Abstract
Globe artichoke roots represent an alternative and sustainable source for inulin extraction and are well-noted for their technological and functional properties. Therefore, the aim of our study was to exploit inulin with high degree of polymerization as a replacement of durum wheat semolina for the production of functional fresh pasta. The effect of increased level of substitution (5, 10, 15%) on cooking, structural, sensory, and nutritional properties were evaluated and compared with a control sample consisting exclusively of durum wheat semolina. Inulin addition caused changes to internal structure as evaluated by scanning electron microscopy. The enriched samples showed a lower swelling index, an increasing cooking time, and values of cooking loss (2.37–3.62%), mainly due to the leaching of inulin into the cooking water. Cooked and raw enriched pasta was significantly darker and firmer than the control, but the sensory attributes were not negatively affected, especially at 5 and 10% of substitution levels. The increase of dietary fiber content in enriched pasta (3.44–12.41 g/100 g) resulted in a significant reduction of glycaemic index (pGI) and starch hydrolysis (HI). After gastrointestinal digestion, inulin-enriched pasta increased prebiotic growth able to significantly reduce E. coli cell density.
Keywords: waste reuse, inulin, high polymerization degree, functional pasta, glycemic index, prebiotics growth
1. Introduction
Globe artichoke (Cynara cardunculus L. subsp. scolymus (L.)) is a perennial plant belonging to the Asteraceae family that is cultivated as a polyannual crop with vegetative propagation. Nevertheless, the length of the crop cycle, which negatively influences yields and the quality of the heads, has led artichoke growers to take an interest in the development of new seed-propagated cultivars for annual crops [1]. Italy represents one of the major producers, accounting for 33% of global production, followed by Spain, France, and Greece [2,3,4,5]. The edible part of the globe artichoke, commonly known as the “heart”, is highly appreciated worldwide and it is consumed fresh, canned, or frozen [6]. However, from the harvesting phase to the processing one, a large amount of waste and by-products (80–85% of the biomass) are produced: roots, stems, bracts, and leaves, which have shown to be a rich source of antioxidant compounds and dietary fiber, mainly as pectin and inulin [6,7,8].
Artichoke roots are an important agricultural waste that, at the end of the harvesting period, remains unexploited in the field [1]. At the same time, their richness in inulin, characterized by a high degree of polymerization [9], makes them an alternative and sustainable source, considering that chicory roots, Jerusalem artichoke, and dahlia are commonly used together to produce commercial inulin [10,11,12]. This fiber is the most abundant reserve polysaccharide after starch, and its structure is characterized by a mixture of oligo and/or polysaccharides consisting of a variable number of d-fructose units bonded with a β-(2 → 1) linkages with a terminal glucose residue. The degree of polymerization can range from 2 to 60 units, which directly influences its physicochemical and nutritional properties and its application as a food ingredient [9,12,13,14]. Generally, short-chain inulin is used as an alternative low-calorie sweetener, with its good solubility contributing to improving the mouthfeel, while long-chain inulin mixed with water or aqueous solution forms a particle gel network that can act as a fat replacer or texture modifier due to its lower solubility and good viscosity stability [12,15].
From the nutritional point of view, the peculiar bond configuration β-(2 → 1) confers to inulin a prebiotic character [16]. Inulin, indeed, reaches the colon unaltered, where it is fermented by beneficial bacteria such as Lactobacillus and Bifidobacterium, determining the release of short-chain fatty acids in the gut and a pH lowering which, in turn, enhances the absorption of minerals (Ca2+ and Mg2+), nutrients [4], and increases the functionality of colonocytes. Moreover, regular consumption of prebiotics has shown several health benefits like modulation of hyperglycemia, reduction of LDL cholesterol and serum lipids, prevention of colorectal cancer, and enhancement of immune system efficiency [17,18,19].
The consumer awareness about the relationship between health and food has led to the spread of functional products, i.e., those foods which contain bioactive compounds that, if taken in suitable quantities, have been shown to be able to prevent disease in addition to their nutritional functions [20,21]. In this regard, pasta seems to be particularly suitable for functional ingredients integration due to its easy preparation and its widespread consumption. Several studies have tried to improve the nutritional profile of pasta by adding soluble and insoluble fiber and evaluating their effect on quality properties [22,23,24,25,26,27,28,29,30].
However, the inclusion of dietary fiber could cause a deterioration of pasta quality in terms of cooking properties and sensory features due to the alteration of protein–starch network integrity, as observed by Foschia et al. [31]. Aravind et al. [32] found that the use of commercial inulin with a low degree of polymerization (DP) had a negative impact on firmness, cooking loss, and sensory acceptability of pasta; instead, the inulin with higher DP provided minimal impact. Similarly, Padalino et al. [33] added inulin at different DP and at two different concentrations (2 and 4%). In particular, the authors found interesting results in that the addition of inulin with a high DP, compared to the low one, had a greater disruptive effect on the starch–protein matrix. Peressini et al. [30] found an increase of firmness in pasta added with Barley Balance (BB), Psyllium seed husk (P) and BB–P, while a lower value than the control when inulin with high DP was used. Moreover, during the in vitro starch digestion, the pasta enriched with both inulin with high and low DP showed the highest reducing sugar release at 20 min compared to other fibers and control sample without fiber. Hence, Garbetta et al. [28] studied the effect of 4% addition of two different types of inulin, namely artichoke roots with high DP and chicory roots with low DP, observing good results in terms of sensory acceptability after the addition of inulin with high DP, suggesting the potential use of inulin-enriched spaghetti as a prebiotic food.
Therefore, an analysis of the literature revealed the influence of the DP of inulin on the technological and functional properties of foods, which needs to be further investigated based on the conflicting results in the literature.
In this framework, our study aimed to enhance the value of globe artichoke roots using them as an alternative source to extract inulin with a high degree of polymerization and their use as a functional ingredient for fresh pasta preparation. The effect of increasing the amount of the extracted inulin on structural, nutritional, and sensory properties of fresh pasta samples was evaluated.
2. Materials and Methods
2.1. Extraction and Characterzsation of Artichoke Root Inulin
2.1.1. Extraction Process
Inulin extraction from artichoke roots (AR) was carried out according to Castellino et al. [10], with slight modifications. Artichoke roots powder (ARP) was mixed with water at a pH of 6.8 with a ratio solid to water of 1:16 (w/w). The extraction took place in a thermostated bath at 80 °C for 2 h, with periodic stirring every 15 min. Afterwards, the sample was filtered with a Buchner funnel using WhatmanTM (Darmstadt, Germany) filters with 11 µm of porosity, and then the filtrate was collected and submitted to precipitation phase through two cycles of freezing and thawing. The precipitate was centrifugated at 7500× g 15 min at 10 °C and the resulting pellet was washed by adding 10 mL of ethanol, centrifugated, dried overnight, and weighted.
Inulin yield was expressed in g per 100 g of ARP.
2.1.2. Moisture and Water Activity of AR Inulin Powder
Moisture was determined with a moisture analyzer (Mod. MAC 110/NP, Rodwang Wagi Elektroniczne, Radom, Poland) at 120 °C until constant weight. Water activity was measured using a hygrometer (Aqua Lab 100–240 V AC, Pullman, WA, USA). Each measurement was carried out in triplicate.
2.1.3. Identification and Quantification of AR Inulin
Identification and quantification of AR inulin was conducted via high-performance liquid chromatography (HPLC) analysis using a 1260 infinity series chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a refractive index detector (RID) and a cationic exchange column 300 × 7.8 mm (RezexTM RCM-Monosaccharide Ca2+, 8 µm, Torrance, CA, USA). The analysis was conducted isocratically using Milli-Q water as a mobile phase with a flow of 0.6 mL/min, column temperature of 80 °C, and RID of 35 °C. For calibration, commercial inulin with a high degree of polymerization and purity ≥98.5% (FibrulineTM XL chicory root fibre, COSUCRA Groupe Warcoing SA, Warcoing, Belgium) was used as standard. Standard inulin solutions were filtered through a 0.45 µm nylon filter and injected in triplicate at different concentrations (0.25 mg/mL, 0.5 mg/mL, and 1.0 mg/mL). AR inulin was properly diluted, filtered, and injected. A calibration curve was obtained for concentration versus area, and AR inulin was identified by coincidences of retention time with standard inulin and quantified through the corresponding calibration curve.
2.1.4. Polymerization Degree and Molecular Weight
Gel permeation chromatography was performed on extracted inulin. The analysis was carried out by using a GPC Max (Viscotek, Malvern, UK) system equipped with a TDA 305 detector (Refractive Index, Low Angle Light Scattering, Right Angle Light Scattering and Viscometer) and UV detector. We used a pre-column TSK PWXL and TSK Gel GMPWXL (Tosoh Bioscience, Griesheim, Germany). The sample was dissolved at 3 mg/mL concentration and eluted in MilliQ water containing 0.2% NaN3. After complete dissolution, the sample was filtered on a membrane having 0.22 μm porosity. The injection volume was 100 μL, and the flow rate was 0.8 mL/min. The chosen method of analysis was universal calibration, using polyethyleneoxide (PEO) standards, ranging from 21.300 kDa to 420 Da. The measurements, performed at 35 °C according to the temperatures of columns and detectors, were ran for 60 min in duplicate.
The polymerization degree (DP) was calculated as follows [34]:
| Mn = 180 + 162 × (DPn − 1) |
and
| Mw = 180 + 162 × (DPw − 1) |
where Mn and Mw are number average molecular weight and weight average molecular weight, respectively.
2.2. Fresh Pasta Preparation and Characterization
2.2.1. Experimental Design
Inulin-enriched fresh pasta was produced by substituting 5% (P5), 10% (P10), and 15% (P15) of durum wheat semolina (Mulino Martimucci, Altamura, Italy) with AR inulin powder. A control sample was produced with 100% durum wheat semolina. Durum wheat semolina and durum-wheat-semolina–inulin were mixed with an adequate amount of water and manually kneaded until obtaining a homogenous dough. The dough was left to rest for 30 min and then laminated (2 mm thickness) and cut with the iPasta (Imperia, Moncalieri, Italy) moulder machine to produce tagliatelle pasta. Afterwards, fresh tagliatelle were put on a wooden vessel, covered with a cotton cloth, and left to dry at room temperature until reaching a moisture content in a range of 26–28% and aw values ranging from 0.92 and 0.97, according with Italian legal requirements for fresh pasta. Two different batches were produced.
2.2.2. Cooking Properties
Pasta samples were cooked in boiling distilled water at 1:10 (w/v) pasta-to-water ratio, without the addition of salt, as reported by Pasqualone et al. [35]. The optimum cooking time (OCT) was determined by removing tagliatelle from boiling water every 30 sec, cutting them, and checking for the white and opaque core to disappear, according to the AACC 16–50 official method [36]. After cooking, pasta samples were drained and rinsed with distilled water and allowed to rest for 5 min. Cooking loss was evaluated by combining cooking and rinsing water, measuring total volume, putting 20 mL in tarred Petri dishes, and evaporating in an air-oven at 105 °C until reaching constant weight. The residue, scaled up to total volume, was expressed as a percentage of the original pasta sample weight. After cooking at their OCT, the samples were analyzed for their water absorption and swelling index (grams of water per gram of dry pasta) according with Bustos et al. [37]. Each determination was carried out in triplicate.
| Wr = weight of raw pasta (g) |
| Wc = weight of cooked pasta (g) |
| Wd = weight of dried pasta (g) |
2.2.3. Inulin Loss in Cooking Water
P5, P10, and P15 were cooked at their OCT with a pasta-to-water ratio of 1:10 (w/v). Afterwards, 15 mL of cooking water was collected and analyzed for its inulin content by a cationic exchange HPLC following the same method described in Section 2.1.3. The inulin cooking water content was expressed as grams of inulin loss during cooking at OCT of 100 g of fresh pasta. The analysis was carried out in triplicate.
2.2.4. Color and Firmness Evaluation
Color of raw and cooked fresh pasta sample at its OCT (red index, corresponding to a*, yellow index, corresponding to b*, and luminosity index, corresponding to L*) was evaluated using a colorimeter CM-600d (Konica Minolta, Osaka, Japan) equipped with SpectraMagicX software. Six measurements were recorded for each pasta sample.
Firmness evaluation of raw and cooked pasta at its OCT was carried out using a Z1.0 TN (Zwick Roell, Ulm, Germany) equipped with a blade set with a guillotine and 50 N load cell. Pasta firmness was evaluated as the force required to cut 5 strands of pasta at a speed of 0.17 mm/s and was expressed as the maximum force (N) required to cut pasta strands. Eight measurements were recorded for each sample.
2.2.5. Microstructure Determination
The microstructure and the surface characteristics of the pasta were studied with a Zeiss Sigma 300 VP (Carl Zeiss NTS GmbH, Oberkochen, Germany) field-emission gun scanning electron microscope (FEG-SEM) equipped with a secondary electrons detector (SE). The analyses were done under vacuum (<10−4 Pa), using an accelerating voltage of 20 kV, an aperture of 30 μA, and a working distance between 4 and 5 mm and magnification of 1000×. Fragments of the different pasta samples were glued onto an aluminium stub with carbon tape. Before the analysis, all the samples were carbon-coated in order to make the surface of the specimen conductive.
2.2.6. Quantitative Descriptive Sensory Analysis
Sensory analyses were conducted by a panel of eight trained tasters (6 females and 2 males) to evaluate the sensory attributes according to Pasqualone et al. [35]. Pasta samples were coded by a three-digit number, cooked at their OCT, and evaluated for color, smell, taste, bulkiness, adhesiveness, hardness, and overall acceptability, using a structured scale ranging from 1 to 10. Color refers to the typical color of durum wheat pasta, where 1 = yellow color and 10 = brown; smell as perceived by olfaction and taste as perceived during mastication, which refer to the typical odor and taste of durum wheat pasta without anomalies, where 1 = very low and 10 = very high; bulkiness refers to adhesion of tagliatelle strands to each other, evaluated both visually and manually by pressing two tagliatelle together and determining the force required for detachment; stickiness, related to the organic matter released during cooking and still adhering to the surface of pasta, evaluated by pressing a single strand against the palate and determining the force require to remove it with the tongue; hardness, which is the resistance of cooked pasta to chewing measured while cutting the spaghetti strand using the front teeth. The analysis was carried out according to Pasqualone et al. [38], following the ethical guidelines of the Laboratory of Food Science and Technology of the Department of Plant, Soil, Food Science of University of Bari, Italy.
2.3. Functional Properties Determination
2.3.1. Proximate Composition of Fresh Pasta
Protein (total nitrogen × 5.7), ash, and lipids content were determined using the AOAC method 979.09, 923.03, and 945.38 F, respectively [39]. Total dietary fiber content was determined by enzymatic gravimetric as described by the AOAC Official Method 991.43. Moisture content was determined by a moisture analyzer (Mod. MAC 110/NP, Radwag Wagi Elektroniczne, Radom, Poland) at 120 °C. The carbohydrate content was determined as difference. The determinations were carried out in triplicate.
2.3.2. In Vitro Starch Hydrolysis
In vitro gastrointestinal digestion of fresh pasta and starch hydrolysis was determined according to Liljeberg et al. [40], simulating the in vivo digestion of starch. Briefly, aliquots of fresh pasta samples, cooked until the optimal cooking time and containing 1 g of starch (determined in cooked fresh pasta), were subjected to an enzymatic process (pancreatic amylase and pepsin-HCl), and the released glucose content was measured with D-fructose/D-glucose Assay Kit (Megazyme, Wicklow, Ireland). Simulated digests were dialyzed (cut-off of the membrane: 12,400 Da) for 180 min. Aliquots of dialysate, containing free glucose, and partially hydrolyzed starch were sampled every 30 min and further treated with amyloglucosidase. Then, free glucose was determined using the above-mentioned enzyme-based kit and finally converted into hydrolyzed (digested) starch in pasta. Control white wheat bread was used as the control to estimate the hydrolysis index (HI = 100). The predicted glycemic index (pGI) was calculated using the equation pGI = 0.549 × HI + 39.71 [41]. Each sample was analyzed in triplicate.
2.3.3. Prebiotic Activity Assay
To evaluate the prebiotic activity of AR inulin-enriched fresh pasta, samples were previously subjected to in vitro gastrointestinal digestion according to the method used by Kamiloglu and Capanoglu [42], with slight modifications. The in vitro gastrointestinal digestion was performed, comprising of a pepsin-HCl digestion for 3 h at 37 °C (to simulate gastric digestion) and pancreatin digestion with pancreatin and bile salts for 3 h at 37 °C (to simulate small intestinal digestion). As reported by Caponio et al. [43], 10 mL of each fresh pasta extract was added to α-amylase (56 mg/mL) (Sigma-Aldrich Chemistry, St. Louis, MO, USA) and to 10 mL of pepsin solution composed of NaCl 125 mM/L + KCl 7 mM/L + NaHCO3 45 mM/L + pepsin (Sigma-Aldrich Chemistry, St. Louis, MO, USA) at 3 g/L. Then, the pH was adjusted to 2 using HCl and incubated at 37 °C for 180 min in a water bath under shaking. After incubation, an aliquot of the gastric-digested extract was added in equal volume to an intestinal solution. The intestinal solution was simulated by dissolving 0.1 g/100 mL of pancreatin (Sigma-Aldrich Chemistry, St. Louis, MO, USA) and 0.15 g/100 mL bile salts (Oxoid™, Hampshire, UK). The pH was adjusted to 8 using NaOH and incubated at 37 °C for 180 min in a water bath under shaking. After incubation, an aliquot of intestinal-digested extract was ultra-filtrated with 3000 Da membrane (Vivaspin 20, Sartorius, Goettingen, Germany) to eliminate free carbohydrates. The retentate fractions were diluted in water and then filtered using 0.45 μm Whatman filter paper and further analyzed for prebiotics activities, as follows.
Twenty-two probiotic strains of probiotics and one strain of Escherichia coli (E. coli) available in the culture collection of the Department of Plant, Soil, Food Science of University of Bari, Italy were used to carry out the experiments in fecal batches. The fecal medium (FM) was constituted as previously described [44] without the addition of glucose. This was labelled as FM (absence of carbohydrates), FMPC (FM + pasta not containing inulin), FMP5 (FM + pasta with 5% of inulin), FMP10 (FM + pasta with 10% of inulin), FMP15 (FM + pasta with 15% of inulin). For those fecal samples containing pasta, this was added in a ratio of 1:5 (w/v) in media after cooking and digestion was simulated as previously described. Viable probiotics and E. coli were inoculated in fecal media at a cell density of 7 UFC/mL (log10), measured through OD at 620 nm. Inoculated batches were incubated in anaerobic conditions for 36 h at 37 °C, under slight stirring (150 rpm). After the incubation, plate counts for lactic acid bacteria and E. coli were respectively made in De Man, Rogosa, and Sharpe agar (MRS) and Violet Red Bile Glucose agar (VRBGA). Both agar media were purchased from Oxoid Ltd. (Basingstoke, Hampshire, England, UK). Probiotic growth was also profiled in terms of ΔpH, as the difference between final (36 h) and initial (pH 7.0 ± 0.02) values of pH.
2.4. Statistical Analysis
The experimental data were subjected to one-way and two-way ANOVA, followed by a Tukey’s HSD test. The two-way ANOVA analysis was carried out considering the rate of substitution and the physical state of pasta (raw and cooked) as factors. Significant differences among the values of all the parameters were determined at p < 0.05 by the Minitab 17 Statistical Software (Minitab, Inc., State College, PA, USA, 2010).
3. Results and Discussion
3.1. Characteristic of AR Inulin Powder
Inulin extraction using hot water is considered the conventional extraction technique and the most-used [45]. Several factors can influence the extraction yield: temperature, time of extraction, solid/liquid ratio [46]. From the data collected in our study, the extraction yield, as the mean of ten measurements, was 23.37% ± 1.55, with a purity level of 89%, estimated in comparison to commercial inulin used as standard, which had a purity of 98.5%. The degree of polymerization (DP) is a fundamental parameter to characterize inulin, being directly correlated to its technological and nutritional properties. AR inulin has shown a DPn and DPw equal to 45 and 60, respectively, much higher than inulin extracted from Jerusalem artichoke (5–19), agave (5–13), and dahlia (17–23) [47,48,49]. This result highlights the possible use of AR inulin as a functional ingredient, since higher DP is associated with the improvement of technological properties in food products [10]. Among the factors that could affect the DP are plant, period of harvesting, storage period, and extraction process [50]. Moreover, the AR inulin powder has shown a moisture content equal to 6% and water activity of 0.40 ± 0.0, values which assure a high glass transition temperature, lower cohesiveness and, consequently, higher physical and microbiological stability [46,51].
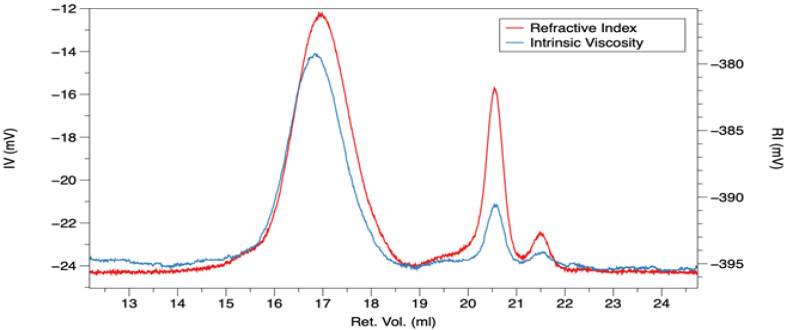
Figure 1 shows the gel permeation chromatography (GPC) profile of AR inulin. Two different peaks were observed, the first peak in the range of 15–19 mL, relative to polysaccharides elution, and a second very sharp peak centred at 20.5 mL, presumably due to the elution of very short polysaccharide chains, monomers, and impurities present in the sample. The ratio between the two peaks was 75/25.
Figure 1.
Gel permeation chromatography (GPC) profile of artichoke roots inulin, considering the refractive index (red line) and the intrinsic viscosity (blue line).
3.2. Quality Characteristics of Fresh Pasta
3.2.1. Cooking Properties of Fresh Pasta
Pasta cooking properties are of great importanceforo ensuring acceptability by consumers. These properties were evaluated by considering parameters such as optimal cooking time (OCT), swelling index (SI), water absorption index (WAI), and solid cooking loss (CL) (Table 1). OCT was set for each pasta sample, observing a slight increase in pasta enriched with 10% and 15% of inulin. The same trend was observed by Foschia et al. [31] in pasta fortified by inulin with high DP and Simonato et al. [52] after the addition of Moringa oleifera L. leaves powder. However, the aforementioned results disagreed with other studies [32,33,53,54], where the addition of inulin caused a significant reduction of OCT due to the disruption of gluten network, which, in turn, caused an easier water penetration into starch granules. The discordant results could be attributed to the different production processes, as well as to the added fibrer and its intrinsic properties, which influence the interaction with other ingredients.
Table 1.
Cooking properties of fresh pasta.
| Sample | OCT (min) | WAI (g/100 g) | SI | CL (g/100 g) | IL (g/100 g) |
|---|---|---|---|---|---|
| PC | 6.30 | 73.20 ± 0.01 a | 1.56 ± 0.03 a | 2.37 ± 0.05 d | - |
| P5 | 6.30 | 73.24 ± 0.10 a | 1.53 ± 0.04 ab | 2.70 ± 0.14 c | 1.01 ± 0.03 c |
| P10 | 6.45 | 72.51 ± 0.62 a | 1.48 ± 0.03 b | 3.11 ± 0.07 b | 1.45 ± 0.07 b |
| P15 | 6.45 | 73.24 ± 0.20 a | 1.47 ± 0.04 b | 3.62 ± 0.06 a | 2.19 ± 0.12 a |
PC, control pasta without inulin addition; P5, pasta with 5% of inulin added; P10, pasta with 10% of inulin added; P15, pasta with 15% of inulin added. OCT, optimal cooking time; WAI, water absorption index, SI, swelling index; CL, cooking losses; IL, inulin losses. The values represent means of triplicates ± standard deviation; different letters in the same column mean significant statistical differences (p < 0.05) to one-way ANOVA followed by Tukey’s HSD test.
Regarding the WAI, no significant differences were found among the samples, whereas P10 and P15 showed a significant lower SI than control (PC). In accordance with our results, Naji-Tabasi et al. [55] and Attanzio et al. [56] noted a significant reduction of SI in pasta samples after the addition of wheat bran, mucilaginous seeds flour and Opuntia cladodes extract. Moreover, Renoldi et al. [57] stated that adding Psyllium fiber led to a lower peak value of storage modulus (G’) of dough, revealing differences in starch swelling of starch granules. The explanation for these results lies in the encapsulation of starch granules into fiber reticule, whose hydroxyl groups compete with starch and protein for water absorption, thus limiting the penetration of water into the starch granules and their consequent swelling [57,58,59]. On the contrary, the addition of whole barley flour to pasta formulation led to an increase of SI, which was related to the presence of β-glucans and their ability to absorb water [60]. Hence, different fibers could have a different effect on starch hydration and swelling.
CL refers to pasta resistance during cooking, and it is strictly connected with the strength of the protein network [61]. Good quality pasta should not have a CL higher than 7–8% [55]. In our study, CL ranged between 2.37% for the control sample (PC) and 3.62% for the P15. However, considering that inulin water solubility increases with high temperature [62], we also estimated the contribution of inulin to the observed CL. Specifically, inulin-imputable CL accounted for 1.01 ± 0.03 g/100 g of pasta in P5, 1.45 ± 0.07 g/100 g of pasta in P10, and 2.19 ± 0.12 g/100 g of pasta in P15. Consequently, making a difference between the CL values of P5, P10, and P15 and the corresponding inulin content found in cooking water, we could assert that in inulin-enriched samples, the solids different from inulin that leach into cooking water are lower than PC. Therefore, the probable formation of fibrous reticulum around starch granules [38] and additional hydrogen bonds and hydrophobic interactions between inulin and glutenin protein could provide extra support to the protein network, reducing the solid loss [63]. However, opposite results were found in several studies in the literature, in which the disruption of protein matrix due to fibers, mainly insoluble, promotes and allows the leaching of starch during cooking, causing an increase in cooking loss value [64,65,66].
3.2.2. Color and Firmness of Fresh Pasta
Color, together with cooking and textural properties, is another key parameter for consumer acceptability: yellow color and high luminosity are associated with high-quality pasta [67]. Table 2 shows the color parameters in raw and cooked pasta samples. According to two-way ANOVA, both the cooking process and the addition of increasing percentages of AR inulin significantly affected the colorimetric parameters. Specifically, both raw and cooked pasta samples with higher rates of AR inulin substitution of durum wheat semolina caused a reduction of luminosity (L*) and yellow index (b*). Regarding cooking, the red index (a*) followed the same trend of L* and b*, whereas the opposite trend was observed for the increasing rate of substitution of durum wheat semolina with AR inulin. After the addition of insoluble fibers, Aravind et al. [68] found the same trend in terms of luminosity (L*), yellow, and red index, while Zarroug et al. [61] and Filipović et al. [21] reported an increase of L* values and a decrease of a* values for pasta enriched with commercial inulin, probably due to the added white color of the commercial inulin.
Table 2.
Color and firmness of fresh pasta.
| L* | a* | b* | Firmness | ||
|---|---|---|---|---|---|
| Raw pasta | PC | 79.65 ± 0.09 a | 2.21 ± 0.07 e | 33.57 ± 0.46 a | 18.25 ± 0.45 b |
| P5 | 74.57 ± 0.19 c | 3.21 ± 0.02 c | 30.49 ± 0.26 b | 19.63 ± 0.50 a | |
| P10 | 72.02 ± 0.38 e | 3.89 ± 0.10 b | 28.52 ± 0.19 c | 19.89 ± 0.05 a | |
| P15 | 70.72 ± 0.25 f | 4.48 ± 0.08 a | 27.62 ± 0.09 cd | 19.77 ± 0.23 a | |
| Cooked pasta | PC | 78.89 ± 0.43 b | 0.30 ± 0.02 f | 27.11 ± 0.56 d | 5.85 ± 0.36 d |
| P5 | 73.69 ± 0.18 d | 2.00 ± 0.11 e | 24.13 ± 0.41 e | 6.22 ± 0.26 d | |
| P10 | 72.24 ± 0.06 e | 2.82 ± 0.11 d | 24.18 ± 0.33 e | 7.37 ± 0.48 c | |
| P15 | 68.12 ± 012 g | 3.79 ± 0.10 b | 22.29 ± 0.43 f | 7.25 ± 0.37 c | |
| p-value | P *C | <0.0001 | <0.0001 | <0.0001 | <0.05 |
PC, control pasta without inulin addition; P5, pasta with 5% inulin added; P10, pasta with 10% inulin added; P15, pasta with 15%= inulin added. P, percentage of inulin addition; C, cooking process. Values are expressed as mean of ± standard deviation; different letters in the same column mean significant statistical differences (p < 0.05) according to two-way ANOVA.
Firmness can be evaluated as the force necessary to cut the pasta strains, and it is strictly connected with the protein matrix development during pasta production and the hydration level of starch granules [64,67]. Cooked P10 and P15 showed a significantly higher firmness (7.37 ± 0.48 and 7.25 ± 0.37 N) than PC and P5 (5.85 ± 0.36 and 6.22 ± 0.26 N), which did not show any significant differences between them (Table 2). According to Chillo et al. [69] the mechanical properties of conventional and unconventional pasta are strictly connected with cooking properties. Therefore, the increase in firmness in cooked P10 and P15 can be reasonably linked to the lower values of the swelling index, supporting the hypothesis of pasta structure rearrangement as discussed in Section 3.2.1 [54]. Moreover, beta-glucan addition by Aravind et al. [70] and Peressini et al. [30] found a firmer pasta than control; however, significantly lower values of firmness were found by Peressini et al. [30] with the addition of 15% of inulin with a high degree of polymerization (DP = 23).
3.2.3. Sensory Evaluation of Fresh Pasta
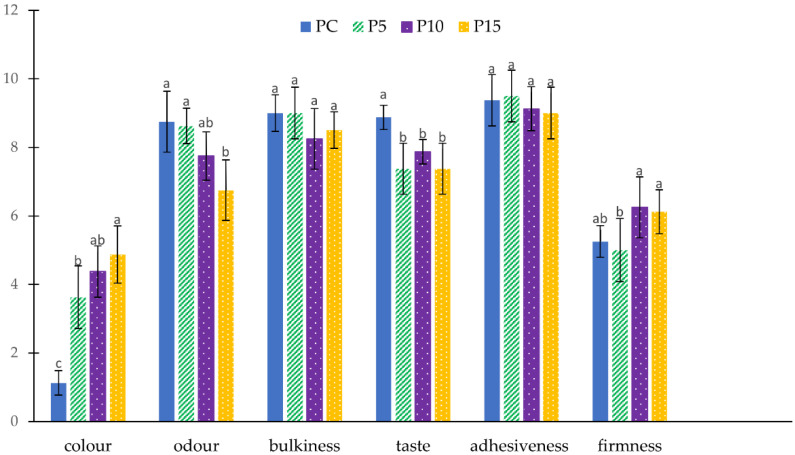
The results of the sensory evaluation are reported in Figure 2. The addition of larger amounts of AR inulin in fresh pasta formulations did not cause significant differences in sensory properties after cooking. The sensory scores for color and firmness have been consistent with instrumental evaluation, with all inulin-enriched pasta samples perceived as browner and P10 and P15 samples slightly firmer than PC. Although brightness and yellowness are linked to high-quality pasta, over the years, consumer attitude has been changing such that darker color is considered a positive trait, as it is associated with high-fiber products [25]. Significant differences were found in the taste of inulin-enriched samples than control, while only P10 odor resulted in a decreased perception rating compared to the typical odor of durum wheat pasta. In conclusion, P10 and P15 were statistically similar to P5; indeed, except for color and odor, no significant differences were highlighted for the other parameters considered. Therefore, it may be possible to add higher amounts of inulin without compromising the sensory properties of pasta.
Figure 2.
Sensory characteristics of control (PC) and inulin-enriched (P5, P10, P15) fresh pasta. Values are expressed as mean ± standard deviation; different letters for each parameters mean significant statistical differences (p < 0.05) to one-way ANOVA followed by Tukey’s HSD test.
3.2.4. Microstructure
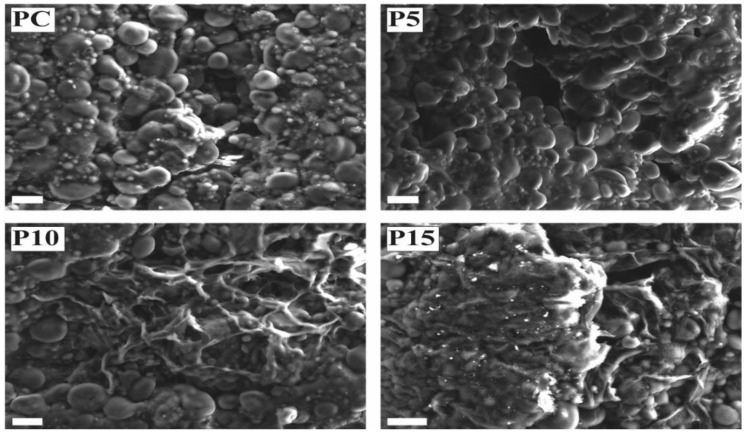
Figure 3 reports the SEM micrographs of cross sections of raw pasta (PC, P5, P10, and P15). Starch granules were well visible in all the samples, and the protein matrix did not appear disrupted. However, in P10 and P15, starch granules seem to be immerged in a more dense and compact structure. Moreover, P10 and P15 exhibit the presence of a reticulated structure, probably formed from the interaction of inulin with the protein network, which could have strengthened the pasta structure and thus giving a higher firmness to the cooked pasta as observed by the instrumental analysis.
Figure 3.
SE-SEM micrographs of control (PC) and inulin enriched (P5, P10, P15) fresh pasta (white bar = 20 µm).
3.3. Functional Properties of Fresh Pasta
3.3.1. Proximate Composition of Fresh Pasta
Table 3 reported the proximate composition of fresh pasta. Significant differences were found among the samples for all the parameters considered. Specifically, inulin-enriched pasta showed a decline in protein content compared to the control (PC) due to a rise in total dietary fiber, which reached values of 3.44 g/100 g in P5, 8.16 g/100 g in P10, and 12.41 g/100 g in P15, exceeding the data observed by Padalino et al. [71] in pasta fortified with tomato byproducts. Therefore, the results in terms of total dietary fiber allow for labelling P5 as a “source of fibre”, while P10 and P15 could be labelled as a pasta having “high fibre content” [72], according to Reg. (EU) 1924/2006, enhancing the nutritional value of fresh pasta. Moreover, higher ash and lower lipid content were found in P10 and P15 than P5 and PC.
Table 3.
Proximate composition of fresh pasta (g/100 g).
| Parameters | PC | P5 | P10 | P15 |
|---|---|---|---|---|
| Ash | 0.41 ± 0.02 b | 0.41 ± 0.01 b | 0.49 ± 0.01 a | 0.50 ± 0.01 a |
| Protein | 10.75 ± 0.03 a | 9.71 ± 0.44 b | 9.25 ± 0.02 b | 9.27 ± 0.08 b |
| Total dietary fiber | 1.47 ± 0.04 d | 3.44 ± 0.10 c | 8.16 ± 0.12 b | 12.69 ± 0.08 a |
| Lipid | 0.23 ± 0.02 a | 0.10 ± 0.01 b | 0.06 ± 0.01 c | 0.08 ± 0.01 c |
| Moisture | 28.21 ± 0.17 a | 27.00 ± 0.21 b | 26.98 ± 0.10 b | 28.24 ± 0.30 a |
| Carbohydrates | 58.94 ± 0.22 a | 59.34 ± 0.25 a | 55.07 ± 0.02 b | 49.20 ± 0.42 c |
PC, control pasta without inulin addition; P5, pasta with 5% of inulin added; P10, pasta with 10% of inulin added; P15, pasta with 15% of inulin added. The values represent means of triplicates ± standard deviation; different letters indicate significant differences; different letters in the same column mean significant statistical differences (p < 0.05) to one way ANOVA followed by Tukey’s HSD test.
3.3.2. In Vitro Starch Hydrolysis
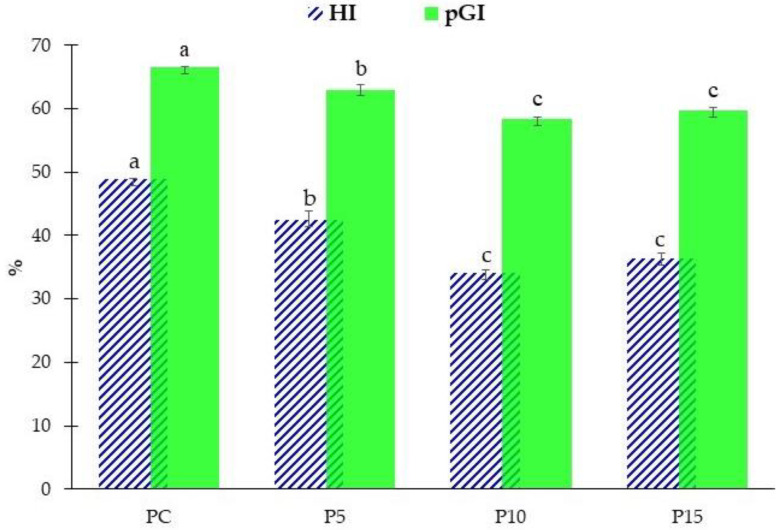
In consideration the pivotal role of nutritional aspects for modern consumers and in recent scientific research aimed at producing foods with a lower glycemic index [73,74], cooked fresh pasta samples were analyzed for in vitro starch hydrolysis. As shown in Figure 4, the addition of AR inulin in fresh pasta promoted the decrease of HI and pGI. On average, the HI and pGI values of fresh pasta samples enriched with AR inulin (P5, P10, and P15) were statistically lower than the control (PC). Specifically, P5 reached a significantly (p < 0.05) lower value of pGI compared to the control (PC): 63.01 and 66.54, respectively. However, a higher concentration of AR inulin resulted in a further decrease of HI and pGI, as shown in P10 and P15. In fact, P10 has a statistically lower pGI value (58.42) compared to both the control (PC) and P5. Nevertheless, no significant differences were observed for P10 and P15 samples in terms of pGI and HI. Data demonstrate a lower pGI response following ingestion of AR inulin-enriched fresh pasta compared to the control.
Figure 4.
Results of hydrolysis index (HI) and predicted glycemic index (pGI) of control (PC) and inulin-enriched (P5, P10, P15) fresh pasta. The values represent means of triplicates ± standard deviation; different letters indicate significant differences (p < 0.05) according to one-way ANOVA followed by Tukey’s HSD test.
Inulin, which is not digestible by humans, has interesting properties as a source of fermentable energy for some intestinal bacteria that produce short-chain fatty acids, which are essential for maintaining the intestinal homeostasis. In addition to stimulating digestion and regularity of intestinal transit, inulin may favor the presence of Bifidobacterium in the microbiota and, at the same time, decrease harmful bacteria [75,76]. Several studies have highlighted the beneficial role of inulin also in glycemic response [41,77,78,79]. Inulin—as a soluble fiber—helps keep blood sugar under control, since the fibers present in complex carbohydrates take longer to release the sugars present in the body: the slow release of glucose prevents glycemic peaks both upwards and downwards by balancing the energy intake and limiting the accumulation of fat due to the excess insulin [31,80]. The gelling effect of these fibers causes the formation of a film on the walls of the stomach and intestines with consequent lower absorption of fats and sugars [81]. In contrast to a previous study on chicory inulin-enriched pasta (from 2.5% to 10%) that did not reduce the pGI compared to the control [28], our results confirmed that AR was able to reduce the pGI and HI already at 5% of its concentration, due to the high DP of inulin used in our pasta samples.
3.3.3. Evaluation of Effects Exerted by AR Inulin, Prebiotics, and Pathogen on Probiotic Growth
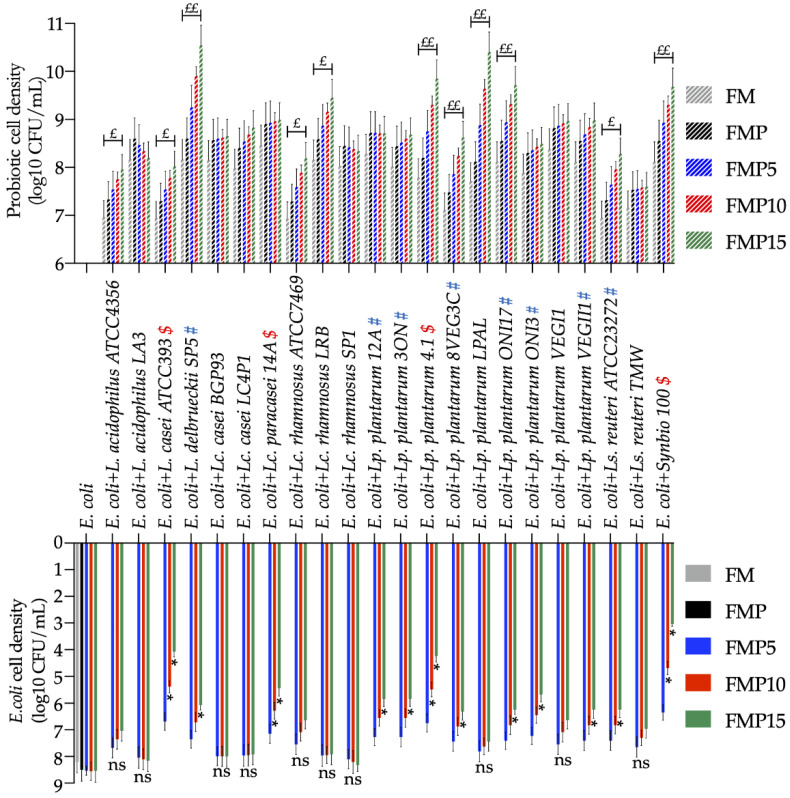
Herein, the prebiotic activity of inulin-enriched pasta was assessed in vitro in terms of probiotics growth. Furthermore, the inhibition of E. coli when co-cultured with probiotics was also determined. Compared to batches not containing carbohydrates (FM), the addition of pasta not containing inulin (FMPC) was sufficient to increase (~0.5 log10 CFU/mL) the cell density of all tested probiotics (Figure 5). This reflects the prebiotic contribution of fructans and arabinoxylans, which are non-digestible oligosaccharides naturally occurring in wheats [82], since their absence in gluten-free diets seems to affect the host microbiome and metabolome [83,84]. However, the presence of 3 g/L of inulin in FMP15 was able to further increase (>0.5 cycle) the cell density by 50% compared to the used probiotics (11 out of 22), while six strains (~27%) showed an increased cell density higher than one cycle. Besides the growth of probiotics, the acidification degree in batches followed the inulin concentration in a dependent manner. Values of ΔpH were, on average, 0.07 ± 0.06, 0.14 ± 0.11, and 0.20 ± 0.17 for FMP5, FMP10, and FMP15, respectively. No significant differences were found comparing the cell densities of E. coli in FMP to those of FMP5 (Figure 5). Oppositely, more than 50% (12 out of 22) of used probiotics were able to significantly decrease the cell density of E. coli in batches containing 3 g/L of inulin (FMP15). Meanwhile, ~36% of probiotics (eight strains) significantly decreased the E. coli cell density in batches containing 2 g/L of inulin (FMP10).
Figure 5.
In vitro prebiotic assay showing the cell density of 22 probiotic lactic acid bacteria co-cultured with E. coli in fecal batches not containing carbohydrates (FM) or made with the addition of pasta without inulin (FMPC) and inulin-enriched pasta at 5 (FMP5), 10 (FMP10), and 15% (FMP15). “£” means >0.5 log10 cycle increased cell density (CFU/mL) of probiotic lactic acid bacte-ria in FMP15 versus FMP; “££” means >1 log10 cycle increased cell density (CFU/mL) of probiotic lactic acid bacteria in FMP15 versus FMP. “#” and “$” indicate those probiotics that had respective-ly determined a significant decrease of E. coli cell density in FMP15 or both FMP15 and FMP10. “*”: significant decrease of E. coli cell density compared to FMP; “ns”: no significant differences.
These results are in line with those previously stated by Kareem and co-workers [85], who reported that the combination of probiotics with prebiotics in vitro exhibited a great inhibition of pathogens due to a synergistic effect. Mechanisms based on microbial cross-feeding are largely distributed within the intestinal lumen [86]. A previous study concerning β-glucans-enriched pasta determined that 3 g of daily fiber supplementation was optimal to increase the saccharolytic metabolism in terms of SCFA profiling and improving the endothelial reactivity in healthy volunteers [87]. Therefore, although some used strains did not directly decrease E. coli growth, evidence suggests that additional taxa belonging to the human gut microbiota (e.g., clostridial cluster IV, XIVa, and Bifidobacterium) can support the metabolism of inulin in SCFA in vivo, eliciting a boosted effect that contributes to the host’s intestinal homeostasis [88].
4. Conclusions
The obtained results show that inulin from globe artichoke represents a promising functional ingredient in terms of technological and nutritional properties. As a matter of fact, adding inulin determined changes in the structure of raw and cooked pasta, which results more compact and firmer than control. The addition of inulin resulted in increasing the optimal cooking time, reducing the swelling index and increasing cooking loss (the latter due to inulin leaching in the cooking water). In terms of color, inulin-enriched samples (P5, P10, and P15) showed lower L* and b* and higher a* than control. The sensory properties did not substantially change compared to control. From the nutritional perspective, the ability of inulin to slow down the release of glucose in the blood was assessed, showing that a significant reduction of HI and pGI occurred in inulin-enriched pasta compared to control. Moreover, the in vitro prebiotic assay demonstrated that FMP10 and FMP15, containing 2 g/L and 3 g/L of inulin, respectively, significantly increased the cell density of prebiotics able to inhibit the E. coli growth. Therefore, the promising results obtained highlight the possibility to upcycle, in a circular perspective, the artichoke roots from agricultural waste to a valuable food ingredient that is useful for preparing high-added-value food products that could satisfy the increasing demand of consumers for more sustainable and functional foods.
Acknowledgments
This work was supported by POR Puglia 2014/2020-Asse X-Azione 10.4 Research for Innovation- REFIN code n. E65BAEEE.
Author Contributions
Conceptualization, G.D. and A.P.; methodology, G.D., G.d.G., G.R.C., M.V., B.I. and A.P.; formal analysis, G.d.G., G.R.C., M.V., I.A. and G.d.P.; writing—original draft preparation, G.D., G.d.G., G.R.C., M.V. and B.I.; writing—review and editing, G.D., G.d.G., G.R.C. and A.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study because data were completely anonymous, with no personal information being collected, and were not considered to be sensitive in nature.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Ministero dell’Istruzione, dell’Università e della Ricerca-Programmi di Ricerca 2017 (2017JTNK78) “GOOD-BY-WASTE. Obtain GOOD products-exploit BY-products-reduce WASTE” (CUP H98D19000940006).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Francavilla M., Marone M., Marasco P., Contillo F., Monteleone M. Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods. 2021;10:112. doi: 10.3390/foods10010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domingo C.S., Rojas A.M., Fissore E.N., Gerschenson L.N. Rheological behavior of soluble dietary fiber fractions isolated from artichoke residues. Eur. Food Res. Technol. 2019;245:1239–1249. doi: 10.1007/s00217-019-03242-y. [DOI] [Google Scholar]
- 3.Villanueva-Suárez M.J., Mateos-Aparicio I., Pérez-Cózar M.L., Yokoyama W., Redondo-Cuenca A. Hypolipidemic effects of dietary fibre from an artichoke by-product in Syrian hamsters. J. Funct. Foods. 2019;56:156–162. doi: 10.1016/j.jff.2019.03.013. [DOI] [Google Scholar]
- 4.Zeaiter Z., Regonesi M.E., Cavini S., Labra M., Sello G., Di Gennaro P. Extraction and Characterization of Inulin-Type Fructans from Artichoke Wastes and Their Effect on the Growth of Intestinal Bacteria Associated with Health. Biomed Res. Int. 2019;2019:1083952. doi: 10.1155/2019/1083952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lattanzio V., Kroon P.A., Linsalata V., Cardinali A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods. 2009;1:131–144. doi: 10.1016/j.jff.2009.01.002. [DOI] [Google Scholar]
- 6.Borsini A.A., Llavata B., Umaña M., Cárcel J.A. Artichoke by Products as a Source of Antioxidant and Fiber: How It Can Be Affected by Drying Temperature. Foods. 2021;10:459. doi: 10.3390/foods10020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasqualone A., Punzi R., Trani A., Summo C., Paradiso V.M., Caponio F., Gambacorta G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultrasound-assisted technology and quality characterisation of the end product. Int. J. Food Sci. Technol. 2017;52:2078–2087. doi: 10.1111/ijfs.13486. [DOI] [Google Scholar]
- 8.Holgado F., Campos-Monfort G., de las Heras C., Rupérez P. In vitro fermentability of globe artichoke by-product by Lactobacillus acidophilus and Bifidobacterium bifidum. Bioact. Carbohydr. Diet. Fibre. 2021;26:100286. doi: 10.1016/j.bcdf.2021.100286. [DOI] [Google Scholar]
- 9.Raccuia S.A., Melilli M.G. Seasonal dynamics of biomass, inulin, and water-soluble sugars in roots of Cynara cardunculus L. Field Crop. Res. 2010;116:147–153. doi: 10.1016/j.fcr.2009.12.005. [DOI] [Google Scholar]
- 10.Castellino M., Renna M., Leoni B., Calasso M., Difonzo G., Santamaria P., Gambacorta G., Caponio F., De Angelis M., Paradiso V.M. Conventional and unconventional recovery of inulin rich extracts for food use from the roots of globe artichoke. Food Hydrocoll. 2020;107:105975. doi: 10.1016/j.foodhyd.2020.105975. [DOI] [Google Scholar]
- 11.Redondo-Cuenca A., Herrera-Vázquez S.E., Condezo-Hoyos L., Gómez-Ordóñez E., Rupérez P. Inulin extraction from common inulin-containing plant sources. Ind. Crops Prod. 2021;170:113726. doi: 10.1016/j.indcrop.2021.113726. [DOI] [Google Scholar]
- 12.López-Molina D., Navarro-Martínez M.D., Rojas-Melgarejo F., Hiner A.N.P., Chazarra S., Rodríguez-López J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.) Phytochemistry. 2005;66:1476–1484. doi: 10.1016/j.phytochem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Lopes S.M.S., Krausová G., Rada V., Gonçalves J.E., Gonçalves R.A.C., de Oliveira A.J.B. Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015;411:15–21. doi: 10.1016/j.carres.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Gominho J., Curt M.D., Lourenço A., Fernández J., Pereira H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy. 2018;109:257–275. doi: 10.1016/j.biombioe.2018.01.001. [DOI] [Google Scholar]
- 15.Giarnetti M., Paradiso V.M., Caponio F., Summo C., Pasqualone A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT Food Sci. Technol. 2015;63:339–345. doi: 10.1016/j.lwt.2015.03.063. [DOI] [Google Scholar]
- 16.Puchkova T.S., Pikhalo D.M., Karasyova O.M. About the universal technology of processing Jerusalem artichoke and chicory for inulin. Food Syst. 2019;2:36–43. doi: 10.21323/2618-9771-2019-2-2-36-43. [DOI] [Google Scholar]
- 17.El-Kholy W.M., Aamer R.A., Ali A.N.A. Utilization of inulin extracted from chicory (Cichorium intybus L.) roots to improve the properties of low-fat synbiotic yoghurt. Ann. Agric. Sci. 2020;65:59–67. doi: 10.1016/j.aoas.2020.02.002. [DOI] [Google Scholar]
- 18.Mitchell C.M., Davy B.M., Halliday T.M., Hulver M.W., Neilson A.P., Ponder M.A., Davy K.P. The effect of prebiotic supplementation with inulin on cardiometabolic health: Rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials. 2015;45:328–337. doi: 10.1016/j.cct.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caponio G.R., Lippolis T., Tutino V., Gigante I., De Nunzio V., Milella R.A., Gasparro M., Notarnicola M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants. 2022;11:1274. doi: 10.3390/antiox11071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivkov M., Kosutic M., Filipovic J., Filipovic V. Spelt pasta with addition of inulin as a functional food: Sensory evaluation and consumer attitudes. Rom. Biotechnol. Lett. 2018;23:13615–13624. [Google Scholar]
- 21.Filipovic J., Pezo L., Filipovic V., Ludajic G. Spelt pasta with inulin as a functional food. Acta Period. Technol. 2015;46:37–44. doi: 10.2298/APT1546037F. [DOI] [Google Scholar]
- 22.Martín-Esparza M., Raga A., González-Martínez C., Albors A. Micronised bran-enriched fresh egg tagliatelle: Significance of gums addition on pasta technological features. Food Sci. Technol. Int. 2018;24:309–320. doi: 10.1177/1082013217750683. [DOI] [PubMed] [Google Scholar]
- 23.Makhlouf S., Jones S., Ye S.-H., Sancho-Madriz M., Burns-Whitmore B., Li Y.O. Effect of selected dietary fibre sources and addition levels on physical and cooking quality attributes of fibre-enhanced pasta. Food Qual. Saf. 2019;3:117–127. doi: 10.1093/fqsafe/fyz010. [DOI] [Google Scholar]
- 24.Bianchi F., Tolve R., Rainero G., Bordiga M., Brennan C.S., Simonato B. Technological, nutritional and sensory properties of pasta fortified with agro-industrial by-products: A review. Int. J. Food Sci. Technol. 2021;56:4356–4366. doi: 10.1111/ijfs.15168. [DOI] [Google Scholar]
- 25.Sobota A., Rzedzicki Z., Zarzycki P., Kuzawińska E. Application of common wheat bran for the industrial production of high-fibre pasta. Int. J. Food Sci. Technol. 2015;50:111–119. doi: 10.1111/ijfs.12641. [DOI] [Google Scholar]
- 26.Costantini M., Summo C., Faccia M., Caponio F., Pasqualone A. Kabuli and Apulian black Chickpea Milling By-Products as Innovative Ingredients to Provide High Levels of Dietary Fibre and Bioactive Compounds in Gluten-Free Fresh Pasta. Molecules. 2021;26:4442. doi: 10.3390/molecules26154442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fradinho P., Oliveira A., Domínguez H., Torres M.D., Sousa I., Raymundo A. Improving the nutritional performance of gluten-free pasta with potato peel autohydrolysis extract. Innov. Food Sci. Emerg. Technol. 2020;63:102374. doi: 10.1016/j.ifset.2020.102374. [DOI] [Google Scholar]
- 28.Garbetta A., D’Antuono I., Melilli M.G., Sillitti C., Linsalata V., Scandurra S., Cardinali A. Inulin enriched durum wheat spaghetti: Effect of polymerization degree on technological and nutritional characteristics. J. Funct. Foods. 2020;71:104004. doi: 10.1016/j.jff.2020.104004. [DOI] [Google Scholar]
- 29.Krawęcka A., Sobota A., Sykut-Domańska E. Physicochemical, Sensory, and Cooking Qualities of Pasta Enriched with Oat β-Glucans, Xanthan Gum, and Vital Gluten. Foods. 2020;9:1412. doi: 10.3390/foods9101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peressini D., Cavarape A., Brennan M.A., Gao J., Brennan C.S. Viscoelastic properties of durum wheat doughs enriched with soluble dietary fibres in relation to pasta-making performance and glycaemic response of spaghetti. Food Hydrocoll. 2020;102:105613. doi: 10.1016/j.foodhyd.2019.105613. [DOI] [Google Scholar]
- 31.Foschia M., Peressini D., Sensidoni A., Brennan M.A., Brennan C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. LWT Food Sci. Technol. 2015;61:41–46. doi: 10.1016/j.lwt.2014.11.010. [DOI] [Google Scholar]
- 32.Aravind N., Sissons M.J., Fellows C.M., Blazek J., Gilbert E.P. Effect of inulin soluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012;132:993–1002. doi: 10.1016/j.foodchem.2011.11.085. [DOI] [Google Scholar]
- 33.Padalino L., Costa C., Conte A., Melilli M.G., Sillitti C., Bognanni R., Raccuia S.A., Del Nobile M.A. The quality of functional whole-meal durum wheat spaghetti as affected by inulin polymerization degree. Carbohydr. Polym. 2017;173:84–90. doi: 10.1016/j.carbpol.2017.05.081. [DOI] [PubMed] [Google Scholar]
- 34.Mensink M.A., Frijlink H.W., van der Voort Maarschalk K., Hinrichs W.L.J. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015;130:405–419. doi: 10.1016/j.carbpol.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Pasqualone A., Gambacorta G., Summo C., Caponio F., Di Miceli G., Flagella Z., Marrese P.P., Piro G., Perrotta C., De Bellis L., et al. Functional, textural and sensory properties of dry pasta supplemented with lyophilized tomato matrix or with durum wheat bran extracts produced by supercritical carbon dioxide or ultrasound. Food Chem. 2016;213:545–553. doi: 10.1016/j.foodchem.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 36.AACC, American Association of Cereal Chemists . Approved Methods of Analysis. 11th ed. AACC International; St. Paul, MN, USA: 2000. [Google Scholar]
- 37.Bustos M.C., Pérez G.T., León A.E. Effect of Four Types of Dietary Fiber on the Technological Quality of Pasta. Food Sci. Technol. Int. 2011;17:213–221. doi: 10.1177/1082013210382303. [DOI] [PubMed] [Google Scholar]
- 38.Pasqualone A., De Angelis D., Squeo G., Difonzo G., Caponio F., Summo C. The effect of the addition of apulian black chickpea flour on the nutritional and qualitative properties of durum wheat-based bakery products. Foods. 2019;8:504. doi: 10.3390/foods8100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.AOAC International, Association of Official Analytical Chemists . Official Method of Analyisis. 17th ed. AOAC International; Gaithersburg, MD, USA: 2006. [Google Scholar]
- 40.Liljeberg H., Åkerberg A., Björck I. Resistant starch formation in bread as influenced by choice of ingredients or baking conditions. Food Chem. 1996;56:389–394. doi: 10.1016/0308-8146(95)00199-9. [DOI] [Google Scholar]
- 41.Capriles V.D., Arêas J.A.G. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013;4:104–110. doi: 10.1039/C2FO10283H. [DOI] [PubMed] [Google Scholar]
- 42.Kamiloglu S., Capanoglu E. In vitro gastrointestinal digestion of polyphenols from different molasses (pekmez) and leather (pestil) varieties. Int. J. Food Sci. Technol. 2014;49:1027–1039. doi: 10.1111/ijfs.12396. [DOI] [Google Scholar]
- 43.Caponio G.R., Noviello M., Calabrese F.M., Gambacorta G., Giannelli G., De Angelis M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants. 2022;11:567. doi: 10.3390/antiox11030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vacca M., Celano G., Lenucci M.S., Fontana S., la Forgia F.M., Minervini F., Scarano A., Santino A., Dalfino G., Gesualdo L., et al. In Vitro Selection of Probiotics, Prebiotics, and Antioxidants to Develop an Innovative Synbiotic (NatuREN G) and Testing Its Effect in Reducing Uremic Toxins in Fecal Batches from CKD Patients. Microorganisms. 2021;9:1316. doi: 10.3390/microorganisms9061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maumela P., van Rensburg E., Chimphango A.F.A., Görgens J.F. Sequential extraction of protein and inulin from the tubers of Jerusalem artichoke (Helianthus tuberosus L.) J. Food Sci. Technol. 2020;57:775–786. doi: 10.1007/s13197-019-04110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubel I.A., Iraporda C., Novosad R., Cabrera F.A., Genovese D.B., Manrique G.D. Inulin rich carbohydrates extraction from Jerusalem artichoke (Helianthus tuberosus L.) tubers and application of different drying methods. Food Res. Int. 2018;103:226–233. doi: 10.1016/j.foodres.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 47.Apolinário A.C., de Carvalho E.M., de Lima Damasceno B.P.G., da Silva P.C.D., Converti A., Pessoa A., da Silva J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017;108:355–362. doi: 10.1016/j.indcrop.2017.06.045. [DOI] [Google Scholar]
- 48.Li W., Zhang J., Yu C., Li Q., Dong F., Wang G., Gu G., Guo Z. Extraction, degree of polymerization determination and prebiotic effect evaluation of inulin from Jerusalem artichoke. Carbohydr. Polym. 2015;121:315–319. doi: 10.1016/j.carbpol.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 49.Petkova N.T., Sherova G., Denev P.P. Characterization of inulin from dahlia tubers isolated by microwave and ultrasound-assisted extractions. Int. Food Res. J. 2018;25:1876–1884. [Google Scholar]
- 50.Xu H., Gunenc A., Hosseinian F. Ultrasound affects physical and chemical properties of Jerusalem artichoke and chicory inulin. J. Food Biochem. 2022;46:1–10. doi: 10.1111/jfbc.13934. [DOI] [PubMed] [Google Scholar]
- 51.Jirayucharoensak R., Khuenpet K., Jittanit W., Sirisansaneeyakul S. Physical and chemical properties of powder produced from spray drying of inulin component extracted from Jerusalem artichoke tuber powder. Dry. Technol. 2019;37:1215–1227. doi: 10.1080/07373937.2018.1492934. [DOI] [Google Scholar]
- 52.Simonato B., Tolve R., Rainero G., Rizzi C., Sega D., Rocchetti G., Lucini L., Giuberti G. Technological, nutritional, and sensory properties of durum wheat fresh pasta fortified with Moringa oleifera L. leaf powder. J. Sci. Food Agric. 2021;101:1920–1925. doi: 10.1002/jsfa.10807. [DOI] [PubMed] [Google Scholar]
- 53.Neylon E., Arendt E.K., Zannini E., Sahin A.W. Fundamental study of the application of brewers spent grain and fermented brewers spent grain on the quality of pasta. Food Struct. 2021;30:100225. doi: 10.1016/j.foostr.2021.100225. [DOI] [Google Scholar]
- 54.Simonato B., Trevisan S., Tolve R., Favati F., Pasini G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT. 2019;114:108368. doi: 10.1016/j.lwt.2019.108368. [DOI] [Google Scholar]
- 55.Naji-Tabasi S., Niazmand R., Modiri-Dovom A. Application of mucilaginous seeds (Alyssum homolocarpum and Salvia macrosiphon Boiss) and wheat bran in improving technological and nutritional properties of pasta. J. Food Sci. 2021;86:2288–2299. doi: 10.1111/1750-3841.15762. [DOI] [PubMed] [Google Scholar]
- 56.Attanzio A., Diana P., Barraja P., Carbone A., Spanò V., Parrino B., Cascioferro S.M., Allegra M., Cirrincione G., Tesoriere L., et al. Quality, functional and sensory evaluation of pasta fortified with extracts from Opuntia ficus-indica cladodes. J. Sci. Food Agric. 2019;99:4242–4247. doi: 10.1002/jsfa.9655. [DOI] [PubMed] [Google Scholar]
- 57.Renoldi N., Brennan C.S., Lagazio C., Peressini D. Evaluation of technological properties, microstructure and predictive glycaemic response of durum wheat pasta enriched with psyllium seed husk. LWT. 2021;151:112203. doi: 10.1016/j.lwt.2021.112203. [DOI] [Google Scholar]
- 58.Brennan C.S., Tudorica C.M. Fresh Pasta Quality as affected by enrichment of nonstarch polysaccharides. J. Food Sci. 2007;72:S659–S665. doi: 10.1111/j.1750-3841.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 59.Xu F., Li X., Li J., Chen J. The interaction between inulin and wheat starch and effects of inulin on frozen storage quality of noodles. Int. J. Food Sci. Technol. 2021;56:2423–2431. doi: 10.1111/ijfs.14870. [DOI] [Google Scholar]
- 60.Zarroug Y., Djebali K., Sfayhi D., Khemakhem M., Boulares M., El Felah M., Mnasser H., Kharrat M. Optimization of barley flour and inulin addition for pasta formulation using mixture design approach. J. Food Sci. 2022;87:68–79. doi: 10.1111/1750-3841.16009. [DOI] [PubMed] [Google Scholar]
- 61.Lucisano M., Cappa C., Fongaro L., Mariotti M. Characterisation of gluten-free pasta through conventional and innovative methods: Evaluation of the cooking behaviour. J. Cereal Sci. 2012;56:667–675. doi: 10.1016/j.jcs.2012.08.014. [DOI] [Google Scholar]
- 62.Li Y., Ma X., Liu X. Physicochemical and rheological properties of cross-linked inulin with different degree of polymerization. Food Hydrocoll. 2019;95:318–325. doi: 10.1016/j.foodhyd.2018.11.026. [DOI] [Google Scholar]
- 63.Liu J., Luo D., Li X., Xu B., Zhang X., Liu J. Effects of inulin on the structure and emulsifying properties of protein components in dough. Food Chem. 2016;210:235–241. doi: 10.1016/j.foodchem.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., Brennan M.A., Brennan C.S., Serventi L. Effect of Vegetable Juice, Puree, and Pomace on Chemical and Technological Quality of Fresh Pasta. Foods. 2021;10:1931. doi: 10.3390/foods10081931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cárdenas-Hernández A., Beta T., Loarca-Piña G., Castaño-Tostado E., Nieto-Barrera J.O., Mendoza S. Improved functional properties of pasta: Enrichment with amaranth seed flour and dried amaranth leaves. J. Cereal Sci. 2016;72:84–90. doi: 10.1016/j.jcs.2016.09.014. [DOI] [Google Scholar]
- 66.Minarovičová L., Lauková M., Kohajdová Z., Karovičová J., Dobrovická D., Kuchtová V. Qualitative properties of pasta enriched with celery root and sugar beet by-products. Czech J. Food Sci. 2018;36:66–72. doi: 10.17221/242/2017-CJFS. [DOI] [Google Scholar]
- 67.Bustos M.C., Perez G.T., Leon A.E. Structure and quality of pasta enriched with functional ingredients. RSC Adv. 2015;5:30780–30792. doi: 10.1039/C4RA11857J. [DOI] [Google Scholar]
- 68.Aravind N., Sissons M., Egan N., Fellows C. Effect of insoluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012;130:299–309. doi: 10.1016/j.foodchem.2011.07.042. [DOI] [Google Scholar]
- 69.Chillo S., Civica V., Iannetti M., Suriano N., Mastromatteo M., Del Nobile M.A. Properties of quinoa and oat spaghetti loaded with carboxymethylcellulose sodium salt and pregelatinized starch as structuring agents. Carbohydr. Polym. 2009;78:932–937. doi: 10.1016/j.carbpol.2009.07.013. [DOI] [Google Scholar]
- 70.Aravind N., Sissons M., Egan N., Fellows C.M., Blazek J., Gilbert E.P. Effect of β-Glucan on Technological, Sensory, and Structural Properties of Durum Wheat Pasta. Cereal Chem. J. 2012;89:84–93. doi: 10.1094/CCHEM-08-11-0097. [DOI] [Google Scholar]
- 71.Padalino L., Amalia C., Lucia L., Desislava L., Vincenzo S., Teresa Maria P., Marco P., Matteo Alessandro Del N. Functional pasta with tomato by-product as a source of antioxidant compounds and dietary fibre. Czech J. Food Sci. 2017;35:48–56. doi: 10.17221/171/2016-CJFS. [DOI] [Google Scholar]
- 72.European Parliament Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. [(accessed on 30 July 2022)];Off. J. Eur. Union. 2006 404:9. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924. [Google Scholar]
- 73.Alongi M., Melchior S., Anese M. Reducing the glycemic index of short dough biscuits by using apple pomace as a functional ingredient. LWT. 2019;100:300–305. doi: 10.1016/j.lwt.2018.10.068. [DOI] [Google Scholar]
- 74.Caponio G.R., Difonzo G., de Gennaro G., Calasso M., De Angelis M., Pasqualone A. Nutritional Improvement of Gluten-Free Breadsticks by Olive Cake Addition and Sourdough Fermentation: How Texture, Sensory, and Aromatic Profile Were Affected? Front. Nutr. 2022;9:830932. doi: 10.3389/fnut.2022.830932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramirez-Farias C., Slezak K., Fuller Z., Duncan A., Holtrop G., Louis P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 76.Le Bastard Q., Chapelet G., Javaudin F., Lepelletier D., Batard E., Montassier E. The effects of inulin on gut microbial composition: A systematic review of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:403–413. doi: 10.1007/s10096-019-03721-w. [DOI] [PubMed] [Google Scholar]
- 77.Brennan C.S., Kuri V., Tudorica C.M. Inulin-enriched pasta: Effects on textural properties and starch degradation. Food Chem. 2004;86:189–193. doi: 10.1016/j.foodchem.2003.08.034. [DOI] [Google Scholar]
- 78.Shoaib M., Shehzad A., Omar M., Rakha A., Raza H., Sharif H.R., Shakeel A., Ansari A., Niazi S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016;147:444–454. doi: 10.1016/j.carbpol.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Romão B., Falcomer A.L., Palos G., Cavalcante S., Botelho R.B.A., Nakano E.Y., Raposo A., Shakeel F., Alshehri S., Mahdi W.A., et al. Glycemic Index of Gluten-Free Bread and Their Main Ingredients: A Systematic Review and Meta-Analysis. Foods. 2021;10:506. doi: 10.3390/foods10030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brennan C.S., Tudorica C.M. Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas. Int. J. Food Sci. Technol. 2008;43:2151–2162. doi: 10.1111/j.1365-2621.2008.01831.x. [DOI] [Google Scholar]
- 81.Kumar M., Nagpal R., Kumar R., Hemalatha R., Verma V., Kumar A., Chakraborty C., Singh B., Marotta F., Jain S., et al. Cholesterol-Lowering Probiotics as Potential Biotherapeutics for Metabolic Diseases. Exp. Diabetes Res. 2012;2012:902917. doi: 10.1155/2012/902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tako E., Glahn R.P., Knez M., Stangoulis J.C. The effect of wheat prebiotics on the gut bacterial population and iron status of iron deficient broiler chickens. Nutr. J. 2014;13:58. doi: 10.1186/1475-2891-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caio G., Lungaro L., Segata N., Guarino M., Zoli G., Volta U., De Giorgio R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients. 2020;12:1832. doi: 10.3390/nu12061832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vacca M., Porrelli A., Calabrese F.M., Lippolis T., Iacobellis I., Celano G., Pinto D., Russo F., Giannelli G., De Angelis M. How Metabolomics Provides Novel Insights on Celiac Disease and Gluten-Free Diet: A Narrative Review. Front. Microbiol. 2022;13:859467. doi: 10.3389/fmicb.2022.859467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kareem K.Y., Hooi Ling F., Teck Chwen L., May Foong O., Anjas Asmara S. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014;6:23. doi: 10.1186/1757-4749-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baxter N.T., Schmidt A.W., Venkataraman A., Kim K.S., Waldron C., Schmidt T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. MBio. 2019;10:e02566-18. doi: 10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cosola C., De Angelis M., Rocchetti M.T., Montemurno E., Maranzano V., Dalfino G., Manno C., Zito A., Gesualdo M., Ciccone M.M., et al. Beta-Glucans Supplementation Associates with Reduction in P-Cresyl Sulfate Levels and Improved Endothelial Vascular Reactivity in Healthy Individuals. PLoS ONE. 2017;12:e0169635. doi: 10.1371/journal.pone.0169635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saa P., Urrutia A., Silva-Andrade C., Martín A.J., Garrido D. Modeling approaches for probing cross-feeding interactions in the human gut microbiome. Comput. Struct. Biotechnol. J. 2022;20:79–89. doi: 10.1016/j.csbj.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available on request.