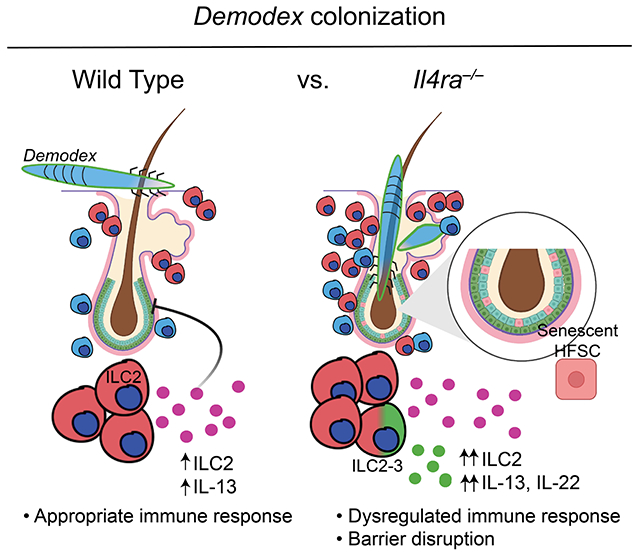
Summary
Demodex mites are commensal parasites of hair follicles (HF). Normally asymptomatic, inflammatory outgrowth of mites can accompany malnutrition, immune dysfunction and aging, but mechanisms restricting Demodex outgrowth are not defined. Here, we show that control of mite HF colonization in mice required innate lymphoid cells (ILC2s), interleukin-13 (IL-13), and its receptor, IL-4Ra-IL-13Ra1. HF-associated ILC2s elaborated IL-13 that attenuated HF and epithelial proliferation at anagen onset; in their absence, Demodex colonization led to increased epithelial proliferation and replacement of gene programs for repair by aberrant inflammation leading to loss of barrier function and HF exhaustion. Humans with rhinophymatous acne rosacea, an inflammatory condition associated with Demodex, had increased HF inflammation with decreased type 2 cytokines, consistent with the inverse relationship seen in mice. Our studies uncover a key role for skin ILC2s and IL-13, which comprise an immune checkpoint that sustains cutaneous integrity and restricts pathologic infestation by colonizing HF mites.
Keywords: Type 2 immunity, ILC2, innate immunity, Demodex mites, tissue immunity, IL-13, hair follicle stem cell, skin homeostasis, barrier function, rhinophyma
eTOC blurb
Type 2 cytokines are well recognized for their role in mediating allergic pathologies in skin but their role in normal skin physiology is unclear. Ricardo-Gonzalez et al. reveal that type 2 immunity restrains skin inflammation in response to injury that is necessary to control hair follicle commensalism by Demodex mites.
Graphical Abstract
Introduction
Type 2 immunity is orchestrated by the production of canonical cytokines, such as interleukin-(IL)-4, IL-5, and IL-13, that are induced during allergic inflammation or infection by parasitic helminths (Gause et al., 2020). Although host adaptive type 2 immunity engages and typically dominates these chronic afflictions, work over the last decade has revealed innate immune cells, particularly Group 2 innate lymphoid cells (ILC2s), as key sources of initial type 2 cytokines in involved tissues. ILC2s develop first during fetal hematopoiesis and position in tissues where they expand perinatally and express effector cytokines like IL-5 and IL-13 at birth (Schneider et al., 2019). Prevalent at mucosal barriers, ILC2s are also in skin (Kobayashi et al., 2019; Ricardo-Gonzalez et al., 2018), where roles in tissue repair have been demonstrated after injury or bacterial invasion (Sakamoto et al., 2021), although contributions of intermittently or constitutively produced type 2 cytokines to basal physiology remain poorly understood.
While investigating an acquired inflammatory skin condition among type 2 immunodeficient mice in the specific pathogen-free (SPF) animal facility at UCSF, we identified a critical role for innate type 2 immunity involving ILC2s in sustaining skin homeostasis in the presence of otherwise benign commensalism by Demodex. Demodex mites have co-evolved over millions of years as obligate commensal ectoparasites inhabiting the hair follicles (HF) and sebaceous glands of mammals (Palopoli et al., 2015; Thoemmes et al., 2014). Relatively short-lived (2-3 weeks), the minute arthropods eat sebum and keratinocyte debris and produce 1-2 dozen eggs that differentiate through intermediate larvae to generate progeny that spread to nearby follicles unless constrained by undefined host factors (Rather and Hassan, 2014). Among humans, prevalence of asymptomatic Demodex commensalism, particularly on the face, reaches essentially 100% with age, but outgrowths can be provoked by skin damage, immunodeficiency and malnutrition, and have been associated with rosacea (Georgala et al., 2001; Zhao et al., 2010), prompting the addition of anti-mite treatment in patients afflicted with inflammation in the setting of a high Demodex burden. Clinically, inflammation of the eyelids (blepharitis, which may overlap with ocular rosacea) observed in patients treated with anti-IL-4Ra for atopic dermatitis (Barbe et al., 2021; Simpson et al., 2016) is associated with increased Demodex spp. infestation involving the dense HF landscape of the eyelid (Heibel et al., 2021; Quint et al., 2020), suggesting that inhibition of basal IL-4Ra signaling might precipitate Demodex overgrowth and HF inflammation.
Using genetic models of adaptive and innate immunity in combination with co-housing and cell transfer experiments, our studies show that loss of type 2 cytokine signaling underlies failure to control HF colonization by Demodex. Patients with rhinophymatous rosacea, an inflammatory dermopathy of the nose associated with heavy Demodex infestation, had increased HF inflammation but reduced IL-13 as compared with HF incidentally commensalized by Demodex among patients without rosacea. Finally, we reveal an unanticipated role for ILC2s and IL-13 in HF and cutaneous homeostasis after perturbation that is activated and sufficient for control of this ubiquitous commensal of mammalian skin.
Results
Demodex outgrowth and inflammatory dermopathy occurs in the absence of type 2 cytokine signaling
Around 8-12 weeks after birth, homozygously-maintained Il4ra−/−, Il4−/−,Il13−/−, and Stat6−/− C57BL/6 mice (here designated type 2 immunodeficient) in our SPF facility developed prominent facial folds and blepharitis, with loss of coat pigmentation that progressed with age (Figure 1A). Microscopic examination of the skin from these type 2 immunodeficient mouse strains revealed acanthosis (thickening of the epidermis), aberrant HF morphology, and enlarged sebaceous glands (Figure 1B). We also observed increased proliferation and numbers of HF stem cells (HFSCs), and increased numbers of HFs (Figure S1, A to D).
Figure 1. Loss of type 2 immunity results in dermatitis associated with Demodex infestation.
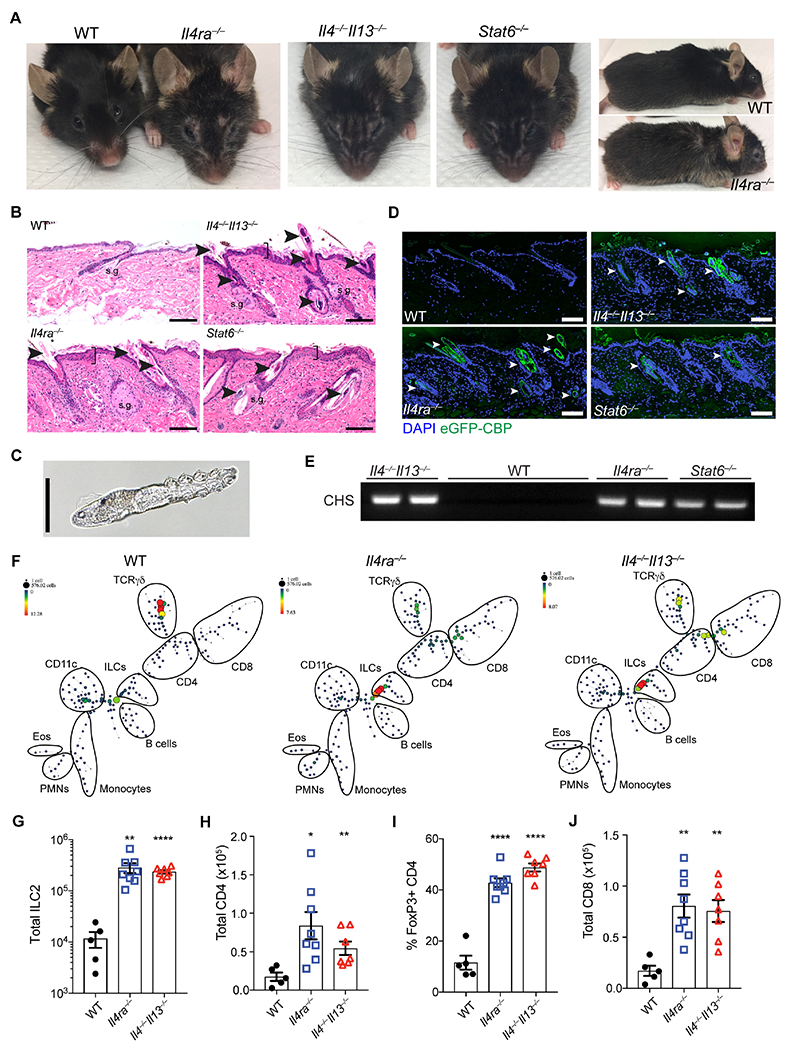
(A) Representative facial and fur phenotype of wild type (WT), Il4ra−/−, Il4−/−,Il13−/− and Stat6−/− mice at homeostasis. Mice shown are retired male breeders at 18-20 months of age. (B) Skin sections from WT, Il4−/−,Il13−/−, Il4ra−/− or Stat6−/− mice stained with H&E. Arrowheads highlight infestation by Demodex mites in hair follicles and sebaceous glands. Brackets emphasize epidermal acanthosis, s.g., sebaceous gland. Scale bar, 100 μm. (C) Brightfield image of a Demodex mite from a fur pluck of Il4ra−/− mice. Scale bar, 50 μm. (D) Skin sections from WT, Il4−/−,Il13−/−, Il4ra−/− or Stat6−/− were stained for chitin (green) using an enhanced GFP chitin-binding domain fusion (eGFP-CBP) and DAPI (blue). Arrowheads highlight the Demodex mites. Scale bar, 100 μm. (E) PCR for Demodex chitin synthase (CHS) from fur plucks. Images are representative of n ≥ 5 mice of both sexes for each genotype. (F) SPADE representation of CD45+ cells from epidermal fraction of 8-10 weeks old WT, Il4ra−/−, and Il4−/−,Il13−/− mice. One representative plot shown, n = 4 individual mice per genotype. (G) Number of ILC2s in skin from WT, Il4ra−−, and Il4−/−,Il13−/− mice. (H) Total CD4 in skin from WT, Il4ra−−, and Il4−/−,Il13−/− mice. (I) Frequencies of skin Treg cells (CD3+CD4+FoxP3+, as a percentage of total CD4) in skin from WT, Il4ra−/−, and Il4−/−,Il13−/− mice at homeostasis. (J) Total CD8 in skin from WT, Il4ra−/−, and Il4−/−,Il13−/− mice. Data are from one representative experiment (F), or pooled from multiple independent experiments (G–J). * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 by two-tailed Student’s t test (vs. WT). Please also see Figure S1.
These alterations were accompanied by infestation with HF-dwelling mites with characteristics of Demodex species (Figure 1B). We used morphologic criteria of mites from hair pluck specimens (Figure 1C) and sequencing of the 18S ribosomal RNA gene to identify the mites as Demodex musculi (Table S1), a relative of human-associated Demodex folliculorum. To localize mites by their chitinous exoskeleton, we generated an enhanced-GFP chitin-binding domain protein (eGFP-CBP) and examined involved skin using immunofluorescence (IF). We observed mites, often multiple, in essentially all HF and sebaceous glands of back skin in type 2 immunodeficient mice (Figure 1D). To assess the Demodex burden, we developed a PCR assay for the highly conserved Demodex chitin synthase gene (Zhao et al., 2012). Affected type 2-deficient mice were PCR positive whereas separately maintained, microscopically negative WT mice were PCR negative (Figure 1E). Thus, mice genetically lacking diverse arms of type 2 immunity developed uncontrolled infestation by an otherwise commensal skin ectoparasite, in line with previous reports of infrequent outbreaks of Demodex mites in mouse facilities (Smith et al., 2016).
We next sought to understand how immune cells and cytokines were altered in affected mice to explore immune mechanisms necessary for restraining Demodex overgrowth. Both flow- and mass cytometry (CyTOF) of immune cells in skin revealed significant expansion of ILC2s, regulatory T (Treg) cells, and CD4+ and CD8+ T cells in type 2 immunodeficient mice as compared to wild-type (WT) C57BL/6 mice (Figures 1, F to J and S1, E to G). Skin ILC2s were expanded 20-30-fold in Demodex-infected type 2 immunodeficient strains. In prior studies, proliferation of resident ILC2s was shown to drive their extrusion from tissues into the blood (Campbell et al., 2019; Huang et al., 2018; Ricardo-Gonzalez et al., 2020). Consistent with marked expansion of skin ILC2s in the presence of high numbers of mites, skin inflammation in Demodex-infested IL4Ra-deficient mice was accompanied by increased circulating ILC2s with the characteristic IL-18R+ skin phenotype (Figure S1, H and I). We also quantified elevated serum IL-5 and IL-13 (in IL-13-sufficient Il4ra−/− mice), prototypical ILC2 cytokines (Figure S1J), but also increased serum IL-22 (Figure S1J), suggesting transition of ILC2s to an inflammatory type 2 and type 3 immunophenotype as reported in other contexts (Bielecki et al., 2021; Reynolds et al., 2021). Indeed, skin ILC2s from Demodex-infected Il4ra−/− mice expressed Il22 and secreted IL-22 when activated in vitro (Figure S1, K and L). Thus, the absence of type 2 cytokines leads to overgrowth by cutaneous Demodex resulting in lymphocytic infiltrates accompanied by marked ILC2 activation, entry into blood and transition to an inflammatory phenotype.
Human rhinophyma with Demodex is associated with folliculitis and reduced IL13
The morphologic facial changes observed in infected type 2 immunodeficient mice resembled changes observed in patients affected by rhinophymatous acne rosacea associated with Demodex (Forton and Seys, 1993). Rhinophyma is a chronic manifestation of rosacea characterized by acanthosis, inflammation and enlargement of the sebaceous glands affecting the nose of older adults (Figure 2A). To investigate correlates of the type 2 immune axis in humans that promote stable commensalism with Demodex, we analyzed excisions of nasal skin from adults with and without rhinophyma. In normal skin, we identified incidental Demodex mite infestation limited to individual HF accompanied by mild inflammatory infiltrates that included more IL13- than IL4-expressing cells as assessed by RNA in situ hybridization (ISH) (Figure 2, B to E). In contrast, patients with rhinophyma had significantly denser inflammatory infiltrates but fewer IL13- and IL4- expressing cells surrounding HF (Figure 2, B to E). Dual IL13 ISH and CD3ε immunohistochemistry (IHC) showed that approximately 40% of IL13-expressing cells in normal skin were CD3ε negative, consistent with production of this cytokine by both T cells (IL13+CD3ε+) and innate lymphocytes (IL13+CD3ε−) (Figure 2F–G). We also assessed the expression of IFNG and IL22 among these patients for evidence of immune deviation and found that IFNG and IL22 expression was increased in rhinophyma compared to normal skin (Figure 2, H to K). These data show that patients with rhinophyma and Demodex, which phenotypically resembles mite infection in type 2 immunodeficient mice, have inflammation and diminished IL13 production, thus encouraging us to investigate further the mechanisms by which type 2 immunity facilitates stable commensalism by this ubiquitous ectoparasite.
Figure 2. Type 2 cytokines expression is associated with healthy Demodex commensalism in humans.
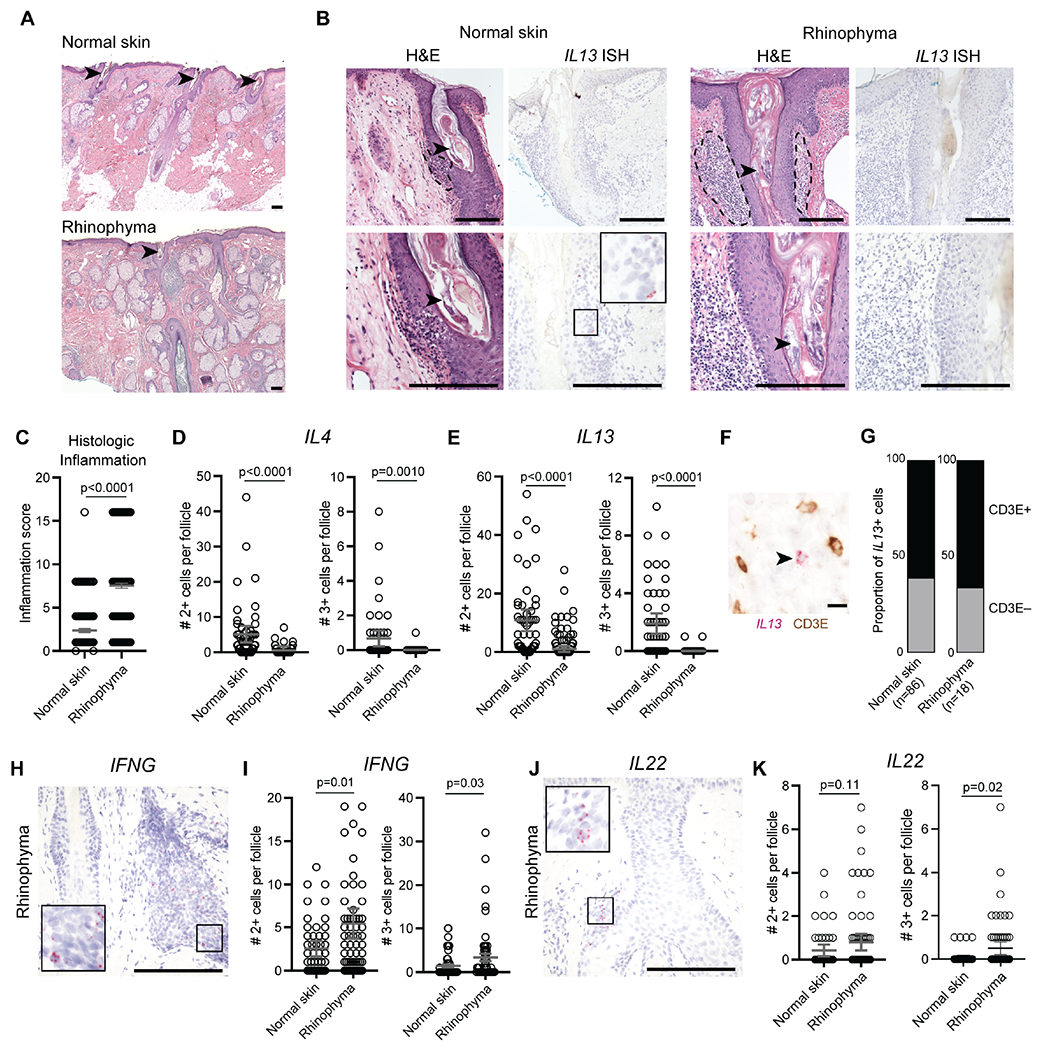
(A) Low power H&E stained sections of normal skin (top) and rhinophyma (bottom). (B) H&E stained sections (left panels) and corresponding IL13 mRNA in situ hybridization (ISH, right panels) in hair follicles infected with Demodex in representative cases of normal skin and rhinophyma. Arrowheads highlight Demodex infection of the hair follicle. Dashed lines highlight perifollicular inflammation. Scale bars, 250 μm. (C to E) Scoring of hair follicle inflammation (C) and quantification of IL4 (D) and IL13 (E) expression by ISH. (F and G) Representative co-stained section for IL13 by ISH and CD3ε by immunohistochemistry (F) and quantification of proportion of CD3+ or CD3− IL13-expressing cells (G). Arrowhead highlights IL13+CD3− cell. Scale bar, 10 μm. (H and I). Representative IFNG mRNA ISH (H) and quantification of IFNG expression (I) in hair follicles infected with Demodex. (J and K). Representative IL22 mRNA ISH (J) and quantification of IL22 expression (K) in hair follicles infected with Demodex. Scale bars, 250 μm. Statistical significance shown by * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 by two-tailed Student’s t test.
Type 2 immunodeficient mice fail to control HF colonization by Demodex
Spontaneous skin inflammation is not typical among type 2 immunodeficient strains in SPF facilities. When affected IL-4Ra-deficient mice were re-derived and bred in separate SPF rooms, none developed dermopathy, skin ILC2 expansion or Demodex infection (Figure 3, A, G, and H). After housing with mite-infested IL-4Ra-deficient mice, however, unaffected IL-4Ra-deficient mice were colonized with Demodex and developed both the dermopathy and the immune phenotypes described above over a period of 6-to-12 weeks (Figures 3, B to G).
Figure 3. Co-housing Demodex-infected with non-infected Il4ra−/− mice transfers the phenotype.
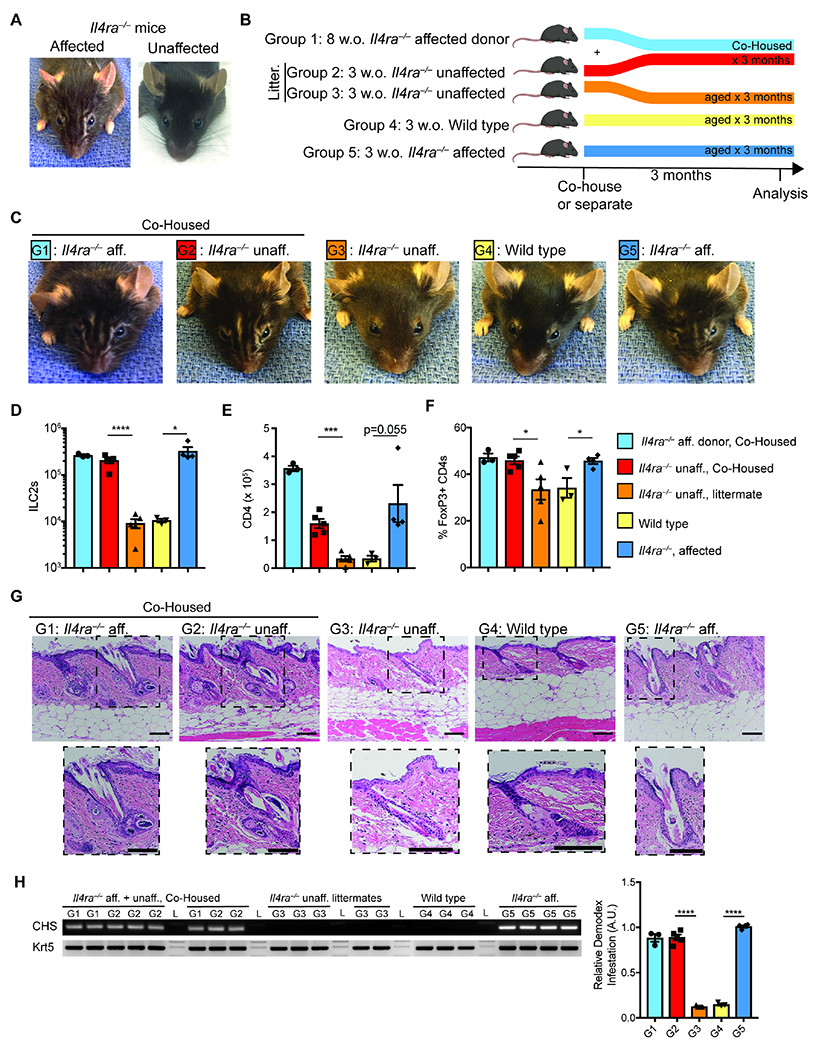
(A) Facial appearance of Il4ra−/− mice infected (affected) or uninfected (unaffected) with Demodex musculi. (B) Schematic of the co-housing experiment. 8 week-old (w.o.) Il4ra−/− mice infected with Demodex (Group 1) were co-housed with unaffected 3 w.o. Il4ra−/− mice (Group 2). Unaffected littermates of co-housed mice (Group 3) were allowed to age concurrently. WT (Group 4) and Il4ra−/− from known Demodex infected parents (Group 5) were aged as independent controls. (C) Representative mice at 3 months after the co-housing experiment. G1-G5 indicate experimental groups as outlined in (B). (D and E) Number of skin ILC2 (D) and CD4+ (E) cells in back skin. (F) Frequency of Treg cells (as percentage of total CD4) in back skin. (G) Sections from back skin were stained with H&E. Inset highlights Demodex infestation of the hair follicle and sebaceous glands. Scale bar, 100 μm. (H) PCR for Demodex chitin synthase gene (CHS, top) or genomic DNA for the keratin 5 gene (Krt5, bottom) from 2mm punches of back skin. Quantification of relative band intensity (CHS/Krt5) is shown on the right. Data are from one representative experiment of two independent experiments. Statistical significance shown by * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 by two-tailed Student’s t test. Please also see Figures S2, S3 and S4.
To further examine transmissibility, we bred uninfected WT mice to Demodex-infected Il4ra−/− mice, which were maintained as homozygotes, and subsequently intercrossed the phenotypically normal-appearing heterozygous F1 littermates to generate WT, heterozygous (Il4ra+/−), and homozygous Il4ra−/− F2 mice. Although the F2 generation was derived from previously affected animals, neither WT, heterozygous, nor genetically ablated (Il4ra−/−) littermates developed the facial or skin inflammation phenotypes when derived from unaffected heterozygous parents descendent from an affected Il4ra−/− grandparent (Figure S2, A to E). However, F1 Il4ra−/− mice derived from the intercross of affected Il4ra−/− and unaffected heterozygous Il4ra+/− mice developed the inflammatory phenotype (Figure S2F), suggesting that direct parental transfer of mites from and to type 2 immunodeficient offspring is important for development of this phenotype. Appearance of the skin and facial phenotypes was accompanied by increases in skin ILC2s, CD4+ T cells and Treg cells, as well as the presence of IL-13 and IL-22 in serum and an increase in follicular mites (Figure S2, G to O). Thus, under these conditions, functional type 2 immunity, even in heterozygous genotypes, suppresses Demodex below what is required for transmission and progression to outgrowth and dermopathic phenotypes in offspring.
Targeted anti-mite but not anti-bacterial therapy prevents the inflammatory dermopathy
Loss of skin ILCs leads to sebaceous gland hypertrophy with altered sebum constituents and effects on the skin bacterial microbiota (Kobayashi et al., 2019). To this end, we performed 16S rDNA amplicon sequencing of skin samples from uninfected WT and Demodex infected and uninfected Il4ra−/− mice (Figure S3A). Although bacterial diversity was similar between phenotypes, analysis of weighted UniFrac distance between samples showed differences in overall community composition (Figure S3B, p = 0.001, PERMANOVA test). Looking at the broad characteristics of the bacteria, we found that the fraction of obligate anaerobes was higher in uninfected mice (p = 0.028, Figure S3C), consistent with the normal anaerobic environment of the healthy HF (Chen et al., 2018). Despite these microbiota differences, treatment of infected Il4ra−/− mice with broad-spectrum antibiotics had no effect on the phenotype (Figure S3D). In contrast, treatment of 6-week-old infected Il4ra−/− littermates (before they show the visual phenotype) with topical anti-mite agents moxidectin and imidacloprid (Nashat et al., 2018), but not vehicle control (ethanol), decreased the mite burden and prevented the skin phenotype, in association with reductions in skin ILC2s and normalization of CD4+ T cells and Treg cells (Figure S4, A to G). We obtained comparable results when treating young (4-5 weeks) Demodex-infected Il4−/−,Il13−/− mice (Figure S4, H to P). Taken together, these data show that targeted anti-mite therapy reverses the immune disruption and prevents the facial phenotype induced by Demodex in the absence of type 2 immunity.
Colonization by Demodex drives accumulation of activated ILC2s in normal skin
Our data suggested that type 2 cytokine signaling is required to restrict Demodex outgrowth and prevent pathology after colonization. Although neither WT nor heterozygous mice develop the inflammatory dermopathy, we used co-housing to ask how ILC2s and type 2 cytokines respond to Demodex. To this end, we co-housed 3-to-5-week old Il4ra−/− mice from infected colonies, before they acquired visible inflammation, with uninfected WT mice. While Il4ra−/− mice developed the cutaneous and facial phenotype with age, as expected, co-housed WT mice remained visually unaffected (Figure 4A). Although Demodex-free at the start of co-housing, however, WT mice were colonized by low numbers of mites as established by histologic exam, eGFP-CBP IF, and PCR for Demodex chitin synthase (Figure 4, B to D). Infected WT mice developed expansion of skin ILC2s and had increased IL-13 and IL-5 expression in skin, but no cytokines in blood; expansion of activated CD4+ T cells and Treg cells was modest (Figure 4, E to H). Activated skin ILC2s appeared by 2 weeks and peaked 1-2 months after infection of WT mice at approximately half the frequency seen in infected IL-4Ra-deficient mice (Figure 4, I to N). Alarmin cytokines like thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 are important for homeostatic activation of tissue ILC2s; IL-18 may play a similar role in skin (Ricardo-Gonzalez et al., 2018). To assess whether typical alarmins are required for activation of skin ILC2s in response to Demodex, we generated Tslp−/−; Il18−/−; Il1rl1−/−; Il25−/− quadruple ablated (Quad-ablated) mice together with Il5Red5 and Il13Smart13 reporters, and co-housed the mice with WT and Demodex-infected IL-4Ra-deficient mice for 8 weeks. At baseline, Quad-ablated mice had reduced numbers of IL-5- and IL-13-expressing skin ILC2s as compared to WT mice (Figure 4, O and P). Although reduced, skin ILC2s from alarmin-deficient mice retained the proportionate capacity to expand and increase IL-5 and IL-13 in response to mites in association with an attenuated but intermediate Demodex burden as compared to WT mice and sufficient to block development of the facial phenotype (Figure 4, O to S). Taken together, these data show that Demodex colonization provokes expansion and activation of skin ILC2s by a mechanism partially dependent on endogenous alarmins responsible for setting basal innate type 2 immune tone, but absolutely dependent on elaboration of type 2 cytokines to restrict mite outgrowth and attenuate the inflammatory dermopathy.
Figure 4. Type 2 immunity sustains non-pathologic Demodex colonization.
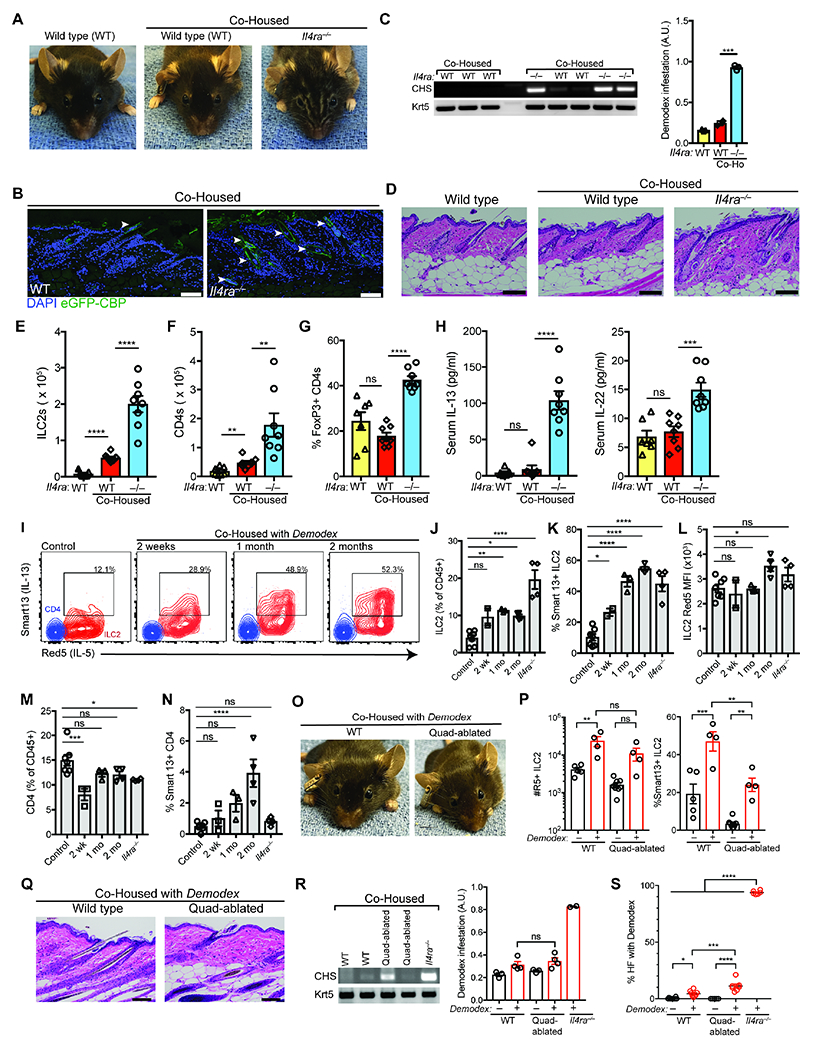
(A) Representative wild type (WT), and co-housed WT with Demodex infested Il4ra−/− mice. Three week old WT and infested Il4ra−/− mice were co-housed for 12 weeks. (B) Skin sections from co-housed WT or Demodex infected Il4ra−/− were stained for chitin (eGFP-CBP, green) and DAPI (blue). Scale bar, 100 μm. (C) PCR for Demodex chitinase synthase gene (CHS, top) or genomic DNA for the keratin 5 gene (Krt5, bottom) from back skin. Quantification of relative Demodex infestation (CHS/Krt5) is shown on the right. (D) H&E-stained sections of back skin from WT, or co-housed WT and Il4ra−/− mice. Scale bar, 100 μm. (E–G) Number of skin ILC2s (E), total CD4 (F), and frequency of Treg cells (CD3+CD4+FoxP3+ T cells) as a percentage of total CD4 (G). (H) Quantification of serum IL-13 and IL-22. (I) IL-5 and IL-13 expression by ILC2s in Demodex infection. WT Il5tdTomato(Red5)/+, I13hCD4(Smart13)/+ littermates were separated (Control) or co-housed with infested Il4ra−/− mice (Co-housed) for 2 weeks (wk), 1 month (mo) or 2 mo. Flow cytometry plots show IL-5 and IL-13 expression by skin ILC2 (Red) or CD4 (blue). (J–L) Quantification of ILC2s (J), and their expression of IL-13 (hCD4, Smart13, (K)), and IL-5 (L). (M–N) Quantification of total CD4 (M) and their expression of IL-13 (hCD4, Smart13, (N)). (O) Representative wild type (WT), and Crlf2−/−,Il1rl1−/−,Il18−/−,Il25−/− (Quadablated) mice co-housed with Demodex infested Il4ra−/− mice. (P) Number of skin ILC2s and IL-13 expressing (Smart13) ILC2s. (Q) H&E-stained sections of back skin from WT, and Quadablated mice co-housed with Demodex-infected Il4ra−/− mice. Scale bar, 100 μm. (R) PCR for Demodex chitinase synthase gene (CHS, top) or genomic DNA for the keratin 5 gene (Krt5, bottom). (S) Quantification of percentage of hair follicles infected with Demodex. Data presented as mean ± s.e.m. Statistical significance shown by * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 by one-way ANOVA. Please also see Figure S5.
Although both IL-4 and IL-13 signal through the IL-4Ra receptor, IL-4-deficient mice (Il4−/−; (Mohrs et al., 2001) controlled Demodex as well as WT mice when co-housed with infected IL-4Ra-deficient mice (Figure S5, A to G) suggesting that IL-13 is necessary for Demodex control. We generated IL-13Ra1-deficient mice and co-housed these mice with mite-infected IL-4Ra-deficient mice. Il13ra1−/− mice were highly susceptible to Demodex outgrowth and developed the dermopathic facial phenotype; heterozygous mice showed partial control (Figure S5, H to J). We additionally co-housed Il4−/− and Il13ra1−/− mice with infected mice to demonstrate the requirement for IL-13 but not IL-4 for control of hair follicle infestation (Figure S5, K to N). Taken together, these experiments in mice and observations from human patients support a key role for type 2 immunity and IL-13 acting through the IL-4Ra and IL-13Ra1 receptor in regulating stable HF colonization by skin mites. In the absence of these constituents of type 2 immunity, Demodex overgrowth appears to be the key trigger driving inflammation and ILC2 activation, immune deviation, and appearance in blood.
The adaptive immune system is not required to control Demodex
In our initial characterization of Demodex-infected type 2 immunodeficient strains, we observed expansion of ILC2s as well as adaptive immune cells. To ascertain a necessary role for the adaptive immune system in Demodex control, we co-housed infected IL-4Ra-deficient mice with uninfected Rag1-deficient mice, which lack adaptive B and T cells, including Treg cells. Like infected WT mice, Rag1-deficient animals remained visually unaffected and restrained Demodex colonization in contrast to co-housed IL-4Ra-deficient mice (Figure S6, A to D). As in co-housed WT mice, IL-18R+ skin ILC2s expanded in cohoused Rag1−/− mice relative to uninfected controls (Figure S6, E to H). These data suggested a role for innate immune cells in controlling Demodex colonization. To explore this hypothesis, we co-housed infected Il4ra−/− mice with uninfected Rag2−/− mice, which lack B and T cells, and Rag2−/−,Il2rg−/− mice, which also lack ILCs. Within 4-6 weeks of co-housing, Rag2−/−,Il2rg−/− mice, but not Rag2−/− cage mates, developed the characteristic facial phenotype, blepharitis and increased mite burden observed in Demodex-infected type 2 immunodeficient mice (Figure 5, A to D). Demodex-infected Rag2-deficient mice had minimal epidermal proliferation (Figure 5E), while Rag2−/−,Il2rg−/− mice developed hyperplasia, hyperkeratosis and epithelial proliferation. Thus, under these conditions, the adaptive immune system is not required for control of Demodex colonization nor for the expansion of skin ILC2s in response to Demodex, whereas innate lymphoid cells are critical for control of mite burden and prevention of the inflammatory dermopathy.
Figure 5. ILC2s are sufficient to restrain Demodex infection.
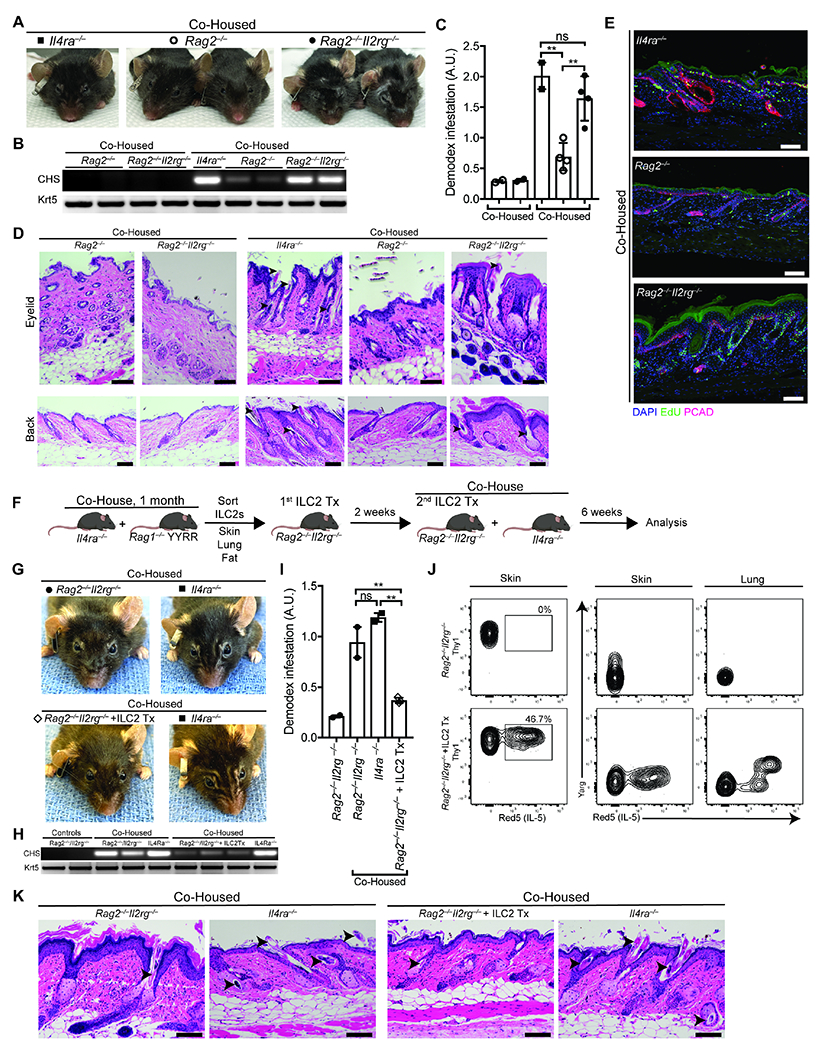
(A) Representative Rag2−/− and Rag2−/−,Il2rg−/− co-housed with Demodex infested Il4ra−/− mice. 5-week old Rag2−/− and Rag2−/−,Il2rg−/− were co-housed with Demodex infested Il4ra−/− for 6 weeks. n = 3 to 5 mice per group, representative of two independent experiments. (B) PCR for Demodex chitinase synthase gene (CHS, top) or genomic DNA for the keratin 5 gene (Krt5, bottom) from back skin. (C) Quantification of relative Demodex infestation (CHS/Krt5) for the PCR in (B). (D) H&E-stained sections of eyelid (top) and back (bottom) skin from unaffected Rag2−/− and Rag2−/−,Il2rg−/− with or without co-housing with a Demodex infested Il4ra−/−. (E) Sections from back skin of co-housed Il4ra−/−, Rag2−/− and Rag2−/−, Il2rg−/− were stained for EdU (green), P-Cadherin (red) and DAPI (blue). (F) Schematic of the adoptive transfer experiment. Il4ra−/− mice infected with Demodex were co-housed with unaffected 4-8 w.o. Rag1−/− ArgYFP/YFP, Il5tdTomato(Red5)/tdTomato(Red5) (Ragl−/− YYRR) mice for 1 month. ILC2s (CD45+Lin− Thy1+Red5+) were sorted from skin, lung, and fat, pooled, and transferred to Rag2−/−,Il2rg−/− mice two weeks prior and at the start of co-housing (see methods). (G) Representative Rag2−/−,Il2rg−/− treated with PBS (top) or with transferred ILC2s (bottom) after 6 weeks of co-housing with Demodex infected Il4ra−/−. (H) PCR for Demodex chitinase synthase gene (CHS, top) or genomic DNA for the keratin 5 gene (Krt5, bottom) from back skin. (I) Quantification of relative Demodex infestation (CHS/Krt5) for the PCR in (H). (J) Flow cytometry plots of ILCs (pre-gated on Live CD45+Lin−Thy1+) showing IL-5 expression (left panels) or Arginase (middle and right panels) on skin and lung as indicated. (K) H&E-stained sections of back skin from Rag2−/−,Il2rg−/− treated with PBS or ILC2 adoptive transfer and co-housed with Demodex infested Il4ra−/−. n = 3 to 5 mice per condition. Arrowheads highlight Demodex mites. Scale bars, 100 μm. For the ILC2 transfer experiments, a representative mouse is shown from 2 independent experiments with n = 2-3 mice per group. Please also see Figure S6.
To test the sufficiency of ILC2s, we sorted ILC2s from Rag1−/− mice (expressing Arg11 and Il5 reporter alleles as described (Bando et al., 2015; Nussbaum et al., 2013) that were co-housed for one month with Demodex-infected Il4ra−/− mice in order to activate and expand ILC2s in vivo (Figure 5F). Primed ILC2s were purified and transferred into Rag2−/−,Il2rg−/− mice prior to and at the start of co-housing with affected Il4ra−/− mice. After 6 weeks, we detected transferred ILC2s in the reconstituted Rag2−/−,Il2rg−/− mice, which had no facial disfigurement, minimal hyperplasia by skin histology, and reduced Demodex burden as compared to colonized Rag2−/−,Il2rg−/− mice that did not receive ILC2s (Figure 5, G to K). Together, these experiments support a fundamental role for skin ILC2s in control of epithelial homeostasis in response to HF colonization by Demodex.
Type 2 immunodeficient mice activate inflammatory rather than repair responses to Demodex colonization
To understand the impact of Demodex HF colonization on skin homeostasis, we analyzed immune and non-immune cells from type 2 immune-sufficient or -deficient mice infected with Demodex using scRNA-seq. In agreement with flow cytometric studies, Demodex HF colonization in WT mice led to expansion of IL-18R+ ILC2s (Figure 6, A and B), which were activated as indicated by expression of Il13, Areg, and Il17a (Figure 6, C to E), and mirroring effector programs associated with tissue repair by skin T cells (Harrison et al., 2019). In type 2 immunodeficient mice, there were increases in ILC2s, Treg cells and CD8+ T cells, and the appearance of an ILC2-ILC3 transitional population, consistent with our prior findings (Figures 6, F to H; 1, F to J; and S1, J to L). Of note, skin lymphocytes in infected WT mice mounted an enhanced type 2 immune response whereas lymphocytes in type 2 immunodeficient strains deviated towards type 1 (Ifng) and type 3 (Il22) responses (Figure 6, H and I).
Figure 6. scRNA-seq of CD45+ cells from Demodex-infected WT and type 2 immunodeficient mice reveal divergent responses.
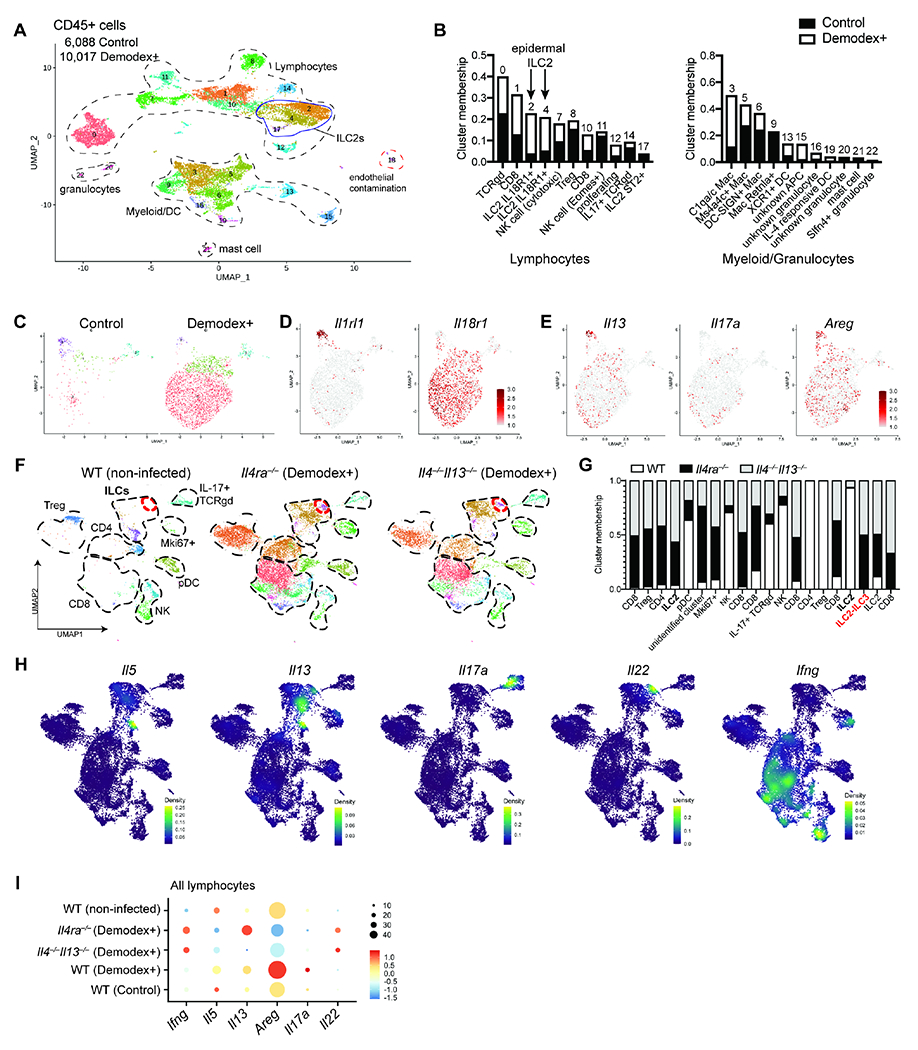
(A) UMAP projection of CD45+ cells from skin of WT uninfected and Demodex-infected mice. (B) Bar plot showing the percentage of cells in each cluster for all lymphocyte (left) and myeloid and granulocyte (right) populations by experimental condition. (C) UMAP projection of ILC subclustering analyses showing experimental condition. (D) Feature plot of ILC subclusters showing expression of genes associated with ILC2 subset identity in skin. (E) Feature plots showing expression of genes for Il13, Il17a, and Areg. (F) UMAP projection of the skin lymphocyte fraction of WT (uninfected), and Il4ra−/− or Il4−/−,Il13−/− (Demodex-infected) mice showing subset of lymphocytes. Red circle denotes ILC2-ILC3 population present only in Il4ra−/− or Il4−/−,Il13−/− samples. (G) Bar plot showing the percentage of cells in each cluster for the samples in (F). (H) Density plots from the combined object as in F, showing the expression for selected cytokines. (H) Dot plot of a combined analysis for all lymphocytes in all samples. Please also see Figure S7.
Analysis of CD45− skin cells from control and Demodex-infected WT mice yielded similar representation between the samples among major skin cell populations (Figure S7, A and B). Gene ontology pathway analysis in Demodex-infected WT mice showed induction of pathways associated with extracellular matrix conferring tensile strength, including induction of collagen proteins which were not as highly expressed in Demodex-infected Il4ra−/− or Il4−/−,Il13−/− mice (Figure S7, C and D). In contrast, infected Il4ra−/− and Il4−/−,Il13−/− mice revealed induction of pathways associated with host defense and inflammation, response to bacteria (S100a8, S100a9, Defb6, Stfa1, Srpinb1a), and antigen presentation (Tap1, H2-M3, H2-D1, Cd74, H2-Q6) (Figure S7, E and F).
To assess skin cells that respond to IL-13, we examined cell populations that expressed Il4ra, Il13ra1 or both, noting that the epithelium had a high correlation of Il4ra and Il13ral co-expression (Figure S7G). Subclustering and biological processes analyses of the epithelia and Lgr5-expressing HF stem cells (HFSCs) revealed a biological response primed for wound healing in Demodex-infected WT mice (Figure S7H). However, this reparative response was absent among epithelia and HFSCs from infected Il4ra−/− and Il4−/−,Il13−/− mice, and replaced by a response characteristic of replicating cells, including DNA synthesis and ribosomal processing (Figure S7, I and J). Together, these data support a role for the ILC2-IL13 pathway in inducing the restorative and regenerative response necessary to sustain anatomic integrity during colonization by HF mites.
Type 2 cytokines reduce HFSC proliferation and delay HF regrowth
To investigate mechanisms by which IL-13 from ILC2s confers protection of HF after mite colonization, we used well-characterized reporter mice for the type 2 cytokines IL-5 and IL-13 (Liang et al., 2011; Nussbaum et al., 2013) to assess the location and activation of ILC2s in resting skin. ILC2s were the most prevalent cells expressing these cytokines under basal conditions, corroborating prior findings (Mayer et al., 2021; Ricardo-Gonzalez et al., 2018; Schneider et al., 2019), and localized predominantly within the epithelial layer in close association with HF (Figure 7, A and B). We observed cyclic IL-13 expression in skin ILC2s, with high expression in the early postnatal period as previously observed (Schneider et al., 2019), and then oscillating expression with phases of the hair cycle, which remains synchronous in the first months of life, with low IL-13 during telogen (rest) and high IL-13 during anagen (growth) (Figure 7C). To confirm that cells from IL-13 reporter mice accurately reflect Il13 gene expression, we sorted ILC2s from back skin of mice in telogen or anagen phases of the hair cycle and confirmed the increase in Il13 during anagen by quantitative PCR (Figure 7D). These observations suggest that ILC2s and IL-13 may support the cyclic growth and turnover of the HF compartment (Naik et al., 2018), particularly at onset of anagen when this compartment may be susceptible to the 2-3 week reproductive cycle of Demodex.
Figure 7. Type 2 immunity sustains skin barrier and HF regeneration after perturbation.
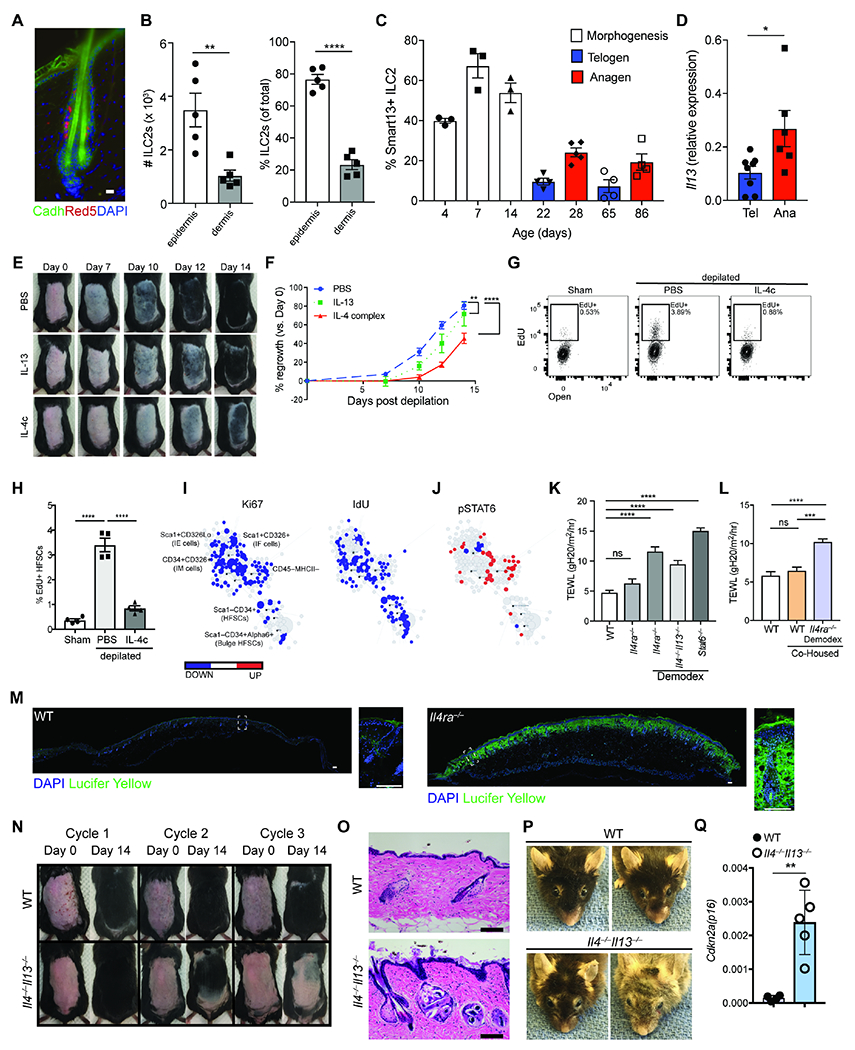
(A) Localization of IL-5 producing ILC2s (red) within the skin epithelium. E-cadherin (green) is used to highlight hair follicles. DAPI is represented in blue. Scale bar, 20 μm. (B) Quantification (left) or percentage (right) of ILC2s (CD45+Lin−Thy1+Red5+) in the epidermis and dermis of adult Il5Red5/+ mice. (C) Quantification of expression of IL-13 by ILC2s throughout the various stages of the hair follicle cycle. (D) Quantitative PCR for Il13 expression by ILC2s (CD45+Lin− Thy1+Red5+) sorted from mice back skin in telogen or anagen stage of the hair cycle. (E) Effect of IL-4 and IL-13 subcutaneous injection in hair growth. Wild type C57BL/6 mice were treated with PBS, IL-13 or IL-4 complex (IL-4c) on the first 2 days after depilation (Days 0 and 1) and their hair regrowth pattern was tracked over 2 weeks. One representative mouse per treatment arm is shown. (F) Quantification of hair regrowth from PBS, IL-4c, or IL-13 treated mice as in (E). (G) Representative flow cytometry panel of hair follicle stem cells (HFSCs, pre-gated as CD45−MHCII−CD34+Alpha6+) incorporation of EdU. Mice were depilated and treated with IL-4 complex (IL-4c) on the first two days after depilation. Mice were harvested on day 4 after depilation. (H) Percent EdU+ HFSCs four days after depilation as in (G). (I) Scaffold maps of Ki67 and IdU for the CD45− epidermal cell fraction as analyzed by CyTOF (see methods). Black nodes represent canonical cell populations identified manually as noted in the top left scaffold map. Blue denotes the population has significant lower expression frequency of the marker (Ki67, or IdU, as denoted in the columns) in the experimental arm (IL-4 complex treated WT mice) vs. WT controls; red denotes significantly higher frequency. n = 4 individual mice per group. (J) Scaffold maps for phospho(p)STAT6 in IL-4 complex treated mice compared to WT controls. Blue and red colors denote statistically significant decrease or increase in expression frequency, respectively, in each cluster compared to the control group (q < 0.05 by SAM). (K) Transepidermal water loss at homeostasis in WT, Il4ra−/− and Demodex-infested Ilra4−/−, Il4−/−Il13−/−, and Stat6−/− mice. (L) Transepidermal water loss at homeostasis in WT or WT mice co-housed with Demodex-infested Il4ra−/− mice. (M) Lucifer yellow barrier assay. WT or Il4ra−/− were shaved and depilated. Two days post-depilation, the epidermal surface was exposed to Lucifer Yellow overnight at 37 °C. Lucifer yellow in green, DAPI in blue. Scale bar, 100 μm. (N) Repeated depilation of wild type (WT) and Il4−/−Il13−/− mice. A representative animal from n = 4 individual mice shown. (O) Sections from back skin of WT and Il4−/−,Il13−/− mice after 3 cycles of depilation were stained with H&E. Scale bar, 100 μm. (P) Representative images of 2 year-old (yo) WT and Il4−/−Il13−/− mice. (Q) qPCR for Cdkn2a(p16) from sorted HFSCs from age-matched 2 yo WT and Il4−/−Il13−/− mice. Data presented as mean ± s.e.m and are representative of at least two independent experiments with n ≥ 3 mice per individual group/time point, pooled from two independent cohorts with n=5-10/group (K, L) or representative from an experiment with n ≥ 4 per group (P, Q). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 as calculated with two-tailed Student’s t test (A to D, Q) or ANOVA (F, H, K, L).
To assess the role of type 2 cytokines on hair growth and HFSCs, we depilated mice to induce anagen and synchronous hair regrowth (Ali et al., 2017; Paus et al., 1999), and then administered IL-13 or IL-4, which signal via the common IL-4Ra receptor. Either IL-13 or IL-4, the latter administered as long-lived antibody complexes, slowed hair regrowth (Figure 7, E and F) and attenuated proliferation of HFSCs (Figure 7, G and H). We used CyTOF to assess the impact of type 2 cytokine signaling on skin epithelia, which express Il4ra and Il13ra1 (Figure S7G), and observed decrease in proliferation of the epithelial fraction as assessed by Ki-67 and uptake of the EdU analog, 5-Iodo-2-deoxyuridine (IdU). IL-4Ra signaling also elicited an increase in phospho-STAT6 (Figure 7, I and J). These findings, in combination with our scRNA-seq data (Figure S7), support a link between type 2 cytokines, epithelia, and HFs in sustaining skin homeostasis that is evident after colonization by mites.
Loss of type 2 immunity compromises cutaneous integrity in the setting of Demodex colonization
The loss of a skin reparative response seen in mite-colonized WT as compared to colonized Il4ra−/− and Il4−/−,Il13−/− mice, together with loss of the normal obligate anaerobes from HF in infected Il4ra−/− mice (Figure S3C), led us to hypothesize that type 2 immunity may be necessary to preserve HF and skin integrity in the setting of perturbation. To test this hypothesis, we measured transepidermal water loss (TEWL) in WT, IL-4Ra-deficient and Demodex-infected IL-4Ra-deficient mice. We noted barrier dysfunction in Demodex-infected IL-4Ra-deficient mice compared to uninfected IL-4Ra-deficient mice (separately housed) or Demodex-colonized WT mice (housed in the same cage as affected IL-4Ra-deficient mice) (Figure 7, K and L). Further, penetration of lucifer yellow applied to depilated skin was constrained to the epidermis and upper HF in WT mice, but diffused throughout the subcutaneous tissues in IL-4Ra-deficient mice, even in the absence of mite infection (Figure 7M).
The finding that depilation alone was sufficient to reveal loss of skin integrity in the absence of type 2 cytokines led us to hypothesize that the repetitive demands of persistent mite HF colonization on tissue repair might drive the appearance of the inflammatory dermopathy among type 2 immunodeficient mice by exceeding the limits for tissue resilience. To test this, we performed sequential depilations in WT or Il4−/−,Il13−/− mice infected with mites. Although WT mice re-grew hair with each cycle as predicted (Paus et al., 1999), Il4−/−,Il13−/− mice had increasingly slower kinetics of regrowth with subsequent depilations (Figure 7N). Histologic exam showed that WT mice maintained normal HF morphology and subcutaneous adipose tissue, whereas Il4−/−,Il13−/− mice had a more fibrotic dermis and HF structures were further compromised, consistent with alterations accompanying tissue senescence (Figure 7O) (Ge et al., 2020). Supporting this, aged Demodex-infected type 2 immunodeficient mice developed premature coat depigmentation as compared to similarly aged WT mice (Figure 7P). Analyses of HFSCs from 20-24 month-old, age-matched WT and Demodex-infected Il4−/−,Il13−/− mice showed an increase of the senescence-associated marker Cdkn2a/p16 in the absence of type 2 cytokines (Figure 7Q). Taken together, these data reveal a key role for skin ILC2s and innate type 2 immunity in enhancing skin resilience following inflammation in response to HF colonization by Demodex.
Discussion
Demodex mites are ubiquitous ectoparasites considered commensals of mammalian HFs (Rosshart et al., 2019; Sastre et al., 2016). As such, Demodex outbreaks remain infrequently reported among SPF mice facilities, but have been noted among strains deficient in type 2 immunity, as occurred here (Smith et al., 2016). As we have shown by co-housing and transfer experiments, activation of ILC2s, IL-13 and signaling through IL-4Ra were necessary and sufficient to limit proliferation and spread of mites once acquired in the pilosebaceous units of the skin. Further study revealed deficits in the ability of type 2 immunodeficient mice to sustain skin homeostasis not only following mite colonization, but also after depilation. Repetitive depilation resulted in evidence for stem cell failure and aged type 2 immunodeficient mice colonized with Demodex developed skin changes consistent with premature HF senescence, uncovering a non-redundant role for type 2 immunity in sustaining cutaneous integrity in the post-birth environment.
Despite their ubiquitous nature and robust reproductive potential, Demodex are maintained at low density on human skin, although mites proliferate in settings of immunodeficiency, malnutrition, and with aging, particularly in sun-exposed areas of the face (Chovatiya and Colegio, 2016; Lacey et al., 2011). Human-associated Demodex possess a reductive genome anticipated by obligate parasites that spend their entire life within the narrow niche of HFs. Demodex share chitinous exoskeletons with other arthropods, and survival in the sebum-rich HF has evolved a rich armamentaria of lipases, proteases and chitinases (Hu et al., 2019), an array of enzymes linked, like chitin itself, with tissue injury, alarmin induction and activation of innate type 2 immunity (Cheng and Locksley, 2014; Gieseck et al., 2018; Van Dyken et al., 2017). As we have shown, loss of canonical alarmins attenuated but did not ablate the ILC2 response, suggesting a prominent role for alarmins in regulating basal ILC2 numbers and tone, but revealing alternative pathways by which ILC2s react to tissue perturbations induced by this evolved HF commensal. Although skin development and basal homeostasis were largely normal in the absence of ILC2s or type 2 cytokines, perturbations like depilation, Demodex and aging resulted in dysregulation and loss of barrier function. In concert with additional signals, including via the epidermal growth fator receptor (EGFR), type 2 cytokines impacted small intestinal epithelial stem cell trajectories, hinting at shared pathways by which type 2 immunity can enhance resilience to perturbations at cutaneous and mucosal barriers (Sanman et al., 2021). Such adaptations in the gut contribute to the phenomenon of concomitant immunity by which helminth-infected hosts become resistant to further infestation by immature forms (Schneider et al., 2018). Whether HF colonization by Demodex imparts similar stem cell and epithelial ‘memory’ and protection against subsequent cutaneous injury will be important areas for further investigation (Gonzales et al., 2021).
Our studies suggest a role for IL-13 in transiently restraining HFSC and epithelial proliferation at anagen onset, revealing barrier abnormalities upon depilation in the absence of this cytokine. Perturbations that impact the skin HF niche by disruptions of cell junctions, migration and adhesion, pathways we show engaged by Demodex colonization, drive precocious anagen entry, resulting in re-directed proliferation of HFSCs towards ‘rescue’ states dedicated to preservation of the HF niche (Gur-Cohen et al., 2019; Harrison et al., 2019; Lay et al., 2018). Precocious anagen entry would be compatible with the increased numbers of proliferating HFSCs and HFs observed in mite-colonized type 2 immunodeficient mice. Of note, the epithelial and HFSC reparative pathways induced by IL-13 in naïve and mite-colonized WT animals were lost in type 2 immunodeficient mice and replaced by epithelial proliferation and an inflammatory cell influx that was not sufficient to control infestation. Given the marked HF anatomic complexities occurring among epithelial and endothelial structures during the transition through anagen (Yang et al., 2017), it is tempting to speculate that effects of IL-13 are critical to keep Demodex compartmentalized to regions of the HF above the isthmus, as compared to the deeper localization of organisms seen in type 2 immunodeficient mice. ILC2s in intestinal mucosa regulate homeostasis with the protozoan, Tritrichomonas (Howitt et al., 2016; Schneider et al., 2018), suggesting a role for innate type 2 immune cells in sustaining tissue function in the presence of barrier-associated eukaryotic pathobionts, perhaps reflecting responsiveness of ILC2s to endogenous damage-associated molecular patterns (DAMPS) rather than to genetically encoded microbial and viral recognition elements. Although we cannot exclude a role for microbiota-mediated inflammation contributing to the inflammatory phenotype, the infection was controlled by topical drugs targeting the mite but not by antibiotics.
Our observations of increased type 2 inflammation in mite-colonized type 2 immunodeficient mice were bolstered by evidence for increased inflammation but diminished IL-4 and IL13-producing innate immune cells among patients with rhinophymatous rosacea associated with Demodex. We cannot exclude contributions by adaptive or unconventional T cells that produce IL-13, particularly with the presence of such cells in human rhinophyma samples under non-SPF conditions with longer life experience as compared to mice. It is possible that the increased prevalence of atopy in the aging population, and perhaps even the early age of onset of type 2 inflammation in atopic skin disease, might represent physiologic efforts to promote tissue integrity and homeostasis during stress induced by senescence and growth, respectively. Efforts to understand the role of traditional immune cells in ‘physiological’ inflammation will be important to anticipate responses to prevalent biologic therapeutics, particularly in aging populations (Medzhitov, 2021). The availability of potent biologics inhibiting type 2 immunity that require continuous therapy may benefit from targeted screening of high-risk patients to curtail symptomatic blepharitis and other cutaneous eruptions while potentially optimizing long-term tissue integrity, particularly in the setting of high Demodex burden.
Limitations of the Study
We characterized an inflammatory dermopathy caused by Demodex among mice at UCSF. Although cases have been noted elsewhere (Smith et al., 2016), the impact of local microbial flora and animal care practices remain unstudied. We analyzed a small human cohort to provide corroborative evidence but further studies are needed across more diverse patient groups to assess the generalizable nature of these findings. Humans are colonized with two types of Demodex and the periodicity of anagen and telogen in humans differs from mice. Although Demodex outgrowths have been noted among patients treated with anti-IL4Ra, controlled trials are needed to assess potential causality linking these drugs with cutaneous outcomes related to Demodex. Finally, increasing information regarding Demodex genomes (Smith et al., 2022) may faciliate identification of moieties or metabolites that sustain quiescent colonization that might represent the target of IL-13-induced host molecules that enable the widespread and successful habitation of the HF niche by these creatures.
STAR Methods
Lead Contact
Further information and requests for reagents may be directed to, and will be fulfilled by, the lead contact Richard Locksley (Richard.Locksley@ucsf.edu).
Materials Availability
All reagents generated or used in this study are available on request from the Lead Contact with a completed Materials Transfer Agreement. Information on reagents used in this study is available in the Key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Brilliant Violet 421™ anti-mouse CD19 Antibody, 50 μg | BioLegend | Cat#115549; RRID:AB_2563066 |
| Brilliant Violet 421™ anti-mouse TER-119/Erythroid Cells Antibody, 50 μg | BioLegend | Cat#116234; RRID:AB_2562917 |
| Brilliant Violet 421™ anti-mouse Ly-6G/Ly-6C (Gr-1) Antibody, 50 μg | BioLegend | Cat#108445; RRID:AB_2562903 |
| Brilliant Violet 421™ anti-mouse/human CD11b Antibody, 500ul | BioLegend | Cat#101236; RRID:AB_11203704 |
| Brilliant Violet 650™ anti-mouse/human CD11b Antibody | BioLegend | Cat#101259; RRID:AB_2566568 |
| Pacific Blue™ anti-mouse CD11c Antibody, 100 μg | BioLegend | Cat#117322; RRID:AB_755988 |
| Pacific Blue™ anti-mouse CD49b (pan-NK cells) Antibody, 100 μg | BioLegend | Cat#108918; RRID:AB_2249376 |
| Brilliant Violet 421™ anti-mouse CD335 (NKp46) Antibody, 50 μg | BioLegend | Cat#137612; RRID:AB_10915472 |
| Brilliant Violet 421™ anti-mouse NK-1.1 Antibody, 50 μg | BioLegend | Cat#108741; RRID:AB_2562561 |
| BrilliantViolet 421™ anti-mouse TCR γ/δ Antibody, 50 μg | BioLegend | Cat#118120; RRID:AB_2562566) |
| Pacific Blue™ anti-mouse FcεRIα Antibody, 100 μg | BioLegend | Cat#134314; RRID:AB_10613298 |
| PE/Cy7 anti-mouse CD3 Antibody, 100 μg | BioLegend | Cat#100220; RRID:AB_1732057 |
| Pacific Blue™ anti-mouse F4/80 Antibody, 100 μg | BioLegend | Cat#123124; RRID:AB_893475 |
| Brilliant Violet 711™ anti-mouse CD45 Antibody, 50 μg | BioLegend | Cat#103147; RRID:AB_2564383 |
| BUV395 Rat Anti-Mouse CD45, 50 μg | BD Biosciences | Cat#564279; RRID:AB_2651134 |
| APC/Cyanine7 anti-mouse CD45 Antibody, 100 μg | BioLegend | Cat#103116; RRID:AB_312981 |
| Brilliant Violet 785™ anti-mouse CD90.2 Antibody, 50 μg | BioLegend | Cat#105331; RRID:AB_2562900 |
| Brilliant Violet 605™ anti-mouse CD90.2 Antibody | BioLegend | Cat#105343; RRID:AB_2632889 |
| Brilliant Violet 711™ anti-mouse CD4 Antibody, 500 μl | BioLegend | Cat#100550; RRID:AB_2562099 |
| Pacific Blue™ anti-mouse CD8a Antibody, 100 μg | BioLegend | Cat#100725; RRID:AB_493425 |
| Brilliant Violet 785™ anti-mouse CD8a Antibody | BioLegend | Cat#100750; RRID:AB_2562610 |
| Pacific Blue™ anti-mouse CD5 Antibody | BioLegend | Cat#100642; RRID:AB_2813916 |
| Brilliant Violet 605™ anti-mouse Ly-6A/E (Sca-1) Antibody, 125 μL | BioLegend | Cat#108133; RRID:AB_2562275 |
| Brilliant Violet 711™ anti-mouse I-A/I-E Antibody | BioLegend | Cat#107643; RRID:AB_2565976 |
| PerCP/Cyanine5.5 anti-mouse CD326 (Ep-CAM) Antibody | BioLegend | Cat#118220; RRID:AB_2246499 |
| FITC anti-human/mouse CD49f Antibody | BioLegend | Cat#313606; RRID:AB_345300 |
| PE/Cyanine7 anti-human/mouse CD49f Antibody | BioLegend | Cat#313622; RRID:AB_2561705 |
| Alexa Fluor® 647 Rat anti-Mouse CD34 | BD Biosciences | Cat#560230; RRID:AB_1645200 |
| CD127 (IL-7R) Monoclonal Antibody (A7R34), APC-eFluor 780 | Invitrogen | Cat#47-1271-82; RRID:AB_1724012 |
| Gata-3 Monoclonal Antibody (TWAJ), eFluor 660, eBioscience™, 100 tests | Invitrogen | Cat#50-9966-42; RRID:AB_10596663 |
| APC anti-human CD4 Antibody | BioLegend | Cat#300514; RRID:AB_314082 |
| FOXP3 Monoclonal Antibody (FJK-16s), eBioscience™ | Invitrogen | Cat#11-5773-82; RRID:AB_465243 |
| BV605 Rat Anti-Mouse IL-33R (ST2), 50 μg | BD Biosciences | Cat#745257; RRID:AB_2742841 |
| CD218a (IL-18Ra) Monoclonal Antibody (P3TUNYA), PerCP-eFluor 710, eBioscience™, 100 μg | Invitrogen | Cat#46-5183-82; RRID:AB_2573764 |
| Living Colors® DsRedPolyclonal Antibody (100μl) | TakaraBio | Cat#632496; RRID:AB_10013483 |
| Ki-67 Monoclonal Antibody (SolA15), FITC, eBioscience™ | Invitrogen | Cat#11-5698-82; RRID:AB_11151330 |
| FITC Mouse Anti-E-Cadherin | BD Biosciences | Cat#612131; RRID:AB_2076677 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 | Invitrogen | Cat#A-21428; RRID:AB_2535849 |
| Anti-human CD3e | Cell Signaling Technology | Cat#85061; RRID:AB_2721019 |
| Purified anti-mouse TER-119/Erythroid Cells (Maxpar® Ready) Antibody | BioLegend | Cat#116241; RRID:AB_2563789 |
| Purified anti-mouse CD45 (Maxpar® Ready) Antibody | BioLegend | Cat#103141; RRID:AB_2562800 |
| BD Transduction Laboratories™ Purified Mouse Anti-Stat3 (pS727) | BD Biosciences | Cat#612542; RRID:AB_399839 |
| Purified anti-mouse CD326 (Ep-CAM) (Maxpar® Ready) Antibody | BioLegend | Cat#118223; RRID:AB_2563743 |
| Purified anti-mouse/human CD11b (Maxpar® Ready) Antibody | BioLegend | Cat#101249; RRID:AB_2562797 |
| Anti-Histone H3 (phospho S28) antibody [HTA28] | Abcam | Cat#ab10543; RRID:AB_2295065 |
| BD Pharmingen™ Purified Hamster Anti-Mouse CD11c | BD Biosciences | Cat#553799; RRID:AB_395058 |
| Phospho-MAPKAPK-2 (Thr334) (27B7) | Cell Signaling Technology | Cat#3007; RRID:AB_490936 |
| Phospho-CREB (Ser133) (87G3) Rabbit mAb | Cell Signaling Technology | Cat#9198; RRID:AB_2561044 |
| BD Transduction Laboratories™ Purified Mouse Anti-Stat1 (pY701) | BD Biosciences | Cat#612232; RRID:AB_399555 |
| BD Transduction Laboratories™ Purified Mouse Anti-Human Stat5 (pY694) | BD Biosciences | Cat#611964; RRID:AB_399385 |
| Phospho-S6 Ribosomal Protein (Ser235/236) (2F9) Rabbit mAb | Cell Signaling Technology | Cat#4856; RRID:AB_2181037 |
| Purified anti-mouse Ly-6C (Maxpar® Ready) Antibody | BioLegend | Cat#128039; RRID:AB_2563783 |
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP® Rabbit mAb | Cell Signaling Technology | Cat#4370; RRID:AB_2315112 |
| Anti-Human CyclinB1 (GNS-1)-153Eu | Fluidigm | Cat#3153009A |
| BD Transduction Laboratories™ Purified Mouse Anti-p38 MAPK (pT180/pY182) | BD Biosciences | Cat#612288; RRID:AB_399605 |
| Purified anti-mouse CD8a (Maxpar® Ready) Antibody | BioLegend | Cat#100755; RRID:AB_2562796 |
| Purified anti-mouse CD4 (Maxpar® Ready) Antibody | BioLegend | Cat#100561; RRID:AB_2562762 |
| BD Pharmingen™ Purified Rat Anti-Mouse CD3 Molecular Complex | BD Biosciences | Cat#555273; RRID:AB_395697 |
| Purified anti-mouse Ly-6G (Maxpar® Ready) Antibody | BioLegend | Cat#127637; RRID:AB_2563784 |
| Phospho-4E-BP1 (Thr37/46) (236B4) Rabbit mAb | Cell Signaling Technology | Cat#2855; RRID:AB_560835 |
| BD Transduction Laboratories™ Purified Mouse Anti-Human ZAP-70 (pY319)/Syk (pY352) | BD Biosciences | Cat#612574; RRID:AB_399863 |
| Phospho-TBK1/NAK (Ser172) (D52C2) XP® Rabbit mAb | Cell Signaling Technology | Cat#5483; RRID:AB_10693472 |
| Purified anti-mouse TCR γ/δ Antibody | BioLegend | Cat#118101; RRID:AB_313826 |
| IκBα (L35A5) Mouse mAb (Amino-terminal Antigen) | Cell Signaling Technology | Cat#4814; RRID:AB_390781 |
| alpha-Fetoprotein/AFP Antibody (SPM334) | Novus | Cat#NBP2-32915 |
| BD Transduction Laboratories™ Purified Mouse Anti-Stat 6 (pY641) | BD Biosciences | Cat#611566; RRID:AB_399012 |
| Anti-pRb [S807/S811] (J112-906)-166Er | Fluidigm | Cat#3166011A |
| FOXP3 Monoclonal Antibody (NRRF-30) | eBioscience | Cat#14-4771-80; RRID:AB_529583 |
| Purified anti-mouse NK-1.1 (Maxpar® Ready) Antibody | BioLegend | Cat#108743; RRID:AB_2562803 |
| Ki-67 Monoclonal Antibody (SolA15) | eBioscience | Cat#14-5698-82; RRID:AB_10854564 |
| BD Transduction Laboratories™ Purified Mouse Anti-Stat4 | BD Biosciences | Cat#610926; RRID:AB_398241 |
| Purified anti-mouse CD62L (Maxpar® Ready) Antibody | BioLegend | Cat#104443; RRID:AB_2562802 |
| BD Pharmingen™ Purified Rat Anti-Mouse Siglec-F | BD Biosciences | Cat#552125; RRID:AB_394340 |
| Purified anti-mouse CD19 (Maxpar® Ready) Antibody | BioLegend | Cat#115547; RRID:AB_2562806 |
| BD Pharmingen™ Purified Rat Anti-Mouse CD34 | BD Biosciences | Cat#553731; RRID:AB_395015 |
| BD Pharmingen™ Purified Rat Anti-Mouse CD44 | BD Biosciences | Cat#553131; RRID:AB_394646 |
| Purified anti-mouse I-A/I-E (Maxpar® Ready) Antibody | BioLegend | Cat#107637; RRID:AB_2563771 |
| Bacterial and virus strains | ||
| Does not apply | ||
| Biological samples | ||
| Does not apply | ||
| Chemicals, peptides, and recombinant proteins | ||
| Recombinant mouse IL-4 | R&D systems | Cat#404-ML-050/CF |
| Recombinant mouse IL-13 | R&D systems | Cat#413-ML-050/CF |
| Recombinant mouse IL-13 | BioLegend | Cat#575908 |
| Recombinant mouse IL-7 | BioLegend | Cat#577804 |
| Recombinant mouse TSLP | R&D systems | Cat#555-TS-010/CF |
| Recombinant mouse IL-18 | BioLegend | Cat#767004 |
| Recombinant mouse IL-33 | BioLegend | Cat#580504 |
| Cell Activation Cocktail (without Brefeldin A) | BioLegend | Cat#423301 |
| InVivoMAb anti-mouse CD16/CD32, Clone 2.4G2 | BioXCell | Cat#BE0307 |
| InVivoMAb anti-mouse IL-4, clone 11B11 | BioXCell | Cat#BE0045 |
| TrueStain FcX (anti-mouse CD16/32 antibody (clone 93) | BioLegend | Cat#101320 |
| Moxidectin | Sigma-Aldrich | Cat#33746-25MG |
| Imidacloprid | Sigma-Aldrich | Cat#37894-100MG |
| Ampicillin | Sigma-Aldrich | Cat#A1593-25G |
| Kanamycin | Sigma-Aldrich | Cat#K1637-25G |
| Neomycin | Sigma-Aldrich | Cat#N1876-100G |
| Vancomycin | Sigma-Aldrich | Cat#V2002-5G |
| Metronidazole | Sigma-Aldrich | Cat#M1547-25G |
| 5-Ethynyl-2’-deoxyuridine (EdU) | Sigma-Aldrich | Cat#900584 |
| Nair depilatory cream | Nivea | N/A |
| RPMI-1640 Medium | Sigma-Aldrich | Cat#R8758 |
| Liberase TL | Sigma-Aldrich | Cat#5401020001 |
| DNAse I | Sigma-Aldrich | Cat#10104159001 |
| Fetal Bovine Serum | Sigma-Aldrich | Cat#F0926-500ML |
| 4’,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) | Roche | Cat#10236276001 |
| Live/Dead fixable dye | Thermofisher | Cat#L34968 |
| Trypsin-EDTA | ThermoFisher | Cat#15400-054 |
| HEPES | Sigma-Aldrich | Cat#H3537-100ML |
| Penicillin/Streptomycin | Fisher | Cat#15140122 |
| 5-Iodo-2′-deoxyuridine (IdU) | Sigma-Aldrich | Cat#I7125 |
| Cisplatin | Sigma-Aldrich | Cat#P4394-25MG |
| Paraformaldehyde | Fisher | Cat#15713 |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | Cat#A7906-500G |
| Goat Serum | Sigma-Aldrich | Cat#G9023-10ML |
| Blocking Reagent | Perkin Elmer | Cat#FP1012 |
| Mounting Media | Vector Laboratories | Cat#H-1000 |
| ProLong Gold Antifade | ThermoFisher | Cat#P36930 |
| 1x DPBS | Life Technologies | Cat#14190-250 |
| 191/193Ir DNA inercalator | Fluidigm | Cat#201192B |
| Q5 High-Fidelity DNA Polymerase | NEB | M0494L |
| Yeast cell lysis buffer | Lucigen | Cat#MPY80200 |
| Lucifer yellow | ThermoFisher | Cat#L453 |
| Optimal Cutting Temperature Compound O.C.T. | VWR | Cat#25608-930 |
| Tris-HCl (1M) | Fisher | Cat#50843267 |
| NaCl (5M) | Fisher | Cat#AM9760G |
| Proteinase K | Thomas Scientific | Cat#C755H28 |
| Citrate buffer (pH 6.0) | ThermoFisher | Cat#00500 |
| 3% hydrogen peroxide | JT Baker | Cat#JT-2186-01 |
| ImmPRESS horseradish peroxidase reagent | Vector Laboratories | Cat#MP-7451 |
| Diaminobenzidine substrate | Vector Laboratories | Cat#SK-4100 |
| Commercial Assays | ||
| CountBright Absolute Counting Beads | Life Technologies | Cat#C36950 |
| Click-IT Plus EdU imaging kit | ThermoFisher | Cat#C10637 |
| Click-IT Plus EdU flow cytometry kit | ThermoFisher | Cat#C10633 |
| MaxPAR antibody conjugation kit | Fluidigm | Cat#201300 |
| DNEasy PowerSoil Pro Kit | Qiagen | Cat#47014 |
| Chromium Single Cell 3’ GEM, Library & Gel Bead Kit v3.1 | 10X Genomics | Cat#PN-1000121 |
| Cytokine Bead Array Flex Sets: IL-4 | BD Biosciences | Cat#558298 |
| Cytokine Bead Array Flex Sets: IL-5 | BD Biosciences | Cat#558302 |
| Cytokine Bead Array Flex Sets: IL-13 | BD Biosciences | Cat#558349 |
| Cytokine Bead Array Flex Sets: IL-17A | BD Biosciences | Cat#560283 |
| LEGEND MAXTMMouse IL-22 ELISA Kit | BioLegend | Cat#436307 |
| RNeasy Micro Plus kit | Qiagen | Cat#74034 |
| Superscript VILO cDNA synthesis kit | ThermoFisher | Cat#11754250 |
| Power SYBR Green PCR master mix | ThermoFisher | Cat#4368702 |
| RNAscope® 2.5 HD Reagent Kit-RED | ACD Bio/Bio-Techne | Cat#322350 |
| RNAscope® Probe- Hs-IL4 | ACD Bio/Bio-Techne | Cat#315191 |
| RNAscope® Probe- Hs-IL5 | ACD Bio/Bio-Techne | Cat#319391 |
| RNAscope® Probe- Hs-IL13 | ACD Bio/Bio-Techne | Cat#586241 |
| RNAscope® Probe- Hs-IL22 | ACD Bio/Bio-Techne | Cat#560811 |
| RNAscope® Probe- Hs-IFNG | ACD Bio/Bio-Techne | Cat#310501 |
| Experimental models: Cell lines | ||
| Does not apply | ||
| Experimental models: Organisms/strains | ||
| Mouse: Wild type: C57BL/6J | The Jackson Laboratory | Stock# 000664 |
| Mouse: Red5: B6(C)-Il5tm1.1(icre)Lky/J | The Jackson Laboratory | Stock# 030926 |
| Mouse: Yarg: B6.129S4-Arg1tm1Lky/J | The Jackson Laboratory | Stock# 015857 |
| Mouse: Smart13: B6.129S4(C)-Il13tm2.1Lky/J | The Jackson Laboratory | Stock# 031367 |
| Mouse: B6.129S2(C)-Stat6tm1Gru/J | The Jackson Laboratory | Stock# 005977 |
| Mouse: B6.Il4ra−/−: BALB/c-Il4ratm1Sz/J | The Jackson Laboratory | Stock# 003514 |
| Mouse: B6.Il4/Il13−/− | Liang et al., 2012 | N/A |
| Mouse: B6.Il4−/−: Il4tm1(CD2)Mmrs(KN2/KN2) | Mohrs et al., 2001 | N/A |
| Mouse: B6.129S7-Rag1tm1Mom/J | The Jackson Laboratory | Stock# 002216 |
| Mouse: B6.129S6-Rag2tm1Fwa N12: ko/ko | Taconic Biosciences | Cat# RAGN12-F |
| Mouse: C57BL/6NTac.Cg-Rag2tm1Fwa Il2rgtm1Wjl: ko/ko;ko/ko (Rag2−/−/Il2rg−/−) | Taconic Biosciences | Cat# 4111-F |
| Mouse: B6.Crlf2−/− | Carpino et al., 2004 | N/A |
| Mouse: B6.Il1rl1−/− | Hoshino et al., 1999 | N/A |
| Mouse: B6.Il25−/−: B6.Il25tm1Anjm | Fallon et al., 2006 | N/A |
| Mouse: B6.Il18−/−:B6.129P2-Il18tm1Aki/J | The Jackson Laboratory | Stock# 004130 |
| Mouse: B6.Il13ra1−/− | This paper | N/A |
| Oligonucleotides | ||
| qPCR Primer: Il13-forward: 5’-CCTGGCTCTTGCTTGCCTT-3’ | PrimerBank | PrimerBank ID:6680403a1 |
| qPCR Primer: Il13-reverse: 5’-GGTCTTGTGTGATGTTGCTCA-3’ | PrimerBank | PrimerBank ID:6680403a1 |
| qPCR Primer: Il22-forward 5’-ATGAGTTTTTCCCTTATGGGGAC-3’ | PrimerBank | PrimerBank ID:21426819a1 |
| qPCR Primer: Il22-reverse 5’-GCTGGAAGTTGGACACCTCAA-3’ | PrimerBank | PrimerBank ID:21426819a1 |
| qPCR Primer: Cdkn2a/p16-forward: 5’-CGCAGGTTCTTGGTCACTGT-3’ | PrimerBank | PrimerBank ID:6753390a1 |
| qPCR Primer: Cdkn2a/p16-reverse: 5’-TGTTCACGAAAGCCAGAGCG-3’ | PrimerBank | PrimerBank ID:6753390a1 |
| qPCR Primer: Rps17-forward: 5’-CCAAGACCGTGAAGAAGGCTG-3’ | PrimerBank | PrimerBank ID:6677801a1 |
| qPCR Primer: Rps17-reverse: 5’-GCTGGGGATAATGGCGATCT-3’ | PrimerBank | PrimerBank ID:6677801a1 |
| Primer: CHS-forward: 5’-GAAGCGGCGAGTAATGTTCATC-3’ | This paper | N/A |
| Primer: CHS-reverse: 5’-CCTGACTCCATCTTTTACGATGTC-3’ | This paper | N/A |
| Primer: Keratin 5-forward: 5’-TCTGCCATCACCCCATCTGT-3’ | PrimerBank | PrimerBank ID:20911031a1 |
| Primer: Keratin 5-reverse: 5’-CCTCCGCCAGAACTGTAGGA-3’ | PrimerBank | PrimerBank ID:20911031a1 |
| Primer: 18S-forward: 5’-TCCAAGGAAGGCAGCAGGCA-3’ | This paper | N/A |
| Primer: 18S-reverse: 5’-CGCGGTAGTTCGTCTTGCGACG-3’ | This paper | N/A |
| 16s rRNA gene forward: 5’-AGAGTTTGATCCTGGCTCAG-3’ | This paper | N/A |
| 16s rRNA gene reverse: 5’-ATTACCGCGGCTGCTGG-3’ | This paper | N/A |
| sgRNA targeting exon 2 of Il13ra1 gene: ACGCTCAAATTCGTCACAGGTGG | This paper | N/A |
| sgRNA targeting exon 2 of Il13ra1 gene: TCCTGAGCCACAGCATGTACTGG) | This paper | N/A |
| Genotyping primer: Il13Ra1-Forward: 5’-AAAGTGAAGCCATCAGTAGTCCCTC-3’ | This paper | N/A |
| Genotyping primer: Il13Ra1-Reverse: 5’-AAATGCAAACCCACCCACCACTAAC-3’ | This paper | N/A |
| Recombinant DNA | ||
| eGFP-CBP plasmid | This study | https://www.addgene.org/169211/ |
| Software and algorithms | ||
| ImageJ Software | https://imagej.net/software/fiji/ | |
| Significance Across Microarrays algorithm | Bair and Tibshirani, 2004; Bruggner et al., 2014 | |
| Scaffold maps R package | https://github.com/spitzerlab/statisticalScaffold | |
| viSNE and Spade analyses | www.Cytobank.org | |
| Flow Cytometric Analysis Program (FCAP) Array software | https://www.beckman.com/flow-cytometry/software | |
| SoftMax Pro software | Molecular Devices | |
| Flowjo (v10.6.1) | https://www.flowjo.com | |
| BD FACSDiva Software | BD Biosciences | |
| GraphPad Prism | https://www.graphpad.com/scientific-software/prism/ | |
| Quantity One (v4.6.1) | Bio-Rad Laboratories | |
| Image Lab (v6.1.0) | Bio-Rad Laboratories | |
| QIIME2 | Bolyen et al., 2019 | |
| DADA2 | Callahan et al., 2016 | |
| Greengenes Reference Database | McDonald et al., 2012 | |
| MAFFT | Katoh and Standley, 2013 | |
| Principal Coordinates Analysis of weighted and unweighted UniFrac distance | Lozupone and Knight, 2005; Lozupone et al., 2007 | |
| scikit-bio | Bokulich et al., 2018 | |
| Seurat V3 or V4 | https://satijalab.org/seurat/ | |
| R package Harmony | https://github.com/immunogenomics/harmony | |
| GOrilla | http://cbl-gorilla.cs.technion.ac.il | |
| Nebulosa Package | https://github.com/powellgenomicslab/Nebulosa | |
| Mass cytometry data normalization algorithm | Finck et al., 2013 | |
| AxioVision Software | Zeiss | |
| NIS Elements Software | Nikon | |
| Other | ||
Data and Code Availability
All the data supporting the findings of the article are available within the main text or supplementary materials. The published article includes datasets generated during this study. Original single cell RNA-seq data has been deposited in GEO (GSE197983). Original 16S rRNA sequencing datasets analyzed in this study are available at the NCBI Sequence Read Archive (SUB11832976).
Mice
Wild-type (C57BL/6J; Stock 000664) mice were purchased from Jackson Laboratories. Il5Red5, Arg1Yarg, and Il13Smart reporter alleles on C57BL/6J (B6) backgrounds were bred and maintained as described (Bando et al., 2013; Ricardo-Gonzalez et al., 2018). For some experiments, mice encoding the Arg1Yarg and/or Il13Smart reporter alleles that do not impact the endogenous gene expression were used as wild-type controls. Il4ra−/− (BALB/c-Il4ratm1Sz/J; 003514) and Stat6−/− (B6.129S2(C)-Stat6tm1Gru/J; 005977) mice were initially purchased from The Jackson Laboratory and backcrossed to C57BL/6J for at least eight generations as described (von Moltke et al., 2016). Additional wild-type C57BL/6J and Il4ra−/− mice on the C57BL/6J background were obtained from the UCSF colony of M.S. Fassett and K.M. Ansel. Il4−/−,Il13−/− mice were generated as previously described and backcrossed C57BL/6J for at least eight generations (Liang et al., 2012). Il4−/− (KN2/KN2 mice) were generated as previously described (Mohrs et al., 2001) and backcrossed to C57BL/6J. Crlf2−/− (Carpino et al., 2004), Il25−/− (Fallon et al., 2006), and Il1rl1−/− (Hoshino et al., 1999) mice on C57BL/6 J backgrounds were intercrossed to generate Crlf2−/−,Il25−/−,Il1rl1−/− triple-deficient mice expressing Il5Red5, Arg1Yarg, and Il13Smart reporter alleles as previously described (Ricardo-Gonzalez et al., 2018; Van Dyken et al., 2016). Triple-deficient mice were crossed to Il18−/− mice (Stock 004130) purchased from The Jackson Laboratory to generate Crlf2−/−,Il25−/−,Il1rl1−/−,Il18−/− quadruple-ablated (Quad-ablated) in combination with Il5Red5, Arg1Yarg, and Il13Smart reporter alleles. Rag1−/− mice (Stock 002216) were purchased from Jackson laboratory, and crossed to Arg1Yarg and Il5Red5 reported alleles. Rag2−/− (Stock RAGN12-F) and Rag2−/−,Il2rg−/− (Stock 4111-F) mice were obtained from Taconic Biosciences. For all experiments, sex-matched mice aged 7-14 weeks were used unless otherwise indicated. Mice were maintained under specific pathogen-free conditions. For in vivo cytokine treatments, mice were injected subcutaneously with IL-4 complex (IL-4c) comprised of rmIL-4 (2 μg, R&D systems) combined with anti-IL-4 antibody (10 μg, Clone Mab 11B11, BioXcell), or rmIL-13 (2 μg, R&D systems) in calcium- and magnesium-free phosphate buffered saline (PBS). For co-housing experiments, mice of the appropriate genotypes were co-housed for 6-12 weeks in the ratios noted in the figure legends. For treatment with topical anti-parasitic agents, a stock solution in 70% ethanol of moxidectin (Sigma 33746) and imidacloprid (Sigma 37894) was prepared and diluted to a working solution of (0.83 mg/ml moxidectin and 3.33 mg/ml imidacloprid) and applied topically to intrascapular skin at 3.3 mg/kg moxidectin and 13.3 mg/kg imidacloprid. Control mice were treated with 70% ethanol. For broad-spectrum antibiotics treatment, mice received drinking water supplemented with ampicillin, kanamycin, and neomycin (all 1 mg/ml), vancomycin (0.5 mg/ml), and metronidazole (2.5 mg/ml) in 1% sucrose. For EdU experiments, mice received a peritoneal injection of 1 μg of EdU (Sigma 900584) 14-18 hours prior to harvesting. All animal procedures were approved by the UCSF Institutional Animal Care and Use Committee.
Generation of IL-13Ra1 deficient mice
Two sgRNAs targeting exon 2 of Il13ra1 gene (ACGCTCAAATTCGTCACAGGTGG and TCCTGAGCCACAGCATGTACTGG) and recombinant Cas9 (PNA Bio) were simultaneously injected as RNPs into the zygotes obtained from C57BL/6 J mice carrying Arg1Yarg/Yarg, Il5Red5, and Il13Smart reporter alleles. Founders were screened by PCR using primers: Il13Ra1-Forward: 5’-AAAGTGAAGCCATCAGTAGTCCCTC-3’ and Il13Ra1-Reverse: 5’-AAATGCAAACCCACCCACCACTAAC-3’ to identify a ~144 bp deletion resulted from the end-joining event of two DSBs at the sgRNA-targeted sites. Since the Il13ra1 gene is an X-linked gene, only positive male founders were chosen to ensure the allelic identity. A male founder was first crossed with C57BL/6 J mice (with Arg1Yarg/Yarg, Il5Red5, and Il13Smart reporter alleles) followed by intercrossing to obtain homozygous female I13ra1−/−.
Depilation Experiment
Mice were anesthetized with isoflurane inhalation. Under anesthesia, the dorsal surface (back) hair of the animals was shaved down to the level of skin with an electric razor. A thin coat (1 fingertip unit) of Nair depilatory cream (Nivea) was applied to the shaved region for a period of 30 s before wiping clean. For monitoring of clinical hair regrowth, standardized pictures were taken with a ruler on the day of depilation (day 0) and then at days 4, 7, 9, 11, 14. Anagen induction was quantified using intensity analysis on ImageJ software (NIH, USA) at each time point or as a percent of pigmented dorsal skin relative to baseline (day 0). For repeat depilation experiments, mice were initially shaved and depilated when they were in second telogen (2 months old). Cycles were repeated once a month once they have completed the anagen and recovery phase. Tissues were analyzed for flow cytometry, stem cell proliferation, and qPCR as described below.
Flow Cytometry and Cell Sorting
Whole skin single cell suspensions were prepared as described previously (Ricardo-Gonzalez et al., 2018). Briefly, back tissue was minced in RPMI-1640 with 5% FBS, then transferred to C tubes (Miltenyi Biotec), containing 5 ml of RPMI-1640 (Sigma R8758) supplemented with Liberase TL (0.25 mg/ml, Sigma 5401020001), and DNAse I (0.1 mg/ml; Sigma 10104159001). Samples were shaken at 250 rpm for 2 hours at 37 °C, then dispersed using an automated tissue dissociator (GentleMACS; Miltenyi Biotec) running program C. Single-cell suspensions were passed through a 70 μm filter and washed twice with RPMI containing 5% FBS. The following antibodies, all from BioLegend (unless otherwise specified, see Key resources table and Supplementary Table S2) were used at 1:300 dilution unless noted: anti-CD3 (17A2, diluted 1:200), anti-CD4 (RM4-5, diluted 1:100), anti-CD5 (53-7.3), anti-CD8α (53-6.7, diluted 1:100), anti-CD11b (M1/70), anti-CD11c (N418), anti-CD19 (6D5), anti-CD25 (PC61, diluted 1:100), anti-CD45 (30F-11, BD Biosciences), anti-CD49b (DX5; eBiosciences), anti-CD127 (A7R34, diluted 1:100), anti-CD218 (P3TUNYA,eBiosciences), anti-F4/80 (BM8), anti-Gr-1 (RB6-8C5), anti-NK1.1 (PK136), anti-NKp46 (29A1.4), anti-Thy1.2 (30-H12; diluted 1:1000); anti-human CD4 (RPA-T4, diluted 1:20; eBiosciences), anti-GATA3 (TWAJ, diluted 1:25), anti-FoxP3 (FJK-16s, Invitrogen, diluted 1:100), anti-T1/ST2 (U29-93; BD Biosciences, diluted 1:200). Live/dead cell exclusion was performed with DAPI (4′,6-diamidine-2′-phenylindole dihydrochloride; Roche) or LIVE/dead fixable dye (Thermofisher). Cell counts were performed using flow cytometry counting beads (CountBright Absolute; Life Technologies) per manufacturer instructions. To assay cell proliferation, the Click-IT EdU flow cytometry or imaging kits (ThermoFisher) were used according to the kit instructions. Sample data were acquired with a 5-laser LSRFortessa X-20 flow cytometer and BD FACSDiva software (BD Biosciences) and analyzed using FlowJo software (Tree Star v10.6.1).
ILC2s were sorted from reporter mice as live (DAPI−), Lin(CD3, CD4, CD8, TCRγδ CD11b, CD11c, CD19, NK1.1, NKp46, Gr-1, F4/80, Ter119, DX5)−CD45+Thy1+Red5+ using a MoFlo XDP (Beckman Coulter). Epithelial cell preparations for flow cytometry or CyTOF analyses were prepared as previously described (Ali et al., 2017; Nagao et al., 2012). Briefly, mice were shaved and dorsal skin was harvested and trimmed of fat. The tissue was floated in 4 ml of 0.25% Trypsin-EDTA (ThermoFisher) and incubated for 90 min at 37 °C. The epidermis was scrapped from the dermis and mechanically separated by pipetting into complete media (RPMI-1640 supplemented with 10% FBS, HEPES, Penicillin/Streptomycin). The cell suspension was passed through a 100 μm strainer and washed with 10 ml of complete media. The cell suspension was then pelleted, filtered through a 40 μm filter and aliquoted for cell staining. The following antibodies were used for staining epithelial fractions: anti-Sca1 (D7, 1:300); anti-MHCII (M5/114.15.2, 1:400); anti-CD326 (G8.8, 1:300); anti-CD45 (30F-11, 1:400); anti-CD34 (RAM34, 1:200); anti-CD49f (GoH3, 1:300).
Adoptive transfer of ILC2s
For adoptive transfer of ILC2s, Demodex-infected Il4ra−/− mice were co-housed with Rag1−/− Arg1Yarg Il5Red5 reporter mice for four weeks to establish Demodex infection. ILC2s (LiveCD45+Lin−Thy1+Red5+) from the Demodex-infected Rag1−/− mice were sorted from skin, lung and fat. Recipient Rag2−/−, Il2rg−/− mice received 2.5 x 104 pooled ILC2 cells intravenously in 200 μl of PBS at two weeks before, and 7 x 104 pooled ILC2s at the start of co-housing. Control mice received PBS. Mice were co-housed with Demodex-infected Il4ra−/− for 6 weeks before analysis.
Mass Cytometry
Sample preparation
Mass cytometry was performed as described (Kleppe et al., 2017). Briefly, single-cell suspension from the epidermal fraction of the various mice strains were prepared. To test the responsiveness to type 2 immune signaling, mice were treated with s.c. IL-4 complex for 30-60 min prior to harvest. IdU (200 μl of 5mM stock solution, Sigma 17125) was injected 20-30 min prior to harvesting. Cells were incubated with 25 μM Cisplatin for 1 min and fixed with paraformaldehyde at 1.6% for 10 min at room temperature (RT). Cell pellets were stored at −80 °C until analysis. Prior to antibody staining, cells were thawed and mass tag cellular barcoding of prepared samples was performed by incubating cells with distinct combinations of isotopically-purified palladium ions chelated by isothiocyanobenzyl-EDTA in 0.02% saponin in PBS as previously described (Behbehani et al., 2014; Zunder et al., 2015). After two washes with staining media (PBS + 0.5% BSA + 0.02% NaN3), sets of 20 barcoded samples were pooled together and washed once more with staining media. Antibody staining was performed using a panel of antibodies and metals that were purchased directly conjugated (Fluidigm) or conjugated using 100 μg of antibody lots combined with the MaxPAR antibody conjugation kit (Fluidigm) according to the manufacturer’s instructions and detailed in Supplementary Table 3. Surface marker antibodies were added to a 500 μL final reaction volumes and stained for 30 min at RT on a shaker. Following staining, cells were washed twice with PBS with 0.5% BSA and 0.02% NaN3. Then cells were permeabilized with 4 °C methanol for 10 min at 4 °C followed by two washes in PBS with 0.5% BSA and 0.02% NaN3 to remove remaining methanol. The intracellular antibodies were added in 500 μL for 30 min at RT on a shaker. Cells were washed twice in PBS with 0.5% BSA and 0.02% NaN3 and stained with 1 mL of 1:4000 191/193Ir DNA intercalator (Fluidigm) diluted in PBS with 1.6% PFA overnight. Cells were washed once in PBS with 0.5% BSA and 0.02% NaN3 and twice with double-deionized (dd)H20. We analyzed 1 x 106 cells per animal, per tissue, per condition consistent with generally accepted practices in the field.
Bead Standard Data Normalization
Just before analysis, the stained and intercalated cell pellet was resuspended in ddH2O containing the bead standard at a concentration ranging between 1 and 2 x 104 beads/ml as described (Finck et al., 2013). The bead standards were prepared immediately before analysis, and the mixture of beads and cells was filtered through filter cap FACS tubes (BD Biosciences) before analysis. Mass cytometry files were normalized together using the mass cytometry data normalization algorithm (Finck et al., 2013), which uses the intensity values of a sliding window of these bead standards to correct for instrument fluctuations over time and between samples.
Scaffold Map Generation
Total live leukocytes (excluding erythrocytes) were used for all analyses. Cells from each tissue for all animals were clustered together (rather than performing CLARA clustering on each file individually as originally implemented in (Spitzer et al., 2015)) Cells were deconvolved into their respective samples. Cluster frequencies or the Boolean expression of Ki67 for each cluster were passed into the Significance Across Microarrays algorithm (Bair and Tibshirani, 2004; Bruggner et al., 2014), and results were tabulated into the Scaffold map files for visualization through the graphical user interface. Cluster frequencies were calculated as a percent of total live nucleated cells (excluding erythrocytes). Scaffold maps were then generated as previously reported (Spitzer et al., 2017; Spitzer et al., 2015). All analyses were performed using the open source Scaffold maps R package available at http://www.github.com/spitzerlab/statisticalScaffold.
Spade and viSNE Generation
viSNE and Spade analyses were performed with Cytobank (www.cytobank.org).
Cytokine quantification
Cytokine in mouse serum or ILC2 culture supernatants were measured according to the manufacturer’s protocol using the following Cytokine Bead Array Flex Sets (BD): IL-4 (#558298), IL-5 (#558302), IL-13 (#558349), and IL-17A (#560283). The data were analyzed using Flow Cytometric Analysis Program (FCAP) Array software (BD). Serum IL-22 was measured using LEGEND MAX™ Mouse IL-22 ELISA Kit (BioLegend, #436307) and analyzed using a SpectraMax M2 microplate reader using SoftMax Pro software (Molecular Devices).
RNA preparation and qRT-PCR
ILC2s were sorted into RLT Plus lysis buffer (Qiagen) and stored at − 80 °C until processing using RNeasy Micro Plus kit (Qiagen) per manufacturer’s protocol. For quantitative reverse transcriptase PCR analyses, RNA was reverse transcribed using Superscript VILO cDNA synthesis kit (ThermoFisher) per the manufacturer’s instructions. The cDNA was used to test the gene expression of selected genes using Power SYBR Green PCR master mix (ThermoFisher) in a StepOnePlus cycler (Applied Biosystems). The following primers sets from PrimerBank were used: Il13: Il13-Forward 5’-CCTGGCTCTTGCTTGCCTT-3’ and Il13-Reverse, 5’-GGTCTTGTGTGATGTTGCTCA-3’; Il22: Il22-Forward 5’-ATGAGTTTTTCCCTTATGGGGAC-3’ and Il22-Reverse 5’-GCTGGAAGTTGGACACCTCAA-3’; Cdkn2a/p16: p16-Forward 5’-CGCAGGTTCTTGGTCACTGT-3’ and p16-Reverse 5’-TGTTCACGAAAGCCAGAGCG-3’; Rps17: Rps17-Forward 5’-CCAAGACCGTGAAGAAGGCTG-3’, Rps17-Reverse 5’-GCTGGGGATAATGGCGATCT-3’. Gene expression was normalized to Rps17 using the ΔCt method.
Chitin Synthase and Demodex 18S rRNA gene PCR
For semi-quantitative analyses of Demodex burden in skin, three random 2 mm punch biopsies of back caudal skin were digested in digest buffer (10 mM Tris pH 8.0, 100 mM NaCl, 10 mM EDTA pH 8.0, 0.5% SDS and 200 μg/ml Proteinase K (Thomas Scientific)) and the genomic DNA was resuspended in 30 μl of TE. The following primers and PCR conditions were used to detect the highly conserved Demodex spp. chitin synthase (CHS) gene (GenBank No. AB080667): CHS-Forward 5’-GAAGCGGCGAGTAATGTTCATC-3’; CHS-Reverse 5’-CCTGACTCCATCTTTTACGATGTC-3’; Cycling conditions 95 °C 3’, (95 °C 30”, 52 °C 30”, 72 °C 30”) x 30 cycles, 72 °C 7’. Amplification of the murine Keratin 5 gene was used as a loading control using the following primers and PCR conditions: Keratin5-Forward: 5’-TCTGCCATCACCCCATCTGT-3’; Keratin5-Reverse: 5’-CCTCCGCCAGAACTGTAGGA-3’, 95 °C 3’, (95 °C 30”, 60 °C 30”, 72 °C 30”) x 30 cycles, 72 °C 7’. PCR products were separated by a 2.5% agarose gel with ethidium bromide and visualized by a GelDoc (Bio-Rad Laboratories). Semi-quantitative analyses of band intensity of CHS and Krt5 was perfomed using Quantity One (version 4.6.1) or Image Lab (version 6.1.0) software (Bio-Rad Laboratories) and reported as the ratio of PCR product of CHS over Krt5. Demodex musculi was confirmed by first amplifying the 18S rRNA gene from DNA samples purified from the hair scrapes alone of affected mice using the universal 18S primers and PCR conditions: 18S-Forward 5’-TCCAAGGAAGGCAGCAGGCA-3’; 18S-Reverse 5’-CGCGGTAGTTCGTCTTGCGACG-3’, 95 °C 3’, (95 °C 30”, 58 °C 30”, 72 °C 30”) x 30 cycles, 72 °C 7’. PCR amplicons (532 bp) were purified and subsequently cloned into pCR4-TOPO plasmid by TOPO-TA cloning (Thermo Fisher) for sequencing. Putative Demodex spp. sequences from individual mouse (>3) were identical to the Demodex musculi 18S rRNA gene (GenBank No. JF834894).
Preparation of eGFP-CBP for immunofluorescence
Enhanced GFP was cloned into pGV358 (gift from Kushol Gupta, Univ. of Pennsylvania), a pETDuet based vector that has an Mxe intein in frame at the C-terminus followed by the non-cleavable hexahistidine tagged chitin-binding domain from Bacillus circulans WL-12 Chitinase A1 (eGFP-CBP). Construct expressing eGFP-CBP (gift from Sy Redding, Univ. of California, San Francisco) was transformed into BL21(DE3) cells and expressed overnight in 50 mL LB media at 37 °C. 10 mL of overnight culture was diluted in 1L LB media and expressed at 37 °C until OD600 = 1-1.2. Culture was induced with 1 mL 1M IPTG, and expression continued overnight at 19 °C. We added protease inhibitor (Roche #11836170001) at the temperature change to minimize proteolysis of the expressed protein. Cells were centrifuged at 4,000 x g at 4 °C for 20 mins. The pelleted cells were resuspended in 50 mL of lysis buffer (100 mM Tris pH 8.5, 150 mM NaCl, 30 mg Lysozyme, protease inhibitor, 1 μL universal nuclease (ThermoFisher Scientific #88700)) per 1 L of culture, then lysed using sonication for 6 mins at 50% power. Lysed cells were centrifuged at 10,000 x g at 4 °C for 15-30 mins. The lysate supernatant was filtered with a 0.22 μm filter. The filtered lysate was bound to a 5 mL HisTrap FF column (Cytiva #17525501), washed with 100 mM Tris pH 8.5, 150 mM NaCl, 50 mM imidazole pH 7.0, and eluted with a gradient into 100 mM Tris pH 8.5, 150 mM NaCl, 500 mM imidazole pH 7.0. Fractions were selected based on presence of molecular weight band at 56.8 kDa, corresponding with eGFP-CBP, as assessed via SDS-PAGE. Fractions were pooled and dialysed overnight into 100 mM Tris pH 8.5, 150 mM NaCl, 5% glycerol w/v. The protein solution was concentrated and separated via size-exclusion chromatography on a HiLoad 16/600 Superdex 75 pg (Cytiva #28989333). Fractions were selected based on purity as assessed via SDS-PAGE.
Fixed tissue preparation and immunofluorescence staining
For immunohistochemistry, 3-4 mm wide cephalad-caudal strips of back skin were cut and fixed in 2% paraformaldehyde for 2-6 hrs at room temperature, followed by 2 washes in D-PBS, and incubation in 30% (w/v) sucrose in D-PBS at 4 °C overnight. Portions of the tissue sections were submitted for routine paraffin embedding and hematoxylin and eosin staining using UCSF Histology and Biomarker Core. For frozen sections, tissues were embedded in Optimal Cutting Temperature Compound (Tissue-Tek) and stored at −80 °C until further processing. Tissue sections (8-12 μm) were cut on a Cryostat (Leica). Immunohistochemistry was performed in Tris/NaCl blocking buffer (0.1 M Tris-HCl, 0.15 M NaCl, 5 μg/ml blocking reagent (Perkin Elmer), pH 7.5) as follows at RT: 1 hour in 5% goat serum, 1-2 hours in primary antibody, 60 min secondary antibody (Goat anti-Rabbit AF555, 1:1000), 5 min of a 1:1000 dilution of DAPI (Stock 5 mg/ml in PBS), Roche) and mounted in Mounting Media (Vector Labs). The following primary antibodies were used in this study: Living colors DsRed Polyclonal antibody (Rabbit, 1:300, Takara Bio #632496); FITC Anti-mouse Ki67 (1:100, Invitrogen #11-5698-82), and FITC Mouse Anti-E-Cadherin (1:400, BD Biosciences #612131). For detection of chitin in skin tissue sections, 4-5 μm thick paraffin-embedded sections were baked at 60 °C for 30 min, treated with xylenes, and washed with 100% ethanol. The sections were incubated with an eGFP-CBP fusion protein (5 μg/ml) and DAPI (5 μg/ml) in PBS at RT for 60 min, washed 3 times with PBS and mounted using ProLong Gold antifade (Thermo Fisher, P36930). For quantification of HF infested by Demodex, two 1.5 to 2 cm strips of back skin from H&E stained sections were evaluated for infestation of hair follicles with a minimum of 70 HF inspected per mouse. Data presented as percentage of HF with at least one mite within the follicle or associated sebaceous gland over total HF visualized. Images were acquired using a Zeiss immunofluorescence AxioImager M2 upright microscope with a Plan Apochromat 20X/0.8NA air objective running AxioVision software (Zeiss) or an inverted Nikon A1R scanning confocal microscope with a Plan Apo λ 20X/0.75NA air objective running NIS Elements software (Nikon).
RNAscope of human tissue samples
Eight cases of normal nose skin were identified by searching the Yale Dermatopathology tissue archive (see Supplementary Table S4). These cases consisted of the tips of elliptical tissue excisions removing non-melanoma skin cancer. Areas that were well away from scar were selected for analysis. Eight cases of rhinophyma excision were similarly identified by searching the Yale Dermatopathology archive. RNA in situ hybridization was performed using a chromogenic RNAscope (Bio-techne) assay according to the manufacturer’s instructions and as previously described (Wang et al., 2021). The probes were purchased directly from the manufacturer: IL4 (315191), IL5 (319391), IL13 (586241), IL22 (560811), and IFNG (310501). CD3ε IHC was performed using an anti-CD3ε antibody (D7A6E) acquired from Cell Signaling Technology (Danvers, MA). Double staining for CD3ε (IHC) and IL13 mRNA ISH were performed as previously described (Wang et al., 2021). Briefly, after detection of IL13 with the RNAscope kit, antigen retrieval was performed with citrate buffer (pH 6.0) (00500, Thermo Fisher Scientific, Waltham, MA). Peroxidase activity was quenched using 3% hydrogen peroxide (JT-2186-01, JT Baker, Phillipsburg, NJ). CD3ε was then hybridized and detected using the Imm-PRESS horseradish peroxidase reagent and diaminobenzidine substrate (Vector Laboratories, Burlingame, CA).
The density of inflammation around the isthmus and infundibular portion of each follicular unit was graded using H&E stained slides and the following scale:
| Histologic description | Semi-quantitative score | Log2 transformation | Score used for statistical analysis |
|---|---|---|---|
| Negligible inflammation | 0 | - | 0 |
| Mild inflammation | 1 | 12 | 1 |
| Modest inflammation | 2 | 22 | 4 |
| Marked inflammation | 3 | 32 | 8 |
| Dense inflammation | 4 | 42 | 16 |
| Very dense inflammation (pseudolymphomatous) | 5 | 52 | 32 |
The amount of positive staining for each RNAscope probe was quantified on a cell-by-cell basis using the manufacturer’s recommendations, with slight modifications. Only inflammatory cells surrounding or within each follicle were scored (there was negligible staining outside these areas). The scoring rubric is summarized below:
| ACD score | Scoring criteria |
|---|---|
| 0 | No staining* |
| 1 | 1-2 dots per cell* |
| 2 | 2-5 dots per cell and no dot clusters |
| 3 | 5-10 dots per cell and/or <10% of dots are in clusters |
| 4 | >10 dots/cell and/or 10% of dots are in clusters |
This intensity of staining is considered background staining and was not included in the quantification.
The slides were scored in a blinded fashion by a board-certified dermatopathologist (W.D.). For CD3ε/IL13 doublestains the number of CD3ε positive and CD3ε negative cells was manually quantitated. The values were entered into GraphPad Prism. These studies were approved by the Yale IRB (ID 1501015235).
Single cell RNA-seq
C57BL/6J female mice were obtained from Jackson Laboratory at 3 weeks of age and divided into the following groups 1 weeks later: WT control (n = 3), and WT co-housed with Demodex-infested Il4ra−/− mice for 1 month (n = 3). In a second experiment, WT (uninfected, n = 3) or Demodex infected Il4ra−/− (n = 3) and Il4−/−, Il13−/− (n = 3) were used for analyses. Single cell suspensions from full thickness skin were stained with antibody to CD45 (to isolate the CD45+ fraction) and integrin alpha 6 to generate a 50:50 representation of CD45−alpha6+ and CD45− alpha6− cells in the CD45− fraction. Cell suspensions were stained with DAPI and live cells (DAPI−) were isolated by FACS using a MoFlo XDP (Beckman Coulter). The concentration of sorted cells was determined using CountBright beads on an LSRFortessa. Single-cell libraries from 25,000 cells per sample were prepared with the Chromium Single Cell 3′ GEM, Library & Gel Bead Kit v3.1 (10 x Genomics PN-1000121) following the manufacturer’s protocol. The libraries were sequenced on the NovaSeq 6000 at the UCSF Institute for Human Genetics with the following number of cycles for each read: Read 1, 28 cycles; i7 Index, 8 cycles; i5 Index, 0 cycles; Read 2, 91 cycles.
Data were analyzed in Seurat V3 or V4 in R using SCTransform aggregation, 0.6 resolution, with the following quality parameters: min.cells =5, nFeature_RNA ≥ 500; nFeature_RNA< 5000; nCount_RNA < 25000; percent mitochondrial reads < 15; percent hemoglobin reads 0.001. Percentage mitochondrial and ribosomal content were regressed out of clustering and aggregation. After initial clustering, cell identities in the CD45− fraction (epithelia, fibroblasts, other stromal cells) were assigned using public data sets (Joost et al., 2020). CD45+ immune clusters were identified by canonical markers and subclustered. Doublets were removed and the populations were clustered again before differential expression (DEG) analysis for cluster-defining genes and differential expression between treatment conditions. Data generated from different experiments (WT control vs. WT Demodex+; WT uninfected vs. Il4ra−/− Demodex+ vs. Il4,Il13−/− Demodex+) were integrated using the R package Harmony prior to clustering cell types. Batch correction was performed by creating a binary batch variable between the two datasets.
For DEG analysis between clusters, genes were detected if they are expressed in at least 10% of cells in a cluster with a log-fold change of at least 0.25. For DEG analysis between comparison groups within each cluster, genes were detected if expressed in at least 10% of cells in the group with a log-fold change of at least 0.25. For DEG analysis comparisons for all cells in aggregate, genes were detected if expressed in at least 10% of cells and with a log fold-change of at least 0.10. Additionally, for within-cluster DEG analysis, analysis was only done for clusters with >30 cells in each comparison group. Gene ontology (GO) term pathway analyses was performed using GOrilla (http://cbl-gorilla.cs.technion.ac.il) by inputting ‘pseudo’-bulk DEGs, from all CD45 negative cells, that were upregulated with a log-fold change of at least 0.2 in Demodex-infected WT, Il4ra−/− or Il4−/− Il13−/− mice vs. the experimental WT control. Additional data visualization was performed using the Nebulosa package in Seurat (https://github.com/powellgenomicslab/Nebulosa).
In Figure S7H–J epidermal stem cells were identified among subclustered epithelial cells by differential expression of Lgr5, Cdh3, Gli1 and/or Mki67. DEGs among all stem/dividing cells between conditions were identified by expression in at least 10% of cells with a log2 fold change of at least 0.10 between condition. GO Term enrichment was performed using EnrichGO in the ClusterProfiler package in R (Yu et al., 2012).
Transepidermal water loss measurements
Transepidermal water loss (TEWL) measurements were performed as described (Mathur et al., 2019). Briefly, mice back were shaved and rested for 18-24 h. Mice were sedated and TEWL was measured on four quadrants of back skin using a Tewameter (TM300, Khazaka Electronic).
Lucifer yellow assay to assess barrier function
Back skin from WT or Il4ra−/− mice was shaved and depilated. Two days after depilation, skin was harvested, washed twice with PBS and placed on a 7 mm Franz-Cell chamber (PermeGear Inc). The epidermal surface was incubated with Lucifer yellow (L453, ThermoFisher, 1 mg/ml) in PBS for 18 h at 37 °C. Samples were fixed for 1 h in 4 % PFA, washed twice with PBS, embedded in OCT and processed for immunofluorescence imaging.
16S rRNA gene sequence analysis
Sample collection
Mice facial and back skin were swabbed at the end of the experiment using a sterile foam tipped applicator (Puritan) that was moistened with yeast cell lysis buffer (Lucigen) and collected into sterile 2 ml tubes (Eppendorf). All samples were immediately snap frozen in dry ice and stored at −80 °C until DNA extraction.
DNA extraction and V1V3 Library Prep
DNA was extracted from samples using the DNEasy PowerSoil Pro Kit (Qiagen) according to the manufacturer’s protocol. Barcoded PCR primers annealing to the V1-V3 region of the 16S rRNA gene (Fwd, 5’-AGAGTTTGATCCTGGCTCAG-3’; Rev, 5-ATTACCGCGGCTGCTGG-3’) were used for library generation. PCR reactions were carried out in quadruplicate using Q5 High-Fidelity DNA Polymerase (NEB, Ipswich, MA). Each PCR reaction contains 0.5 μM of each primer, 0.34 U Q5 Pol, 1X Buffer, 0.2 mM dNTPs, and 5.0 μl DNA in a total volume of 25 μl. Cycling conditions are as follows: 1 cycle of 98 °C for 1 min; 30 cycles of 98 °C for 10 sec, 56 °C for 20 sec, and 72 °C for 20 sec; 1 cycle of 72 °C for 8 min. After amplification, quadruplicate PCR reactions were pooled and then purified using a 1:1 volume of SPRI beads. DNA in each sample was quantified using PicoGreen and pooled in equal molar amounts. The resulting library was sequenced on the Illumina MiSeq using 2x300 bp chemistry. Extraction blanks and DNA free water were subjected to the same amplification and purification procedure to allow for empirical assessment of environmental and reagent contamination.
Bioinformatics processing
Sequence data were processed using QIIME2 (Bolyen et al., 2019). Read pairs were processed to identify amplicon sequence variants with DADA2 (Callahan et al., 2016). Taxonomic assignments were generated by comparison to the Greengenes reference database (McDonald et al., 2012), using the naive Bayes classifier implemented in scikit-bio (Bokulich et al., 2018). A phylogenetic tree was inferred from the sequence data using MAFFT (Katoh and Standley, 2013).
Statistical analysis for microbiome data
Data files from QIIME were analyzed in the R environment for statistical computing, using the QIIMER library, which we developed (http://cran.r-project.org/web/packages/qiimer). Global differences in bacterial community composition were visualized using Principal Coordinates Analysis of weighted and unweighted UniFrac distance (Lozupone and Knight, 2005; Lozupone et al., 2007). Community-level differences between sample groups were assessed using the PERMANOVA test, which allows sample-sample distances to be applied to an ANOVA-like framework (Anderson, 2001). Linear mixed-effects models were used to ascertain differences in alpha diversity and taxonomic abundances.
Statistical analysis
All experiments were performed using randomly assigned mice without investigator blinding. All data points and n values reflect biological (individual mice) replicates. Experiments were pooled whenever possible, and all data were analyzed using Prism 7 (GraphPad Software) by comparison of means using unpaired two-tailed Student’s t tests or one-way ANOVA for multiple comparison as indicated in the figure legends. Data in all figures displayed as mean ± s.e.m. unless otherwise indicated.
Supplementary Material
Highlights.
Type 2 innate immunity is critical for normal commensalism with Demodex
In the absence of type 2 immunity, Demodex causes aberrant inflammation
IL-13 from ILC2s is coupled to the hair cycle and restrains stem cell proliferation
Decreased type 2 cytokine expression is observed in patients with rhinophyma
Acknowledgments
We thank M. Ji and M. Conseco for technical expertise and mouse colony maintenance, Z. Wang for cell sorting, J. Bolen and X.Y. Jiang and the UCSF Biomarkers and Histology Core for assistance with histology. We thank members of the Locksley laboratory and M. D. Rosenblum, A. L. DeFranco, J. von Moltke, and C. Schneider for comments on the manuscript. Figure diagrams were created with BioRender.
Funding
This work was supported by the National Institutes of Health (AR007175 to R.R.R.G. and M.S.F, AR075880 to R.R.R.G, HL140868 to M.E.K., DK121476 to C.E.O., AI159229 to W.D., AR074556 to M.S.F., AI026918 and HL107202 to R.M.L.), Dermatology Foundation (R.R.R.G., M.S.F.), A.P. Giannini Foundation (R.R.R.G, M.E.K.), Robert Wood Johnson Foundation (R.R.R.G.), Howard Hughes Medical Institute (R.M.L.), University of California Tobacco-Related Disease Research Program (T29IP0554 to J.S.F.), and the Sandler Asthma Basic Research Center at the University of California San Francisco. R.E. Díaz was supported by NSF GRFP (#1650113). R.E. Díaz is a Howard Hughes Medical Institute Gilliam Fellow (#GT11377). R.R.R.G. and M.H.S. are Chan Zuckerberg Investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Inclusion and Diversity Statement
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper received support from a program designed to increase minority representation in science.
Declaration of interests
R.M.L. is a member of the Scientific Resource Board at Genentech and serves on the Advisory Board at Immunity. The other authors declare no competing interests.
References
- Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, et al. (2017). Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 169, 1119–1129 e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology 26, 32–46. [Google Scholar]
- Bair E, and Tibshirani R (2004). Semi-supervised methods to predict patient survival from gene expression data. PLoS Biol 2, E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando JK, Liang HE, and Locksley RM (2015). Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol 16, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando JK, Nussbaum JC, Liang HE, and Locksley RM (2013). Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J Leukoc Biol 94, 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe J, Poreaux C, Remen T, Schoeffler A, Cloche V, Schmutz JL, Escobar G, and Busztejn AC (2021). Prevalence of ocular disease during dupilumab treatment for atopic dermatitis: a bicentric retrospective comparative cohort study. Int J Dermatol 60, 1520–1528. [DOI] [PubMed] [Google Scholar]
- Behbehani GK, Thom C, Zunder ER, Finck R, Gaudilliere B, Fragiadakis GK, Fantl WJ, and Nolan GP (2014). Transient partial permeabilization with saponin enables cellular barcoding prior to surface marker staining. Cytometry A 85, 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki P, Riesenfeld SJ, Hutter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, Lian M, Amezcua Vesely MC, Kroehling L, Xu H, et al. (2021). Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 592, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, and Gregory Caporaso J (2018). Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggner RV, Bodenmiller B, Dill DL, Tibshirani RJ, and Nolan GP (2014). Automated identification of stratifying signatures in cellular subpopulations. Proc Natl Acad Sci U S A 111, E2770–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, and Holmes SP (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13, 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Hepworth MR, Whittingham-Dowd J, Thompson S, Bancroft AJ, Hayes KS, Shaw TN, Dickey BF, Flamar AL, Artis D, et al. (2019). ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J Exp Med 216, 2714–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, and Ihle JN (2004). Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol 24, 2584–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YE, Fischbach MA, and Belkaid Y (2018). Skin microbiota-host interactions. Nature 553, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, and Locksley RM (2014). Allergic inflammation--innately homeostatic. Cold Spring Harb Perspect Biol 7, a016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovatiya RJ, and Colegio OR (2016). Demodicosis in Renal Transplant Recipients. Am J Transplant 16, 712–716. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, Mcllgorm A, Jolin HE, and McKenzie AN (2006). Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 203, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, Pe’er D, Nolan GP, and Bendall SC (2013). Normalization of mass cytometry data with bead standards. Cytometry A 83, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton F, and Seys B (1993). Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol 128, 650–659. [DOI] [PubMed] [Google Scholar]
- Gause WC, Rothlin C, and Loke P (2020). Heterogeneity in the initiation, development and function of type 2 immunity. Nat Rev Immunol 20, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Miao Y, Gur-Cohen S, Gomez N, Yang H, Nikolova M, Polak L, Hu Y, Verma A, Elemento O, et al. (2020). The aging skin microenvironment dictates stem cell behavior. Proc Natl Acad Sci U S A 117, 5339–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgala S, Katoulis AC, Kylafis GD, Koumantaki-Mathioudaki E, Georgala C, and Aroni K (2001). Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. J Eur Acad Dermatol Venereol 15, 441–444. [DOI] [PubMed] [Google Scholar]
- Gieseck RL 3rd, Wilson MS, and Wynn TA (2018). Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 18, 62–76. [DOI] [PubMed] [Google Scholar]
- Gonzales KAU, Polak L, Matos I, Tierney MT, Gola A, Wong E, Infarinato NR, Nikolova M, Luo S, Liu S, et al. (2021). Stem cells expand potency and alter tissue fitness by accumulating diverse epigenetic memories. Science 374, eabh2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, and Fuchs E (2019). Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OJ, Linehan JL, Shih HY, Bouladoux N, Han SJ, Smelkinson M, Sen SK, Byrd AL, Enamorado M, Yao C, et al. (2019). Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363, eaat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heibel HD, Hendricks AJ, Foshee JP, and Shi VY (2021). Rosacea associated with dupilumab therapy. J Dermatolog Treat 32, 114–116. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, Matsuyama T, Takeda K, and Akira S (1999). The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med 190, 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al. (2016). Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science (New York, N.Y.) 351, 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Zhao Y, Niu D, Gong X, and Yang R (2019). De novo transcriptome sequencing and differential gene expression analysis of two parasitic human Demodex species. Parasitol Res 118, 3223–3235. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, Usher N, Zhu J, Urban JF Jr., Paul WE, and Germain RN (2018). S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U, Sequeira I, Sandberg R, and Kasper M (2020). The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell Stem Cell 26, 441–457 e447. [DOI] [PubMed] [Google Scholar]
- Katoh K, and Standley DM (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe M, Spitzer MH, Li S, Hill CE, Dong L, Papalexi E, De Groote S, Bowman RL, Keller M, Koppikar P, et al. (2017). Jak1 Integrates Cytokine Sensing to Regulate Hematopoietic Stem Cell Function and Stress Hematopoiesis. Cell Stem Cell 21, 489–501 e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo JH, Shih HY, Truong A, Doebel T, Sakamoto K, Cui CY, et al. (2019). Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 176, 982–997 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey N, Ni Raghallaigh S, and Powell FC (2011). Demodex mites--commensals, parasites or mutualistic organisms? Dermatology 222, 128–130. [DOI] [PubMed] [Google Scholar]
- Lay K, Yuan S, Gur-Cohen S, Miao Y, Han T, Naik S, Pasolli HA, Larsen SB, and Fuchs E (2018). Stem cells repurpose proliferation to contain a breach in their niche barrier. Elife 7, e41661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H-E, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, and Locksley RM (2012). Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nature Immunology 13, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, and Locksley RM (2011). Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol 13, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, and Knight R (2005). UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71, 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Hamady M, Kelley ST, and Knight R (2007). Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol 73, 1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Zirak B, Boothby IC, Tan M, Cohen JN, Mauro TM, Mehta P, Lowe MM, Abbas AK, Ali N, and Rosenblum MD (2019). Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity 50, 655–667 e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JU, Hilligan KL, Chandler JS, Eccles DA, Old SI, Domingues RG, Yang J, Webb GR, Munoz-Erazo L, Hyde EJ, et al. (2021). Homeostatic IL-13 in healthy skin directs dendritic cell differentiation to promote TH2 and inhibit TH17 cell polarization. Nat Immunol 22, 1538–1550. [DOI] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, and Hugenholtz P (2012). An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R (2021). The spectrum of inflammatory responses. Science 374, 1070–1075. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, and Locksley RM (2001). Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311. [DOI] [PubMed] [Google Scholar]
- Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, et al. (2012). Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 13, 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Cowley CJ, and Fuchs E (2018). Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell 175, 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashat MA, Ricart Arbona RJ, Lepherd ML, Santagostino SF, Livingston RS, Riedel ER, and Lipman NS (2018). Ivermectin-compounded Feed Compared with Topical Moxidectin-Imidacloprid for Eradication of Demodex musculi in Laboratory Mice. J Am Assoc Lab Anim Sci 57, 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, and Locksley RM (2013). Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palopoli MF, Fergus DJ, Minot S, Pei DT, Simison WB, Fernandez-Silva I, Thoemmes MS, Dunn RR, and Trautwein M (2015). Global divergence of the human follicle mite Demodex folliculorum: Persistent associations between host ancestry and mite lineages. Proc Natl Acad Sci U S A 112, 15958–15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, and Handjiski B (1999). A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 113, 523–532. [DOI] [PubMed] [Google Scholar]
- Quint T, Brunner PM, Sinz C, Steiner I, Ristl R, Vigl K, Kimeswenger S, Neubauer K, Pirkhammer D, Zikeli M, et al. (2020). Dupilumab for the Treatment of Atopic Dermatitis in an Austrian Cohort-Real-Life Data Shows Rosacea-Like Folliculitis. J Clin Med 9, 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather PA, and Hassan I (2014). Human demodex mite: the versatile mite of dermatological importance. Indian J Dermatol 59, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G, Vegh P, Fletcher J, Poyner EFM, Stephenson E, Goh I, Botting RA, Huang N, Olabi B, Dubois A, et al. (2021). Developmental cell programs are co-opted in inflammatory skin disease. Science 371, eaba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Gonzalez RR, Schneider C, Liao C, Lee J, Liang HE, and Locksley RM (2020). Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J Exp Med 217, e20191172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, Vaka D, Eckalbar WL, Molofsky AB, Erie DJ, and Locksley RM (2018). Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 19, 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosshart SP, Herz J, Vassallo BG, Hunter A, Wall MK, Badger JH, McCulloch JA, Anastasakis DG, Sarshad AA, Leonardi I, et al. (2019). Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, eaaw4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Jin SP, Goel S, Jo JH, Voisin B, Kim D, Nadella V, Liang H, Kobayashi T, Huang X, et al. (2021). Disruption of the endopeptidase ADAM10-Notch signaling axis leads to skin dysbiosis and innate lymphoid cell-mediated hair follicle destruction. Immunity 54, 2321–2337 e2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanman LE, Chen IW, Bieber JM, Steri V, Trentesaux C, Hann B, Klein OD, Wu LF, and Altschuler SJ (2021). Transit-Amplifying Cells Coordinate Changes in Intestinal Epithelial Cell-Type Composition. Dev Cell 56, 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre N, Francino O, Curti JN, Armenia TC, Fraser DL, Kelly RM, Hunt E, Silbermayr K, Zewe C, Sanchez A, and Ferrer L (2016). Detection, Prevalence and Phylogenetic Relationships of Demodex spp and further Skin Prostigmata Mites (Acari, Arachnida) in Wild and Domestic Mammals. PLoS One 11, e0165765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Lee J, Koga S, Ricardo-Gonzalez RR, Nussbaum JC, Smith LK, Villeda SA, Liang HE, and Locksley RM (2019). Tissue-Resident Group 2 Innate Lymphoid Cells Differentiate by Layered Ontogeny and In Situ Perinatal Priming. Immunity 50, 1425–1438 e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, O’Leary CE, Moltke J.v., Liang H-E, Ang QY, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, and Locksley RM (2018). A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 12, 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, Silverberg JI, Deleuran M, Kataoka Y, Lacour JP, et al. (2016). Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med 375, 2335–2348. [DOI] [PubMed] [Google Scholar]
- Smith G, Manzano Marin A, Reyes-Prieto M, Ribeiro Antunes CS, Ashworth V, Goselle ON, Jan AAA, Moya A, Latorre A, Perotti MA, and Braig HR (2022). Human follicular mites: Ectoparasites becoming symbionts. Mol Biol Evol 39, msac125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PC, Zeiss CJ, Beck AP, and Scholz JA (2016). Demodex musculi Infestation in Genetically Immunomodulated Mice. Comp Med 66, 278–285. [PMC free article] [PubMed] [Google Scholar]
- Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, et al. (2017). Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell 168, 487–502 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer MH, Gherardini PF, Fragiadakis GK, Bhattachaiya N, Yuan RT, Hotson AN, Finck R, Carmi Y, Zunder ER, Fantl WJ, et al. (2015). IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science 349, 1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoemmes MS, Fergus DJ, Urban J, Trautwein M, and Dunn RR (2014). Ubiquity and diversity of human-associated Demodex mites. PLoS One 9, e106265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Liang HE, Naikawadi RP, Woodruff PG, Wolters PJ, Erie DJ, and Locksley RM (2017). Spontaneous Chitin Accumulation in Airways and Age-Related Fibrotic Lung Disease. Cell 169, 497–509 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, et al. (2016). A tissue checkpoint regulates type 2 immunity. Nat Immunol 17, 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, and Locksley RM (2016). Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Fogel AL, Murphy MJ, Panse G, McGeary MK, McNiff JM, Bosenberg M, Vesely MD, Cohen JM, Ko CJ, et al. (2021). Cytokine RNA In Situ Hybridization Permits Individualized Molecular Phenotyping in Biopsies of Psoriasis and Atopic Dermatitis. JID Innovations 1, 10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Adam RC, Ge Y, Hua ZL, and Fuchs E (2017). Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell 169, 483–496 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, and He QY (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YE, Wang ZH, Xu Y, Xu JR, Liu WY, Wei M, and Wang CY (2012). Cloning and sequence analysis of chitin synthase gene fragments of Demodex mites. J Zhejiang Univ Sci B 13, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YE, Wu LP, Peng Y, and Cheng H (2010). Retrospective analysis of the association between Demodex infestation and rosacea. Arch Dermatol 146, 896–902. [DOI] [PubMed] [Google Scholar]
- Zunder ER, Lujan E, Goltsev Y, Wernig M, and Nolan GP (2015). A continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell 16, 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of the article are available within the main text or supplementary materials. The published article includes datasets generated during this study. Original single cell RNA-seq data has been deposited in GEO (GSE197983). Original 16S rRNA sequencing datasets analyzed in this study are available at the NCBI Sequence Read Archive (SUB11832976).


