Abstract
A standard method for diagnosing measles is to detect measles-specific immunoglobulin M (IgM) in the serum of infected persons. Interpreting a positive IgM result from a person with suspected measles can be difficult if the person has recently received a measles vaccine. We have previously demonstrated that measles-specific IgM may persist for at least 8 weeks after primary vaccination, but it is unknown how quickly IgM appears. This study determined the timing of the rise of measles-specific IgM and IgG after primary measles vaccination with Schwartz vaccine. Two hundred eighty 9-month-old children from Ethiopia presenting for routine measles vaccination were enrolled. Sera were collected before and either 1, 2, 3, or 4 weeks after vaccination and tested for measles-specific antibodies by an IgM capture enzyme immunoassay (EIA) and by an indirect IgG EIA. A total of 209 of the 224 children who returned for the second visit had prevaccination sera that were both IgM and IgG negative. The postvaccination IgM positivity rates for these 209 children were 2% at 1 week, 61% at 2 weeks, 79% at 3 weeks, and 60% at 4 weeks. The postvaccination IgG positivity rates were 0% at 1 week, 14% at 2 weeks, 81% at 3 weeks, and 85% at 4 weeks. We conclude that an IgM-positive result obtained by this antibody capture EIA is difficult to interpret if serum is collected between 8 days and 8 weeks after vaccination; in this situation, the diagnosis of measles should be based on an epidemiologic linkage to a confirmed case or on the detection of wild-type measles virus.
The detection of measles-specific immunoglobulin M (IgM) has become a standard diagnostic method for the laboratory confirmation of measles infection. A previous study of persons who developed detectable measles IgM after natural measles infection demonstrated that 77% developed IgM within 72 h after rash onset and 100% developed IgM at 4 to 11 days after rash onset. Moreover, more than 90% of persons remained IgM positive for 28 days (7). The interpretation of positive IgM results from persons with suspected natural measles infections becomes more difficult if these persons have been vaccinated recently. The timing of the rise and decline of measles IgM in the first 4 weeks after primary vaccination has not been well documented. We recently demonstrated that measles IgM declines rapidly between 4 and 8 weeks after primary measles vaccination (6). However, the rate of IgM positivity was lower than expected at 4 weeks, raising the possibility that IgM is already declining by 4 weeks. In this report, we evaluate the kinetics of measles-specific IgM and IgG in the first 4 weeks after primary measles vaccination.
MATERIALS AND METHODS
The sera for this report were part of a study to evaluate the comparative detection of measles-specific IgM antibodies in serum and oral fluid samples after primary measles vaccination. The study group consisted of 280 9-month-old infants presenting for routine measles vaccination to Tekle Haimanot Health Centre, Addis Ababa, Ethiopia, between August 1996 and January 1997. After informed consent was obtained, sera (by heel stick) and oral fluid samples were collected before and either 1, 2, 3, or 4 weeks after routine measles vaccination (Schwartz vaccine). Infants were enrolled into sequential weeks according to when they presented for measles vaccination (e.g., subjects 1 through 4 were enrolled into weeks 1 through 4, respectively; subjects 5 through 8 were enrolled into weeks 1 through 4, etc.). Samples were frozen at −70°C and shipped to the Centers for Disease Control and Prevention (CDC) on dry ice. The comparison of serum and oral fluid samples will be reported separately in the future.
At CDC, serum samples were tested for measles-specific IgM antibodies by using a monoclonal-based antibody-capture enzyme immunoassay (EIA) (8). Microtiter plates were coated with goat anti-human IgM antibodies diluted in phosphate-buffered saline (PBS), incubated for 1 h at 37°C, and then washed. Next, serum diluted 1:200 in PBS with 0.5% gelatin and 0.15% Tween 20 (PBS-GT) was added to four consecutive wells. The plates were then incubated for 1 h at 37°C and washed. Baculovirus-measles virus nucleoprotein or sf9-uninfected cell control lysate diluted in PBS-GT with 4% normal goat serum and 0.3% sodium deoxycholate was added to duplicate wells. The plates were then incubated for 2 h at 37°C and washed. Biotinylated monoclonal antibody (83VIIKK2) in PBS-GT was added to the plates, and the plates were incubated for 1 h at 37°C and washed. The plates were then incubated at 37°C with streptavidin-peroxidase in PBS-GT for 20 min and washed again. Tetramethylbenzidine substrate was added for 15 min, and the reaction was stopped by acidification. Finally, optical densities for antigen-positive and antigen-negative wells were determined photometrically.
IgM-EIA results were expressed as the average difference in measured optical density values between duplicate wells of positive antigen (P) and negative tissue culture control antigen (N). Although the presence of prevaccination sera made it possible to calculate study-specific cutoff values, we chose to use standard cutoff values to be consistent with our reporting practices of individual samples received for diagnostic testing. These standard cutoff values were calculated previously to maximize specificity and to take into account run-to-run variability. Specifically, a sample was considered IgM positive if P − N was ≥0.10 and P/N was ≥3. A sample was considered IgM borderline if either (i) P − N was ≥0.09 but <0.10 and P/N was ≥3 or (ii) P − N was ≥0.10 and P/N was ≥2 but <3. Sera were also tested for the presence of measles-specific IgG antibodies by using an indirect EIA (3, 8). The standard IgG cutoff values used were as follows. A sample was considered positive if P − N was ≥0.09 and P/N ≥3, and a sample was considered borderline if either (i) P − N was ≥0.08 but <0.09 and P/N was ≥3 or (ii) P − N was ≥0.09 and P/N was ≥2 but <3.
RESULTS
Two hundred twenty-four (80%) of the 280 enrolled children returned and had serum collected during the second visit. The median age at vaccination was 9.4 months (range, 7.3 to 13.0 months), and 111 (49.6%) of the children were males.
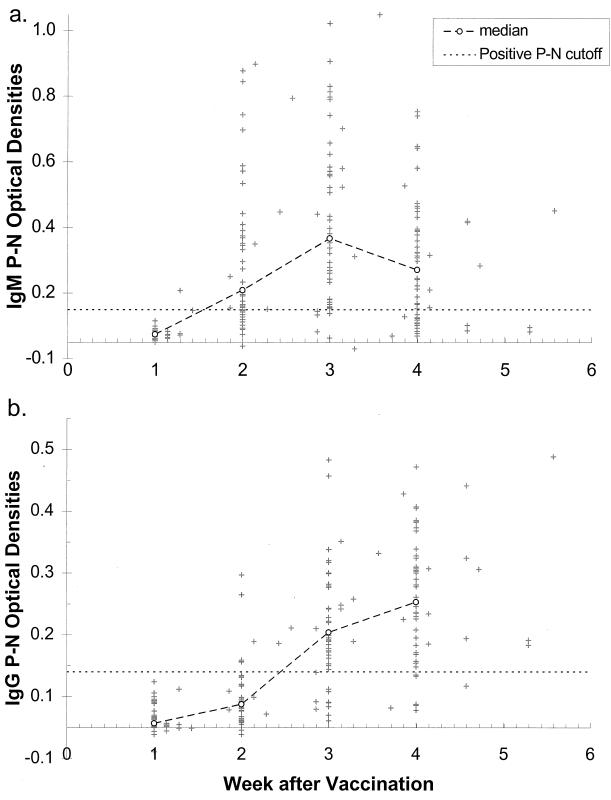
Two hundred and nine (93.3%) of the 224 children were both IgM and IgG negative prior to vaccination. Table 1 displays IgM and IgG results by week after vaccination for these 209 children. Approximately 50 children returned for follow-up visits each week during the first 4 weeks after vaccination. In week 1, 98% and 100% of samples were IgM negative and IgG negative, respectively. The ratio of IgM-positive samples reached 61% at week 2, peaked at 79% in week 3, and declined to 60% in week 4. As expected, the highest rate of IgG positivity occurred in weeks 4 and 5. The timing of the IgM and IgG responses is demonstrated graphically in Fig. 1a and b, which show the optical densities (P − N) for IgM and IgG by time since vaccination for the 209 children. The optical densities peaked at week 3 for IgM and were declining at week 4. As expected, the IgG optical densities rose more slowly.
TABLE 1.
Percentage of persons with measles-specific IgM and IgG by week after measles vaccination for the 209 children who were IgM- and IgG-negative prior to vaccination
| EIA result | Rate of result (%)
from:
|
||||
|---|---|---|---|---|---|
| Week 1 (n = 52)a | Week 2 (n = 49) | Week 3 (n = 52) | Week 4 (n = 48) | Weeks 5 and 6 (n = 8) | |
| IgM | |||||
| Negative | 98 | 31 | 15 | 29 | 50 |
| Positive | 2 | 61 | 79 | 60 | 50 |
| Borderline | 0 | 8 | 6 | 10 | 0 |
| IgG | |||||
| Negative | 100 | 80 | 17 | 13 | 13 |
| Positive | 0 | 14 | 81 | 85 | 88 |
| Borderline | 0 | 6 | 2 | 2 | 0 |
| Median no. of days after vaccination | 7 | 14 | 21 | 28 | 32.5 |
| Range | 7–10 | 13–17 | 18–23 | 25–29 | 32–39 |
Number of children who returned for follow-up visit.
FIG. 1.
(a) Difference in IgM optical densities of positive and negative (P − N) wells for each infant by week after measles vaccination. The larger dashed line represents the 50th percentile for each week (a week was defined as a multiple of 7 ± 3 days; e.g., week 2 = 11 to 17). The horizontal dashed line represents the cutoff P − N value of 0.10, which was used in combination with the P/N ratio to determine whether the specimen was considered IgM positive, IgM borderline, or IgM negative. (b) Difference in IgG optical densities of P − N wells for each infant by week after vaccination. As in panel a, the larger dashed line represents the 50th percentile for each week, and the horizontal dashed line represents the cutoff P − N value of 0.09. Both graphs include only data for infants who were both IgM and IgG negative before vaccination.
Fifteen children had prevaccination samples that were not negative for both IgM and IgG: three had uninterpretable IgG results because of high background; five were IgG positive and four had borderline IgG results (all nine were IgM negative); two were IgM positive but IgG negative; and one was both IgM positive and IgG positive. After vaccination, three children continued to have uninterpretable IgG results (one was IgM negative in week 1 after vaccination and two were IgM positive in week 3 after vaccination). Ten of the remaining 12 children were IgG positive after vaccination, and the other two children who were not IgG positive were IgM positive (for the 12 children, 0 of 3, 1 of 3, and 2 of 4 were IgM positive at weeks 1, 2, and 3 after vaccination, respectively). The exclusion of these 15 children likely did not bias the results, given the high rates of IgM and IgG positivity after vaccination.
DISCUSSION
In this study, only 2% of persons had measles-specific IgM in the first 7 days after primary measles vaccination. In addition, measles-specific IgM peaked at week 3 and was already declining by week 4. This finding explains the unexpectedly low IgM positivity rate of 73% we found at week 4 after vaccination in our previous study conducted in the United States (7).
Only 14% of persons were IgG positive at 14 days after vaccination, with the rate rising sharply at week 3 (81%) and reaching 85% by week 4. Although this rate of 85% is consistent with the expected seroconversion rate of 85% in 9-month-old children from developing countries (4, 5), data from Carson and colleagues (2) suggest that seroconversion rates may be higher when sera are collected 6 to 8 weeks after vaccination compared with 4 to 5 weeks after vaccination. Because we collected few serum samples after week 4 in this study, we do not know how many of the IgG-negative persons would have developed measles-specific IgG later than 4 weeks.
This study was conducted in a country where routine measles vaccination is given earlier than in the United States (9 months versus 12 to 15 months) (1), with a subsequent lower seroconversion rate. It is possible that in addition to the seroconversion rate, the timing of IgM and IgG responses is affected by factors such as age, nutritional status, and the type of vaccine used. Therefore, it would be useful to repeat this study in the United States to verify the generalizability of the findings. Even so, the results may still be helpful when designing vaccine trials to optimize the likelihood of finding measles IgM or IgG.
These findings may also help to guide the interpretation of positive IgM results from persons with suspected measles who have been vaccinated recently. For instance, the findings from this study suggest that if persons with suspected measles have been vaccinated within 7 days of the onset of rash and are IgM positive according to this antibody-capture IgM EIA, this IgM is probably due to wild-type measles infection. Because blood samples were collected weekly, we have no information about IgM results on days 8–13 after vaccination, and therefore must assume for now that vaccine-induced IgM may be present as early as 8 days after primary vaccination. We have previously demonstrated in a U.S. cohort that IgM may persist until at least 8 weeks after primary measles vaccination for 10% of persons (6). Combining the results from the previous study with the current study, we conclude that an IgM-positive result obtained by this antibody-capture IgM EIA may be difficult to interpret if sera are collected between 8 days and 8 weeks after vaccination; in this situation, the diagnosis of measles should be based on an epidemiologic link to a confirmed case or on detection of wild-type measles virus.
ACKNOWLEDGMENTS
We acknowledge James Alexander for his assistance in study design, Elizabeth Behaimanot for subject enrollment and data collection, Kidane Woldeyesus for data entry, John O’Connor for his editorial support, and Alisa Murray for her laboratory assistance.
REFERENCES
- 1.American Academy of Pediatrics. Measles. In: Peter G, editor. 1997 Red Book: report of the Committee on Infectious Diseases. Elk Grove Village, Ill: American Academy of Pediatrics; 1997. pp. 344–357. [Google Scholar]
- 2.Carson M M, Spady D W, Albrecht P, Beeler J A, Thipphawong J, Barreto L, Grimsrud K M, Pabst H F. Measles vaccination of infants in a well-vaccinated population. Pediatr Infect Dis J. 1995;14:17–22. doi: 10.1097/00006454-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Erdman D, Anderson L, Adams D, Stewart J, Markowitz L, Bellini W. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991;29:1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halsey N A. The optimal age for administering measles vaccine in developing countries. In: Halsey N A, De Quadros C, editors. Recent advances in immunization. A bibliographic review. Washington, D.C: Pan American Health Organization; 1983. pp. 4–17. [Google Scholar]
- 5.Halsey N A, Boulos R, Mode F, Andre J, Bowman L, Yaeger R G, Troureau S, Rohde J, Boulos C. Response to measles vaccine in Haitian infants 6 to 12 months old. Influence of maternal antibodies, malnutrition, and concurrent illnesses. N Engl J Med. 1985;313:544–549. doi: 10.1056/NEJM198508293130904. [DOI] [PubMed] [Google Scholar]
- 6.Helfand R F, Gary H E, Jr, Atkinson W L, Nordin J D, Keyserling H L, Bellini W J. Decline of measles-specific immunoglobulin M antibodies after primary measles, mumps, and rubella vaccination. Clin Diagn Lab Immunol. 1998;5:135–138. doi: 10.1128/cdli.5.2.135-138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helfand R F, Heath J L, Anderson L J, Maes E F, Guris D, Bellini W J. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis. 1997;175:195–199. doi: 10.1093/infdis/175.1.195. [DOI] [PubMed] [Google Scholar]
- 8.Hummel K, Erdman D, Heath J, Bellini W. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol. 1992;30:2874–2880. doi: 10.1128/jcm.30.11.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]