Abstract
Since the very beginning of the coronavirus disease 2019 (COVID-19) pandemic in early 2020, it was evident that patients with cardiovascular disease (CVD) were at an increased risk of developing severe illness, and complications spanning cerebrovascular disorders, dysrhythmias, acute coronary syndrome, ischemic and non-ischemic heart disease, pericarditis, myocarditis, heart failure, thromboembolic disease, stroke, and death. Underlying these was excessive systemic inflammation and coagulopathy due to SARS-COV-2 infection, the effects of which also continued long-term as evidenced by post-COVID-19 cardiovascular complications.
The acute and chronic cardiovascular effects of COVID-19 occurred even among those who were not hospitalized and had no previous CVD or those with mild symptoms.
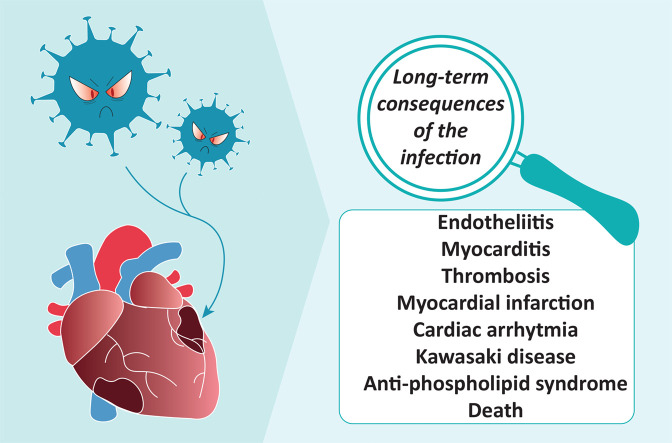
This comprehensive review summarizes the current understanding of molecular mechanisms triggered by the SARS-CoV-2 virus on various cells that express the angiotensin-converting enzyme 2, leading to endothelial dysfunction, inflammation, myocarditis, impaired coagulation, myocardial infarction, arrhythmia and a multisystem inflammatory syndrome in children or Kawasaki-like disease.
Keywords: COVID-19, Endothelial dysfunction, Inflammation, Oxidative stress, Thrombosis, Myocardial injury
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a global emergency on March 11, 2020, by the World Health Organization [1]. As of August 11th 2022 there have been 584,065,952 confirmed cases of COVID-19, including 6,418,958 deaths, reported to the WHO (Ref: https://covid19.who.int/) [2]. While a spectrum of clinical manifestations occur, and the majority of individuals with COVID-19 have mild to moderate symptoms, a small proportion develop severe forms of COVID-19, often accompanied by respiratory and multi-organ failure requiring intensive care [3]. This has held true even with the selection and increased prevalence of new strains, such as the Omicron (B.1.1.529), where the lower pathogenicity is counterbalanced with much higher infectivity [4].
At the beginning of the pandemic, epidemiologic studies showed that individuals of advanced age, male gender, and with preexisting illnesses such as cancer or cardiovascular diseases (CVD), metabolic disorders, diabetes, and obesity, were at higher risk of developing complications and severe forms of COVID-19, and were associated with unfavorable outcomes [5]. CVD is one of the leading causes of mortality in the world, whose prevalence is growing due to the aging population and the increase in age-related diseases such as diabetes mellitus, obesity, dyslipidemia, and hypertension, which are risk factors for heart disease [6]. In addition, poor life choices such as inadequate diet and sedentary lifestyle coupled with stress contribute to an even greater number of CVD cases [6]. The reason why patients with CVD are generally prone to develop severe forms of infective disorders lies in the already present impaired endothelial function and activity, deteriorated immune response and platelet hyperreactivity [7], [8]. Therefore, patients with CVD are already predisposed to develop systemic inflammation, impaired coagulation and thrombotic complications after SARS-CoV-2 infection [7], [8]. This finding is of utmost value, pointing out that clinicians should pay special attention to individuals with CVD since they represent the group of high-risk patients. Even more intriguing are the findings of recent studies that have shown that both asymptomatic and symptomatic SARS-CoV-2 infections have a negative impact on cardiovascular health during one-year follow-up and not only among hospitalized individuals with severe clinical picture. In the following text we will discuss these two interesting studies in more details [9], [10].
In this review, we summarise the latest knowledge regarding the pathophysiological effects of the SARS-CoV-2 virus on blood vessels and the heart, the processes and sequence of events that lead to cardiovascular complications during the acute phase of the COVID-19 and the long-lasting harmful effects and consequences long after recovery from SARS-CoV-2 infection.
2. How does SARS-CoV-2 infect cells and cause renin-angiotensin system imbalance?
The interaction between the SARS-CoV-2 Spike (S) protein with a host cell via the angiotensin-converting enzyme 2 (ACE2) is critical for the internalization of the viral particles. Briefly, the S protein is a trimer composed of 3 receptor binding subunits (S1) and 3 fusogenic stalks (S2). The interaction between S and ACE2 occurs at the receptor-binding domain of the S1 subunit. Proteolytical cleavage of the S protein by furin, TMPRSS2 and other proteases modify the ability of the virus to interact with the target cells and fuse with them [11], [12]. However, mutations of the gene coding for the S protein have modulated infectivity, mode of entrance and viral tropism [4]. In line, Omicron displays an increased affinity for ACE2 receptor and reduced TMPRSS2 dependence for cell entry [13]. Indeed, mutations of the S protein decreased fusion-dependent entry (increasing the endocytic pathway), increased immune evasion, and modified the cell tropism. Indeed, in vitro Omicron replicates faster in bronchial cells, while Delta in cells of the lower respiratory tract. This is paralleled by a decreased ability of the Omicron to alter the lung function of animal models [4].
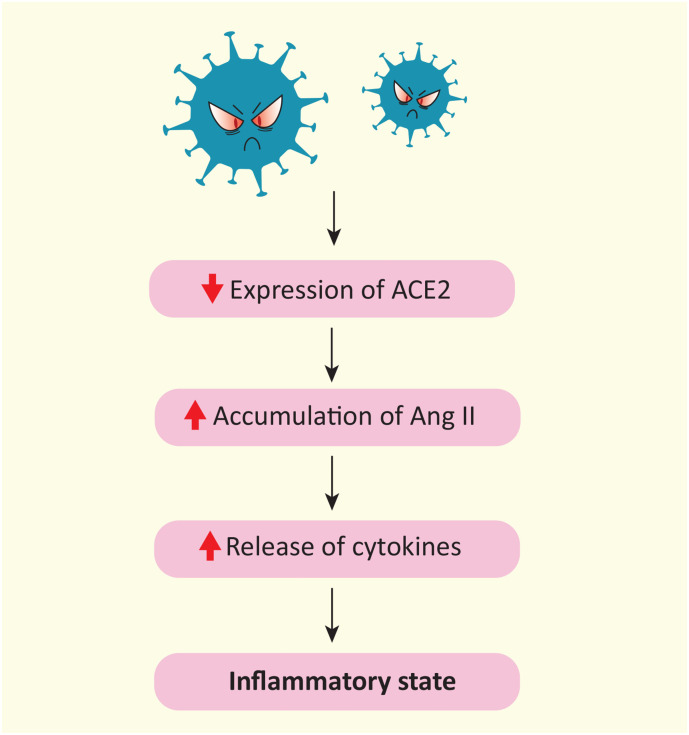
The SARS-CoV-2 S protein is responsible for reducing ACE2 expression on target cells, promoting the degradation of its mRNA [14]. ACE2 is a component of the renin-angiotensin system (RAS). In particular, ACE2 cuts angiotensin II (Ang II) generating the peptide Ang (1–7), which has antioxidant, antithrombotic, and anti-inflammatory properties after binding to the membrane G-protein-coupled Mas receptor [5]. In addition to producing the active peptide Ang (1–7), ACE2 helps control the concentration of Ang II, which is a potent vasoconstrictor and coagulation regulator. The accumulation of Ang II is responsible for sustaining the inflammatory state through the release of cytokines (Fig. 1 ). However, while some contradictory findings are reported in the literature on Ang II levels in COVID-19 patients, either increased Ang II or lower Ang (1–7) levels have been associated with a more severe outcome [15], [16]. Functionally, Ang II has been shown to promote the death of pulmonary artery endothelial cells in vitro, while in pigs it increases pulmonary pressure, increases coagulability, reduces blood oxygenation and pulmonary perfusion, mimicking several aspects of COVID-19 [17]. Therefore, through the misbalance of RAS, SARS-CoV-2 increases the risk of cardiovascular complications by interfering with hemodynamic regulation and promoting both oxidative stress and an inflammatory state [18].
Fig. 1.
Schematic representation of the effects of SARS-CoV-2 on the renin-angiotensin system. SARS-CoV-2 reduces the expression of ACE2 and as a consequence leads to the accumulation of Ang II. Ang II promotes an inflammatory state through the release of cytokines. ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II.
3. Molecular mechanisms of SARS-CoV-2-induced cardiac microvascular damage
The SARS-CoV-2 virus spreads through droplets usually exhaled by an infected individual and the respiratory system is the first site of interaction with the host. The bronchial epithelium and the lungs are the most susceptible tissues due to their high levels of ACE2 expression [4]. Lung infection causes alveolar damage and an increase in microvessels permeability, which could potentially contribute to the presence of SARS-CoV-2 in the bloodstream. Since the heart receives blood from the lungs via the pulmonary circulation, it is likely a primary target. Several molecular mechanisms of tissue damage, endotheliitis, and thrombosis, have been proposed.
3.1. SARS-CoV-2 triggers endotheliitis and thrombosis
There is evidence that SARS-CoV-2 triggers the inflammation of the endothelium also known by the term “endotheliitis”. However, ACE2 is not expressed uniformly on endothelial cells (ECs). Its expression is higher in small epicardial cells and capillaries and decreases, or is null, in major coronary arteries [19]. This heterogeneity could indicate a different susceptibility of ECs across the coronary tree to SARS-CoV-2 infection, shedding new light on how small vessel dysfunction is central to the pathogenesis of the virus. Intriguingly, heart failure (HF) was shown to increase the expression of ACE2 both in cardiomyocytes and fibroblast, exerting a neutral effect on ECs [20]. However, the expression of TMPRSS2 (that conditioned SARS-CoV-2 viral entry until the emergence of the Omicron strain) is more heterogeneous in heart cells, and some authors failed to identify the protein in human hearts [20].
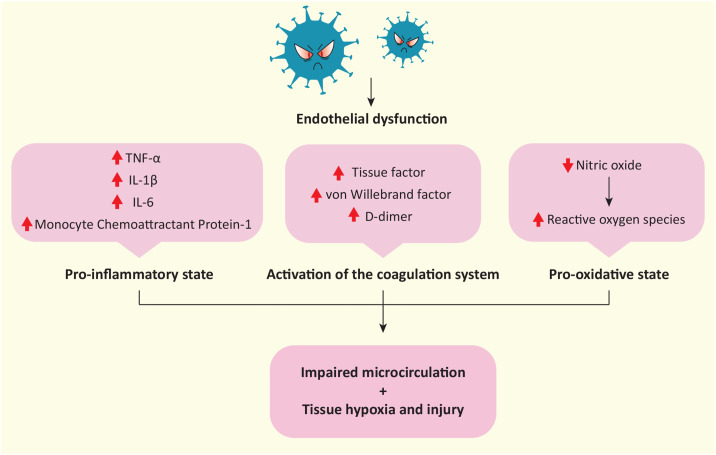
Consistently, perivascular cells (Pc) that embrace capillary ECs, regulating vascular permeability and stabilizing vessels, express the highest levels of ACE2, proved by in vivo studies [21], [22], [23]. Nonetheless, although in vitro data did not support the infectivity of cardiac Pc either [24], the presence of viral dsRNA was shown in brain Pc of patients that died of COVID-19 [25]. Therefore, pathogenetic mechanisms could be attributable to direct endothelial damage by SARS-CoV-2, although there are indications of an indirect inflammation-mediated mechanism, which is likely multifactorial [19], [26]. Indeed, the S protein can directly damage both ECs and Pc, activating pro-inflammatory, matrix-degrading pathways, increasing Pc motility and altering the cell permeability [21], [24] via alternate receptors, such as CD147. More precisely, although regarded as controversial findings, S protein by engaging CD147 triggers extracellular signal-regulated kinase 1/2 signaling, and promotes the release of proinflammatory cytokines interleukin (IL)-1β, tumor necrosis factor α (TNF-α), IL-6, and Monocyte Chemoattractant Protein-1 [24]. After SARS-CoV-2 infection, the decrease in ACE2 concentration contributes to an increase in Ang II whose consequences are not limited to the impairment of vasocontractility and permeability but include the reduction of nitric oxide (NO), fundamental for ECs functionality, and the increase in reactive oxygen species (ROS) [27], [28], which all together contribute to endothelial dysfunction [29]. Therefore, endotheliitis alters the microcirculation, resulting in tissue hypoxia and injury (Fig. 2 ) [30]. These ischemic events could result in the necrosis of cardiomyocytes, a hypothesis consistent with the frequent elevation of cardiac troponins in COVID-19 patients, possibly worsening HF [31], [32], which explains why patients with both CVD and SARS-CoV-2 infection are more likely to develop the severe form of COVID-19.
Fig. 2.
Schematic representation of SARS-CoV-2-mediated endothelial dysfunction. An increase in cytokine production, coagulation factors, and a decrease in nitric oxide lead to altered microcirculation, tissue hypoxia, and injury.
Furthermore, cytokine release and endothelial dysfunction turn ECs into an activated phenotype that is often coupled with a pro-coagulant state [33]. Activation of ECs also include the expression of tissue factor, which contributes to the activation of the coagulation system, and the release of the von Willebrand factor for platelets binding. In patients with COVID-19, elevated levels of fibrinogen, von Willebrand factor, and D-dimer, indicate a hypercoagulative state, positively correlated with the severity of the disease [18], [34]. Recent findings suggest that, in thrombi, the association between platelets and neutrophils stimulates the latter to form extracellular traps (NETs), which increase the tendency for platelet aggregation and negatively affect the prognosis of patients after SARS-CoV-2 infection [33], [35], [36]. At autopsy, the heart tissue of individuals who had COVID-19 exhibited vascular inflammation and extensive thrombosis both in the microcirculation and in the coronary arteries [26], [37].
Although severe inflammation is one of the known mechanisms of endothelial activation [35], Johnson et al. have recently found no evidence of cytokine-mediated activation, nor the expression of adhesion proteins such as intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) in heart tissue [38]. Furthermore, in an in vitro study, exposure of ECs culture from the coronary artery to the viral nucleocapsid protein stimulates the expression of ICAM-1 and VCAM-1 [39]. Therefore, further investigation is required to clarify this aspect.
4. Pathogenic effects of SARS-CoV-2 infection on the heart
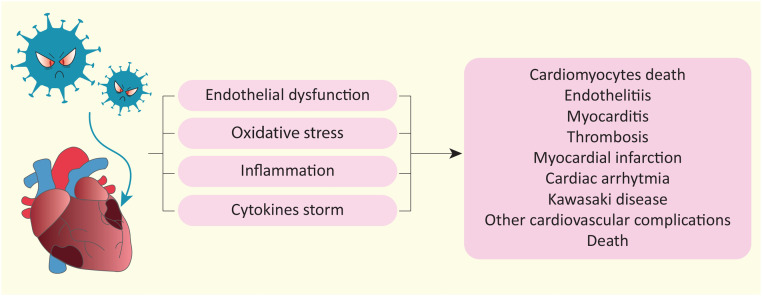
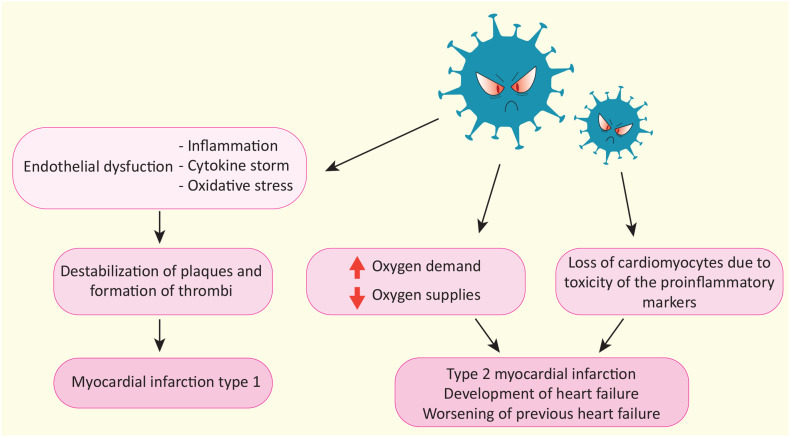
SARS-CoV-2 infection is associated with an increased risk of myocardial injury that can lead to cardiac complications such as HF, arrhythmia, cardiocirculatory arrest and death (Fig. 3 ).
Fig. 3.
Cardiovascular complications due to SARS-CoV-2 infection. Schematic representation of molecular mechanisms induced by SARS-CoV-2 and the subsequent cardiac complications whose incidence is increased in the population after infection.
4.1. SARS-CoV-2 and myocarditis followed by myocardial injury and arrhythmia
An increase in arrhythmic complications has been described in patients after recovery from acute COVID-19, and their long-term frequency is still under study [40]. In particular, based on current data, atrial arrhythmias and bradyarrhythmia are the most common in the acute setting of COVID-19, with an incidence of 13 % and 12.8 % respectively. Furthermore, the atrioventricular block has been observed with an incidence of 8.6 %, while ventricular arrhythmia was reported in 5.9 % of cases, being the less predominant form [41]. Since arrhythmic events have been reported in patients with no previous clinical history [41], increased arrhythmic complications should be sought in sequelae of SARS-CoV-2 on the heart. Several mechanisms have now been proposed and myocarditis is plausible [42], [43]. Although there is a desperate need to understand the pathological mechanisms triggered by the virus, the investigation is challenging due to pauci-symptomatic or asymptomatic cases, which leads to underestimating the real incidence of myocarditis [43]. Whilst the literature in this field has been conflicting at times, one of most comprehensive analysis to date has estimated a prevalence for definite/probable acute myocarditis (AM) of 2.4 per 1000 hospitalizations [44] that increases to 4.1 considering also possible AM cases.
Cardiac magnetic resonance (CMR) imaging is a potent tool for the detection of myocarditis as it allows the differentiation between ischemic and non-ischemic injury, while histological examination provides more detailed information. Additional data come from a recent prospective study by Luetkens et al. which evaluated CMR features of patients with suspected acute COVID-19 myocarditis. Notably, this study demonstrated the presence of diffuse myocardial edema associated with a lower burden of late gadolinium enhancement lesions in patients with suspected SARS-CoV-2-induced myocarditis when compared to patients with acute myocarditis caused by common cardiotropic viruses [45].
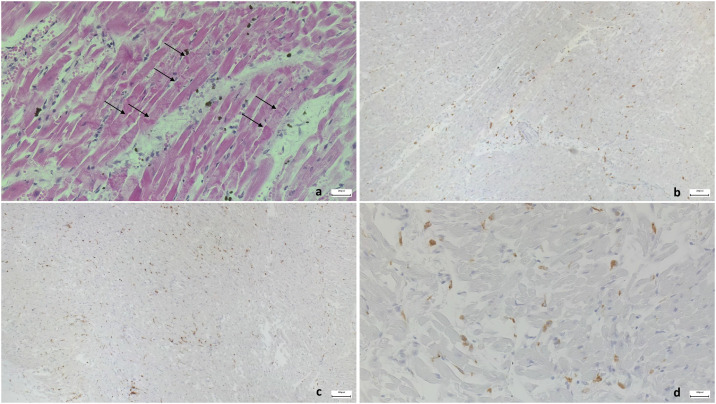
Bräninger et al. have found that active SARS-CoV-2 replication is localized mainly in ECs, while its localization in cardiomyocytes is sporadic [46]. These results confirm that ECs are at the forefront in the response to the infection. Furthermore, although Bräninger et al. found no increase in cell infiltrates, this feature has been identified in numerous previous published papers [26], [46]. Cell infiltrates, including macrophages CD68+ and lymphocytes T CD8+, are often associated with vessels and cardiomyocyte necrosis [47] (Fig. 4 ).
Fig. 4.
Histopathological representation of infiltrated immune response cells. (a) Early contraction band necrosis. Hypercontraction of the myofibers with myofibrillar break and formation of cross bands (H&E 40×); (b) infiltrates of CD45+ cells (10×); (c) infiltrates of CD68+ macrophages (10×); (d) infiltrates with CD4+ T cells (40×).
Overall, the aforementioned characteristics lead to myocardial damage, thus inducing remodeling and fibrosis that contribute to conduction system dysfunction as emerged at echocardiogram evaluation [43], [48]. Furthermore, nerve inflammation, or inflammatory neuropathy, is involved in the degeneration of ganglia and neuronal fibers increasing the incidence of arrhythmic complications [47], [49]. Finally, as for vessels and cardiomyocytes, inflammatory cell infiltration is reported as a cause of conduction tissue necrosis in the His-Purkinje system [47].
4.2. Myocardial injury due to SARS-CoV-2 infection
The prevalence of myocardial injury among patients hospitalized for the severe form of COVID-19 is approximately 13–47 % characterized by the elevated concentration of cardiac troponin [50]. As above illustrated, the exact mechanisms leading to myocardial damage associated with SARS-CoV-2 infection are complex and not fully understood. The most likely explanation proposed is that multiple deterioration processes are involved in myocardial injury. One of the proposed hypotheses is the previously explained endothelial dysfunction, that activate serial processes resulting in impaired coagulation, thrombosis and destabilization of atherosclerotic plaques [51]. Inflammatory molecules and components of oxidative stress activate inflammatory cells in plaques such as macrophages that produce metalloproteinases and peptidases that break down components of the extracellular matrix, hamper the integrity of the surface of the plaques and further deepen the pro-inflammatory state and increased production of molecules of oxidative stress. The latter events lead to the formation of thrombus and in some cases the myocardial infarction type 1 [52]. Therefore, this in one of the provided explanations of the complications that can occur in patient with coronary artery disease during acute phase of SARS-CoV-2 infection.
There is also a hypothesis that point out the direct influence of virus on cardiomyocytes. A study by Negron et al. demonstrated that S protein, more specifically the S1 subunit, causes the heart damage by triggering the cardiomyocyte innate immune machinery interacting with Toll-like receptor 4 (TLR4), thus activating nuclear factor kappa B and represents TLR-4 alarmin [53]. In the same study, the researchers cloned the S1 subunit into the vector and introduced it to laboratory mice in order to activate the S1 subunit in cardiomyocytes. The results showed that S1 subunits were able to induce hypertrophic remodeling, cardiac dysfunction and inflammation, indicating that S1 is toxic to cardiac cells. [53]. Thus, these findings demonstrated that in addition to being directly damaging to the cardiomyocyte, S protein is also highly inflammatory and indirectly causes heart damage. Another study that pointed out the myocardial involvement in COVID-19 by Duerr et al., demonstrated that pericardial effusion and higher CD8/Treg/monocyte ratio predicted severe forms of disease and adverse outcomes [54].
Last, we should mention that induced pluripotent stem cells-derived cardiomyocytes can be productively infected by SARS-CoV-2 in vitro. Cardiomyocyte infection in vitro results in the perturbation of their transcriptome, myofibrillar fragmentation and nuclear disruption [55]. In this context, changes in the transcription pattern refers to the enhance in the inflammatory response via interferon I and significant down-regulation of genes encoding the structural protein in cardiomyocytes, which compromises cardiomyocytes' functionality and integrity [46]. Intriguingly, evidence of cardiomyocyte infection has also been obtained in vivo in hamsters. In that work, Yang et al. showed that infected myocytes were able to secrete the monocyte chemoattractant protein CCL2, recruiting these cells to the infected hearts, where they could limit myocyte infection [56]. On other side, a recent study by Filman et al. which used plasma proteomic analysis compering tissue-specific and cell-type-specific death signatures and ACE2 expression between healthy and COVID-19-infected individuals, pointed out that heart damage might be an indirect consequence of the COVID-19 [57].
Furthermore, the imbalance between increased myocardial oxygen demand and reduced oxygen supply during acute infection can cause myocardial hypoxia and ischemia followed by cardiomyocytes death, and in some cases result in myocardial infarction type 2 or worsening of cardiovascular diseases such as coronary artery disease, and congestive HF [58]. In addition, it is important to acknowledge that increased levels of cytokines due to inflammation are toxic to the heart and can lead as well to cardiomyocytes death [58], as well as endothelial dysfunction of microcirculation can cause cardiac tissue hypoxia and injury (Fig. 5 ).
Fig. 5.
Myocardial infarction as a consequence of SARS-CoV-2 infection. Schematic representation of how SARS-CoV-2 by multiple mechanisms causes myocardial infarction.
4.3. Cardiocirculatory arrest in SARS-CoV-2 infection
Numerous reports from all over the world have documented an excess of out-of-hospital cardiac arrests (OHCA) during the COVID-19 pandemic [59], [60], [61]. This evidence is supported by solid pathophysiological bases: SARS-CoV-2-related myocardial injury can result in ventricular dysfunction and fibrosis, thus promoting new-onset re-entrant arrhythmias, as well as severe hypoxia due to acute respiratory failure and pulmonary embolism, which increase the risk of cardiac arrest [62]. Furthermore, the occurrence of a COVID-19 cytokine release can evoke an unchecked immune response and sub-sequent multiorgan damage that could lead to refractory shock and cardiac arrest [63]. Finally, in a cohort study of 9 autopsy cases of individuals with COVID-19, microscopic examination demonstrated inflammatory infiltrates in subepicardial ganglia in all three patients deceased for ventricular tachyarrhythmias, therefore suggesting neuronal cell death as additional trigger of arrhythmic instability [47].
Aside from the direct impact of virus infection, the increased incidence of cardiac arrests during the pandemic outbreak may be attributed to the strong decrement in hospital admissions for acute cardiovascular syndromes and critical issues in the healthcare delivery system [64]. Furthermore, multiple studies have recognized the prognostic power of SARS-CoV-2 infection in the specific setting of out-of-hospital cardiac arrest. Of note, a recent meta-analysis involving more than 7000 OHCA individuals showed lower rates of shockable rhythms and restoration of spontaneous circulation among patients suffering from SARS-CoV-2 infection in comparison with uninfected patients [65].
4.4. SARS-CoV-2 as a systemic disease: the example of Kawasaki disease and Kawasaki-like illness
COVID-19 is a systemic disease and there is growing evidence about post-viral immunological reactions, especially among pediatric population. During the first phases of COVID-19 pandemic, children seemed to be less susceptible and to contract milder forms of infection. Later, a growing number of children developed inflammatory systemic symptoms that appeared weeks after the initial infection of COVID-19. This clinical entity, defined as multisystem inflammatory syndrome in children (MIS-C) or Kawasaki-like disease, includes prolonged fever, gastrointestinal symptoms, cutaneous signs, neurological alterations and cardiovascular manifestations such as myocarditis with ventricular dysfunction, coronary artery aneurysms, arrhythmias [66], [67]. MIS-C is burdened by high possibility of morbidity, complications, and adverse outcomes [67], but its incidence is still not well defined [68], [69].
MIS-C shares many characteristics of Kawasaki disease (KD), which is the most common form of systemic vasculitis of medium and small arteries among children, and the main cardiac manifestations are coronary aneurysm or dilatation and secondary myocardial dysfunction. The certain etiology of KD has not been determined yet, but viruses have been suspected to be a causal factor due to the seasonal epidemic trend of the disease [70]. Coronavirus family has been proposed as possible triggers and some studies reported an increase in KD cases during COVID-19 pandemic [71], [72], [73].
Several differences between KD and MIS-C have been observed. For example, MIS-C affects a broad age range from early childhood to late adolescence, determines thrombocytopenia and capillary leak syndrome and evolves more frequently to cardiogenic shock [74], [75]. MIS-C pathophysiology is still not well defined, although the most accredited hypothesis is that there is an abnormal immune response to the virus, with some similarities to KD and other hyperinflammatory syndromes. Some authors reported that anti-spike antibodies may stimulate host cytokine release and inflammatory response through immune cellular stimulation or the formation and deposition of immune complexes into vascular walls [76], [77]. This kind of pathophysiological mechanism and inflammatory activation are similar to the one observed in KD and might cause coronary aneurism [78], [79]. For this reason, differentiating KD from MIS-C remains challenging in many cases. The hypothesis that MIS-C is linked to a post-infective immune dysregulation, rather than to the infection itself, is supported by the presence of positive serology with negative polymerase chain reaction for COVID-19 reported in many affected children [80]. Moreover, many authors reported an increase of MIS-C in many countries several weeks after the peak of COVID-19 cases [81], [82]. The timing of these events represents a further element suggesting a post-viral immune response.
Treatment for these hyperinflammatory responses is mostly supportive [81]. Further studies are needed in order to identify the best therapeutic strategy and to explore the pathophysiology and mechanisms for the systemic immune response caused by COVID-19.
4.5. SARS-CoV-2 and antiphospholipid syndrome
Recent evidence suggests a similarity between COVID-19 and acquired antiphospholipid syndrome (APS), due to increased risk of thromboembolic events in both. APS is an immune disease already known to be associated with infective disorders and characterized by the presence of antiphospholipids antibodies (aPLs) including lupus anticoagulant [83]. A recent study has reported the detection of lupus anticoagulants in COVID-19 patients with prolonged activated partial thromboplastin time, thus suggesting that APS could be a contributor to thrombogenesis in COVID-19 patients [84]. In the context of infective diseases, acquired thrombogenesis is explained by mechanisms of molecular mimicry and endothelin dysfunction. The SARS-CoV-2 infection could trigger the production of aPLs with two mechanisms. The first mechanism is molecular mimicry with S protein subunits acting as a phospholipid-like epitope and therefore inducing the production of aPLs. The second mechanism consists in the formation of a neoepitope due to a change in the conformation of host cells β2-glycoprotein I as a consequence of the oxidative stress related to COVID-19 [83]. However, the presence of aPLs alone is not enough to determine thrombosis and additional factors are needed. In the context of COVID-19, the activation of aPLs with consequent thrombogenesis may be linked either to endothelial injuries or the imbalance between oxidative stress and protective antioxidant pathways [83]. The fact that the presence of aPLs is not per se thrombogenic could explain why some studies have found no correlation between aPLs presence and thrombotic events which also needs the presence of systemic inflammation [85].
The finding of aPLs including lupus anticoagulants could help identify patients at higher risk for thrombosis and they should be routinely checked in COVID-19 patients [86]. Taking into consideration COVID-19 treatment strategies, anticoagulation and corticosteroids have proved to have good results. Furthermore, it needs to be clarified whether plasmapheresis may play a role in patients with high aPL antibodies titers [87].
4.6. Persistent post-COVID-19 asthenia
Fatigue is one of the most common symptoms following acute COVID-19 [88] and it could be related to endocrine system involvement. In particular, although current data have shown adequate stress response with no evidence of adrenal insufficiency in acute COVID-19, the possibility of adrenal involvement at follow-up cannot be completely ruled out [89], [90]. Indeed, persistent asthenia could be compared to that experienced by patients with primary and secondary adrenal insufficiency. The results from a recent study indicate no changes in cortisol levels in patients with persistent fatigue after three months from the infection [88]. Therefore, the reason for post-COVID-19 asthenia should be traced to other determinants such as the consequences of inflammation, poor nutritional status, respiratory complications and cardiovascular damage [91]. Despite what has been described so far, COVID-19 could contribute to the progression of adrenal insufficiency as reported in the case of Addison's disease, a primary endocrine disorder caused by the insufficient production of adrenocortical hormones [92]. Therefore, SARS-CoV-2 could be a trigger for this autoimmune disease, as in the case of other immune disorders such as antiphospholipid disease. Conversely, patients with Addison's disease are not more susceptible to the infection [93].
5. Development of cardiovascular complications in post-COVID-19 period
Given that the pandemic has been going on for over 32 months, it is possible to assess the post-acute effects of COVID-19 in mid- and long-term follow-up and evaluate the lasting deteriorating effect of SARS-CoV-2 infection on cardiovascular health.
After the acute phase of SARS-CoV-2 infection, a relevant population prevalence of persistent cardiorespiratory symptoms, such as palpitations, non-specific chest pain, breathlessness, exercise intolerance, and orthostatic hypotension it have been demonstrated [94]. These heterogeneous clinical manifestations, currently summarized with the acronym “PASC” (post-acute sequelae of SARS-CoV-2 infection), are attributed to various possible mechanisms, including persistent inflammation and catabolic state, reactivation of latent virus with pulmonary fibrotic changes, RAS dysregulation, and deep cardiovascular deconditioning [62].
Several studies have shown an increased risk of cardiovascular complications during the post-hospital period due to COVID-19 [9], [95], [96]. In comparison with the general population not exposed to SARS-CoV-2 infection, patients that were hospitalized with COVID-19 between January 1st to August 31st 2020 displayed, months after admission, higher rates of diabetes, respiratory disease, cardiovascular disease, chronic kidney and liver disease. Interestingly, this increase in risk is not uniquely affecting the oldest patients [95]. Negreira-Caamano et al. in their study showed that one-third of patients hospitalized between March 10th and May 4th 2020, for COVID-19 suffered from various cardiovascular events such as acute coronary syndrome, cerebrovascular event, thrombosis, hospitalization for HF and death during the first 30 days after the discharge, while one patient in sixteen during the first year after hospitalization [96].
A large study by Xie et al. that enrolled more than eleven million subjects yield fascinating results taking into consideration all patients presenting (between March 1st 2020 and January 15th 2021) with COVID-19, not just hospitalized patients with critical clinical pictures [9]. Specifically, the risk of developing CVD following the SARS-CoV-2 infection was assessed by comparing the clinical parameters of individuals diagnosed with COVID-19 and parameters of two sets of controls, individuals without COVID-19 enrolled in the study contemporarily with the previous cohort and during 2017, respectively [9]. The study pointed out that individuals after SARS-CoV-2 infection have an increased risk of developing cardiovascular disorders such as dysrhythmias, pericarditis and myocarditis, angina, acute coronary disease, myocardial infraction, ischemic and non-ischemic cardiomyopathy, thromboembolic disorders, HF and cardiac arrest in comparison with healthy controls [9]. Remarkably, an increased risk of developing CVD has been observed even in persons without previous diseases, which indicates a negative impact of SARS-CoV-2 on patients at low risk of cardiovascular disorders [9]. Therefore, an increased risk was observed among patients who were not hospitalized during acute infection and, as expected, the risk increased proportionally from non-hospitalized, hospitalized to intensive care patients in line with the severity of COVID-19 [9]. Another recent prospective study, including only patients with mild COVID-19 symptoms and without previous heart conditions yield similar results. After serial cardiovascular measuring, the data after almost a year of follow-up pointed out that 57 % of individuals have persistent cardiac symptoms suggestive for post-COVID-19 inflammatory cardiac involvement [97]. Furthermore, study by Tereshchenko et al. demonstrated an increased risk of CVD events and poor outcomes in both asymptomatic and symptomatic patients at least 30 days after the SARS-CoV-2 infection [10], emphasizing the importance of taking into account the presence of infection as a risk factor for CVD development in the future assessment of the patient's physical health.
It is important to point out that these studies were conducted between January 1st 2020 and January 15th 2021, when the most prevalent SARS-CoV-2 strains were the Wuhan Hu-1, followed by the Alpha (B.1.1.7, first identified in the UK in late December 2020) and Delta (B.1.617.2, first reported in India in December 2020) Variants of Concern (VOC) [98]. This fact should be emphasized since both transmissibility and pathogenicity greatly vary among the different strains. Indeed, while Delta showed increased pathogenicity with respect to the Alpha VOC, both in experimental animal models and in humans, Omicron (B.1.1.529) VOC showed an opposite behavior [98], [99], [100]. Last, the vaccination campaign, which started at the end of 2020, has modified the natural history of COVID-19, reducing the incidence of severe clinical manifestations [101], [102].
6. Conclusions
Accumulating evidence after more than two years of the pandemic indicates that COVID-19 is not just the flu as some individuals initially claimed. Recent studies demonstrated that all patients with COVID-19 and not only those hospitalized due to the severe form of the disease, weeks and months after admission displayed higher rates of respiratory disease, cardiovascular disease, chronic kidney and liver disease in comparison with the general population not exposed to SARS-CoV-2 infection. What is even more fascinating is the fact that an increased rate of cardiovascular events was noted even among healthy individuals without previous history of CVD, with a low risk of cardiac diseases. Of course, as expected, patients with CVD had a higher risk of complications and mortality due to the already present impaired endothelial function, altered immune response and platelet hyperreactivity. In addition, studies demonstrated that even children, who were thought not to be susceptible to the SARS-CoV-2 virus, in some cases developed a multisystem inflammatory syndrome or Kawasaki-like disease, weeks after the initial infection of SARS-CoV-2. Therefore, this novel data definitely proved that SARS-CoV-2 infection orchestrates even after a long-term period multiple mechanisms that lead to persistent inflammation, oxidative stress, endothelial dysfunction, tissue and heart damage. For these reasons, it has been already suggested to consider the presence of the previous SARS-CoV-2 infection as a high-risk factor for cardiovascular outcomes.
CRediT authorship contribution statement
Aneta Aleksova and Milijana Janjusevic contributed to conception of the manuscript.
Aneta Aleksova, Alessandra Lucia Fluca, Giulia Gagno, Alessandro Pierri, Laura Padoan, Agnese Derin, Rita Moretti, Elena Aleksova Noveska, Eros Azzalini, Stefano D’Errico and Milijana Janjusevic wrote and prepared the original draft.
Aneta Aleksova, Alessandra Lucia Fluca, Antonio Paolo Beltrami, Alimuddin Zumla, Giuseppe Ippolito, Gianfranco Sinagra and Milijana Janjusevic reviewed and edited the manuscript.
Alessandra Lucia Fluca, Stefano D’Errico and Milijana Janjusevic created the images.
All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Regione Friuli Venezia Giulia (grant for the project “Lo scompenso cardiaco quale morbo di Alzheimer del cuore: opportunità diagnostiche e terapeutiche—HEARTzheimer”).
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Declaration of competing interest
The authors declare no conflict of interest.
Data availability
Not applicable.
References
- 1.Organization W.H. 2020. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Geneva, Switzerland. [Google Scholar]
- 2.Organization, W.H. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int. Accessed 19 Sep 2022.
- 3.Dries D.J. Coronavirus disease 2019: from intensive care unit to the long haul-part 2. Air Med. J. 2021;40(5):298–302. doi: 10.1016/j.amj.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balint G., Voros-Horvath B., Szechenyi A. Omicron: increased transmissibility and decreased pathogenicity. Signal Transduct. Target Ther. 2022;7(1):151. doi: 10.1038/s41392-022-01009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleksova A., Gagno G., Sinagra G., Beltrami A.P., Janjusevic M., Ippolito G., Zumla A., Fluca A.L., Ferro F. Effects of SARS-CoV-2 on cardiovascular system: the dual role of angiotensin-converting enzyme 2 (ACE2) as the virus receptor and homeostasis regulator-review. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22094526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aleksova A., Janjusevic M., Gagno G., Pierri A., Padoan L., Fluca A.L., Carriere C., Beltrami A.P., Sinagra G. The role of exercise-induced molecular processes and vitamin D in improving cardiorespiratory fitness and cardiac rehabilitation in patients with heart failure. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.794641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu S.X., Tyagi T., Jain K., Gu V.W., Lee S.H., Hwa J.M., Kwan J.M., Krause D.S., Lee A.I., Halene S., Martin K.A., Chun H.J., Hwa J. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021;18(3):194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacca M., Shah A.M. The pathological maelstrom of COVID-19 and cardiovascular disease. Nat. Cardiovasc. Res. 2022:1–11. doi: 10.1038/s44161-022-00029-5. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tereshchenko L.G., Bishop A., Fisher-Campbell N., Levene J., Morris C.C., Patel H., Beeson E., Blank J.A., Bradner J.N., Coblens M., Corpron J.W., Davison J.M., Denny K., Earp M.S., Florea S., Freeman H., Fuson O., Guillot F.H., Haq K.T., Kim M., Kolseth C., Krol O., Lin L., Litwin L., Malik A., Mitchell E., Mohapatra A., Mullen C., Nix C.D., Oyeyemi A., Rutlen C., Tam A.E., Van Buren I., Wallace J., Khan A. Risk of cardiovascular events after COVID-19. Am. J. Cardiol. 2022;179:102–109. doi: 10.1016/j.amjcard.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustafa Z., Zhanapiya A., Kalbacher H., Burster T. Neutrophil elastase and proteinase 3 cleavage sites are adjacent to the polybasic sequence within the proteolytic sensitive activation loop of the SARS-CoV-2 spike protein. ACS Omega. 2021;6(10):7181–7185. doi: 10.1021/acsomega.1c00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng B., Abdullahi A., Ferreira I., Goonawardane N., Saito A., Kimura I., Yamasoba D., Gerber P.P., Fatihi S., Rathore S., Zepeda S.K., Papa G., Kemp S.A., Ikeda T., Toyoda M., Tan T.S., Kuramochi J., Mitsunaga S., Ueno T., Shirakawa K., Takaori-Kondo A., Brevini T., Mallery D.L., Charles O.J., Collaboration C.-N.B.C., Genotype to Phenotype Japan C. Ecuador C.C., Bowen J.E., Joshi A., Walls A.C., Jackson L., Martin D., Smith K.G.C., Bradley J., Briggs J.A.G., Choi J., Madissoon E., Meyer K.B., Mlcochova P., Ceron-Gutierrez L., Doffinger R., Teichmann S.A., Fisher A.J., Pizzuto M.S., de Marco A., Corti D., Hosmillo M., Lee J.H., James L.C., Thukral L., Veesler D., Sigal A., Sampaziotis F., Goodfellow I.G., Matheson N.J., Sato K., Gupta R.K. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706–714. doi: 10.1038/s41586-022-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X., Zhang S., Gou J., Zhou G., Xu G., Zhang Z. Spike-mediated ACE2 down-regulation involved in the pathogenesis of SARS-CoV-2 infection. J. Infect. 2022 doi: 10.1016/j.jinf.2022.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care. 2020;24(1):290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry B.M., Benoit J.L., Berger B.A., Pulvino C., Lavie C.J., Lippi G., Benoit S.W. Coronavirus disease 2019 is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1,7. J. Med. Virol. 2021;93(2):678–680. doi: 10.1002/jmv.26479. [DOI] [PubMed] [Google Scholar]
- 17.Rysz S., Al-Saadi J., Sjostrom A., Farm M., Campoccia Jalde F., Platten M., Eriksson H., Klein M., Vargas-Paris R., Nyren S., Abdula G., Ouellette R., Granberg T., Jonsson Fagerlund M., Lundberg J. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 2021;12(1):2417. doi: 10.1038/s41467-021-22713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinci R., Pedicino D., Andreotti F., Russo G., D'Aiello A., De Cristofaro R., Crea F., Liuzzo G. From angiotensin-converting enzyme 2 disruption to thromboinflammatory microvascular disease: a paradigm drawn from COVID-19. Int. J. Cardiol. 2021;326:243–247. doi: 10.1016/j.ijcard.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maccio U., Zinkernagel A.S., Shambat S.M., Zeng X., Cathomas G., Ruschitzka F., Schuepbach R.A., Moch H., Varga Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vukusic K., Thorsell A., Muslimovic A., Jonsson M., Dellgren G., Lindahl A., Sandstedt J., Hammarsten O. Overexpression of the SARS-CoV-2 receptor angiotensin converting enzyme 2 in cardiomyocytes of failing hearts. Sci. Rep. 2022;12(1):965. doi: 10.1038/s41598-022-04956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Rooij L., Becker L.M., Carmeliet P. A role for the vascular endothelium in post-acute COVID-19? Circulation. 2022;145(20):1503–1505. doi: 10.1161/CIRCULATIONAHA.122.059231. [DOI] [PubMed] [Google Scholar]
- 22.Muhl L., He L., Sun Y., Andaloussi Mae M., Pietila R., Liu J., Genove G., Zhang L., Xie Y., Leptidis S., Mocci G., Stritt S., Osman A., Anisimov A., Hemanthakumar K.A., Rasanen M., Hansson E.M., Bjorkegren J., Vanlandewijck M., Blomgren K., Makinen T., Peng X.R., Hu Y., Ernfors P., Arnold T.D., Alitalo K., Lendahl U., Betsholtz C. The SARS-CoV-2 receptor ACE2 is expressed in mouse pericytes but not endothelial cells: implications for COVID-19 vascular research. Stem Cell Rep. 2022;17(5):1089–1104. doi: 10.1016/j.stemcr.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avolio E., Carrabba M., Milligan R., Kavanagh Williamson M., Beltrami A.P., Gupta K., Elvers K.T., Gamez M., Foster R.R., Gillespie K., Hamilton F., Arnold D., Berger I., Davidson A.D., Hill D., Caputo M., Madeddu P. The SARS-CoV-2 spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. (Lond) 2021;135(24):2667–2689. doi: 10.1042/CS20210735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bocci M., Oudenaarden C., Saenz-Sarda X., Simren J., Eden A., Sjolund J., Moller C., Gisslen M., Zetterberg H., Englund E., Pietras K. Infection of brain pericytes underlying neuropathology of COVID-19 patients. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiese A., Frati P., Del Duca F., Santoro P., Manetti A.C., La Russa R., Di Paolo M., Turillazzi E., Fineschi V. Myocardial pathology in COVID-19-associated cardiac injury: a systematic review. Diagnostics (Basel) 2021;11(9) doi: 10.3390/diagnostics11091647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gul R., Kim U.H., Alfadda A.A. Renin-angiotensin system at the interface of COVID-19 infection. Eur. J. Pharmacol. 2021;890 doi: 10.1016/j.ejphar.2020.173656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin J., Wang S., Liu Y., Chen J., Li D., Xu T. Coronary microvascular dysfunction pathophysiology in COVID-19. Microcirculation. 2021;28(7) doi: 10.1111/micc.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moretti R., Janjusevic M., Fluca A.L., Saro R., Gagno G., Pierri A., Padoan L., Restivo L., Derin A., Beltrami A.P., Caruso P., Sinagra G., Aleksova A. Common shared pathogenic aspects of small vessels in heart and brain disease. Biomedicines. 2022;10(5) doi: 10.3390/biomedicines10051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021;9(3) doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topol E.J. COVID-19 can affect the heart. Science. 2020;370(6515):408–409. doi: 10.1126/science.abe2813. [DOI] [PubMed] [Google Scholar]
- 32.Bugert C.L., Kwiat V., Valera I.C., Bugert J.J., Parvatiyar M.S. Cardiovascular injury due to SARS-CoV-2. Curr Clin Microbiol Rep. 2021:1–11. doi: 10.1007/s40588-021-00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., Dentali F., Montecucco F., Massberg S., Levi M. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Tecson K.M., McCullough P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020;21(3):315–319. doi: 10.31083/j.rcm.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 35.Nappi F., Giacinto O., Ellouze O., Nenna A., Avtaar Singh S.S., Chello M., Bouzguenda A., Copie X. Association between COVID-19 diagnosis and coronary artery thrombosis: a narrative review. Biomedicines. 2022;10(3):702. doi: 10.3390/biomedicines10030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canzano P., Brambilla M., Porro B., Cosentino N., Tortorici E., Vicini S., Poggio P., Cascella A., Pengo M.F., Veglia F., Fiorelli S., Bonomi A., Cavalca V., Trabattoni D., Andreini D., Omodeo Sale E., Parati G., Tremoli E., Camera M. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic. Transl. Sci. 2021;6(3):202–218. doi: 10.1016/j.jacbts.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Errico S., Zanon M., Montanaro M., Radaelli D., Sessa F., Di Mizio G., Montana A., Corrao S., Salerno M., Pomara C. More than pneumonia: distinctive features of SARS-Cov-2 infection. From autopsy findings to clinical implications: a systematic review. Microorganisms. 2020;8(11) doi: 10.3390/microorganisms8111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson J.E., McGuone D., Xu M.L., Jane-Wit D., Mitchell R.N., Libby P., Pober J.S. Coronavirus disease 2019 (COVID-19) coronary vascular thrombosis: correlation with neutrophil but not endothelial activation. Am. J. Pathol. 2022;192(1):112–120. doi: 10.1016/j.ajpath.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossouw T.M., Anderson R., Manga P., Feldman C. Emerging role of platelet-endothelium interactions in the pathogenesis of severe SARS-CoV-2 infection-associated myocardial injury. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.776861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satterfield B.A., Bhatt D.L., Gersh B.J. Publisher correction: cardiac involvement in the long-term implications of COVID-19. Nat. Rev. Cardiol. 2022;19(5):342. doi: 10.1038/s41569-021-00641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavelle M.P., Desai A.D., Wan E.Y. Arrhythmias in the COVID-19 patient. Heart Rhythm O2. 2022;3(1):8–14. doi: 10.1016/j.hroo.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaine S., Devitt P., Coughlan J.J., Pearson I. COVID-19-associated myocarditis presenting as new-onset heart failure and atrial fibrillation. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-244027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali M., Shiwani H.A., Elfaki M.Y., Hamid M., Pharithi R., Kamgang R., Egom C.B., Oyono J.L.E., Egom E.E. COVID-19 and myocarditis: a review of literature. Egypt Heart J. 2022;74(1):23. doi: 10.1186/s43044-022-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ammirati E., Lupi L., Palazzini M., Hendren N.S., Grodin J.L., Cannistraci C.V., Schmidt M., Hekimian G., Peretto G., Bochaton T., Hayek A., Piriou N., Leonardi S., Guida S., Turco A., Sala S., Uribarri A., Van de Heyning C.M., Mapelli M., Campodonico J., Pedrotti P., Barrionuevo Sanchez M.I., Ariza Sole A., Marini M., Matassini M.V., Vourc'h M., Cannata A., Bromage D.I., Briguglia D., Salamanca J., Diez-Villanueva P., Lehtonen J., Huang F., Russel S., Soriano F., Turrini F., Cipriani M., Bramerio M., Di Pasquale M., Grosu A., Senni M., Farina D., Agostoni P., Rizzo S., De Gaspari M., Marzo F., Duran J.M., Adler E.D., Giannattasio C., Basso C., McDonagh T., Kerneis M., Combes A., Camici P.G., de Lemos J.A., Metra M. Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation. 2022;145(15):1123–1139. doi: 10.1161/CIRCULATIONAHA.121.056817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luetkens J.A., Isaak A., Ozturk C., Mesropyan N., Monin M., Schlabe S., Reinert M., Faron A., Heine A., Velten M., Dabir D., Boesecke C., Strassburg C.P., Attenberger U., Zimmer S., Duerr G.D., Nattermann J. Cardiac MRI in suspected acute COVID-19 myocarditis. Radiol. Cardiothorac. Imaging. 2021;3(2) doi: 10.1148/ryct.2021200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brauninger H., Stoffers B., Fitzek A.D.E., Meissner K., Aleshcheva G., Schweizer M., Weimann J., Rotter B., Warnke S., Edler C., Braun F., Roedl K., Scherschel K., Escher F., Kluge S., Huber T.B., Ondruschka B., Schultheiss H.P., Kirchhof P., Blankenberg S., Puschel K., Westermann D., Lindner D. Cardiac SARS-CoV-2 infection is associated with pro-inflammatory transcriptomic alterations within the heart. Cardiovasc. Res. 2022;118(2):542–555. doi: 10.1093/cvr/cvab322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Nonno F., Frustaci A., Verardo R., Chimenti C., Nicastri E., Antinori A., Petrosillo N., Lalle E., Agrati C., Ippolito G., Group I.C.s. Virus-negative myopericarditis in human coronavirus infection: report from an autopsy series. Circ. Heart Fail. 2020 doi: 10.1161/CIRCHEARTFAILURE.120.007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdi A., AlOtaiby S., Badarin F.A., Khraibi A., Hamdan H., Nader M. Interaction of SARS-CoV-2 with cardiomyocytes: insight into the underlying molecular mechanisms of cardiac injury and pharmacotherapy. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbasi J. The COVID heart-one year after SARS-CoV-2 infection, patients have an Array of increased cardiovascular risks. JAMA. 2022;327(12):1113–1114. doi: 10.1001/jama.2022.2411. [DOI] [PubMed] [Google Scholar]
- 50.Friedrich M.G., Cooper L.T., Jr. What We (Don’t) Know About Myocardial Injury After COVID-19. Oxford University Press; 2021. pp. 1879–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam M.S., Czajkowsky D.M. SARS-CoV-2 infection and oxidative stress: pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 2022;63:44–57. doi: 10.1016/j.cytogfr.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiavone M., Gobbi C., Biondi-Zoccai G., D'Ascenzo F., Palazzuoli A., Gasperetti A., Mitacchione G., Viecca M., Galli M., Fedele F., Mancone M., Forleo G.B. Acute coronary syndromes and Covid-19: exploring the uncertainties. J. Clin. Med. 2020;9(6) doi: 10.3390/jcm9061683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negron S.G., Kessinger C.W., Xu B., Pu W.T., Lin Z. bioRxiv; 2021. Selectively Expressing SARS-CoV-2 Spike Protein S1 Subunit in Cardiomyocytes Induces Cardiac Hypertrophy in Mice. [DOI] [Google Scholar]
- 54.Duerr G.D., Heine A., Hamiko M., Zimmer S., Luetkens J.A., Nattermann J., Rieke G., Isaak A., Jehle J., Held S.A.E., Wasmuth J.C., Wittmann M., Strassburg C.P., Brossart P., Coburn M., Treede H., Nickenig G., Kurts C., Velten M. Parameters predicting COVID-19-induced myocardial injury and mortality. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez-Bermejo J.A., Kang S., Rockwood S.J., Simoneau C.R., Joy D.A., Silva A.C., Ramadoss G.N., Flanigan W.R., Fozouni P., Li H., Chen P.Y., Nakamura K., Whitman J.D., Hanson P.J., McManus B.M., Ott M., Conklin B.R., McDevitt T.C. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci. Transl. Med. 2021;13(590) doi: 10.1126/scitranslmed.abf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L., Nilsson-Payant B.E., Han Y., Jaffre F., Zhu J., Wang P., Zhang T., Redmond D., Houghton S., Moller R., Hoagland D., Carrau L., Horiuchi S., Goff M., Lim J.K., Bram Y., Richardson C., Chandar V., Borczuk A., Huang Y., Xiang J., Ho D.D., Schwartz R.E., tenOever B.R., Evans T., Chen S. Cardiomyocytes recruit monocytes upon SARS-CoV-2 infection by secreting CCL2. Stem Cell Rep. 2021;16(10):2565. doi: 10.1016/j.stemcr.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filbin M.R., Mehta A., Schneider A.M., Kays K.R., Guess J.R., Gentili M., Fenyves B.G., Charland N.C., Gonye A.L.K., Gushterova I., Khanna H.K., LaSalle T.J., Lavin-Parsons K.M., Lilley B.M., Lodenstein C.L., Manakongtreecheep K., Margolin J.D., McKaig B.N., Rojas-Lopez M., Russo B.C., Sharma N., Tantivit J., Thomas M.F., Gerszten R.E., Heimberg G.S., Hoover P.J., Lieb D.J., Lin B., Ngo D., Pelka K., Reyes M., Smillie C.S., Waghray A., Wood T.E., Zajac A.S., Jennings L.L., Grundberg I., Bhattacharyya R.P., Parry B.A., Villani A.C., Sade-Feldman M., Hacohen N., Goldberg M.B. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep. Med. 2021;2(5) doi: 10.1016/j.xcrm.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aikawa T., Takagi H., Ishikawa K., Kuno T. Myocardial injury characterized by elevated cardiac troponin and in-hospital mortality of COVID-19: an insight from a meta-analysis. J. Med. Virol. 2021;93(1):51–55. doi: 10.1002/jmv.26108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baldi E., Sechi G.M., Mare C., Canevari F., Brancaglione A., Primi R., Klersy C., Palo A., Contri E., Ronchi V., Beretta G., Reali F., Parogni P., Facchin F., Bua D., Rizzi U., Bussi D., Ruggeri S., Oltrona Visconti L., Savastano S., Lombardia C.R. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N. Engl. J. Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marijon E., Karam N., Jost D., Perrot D., Frattini B., Derkenne C., Sharifzadehgan A., Waldmann V., Beganton F., Narayanan K., Lafont A., Bougouin W., Jouven X. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health. 2020;5(8):e437–e443. doi: 10.1016/S2468-2667(20)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holland M., Burke J., Hulac S., Morris M., Bryskiewicz G., Goold A., McVaney K., Rappaport L., Stauffer B.L. Excess cardiac arrest in the community during the COVID-19 pandemic. JACC Cardiovasc. Interv. 2020;13(16):1968–1969. doi: 10.1016/j.jcin.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siripanthong B., Asatryan B., Hanff T.C., Chatha S.R., Khanji M.Y., Ricci F., Muser D., Ferrari V.A., Nazarian S., Santangeli P., Deo R., Cooper L.T., Jr., Mohiddin S.A., Chahal C.A.A. The pathogenesis and long-term consequences of COVID-19 cardiac injury. JACC Basic Transl. Sci. 2022;7(3):294–308. doi: 10.1016/j.jacbts.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.VasanthiDharmalingam P., Karuppagounder V., Watanabe K., Karmouty-Quintana H., Palaniyandi S.S., Guha A., Thandavarayan R.A. SARS-CoV-2 mediated hyperferritinemia and cardiac arrest: preliminary insights. Drug Discov. Today. 2021;26(5):1265–1274. doi: 10.1016/j.drudis.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatt A.S., Moscone A., McElrath E.E., Varshney A.S., Claggett B.L., Bhatt D.L., Januzzi J.L., Butler J., Adler D.S., Solomon S.D., Vaduganathan M. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;76(3):280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scquizzato T., Landoni G., Scandroglio A.M., Franco A., Calabro M.G., Paoli A., D'Amico F., Yavorovskiy A., Zangrillo A. Outcomes of out-of-hospital cardiac arrest in patients with SARS-CoV-2 infection: a systematic review and meta-analysis. Eur. J. Emerg. Med. 2021;28(6):423–431. doi: 10.1097/MEJ.0000000000000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lami F., Scalabrini I., Lucaccioni L., Iughetti L. The, "perfect" storm: current evidence on pediatric inflammatory multisystem disease during SARS-CoV-2 pandemic. Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jatczak-Pawlik I., Lewek J., Czkwianianc E., Blomberg A., Krysiak N., Zeman K., Jankowski P., Banach M., Study L.A.-C.-K. Biochemical and cardiovascular predictors of PIMS-TS risk in children after COVID-19 recovery: preliminary results of the LATE-COVID-Kids Study. Arch. Med. Sci. 2022;18(2):545–552. doi: 10.5114/aoms/146827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mardi P., Esmaeili M., Iravani P., Abdar M.E., Pourrostami K., Qorbani M. Characteristics of children with Kawasaki disease-like signs in COVID-19 pandemic: a systematic review. Front. Pediatr. 2021;9 doi: 10.3389/fped.2021.625377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Payne A.B., Gilani Z., Godfred-Cato S., Belay E.D., Feldstein L.R., Patel M.M., Randolph A.G., Newhams M., Thomas D., Magleby R., Hsu K., Burns M., Dufort E., Maxted A., Pietrowski M., Longenberger A., Bidol S., Henderson J., Sosa L., Edmundson A., Tobin-D'Angelo M., Edison L., Heidemann S., Singh A.R., Giuliano J.S., Jr., Kleinman L.C., Jr., Tarquinio K.M., Jr., Walsh R.F., Jr., Fitzgerald J.C., Jr., Clouser K.N., Jr., Gertz S.J., Jr., Carroll R.W., Jr., Carroll C.L., Jr., Hoots B.E., Jr., Reed C., Jr., Dahlgren F.S., Jr., Oster M.E., Jr., Pierce T.J., Jr., Curns A.T., Jr., Langley G.E., Jr., Campbell A.P., Jr., Group M.-C.I.A., Jr., Balachandran N., Jr., Murray T.S., Jr., Burkholder C., Jr., Brancard T., Jr., Lifshitz J., Jr., Leach D., Jr., Charpie I., Jr., Tice C., Jr., Coffin S.E., Jr., Perella D., Jr., Jones K., Jr., Marohn K.L., Jr., Yager P.H., Jr., Fernandes N.D., Jr., Flori H.R., Jr., Koncicki M.L., Walker K.S., Di Pentima M.C., Li S., Horwitz S.M., Gaur S., Coffey D.C., Harwayne-Gidansky I., Hymes S.R., Thomas N.J., Ackerman K.G., Cholette J.M. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw. Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soni P.R., Noval Rivas M., Arditi M. A comprehensive update on Kawasaki disease Vasculitis and myocarditis. Curr. Rheumatol. Rep. 2020;22(2):6. doi: 10.1007/s11926-020-0882-1. [DOI] [PubMed] [Google Scholar]
- 71.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D'Antiga L. An outbreak of severe Kawasaki-like disease at the italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Licciardi F., Pruccoli G., Denina M., Parodi E., Taglietto M., Rosati S., Montin D. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146(2) doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 73.Ouldali N., Pouletty M., Mariani P., Beyler C., Blachier A., Bonacorsi S., Danis K., Chomton M., Maurice L., Le Bourgeois F., Caseris M., Gaschignard J., Poline J., Cohen R., Titomanlio L., Faye A., Melki I., Meinzer U. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc. Health. 2020;4(9):662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bukulmez H. Current understanding of multisystem inflammatory syndrome (MIS-C) following COVID-19 and its distinction from Kawasaki disease. Curr. Rheumatol. Rep. 2021;23(8):58. doi: 10.1007/s11926-021-01028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoepel W., Chen H.-J., Allahverdiyeva S., Manz X., Aman J., <collab>COVID A.U.</collab>, Bonta P., Brouwer P., de Taeye S., Caniels T. BioRxiv; 2020. Anti-SARS-CoV-2 IgG From Severely Ill COVID-19 Patients Promotes Macrophage Hyper-inflammatory Responses. [Google Scholar]
- 77.Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., Klein J.D., Bhutta Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dimitriades V.R., Brown A.G., Gedalia A. Kawasaki disease: pathophysiology, clinical manifestations, and management. Curr. Rheumatol. Rep. 2014;16(6):423. doi: 10.1007/s11926-014-0423-x. [DOI] [PubMed] [Google Scholar]
- 79.Menikou S., Langford P.R., Levin M. Kawasaki disease: the role of immune complexes revisited. Front. Immunol. 2019;10:1156. doi: 10.3389/fimmu.2019.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shakeel S., Ahmad Hassali M.A. Post-COVID-19 outbreak of severe Kawasaki-like multisystem inflammatory syndrome in children. Malays J Med Sci. 2021;28(1):109–116. doi: 10.21315/mjms2021.28.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abrams J.Y., Godfred-Cato S.E., Oster M.E., Chow E.J., Koumans E.H., Bryant B., Leung J.W., Belay E.D. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J. Pediatr. 2020;226(45–54) doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bustos B.R., Jaramillo-Bustamante J.C., Vasquez-Hoyos P., Cruces P., Diaz F. Pediatric inflammatory multisystem syndrome associated with SARS-CoV-2: a case series quantitative systematic review. Pediatr. Emerg. Care. 2021;37(1):44–47. doi: 10.1097/PEC.0000000000002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tung M.L., Tan B., Cherian R., Chandra B. Anti-phospholipid syndrome and COVID-19 thrombosis: connecting the dots. Rheumatol. Adv. Pract. 2021;5(1) doi: 10.1093/rap/rkaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., Grossi C., Blengino S., Tincani A., Franceschini F., Andreoli L., Lazzaroni M.G., Piantoni S., Masneri S., Crisafulli F., Brugnoni D., Muiesan M.L., Salvetti M., Parati G., Torresani E., Mahler M., Heilbron F., Pregnolato F., Pengo M., Tedesco F., Pozzi N., Meroni P.L. medRxiv; 2020. Anti-phospholipid Antibodies in COVID-19 Are Different From Those Detectable in the Anti-phospholipid Syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodríguez Y., Novelli L., Rojas M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M., Yadav V., Wang J., Woodard W., Lezak S.P., Lugogo N.L., Smith S.A., Morrissey J.H., Kanthi Y., Knight J.S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12(570) doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clarke S.A., Phylactou M., Patel B., Mills E.G., Muzi B., Izzi-Engbeaya C., Choudhury S., Khoo B., Meeran K., Comninos A.N., Abbara A., Tan T., Dhillo W.S. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J. Clin. Endocrinol. Metab. 2021;106(8):2208–2220. doi: 10.1210/clinem/dgab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan T., Khoo B., Mills E.G., Phylactou M., Patel B., Eng P.C., Thurston L., Muzi B., Meeran K., Prevost A.T., Comninos A.N., Abbara A., Dhillo W.S. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montani D., Savale L., Noel N., Meyrignac O., Colle R., Gasnier M., Corruble E., Beurnier A., Jutant E.M., Pham T., Lecoq A.L., Papon J.F., Figueiredo S., Harrois A., Humbert M., Monnet X., Group C.S. Post-acute COVID-19 syndrome. Eur. Respir. Rev. 2022;31(163) doi: 10.1183/16000617.0185-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azzolino D., Cesari M. Fatigue in the COVID-19 pandemic. Lancet Healthy Longev. 2022;3(3):e128–e129. doi: 10.1016/S2666-7568(22)00029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanchez J., Cohen M., Zapater J.L., Eisenberg Y. Primary adrenal insufficiency after COVID-19 infection. AACE Clin. Case Rep. 2022;8(2):51–53. doi: 10.1016/j.aace.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sabbadin C., Betterle C., Scaroni C., Ceccato F. Frequently asked questions in patients with adrenal insufficiency in the time of COVID-19. Front. Endocrinol. (Lausanne) 2021;12 doi: 10.3389/fendo.2021.805647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whitaker M., Elliott J., Chadeau-Hyam M., Riley S., Darzi A., Cooke G., Ward H., Elliott P. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people. Medrxiv. 2021 doi: 10.1101/2021.06.28.21259452. [DOI] [Google Scholar]
- 95.Ayoubkhani D., Khunti K., Nafilyan V., Maddox T., Humberstone B., Diamond I., Banerjee A. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Negreira-Caamano M., Martinez-Del Rio J., Aguila-Gordo D., Mateo-Gomez C., Soto-Perez M., Piqueras-Flores J. Cardiovascular events after COVID-19 hospitalization: long-term follow-up. Rev. Esp. Cardiol. (Engl Ed) 2022;75(1):100–102. doi: 10.1016/j.rec.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puntmann V.O., Martin S., Shchendrygina A., Hoffmann J., Ka M.M., Giokoglu E., Vanchin B., Holm N., Karyou A., Laux G.S., Arendt C., De Leuw P., Zacharowski K., Khodamoradi Y., Vehreschild M., Rohde G., Zeiher A.M., Vogl T.J., Schwenke C., Nagel E. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022 doi: 10.1038/s41591-022-02000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Twohig K.A., Nyberg T., Zaidi A., Thelwall S., Sinnathamby M.A., Aliabadi S., Seaman S.R., Harris R.J., Hope R., Lopez-Bernal J., Gallagher E., Charlett A., De Angelis D., Presanis A.M., Dabrera G., Consortium C.-G.U. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22(1):35–42. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saito A., Irie T., Suzuki R., Maemura T., Nasser H., Uriu K., Kosugi Y., Shirakawa K., Sadamasu K., Kimura I., Ito J., Wu J., Iwatsuki-Horimoto K., Ito M., Yamayoshi S., Loeber S., Tsuda M., Wang L., Ozono S., Butlertanaka E.P., Tanaka Y.L., Shimizu R., Shimizu K., Yoshimatsu K., Kawabata R., Sakaguchi T., Tokunaga K., Yoshida I., Asakura H., Nagashima M., Kazuma Y., Nomura R., Horisawa Y., Yoshimura K., Takaori-Kondo A., Imai M., Tanaka S., Nakagawa S., Ikeda T., Fukuhara T., Kawaoka Y., Sato K., Genotype to Phenotype Japan C Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature. 2022;602(7896):300–306. doi: 10.1038/s41586-021-04266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan S., Ye Z.W., Liang R., Tang K., Zhang A.J., Lu G., Ong C.P., Man Poon V.K., Chan C.C., Mok B.W., Qin Z., Xie Y., Chu A.W., Chan W.M., Ip J.D., Sun H., Tsang J.O., Yuen T.T., Chik K.K., Chan C.C., Cai J.P., Luo C., Lu L., Yip C.C., Chu H., To K.K., Chen H., Jin D.Y., Yuen K.Y., Chan J.F. Pathogenicity, transmissibility, and fitness of SARS-CoV-2 omicron in Syrian hamsters. Science. 2022 doi: 10.1126/science.abn8939. [DOI] [PubMed] [Google Scholar]
- 101.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Jr., Tureci O., Jr., Nell H., Jr., Schaefer A., Jr., Unal S., Jr., Tresnan D.B., Jr., Mather S., Jr., Dormitzer P.R., Jr., Sahin U., Jr., Jansen K.U., Jr., Gruber W.C., Jr., Group C.C.T. Safety and efficacy ofthe BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., Group C.S. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.