Abstract
Introduction
Three per cent of all infants are born in breech presentation, still the preferred way to deliver them remains controversial. The objective of this systematic review was to assess the safety for the mother and child depending on intended mode of delivery when the baby is in breech position at term.
Material and Methods
The population (P) was pregnant women with a child in breech presentation, from gestational week 34+0. The intervention (I) was the intention to deliver by cesarean section, the comparison (C) was the intention to deliver vaginally. Outcomes (O) were perinatal mortality, perinatal morbidity, maternal mortality, maternal morbidity, conversion of delivery mode, and the mother's experience. Systematic literature searches were performed. We included randomized trials, cohort studies with more than 500 women/group and case series for more than 15 000 women published between 1990 and October 2021, written in English or the Nordic languages. The certainty of evidence was assessed using the GRADE approach and data were pooled in meta‐analyses. PROSPERO registration number: CRD42020209546.
Results
Thirty‐two articles were included (with 530 604 women). The certainty of evidence was moderate or low because the study designs were mostly retrospective cohort studies. The only randomized trial showed reduced risk of perinatal mortality for planned cesarean section, risk ratio (RR) 0.27 (95% confidence interval [CI] 0.08–0.97, 2078 women, low certainty of evidence), stillbirths excluded. A meta‐analysis of cohort studies resulted in a similar estimate, RR 0.36 (95% CI 0.25–0.51, 21 studies, 388 714 women, low certainty of evidence). We also found reduced risk for outcomes representing perinatal morbidity 0–28 days: 5‐min Apgar score less than 7 in one randomized controlled trial: RR 0.27 (95% CI 0.12–0.58, 2033 women, moderate certainty of evidence), and in a meta‐analysis: RR 0.1 (95% CI 0.14–0.26, 18 studies, 217 024 women, moderate certainty of evidence); APGAR score less than 4 at 5 min: RR 0.39 (95% CI 0.19–0.81, five studies, 44 498 women, low certainty of evidence); and pH less than 7.0: RR 0.23 (95% CI 0.12–0.43, four studies, 13 440 women, low certainty of evidence). Outcomes for the mother were similar in the groups except for reduced risk for experience of urinary incontinence in the group of planned cesarean section: RR 0.62 (95% CI 0.41–0.93, one study, 1940 women, low certainty of evidence). The conversion rate from planned vaginal delivery to emergency cesarean section ranged from 16% to 51% (median 41.8%, 10 studies, 50 763 women, moderate certainty of evidence).
Conclusions
Intended cesarean section may reduce the risk of perinatal mortality and perinatal as well as some maternal morbidity compared with intended vaginal delivery. It is uncertain whether there is any difference in maternal mortality. The conversion rate from intended vaginal delivery to emergency cesarean section is high.
Keywords: breech presentation, cesarean section, mode of delivery, morbidity, perinatal mortality, term birth, vaginal delivery
Abbreviations
- CI
confidence interval
- CS
cesarean section
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- RCT
randomized controlled trial
- RR
risk ratios
- TBT
Term Breech Trial
Key message.
Intended cesarean section as mode of delivery of a singleton term infant in breech presentation may reduce the risk of perinatal mortality and short‐term perinatal morbidity.
1. INTRODUCTION
Approximately 3% of all infants are born in breech presentation with bottom first, sometimes a foot or knee is leading. 1 , 2 , 3 The risk of breech presentation is sometimes increased, for example, in malformations of the child or the uterus; however, most of the infants and mothers among breech deliveries are totally healthy. 4 Vaginal delivery in breech compared with cephalic presentation seems to be associated with greater risks of complications for the baby due to factors such as cord prolapse, brachial plexus injury, or difficulties delivering the after‐coming head. 5 These complications can cause perinatal death as well as permanent illness and disability. An intended cesarean section (CS) may reduce these risks for the baby. On the other hand, a CS carries higher potential risks for the mother perioperatively, and also in future pregnancies where the frequency of uterine rupture, invasive placental growth, and hysterectomy is increased. 6 , 7 The contradictory risks for mother and child depending on delivery mode in breech presentation create an ethical conflict between the mother and her unborn child. To plan for vaginal breech delivery or intended CS is a complex decision for the patient and the obstetrician. The proportion of breech babies delivered vaginally differ largely among the Nordic countries from 10% in Denmark, 7% in Sweden, to 36% in Norway. 8 , 9 , 10 Accurate studies are needed to obtain reliable data about these rare but serious complications in order to correctly inform the pregnant woman about her choices.
It has long been documented that intended vaginal delivery for term infants in breech presentation was associated with higher rates of perinatal morbidity and mortality compared with intended cesarean delivery. 11 In 2000, the results from the Term Breech Trial (TBT) were published. This multicenter randomized controlled trial (RCT) included 2088 patients and found significantly higher risk for perinatal death or serious perinatal morbidity after intended vaginal delivery. 12 This led to a remarkable increase of planned CS of breech babies both in Europe and the USA. 13 , 14 , 15 , 16 As previously stated by other authors there are serious limitations in the TBT publications. 17 , 18 First, only 2088 women from 121 centers in 26 countries were included during the study, implying that it is a highly selected group although this is poorly discussed in the TBT publications. Second, the primary outcome is a composite of mortality and a wide range of morbidity for the child. This composite outcome was reported as statistically significantly decreased by planned CS, using a one‐sided test. With an appropriate use of a two‐sided test, the p value would be 0.06.
The preferred way to deliver an infant in breech presentation hence remains controversial. A French/Belgian prospective observational study 19 concluded that vaginal deliveries of singleton fetuses in breech presentation at term remains a safe option, provided that strict criteria are met before and during labor. After the Cochrane review published in 2015, 4 some extensive cohort studies with intention‐to‐treat design have been published from the Nordic countries. 3 , 20 , 21 In a Swedish study including 23 357 breech presentations, Ekéus et al. reported that vaginal breech deliveries had surprisingly high complication rates (odds ratio [OR] neonatal death 7.6, OR Apgar <7 at 5 min 13.3, and OR plexus injury 23.8), despite an appropriately selected group of women for vaginal breech delivery according to strict clinical criteria. 3 The interpretation of this study, like several other retrospective cohorts, was difficult as it was impossible to distinguish between planned and unplanned CS. In this review we only included studies that differentiated between intended CS and unplanned CS, because the intended delivery mode is crucial in clinical praxis.
The discussion concerning which delivery mode should be preferred in case of breech is still vivid among professionals. The objective of this systematic review was to evaluate if the intention to deliver a term infant in breech presentation by planned CS compared with intended vaginal delivery, affects perinatal and maternal mortality and morbidity and long‐term outcomes for mother and child.
2. MATERIAL AND METHODS
This systematic review and meta‐analysis was performed according to the guidelines for Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) 22 with PROSPERO registrations number: CRD42020209546. We used the PICO model to define the Population, Intervention, Comparison, and Outcome. The population (P) was pregnant women with a child in breech presentation, from gestational week 34+0. The intervention (I) was the intention to deliver by cesarean section and the comparison (C) was the intention to deliver vaginally. Outcomes (O) was perinatal mortality, short‐term perinatal outcome (stay at neonatal care unit, hypoxic ischemic encephalopathy, intracranial bleeding, asphyxia defined as Apgar score <7 or <4 at 5 min, pH ≤7.05 or base excess ≤ −12, or traumatic birth injury), long term child outcome (neurodevelopmental or physical problems), maternal mortality, short‐term maternal outcome (infection, bleeding, thrombosis, delivery tract trauma), long‐term maternal outcome (pelvic floor dysfunction, placenta previa/accreta, or uterine rupture in subsequent pregnancies), conversion of delivery mode, the mother's experience of delivery.
2.1. Data sources and eligibility criteria
Literature searches were performed in PubMed, Embase, the Cochrane Library, and Cinahl January 26, 2017, and updated October 5, 2021. Searches in Clinicaltrials.gov and WHO International Clinical Trials Registry Platform on May 6, 2021did not find any trials relevant for our question. A medical librarian performed the searches using a combination of controlled vocabulary and free‐text words (Figure S1). Eligibility criteria for included studies were RCTs, cohort studies with more than 500/group and case series with more than 15 000 patients. The languages included were English, Swedish, Danish, and Norwegian and a time limit was set at the year 1990. Reference lists of relevant articles were scrutinized for additional references. In an initial screening process, two reviewers read all abstracts independently of each other and full‐length articles if needed, disagreements were resolved in consensus. The remaining potentially eligible articles were sent to all authors for assessment. Reasons for exclusion were not fulfilling PICO, poster or conference abstract, other types of publication than an original article, intended mode of delivery not described. Controlled studies were critically appraised with regards to directness (external validity), risk of bias (internal validity), and precision (Tables S2 and S3) using validated checklists for RCTs 23 and non‐RCTs 24 , 25 obtained from The Swedish Agency for Health Technology Assessment and the Assessment of Social Services, which are the standard risk‐of‐bias tools in Sweden. Any disagreements in the assessment of articles were solved in consensus between the authors.
2.2. Data collection and analysis
The individual data for all outcomes were extracted independently by two authors. We assumed that groups named “Emergency CS” and “Actual vaginal delivery” together constituted the group “Planned vaginal delivery”, when such a group was not presented. Raw data from the cohort studies were pooled in meta‐analyses, 26 with the Mantel–Haenszel method, random effects model, and displayed as forest plots in revman 5.2. Point estimates were presented as risk ratios (RR) with 95% confidence intervals (CI). Publication bias was assessed and presented in funnel plots for outcomes with at least 10 studies (Figure S2). The certainty of evidence was assessed using the GRADE approach. 27 No authors were contacted. Any material including template data collection forms can be acquired from the authors upon request.
3. RESULTS
The systematic literature search identified 2340 articles after removal of duplicates. Two authors made 2200 exclusions on the abstract level and 44 on the full‐text level (Figure 1). At least two of the other authors assessed the 96 remaining articles. In the final assessment 32 articles (comprising 530 604 women) were included (five publications from the same RCT material, 12 , 28 , 29 , 30 , 31 25 cohort studies 5 , 7 , 9 , 13 , 14 , 15 , 19 , 20 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 and two case series 49 , 50 ). The included studies are presented in Table 1 along with their study characteristics. Table S3 lists the excluded articles and reasons for exclusion. Only two of 25 cohort studies were prospective. 19 , 36 The outcome data and its quality assessment are presented in the summary of findings in Table 2 and Tables [Link], [Link]. Overall, the certainty of evidence was moderate or low, naturally because the study designs were mostly retrospective cohort studies. No obvious publication bias was detected but it cannot be ruled out.
FIGURE 1.
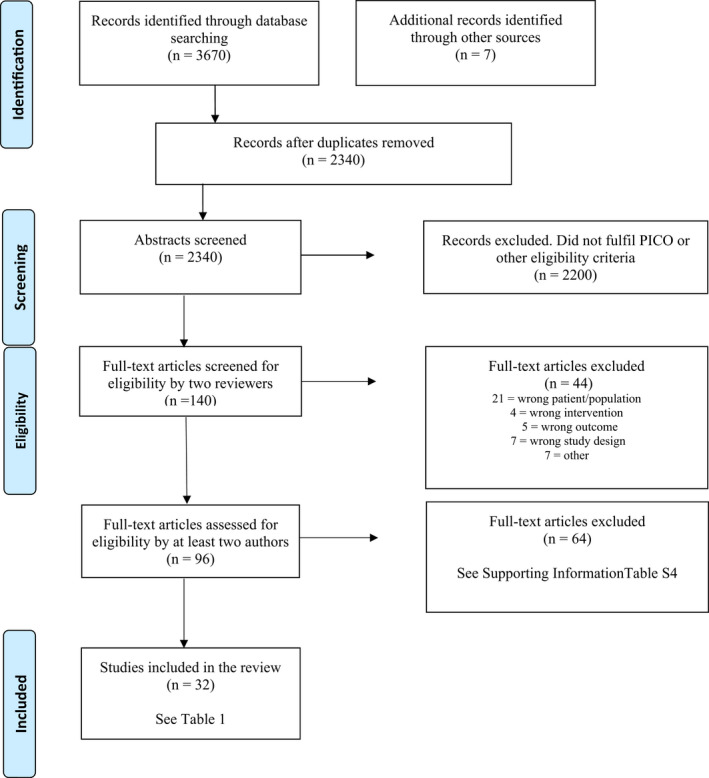
Flow diagram presenting the selection process after the literature search.
TABLE 1.
Included articles
| Author, year, country | Study design | Study duration (years) | Study groups; intervention vs. control | Patients (n before dropout) | Malformations excluded | Outcome variables |
|---|---|---|---|---|---|---|
| Hannah, 12 2000, Canada | Randomized controlled trial (RCT) | 1997–2000 |
I = planned cesarean section (1043) C = planned vaginal birth (1045) |
2088 | Yes |
Perinatal mortality Perinatal morbidity <28 days Maternal mortality Maternal morbidity, first year Conversion |
| Hannah, 28 2002, Canada | RCT | 1997–2000 |
I = planned cesarean section (798) C = planned vaginal birth (798) |
1940 | Yes |
Perinatal morbidity 28 days to 2 years Maternal morbidity, first year Mother's experience |
| Hannah, 29 2004, Canada | RCT | 1997–2000 |
I = planned cesarean section (457) C = planned vaginal birth (460) |
1159 | Yes | Maternal morbidity, long term |
| Hodnett, 30 2005, Canada | RCT | 1997–2000 |
I = planned cesarean section (457) C = planned vaginal birth (460) |
1159 | Yes | Mother's experience |
| Whyte, 31 2004, Canada | RCT | 1997–2000 |
I = planned cesarean section (457) C = planned vaginal birth (463) |
1159 | Yes | Perinatal morbidity >2 years |
|
Adjaoud, 32 2017, France |
Cohort | 2000–2011 |
I = planned cesarean section (876) C = planned vaginal birth (665) |
1541 | No |
Perinatal mortality Perinatal morbidity <28 days Conversion |
| Bin, 2016a, 47 Australia | Cohort | 2001–2012 |
I = planned cesarean section (11 339) C = planned vaginal birth (1183) (2759 intention uncertain) |
15 281 | Yes |
Perinatal mortality Perinatal morbidity >2 years |
| Bin, 2016b, 48 Australia | Cohort | 2009–2012 |
I = planned cesarean section (3970) C = planned vaginal birth (352) (875 intention uncertain) |
5197 | Yes |
Perinatal morbidity <28 days Maternal morbidity, first year Conversion |
| Bjellmo, 20 2017, Norway | Cohort | 1999–2009 |
I = planned cesarean section (8780) C = planned vaginal birth (7873) |
16 700 | Yes |
Perinatal mortality Perinatal morbidity >2 years Conversion |
| Burgos, 33 2015, Spain | Cohort | 2003–2012 |
I = planned cesarean section (793) C = planned vaginal birth (891) |
1684 | Yes |
Perinatal mortality Perinatal morbidity <28 days Conversion |
| Gilbert, 13 2003, USA | Cohort | 1991–1999 |
I1 = planned cesarean section (60418) I2 = emergency cesarean section (35 297) C = vaginal birth (4952) |
100 667 | Yes | Perinatal mortality |
| Goffinet, 19 2006, France and Belgium | Cohort (prospective) | 2001–2002 |
I = planned cesarean section (5579) C = planned vaginal birth (2526) |
8105 | No |
Perinatal mortality Perinatal morbidity <28 days |
| Hartnack, 15 2011, Denmark | Cohort | 1997–2008 |
I = planned cesarean section (14 764) C = planned vaginal birth (7039) |
23 789 | Yes |
Perinatal mortality Perinatal morbidity <28 days |
| Henriksen, 34 2019, Norway | Cohort | 2000–2012 |
I = planned cesarean section (1528) C = planned vaginal birth (1458) |
2986 | Yes |
Perinatal morbidity <28 days Conversion |
| Herbst, 9 2005, Sweden | Cohort | 1991–2001 |
I = planned cesarean section (9749) C = planned vaginal birth (6839) |
22 549 | Yes |
Perinatal mortality Perinatal morbidity <28 days Study B is excluded, few cases |
| Hinnenberg, 35 2019, Finland | Cohort | 2004–2014 |
I = planned cesarean section (804) C = planned vaginal birth (1037) |
1841 | No |
Perinatal mortality Perinatal morbidity <28 days Conversion |
|
Korb, 36 2020, France and Belgium |
Cohort (prospective) | 2001–2002 |
I = planned cesarean section (5098) C = planned vaginal birth (2466) |
8105 | No |
Maternal mortality Maternal morbidity, first year Conversion |
| Krebs, 37 2003, Denmark | Cohort | 1982–1998 |
I1 = planned cesarean section (7503) I2 = emergency cesarean section (5575) C = actual vaginal birth (2363) |
15 441 | No |
Maternal mortality Maternal morbidity, first year Maternal morbidity, long term |
| Krebs, 38 1995, Denmark | Cohort | 1982–1990 |
I1 = planned cesarean section (7106) I2 = emergency cesarean section (5356) C = actual vaginal birth (2363) |
15 718 | Yes |
Perinatal mortality Perinatal morbidity <28 days |
|
Macharey, 46 2017, Finland “Neurodevelopmental outcome” |
Cohort | 2004–2010 |
I = planned cesarean section (4467) C = planned vaginal birth (3907) |
8374 | Yes |
Perinatal mortality Perinatal morbidity <28 days Perinatal morbidity >2 years Conversion |
| Pasupathy, 39 2009, Scottland | Cohort | 1985–2004 |
I1 = planned cesarean section (19 832) I2 = pre‐labor emergency cesarean section (4108) I3 = post‐labor emergency cesarean section (4910) C = planned vaginal birth (3926) |
32 776 | Yes | Perinatal mortality |
| Pradhan, 40 2005, UK | Cohort | 1991–2000 |
I = planned cesarean section (552) C = planned vaginal birth (881) |
1433 | No |
Perinatal mortality Perinatal morbidity <28 days Perinatal morbidity >2 years |
| Rietberg, 41 2003, The Netherlands | Cohort | 1995–1999 |
I = planned cesarean section (6840) C = planned vaginal birth (24 391) |
33 824 | Yes |
Perinatal mortality Perinatal morbidity <28 days |
| Rietberg, 14 2005, The Netherlands | Cohort | 1998–2002 |
I1 = planned cesarean section (8682) I2 = emergency cesarean section (2731) C = actual vaginal birth (2835) |
14 258 | Yes |
Perinatal mortality Perinatal morbidity <28 days |
| Roman, 5 1998, Sweden | Cohort | 1987–1993 |
I1 = planned cesarean section (6031) I2 = emergency cesarean section (3011) I3 = unspecified cesarean section (879) C = actual vaginal birth (5897) |
15 818 | Yes |
Perinatal mortality Perinatal morbidity <28 days Maternal mortality Maternal morbidity, first year |
| Thorpe‐Beeston, 42 1992, England | Cohort | 1988–1990 |
I1 = planned cesarean section (1457) I2 = emergency cesarean section (1029) C = actual vaginal birth (961) |
3447 | Yes |
Perinatal mortality Perinatal morbidity <28 days |
| Ulander, 43 2004, Finland | Cohort | 1987–1989 |
I = planned cesarean section (1640) C = planned vaginal birth (1270) |
2910 | Yes |
Perinatal mortality Perinatal morbidity <28 days Perinatal morbidity >2 years Maternal mortality Maternal morbidity, first year |
| Venditelli, 44 2006, France | Cohort | 1994–2000 |
I = planned cesarean section (879) C = planned vaginal birth (1216) |
2136 | Yes |
Perinatal mortality Perinatal morbidity <28 days Conversion |
| Vistad, 7 2015, Norway | Cohort |
1991–2011 |
I = planned cesarean section (13 361) C = planned vaginal birth (17 500) |
30 861 | Yes |
Perinatal mortality Perinatal morbidity <28 days |
| Vlemmix, 45 2014, The Netherlands | Cohort | 1999–2007 |
I = planned cesarean section (30 503) C = planned vaginal birth (27 817) |
58 320 | Yes |
Perinatal mortality Perinatal morbidity <28 days |
|
Liu, 49 2007, Canada |
Case report | 1991–2005 | I = planned cesarean section (46 766) | 68 404 | Yes |
Maternal mortality Maternal morbidity, first year |
|
Schutte, 50 2007, The Netherlands |
Case report | 2000–2002 | I = planned cesarean section (16 351) | 16 351 | No | Maternal mortality |
TABLE 2.
Summary of findings comparing intended cesarean section with vaginal delivery
| Outcomes | Study design (number of patients) |
Relative effect Risk ratio (95% CI) |
Absolute effects a | Certainty of evidence GRADE b |
|---|---|---|---|---|
| Perinatal outcomes | ||||
| Perinatal mortality |
1 RCT (n = 2078) 21 non‐RCT (n = 388 714) |
RR 0.27 c (0.076; 0.974) RR 0.36 (0.25; 0.51) |
0.3 vs. 1.1% 0.05% vs. 0.19% |
Low d ⊕⊕⃝⃝ |
| Apgar score <7 at 5 min |
1 RCT (n = 2033) 18 non‐RCT (n = 217 024) |
RR 0.27 (0.12; 0.58) RR 0.1 (0.14; 0.26) |
0.8% vs. 3.0% 0.45% vs. 2.50% |
Moderate e ⊕⊕⊕⃝ |
| Apgar score <4 at 5 min | 5 non‐RCT (n = 44 498) | RR 0.39 (0.19; 0.81) | 0.20% vs. 0.55% | Low ⊕⊕⃝⃝ |
| Umbilical cord pH <7.0 (in our PICO <7.05 but <7.0 was reported in all articles) | 4 non‐RCT (n = 13 440) | RR 0.23 (0.12; 0.43) | 0.26% vs. 1.23% | Low f ⊕⊕⃝⃝ |
| Base excess < −12 | 1 non‐RCT (n = 1684) | RR 0.14 (0.08; 0.24) | 1.7% vs. 11.9% | Low ⊕⊕⃝⃝ |
| Admission to Neonatal Intensive Care Unit, >4 days |
1 RCT (n = 2000) 4 non‐RCT (n = 46 055) |
RR 0.67 (0.19; 2.36) RR 0.73 (0.57; 0.92) |
0.4% vs. 0.6% 2.45% vs. 3.72% |
Low g ⊕⊕⃝⃝ |
| Traumatic birth injury |
1 RCT (n = 2000) 9 non‐RCT (n = 145 068) |
RR 0.43 (0.17; 1.11) RR 0.18 (0.13; 0.27) |
0.6% vs. 1.4% 0.20% vs. 0.67% |
Low g ⊕⊕⃝⃝ |
| Hypoxic ischemic encephalopathy | Not reported | |||
| Cerebral palsy >2 years | 3 non‐RCT (n = 26 155) | RR 1.03 (0.53; 2.01) | 0.13% vs. 0.13% | Low ⊕⊕⃝⃝ |
| Intracranial bleeding | 3 non‐RCT (n = 27 192) | RR 0.24 (0.05; 1.08) | 0.02% vs. 0.11% | Low ⊕⊕⃝⃝ |
| Neurodevelopmental problems | Reported in a wide variation of outcomes, see Table S2. | |||
| Maternal outcomes | ||||
| Maternal mortality |
1 RCT (n = 2083) 4 non‐RCT (n = 40 854) |
RR 0.33 (0.01; 8.18) RR 0.15 (0.01; 2.93) |
0% vs. 0.10% 0% vs. 0.02% |
Very low h ⊕⃝⃝⃝ |
| Thrombo‐embolic event | 4 non‐RCT (n = 40 854) |
RR 1.25 (0.56; 2.80) |
0.08% vs. 0.05% |
Low ⊕⊕⃝⃝ |
| Pelvic infection | 2 non‐RCT (n = 30 380) |
RR 1.03 (0.67; 1.56) |
1.2% vs. 1.2% |
Low ⊕⊕⃝⃝ |
| Severe bleeding | Reported in a wide variation of outcomes, see Table S3. | |||
| Delivery tract trauma | Reported in a wide variation of outcomes, see Table S3. | |||
| Pelvic floor dysfunction (3 months and 2 years follow up) |
Reported in a wide variation of outcomes, see Table S3. The only significant differences were “having experienced urinary incontinence or incontinence of flatus” at 3 months. |
|||
| Placental or uterine complications in following pregnancies | Reported in a wide variation of outcomes, see Table S3. | |||
| Other outcomes | ||||
| Conversion of delivery mode from cesarean section to vaginal delivery | 1 RCT (n = 2088) | Not relevant | 9.6% | Low i ⊕⊕⃝⃝ |
| Conversion of delivery mode from vaginal delivery to cesarean section |
1 RCT (n = 2088) 9 non‐RCT (n = 48 675) |
Not relevant |
43.3% 16%–51% (mean 38.0% median 41.8%) |
Moderate j ⊕⊕⊕⃝ |
| The mother's experience of delivery |
Reported in a wide variation of outcomes, see Table S3. No significant differences at 3 months and 2 years follow up. |
|||
| Certainty of evidence | |
|---|---|
| High certainty ⊕⊕⊕⊕ | We are very confident that the true effect lies close to that of the estimate of the effect |
| Moderate certainty ⊕⊕⊕⃝ | We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low certainty⊕⊕⃝ ◯ | Confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect |
| Very low certainty ⊕⃝ ◯⃝ | We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |
Absolute effects for event rates, presented as the sum of all events/the total numbers of participants, across the RCTs or cohort studies, respectively.
GRADE based primary on RCTs, or when not available on non‐RCTs.
Stillbirths excluded by us (n = 2).
Downgraded two levels due to serious limitations in study design and analysis, and serious imprecision.
Downgraded one level due to serious limitations in study design and uncertain precision.
Downgraded one level due to some limitations in study design, some uncertainty about directness, and uncertain precision.
Downgraded two levels due to serious limitations in study design, serious imprecision, and some uncertainty about directness.
Downgraded three levels due to serious limitations in study design, some uncertainty about directness, and very serious imprecision.
Downgraded two levels due to some limitations in study design and very serious indirectness.
Downgraded one level due to some limitations in study design.
3.1. Perinatal outcomes
Perinatal mortality was reported in one RCT showing reduced risk of perinatal mortality for planned CS (RR 0.27, 95% CI 0.08–0.97, 2078 women, low certainty of evidence), stillbirths were excluded by us. 12 A meta‐analysis of cohort studies resulted in similar estimate (RR 0.36, 95% CI 0.25–0.51, 21 studies, 388 714 women, low certainty of evidence). 5 , 7 , 9 , 13 , 14 , 15 , 19 , 20 , 32 , 33 , 35 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 Most studies define perinatal mortality as death during days 0–28, stillbirths excluded, but a few studies report deaths during days 0–7 (listed in Table S2). The RCT and the pooled estimate of the cohort studies showed a significant reduction in perinatal mortality after intended CS compared with intended vaginal delivery (Figure 2). However, the certainty of evidence was low, based on moderate problems with directness, risk of bias, and precision in the RCT and the inherent risk of selection bias in the cohort studies.
FIGURE 2.
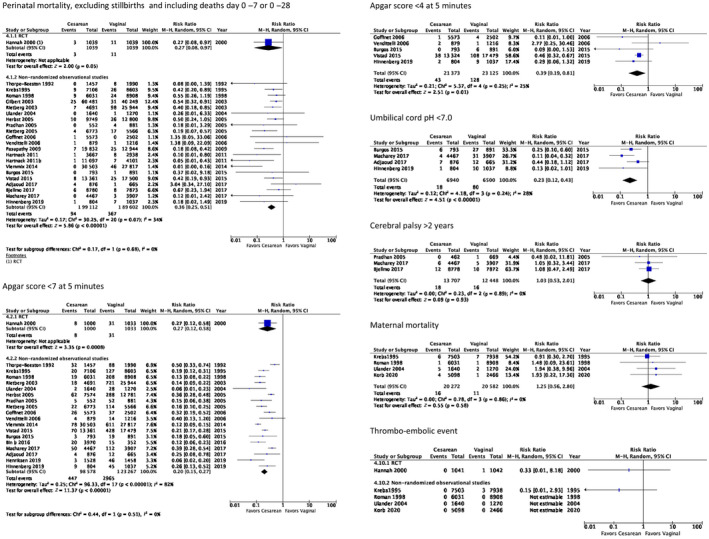
Meta‐analyses of studies comparing intended cesarean section with intended vaginal delivery of term breech babies presenting most important perinatal and maternal outcomes.
Perinatal morbidity in days 0–28 consists of heterogeneous outcomes reported in one RCT 12 and 19 cohort studies. 5 , 7 , 9 , 14 , 15 , 19 , 32 , 33 , 34 , 35 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 48 In Table S2 the studies are tabulated for each outcome available. Some of the outcomes could be pooled in meta‐analyses (Figure 2 and Figure S3). All these perinatal outcomes demonstrated reduced risk in the group with planned CS. A 5‐min Apgar score below 7 was reported in one RCT as RR 0.27 (95% CI 0.12–0.58, 2033 women, moderate certainty of evidence). The meta‐analysis of cohort studies showed RR 0.1 (95% CI 0.14–0.26, 18 studies, 221 845 women, moderate certainty of evidence). Five‐minute Apgar score less than 4 was pooled in a meta‐analysis showing RR 0.39 (95% CI 0.19–0.81, five studies, 44 498 women, low certainty of evidence). Umbilical pH below 7.0 was pooled in a meta‐analysis showing RR 0.23 (95% CI 0.12–0.43, four studies, 13 440 women, low certainty of evidence). Base excess less than −12 was reported in one RCT as RR 0.14 (95% CI 0.08–0.24, 1684 women, low certainty of evidence). NICU admission for longer than 4 days was reported in one RCT as RR 0.67 (95% CI 0.19–2.36, 2000 women, low certainty of evidence). The meta‐analysis of cohort studies showed RR 0.73 (95% CI 0.57–0.92, four studies, 46 055 women, low certainty of evidence). Traumatic birth injury was reported in one RCT as RR 0.43 (95% CI 0.17–1.11, 2000 women, low certainty of evidence). The meta‐analysis of cohort studies showed RR 0.18 (95% CI 0.13–0.27, nine studies, 145 068 women, low certainty of evidence). Intracranial bleeding was pooled in a meta‐analysis showing RR 0.24 (95% CI 0.05–1.08, three studies, 27 192 women, low certainty of evidence). It has been stated earlier that a 5‐min Apgar score less than 4 instead of less than 7 is a stronger predictor for long‐term sequelae. 51 The outcome hypoxic ischemic encephalopathy was not reported in any publication.
Perinatal morbidity from 28 days to 2 years was reported in the 3 months follow up of the TBT and showed no difference in health of the infant such as breastfeeding, readmission to hospital, or need to see a physician at 3 months. 28 The certainty of evidence for the outcome perinatal morbidity from 28 days to 2 years was low.
Perinatal morbidity after 2 years consists of heterogeneous outcomes reported in one RCT 31 and five cohort studies. 20 , 40 , 43 , 46 , 47 Cerebral palsy after 2 years was pooled in a meta‐analysis showing RR 1.03 (95% CI 0.53–2.01, three studies, 26 155 women, low certainty of evidence) (Figure 2). No difference in the risk of long‐term perinatal morbidity was reported, the certainty of evidence was low.
3.2. Maternal outcomes
Maternal mortality was reported in one RCT showing RR 0.33 (95% CI 0.01–8.18, 2083 women, very low certainty of evidence). 12 A meta‐analysis of cohort studies resulted in RR 0.15 (95% CI 0.01–2.93, four studies, 40 854 women, very low certainty of evidence) (listed in Table S3). 5 , 36 , 37 , 43 Maternal mortality was also reported in two case series. 49 , 50 One of the case series 50 reported three deaths in the group with planned CS (0.04%) and one death in the group with planned vaginal delivery (0.01%). The other case series 49 had no maternal deaths in any of the groups. These extremely rare events are depicted in a forest plot (Figure 2).
Maternal morbidity during the first year was reported as heterogeneous outcomes in two RCTs, 12 , 28 five cohort studies, 5 , 36 , 37 , 43 , 48 and one case series. 49 The 3 months follow up of the TBT showed significantly reduced risk for experience of urinary incontinence (RR 0.62, 95% CI 0.41–0.93, one study, 1940 women, low certainty of evidence). 28 The only outcome possible to review in a meta‐analysis was the risk of having a thromboembolic event, demonstrating a non‐significant difference (RR 1.25, 95% CI 0.56–2.80, four studies, 40 854 women, low certainty of evidence) (Figure 2). The short‐term maternal outcomes of infection, bleeding, and delivery tract trauma were reported separately in some studies and combined as a composite outcome in others. Due to the heterogenicity in how the results were reported we could not draw any firm conclusions.
Maternal morbidity after the first year was reported in one RCT (917 women) 29 and one retrospective cohort study (10 588 women). 37 None of the studies reported any statistically significant difference in long‐term maternal morbidity such as pain, incontinence, problems when having sex etc. The cohort study also reported placental or uterine complications in subsequent pregnancies. Uterine rupture occurred in 0.1% of patients with previously planned CS vs. 0.04% if previously planned vaginal delivery but the study was underpowered and could not prove any significant difference. Such rare events are better studied in a review comprising all types of CS because the complications are not specific for CS on breech indication. The certainty of evidence for the outcome long‐term maternal morbidity was low.
3.3. Other outcomes
Conversion from planned vaginal delivery to unplanned CS was reported in one RCT (2088 women) 12 and nine cohort studies (50 763 women) (listed in Table S3). 20 , 32 , 33 , 34 , 35 , 36 , 44 , 46 , 48 The conversion rate ranged from as low as 16% 32 to 51%. 34 The mean was 38.0% and the median 41.8%, indicating a generally high conversion rate. The certainty of evidence for the outcomes of conversion from planned vaginal delivery to unplanned CS was moderate.
The mother´s experience of delivery was reported in two follow‐up studies from the TBT, after 3 months (1940 women) and 2 years. 28 , 30 There were no significant differences in any of them, except for less worry about the child´s health in the group with planned CS. This outcome was reported after 3 months as RR 0.60 (95% CI 0.50%–0.73%, 1940 women) and after 2 years as RR 0.53 (95% CI 0.41%–0.69%, 1159 women). 28 , 30 The certainty of evidence for the outcome of the mother´s experience of delivery was moderate.
4. DISCUSSION
This systematic review and meta‐analysis identified 32 articles: five papers based on one RCT, 25 cohort studies and two case series providing data from 530 604 women. The incidence of adverse outcomes was low, and the certainty of evidence was predominantly low, partly because the majority of the studies were cohort studies. The data show that intended CS as mode of delivery of a singleton term infant in breech presentation may reduce the risk of perinatal mortality compared with intended vaginal delivery. Intended CS may also reduce the short‐term child morbidity, whereas there may be little or no difference in child morbidity after 3 months or at long‐term follow up. Considering long‐term child morbidity, no firm conclusions could be drawn, making consequences for a child delivered by CS largely unknown. Maternal mortality events were very few in both groups, but CS compared with vaginal delivery may reduce the risk for urinary incontinence. When interpreting the results of our meta‐analyses it is important to keep in mind that the group of intended vaginal delivery also consists of many unplanned CS during labor, because the conversion rate from intended vaginal delivery to unplanned CS is approximately 40% (Table S3).
Five of the included papers 12 , 28 , 29 , 30 , 31 are based on the RCT named the Term Breech Trial (TBT) including 2078 women published in 2000. The TBT was conducted in both high‐income and low‐income countries where the primary outcome was a composite of mortality and serious morbidity for the child. The result regarding the primary outcome was reported as statistically significant but when two stillbirths were excluded from the group with intended vaginal delivery the difference in the composite outcome was no longer significant. Future RCTs are not to be expected because of ethical issues, making well‐made cohort studies and systematic reviews with meta‐analysis extremely important. Two of the included papers 19 , 36 are based on the PREMODA study, the only prospective cohort study in the review. Limitations of the PREMODA study are differences in baseline data, although the study design is prospective, and confounders are not adjusted for in the statistical analysis. Four previous systematic reviews were also identified. A Cochrane review published in 2015 (comprising three RCTs with a total of 2396 women, two of them excluded by us because of age) points out that the short‐term perinatal benefits of intended CS must be weighed against a number of factors, eg increased risk in future pregnancies for uterine rupture and placental ingrowth, maternal surgical complications, and long‐term pediatric health problems. 4 At 2 years the maternal outcomes were similar in the two groups. The reviewers also stated that routine intended CS was widespread in developed countries, without clear evidence that such a policy was preferable. Three RCTs were included in the mentioned Cochrane review but in numbers the TBT trial dominates. In the systematic review from 2016 by Berhan and Haileamlak, 52 27 articles with a total of 258 953 women were included. The relative risk of perinatal mortality and morbidity was analyzed to be two to five times higher if a breech baby was delivered vaginally compared with planned CS. The other systematic reviews had different inclusion criteria than ours, preterm population, 53 and articles published before 1990. 54
Perinatal data from cohort studies (Table S2) were used to calculate the numbers needed to treat (NNT = 1/Absolute Risk reduction), unless the articles did not state it specifically. 55 NNT for perinatal death was 406 in Denmark. 37 In the Netherlands it was 338 45 and in Sweden it was 400. 9 This means that approximately 400 CS were needed to save one baby from dying. NNT to avoid an Apgar score at 5 min of less than 7 in the Danish and Swedish studies were 84 and 34, respectively. 9 , 37 For the five cohort studies reporting Apgar score below 4 at 5 min in this review the calculated NNT was 284. 7 , 19 , 33 , 35 , 44 Several Nordic studies indicate that when the rate of intended vaginal breech deliveries decreased after the TBT the neonatal mortality rate also decreased. In 1998, Roman et al. 5 illustrated the situation in Sweden before (56% of breech presentations planned for vaginal delivery, 0.22% perinatal death) and Ekéus et al. 3 after the TBT (6.4% of breech presentations actual vaginal delivery, 0.07% perinatal death). In Denmark, the rate of intended vaginal delivery was 41% and of neonatal death was 0.13% before the TBT; changing to 27% and 0.05%, respectively, after the TBT. 15 In Norway, the trend was the same, before the TBT 66% of breech presentations were planned for vaginal delivery and neonatal death was 0.14%, after the TBT the numbers were 49% and 0.07%, respectively. 7 Also figures from Holland show the same trend: after TBT was published the vaginal breech delivery rate decreased from 50% to 20%, and this was associated with a significant decrease in perinatal mortality from 0.35% to 0.18%, a decrease of the incidence of a 5‐min Apgar score below 7 from 2.4% to 1.1% and a decrease of birth trauma from 0.29% to 0.08%. 14 According to a report from the Swedish National Board of Health and Welfare, death during delivery due to non‐cephalic presentation constituted 2.4% of all deaths during delivery. 56 Of course, many other factors in obstetric and neonatal care were also improved during this time but the change to more planned CS in term breech deliveries was most likely important. Even if vaginal breech delivery has remained a more common practice in Norway than in the other Nordic countries, they do not present fewer complications in planned vaginal breech deliveries, suggesting that larger volume and more experience do not result in better outcomes. The results in this review are consistent not only with Nordic findings but also large North American studies. 57 , 58
There are contradictory findings whether parity affects the risk associated with vaginal breech delivery. According to Kielland et al, 59 the rate of emergency CS during labor was significantly higher in the group of nulliparous women compared with previously vaginally delivered women (41% vs. 17%) but there was no difference in neonatal mortality or morbidity. On the other hand, Gilbert et al. 13 found increased neonatal morbidity after vaginal breech delivery compared with prelabor CS in both groups but increased neonatal mortality only for nulliparous. Macharey et al. 21 stated that nulliparity is a risk factor for severe adverse neonatal outcome in trial of vaginal breech birth compared with CS (adjusted OR 1.84).
An important reason to avoid CS is the risk of complications in a subsequent pregnancy. Only one study in this review reported this as an outcome. 37 The cohort consisted of 10 608 women and showed placental or uterine complications in subsequent pregnancy in 0.4% after actual vaginal delivery, 0.6% after intended CS, and 0.8% after emergency CS. The difference between the groups was not significant. According to their study data the NNH (Number Needed to Harm = number of CS needed to cause one placental complication) was 6147 for placenta previa and 2410 for uterine rupture (numbers of performed CS to cause a placental complication in a subsequent pregnancy). Hesselman et al. 60 studied 7683 women attempting vaginal birth after previous CS. Complications strongly associated with previous CS were uterine rupture during labor (1.3%), placenta previa, placenta accreta, increased risks of hemorrhage, peripartum hysterectomy, and maternal mortality. Silver et al. 61 found the rate of placenta accreta in a subsequent pregnancy to be 0.24% after one previous CS compared with 6.74% after six previous CS. They also found the rate of placenta previa to be 6.42% after one previous CS compared with 0.23% in women without a previous CS. These findings underline the importance of taking wish for future pregnancies into account when considering a CS.
The strength of this systematic review is the large number of included studies and hence the number of patients decreasing the risk of a type II error related to uncommon outcomes. The approach was systematic and used the PRISMA guidelines and only studies that stated intended mode of delivery were included. The majority of included articles in the present systematic review were cohort studies (n = 25) and drawing conclusions from observational studies might exaggerate the expected effect as opposed to data drawn from RCTs. Our inclusion limits were set to avoid smaller studies at an increased risk, for example for type 2 errors. Only three minor cohort studies were thus excluded from our full‐length search due to this and the results of this systematic review and meta‐analysis would be unlikely to change if these were to be included. As breech presentation is uncommon and severe perinatal morbidity and mortality are rare, very large sample sizes are required to perform randomized trials. Future RCTs are highly unlikely to be conducted also due to ethical considerations. Details that can affect the applicability of the results of this systematic review are, for example, type of breech presentation, induction, and use of oxytocin. Many of the included studies in this review do not report such details. One limitation of this systematic review is that we included articles from 1990, since when delivery care has developed extensively. Furthermore, we have not contacted any authors for non‐published data.
5. CONCLUSION
Intended CS compared with intended vaginal delivery of term breech babies may reduce the perinatal mortality and short‐term child morbidity, whereas there is no evident difference in morbidity for mother or child at long‐term. Short‐term maternal morbidity such as thromboembolic events and urinary incontinence may be reduced by intended CS. It is uncertain whether there is any difference in the rare outcome maternal mortality.
The available evidence regarding long‐term risks for the mother and the infant after intended CS vs. vaginal delivery is sparse. Obstetricians should give the pregnant woman individualized information for her to be able to make an informed decision regarding mode of delivery.
AUTHOR CONTRIBUTIONS
Julia Wängberg Nordborg contributed to study set‐up, selection process, quality assessment of the articles, extracting of data, interpreting the data, and writing the manuscript. Therese Svanberg contributed to literature search, selection process, and revising the manuscript. Annika Strandell contributed to study set‐up, selection process, supervising the meta‐analyses, interpreting the data, and writing the manuscript. Ylva Carlsson contributed to study set‐up, selection process, quality assessment of the articles, extracting of data, performing meta‐analyses, interpreting the data, and writing the manuscript. All authors have read and approved the final version for publication.
FUNDING INFORMATION
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG‐77860).
CONFLICT OF INTEREST
None.
Supporting information
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
We thank Mårten Alkmark, Monica Eriksson Orrskog, Lennart Jivegård, and Ida Stadig for their work with literature search, selecting articles, and extraction of data in 2017.
Wängberg Nordborg J, Svanberg T, Strandell A, Carlsson Y. Term breech presentation—Intended cesarean section versus intended vaginal delivery—A systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2022;101:564‐576. doi: 10.1111/aogs.14333
REFERENCES
- 1. Hickok DE, Gordon DC, Milberg JA, Williams MA, Daling JR. The frequency of breech presentation by gestational age at birth: a large population‐based study. Am J Obstet Gynecol. 1992;166(3):851‐852. [DOI] [PubMed] [Google Scholar]
- 2. Socialstyrelsen. Statistik om graviditeter, förlossningar och nyfödda barn . Stockholm: Socialstyrelsen; 2017 [Sveriges officiella statistik: Hälso‐och sjukvård; March 3, 2017 http://www.socialstyrelsen.se/publikationer/‐3‐3]. 2015.
- 3. Ekeus C, Norman M, Aberg K, Winberg S, Stolt K, Aronsson A. Vaginal breech delivery at term and neonatal morbidity and mortality—a population‐based cohort study in Sweden. J Matern Fetal Neonatal Med. 2019;32:265‐270. [DOI] [PubMed] [Google Scholar]
- 4. Hofmeyr GJ, Hannah M, Lawrie TA. Planned caesarean section for term breech delivery. Cochrane Database Syst Rev. 2015;2015(7):CD000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roman J, Bakos O, Cnattingius S. Pregnancy outcomes by mode of delivery among term breech births: Swedish experience 1987–1993. Obstet Gynecol. 1998;92(6):945‐950. [DOI] [PubMed] [Google Scholar]
- 6. Lawson GW. The term breech trial ten years on: primum non nocere? Birth. 2012;39(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 7. Vistad I, Klungsøyr K, Albrechtsen S, Skjeldestad FE. Neonatal outcome of singleton term breech deliveries in Norway from 1991 to 2011. Acta Obstet Gynecol Scand. 2015;94(9):997‐1004. [DOI] [PubMed] [Google Scholar]
- 8. Pyykönen A, Gissler M, Løkkegaard E, et al. Cesarean section trends in the Nordic Countries—a comparative analysis with the Robson classification. Acta Obstet Gynecol Scand. 2017;96(5):607‐616. [DOI] [PubMed] [Google Scholar]
- 9. Swedish Collaborative Breech Study Group . Term breech delivery in Sweden: mortality relative to fetal presentation and planned mode of delivery. Acta Obstet Gynecol Scand. 2005;84(6):593‐601. [DOI] [PubMed] [Google Scholar]
- 10. Krebs . Underkropspraesentation [Lower body presentation] In Swedish. 2010. https://static1.squarespace.com/static/5467abcce4b056d72594db79/t/5ead83d5c2d94e4ef4d8f178/1588429789157/200403+Breech_final.pdf
- 11. Cheng M, Hannah M. Breech delivery at term: a critical review of the literature. Obstet Gynecol. 1993;82(4 Pt 1):605‐618. [PubMed] [Google Scholar]
- 12. Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375‐1383. [DOI] [PubMed] [Google Scholar]
- 13. Gilbert WM, Hicks SM, Boe NM, Danielsen B. Vaginal versus cesarean delivery for breech presentation in California: a population‐based study. Obstet Gynecol. 2003;102(5 Pt 1):911‐917. [DOI] [PubMed] [Google Scholar]
- 14. Rietberg CC, Elferink‐Stinkens PM, Visser GH. The effect of the Term Breech Trial on medical intervention behaviour and neonatal outcome in The Netherlands: an analysis of 35,453 term breech infants. BJOG. 2005;112(2):205‐209. [DOI] [PubMed] [Google Scholar]
- 15. Hartnack Tharin JE, Rasmussen S, Krebs L. Consequences of the term breech trial in Denmark. Acta Obstet Gynecol Scand. 2011;90(7):767‐771. [DOI] [PubMed] [Google Scholar]
- 16. Socialstyrelsen . Graviditeter, förlossningar och nyfödda barn: Medicinska födelseregistret 1973–2010 Assisterad befruktning 1991–2009 [Internet] Stockholm: Socialstyrelsen; 2012. https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18669/2012‐4‐2.pdf
- 17. Jensen VM, Wüst M. Can Caesarean section improve child and maternal health? The case of breech babies. J Health Econ. 2015;39:289‐302. [DOI] [PubMed] [Google Scholar]
- 18. Mackay DF, Wood R, King A, et al. Educational outcomes following breech delivery: a record‐linkage study of 456947 children. Int J Epidemiol. 2015;44(1):209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goffinet F, Carayol M, Foidart JM, et al. Is planned vaginal delivery for breech presentation at term still an option? Results of an observational prospective survey in France and Belgium. Am J Obstet Gynecol. 2006;194(4):1002‐1011. [DOI] [PubMed] [Google Scholar]
- 20. Bjellmo S, Andersen GL, Martinussen MP, et al. Is vaginal breech delivery associated with higher risk for perinatal death and cerebral palsy compared with vaginal cephalic birth? Registry‐based cohort study in Norway. BMJ Open. 2017;7:e014979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Macharey G, Gissler M, Ulander VM, et al. Risk factors associated with adverse perinatal outcome in planned vaginal breech labors at term: a retrospective population‐based case‐control study. BMC Pregnancy Childbirth. 2017;17:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Checklist from SBU regarding randomized controlled trials [cited 2020 March 11, 2020]. https://www.sbu.se/globalassets/ebm/bedomning_randomiserade_studier_fullfolja.pdf
- 24. Statens beredning för medicinsk och social utvärdering (SBU) . Bedömmning icke randomiserade kontrollerade studier [Assessment of non‐randomized controlled trials], in Swedish. https://www.sbu.se/globalassets/ebm/bedomning_icke_randomiserade_studier_tilldelas.pdf
- 25. Mall för kvalitetsgranskning av studier med kvalitativ forskningsmetodik—patientupplevelser SBU . Utvärdering av metoder i hälso‐och sjukvården och insatser i socialtjänsten: en metodbok. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU). Statens beredning för medicinsk utvärdering SBU. 2014 [cited 2020]. http://www.sbu.se/met
- 26. The Cochrane Collaboration . Review Manager (RevMan) 5.3. ed. Review Manager (RevMan) [Computer program]. Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. https://training.cochrane.org/online‐learning/core‐software‐cochrane‐reviews/revman2014
- 27. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hannah ME, Hannah WJ, Hodnett ED, et al. Outcomes at 3 months after planned cesarean vs. planned vaginal delivery for breech presentation at term: the international randomized Term Breech Trial. JAMA. 2002;287(14):1822‐1831. [DOI] [PubMed] [Google Scholar]
- 29. Hannah ME, Whyte H, Hannah WJ, et al. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917‐927. [DOI] [PubMed] [Google Scholar]
- 30. Hodnett ED, Hannah ME, Hewson S, et al. Mothers' views of their childbirth experiences 2 years after planned Caesarean versus planned vaginal birth for breech presentation at term, in the international randomized Term Breech Trial. J Obstet Gynaecol Can. 2005;27(3):224‐231. [DOI] [PubMed] [Google Scholar]
- 31. Whyte H, Hannah ME, Saigal S, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):864‐871. [DOI] [PubMed] [Google Scholar]
- 32. Adjaoud S, Demailly R, Michel‐Semail S, et al. Is trial of labor harmful in breech delivery? A cohort comparison for breech and vertex presentations. J Gynecol Obstet Hum Reprod. 2017;46:445‐448. [DOI] [PubMed] [Google Scholar]
- 33. Burgos J, Rodríguez L, Cobos P, et al. Management of breech presentation at term: a retrospective cohort study of 10 years of experience. J Perinatol. 2015;35(10):803‐808. [DOI] [PubMed] [Google Scholar]
- 34. Henriksen L, Knutsen H, Laine K. Obstetric care for breech presentations at Oslo University Hospital, Ullevål in the period 2000–2012. Norwegian J Clin Nurs/Sykepleien Forskning. 2019;1‐24. [Google Scholar]
- 35. Hinnenberg P, Toijonen A, Gissler M, Heinonen S, Macharey G. Outcome of small for gestational age‐fetuses in breech presentation at term according to mode of delivery: a nationwide, population‐based record linkage study. Arch Gynecol Obstet. 2019;299:969‐974. [DOI] [PubMed] [Google Scholar]
- 36. Korb D, Schmitz T, Alexander S, et al. Association between planned mode of delivery and severe maternal morbidity in women with breech presentations: a secondary analysis of the PREMODA prospective general population study. J Gynecol Obstet Hum Reprod. 2020;49:101662. [DOI] [PubMed] [Google Scholar]
- 37. Krebs L, Langhoff‐Roos J. Elective cesarean delivery for term breech. Obstet Gynecol. 2003;101(4):690‐696. [DOI] [PubMed] [Google Scholar]
- 38. Krebs L, Langhoff‐Roos J, Weber T. Breech at term—mode of delivery? A register‐based study. Acta Obstet Gynecol Scand. 1995;74(9):702‐706. [DOI] [PubMed] [Google Scholar]
- 39. Pasupathy D, Wood AM, Pell JP, Fleming M, Smith GC. Time trend in the risk of delivery‐related perinatal and neonatal death associated with breech presentation at term. Int J Epidemiol. 2009;38(2):490‐498. [DOI] [PubMed] [Google Scholar]
- 40. Pradhan P, Mohajer M, Deshpande S. Outcome of term breech births: 10‐year experience at a district general hospital. BJOG. 2005;112(2):218‐222. [DOI] [PubMed] [Google Scholar]
- 41. Rietberg CC, Elferink‐Stinkens PM, Brand R, van Loon AJ, Van Hemel OJ, Visser GH. Term breech presentation in The Netherlands from 1995 to 1999: mortality and morbidity in relation to the mode of delivery of 33824 infants. BJOG. 2003;110(6):604‐609. [PubMed] [Google Scholar]
- 42. Thorpe‐Beeston JG, Banfield PJ, Saunders NJ. Outcome of breech delivery at term. BMJ. 1992;305(6856):746‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ulander VM, Gissler M, Nuutila M, Ylikorkala O. Are health expectations of term breech infants unrealistically high? Acta Obstet Gynecol Scand. 2004;83(2):180‐186. [DOI] [PubMed] [Google Scholar]
- 44. Vendittelli F, Pons JC, Lemery D, Mamelle N, Network OotAS . The term breech presentation: neonatal results and obstetric practices in France. Eur J Obstet Gynecol Reprod Biol. 2006;125(2):176‐184. [DOI] [PubMed] [Google Scholar]
- 45. Vlemmix F, Bergenhenegouwen L, Schaaf JM, et al. Term breech deliveries in the Netherlands: did the increased cesarean rate affect neonatal outcome? A population‐based cohort study. Acta Obstet Gynecol Scand. 2014;93(9):888‐896. [DOI] [PubMed] [Google Scholar]
- 46. Macharey G, Vaisanen‐Tommiska M, Gissler M, et al. Neurodevelopmental outcome at the age of 4 years according to the planned mode of delivery in term breech presentation: a nationwide, population‐based record linkage study. J Perinat Med. 2018;46:323‐331. [DOI] [PubMed] [Google Scholar]
- 47. Bin YS, Ford JB, Nicholl MC, Roberts CL. Long‐term childhood outcomes of breech presentation by intended mode of delivery: a population record linkage study. Acta Obstet Gynecol Scand. 2017;96(3):342‐351. [DOI] [PubMed] [Google Scholar]
- 48. Bin YS, Roberts CL, Ford JB, Nicholl MC. Outcomes of breech birth by mode of delivery: a population linkage study. Aust N Z J Obstet Gynaecol. 2016;56(5):453‐459. [DOI] [PubMed] [Google Scholar]
- 49. Liu S, Liston RM, Joseph KS, et al. Maternal mortality and severe morbidity associated with low‐risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176(4):455‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schutte JM, Steegers EA, Santema JG, Schuitemaker NW, van Roosmalen J, Maternal Mortality Committee Of The Netherlands Society Of Obstetrics . Maternal deaths after elective cesarean section for breech presentation in the Netherlands. Acta Obstet Gynecol Scand. 2007;86(2):240‐243. [DOI] [PubMed] [Google Scholar]
- 51. EShdoAP . Committee opinion no. 644: the apgar score. Obstet Gynecol. 2015;126(4):e52‐e55. [DOI] [PubMed] [Google Scholar]
- 52. Berhan Y, Haileamlak A. The risks of planned vaginal breech delivery versus planned caesarean section for term breech birth: a meta‐analysis including observational studies. BJOG. 2016;123(1):49‐57. [DOI] [PubMed] [Google Scholar]
- 53. Bergenhenegouwen LA, Meertens LJ, Schaaf J, et al. Vaginal delivery versus caesarean section in preterm breech delivery: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2014;172:1‐6. [DOI] [PubMed] [Google Scholar]
- 54. Håheim LL, Albrechtsen S, Berge LN, et al. Breech birth at term: vaginal delivery or elective cesarean section? A systematic review of the literature by a Norwegian review team. Acta Obstet Gynecol Scand. 2004;83(2):126‐130. [DOI] [PubMed] [Google Scholar]
- 55. Wängberg Nordborg J. Term Breech Presentation—Caesarean Section Versus Vaginal Delivery. HTA; 2017:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Socialstyrelsen . Dödfödda barn; 2018.
- 57. Lee K‐S, Khoshnood B, Sriram S, et al. Relationship of cesarean delivery to lower birth weight‐specific neonatal mortality in singleton breech infants in the United States. Obstet Gynecol. 1998;92:769‐774. [DOI] [PubMed] [Google Scholar]
- 58. Kotaska A, Menticoglou S. No. 384‐management of breech presentation at term. J Obstet Gynaecol Can. 2019;41:1193‐1205. [DOI] [PubMed] [Google Scholar]
- 59. Kielland‐Kaisen U, Paul B, Jennewein L, et al. Maternal and neonatal outcome after vaginal breech delivery of nulliparous versus multiparous women of singletons at term‐A prospective evaluation of the Frankfurt breech at term cohort (FRABAT). Eur J Obstet Gynecol Reprod Biol. 2020;252:583‐587. [DOI] [PubMed] [Google Scholar]
- 60. Hesselman S, Högberg U, Ekholm‐Selling K, Råssjö EB, Jonsson M. The risk of uterine rupture is not increased with single‐compared with double‐layer closure: a Swedish cohort study. BJOG. 2015;122(11):1535‐1541. [DOI] [PubMed] [Google Scholar]
- 61. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226‐1232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3
Table S4


