Abstract
Background
Trastuzumab given intravenously in combination with chemotherapy is standard of care for patients with early HER2-positive breast cancer (BC). Different randomised studies have shown equivalent efficacy of a subcutaneous injection into the thigh compared to the intravenous formulation. Other body regions for injection have not been investigated but might be more convenient for patients.
Methods
After surgery, patients were randomised to receive either subcutaneous trastuzumab into the thigh or into the abdominal wall (AW). Patient preferences were evaluated using validated questionnaires (PINT). Primary objectives of this multicentre, non-blinded, randomised substudy of the GAIN-2 study were to investigate pharmacokinetics of the injection into the thigh versus AW and to determine patients' preferences of either administration site versus the previously received intravenous application.
Results
226 patients were randomised and 219 patients (thigh: N = 110; AW: N = 109) formed the modified intent-to-treat (mITT). Overall, 83.5% (out of N = 182 with information about patients’ preference) preferred subcutaneous over previous intravenous application or had no preference. Preference was similar between both administration sites (thigh: 80.6%; AW: 86.5; p = 0.322). Pharmacokinetic analysis included 30 patients. Geometric means of Cmax and AUC0-21d were higher in thigh than in AW group (geometric mean ratio with body weight adjustment: Cmax: 1.291, 90%-CI 1.052–1.584; AUC0-21d: 1.291, 90%-CI 1.026–1.626). Safety profile was in line with previous reports of subcutaneous trastuzumab.
Conclusion
Subcutaneous trastuzumab into the thigh showed an approximately 30% higher bioavailability. Injections were well tolerated and preferred over intravenous administration. The subcutaneous injection into the thigh should remain the standard of care.
Keywords: Breast cancer, Trastuzumab subcutaneous, patients' preference, Pharmacokinetic, Thigh or abdominal wall, Safety
Highlights
-
•
The bioavailability of subcutaneous trastuzumab was 30% higher when injected the thigh vs the abdominal wall.
-
•
Patients preferred subcutaneous over intravenous injection of trastuzumab.
-
•
Patient preference was comparable between abdominal wall or thigh subcutaneous injection.
-
•
There were no new safety signals for subcutaneous trastuzumab injection.
1. Introduction
Trastuzumab given intravenously (i.v.) every three weeks (q3w) in combination with chemotherapy is standard of care for patients with early HER2-positive breast cancer (BC) [1,2] and has substantially improved pathological complete response (pCR) [[3], [4], [5]], disease free survival and overall survival [6]. The randomised phase III HannaH study showed equivalent efficacy of a (neo)adjuvant trastuzumab subcutaneous (s.c.) formulation into the thigh compared to trastuzumab i.v. in female patients with HER2-positive BC [7,8]. The study reported non-inferiority on pCR rates, comparable pharmacokinetics (PK) and safety results. Other studies support these findings on comparable efficacy and safety for patients with HER2-positive early [[9], [10], [11]] and metastatic disease [12,13]. The reported advantages of s.c. over i.v. administration are shorter treatment times of about 5 min versus 30–90 min, a reduced use of health care resources, an increased convenience for patients, all of which lead to a greater patient preference [10,12,13]. Moreover, the s.c. formulation is a fixed dose and does not need to be calculated per patient allowing for easier use and accessibility with less preparation time and a decreased risk for dosing mistakes. Tolerability and immunogenicity were reported to be equivalent to trastuzumab i.v [7,14]. Taking these data into account, trastuzumab s.c. into the thigh has been approved by the European Medical Agency (EMA) in 2013 for the treatment of early and advanced BC. The Federica study [15] investigated the PK, efficacy, and safety and the PHranceSCa study [16] investigated the preference of trastuzumab and pertuzumab as one fixed dose combination. Both studies showed that s.c. injection was not inferior to i.v. administration and despite the higher volume of injection, patients continued to prefer -s.c. administration. None of these studies investigated so far other body regions for s.c. injection besides the thigh. Injections into the abdominal wall (AW) might be more convenient, is standard in many other s.c. medications, and allows easier access for health care professionals as partly undressing is not necessary.
Therefore, PK profiles, safety and patients’ preference of the two different trastuzumab s.c. injection sites (AW versus thigh) were evaluated in a substudy of the multicentre, randomised phase III GAIN-2 study [17].
2. Patients and methods
2.1. Study design
This study was a substudy of the phase III multicentre, randomised GAIN-2 study (NCT01690702) [17]. The design of GAIN-2 study has been published elsewhere [[17], [18], [19], [20]] (Fig. S1). Patients with HER2-positive BC had received (neo)adjuvant chemotherapy and adequate surgery within the main study when entering this substudy. The chemotherapy consisted of 3 cycles epirubicin, followed by 3 cycles of nab-paclitaxel followed by 3 cycles of cyclophosphamide q2w (EnPC) or 4 cycles of dose-tailored/dose-dense epirubicin and cyclophosphamide q2w followed by 4 cycles of docetaxel q2w (dtEC-dtD). Patients had also received i.v. anti-HER2 directed therapy q3w simultaneously to chemotherapy.
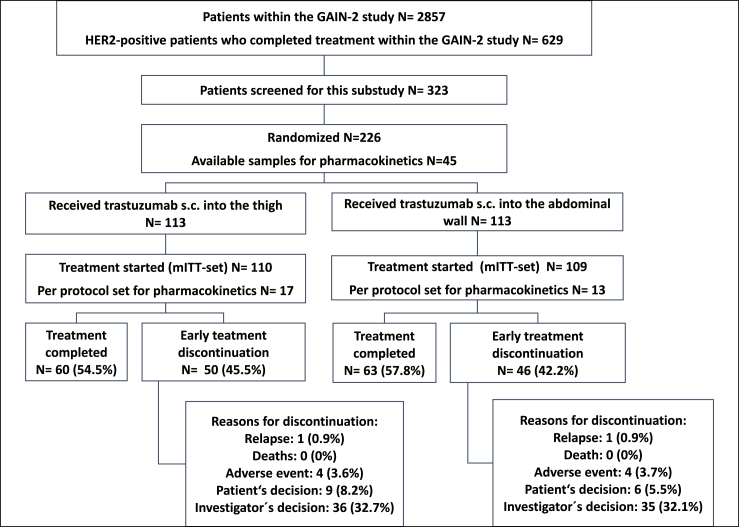
Following the main study treatment, patients were randomised in a 1:1 ratio to receive either trastuzumab 600 mg fixed dose injected q3w s.c. into the thigh or into the AW for the total of 18 administrations (Fig. 1). Trastuzumab s.c. was administered by using a hand-held syringe as per manufactures recommendation at a steady rate over about 5 min by a health care professional. Last i.v. trastuzumab administration had to be 3 weeks prior to randomization. Randomization was stratified according to chemotherapy arm and age (≤50 versus >50 years).
Fig. 1.
Consort statement, mITT, modified intent-to-treat; s.c., subcutaneous.
Patient preferences were evaluated by using validated, study-specific patient interview (PINT) questionnaires before randomization (PINT1) and after the end of cycle 8 of s.c. trastuzumab (PINT2) [21].
The co-primary objectives of thissubstudy were to investigate PK of trastuzumab injection either into the AW or thigh, and to determine patients' preferences of either administration region versus the previously received i.v. application. Further objectives were safety and compliance. The study was approved by the ethics committees/institutional review boards and the relevant health authorities.
2.2. Patient population
Women with HER2-positive BC and active participant in the GAIN-2 study were eligible. Previous treatment with i.v. trastuzumab for 12 weeks in the EnPC arm and 8 weeks in the dtEC-dtD arm within the GAIN-2 study was necessary for participation or minimum of 9 (EnPC) or 6 (dtEC-dtD) weeks of trastuzumab treatment in case chemotherapy was discontinued early. Last i.v. trastuzumab administration within 3 weeks prior to randomization and PINT1 needed to be done prior to randomization. Adjuvant radiotherapy or endocrine therapy was applied according to local standards. For study participation, patients should have had a left ventricular ejection faction (LVEF) of minimum ≥55% when entering this study.
A modified intention-to-treat (mITT) analysis was conducted for all patients who have been randomised into the substudy and have received at least one dose of trastuzumab s.c. Patients in the PK substudy were not allowed to have more than two missing samples and patients with dosing delays of more than 7 days in the last 3 cycles prior to PK assessments in cycle 7 (i.e. in cycles 4, 5, 6) were excluded from the per-protocol analysis.
2.3. Statistical analysis on pharmacokinetic
Based on the variability observed in the HannaH study [7] and using 15 patients per group available with complete samples, the simulated two-sided 90% confidence interval (CI) of the area under the plasma concentration-time curve (AUC0-21d) was (0.79–1.27), (0.77–1.30) for the peak drug concentration (Cmax) and (0.73, 1.38) for the concentration at the end of the dosage interval (Cthrough) assuming a true geometric mean ratio (GMR) of 1.0. Allowing for a dropout rate of 15%, 18 patients per group were planned to be included in the PK substudy. Blood samples collected before cycle 7 and on days (d) 2, 4, 8, 15 and 21 of cycle 7 were evaluated. One-way analyses of covariance (ANCOVA) were performed to compare AUC0-21d and Cmax between thigh versus AW and adjusted for body weight at baseline including stratification factors. Sensitivity analyses without body weight adjustment were applied. The ANCOVA was performed with the logarithmically transformed PK parameters and later transformed back to the original scale. Routes of administration could be considered comparable if the observed 90% CIs of the GMR would lie entirely within the estimated CIs. Invasive disease-free survival (iDFS) was estimated using Kaplan-Meier method and compared between arms using the log-rank test and a univariate Cox proportional hazard model to estimate the hazard ratio with 95% CI.
2.4. Statistical analysis on patients' preference and safety
PINT1 was performed −20 to −1 days prior to randomization of the substudy. Patients were assessed regarding the factors influencing preference after having received i.v. trastuzumab within GAIN-2 study [21]. PINT2 was performed at the end of cycle 8 of s.c. trastuzumab, concluding with a single binary assessment of preference [21].
The primary efficacy endpoint was the proportion of patients preferring s.c. trastuzumab (thigh versus AW) compared to previous i.v. trastuzumab, assessed by the question on PINT2: “All things considered, which method of administration do you prefer?” (“i.v.“, “s.c.“, “no preference”), compared by Fisher's exact test. Secondary objectives were to validate factors potentially influencing patients' preference of s.c. trastuzumab as detected in the PrefHer study [10,21] and have been validated elsewhere [21,22] by univariate logistic regression analysis.
3. Results
3.1. Patients
Between 11/2013 and 8/2017, 226 female patients were randomised within 71 centres in Germany. 219 patients formed the mITT analysis set for patients’ preferences (thigh N = 110; AW N = 109). Seven randomised patients did not start therapy and were excluded from the mITT set (patients' decision: N = 6; progress: N = 1). 123/219 (56.2%) patients completed treatment: 60/110 (54.5%) in the thigh and 63/109 (57.8%) in the AW group (Fig. 1).
Baseline tumour and patients' characteristics were balanced between the arms for the whole cohort and the patients analysed for PK (Table 1). Median age was 50 years (range 25–69), 102 (46.6%) patients were >50 years. The majority had T2 (45.7%), G3 (60.7%), node negative (11.4%) disease. 57 (26%) patients were included after neoadjuvant and 162 (74.0%) after adjuvant chemotherapy.
Table 1.
Baseline characteristics.
| Parameter | Category | Thigh N = 110; N (%) |
Abdominal wall N = 109; N (%) | Overall N = 219; N (%) |
|---|---|---|---|---|
| Age, years | Median | 50.0 | 50.0 | 50.0 |
| Min, Max | 25.0, 69.0 | 30.0, 67.0 | 25.0, 69.0 | |
| BMI (kg/m2) | Median | 24.0 | 24.6 | 24.2 |
| Min, Max | 17.4, 50.7 | 18.4, 43.2 | 17.4, 50.7 | |
| Pre-study trastuzumab treatment (i.v.), weeks | Median Min, Max |
12.4 7.0, 26.9 |
12.2 6.0, 23.9 |
12.3 6.0, 26.9 |
| missing | 0 | 1 | 1 | |
| Tumour stage (all) | c/pT1 | 53 (48.2) | 42 (38.5) | 95 (43.4) |
| c/pT2 | 43 (39.1) | 57 (52.3) | 100 (45.7) | |
| c/pT3 | 11 (10.0) | 7 (6.4) | 18 (8.2) | |
| c/pT4 | 3 (2.7) | 3 (2.8) | 6 (2.7) | |
| Nodal status (all) | c/pN0 | 14 (12.7) | 11 (10.1) | 25 (11.4) |
| c/pN1 | 59 (53.6) | 64 (58.7) | 123 (56.2) | |
| c/pN2 | 26 (23.6) | 14 (12.8) | 40 (18.3) | |
| c/pN3 | 11 (10.0) | 20 (18.3) | 31 (14.2) | |
| ER/PR central testing | both ER and PR negative ER and/or PR positive |
35 (79.5) 9 (20.5) |
30 (81.1) 7 (18.9) |
65 (80.2) 16 (19.8) |
| missing | 66 | 72 | 138 | |
| Ki67, central testing | ≤20% | 17 (15.5) | 18 (16.5) | 35 (16.0) |
| >20% | 93 (84.5) | 91 (83.5) | 184 (84.0) | |
| Setting of chemotherapy | neoadjuvant | 30 (27.3) | 27 (24.8) | 57 (26.0) |
| adjuvant | 80 (72.7) | 82 (75.2) | 162 (74.0) | |
| Treatment arm | EnPC dtEC-dtD | 57 (51.8) 53 (48.2) |
54 (49.5) 55 (50.5) |
111 (50.7) 108 (49.3) |
| Histological tumour type | Ductal/ductal-lobular Lobular missing |
84 (76.4) 3 (2.7) |
84 (77.1) 2 (1.8) |
168 (76.7) 5 (2.3) |
| Within the PK cohort | N = 17 | N = 13 | N = 30 | |
| Treatment arma | EnPC | 9 (52.9) | 7 (53.8) | 16 (53.3) |
| dtEC-dtD | 8 (47.1) | 6 (46.2) | 14 (46.7) | |
| Age groupa | ≤50 years | 11 (64.7) | 8 (61.5) | 19 (63.3) |
| >50 years | 6 (35.3) | 5 (38.5) | 11 (36.7) | |
| BMI | Median | 25.4 | 26.2 | 25.9 |
| Min, Max | 18.8, 32.0 | 20.7, 42.0 | 18.8, 42.0 |
BMI, body mass index; dt EC-dtD, 4 cycles of dose dense dose tailored epirubicin and cyclophosphamide followed by 4 cycles of docetaxel q2w; EnPC, epirubicin (150 mg/m2), followed by 3 cycles of nab-paclitaxel (330 mg/m2) followed by 3 cycles of cyclophosphamide (2000 mg/m2) q2w; ER, estrogen receptor; PK, pharmacokinetics; PR, progesterone receptor.
Stratification factors for randomization in the pharmacokinetic part of the substudy.
Pharmacokinetics
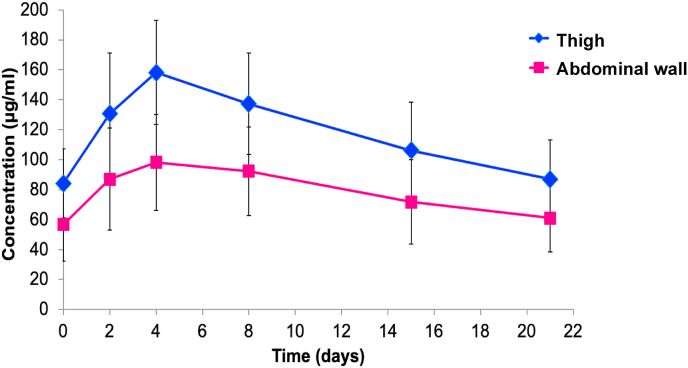
PK details between the site of injection were investigated in 30 patients in the per-protocol set (thigh N = 17; AW N = 13). The mean plasma concentration-time profiles of the s.c. trastuzumab administered into the thigh and AW are presented in Fig. 2. The Cmax was reached on day 4 after injection and declined thereafter.
Fig. 2.
Mean plasma concentration-time profile of the s.c. trastuzumab, s.c., subcutaneous.
Furthermore, the mean time-to-peak drug plasma concentrations (Tmax) did not differ between the two groups (Table 2). Variability as measured by the coefficient of variation (CV) for the PK parameters was higher in the AW than in the thigh group (Table 2). The GMRs of Cmax and AUC0-21d with body weight adjustment were higher in the thigh than in the AW group (1.29 [90% CI 1.05; 1.58] and 1.29 [90% CI 1.03; 1.63], respectively) and the 90% CI was not entirely within the simulated CI.
Table 2.
Pharmacokinetic details of the per-protocol cohort of the s.c. trastuzumab.
| Parameter | Category | Thigh (N = 17) | Abdominal wall (N = 13) |
|---|---|---|---|
| Cmax | Mean (SD) | 150.73 (44.81) | 100.00 (31.14) |
| (μg/ml) | CV% | 29.73 | 31.14 |
| Geo-Mean (SD) | 142.08 (1.49) | 94.00 (1.49) | |
| GLSM (95%CI) | 129.70 (113.77; 147.87) | 100.48 (85.96; 117.47) | |
| AUC(0-21d) | Mean (SD) | 2377.05 (639.24) | 1589.95 (568.96) |
| (μg day/ml) | CV% | 26.89 | 35.78 |
| Geo-Mean (SD) | 2246.41 (1.50) | 1468.45 (1.57) | |
| GLSM (95%CI) | 2060.38 (1777.64; 2388.32) | 1595.50 (1338.29; 1902.35) | |
| Tmax | Mean (SD) | 5.18 (2.24) | 5.23 (2.39) |
| (day) | Median | 4 | 4 |
| Min; Max | 2; 8 | 2; 8 | |
| C(trough) | Mean (SD) | 87.02 (26.05) | 58.67 (23.23) |
| (μg/ml) | CV% | 29.94 | 39.60 |
| Geo-Mean (SD) | 81.24 (1.56) | 54.31 (1.52) | |
| GLSM (95%CI) | 76.67 (64.34; 91.38) | 58.26 (47.27; 71.79) |
AUC(0-21d), area under the plasma concentration-time curve from day 0 to day 21; Cmax peak drug concentration; C(trough), concentration at the end of the dosage interval; CV, coefficient of variation; Geo-Mean, back-transformed geometric mean of log transformed values; GLSM, model-adjusted geometric least square means; SD, standard deviation; Tmax, time-to-peak drug plasma concentrations.
Similarly, the GMR of Ctrough, was higher in the thigh than in the AW group (1.32 [90%CI 1.00; 1.73]). Bioavailability of s.c. trastuzumab as reflected by the selected PK parameters (Cmax, AUC(0-21d) and Ctrough) measured in cycle 7 was approximately 30% higher using body weight adjustment in the analysis. After a median follow up of 45.8 months iDFS between the thigh or AW group was comparable (Fig. S2).
3.2. Patient preference
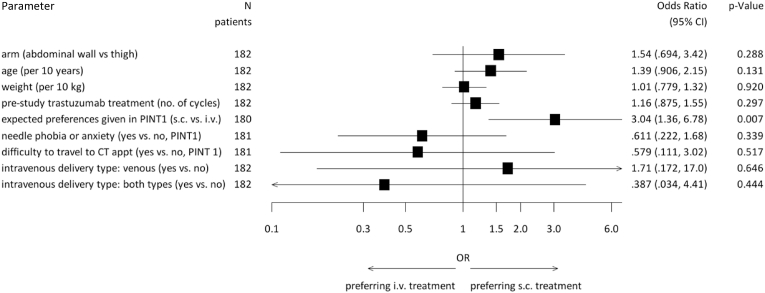
Considering all possibilities, information about patients’ preference was available in 182/219 (83.1%) patients and 152 (83.5%) of them preferred either s.c. over previous i.v. injection or had no preference. None of the s.c. sites of injection were preferred over the other (thigh: N = 93 (80.6% [95% CI 72.6, 88.7]); AW: N = 89 (86.5% [95% CI 79.4, 93.6]), p = 0.322; odds ratio (OR) 1.54 [95% CI 0.69–3.42], p = 0.288). 23 patients (10.5%) (thigh N = 15; AW N = 8) had no preference regarding s.c. or i.v. treatment and in 14 patients (6.4%) the answers were missing. In univariate logistic regression analyses the expected preferences given in PINT1 (i.v./no preference versus s.c.) showed a significant influence on the preference of the application site given in PINT2 (OR 3.04 [95% CI 1.36–6.78]; p = 0.007, Fig. 3). Shorter administration time was the main reason for preferring s.c. over i.v. administration. Both ways of s.c. injections led to a high rate of acceptance compared to the i.v. administration (overall N = 149, 82.3%; AW N = 74, 84.1%; thigh N = 75, 80.6%; p = 0.014). The s.c. injections caused less pain in the AW than in the thigh (p = 0.01) and irritations around the s.c. injection site was more often a problem in the thigh than in the AW (p = 0.033) (Table 3).
Fig. 3.
Forest Plot of univariate logistic regressions for patient preference to s.c. versus i.v./no preference trastuzumab, CT appt, chemotherapy appointments (place where the chemotherapy was applied); i.v., intravenous; PINT1, patient interview at timepoint 1 = 20 to −1 days prior to randomization; s.c., subcutaneous.
Table 3.
Summary of analysis on PINT2 regarding patient preference of s.c. versus i.v. trastuzumab.
| Parameter | Category | Thigh N = 110 N (%) |
Abdominal wall N = 109 N (%) |
Overall N = 219 N (%) |
p-value |
|---|---|---|---|---|---|
| In how far were irritations around the s.c. injection site a problem for you? | very | 2 (2.2) | 0 (0.0) | 2 (1.1) | 0.033 |
| a bit | 23 (24.7) | 11 (12.4) | 34 (18.7) | ||
| never | 68 (73.1) | 78 (87.6) | 146 (80.2) | ||
| missing | 17 | 20 | 37 | ||
| How often were the reactions a problem for you? | only at the first and/or second administration | 12 (54.5) | 3 (20.0) | 15 (40.5) | 0.036 |
| in most of the administrations | 10 (45.5) | 12 (80.0) | 22 (59.5) | ||
| missing | 88 | 94 | 182 | ||
| How would you generally describe the s.c. administrations? | very uncomfortable | 1 (1.1) | 7 (8.0) | 8 (4.4) | 0.014 |
| a bit uncomfortable | 17 (18.3) | 7 (8.0) | 24 (13.3) | ||
| acceptable | 75 (80.6) | 74 (84.1) | 149 (82.3) | ||
| missing | 17 | 21 | 38 | ||
| Which method of administration caused the least pain? | i.v. | 20 (21.5) | 11 (12.4) | 31 (17.0) | 0.010 |
| s.c. | 19 (20.4) | 36 (40.4) | 55 (30.2) | ||
| no difference | 54 (58.1) | 42 (47.2) | 96 (52.7) | ||
| missing | 17 | 20 | 37 | ||
| Which method of administration caused less pain due to bruises around the injection site? | i.v. | 13 (14.0) | 5 (5.7) | 18 (9.9) | 0.083 |
| s.c. | 9 (9.7) | 15 (17.0) | 24 (13.3) | ||
| no difference | 71 (76.3) | 68 (77.3) | 139 (76.8) | ||
| missing | 17 | 21 | 38 |
s.c., subcutaneous; i.v. intravenous.
3.3. Safety and compliance
The study compliance was comparable between arms. The number of patients experiencing treatment related adverse events (AEs) of any grade (thigh N = 81 (73.6%); AW: N = 80 (73.4%); p = 1.000) or high-grade (3–4) (thigh N = 10 (9.1%); AW: N = 15 (13.8%); p = 0.296) were comparable between arms. Treatment related haematological toxicity any grade (thigh N = 8 (7.3%); AW N = 3 (2.8%); p = 0.215), high-grade (thigh N = 0 (0%); AW N = 1 (0.9%); p = 0.498) as well as treatment related non-haematological toxicity any grade (thigh N = 81 (73.6%); AW N = 80 (73.4%); p = 1.000) and high-grade (thigh N = 10 (9.1%); AW N = 14 (12.8%); p = 0.396) were also comparable (Table 4). Local side reactions (any grade thigh N = 21 (19.1%); AW N = 18 (16.5%); p = 0.724) did not differ between arms. No fatal AE was reported.
Table 4.
Summary of the most frequent adverse events per patients.
| AE | Grade | Thigh N (%) |
Abdominal wall N (%) |
Overall N (%) |
p-value |
|---|---|---|---|---|---|
| Any AE | any | 107 (97.3) | 108 (99.1) | 215 (98.2) | 0.622 |
| 3–4 | 24 (21.8) | 27 (24.8) | 51 (23.3) | 0.634 | |
| Any hematological AE | any | 99 (90.0) | 99 (90.8) | 198 (90.4) | 1.00 |
| 3–4 | 8 (7.3) | 11 (10.1) | 19 (8.7) | 0.483 | |
| Anemia | any | 84 (76.4) | 76 (69.7) | 160 (73.1) | 0.289 |
|
3–4 |
0 (0.0) |
1 (0.9) |
1 (0.5) |
0.498 |
|
| Leukopenia | Any | 86 (78.2) | 85 (78.0) | 171 (78.1) | 1.00 |
|
3–4 |
4 (3.6) |
5 (4.6) |
9 (4.1) |
0.748 |
|
| Any non-hematological AE | any | 101 (91.8) | 102 (93.6) | 203 (92.7) | 0.796 |
| 3–4 | 17 (15.5) | 21 (19.3) | 38 (17.4) | 0.480 | |
| Increased ASAT | any | 27 (24.5) | 26 (23.9) | 53 (24.2) | 1.00 |
| 3–4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | n.a. | |
| Increased ALAT | any | 34 (30.9) | 33 (30.3) | 67 (30.6) | 1.00 |
| 3–4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | n.a. | |
| Fatigue | any | 49 (44.5) | 51 (46.8) | 100 (45.7) | 0.787 |
| 3–4 | 3 (2.7) | 0 (0.0) | 3 (1.4) | 0.247 | |
| Peripheral neuropathy | any | 54 (49.1) | 53 (48.6) | 107 (48.9) | 1.000 |
|
3–4 |
4 (3.6) |
4 (3.7) |
8 (3.7) |
1.000 |
|
| Hypersensitivity | any | 3 (2.7) | 9 (8.3) | 12 (5.5) | 0.083 |
| 3–4 | 0 (0.0) | 1 (0.9) | 1 (0.5) | 0.498 | |
| Serious adverse event | 10 | 9 | 19 | n.a. |
AE, adverse event; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase.
The LVEF did not significantly change along the study according to the administration site. Two patients in the thigh group and one in the AW group experienced a LVEF between 40% and 50% or a decrease of ≥10% since baseline measurement (p = 0.594).
96 (43.8%) patients stopped study treatment early (thigh N = 50, 45.5%; AW N = 46, 42.2%; p = 0.683), 71 (32.4%) of those patients (thigh N = 36, 32.7%; AW N = 35, 32.1%) discontinued therapy on investigator's discretion, typically because the overall number of 18 foreseen administrations of trastuzumab were already achieved (including trastuzumab i.v. treatment in the main study). One patient in each arm (overall 0.9%) discontinued early due to progress; 8 (4 in each arm; overall 3.7%) due to AEs (3 patients due to cardiac toxicity and 4 due to non-haematological toxicity, one patient with no reason given), and 15 patients (thigh N = 9, 8.2%; AW N = 6, 5.5%; overall 6.8%) wished to stop participating. A delay of s.c. administration was observed in 200 patients (thigh N=101, 91.8%; AW N=99, 90.8%), 100 of them due to organizational reasons (thigh N = 48, 47.5%; AW N = 52, 52.5%). No delay occurred due to haematological toxicity but in two patients due to cardiac toxicity (one in each group).
4. Discussion
This is the first study investigating pharmacokinetics, patients' preferences and safety of different body areas of administration of s.c. trastuzumab. As there is a variety of different s.c. drugs on the market which are all approved for AW injections, further investigation of the safety and patients' preference regarding the injection into the AW compared to the thigh seemed appropriate.
The bioavailability of the s.c. trastuzumab as reflected by peak and total exposure measured in cycle seven was approximately 30% higher in patients receiving the injection into the thigh compared to the AW. The two investigated sites of administration can therefore not be seen as equivalent. The PK parameters trastuzumab s.c. administered into the thigh were in line with those from the HannaH study [7]. The body mass index had no influence on the PK parameters supporting the fixed dosing of s.c. trastuzumab.
This analysis confirmed the results previously demonstrated by others [7,8,10,11] regarding the preference of the s.c. over the i.v. administration. The main reason of preferring the s.c. administration was the shorter administration time which is also in line with other reports [10,12,16]. However, we investigated in addition the preference of patients regarding different s.c. injection sites, which has not been done before. Overall, the patients' preference between s.c. injection into the thigh or the AW was comparable.
The expected preferences given at study start (PINT1; i.v./no preference versus s.c.) seemed to be the most significant influence on the patient's preference of the application site along the treatment duration (PINT2). A similar correlation has also been reported in the MetaspHer study [12].
The combination of trastuzumab and pertuzumab is standard of care in patients with HER2-positive BC. The non-inferiority, safety [15] and patients' preference [16] of the s.c. administration as a fixed dose combination have been investigated. Despite the combination of two drugs as one single s.c. injection with a higher volume, patients prefer the s.c. administration. The studies showed comparable safety and efficacy results, which was consistent to previous studies with only a single drug injection.
The reported toxicity were consistent with the known safety profiles of trastuzumab [23]. The site of administration did not lead to a difference in the reported AEs and were in line with previous reports [[7], [8], [9],11,12].
Limitations of our study are that no cross-over design was used and that the number of patients satisfying criteria for the per-protocol set were different in the groups. Nevertheless, significant differences were detected within the cohort and the analysis was adequately powered for this objective.
In conclusion, trastuzumab s.c. was preferred over i.v. injection and well tolerated regardless of the site of subcutaneous administration. No new safety signals were reported. The s.c. injection of trastuzumab into the thigh led to a higher bioavailability and should therefore be kept as the standard injection site.
Support
The GAIN-2 study was supported by Amgen and BMS (Celgene). The substudy on s.c. trastuzumab was financially supported by F. Hoffman-La Roche.
Role of funding sources
The funders had no role in the collection, analysis, or interpretation of the data, and had no access to the study data. The study statisticians (JR, VN) had access to the raw data. The report was reviewed by all authors. The sponsor (GBG Forschungs GmbH) has developed the study design and written the report together with the members of the neoadjuvant/adjuvant subboard of the German Breast Group (GBG) and AGO-B. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contribution
This substudy was designed by the members of the neoadjuvant/adjuvant subcommittee of the GBG (MR, MU, CJ, TR, FM, SSch, MS, BVS, WJ, CD, LM, ES, VM and SL). The substudy resources and data collections were provided by MR, RM, TH, MA, H-JL, MS, EL, PK, TG, ES and NK. The data were analysed by NB and JR. MR and SL had full access to all data in the substudy and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors interpreted the data. The first draft of the manuscript was written by MR. The decision to submit the manuscript for publication was made by all the authors. All authors contributed to the review of the manuscript. No persons other than the listed authors contributed to the writing of the manuscript.
Declaration of competing interest
MR reports travel support from AstraZeneca, Celgene, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, Seagen, Somatex and Roche. MU reports personal fees for lectures and/or consultancy from Abbvie, Amgen, Astra Zeneca, BMS, Celgene, Daiji Sankyo, Gilead, GSK, Lilly, MSD Merck, Mundipharma, Myriad Genetics, Novartis, Pierre Fabre, Pfizer, Roche, Sanofi Aventis, Saegen.
MS reports personal fees from AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Roche, and SeaGen outside the submitted work. Institutional research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre Fabre, and SeaGen; has a patent for EP 2390370 B1 issued and a patent for EP 2951317 B1issued. SL reports other from Amgen, other from BMS, grants and other from Celgene, grants, non-financial support and other from Roche, during the conduct of the study; grants and other from Abbvie, grants and other from AstraZeneca, other from Eirgenix, other from GSK, grants, non-financial support and other from Gilead, other from Lilly, other from Merck, grants, non-financial support and other from Novartis, grants, non-financial support and other from Pfizer, other from Pierre Fabre, non-financial support and other from Seagen, grants, non-financial support and other from Daiichi-Sankyo, other from Sanofi, outside the submitted work; In addition; has a patent EP14153692.0 pending, a patent EP21152186.9 pending, a patent EP15702464.7 issued, a patent EP19808852.8 pending, and a patent Digital Ki67 Evaluator with royalties paid. ES reports honoraria from Pfizer, Roche, AstraZeneca, MSD, Gilead, Daiichi-Sankyo, Novartis, Seagen, Lilly, Pierre Fabre. SaS reports grants and non-financial support from F. Hoffman-La Roche, grants from BMS (Celgene), Amgen, during the conduct of the study; personal fees from Abbvie, outside the submitted work. CJ reports Honoraria from Amgen, AstraZeneca, Roche, Lilly, Novartis, Pfizer, Exact Sciences, Pierre-Fabré, Molecular Health. FM reports personal fees from Roche, AstraZeneca, Pfizer, Tesaro, Novartis, Amgen, PharmaMar, GenomicHealth, CureVac, EISAI outside the submitted work. CD reports stock and other ownership interest from Sividon Diagnostics (until 2016); consulting or advisor role from MSD Oncology, Daiichi Sankyo, Molecular Health, AstraZeneca, Merck, Roche, Lilly; research funding from Myriad Genetics, Roche; patents, royalties or other intellectual property from VMScope digital pathology software, patent applications WO2015114146A1 and WO2010076322A1-therapy response, patent application WO2020109570A1-immunotherapy. No other potential conflict of interest relevant to this article was reported.
Acknowledgements
We would like to thank all patients, investigators and study personnel who supported the study, and Dr. Bianca Lederer and Dr. Valentina Vladimirova for editorial assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.10.002.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Lurie R.H., Anderson B.O., Abraham J., Aft R., Agnese D., Allison K.H., et al. 2020. NCCN guidelines version 3.2020 breast cancer. [DOI] [Google Scholar]
- 2.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., et al. 2019. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. [DOI] [PubMed] [Google Scholar]
- 3.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384 doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 4.von Minckwitz G., Untch M., Blohmer J.-U., Costa S.D., Eidtmann H., Fasching P.A., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 5.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389 doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 6.Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M., Goldhirsch A., de Azambuja E., et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389 doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismael G., Hegg R., Muehlbauer S., Heinzmann D., Lum B., Kim S.B., et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 8.Jackisch C., Stroyakovskiy D., Pivot X., Ahn J.S., Melichar B., Chen S.-C., et al. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol. 2019;5 doi: 10.1001/jamaoncol.2019.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pivot X., Verma S., Fallowfield L., Müller V., Lichinitser M., Jenkins V., et al. Efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab as part of adjuvant therapy for HER2-positive early breast cancer: final analysis of the randomised, two-cohort PrefHer study. Eur J Cancer. 2017;86:82–90. doi: 10.1016/j.ejca.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Pivot X., Gligorov J., Müller V., Barrett-Lee P., Verma S., Knoop A., et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14:962–970. doi: 10.1016/S1470-2045(13)70383-8. [DOI] [PubMed] [Google Scholar]
- 11.Gligorov J., Ataseven B., Verrill M., De Laurentiis M., Jung K.H., Azim H.A., et al. Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2-positive early breast cancer: SafeHer phase III study's primary analysis of 2573 patients. Eur J Cancer. 2017;82:237–246. doi: 10.1016/j.ejca.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Pivot X., Spano J.P., Espie M., Cottu P., Jouannaud C., Pottier V., et al. Patients' preference of trastuzumab administration (subcutaneous versus intravenous) in HER2-positive metastatic breast cancer: results of the randomised MetaspHer study. Eur J Cancer. 2017;82:230–236. doi: 10.1016/j.ejca.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Zambetti M., Montemurro F., Morandi P., Zamagni C., Brandes A.A., Bisagni G., et al. Safety profile of subcutaneous trastuzumab for the treatment of patients with HER2-positive early or locally advanced breast cancer: primary analysis of the SCHEARLY study. Eur J Cancer. 2018;105:61–70. doi: 10.1016/j.ejca.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Pimentel F.F., Morgan G., Tiezzi D.G., de Andrade J.M. Development of new formulations of biologics: expectations, immunogenicity, and safety for subcutaneous trastuzumab. Pharmaceut Med. 2018;32:319–325. doi: 10.1007/s40290-018-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan A.R., Im S.-A., Mattar A., Colomer R., Stroyakovskii D., Nowecki Z., et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021;22:85–97. doi: 10.1016/S1470-2045(20)30536-2. [DOI] [PubMed] [Google Scholar]
- 16.O'Shaughnessy J., Sousa S., Cruz J., Fallowfield L., Auvinen P., Pulido C., et al. 165MO Patient (pt) preference for the pertuzumab-trastuzumab fixed-dose combination for subcutaneous use (PH FDC SC) in HER2-positive early breast cancer (EBC): primary analysis of the open-label, randomised crossover PHranceSCa study. Ann Oncol. 2020;31:S306–S307. doi: 10.1016/j.annonc.2020.08.287. [DOI] [Google Scholar]
- 17.Möbus V., Lück H.-J.J., Ladda E., Klare P., Schmidt M., Schneeweiss A., et al. Phase III randomised trial comparing intense dose-dense chemotherapy to tailored dose-dense chemotherapy in high-risk early breast cancer (GAIN-2) Eur J Cancer. 2021;156:138–148. doi: 10.1016/j.ejca.2021.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Möbus V., Mahlberg R., Janni W., Tomé O., Marmé F., Forstbauer H., et al. Abstract P5-20-09: pharmacokinetic results of a subcutaneous injection of trastuzumab into the thigh versus into the abdominal wall in patients with HER2-positive primary breast cancer (BC) treated within the neo-/adjuvant GAIN-2 study. Poster Session Abstracts, American Association for Cancer Research. 2018 doi: 10.1158/1538-7445.SABCS17-P5-20-09. P5-20-09-P5-20–09. [DOI] [Google Scholar]
- 19.Moebus V., Noeding S., Ladda E., Klare P., Schmidt M., Schneeweiss A., et al. Neo-/adjuvant phase III trial to compare intense dose-dense (idd) treatment with EnPC to tailored dose-dense (dt) therapy with dtEC-dtD for patients with high-risk early breast cancer: results on pathological complete response (pCR) for patients treated within the neoadjuvant setting. J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.568. 568–568. [DOI] [Google Scholar]
- 20.Reinisch M., Untch M., Reimer T., Mahlberg R.J.C., Aydogdu M., Hitschold T., et al. 86P Patients (pts) preference for different administration methods of trastuzumab (T) in pts with HER2+ early breast cancer (BC) treated within the GAIN-2 trial. Ann Oncol. 2020;31:S44. doi: 10.1016/j.annonc.2020.03.026. [DOI] [Google Scholar]
- 21.Fallowfield L., Osborne S., Langridge C., Monson K., Kilkerr J., Jenkins V. Implications of subcutaneous or intravenous delivery of trastuzumab; further insight from patient interviews in the PrefHer study. Breast. 2015;24:166–170. doi: 10.1016/j.breast.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Theodore-Oklota C., Humphrey L., Wiesner C., Schnetzler G., Hudgens S., Campbell A. Validation of a treatment satisfaction questionnaire in non-Hodgkin lymphoma: assessing the change from intravenous to subcutaneous administration of rituximab. Patient Prefer Adherence. 2016;10:1767–1776. doi: 10.2147/PPA.S108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianni L., Eiermann W., Semiglazov V., Lluch A., Tjulandin S., Zambetti M., et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15 doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.