Abstract
The objective of this study was to investigate whether the superantigen staphylococcal enterotoxin A (SEA), which binds to HLA class II and T-cell receptor Vβ chains, can direct cytotoxic T cells to lyse cytokine-stimulated endothelial cells (EC). In addition, we wanted to determine whether SEA-primed cytotoxic T cells could be targeted to EC surface molecules as a means of a novel cancer immunotherapy. Human umbilical vein EC (HUVEC), dermal microvascular EC (HMVEC), or the EC line EA.hy926 stimulated with gamma interferon (IFN-γ) or tumor necrosis factor alpha (TNF-α) displayed upregulated HLA class II and adhesion molecule (CD54 and CD106) expression, respectively. SEA-primed T cells induced a strong cytotoxicity against IFN-γ- and TNF-α-activated EA.hy926 which had been preincubated with SEA. Blocking of CD54 completely abrogated the T-cell attack. SEA-D227A, which has a mutated class II binding site, did not promote any cytotoxicity. A strong lysis was observed when a fusion protein consisting of protein A and SEA-D227A was added together with T cells to TNF-α-induced EA.hy926 and HUVEC precoated with monoclonal antibodies (MAb) directed against HLA class I, CD54, or CD106 molecules. Finally, an scFv antibody fragment reactive with an unknown EC antigen was fused with SEA-D227A. Both EA.hy926 and HMVEC were efficiently lysed by scFv-SEA-D227A-triggered cytotoxic T cells. Taken together, superantigen-activated T-cell-dependent EC killing was induced when EC expressed an inflammatory phenotype. Moreover, specific MAb targeting of the superantigen to surface antigens induced EC lysis. Our data suggest that directed T-cell-mediated lysis of unwanted proliferating EC, such as those in the tumor microvasculature, can be clinically useful.
Endothelial cells (EC) line the blood vessels and form a barrier between blood components and the tissues; they also play a crucial role in inflammatory responses, immune reactions, and vascular hemostasis (24). The cytokines interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) are secreted by leucocytes in response to various microorganisms during the early phase of an inflammatory response. This results in the activation of EC and production of autacoids, including prostanoids, platelet-activating factor, and nitric oxide. Activated EC display an increased cell surface expression of adhesion molecules, such as E-selectin (CD62E), ICAM-1 (CD54), PECAM-1 (CD31), and VCAM-1 (CD106), which facilitate the extravasation of leukocytes from the microvasculature to inflammatory sites in the peripheral tissues (14, 21). Increased concentrations of gamma interferon (IFN-γ) are also detected during the later stages of an inflammatory response and may result in the induction of HLA class II surface expression, upregulation of HLA class I density, and enhanced peptide transport capacity in EC (6, 23). These phenotypic changes allow EC to serve as antigen-presenting cells (APC) and suggest that EC plays an active role during several phases of an immune response.
Certain strains of Staphylococcus aureus produce immunostimulatory exotoxins, such as toxic shock syndrome (TSS) toxin 1, staphylococcal enterotoxin A (SEA), SEB, and SEC, all of which are associated with food poisoning and TSS (for a review, see reference 31). These exotoxins have been denominated superantigens (SAg) due to their ability to activate a high frequency of T lymphocytes. SAg bind as unprocessed proteins to HLA class II molecules on APC and oligoclonally activate T cells expressing particular T-cell receptor Vβ chains (25). In vivo exposure to excessive amounts of SAg results in a strong cytokine production, including IL-2, TNF-α, and IFN-γ, which are associated with a toxic shock-like syndrome (15, 27, 34).
Interestingly, SAg binds to not only professional APC but also to other HLA class II-bearing cells, such as activated human umbilical vein EC (HUVEC) (37). It has been demonstrated that bacterial SAg efficiently bind HLA class II-positive, activated EC and subsequently trigger human T cells to proliferate and produce cytokines (2, 17). SAg- and EC-induced T-cell activation appears to be strongly inhibited by monoclonal antibodies (MAb) to CD2, CD11a, CD28, ICAM-1, and VCAM-1, suggesting that multiple adhesion pathways contribute to EC–T-cell interactions (17).
In the present study, we show that the SAg SEA was able to induce T-cell-directed cytotoxicity against activated HLA class II-positive EC (SAg-dependent cellular cytotoxicity [SDCC]). SEA-directed cytotoxic T lymphocytes (CTL) efficiently lysed established HLA class II-positive EC lines as well as primary HUVEC and human microvascular endothelial cells (HMVEC). In addition to the SDCC against EC, we demonstrate that attenuated and mutated SEA proteins that fail to bind HLA class II proteins, can be linked to EC-reactive MAb, and target CTL to lyse EC. An scFv-SEA chimeric protein, which is selectively reactive to activated EC, may have a therapeutic potential for inhibition of pathological vascular growth, such as neoangiogenic processes in solid tumors.
MATERIALS AND METHODS
Cells and reagents.
The EA.hy926 cell line was obtained from F. Lupu (Thrombosis Research Institute, London, United Kingdom) (11). The immortalized cell line was maintained in RPMI 1640 (Gibco-BRL, Paisley, United Kingdom) supplemented with gentamicin (12 μg/ml), l-glutamine, and 10% fetal calf serum. Primary HUVEC and dermal HMVEC were obtained from Biowhittaker (Walkersville, Md.) and grown in media as specified by the supplier. All EC except ECV304 were found to be positive for CD31 as revealed by flow cytometry analysis. The cytokines IL-2, TNF-α, and IFN-γ were purchased from Genzyme (Cambridge, Mass.).
Human peripheral blood lymphocytes were isolated from buffy coats with citrate by centrifugation on a step gradient of Ficoll-Isopaque (Lymphoprep; Pharmacia, Uppsala, Sweden). SEA-reactive T-cell lines were established by stimulation of 2 × 106 cells/ml with mitomycin-treated, SEA-coated, B-cell lymphoma cells as previously described (8). The T-cell lines were restimulated every second week with 20 U of human recombinant IL-2 per ml and weekly with mitomycin-treated B-cell lymphoma cells. T cells were maintained in RPMI 1640 medium with additives as described above. All cells were kept at 37°C in a humidified atmosphere of 5% CO2–95% air.
SAg expression and purification.
Wild-type SEA and SEA-D227A were expressed in Escherichia coli K-12 strain UL635 and purified as previously described (1). To allow binding of SEA molecules to immunoglobulin G (IgG)-coated EC; we produced a fusion protein consisting of protein A and SEA by linking the carboxy terminus of the protein A fragment to the amino terminus of SEA or SEA-D227A. The gene fragment encoding the IgG-binding Z regions was isolated from the protein A gene, and two repeat units of the Z domain were fused to the SEA gene. The protein A-SEA fusion gene was driven by a protein A promoter, and secretion was directed by a protein A signal sequence (28). In addition, a transcription terminator was introduced following the expression cassette. The gene fragments were assembled in pUc, and a kanamycin gene was included in the plasmid pKP1120 to replace the original amp gene (28). The fusion gene was expressed in E. coli K-12 strain UL635, and the secreted protein was applied to an anti-SEA IgG affinity column and eluted at a low pH. The protein A-SEA and protein A-SEA-D227A proteins were obtained at >95% purity as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The scFv antibody K594 was selected by melanoma tissue-based selection from an antibody phage library generated from Cynomolgus monkeys immunized with human metastatic melanoma tissue (36). Vascular reactivity was confirmed by immunohistochemistry, and the scFv gene was subcloned into an expression vector containing SEA-D227A. Recombinant protein was produced as described and purified by affinity chromatography (1).
Flow cytometry and antibodies.
Mouse anti-human ICAM-1, VCAM-1, HLA class I, and HLA-DR MAb were from Dakopatts (Glostrup, Denmark). Fluorescein isothiocyanate-conjugated swine anti-rabbit Ig and sheep anti-mouse Ig were used as detection antibodies, and mouse IgG1 and mouse IgG2a MAb were included as negative controls (Dakopatts). EC were stained according to standard protocols and analyzed in a FACSort flow cytometry device (Becton Dickinson, San Jose, Calif.).
Cytotoxic 51Cr release assay.
Cytotoxicity was measured in a standard 4-h 51Cr release assay (7) using EC and the human B-lymphoma cell line Raji (positive control) as target cells. Briefly, 51Cr-labeled target cells (2,500 cells/200 μl) were incubated in complete medium in V-bottom 96-well microtiter plates. Effector cells were added at an effector-to-target (E:T) cell ratio of 40:1. SEA, SEA-D227A, or K594ScFv-SEA-D227A was added at various concentrations as indicated, and 51Cr release was measured in a gamma counter. The percentage specific cytotoxicity was calculated as 100 × [(counts per minute for experimental release − counts per minute for background release)/(counts per minute for total release − counts per minute for background release)].
RESULTS
The HUVEC-derived EC line EA.hy926 displays an inflammatory phenotype when induced by cytokines.
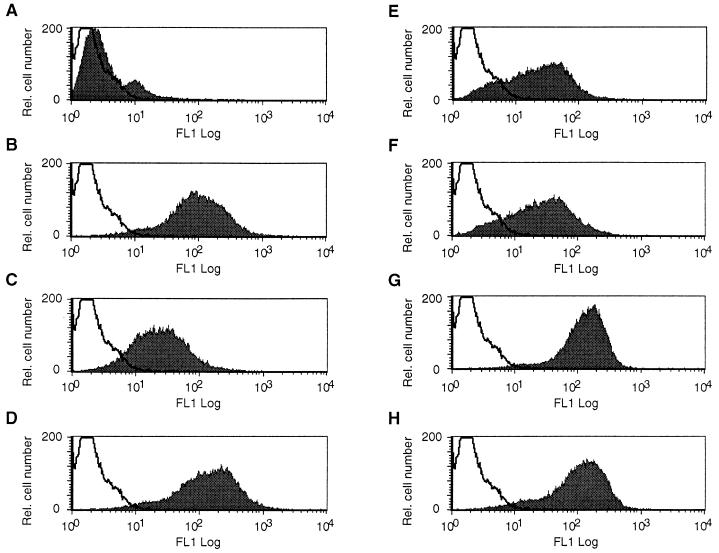
Activated EC express HLA class II and are thus able to bind SAg and present these molecules to T cells. To mimic an inflammatory phenotype, the HUVEC-derived EC line EA.hy926 was activated with either TNF-α, IFN-γ, or a combination of the two cytokines. In addition to the adhesion molecules ICAM-1 (CD54) and VCAM-1 (CD106), HLA class I and II were analyzed by flow cytometry. When EA.hy926 was incubated with TNF-α for 18 h, a 10-fold increase in CD54 expression was detected compared to untreated control cells (Fig. 1A and B). After 48 h of IFN-γ treatment, a 3-fold increase in CD54 density was measured, while a further significant upregulation (12-fold) was observed when TNF-α was added in combination with IFN-γ (Fig. 1D). In contrast, TNF-α and IFN-γ stimulation only slightly increased CD106 expression above background levels (1.8-fold; FACSort profile not shown) and no significant CD106 upregulation was observed when TNF-α or IFN-γ was added separately compared to control cultures.
FIG. 1.
Cytokine activation increases CD54 and HLA class II expression. Flow cytometry profiles are shown for ICAM-1 (CD54) (A to D) and HLA class II (E to H). Unstimulated EA.hy926 (A and E) were compared to cultures activated with TNF-α (B and F), IFN-γ (C and G), or TNF-α and IFN-γ in combination (D and H). EC were treated with recombinant IFN-γ (100 U/ml) and/or TNF-α (250 U/ml) for 48 and 18 h, respectively. Cytokine induction was followed by staining with mouse MAb directed against CD54 or HLA class II for 30 min on ice. A fluorescein isothiocyanate-conjugated sheep anti-mouse polyclonal antibody was added as a secondary layer, and specific fluorescence was analyzed by fluorescence-activated cell sorting. FL1 Log, log fluorescence; Rel., relative.
Cytokine treatment also increased expression of HLA class I on EA.hy926 cells. When the EC line was incubated with TNF-α or IFN-γ, HLA class I levels were increased 1.2- and 1.3-fold, respectively, compared to untreated controls (data not shown). Up to 1.8-fold more HLA class I was detected when cells were grown in a combination of the two cytokines. As expected, HLA class II expression was strongly enhanced in the presence of IFN-γ (Fig. 1G), while TNF-α activation failed to increase HLA class II density (Fig. 1F).
SEA-induced T-cell-dependent cytotoxicity against cytokine-activated EC.
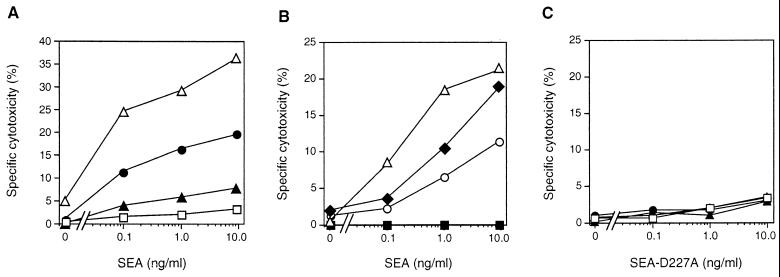
SAg such as SEA bind to HLA class II on APC and direct CTL bearing particular T-cell-receptor Vβ families. To investigate the sensitivity of HLA class II-positive EC to SAg-targeted CTL, activated EA.hy926 loaded with 51Cr were incubated with SEA-primed human T cells and the SAg SEA. As can be seen in Fig. 2A, SEA induced a dose-dependent and specific SDCC against HLA class II-positive EA.hy926 cells that had been preactivated with IFN-γ with or without supplementation of TNF-α. A moderate cytotoxicity (20%) was observed after IFN-γ stimulation, while combined IFN-γ and TNF-α activation induced a strong SDCC (37%). In contrast, TNF-α alone induced only a low sensitivity to SEA-directed CTL (6%).
FIG. 2.
Cytokine-treated EC were lysed by SEA-primed T cells, and cytotoxicity was inhibited by specific MAb. (A) SDCC against EA.hy926 cells activated with TNF-α (▴), IFN-γ (•), or a combination of the two cytokines (▵) was compared to that against control cells in the absence of stimulation (□). (B) Cytokine (TNF-α and IFN-γ)-activated EA.hy926 cells were preincubated with MAb (20 μg/ml) directed against CD54 (■) or CD31 (○) and compared to an isotype-matched control MAb (CD10; ⧫). Control cells without any MAb addition were also included (▵). (C) Data are presented from SDCC experiments with the SEA-D227A mutant, which displays a low binding to HLA class II. Symbols are as given for panel A. For panels A and C, EC were treated with IFN-γ and/or TNF-α as indicated in the legend to Fig. 1. EA.hy926 was loaded with 51Cr, and a SEA-reactive T-cell line was used as a source of effector cells at an E:T ration of 40:1. SEA was used at 0.1 to 10 ng/ml. For all panels, the data presented are representative of at least three separate experiments.
To dissect the role of the different costimulatory molecules involved in SDCC, we pretreated EA.hy926 cells with TNF-α and IFN-γ and included antibodies directed against CD31 and CD54 in the CTL assay. SDCC was completely abrogated by CD54-reactive MAb (Fig. 2B), while blocking of CD31 only partly decreased the SEA-induced cytotoxicity compared to the CD10 control antibody. Finally, to confirm that the SEA-dependent effects were caused by HLA class II binding, the SEA-D227A molecule was included in the study. SEA-D227A contains a mutated class II binding site and has been shown to have an impaired affinity for HLA class II and consequently fails to activate T cells (1). SEA-D227A-induced SDCC was less than 5%, proving that the SEA-induced cytotoxicity was indeed specifically due to HLA class II binding (Fig. 2C). Taken together, the results show that the SAg SEA that is synthesized by certain strains of staphylococci, is a powerful inducer of cytotoxic responses against HLA class II (and CD54)-expressing EC.
MAb targeting of SEA-D227A to EC promotes T-cell-mediated cytotoxicity.
We have previously shown that antibody-targeted SAg reduce the majority of metastases in tumor-bearing mice and that this is mainly due to the cytotoxic killing of tumor cells (30). However, although the treatment is very efficient, a complete cure has not been achieved. Since blood supply is crucial for all tumors, targeting and lysis of EC would be beneficial to tumor treatment. To reduce nonspecific HLA class II binding, the mutant SEA-D227A was chosen and linked to staphylococcal protein A (SpA). The resulting SpA-SEA-D227A chimeric protein made it possible to target the mutated SEA to EC coated with specific MAb directed against cell surface molecules.
To investigate SpA-SEA-D227A-induced cytotoxicity, EA.hy926 cells were loaded with 51Cr and incubated with an anti-HLA class I MAb for 30 min, followed by the addition of SpA-SEA-D227A. After 4 h of incubation with SEA-primed T cells, released 51Cr was measured and the specific cytotoxicity was calculated. Interestingly, up to 20% of the SAg antibody-dependent cellular cytotoxicity (SADCC) was induced against EA.hy926 cells when SpA-SEA-D227A was targeted to HLA class I molecules (Fig. 3A). In contrast, when EC were preactivated overnight with TNF-α, more than 40% of the EC were lysed. To determine the cytotoxic efficacy, cells (EA.hy926) were incubated at different E:T ratios with a fixed concentration of SpA-SEA-D227A. A dose-dependent increase in cytotoxicity was observed both when unstimulated or TNF-α-activated EA.hy926 cells were used (Fig. 3B). No activity was observed in the absence of anti-HLA class I MAb, indicating the importance of cell surface targeting.
FIG. 3.
SpA-SEA-D227A-induced CTL lyse MAb-coated EC. (A) A strong SADCC was induced by SpA-SEA-D227A targeted to HLA class I-expressing EA.hy926 cells that had been preactivated in the presence of TNF-α (▵) compared to unstimulated controls (■). (B) E:T ratios are shown for SADCC induced by SpA-SEA-D227A (1.0 ng/ml) by using anti-HLA class I-coated target cells with (▵) and without (■) TNF-α preactivation. (C) Cytotoxicity was induced when SpA-SEA-D227A was targeted to the cell surface by using anti-CD54 MAb. TNF-α-activated EA.hy926 cells (⧫ and ▵) were compared to unstimulated cells (■ and ○). The results of two separate experiments are indicated. Experimental procedures were as indicated in the legend to Fig. 2. In panels A and B, the results of one out of three representative experiments are shown.
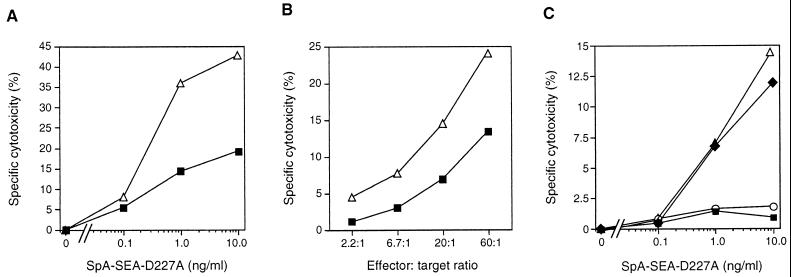
In another set of experiments, EA.hy926 cells were activated with TNF-α and incubated with a MAb against CD54, this was followed by the addition of SpA-SEA-D227A and SEA-primed T cells. Significant SADCC was observed at an SpA-SEA-D227A concentration of ∼1 ng/ml (Fig. 3C). Unstimulated cells did not bind any anti-CD54 MAb, and consequently no specific cytotoxicity was measured. Thus, targeting SEA-D227A to surface molecules on the immortalized HUVEC cell line EA.hy926 promoted cytotoxic T-cell lysis of EC.
Primary derived EC are lysed by SAg-directed CTL.
To confirm our findings in primary cell systems, freshly prepared HUVEC were stimulated with TNF-α for 18 h and analyzed for CD54 and CD106 expression by flow cytometry (Fig. 4A to D). The expression levels for CD54 and CD106 increased 13- and 9.5-fold, respectively, compared to unstimulated controls. In contrast to EA.hy926, HUVEC displayed higher basal levels of CD54 in the absence of prior cytokine activation (compare Fig. 1A and 4A). TNF-α-treated HUVEC expressed 10% more HLA class I molecules, which was a slightly smaller increase than that seen on EA.hy926 cells (not shown).
FIG. 4.
SADCC was induced against primary EC coated with MAb directed against HLA class I or CD106. Cellular adhesion molecules CD54 (A and B) and CD106 (C and D) were upregulated on HUVEC when activated with the cytokine TNF-α (B and D) compared to unstimulated cells (A and C). (E) TNF-α-stimulated HUVEC (▵) or unstimulated cells (■) were incubated with MAb against HLA class I followed by the addition of SpA-SEA-D227A and CTL. (F) A specific SADCC was also induced against TNF-α-activated HUVEC expressing CD106 (▵) compared to unstimulated controls (■). The experimental procedure and abbreviations are outlined in the legend to Fig. 2. The results of one out of two similar experiments are shown.
In order to determine the SADCC to primary cells, SpA-SEA-D227A was targeted to HLA class I on unstimulated or activated HUVEC. Up to 20% of the EC were lysed when CTL were directed towards HLA class I on TNF-α-treated cells (Fig. 4E). In contrast, only 8% SADCC was observed when unstimulated HUVEC were used as targets. As can be seen in Fig. 4F, it was also possible to direct SpA-SEA-D227A to the VCAM-1 on activated EC by using a specific VCAM-1-reactive MAb. In these experiments, 11% lysis was obtained with activated cells. No lysis was recorded in uninduced control cultures.
An scFv-SEA-D227A fusion protein targets CTL against EC.
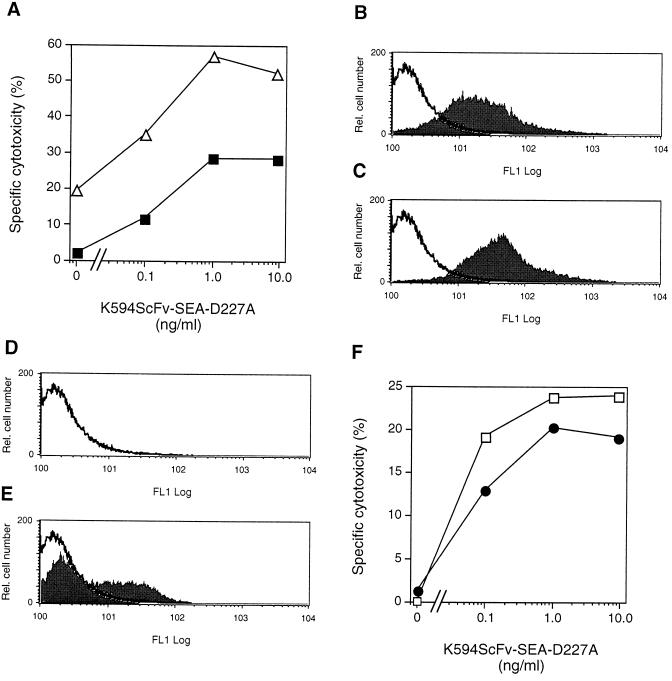
To efficiently direct CTL against EC, we constructed a recombinant fusion protein consisting of the mutant SEA-D227A and an scFv antibody fragment (K594ScFv) directed toward an unknown EC antigen. The HUVEC-derived cell line EA.hy926 was challenged with TNF-α, loaded with 51Cr, and incubated with K594ScFv-SEA-D227A in addition to SEA-primed T cells. Up to 55% cell killing was observed with the chimeric SEA mutant at 1.0 ng/ml (Fig. 5A). The corresponding value for unstimulated and consequently CD54-negative cells was 25%.
FIG. 5.
The K594ScFv-SEA-D227A fusion protein targets CTL to lyse EA.hy926 cells and primary dermal HMVEC. (A) TNF-α-activated EA.hy926 cells (▵) were incubated with K594ScFv-SEA-D227A (0.1 to 10 ng/ml) and CTL. For comparison, unstimulated EA.hy926 cells were included (■). Dermal HMVEC constitutively expressed CD54 (B), and only a slight upregulation was observed upon TNF-α activation (C). HMVEC were negative for CD106 (D) and displayed increased levels when stimulated with TNF-α (E). (F) TNF-α-activated HMVEC were incubated with CTL in the presence of K594ScFv-SEA-D227A (•) and compared to unstimulated cells (□). Experimental conditions were as described in the legend to Fig. 2. The results of one out of two representative experiments are shown.
Most tumor blood vessels are of microvascular origin. To determine the sensitivity of HMVEC to SEA-induced CTL, we treated primary HMVEC isolated from human skin with TNF-α overnight and performed a cytotoxicity assay. Interestingly, HMVEC expressed CD54 in the absence of any prior cytokine stimulation (Fig. 5B). However, a slight upregulation of CD54 was observed upon activation (Fig. 5C). In contrast, these primary cells were negative for CD106 (Fig. 5D), but exhibited an enhanced CD106 expression upon TNF-α challenge (Fig. 5E). When dermal HMVEC were challenged with K594ScFv-SEA-D227A together with CTL, a maximum lysis of 24% was recorded (Fig. 5F), however no significant difference was observed between unstimulated or TNF-α-activated cells. In addition, SEA-D227A without the K594ScFv fragment was included as a negative control. Neither unstimulated nor TNF-α-activated HMVEC were sensitive to SEA-D227A-triggered T cells, as judged by the absence of any SDCC (not shown). In conclusion, both HMVEC and the HUVEC-derived cell line EA.hy926 were lysed by CTL induced by the fusion protein K594ScFv-SEA-D227A.
DISCUSSION
Many different cytokines are secreted at inflammatory sites and are able to markedly influence the EC phenotype in a number of ways, including upregulation of adhesion molecule expression, HLA molecules, and procoagulant factors (24). In the present study, we show cytokine-stimulated EC to be sensitive to SEA-targeted CTL; however, to obtain a strong and specific EC killing, preactivation with IFN-γ and TNF-α was required. A clear pattern of induction of adhesion molecule expression was observed; TNF-α mainly increased CD54 (ICAM-1) expression on the HUVEC-derived cell line EA.hy926 but also strongly upregulated CD106 (VCAM-1) on primary HUVEC (Fig. 1 and 4). Primary skin-derived HMVEC constitutively expressed CD54, and the costimulatory molecule was only slightly upregulated in the presence of TNF-α (Fig. 5). In contrast, activation with IFN-γ induced HLA class II on EA.hy926 (Fig. 1). Interestingly, it has been shown that IFN-γ strongly enhances TNF-α-induced endothelial CD54 expression and that the two cytokines exert a synergistic effect on HLA expression (10, 18). However, our flow cytometry analyses of EA.hy926 cells activated with IFN-γ and TNF-α in combination revealed only a slight CD54 upregulation, while HLA class II density was not further increased (Fig. 1).
A significantly enhanced SDCC was induced against EC activated by a combination of IFN-γ and TNF-α compared to EC stimulated with the individual cytokines (Fig. 2A). Both a sufficient number of HLA class II molecules to promote the SEA binding and a significant density of CD54 were crucial to obtain a strong EC lysis. The importance of class II binding was further demonstrated when the mutant SEA molecule SEA-D227A, with decreased HLA class II affinity, was included in the study (Fig. 2C). Several reports exist on SAg induction of T lymphocytes, and it has been shown that S. aureus-derived SEA strongly activates T cells to produce TH1 cytokines such as IL-2, IFN-γ, and TNF-α (15, 25, 27, 33, 34). These findings together with our present data imply that cytokines secreted by SAg-activated T cells and CTL induced by SAg act in a sequential manner to activate EC and subsequently lyse EC displaying an inflammatory phenotype (consisting of HLA class II and CD54).
Blocking of CD54 with specific MAb completely abrogated the SEA-induced EC lysis compared to SDCC against EC that were precoated with an isotype-matched MAb (Fig. 2B). A key role for CD54 in T-cell-induced SAg-dependent cytotoxicity against fibroblasts and B cells has been extensively documented in previous studies (9, 13). When CD54 is blocked by specific MAb, SDCC against HLA-DR2 and CD54 expressing L-cell transfectants significantly decreases (9). Consequently, blocking of the T-cell integrin CD11a/CD18, which is the major CD54 receptor on T cells, results in up to 80% inhibition of cytotoxicity. Our EC line EA.hy926 expressed low levels of CD106 that increased slightly upon cytokine induction. In contrast to CD54, CD106 expression did not interfere with the SDCC; i.e., any additional interaction of CD106 with CTL was not detected when CD54 was blocked (Fig. 2B). However, CD106 plays an important role as a costimulatory molecule in T-cell proliferation. In an elaborate study with HUVEC as APC, the importance of CD54 and CD106 in SEB-induced T-cell proliferation has been dissected (17). When the adhesion molecules CD54 and CD106 are blocked, a pronounced decrease in proliferation is seen, confirming an important role for both adhesion molecules during interactions between SAg-activated T cells and EC. Furthermore, it has been demonstrated that lipopolysaccharide-induced and neutrophil-mediated endothelial cytotoxicity is enhanced when T lymphocytes are present and that this increase is associated with an augmented expression of both CD54 and CD106 (35).
In contrast to inhibition of the CD54-CD11a/CD18 interaction, CD31 blocking was less efficient and reduced the SDCC by only 50% (Fig. 2B). In conformation with our results, it has been reported that blockade of human CD31 by addition of MAb or a specific peptide dose dependently abrogates the mixed-lymphocyte reaction (see reference 5 and references therein). Interestingly, when CD31 is blocked, up to 50% inhibition of CTL activity is also observed. Furthermore, by using a CD31-Ig fusion protein, the proliferative response and cytokine (IL-4, IFN-γ, and TNF-α) production are successfully inhibited in the CD4-CD31 T-cell population (29). Although the CD31 ligand on T cells has not yet been isolated, murine T cells have been demonstrated to express the αvβ3 integrin, which might be a ligand for CD31 (3, 12). Thus, available data from the literature demonstrate that CD31 is important for T-cell activation but is also involved in CTL function, thus supporting our findings with the SEA-primed T cells and their EC target (Fig. 2B).
The implications of SAg-mediated EC lysis for human disease pathology are intriguing. Excessive exposure to SAg during infection by certain gram-positive bacteria may lead to a TSS that is characterized by fever, hypotension, pulmonary edema, and extravasation of leukocytes. In addition to TSS, Kawasaki syndrome is also suspected to be caused by bacterial toxins (26). In this obscure disease, a multisystem vasculitis may occur that in some cases leads to complications such as coronary artery aneurysms and ectasia (20). When examining the cellular pathophysiology in Kawasaki syndrome, a selective expansion of Vβ2-positive T cells in the peripheral blood has been observed during acute disease in most patients (19). Thus, it is tempting to suggest that EC killing by SAg-induced T cells causes tissue damage that contributes to the general pathophysiology (including vasculitis) observed in patients infected with enterotoxin-producing S. aureus.
To target an immune attack against tumor cells, we have genetically engineered tumor-reactive SAg by constructing a fusion protein between the SAg SEA and a Fab fragment reacting with colon carcinoma cells. These Fab-SEA proteins show strong antitumor effects in experimental tumor models (7, 8, 22, 33). Fab-SEA administered to mice carrying B16 melanoma lung metastases induces recruitment of pseudospecific tumor-infiltrating SEA-reactive T cells to the tumor site, resulting in the eradication of lung micrometastases (8). MAb-based therapy for a solid tumor is, however, limited by the poor penetration of antibodies. A more favorable approach for attacking tumors by protein therapeutics may be to directly target the highly accessible tumor microvasculature and thereby strangle the supply of nutrients and oxygen to the vast majority of tumor cells. Indeed, an interesting approach consisting of targeting a ricin A toxin to EC in the tumor tissue has been presented by Burrows and Thorpe (4). Recently, the same group improved the concept by directing a truncated form of the coagulation-promoting cell surface protein, tissue factor, to the tumor vasculature (16). The tissue factor-dependent activation of coagulation factors FVII and FX, which results in fibrin clot formation, appears to be an efficient strategy for inhibition of tumor growth, since complete tumor regression were observed in a large fraction of treated but not untreated mice. Thus, EC targeting and subsequent destruction of the vasculature has been proven to be an effective approach for cell-targeting tumor therapy.
To investigate SAg targeting to EC in vitro, we constructed a chimeric protein consisting of the mutant SEA-D227A fused with protein A. The resulting SpA-SEA-D227A was targeted to EC by specific MAb directed to EC surface molecules. Constitutively expressed HLA class I and inducible CD54 were chosen as targets on the HUVEC-derived EA.hy926 cell line. A strong SADCC was induced against EC when SpA-SEA-D227A was targeted to class I (Fig. 3A and B), while targeting to CD54 revealed a slightly weaker SADCC (Fig. 3C). Two possible explanations exist for the increased SADCC against HLA class I on TNF-α-activated EA.hy926 compared to unstimulated cells. Firstly, TNF-α-activated cells express a higher density of CD54, thus promoting CTL activity, as demonstrated in the experiments with wild-type SEA and SDCC (Fig. 1B and Fig. 2A). Secondly, the HLA class I expression is increased upon TNF-α challenge, and thus more target molecules are displayed. In parallel with EA.hy926, HUVEC were lysed when SpA-SEA-D227A was targeted to HLA class I (Fig. 4E). As HUVEC were found to display CD106 upon activation with TNF-α, we also successfully targeted the fusion protein to this adhesion molecule (Fig. 4F). Taken together, SEA-D227 induced SADCC when specifically directed to HLA class I, CD54, or CD106.
To further refine our experimental system, a chimeric protein consisting of an scFv antibody fragment was linked to the mutant SEA-D227A. This K594ScFv-SEA-D227A recombinant fusion protein was targeted to both the HUVEC-derived cell line EA.hy926 and HMVEC isolated from the dermis. A strong SADCC was induced when EA.hy926 cells were preactivated by TNF-α (Fig. 5A). In contrast, HMVEC constitutively expressed CD54 (Fig. 5C). However, no additional SADCC was observed when HMVEC were stimulated (Fig. 5F), revealing that the (presently unknown) EC surface molecule targeted by K594ScFv-SEA-D227A is not upregulated by TNF-α. From a therapeutic point of view, HMVEC are the most attractive target in that they resemble the type of microvasculature detected in tumors. Thus, SAg targeted to EC that are derived from the microvasculature can be used for directing and activating CTL.
A large problem associated with immunotoxin-based therapies with the goal of targeting drugs to the vasculature is to find suitable EC surface markers that are tumor EC-specific and do not exhibit cross-reactivity with normal blood vessels. Recently, Seon et al. used a MAb directed against endoglin to specifically target EC (32). The antiendoglin MAb was chemically fused to ricin A to allow a potent effector function. The MAb ricin A conjugate showed a remarkable antitumor efficacy in SCID mice inoculated with the human breast carcinoma cell line MCF-7. Vascular endothelial growth factor receptor 2 and the integrin αvβ3 are other examples of EC targets that may be valid for testing in animal models. Interestingly, B16 melanoma-bearing mice treated with C215Fab-SEA show a strong upregulation of CD106 on EC in the tumor area (22). A significant SADCC was demonstrated in this study when a MAb against CD106 was used to direct SpA-SEA-D227A to the cell surface of HUVEC (Fig. 4). Although CD106 is probably too nonspecific as a target in a clinical setting, the use of anti-CD106-SEA fusion proteins to validate the concept of T-cell-mediated lysis of tumor vasculature may be tested in the B16 melanoma mouse model.
ACKNOWLEDGMENTS
We are grateful to Lena Evilevitch and Ann Åberg for excellent technical assistance. We also thank F. Lupu for providing us with cells.
REFERENCES
- 1.Abrahmsen L, Dohlsten M, Segren S, Björk P, Jonsson E, Kalland T. Characterization of two distinct MHC class II binding sites in the superantigen staphylococcal enterotoxin A. EMBO J. 1995;14:2978–2986. doi: 10.1002/j.1460-2075.1995.tb07300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araake M, Uchiyama T, Imanishi K, Yan X J. Activation of human vascular endothelial cells by IFN-gamma: acquisition of HLA class II expression, TSST-1-binding activity and accessory activity in T cell activation by the toxin. Int Arch Allergy Appl Immunol. 1991;96:55–61. doi: 10.1159/000235535. [DOI] [PubMed] [Google Scholar]
- 3.Buckley C D, Doyonnas R, Newton J P, Blystone S D, Brown E J, Watt S M, Simmons D L. Identification of alpha v beta 3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- 4.Burrows F J, Thorpe P E. Eradication of large solid tumors in mice with an immunotoxin directed against tumor vasculature. Proc Natl Acad Sci USA. 1993;90:8996–9000. doi: 10.1073/pnas.90.19.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Schlegel P G, Tran N, Thompson D, Zehnder J L, Chao N J. Administration of a CD31-derived peptide delays the onset and significantly increases survival from lethal graft-versus-host disease. Blood. 1997;89:1452–1459. [PubMed] [Google Scholar]
- 6.Collins T, Korman A J, Wake C T, Boss J M, Kappes D J, Fiers W, Ault K A, Gimbrone M A, Jr, Strominger J L, Pober J S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci USA. 1984;81:4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohlsten M, Abrahmsen L, Bjork P, Lando P A, Hedlund G, Forsberg G, Brodin T, Gascoigne N R, Forsberg C, Lind P, et al. Monoclonal antibody-superantigen fusion proteins: tumor-specific agents for T-cell-based tumor therapy. Proc Natl Acad Sci USA. 1994;91:8945–8949. doi: 10.1073/pnas.91.19.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohlsten M, Hansson J, Ohlsson L, Litton M, Kalland T. Antibody targeted superantigens are potent inducers of tumor-infiltrating T lymphocytes in vivo. Proc Natl Acad Sci USA. 1995;92:9791–9795. doi: 10.1073/pnas.92.21.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohlsten M, Hedlund G, Lando P A, Trowsdale J, Altmann D, Patarroyo M, Fischer H, Kalland T. Role of the adhesion molecule ICAM-1 (CD54) in staphylococcal enterotoxin-mediated cytotoxicity. Eur J Immunol. 1991;21:131–135. doi: 10.1002/eji.1830210120. [DOI] [PubMed] [Google Scholar]
- 10.Doukas J, Pober J S. IFN-gamma enhances endothelial activation induced by tumor necrosis factor but not IL-1. J Immunol. 1990;145:1727–1733. [PubMed] [Google Scholar]
- 11.Edgell C-J S, McDonald C C, Graham J B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber D J, Pereira P, Huang S Y, Pelletier C, Tonegawa S. Expression of alpha v and beta 3 integrin chains on murine lymphocytes. Proc Natl Acad Sci USA. 1996;93:14698–14703. doi: 10.1073/pnas.93.25.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gidlöf C, Dohlsten M, Kalland T, Tötterman T H. Antibodies are capable of directing superantigen-mediated T cell killing of chronic B lymphocytic leukemia cells. Leukemia. 1995;9:1534–1542. [PubMed] [Google Scholar]
- 14.Haraldsen G, Kvale D, Lien B, Farstad I N, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996;1:2558–2565. [PubMed] [Google Scholar]
- 15.Holzer U, Orlikowsky T, Zehrer C, Bethge W, Dohlsten M, Kalland T, Niethammer D, Dannecker G E. T-cell stimulation and cytokine release induced by staphylococcal enterotoxin A (SEA) and the SEAD227A mutant. Immunology. 1997;90:74–80. doi: 10.1046/j.1365-2567.1997.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Molema G, King S, Watkins L, Edgington T S, Thorpe P E. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science. 1997;24:547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
- 17.Krakauer T. Costimulatory receptors for the superantigen staphylococcal enterotoxin B on human vascular endothelial cells and T cells. J Leukoc Biol. 1994;56:458–463. doi: 10.1002/jlb.56.4.458. [DOI] [PubMed] [Google Scholar]
- 18.Lapierre L A, Fiers W, Pober J S. Three distinct classes of regulatory cytokines control endothelial cell MHC antigen expression. Interactions with immune gamma interferon differentiate the effects of tumor necrosis factor and lymphotoxin from those of leukocyte alpha and fibroblast beta interferons. J Exp Med. 1988;1:794–804. doi: 10.1084/jem.167.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung D Y. Superantigens related to Kawasaki syndrome. Springer Semin Immunopathol. 1996;17:385–396. doi: 10.1007/BF01795136. [DOI] [PubMed] [Google Scholar]
- 20.Leung D Y, Sullivan K E, Brown-Whitehorn T F, Fehringer A P, Allen S, Finkel T H, Washington R L, Makida R, Schlievert P M. Association of toxic shock syndrome toxin-secreting and exfoliative toxin-secreting Staphylococcus aureus with Kawasaki syndrome complicated by coronary artery disease. Pediatr Res. 1997;42:268–272. doi: 10.1203/00006450-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Liao F, Ali J, Greene T, Muller W A. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J Exp Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litton M J, Dohlsten M, Hansson J, Rosendahl A, Ohlsson L, Kalland T, Andersson J, Andersson U. Tumor therapy with an antibody-targeted superantigen generates a dichotomy between local and systemic immune responses. Am J Pathol. 1997;150:1607–1618. [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W, Lehner P J, Cresswell P, Pober J S, Johnson D R. Interferon-gamma rapidly increases peptide transporter (TAP) subunit expression and peptide transport capacity in endothelial cells. J Biol Chem. 1997;27:16585–16590. doi: 10.1074/jbc.272.26.16585. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, Bussolino F, Introna M. Cytokine regulation of endothelial cell function: from the molecular level to the bedside. Immunol Today. 1997;18:231–240. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- 25.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 26.Melish M E. Kawasaki syndrome. Pediatr Rev. 1996;17:153–162. doi: 10.1542/pir.17-5-153. [DOI] [PubMed] [Google Scholar]
- 27.Miethker T, Wahl C, Heeg K, EchtenAcher B, Krammer P, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson B, Moks T, Jansson B, Abrahmsen L, Elmblad A, Holmgren E, Henrichson C, Jones T A, Uhlen M A. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;1:107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 29.Prager E, Sunder-Plassmann R, Hansmann C, Koch C, Holter W, Knapp W, Stockinger H. Interaction of CD31 with a heterophilic counterreceptor involved in downregulation of human T cell responses. J Exp Med. 1996;184:41–50. doi: 10.1084/jem.184.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosendahl A, Kristensson K, Riesbeck K, Kalland T, Dohlsten M. Perforin and cytokines are crucial in superantigen directed therapy of B16 melanoma bearing mice. J Immunol. 1998;160:5309–5313. [PubMed] [Google Scholar]
- 31.Schafer R, Sheil J M. Superantigens and their role in infectious disease. Adv Pediatr Infect Dis. 1995;10:369–390. [PubMed] [Google Scholar]
- 32.Seon B K, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in DCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997;3:1031–1044. [PubMed] [Google Scholar]
- 33.Sogaard M, Hansson J, Litton M J, Ohlsson L, Rosendahl A, Lando P A, Antonsson P, Kalland T, Dohlsten M. Antibody-targeted superantigens in cancer immunotherapy. Immunotechnology. 1996;2:151–162. doi: 10.1016/s1380-2933(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 34.Sundstedt A, Dohlsten M, Hedlund G, Hoiden I, Björklund M, Kalland T. Superantigens anergize cytokine production but not cytotoxicity in vivo. Immunol. 1994;82:117–125. [PMC free article] [PubMed] [Google Scholar]
- 35.Tennenberg S D, Weller J J. Endotoxin-induced, neutrophil-mediated endothelial cytotoxicity is enhanced by T-lymphocytes. J Surg Res. 1997;69:11–13. doi: 10.1006/jsre.1996.4996. [DOI] [PubMed] [Google Scholar]
- 36.Tordsson J, Abrahamsén L, Kalland T, Ljung C, Ingvar C, Brodin T. Efficient selection of scFv antibody phage by adsorption to in situ expressed antigens in tissue sections. J Immunol Methods. 1997;210:11–23. doi: 10.1016/s0022-1759(97)00165-8. [DOI] [PubMed] [Google Scholar]
- 37.Uchiyama T, Araake M, Yan X J, Miyanaga Y, Igarashi H. Involvement of HLA class II molecules in acquisition of staphylococcal enterotoxin A-binding activity and accessory cell activity in activation of human T cells by related toxins in vascular endothelial cells. Clin Exp Immunol. 1992;87:322–328. doi: 10.1111/j.1365-2249.1992.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]