Abstract
Endometriosis that afflicts one in 10 women of reproductive age is characterized by growth of endometrial tissue in the extra-uterine sites and encompasses metabolic-, immunologic-, and endocrine-disruption. Importantly, several comorbidities are associated with endometriosis, especially autoimmune disorders such as inflammatory bowel disease. Primarily thought of as a condition arising from retrograde menstruation, emerging evidence uncovered a functional link between the gut microbiota and endometriosis. Specifically, recent findings revealed altered gut microbiota profiles in endometriosis and in turn this altered microbiota appears to be causal in the disease progression, implying a bidirectional crosstalk. In this review, we discuss the complex etiology and pathogenesis of endometriosis, emphasizing on this recently recognized role of gut microbiome. We review the gut microbiome structure and functions and its complex network of interactions with the host for maintenance of homeostasis that is crucial for disease prevention. We highlight the underlying mechanisms on how some bacteria promote disease progression and others protect against endometriosis. Furthermore, we highlight the areas that require future emphases in the gut microbiome–endometriosis nexus and the potential microbiome-based therapies for amelioration of endometriosis.
Keywords: gut microbiome, endometriosis, female reproductive health
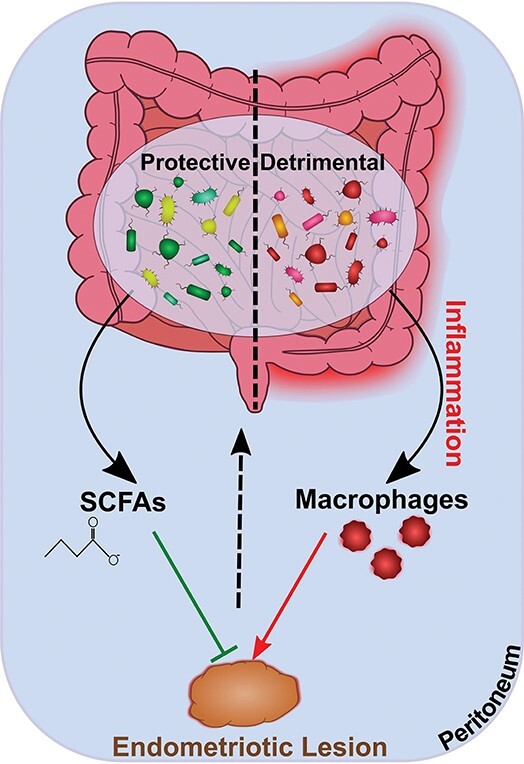
The commensal gut microbes prevent endometriotic lesions formation through protective effect of short chain fatty acids (SCFAs) while other gut microbes may promote lesion formation in the state of dysbiosis through disruption of gut barrier integrity and the resulting macrophage activation.
Graphical abstract
Introduction
The earliest notions of the genotype shaping the organismal biology singularly are overthrown with dramatic advances in our understanding of the “microbiome,” and its staggering role in shaping the physiological, psychological, and reproductive phenotypes of the host. The resident microorganisms and their combined gene pool expand the host functions multitudinally, such that the microbiome is essentially recognized as an “extended human genome” [1]. Majority of these microbes are harbored in the gastrointestinal tract with explicit roles in virtually all host biological processes. In turn, the gut microbiota is impacted by several host-associated and environmental factors involving but not limited to diet, immune status, and exposure to toxins and antibiotics. These individual variations muddle our understanding of the microbiome, yet each study inch us closer to the association between gut microbiome perturbations and disease resistance or susceptibility, leading us to argue upon how we explore our genetic fitness landscapes. The importance of host–microbial balance has been widely elaborated in metabolic and immunological as well as neuronal processes. On the other hand, the gut microbiome–reproductive health nexus remains underappreciated. Nevertheless, most of the reproductive tract pathologies are regulated by hormonal imbalance that apparently result, in part, from the interactions of microbiota with gut milieu and the resulting influences on immunologic signaling.
Reproductive health, particularly in females, is of major concern that can lead to several diseases, such as endometriosis, polycystic ovary syndrome (PCOS), pregnancy complications, infertility, and cancer. These diseases have distinct clinical manifestations, but all have been linked to the underlying gut microbiome perturbations and loss of intestinal integrity. Under this scenario, corrections of perturbed gut microbiota are being viewed as an alternative strategy to prevent and treat several female reproductive pathologies. Endometriosis is one of the most frequently encountered pathological conditions with incidences reported in one out of every 10 females in the reproductive age (18–52 years) and affecting up to ~196 million women worldwide [2]. The condition is of serious global concern as up to half of the women (35–50%) presenting for infertility are afflicted with endometriosis [3]. The disease is characterized by the growth of endometrium that normally lines the uterus outside, mainly in ovaries, fallopian tubes and pelvic tissues. Intriguingly, the condition arises in only 10% of the women, even when a small degree of retrograde menstruation is experienced by almost all women [4]. Recent studies have revealed altered gut microbiota profiles during endometriosis and effects of microbial metabolic by-products on disease progression [5, 6]. This arise the possibility of targeting the commensal gut microbes to re-establish homeostasis in this hormone-mediated inflammatory disease [7–10]. Here, we summarize the theories concerning the origin of endometriosis and the underlying mechanisms of disease progression. We discuss the gut microbiome and complex network of its interactions with the host encompassing metabolic, immune-, and neuroendocrine-modulatory effects that, if disrupted, may all contribute to endometriosis progression in women. Besides structural changes in gut microbiota due to endometriosis, we review the recent knowledge gained on how gut microbiome may affect and potentially contribute to disease progression in this bidirectional crosstalk. Of clinical relevance, we discuss the potential of microbiome-based therapeutic strategies that can be capitalized upon for the treatment of endometriosis.
Endometriosis
Endometriosis is a poorly understood disease characterized by the growth of endometrial glands and stroma tissue outside of the uterus in the pelvic peritoneal cavity, ovaries, bowel, uterosacral ligaments, pouch of Douglas, rectovaginal septum, and even extra-pelvic sites [11–13]. These extra-uterine implants act much like eutopic endometrium bleeding cyclically in response to estrogen and result in acute chronic inflammation that causes severe pelvic pain [14].
Prevalence and theories of origin
At a global prevalence rate of 10%, endometriosis is a much common, but major concern in women of reproductive age. Five out of every 10 women with pelvic pain or infertility are affected with endometriosis [15]. The incidences of the condition are reported in about 2–7/1000 women per year [15]. This number, however, is an underestimate and a further 11% of the asymptomatic cases remain undiagnosed or are not reported in general population [16]. Moreover, it is suggested to be a transient phenomenon and that all women have endometriosis at some stage of their reproductive cycle [17].
Multiple hypotheses to describe the origin of endometrium-like tissue at ectopic sites have been proposed, all of which are based on two opposing ideas. First, endometriosis develops on the site where it is found (in situ development), and second, endometrial tissue is transplanted and subsequently implanted onto extra-uterine sites (transplantation theory). The theories of “coelomic metaplasia” and “induction” support the idea of in situ development of endometriosis through metaplastic transformations of coelomic membrane [18] or induction from unknown substances that are released from degenerating endometrium in menstruating women [19]. These theories, however, lack experimental evidence to support the development of endometrial stroma at ectopic sites from induced metaplastic changes. The most widely accepted theory describing the cause of endometriosis is Sampson theory of retrograde menstruation [4, 20] that supports the idea of “transplantation or implantation.” The theory proposes that the endometrial tissue, which is otherwise expelled out of the uterus through vagina, flows backward through the fallopian tubes into the peritoneal cavity [4, 20, 21] and that allows for the entry of endometrial cells in the ectopic space. Building upon the concept of retrograde menstruation, the “lymphatic dissemination” of the endometrial fragments for implantation to distant ectopic sites is also proposed [4]. All hypotheses are based on the different genetic, epigenetic, hormonal, immunologic, inflammatory, angiogenic, and lymphangiogenic components involved in the pathogenesis, yet none is singly able to explain the different manifestations of the disease. In this view, composite theories such as those combining implantation and lymphatic dissemination are also proposed [22].
Pathogenesis of endometriosis
Much like its origin, the pathogenesis of the disease remains mystifyingly elusive. The disease presents itself in heterogenous manifestations based on where the endometriotic tissue implants: (a) superficial peritoneal endometriosis—the most common type of endometriosis when atypical lesions grow superficially on the peritoneum; (b) ovarian endometrioma—endometrial tissue implant into ovaries progressing from very early lesions to typical dark, fluid-filled cysts with adhesions; and (c) deeply infiltrating endometriosis—a less common manifestation when endometriotic tissue implants >5 mm below the peritoneum [11]. These multiple manifestations of the disease and a substantial number of women that experience retrograde menstruation yet do not develop endometriosis suggest that a range of factors that may be genetic, lifestyle-related, or environmental, hormonal, immunological are involved in its pathogenesis. These factors and the aberrant expression of genes/proteins under each of these classes are summarized in Table 1. Genome-wide association studies to identify causal variants that may predispose women to this disease implicate genes for estrogen-induced cell growth, inflammatory cytokines, steroid hormone receptors, adhesion molecules, cellular damage, and growth and differentiation [14, 23–27]. Epigenetic modifications that result in reduced expression of genes involved in homeostasis are also seen in women with endometriosis [28–30]. The dietary components that exert pro-inflammatory and oxidative effects are also thought to play significant roles in endometriosis development. For instance, consumption of fruits and green vegetables reduces the risk of endometriosis due to anti-inflammatory and antioxidant effects of vitamins A and C, whereas red meat consumption increases the risk by promoting higher estrogen levels [31, 32]. Exposure to environmental toxins such as dioxins and bisphenol A promote endometriosis possibly through epigenetic modifications and altering uterine structure to promote retrograde menstruation [33–38]. However, an indefinitive diagnosis limits the studies concerning causal relationship between endometriosis and diet or environmental toxins [39].
Table 1.
Factors involved in pathogenesis of endometriosis
| Factor | Mechanism | Dysregulated proteins/pathways | References |
|---|---|---|---|
| Cellular adhesion and invasion | Endometrial expression of cell adhesion molecules is increased for attachment of endometrial tissue to the peritoneum | ↑Integrins, MMPs, VEGF, peritoneal macrophages, pro-inflammatory cytokines ↓Selectins, TIMPs |
[166, 167] |
| Hormonal (estrogen/progesterone) imbalance | Estradiol synthesis in endometrium is increased; decreased estrogen inactivation; progesterone resistance and inaction | ↑Pro-inflammatory cytokines, NF-kappaB signaling, Akt phosphorylation, E2, ER-beta, COX-2, aromatase, PGE2 ↓P4, ER-alpha, 17beta-HSD2, PR- beta |
[41, 43, 168] |
| Immune dysfunction and inflammation | Enhanced expression of pro-inflammatory cytokines; compromised immunosurveillance, chronic inflammation | ↑Peritoneal macrophages and other immune cells, pro-inflammatory cytokines- IL-6, IL-8, TNF-alpha, IL-1beta, TGF-beta; NO, COX-2, iNOS, PGE2 | [168–171] |
| Cell proliferation and survival | Reduced endometrial apoptosis; enhanced stromal and epithelial cell proliferation | ↑NF-kappaB, bcl-2, FASL ↓Caspase, BAX, FAS |
[172–174] |
| Angiogenesis, lymphangiogenesis, and neuroangiogenesis | Increased angiogenic and lymphangiogenic potential in eutopic endometrium to facilitate survival at ectopic locations; abnormal growth of nerve fibers, leading to pain | ↑Epithelial to mesenchymal transition (EMT), VEGF, PDGF, FGF, HIF-1alpha, Ang-1, Ang-2, Tie-2, SDF-1, erythropoietin, neutrophins, nerve fibers | [175–181] |
| Oxidative stress | Disrupted balance of ROS production and antioxidant defense | ↑ROS, RNS, iNOS, eNOS, pro-oxidant enzymes ↓Antioxidant enzymes: SOD, GPx |
[25, 182] |
| Genetic and epigenetic | Cellular functions are altered for lesion growth and immune evasion | Hypermethylation of PR-beta and HOXA-10 genes promoter; hypomethylation of ER-beta and SF-1 genes promoter; ↑PR gene polymorphisms (PROGINS), mir-196a, mir-29c ↓mir-9, mir-34 |
[14, 27, 183, 184] |
| Diet and environment | External factors that exert pro-inflammatory and oxidative effects | ↑Endometriosis risk—red meat, alcohol, environmental toxins such as dioxins, PCBs, BPA, DES ↓Endometriosis risk—PUFAs, Vitamins A and C -No effect—Smoking |
[31–33, 35–38] |
MMPs, matrix metalloproteinases; VEGF, vascular endothelial growth factor; TIMPs, tissue inhibitors of metalloproteinases; E2, estrogen; ER-beta, estrogen receptor beta; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; P4, progesterone; ER-alpha, estrogen receptor alpha; 17beta-HSD2, 17beta-hydroxysteroid dehydrogenase 2; PR-B, progesterone receptor beta; TNF-alpha, tumor necrosis factor alpha; TGF-beta, transforming growth factor beta; NO, nitric oxide; iNOS, inducible nitric oxide synthase; bcl-2, B-cell lymphoma 2; FASL, Fas ligand; BAX, BCL2-associated X; FAS, Fas cell surface death receptor; PDGF, platelet-derived growth factor; FGF, fibroblast growth factor; HIF-1alpha, hypoxia-inducible factor 1-alpha; Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; SDF-1, stromal cell-derived factor 1; ROS, reactive oxygen species; RNS, reactive nitrogen species; eNOS, endothelial nitric oxide synthase; SOD, superoxide dismutase; GPx, glutathione peroxidase; HOXA-10, homeobox A10; PCBs, polychlorinated biphenyls; BPA, bisphenol A; DES, diethylstilbesterol; PUFAs, polyunsaturated fatty acids.
Regarded as estrogen-dominant condition, the disease progression is known to be mediated by high expression of enzymes that convert androgens to estrogens and deficiency of enzymes that convert estrogen to weak estrone [40–42]. The high estrogen levels induce expression of these enzymes to enable a positive feedback loop that exacerbates further estrogen production [41]. Synergistic to estrogen production is the suppression as well as resistance to progesterone that, in general, acts to halt estrogen-driven endometrial proliferation, elicits differentiation, and reduces inflammation [43–45]. There is increased adhesion, aberrant expression of growth factors and proteolytic enzymes, apoptotic suppression, and parallel angiogenesis and neuroangiogenesis during pathogenesis of endometriosis that promote lesion growth at ectopic sites [46–48]. Of these, the chronic inflammation resulting from defective immune clearance of sloughed endometrium is considered the most important hallmark central to the disease progression.
Immune dysfunction and inflammation in endometriosis
The refluxed endometrial tissue at the ectopic sites is processed through adhesion, acute inflammation, macrophage infiltration, and tissue remodeling. The initial attachment is mediated by increased adhesive properties of the endometrial cells resulting from enhanced expression of integrins [49]. This adhesion is further promoted by chronic inflammatory peritoneal environment, partly achieved from high estrogen levels [50]. Macrophages are key immunologic cells that are recruited in both eutopic endometrium and ectopic lesions in women with endometriosis independent of hormonal cycle [51]. The high levels of macrophages help secrete pro-inflammatory cytokines such as transforming growth factor-beta1 (TGF-beta1), interleukin-8 (IL-8), IL-6, tumor necrosis factor-alpha (TNF-alpha), and IL-1beta for activation of nuclear factor kappa light chain enhancer of activated B cells (NF-kappaB) signaling [52–54]. Surges in cytokines promote the recruitment of immune cells and production of more cytokines, permitting a persistent inflammatory peritoneal milieu to favor angiogenesis [55–57]. Consequent activation of vascular endothelial growth factor (VEGF) expression, cell cycle, and anti-apoptotic gene B-cell lymphoma 2 (bcl-2) also mediate pathogenesis [58]. Angiogenesis is further favored by the chronic inflamed state that exposes damaged tissues to provide for an adequate blood supply to the growing implants [59]. The estrogen receptor beta, Erbeta, is involved in the (1) inhibition of TNF-alpha-induced apoptosis signaling [60], (2) direct activation of the NF-kappaB pathway and radical oxygen species detoxification system for enhanced cell survival and escape from immune clearance, (3) upregulation of hypoxia-induced signaling, and (4) epithelial mesenchymal transition signaling, which are all involved in progression of endometriosis [61].
Intriguingly, women with endometriosis are shown more likely to have comorbidities that are associated with heightened inflammation. These include inflammatory bowel disease (IBD) and metabolic disorders—obesity and diabetes—while it has been also linked with other autoimmune disorders such as systemic lupus, multiple sclerosis [62, 63], atopic diseases [62], neurological disorders [64, 65], as well as cancers [66]. The molecular mechanisms linking the condition with these chronic diseases are still unknown; however, a recent view embraces a central role of gut microbiome in the onset and progression of endometriosis. The two primary drivers of endometriosis are hormonal and immune dysregulation, both of which are profoundly governed by microbial members in the gut. The sex steroid hormones, estrogen and progesterone, are well known to mediate bidirectional crosstalk between host and microorganisms [67]. Aberrant gut microbiota profiles have also been linked to the disruption of immune function including elevation of pro-inflammatory cytokines, compromised immunosurveillance, and altered immune cell profiles, all of which are implicated in pathogenesis of endometriosis. Whether these altered microbiome profiles result from the disease or are instead involved in its causation is work in progress. Therefore, the role of gut microbiota in the complex multifactorial etiology of endometriosis involving genetic predisposition, prenatal endocrine disruption, immune dysfunction, and the sex hormones must be explored.
Gut microbiome
Exploring the role of microorganisms that live in close association with human hosts has been of tremendous interest in the last two decades. Since the human microbiome project, the estimated count of these microbial partners was estimated to be 10–100 trillion, exceeding the human cells by a factor of 10 with a breadth of genes that dwarfs that in human genome [68]. Even though the numbers have been revised to a 1:1 ratio of human and microbial cells [69], the latter are rightly perceived to be our “last organ” [70]. The complex assemblage of microbes, including bacteria, archaea, viruses, phages, yeast, and fungi, that occupy the body space are referred to as the “microbiota,” with their genes together constituting the “microbiome” [71]. Human intestinal tract harbors most of these microbes, providing one of the largest interfaces for interactions with environmental factors, especially the large intestine with conditions optimum for microbial growth such as slow flow rates and neutral to mildly acidic pH [72]. The advances of molecular biology techniques, including 16S ribosomal RNA sequencing and metagenomics, have been instrumental in identifying the large gut microbiota diversity. Interestingly, the adult human gut microbiota is not as diverse as that from other bodily sites and reveals a high degree of functional redundancy [73, 74]. Typically, it is dominated by bacteria of the phyla Bacteroidetes and Firmicutes (together representing up to 90% of gut microbiota), Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia [75–77]. The members have been further identified at different taxonomic levels, with Firmicutes largely represented by genera such as Clostridium, Lactobacillus, Bacillus, Enterococcus, and Ruminococcus [10, 75]; Bacteroides and Prevotella predominantly represent Bacteroidetes; Proteobacteria involve Escherichia and Desulfovibrio; and the less abundant Actinobacteria is represented by Bifidobacterium, whereas Verrucomicrobia by Akkermansia spp. [75]. Despite the established beneficial roles of several commensal bacteria, there is not a defined composition of healthy human gut microbiota but a host–microbe balance that is crucial for disease prevention. Any change in the structural and/or functional profiles of gut microbiome that disrespects this homeostatic balance (eubiosis) can cause dysbiosis and a range of disorders.
Gut microbiome and host physiology
Gut microbiome encodes the ability to perform a variety of functions that are implicated in host physiology and diseases (Figure 1). These functions range from synthesis of micronutrients to metabolism of complex carbohydrates as well as regulation of signaling pathways. Importantly, these microbial catalytic pathways produce short chain fatty acids (SCFAs) and anti-inflammatory lipids as important energy sources for gut epithelial cells [78]. In addition, microbial regulation of host metabolism helps in release of gut hormones such as cholecystokinin, peptide YY, glucagon-like peptide-1 from enteroendocrine cells regulating insulin sensitivity, glucose tolerance, fat storage, and appetite [79]. Gut commensals also prevent bacterial invasion by maintaining the intestinal epithelium integrity [80] and respond to perturbations in the niche via secretion of a wide range of bioactive molecules involved in microbe–microbe–host communications through complex antimicrobial and immunomodulatory activities [81]. Through these responses, gut microbiome modulates almost all major host metabolic and physiologic functions, even expanding to crucial gut–lung and gut–brain axes and controlling several human pathologies.
Figure 1.
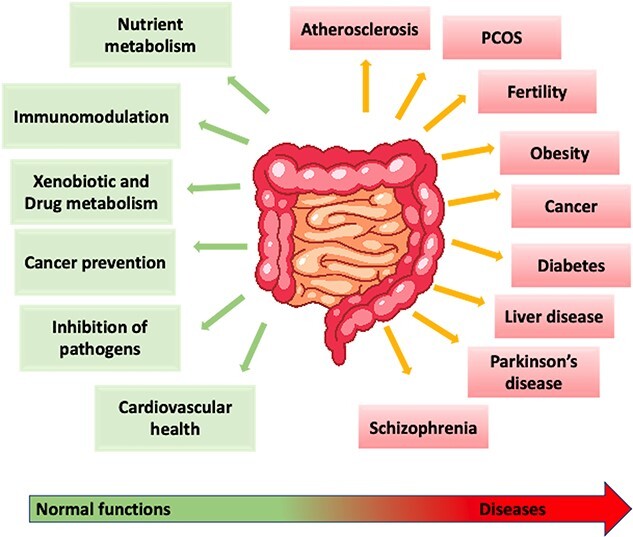
Impact of gut microbiome on host physiology. The gut microbiome influences several host metabolic pathways in a healthy state of normobiosis (green), whereas an imbalance in its composition or dysbiosis (red) is implicated in several diseases.
The two dominant phyla: gram-positive Firmicutes and gram-negative Bacteroidetes play pivotal roles in gut microbiota-mediated functions such that changes in their ratios markedly influence host health status [82]. Members of these phyla form three main groups of strict gut extremophile anaerobes: Bacteroides, Clostridium cluster XIVa (Clostridium coccoides group), and Clostridium cluster IV (Clostridium leptum group) [83, 84]. Bacteroides spp. function as “generalists” that can grow on a variety of substrates for making them available to other non-utilizing commensals [85]. Through their complex starch utilization systems (sus), Bacteroides spp. fundamentally process complex dietary carbohydrates and host-derived glycans into simpler ones [85]. For instance, Bacteroides thetaiotaomicron encodes several sus-like polysaccharide utilization loci with carbohydrate-active enzymes to degrade a wide range of glycan substrates [86]. More nutritionally specialized members from phyla Firmicutes, Actinobacteria, and Verrucomicrobia play critical roles within the community by degradation of complex plant cell walls, starch, as well as mucin [87]. Several Firmicutes spp. produce SCFAs from complex glycans. For instance, Clostridium spp. of the groups IV and XIVa produce most of the butyrate, which is the main energy source of gut epithelial cells [84]. Besides, Actinobacteria, importantly Bifidobacterium spp., functions as probiotics [88], whereas mucin-degrading members of Verrucomicrobia, in particular, Akkermansia muciniphila hydrolyzes up to 85% of mucin and serves to fortify gut epithelial integrity [89]. A large number of other microbes including those with yet undefined functions maintain host physiology and homeostasis through intricate communications while they reside within the host.
Immune homeostasis
Gut microbiota closely interact and co-evolve with intestinal immunologic cells to protect the host against pathogens while simultaneously conditioning them to develop tolerance to the healthy gut microbiota [90]. This phenomenon is crucial for maintaining immune homeostasis. For example, macrophages within gut develop “inflammation anergy,” which maintain a non-inflammatory profile when they encounter microbial stimuli such as Toll-like receptor (TLR) ligands in homeostatic conditions [91]. The strong communication between microbes and intestinal immunologic cells is observed through the deficient profiles of secretory immunoglobulin A (IgA) and cytotoxic T cells in gut-associated lymphoids due to microbial absence [92] and lower mast cell densities in germ-free mice [93]. Certain groups of gut commensals are also involved in maintaining adaptive immune homeostasis (Figure 2). For example, segmented filamentous bacteria potently induce Th17 cells [94]. Clostridia of cluster IV and XIVa can induce regulatory T cells (Tregs) [95], whereas polysaccharide A of Bacteroides fragilis can mediate suppression of systemic Th1 response by signaling through TLR2 on Tregs [96] (Figure 2). Moreover, colonic Tregs possess a unique repertoire of T-cell receptors (TCR) optimized for recognition of colonic bacteria [97] (Figure 2). Thus, colonic Tregs are educated by gut microbial commensals such that altered gut microbiota structure may lead to a pathological outcome. In addition, DNA of microbial origin is also shown to maintain immune homeostasis by limiting Treg cell conversion through TLR9 signaling in the intestinal sites [98]. The gut microbiome also condition CD8+ T cells to modulate other peripheral immune cells, such as marginal zone B cells, plasmacytoid dendritic cells (DCs), and invariant natural killer T cells [99, 100]. Therefore, gut microbiome and immune homeostasis maintain a delicate balance that, if disrupted, may trigger inflammation and, subsequently, a number of pathologies.
Figure 2.
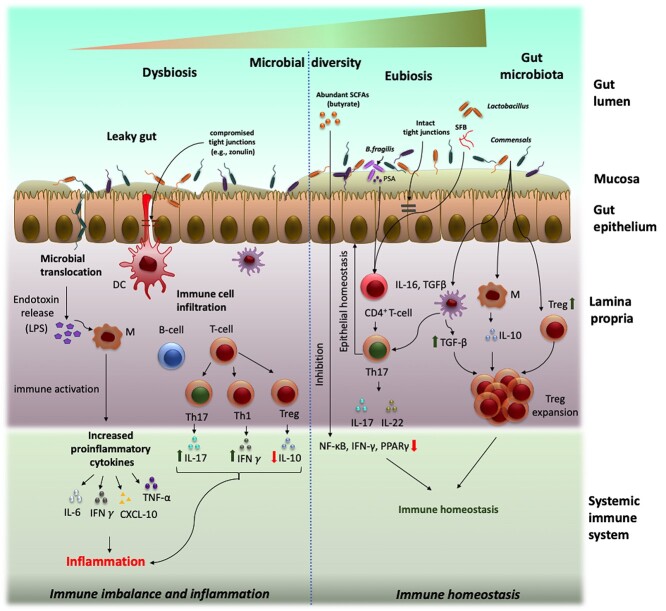
Gut–microbiome barrier integrity and dysfunction. Gut microbiome participates in a complex network of communications with the host and orchestrates immune responses and maintains gut barrier integrity implicated in host health and diseases. Under healthy state or “eubiosis” (right), the high gut microbial diversity exhibits colonization resistance and checks pathogenic population. The intact mucus layer prevents microbial translocation while interactions with select commensals and with their metabolites (such as SCFAs) at the interface educate the host immune system for maintenance of homeostasis. Under conditions of “dysbiosis” (left), the integrity of gut epithelial layer is compromised due to reduction in gut commensals that leads to microbiota translocation through the “leaky gut,” thereby activating immune responses and a chronic inflammatory state.
Intestinal integrity
The gut epithelial layer, together with the biochemical mucus layer, the tight junction proteins, the vascular and cellular immune systems, acts as a barrier, whereas the gut microbes orchestrate intestinal immune responses. The integrity of the epithelial layer is pivotal to maintaining this gut–microbial immune homeostasis. A compromised barrier integrity, referred to as the “leaky-gut,” may arise from gut–microbe imbalance and may lead to systemic entry of harmful microbes and abnormal perpetuation of mucosal and intestinal immune responses (as illustrated in Figure 2). In this context, the mucus layer of the gut offers first line of defense and functions at the interface to prevent the luminal microbes and limit pathogenic interactions. The gut microbes inhabit the outer mucosal layer (luminal), whereas the inner sterile mucosa harbors abundant immunologic cells [101]. To favor or limit a species’ colonization is selected for by the host through the mucus layer, for instance, by regulating mucin glycosylation that mediates adhesion [102]. Alternatively, the mucus layer acts as carbohydrate source for many mucin-degrading gut bacteria, such as B. thetaiotaomicron [103], A. muciniphila [104], and B. fragilis [105]. Mucin 2 (Muc2), a major component of mucus, also facilitates Treg cell differentiation and immune tolerance and suppression [106, 107]. Next, the tight junctions, adherent junctions, and desmosomes together with transmembrane (claudin, occludin) and peripheral membrane proteins (zonula occludens) connect epithelial cells tightly and control gut permeability through molecular signaling [108, 109]. The lamina propria underneath the epithelial layer nurtures crosstalk between the immune cells and microbial counterparts to maintain intestinal homeostasis [110]. The gut vascular system composed of endothelial cells, pericytes, and fibroblast is also crucial for gut wall integrity [111].
Increasing evidence suggests intricate involvement of gut microbial commensals in maintaining barrier integrity by acting as energy sources for gut epithelial cells and regulating tight junction expression [112]. The SCFAs, for example, butyrate that derives from bacterial anaerobic fermentation of complex dietary polysaccharides, serve as a major energy source for the colonic epithelial cells [113]. Other SCFAs such as propionate are involved in producing glucose in the liver and small intestine and acetate is important for energy production and synthesis of lipids [113]. Furthermore, these metabolites promote anti-inflammatory environment within the gut by exerting pleiotropic effects on different immunologic cells, including macrophages, DCs, B and T lymphocytes [114] (Figure 2). Microbiota-derived butyrate is also shown to augment barrier function by increasing epithelial oxygen consumption and thereby stabilizing hypoxia-inducible factor, a transcription factor that coordinates barrier protection [78]. Microbial metabolites derived from dietary tryptophan also reinforce intestinal barrier function via aryl hydrocarbon receptor [115]. Several gut bacteria produce antibacterial lectins and secretory IgA that promote host–bacterial segregation and maintain homeostatic spatial relationships between host and microbiota [116, 117]. In contrast, direct epithelial interaction with certain microbiota-derived components also contributes to maintaining intestinal homeostasis. For instance, epithelial recognition of bacterial lipopolysaccharide (LPS), a major component of gram-negative cell wall, triggers inflammation through TLR signaling, compromising intestinal integrity [118]. LPS-induced endotoxemia is a major underlying cause involved in the onset of insulin resistance and obesity [119]. At the gut–microbiome interface, the epithelium-associated inflammosomes sense endogenous or exogenous damage-associated molecular patterns and maintain colonic homeostasis [120, 121]. Specifically, the NOD-like receptor family pyrin domain containing 6 (NLRP6) inflammasome stimulates mucus synthesis for barrier function, IL-18 secretion, and anti-microbial peptides production for shaping the gut microbiota [121]. Nlrp6 inflammasome-deficient mice show an aberrant microbiota, with an overgrowth of Bacteroidetes, particularly Prevotellaceae, and a decrease in Lactobacillaceae [120, 122]. The subsequent dysbiosis enhances inflammatory responses in the intestine, thereby predisposing the host to develop IBD [120]. Intriguingly, endometriotic women co-develop autoimmune disorders, most commonly IBD, which share similar gut microbiota alterations with a general loss of diversity and imbalance of Firmicutes/Bacteroides ratio. Research on the association of endometriosis and gut microbiome is a fledgling field that lies far behind that of IBD. However, recent studies suggest a “leaky gut” through perturbed gut microbiota as the possible conduit to endometriosis disease progression, owing to its pleiotropic effects on host functions.
Gut microbiota–endometriosis crosstalk
The gut microbiota profiles are long known to be associated with endometriosis in rhesus monkeys [123]. More recently, Huang et al. [124] suggested that gut microbiota profiles may critically diagnose the debilitating condition in humans, and which even exceed the diagnostic potential of cervical microbiome. This is explained by the metabolic and inflammatory changes originating from changes in gut microbiome that may govern development of a disease both inside and outside of the intestinal tract. Although the field is nascent, a strong association between gut microbiome and endometriosis is suggested from limited data from human subjects and through mouse and rat models of the disease [125–128]. The recent insights gained on this bidirectional crosstalk of gut microbiome and endometriosis and the possible underlying mechanisms are summarized in Figure 3. For elucidating the potential link between the two, we need to explore (1) perturbations in gut microbiota profiles that arise during endometriosis and (2) how these perturbations in gut microbiome regulate onset and/or progression of the disease. Although most studies address the alterations in gut microbiota, focus on deciphering the latter needs to pace up (summarized in Table 2). Currently, only two studies from our group describe the regulation of endometriosis by the gut microbiota [6, 127]. If we are to address the disease through gut microbiome-based therapies, further studies emphasizing on the latter are needed to unveil crucial insights. Furthermore, the causal direction between gut microbiota alterations and endometriosis development is a challenging question that needs to be addressed.
Figure 3.
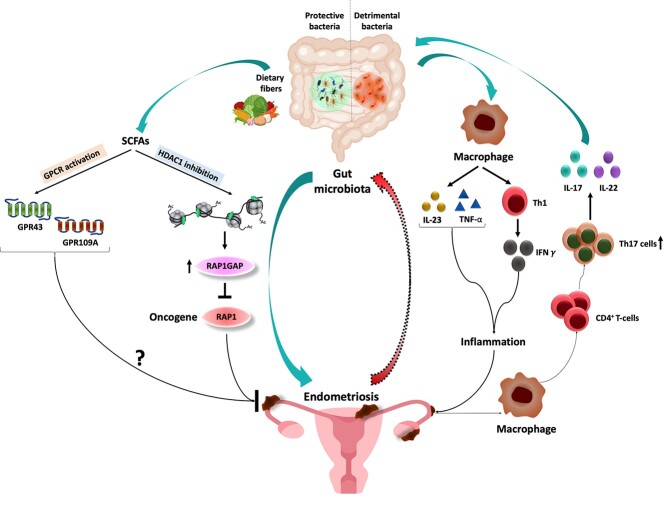
Schematic illustration of the mechanisms underlying the gut microbiome–endometriosis crosstalk. The gut microbiota structure is significantly altered (detrimental) in endometriosis that may be greatly involved in promoting the disease through disruption of gut barrier integrity and the resulting activation of macrophages and inflammation (shown on the right). The endometriotic lesions may, in turn, activate macrophages and the Th17/IL22 axis to promote conditions conducive to gut inflammation. On the other hand, the commensal gut microbes (protective) are shown to prevent endometriotic lesion formation and disease progression (shown on the left). This latter protective effect is shown to be mediated by gut microbiota-derived SCFAs in a G-protein-coupled receptors (GPCRs)-mediated signaling-dependent mechanisms through inhibition of Class I and Class II histone deacetylases (HDACs) and upregulation of RAP1GAP (tumor suppressor gene) signaling.
Table 2.
Key findings from the studies concerning endometriosis and gut microbiome
| Reference | Question | Sample Size/Study design | Findings | Strengths | Limitation |
|---|---|---|---|---|---|
| Studies in humans | |||||
| [124] | Robustness of gut, cervical mucus and peritoneal fluid microbiota for identifying microbial classifiers of EMS | EMS (n = 21); C (n = 20); 16S ribosomal RNA (V4 regions) sequencing |
• Distinct gut and PF microbiota in EMS • ↑ pathogen abundance in EMS peritoneal fluid (PFE) • ↓ alpha and beta diversity • ↓ Clostridia and Ruminococcus and Lachnospiraceae • ↑ Eggerthella lenta and Eubacterium dolichum. • F ecal microbiota differs in early and advanced stages of EMS; exceeds cervical microbiota in diagnosing EMS |
Control group was diagnosed with benign gynecology disease although peritoneal microenvironment was not determined as the root cause | |
| [132] | Association between EMS and gut microbiota | EMS (n = 66); C (n = 198); 16S ribosomal RNA (V1–V3 regions) sequencing | • ↓ alpha diversity; beta diversity marginally reduced. • ↑ Enterobacteriaceae (non-significant), Escherichia/Shigella not identified at genus level • ↑ Bacteroides and Parabacteroides (Bacteroidia) and, Oscillospira and Coprococccus (Clostridia) • ↓ Paraprevotella (Bacteroidia), Lachnospira (Clostridia) and Turicibacter (Bacilli) |
Each patient matched with three controls according to sex (only women), age, BMI and smoking habits, and excluded lactose intolerant celiac disease, CD, UC or IBS subjects. | Findings not significant after correction for multiple testing |
| Involvement of gut microbiota components with different EMS symptoms | • Lactococcus (Firmicutes) and Prevotella (Bacteroidetes) associated with gastrointestinal symptoms in EMS; Prevotella correlated with constipation, bloating and flatulence, vomiting and nausea symptoms. |
||||
| [133] | Changes in gut microbiota profiles EMS | EMS (n = 12; stage 3/4); C (n = 12); 16S ribosomal RNA (V1–V3 regions) sequencing | • ↓ alpha diversity • ↑ Firmicutes/Bacteroidetes ratio • ↑Actinobacteria, Cyanobacteria, Saccharibacteria, Fusobacteria, Acidobacteria • ↓Tenericutes • ↑ Prevotella (Bacteroidetes); Coprococcus (Firmicutes) in highest abundance in healthy gut. • ↑Bifidobacterium, Blautia, Dorea, Streptococcus • ↓Lachnospira, and Subdolibacterium |
Small sample size of the study; No laparoscopic confirmation of healthy controls | |
| Changes in gut microbiota functions related to EMS | • ↑ environmental information processing, endocrine system, and immune system functions in EMS • ↑ RIG-I-like receptor signaling pathway for transcription of NF-kB target genes, associated with inflammatory response (IL-8 and TNFα), apoptosis (Bax and Fas), proliferation (epidermal growth factor), and angiogenesis (VEGF) |
||||
| [134] | Gut and vaginal microbiome profiles in endometriosis | EMS (n = 24); C (n = 35); 16S ribosomal RNA (V4 regions) sequencing Patients with identified rASRM EMS stages – 1 (n = 9), 2 (n = 12), 3 (n = 4), 4 (n = 10) Rectal and vaginal samples were collected 2 months prior to surgery and during two different phases of the menstrual cycle—menstrual period (days 1–3 of the cycle) and follicular phase (days 8–12 of the cycle). |
• No difference in the vaginal and gut microbiome profiles between EMS and control groups during follicular and menstrual phases |
EMS laparoscopically confirmed; Patients on hormones/ antibiotics in 3 months prior to study excluded; and in cases of systemic infection, a history of autoimmune diseases history, active vaginosis, or history of sexually transmitted diseases; and if they had acquired or primary immunodeficient diseases, pregnancy, and malignant neoplasia; Controls also underwent surgery although for gynecoloogic conditions other than EMS |
Small sample size; statistically significant differences among groups regarding clinical EMS symptoms; high inter-subject and intra-subject variability of the gut and vaginal microbiomes |
| To explore potential of gut and vaginal microbiome to predict rASRM stage of EMS | • EMS could not be distinguished from control groups based on both gut and vaginal microbiomes; vaginal microbiomes, were predictive of EMS severity in advanced stages 3/4 |
||||
| [129] | Vaginal, cervical and gut microbiota alterations in stage 3/4 EMS patients | EMS (n = 14); C (n = 14); 16S ribosomal RNA (V1–V3 regions) sequencing |
• Genus level dissimilarities in vaginal, cervical and gut microbiota profiles in stage 3/4 EMS • ↑ Shigella/Escherichia in stage 3/4 EMS • ↓ Sneathia, Barnesella and Gardnerella • Cervical microbiota not dissimilar in EMS; Lactobacillus and Gardnerella dominant |
Only women with histology proven stage 3/4 EMS involved | No definitive laparoscopic confirmation of EMS absence in the control group |
| Studies in mice | |||||
| [136] | To study and compare the effects of EMS and alpha-linolenic acid (ALA) treatment on gut microbiota | Gut microbiota compared (i) Control (ii) EMS mice sampled after 21 days (iii) EMS mice treated with ALA every two days for two weeks starting from 1 week of EMS establishment; 16S rRNA (V3-V4 regions) gene sequencing |
• ↑ Firmicutes, ↓ Bacteroidota • ↑ Firmicutes/Bacteroidetes ratio • ↑ Actinobacteriota and Patescibacteria • ↓ Deferribacterota, Campilobacterota and Desulfobacterota Genus level • ↑ Lactobacillus, Clostridium_sensu_stricto_1, Bifidobacterium and Candidatus_Saccharimonas • ↓ Bacteroides, Dubosiella and Muribaculum • ALA restored normal gut microbiota and regulated abundance of abnormal pathways. |
||
| Effect of healthy and EMS gut microbiome on intestinal barrier and peritoneal environment | Fecal microbiota transplantation (FMT) from EMS and control mice on mice treated with antibiotic cocktail | • ↓ ZO-2 protein expression in the intestinal wall and ↑ LPS in the abdominal cavity, after FMT from EMS mice |
|||
| Effects of ALA on the gut microbiota and the abdominal environment in EMS. | Claudin 4 and ZO-2 protein levels in the intestinal wall and level of LPS in the abdominal cavity were detected | • ↑ ZO-2 expression in the intestinal wall of EMS mice upon ALA administration; little effect on Claudin 4 expression. • ↓ peritoneal LPS in EMS group upon ALA treatment. |
|||
| [6] | Effect of gut bacteria on endometriotic lesion growth | EMS induced in microbiota-depleted (MD) mice and lesions examined upon 21 days | • ↓ lesion size & ↓ proliferative cells in lesions in MD mice following EMS induction – Gut microbiota drive lesion progression. |
Used microbiota-depleted mice in place of germ-free to study effect on the educated immune system and eliminate any developmental defects | Findings are from a mouse model of surgically-induced endometriosis; conclusions may differ in natural EMS scenario. |
| Whether reduced growth of EMS lesions in MD mice due to altered gut bacteria? | MD mice, injected uterine fragments from control donor mice, and then FMT from mice with and without EMS | • FMT from healthy controls developed smaller lesions than those that received feces from mice with EMS. |
|||
| Fecal SCFA concentrations in mice with and without EMS | EMS and sham mice feces tested for relative concentrations of 10 SCFAs | • ↓ n-butyrate, iso-butyrate, and valerate in EMS; • ↓ acetate and propionate (non-significant) in EMS |
|||
| Effects of SCFAs—acetate, propionate, and n-butyrate on EMS lesion growth | EMS induced mice administered with acetate, propionate, or n-butyrate | • Acetate or propionate administration - ↓ lesion size non-significantly • n-butyrate administration - ↓ lesion size, ↓ proliferative cells in lesions, ↓ macrophages |
|||
| Effects of n-butyrate, acetate and propionate in vitro on cells derived from human EMS lesions | Immortalized human endometriotic epithelial cells expressing luciferase (iHEECs/Luc) and primary human endometriotic stromal cells (HEnSCs) treated with n-butyrate, acetate or propionate | • n-butyrate inhibited in vitro growth of both iHEECs/Luc and HEnSCs; • Modest effect of acetate and propionate on cellular proliferation at later time points only |
|||
| Effect of n-butyrate on human derived EMS lesions in vivo | iHEECs/Luc and iHESCs/Luc injected into the peritoneal space of immunocompromised mice followed by n-butyrate administration for 21 d. | • Lesions with fewer proliferative cells and macrophages in vivo |
|||
| [126] | Correlation of fecal metabolomics and gut microbiota with EMS | EMS induced and samples collected upon 21 days; 16S rRNA (V3-V4 regions) gene sequencing and fecal metabolomics | • ↓ alpha diversity reduced in EMS • ↑ Firmicutes/Bacteroides ratio; Proteobacteria and Verrucomicrobia • ↓ Bacteroides and Firmicutes Genus level • ↑ Allobaculum, Akkermansia, Parasutterella, and Rikenella • ↓ Lactobacillus, Bacteroides Functional pathways • ↑ bile acid biosynthesis; primary chenodeoxycholic acid (CDCA) and secondary ursodeoxycholic acid (UDCA) • ↓ alpha-linolenic acid (ALA) metabolism, positively correlated with Helicobacter and Ruminococcus; • ↓ 12,13-EOTrE (positively correlated with Candidatus_Stoquefichus) |
At 21-day time point after EMS induction, gut microbiota is significantly different, and the overall time is not too long; Non-targeted metabolomics combined with function prediction of the gut microbiota to analyze the important differential metabolites in the feces and the relative KEGG pathways |
|
| [137] | Effect of broad-spectrum antibiotics on early endometriotic lesion growth | VNMA (vancomycin, neomycin, metronidazole, and ampicillin) administration followed by EMS induction | • VNMA administration prior to EMS induction reduced endometriotic lesion size. |
Findings are from a mouse model of surgically-induced EMS; conditions may differ in natural EMS scenario. | |
| Effect of antibiotics on the progression of established endometriotic lesions | EMS induced first followed by treatment with antibiotics | • Lesions were smaller, had significantly less epithelial cells that were positive for the proliferation marker Ki-67, and contained less macrophages upon VNMA treatment. |
|||
| Effect of antibiotics on gut microbiota structure | 16 S rRNA (V4 region) gene sequencing of DNA isolated from faecal samples from endo-vehicle and endo-VNMA and non-endo mice. | • ↓ alpha diversity in antibiotic treated mice. • ↓ Bacteroidetes and Firmicutes; ↑ Proteobacteria upon antibiotic treatment. |
|||
| Effects of metronidazole (that target Bacteroides spp.) and neomycin individually on lesion growth | Known susceptibility and resistance of Bacteroides spp. towards to metronidazole and neomycin exploited to study their individual effects on lesion growth upon treatment. | • ↓ lesion size with reduced glands and epithelium, and ↓ macrophages upon metronidazole treatment. • ↓ IL-1β in peritoneal fluid upon metronidazole treatment. • No effect of neomycin on ectopic lesions |
|||
| Altered microbiota due to EMS in disease progression | Induced EMS on day 0, administered metronidazole on days 1 through 5, FMT from mice with or without EMS on days 7 and 14, and examined lesions on day 28 | • FMT from EMS to metronidazole-treated mice developed similar lesions as controls - ↑ size, ↑ macrophages and ↑ IL-1β in peritoneal fluid. |
|||
| [128] | Effect of new EMS lesions formation on gut microbiota structure | EMS induced; samples collected 3 days before as well as 7 and 21 days after the induction; 16S ribosomal RNA (V4-V5 regions) sequencing | • No significant differences observed, neither on genus nor on family level; EMS induction has no effect on gut microbiota • Stable alpha and beta diversities • ↑ community diversity • ↑ Bacteroidales S24–7 group, Lactobacillus, Prevotellaceae UCG-001 group and Lachnospiraceae NK4A136. |
Stringent quality control, singleton removal and FDR correction may have contributed to the lack of significant differences | |
| [5] | Trends in gut microbiota changes during EMS development | EMS induced and samples collected upon 7, 14, 28 and 42 days; 16S ribosomal RNA (V4 regions) sequencing | • Distinct gut microbiota developed upon 42 days of EMS lesions persistence. • ↑ Firmicutes/Bacteroidetes ratio (two folds) • ↑ Ruminococcus (Firmicutes), Bifidobacterium (Actinobacteria), Parasutterella (Proteobacteria) |
Results only indicate changes after 42 days, long term effects on gut microbiota were out of the scope of the study; more control groups needed to clarify the specificity of EMS induced dysbiosis. | |
| Study in rats | |||||
| [135] | Effects of letrozole (lowers estrogen) and Chinese herbal medicine, Shaofu Zhuyu decoction (SFZYD) on gut microbiota in EMS | Ectopic lesion size and COX-2 expression in the endometrium and lesions were compared; 16S rRNA gene sequencing. | • ↓ size of ectopic lesions, ↓ COX-2 expression • ↓ alpha-diversity in EMS group • ↑ Firmicutes/Bacteroidetes ratio in EMS • ↓ Ruminococcaceae • Letrozole and SFZY restored alpha diversity • Letrozole diminished Firmicutes/Bacteroidetes ratio compared to the control group; • SFZY lowered Firmicutes/Bacteroidetes ratio, and ↑ Ruminococcaceae |
||
| Study in baboons | |||||
| [185] | Effect of EMS induction on peripheral immune cell populations | EMS induced in olive baboons (n = 8); identified `natural' Tregs (nTregs) (CD4+CD25+Foxp3+), induced Tregs (iTregs) (CD4+CD25−Foxp3+) and Th17 cell populations in blood samples |
• Systemic inflammation through an alteration of tolerant and inflammatory T cell populations • ↓ peripheral nTregs at 3 & 9 months post-inoculation; and ↓ iTregs at 3 months post-inoculation and later time points • ↑ peripheral Th17 at each post-inoculation timepoint • ↑ Th17/Tregs ratio at 3 months post-inoculation and afterwards • ↑ Foxp3 (Tregs) and RORγt (Th17) transcripts levels in the eutopic & ectopic endometrium post EMS induction |
All animals were confirmed disease free laparoscopically;Baboon model provides physiologic similarity to humans and reproducibility of experimental results; Environmental factors such as diet, infections, antibiotics, and genetic background were controlled; Pre-inoculation stage was used as control to establish an internal baseline to reduce animal variability and error | No control animals taken in the study; Less sample size |
| Gut microbiota alterations during EMS | 16S rRNA gene (V4 region) sequencing | • Gut microbiota but not vaginal and peritoneal fluid microbiota altered upon EMS induction • ↓ Succinivibrio, Prevotella, Megasphaera, Lactobaccillus and CF231 at 3 months post-induction, but Succinivibrio, Prevotella, and CF231 increased throughout disease progression from 6 to 9 months post-induction |
|||
| Impact of microbial dynamics on immune status | Altered microbiota correlation with immune cells | • Immune tolerant cells (Treg) associated with gut microbial diversity during early and later stages of disease progression • Inflammatory cells (Th17) associated with microbial diversity in the middle of disease progression |
|||
| Association between microbial species and peripheral immune cells with EMS induction | Pearson’s correlation coefficient for each bacterial site with immune cells | • Actinobacteria, Euryarchaeota, Fusobacteria, Lentisphaerae, Spirochaetes, and Synergistetes positively correlated with nTregs and Th17 populations upon EMS induction • Porphyromonas, Prevotella, and WAL were negatively correlated with the level of iTregs |
|||
Gut microbiota alterations in endometriosis
The gut, peritoneal cavity, and female reproductive tract are known to harbor unique microbial communities where the peritoneal flora may itself derive from the gut or lower reproductive tract [129–131]. Studies in mice show that endometriosis drives changes in gut microbial flora that develops a distinct profile following 21 and 42 days of endometriosis induction [5, 127]. Out of the few works that studied association between gut microbiota and endometriosis, even fewer studied this link in human subjects (Table 2). A reduced overall microbial diversity within gut of women with endometriosis is generally observed [124, 132, 133]. Studies in mice also found a decrease in gut microbial diversity associated with endometriosis [126, 127]. However, the results are not consistent as Hantschel et al. [128] reported no effect of endometriosis on gut microbiota diversity, neither on genus nor on family level. Perrotta et al. [134] also found this in humans and reported no difference in gut microbiomes in patients and healthy groups. Although the study used stringent control measures, the bias in results that may arise from sample size, difficult diagnosis, and unmatched controls calls for careful analyses before drawing conclusions from such studies. The ratio of Firmicutes/Bacteroidetes, which is an important feature of dysbiosis, is elevated in the disease [5, 126, 133, 135, 136]. Proteobacteria, Actinobacteria, Cyanobacteria, Saccharibacteria, Fusobacteria, Acidobacteria, and Patescibacteria are significantly increased in endometriosis and Tenericutes are reported to be significantly decreased in the patients [5, 133, 136]. At genus level, Huang et al. [124] reported increase in populations of harmful Eggerthella lenta and Eubacterium dolichum, whereas a marked decrease in other protective microbes in gut of women with endometriosis. Particularly, they found reduced abundances of Clostridia and Ruminococcus and Lachnospiraceae. These commensals produce SCFAs regulating the intestinal integrity and are implicated in various other diseases linked with gut–microbiome dysbiosis such as Crohn disease [124]. Hormonal treatment of endometriosis is shown to increase Ruminococcus and other SCFA producers, Blautia and Butyricimonas, in endometriosis patients [132]. Contrarily, Shan et al. [133] observed increased abundances of Blautia in endometriosis patients that correlated with increased estradiol levels in blood. Genera Sneathia, Barnesella, and Gardnerella are shown to be significantly reduced in advanced stage 3/4 endometriosis gut [129]. The dominance of genera Shigella and Escherichia is reported in endometriosis-afflicted group [129]. However, a subsequent study only reported a non-significant enrichment of Enterobacteriaceae, and Shigella and Escherichia could not be detected at genus level [132]. Prevotella (Bacteroidetes) is found in high abundance in endometriosis groups [133] and in patients with gastrointestinal symptoms, Prevotella (Bacteroidetes) correlates with symptoms of constipation, bloating, flatulence, vomiting, and nausea [132].
Functional profiles of gut microbiomes in endometriosis are enriched in pathways for environmental information processing, endocrine system, and immune system functions as compared with healthy gut microbiomes [133]. Altered gut microbiota in endometriosis is closely related to enrichment of Retinoic acid-inducible gene I (RIG-I)-like receptor signaling pathway for transcription of NF-kB target genes, associated with inflammatory response (IL-8 and TNF-alpha), apoptosis (Bax and Fas), proliferation (epidermal growth factor), and angiogenesis (VEGF) [133]. Analysis of differential metabolites in endometriosis and healthy mice groups also suggest decreased abundances of alpha-linolenic acid in endometriosis (positively correlated with Helicobacter and Ruminococcus) and increased abundances of primary and secondary bile acids chenodeoxycholic acid (CDCA; negatively correlated with the abundance of Blautia) and ursodeoxycholic acid (UDCA) that are known to exert anti-inflammatory effects [136]. Although it is evident that gut microbiome is altered during endometriosis, yet conflicting results from limited data make it difficult to develop a consensus gut microbiota profile linked with endometriosis. Other than itself being altered due to endometriosis, data from mouse models of endometriosis also suggest an inverse relationship, suggesting that gut microbiome alterations may also drive progression of the disease.
Role of gut microbiome in endometriosis disease progression
Gut microbiome dysbiosis is linked with progression of several pathologies centrally originating from compromised gut barrier integrity. An interesting question to address is if the gut microbiome alterations could potentially drive the progression of disease in non-endometriosis models. To test this potential, previous studies from our group used microbiota-depleted mice and observed smaller lesions in these mice and that had fewer proliferative cells (positive for Ki-67 marker) as compared with control mice [127]. Depletion of gut microbiota through antibiotic cocktail resulted in smaller lesions as compared with mice that received only the vehicle [6]. Whether smaller lesions only resulted from antibiotics treatment was further confirmed through fecal transfer from mice with endometriosis (endo-feces) to mice treated with metronidazole (endo-metronidazole) that developed lesions that were similar in mass and volume to those in endo-vehicle mice, contained more macrophages in lesions and more IL-1beta in the peritoneal fluid. These findings highlight a potentially significant role of gut microbiome in endometriosis progression. The pro-inflammatory potential of microbiota members was revealed from high numbers of macrophages in lesions from control mice with higher peritoneal IL-1beta, TNF-alpha, IL-6, and TGF-beta1 levels than mice treated with antibiotics [6]. These provide substantial evidence for a casual role of gut microbiome dysbiosis in endometriosis disease progression. Interestingly, in certain diseases, gut microbiome imbalance results in microbiota-derived metabolite alteration, including gut-derived SCFAs (Figure 3). Thus, our group further measured the concentrations of different SCFAs in feces of mice with or without endometriosis. Interestingly, endometriotic mice were found to have significantly reduced n-butyrate concentrations. Although acetate and propionate concentrations were also non-significantly reduced, their supplementation through diet had only modest effect on lesion reduction, whereas butyrate supplementation orally resulted in greatly reduced sizes of lesions and inflammatory cell infiltration in mice with endometriosis [6]. n-butyrate also demonstrated similar inhibitory effect on immortalized human endometriotic epithelial cells (iHEECs) and primary human endometriotic stromal cells in vitro and on lesions developed in pre-clinical mouse models injected with iHEECs and immortalized human endometrial stromal cells. Another interesting question to address appears to be whether n-butyrate exerts protective effect through the well-known G-protein-coupled receptor (GPCR) signaling. This was tested in iHEECs cells pre-treated with antagonists of either or both known GPCR receptors (GPR43 and GPR109A) of n-butyrate. Treatment with either or both receptor antagonists resulted in increased proliferation of these cells as compared with non-pretreated endometriotic cells. Furthermore, when these cells were transfected with either or both GPR43 or GPR109A silencing RNA and followed by n-butyrate treatment, knockdown of either or both receptors could restore the viability of n-butyrate-treated cells [6]. Endometriotic mice that were injected with GPR43 and GPR109A antagonists before treatment with n-butyrate developed larger lesions than those that were given only n-butyrate. However, blocking the receptors with antagonists only partially prevented n-butyrate-derived protection that suggested other mechanisms independent of the GPCR signaling. Hence, inhibition of histone deacetylases (HDAC) by n-butyrate was tested as another possible mechanism contributing to its protective actions. Treatment of iHEECs with pan-HDAC inhibitors inhibited their viability to a great extent than did treatment with n-butyrate. Further intraperitoneal injections of HDAC inhibitors in endometriotic mice resulted in similar lesions as in control mice as compared with mice that received n-butyrate in addition to HDAC inhibitors [6]. Therefore, it is apparent that n-butyrate-mediated inhibition of HDAC activity also contributes as one of the underlying mechanisms through which gut microbiota-derived n-butyrate suppresses growth of endometriotic lesions. From RNA-seq analysis of iHEECs treated with n-butyrate, Repressor/activator protein 1 (RAP1) signaling was observed as one of the upregulated pathways. RAP1 regulates cell proliferation and tumor cell migration and invasion and is inactivated by RAP1GTPase-activating protein (RAP1GAP). n-butyrate treatment of iHEECs upregulated RAP1GAP possibly through inhibition of HDAC1. Further n-butyrate-treated cells in which RAP1GAP was knocked down proliferate significantly more than n-butyrate-treated cells transfected with control siRNA [6]. Data from these studies support the hypothesis that whereas some gut bacteria promote endometriosis by inducing macrophage-mediated inflammation, others protect against endometriosis by fermenting fiber to produce SCFAs.
Whether gut microbiome or endometriosis affects the other is unclear, yet certain groups of bacteria may aggravate the pathological condition as observed through reduced endometriotic lesions and inflammation following antibiotic treatment in mice [127]. It appears that a dysbiosis of gut microbial diversity (reduced) resulting from prolonged inflammation from retrogradely shed endometrial tissues may impair intestinal barrier function that may lead to disease progression and further promote gut microbiome dysbiosis in a vicious cycle (Figure 3). The insignificant differences in the hitherto published studies suggest that research in this field has yet to deliver an enormous amount of mechanistic understanding necessary to unveil the causal relationship between endometriosis and gut microbiome and for the development of gut microbiome-based treatment options of the disease.
Potential gut microbiome-based therapies for endometriosis
With understanding of the multifarious roles of gut microbiota in range of disorders, the gut microbiome research has advanced to its targeted exploitation and/or manipulation that can also be disseminated to endometriosis treatment or, in part, its symptoms. The broad aim remains the enteric reconstitution of commensal microflora to re-establish homeostasis, while the targeted microbial members might differ in different disorders. The idea behind microbiome-based therapies is to restore beneficial microbiome functions including strengthening of gut mucosal barrier, reinforcing gut colonization resistance, and restoring healthy microbial–immune cells crosstalk. We discuss the potential of widely accepted (i) microbiota-derived metabolites, (ii) whole fecal microbiota transplantation (FMT), (iii) modulation of diet and probiotics, and (iv) use of target microbial consortia, as emerging therapeutic options for endometriosis.
Microbiota-derived SCFAs and other metabolites
The SCFAs and secondary bile acids are recognized as important classes of commensal-derived homeostasis effectors and restoring their healthy levels warrants protection from diseases [137]. Significantly, butyrate, propionate, and acetate interact locally with colonic epithelial cells [138] and most notably target the mammalian G protein-coupled receptor pair of GPR41 and GPR43, inhibit the activity of HDACs, mediating anti-inflammatory activities of intestinal epithelial cells (IECs), macrophages, and DCs, and further promote the development of Treg cells [139, 140]. Reduced concentrations of these SCFAs are central to pathogenesis of several disorders including endometriosis [6]. The reduction in lesion growth observed upon n-butyrate administration in endometriotic mice highlight its therapeutic potential of n-butyrate largely produced by commensals of families Lachnospiraceae and Ruminococcaceae of the eubiotic gut microbiome [141, 142].
An additional class of microbiota-derived protective metabolites is represented by secondary bile acids that are produced from microbial modulation of primarily bile acids. For example, deoxycholic acid and lithocholic acids are generated from cholate and chenodeoxycholate, respectively, by gut commensals. The modified isodeoxycholic acid downregulates immunostimulatory properties of DCs, inducing proliferation of Treg cells [143]. In mouse models of endometriosis, Ni et al. [126] reported an increase in bile acids CDCA and UDCA synthesis pathways. CDCA is a primary bile acid produced in liver, a small part of which remains in the gut where it serves as substrate for modification by the commensals [144]. On the other hand, the secondary bile acid UDCA is generated through 7alpha-dehydroxylation by gut microbiota. Both CDCA and UDCA exert anti-inflammatory effects by promoting intestinal barrier function. CDCA blocks LPS-induced activation of the myosin light chain kinase pathway, thereby protecting the intestinal epithelial barrier function [145, 146], whereas UDCA protects the intestinal barrier through epidermal growth factor receptor (EGFR) and cyclooxygenase-2-dependent mechanisms, thus alleviating the inflammatory response [147, 148]. The increased CDCA and UDCA in the intestine of endometriotic mice is demonstrated to build an effective protective wall between gut microbiota dysbiosis and endometriosis [126]. Although these metabolites offer host protection, numerous challenges due to their functional versatility need to be overcome for devising effective microbiome-based therapeutic strategies.
Fecal microbiota transplantation (FMT)
FMT from healthy subjects to the patients of serious illnesses appears the most likely approach in microbiome-based treatments of human diseases [149, 150]. In general, feces from healthy subjects are screened for absence of any pathogens prior to transplantation. Pertaining to efficacy of FMT in endometriosis, our group previously showed that endometriotic mice transplanted with feces from healthy mice exhibit reduced lesion growth as compared with those transplanted with feces from endometriotic mice [6]. Similar findings were recently reported by Ni et al. [136] that showed reduced zona occludens-2 (ZO-2) expression upon fecal administration from endometriotic mice to healthy mice. These preliminary studies highlight the potential of FMT in mitigating endometriosis through reinforcement of barrier integrity. If not treatment per se, FMT can be used as an adjunctive treatment of this yet incurable condition. FMT has received much attention in treatment of Clostridium difficile infection (CDI) in humans with the curative symbiotic microbial consortium identified to up to strain level [151–153]. Its potential in treatment of other acute and inflammatory disorders of female reproductive tract has also been identified such as in PCOS [154]. FMT from healthy rats in PCOS rats resulted in increased populations of gut commensals Lactobacillus and Clostridium and decreased Prevotella numbers. It also resulted in improved estrous cycles and ovarian morphologies and decreased androgen biosynthesis in the FMT groups [154]. Endometriosis development and progression is also mediated by estrogen that is modulated by estrobolome, the component of gut microbiome involved in estrogen metabolism. Theoretical logic aside, the strategy is challenged by undefined microbiota composition and unexplored pathogenic strains in the transplanted feces. Furthermore, the immune status and inflammatory state that underpins the stage of the disease is important for persistence of the transplanted microbiota in the recipient endometriotic patient.
Modulation of diet and use of probiotics
Very few studies report the effects of diet on endometriosis, although their probable correlation is frequently noted. A high intake of omega-3 polyunsaturated fatty acids (PUFAs) is known to lower the risk of endometriosis in women that can be attributed to underlying modification of gut microbiota [155, 156]. In mouse models, PUFA-rich diet exhibits anti-inflammatory effects through reducing TNF-alpha and IL-6 inflammatory markers [52, 157–159] and suppresses the formation of endometriotic lesions [160, 161]. Diets rich in PUFAs and probiotic supplements are also demonstrated to alter the gut flora and prevent various diseases, such as obesity [162, 163]. Apparently, plant-based diets are shown to result in dominance of SCFA-producing Firmicutes, as opposed to animal-based diets that increase the frequencies of Bacteroidetes [164]. The preliminary results are, however, insufficient and more empirical data needs to be generated to conclude the role of diet and identification of pre- and probiotics for mitigating endometriosis.
Use of defined commensal consortia
Another potential microbiome-based mitigation of the disease can be viewed in the use of a defined microbial consortium of commensals that significantly impact endometriosis progression. The approach appears to be a safer than FMT with undefined microbiota components and has been successfully applied in various bowel disorders such as CDI [165]. Using target microbial consortium to increase the production of n-butyrate and other beneficial SCFAs, for reducing intestinal inflammation, and reinforcing barrier maintenance and colonization resistance may substantially contribute to endometriosis treatment. However, the structural and functional dynamics of the gut microbiome in endometriosis need to be fully elucidated to exploit the potential of commensal microbiota within the community.
Summary and future perspectives
Although the two fields are not very well connected, a consistent trend of evidence of gut microbiome–endometriosis relationship is revealed from recent studies. Most importantly, a reduced gut microbiome diversity and an elevated Firmicutes/Bacteroidetes ratio have mostly been linked with increased endometriosis risk. Reduced populations of Gardnerella, Lachnospira, Paraprevotella, Sneathia and increased abundances of Bifidobacterium, Blautia, Dorea, Parabacteroides, and Enterobacteriaceae, mainly Escherichia/Shigella, at genus level are reported alterations in gut microbiota of patients. Contradictory observations from a smaller number of studies highlight the need for extensive research required to draw conclusions. We are yet to understand if dysbiosis of the gut microbiome drives the onset of the disease or is itself critically impacted during diseased state. Fecal microbiota transplants from endometriotic mice to healthy groups promote lesion growth. Broad spectrum antibiotics such as metronidazole are shown to significantly impede lesion growth, opposed to the reduced gut microbial diversity observed in endometriosis. In this context, it is noteworthy to remember that microbial residents of the gut reside in complex assemblages interacting and communicating with each other and with the gut epithelial cells. A balance of this crosstalk is, therefore, critical to maintaining the functional dynamics characteristic of eubiosis. Nonetheless, the understanding put forth enormous opportunities to mitigate the condition through modulation of the gut microbiome and derived metabolites. Identifying the effects of microbiota-derived SCFAs, primary and secondary bile acids, as well as altered metabolic pathways in endometriotic physiological settings in human subjects can be rewarding. The field may benefit from understanding of gut microbiome perturbations in other diseases that often accompany endometriosis in patients and correlating with the disease severity in longitudinal studies. Certainly, the challenge that requires immediate attention is small sample sizes due to lack of non-invasive definitive diagnostic methods and the lack of robust methods for inclusion of asymptomatic healthy controls in the studies. An additional challenge in tailoring the gut microbiome for remission of endometriosis would be to leverage specific ecological concepts in the healthy gut microbiota–endometriosis crosstalk. Nonetheless, an understanding of the key microbiota-derived metabolites associated with endometriosis would be of interest to evaluate and design future microbial consortium-based therapies. Specially, identifying bacterial candidates that promote or protect against endometriosis in reproductive age women will accelerate efforts to diagnose, prevent, and treat endometriosis.
Authors’ contributions
CT and RK wrote the manuscript. VS prepared the figures. RK conceived the idea for graphical abstract. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
We thank the InPrint service at Washington University, St. Louis, MO for assisting with preparation of the graphical abstract.
Contributor Information
Chandni Talwar, Department of Pathology and Immunology, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, TX, USA.
Vertika Singh, Department of Pathology and Immunology, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, TX, USA.
Ramakrishna Kommagani, Department of Pathology and Immunology, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, TX, USA.
Funding
RK received the funding from National Institutes of Health/National Institute of Child Health and Human Development (grants R01HD102680 and R01HD065435) and a Research Scholar Grant from the American Cancer Society.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Grice EA, Segre JA. The human microbiome: our second genome. Annu Rev Genomics Hum Genet 2012; 13:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010; 362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril 2009; 92:68–74. [DOI] [PubMed] [Google Scholar]
- 4. Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927; 14:442–469. [Google Scholar]
- 5. Yuan M, Li D, Zhang Z, Sun H, An M, Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod 2018; 33:607–616. [DOI] [PubMed] [Google Scholar]
- 6. Chadchan SB, Popli P, Ambati CR, Tycksen E, Han SJ, Bulun SE, Putluri N, Biest SW, Kommagani R. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci Alliance 2021; 4:e202101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, Cone RA, Tachedjian G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 2015; 6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quaranta G, Sanguinetti M, Masucci L. Fecal microbiota transplantation: a potential tool for treatment of human female reproductive tract diseases. Front Immunol 2019; 10:2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, Strahilevitz J, Moses AE, Shapiro H, Yagel S, Elinav E. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med 2019; 25:1500–1504. [DOI] [PubMed] [Google Scholar]
- 10. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019; 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 1997; 68:585–596. [DOI] [PubMed] [Google Scholar]
- 12. Farquhar CM. Extracts from the “clinical evidence”. Endometriosis. BMJ 2000; 320:1449–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Machairiotis N, Stylianaki A, Dryllis G, Zarogoulidis P, Kouroutou P, Tsiamis N, Katsikogiannis N, Sarika E, Courcoutsakis N, Tsiouda T, Gschwendtner A, Zarogoulidis K et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013; 8:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers 2018; 4:9. [DOI] [PubMed] [Google Scholar]
- 15. Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A, Giudice LC, ENDO Study Working Group . Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 2011; 96:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am 2003; 30:1–19. [DOI] [PubMed] [Google Scholar]
- 17. Evers JL. Is adolescent endometriosis a progressive disease that needs to be diagnosed and treated? Hum Reprod 2013; 28:2023. [DOI] [PubMed] [Google Scholar]
- 18. Novak E. Pelvic endometriosis. Spontaneous rupture of endometrial cysts, with a report of three cases. Am J Obstet Gynecol 1931; 22:826–837. [Google Scholar]
- 19. Levander G, Normann P. The pathogenesis of endometriosis. An experimental study. Acta Obstet Gynecoi Scand 1955; 34:366–398. [DOI] [PubMed] [Google Scholar]
- 20. Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol 1940; 40:549–557. [Google Scholar]
- 21. Haney AF. The pathogenesis and aetiology of endometriosis. In: Thomas EJ, Rock JA (eds.), Modern Approaches to Endometriosis. Dordrecht, Boston, London: Kluwer Academic Publishers; 1991: 3–19. [Google Scholar]
- 22. Javert C. Pathogenesis of endometriosis based on endometrial homeoplasia, direct extension, exfoliation and implantation, lymphatic and hematogenous metastasis. Cancer 1949; 2:399–410. [DOI] [PubMed] [Google Scholar]
- 23. Viganò P, Infantino M, Lattuada D, Lauletta R, Ponti E, Somigliana E, Vignali M, DiBlasio AM. Intercellular adhesion molecule-1 (ICAM-1) gene polymorphisms in endometriosis. Mol Hum Reprod 2003; 9:47–52. [DOI] [PubMed] [Google Scholar]
- 24. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update 2011; 17:637–653. [DOI] [PubMed] [Google Scholar]
- 25. Augoulea A, Alexandrou A, Creatsa M, Vrachnis N, Lambrinoudaki I. Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress. Arch Gynecol Obstet 2012; 286:99–103. [DOI] [PubMed] [Google Scholar]
- 26. Fung JN, Rogers PA, Montgomery GW. Identifying the biological basis of GWAS hits for endometriosis. Biol Reprod 2015; 92:87. [DOI] [PubMed] [Google Scholar]
- 27. Borghese B, Zondervan KT, Abrao MS, Chapron C, Vaiman D. Recent insights on the genetics and epigenetics of endometriosis. Clin Genet 2017; 91:254–264. [DOI] [PubMed] [Google Scholar]
- 28. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006; 1:106–111. [DOI] [PubMed] [Google Scholar]
- 29. Wu Y, Shi X, Guo S. The knockdown of progesterone receptor isoform B (PR-B) promotes proliferation in immortalized endometrial stromal cells. Fertil Steril 2008; 90:1320–1323. [DOI] [PubMed] [Google Scholar]
- 30. Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol 2005; 193:371–380. [DOI] [PubMed] [Google Scholar]
- 31. Parazzini F, Chiaffarino F, Surace M, Chatenoud L, Cipriani S, Chiantera V, Benzi G, Fedele L. Selected food intake and risk of endometriosis. Hum Reprod 2004; 19:1755–1759. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto A, Harris HR, Vitonis AF, Chavarro JE, Missmer SA. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol 2018; 219:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giampaolino P, Della CL, Foreste V, Barra F, Ferrero S, Bifulco G. Dioxin and endometriosis: a new possible relation based on epigenetic theory. Gynecol Endocrinol 2020; 36:279–284. [DOI] [PubMed] [Google Scholar]
- 34. Cummings AM, Hedge JM, Birnbaum LS. Effect of prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol Sci 1999; 52:45–49. [DOI] [PubMed] [Google Scholar]
- 35. Nap AW, Groothuis PG, Demir AY, Evers JLH, Dunselman GAJ. Pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol 2004; 18:233–244. [DOI] [PubMed] [Google Scholar]
- 36. Signorile PG, Spugnini EP, Mita L, Mellone P, D’Avino A, Bianco M, Diano N, Caputo L, Rea F, Viceconte R, Portaccio M, Viggiano E et al. Prenatal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol 2010; 168:318–325. [DOI] [PubMed] [Google Scholar]
- 37. Wei M, Chen X, Zhao Y, Cao B, Zhao W. Effects of prenatal environmental exposures on the development of endometriosis in female offspring. Reprod Sci 2016; 23:1129–1138. [DOI] [PubMed] [Google Scholar]
- 38. Pivonello C, Muscogiuri G, Nardone A, Garifalos F, Provvisiero DP, Verde N, de Angelis C, Conforti A, Piscopo M, Auriemma RS, Colao A, Pivonello R. Bisphenol A: an emerging threat to female fertility. Reprod Biol Endocrinol 2020; 18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Helbig M, Vesper AS, Beyer I, Fehm T. Does nutrition affect endometriosis? Geburtshilfe Frauenheilkd 2021; 81:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, Fushiki S, Osawa Y, Honjo H. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod 1997; 57:514–519. [DOI] [PubMed] [Google Scholar]
- 41. Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab 1996; 81:174–179. [DOI] [PubMed] [Google Scholar]
- 42. Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B. Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinol Metab 1998; 83:4474–4480. [DOI] [PubMed] [Google Scholar]
- 43. McKinnon B, Mueller M, Montgomery G. Progesterone resistance in endometriosis: an acquired property? Trends Endocrinol Metab 2018; 29:535–548. [DOI] [PubMed] [Google Scholar]
- 44. Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol 2012; 25:208–215. [DOI] [PubMed] [Google Scholar]
- 45. Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update 2015; 21:155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update 2013; 19:558–569. [DOI] [PubMed] [Google Scholar]
- 47. Taylor RN, Yu J, Torres PB, Schickedanz AC, Park JK, Mueller MD, Sidell N. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci 2009; 16:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mechsner S, Kaiser A, Kopf A, Gericke C, Ebert A, Bartley J. A pilot study to evaluate the clinical relevance of endometriosis-associated nerve fibers in peritoneal endometriotic lesions. Fertil Steril 2009; 92:1856–1861. [DOI] [PubMed] [Google Scholar]
- 49. Klemmt PA, Carver JG, Koninckx P, McVeigh EJ, Mardon HJ. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: towards a mechanistic model for endometriosis progression. Hum Reprod 2007; 22:3139–3147. [DOI] [PubMed] [Google Scholar]
- 50. Johansson HKL, Damdimopoulou P, van Duursen MBM, Boberg J, Franssen D, de Cock M, Jääger K, Wagner M, Velthut-Meikas A, Xie Y, Connolly L, Lelandais P et al. Putative adverse outcome pathways for female reproductive disorders to improve testing and regulation of chemicals. Arch Toxicol 2020; 94:3359–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agostinis C, Balduit A, Mangogna A, Zito G, Romano F, Ricci G, Kishore U, Bulla R. Immunological basis of the endometriosis: the complement system as a potential therapeutic target. Front Immunol 2021; 11:599117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shephard RJ. Cytokine responses to physical activity, with particular reference to IL-6: sources, actions, and clinical implications. Crit Rev Immunol 2002; 22:165–182. [PubMed] [Google Scholar]
- 53. Choi HJ, Park MJ, Kim BS, Choi HJ, Joo B, Lee KS, Choi JH, Chung TW, Ha KT. Transforming growth factor β1 enhances adhesion of endometrial cells to mesothelium by regulating integrin expression. BMB Rep 2017; 50:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mu F, Harris HR, Rich-Edwards JW, Hankinson SE, Rimm EB, Spiegelman D, Missmer SA. A prospective study of inflammatory markers and risk of endometriosis. Am J Epidemiol 2018; 187:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M, Tayade C. The immunopathophysiology of endometriosis. Trends Mol Med 2018; 24:748–762. [DOI] [PubMed] [Google Scholar]
- 56. Cosín R, Gilabert-Estellés J, Ramón LA, Gómez-Lechón MJ, Gilabert J, Chirivella M, Braza-Boïls A, España F, Estellés A. Influence of peritoneal fluid on the expression of angiogenic and proteolytic factors in cultures of endometrial cells from women with endometriosis. Hum Reprod 2010; 25:398–405. [DOI] [PubMed] [Google Scholar]
- 57. Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, Terakawa N. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab 2000; 85:824–829. [DOI] [PubMed] [Google Scholar]
- 58. Delbandi AA, Mahmoudi M, Shervin A, Heidari S, Kolahdouz-Mohammadi R, Zarnani AH. Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health 2020; 20:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014; 10:261–276. [DOI] [PubMed] [Google Scholar]
- 60. Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O'Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015; 163:960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han SJ, Lee JE, Cho YJ, Park MJ, O’Malley BW. Genomic function of estrogen receptor β in endometriosis. Endocrinology 2019; 160:2495–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod 2002; 17:2715–2724. [DOI] [PubMed] [Google Scholar]
- 63. Nielsen NM, Jørgensen KT, Pedersen BV, Rostgaard K, Frisch M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum Reprod 2011; 26:1555–1559. [DOI] [PubMed] [Google Scholar]
- 64. Chen LC, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, Yang AC, Chang WH, Chen TJ, Tsai SJ, Chen MH. Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study. J Affect Disord 2016; 190:282–285. [DOI] [PubMed] [Google Scholar]
- 65. Laganà AS, La Rosa V, Petrosino B, Vitale SG. Comment on “Risk of developing major depression and anxiety disorders among women with endometriosis: A longitudinal follow-up study”. J Affect Disord 2017; 208:672–673. [DOI] [PubMed] [Google Scholar]
- 66. Dawson A, Fernandez ML, Anglesio M, Yong PJ, Carey MS. Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018; 12:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vom Steeg LG, Klein SL. Sex steroids mediate bidirectional interactions between hosts and microbes. Horm Behav 2017; 88:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016; 14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect 2012; 18:2–4. [DOI] [PubMed] [Google Scholar]
- 71. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev 2012; 70:S38–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012; 9:577–589. [DOI] [PubMed] [Google Scholar]
- 73. Schluter J, Foster KR, Ellner SP. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol 2012; 10:e1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, Neulinger SC, Däumer C. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 2013; 62:1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N et al. Enterotypes of the human gut microbiome. Nature 2011; 473:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science 2006; 312:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wexler AG, Goodman AL. An insider’s perspective: Bacteroides as a window into the microbiome. Nat Microbiol 2017; 2:17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015; 17:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Martin AM, Sun EW, Rogers GB, Keating DJ. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front Physiol 2019; 10:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci 2020; 21:6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Donia MS, Fischbach MA. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 2015; 349:1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020; 8:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog 2013; 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo P, Zhang K, Ma X, He P. Clostridium species as probiotics: potentials and challenges. J Animal Sci Biotechnol 2020; 11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zafar H, Saier MH Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021; 13:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lapébie P, Lombard V, Drula E, Terrapon N, Henrissat B. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat Commun 2019; 10:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tailford LE, Crost EH, Kavanaugh D, Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet 2015; 6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li J, Si H, Du H, Guo H, Dai H, Xu S, Wan J. Comparison of gut microbiota structure and Actinobacteria abundances in healthy young adults and elderly subjects: a pilot study. BMC Microbiol 2021; 21:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trastoy B, Naegeli A, Anso I, Sjögren J, Guerin ME. Structural basis of mammalian mucin processing by the human gut O-glycopeptidase OgpA from Akkermansia muciniphila. Nat Commun 2020; 11:4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res 2020; 30:492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smythies LE, Shen R, Bimczok D, Novak L, Clements RH, Eckhoff DE, Bouchard P, George MD, Hu WK, Dandekar S, Smith PD. Inflammation anergy in human intestinal macrophages is due to Smad-induced IkappaBalpha expression and NF-kappaB inactivation. J Biol Chem 2010; 285:19593–19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012; 336:1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schwarzer M, Hermanova P, Srutkova D, Golias J, Hudcovic T, Zwicker C, Sinkora M, Akgün J, Wiedermann U, Tuckova L, Kozakova H, Schabussova I. Germ-free mice exhibit mast cells with impaired functionality and gut homing and do not develop food allergy. Front Immunol 2019; 10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sittipo P, Lobionda S, Choi K, Sari IN, Kwon HY, Lee YK. Toll-like receptor 2-mediated suppression of colorectal cancer pathogenesis by polysaccharide A from Bacteroides fragilis. Front Microbiol 2018; 9:1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wiechers C, Zou M, Galvez E, Beckstette M, Ebel M, Strowig T, Huehn J, Pezoldt J. The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell Mol Immunol 2021; 18:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 2008; 29:637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wei B, Su TT, Dalwadi H, Stephan RP, Fujiwara D, Huang TT, Brewer S, Chen L, Arditi M, Borneman J, Rawlings DJ, Braun J. Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells. Eur J Immunol 2008; 38:3411–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, Borneman J, Kronenberg M, Braun J. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J Immunol 2010; 184:1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li H, Limenitakis J, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, Stolp B, Stein JV et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun 2015; 6:8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016; 16:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 2003; 299:2074–2076. [DOI] [PubMed] [Google Scholar]
- 104. Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov, sp. nov, a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004; 54:1469–1476. [DOI] [PubMed] [Google Scholar]
- 105. Macfarlane GT, Gibson GR. Formation of glycoprotein degrading enzymes by Bacteroides fragilis. FEMS Microbiol Lett 1991; 61:289–293. [DOI] [PubMed] [Google Scholar]
- 106. Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002; 295:1726–1729. [DOI] [PubMed] [Google Scholar]
- 107. Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013; 342:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nagpal R, Yadav H. Bacterial translocation from the gut to the distant organs: an overview. Ann Nutr Metab 2017; 71:11–16. [DOI] [PubMed] [Google Scholar]
- 109. Barreau F, Hugot JP. Intestinal barrier dysfunction triggered by invasive bacteria. Curr Opin Microbiol 2014; 17:91–98. [DOI] [PubMed] [Google Scholar]
- 110. Eri R, Chieppa M. Messages from the inside. The dynamic environment that favors intestinal homeostasis. Front Immunol 2013; 4:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015; 350:830–834. [DOI] [PubMed] [Google Scholar]
- 112. Paradis T, Bègue H, Basmaciyan L, Dalle F, Bon F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int J Mol Sci 2021; 22:2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kim M, Friesen L, Park J, Kim HM, Kim CH. Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. Eur J Immunol 2018; 48:1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 2017; 26:110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A 2020; 117:19376–19387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011; 334:255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 2012; 12:503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tulkens J, Vergauwen G, Van Deun J, Geeurickx E, Dhondt B, Lippens L, De Scheerder MA, Miinalainen I, Rappu P, De Geest BG, Vandecasteele K, Laukens D et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020; 69:191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dalby MJ, Aviello G, Ross AW, Walker AW, Barrett P, Morgan PJ. Diet induced obesity is independent of metabolic endotoxemia and TLR4 signalling, but markedly increases hypothalamic expression of the acute phase protein, SerpinA3N. Sci Rep 2018; 8:15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011; 145:745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Levy M, Shapiro H, Thaiss CA, Elinav E. NLRP6: a multifaceted innate immune sensor. Trends Immunol 2017; 38:248–260. [DOI] [PubMed] [Google Scholar]
- 122. Elinav E, Thaiss CA, Flavell RA. Analysis of microbiota alterations in inflammasome-deficient mice. Methods Mol Biol 2013; 1040:185–194. [DOI] [PubMed] [Google Scholar]
- 123. Bailey M, Coe C. Endometriosis is associated with an altered profile of intestinal microflora in female Rhesus monkeys. Hum Reprod 2002; 17:1704–1708. [DOI] [PubMed] [Google Scholar]
- 124. Huang L, Liu B, Liu Z, Feng W, Liu M, Wang Y, Peng D, Fu X, Zhu H, Cui Z, Xie L, Ma Y. Gut microbiota exceeds cervical microbiota for early diagnosis of endometriosis. Front Cell Infect Microbiol 2021; 11:788836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wei W, Zhang X, Tang H, Zeng L, Wu R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann Clin Microbiol Antimicrob 2020; 19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ni Z, Sun S, Bi Y, Ding J, Cheng W, Yu J, Zhou L, Li M, Yu C. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am J Reprod Immunol 2020; 84:e13307. [DOI] [PubMed] [Google Scholar]
- 127. Chadchan SB, Cheng M, Parnell LA, Yin Y, Schriefer A, Mysorekar IU, Kommagani R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Hum Reprod 2019; 34:1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hantschel J, Weis S, Schäfer KH, Menger MD, Kohl M, Egert M, Laschke MW. Effect of endometriosis on the fecal bacteriota composition of mice during the acute phase of lesion formation. PLoS One 2019; 14:e0226835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A, Urman B. The endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep 2019; 9:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Akiyama K, Nishioka K, Khan KN, Tanaka Y, Mori T, Nakaya T, Kitawaki J. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am J Reprod Immunol 2019; 82:1–9. [DOI] [PubMed] [Google Scholar]
- 131. Baker JM, Chase DM, Herbst-Kralovetz MM. Uterine microbiota: residents, tourists, or invaders? Front Immunol 2018; 9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Svensson A, Brunkwall L, Roth B, Orho-Melander M, Ohlsson B. Associations between endometriosis and gut microbiota. Reprod Sci 2021; 28:2367–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shan J, Ni Z, Cheng W, Zhou L, Zhai D, Sun S, Yu C. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch Gynecol Obstet 2021; 304:1363–1373. [DOI] [PubMed] [Google Scholar]
- 134. Perrotta A, Borrelli GM, Martins CO, Kallas EG, Sanabani SS, Griffith LG, Alm EJ, Abrao MS. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: a pilot study. Reprod Sci 2020; 27:1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cao Y, Jiang C, Jia Y, Xu D, Yu Y. Letrozole and the traditional Chinese medicine, Shaofu Zhuyu decoction, reduce endometriotic disease progression in rats: a potential role for gut microbiota. Evid Based Complement Alternat Med 2020; 2020:3687498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ni Z, Ding J, Zhao Q, Cheng W, Yu J, Zhou L, Sun S, Yu C. Alpha-linolenic acid regulates the gut microbiota and the inflammatory environment in a mouse model of endometriosis. Am J Reprod Immunol 2021; 86:e13471. [DOI] [PubMed] [Google Scholar]
- 137. Chen H, Nwe PK, Yang Y, Rosen CE, Bielecka AA, Kuchroo M, Cline GW, Kruse AC, Ring AM, Crawford JM, Palm NW. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell 2019; 177:1217–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Koh A, De Vadder F, Kovatcheva- Datchary P, Backhed F. From dietary fiber to host physiology: short- chain fatty acids as key bacterial metabolites. Cell 2016; 165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 139. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504:446–450. [DOI] [PubMed] [Google Scholar]
- 140. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 2019; 16:461–478. [DOI] [PubMed] [Google Scholar]
- 141. Sorbara MT, Littmann ER, Fontana E, Moody TU, Kohout CE, Gjonbalaj M, Eaton V, Seok R, Leiner IM, Pamer EG. Functional and genomic variation between human-derived isolates of Lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe 2020; 28:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Env Microbiol 2017; 19:29–41. [DOI] [PubMed] [Google Scholar]
- 143. Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante S, Ramos RJ, Cross JR et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020; 581:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 2016; 24:41–50. [DOI] [PubMed] [Google Scholar]
- 145. Song M, Ye J, Zhang F, Su H, Yang X, He H, Liu F, Zhu X, Wang L, Gao P, Shu G, Jiang Q et al. Chenodeoxycholic Acid (CDCA) protects against the lipopolysaccharide-induced impairment of the intestinal epithelial barrier function via the FXR-MLCK pathway. J Agric Food Chem 2019; 67:8868–8874. [DOI] [PubMed] [Google Scholar]
- 146. Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011; 60:463–472. [DOI] [PubMed] [Google Scholar]
- 147. Golden JM, Escobar OH, Nguyen MVL, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G et al. Ursodeoxycholic acid protects against intestinal barrier breakdown by promoting enterocyte migration via EGFR- and COX-2-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol 2018; 315:G259–G271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ko WK, Kim SJ, Jo MJ, Choi H, Lee D, Kwon IK, Lee SH, Han IB, Sohn S. Ursodeoxycholic acid inhibits inflammatory responses and promotes functional recovery after spinal cord injury in rats. Mol Neurobiol 2019; 56:267–277. [DOI] [PubMed] [Google Scholar]
- 149. Filip M, Tzaneva V, Dumitrascu DL. Fecal transplantation: digestive and extradigestive clinical applications. Clujul Med 2018; 91:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017; 66:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ianiro G, Masucci L, Quaranta G, Simonelli C, Lopetuso LR, Sanguinetti M, Gasbarrini A, Cammarota G. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment Pharmacol Ther 2018; 48:152–159. [DOI] [PubMed] [Google Scholar]
- 152. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407–415. [DOI] [PubMed] [Google Scholar]
- 153. Smillie CS, Sauk J, Gevers D, Friedman J, Sung J, Youngster I, Hohmann EL, Staley C, Khoruts A, Sadowsky MJ, Allegretti JR, Smith MB et al. Strain tracking reveals the determinants of bacterial engraftment in the human gut following fecal microbiota transplantation. Cell Host Microbe 2018; 23:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, Tang L. Association between polycystic ovary syndrome and gut microbiota. PLoS One 2016; 11:e0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, Barbieri RL, Willett WC, Hankinson SE. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod 2010; 25:1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hopeman MM, Riley JK, Frolova AI, Jiang H, Jungheim ES. Serum polyunsaturated fatty acids and endometriosis. Reprod Sci 2015; 22:1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001; 280:E745–E751. [DOI] [PubMed] [Google Scholar]
- 158. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95:2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tomio K, Kawana K, Taguchi A, Isobe Y, Iwamoto R, Yamashita A, Kojima S, Mori M, Nagamatsu T, Arimoto T et al. Omega-3 polyunsaturated fatty acids suppress the cystic lesion formation of peritoneal endometriosis in transgenic mouse models. PLoS One 2013; 8:e73085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Attaman JA, Stanic AK, Kim M, Lynch MP, Rueda BR, Styer AK. The anti-inflammatory impact of omega-3 polyunsaturated fatty acids during the establishment of endometriosis-like lesions. Am J Reprod Immunol 2014; 72:392–402. [DOI] [PubMed] [Google Scholar]
- 162. Ilich JZ, Kelly OJ, Kim Y, Spicer MT. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arch Ind Hyg Toxicol 2014; 65:139–148. [DOI] [PubMed] [Google Scholar]
- 163. Kelly OJ, Gilman JC, Kim Y, Ilich JZ. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr Res 2013; 33:521–533. [DOI] [PubMed] [Google Scholar]
- 164. Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr 2019; 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tvede M, Rask-Madsen J. Bacteriotherapy for Clostridium difficile diarrhoea. Lancet 1990; 335:110. [DOI] [PubMed] [Google Scholar]
- 166. Finas D, Altevogt P, Fogel M, Hornung D, Huszar M, Agic A, Dogan S, Kiefel H, Riedle S, Gast D, Marcovich R, Noack F. L1 cell adhesion molecule (L1CAM) as a pathogenetic factor in endometriosis. Hum Reprod 2008; 23:1053–1062. [DOI] [PubMed] [Google Scholar]
- 167. Delbandi A, Mahmoudi M, Shervin A, Akbari E, Tehrani M, Sankian M, Kazemnejad S, Zarnani A. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil Steril 2013; 100:761–769. [DOI] [PubMed] [Google Scholar]
- 168. Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser I. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod 2009; 24:325–332. [DOI] [PubMed] [Google Scholar]
- 169. Khan K, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril 2004; 81:652–661. [DOI] [PubMed] [Google Scholar]
- 170. Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser I. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod 2009; 24:1695–1703. [DOI] [PubMed] [Google Scholar]
- 171. Berbic M, Hey-Cunningham A, Ng C, Tokushige N, Ganewatta S, Markham R, Russell P, Fraser I. The role of Foxp3+ regulatory T-cells in endometriosis: a potential controlling mechanism for a complex, chronic immunological condition. Hum Reprod 2010; 25:900–907. [DOI] [PubMed] [Google Scholar]
- 172. Hapangama DK, Turner MA, Drury J, Heathcote L, Afshar Y, Mavrogianis PA, Fazleabas AT. Aberrant expression of regulators of cell-fate found in eutopic endometrium is found in matched ectopic endometrium among women and in a baboon model of endometriosis. Hum Reprod 2010; 25:2840–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Szymanowski K. Apoptosis pattern in human endometrium in women with pelvic endometriosis. Eur J Obstet Gynecol 2007; 132:107–110. [DOI] [PubMed] [Google Scholar]
- 174. Wingfield M, Macpherson A, Healy D, Rogers P. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil Steril 1995; 64:340–346. [DOI] [PubMed] [Google Scholar]
- 175. Gilbert-Estelles J, Ramon L, Espana F, Gilbert J, Vila V, Reganon E, Castello R, Chirivella M, Estelles A. Expression of angiogenic factors in endometriosis: relationship to fibrinolytic and metalloproteinase systems. Hum Reprod 2007; 22:2120–2127. [DOI] [PubMed] [Google Scholar]
- 176. Healy D, Rogers P, Hii L, Wingfield M. Angiogenesis: a new theory for endometriosis. Hum Reprod 1998; 4:736–740. [DOI] [PubMed] [Google Scholar]
- 177. Jerman LF, Hey-Cunningham AJ. The role of the lymphatic system in endometriosis: a comprehensive review of the literature. Biol Reprod 2015; 92:64. [DOI] [PubMed] [Google Scholar]
- 178. Al-Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe G, Markham R, Fraser I. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Hum Reprod 2009; 24:3019–3024. [DOI] [PubMed] [Google Scholar]
- 179. Bokor A, Kyama C, Vercruysse L, Fassbender A, Gevaert O, Vodolazkaia A, Moor B, Fulop V, D'Hooghe T. Density of small diameter sensory nerve fibres in endometrium: a semi-invasive diagnostic test for minimal to mild endometriosis. Hum Reprod 2009; 24:3025–3032. [DOI] [PubMed] [Google Scholar]
- 180. Browne AS, Yu J, Huang R-P, Francisco AMC, Sidell N, Taylor RN. Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis. Fertil Steril 2012; 98:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Tokushige N, Markham R, Russell P, Fraser I. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod 2006; 21:782–787. [DOI] [PubMed] [Google Scholar]
- 182. Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, Greco P, Nappi L. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev 2017; 2017:7265238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017; 96:623–632. [DOI] [PubMed] [Google Scholar]
- 184. Pabalan N, Salvador A, Jarjanazi H, Christofolini DM, Barbosa CP, Bianco B. Association of the progesterone receptor gene polymorphism (PROGINS) with endometriosis: a meta-analysis. Arch Gynecol Obstet 2014; 290:1015–1022. [DOI] [PubMed] [Google Scholar]
- 185. Le N, Cregger M, Fazleabas A, Braundmeier-Fleming A. Effects of endometriosis on immunity and mucosal microbial community dynamics in female olive baboons. Sci Rep 2022; 12:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]