Abstract
Purpose
To evaluate the safety and preliminary efficacy of THR-687 in patients with center-involved diabetic macular edema (DME).
Design
Phase 1, open-label, multicenter, 3 + 3 dose-escalation study with 3-month follow-up.
Participants
Patients 18 years of age or older with visual impairment resulting from DME.
Methods
Single intravitreal injection of THR-687 (0.4 mg, 1.0 mg, or 2.5 mg).
Main Outcome Measures
The primary outcome measure was the incidence of dose-limiting toxicities (DLTs). The secondary outcome measure was the incidence of adverse events (AEs), including the occurrence of laboratory abnormalities. Exploratory outcome measures included changes from baseline in best-corrected visual acuity (BCVA) and central subfield thickness (CST), assessments of ischemia and leakage on fluorescein angiography, and THR-687 levels in plasma.
Results
Twelve patients were treated: 3 patients received 0.4 mg of THR-687, 3 patients received 1.0 mg of THR-687, and 6 patients received 2.5 mg of THR-687. Most patients were men (9/12 patients). Their mean age was 57.8 years. No DLTs or serious AEs were reported at any of the dose levels tested. Overall, 9 AEs in the study eye were reported for 5 of 12 patients. Of those, 4 AEs in 3 of 12 patients were deemed treatment related by the investigator, all of which were mild, started on the day of the injection, and had resolved within 28 days without treatment. Overall, mean gains from baseline in BCVA were observed at all study visits with a rapid onset (7.2 Early Treatment Diabetic Retinopathy Study [ETDRS] letters at day 7) and a durability up to the end of the study (8.3 ETDRS letters at month 3). A mean decrease in CST was observed up to month 1. Overall, the mean BCVA gains and CST decreases were highest at the highest THR-687 dose level tested. THR-687 was undetectable in plasma at 7 days after the injection.
Conclusions
At all dose levels tested, a single intravitreal injection of THR-687 was safe and well tolerated. Preliminary efficacy was observed by a rapid gain in BCVA with 3 months’ durability and a decrease in CST up to 1 month after the injection.
Keywords: DME, Integrin antagonist, Intravitreal injection, Phase 1, THR-687
Abbreviations and Acronyms: AE, adverse event; BCVA, best-corrected visual acuity; CI, confidence interval; CST, central subfield thickness; DLT, dose-limiting toxicity; DME, diabetic macular edema; ETDRS, Early Treatment Diabetic Retinopathy Study; FA, fluorescein angiography; NPDR, nonproliferative diabetic retinopathy; RGD, arginine-glycine-aspartic acid; SD-OCT, spectral-domain OCT; VEGF, vascular endothelial growth factor
One of the most common microvascular manifestations of diabetes is the development of diabetic eye disease, including diabetic macular edema (DME). Diabetic eye disease is a principal cause of blindness among working-age adults.1 Worldwide, diabetic eye disease accounted for 2.6% of all cases of blindness in 2010, and the number of affected individuals is expected to increase in the coming years.1, 2, 3, 4 The number of adults 20 to 79 years of age with diabetes worldwide was estimated to be 463 million in 2019 and is estimated to reach 700 million by 2045.5 In addition to the burden on the health care economy, DME negatively impacts the quality of life of those with the disorder.5, 6, 7
Diabetic macular edema is characterized by a dysfunction in the blood–retinal barrier that leads to fluid accumulation in the macula. Hallmarks of the disease include oxidative stress, inflammation, and vascular dysfunction, as well as upregulation of cytokines and growth factors such as vascular endothelial growth factor (VEGF).8 Intravitreal anti-VEGF therapy has been the standard of care for center-involved DME for almost a decade.9, 10, 11, 12 However, the risk of nonadherence in long-term anti-VEGF therapy with frequent repeat injections is high. Nonadherence negatively affects visual acuity outcome for the patients and worsens the burden on the health care system.13,14 Although adverse events (AEs) related to anti-VEGF therapy are uncommon and mostly are related to the need of repeated intravitreal injections; long-term treatment has been associated with arterial thrombotic events.15, 16, 17, 18 More importantly, 20% to 40% of patients do not respond optimally to anti-VEGF therapy.19, 20, 21, 22, 23 Intravitreal corticosteroids often are used as a second-line therapy for DME because of their ability to modulate numerous cytokines while also blocking VEGF.9 However, intravitreal corticosteroids are associated with well-documented AEs, including cataract development and intraocular pressure elevation, which limits their usefulness in patients unresponsive to anti-VEGF therapy.9,24,25 The suboptimal response rate seen with anti-VEGF therapies, as well as the safety concerns related to second-line treatments, highlight the need for alternative treatment options that target additional pathways involved in DME pathogenesis.
Integrins constitute a family of transmembrane cell surface receptors that can mediate cell–cell and cell–extracellular matrix interactions. Integrins are involved in various biological processes, including cell differentiation, adhesion, migration, motility, invasion, proliferation, and survival.26,27 Integrin receptors exist as heterodimers of α and β subunits. To date, a total of 18 α subunits and 8 β subunits have been identified, which combine to form 24 known human integrin receptors.28 Based on ligand binding properties, integrins can be grouped into 4 classes, including receptors that recognize an arginine-glycine-aspartic acid (RGD) binding motif. In the eye, the interaction of specific RGD integrins with the extracellular matrix can lead to neovascularization (αvβ3, αvβ5, and α5β1)29, 30, 31 and vascular permeability (αvβ3, αvβ5, and α5β1).32
THR-687 is a small (750 Dalton; Oxurion), highly selective integrin antagonist with low nanomolar affinity toward many RGD integrins.33 Potent antiangiogenic activity of THR-687 has been shown in preclinical experiments in vitro and in vivo.33 In vivo, combined intravitreal and intraperitoneal administration of THR-687 inhibited VEGF-induced vascular leakage in the mouse retina, and intravitreal administration of THR-687 potently inhibited choroidal neovascularization-induced leakage in a cynomolgus monkey model of choroidal neovascularization.33 Herein, the authors present safety and preliminary efficacy data from a phase 1 study of THR-687 in patients with center-involved DME.
Methods
This study (ClinicalTrials.gov identifier, NCT03666923) was conducted according to the principles of the Declaration of Helsinki, the International Council for Harmonisation guideline for Good Clinical Practice, and all applicable local regulatory requirements. Institutional review board approval was obtained from the Copernicus Group IRB. All patients provided written informed consent before screening.
Study Population
The study population consisted of patients with visual impairment resulting from center-involved DME who had a history of response to anti-VEGF agents, corticosteroids, or both and, in the opinion of the investigator, remained responsive to treatment.
The study enrolled patients of both sexes who were 18 years of age or older with center-involved DME and central subfield thickness (CST) of 320 μm or more. Patients were required to have best-corrected visual acuity (BCVA) of 62 or fewer and 23 or more ETDRS letters in the study eye. Exclusion criteria included comorbid conditions or treatments that could confound the study results. The key inclusion and exclusion criteria are provided in Supplemental Table 1.
Study Design and Treatment
This was a phase 1, open-label, multicenter study in the United States with a 3 + 3 dose-escalation design. Three patients were treated at each THR-687 dose level, starting at the lowest dose level. Dose escalation occurred on review of all available safety data by the internal safety monitoring committee. In addition, as per protocol, 3 additional patients were treated at the highest dose level after the review by the safety monitoring committee.
Patients received a single intravitreal injection of THR-687 at 0.4 mg, 1.0 mg, or 2.5 mg (dose levels expressed as THR-687 salt form [all THR-687]; these correspond to 0.3 mg, 0.8 mg, and 2.1 mg THR-687 free base form [active THR-687], respectively). Standard-of-care rescue treatment in the study eye was allowed during the study if deemed necessary by the investigator and if protocol-specified criteria were met. The type of rescue treatment was determined by the investigator, as per local clinical practice.
Outcome Measures
The primary outcome measure was the incidence of dose-limiting toxicities (DLTs). The secondary outcome measure was the incidence of systemic and ocular AEs from the day of study treatment up to month 3, including the occurrence of hematologic, biochemical, or urinary laboratory abnormalities. Exploratory outcome measures included change from Baseline in BCVA and CST (based on spectral-domain [SD] OCT) by study visit, THR-687 levels in plasma by day 7, and assessments of ischemia and leakage (based on widefield fluorescein angiography [FA]) by month 3.
Outcome Assessments
Dose-limiting toxicities included clinically relevant decreases in BCVA and ocular inflammation occurring from the day of the injection up to 14 days thereafter. Adverse events were reported by the patient or detected by the investigator by performing study assessments (e.g., full ophthalmic examination). For each AE, the investigator indicated whether he or she deemed it to be treatment related. Treatment-related AEs either were related to THR-687, to the intravitreal injection procedure, or both. Best-corrected visual acuity was recorded as ETDRS letter score.
Spectral-domain OCT with Heidelberg Spectralis software version 5.1 or beyond was used to assess retinal properties, including CST. Widefield FA (Optomap) with Vantage software version 3.4 or beyond or Advance software was used to assess nonperfusion index, vascular leakage index, and macular capillary leakage. Color fundus photography with an Optos device was used to assess the severity of diabetic retinopathy. To ensure accuracy and standardization of ophthalmic assessments across the study sites, both the imaging equipment and the photographers were certified by the central reading center (Duke Reading Center), which also performed the grading of the images. THR-687 levels in plasma were analyzed at Charles River Laboratories by using a liquid chromatography-tandem mass spectrometry method.
Schedule of Assessments
Eight study visits were scheduled: screening (maximum 28 days before the date of the study treatment), the day of the study treatment (day 0), day 1, day 7, day 14, month 1, month 2, and month 3. Eligibility was assessed at the screening visit based on demography, medical history, blood pressure, hematologic and biochemistry blood analysis (including glycated hemoglobin A), urine analysis, urine pregnancy test (for women of childbearing age), full ophthalmic examination (slit-lamp, intraocular pressure, and dilated fundus examination), BCVA, SD-OCT, FA, and color fundus photography imaging. Eligibility was reconfirmed by the investigator at day 0 before study treatment. Refer to Supplemental Table 1 for the key inclusion and exclusion criteria.
After the study treatment, a postinjection assessment of the study eye was performed at least once within 60 minutes after the injection and was repeated as many times as needed at the discretion of the investigator to exclude central retinal artery nonperfusion or other complications. Urine and hematologic and biochemistry blood analyses were performed at screening, day 1, day 7, month 1, and month 3. At each visit, BCVA was assessed and a full ophthalmic examination was performed. Spectral-domain OCT was performed at each visit except for day 0 (by consequence, for CST, the day 1 value was considered the baseline value because this was the measurement obtained the closest to the injection). Widefield FA and color fundus photography were performed at screening and at month 3. THR-687 levels in plasma were determined at screening, day 1, and day 7.
Statistical Analysis
The all treated patients analysis set was used for all analyses and included data from all patients who received the intravitreal injection with THR-687. Descriptive statistics were presented by dose level and across dose levels. Missing data were not imputed, and the observed cases principle was used. Analyses of BCVA and SD-OCT parameters were adjusted to account for rescue treatment. This was carried out by replacing all BCVA and SD-OCT values after rescue treatment by the last value before or on the day of rescue treatment (last value before or on the day of rescue treatment carried forward). Descriptive summary statistics for continuous variables included mean, standard deviation, and range, as applicable. A 95 % confidence interval (CI) of the mean was calculated based on the t distribution. Categorical data were expressed in count and percentage with the 95 % CI using the exact binomial Clopper-Pearson method.
Results
Patient Disposition and Baseline Characteristics
Thirty-five patients were screened at 7 sites; 23 of these screenings resulted in failure. The most common reason for screening failure was not meeting the BCVA inclusion criterion. Twelve patients were treated at 6 sites. All patients received a single intravitreal injection of THR-687: 3 patients received 0.4 mg, 3 patients received 1.0 mg, and 6 patients received 2.5 mg of THR-687 (further referred to as low-dose, middle-dose, and high-dose levels, respectively). All patients attended each study visit and completed the study; the total duration of follow-up after the intravitreal injection ranged from 79 to 93 days.
Demographics and baseline characteristics are summarized in Table 1. Patient demographics were well-balanced across the different dose levels. The mean age of the patients was 57.8 years (standard deviation, 10.4 years) and ranged between 38 and 72 years. Most patients were men and White (9/12 patients each). No relevant imbalances were found between dose levels in baseline BCVA; however, mean baseline CST was lower in the patients treated at the high-dose level. Most patients (9/12) had moderate nonproliferative diabetic retinopathy (NPDR). The other 3 patients (all treated at the low or medium dose level), had severe NPDR or proliferative diabetic retinopathy. All patients had received previous treatment with anti-VEGF agents, with the last injection administered between 1.5 and 16 months before study treatment. The number of prior anti-VEGF injections ranged from 3 to 19. Two of 12 patients also had received previous treatment with corticosteroids.
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristic | THR-687 Dose Level |
Overall (n = 12) | ||
|---|---|---|---|---|
| Low (n = 3) | Middle (n = 3) | High (n = 6) | ||
| Age (yrs) | ||||
| Mean (SD) | 58.0 (9.54) | 59.7 (8.08) | 56.8 (13.14) | 57.8 (10.41) |
| Minimum–maximum | 47–64 | 51–67 | 38–72 | 38–72 |
| Male, no. (%) | 2 | 2 | 5 | 9 (75.0) |
| White race, no. (%) | 2 | 2 | 5 | 9 (75.0) |
| HbA1c (%) | ||||
| Mean (SD) | 6.87 (0.709) | 8.47 (1.815) | 8.93 (2.399) | 8.30 (2.023) |
| Minimum–maximum | 6.1–7.5 | 6.4–9.8 | 6.8–12.5 | 6.1–12.5 |
| Diabetic retinopathy scale, no. (%) | ||||
| Moderate NPDR | 1 | 2 | 6 | 9 (75.0) |
| Severe NPDR | 1 | 0 | 0 | 1 (8.3) |
| PDR | 1 | 1 | 0 | 2 (16.7) |
| BCVA (ETDRS letters) | ||||
| Mean (SD) | 59.3 (2.08) | 54.7 (2.31) | 55.7 (8.26) | 56.3 (6.02) |
| Minimum–maximum | 57–61 | 52–56 | 39–61 | 39–61 |
| CST (μm) | ||||
| Mean (SD) | 557.7 (123.50) | 538.3 (41.30) | 429.5 (78.11) | 488.8 (98.68) |
| Minimum–maximum | 434–681 | 491, 567 | 330, 524 | 330, 681 |
| Prior treatment for DME, no. (%)∗ | ||||
| Anti-VEGF | 3 | 3 | 6 | 12 (100.0) |
| Corticosteroids | 1 | 0 | 1 | 2 (16.7) |
BCVA = best-corrected visual acuity; CST = central subfield thickness; DME = diabetic macular edema; ETDRS = Early Treatment Diabetic Retinopathy Study; HbA1c = glycated hemoglobin A; NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy; SD = standard deviation; VEGF = vascular endothelial growth factor.
All subjects received prior anti-VEGF and/or corticosteroids treatment, per study inclusion criteria.
Safety Outcomes
THR-687 was well tolerated, and no DLTs were reported at any dose level. All ocular AEs are summarized in Table 2. No serious AEs occurred, and no AEs led to discontinuation from the study. Overall, 5 patients (41.7 %) experienced 9 AEs in the study eye. No AEs occurred in the fellow eye. One nonocular AE occurred: food poisoning.
Table 2.
Summary of Ocular Adverse Events in the Study Eye
| Characteristic | THR-687 Dose Level |
Overall (n = 12) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (n = 3) |
Middle (n = 3) |
High (n = 6) |
|||||||
| n | No. of Events | n | No. of Events | n | No. of Events | No. (%) | 95 % Confidence Interval | No. of Events | |
| AE in the study eye | 1 | 2 | 1 | 3 | 3 | 4 | 5 (41.7) | 15.2–72.3 | 9 |
| Conjunctival hemorrhage∗ | 1 | 1 | 1 | 1 | 0 | 0 | 2 (16.7) | 2 | |
| Diabetic retinal edema | 0 | 0 | 1 | 1 | 2 | 2 | 3 (25.0) | 3 | |
| Eye pain∗ | 0 | 0 | 0 | 0 | 1 | 1 | 1 (8.3) | 1 | |
| IOP increased∗ | 1 | 1 | 0 | 0 | 0 | 0 | 1 (8.3) | 1 | |
| Ocular hypertension | 0 | 0 | 1 | 1 | 0 | 0 | 1 (8.3) | 1 | |
| Vision blurred | 0 | 0 | 0 | 0 | 1 | 1 | 1 (8.3) | 1 | |
AE = adverse event; IOP = intraocular pressure.
Deemed treatment related (THR-687 or intravitreal injection related, or both) by the investigator.
For 3 patients (1 patient at each dose level), 4 AEs occurred in the study eye that were deemed treatment related by the investigator. These AEs were conjunctival hemorrhage (1 patient in the low-dose group and 1 patient in the middle-dose group), eye pain (1 patient in the high-dose group), and increased intraocular pressure (the same patient in the low-dose group). All treatment-related events were mild, started on the day of the injection, and resolved within 28 days without treatment. Considering the nature of these AEs, they are deemed related to the intravitreal injection procedure.
Other (nontreatment-related) AEs reported in the study eye were ocular hypertension, vision blurred, and diabetic retinal edema (worsening of DME). Diabetic retinal edema was reported in 1 patient at the low-dose level and 2 patients at the high-dose level. This AE was considered related to the underlying disease progression. In line with this, all patients for whom diabetic retinal edema was reported as an AE also received rescue treatment. The patient in the low-dose group received bevacizumab between the month 1 and month 2 visit, whereas both patients in the high-dose group received aflibercept, 1 patient at the month 1 visit and the other patient at the month 2 visit. None of the other patients in the study received rescue treatment.
In general, individual glycated hemoglobin A values did not change during the study. Any abnormal laboratory values were in line with the underlying or concomitant disease(s), and accordingly, no AEs were reported for laboratory parameters.
THR-687 levels were detectable in plasma in a dose level-dependent manner on the day after study treatment, with mean plasma levels of 0.33 ng/ml (standard deviation, 0.027 ng/ml), 0.79 ng/ml (standard deviation, 0.601 ng/ml), and 2.25 ng/ml (standard deviation, 0.895 ng/ml) in the low-, middle-, and high-dose groups, respectively. By day 7 after injection, THR-687 levels were below the lower limit of detection in all patients.
Exploratory Efficacy Outcomes
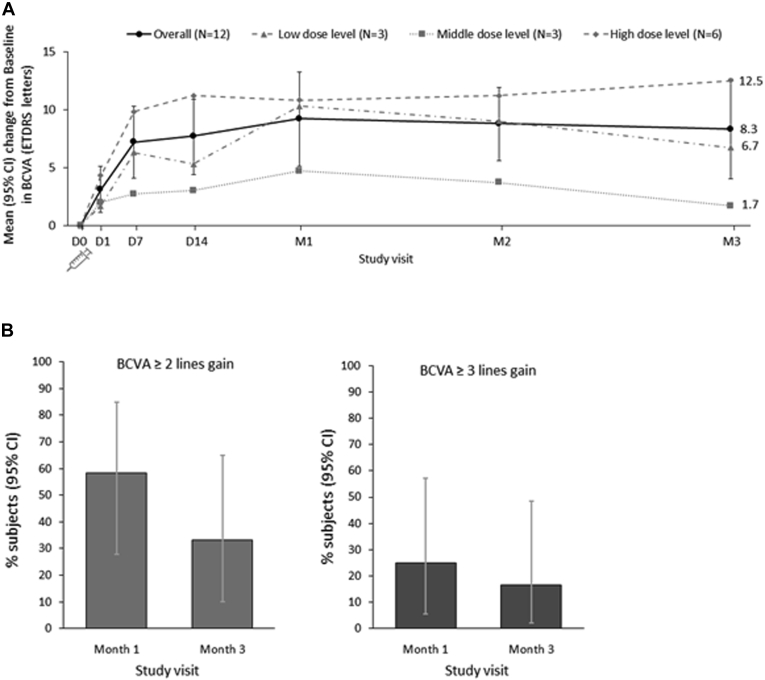
Mean changes from baseline in BCVA for all patients combined and by dose level are shown in Figure 1A. Overall, a mean gain of 7.2 letters (95 % CI, 4.1–10.3 letters) in BCVA was observed at day 7. The gain was the highest at month 1, with a mean gain of 9.2 letters (95 % CI, 5.1–13.2 letters). The gain in BCVA was maintained up to the end of the study (month 3), with a mean gain of 8.3 letters (95 % CI, 4.0–12.6 letters). When assessed by dose level, the highest mean gains in BCVA were seen at all study visits for the high-dose level.
Figure 1.
Graphs showing mean change in best-corrected visual acuity (BCVA) from baseline. A, Overall mean (95 % confidence interval [CI]) changes from baseline in BCVA Early Treatment Diabetic Retinopathy Study (ETDRS) letters over time, accounting for rescue treatment,∗ for all treated patients and by dose level. B, Percentage (95 % CI) of patients with a 2-line or more or 3-line or more gain in BCVA from baseline, accounting for rescue treatment,∗ for all treated patients (n = 12). ∗Last value before or on the day of rescue treatment carried forward. D = day; M = month.
Figure 1B shows the number of patients gaining 2 or more lines and 3 or more lines of vision at month 1 and month 3. A 2-line or more gain in BCVA from baseline was observed in 7 of 12 patients (58.3 %) at month 1 and 4 of 12 patients (33.3 %) at month 3. A 2-line or more gain in BCVA was observed more frequently in patients treated at the high-dose level: 4 of 7 patients with BCVA gain of 2 lines or more at month 1 were treated at the high-dose level and 4 of 4 patients at month 3. A 3-line or more gain in BCVA from baseline was observed in 3 of 12 patients (25 %) at month 1 and 2 of 12 patients (16.7 %) at month 3, all of which were treated at the high-dose level. A 1-line or more decline in BCVA from baseline was not observed (in any of the study patients, at any of the study visits).
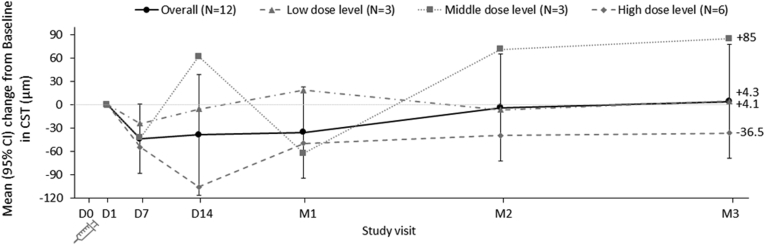
Mean changes from baseline in CST for all patients combined and by dose level are shown in Figure 2. Overall, the mean CST decreased marginally up to month 1. The largest decrease was observed at day 7 with a mean of –43.9 μm (95 % CI, –88.7 to 0.9 μm). A mean increase of 4.1 μm (95 % CI, –69.4 to 77.6 μm) was seen at month 3. When assessed by dose level, the highest mean decreases in CST were seen for the high-dose level.
Figure 2.
Graph showing mean change from baseline in central subfield thickness (CST). Overall mean (95 % confidence interval [CI]) changes from baseline∗ in CST over time, accounting for rescue treatment,† for all treated patients and by dose level. ∗Spectral-domain OCT was not performed at day 0; therefore, the measurement closest to the injection was considered as the baseline (i.e., day 1). †Last value before or on the day of rescue treatment carried forward. D = day; M = month.
No clinically meaningful changes from baseline were observed for any of the FA assessments at month 3. None of the patients showed worsening from baseline in diabetic severity level at month 3. For 1 of 12 patients (8.3 %), the diabetic retinopathy severity level improved from severe NPDR at baseline to moderate NPDR at month 3.
Discussion
In this first-in-human phase 1 study, the safety and preliminary efficacy of a single intravitreal injection of THR-687 was assessed in patients with center-involved DME who had received anti-VEGF treatment, corticosteroid therapy, or both and, in the opinion of the investigator, remained responsive to treatment. This population was selected to ensure that the patients were able to respond to treatment for DME. THR-687 was safe and well tolerated at all dose levels tested. No DLTs occurred and no safety signals of concern were seen during the 3-month follow-up period after the single intravitreal administration of THR-687. All treatment-related AEs in the study eye were mild, resolved without treatment, and likely were related to the injection procedure, rather than to THR-687 itself.
Preliminary efficacy was observed by gain in BCVA. Overall, across dose levels, a rapid onset of action occurred, with a mean gain of 7.2 letters from baseline in BCVA seen at day 7. Over time, the largest gain in BCVA was observed at month 1 with a mean gain of 9.2 letters. The effect of THR-687 was durable, with an overall mean BCVA gain of 8.3 letters at the end of the study (month 3). At all study visits, the highest BCVA gains were seen at the high-dose level. Overall, across dose levels, mean decreases from baseline in CST were marginal up to month 1 and had returned to baseline values by month 2. For patients treated at the high-dose level, more clinically relevant decreases in CST were observed.
Before this study, other integrin inhibitors also had been studied in patients with DME.26 Risuteganib (Luminate [formerly ALG-1001]; Allegro Ophthalmics) is a synthetic peptide that blocks several RGD integrins. Its safety and efficacy have been examined in the randomized, prospective, double-masked phase 2b DEL MAR study. In stage 1 of the DEL MAR study, 138 patients were treated with 1 of 3 dose levels of risuteganib (1.0 mg, 2.0 mg, or 3.0 mg) or to bevacizumab (control arm). All patients received 3 monthly intravitreal injections at day 0, month 1, and month 2. Patients in the bevacizumab arm could receive pro re nata injections of bevacizumab month 3 and month 4. Efficacy was assessed at 3 months after the third monthly injection (at month 5). Risuteganib 1.0 mg was found to be noninferior to bevacizumab in terms of gains in BCVA and central macular thickness (ClinicalTrials.gov identifier, NCT02348918).34,35
In stage 2 of the DEL MAR study, 80 patients were randomized to arms in which risuteganib (0.5 mg or 1.0 mg) was given either as 3 monthly intravitreal injections at week 1, month 1, and month 2 after a single intravitreal injection of bevacizumab at day 0 (sequential treatment); as 3 monthly intravitreal injections of combined risuteganib/bevacizumab at week 1, month 1, and month 2 after a single intravitreal injection of bevacizumab at day 0 (combination treatment); or as bevacizumab alone given as 3 monthly intravitreal injections at week 1, month 1, and month 2 followed by pro re nata injections at month 3 and month 4 (control arm). Efficacy was assessed at 3 months after the third monthly injection (at month 5). Risuteganib 1.0 mg was found to be noninferior to bevacizumab in terms of BCVA gain when it was given as a sequential treatment after bevacizumab.34,36 Risuteganib administered in combination with bevacizumab was inferior to sequential therapy and to bevacizumab alone. The durable gain in BCVA observed at 3 months after the third injection with risuteganib in the sequential treatment arm did not seem to correlate with changes in retinal architecture. Specifically, the observed mean decreases from baseline in central retinal thickness were the largest at 1 month after the third injection with risuteganib (at month 3) and were not maintained at month 4 and month 5, even as BCVA gains were maintained.
Although from a mechanistic point of view risuteganib and THR-687 are very similar, they differ in terms of potency. This was reflected in an in vitro functional cellular assay in which THR-687 inhibited endothelial cell migration with a half maximum inhibitory concentration at micrometer concentration (Oxurion internal data), whereas the half maximum inhibitory concentration of ALG-1001 was at micrometer concentration in a similar assay (3 logs difference). In addition, the DEL MAR study of risuteganib differed in many ways from the phase 1 study of THR-687 reported herein. Both stages of this study examined the effects of 3 administrations of risuteganib, whereas the study reported herein examined the effects of a single administration of THR-687. Nevertheless, the BCVA gains from baseline shown at 3 months after a single injection of THR-687 (8.3 letters) seem as durable as gains in BCVA from baseline 3 months after the last of 3 injections of risuteganib (7.1 letters in DEL MAR stage 2 sequential treatment with risuteganib 1.0 mg). However, it also should be noted that baseline BCVA in the DEL MAR stage 2 study was measured before the bevacizumab injection at day 0, and a gain of 4.1 letters already had occurred before the first administration of risuteganib. The durability of response to both risuteganib and THR-687 suggest that integrin inhibitors may be able to decrease the treatment frequency required with current anti-VEGF therapies.
SF0166 (SciFluor Life Sciences) is a topical (eye drop) antagonist of the RGD integrin αvβ3 that has been examined in a phase 1/2 study in 40 patients with DME.37 Patients were randomized to a dose of 2.5 % or 5 % eye drop formulation, self-administered twice daily for 28 days. Both doses of SF0166 exhibited therapeutic efficacy, with 53 % of the patients displaying a decline in retinal thickness and BCVA gain. Durability of retinal thickness response to SF0166 treatment was observed during the 28-day follow-up period after discontinuing treatment.34 Although the data from the phase 1/2 study of SF0166 thus did seem to indicate a biological effect, no further development of this compound has been reported to date.
The principal limitation of the phase 1 study reported herein is the small number of patients included. Nevertheless, the data reported indicate that THR-687 is safe and well tolerated after a single intravitreal administration. All patients attended every study visit. No DLTs or serious AEs occurred. Preliminary evidence of efficacy was observed by rapid and durable gains in BCVA, especially at the high-dose level. At the high-dose level, decreases in CST were seen. Taken together, these results support the further development of THR-687 as a potential, non-VEGF–targeted therapy for patients with DME. A phase 2 study is planned in which the safety and efficacy of multiple injections of THR-687 will be evaluated in patients with center-involved DME.
Manuscript no. D-21-00051.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): A.M.K.: Financial support – Genentech, Novartis, Allergan, Opthea, Chengdu Kanghong, Kodiak, Adverum, Regenexbio, PolyPhotonix
S.S.P.: Consultant – AiViva, Allergan, Allgenesis, Genentech-Roche, Kala, Kodiak Sciences; Advisory board – Allergan, Genentech-Roche, Kodiak Sciences; Financial support – Aerie, Aerpio, Allergan, Apellis, Boehringer Ingelheim, Chengdu Kanghong, Clearside, Genentech-Roche, Ionis Pharmaceuticals, IVERIC Bio, KalVista, Kodiak Sciences, Mylan, Novartis, Opthea, Ora, Oxurion, Regeneron, Samsung, Stealth Biotherapeutics, ThromboGenics, Xbrane Biopharma
G.J.J.: Financial support (to institution) – Oxurion, Roche/Genentech; Consultant – Regeneron
P.K.: Employee – Oxurion
P.U.D.: Financial support – Oxurion
Supported by Oxurion NV (formerly ThromboGenics), Leuven, Belgium. The sponsor participated in the design and conduct of the study; the collection, analysis, and interpretation of data; and review and approval of the manuscript.
Presented at: American Academy of Ophthalmology Annual Meeting, November 2020, virtual meeting.
HUMAN SUBJECTS: Human subjects were included in this study. This study (ClinicalTrials.gov Identifier NCT03666923) was conducted according to the principles of the Declaration of Helsinki, the International Council for Harmonisation guideline for Good Clinical Practice, and all applicable local regulatory requirements. Institutional Review Board (Copernicus Group IRB) approval was obtained. All subjects provided written informed consent prior to screening.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Khanani, Kozma
Analysis and interpretation: Khanani, Kozma
Data collection: Khanani, Patel, Gonzalez, Moon, Jaffe, Wells, Kozma, Dugel, Maturi
Obtained funding: N/A; Study was performed as part of regular employment duties at Oxurion. No additional fundung was provided.
Overall responsibility: Khanani, Kozma
Supplementary Data
References
- 1.Bourne R.R., Stevens G.A., White R.A., et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–e349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 2.Ting D.S., Cheung G.C., Wong T.Y. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Global report on diabetes. https://www.who.int/diabetes/global-report/en/ Available at: Accessed October 30, 2016.
- 4.International Diabetes Federation, International Diabetes Federation Diabetes Atlas, 9th ed., 2019, International Diabetes Federation. Available at: https://www.diabetesatlas.org/en/resources/. Accessed October 30, 2020.
- 5.Gonder J.R., Walker V.M., Barbeau M., et al. Costs and quality of life in diabetic macular edema: Canadian Burden of Diabetic Macular Edema Observational Study (C-REALITY) J Ophthalmol. 2014;2014:939315. doi: 10.1155/2014/939315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiss S., Chandwani H.S., Cole A.L., et al. Comorbidity and health care visit burden in working-age commercially insured patients with diabetic macular edema. Clin Ophthalmol. 2016;10:2443–2453. doi: 10.2147/OPTH.S114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook R.A., Kleinman N.L., Patel S., et al. United States comparative costs and absenteeism of diabetic ophthalmic conditions. Postgrad Med. 2015;127(5):455–462. doi: 10.1080/00325481.2014.994468. [DOI] [PubMed] [Google Scholar]
- 8.Das A., McGuire P.G., Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122(7):1375–1394. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Bakri S.J., Wolfe J.D., Regillo C.D., et al. Evidence-based guidelines for management of diabetic macular edema. J Vitreoretina Dis. 2019;3(3):145–152. [Google Scholar]
- 10.Nguyen Q.D., Brown D.M., Marcus D.M., et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Erfurth U., Garcia-Arumi J., Bandello F., et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237(4):185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 12.Flaxel C.J., Adelman R.A., Bailey S.T., et al. Diabetic retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127(1):P66–P145. doi: 10.1016/j.ophtha.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Ehlken C., Helms M., Bohringer D., et al. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. doi: 10.2147/OPTH.S151611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss M., Sim D.A., Herold T., et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38(12):2293–2300. doi: 10.1097/IAE.0000000000001892. [DOI] [PubMed] [Google Scholar]
- 15.Avery R.L., Gordon G.M. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134(1):21–29. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 16.Brown D.M., Nguyen Q.D., Marcus D.M., et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Korobelnik J.F., Kleijnen J., Lang S.H., et al. Systematic review and mixed treatment comparison of intravitreal aflibercept with other therapies for diabetic macular edema (DME) BMC Ophthalmol. 2015;15:52. doi: 10.1186/s12886-015-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell P., Bandello F., Schmidt-Erfurth U., et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Blinder K.J., Dugel P.U., Chen S., et al. Anti-VEGF treatment of diabetic macular edema in clinical practice: effectiveness and patterns of use (ECHO study report 1) Clin Ophthalmol. 2017;11:393–401. doi: 10.2147/OPTH.S128509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bressler S.B., Ayala A.R., Bressler N.M., et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol. 2016;134(3):278–285. doi: 10.1001/jamaophthalmol.2015.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells J.A., Glassman A.R., Ayala A.R., et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–1359. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bressler N.M., Beaulieu W.T., Glassman A.R., et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257–269. doi: 10.1001/jamaophthalmol.2017.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bressler N.M., Beaulieu W.T., Maguire M.G., et al. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. Am J Ophthalmol. 2018;195:93–100. doi: 10.1016/j.ajo.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer D.S., Yoon Y.H., Belfort R., Jr., et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Maturi R.K., Pollack A., Uy H.S., et al. Intraocular pressure in patients with diabetic macular edema treated with dexamethasone intravitreal implant in the 3-year Mead study. Retina. 2016;36(6):1143–1152. doi: 10.1097/IAE.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 26.Bhatwadekar A.D., Kansara V., Luo Q., Ciulla T. Anti-integrin therapy for retinovascular diseases. Expert Opin Invest Drugs. 2020;29(9):935–945. doi: 10.1080/13543784.2020.1795639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 28.Barczyk M., Carracedo S., Gullberg D. Integrins.Cell Tissue Res. 2010;339(1):269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedlander M., Theesfeld C.L., Sugita M., et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A. 1996;93(18):9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramakrishnan V., Bhaskar V., Law D.A., et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5(4):273–286. [PubMed] [Google Scholar]
- 31.Umeda N., Kachi S., Akiyama H., et al. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol. 2006;69(6):1820–1828. doi: 10.1124/mol.105.020941. [DOI] [PubMed] [Google Scholar]
- 32.Santulli R.J., Kinney W.A., Ghosh S., et al. Studies with an orally bioavailable alpha V integrin antagonist in animal models of ocular vasculopathy: retinal neovascularization in mice and retinal vascular permeability in diabetic rats. J Pharmacol Exp Ther. 2008;324(3):894–901. doi: 10.1124/jpet.107.131656. [DOI] [PubMed] [Google Scholar]
- 33.Hu T.T., Vanhove M., Porcu M., et al. The potent small molecule integrin antagonist THR-687 is a promising next-generation therapy for retinal vascular disorders. Exp Eye Res. 2019;180:43–52. doi: 10.1016/j.exer.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Dugel P.U. The scoop on anti-integrin therapy for DME. Retina Today. 2019:30–32. [Google Scholar]
- 35.Shaw L.T., Mackin A., Shah R., et al. Risuteganib-a novel integrin inhibitor for the treatment of non-exudative (dry) age-related macular degeneration and diabetic macular edema. Expert Opin Invest Drugs. 2020;29(6):547–554. doi: 10.1080/13543784.2020.1763953. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser P.K., Kuppermann B.D., Heier J.S., et al. Randomized, prospective, double-masked, controlled phase 2B trial on the safety and efficacy of Alg-1001 (Luminate) in DME. 2018 [Google Scholar]
- 37.MacDougall Biomedical Communications . MacDougall Biomedical Communications; 2017. SciFluor announces positive results of phase 1/2 study of SF0166 topical ophthalmic solution in diabetic macular edema patients.https://www.businesswire.com/news/home/20170928005321/en/SciFluor-Announces-Positive-Results-of-Phase-12-Study-of-SF0166-Topical-Ophthalmic-Solution-in-Diabetic-Macular-Edema-Patients [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.