Abstract
Aim.
To identify skeletal muscle relaxant (SMR) drug-drug-drug (3DI) signals associated with increased rates of unintentional traumatic injury.
Methods.
We conducted automated high-throughput pharmacoepidemiologic screening of 2000–2019 healthcare data for members of United States commercial and Medicare Advantage health plans. We performed a self-controlled case series study for each drug triad consisting of an SMR base-pair (i.e., concomitant use of an SMR with another medication), and a co-dispensed medication (i.e., candidate interacting precipitant) taken during ongoing use of the base-pair. We included patients aged ≥16 years with an injury occurring during base-pair-exposed observation time. We used conditional Poisson regression to calculate adjusted rate ratios (RRs) with 95% confidence intervals (CIs) for injury with each SMR base-pair + candidate interacting precipitant (i.e., triad) versus the SMR-containing base-pair alone.
Results.
Among 58,478 triads, 29 were significantly positively associated with injury; confounder-adjusted RRs ranged from 1.39 (95% CI=1.01–1.91) for tizanidine+omeprazole with gabapentin to 2.23 (95% CI=1.02–4.87) for tizanidine+diclofenac with alprazolam. Most identified 3DI signals are new and have not been formally investigated.
Conclusion.
We identified 29 SMR 3DI signals associated with increased rates of injury. Future etiologic studies should confirm or refute these SMR 3DI signals.
Keywords: Muscle relaxants, drug interactions, injury, pharmacoepidemiology, population health, self-controlled case series
INTRODUCTION
Skeletal muscle relaxants (SMRs) are a group of structurally diverse medications approved by the United States (US) Food and Drug Administration (FDA) for relieving muscle tone or treating acute muscle spasms [1]. US ambulatory care visits resulting in new or continued therapy with an SMR doubled from 15.5 million in 2005 to 30.7 million in 2016 [2]. In 2018, approximately 1 in 15 commercially insured Americans was treated with an SMR [3]. This increasing and widespread SMR use is concerning, given their uncertain efficacy for off-label indications and high-risk safety profiles [1]. Most SMRs have central nervous system (CNS) sedation effects causing dizziness, ataxia, and confusion, thereby potentially precipitating accidental falls, unsafe operation of motor vehicles, and traumatic injuries [4–6]. In fact, SMR use has been linked to a 5-fold increased risk of falls in multiple sclerosis patients [4], a 2.25-fold increased risk of fracture in older adults [5], and a 3.7-fold increased risk of traffic accidents in adults [6].
SMRs are frequently used with other medications [2, 3, 5]. In 2016, two-thirds of US ambulatory care visits that continued SMR therapy documented opioid use [2]. In a cohort of older US veterans newly prescribed SMRs, 72% received six or more medications [5]. SMR use in the setting of polypharmacy raises further concerns for unintentional traumatic injury, as concomitant use of SMRs with other medications may lead to pharmacokinetic and/or pharmacodynamic interactions and amplify SMRs’ inherent risks [7]. Previous research on such risks has focused on pairwise interactions (i.e., drug-drug interactions (DDIs)) between SMRs and a single interacting drug (i.e., precipitant drug), such as the SMR-benzodiazepine combination [7]. An intrinsic limitation of these studies was their inability to identify higher-order interactions, such as drug-drug-drug interactions (3DIs). While some 3DIs could be theoretically postulated based on known pairwise DDIs, the interplay among the three drugs may be more complex in routine clinical practice and warrant independent examination [8]. Investigating SMR 3DIs is particularly vital given the high prevalence of polypharmacy among SMR users [5] as well as specific concern about the “Holy Trinity”—combined use of SMRs, opioids, and benzodiazepines [9]. Indeed, in a sample of state of Florida residents, 30% of SMR users received concomitant SMRs, opioids, and benzodiazepines, raising concerns about adverse events due to additive or synergistic CNS depression [10].
To fill the knowledge gap regarding higher-order SMR drug interactions, we conducted high-throughput pharmacoepidemiologic screening studies to identify potential signals of SMR 3DIs associated with unintentional traumatic injury, so that future etiologic studies can be prioritized accordingly to test whether these signals represent true 3DIs.
METHODS
Data source
We used Optum’s de-identified Clinformatics® Data Mart administrative data from May 1, 2000, through June 30, 2019. The data contain individual-level information on enrollment status, demographics, and healthcare billing records of >71 million US commercially insured and Medicare Advantage beneficiaries (see details in eMethods) [11]. The study protocol (#831486) was approved by the University of Pennsylvania’s Office of Regulatory Affairs with exemption from institutional review board review.
Study design overview
We performed high-throughput pharmacoepidemiologic screening analyses to identify potential signals of injury associated with 3DI triads comprised of a) SMRs (i.e., object drugs or, primary affected drugs of 3DI triads) + co-dispensed drugs (i.e., base-pairs), and b) candidate interacting precipitants (i.e., primary affecting drugs of 3DI triads). For each of thousands of base-pairs, we conducted a self-controlled case series (SCCS) study to compare injury rates during triad-exposed versus base-pair-only-exposed observation time, and deemed triads with significantly increased injury rates as potential 3DI signals. Figure 1 provides a graphical representation of the design.
Figure 1. Example of skeletal muscle relaxant object + co-dispensed drug of the base-pair episode eligible for inclusion.
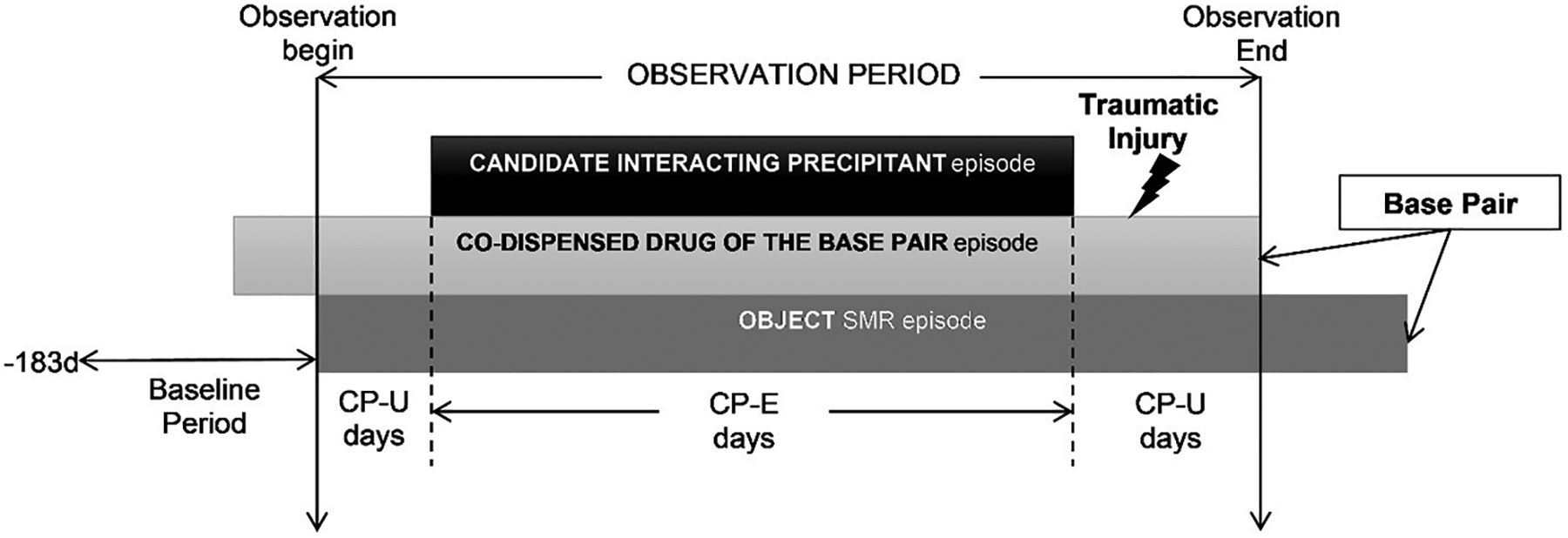
CP-E=candidate interacting precipitant-exposed; CP-U=candidate interacting precipitant-unexposed; SMR=skeletal muscle relaxant.
For each eligible SMR initiator (defined as having a ≥183-day washout period without SMR use before the first SMR prescription dispensing), the observation period began on the first day with supplies of both the SMR and the co-dispensed drug of the base-pair. The observation period consisted exclusively of days with continuous base-pair use. Days were categorized as candidate interacting precipitant exposed (CP-E) or unexposed (CP-U) based on whether the study individual had or did not have supply of a candidate interacting precipitant. The study individual was also required to experience a traumatic injury during the observation period. The comparison of interest was the rate of injury during CP-E days versus CP-U days.
We selected the within-person, case-only SCCS design because: a) it automatically controls for measured and unmeasured confounders that are stable over the observation period, such as sex, race, and chronic conditions; b) measured confounders that change over time can be addressed via statistical adjustment; c) restricting the study sample to persons experiencing an outcome and the lack of needing to identify control series is highly computationally efficient and suitable for high-throughput screening, and d) it is not prone to exposure-trend bias [12]. We have used this epidemiologic method to study opioid and antidepressant DDIs leading to injury [13, 14], insulin secretagogue DDIs leading to hypoglycemia [15–17], anticoagulant DDIs leading to thromboembolism [18, 19], and anticoagulant and antiplatelet DDIs leading to bleeding [20–22].
Constructing the study sample for each base-pair
We constructed separate study samples for each base-pair by identifying patients aged ≥16 years who: a) were new users of the base pair, defined as first day with supplies of both SMR and co-dispensed drug of the base-pair; and b) experienced an outcome while continuously exposed to the base-pair. The first criterion was intended to capture incident triad exposure and thereby minimize depletion of susceptible person-time [23]; the second criterion was required by the SCCS design [12]. To identify new users of the base-pair, we first used pharmacy claim files to identify patients initiating the following SMRs: baclofen; carisoprodol; chlorzoxazone; cyclobenzaprine; dantrolene; metaxalone; methocarbamol; orphenadrine; and tizanidine. SMR initiation was defined as filling a prescription for a given SMR without filling it during the prior 183-day continuously enrolled period (i.e., baseline). Since SMRs may be used to treat injury-induced muscle pain, to minimize reverse causation, we pushed the dispensing dates of SMRs forward by one day. From these SMR initiators, we then identified new users of the base-pair as those who received the co-dispensed drug during continuous periods of SMR use. Patients may have had previous exposure to an alternative SMR or the co-dispensed drug in the base-pair during the baseline period. We classified all medications using Lexicon Plus (Cerner Multum: Denver, Colorado, US).
Defining observation periods
Patients contributed person-days from new use of the base-pair until the earliest of the following: a) lapsed exposure to the base-pair, defined as at least one day without either SMR or the co-dispensed drug in the base-pair, after extending days’ supply by 20% to account for imperfect adherence; b) switching from solid to liquid formulation of SMR [24]; c) disenrollment from health plan; or d) end of the study period (June 30, 2019). To meet an assumption of the SCCS design [12], we did not censor upon outcome occurrence, but included prior outcome occurrence as a covariate.
Identifying candidate interacting precipitants and categorizing observation periods
During periods of base-pair use, we identified use of candidate interacting precipitants, operationalized as any orally administered medications co-dispensed with the base-pair. We categorized each day of the observation period as candidate precipitant-exposed if covered by the base-pair and the candidate precipitant, and candidate precipitant-unexposed if it was covered by the base-pair only. Since co-dispensed drugs (e.g., A and B) used with a SMR can be classified as either the co-dispensed drug of the base-pair or candidate interacting precipitant, we examined both scenarios, i.e., 1) SMR+drug A (base-pair) with drug B (candidate interacting precipitant) vs. without drug B, and 2) SMR+drug B (base-pair) with drug A (candidate interacting precipitant) vs. without drug A.
Ascertaining outcomes
We ascertained outcomes occurring during the observation periods. The primary outcome of interest was unintentional traumatic injury, defined as an emergency department (ED) or inpatient hospitalization with principal diagnosis indicative of fracture, dislocation, sprain/strain, intracranial injury, internal injury of thorax, abdomen, or pelvis, open wound, injury to blood vessels, crushing injury, injury to nerves or spinal cord, or certain traumatic complications and unspecified injuries [25]. Secondary outcomes included: a) hip fracture, defined as having a principal inpatient discharge diagnosis indicative of typical open and closed hip fractures; and b) motor vehicle crash while individual was driving, defined as having an unintentional traumatic injury (see primary outcome above) accompanied by an external cause of injury code for unintended traffic or nontraffic accident. We provide diagnostic codes, their performances, and support for their use in eTable 1.
Measuring covariates
The SCCS design inherently controls for time-invariant, but not time-varying, confounders [12]. We, therefore, included in each regression model the following time-varying covariates assessed during each day of observation time: a) average daily dose of SMR categorized as ≤median or >median dose; b) follow-up month categorized as 1 or ≥2; and c) ever having a prior traumatic injury of interest (yes/no), as prior injury is likely to increase the risk for subsequent injury [12].
Statistical analysis
To identify 3DI signals for each base-pair associated with an outcome, we used conditional Poisson regression to estimate rate ratios (RRs) and 95% confidence intervals (CIs) to compare outcome rates during candidate precipitant-exposed vs. candidate precipitant-unexposed days, i.e., , adjusting for the aforementioned covariates. To ensure statistical stability, we only estimated RRs when: a) there were ≥5 candidate precipitant-exposed persons; and b) the variance of the beta estimate for the parameter of interest (calculated using conventional asymptotic theory) was <10. To reduce the chance of type 1 statistical error due to testing multiple exposures simultaneously, we adjusted RRs using semi-Bayes shrinkage, which minimizes false-positive findings by shrinking the extreme effect estimates towards the overall average effect (see details in eMethods) [26, 27].
RESULTS
Table 1 summarizes characteristics of persons constituting SMR samples for analyses of unintentional traumatic injury. The majority of the study samples were Caucasian (64.2–70.2%) and female (50.0–67.8%). For the four most used SMRs, we included 35,940, 12,167, 7,981, and 6,054 users of cyclobenzaprine, tizanidine, baclofen, and methocarbamol, and observed them for a median duration of 19, 37, 37, and 19 days, respectively. In analyses of secondary outcomes, we included a total of 1,308 and 844 SMR users for typical hip fracture and motor vehicle crash, respectively; eTable 2 and eTable 3 summarize characteristics for persons experiencing these secondary outcomes.
Table 1.
Characteristics of study samples examining unintentional traumatic injury, by skeletal muscle relaxant
| Skeletal Muscle Relaxant | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baclofen | Carisoprodol | Chlorzoxazone | Cyclobenzaprine | Dantrolene | Metaxalone | Methocarbamol | Orphenadrine | Tizanidine | |
| Number of persons | 7,981 | 5,726 | 629 | 35,940 | 42 | 4,626 | 6,054 | 1,492 | 12,167 |
| Days of observation period per person, median (Q1–Q3) | 37.0 (28.0–123.0) | 37.0 (13.0–71.0) | 19.0 (13.0–37.0) | 19.0 (13.0–37.0) | 104.0 (37.0–256.0) | 13.0 (10.0–37.0) | 19.0 (10.0–37.0) | 13.0 (10.0–35.0) | 37.0 (23.0–109.0) |
| Total number of unintentional traumatic injuriesa | 8,978 | 6,133 | 693 | 38,856 | 46 | 4,772 | 6,545 | 1,548 | 13,738 |
| Age in years, median (Q1–Q3) | 65.0 (53.4–74.5) | 49.5 (39.7–60.1) | 51.0 (39.9–62.9) | 51.8 (39.8–64.8) | 55.1 (43.1–66.6) | 47.0 (37.2–57.9) | 51.7 (39.6–63.8) | 47.9 (36.7–60.5) | 61.6 (50.4–71.9) |
| Female, n (%) | 5,296 (66.4) | 3,403 (59.4) | 386 (61.4) | 21,512 (59.9) | 21 (50.0) | 2,746 (59.4) | 3,590 (59.3) | 839 (56.2) | 8,244 (67.8) |
| Race, n (%) | |||||||||
| Caucasian | 5,125 (64.2) | 3,863 (67.5) | 420 (66.8) | 24,491 (68.1) | 27 (64.3) | 3,208 (69.3) | 4,252 (70.2) | 1,011 (67.8) | 7,968 (65.5) |
| African American | 1,008 (12.6) | 543 (9.5) | 73 (11.6) | 3,674 (10.2) | 5 (11.9) | 312 (6.7) | 586 (9.7) | 134 (9.0) | 1,775 (14.6) |
| Hispanic | 839 (10.5) | 453 (7.9) | 43 (6.8) | 2,981 (8.3) | 4 (9.5) | 314 (6.8) | 477 (7.9) | 106 (7.1) | 866 (7.1) |
| Asian | 93 (1.2) | 98 (1.7) | 10 (1.6) | 641 (1.8) | 0 (0.0) | 63 (1.4) | 111 (1.8) | 16 (1.1) | 136 (1.1) |
| Unknown | 916 (11.5) | 769 (13.4) | 83 (13.2) | 4,153 (11.6) | 6 (14.3) | 729 (15.8) | 628 (10.4) | 225 (15.1) | 1,422 (11.7) |
| Region, n (%) | |||||||||
| New England | 249 (3.1) | 162 (2.8) | 10 (1.6) | 1,479 (4.1) | 1 (2.4) | 143 (3.1) | 251 (4.1) | 40 (2.7) | 257 (2.1) |
| Middle Atlantic | 408 (5.1) | 180 (3.1) | 27 (4.3) | 1,928 (5.4) | 2 (4.8) | 311 (6.7) | 218 (3.6) | 50 (3.4) | 458 (3.8) |
| East North Central | 973 (12.2) | 476 (8.3) | 151 (24.0) | 6,388 (17.8) | 4 (9.5) | 840 (18.2) | 978 (16.2) | 400 (26.8) | 1,869 (15.4) |
| West North Central | 641 (8.0) | 314 (5.5) | 63 (10.0) | 3,801 (10.6) | 11 (26.2) | 381 (8.2) | 525 (8.7) | 190 (12.7) | 1,009 (8.3) |
| South Atlantic | 2,598 (32.6) | 1,542 (26.9) | 151 (24.0) | 9,282 (25.8) | 14 (33.3) | 1,341 (29.0) | 1,397 (23.1) | 397 (26.6) | 3,991 (32.8) |
| East South Central | 460 (5.8) | 332 (5.8) | 45 (7.2) | 1,371 (3.8) | 1 (2.4) | 218 (4.7) | 419 (6.9) | 67 (4.5) | 1,002 (8.2) |
| West South Central | 999 (12.5) | 1,035 (18.1) | 110 (17.5) | 4,967 (13.8) | 3 (7.1) | 731 (15.8) | 876 (14.5) | 175 (11.7) | 1,908 (15.7) |
| Mountain | 813 (10.2) | 771 (13.5) | 45 (7.2) | 3,452 (9.6) | 3 (7.1) | 391 (8.5) | 608 (10.0) | 139 (9.3) | 1,019 (8.4) |
| Pacific | 814 (10.2) | 876 (15.3) | 26 (4.1) | 3,143 (8.7) | 3 (7.1) | 265 (5.7) | 758 (12.5) | 33 (2.2) | 631 (5.2) |
| Unknown | 26 (0.3) | 38 (0.7) | 1 (0.2) | 129 (0.4) | 0 (0.0) | 5 (0.1) | 24 (0.4) | 1 (0.1) | 23 (0.2) |
Q = quartile
Per the self-controlled case series design, all included individuals had to have at least one injury during the observation period.
Table 2 provides summary data on RRs for unintentional traumatic injury, before and after confounder adjustment. For the four most used SMRs—cyclobenzaprine, tizanidine, baclofen, and methocarbamol—we examined 16610, 12330, 9363, and 3223 sets of triads in confounder-adjusted analyses, and observed a total of 29 sets (i.e., 9, 14, 5, and 1, respectively) to have statistically significantly elevated RRs after semi-Bayes shrinkage. No elevated RRs were observed for triads involving other SMRs. Therefore, we deemed these 29 triads as potential 3DI signals. Volcano plots in Figure 2 graphically depict semi-Bayes shrunk confounder-adjusted RRs for the unintentional traumatic injury of these four most used SMRs; secondary analyses using an alternative variance parameter for semi-Bayes shrinkage yielded similar findings (eFigure 1). We present results of secondary outcomes in supplemental materials. eTable 4 and eTable 5 provide summary data on RRs for typical hip fracture and motor vehicle crash, respectively; eFigure 2 and eFigure 3 depict semi-Bayes shrunk confounder-adjusted RRs for motor vehicle crash for cyclobenzaprine—the only SMR sample with statistically stable models for this outcome, using two different variance parameters for semi-Bayes shrinkage. No statistically stable models were generated for hip fracture; thus, plots were not produced for this outcome.
Table 2.
Summary data on rate ratios for unintentional traumatic injury, by skeletal muscle relaxant
| Skeletal Muscle Relaxant | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baclofen | Carisoprodol | Chlorzoxazone | Cyclobenzaprine | Dantrolene | Metaxalone | Methocarbamol | Orphenadrine | Tizanidine | |
| Unadjusted analyses | |||||||||
| Number of base-pair-candidate interacting precipitant triads examined | 11,942 | 4,777 | 223 | 18,725 | No models runa | 2,449 | 4,957 | 535 | 14,870 |
| Range of RRs after semi-Bayes shrinkage | 0.35 – 2.02 | 0.44 – 2.15 | 0.61 – 1.26 | 0.37 – 2.13 | 0.43 – 1.64 | 0.46 – 1.95 | 0.52 – 1.55 | 0.34 – 2.13 | |
| Number of statistically significantly elevated ratio of RRs | 2 | 1 | 0 | 12 | 0 | 2 | 0 | 8 | |
| Confounder-adjusted analyses b | |||||||||
| Number of base-pair-candidate interacting precipitant triads examined | 9,363 | 3,609 | 21 | 16,610 | No models runa | 975 | 3,223 | 17 | 12,330 |
| Range of RRs after semi-Bayes shrinkage | 0.38 – 2.06 | 0.41 – 2.05 | 0.55 – 0.84 | 0.33 – 2.20 | 0.44 – 1.69 | 0.43 – 2.14 | 0.79 – 1.68 | 0.33 – 2.28 | |
| Number of statistically significantly elevated ratio of RRs | 5 | 0 | 0 | 9 | 0 | 1 | 0 | 14 | |
| Final number of potential 3DI signals | 5 | 0 | 0 | 9 | n/a | 0 | 1 | 0 | 14 |
RR = rate ratio
No models were run because no base-pair-candidate interacting precipitant combinations had ≥5 exposed patients.
The number of triads decreased in the confounder-adjusted analyses compared to the unadjusted analyses because the adjusted models included time-varying covariates (i.e., average daily dose of SMR, follow-up month, and ever having a prior traumatic injury of interest). In some confounder-adjusted models, the estimates for one or more of the covariates were unstable; these models were considered invalid and not reported in confounder-adjusted analyses.
Figure 2. Commonly prescribed skeletal muscle relaxant + co-dispensed drug of the base-pair with candidate interacting precipitant associations with unintentional traumatic injury.
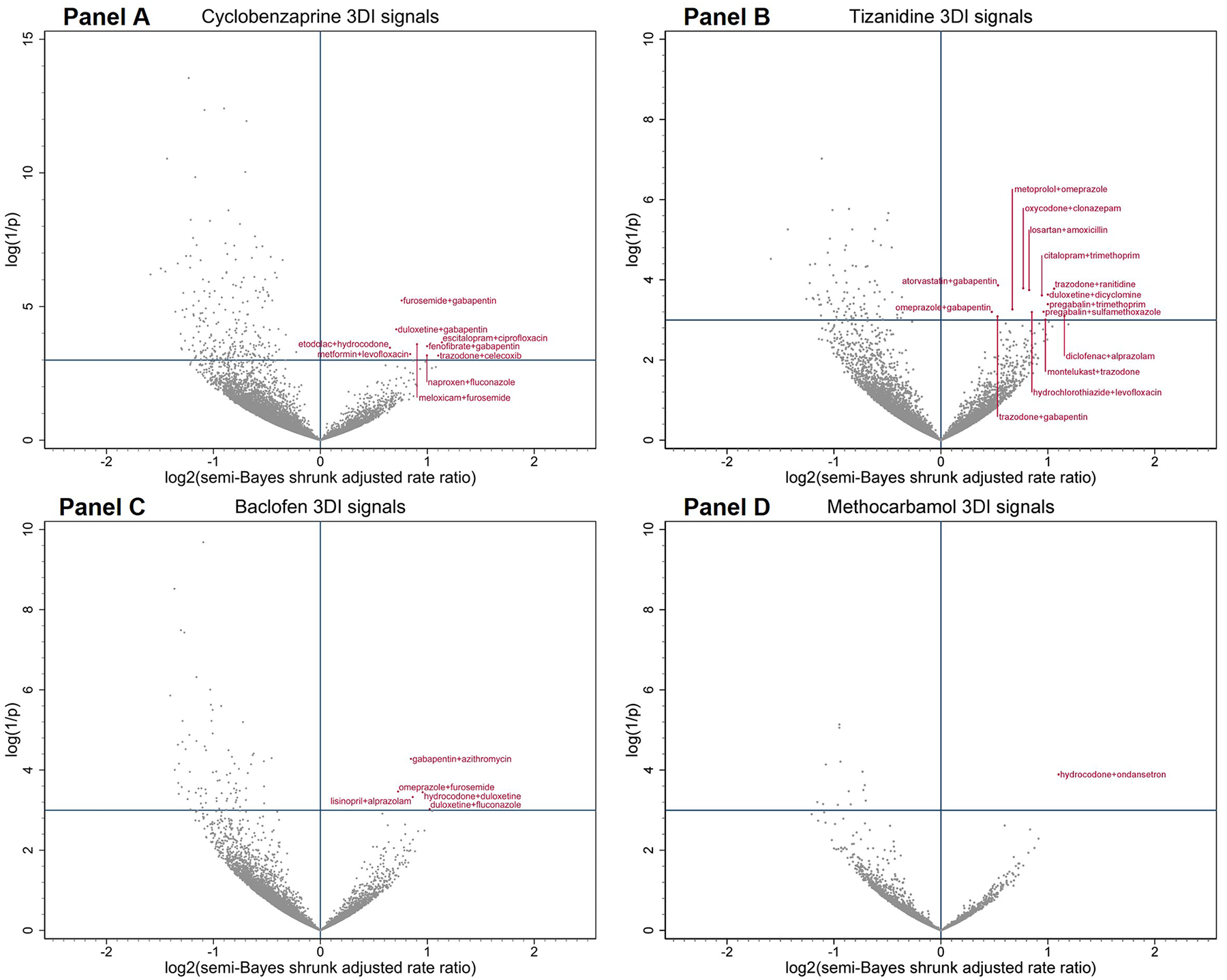
Panels A–D depict associations between 3DIs and unintentional traumatic injury when the object skeletal muscle relaxant of interest was cyclobenzaprine, tizanidine, baclofen, and methocarbamol, respectively. Semi-Bayes shrinkage prespecified a variance of 0.25, assuming that 95% of true rate ratios would fall within an unspecified 7-fold range of each other. The x-axis is the log base 2 of the semi-Bayes shrunk adjusted rate ratio (RR) comparing skeletal muscle relaxant + co-dispensed drug of base-pair + candidate interacting precipitant vs. skeletal muscle relaxant + co-dispensed drug of base-pair. The value of the X represents the magnitude of the RR, whereas the value of Y represents the level of statistical significance. Data points in the upper right quadrant represent statistically significant elevated rate ratios for the association between skeletal muscle relaxant + co-dispensed drug of base-pair + candidate interacting precipitant (vs. skeletal muscle relaxant + co-dispensed drug of base-pair) and injury (i.e., putative 3DI signals).
Table 3 lists the 29 potential 3DI signals by therapeutic category. The most common potentially interacting co-dispensed drugs in the base-pair with candidate interacting precipitants were CNS with CNS agents (N=8), CNS with anti-infective agents (N=7), and cardiovascular with CNS agents (N=3). Statistically elevated adjusted RRs for unintentional traumatic injury after semi-Bayes shrinkage ranged from 1.39 (95% CI=1.01–1.91) for tizanidine+omeprazole with gabapentin (vs. tizanidine+omeprazole without gabapentin) to 2.23 (95% CI=1.02–4.87) for tizanidine+diclofenac with alprazolam (vs. tizanidine+diclofenac without alprazolam).
Table 3.
Skeletal muscle relaxant drug-drug-drug interaction signals with statistically significantly increased rates of unintentional traumatic injury, by commonly used object drug, by therapeutic category of co-dispensed drug of the base-pair
| Object | Therapeutic Category | Co-Dispensed Drug of the Base-Pair | Candidate Interacting Precipitant | Rate ratio, semi-Bayes shrunk and adjusteda | 95% confidence interval |
|---|---|---|---|---|---|
| BACLOFEN | Cardiovascular | lisinopril | alprazolam | 1.82 | 1.04–3.18 |
| Central nervous system | duloxetine | fluconazole | 2.03 | 1.00–4.10 | |
| gabapentin | azithromycin | 1.80 | 1.13–2.87 | ||
| hydrocodone | duloxetine | 1.94 | 1.06–3.55 | ||
| Gastrointestinal | omeprazole | furosemide | 1.65 | 1.05–2.62 | |
| CYCLOBENZAPRINE | Cardiovascular | fenofibrate | gabapentin | 2.00 | 1.07–3.73 |
| Central nervous system | duloxetine | gabapentin | 1.64 | 1.10–2.44 | |
| escitalopram | ciprofloxacin | 2.20 | 1.10–4.39 | ||
| etodolac | hydrocodone | 1.57 | 1.04–2.37 | ||
| meloxicam | furosemide | 1.87 | 1.07–3.27 | ||
| naproxen | fluconazole | 2.00 | 1.03–3.88 | ||
| trazodone | celecoxib | 2.15 | 1.03–4.49 | ||
| Endocrine and metabolic | metformin | levofloxacin | 1.79 | 1.03–3.12 | |
| Renal and genitourinary | furosemide | gabapentin | 1.69 | 1.17–2.46 | |
| METHOCARBAMOL | Central nervous system | hydrocodone | ondansetron | 2.14 | 1.13–4.09 |
| TIZANIDINE | Cardiovascular | atorvastatin | gabapentin | 1.45 | 1.06–1.98 |
| losartan | amoxicillin | 1.77 | 1.08–2.91 | ||
| metoprolol | omeprazole | 1.59 | 1.03–2.46 | ||
| Central nervous system | citalopram | trimethoprim | 1.92 | 1.08–3.44 | |
| diclofenac | alprazolam | 2.23 | 1.02–4.87 | ||
| duloxetine | dicyclomine | 2.00 | 1.08–3.69 | ||
| oxycodone | clonazepam | 1.71 | 1.08–2.70 | ||
| pregabalin | sulfamethoxazole | 1.95 | 1.03–3.68 | ||
| pregabalin | trimethoprim | 2.00 | 1.06–3.79 | ||
| trazodone | gabapentin | 1.44 | 1.01–2.07 | ||
| trazodone | ranitidine | 2.08 | 1.11–3.92 | ||
| Gastrointestinal | omeprazole | gabapentin | 1.39 | 1.01–1.91 | |
| Renal and genitourinary | hydrochlorothiazide | levofloxacin | 1.80 | 1.02–3.17 | |
| Respiratory | montelukast | trazodone | 1.97 | 1.00–3.87 |
Rate ratio was calculated as outcome rates during candidate precipitant-exposed person-time divided by outcome rates during candidate precipitant-unexposed days, i.e., , adjusting for the following time-varying covariates: average daily dose of SMR, follow-up month, and ever having a prior traumatic injury of interest.
DISCUSSION
We conducted high-throughput pharmacoepidemiologic screening using real-world health care data to identify potential 3DIs involving SMRs that were associated with increased rates of unintentional traumatic injury. Among 58,478 base-pairs (i.e., SMR plus concomitant medication) coupled with a candidate interacting precipitant, we identified 29 potential signals of 3DIs, all involving one of the four most used SMRs (cyclobenzaprine, tizanidine, baclofen, and methocarbamol).
Despite the high prevalence of polypharmacy among SMR users [5], few prior studies have examined 3DIs involving SMRs. One exception includes investigations of the combination of SMRs, opioids, and benzodiazepines, which has been associated with an elevated risk of overdose and ED visits, compared with use of two of these drugs alone [3, 28]. Aligned with these findings, we found that use of clonazepam was associated with a 1.7-fold increased rate of unintentional traumatic injury among patients receiving tizanidine+oxycodone. Interactions among tizanidine, oxycodone, and clonazepam are biologically plausible given additive or synergistic pharmacodynamic effects (e.g., CNS depression) from all three drugs, and the potential pharmacokinetic interplay between clonazepam and oxycodone. Indeed, concomitant clonazepam was theorized to increase the plasma concentration of oxycodone by inhibiting its metabolism via the cytochrome P450 (CYP) 3A4 isozyme [29], potentially enhancing the pharmacodynamic interactions between oxycodone and tizanidine. We also identified several other SMR-opioid-benzodiazepine triads with numerically elevated, yet statistically imprecise, rates of injury (e.g., baclofen+morphine with alprazolam, RR=1.47, 95% CI=0.72–3.02, data not shown; carisoprodol+diazepam with hydrocodone, RR=1.37, 95% CI=0.79–2.38, data not shown). The lack of expected signaling for these triads was likely driven by the limited sample of individuals on these drug triads. On the other hand, this may imply that our approach to semi-Bayes shrinkage was conservative in that specificity was prioritized over sensitivity. Given our aim was to generate hypotheses for future etiologic studies, such conservativeness may be appropriate.
Almost one-third (8 of 29) of the identified 3DI signals were SMR+CNS agent with another CNS agent. The most obvious mechanism underlying these signals may be the pharmacodynamic interaction among the three drugs, i.e., the addition or synergy of the CNS depressant effects. In select cases, these pharmacodynamic effects may be compounded by the pharmacokinetic interplay between the interacting CNS drug with either the SMR or the co-dispensed drug in the base-pair. For example, the observed RR of 2.15 for cyclobenzaprine+trazodone and celecoxib may be plausible if celecoxib increases the serum concentration of trazodone by inhibiting its metabolism via CYP2D6 [30, 31], and potentiates the pharmacodynamic interaction between trazodone and cyclobenzaprine. The plausible biological mechanism of these observed 3DI signals supports the overall validity of our screening approach.
Several identified 3DI signals deserve further investigation, given their relatively high RRs and biological plausibility. We discuss these signals based on putative mechanisms. First, pharmacodynamic effects of the candidate interacting precipitant alone may contribute to injury risk. For example, the observation that gabapentin was identified as the candidate interacting precipitant in several 3DI signals (e.g., cyclobenzaprine+fenofibrate with gabapentin, RR=2.00; cyclobenzaprine+furosemide with gabapentin, RR=1.69) may be explained by the side effects such as dizziness, somnolence, sedation, and ataxia from gabapentin administration, which could worsen the symptoms of CNS depression from SMR. Second, the candidate interacting precipitant may pharmacodynamically and pharmacokinetically interact with the co-dispensed drug in the base-pair, thereby adding to SMRs’ inherent injury risks. For example, the RR of 1.79 for cylobenzaprine+metformin with levofloxacin can be explained by: a) glucose-lowering effects from levofloxacin, which potentially magnifies the risk of hypoglycemia from metformin use [32, 33]; and b) a pharmacokinetic interaction between metformin and levofloxacin, in which levofloxacin inhibits the organic cation transporters [34] of metformin and potentially increases the serum concentration of and risks from metformin use; both pathways may compound the risk of injury resulting from cyclobenzaprine use. As the resources to formally examine the risks associated with 3DIs are likely limited, future etiologic studies may be prioritized to study the above interactions with relatively high RRs and intuitive biologic pathways.
We also identified signals with an anti-infective as the candidate interacting precipitant, at least one of which may be supported by a plausible biologic mechanism. Ciprofloxacin may increase the concentration and CNS depressant effects of escitalopram by inhibiting [35, 36] its metabolism via CYP3A4 [37] and potentiate the pharmacodynamic interaction between escitalopram and cyclobenzaprine, potentially explaining our finding of a RR of 2.20. However, mechanisms underlying most SMR-anti-infective signals remain unknown. Reverse causation may partially explain such signals, since infection and pain may result from traumatic injury that warranted anti-infective use and SMR treatment, respectively. In addition, protopathic bias may have resulted if anti-infectives were prescribed for prophylaxis of infection due to an injury that later resulted in ED presentation or hospitalization.
Our study has notable strengths. First, the very large sample size allowed us to study the 3DIs associated with traumatic injury that are hard to study in smaller settings. Second, by comparing the individuals to themselves, we eliminated between-person and decreased within-person confounding. Third, we focused on signals potentially leading to traumatic injury, a clinically relevant outcome identified by validated algorithms. Finally, we were able to reduce type I errors and increase the specificity of our findings via semi-Bayes shrinkage.
Our hypothesis-generating screening should be interpreted in light of several limitations. First, the pharmacy claims capture only prescription dispensings reimbursed by health insurance without information on over-the-counter medications and contain no data on whether individuals took the drug as directed. Lack of such information may introduce exposure misclassification and preclude us from controlling for time-varying use of over-the-counter medications that may interact with the base-pair. Second, our primary analysis focused on injury rates during SMR base-pair time while exposed vs. unexposed to a candidate interacting precipitant. Some observed signals may represent pairwise interactions between the candidate interacting precipitant and the co-dispensed drug of the base-pair, or the effect of candidate interacting precipitant itself, rather than a true 3DI. Third, given the high-throughput nature of our hypothesis-generating work, it was infeasible to differentiate the initiating order between SMR, co-dispensed drug of the base-pair, and the candidate interacting precipitant drug. Future etiologic studies that examine the robustness of these potential 3DI signals should consider different initiating orders of the SMR triads. Fourth, analyses for secondary outcomes were underpowered, due to insufficient number of eligible samples experiencing these events. Fifth, one key assumption of SCCS is that the outcome does not alter the probability of subsequent exposure [12]. Given that traumatic injury may result in death, there is a chance that this probability is brought down to zero, resulting in bias in either direction. However, using disenrollment from health plans as a proxy measure for death, we estimated the proportion of possible deaths following an injury was <3.8% in our sample. Due to this low mortality rate from injury, bias from possible violation of the assumption is likely negligible [12]. Finally, given that the median duration of SMR use was relatively short in our study sample, our findings may not be generalizable to long-term SMR users.
CONCLUSION
We identified 29 potential SMR-related drug-drug-drug interactions that are associated with elevated rates of unintentional traumatic injury. These signals involved the four most used SMRs—cyclobenzaprine, tizanidine, baclofen, and methocarbamol. Our findings may provide important targets to guide hypothesis generation and prioritize future etiologic investigations into higher-order SMR interactions and risk for unintentional traumatic injury.
Supplementary Material
What is already known about this subject?
Skeletal muscle relaxants (SMRs) are commonly co-prescribed with potentially interacting medications that may contribute to increased risk of unintentional traumatic injury.
Prior research mainly focused on health outcomes of pairwise SMR drug-drug interactions.
Health outcomes of drug-drug-drug interactions involving SMRs remain understudied.
What this study adds?
We conducted high-throughput pharmacoepidemiologic screening to generate hypotheses regarding potential drug-drug-drug interactions involving skeletal muscle relaxants (SMRs).
Among 58,478 drug triads examined, 29 showed increased rates of unintentional traumatic injury.
These 29 SMR drug triads may suggest potential drug-drug-drug interactions that warrant future research.
List of Hyperlinks for Crosschecking.
Funding statement
This research was supported by the United States National Institutes of Health ([grant numbers R01AG060975 [PI: Dr.Leonard], R01DA048001 [PI: Dr.Hennessy], R01AG064589 [PI: Dr.Hennessy], and R01AG025152] [PI: Dr.Hennessy].
Conflicts of interest statement
Dr. Leonard is an Executive Committee Member of and Dr. Hennessy directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training. The Center receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. Dr. Leonard recently received honoraria from the American College of Clinical Pharmacy Foundation, the University of Florida, the University of Massachusetts, and the Scientific and Data Coordinating Center for the NIDDK-funded Chronic Renal Insufficiency Cohort Study. Dr. Leonard is a Special Government Employee of the United States (US) Food and Drug Administration and consults for their Reagan-Udall Foundation. Dr. Leonard receives travel support from John Wiley & Sons. Dr. Leonard’s spouse is an employee of Merck; neither he nor she own stock in the company. Dr. Horn is coauthor and publisher of The Top 100 Drug Interactions: A Guide to Patient Management and a consultant to Urovant Sciences and Seegnal US. Dr. Bilker serves on multiple data safety monitoring boards for Genentech. Dr. Dublin has research funding from GlaxoSmithKline. Dr. Hennessy has consulted for multiple pharmaceutical companies. Dr. Pham Nguyen receives support from Acadia Pharmaceuticals Inc., unrelated to this project. All other authors declared no competing interests for this work.
Data availability statement
The data that support the findings of this study are available from Optum, Inc. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Optum, Inc.
Reference
- 1.Cashin AG, Folly T, Bagg MK, Wewege MA, Jones MD, Ferraro MC, Leake HB, Rizzo RR, Schabrun SM, Gustin SM. Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: systematic review and meta-analysis. bmj 2021; 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soprano SE, Hennessy S, Bilker WB, Leonard CE. Assessment of physician prescribing of muscle relaxants in the United States, 2005–2016. JAMA network open 2020; 3: e207664–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Delcher C, Reisfield GM, Wei Y-J, Brown JD, Winterstein AG. Utilization patterns of skeletal muscle relaxants among commercially insured adults in the United States from 2006 to 2018. Pain Medicine 2021. [DOI] [PubMed] [Google Scholar]
- 4.Comber L, Quinn G, McGuigan C, Galvin R, Coote S. Medication usage and falls in people with multiple sclerosis. Multiple Sclerosis Journal 2018; 24: 995–98. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez CA, Mortensen EM, Makris UE, Berlowitz DR, Copeland LA, Good CB, Amuan ME, Pugh MJV. Association of skeletal muscle relaxers and antihistamines on mortality, hospitalizations, and emergency department visits in elderly patients: a nationwide retrospective cohort study. BMC geriatrics 2015; 15: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramness JG, Skurtveit S, Mørland J, Engeland A. The risk of traffic accidents after prescriptions of carisoprodol. Accident Analysis & Prevention 2007; 39: 1050–55. [DOI] [PubMed] [Google Scholar]
- 7.Golden AG, Ma Q, Nair V, Florez HJ, Roos BA. Risk for fractures with centrally acting muscle relaxants: an analysis of a national Medicare Advantage claims database. Annals of Pharmacotherapy 2010; 44: 1369–75. [DOI] [PubMed] [Google Scholar]
- 8.Horn JR. Triple Drug Interactions. In, Pharmacy Times, 2011. [Google Scholar]
- 9.Horsfall JT, Sprague JE. The pharmacology and toxicology of the ‘Holy Trinity’. Basic & clinical pharmacology & toxicology 2017; 120: 115–19. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol:“Holy Trinity” prescribing in the state of Florida. Drug and alcohol dependence 2019; 205: 107693. [DOI] [PubMed] [Google Scholar]
- 11.Optum Inc. Clinformatics Data Mart. In.
- 12.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. bmj 2016; 354. [DOI] [PubMed] [Google Scholar]
- 13.Leonard CE, Brensinger CM, Nguyen TPP, Horn JR, Chung S, Bilker WB, Dublin S, Soprano SE, Dawwas GK, Oslin DW. Screening to identify signals of opioid drug interactions leading to unintentional traumatic injury. Biomedicine & Pharmacotherapy 2020; 130: 110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard CE, Brensinger CM, Acton EK, Miano TA, Dawwas GK, Horn JR, Chung S, Bilker WB, Dublin S, Soprano SE. Population‐based signals of antidepressant drug interactions associated with unintentional traumatic injury. Clinical Pharmacology & Therapeutics 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X, Chiang C, Leonard CE, Bilker WB, Brensinger CM, Li L, Hennessy S. Biomedical informatics approaches to identifying drug-drug interactions: application to insulin secretagogues. Epidemiology (Cambridge, Mass) 2017; 28: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam YH, Brensinger CM, Bilker WB, Leonard CE, Han X, Hennessy S. Serious hypoglycemia and use of warfarin in combination with sulfonylureas or metformin. Clinical Pharmacology & Therapeutics 2019; 105: 210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hee Nam Y, Brensinger CM, Bilker WB, Flory JH, Leonard CE, Hennessy S. Angiotensin‐Converting Enzyme Inhibitors Used Concomitantly with Insulin Secretagogues and the Risk of Serious Hypoglycemia. Clinical Pharmacology & Therapeutics 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou M, Leonard CE, Brensinger CM, Bilker WB, Kimmel SE, Hecht TE, Hennessy S. Pharmacoepidemiologic screening of potential oral anticoagulant drug interactions leading to thromboembolic events. Clinical Pharmacology & Therapeutics 2020; 108: 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard CE, Brensinger CM, Bilker WB, Kimmel SE, Whitaker HJ, Hennessy S. Thromboembolic and neurologic sequelae of discontinuation of an antihyperlipidemic drug during ongoing warfarin therapy. Scientific reports 2017; 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard CE, Zhou M, Brensinger CM, Bilker WB, Soprano SE, Pham Nguyen TP, Nam YH, Cohen JB, Hennessy S. Clopidogrel drug interactions and serious bleeding: generating real‐world evidence via automated high‐throughput pharmacoepidemiologic screening. Clinical Pharmacology & Therapeutics 2019; 106: 1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam YH, Han X, Brensinger CM, Bilker WB, Leonard CE, Hennessy S. Sulfonylureas and Metformin Were Not Associated With an Increased Rate of Serious Bleeding in Warfarin Users: A Self‐Controlled Case Series Study. Clinical Pharmacology & Therapeutics 2020; 108: 1010–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen TPP, Brensinger CM, Bilker WB, Hennessy S, Leonard CE. Evaluation of serious bleeding signals during concomitant use of clopidogrel and hypnotic drugs. Biomedicine & Pharmacotherapy 2021; 139: 111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennessy S, Leonard C, Gagne J, Flory J, Han X, Brensinger C, Bilker W. Pharmacoepidemiologic methods for studying the health effects of drug–drug interactions. Clinical Pharmacology & Therapeutics 2016; 99: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner JG. Biopharmaceutics: 8. Relationship between Dosage Forms, Dosage Regimens and Pharmacokinetics. Drug Intelligence 1969; 3: 21–27. [Google Scholar]
- 25.Surgeons ACo. ACS NTB National Trauma Data Standard: data dictionary. In.
- 26.Greenland S. A semi‐Bayes approach to the analysis of correlated multiple associations, with an application to an occupational cancer‐mortality study. Statistics in medicine 1992; 11: 219–30. [DOI] [PubMed] [Google Scholar]
- 27.Strömberg U. Empirical Bayes and semi-Bayes adjustments for a vast number of estimations. European journal of epidemiology 2009; 24: 737–41. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe JH, Yang J. Association of combination opioid, benzodiazepine, and muscle relaxant usage with emergency department visits in a nationwide cohort in the United States. International journal of clinical pharmacy 2021; 43: 358–64. [DOI] [PubMed] [Google Scholar]
- 29.Burrows DL, Hagardorn AN, Harlan GC, Wallen ED, Ferslew KE. A fatal drug interaction between oxycodone and clonazepam. Journal of forensic sciences 2003; 48: 683–86. [PubMed] [Google Scholar]
- 30.Werner U, Werner D, Rau T, Fromm MF, Hinz B, Brune K. Celecoxib inhibits metabolism of cytochrome P450 2D6 substrate metoprolol in humans. Clinical Pharmacology & Therapeutics 2003; 74: 130–37. [DOI] [PubMed] [Google Scholar]
- 31.CELEBREX ® (celecoxib) [package insert], G.D. Searle LLC; Dividion of Pfizer Inc, NY, NY: 10017. [Google Scholar]
- 32.Friedrich LV, Dougherty R. Fatal hypoglycemia associated with levofloxacin. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2004; 24: 1807–12. [DOI] [PubMed] [Google Scholar]
- 33.Micheli L, Sbrilli M, Nencini C. Severe hypoglycemia associated with levofloxacin in Type 2 diabetic patients receiving polytherapy: two case reports. International journal of clinical pharmacology and therapeutics 2012; 50: 302–06. [DOI] [PubMed] [Google Scholar]
- 34.Parvez MM, Kaisar N, Shin HJ, Jung JA, Shin J-G. Inhibitory interaction potential of 22 antituberculosis drugs on organic anion and cation transporters of the SLC22A family. Antimicrobial agents and chemotherapy 2016; 60: 6558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLellan RA, Drobitch RK, Monshouwer M, Renton KW. Fluoroquinolone antibiotics inhibit cytochrome P450-mediated microsomal drug metabolism in rat and human. Drug metabolism and disposition 1996; 24: 1134–38. [PubMed] [Google Scholar]
- 36.von Moltke LL, Greenblatt DJ, Giancarlo GM, Granda BW, Harmatz JS, Shader RI. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metabolism and Disposition 2001; 29: 1102–09. [PubMed] [Google Scholar]
- 37.Rao N. The clinical pharmacokinetics of escitalopram. Clinical pharmacokinetics 2007; 46: 281–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Optum, Inc. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Optum, Inc.


