Abstract
There is great interest in characterizing the proteins of the gastric pathogen Helicobacter pylori, especially those to which humans respond immunologically, because of the potential importance of such proteins in diagnosis and vaccine development. Two-dimensional gel electrophoresis was used to separate and identify potential antigens of H. pylori ATCC 43504. Over 30 proteins were reactive in Western blots with pooled sera from 14 infected patients. These proteins were analyzed by N-terminal sequence analysis. Fourteen proteins were determined to be distinct from any proteins previously described from H. pylori; the others were previously isolated and characterized proteins. Analysis of eight distinct H. pylori strains showed that most of these antigens were produced by all of the strains. We propose that collection of new antigens such as those recognized here will be useful in serologic tests for detecting and monitoring H. pylori infection and may also serve as potential targets for antimicrobial agent or vaccine development.
Helicobacter pylori is a gram-negative bacterium that chronically infects the gastric mucosa of more than half of all humans worldwide and is a major cause of gastritis and peptic ulcer disease and an early risk factor for gastric cancer (6). Only some 10 to 20% of infections, however, result in overt disease. DNA typing has established that H. pylori is extremely diverse as a species, and it is likely that the varied outcomes of infection reflect differences in bacterial genotype, human host genotype, and physiologic, immunologic, and environmental factors (25). These considerations make it valuable to thoroughly characterize the proteins and other antigens that H. pylori produces and the human responses to them.
Factors important for H. pylori colonization or virulence are just beginning to be identified. Some of the more prominent factors include (i) flagellae, which allow the organism to move in the mucous layer (15); (ii) urease complex, which may help maintain a neutral micro pH environment in the face of gastric acidity (11); (iii) the VacA protein, which generates vacuoles in eukaryotic epithelial cells (2); and (iv) the cag pathogenicity island, some of whose encoded proteins help trigger severe inflammatory responses and which, like VacA toxigenicity, is disease associated (1). Several other H. pylori proteins with known activities, or which are related to similar proteins of known function in other organisms, have been isolated. Most recently, the complete genomic DNA sequence of H. pylori 26695 has been reported (28). However, many of the proteins inferred from this DNA sequence have no known function, and this DNA sequence clone does not always predict which open reading frames are likely to encode virulence factors or antigens suitable for diagnostic or vaccine studies.
A number of studies have begun to address associations of specific H. pylori antigens to antibodies in patients with particular gastroduodenal pathologies and of possible autoimmune components to H. pylori-associated disease. There is very little information, however, regarding the long-term evolution and clinical implications of these human responses before and after the eradication of H. pylori by antibiotic treatment regimens.
Here we have identified 30 well-conserved proteins that are strongly recognized by sera of infected individuals. Fourteen of these 30 proteins had not been identified previously.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions for H. pylori.
Clinical isolates were from the Berg laboratory collection. Initial two-dimensional (2D) characterization and isolation of H. pylori antigens were performed with strain ATCC 43504 (type strain, NCTC 11637), which was isolated from a peptic ulcer patient at Royal Perth Hospital, Perth, Australia. Strains used for comparative purposes were as follows: 26695, the strain whose sequence was fully determined (28), originally from an English gastritis patient; Chico, from a symptomatic male patient from Feather River Hospital, Chico, Calif.; J170, from a gastric ulcer patient in Tennessee and used by DuBois et al. (3a) for monkey colonization experiments; 4655/1, from a symptomatic Gambian child; Rus-95, from a Russian citizen in the United States; Peru #9, from a symptomatic patient in Lima, Peru; C-3c, from a symptomatic Lithuanian patient, and A-1c, an unrelated strain from a Lithuanian gastric cancer patient; and 96-212, from an Aleut (native Alaskan) male with gastric cancer. All H. pylori strains were cultured on campylobacter agar Skirrow (Difco) plates supplemented with 10% defibrinated sheep’s blood (Quad 5, Helena, Mont.) in chambers that had been made microaerobic by the CampyPak system (BBL). Cells harvested from Skirrow blood agar plates were washed with phosphate-buffered saline (PBS) and lysed according the procedure of Panini et al. (23).
2D gel electrophoresis (pH 4 to 8).
2D electrophoresis was performed according to the method of O’Farrell (20), as follows. Isoelectric focusing was carried out in glass tubes of inner diameter 2.0 mm with 2% ampholines (BDH; Hofer Scientific Instruments, San Francisco, Calif.), pH 4 to 8, for 9,600 V · h. The final tube gel pH gradient as measured by a surface pH electrode is shown in the figure. After equilibration for 10 min in buffer O (10% glycerol, 50 mM dithiothreitol, 2.3% sodium dodecyl sulfate (SDS), and 62.5 mM Tris [pH 6.8]), the tube gel was sealed to the top of the stacking gel, which was placed on top of a 10% acrylamide slab gel (0.75 mm thick), and SDS slab gel electrophoresis was carried out for 4 h at 12.5 mA/gel. The slab gels were fixed in a solution of 10% acetic acid–50% methanol overnight. The following proteins were added as molecular size standards (Sigma) to the agarose which sealed the tube gel to the slab gel: myosin (220 kDa), phosphorylase A (94 kDa), catalase (60 kDa), actin (43 kDa), carbonic anhydrase (29 kDa), and lysozyme (14 kDa). These standards appear as horizontal lines on the silver-stained 10% acrylamide slab. The silver-stained gel was dried between sheets of cellophane paper with the acid edge to the left.
2D gel electrophoresis (pH 8 to 13).
2D electrophoresis adapted for resolution of basic proteins was performed according to the method of O’Farrell et al. (21), as follows. Nonequilibrium pH gradient electrophoresis with 1.5% pH 3.5 to 10 and 0.25% pH 9 to 11 ampholines (Pharmacia Biotechnology, Piscataway, N.J.) was carried out at 140 V for 12 h. Purified tropomyosin, lower spot (33 kDa and pI 5.2), and purified lysozyme (14 kDa and pI 10.5 to 11) (Sigma) were added to the samples as internal pI markers. After equilibration for 10 min in buffer O, the tube gel was sealed to the top of the stacking gel, which was placed on top of a 10% acrylamide slab gel (0.75 mm thick), and SDS slab gel electrophoresis was carried out for 4 h at 12.5 mA/gel. The slab gels were fixed in a solution of 10% acetic acid–50% methanol overnight. As with the low-pH 2D gel, the following proteins were added as molecular size standards to the agarose which sealed the tube gel to the slab gel: myosin (220 kDa), phosphorylase A (94 kDa), catalase (60 kDa), actin (43 kDa), carbonic anhydrase (29 kDa), and lysozyme (14 kDa). These standards appear as horizontal lines on the silver-stained 10% acrylamide slab. The silver-stained gel was dried between sheets of cellophane paper with the acid edge to the left.
Western blotting.
Following slab gel electrophoresis, the gel was placed in transfer buffer (12.5 mM Tris [pH 8.8], 86 mM glycine, 10% methanol) and proteins were transblotted onto polyvinylidene difluoride (PVDF) paper overnight at 200 mA (approximately 50 V/gel). The blot was blocked for 2 h in 2% bovine serum albumin (BSA) in 1% Tween–Tris-buffered saline (vol/vol) (TTBS), rinsed in TTBS, incubated with primary antibody diluted 1:2,500 in 1% BSA–TTBS for 2 h, rinsed in TTBS, and incubated with a secondary antibody (anti-human immunoglobulin G–horseradish peroxidase [Zymed] diluted 1:5,000 in TTBS) for 1 h. The blot was rinsed with TTBS, treated with ECL (Amersham), and exposed to X-ray film.
N-terminal sequencing.
The PVDF blot was stained with Coomassie brilliant blue. Spots corresponding to Western blot-positive spots were excised by scalpel and sequenced directly with a Hewlett-Packard G1005A N-terminal sequencer. The instrument gave a high repetitive yield (typically 93 to 98%), with a detection limit of approximately 100 to 200 fmol. All sequences were compared to data available on 13 September 1997 in the PIR, NRDB, GenBank, EMBL, and Swiss Protein databases.
Serum pools.
The positive serum pool was derived from pooled sera obtained from 14 patients identified by endoscopy as H. pylori positive. The negative serum pool was derived from 14 volunteers whose sera were negative by Helico Blot 2.0 (Genelabs Diagnostics, Ltd., Singapore, Singapore).
RESULTS
2D SDS-polyacrylamide gel electrophoresis (PAGE) (pH 4 to 8).
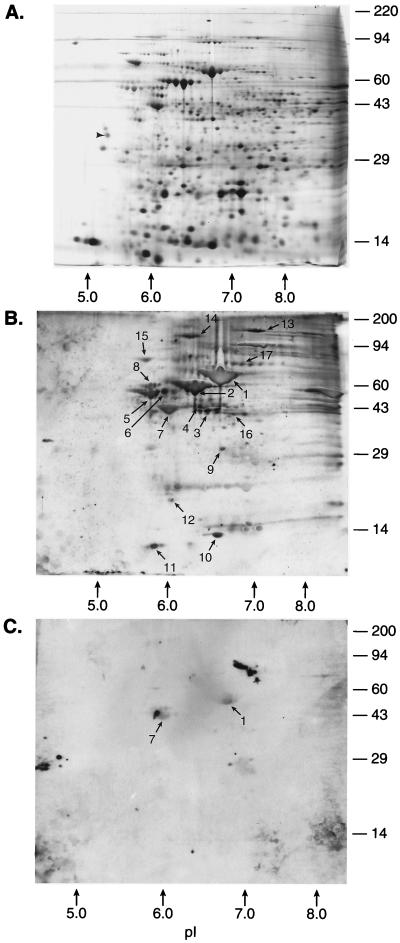
The proteins from lysed cell pellets of H. pylori ATCC 43504 were separated on a series of 2D gels run in parallel with an initial pH gradient of pH 4 to pH 8. The silver-stained gel (Fig. 1A) revealed prominent individual proteins, with several protein “families”—most notably as clusters of bands at approximately 89, (pI 6.8), 66, and 58 kDa (pI 6.5). The proteins from these 2D gels were transferred to PVDF membranes and incubated with a positive serum pool (Fig. 1B) or a negative serum pool (Fig. 1C). Western blot data revealed at least 17 spots or groups of spots which were recognized by antibodies in the infected patient serum pool. Transblotted 2D spots from the pH 4 to 8 gel were sequenced by Edman-type amino acid analysis, with the protein within selected spots evaluated further for internal sequence information. The sequences from these spots were compared with sequences in available databases (Table 1). Briefly, spots 1 and 2 corresponded to the H. pylori urease b subunit (4) and the urease b-associated chaperonin GroEL (5), respectively. Spot 3 consisted of two proteins: the major species was pyruvate flavidoxin oxidoreductase (13), and the minor protein species corresponded to the previously described H. pylori hypothetical protein 2, or HP0154 (26, 28). Spot 4 corresponded to HP0537, from the cag region (28). Spots 5, 6, and 8 corresponded to flagellin proteins (15). Spot 7 consisted of two proteins which did not match any previously reported sequences from H. pylori. The major component, however, has 90% homology with the Escherichia coli TufB protein (possibly HP1205), and the minor component has some sequence homology with various ATPase proton pumps (10, 14). Spot 9 was homologous to monomine oxidase from various species (27). Spot 10 corresponded to the neutrophil-activating protein (8). Spot 11 corresponded to HP1199, a ribosomal protein (28). Spot 12 had homology with the ClpP protease from various bacteria (19). The sequencing signals of spots 13 and 14 were too low to be read with confidence. Spots 15 and 16 (major) corresponded to HP0109 (Hsp 70) and HP0589 (ferrodoxin oxidoreductase), respectively (28). Spots 16 (minor) and 17 corresponded to a protein previously isolated by O’Toole et al. (22). In the control blot with sera from H. pylori-negative persons, only the urease b subunit (spot 1), likely due to cross-reaction with ureases of intestinal bacteria, and the spot 7 proteins showed cross-reactivity.
FIG. 1.
H. pylori 2D map (pH gradient electrophoresis, pH 4 to 8) with identified proteins (listed in Table 1). Strain 43504 was grown as described in Materials and Methods, and 200 μg of protein extract was loaded in the first dimension. Identified proteins are indicated by spot numbers in Table 1. Molecular size markers are indicated on the right (in kilodaltons). (A) Silver-stained 2D gel. Fifty nanograms of tropomyosin was added as an internal IEF standard. This protein migrates as a doublet with a polypeptide spot of 33 kDa and pI 5.2. (B) Western blot of a duplicate 2D gel with an H. pylori-positive serum pool. (C) Western blot of a duplicate 2D gel with an H. pylori-negative (control) serum pool.
TABLE 1.
Identification of Western blot-positive proteins by N-terminal sequence analysis (pH 4 to 8)
| Spot no. | Observed molecu- lar size (kDa) | pI | N-terminal sequence | Antigen, if known (reference) |
|---|---|---|---|---|
| 1 | 62 | 6.7 | MKKIS | Urease b subunit (4) |
| 2 | 58 | 6.3 | AKEIK | Urease b-associated chaperonin (5) |
| 3 (major) | 42 | 6.5 | AKSIELQEIE | Pyruvate flavidoxin reductase (13) |
| 3 (minor) | 42 | 6.5 | MLTXKDIHAL | HP0154 (enolase) (26, 28) |
| 4 | 41 | 6.4 | XTKIVF | HP0537 (Cag 16) (28) |
| 5 | 56 | 5.8 | AFQVN | Flagellin a protein (15) |
| 6 | 58 | 6.0 | AFQVN | Flagellin a protein (15) |
| 7 (major) | 42 | 6.1 | XKEKFNRTKP | E. coli TufB protein (10) |
| 7 (minor) | 42 | 6.1 | MXGXIIQVLG | ATPase proton pump (14) |
| 8 | 60 | 5.8 | SFRINTNIAA | Flagellin b precursor (15) |
| 9 | 31 | 6.7 | MIDXAIIGGG | Monomine oxidase (27) |
| 10 | 12 | 6.8 | MKTFEILKHL | Neutrophil-activating protein (8) |
| 11 | 6 | 5.7 | AISKEEVLEY | HP1199 (ribosomal pro- tein L7/L12) (28) |
| 12 | 20 | 6.0 | MYIPYVIEN | ClpP protein (19) |
| 13 | 159 | 7.1 | MKLI | (Signal too low) |
| 14 | No sequence | |||
| 15 | 79 | 5.7 | GKVIGIDLGT | HP0109 (heat shock protein 70) (28) |
| 16 (major) | 42 | 6.8 | MREIIXDGNE | HP0589 (ferredoxin oxidoreductase) (28) |
| 16 (minor) | MKLLE | O’Toole protein (22) | ||
| 17 | 78 | 6.9 | MKLLEE | O’Toole protein (22) |
2D SDS-PAGE (pH 8 to 13).
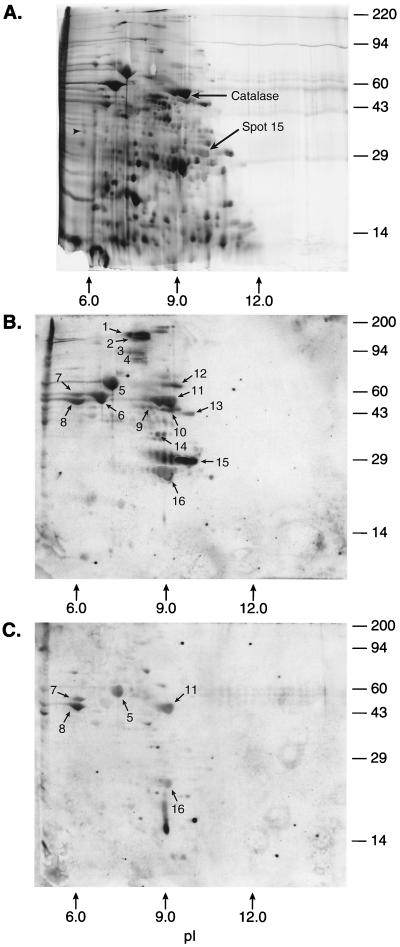
Additional unique proteins were found by SDS-PAGE with a nonequilibrium gel, even though fewer proteins, overall, were resolved (Fig. 2). Spots 1 through 4 were present in very low quantities; therefore, a clear N-terminal sequence could not be determined with confidence (Table 2). Spot 5 was the urease b subunit also seen in the pH 4 to 8 2D gels. Likewise, spots 6, 7, and 8 corresponded to urease b-associated chaperonin, flagellin b precursor, and flagellin a protein, respectively, which were also separated on the pH 4 to 8 2D gel. Spot 9 (major) corresponded to HP0027 (isocitrate dehydrogenase) (28), with spot 9 (minor) representing a possible contaminant in the sequencing sample. Spot 10 corresponded to an open reading frame from HP1018, an open reading frame with no known database homologs. Spot 11 corresponded to H. pylori catalase (12). Spot 12 contained an N-terminal sequence which has been found in several Omp’s (Omp 5, 8, 9, 19, and 27) (see reference 28). Spot 13 corresponded to HP1350, a putative protease (28). Spot 14 was the previously reported HopC protein, and spot 16 was the urease a subunit (7, 9). The sequence yields from transblotted spot 15 were low (in the mid-femtomole range), suggesting that the protein was blocked. The sequence information derived from spot 15 gave an N-terminal amino acid sequence which did not match any known protein sequences. This suggested that the protein(s) in this spot might be modified at the amino terminus, as sequencing yields were low despite the protein(s) being clearly visible on a silver-stained gel.
FIG. 2.
H. pylori 2D map (nonequilibrium pH gradient electrophoresis, pH 8 to 13) with identified proteins (listed in Table 2). Strain 43504 was grown as described in Materials and Methods, and 200 μg of protein extract was loaded in the first dimension. Identified proteins are indicated by spot numbers in Table 2. Molecular size markers are indicated on the right (in kilodaltons). (A) Silver-stained 2D gel. Fifty nanograms of tropomyosin was added as an internal IEF standard. This protein migrates as a doublet with a polypeptide spot of 33 kDa and pI 5.2. Purified lysozyme (14 kDa, pI 10.5 to 11.0) was also added as an internal pI standard. (B) Western blot of a duplicate 2D gel with an H. pylori-positive serum pool. (C) Western blot of a duplicate 2D gel with an H. pylori-negative (control) serum pool.
TABLE 2.
Identification of Western blot-positive proteins by N-terminal sequence analysis (pH 8 to 13)
| Spot no. | Observed molecu- lar size (kDa) | pI | N-terminal sequence | Antigen, if known (reference) |
|---|---|---|---|---|
| 1 | 180 | 8.2 | NPP | (Signal too low) |
| 2 | 180 | 8.3 | MDXY | (Signal too low) |
| 3 | 88 | 8.3 | NKITY | (Signal too low) |
| 4 | 82 | 8.3 | ALXTY | (Signal too low) |
| 5 | 62 | 6.7 | MKKIS | Urease b subunit (4) |
| 6 | 58 | 6.3 | AKEIK | Urease b-associated chaperonin (5) |
| 7 | 60 | 6.0 | SFRIN | Flagellin b precursor (15) |
| 8 | 58 | 6.0 | AFQVN | Flagellin a precursor (15) |
| 9 (major) | 46 | 8.5 | AYNPK | HP0027 (isocitrate dehydrogenase) (28) |
| 9 (minor) | AVTLI | (Possible contaminant) | ||
| 10 | 45 | 9.1 | XNIQIQNMPK | HP1018 (28) |
| 11 | 58 | 9.1 | MVNKD | Catalase (12) |
| 12 | 65 | 9.4 | EDDGFYTSVG | Omp 5, 8, 9, 19, 27 (28) |
| 13 | 43 | 9.7 | KEVKEKKA | HP1350 (28) |
| 14 | 38 | 8.8 | XDDGGFFTVG | HopC protein (9) |
| 15 (major) | 28.5 | 9.8 | HECNAAFVAI | Novel (possibly blocked) |
| 15 (minor) | GPKHNXEAGD | Novel (possibly blocked) | ||
| 16 | 28 | 9.0 | MKLTP | Urease a subunit (7) |
Comparisons of strains.
While the protein profiles of various strains obtained by using 2D gels with the initial focusing gel from pH 4 to 8 were similar by silver stain analysis of whole-cell lysates, the Western blot profiles showed subtle differences (Table 3). 2D spots from ATCC 43504 which were reactive with the positive serum pool were isolated and sequenced. These spots were compared in eight other strains: Chico, 26695, 96-212, 4655/1, J170, A-1c, Rus-95, and Peru #9. Most notably, the presence of flagellins was not apparent in the 26695 2D Western blot profile. Several of the identified spots from ATCC 43504 were also missing in the A-1c lysate.
TABLE 3.
Comparison of H. pylori strains by 2D PAGE (pH 4 to 8)
| Spot from ATCC 43504 |
H. pylori strain
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chico | 26695 | 96-212 | 4655/1 | J170 | A-1c | Rus-95 | Peru #9 | C-3c | |
| 1 | + | + | + | + | + | + | + | + | + |
| 2 | + | + | + | + | + | + | + | + | + |
| 3 (major) | + | + | + | + | ++ | + | + | + | + |
| 3 (minor) | + | + | + | + | ++ | + | + | + | + |
| 4 | + | + | + | − | + | − | + | + | + |
| 5 | + | +/− | + | + | + | + | + | + | + |
| 6 | + | +/− | + | + | + | + | + | + | + |
| 7 (major) | + | + | + | + | + | − | + | + | + |
| 7 (minor) | + | + | + | + | + | − | + | + | + |
| 8 | + | − | + | + | + | + | + | + | + |
| 9 | + | + | + | − | + | − | − | + | + |
| 10 | + | + | + | + | + | − | +/− | + | + |
| 11 | + | + | + | + | + | + | +/− | + | + |
| 12 | − | + | + | + | ++ | − | + | + | + |
| 13 | + | + | + | + | + | + | + | + | + |
| 14 | + | + | + | + | + | − | + | + | + |
| 15 | + | + | + | + | + | + | + | + | + |
| 16 (major) | + | + | + | + | + | + | + | + | + |
| 16 (minor) | + | + | + | + | + | + | + | + | + |
| 17 | + | + | + | + | + | ++ | + | + | + |
When the isoelectric focusing (IEF) gel was from pH 8 to 13, the most obvious difference in Western blot profiles was in the lack of reactivity of the 26695 strain catalase with the disease-positive serum pool (Table 4). The silver-stained gel also showed a noticeable lack of catalase compared to the silver-stained gels of other strains. It is possible that this gene has been down regulated, or mutated, during laboratory passage, although we have not tested this explicitly. The 26695 strain was evaluated for catalase activity by smearing in 3% H2O2. The 26695 strain showed noticeably less activity than in a control (43504) sample (data not shown). Loss of catalase can be fairly common. Westblom et al. (29) investigated catalase-negative mutants of H. pylori and found that growth characteristics in vitro were unaffected by the mutations, showing that catalase was not essential for growth of H. pylori. It was concluded that catalase-negative mutants of H. pylori occurred spontaneously in vitro but had not yet been observed in vivo. The paucity of such catalase-negative strains in clinical specimens may mean that catalase is a virulence factor in vivo that puts mutants at a selective disadvantage.
TABLE 4.
Comparison of H. pylori strains by 2D PAGE (pH 8 to 13)
| Spot from ATCC 43504 |
H. pylori strain
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chico | 26695 | 96-212 | 4655/1 | J170 | A-1c | Rus-95 | Peru #9 | C-3c | |
| 1 | + | + | + | + | + | + | + | + | + |
| 2 | + | + | + | + | + | + | + | + | + |
| 3 | + | + | + | − | + | − | − | − | + |
| 4 | + | + | + | − | + | + | − | − | + |
| 5 | + | + | + | + | + | + | + | + | + |
| 6 | + | + | + | + | + | + | + | + | + |
| 7 | + | + | + | + | + | + | + | + | + |
| 8 | + | + | + | + | + | + | + | + | + |
| 9 (major) | + | + | + | + | + | − | + | + | + |
| 9 (minor) | + | + | + | + | + | − | + | + | + |
| 10 | + | + | + | + | + | + | + | + | + |
| 11 | + | − | + | + | + | + | + | + | + |
| 12 | + | + | + | + | + | − | + | + | + |
| 13 | + | + | + | + | + | + | + | + | + |
| 14 | + | + | + | + | + | + | + | + | + |
| 15 (major) | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 15 (minor) | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 16 | + | + | + | + | + | + | + | + | + |
The only other observed differences between strains involved spots which present in quantities too small to be sequenced.
DISCUSSION
The demonstration that H. pylori is a major gastroduodenal pathogen and the realization that strains differ in virulence has created a continuing need for new and improved methods of diagnosis and treatment of infection. Five types of test are in general use to detect H. pylori infection: three are invasive, requiring endoscopy (culture, histologic detection, and gastric urease in biopsy [CLO test]), and two are noninvasive (detection of antibodies against H. pylori antigens in sera and detection of CO2 in breath generated from ingested urea by gastric urease). The noninvasive and invasive tests can be of similar accuracy, and noninvasive tests are particularly important for preliminary diagnosis of any possible H. pylori infection and in large-scale population surveys, because they are much less costly and disruptive than invasive tests. Many serologic tests have been developed, most based on pooled H. pylori antigen; their performance varies, however, with the antigens chosen, the population from which reference sera are drawn, and age, ethnicity, and the risk of infection by other organisms with cross-reacting antigens in the population studied. Most standardization of serologic tests has been done with adults in Western (industrialized) countries; for children, in particular, there is still considerable uncertainty concerning standards and cutoff values. H. pylori strains from different geographic areas may differ greatly in genotype; hence, antigen selection is particularly important in comparisons of immigrant and native populations in a single area or of societies in different regions of the world. While several rapid serological tests have been marketed, none are based upon purified recombinant antigens; the present identification of major, highly conserved H. pylori antigens should lead to the development of diagnostic tests that are of much greater sensitivity and specificity than any currently available. A start has been made with Helico Blot 2.0 and also with an assay for detection of CagA (3), which is important because CagA is linked to virulence. However, CagA proteins may differ in strains from different human populations, and so use of this antigen from one strain may well result in underreporting of Cag+ frequencies from other regions of the world. This may explain why Cag+ phenotypes are relatively infrequent in China (24), although direct tests indicate that all H. pylori strains are Cag+.
Our experiments were motivated by the great need for an effective anti-H. pylori vaccine, especially in Third World, high-risk populations where H. pylori eradication by standard antimicrobial therapies is often followed by reinfection. Much attention has been focused on urease-based vaccines because of the essentiality of urease and some encouraging results with mouse Helicobacter felis models. VacA has also been considered a candidate based upon results with a mouse H. pylori model (17, 18); these mouse models, however, may not adequately mimic the human condition. Clinical trials of urease have been only marginally encouraging (17a). This reinforces the sense that other or additional antigens may be needed for a truly effective vaccine.
Our experiments illustrate that 2D gel electrophoresis can give a global view of the abundant proteins of H. pylori. The identification of large numbers of proteins and their characterization with defined serum pools raises the possibility of rapid screening for potential vaccine, as well as diagnostic, candidates. Amino-terminal sequencing and/or proteolytic mass spectral mapping on isolated spots allows for efficient characterization of these potential antigens. Peptidomimetic analysis in parallel with libraries of cloned DNA fragments can provide additional information for the construction of specific vaccine clones or diagnostic recombinant “mosaic” antigens. This is especially important in the case of pathogens whose genomes have not yet been sequenced. One of the many advantages in using “proteome”-type technologies, as here, as opposed to traditional molecular biology (DNA) library approaches, stems from information about likely functionality and utility that comes from initial screening and that is refined as candidate antigens are discovered.
ACKNOWLEDGMENTS
These studies were supported in part by NIH grants AI138166 and DK48029 to D.E.B.
REFERENCES
- 1.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 3.Cutler A F, Havstad S, Ma C K, Blaser M J, Perez-Perez G I, Schubert T T. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136–141. doi: 10.1016/0016-5085(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 3a.DuBois, A., et al. Unpublished data.
- 4.Dunn B E, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 5.Dunn B E, Roop R, Sung C-C, Sharma S, Perez-Perez G I, Blaser M J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eck M, Schmausser B, Haas R, Greiner A, Czub S, Muller-Hermelink H. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 7.Evans D J, Jr, Evans D G, Kirkpatrick S S, Graham D Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 8.Evans D J, Jr, Evans D G, Lampert H C, Nakano H. Identification of four new prokaryotic bacterioferritins, from Helicobacter pylori, Anabaena variabilis, Bacillus subtilis and Treponema pallidum, by analysis of gene sequences. Gene. 1995;153:123–127. doi: 10.1016/0378-1119(94)00774-m. [DOI] [PubMed] [Google Scholar]
- 9.Exner M M, Doig P, Trust T J, Hancock R E. Isolation and characterization of a family of porin proteins from Helicobacter pylori. Infect Immun. 1995;63:1567–1572. doi: 10.1128/iai.63.4.1567-1572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukui Y, Saito I, Shiroki K, Shimojo H, Takebe Y, Kaziro Y. The 19-kDa1 protein encoded by early region 1b of adenovirus type 12 is synthesized efficiently in Escherichia coli only as a fused protein. Gene. 1983;23:1–13. doi: 10.1016/0378-1119(83)90211-1. [DOI] [PubMed] [Google Scholar]
- 11.Graham D Y, Go M F, Evans D J., Jr Urease, gastric ammonium/ammonia, and Helicobacter pylori—the past, the present, and recommendations for future research. Aliment Pharmacol Ther. 1992;6:659–669. doi: 10.1111/j.1365-2036.1992.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 12.Hazell S L, Evans D J, Jr, Graham D Y. Helicobacter pylori catalase. J Gen Microbiol. 1991;137:57–61. doi: 10.1099/00221287-137-1-57. [DOI] [PubMed] [Google Scholar]
- 13.Hughes N J, Chalk P A, Clayton C L, Kelly D J. Identification of carboxylation enzymes and characterization of a novel four-subunit pyruvate:flavodoxin oxidoreductase from Helicobacter pylori. J Bacteriol. 1995;177:3953–3959. doi: 10.1128/jb.177.14.3953-3959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasimoglu E, Park S-J, Malek J, Tseng C P, Gunsalus R P. Transcriptional regulation of the proton-translocating ATPase (atpIBEFHAGDC) operon of Escherichia coli: control by cell growth rate. J Bacteriol. 1996;178:5563–5567. doi: 10.1128/jb.178.19.5563-5567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostrzynska M, Betts J D, Austin J W, Trust T J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173:937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kostrzynska M, O’Toole P W, Taylor D E, Trust T J. Molecular characterization of a conserved 20-kilodalton membrane-associated lipoprotein antigen of Helicobacter pylori. J Bacteriol. 1994;176:5938–5948. doi: 10.1128/jb.176.19.5938-5948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A. Animal models and vaccine development. Bailliere’s Clin Gastroenterol. 1995;9:615–632. doi: 10.1016/0950-3528(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 17a.Lee C. Presented at the 97th General Meeting of the American Society for Microbiology, Miami Beach, Fla., 4 to 8 May 1997. 1997. [Google Scholar]
- 18.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 19.Missiakas D, Schwager F, Betton J M, Georgopoulos C, Raina S. Identification and characterization of HsIV HsIU (ClpQ ClpY) proteins involved in overall proteolysis of misfolded proteins in Escherichia coli. EMBO J. 1996;15:6899–6909. [PMC free article] [PubMed] [Google Scholar]
- 20.O’Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 21.O’Farrell P Z, Goodman H M, O’Farrell P H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 22.O’Toole P W, Logan S M, Kostrzynska M, Wadstrom T, Trust T J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991;173:505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panini E, de Bernard M, Milia E, Bugnoli M, Zerial M, Rappuoli R, Montecucco C. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc Natl Acad Sci USA. 1994;91:9720–9724. doi: 10.1073/pnas.91.21.9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci V, Ciacci C, Zarrilli R, Sommi P, Tummuru M K, Del Vecchio B C, Bruni C B, Cover T L, Blaser M J, Romano M. Effects of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996;64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riegg S J, Dunn B E, Blaser M. Microbiology and pathogenesis of Helicobacter pylori. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 535–550. [Google Scholar]
- 26.Schmitt W, Odenbreit S, Heuermann D, Haas R. Cloning of the Helicobacter pylori recA gene and functional characterization of its product. Mol Gen Genet. 1995;248:563–572. doi: 10.1007/BF02423452. [DOI] [PubMed] [Google Scholar]
- 27.Strolin Q, Benedetti M, Thomassin J, Tocchetti P, Dostert P, Kettler R, Da Prada M. Species differences in changes of heart monoamine oxidase activities with age. J Neural Transm Suppl. 1994;41:83–87. doi: 10.1007/978-3-7091-9324-2_10. [DOI] [PubMed] [Google Scholar]
- 28.Tomb J-F, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 29.Westblom T U, Phadnis S, Langenberg W, Yoneda K, Madan E, Midkiff B R. Catalase negative mutants of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1992;6:522–526. doi: 10.1007/BF01960807. [DOI] [PubMed] [Google Scholar]