Abstract
The Toxoplasma gondii rhoptry protein Rop2 was expressed in Escherichia coli as a fusion protein containing 44 kDa of the 55-kDa mature Rop2, supplied with six histidyl residues at the N-terminal end (Rop2196–561). Humoral response during Toxoplasma infection of humans was analyzed by immunoglobulin G (IgG), IgA, and IgM enzyme-linked immunosorbent assay with Rop2196–561 as the antigen substrate. The analyzed sera were divided according to T. gondii-specific serological tests (IgG, IgA, or IgM indirect immunofluorescence and IgA or IgM immunosorbent agglutination assay) as group A (IgG+ IgA− IgM−; n = 35), group B (IgG+ IgA+ IgM+; n = 21), group C (IgG+ IgA+ IgM−; n = 5), and group D (IgG+ IgA− IgM+; n = 16). Twenty-six T. gondii-seronegative sera from individuals with other infections were also included (group E). Anti-Rop2 IgG antibodies were detected in 82.8% of group A sera and in 97.6% of the sera with acute-phase marker immunoglobulins (groups B, C, and D). The percentage of IgA antibody reactivity against Rop2196–561 was 17.1% in group A, 50% in group D, and 80.8% in groups B and C. The percentage of IgM antibody reactivity was 0% in groups A and C and 62% in groups B and D. Sera from group E failed to show IgA, IgM, or IgG antibody reactivity. Since T. gondii Rop2 elicits a strong humoral response from an early stage of infection, it is suggested that recombinant Rop2196–561 would be suitable for use in diagnostic systems, in combination with other T. gondii antigens, to detect specific IgG, IgA, and IgM antibodies.
The coccidian protozoan Toxoplasma gondii is an obligate intracellular parasite of humans and other warm-blooded animals. It is a significant hazard to the fetuses of mothers who acquire the infection during pregnancy, and it has been established as a cause of life-threatening disease in immunocompromised individuals. Diagnosis of T. gondii infection is of considerable importance, since there are specific anti-T. gondii therapies.
In recent years, molecular biology techniques, such as PCR or dot blot analysis, have been applied for the detection of T. gondii DNA in clinical samples (3, 22). However, serological tests are the basic approach for screening purposes or to determine the infection phase. On the one hand, the detection of specific immunoglobulin G (IgG) antibodies and the absence of the acute-phase markers IgM and IgA allow diagnosis of the chronic stage of infection (29) or of past exposure to T. gondii. On the other hand, in spite of the difficulty of establishing the time of acquisition, the detection of IgM and IgA could suggest active infection (26, 29). Immunoglobulins belonging to class A are considered to be markers of interest in acquired toxoplasmosis because the kinetics of this isotype is faster than that of IgM antibodies and because naturally reacting IgA is not found in seronegative subjects. At present, studies on the value of specific IgE antibody detection for serological diagnosis of acute T. gondii infection are being developed, with promising results (11, 20).
Most serological tests for Toxoplasma require the preparation of parasite antigens from tachyzoites harvested from mice or cell culture systems. However, the use of whole-tachyzoite antigens can result in false-positive reactions (13, 27). Therefore, recent advances have been made in generating recombinant antigens of T. gondii which are less expensive and easier to standardize in IgG and IgM serological tests (1, 8, 12, 14, 15, 19, 21, 28).
Since the main mode of transmission of Toxoplasma infection is by ingestion of cysts or oocysts, IgA antibodies against this parasite should be strongly displayed by hosts. Chardes et al. (7) used Western blotting to analyze sera, intestinal secretions, and milk from mice orally infected with T. gondii cysts, finding specific IgA reactivity in intestinal secretions against proteins comigrating with p22, p30 (SAG1), p28 (GRA4), and the 55- and 60-kilodalton rhoptry proteins, among others. In addition, the cellular distribution of IgA epitopes on tachyzoites has been analyzed in the course of human acute, chronic, and congenital toxoplasmosis, showing high rhoptry immunolabeling in all cases (16).
Among rhoptry antigens, the antigenic value of Rop2 has been studied. Van Gelder et al. (28) constructed a fusion protein containing the 330 carboxy terminal residues of Rop2 supplied with six histidyl residues plus 7 kDa of Cro-LacI polypeptide at the N-terminal end. They found IgG reactivity against the recombinant Rop2 in 78% of sera from chronically infected individuals (IgG+ IgM−) and in 89% of sera with Toxoplasma-specific anti-IgG and -IgM antibodies, but they did not search for specific early immunoglobulins, such as IgA or IgM.
In order to determine whether Rop2 is involved in the antigenic fraction that elicits Toxoplasma-specific early immunoglobulins, a recombinant Rop2 (Rop2196–561) was designed and expressed in Escherichia coli. Rop2196–561 has the 365 carboxy-terminal amino acids of the 55-kDa mature protein, which excludes a potential processing site located at positions 111 to 116 (5). It is supplied with only six histidyl residues at the N-terminal end. This construction includes the main T-cell epitope, positions 197 to 216 (23, 24), for potential immunization uses. The fusion protein was purified and tested with diverse groups of human sera. Here we discuss the antigenic value of Rop2196–561 based on the detection of Toxoplasma-specific IgG, IgM, and IgA during human T. gondii infection and its use as a serological tool.
MATERIALS AND METHODS
Sera.
Seventy-seven sera were obtained either from pregnant immunocompetent asymptomatic women (n = 49) at the first (20.5%), second (63.2%), and third (16.3%) gestational stages for prenatal screening or from immunocompetent women and men (n = 28) who presented clinical data compatible with acute toxoplasmosis. The sera were collected by a national institute and hospitals in two countries and analyzed with highly sensitive and referenced methods: IgG, IgA, and IgM indirect immunofluorescence (IIF) and IgA and IgM immunosorbent agglutination assay (ISAGA). IgM ISAGA (BioMerieux) was performed according to the manufacturer’s instructions, whereas IgA ISAGA was adapted from the IgM ISAGA as described previously (9).
Twenty-seven sera were collected at the José de San Martín Clinical Hospital, Buenos Aires, Argentina, for routine diagnosis. The sera were tested by IIF with total IgG, -A, and -M. The presence of IgM and IgA was detected by IgA IIF and IgM IIF with sera previously absorbed at a 1:10 ratio with goat anti-human IgG (The Binding Site, Birmingham, United Kingdom) as described previously (4), as well as by IgA and IgM ISAGA.
Twenty-six sera were collected at the Evandro Chagas Hospital, Rio de Janeiro, Argentina. All patients had clinical symptoms or previous serological data suggestive of acute toxoplasmic infection, and most presented lymphadenopathy. All sera were tested by IgG IIF, IgM IIF, IgM ISAGA, and IgA ISAGA.
Twenty-four sera were collected at the ANLIS Dr. Carlos G. Malbran, Buenos Aires, Argentina, for routine diagnosis. All sera were tested by IgG IIF, IgM IIF, IgM ISAGA, and IgA ISAGA.
Twenty-six T. gondii-seronegative sera were collected from immunocompetent subjects who had no toxoplasmic infections. These sera were also provided by ANLIS Dr. Carlos G. Malbran and checked for anti-Toxoplasma antibodies by IgG IIF and IgA and IgM ISAGA after specific diagnosis. Sera were also obtained from patients with cutaneous leishmaniasis (n = 3), Chagas’ disease (n = 5), syphilis (n = 4), hydatidosis (n = 2), trichinosis (n = 6), toxocariasis (n = 2), and Plasmodium vivax malaria (n = 4).
Serum samples were classified into five groups. None of the patients providing serum samples were human immunodeficiency virus positive. Group A comprised 35 serum samples with positive serological results by IgG IIF and an absence of specific IgA and IgM (IgG+ IgA− IgM−). Thirty of the 35 sera came from women. Twenty-five of the sera were from women who were pregnant at different gestational stages; the remaining 10 sera were from individuals with lymphadenopathy (n = 8) and fever (n = 2). In the latter cases specific IgM had been detected 2 to 3 months before. Group B consisted of 21 serum samples (IgG+ IgA+ IgM+), 16 of which came from women. Twelve of the sera were from women who were pregnant at different gestational stages. The remaining nine sera were obtained from patients with lymphadenopathy, and in two cases specific IgM had been detected 1 to 2 months before. Group C consisted of five serum samples (IgG+ IgA+ IgM−) from pregnant women. Group D consisted of 16 serum samples (IgG+ IgA− IgM+), 12 of which came from women. Six of the sera were from women who were pregnant at different gestational stages. The remaining 10 sera were obtained from patients with lymphadenopathy, and in three cases specific IgM had been detected 1, 2, and 5 months before. Group E consisted of 26 serum samples (IgG− IgA− IgM−) from women (34.7%) and men (65.3%) with other nontoxoplasmic infections as described above.
Sera from five healthy subjects negative for Toxoplasma infection were used as negative controls for IgG, IgA, and IgM antibodies.
Construction of plasmids.
A Rop2-containing pBluescript plasmid (obtained from K. A. Joiner, Yale University School of Medicine [4]) was digested with BamHI and religated in order to create a plasmid containing a Rop2B subfragment, which codes for a fusion protein, Rop2196–561 (from amino acids 196 to 561, the end of Rop2). Plasmid pRop2B was sequenced and digested with BamHI and HindIII. The resulting 1,212-bp DNA fragment was purified from agarose (Qiagen) and cloned into BamHI-HindIII-digested plasmid pQE31 (Qiagen) to create plasmid pQE-Rop2B.
Expression and purification of recombinant protein.
E. coli M15 cells containing pQE-Rop2B from an overnight culture diluted 1:40 were grown in Luria broth supplemented with ampicillin (100 μg/ml) and kanamycin (10 μg/ml) for 3 h at 37°C. The fusion protein Rop2196–561 was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) at a final concentration of 5 mM for 2 h at 37°C. The bacteria were harvested by centrifugation at 4°C. The pellet was resuspended in lysis solution (8 M urea, 0.1 M NaH2PO4, 10 mM Tris-HCl) at pH 8 and sonicated at high frequencies for 1 min on ice three times. The fusion protein was passed through a Ni2+-nitrilotriacetic acid resin (Qiagen), and specific bacterial protein was eluted from the column with washing solution (lysis solution at pH 6). Finally, fusion protein Rop2196–561 was eluted from the column with purification solution (lysis solution at pH 4.2). The purified protein sample was desalted by dialysis against 0.1× phosphate-buffered saline (PBS) solution and then lyophilized.
T. gondii protein sample.
Tachyzoites of the RH strain were grown in the peritoneal cavities of CF1 mice. Harvested tachyzoites were washed three times with PBS by centrifugation. The pellet was resuspended in distilled water and sonicated three times at high frequencies for 1 min on ice. Parasite proteins were quantified by Bradford’s method (6).
Rop2196–561 ELISA.
Each well of the microtiter plate (Immuno Plate Maxisorp; Nunc) was coated overnight at room temperature with 100 μl of the recombinant protein diluted in 0.05 M carbonate buffer (pH 9.6) at the optimal concentration of 3 μg/ml. After being coated, the wells were washed three times with PBS–0.25% Tween 20 (PBS-T) and blocked with 200 μl of 5% skim milk in PBS-T (blocking solution) for 2 h at 37°C. The plates were then washed as described above, and 100 μl of test or control serum was applied to each well. To test for anti-Rop2 IgG, the sera were diluted 1:200 in blocking solution. In the case of anti-Rop2 IgM or IgA detection, the sera were diluted at the optimal 1:50 dilution in blocking solution. The plates were incubated for 2 h at room temperature and washed as described above. Goat anti-human (H+L) IgG, IgM, or IgA horseradish peroxidase-labeled antibodies (Jackson ImmunoResearch Laboratories Inc.) diluted 1:2,000 were used as the secondary antibody. After being incubated for 1 h at room temperature and washed, immune complexes were developed with ortho-phenylenediamine as the chromogenic substrate. Absorbance at 410 nm (A410) was measured with an automatic enzyme-linked immunosorbent assay (ELISA) reader (Dynatech MR4000). ELISA results were determined for each serum in duplicate. At least two independent ELISAs were performed for each serum. The cutoff point was established as the mean value of reactivity (plus 3 standard deviations) of the negative controls.
PAGE and immunoblot analysis.
Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% acrylamide gels (17) in the Mini-Protean system (Bio-Rad). After electrophoresis, the proteins were transferred to nitrocellulose membranes (Bio-Rad) for immunoblot analysis. Transfers were blocked with blocking solution as described for ELISA. The filters were sequentially probed with primary and secondary antisera diluted in blocking solution (1:200 and 1:2,000, respectively). A peroxidase immunoconjugate (Jackson ImmunoResearch Laboratories Inc.) was used as the second antibody, and specific binding was developed with diaminobenzidine as a chromogenic substrate.
Immunization.
Five NIH mice (2 months old) were immunized four times intraperitoneally at days 0, 15, 25, and 28. The immunization doses and boosters contained 10 μg of purified Rop2196–561 emulsified with Freund’s complete adjuvant (1:9). Two mice from the control group were immunized with a Rop2196–561-free preparation. At days 31 and 35 approximately 2 to 5 ml of ascitic fluid per mouse was collected, except in the control group, where only 50 to 200 μl could be collected. The titer of hyperimmune ascitic fluid (HAF) was determined by Rop2196–561 ELISA with anti-mouse (H+L) IgG conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories Inc.) as the secondary antibody, diluted 1:2,000.
RESULTS
Expression of T. gondii recombinant Rop2196–561 protein.
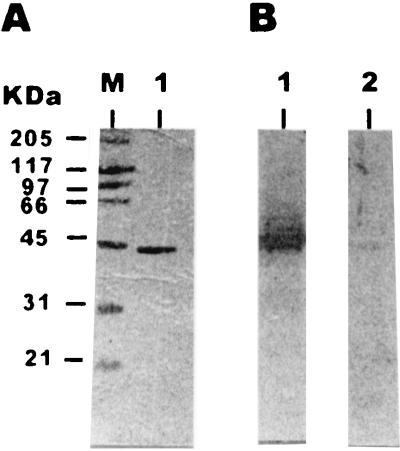
In order to determine the reactivity of human sera against T. gondii Rop2 protein, a 1,212-bp DNA fragment which encodes amino acids from 196 to the end of the protein (amino acid 561) was cloned in the expression vector pQE to generate the fusion protein Rop2196–561. Then the recombinant protein was affinity purified, and its purity was checked by SDS-PAGE (Fig. 1A). Coomassie blue staining of the purified Rop2196–561 showed that the fusion protein appeared at the 44-kDa region, which was close to the expected mass (43.6 kDa) according to the amino acid sequence. Furthermore, after Western blotting, Rop2196–561 was probed with human sera (Fig. 1B). It was observed that only the serum which contained anti-T. gondii IgG reacted against the fusion protein.
FIG. 1.
Purified fusion protein Rop2196–561 resolved by SDS-PAGE on 10% acrylamide gel. (A) Coomassie blue staining of the gel. Lanes: M, molecular mass markers; 1, 2 μg of Rop2196–561. (B) Immunoblots of 2 μg of fusion protein Rop2196–561 with an anti-T. gondii-positive human serum (lane 1) and a Toxoplasma-seronegative human serum (lane 2). Molecular masses are given on the left. The sera were used at a dilution of 1:200.
Reactivity of HAF from Rop2196–561-immunized mice against T. gondii antigens.
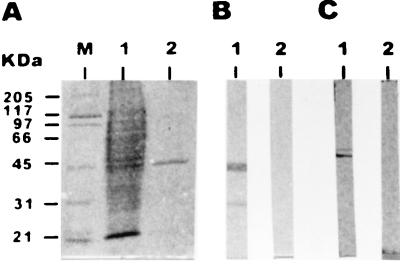
To determine whether recombinant Rop2 elicits antibodies that recognize authentic Rop2 protein, mice were immunized intraperitoneally with Rop2196–561 mixed with Freund’s complete adjuvant. All five mice displayed serological responses against Rop2196–561, either by immunoblotting (Fig. 2B) or by ELISA. Their HAFs had Rop2196–561 ELISA titers as follows: M1, 5,000; M2, 25,000; M3, 2,500; M4, 50,000, and M5, 1,000. Preimmune sera, HAF, and sera from nonimmunized controls were tested by immunoblotting against tachyzoite homogenate. Only HAF recognized a 55-kDa polypeptide (Fig. 2C), which is the apparent mass found for Rop2 in SDS-PAGE. An additional antigen with a Mr of 60,000 was detected, most likely another Rop protein, such as Rop3 or -4 (18, 25). M2 and M4 HAFs were assayed by immunofluorescence on fresh tachyzoites treated with Triton X-100 in order to confirm the Rop2-like cellular localization. The pattern of both HAFs was unipolar and occupied approximately one-third of the organism (data not shown), as described for an anti-Rop2 monoclonal antibody (T3 4A7) in a similar immunofluorescence assay (18).
FIG. 2.
Western blot profiles of HAFs from Rop2196–561-immunized mice and preimmune sera against tachyzoite homogenate. (A) SDS-PAGE gel stained with Coomassie blue. Lanes: M, molecular mass markers; 1, whole-tachyzoite protein (107 tachyzoites); 2, 2 μg of Rop2196–561. (B) Immunoblots of 2 μg of fusion protein Rop2196–561 with M2 HAF (lane 1) and preimmune serum (lane 2). (C) Immunoblots of 107 tachyzoites with M2 HAF (lane 1) and preimmune serum (lane 2). Molecular masses are given on the left. The sera were used at a dilution of 1:500.
Specificity of a Rop2196–561 ELISA.
An ELISA was performed with Rop2196–561 as the coating antigen to detect IgG, IgA, and IgM antibodies in human serum. Sera from five healthy individuals (negative controls) were used to obtain relative absorbance (Ar) for each serum and the cutoff value. The latter was defined as the mean value of negative control sera plus 3 standard deviations, resulting in 1.9 for IgG ELISA, 1.8 for IgA ELISA, and 1.7 for IgM ELISA.
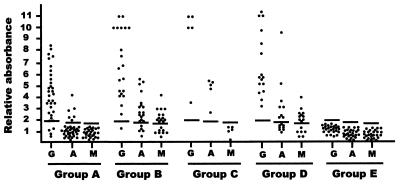
The specificity of a Rop2196–561 ELISA was analyzed by measuring the reactivities of sera from group E. No cross-reactivity was detected in any of the 26 control sera by IgG, IgA, and IgM ELISA sensitized with Rop2196–561, since none of them showed Ar values above their respective cutoff values (Fig. 3).
FIG. 3.
Immunoreactivity of the recombinant Rop2196–561 with a collection of sera from humans infected with T. gondii and other pathogens. The sera were divided into groups according to IgG IIF and IgA and IgM ISAGA or IIF with absorbed IgG sera. Groups A (n = 35), B (n = 21), C (n = 5), D (n = 16), and E (n = 26) are defined in Materials and Methods. All sera were tested by ELISA sensitized with 3 μg of Rop2196–561/ml. Bound antibodies were developed with horseradish peroxidase-labeled goat anti-human IgG (G), IgA (A), and IgM (M) and the chromogenic substrate. The relative absorbance is the mean absorbance value of test sera divided by the mean absorbance value of sera from negative controls (n = 5). The sera were used at an optimal dilution of 1:200 for IgG detection, with a cutoff value of 1.9, and an optimal dilution of 1:50 for IgA and IgM detection, with cutoff values of 1.8 (A) and 1.7 (M).
Reactivity of group A sera (IgG+ IgA− IgM−) against Rop2196–561.
The detection of anti-Rop2 IgG antibodies in sera from group A was analyzed by Rop2196–561 ELISA. IgG antibody reactivity was observed in 29 of the 35 sera (82.8%), and in 2 of the 35 sera (5.7%) the results were equal to the cutoff value (doubtful) (Fig. 3). The mean Ar value of the 35 sera was 4.0, with a standard deviation of 1.46.
In this group, 6 of the 35 sera (17.1%) showed IgA reactivity against recombinant Rop2 (Fig. 3). These six sera were obtained from patients who had clinical data compatible with acute Toxoplasma infection: lymphadenopathy, fever, and headache (n = 4) and fever with a clinical history showing anti-T. gondii IgM detected 2 or 3 months before the time of serum collection (n = 2). On the other hand, none of the 35 sera reacted against recombinant Rop2 by IgM ELISA. The mean Ar value of IgA ELISA sensitized with Rop2196–561 was 1.3, with a standard deviation of 0.8, whereas for IgM ELISA it was 1.0, with a standard deviation of 0.4.
Evaluation of IgA- and/or IgM-positive sera by Rop2196–561 ELISA (groups B, C, and D).
Based on the high IgG IIF titers of most sera, 41 of the 42 (97.6%) reacted by IgG ELISA against Rop2196–561 (Fig. 3), with a mean Ar value of 7.1 and a standard deviation of 3.1.
To study anti-Rop2 IgA and IgM reactivity, the sera were grouped according to IgA and/or IgM ISAGA results. Twenty-one of 26 (80.8%) IgA-positive sera (groups B and C) reacted by IgA ELISA against the recombinant Rop2 (Fig. 3), with a mean Ar value of 3.2 and a standard deviation of 1.6. Although group D had negative results by IgA ISAGA, 8 of 16 (50%) sera showed IgA reactivity against Rop2196–561 ELISA, with a mean Ar value of 2.6 and a standard deviation of 2.1. Four of these eight anti-Rop2 IgA-positive sera were obtained from patients with lymphadenopathy, fever, and headache who had IgG IIF serum titers of 1,024 (n = 2) and 4,096 (n = 2). In one of the patients, specific IgM had been detected 1 month before serum collection. The other four sera came from pregnant women who had IgG IIF serum titers of 512, 2,048, 8,192, and 16,384.
Twenty-three of 37 (62.1%) IgM-ISAGA-positive sera (groups B and D) reacted by IgM ELISA against the recombinant Rop2 (Fig. 3), with a mean Ar value of 1.9 and a standard deviation of 0.9. In group C (IgG+ IgA+ IgM−) there was no reactivity against Rop2196–561 by IgM ELISA.
Taking into account the detection of acute-phase immunoglobulins, 35 of the 42 IgM- and/or IgA-ISAGA-positive sera (groups B, C, and D) reacted either by IgA or IgM ELISA sensitized with Rop2196–561, giving 83.3% reactivity.
Reactivity of sera against a Leishmania fusion protein supplied with six histidyl residues.
In order to rule out the existence of antibodies against the six histidyl residues of our recombinant protein, all anti-Rop2196–561-positive sera were tested against a recombinant protein of Leishmania infantum called LiB1, which was expressed in E. coli from pQE plasmid (2). The recombinant protein is also supplied with six histidyl residues at the amino termini. LiB1 corresponds to the region of L. Infantum Hsp83 protein which is less conserved than those of other homologous proteins. None of the sera which recognized Rop2196–561 reacted against LiB1, developed either with anti-IgG, anti-IgA, or peroxidase-conjugated anti-IgM antibodies.
DISCUSSION
The results shown here demonstrate that during human Toxoplasma infection Rop2 antigen elicits a humoral response that involves the acute-phase markers IgA and IgM, as well as the acute- and chronic-phase marker IgG antibodies. The reactivity of the recombinant protein Rop2196–561 with the acute-phase immunoglobulins is not unexpected. Rop2 is a T. gondii protein detected in all three parasite stages (25), and rhoptries are secretory organelles of antigens with IgA reactivity during acute, chronic, and congenital stages in human T. gondii infection (16). Moreover, intestinal and serum IgA antibodies from orally infected mice were shown to react against antigens comigrating with the 55- and 60-kDa rhoptry proteins (7).
Van Gelder et al. (28) studied the humoral response against Rop2 by using a recombinant protein to develop an IgG ELISA. They found IgG reactivity in 89% of human sera (IgM+ or IgM−). This recombinant protein was expressed in E. coli as a fusion protein containing in the N-terminal end the 330 carboxy-terminal residues of Rop2 supplied with six histidyl residues followed by a 48-amino-acid sequence derived from the phage lambda protein Cro and the E. coli protein LacI.
In an effort to determine the antigenic value of Rop2 for serological diagnosis of toxoplasmic infection, we constructed a new recombinant Rop2 (Rop2196–561) containing the 365 carboxy-terminal residues of Rop2 supplied with six histidyl residues. Saavedra et al. (24) found three potential epitopes recognized by human T cells in Rop2 antigen, the most frequently recognized in proliferation assays being the selected peptides 197 to 216 and 501 to 524 (45 and 36%, respectively). Rop2196–561 has the three epitopes and therefore retains its potential utility as a T-cell repertoire stimulator.
Here, the antigenicity of Rop2 was evaluated with human sera by IgG, IgA, and IgM ELISAs sensitized with Rop2196–561. The specificity of Rop2196–561 ELISA was demonstrated by the low reactivity shown by sera from patients seronegative for toxoplasmosis but with other parasitic or nonparasitic infections. In all the sera from Toxoplasma-infected subjects that were studied, IgG antibody reactivity against Rop2196–561 was 91%. The reactivity was slightly higher for the sera that contained T. gondii-specific IgA or IgM (groups B, C, and D) than for sera which were devoid of such antibodies (group A) (97.6 and 82.8%, respectively). These results were similar to those reported previously by others (28).
Regarding the detection of acute-phase immunoglobulins, Rop2196–561 proved to be more readily recognized by anti-T. gondii IgA than by IgM antibodies: 80.8 and 62.1% (groups B and C and B and D, respectively). Anti-Rop2 class A immunoglobulins were not detected by T. gondii-seronegative sera (group E) but were detected in some negative sera by IgA ISAGA and IgA IIFs: i.e., sera of group A (17.1%) and group D (50%). Some of these results could be explained by previous results reported by Kumolosasi et al. (16), who showed that rhoptry antigens were recognized throughout IgA kinetics even when IgA tests, such as IgA ELISA or immunocapture IgA, were negative. Further studies should be done to shed light on this point.
Several T. gondii antigens have already been used as fusion proteins to develop serological tools based only on the detection of acute and chronic IgG and IgM antibodies (1, 12, 14, 15, 19, 21, 28). Among them, recombinant P22 (glutathione S-transferase–P22 fusion) proved to be an acute-infection marker, detecting anti-P22 IgG in sera from acutely infected individuals more strongly than in those from chronically infected individuals (19). However, recombinant P22 proved to be of little value in detecting anti-P22 IgM or IgA (19). Although antigen P28 was regarded as a marker of chronic infection, recombinant P28 (β-galactosidase–P28 fusion protein) reacted with IgG antibodies in sera from both chronically and acutely infected individuals (21). The recombinant T. gondii antigens H4 and H11 (a carboxyl-terminal fragment of GRA4) both fused to glutathione S-transferase, reacted more sensitively with sera from patients with acute toxoplasmosis than with those from patients with chronic infection (15). Andrews et al. (1) obtained similar results with swine sera tested by H4 and H11 ELISA. Harning et al. (12) designed a construction which generates a recombinant protein, SAG1, supplied with six histidyl residues at the N-terminal end. This recombinant SAG1 must be purified in nonreduced conditions to be recognized by a monoclonal antibody against a conformational epitope. Thus, recombinant SAG1 proved to be suitable for use in diagnostic systems to detect anti-SAG1 acute-phase IgM and chronic-phase IgG. Previously, specific anti-SAG1 IgA antibodies had been demonstrated in human sera (10).
Based on the data obtained from other recombinant antigens, Rop2196–561 proved to be a powerful tool for the development of serological diagnostic systems to diagnose either chronic or acute T. gondii infections, in both cases in combination with other recombinant antigens.
ACKNOWLEDGMENTS
We are grateful to K. A. Joiner (Yale University School of Medicine) for Rop2 plasmid, to Christian Diaz for photographic work, and to E. Arzt, A. Sequeira, V. Pszenny, and E. Bontempi for helpful suggestions.
Sergio O. Angel and Juan C. Garberi are members of the National Research Council (CONICET), Argentina.
This work was supported by grants from CONICET (PEI 0039-97) and ANLIS Dr. Carlos G. Malbran.
REFERENCES
- 1.Andrews C D, Dubey J P, Tenter A M, Webert D W. Toxoplasma gondii recombinant antigens H4 and H11: use in ELISAs for detection of toxoplasmosis. Vet Parasitol. 1997;70:1–11. doi: 10.1016/s0304-4017(96)01154-5. [DOI] [PubMed] [Google Scholar]
- 2.Angel S O, Requena J M, Soto M, Criado D, Alonso C. During canine leishmaniasis a protein belonging to the 83-kDa heat-shock protein family elicits a strong humoral response. Acta Trop. 1996;62:45–56. doi: 10.1016/s0001-706x(96)00020-4. [DOI] [PubMed] [Google Scholar]
- 3.Angel S O, Matrajt M, Margarit J, Nigro M, Illescas E, Pszenny V, Amendoeira M R R, Guarnera E, Garberi J C. Screening for active toxoplasmosis in patients by DNA hybridization with ABGTg7 probe in blood samples. J Clin Microbiol. 1997;35:591–595. doi: 10.1128/jcm.35.3.591-595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcavi M, Orfus G, Griemberg G. Diagnosis of toxoplasmosis by joint detection of immunoglobulin A and immunoglobulin M. J Clin Microbiol. 1997;35:1450–1453. doi: 10.1128/jcm.35.6.1450-1453.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckers C J M, Dubremetz J F, Mercereau-Puijalon O, Joiner K A. The Toxoplasma gondii rhoptry protein Rop 2 is inserted onto the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Chardes T, Bourguin I, Mevelec M N, Dubremetz J F, Bout D. Antibody responses to Toxoplasma gondii in sera, intestinal secretions, and milk from orally infected mice and characterization of target antigens. Infect Immun. 1990;58:1240–1246. doi: 10.1128/iai.58.5.1240-1246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darcy F, Torpier G, Cesbron-Delauw M F, Decoster A, Ridel P R, Duquesne V, Charif H, Godard I, Pierce R J, Auriault C, Capron A. Toxoplasma gondii: new strategies for the identification of antigens of interest in diagnosis and as potential vaccines. Ann Biol Clin. 1989;47:451–457. [PubMed] [Google Scholar]
- 9.Desmonts G, Naot Y, Remington J S. M-immunosorbent assay for diagnosis of infectious disease: diagnosis of acute congenital and acquired Toxoplasma infection. J Clin Microbiol. 1981;14:486–491. doi: 10.1128/jcm.14.5.486-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross U, Roos T, Appoldt D, Heesemann J. Improved serological diagnosis of Toxoplasma gondii infection by detection of immunoglobulin A (IgA) and IgM antibodies against P30 by using the immunoblot technique. J Clin Microbiol. 1992;30:1436–1441. doi: 10.1128/jcm.30.6.1436-1441.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross U, Kessel O, Darde M L. Value of detecting immunoglobulin E antibodies for the serological diagnosis of Toxoplasma gondii infection. Clin Diagn Lab Immunol. 1997;4:247–251. doi: 10.1128/cdli.4.3.247-251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harning D, Spenter J, Metsis A, Vuust J, Petersen E. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin Diagn Lab Immunol. 1996;3:355–357. doi: 10.1128/cdli.3.3.355-357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassl A, Müller W A, Aspöck H. An identical epitope in Pneumocystis carinii and Toxoplasma causing serological cross-reactions. Parasitol Res. 1991;77:351–352. doi: 10.1007/BF00930914. [DOI] [PubMed] [Google Scholar]
- 14.Johnson A M, Illana S. Cloning of T. gondii gene fragments encoding diagnostic antigens. Gene. 1991;99:127–132. doi: 10.1016/0378-1119(91)90044-c. [DOI] [PubMed] [Google Scholar]
- 15.Johnson A M, Roberts H, Tenter A M. Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J Med Microbiol. 1992;37:404–409. doi: 10.1099/00222615-37-6-404. [DOI] [PubMed] [Google Scholar]
- 16.Kumolosasi E, Bonhome A, Beorchia A, Foudrinier F, Marx C, Pluot M, Pinon J M. Kinetics study of the localization and quantitation of target antigens of immunoglobulin A antibodies in acquired and congenital toxoplasmosis. Parasitol Res. 1996;82:402–409. doi: 10.1007/s004360050136. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Leriche M A, Dubremetz J F. Characterization of the protein contents of rhoptries and dense granules of Toxoplasma gondii tachyzoites by subcellular fractionation and monoclonal antibodies. Mol Biochem Parasitol. 1991;45:249–259. doi: 10.1016/0166-6851(91)90092-k. [DOI] [PubMed] [Google Scholar]
- 19.Parmley S F, Sgarlato G D, Mark J, Prince J B, Remington J S. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol. 1992;30:1127–1133. doi: 10.1128/jcm.30.5.1127-1133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinon J M, Toubas D, Marx C, Mougeot G, Bonnin A, Bonhomme A, Villaume M, Foudrinier F, Lepan H. Detection of specific immunoglobulin E in patients with toxoplasmosis. J Clin Microbiol. 1990;28:1739–1743. doi: 10.1128/jcm.28.8.1739-1743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince J B, Araujo F G, Remington J S, Burg J L, Boothroyd J C, Sharma S D. Cloning of cDNAs encoding a 28 kilodalton antigen of Toxoplasma gondii. Mol Biochem Parasitol. 1989;34:3–14. doi: 10.1016/0166-6851(89)90014-5. [DOI] [PubMed] [Google Scholar]
- 22.Robert F, Ganivet M F, Tourte-Scaefer C, Dupuoy-Camet J. Intérets et limites de la polymerase chain reaction dans le diagnostic de la toxoplasmose. Immunoanal Biol Spéc. 1996;11:176–182. [Google Scholar]
- 23.Saavedra R, de Meuter F, Decourt J L, Herion P. Human T cell clone identifies a potentially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J Immunol. 1991;147:1975–1982. [PubMed] [Google Scholar]
- 24.Saavedra R, Becerril M A, Dubeaux C, Lippens R, De Vos M-J, Hérion P, Bollen A. Epitopes recognized by human T lymphocytes in the ROP2 protein antigen of Toxoplasma gondii. Infect Immun. 1996;64:3858–3862. doi: 10.1128/iai.64.9.3858-3862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadak A, Taghy Z, Fortier B, Dubremetz J F. Characterization of a family of rhoptry proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1988;29:203–212. doi: 10.1016/0166-6851(88)90075-8. [DOI] [PubMed] [Google Scholar]
- 26.Stepick-Bick P, Thulliez P, Araujo F G, Remington J S. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis. 1990;162:270–273. doi: 10.1093/infdis/162.1.270. [DOI] [PubMed] [Google Scholar]
- 27.Taylor D W, Evans C B, Aley S B, Barta J R, Danforth H D. Identification of an apically-located antigen that is conserved in sporozoan parasites. J Protozool. 1990;37:540–545. doi: 10.1111/j.1550-7408.1990.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Gelder P, Bosman F, de Meuter F, Van Heuverswyn H, Hérion P. Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli. J Clin Microbiol. 1993;31:9–15. doi: 10.1128/jcm.31.1.9-15.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong S Y, Remington J S. Toxoplasmosis in pregnancy. Clin Infect Dis. 1994;18:727–729. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]