Abstract
Cabbage, cauliflower and broccoli are well-known vegetables from the Brassica family having functional effects on human health. This study was carried out to identify different antioxidant properties and to quantify phenolic compounds by HPLC-DAD in different extracts (methanol, ethanol and water: acetic acid: acetone) of these vegetables. The results showed that, the methanolic dry extract of cabbage possessed the highest antioxidant activity (549 ± 7.30 μg/g) and IC50 was 90 ± 2.52 μg/mL than others. Whereas the ethanolic dry extract of cauliflower had 348 ± 5.20 μg/g of flavonoid, which was the highest among all. The maximum levels of total tannin (414 ± 5.20 μg/g) and total phenolic content (465 ± 3.25 μg/g) was found in broccoli dry extract. Several polyphenolic compounds were identified in different extracts of the vegetables and they were Cauliflower (8) > Cabbage (10) > Broccoli (9) in total. Therefore, the use of total vegetables rather than extracts in the food industry would be more appropriate to get greater health benefit.
Keywords: Cabbage, Cauliflower, Broccoli, Brassica vegetables, Polyphenols, Antioxidant activity, Functional activity
Cabbage; Cauliflower; Broccoli; Brassica vegetables; Polyphenols; Antioxidant activity; Functional activity.
1. Introduction
Cauliflower, cabbage and broccoli are three popular winter vegetables of the Brassicaceae genus in the Cruciferae family. Cauliflower (Brassica oleracea var. botrytis) is served as a curry, soup, or fried dish. It includes cancer-fighting sulforaphane, as well as glucosinolates, carotenoids, indole-3-carbinol, isothiocyanates, dithiolethiones, and phenols, which improves DNA repair, functions as an estrogen antagonist and reduces cancer cell proliferation [1]. Additional bioactive breakdown products, including nitriles, thiocyanates, epithionitriles, and oxazolidines, are produced when cauliflower myrosinase hydrolyzes glucosinolates during cellular disruption [2]. The most prevalent hydrophilic compound in cauliflower is ascorbic acid, which is known to detoxify reactive oxygen species directly [3].
Cabbage (Brassica oleracea var. capitata) is another common vegetable consumed either raw as salads or processed in different ways, e.g., boiled or, fermented. Cancer patients who follow such diets also benefit from an increase in the bioavailable content of non-heme iron and from the use of complementary and alternative medicine [4]. Clinical research also shows that eating cabbage can help with peptic ulcer healing and lowering LDL levels in the blood [5]. Moreover, they have been used for centuries in traditional medicine to treat a variety of conditions, including minor cuts and wounds, mastitis, and gastrointestinal disorders such as gastritis, peptic and duodenal ulcers, and irritable bowel syndrome.
Broccoli (Brassica oleracea L. var. italica) sprouts, florets, flour, fiber, flakes, powder, crisps and so on are gaining popularity for their preventive role in noncommunicable diseases like hypertension, atherosclerosis and cancer [6]. It is also a good source of isothiocyanates as well as sulforaphane (SF), known for its chemopreventive properties [7]. It is also rich in vitamins (C and K), beta-carotenes, dietary fiber, polyphenols and fatty acids, with considerable beneficial health effects [6, 8]. Consumption of this vegetable may decrease the risk of gastric cardiac and esophageal adenocarcinomas [9], but also colon and colorectal cancers in human [10].
Various structural components like lignin, cellulose is present in these vegetables. Among them, cellulose is important, which contains a long chain of covalently linked glucose units and gives tensile strength in plant cells [11]. This cellulose is important as a coating material in food processing techniques, especially in the microencapsulation process. Moreover, vegetables are a good source of bioactive polyphenols which show different functional properties. There is not enough research on the polyphenolic content and antioxidant properties of these three vegetables of the brassica genus extracted with both polar and non-polar solvents. Hence, this study was designed to determine the polyphenolic compounds and antioxidant profiles of these three vegetables extracted in three different solvents based on polarity. In the end, this will give a thorough summary of bioactive polyphenols with functional qualities as well as the antioxidant activities of extracts of broccoli, cabbage, and cauliflower.
2. Materials and methods
2.1. Sample collection
The cabbage (n = 25), broccoli (n = 28), cauliflower (n = 21) were collected from different wholesale markets around Dhaka city, where vegetables were gathered from different agricultural regions of Bangladesh and the cultivars were identified by a botanist. Then, those samples were cut into about 2cm pieces and dried at 60 °C in an oven (Memmert UNB 100). After drying, samples were ground in a blender and the powder was kept in an air-tight container. A single composite sample of a homogeneous mix was prepared from of same type of item.
2.2. Sample extraction
The maceration process was employed to extract bioactive chemicals from dry materials based on the polarity of the solvents [12]. It has long been recognized that ethanol, which is safe for consumption, makes a good solvent for extracting polyphenols. Lower molecular weight polyphenols can generally be extracted more effectively with methanol, whilst higher molecular weight can be extracted more effectively with aqueous acetone [13].
2.2.1. Methanol extract (ME) and ethanol extract (EE)
About 25 g of dry powder of each sample was put into 250 mL of methanol and ethanol separately in a conical flask. Then, flasks were put on the shaker (GFL orbital shaker 3005) and were shaken for 72 h. Solutions were filtered through filter paper (Whatman, no. 1) and filtrates were evaporated in a rotary evaporator (IKA RV 10, USA) at 60 °C. Dried extracts of broccoli (BCME, BCEE), cabbage (CAME, CAEE) and cauliflower (CUME, CUEE) were obtained after evaporation and were stored at −20 °C [14].
2.2.2. Water, acetic acid and acetone extract (WAA)
Solvent polarity is a key factor in enhancing phenolic solubility. Hexane, dichloromethane (ratio = 50:50), and acetone: water: acetic acid (70: 29.5: 0.5) were utilized subsequently to boost the extraction of water-soluble polyphenols. The disruption of the cell matrix of the sample for maximum extraction was enhanced by acetone: water: acetic acid solvent [14, 15]. About 25–30 g of dried powder was put into 250 mL of solvent (hexane: dichloromethane = 50:50) in a conical flask and was shaken on a shaker for 72 h. The solution was filtered through filter paper (Whatman, no. 1) and the residue was dried in an oven at 60 °C. Then this dried powder was mixed with 200 mL of solvent (water:acetic acid:acetone = 0.5:29.5:70) and was put on shaker for 72 h [16]. After filtration, the filtrate was dried using a freeze dryer at −42 °C (ThermoFisher Modulyod-230). Properly dried extracts of broccoli (BCWAA), cabbage (CAWAA) and cauliflower (CUWAA) were powdered and were stored at −20 °C.
2.3. Chemicals
The following ingredients were acquired from Sigma (St. Louis, Mo, USA): 1,1-Diphenyl-2-Picryl Hydrazyl (DPPH), Folin–Ciocalteu reagent, Aluminum Chloride (AlCl3), Sodium Acetate, Ascorbic acid, Tri-Sodium Hydrogen Phosphate (Na3PO4), Sodium Carbonate (Na2CO3), Ammonium Molybdate, H2SO4, Potassium Di-Hydrogen Phosphate (KH2PO4), HPLC grade solvent (acetonitrile, methanol, acetic acid, IPA), and standards. Gallic acid, chlorogenic acid, catechol, catechin hydrate, vanillic acid, caffeic acid, (−) epicatechin, vanillin, trans-ferulic acid, p-coumaric acid, rutin hydrate, rosmarinic acid, myricetin, quercetin, trans-cinnamic acid, naringenin, and kaempferol were used as standards.
2.4. Yield determination
The yield percentage was determined to observe the effect of the solvent system on the extraction. The yield was calculated using the equation, Yield (%) = 100∗(A-B)/W, where A = weight of flask containing extract after evaporation, B = weight of dry empty flask, W = weight of dry sample.
2.5. Determination of antioxidant properties
2.5.1. Sample preparation
The dried extract was dissolved in a corresponding solvent (methanol, ethanol or water: acetic acid: acetone) to prepare a 10 mg/mL concentration, and the solution was vigorously shaken and sonicated. This solution was used as a stock solution and kept at 4 °C.
2.5.2. Determination of total flavonoid content (TFC)
Total flavonoid content was determined colorimetrically [17]. First, 0.3325 g of AlCl3 and 1 g of sodium acetate were mixed in 100 mL of DI water. The thawed sample (0.2 mL) was mixed with 4.8 mL of water and the mixture was kept for 5–6 min after adding the 2.5 mL of reagent mix. A Thermo Scientific double beam UV-VIS spectrophotometer (Model: Evolution 300) was used to measure absorbance at 430 nm. Quercetin (0.01 g) was dissolved in 100 mL of methanol for the preparation of the calibration curve. Total flavonoids were expressed as μg of Quercetin equivalent per gram of dry extract.
2.5.3. Determination of total tannin content (TTC)
The method used by Haile & Kang (2019) with a few modifications was used to determine the tannins [18]. A sample extract (0.5 mL) was combined with 8.5 mL of distilled water and 0.5 mL of the Folin-Ciocalteu Phenol reagent and maintained at room temperature for 5 min. A 35% sodium carbonate solution (1 mL) was then added, and it was allowed to sit at room temperature for 20 min after thoroughly stirring the mixture. The absorbance at 725 nm was measured and the total tannin concentration was stated as μg of tannic acid equivalent per gram of dry extract.
2.5.4. Determination of total phenolic content (TPC)
Samples were made according to section 2.5.3’s, instructions. After 20 min of incubation at room temperature, the absorbance was measured at 765 nm. A blank was read against a collection of Gallic acid standard solutions and the phenolic values were represented in terms of Gallic acid in μg/g of dry extract [19].
2.5.5. Determination of total antioxidant activity (TAA)
The phosphomolybdenum assay method, which is based on the reduction of Mo (VI) to Mo (V) and the subsequent formation of a green phosphate-Mo (V) complex in acidic conditions, was used to assess the extract’s overall antioxidant activity [20]. The sample extract (0.5 mL) was mixed with 3.0 mL of reagent solution (0.6 M H2SO4, 28 mM Na3PO4, 4 mM ammonium molybdate) and incubated at 95 °C for 90 min. The absorbance of the solution was measured at 695 nm against a reagent blank, and the ascorbic acid equivalent per gram of dry extract was used to evaluate antioxidant activity.
2.5.6. Determination of DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity
The experiment that investigated the DPPH radical-scavenging activity was carried out using the modified approach described by Sanna et al. (2012) [21]. During this procedure, 2 mL of 0.2 mM methanol DPPH solution was added to 2 mL of extract solution at varied concentrations. The solutions were left in a dark, room-temperature setting for 30 min to react and the absorbance at 517 nm was measured after 10 min.
DPPH radical-scavenging activity (%) = (Ac–As)/Ac × 100, where Ac and As is the absorbance of control solution’s and DPPH solution with plant extracts respectively. The IC50 was estimated by plotting DPPH radical-scavenging activity (%) versus sample extract concentration [22]. Positive control in this investigation was ascorbic acid.
2.6. Identification of bioactive polyphenols
2.6.1. Standard preparation
A stock standard solution (100 μg/mL) of each phenolic compound was prepared in methanol. The mixed standard solution was prepared by diluting the stock standard solutions in methanol to give a concentration of 5 μg/mL for each polyphenol. All standard solutions were stored in the dark at 4 °C.The calibration curves of the standards were made by a dilution of the stock standards (five sets of standard dilutions). The calibration curves were constructed from chromatograms as peak area vs. concentration of standard [14].
2.6.2. HPLC system
Thermo Scientific Dionex UltiMate 3000 Rapid Separation Liquid Chromatography (RSLC) system (Thermo Fisher Scientific Inc., MA, USA) was used for the chromatographic analyses. It is equipped with a quaternary rapid separation pump (LPG-3400RS), Ultimate 3000RS autosampler (WPS-3000), and rapid separation diode array detector (DAD-3000RS). An Acclaim® C18 (4.6 × 250 mm; 5 m; 120 A°) column (Dionix, USA) was used to separate phenolic compounds. The temperature of the temperature-controlled column compartment (TCC-3000) was maintained at 30 degrees Celsius. For data acquisition, peak integration, and calibrations, the Dionix Chromeleon software (Version 6.80 RS 10) was used.
The phenolic content was determined using Sarunya and Sukon’s (2006) approach [23]. Acetonitrile (solvent A), acetic acid solution (solvent B), methanol (solvent C), and IPA (solvent D) were used in the mobile phase. The system was run with the gradient elution program described in Table 1. A 5-minute post-run at initial conditions was performed to equilibrate the column. The flow rate varied throughout the analysis from 1 to 0.75 mL/min, and the injection volume was 20 μL. The wavelength program was adjusted to monitor phenolic chemicals at 280 nm for PDA detection. Calibration curves were generated using standards, and the R2 for these curves was more than 0.995%.
Table 1.
Chromatographic conditions.
| Sl no. | Retention [min] | Flow [mL/min] | A% | %B | %C |
|---|---|---|---|---|---|
| 1 | 0.000 | 1.000 | 0.00 | 100 | 0.0 |
| 2 | 4.000 | 1.000 | 3.00 | 95.0 | 2.0 |
| 3 | 10.00 | 1.000 | 3.00 | 92.0 | 2.0 |
| 4 | 14.00 | 0.800 | 6.00 | 90.0 | 4.0 |
| 5 | 20.00 | 0.800 | 10.00 | 85.0 | 5.0 |
| 6 | 24.00 | 0.750 | 14.00 | 80.0 | 6.0 |
| 7 | 30.000 | 0.750 | 15.00 | 75.0 | 10.0 |
| 8 | 39.00 | 0.750 | 20.00 | 65.0 | 15.0 |
| 9 | 45.00 | 0.750 | 25.00 | 55.0 | 20.0 |
| 10 | 55.00 | 1.00 | 0.00 | 100 | 0.0 |
Where, A = Acetonitrile, B= Acetic acid solution of pH 3.0, C = Methanol.
2.7. Statistical analysis
All experimental results were expressed as mean ± standard deviations (SD). Data was analyzed by ANOVA, followed by the Tukey HSD test to analyze multiple comparisons using IBM SPSS Statistics (version 25). P < 0.05 was regarded as statistically significant. A Pearson correlation analysis was used to determine the association of test parameters. The figures were plotted with RStudio 2022.02.3 + 492 for Windows.
3. Results and discussion
3.1. Yield of the extract
The solvent system affects the yield percentage of the extract. The yield percentage based on the solvent follows the order: methanol > ethanol > acetone: water: acetic acid (Table 2). The highest yield percentage was observed for broccoli: methanol (18.54%), ethanol (16.02%), and acetone: water: acetic acid (15.29%). The results of the study were in good accord with Do et al. (2014)’s investigations on the role of different polarity solvents in bioactive component extraction [13].
Table 2.
Yield and DPPH radical-scavenging activities of different extracts.
| Samples | Yield (%) | DPPH scavenging activity (IC50, μg/mL) | Regression equation, Inhibition (R2) |
|---|---|---|---|
| CUME | 15.14 ± 1.52ab | 380 ± 4.50b | y = 21.834ln(x) - 80.54 R2 = 0.9927 |
| CUEE | 14.69 ± 1.2bc | 160±5fg | y = 14.385ln(x) - 22.24 R2 = 0.9904 |
| CUWAA | 12.69 ± 0.69cd | 400 ± 10a | y = 20.122ln(x) - 72.512 R2 = 0.9812 |
| CAME | 14.73 ± 0.84bcd | 90 ± 2.52h | y = 28.317ln(x) -77.61 R2 = 0.995 |
| CAEE | 13.62 ± 0.76cd | 180 ± 4.05d | y = 23.073ln(x) -70.73 R2 = 0.9657 |
| CAWAA | 12.15 ± 0.42d | 200±5c | y = 14.385ln(x) - 22.24 R2 = 0.9904 |
| BCME | 18.54 ± 1.29a | 170 ± 3.60e | y = 15.033ln(x) - 26.62 R2 = 0.9596 |
| BCEE | 16.02 ± 1.29ab | 165 ± 2.51f | y = 13.193ln(x) - 18.77< R2 = 0.9717 |
| BCWAA | 15.29 ± 1.36bc | 155 ± 1.73g | y = 18.302ln(x) - 43.21 R2 = 0.9523 |
| Ascorbic Acid | - | 19.5 ± 0.15i | y = 16.082ln(x) + 3.33 R2 = 0.8538 |
Values are mean ± SD, n = 3 (three independent extractions).
Means containing same letter (s) in the column did not differ significantly at 5% level of significance.
Where, CAME = Methanol extract of cabbage, CAEE = Ethanol extract of cabbage, CAWAA = water, acetone and acetic acid extract of cabbage, CUME = Methanol extract of cauliflower, CUEE = Ethanol extract of cauliflower, CUWAA = water, acetone and acetic acid extract of cauliflower, BCME = Methanol extract of broccoli, BCEE = Ethanol extract of broccoli, BCWAA = water, acetone and acetic acid extract of broccoli.
nd = not detected.
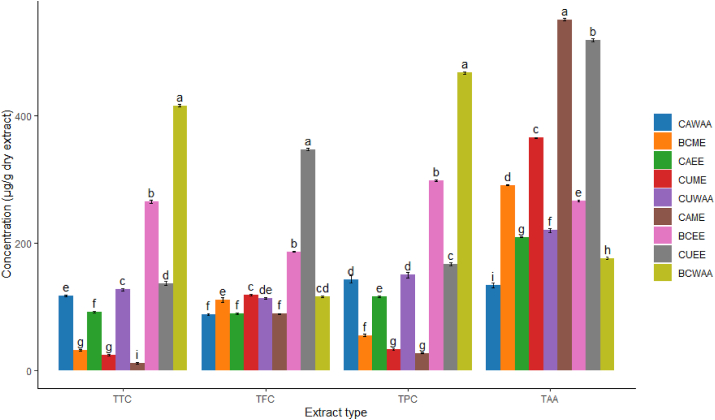
3.2. Total flavonoid content (TFC)
The total amount of flavonoid content varied according to plant extract and solvent (Figure 1). Ethanolic extract of cauliflower had significantly higher amounts of TFC than other vegetables (p = 0.03). CUEE contains the highest amount of TFC among all the extracts (348 ± 5.20 μg/g dry extract). Whereas, flavonoid content in CUME and CUWAA were 118 ± 3.20 μg/g and 113 ± 2.20 μg/g, respectively. Drabińska et al. (2021) showed 290 μg/g of TFC in methanolic extract of cauliflower [24]. Broccoli had moderate amount of TFC and the amount in BCEE, BCME, and BCWAA was 186 ± 7.10 μg/g, 115 ± 3.2 μg/g and 109 ± 5.6 μg/g respectively. Bhandari et al. (2018) showed the level of TFC value ranged from 20 to 80 μg/g in methanolic extract of broccoli, which supported the findings of the current study [25]. Cabbage showed a significantly lower amount of TFC than the other two brassica vegetables. CAWWA illustrated the lowest amount of TFC (87 ± 5.20 μg/g) than CAME (88 ± 3.20 μg/g) and CAEE (89 ± 2.20 μg/g). A study by Singh et al. (2006) reported similar levels of total phenolic content in ethanolic extract of cabbage [26].
Figure 1.
Antioxidant properties of cabbage, cauliflower and broccoli extracts. The same letter (s) on the top of the bar of the same experiment did not differ significantly at the 5% level of significance. Where, CAME = Methanol extract of cabbage, CAEE = Ethanol extract of cabbage, CAWAA = water, acetone and acetic acid extract of cabbage, CUME = Methanol extract of cauliflower, CUEE = Ethanol extract of cauliflower, CUWAA = water, acetone and acetic acid extract of cauliflower, BCME = Methanol extract of broccoli, BCEE = Ethanol extract of broccoli, BCWAA = water, acetone and acetic acid extract of broccoli.
3.3. Total tannin content (TTC)
Broccoli demonstrated a significantly higher amount of TTC than cabbage and cauliflower (p = 0.04) in all types of extracts (Figure 1). BCWAA had the highest concentration of TTC (414 ± 5.20 μg/g dry extract). Whereas, the tannin content of BCME and BCEE were 31 ± 1.36 μg/g and 262 ± 5.70 μg/g respectively. A previous study conducted by Manchali et al. (2012) showed 410 μg/g of TTC in ethanolic extract, which concurred with the findings of the current study [27]. Cauliflower had moderate amounts of TTC in CUEE, CUME and CUWAA, which were 139 ± 8.10 μg/g, 25 ± 1.2 μg/g and 127 ± 5.6 μg/g respectively. However, in a study in Algeria showed higher levels of tannin (526 μg/g) in methanolic extract of cauliflower [28]. Cabbage had a significantly (p = 0.02) lower amount of TTC than the other two extracts, and the amount in CAME was 11 ± 1.25 μg/g. The TTC in CAEE and CAWAA were 90 ± 3.70 μg/g and 116 ± 5.30 μg/g respectively. Although Deepa et al. (2020) found almost double TTC (285 μg/g) in ethanolic extract of cabbage [29].
3.4. Total phenolic content (TPC)
BCWAA contains the highest amount of TPC among all the extracts and it was 465 ± 3.25 μg/g dry extract (Figure 1). TPC in BCME and BCEE were 54 ± 1.75 μg/g and 297 ± 4.55 μg/g respectively. Jaiswal et al. (2012) reported that methanolic and ethanolic extracts of broccoli showed TPC value of 236 μg/g and 195 μg/g, respectively [30].
Cauliflower showed moderate TPC values in CUEE, CUME and CUWAA, which were 165 ± 3.25 μg/g, 33 ± 2.25 μg/g and 146 ± 2.35 μg/g respectively. Ahmed et al. (2013) and Drabińska et al. (2021) found 267 μg/g and 276 μg/g of TPC in methanolic extracts of cauliflower, respectively [24, 31]. Cabbage showed significantly (p = 0.02) lower amount of TPC in CAME (27 ± 1.15 μg/g) than CAEE (115 ± 2.45 μg/g) and CAWAA (140 ± 3.25 μg/g). Whereas, Jaiswal et al. (2012) reported that the TPC of methanolic extract of cabbage was 187 μg/g [30]. In addition, TTC and TPC showed strong positive (r = 0.953) correlation in all extracts.
3.5. Total antioxidant activity (TAA)
TAA varied according to vegetable and solvent (Figure 1). The ethanolic extract of cabbage showed significantly higher amounts of TAA than the other two vegetables (p = 0.04). The highest amount of TAA was found in CAME, which was 549 ± 7.30 μg/g. Whereas, the TAA of CAEE and CAWAA were 209 ± 5.70 μg/g and 131 ± 3.30 μg/g, respectively. On the other hand, the TAA of CUEE, CUME and CUWAA were 515 ± 5.25 μg/g, 364 ± 6.15 μg/g and 217 ± 3.65 μg/g respectively. Moreover, the antioxidant activity of BCEE, BCME and BCWAA were 265 ± 3.20 μg/g, 290 ± 4.20 μg/g, 175 ± 5.25 μg/g respectively. Bahorun et al. (2004) presented 188 μg/g, 748 μg/g, 499 μg/g of TAA in methanolic extracts of cabbage, broccoli, and cauliflower [32]. Podse et al. (2006) also showed 250 μg/g of TAA in methanolic extract of cabbage, which supports the findings of our study [33].
3.6. DPPH radical scavenging activity
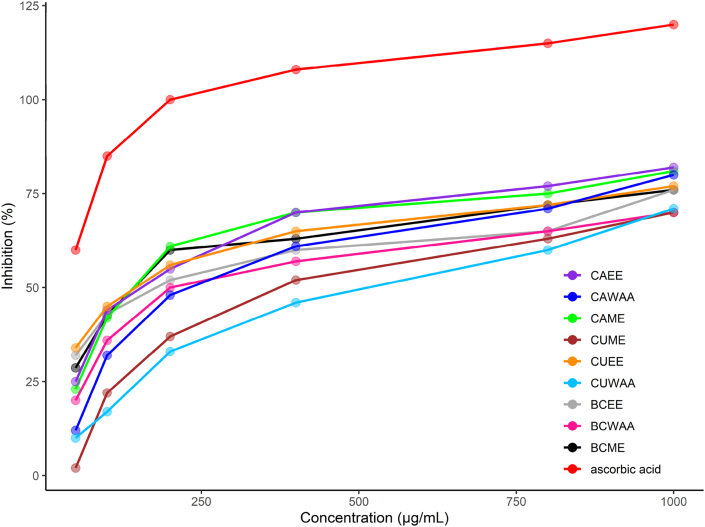
At the tested levels, all of the vegetable extracts and the reference compound (ascorbic acid) were capable of directly interacting with and quenching the DPPH radical (Table 2). In the case of cabbage, methanolic extract was found to have the highest DPPH radical–scavenging capacity with an IC50 value of 90 ± 2.52 μg/mL (Figure 2). On the other hand, Cauliflower was found to have the lowest DPPH radical–scavenging capacity with IC50 400 ± 10 μg/mL in CUWAA followed by 380 ± 4.50 μg/mL in CUME. There was no significant difference in IC50 value between BCME, BCEE and BCWAA. According to Turkmen et al. (2006), solvent polarity may be possible reason of efficiency of different extracts on scavenging of free radicals [34]. This antiradical activity was similar to the extracts of broccoli, cabbage and cauliflower [35, 36, 37, 38].
Figure 2.
Logarithmic trendline for DPPH radical-scavenging activity of cabbage, cauliflower and broccoli extracts and Ascorbic acid. Where, CAME = Methanol extract of cabbage, CAEE = Ethanol extract of cabbage, CAWAA = water, acetone and acetic acid extract of cabbage, CUME = Methanol extract of cauliflower, CUEE = Ethanol extract of cauliflower, CUWAA = water, acetone and acetic acid extract of cauliflower, BCME = Methanol extract of broccoli, BCEE = Ethanol extract of broccoli, BCWAA = water, acetone and acetic acid extract of broccoli.
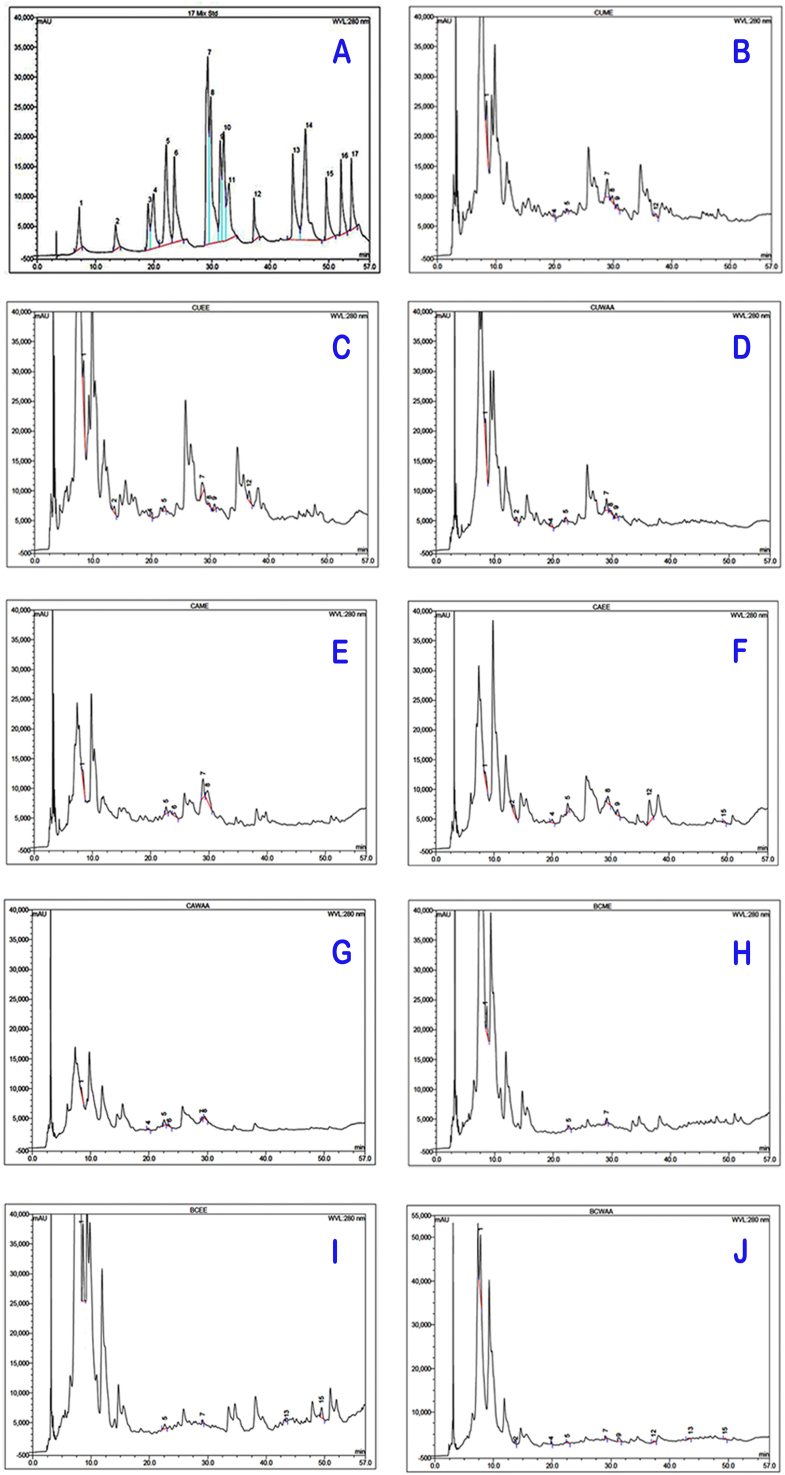
3.7. Bioactive polyphenols
The various phenolic compounds that were found in the extracts of these three brassica vegetables are presented in Table 3, and the corresponding chromatograms are shown in Figure 3. The panels labeled A–J of Figure 3 represent the standards, CUME, CUEE, CUWAA, CAME, CAEE, CAWAA, BCME, BCEE, and BCWAA, respectively. Gallic acid was the most abundant phenolic acid in all types of extracts. CUWAA had significantly (p = 0.03) the highest amount of Gallic acid (881 ± 4.24 μg/g) than others. CAWAA had the lowest amount of Gallic acid (112 ± 2.31 μg/g). A study by Bahorun et al. (2004) found 270 μg/g, 150 μg/g of Gallic acid in methanolic extracts of cauliflower and cabbage respectively [32].
Table 3.
Bioactive polyphenolic compounds (μg/g dry extract) of different extract of three vegetables.
| Compounds (μg/g dry extract) | Retention time (minute) | CUME | CUEE | CUWAA | CAME | CAEE | CAWAA | BCME | BCEE | BCWAA |
|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | 8.33 | 451 ± 3.26c | 518 ± 6.2b | 881 ± 4.24a | 141 ± 3.5g | 122 ± 1.23h | 112 ± 2.31c | 245 ± 2.5f | 290 ± 2.30e | 320 ± 4.15d |
| Chlorogenic Acid | 13.70 | nd | 502 ± 1.7a | 282 ± 1.24c | nd | 115 ± 1.25d | nd | nd | nd | 331 ± 3.60b |
| Catechol | 19.20 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Catechin hydrate | 22.6 | 48 ± 2.51e | 89 ± 1.5d | 113 ± 2.94c | 115 ± 1.5bc | 125 ± 2.5b | 90 ± 2.84d | 440 ± 1.45a | 92 ± 1.20d | 35 ± 1.20f |
| Vanillic acid | 20.10 | 80 ± 3.54b | 540 ± 2.31a | 40 ± 5.1c | nd | 35 ± 3.26d | 20 ± 2.59f | nd | nd | 22 ± 0.50e |
| Caffeic acid | 24.06 | nd | nd | nd | 31 ± 1.45a | nd | 10 ± 1.51b | nd | nd | nd |
| (-) Epicatechin | 29.08 | 158 ± 2.1b | 135 ± 1.36c | 90 ± 1.24e | 210 ± 1.05a | nd | 26 ± 3.20g | 36 ± 1.30f | 29 ± 1.10g | 94 ± 1.05d |
| Vanillin | 31.87 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Trans-Ferulic acid | 32.81 | Nd | nd | nd | nd | nd | nd | nd | nd | nd |
| p-Coumaric acid | 31.34 | 58 ± 0.76e | 340 ± 1.59b | 286 ± 1.7c | nd | 380 ± 3.25a | nd | nd | nd | 65 ± 1.1d |
| Rutin Hydrate | 37.28 | 63 ± 1.23c | 350 ± 2.31b | nd | nd | 551 ± 2.12a | nd | nd | nd | 21 ± 0.75d |
| Rosmarinic acid | 29.76 | 122 ± 1.23d | 202 ± 1.24a | 53 ± 0.81f | 150 ± 2.10b | 142 ± 1.50c | 75 ± 3.5e | nd | nd | nd |
| Myricetin | 43.59 | nd | nd | nd | nd | nd | nd | nd | 34 ± 1.20b | 52 ± 0.65a |
| Quercetin | 49.47 | nd | nd | nd | nd | 33 ± 0.90b | nd | nd | 186 ± 1.5a | 22 ± 0.75c |
| trans-Cinnamic acid | 46.05 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Naringenin | 52.03 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Kaempferol | 53.80 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
Values are mean ± SD, n = 3 (three independent extractions).
Means containing same letter (s) in row did not differ significantly at 5% level of significance.
Where, CAME = Methanol extract of cabbage, CAEE = Ethanol extract of cabbage, CAWAA = water, acetone and acetic acid extract of cabbage, CUME = Methanol extract of cauliflower, CUEE = Ethanol extract of cauliflower, CUWAA = water, acetone and acetic acid extract of cauliflower, BCME = Methanol extract of broccoli, BCEE = Ethanol extract of broccoli, BCWAA = water, acetone and acetic acid extract of broccoli.
nd = not detected.
Figure 3.
Chromatogram of polyphenols: A) Standards, B) CUME, C) CUEE, D) CUWAA, E) CAME, F) CAEE, G) CAWAA, H) BCME, I) BCEE, J) BCWAA. Where, 1 = Gallic acid, 2 = Chlorogenic Acid, 3 = Catechol, 4 = Vanillic acid, 5 = Catechin hydrate, 6 = Caffeic acid, 7= (-) Epicatechin, 8 = Rosmarinic acid, 9 = p-Coumaric acid, 10 = Vanillin, 11 = Trans-Ferulic acid, 12 = Rutin Hydrate, 13 = Myricetin, 14 = trans-Cinnamic acid, 15 = Quercetin, 16 = Naringenin, 17 = Kaempferol.
Chlorogenic acid was found in cabbage and cauliflower. CUEE contained 540 ± 2.31 μg/g of Chlorogenic acid which was the highest among all. The level of Chlorogenic acid in cabbage was 115 ± 1.25 μg/g. In a similar report, Park et al. (2019) showed that Chlorogenic acid in cabbage was 37 μg/g [39].
Catechin hydrate is the second most available phenolic compound in all types of extracts. The highest amount of Catechin hydrate was found in BCME (440 ± 1.45 μg/g). Cabbage contained Catechin hydrate in the range of 113–125 μg/g dry extract. Park et al. (2019) reported that the methanolic extract of cabbage had 11 μg/g of Catechin hydrate [39]. The level of Vanillic acid in CUEE (540 ± 2.31 μg/g) was higher than other extracts. Ahmed and Ali (2013) reported 119 μg/g of Vanillic acid in methanolic extract of cauliflower [31]. Caffeic acid was identified only in cabbage and the amount in CAME and CAWAA was 31 ± 1.45 μg/g, 10 ± 1.51 μg/g respectively. Park et al. (2019) also found similar levels of Caffeic acid (5 μg/g) in the methanolic extract of cabbage [39]. CAME had the highest amount of Epicatechin (210 ± 1.05 μg/g) followed by CUME (158 ± 2.1 μg/g). Park et al. (2019) found 35 μg/g of Epicatechin in methanolic extract of cabbage [39]. P-Coumaric acid was found in all vegetables of the Brassicaceae but it was mostly abundant in ethanolic extracts of cabbage and cauliflower. CAEE had 380 ± 3.25 μg/g of P-Coumaric acid, which was the highest amount of all and was followed by CUEE (340 ± 1.59 μg/g). Whereas, CUWAA (286 ± 1.7 μg/g) contained the highest P-Coumaric acid, followed by BCWAA (65 ± 1.1 μg/g). Paśko et al. (2018) found 270 μg/g of p-Coumaric acid in methanolic extract of broccoli [40]. The level of p-Coumaric acid in CUEE was greater than the findings of Ahmed and Ali (2013), which was 70 μg/g [31]. The highest amount of Rutin Hydrate was found in CAEE (551 ± 2.125 μg/g) followed by CUEE (350 ± 2.31 μg/g). Conversely, Park et al. (2019) showed that cabbage contained 50 μg/g of Rutin Hydrate [39]. Broccoli had small amount of Rutin Hydrate in BCWAA (21 ± 0.75 μg/g). Rosmarinic acid was not detected in broccoli, but nevertheless found in both cabbage and cauliflower. The highest level of Rosmarinic acid was determined in CAME (150 ± 2.10 μg/g) and the lowest in CUWAA (53 ± 0.81 μg/g). Ahmed and Ali (2013) showed that 17.3 μg/g of Rosmarinic acid was present in methanolic extract of cauliflower [31]. Myricetin was found only in Broccoli, ranging from 34 ± 1.20 μg/g (BCEE) to 52 ± 0.65 μg/g (BCWAA). Miean et al. (2001) expressed that 62.5 μg/g of myricetin was estimated in a methanolic extract of broccoli [41]. Quercetin was not detected in Cauliflower but was found in both cabbage and broccoli. The highest amount of Quercetin was recorded in BCEE (186 ± 1.5 μg/g). USDA (2022) claimed 28 μg/g and 33 μg/g of quercetin in methanolic extracts of cabbage and broccoli [42]. Catechol, Vanillin, trans-Cinnamic acid, trans-Ferulic acid, Naringenin and Kaempferol were not detected in any of the extracts of three vegetables.
In addition, Caffeic acid had a strong positive association (r = 0.932) with Gallic acid. At the same time, Gallic acid also had a well-built positive association (r = 0.932) with Myricetin. Whereas, Caffeic acid showed a strong affirmative association (r = 0.932) with Epicatechin and Catechin hydrate. Moreover, Epicatechin showed a moderate positive association (r = 0.602) with Chlorogenic Acid and Quercetin. Catechin hydrate showed a strong positive association (r = 0.952) with p-Coumaric acid and a moderate negative association (r = -0.522) with Quercetin and Myricetin.
In the case of cabbage and broccoli, aqueous extract shows more efficiency in extracting antioxidant compounds, whereas ethanol has good properties for cauliflower. The combination of organic solvent and water may help extract all constituents that are soluble in both water and organic solvents, which may explain why overall extraction efficiency improved. Several variables, including the variation in plant matrix, the composition of antioxidant compounds, the amount of hydroxyl groups in components, the temperature and duration of extraction, as well as solvent polarity, affect the antioxidant capabilities and bioactive compound extraction [13].
Different epidemiological studies over recent years and their linked meta-analyses strongly recommended that intake of diets loaded with plant polyphenols for the long term provides defense against the development of non-communicable chronic health problems like cancers, cardiovascular diseases, diabetes, osteoporosis and neurodegenerative diseases [43]. A short review of the functional activity of the analyzed phenolic compounds was presented in Table 4 and as well depicting the scenario of available polyphenols in different extract to understand the extraction behavior of extracts.
Table 4.
Review on the functional properties of bioactive polyphenols available in the extract of three brassica vegetables (cabbage, cauliflower and broccoli).
| Polyphenol compounds | Structure | Source | Functional properties |
|---|---|---|---|
| Gallic acid | 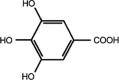 |
Cabbage (CAME, CAEE and CAWAA) Cauliflower (CUME, CUEE and CUWAA) Broccoli (BCME, BCEE and BCWAA) |
|
| Chlorogenic Acid |  |
Cabbage (CAME, CAEE and CAWAA) Cauliflower (CUEE) Broccoli (BCWAA) |
|
| Catechin hydrate |  |
Cabbage (CAME, CAEE and CAWAA) Cauliflower (CUME, CUEE and CUWAA) Broccoli (BCME, BCEE and BCWAA) |
|
| Vanillic acid |  |
Cabbage (CAEE and CAWAA) Cauliflower (CUME, CUEE and CUWAA) Broccoli (BCWAA) |
|
| Caffeic acid |  |
Cabbage (CAME and CAWAA) |
|
| (-) Epicatechin | 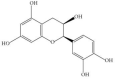 |
Cabbage (CAME and CAWAA) Cauliflower (CUME, CUEE and CUWAA) Broccoli (BCME, BCEE and BCWAA) |
|
| p-Coumaric acid | 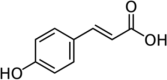 |
Cabbage (CAEE) Cauliflower (CUME, CUEE and CUWAA) Broccoli (BCWAA) |
|
| Rutin Hydrate | 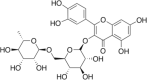 |
Cabbage (CAEE) Cauliflower (CUME and CUEE) Broccoli (BCWAA) |
|
| Rosmarinic acid | 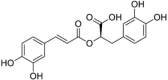 |
Cabbage (CAME, CAEE and CAWAA) Cauliflower (CUME, CUEE and CUWAA) |
|
| Myricetin | 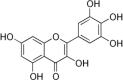 |
Broccoli (BCEE and BCWAA) | |
| Quercetin | 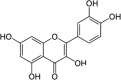 |
Cabbage (CAEE) Broccoli (BCEE and BCWAA) |
|
4. Conclusion
Cauliflower, cabbage and broccoli contained bioactive polyphenols along with remarkable in vitro antioxidant properties. The uniqueness of this work was that it presents a polarity-based comparison of the polyphenolic components and antioxidant profiles of these three brassica vegetables that were extracted in three different solvents. The discussion revealed that the relative polarity of the solvent had an impact on the extraction ability of particular bioactive polyphenols. The funding of this study supported the functional properties of brassica vegetables which might play an important role in human health. It could be said in support of the literature that these vegetables as a whole are perhaps more appropriate to the value-added food products in the food industry. In order to promote the health advantages of such veggies, the extraction solvent may choose to produce functional food by focusing on a particular bioactive polyphenol.
Declarations
Author contribution statement
Mohammad Mahfuzur Rahman: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Abu Tareq Mohammad Abdullah: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Miskat Sharif: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sharmin Jahan; Md. Alamgir Kabir; Md. Motalab: Performed the experiments; Analyzed and interpreted the data.
Tanzir Ahmed Khan: Performed the experiments; Aanalyzed and interpreted the data; Contributed reagents, materials, analysis tools.
Funding statement
Mohammad Mahfuzur Rahman was supported by Bangladesh Council of Scientific and Industrial Research, Bangladesh [39.02.0000.011.14.128.2020/636; dated: 29/12/2020].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ambrosone C.B., Tang L. Cruciferous vegetable intake and cancer prevention: role of nutrigenetics. Cancer Prev. Res. 2009;2:298–300. doi: 10.1158/1940-6207.CAPR-09-0037. [DOI] [PubMed] [Google Scholar]
- 2.Cabello-Hurtado F., Gicquel M., Esnault M.A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem. 2012;132:1003–1009. [Google Scholar]
- 3.Davey M.W., Van Montagu M., Inze D., Sanmartin M., Kanellis A., Smirnoff N., Benzie I.J.J., Strain J.J., Favell D., Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. 2000;80 [Google Scholar]
- 4.Maritess C., Small S., Waltz-Hill M. Alternative nutrition therapies in cancer patients. Semin. Oncol. Nurs. 2005;21:173–176. doi: 10.1016/j.soncn.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Suido H., Tanaka T., Tabei T., Takeuchi A., Okita M., Kishimoto T., Kasayama S., Higashino K. A mixed green vegetable and fruit beverage decreased the serum level of low-density lipoprotein cholesterol in hypercholesterolemic patients. J. Agric. Food Chem. 2002;50:3346–3350. doi: 10.1021/jf0116698. [DOI] [PubMed] [Google Scholar]
- 6.Moon J.K., Kim J.R., Ahn Y.J., Shibamoto T. Analysis and anti- helicobacter activity of sulforaphane and related compounds present in Broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010;58(11):6672–6677. doi: 10.1021/jf1003573. [DOI] [PubMed] [Google Scholar]
- 7.Herr I., Büchler M.W. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36:377–383. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Evans P.C. The influence of sulforaphane on vascular health and its relevance to nutritional approaches to prevent cardiovascular disease. EPMA J. 2011;2:9–14. doi: 10.1007/s13167-011-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q.J., Yang Y., Vogtmann E., Wang J., Han L.H., Li H.L., Xiang Y.B. Crucif. Veg. Intake risk color. Cancer a meta-analysis obs. For. Stud. 2012;24:1079–1087. doi: 10.1093/annonc/mds601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steevens J., Schouten L.J., Goldbohm R.A., van den Brandt P.A. Veg. Fruits consum. Risk esophageal gastric cancer subtypes Netherlands Cohort study. Randomized Contr. Trial. 2011;129:2681–2693. doi: 10.1002/ijc.25928. [DOI] [PubMed] [Google Scholar]
- 11.Alberts B., Johnson A., Lewis J. fourth ed. GarlandScience.Availablefrom; New York: 2002. Molecular Biology of the Cell.https://www.ncbi.nlm.nih.gov/books/NBK26928 [Google Scholar]
- 12.Ben Attia I., Zucca P., Cesare Marincola F., Piras A., Rosa A., Chaieb M., Rescigno A. Chemical composition and antioxidant potential differences between Cynomorium coccineum L. Growing in Italy and in Tunisia: effect of environmental stress. Diversity. 2018;10 [Google Scholar]
- 13.Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan T., Ipshita A., Mazumdar R., Abdullah A., Islam G., Rahman M. Bioactive polyphenol profiling and in-vitro antioxidant activity of Tinospora cordifolia Miers ex Hook F and Thoms : a potential ingredient for functional food development. Bangladesh J. Sci. Ind. Res. 2020;55:23–34. [Google Scholar]
- 15.Shaheen N., Goto M., Watanabe J., Takano-Ishikawa Y. Antioxidant capacity and total phenol content of commonly consumed indigenous foods of Asian tropical regions. J. Food Sci. Eng. 2012;2:187. [Google Scholar]
- 16.Mamun S., Shaheen N., Basak Tukun A., Mohiduzzaman M., Banu C.P., Takano-Ishikawa Y. Hydrophilic antioxidant capacities and total phenol content of seasonal fruits of Bangladesh. Malays. J. Nutr. 2012;18:355–362. [PubMed] [Google Scholar]
- 17.Chang S.T., Wu J.H., Wang S.Y., Kang P.L., Yang N.S., Shyur L.F. Antioxidant activity of extracts from acacia confusa Bark and Heartwood. J. Agric. Food Chem. 2001;49:3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- 18.Haile M., Kang W.H. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation. 2019;5 [Google Scholar]
- 19.Johari M.A., Khong H.Y. Total phenolic content and antioxidant and antibacterial activities of pereskia bleo. Adv. Pharmacol. Sci. 2019;2019:1–4. doi: 10.1155/2019/7428593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jan S., Khan M.R., Rashid U., Bokhari J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of monotheca buxifolia fruit. Osong Publ. Heal. Res. Perspect. 2013;4:246–254. doi: 10.1016/j.phrp.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanna D., Delogu G., Mulas M., Schirra M., Fadda A. Determination of free radical scavenging activity of plant extracts through DPPH assay: an EPR and UV-vis study. Food Anal. Methods. 2012;5:759–766. [Google Scholar]
- 22.Sembiring E.N., Elya B., Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018;10:123–127. [Google Scholar]
- 23.Chuanphongpanich S., Phanichphant S. Method development and determination of phenolic compounds in broccoli seeds samples. Chiang Mai J. Sci. 2006;33:103–107. [Google Scholar]
- 24.Drabińska N., Jeż M., Nogueira M. Variation in the accumulation of phytochemicals and their bioactive properties among the aerial parts of cauliflower. Antioxidants. 2021;10:1597. doi: 10.3390/antiox10101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhandari S.R., Kwak J.H., Jo J.S., Lee J.G. Changes in phytochemical content and antioxidant activity during inflorescence development in broccoli. Chil. J. Agric. Res. 2019;79:36–47. [Google Scholar]
- 26.Singh J., Upadhyay A.K., Bahadur A., Singh B., Singh K.P., Rai M. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata) Sci. Hortic. (Amsterdam) 2006;108:233–237. [Google Scholar]
- 27.Manchali S., Murthy K.C., Patil B. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct.Foods. 2012;4:94–106. [Google Scholar]
- 28.Djenidi H., Khennouf S., Bouaziz A. Antioxidant activity and phenolic content of commonly consumed fruits and vegetables in Algeria. Prog. Nutr. 2020;22:224–235. [Google Scholar]
- 29.Deepa P., Sowndhararajan K., Park S.J. 2020. Polyphenolic Contents and Antioxidant Activity of Brassicaceae Sprouts Cultivated in the Plant Factory System. [Google Scholar]
- 30.Jaiswal A.K., Abu-Ghannam N., Gupta S. A comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012;47:223–231. [Google Scholar]
- 31.Ahmed F.A., Ali R.F.M. Bioactive compounds and antioxidant activity of fresh and processed white cauliflower. BioMed Res. Int. 2013;2013:9–20. doi: 10.1155/2013/367819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahorun T., Luximon-Ramma A., Crozier A., Aruoma O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritius vegetables. J. Sci. Food Agric. 2004;84:1553–1561. [Google Scholar]
- 33.Podse˛ dek A., Sosnowska D., Redzynia M., Anders B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Int. J. Food Sci. Technol. 2006;41:49–58. [Google Scholar]
- 34.Turkmen N., Sari F., Velioglu Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006;99:835–841. [Google Scholar]
- 35.Miller H.E., Rigelhof F., Marquart L., Prakash A., Kanter M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J. Am. Coll. Nutr. 2000;19:312S–319S. doi: 10.1080/07315724.2000.10718966. [DOI] [PubMed] [Google Scholar]
- 36.Guo J.-T., Lee H.-L., Chiang S.-H., Lin F.-I., Chang C.-Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001;9 [Google Scholar]
- 37.Lin C.H., Chang C.Y. Textural change and antioxidant properties of broccoli under different cooking treatments. Food Chem. 2005;90:9–15. [Google Scholar]
- 38.Llorach R., Espín J.C., Tomás-Barberán F.A., Ferreres F. Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics. J. Agric. Food Chem. 2003;51:2181–2187. doi: 10.1021/jf021056a. [DOI] [PubMed] [Google Scholar]
- 39.Park C.H., Yeo H.J., Park S.Y., Kim J.K., Park S.U. Comparative phytochemical analyses and metabolic profiling of different phenotypes of Chinese cabbage (Brassica rapa ssp. Pekinensis) Foods. 2019;8:587. doi: 10.3390/foods8110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paśko P., Tyszka-Czochara M., Galanty A., Gdula-Argasińska J., Żmudzki P., Bartoń H., Zagrodzki P., Gorinstein S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018;73:95–100. doi: 10.1007/s11130-018-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 42.USDA Database for the Flavonoid Content of Selected Food. 2022. www.ars.usda.gov/arsuserfiles/80400525/data/flav/flav_r03.pdf
- 43.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanwar A.A., Badole S.L., Shende P.S., Hegde M.V., Bodhankar S.L. Role of gallic acid in cardiovascular disorders. Polyphenols Hum. Heal. Dis. 2013:1045–1047. [Google Scholar]
- 45.Tajik N., Tajik M., Mack I., Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur. J. Nutr. 2017;56:2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- 46.Kim J.M., Heo H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022:1–14. doi: 10.1007/s10068-022-01069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H.J., Il Jeong Y., Lee T.H., Jung I.D., Lee J.S., Lee C.M., Il Kim J., Joo H., Lee J.D., Park Y.M. Rosmarinic acid inhibits indoleamine 2,3-dioxygenase expression in murine dendritic cells. Biochem. Pharmacol. 2007;73:1412–1421. doi: 10.1016/j.bcp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Calixto-Campos C., Carvalho T.T., Hohmann M.S.N., Pinho-Ribeiro F.A., Fattori V., Manchope M.F., Zarpelon A.C., Baracat M.M., Georgetti S.R., Casagrande R., Verri W.A. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015;78:1799–1808. doi: 10.1021/acs.jnatprod.5b00246. [DOI] [PubMed] [Google Scholar]
- 49.Birková A., Hubková B., Bolerázska B., Mareková M., Čižmárová B. Caffeic acid: a brief overview of its presence, metabolism, and bioactivity. Bioact. Compd. Heal. Dis. 2020;3:74–81. [Google Scholar]
- 50.Abdulkhaleq L.A., Assi M.A., Noor M.H.M., Abdullah R., Saad M.Z., Taufiq-Yap Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World. 2017;10:869–872. doi: 10.14202/vetworld.2017.869-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pei K., Ou J., Huang J., Ou S. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016;96:2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- 52.Ganeshpurkar A., Saluja A. The pharmacological potential of catechin. Indian J. Biochem. Biophys. 2020;57:505–511. [Google Scholar]
- 53.Alagawany M., El-Hack A., Farag M., Gopi M., Karthik M., Malik K.Y., Dhama K. Rosmarinic acid modes action. Med. Values Heal. Benefits. 2017;18(n.d.):167–176. doi: 10.1017/S1466252317000081. [DOI] [PubMed] [Google Scholar]
- 54.Semwal D.K., Semwal R.B., Combrinck S., Viljoen A. Myricetin: a dietary molecule with diverse biological activities. Nutrients. 2016;8:90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.