Abstract
Bacterial biofilms are communities living in a matrix consisting of self-produced, hydrated extracellular polymeric substances. Most microorganisms adopt the biofilm lifestyle since it protects by conferring resistance to antibiotics and physico-chemical stress factors. Consequently, mechanical removal is often necessary but rendered difficult by the biofilm’s complex, viscoelastic response, and adhesive properties. Overall, the mechanical behaviour of biofilms also plays a role in the spreading, dispersal and subsequent colonization of new surfaces. Therefore, the characterization of the mechanical properties of biofilms plays a crucial role in controlling and combating biofilms in industrial and medical environments. We performed in situ shear rheological measurements of Bacillus subtilis biofilms grown between the plates of a rotational rheometer under well-controlled conditions relevant to many biofilm habitats. We investigated how the mechanical history preceding rheological measurements influenced biofilm mechanics and compared these results to the techniques commonly used in the literature. We also compare our results to measurements using interfacial rheology on bacterial pellicles formed at the air–water interface. This work aims to help understand how different growth and measurement conditions contribute to the large variability of mechanical properties reported in the literature and provide a new tool for the rigorous characterization of matrix components and biofilms.
Keywords: rheology, biofilm, Bacillus subtilis, biofilm mechanics
1. Introduction
Bacteria commonly exist in surface-attached aggregates of cells embedded in a matrix, called biofilms [1–3]. In biofilms, the cells are surrounded by a self-produced extracellular matrix, mainly consisting of hydrated extracellular polymeric substances (EPS) [4–6]. Viewed from the perspective of soft material science, a biofilm can be treated as a soft material composite where bacteria act as solid inclusions, and the extracellular matrix is a cross-linked polymer network [7,8]. Additionally, biofilms often exhibit complex three-dimensional structures and spatial heterogeneity that favour the transport of nutrients and oxygen [9–12].
Biofilms are undesired in a wide range of industrial and medical settings, causing problems ranging from industrial biofouling to chronic infections of medical implants [13–16]. In most cases, the biofilm forms on a solid substrate and is exposed to fluid flow, which influences the transport of nutrients, signal molecules and metabolites, as well as the structure of biofilms through drag stresses [17–24]. Biofilms exhibit viscoelastic characteristics, and, like most gels, they are sensitive to their mechanical history [25]. Their solid-like elastic properties play a role in the biofilms’ survival, and their understanding is important when it comes to resistance to biofilm removal in industry and medical applications [26,27]. For instance, lung clearance of cystic fibrosis patients is impeded if the elasticity of the lung mucus is increased due to an infection with biofilm-producing Pseudomonas aeruginosa [28]. On the other hand, the viscous nature enables the bacterial community to flow along surfaces when exposed to shear stresses. Under strong flow, the bacteria can even form filament-like structures called streamers [29–31]. This makes biofilm growth in flows, such as in industrial pipelines or ship hulls, possible and difficult to prevent [14,32]. It becomes obvious that precise characterization of biofilm mechanics under relevant conditions is crucial in understanding biofilm formation and evolution as well as battling biofilm contaminations. In this work, we exploit millifluidic techniques to grow bacterial biofilms in conditions relevant to many industrial and medical settings and present an improved method to measure the bulk shear rheology in situ.
In the past two decades, bulk shear rheology has been increasingly used to characterize bacterial biofilms. Several approaches have been used to measure biofilms’ rheological, viscoelastic properties [33]. While in situ measurements in liquid are limited to Staphylococcus epidermidis biofilms, the most common technique involves agar plates as a growth substrate and is applicable to many different microorganisms [34]. After biofilm formation at the agar–air interface, the biofilm is carefully scraped off the agar plates with a glass slide and placed on a standard rotational rheometer (plate–plate or cone and plate), where the biofilm is either compressed to a fixed gap [28,35–42] or to a fixed normal force [43–45]. To improve the reproducibility of the transfer from the agar plate to the rheometer, Körstgens et al. developed a technique where the biofilm is grown on a membrane placed on top of an agar plate [46]. This method was adapted for different bacterial species [28,47,48]. Alternatively, the agar plate with the biofilm can be directly used as a bottom plate on the rheometer [49,50]. Similarly, Walker et al. developed a non-destructive method where the bottom plate of the rheometer consists of a Petri dish, within which the biofilm is grown in liquid culture [51]. However, in these methods, the biofilm is grown on a moist substrate or in a liquid medium, and liquid flow is absent. In order to introduce flow during growth, the biofilm was grown on rotating discs in bioreactors filled with a nutrient solution, and the rotation of the discs introduced shear stresses. The biofilm-covered discs were then transferred to the rheometer, where the biofilm was brought into contact with the bottom plate and characterized [52–56]. As a drawback, in all the aforementioned methods, biofilms are exposed to mechanical stresses before the measurement, either through scraping from the agar plate or compression during loading on the rheometer. These mechanical stresses might introduce changes to the biofilm structure and influence the mechanical properties of the biofilm. Hence, a method to measure the biofilm in the unperturbed, as-grown state is needed to quantify the mechanical properties under relevant conditions.
This work presents a novel set-up to measure the in situ rheology of bacterial biofilms grown under relevant environmental conditions using a standard rotational rheometer. The experimental procedure allows us to precisely control the flow rate, hence the nutrient availability and hydrodynamic stresses experienced by the biofilm, and measure the mechanical properties during growth, as the biofilm is grown directly in the rheometer geometry. This avoids the exposure of the biofilm to strong mechanical stresses in the form of scraping, consolidation, or compression during collection, and transfer to the measuring device. We compare undisturbed biofilm grown in flow and measured in situ with scraped biofilms grown on agar plates and show how the mechanical properties, as well as the biological variability, differ. Furthermore, we demonstrate that exposing the biofilm to mechanical stresses before the measurement can have detrimental effects on the mechanical properties of the biofilm. These findings emphasize the influence of the mechanical history on the rheological properties of the biofilm and the need for careful experimental procedures to probe relevant properties of the biofilm.
2. Results
2.1. Biofilm grown on agar plates, collected via scraping
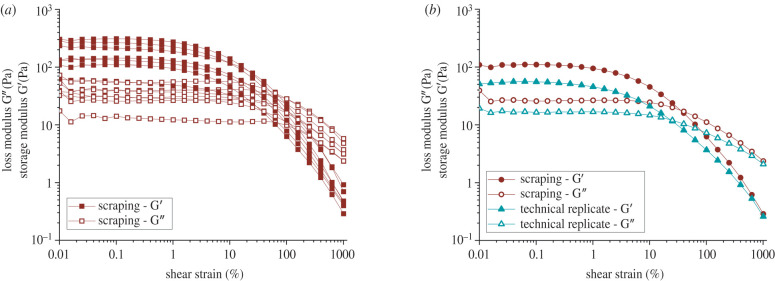
The bulk rheological measurements of biofilms grown on agar plates revealed their viscoelastic behaviour, as inferred from measurements of their linear viscoelastic moduli. However, a large variability is observed in biological replicates, where measurements are performed on different biofilms grown under identical conditions, as well as technical replicates, where two identical measurements are performed on the same biofilm sample. Bacillus subtilis NCIB 3610 biofilms were grown on 1% LBGM agar plates (see §4.1) and collected via careful scraping with a microscopy glass slide to transfer the sample onto the rheometer. Figure 1a shows the results from amplitude sweeps performed at 1 rad s−1 on seven biological replicates. The (elastic) storage modulus G’ and the (viscous) loss modulus G” were measured as a function of the shear strain amplitude. All biological replicates showed viscoelastic behaviour with a linear viscoelastic regime up to a shear strain of 1–5% where the biofilm behaved elastically as G’ > G”. The results of the biological replicates were subject to large variability. For instance, the plateau value of the storage modulus G’ taken at varied for different measurements by a factor of 6 from about 56 Pa to 309 Pa, and the loss modulus G” by a factor of 4 from 14 Pa to 55 Pa. By contrast, measurements on a reference viscoelastic material in our laboratory deviate about 3% between the magnitudes of the moduli when standard operating procedures are pursued.
Figure 1.
Storage (G’) and loss (G”) moduli of Bacillus subtilis NCIB 3610 biofilms as a function of strain amplitude. These biofilms were grown on 1% LBGM agar plates at 30°C for 24 h and collected via gentle scraping with a microscopy glass slide. (a) Amplitude sweeps at 1 rad s−1 of seven biological replicates. (b) Recovery: amplitude sweeps of a freshly scraped biofilm and a technical replicate of the same sample. Between the measurements, the biofilm was allowed to relax for 5 min.
Repeat measurements of the same biofilm sample (being exposed to strain amplitudes in the nonlinear response regime) consistently led to an ever lower loss and storage modulus. Figure 1b shows the moduli in the initial amplitude sweep (red) performed on the biofilm immediately after scraping and transferring the sample to the rheometer, as well as the technical replicate of the same sample (green), which has hence previously been exposed to an oscillatory strain with a maximum amplitude of 10 strain units (or 1000%) at 1 rad s−1. Between these two measurements, the sample was allowed to rest in the rheometer for five minutes. Both the loss and storage modulus values were considerably lower when the same sample was measured for a second time. These technical replicates were performed for three biofilms, and for all measurements, the linear viscoelastic moduli at small strain amplitudes subsided on average by a factor of 2. These findings show that biofilms exposed to shear in the nonlinear viscoelastic regime exhibited a substantial decrease in mechanical properties. Within the timescale of our experiment, we did not see any recovery of the mechanical properties (electronic supplementary material, figure S1) and therefore attributed this decrease in the shear modulus to structural changes, which are effectively irreversible with respect to the timescale of our experiment. In summary, the mechanical history of the biofilms played a crucial role in the quantification of the mechanical properties of the biofilm; even a large strain amplitude simple shear flow causes substantial effects. In order to avoid transfer and loading onto the rheometer, a new growth protocol was needed to probe the rheology of unperturbed samples of biofilm.
2.2. Rheology of biofilm grown in flow
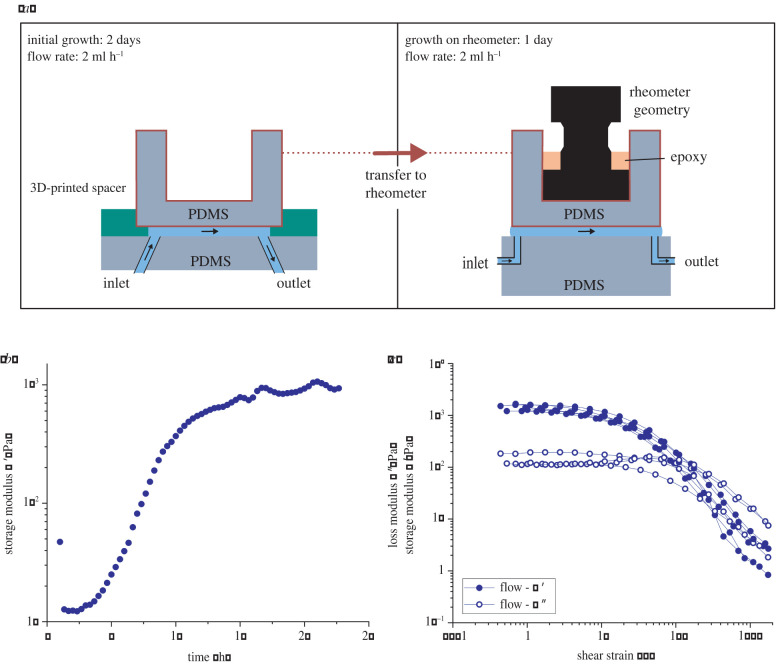
We developed an experimental protocol to grow biofilm in flow in situ in a rheometer geometry to avoid interference of mechanical stresses during sample transfer. First, the linear viscoelastic properties of the biofilm during growth were studied up to when it was fully developed. Figure 2a shows a schematic of the experimental procedure. The B. subtilis biofilm was allowed to grow for 3 days in the rheometer geometry with a continuous flow of nutrients, while the growth was divided into two stages (stages 1 and 2) and a final measurement stage (stage 3).
Figure 2.
(a) Schematic of the experimental set-up for the biofilm grown in flow (in LBGM medium at 30°C with flow speed v = 346 μm s−1) for a total of 3 days; 2 days in the millifluidic device, 1 day on the rheometer. (b) Representative small amplitude oscillatory shear measurements over time for B. subtilis biofilms grown in flow on the rheometer. (c) Amplitude sweeps of five biological replicates of biofilms grown in flow on the rheometer.
Stage 1: During the initial stage, the biofilm was grown under constant flow in a circular millifluidic device, with a diameter of 18.5 mm and a height of 200 μm made of polydimethylsiloxane (PDMS) with a detachable upper part. The growth conditions were kept constant in LBGM medium at 30°C with flow speed v = 346 μm s−1. After 2 days, a stable biofilm formed on the PDMS surfaces, distinguishable by eye as a uniform, white layer. The development of the biofilm in the flow cell during stage 1 was also studied in situ by phase contrast microscopy.
Stage 2: Subsequently, an aluminium rheometer geometry, compatible with an Anton Paar MCR rheometer disposable geometry measuring system, was attached to the upper, detachable part of the millifluidic device using epoxy that was allowed to dry for several hours. Then, the whole upper part of the millifluidic cell with the attached biofilm was transferred to the rheometer, gently brought into contact with a PDMS bottom plate, and retracted to establish a gap of 200–250 μm. In the course of this paper, we will refer to this time point as t = 0 h, as it is also the starting point of the rheological measurements. After the transfer, the biofilm was allowed to grow for another day under steady nutrient flow (in LBGM medium at 30°C with flow speed v = 346 μm s−1), on the rheometer. During this growth stage on the rheometer, G’ and G” were probed with a time sweep in the linear viscoelastic regime at a constant frequency (ω = 1 rad s−1). It was observed that the results for the moduli in stages 2 and 3 are frequency independent (see further) in the accessible range of frequencies. This suggests that biofilm formation in stages 2 and 3 is not a critical gelation process, as described by Winter & Chambon [57]. So for this growth stage, a time sweep at a single frequency suffices to characterize the time evolution of the biofilm with minimal interference.
Stage 3: After the formation of a steady-state biofilm on the rheometer, strain amplitude sweeps from at 1 rad s−1 were performed, or for some films frequency sweeps in the linear response regime. During the amplitude and frequency sweeps, the fluid flow was stopped to avoid any interference with the oscillatory measurements.
The rheometer set-up consisted of the upper part of the first millifluidic cell as the ceiling and of a PDMS ‘floor’. The PDMS floor was equipped with an inlet and outlet that allowed us to drive fluid flow during the final growth stage. The laterally open set-up permitted rheological measurements during all growth stages, while the surface tension of the nutrient solution ensured a nearly constant shape at the edge of the parallel plates. Special care was taken to ensure centricity and parallelism of the upper and lower parts of the rheometer set-up. To validate the set-up, a solution of a reference material, a wormlike micellar solution (100 mmol l−1 cetylpyridiniumchloride in a aqueous solution containing 100 mmol NaCl and 60 mmol sodium salicylate), with a single relaxation time was used to perform calibration measurements, which revealed a good agreement with the literature as well as commercial measuring systems using a standard 40 mm parallel plate configuration on the same rheometer (electronic supplementary material, figure S2) [58]. In the relevant frequency range (between 1 and 10 rad s−1), the moduli measured with the PDMS set-up deviated by no more than 10% from the moduli measured with the commercial measuring system. The experimental design with locally distinct stages, stage 1 outside of the rheometer and stages 2 and 3 on the rheometer, enabled us to effectively use instrument time and perform more biological replicates, even with long growth times of up to several days. Additionally, the use of a closed millifluidic device avoided contamination and, at the same time, provided consistent and well-controlled growth conditions over the whole growth period.
One of the main benefits of this experimental approach is the ability to measure the mechanical properties during growth without altering the biofilm structure, using small amplitude oscillatory shear. Figure 2b shows a representative evolution of the storage modulus G’ of B. subtilis biofilm grown in flow over time, for strain amplitudes in the linear response regime. The measurement showed that after an initial plateau of 2–3 h, G’ steeply increased until another plateau was reached after 10–12 h of growth on the rheometer. This plateau indicated that the biofilm could be considered fully developed, or at steady state. It is important to note that G’ > G” was observed during the entire course of this measurement. This demonstrated that a network with a weak elastic response, with magnitudes of the torque signal close to the resolution limit of the rheometer set-up, was formed early after the transfer of the biofilm onto the rheometer. The subsequent increase of G’ seems to be due to homogeneous growth and densification of an already developed network spanning the rheometer gap.
The biofilms grown in the millifluidic cell under flow conditions showed a much smaller variability among biological replicates compared to those prepared by scraping. Figure 2c shows the results of amplitude sweeps of five biological replicates of biofilms grown in flow. The measurements were performed on fully formed biofilms, characterized by a plateau in G’ at small strain amplitudes measured over time (figure 2b).
The biofilm grown in the millifluidic cell does show qualitatively similar behaviour to the scraped biofilm grown on agar, a viscoelastic behaviour with an elastically dominated linear regime for and an average G’ small strain amplitude linear viscoelastic modulus, which now is observed to have a much higher value of G′ ≈ 1300 Pa (factor 5–20 higher when compared to scraped biofilm). In contrast with the scraped biofilms, G’ varied only by a factor of 1.325 from 1200 Pa to 1590 Pa at , and G” varied by a factor of 2 from 110 Pa and 230 Pa. These results correspond to a small variability in biological replicates compared to scraped biofilms where G’ varied by a factor of 5.5, G” by a factor of 4. The reduction of the variability when comparing data from figure 2c and figure 1a clearly shows that the variability observed in figure 1a came from the mechanical scraping procedure.
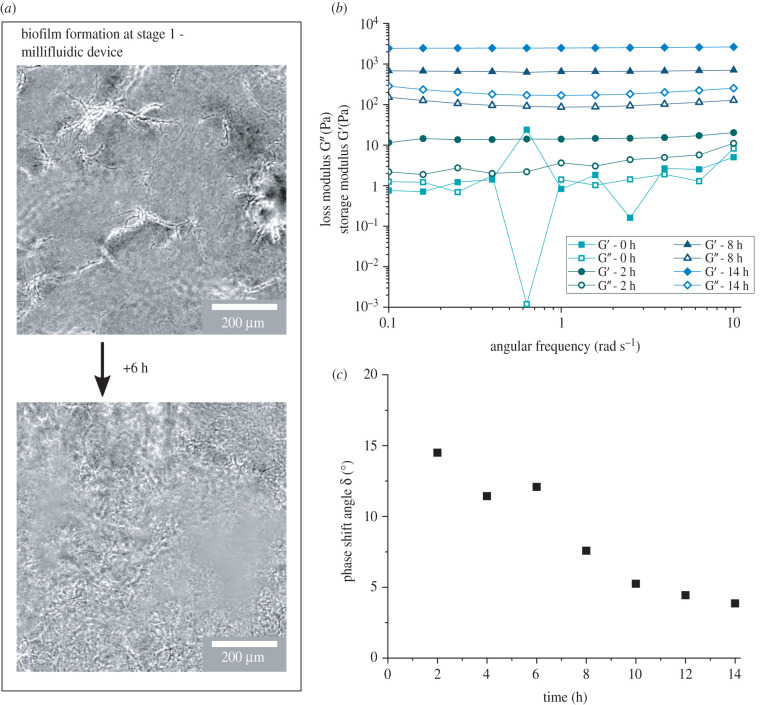
To visualize the formation of the biofilm during stage 1, phase contrast images of the biofilm growth in the millifluidic device (initial growth, stage 1) were taken at the midplane at least 100 μm away from the vertical and horizontal channel surfaces. The images in figure 3a show the temporal evolution of the biofilm where first a weak, percolating network was formed before it was filled in, strengthened and densified through growth. After approximately 8 h (figure 3a, upper image) in the millifluidic device, single strands of bacteria and biofilm matrix were forming a first gap spanning network, clearly visible in the focal plane located at the midplane of the device. In the following hours, the network densified through growth until the whole field of view was filled with biofilm, and an elastic, strong network was formed (figure 3a, lower image). Although the process of biofilm formation on the rheometer was optically not accessible, the images of the biofilm at the late stages in the millifluidic device showed qualitative indication of gap filling network formation of B. subtilis biofilms. These findings are in good agreement with previous, more detailed confocal microscopy studies on related strains that revealed that B. subtilis forms a three-dimensional, fibrillar structure of bacterial cells connected by eDNA and EPS [59,60].
Figure 3.
Development of B. subtilis biofilm grown in flow (LBGM medium at 30°C with flow speed v = 346 μm s−1) over time. (a) Growth during stage 1: phase contrast microscopy images of the network formation of B. subtilis biofilm during stage 1 in the millifluidic device, which holds the detachable measurement geometry (depicted in figure 2a). Images were taken at the midplane, 100 μm away from the vertical and horizontal channel surfaces. In phase contrast, the cells appear black while the biofilm matrix appears white. (b) Frequency sweeps of biofilms grown with constant nutrient flow during the growth phase on the rheometer, after 2 days of growth in the separate millifluidic device. (c) Phase shift angle taken at ω = 1 rad s−1 from the frequency sweeps, which were performed every 2 h during growth.
Frequency sweeps within the linear viscoelastic response regime during the growth phase of the biofilm upon transfer to the rheometer reveal how B. subtilis biofilms developed a solid-like response from the start, when first grown in flow, with no dependence on the frequency of the elastic moduli. The solid-like response stems from the fact that B. subtilis establishes a space-filling three-dimensional, elastic network early after the transfer to the rheometer. The initially weak network was strengthened through growth as the level of the moduli increased as a function of time. Figure 3b shows that at the beginning of growth during stage 2 on the rheometer, t = 0 h, the values of G’ and G” are small and close to the resolution limit of the rheometer set-up, where small differences in the phase angle can have significant effects on G’ and G” and lead to outliers (such as the drop in G” at ω = 0.6 rad s−1). However, 2 h after the transfer to the rheometer, an elastic response is clearly observed due to the formation of a gel network which leads to G′ > G″. The small, constant slope of the shear moduli with respect to the frequency indicated a power-law regime, which shows that initially relaxation processes are still possible. However, a continual strengthening of the network over time, an increase in both the storage and loss moduli, as well as a transition from the power-law regime to true solid-like behaviour with frequency-independent moduli (in the accessible frequency range) were observable and correspond to the response of a well-developed elastic network [61]. This increase in strength over 2–3 orders of magnitude and the transition to a solid-like material were also reflected in the evolution of the phase shift angle δ at ω = 1 rad s−1 over time (figure 3c). The decrease in the phase shift angle indicates that the elastic contribution became increasingly more dominant compared to the viscous contribution. At t = 10–12 h a true plateau in δ was reached, which is in agreement with the oscillatory measurements over time (figure 2b), where a plateau in G’ was reached after t ≈ 10 h.
2.3. Comparison of properties of scraped, in flow grown biofilm and bacterial pellicles
Bacillus subtilis is known to form biofilm at the air–liquid interface in the form of pellicles which can be measured with interfacial rheology [62–64]. We found that these pellicles showed viscoelastic behaviour qualitatively and quantitatively similar to the in flow grown biofilm measured in situ. The pellicle was grown on an interfacial rheometer with a double wall ring in stagnant conditions without liquid flow. During the growth of the pellicle, the mechanical properties were measured through small amplitude oscillatory shear measurements. The evolution of the interfacial storage modulus in figure 4 showed similar behaviour to the evolution of the bulk material’s shear modulus (G’) in time (figure 2b). At the beginning of the experiment, the signal was below the sensitivity of the rheometer until after 7.5 h started to increase measurably. The interfacial storage modulus reached a steady plateau after 11 h of growth.
Figure 4.

Representative small amplitude oscillatory shear measurement over time for B. subtilis pellicles grown at the air–liquid interface in LBGM medium at 30°C on an interfacial rheometer. Measured using a double wall-ring geometry at 1 rad s−1 and a strain amplitude of 0.1%.
During the growth of the pellicle, we assume that similar to the growth in flow, the nutrients are not limited as the volume of the nutrient solution is large (20 ml). Therefore, we do not expect complex growth or nutrient gradients in the pellicle as could occur in the biofilm grown on agar plates. Once the pellicle was fully developed, indicated by the levelling off of , an amplitude sweep was performed to compare the rheological properties of the pellicle with those of biofilms obtained by scraping or grown in the millifluidic geometry. The rheological properties of the pellicle were measured in situ, without transferring or disturbing the biofilm at all, before the measurement. To compare the interfacial and bulk three-dimensional material properties, equivalent bulk shear moduli of the pellicles can be estimated by dividing the measured interfacial moduli by the order of magnitude of the thickness of the pellicle, which was found to be 150 μm. The amplitude sweeps of four biological replicates and the results for the storage modulus are shown in figure 5. The plateau values of the equivalent G’ (obtained from the interfacial measurements) varied by a factor of 1.65 from 1150 Pa to 1900 Pa. These results are similar to the mechanical properties of the in flow grown biofilm, in which G’ plateau values ranged between 1200 Pa and 1590 Pa, varying by a factor 1.325.
Figure 5.
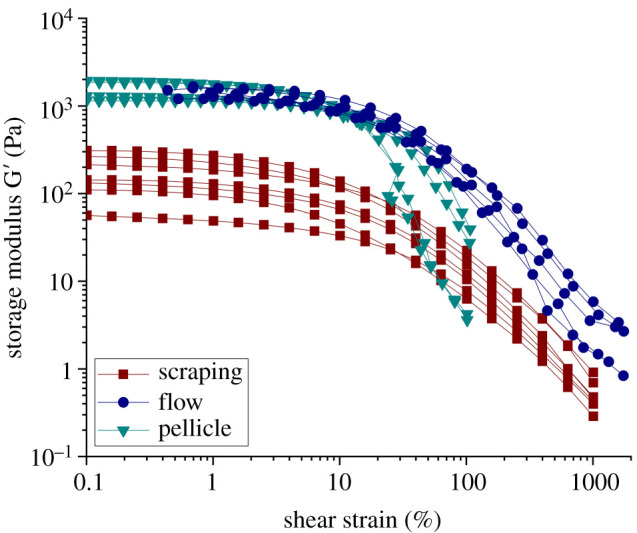
Comparison of storage modulus G’ of B. subtilis biofilms grown on agar plates and collected via scraping (red), grown in flow directly on the rheometer (blue) and apparent moduli calculated from the interfacial moduli obtained on pellicles grown at the liquid–air interface (green).
To compare the effects of the different growth conditions (plate/flow/interface) in more detail, we measured the solid content of B. subtilis biofilms formed under these three different growth conditions and found that the solid content varied greatly (table 1). While biofilms grown under static conditions on agar plates showed a high solid content with 18.3%, the biofilms grown in flow had a distinctly lower solid content with 4.8%. The biofilms grown at the air–liquid interface, which is also formed under static conditions but without a solid substrate, had a solid content of 8.4%, representing a more open structure compared to biofilms grown on agar. The standard deviation for the solid content measurements was between 0.7 and 1.2, with at least five samples per growth condition. Although the solid content was measured for different growth conditions, the nutrient solution, temperature and microorganism were identical for all measurements. Contrary to polymer gels, where an increase in solid content often leads to an increase in the mechanical properties, solid content of biofilms under different growth conditions is hence not a clear indicator of mechanical properties as an increase in solid content is not necessarily correlated to an increase in G’. Biofilms grown on agar showed the highest solid content, but also the lowest G’ plateau values with the largest variability. This large variability in mechanical properties was not reflected in the variability in solid content between biological replicates, which was small and similar for all growth conditions.
Table 1.
Solid content of B. subtilis biofilms under various growth conditions, presented in figures 1, 2 and 4. The statistical difference was computed with a t-test and is indicated in the table (*p < 0.01).
 |
Our experiments revealed that B. subtilis biofilms measured in situ exhibited a clear increase in their shear moduli as well as a smaller variability in their mechanical properties compared to biofilms that have been exposed to complex mechanical histories prior to the measurement. We compared the storage modulus G’ of biofilm grown in flow, grown on agar plates, and the equivalent bulk storage modulus for pellicles grown at the air–liquid interface (figure 5). For the biofilms grown in flow and the pellicles, both directly grown and measured on the rheometer, the values for G’ and the equivalent modulus inferred from the interfacial ones agree surprisingly well. On the other hand, the biofilm grown on agar plates with a mechanical history during scraping and transfer to the rheometer showed a decrease in the storage modulus G’ with larger variability. For all three growth conditions, the response was viscoelastic with a linear regime where G’ > G” for strain amplitudes of only a few per cent, consistent with a polymer gel network. The pellicle and the in flow grown biofilm were characterized by equivalent G’ or G’ plateau values of 1–2 kPa. By contrast, the average G’ plateau value of scraped biofilm grown on agar was one order of magnitude lower, due to the measurement protocol used. Additionally, measurements with scraped biofilm were subject to large variability between biological replicates as the G’ plateau values varied by a factor of 5.5, while values for the pellicle and in flow grown biofilm varied by a factor of 1.65. These results show that a mechanical deformation history of the biofilm drastically influences the rheological properties of the biofilm measured through shear rheology.
3. Discussion and conclusion
In the present work, the importance of characterizing the rheological properties of biofilms in their unperturbed state was shown, as a history of mechanical perturbations due to biofilm removal and sample loading lead to a decrease in mechanical properties, which are effectively irreversible. To this end, a novel technique to measure the rheology of bulk, three-dimensional bacterial biofilms grown under continuous flow conditions was developed. This set-up enables one to measure the bulk rheology of biofilm in a near pristine condition, as no scraping or compression was necessary during the transfer onto the rheometer. Other studies investigating the rheology of B. subtilis biofilms found similar values for the biofilm elasticity, but under various temperature, nutrient and growth conditions [37,39,42,45,48]. Our results present the first direct comparison of growth conditions, in flow, on agar plates, and as pellicles, under otherwise identical growth temperatures and nutrient conditions. To this end, we found a clear difference in mechanical properties between biofilms that have been subject to scraping before the rheological characterization and biofilms that have been measured as grown, in situ. We compared three different growth conditions, biofilm grown on agar plates, biofilm grown in situ in a flow-through device at the solid–liquid interface, and biofilm in the form of pellicles at the air–liquid interface. While the biofilm grown in flow and the pellicle were grown directly in the measurement geometry, the biofilm grown on agar plates was subject to scraping and compression during the transfer and loading on the rheometer. For biofilms grown directly on the rheometer, we find order of magnitude higher G’ plateau values compared to biofilms that were subjected to scraping before the measurement. Since pellicles, where flow is absent, as well as biofilms grown in flow, exhibit very similar G’ plateau values, we conclude that in B. subtilis biofilms the mechanical history preceding the rheological measurement is of major importance. Therefore, we hypothesize that the biofilm grown on agar experiences mechanical deformation during scraping, leading to irreversible structural changes that negatively impact the mechanical properties. A further decrease of the G’ plateau value for a technical replicate on the same sample, which had been exposed to large deformations, is in good agreement with this hypothesis. The rheological properties of biofilms subjected to large deformations in the nonlinear regime are hence associated with possibly irreversible structural changes of the biofilm itself. Furthermore, since scraping is performed by hand and therefore inadvertently in a non-uniform manner, each sample is subject to a different mechanical history. This difference in mechanical history would explain the large variability in biological replicates observed in scraped biofilms.
We find a clear difference between the solid content of the three biofilms studied, which can help interpret the factors determining the mechanical properties. Although the biofilm grown in flow and the pellicle exhibit a larger G’ plateau value, the solid mass is considerably smaller when compared to biofilms grown on agar plates. Therefore, as an increase in solid content not necessarily leads to an increase in mechanical strength, we hypothesize that only some biofilm components contribute to the network strength and elasticity. Recent work found that P. fluorescens exposed to hydrodynamic stresses formed mechanically stronger biofilms compared to those formed in static conditions, and this was attributed specifically to an increase in EPS content [65]. This increase in matrix components was hypothesized to lead to an increase in cross-linking density in the biofilm. However, our results show that B. subtilis exposed to hydrodynamic stress formed stronger biofilms but with a lower solid content compared to static conditions on agar. We argue that for B. subtilis biofilms an increase in solid mass does not necessarily correspond to an increase in cross-links. In B. subtilis biofilms grown in flow, loosely bound, non-contributing components could be simply washed away, which leads to a lower solid content () compared to biofilm grown on agar. In pellicles, some loosely bound components may be dispersed in the underlying liquid, which leads to a lower biofilm solid content () compared to biofilm grown on agar. Nevertheless, the solid content of in-flow-grown biofilm is clearly lower compared to pellicles as the advective process of washing out unbound components with flow is more effective than passive dispersal in the underlying, stagnant liquid. However, the effective shear moduli of the pellicle are similar to the rheological properties of in-flow-grown biofilms. We attribute this to our measurement method, which avoids mechanically altering the biofilm before the measurement. Conversely, in biofilms grown on agar, the loosely bound biofilm components cannot relocate as the agar substrate is solid, and the components remain in the biofilm without contributing to strengthening the network. Therefore, we assume that the mechanical properties of the biofilm are dominated by a subset of bound components constituting the percolating network we characterized in the in-flow-grown biofilm.
Our experimental set-up allows us to grow biofilm in flow directly on the rheometer and probe the biofilm during its growth phase. Shear rheology during growth in combination with optical microscopy data as well as microstructural analyses from previous studies indicates that B. subtilis biofilms grown in flow form a weak, percolating network that increases in strength as they grow [59,60,66]. This emphasizes that rheological characterizations are not limited to studying the biofilm's mechanical properties, but can also shed light on more complex and time-dependent properties, such as network formation kinetics. Additionally, the experimental set-up presented in this work opens up the possibility of studying the bulk rheology of biofilms of other organisms. Furthermore, the growth conditions are well controlled and adjustable, enabling future works to study biofilms grown under different flow rates, growth media or temperature.
4. Methods
4.1. Culture conditions and growth on agar plates
In this work, Bacillus subtilis NCIB 3610 wild-type was studied. Bacillus subtilis NCIB 3610 was grown overnight in lysogenic broth (LB) with glycerol and manganese sulfate (LBGM media, 20 g LB, 0.1 mM MnSO4 and 1% v/v glycerol), inoculated from plates at 30°C while shaking at 200 r.p.m. LBGM plates with 1% agar were inoculated from an overnight culture (100 μl) using an L-shaped spreader to evenly distribute the inoculum on the agar surface. The plates were placed in an incubator at 30°C for 24 h.
4.2. Culture conditions and growth in flow
Bacillus subtilis NCIB 3610 was grown overnight in LBGM media (20 g LB, 0.1 mM MnSO4 and 1% v/v glycerol), inoculated from overnight stock, at 30°C with shaking at 200 r.p.m. After 20 h, a subculture was made by diluting the overnight culture 1:100 and incubated during 3 h at 30°C while shaking at 200 r.p.m. This early-exponential bacterial solution was then used to inoculate the millifluidic device by withdrawing 1 ml. The bacteria were left at 30°C for 3 h before turning on the flow of LBGM medium with a constant flow rate of Q = 2 ml h−1.
4.3. Culture conditions and growth of pellicles
Bacillus subtilis NCIB 3610 was grown overnight in LBGM media (20 g LB, 0.1 mM MnSO4 and 1% v/v glycerol), inoculated from overnight stock at 30°C while shaking at 200 r.p.m. After 20 h, a subculture was made by diluting the overnight culture 1 : 100 and incubated during 3 h at 30°C while shaking at 200 r.p.m. This early-exponential bacterial solution was then used to inoculate the double wall-ring cup with 5 ml pipetted into the cup. The temperature was held constant at 30°C during the growth phase of 20 h.
4.4. Bulk rheology of scraped biofilms
The viscoelastic response of each biofilm was quantified using an Anton Paar MCR302 rheometer. Briefly, biofilm colonies were grown on LBGM plates with a 1% agar concentration as described above. After 24 h incubation at 30°C the biofilms were transferred to the rheometer via gentle scraping with a glass microscopy slide. For each measurement, the biofilm was collected from 2 to 10 plates to accumulate sufficient biomass (approx. 1 ml). To minimize slip during the rheology measurement, grit paper was added to the bottom plate of the rheometer using double-sided tape. All measurements were performed using a 25 mm hatched parallel plate geometry. For each measurement, the temperature was set at 30 ± 0.1°C. Oscillatory amplitude sweeps were performed at a frequency of ω = 1 rad s−1 and strain range of . A total of seven biological replicates were performed.
4.5. Millifluidic device and growth in flow
The growth of the biofilm in flow was separated into two parts (figure 2). The initial phase of the growth was done in a millifluidic device, where the bottom and top surface consisted of PDMS (Sylgard 184 Silicone Elastomer Kit, Dow Corning, Midland, MI, USA) to ensure optimal oxygen permeability. To minimize slip during the rheology measurements the PDMS for the top of the millifluidic device was cast on sanding paper and thoroughly cleaned with soap and isopropanol. The top and bottom were connected with circular, 3D-printed, 200 μm thick spacers. Everything was connected and sealed using PDMS, which ultimately resulted in a circular millifluidic device with a diameter of 18.5 mm and a height of 200 μm. Flow was introduced by a syringe pump (Standard PHD Ultra syringe pump, Harvard Aparatus) through opposing inlet and outlet with a diameter of 1.5 mm and a flow rate of Q = 2 ml h−1. This leads to an average shear rate of 9.38 s−1 and an average flow speed of 346 μm s−1 in the millifluidic device. Before bacterial inoculation, the device was flushed with 5 ml of LBGM medium. The temperature was held constant at 30°C during the 2 days of growth.
The biofilm was allowed to grow for 2 days before the top was transferred to the rheometer (figure 2). Before the transfer, a disposable rheometer measuring system (D-PP12, Anton Paar) was added to the top of the millifluidic device and secured with epoxy. Subsequently, the PDMS top was separated from the millifluidic device and transferred to the rheometer with the adhering biofilm. The biofilm was brought into contact with the bottom plate until a normal force of was registered. Afterwards, the upper geometry was retracted to ensure a gap size of 200–250 μm. The bottom plate of the rheometer set-up consisted of a PDMS cylinder that was cast on sanding paper to ensure a rough surface to minimize slip during the measurement. The cylinder was equipped with opposing inlet and outlet of 1.5 mm. During the growth phase, the biofilm was exposed to a flow rate of Q = 2 ml h−1 driven by a syringe pump infusing LBGM medium at the inlet and withdrawing at the same flow rate at the outlet. The temperature was held constant at 30°C.
4.6. Bulk rheology of biofilms grown in flow
After the 2 days growth phase in the millifluidic device, the biofilm was transferred to a modular compact rheometer (MCR-302, Anton Paar). During the growth phase on the rheometer, oscillatory shear with at ω = 1 rad s−1 was applied to probe the biofilm during growth. Additionally, every 2 h a frequency sweep at was performed over a range of ω = 0.1–10 rad s−1. These measurements were performed while flow was induced with the syringe pump. After 15–20 h, when a plateau in the storage modulus G’ of the growth curve (figure 2) was reached, flow was turned off and oscillatory amplitude sweeps were performed at a frequency of ω = 1 rad s−1 and strain range of γ ≈ 1–1000%. After the measurement, the biofilm was removed from the rheometer to determine the solid content as explained in the following. Finally, after the removal of the sample, the exact gap size was determined and used to calculate the exact shear rate at which the experiment was carried out.
4.7. Interfacial shear rheology of pellicles
For the interfacial rheology of pellicles, a DHR-3 (Texas Instruments) with a double wall-ring geometry (outer radius of 35.5 mm) was used. The cup was equipped with a subface connection, where new LBGM medium was supplied at a rate of 0.3 ml h−1 to account for evaporation during the growth phase using a syringe pump. During the growth phase on the rheometer, oscillatory shear with at ω = 1 rad s−1 was applied to probe the biofilm during growth. After 15 h, nutrient supply was turned off and oscillatory amplitude sweeps were performed at a frequency of ω = 1 rad s−1 and strain range of γ = 0.01–100%. A microscopy slide was used to transfer the pellicle onto a thin layer of LBGM medium in a Petri dish and light microscopy was used to determine the order of magnitude of thickness of the pellicle to be approximately 150 μm. The bulk loss and storage moduli were then calculated dividing the results from the interfacial shear rheology by the thickness of the pellicles.
4.8. Solid content measurements
The solid content of the biofilm colonies was obtained by growing the biofilm on LBGM plates. LBGM plates were inoculated from an overnight culture (100 ml) using an L-shaped spreader to evenly distribute the inoculum around the agar surface. After 24 h incubation at 30°C, the biofilms were gently scraped off the agar plates and transferred to glass slides. The samples were weighed on a high precision scale (XS105, Mettler Toledo) to determine the weight of the hydrated biomass and placed in an oven at 110°C under vacuum (10 mbar during 20 h). The dry weight was then measured on the scale. The solid content was calculated with the following equation:
| 4.1 |
with wdry being the dry weight of the sample, wg being the weight of the glass slide and whydrated being the hydrated weight of the sample.
4.9. Optical microscopy
Light microscopy images were taken using a Nikon Eclipse Ti2-A in phase contrast configuration, equipped with a Hamamatsu ImageEM-X2 CCD camera and a 10× objective. The biofilm was grown in flow in a millifluidic device as described above.
Acknowledgements
The authors acknowledge Dorothee Kurz and Dr Samuel Charlton (ETH Zurich) for producing the agar plates and cultivating biofilm on the plates; Giovanni Savorana (ETH Zurich) for supporting us with calculations on the average wall shear stress and flow speed; and Kirill ‘McLovin’ Feldman for the experimental support on the MCR 302.
Data accessibility
The rheology data are available in a publicly accessible database: https://doi.org/10.3929/ethz-b-000560007 [67].
The data are provided in electronic supplementary material [68].
Authors' contributions
S.G.: conceptualization, data curation, formal analysis, investigation, visualization, writing—original draft, writing—review and editing; E.S.: conceptualization, investigation, methodology, supervision, writing—original draft, writing—review and editing; J.V.: conceptualization, funding acquisition, investigation, methodology, resources, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported from SNSF PRIMA grant no. 179834 (to E.S.).
References
- 1.Nadell CD, Ricaurte D, Yan J, Drescher K, Bassler BL. 2017. Flow environment and matrix structure interact to determine spatial competition in Pseudomonas aeruginosa biofilms. eLife 6, e21855. ( 10.7554/eLife.21855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563-575. ( 10.1038/nrmicro.2016.94) [DOI] [PubMed] [Google Scholar]
- 3.Flemming HC, Wuertz S. 2019. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 17, 247-260. ( 10.1038/s41579-019-0158-9) [DOI] [PubMed] [Google Scholar]
- 4.Lasa I. 2006. Towards the identification of the common features of bacterial biofilm development. Int. Microbiol. 9, 21-28. [PubMed] [Google Scholar]
- 5.Frolund B, Palmgren R, Keiding K, Nielsen PH. 1996. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 30, 1749-1758. ( 10.1016/0043-1354(95)00323-1) [DOI] [Google Scholar]
- 6.Flemming HC, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8, 623-633. ( 10.1038/nrmicro2415) [DOI] [PubMed] [Google Scholar]
- 7.Wilking JN, Angelini TE, Seminara A, Brenner MP, Weitz DA. 2011. Biofilms as complex fluids. MRS Bull. 36, 385-391. ( 10.1557/mrs.2011.71) [DOI] [Google Scholar]
- 8.Ido N, Lybman A, Hayet S, Azulay DN, Ghrayeb M, Liddawieh S, Chai L. 2020. Bacillus subtilis biofilms characterized as hydrogels. Insights on water uptake and water binding in biofilms. Soft Matter 16, 6180-6190. ( 10.1039/D0SM00581A) [DOI] [PubMed] [Google Scholar]
- 9.Stoodley P, Boyle JD, Debeer D, Lappin-Scott HM. 1999. Evolving perspectives of biofilm structure. Biofouling 14, 75-90. ( 10.1080/08927019909378398) [DOI] [Google Scholar]
- 10.Epstein AK, Pokroy B, Seminara A, Aizenberg J. 2011. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc. Natl Acad. Sci. USA 108, 995-1000. ( 10.1073/pnas.1011033108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okegbe C, Price-Whelan A, Dietrich LE. 2014. Redox-driven regulation of microbial community morphogenesis. Curr. Opin. Microbiol. 18, 39-45. ( 10.1016/j.mib.2014.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madsen JS, et al. 2015. Facultative control of matrix production optimizes competitive fitness in Pseudomonas aeruginosa PA14 biofilm models. Appl. Environ. Microbiol. 81, 8414-8426. ( 10.1128/AEM.02628-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh T, Ngo TD, Kumar A, Ayranci C, Tang T. 2019. Cleaning carbohydrate impurities from lignin using Pseudomonas fluorescens. Green Chem. 21, 1648-1659. ( 10.1039/C8GC03341B) [DOI] [Google Scholar]
- 14.Schultz MP, Bendick JA, Holm ER, Hertel WM. 2011. Economic impact of biofouling on a naval surface ship. Biofouling 27, 87-98. ( 10.1080/08927014.2010.542809) [DOI] [PubMed] [Google Scholar]
- 15.Bixler GD, Bhushan B. 2012. Review article: biofouling: lessons from nature. Phil. Trans. R. Soc. A 370, 2381-2417. ( 10.1098/rsta.2011.0502) [DOI] [PubMed] [Google Scholar]
- 16.Caldara M, Belgiovine C, Secchi E, Rusconi R. 2022. Environmental, microbiological, and immunological features of bacterial biofilms associated with implanted medical devices. Clin. Microbiol. Rev. 35, e00221-20. ( 10.1128/cmr.00221-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purevdorj B, Costerton JW, Stoodley P. 2002. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 68, 4457-4464. ( 10.1128/AEM.68.9.4457-4464.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krsmanovic M, Biswas D, Ali H, Kumar A, Ghosh R, Dickerson AK. 2021. Hydrodynamics and surface properties influence biofilm proliferation. Adv. Colloid Interface Sci. 288, 102336. ( 10.1016/j.cis.2020.102336) [DOI] [PubMed] [Google Scholar]
- 19.Conrad JC, Poling-Skutvik R. 2018. Confined flow: consequences and implications for bacteria and biofilms. Annu. Rev. Chem. Biomol. Eng. 9, 175-200. ( 10.1146/annurev-chembioeng-060817-084006) [DOI] [PubMed] [Google Scholar]
- 20.Stoodley P, Dodds I, Boyle JD, Lappin-Scott HM. 1999. Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. Symp. Suppl. 85, 19-28. ( 10.1111/j.1365-2672.1998.tb05279.x) [DOI] [PubMed] [Google Scholar]
- 21.Stoodley P, Lewandowski Z, Boyle JD, Lappin-Scott HM. 1999. The formation of migratory ripples in a mixed species bacterial biofilm growing in turbulent flow. Environ. Microbiol. 1, 447-455. ( 10.1046/j.1462-2920.1999.00055.x) [DOI] [PubMed] [Google Scholar]
- 22.Pearce P, et al. 2019. Flow-induced symmetry breaking in growing bacterial biofilms. Phys. Rev. Lett. 123, 258101. ( 10.1103/PhysRevLett.123.258101) [DOI] [PubMed] [Google Scholar]
- 23.Hartmann R, Singh PK, Pearce P, Mok R, Song B, Díaz-Pascual F, Dunkel J, Drescher K. 2019. Emergence of three-dimensional order and structure in growing biofilms. Nat. Phys. 15, 251-256. ( 10.1038/s41567-018-0356-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savorana G, Slomka J, Stocker R, Rusconi R, Secchi E. 2022. A microfluidic platform for characterizing the structure and rheology of biofilm streamers. Soft Matter 18, 3878-3890. ( 10.1039/D2SM00258B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mewis J, Wagner NJ. 2009. Thixotropy. Adv. Colloid Interface Sci. 147–148, 214-227. ( 10.1016/j.cis.2008.09.005) [DOI] [PubMed] [Google Scholar]
- 26.Gloag ES, Fabbri S, Wozniak DJ, Stoodley P. 2020. Biofilm mechanics: implications in infection and survival. Biofilm 2, 100017. ( 10.1016/j.bioflm.2019.100017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson BW, et al. 2015. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 39, 234-245. ( 10.1093/femsre/fuu008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gloag ES, German GK, Stoodley P, Wozniak DJ. 2018. Viscoelastic properties of Pseudomonas aeruginosa variant biofilms. Sci. Rep. 8, 9691. ( 10.1038/s41598-018-28009-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusconi R, Lecuyer S, Autrusson N, Guglielmini L, Stone HA. 2011. Secondary flow as a mechanism for the formation of biofilm streamers. Biophys. J. 100, 1392-1399. ( 10.1016/j.bpj.2011.01.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rusconi R, Lecuyer S, Guglielmini L, Stone HA. 2010. Laminar flow around corners triggers the formation of biofilm streamers. J. R. Soc. Interface 7, 1293-1299. ( 10.1098/rsif.2010.0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Secchi E, Savorana G, Vitale A, Eberl L, Stocker R, Rusconi R. 2022. The structural role of bacterial eDNA in the formation of biofilm streamers. Proc. Natl Acad. Sci. USA 119, e2113723119. ( 10.1073/pnas.2113723119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunsmore BC, Jacobsen A, Hall-Stoodley L, Bass CJ, Lappin-Scott HM, Stoodley P. 2002. The influence of fluid shear on the structure and material properties of sulphate-reducing bacterial biofilms. J. Ind. Microbiol. Biotechnol. 29, 347-353. ( 10.1038/sj.jim.7000302) [DOI] [PubMed] [Google Scholar]
- 33.Boudarel H, Mathias JD, Blaysat B, Grédiac M. 2018. Towards standardized mechanical characterization of microbial biofilms: analysis and critical review. npj Biofilms Microbiomes 4, 17. ( 10.1038/s41522-018-0062-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlovsky L, Younger JG, Solomon MJ. 2013. In situ rheology of Staphylococcus epidermidis bacterial biofilms. Soft Matter 9, 122-131. ( 10.1039/C2SM27005F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieleg O, Caldara M, Baumgärtel R, Ribbeck K. 2011. Mechanical robustness of Pseudomonas aeruginosa biofilms. Soft Matter 7, 3307-3314. ( 10.1039/c0sm01467b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovach K, et al. 2017. Evolutionary adaptations of biofilms infecting cystic fibrosis lungs promote mechanical toughness by adjusting polysaccharide production. npj Biofilms Microbiomes 3, 1. ( 10.1038/s41522-016-0007-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kretschmer M, Schüßler CA, Lieleg O. 2021. Biofilm adhesion to surfaces is modulated by biofilm wettability and stiffness. Adv. Mater. Interfaces 8, 2001658. ( 10.1002/admi.202001658) [DOI] [Google Scholar]
- 38.Yan J, et al. 2018. Bacterial biofilm material properties enable removal and transfer by capillary peeling. Adv. Mater. 30, 1804153. ( 10.1002/adma.201804153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klotz M, Kretschmer M, Goetz A, Ezendam S, Lieleg O, Opitz M. 2019. Importance of the biofilm matrix for the erosion stability of Bacillus subtilis NCIB 3610 biofilms. RSC Adv. 9, 11 521-11 529. ( 10.1039/C9RA01955C) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horvat M, Pannuri A, Romeo T, Dogsa I, Stopar D. 2019. Viscoelastic response of Escherichia coli biofilms to genetically altered expression of extracellular matrix components. Soft Matter 15, 5042-5051. ( 10.1039/C9SM00297A) [DOI] [PubMed] [Google Scholar]
- 41.Houari A, Picard J, Habarou H, Galas L, Vaudry H, Heim V, Di Martino P. 2008. Rheology of biofilms formed at the surface of NF membranes in a drinking water production unit. Biofouling 24, 235-240. ( 10.1080/08927010802023764) [DOI] [PubMed] [Google Scholar]
- 42.Kesel S, Grumbein S, Gümperlein I, Tallawi M, Marel AK, Lieleg O, Opitz M. 2016. Direct comparison of physical properties of Bacillus subtilis NCIB 3610 and B-1 biofilms. Appl. Environ. Microbiol. 82, 2424-2432. ( 10.1128/AEM.03957-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero CM, Martorell PV, López AG, Peñalver CN, Chaves S, Mechetti M. 2018. Architecture and physicochemical characterization of Bacillus biofilm as a potential enzyme immobilization factory. Colloids Surf. B 162, 246-255. ( 10.1016/j.colsurfb.2017.11.057) [DOI] [PubMed] [Google Scholar]
- 44.Charlton SG, White MA, Jana S, Eland LE, Jayathilake PG, Burgess JG, Chen J, Wipat A, Curtis TP. 2019. Regulating, measuring, and modeling the viscoelasticity of bacterial biofilms. J. Bacteriol. 201, e00101-19. ( 10.1128/JB.00101-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jana S, Charlton SG, Eland LE, Burgess JG, Wipat A, Curtis TP, Chen J. 2020. Nonlinear rheological characteristics of single species bacterial biofilm. npj Biofilms Microbiomes 6, 19. ( 10.1038/s41522-020-0126-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Körstgens V, Flemming HC, Wingender J, Borchard W. 2001. Influence of calcium ions on the mechanical properties of a model biofilm of mucoid Pseudomonas aeruginosa. Water Sci. Technol. 43, 49-57. ( 10.2166/wst.2001.0338) [DOI] [PubMed] [Google Scholar]
- 47.Wloka M, Rehage H, Flemming HC, Wingender J. 2005. Structure and rheological behaviour of the extracellular polymeric substance network of mucoid Pseudomonas aeruginosa biofilms. Biofilms 2, 275-283. ( 10.1017/S1479050506002031) [DOI] [Google Scholar]
- 48.Yannarell SM, Grandchamp GM, Chen SY, Daniels KE, Shank EA. 2019. A dual-species biofilm with emergent mechanical protective properties. J. Bacteriol. 201, e00670-18. ( 10.1128/JB.00670-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Stefano A, et al. 2009. Viscoelastic properties of Staphylococcus aureus and Staphylococcus epidermidis mono-microbial biofilms. Microb. Biotechnol. 2, 634-641. ( 10.1111/j.1751-7915.2009.00120.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell LC, et al. 2013. The effect of alginate oligosaccharides on the mechanical properties of Gram-negative biofilms. Biofouling 29, 413-421. ( 10.1080/08927014.2013.777954) [DOI] [PubMed] [Google Scholar]
- 51.Daalkhaijav U, Walker TW. 2017. Developing a nondestructive technique for measuring bulk rheology of Pseudomonas aeruginosa biofilm. Appl. Rheol. 27, 64033. ( 10.3933/ApplRheol-27-64033) [DOI] [Google Scholar]
- 52.Vinogradov AM, Winston M, Rupp CJ, Stoodley P. 2004. Rheology of biofilms formed from the dental plaque pathogen Streptococcus mutans. Biofilms 1, 49-56. ( 10.1017/S1479050503001078) [DOI] [Google Scholar]
- 53.Towler BW, Rupp CJ, Cunningham ALB, Stoodley P. 2003. Viscoelastic properties of a mixed culture biofilm from rheometer creep analysis. Biofouling 19, 279-285. ( 10.1080/0892701031000152470) [DOI] [PubMed] [Google Scholar]
- 54.Brugnoni LI, Tarifa MC, Lozano JE, Genovese D. 2014. In situ rheology of yeast biofilms. Biofouling 30, 1269-1279. ( 10.1080/08927014.2014.981165) [DOI] [PubMed] [Google Scholar]
- 55.Kim B, Perez-Calleja P, Li M, Nerenberg R. 2020. Effect of predation on the mechanical properties and detachment of MABR biofilms. Water Res. 186, 116289. ( 10.1016/j.watres.2020.116289) [DOI] [PubMed] [Google Scholar]
- 56.Patsios SI, Goudoulas TB, Kastrinakis EG, Nychas SG, Karabelas AJ. 2015. A novel method for rheological characterization of biofouling layers developing in membrane bioreactors (MBR). J. Membr. Sci. 482, 13-24. ( 10.1016/j.memsci.2015.02.016) [DOI] [Google Scholar]
- 57.Winter HH, Chambon F. 1986. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 30, 367-382. ( 10.1122/1.549853) [DOI] [Google Scholar]
- 58.Snijkers F, D’Avino G, Maffettone PL, Greco F, Hulsen M, Vermant J. 2009. Rotation of a sphere in a viscoelastic liquid subjected to shear flow. Part II. Experimental results. J. Rheol. 53, 459-480. ( 10.1122/1.3073052) [DOI] [Google Scholar]
- 59.Bridier A, Meylheuc T, Briandet R. 2013. Realistic representation of Bacillus subtilis biofilms architecture using combined microscopy (CLSM, ESEM and FESEM). Micron 48, 65-69. ( 10.1016/j.micron.2013.02.013) [DOI] [PubMed] [Google Scholar]
- 60.Peng N, Cai P, Mortimer M, Wu Y, Gao C, Huang Q. 2020. The exopolysaccharide-eDNA interaction modulates 3D architecture of Bacillus subtilis biofilm. BMC Microbiol. 20, 115. ( 10.1186/s12866-020-01789-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larson RG 1999. The structure and rheology of complex fluids. Oxford, UK: Oxford University Press. [Google Scholar]
- 62.Krajnc M, Stefanic P, Kostanjšek R, Mandic-Mulec I, Dogsa I, Stopar D. 2022. Systems view of Bacillus subtilis pellicle development. npj Biofilms Microbiomes 8, 25. ( 10.1038/s41522-022-00293-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rühs PA, Böni L, Fuller GG, Inglis RF, Fischer P. 2013. In-situ quantification of the interfacial rheological response of bacterial biofilms to environmental stimuli. PLoS ONE 8, e78524. ( 10.1371/journal.pone.0078524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qi L, Christopher GF. 2021. Rheological variability of Pseudomonas aeruginosa biofilms. Rheol. Acta 60, 219-230. ( 10.1007/s00397-021-01260-w) [DOI] [Google Scholar]
- 65.Jara J, et al. 2021. Self-adaptation of Pseudomonas fluorescens biofilms to hydrodynamic stress. Front. Microbiol. 11, 588884. ( 10.3389/fmicb.2020.588884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnaouteli S, Bamford NC, Stanley-Wall NR, Kovács ÁT. 2021. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 19, 600-614. ( 10.1038/s41579-021-00540-9) [DOI] [PubMed] [Google Scholar]
- 67.Geisel S. 2022. Experimental Challenges in Determining the Rheological Properties of Bacterial Biofilms. ETH Zurich. ( 10.3929/ethz-b-000560007) [DOI] [PMC free article] [PubMed]
- 68.Geisel S, Secchi E, Vermant J. 2022. Experimental challenges in determining the rheological properties of bacterial biofilms. Figshare. ( 10.6084/m9.figshare.c.6145914) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Geisel S. 2022. Experimental Challenges in Determining the Rheological Properties of Bacterial Biofilms. ETH Zurich. ( 10.3929/ethz-b-000560007) [DOI] [PMC free article] [PubMed]
- Geisel S, Secchi E, Vermant J. 2022. Experimental challenges in determining the rheological properties of bacterial biofilms. Figshare. ( 10.6084/m9.figshare.c.6145914) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The rheology data are available in a publicly accessible database: https://doi.org/10.3929/ethz-b-000560007 [67].
The data are provided in electronic supplementary material [68].