-tropic HIV-1 isolates utilize the β-chemokine receptor (CCR-5) as an entry cofactor (1, 8, 9, 21). A 32-bp deletion in the CCR-5 receptor gene apparently alters the structure of the translated protein so as to prevent HIV-1 entry; thus, CCR-5 polymorphisms are thought to play an important role in HIV-1 transmission and pathogenesis (7, 21). Similarly, SDF-1 (stromal cell-derived cofactor 1), an α-chemokine which acts as an extremely efficacious chemoattractant for T lymphocytes, was identified as the natural ligand for CXC-chemokine receptor 4 (CXCR-4) and acts as the second receptor for T-cell line-tropic, but not M-tropic, HIV isolates (4, 19).
Because the β-chemokines (MIP-1α, MIP-1β, and RANTES) are major components of the HIV-suppressive soluble factors in vitro (5, 10), the present study was undertaken to study the relationship between concentrations of β-chemokines in circulation and viral load in HIV-infected subjects.
MATERIALS AND METHODS
Plasma.
Fifty-nine plasma samples for the control group were obtained from normal healthy volunteers, mostly Specialty Laboratories employees. All plasma donors were remunerated. The plasma samples were frozen at −20°C until tested. One hundred and forty plasma samples for the HIV-positive (HIV+) group were remnants of samples sent to Specialty Laboratories for routine clinical testing for HIV-1 viral load by quantitative PCR. The plasma samples were collected according to the collection procedure recommended by our clinical laboratory to ensure accurate quantitation of viral RNA. Plasma was separated by centrifugation of EDTA-blood immediately after draw to minimize the contamination of platelets. The plasma samples were frozen at ≤20°C and shipped on dry ice by overnight courier. The samples were stored in our serum bank at −20°C. The samples with fewer than 400 copies of HIV-1 RNA per ml were considered negative for HIV-1 RNA. However, we tested all of the plasma samples with fewer than 400 copies of HIV-1 RNA per ml by enzyme-linked immunosorbent assay (ELISA) for anti-HIV antibodies. Among 41 samples tested for anti-HIV-1 antibodies, 39 were positive, and the 2 negative samples were excluded from our data analysis.
HIV-1 RNA quantitation.
HIV-1 RNA in plasma was quantitated with the use of the Amplicor HIV-1 Monitor test kits (Roche Diagnostic Systems, Inc., Branchburg, N.J.). Briefly, HIV-1 RNA was extracted from 200 μl of plasma from random HIV-1+ specimens. Subsequently, the RNA was amplified and quantitated with a microtiter detection system. An internal quality standard was used to normalize for amplification and extraction.
HIV-1 immunoglobulin G antibodies.
Qualitative analysis for antibodies to HIV-1 was conducted with the HIVAB HIV-1 enzyme immunoassay (EIA) kit (Abbott Laboratories, Abbott Park, Ill.).
Chemokines.
Quantitation of MIP-1α, MIP-1β, and RANTES was performed with Quantikine EIA kits (R&D Systems, Inc., Minneapolis, Minn.) according to the manufacturer’s suggestions. Briefly, for MIP-1α, 200 μl of standard or sample was added to wells of microtiter plates coated with antibody to MIP-1α. For MIP-1β and RANTES, 100 μl of standard or sample was added to respective microtiter plates coated with antibody to MIP-1β and RANTES. Following incubation and washing, 200 μl of enzyme (conjugated with the respective antibodies) was added. After incubation and washing, 200 μl of substrate was added per well, and color development was stopped by the addition of 2 N sulfuric acid. The plates were read by an ELISA reader at 450 nM, and data were obtained by four-parameter analysis with standard samples (Softmax; Molecular Devices Corporation, Sunnyvale, Calif.).
Statistical analysis.
Student’s t test was employed to analyze differences between control and patient groups.
RESULTS
MIP-1α concentrations in HIV+ patients.
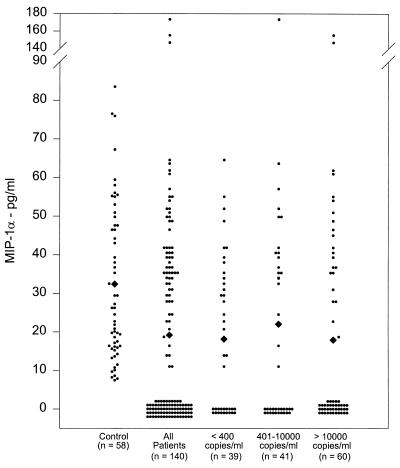
The 140 HIV+ patients had significantly lower concentrations of MIP-1α (19.2 ± 2.5 pg/ml; P < 0.0005) than the 58 control samples (32.4 ± 2.6 pg/ml) (Fig. 1). In addition, among 140 patient samples, 76 lacked detectable levels of MIP-1α (54%) compared to the control group (0%).
FIG. 1.
MIP-1α concentrations in HIV+ patients. Plasma samples from healthy controls and HIV+ patients were analyzed for MIP-1α concentrations by EIA. Means are indicated (diamonds).
The patient group was divided into three subgroups based on their HIV-1 viral load in plasma: group I patients (n = 39) were positive for anti-HIV immunoglobulin G by ELISA but had fewer than 400 copies of HIV RNA per ml, group II patients (n = 41) had 401 to 10,000 copies of HIV RNA per ml, and group III patients (n = 60) had more than 10,000 copies of HIV RNA per ml. All three groups had significantly lower concentrations of MIP-1α than the control group (control group, 32.4 ± 2.6 pg/ml; group I, 18.1 ± 3.2 pg/ml [P < 0.0005]; group II, 22 ± 5.0 pg/ml [P < 0.05]; group III, 17.9 ± 4.1 pg/ml [P < 0.005]) (Fig. 1). There was no significant difference in plasma MIP-1α concentrations among the three patient subgroups.
MIP-1β concentrations in HIV+ patients.
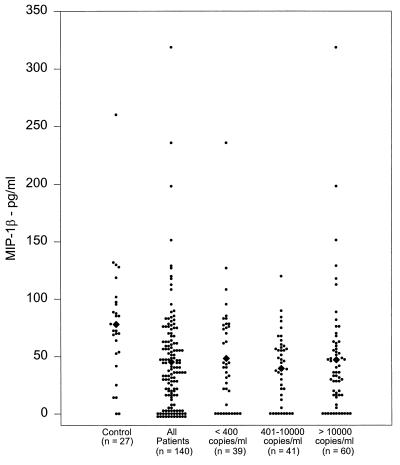
Similar to MIP-1α concentrations in plasma samples, MIP-1β concentrations in 140 patients were significantly lower (45.1 ± 3.9 pg/ml; P < 0.005) than those in the control plasma samples (77.9 ± 10 pg/ml) (Fig. 2). Of the 140 patients’ plasma samples, 29 (21%) had no detectable level of MIP-1β, compared to only 2 control samples (7%). Furthermore, when patients’ samples were analyzed on the basis of HIV RNA copies, all three subgroups had significantly lower concentrations of MIP-1β than the control group (group I, 48.2 ± 7.6 pg/ml [P < 0.025]; group II, 39.4 ± 4.7 pg/ml [P < 0.0005]; group III, 46.9 ± 6.9 pg/ml [P < 0.001]) (Fig. 2). There was no significant difference in plasma MIP-1β concentrations among the three patient subgroups.
FIG. 2.
MIP-1β concentrations in HIV+ patients. The plasma samples from healthy controls and HIV+ patients were analyzed for MIP-1β concentrations by EIA. Means are indicated (diamonds).
RANTES concentrations in HIV+ patients.
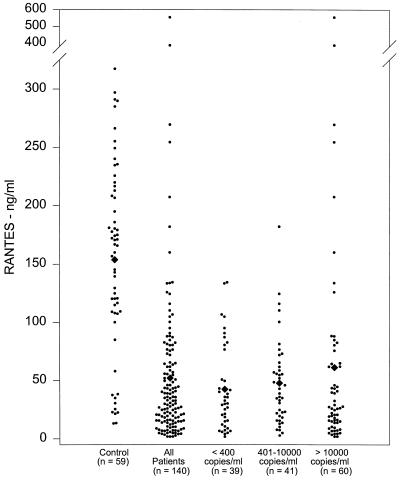
RANTES concentrations in plasma samples from both control and HIV+ patient groups were relatively higher than the concentrations of the other β-chemokines tested (MIP-1α and MIP-1β). However, the patient group had significantly lower concentrations of RANTES (52.2 ± 5.9 ng/ml; P < 0.0005) than the control samples (153.8 ± 10.6 ng/ml) (Fig. 3). Subsequently, when the patient samples were subdivided into three groups based on the viral RNA load, all three subgroups had significantly reduced concentrations of RANTES compared to the control group (group I, 42.7 ± 6 ng/ml [P < 0.0005]; group II, 47.9 ± 5.9 ng/ml [P < 0.0005]; group III, 61.2 ± 12.5 ng/ml [P < 0.0005]) (Fig. 3); again, there was no significant difference in plasma RANTES concentrations among the three patient subgroups.
FIG. 3.
RANTES concentrations in HIV+ patients. The plasma samples from healthy controls and HIV+ patients were analyzed for RANTES concentrations by EIA. Means are indicated (diamonds).
Lack of correlation between viral load and the concentrations of β-chemokines in HIV+ patients.
There was no significant positive or negative correlation between the concentrations of β-chemokines and viral load (r2, 0.26 for MIP-1α, 0.07 for MIP-1β, and −0.11 for RANTES).
DISCUSSION
The β-chemokines MIP-1α, MIP-1β, and RANTES are the major HIV-suppressive factors produced by CD8+ T cells (5). In addition to CD8+ T cells, CD4+ T cells from HIV-infected individuals also produce comparable concentrations of β-chemokines in vitro (13). The present study explored the possible relationship between the concentrations of the β-chemokines and HIV viral load in circulation. Our results demonstrate that HIV-infected patients have significantly reduced concentrations of MIP-1α, MIP-1β, and RANTES in plasma compared to uninfected individuals. Moreover, there was no correlation between the concentration of each of the chemokines and the number of HIV RNA copies in plasma.
Our findings support the conclusion that β-chemokines cannot alone be responsible for the CD8+ T-cell-mediated suppression of HIV replication in peripheral blood mononuclear cells from HIV-infected individuals (13, 23). The significant reductions in the concentrations of the β-chemokines in plasma in HIV-infected individuals observed in our study were not due to degradation of the chemokines by freezing and thawing. Two cycles of freeze-thawing of normal and patient plasma samples (eight in each group) resulted in minimal variation (percent coefficient of variation of mean values, 5% for MIP-1α, 6% MIP-1β, and 6% for RANTES).
The observed low concentrations of β-chemokines might reflect either reduction in the number of CD8+ T cells, which secrete the β-chemokines, or decreased production of the β-chemokines by CD8+ T cells. However, it is well known that the total number of CD8+ T cells is increased in most HIV-infected individuals (22), and CD8+ T cells from HIV-infected subjects produced concentrations of the β-chemokines comparable to those in CD8+ T cells from uninfected individuals in vitro (23). Additionally, declining numbers of CD4+ T cells may also have directly contributed to the reduction in the chemokine levels to some extent because of their ability to secrete chemokines (13). However, this contribution may be minimal, because of the observed absence of correlation between chemokines and viral load, which in turn has been shown to be indirectly related to CD4+ T-cell count (12). Alternatively, it may be possible that the β-chemokines produced are simply binding to CD4+ T cells and are subsequently eliminated from the circulation due to increased turnover of CD4+ T cells. Although our results do not support this hypothesis, recent findings that β-chemokine receptor (CCR5) expression in activated CD4+ T cells is 20-fold higher in normal individuals than in individuals homozygous for defective CCR5 alleles indirectly support our speculation (17).
The mechanism originally proposed for β-chemokine-mediated inhibition of M-tropic HIV-1 replication was that the β-chemokines bind to chemokine receptors that serve as a fusion cofactor for M-tropic HIV-1 and prevent virus-cell fusion, subsequent to CD4 binding (5, 20). This phenomenon was confirmed by various laboratories by identifying the β-chemokine receptor of CCR-5 as the second receptor for entry of M-tropic HIV isolates (1, 2, 8, 9). Biochemical studies on the interaction of CD4, gp120, and β-chemokine receptor showed that both the direct interaction of gp120 with the CCR-5 receptor and its affinity are greatly enhanced in the presence of CD4 (24, 25). The V3 domain of gp120 is the critical component of chemokine-mediated suppression of HIV-1 infection (6). Indeed, the region of the 32-bp deletion in the defective CCR-5 alleles corresponds to the second extracellular loop of CCR-5, the mutated form of which offers protection against HIV-1 infection (7, 21). The recent demonstration that MIP-1α, MIP-1β, and RANTES levels do not distinguish patients with AIDS from patients with nonprogressing HIV infection (16) indirectly supports our current findings. In contrast, Krowka et al. (14) showed increased RANTES concentrations in seroconverted patients compared to healthy controls. The concentrations of RANTES in those patients are quite comparable to our results. However, RANTES concentrations in their healthy control group were significantly lower than those in our control samples. The reason for this difference is unclear, although it may be due to variations among the individuals studied. Another difference in their study was the fewer number of individuals in each group. However, their conclusion is in agreement with our results showing that there is no significant association between β-chemokines and viral load (14). Additionally, in vitro studies on the production of β-chemokines by CD4+ and CD8+ T cells from HIV-infected and uninfected individuals did not provide evidence of a substantial protective role of β-chemokines, in spite of their control over the replication of primary non-syncytium-inducing, but not syncytium-inducing, HIV isolates (15). The absence of correlation between the concentrations of β-chemokines and HIV-1 viral load in plasma in HIV-infected subjects does not rule out the role of a factor(s) other than the β-chemokines in the suppression of HIV-1 replication in vitro.
ACKNOWLEDGMENTS
We thank Terry Robins for providing the clinical samples and Ashu Kumar, Foaad Hanna, and Narsis Bhasharkhah for excellent technical assistance. We also thank Ronald Blum for reviewing the manuscript.
Footnotes
Corresponding author. Mailing address: Specialty Laboratories, 2211 Michigan Ave., Santa Monica, CA 90404-3900. Phone: (310) 828-6543. Fax: (310) 828-5173. E-mail: VKakkanaiah@specialtylabs.com.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 4.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 7.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–667. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza M P, Harder V A. Chemokines and HIV-1 second receptors: confluence of two fields generates optimism in AIDS research. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 11.Hedrick J A, Zlotnik A. Chemokines and lymphocyte biology. Curr Opin Immunol. 1996;8:343–347. doi: 10.1016/s0952-7915(96)80123-3. [DOI] [PubMed] [Google Scholar]
- 12.Iuliano R, Forastieri G, Brizzi M, Mecocci L, Mazzotta F, Ceccherini-Nelli L. Correlation between plasma HIV-1 RNA levels and the rate of immunologic decline. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;14:408–414. doi: 10.1097/00042560-199704150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kinter A L, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci A S. HIV replication in CD4+ T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous β-chemokines and the viral inductive effects of other endogenous cytokines. Proc Natl Acad Sci USA. 1996;93:14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krowka J F, Gesner M L, Ascher M S, Sheppard H W. Lack of associations of chemotactic cytokines with viral burden, disease progression, or lymphocyte subsets in HIV-infected individuals. Clin Immunol Immunopathol. 1997;85:21–27. doi: 10.1006/clin.1997.4411. [DOI] [PubMed] [Google Scholar]
- 15.Mackewicz C E, Barker E, Greco G, Reyes-Teran G, Levy J A. Do β-chemokines have clinical relevance in HIV infection? J Clin Invest. 1997;100:921–930. doi: 10.1172/JCI119608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie S W, Dallalio G, North M, Frame P, Means R T., Jr Serum chemokine levels in patients with non-progressing HIV infection. AIDS. 1996;10:F29–F33. doi: 10.1097/00002030-199610090-00001. [DOI] [PubMed] [Google Scholar]
- 17.Moore J P. Coreceptors: implications for HIV pathogenesis and therapy. Science. 1997;276:51–52. doi: 10.1126/science.276.5309.51. [DOI] [PubMed] [Google Scholar]
- 18.Murphy P M. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 19.Oberlin E, Amara A, Bacherlerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 20.Oravecz T, Pall M, Norcross M A. β-Chemokine inhibition of monocytotropic HIV-1 infection: interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 21.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 22.Roederer M, Dub J G, Anderson M T, Raju P A, Herzenberg L A, Herzenberg L A. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;5:2061–2069. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubbert A, Weissman D, Combadiere C, Pettrone K A, Daucher J A, Murphy P M, Fauci A S. Multifactorial nature of noncytolytic CD8+ T cell-mediated suppression of HIV replication: β-chemokine-dependent and -independent effects. AIDS Res Hum Retroviruses. 1997;13:63–69. doi: 10.1089/aid.1997.13.63. [DOI] [PubMed] [Google Scholar]
- 24.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 25.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newmann W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]