Abstract
This paper reports various types of cancer, their incidence, and prevalence all over the globe. Along with the discovery of novel natural drugs for cancer treatment, these present a promising option which are eco-friendly, safe, and provide better acceptability in comparison to synthetic agents that carries multiple side effects. This paper provides an idea about various nanocarriers and phytochemicals, along with how their solubility and bioavailability can be enhanced in nanocarrier system. This report combines the data from various literature available on public domain including PubMed on research articles, reviews, and along with report from various national and international sites. Specialized metabolites (polyphenols, alkaloids, and steroids etc) from medicinal plants are promising alternatives to existing drugs. Studies have suggested that the treatment of cancer using plant products could be an alternative and a safe option. Studies have shown with the several cell lines as well as animal models, that phytomolecules are important in preventing/treating cancer. Phytochemicals often outperform chemical treatments by modulating a diverse array of cellular signaling pathways, promoting cell cycle arrest, apoptosis activation, and metastatic suppression, among others. However, limited water solubility, bioavailability, and cell penetration limit their potential clinical manifestations. The development of plant extract loaded nanostructures, rendering improved specificity and efficacy at lower concentrations could prove effective. Nanocarriers, such as liposomes, nanostructured lipids, polymers, and metal nanoparticles, have been tested for the delivery of plant products with enhanced effects. Recent advances have achieved improvement in the the stability, solubility, bioavailability, circulation time, and target specificity by nanostructure-mediated delivery of phytochemicals. Nanoparticles have been considered and attempted as a novel, targeted, and safe option. Newer approaches such as phyto-nanocarriers with carbohydrates, lignin, and polymers have been considered even more selective and effective modes of drug delivery in biomedical or diagnostic applications.
Keywords: Cancer, Phytochemical, Phyto-nanocarriers, Nanoparticles, Phytotherapeutics, Polyphenols, Alkaloids, Steroids, Macromolecules, Anticancer
Introduction
Cancer is a genetic disease that can be characterized by the alteration of genes. Each year, cancer ranks as the second leading cause of fatalities (Jubeen et al. 2019). Uncontrolled development and extension of the cell, due to which cell stop responding on their checkpoints, which in turn leads to growth of tumor and metastasis is known as cancer (Rai et al. 2014). It is a Non-Communicable Disease (NCD) and is widely recognized as a global threat (Christina et al. 2019). Every fourth person is at risk of their life due to cancer, which is an alarming situation (Jemal et al. 2011). A person, who is prone to cancer, has weak immunity or suppressed immune system. The factors, such as older age, stress conditions, chronic devitalizing ailments, history of chemotherapy, as well as drug resistance, all these factors may further increase the risk of cancer development (Hanahan and Weinberg 2011).
A load of cancer all over the world in 2020 is raised approximately 19.3 million new cases and about 10 million deaths. There are more than thirty types of cancer that are reported to affect both men and women, for example, breast, lung, liver, stomach cancer, etc. (Bray et al. 2018). Cervical cancer is one of the most prevalent cancers and a leading cause of death in women worldwide (Bray et al. 2018). The prevalence and fatality rate have risen despite recent advancements in early identification and advance therapy approaches. Nearly 7,60,000 cases of stomach cancer are identified globally, making gastric cancer (GC) the second most common cause of cancer-related deaths (Gurunathan et al. 2015). The most lethal of all female-specific reproductive malignancies is ovarian cancer. It is thought to be the sixth most common reason for cancer deaths in women worldwide, and it roughly accounts for 4% of fatalities in women worldwide. Hepatocellular carcinoma (HCC), also known as liver cancer, is one of the most frequent malignant tumors across worldwide (Anand et al. 2008). One of the most prevalent malignancies worldwide, but primarily in western countries, is colon cancer (Jemal et al. 2011). The elevated occurrences are frequently linked to diet and dietary consumption habits. A prevalent male malignancy that is spreading across the globe is prostate cancer (Almatroodi et al. 2021). With an excepted 2.3 million new cases, female breast cancer has the most commonly diagnosed malignancy. With a projected 1.8 million fatalities, lung cancer remained the largest cause of cancer mortality, followed by colorectal, liver, stomach, and female breast cancers. As per the estimate, in 2040, the world’s cancer burden is anticipated to reach 28.4 million cases (Sung et al. 2021). In developed countries, like the USA, new cervical cancer cases were approx. 12,990 with 4120 deaths in the year 2016 (ACS 2016). Developing countries stand 2nd rank while developed countries stand 4th rank in cases of cervical cancer (Bray et al. 2018). The risk of cervical cancer is increased by having more full-term births, immunosuppressants (especially for HIV), smoking, oral contraceptive usage, and low socioeconomic levels, all of which are essentially causing impact on onset of disease (Bray et al. 2018). India alone contributes 25% of cervical cancer death cases among developing countries. According to the Population Cancer Registry of ICMR report, the occurrence and death were lesser in other parts of the countries compared to the northeastern region where this scale is highest (Zargar et al. 2021). Adverse medication reactions caused by chemical cancer treatments as well are substantial contributor to morbidity and mortality worldwide.
Characteristics of cancer cells
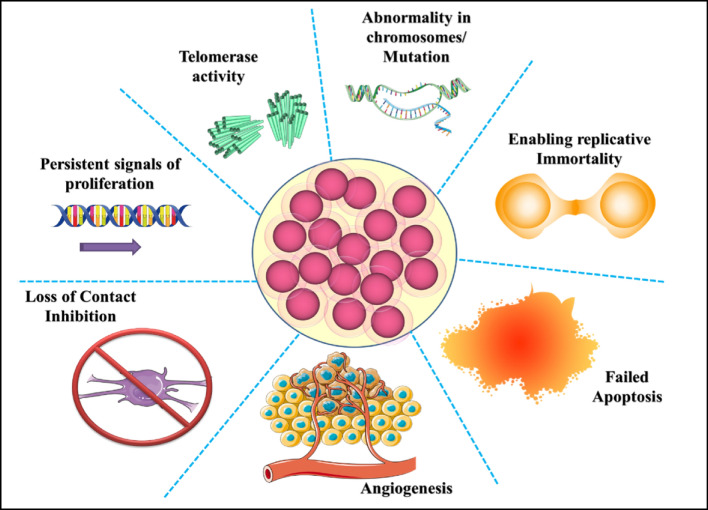
The cancer cells spread to tissues and organs outside of the site of the primary tumor through blood circulation (Jubeen et al. 2020) and may lead to the development of new tumors (Dhupal and Chowdhary 2020). Tumor cells do not depend on any growth factor, unlike the normal cell, to reach a proliferative state from a quiescent state, and also lack contact inhibition properties (Cheng et al. 2021), resulting in uncontrolled growth (Basker et al. 2010). No programmed cell death phenomenon, i.e., apoptosis (it is a kind of silent killing which does not harm nearby cells), is performed by cancerous cell (Bao et al. 2014). These cells are genetically unstable cells because of loss of gene function by mutator phenotype and oncogene-induced DNA replication stress model (Yao and Dai 2014) and do not maintain diploid chromosome complements on dividing by deletion, addition, or translocation (Cheng et al. 2021). Cancer cells are energy-hungry cells that have high metabolic requirements, so they mainly perform glycolysis. There is an enzyme known as telomerase which is responsible for maintaining the end of the chromosome that allows a cell to divide. Telomerase activity is lost in the case of normal cells (Hanahan and Weinberg 2011).The blood vessels nearby the tumor can offer a pathway for the detached cells to enter the circulatory system and metastasis to distant sites (Cheng et al. 2021); there are some characteristics as shown in Fig. 1 that may contribute to tumorigenesis and differentiate cancer cells from healthy individuals (Huynh et al. 2017).
Fig. 1.
Characteristics of cancerous cells
Causes of cancer
As per an estimate and statistical analysis revealed that in developed countries, environmental and lifestyle factors are the key cause of 90–95% of cancer cases (Elisabete 2010). The lifestyle (Karikas 2010) variables include cigarette smoking (25–30% abnormalities are caused by cigarettes) (Jemal et al. 2011), diet, fried food (35% are related to food) (Boutayeb and Boutayeb 2005), red meat (Jemal et al. 2011), alcohol (Boutayeb and Boutayeb 2005; Jemal et al. 2011), sun exposure (Firdhouse and Lalitha 2015), environmental pollutants (Sunget al. 2021), infections (15–20% are related to infections) (Huynh et al. 2017), stress (Anand et al. 2008), obesity (Jemal et al. 2011), and physical inactivity (Jemal et al. 2011); overall 5–10% of all cancer cases can be attributable to genetic abnormalities, and change in cell signaling pathways (Xia et al. 2018).
Tobacco
Tobacco assigns to the development of around 20 types of cancers and has become the leading aspect of cancer globally (Sung et al. 2021). More than 70% cases of lung cancer around the globe are because of tobacco consumption (Gaziano et al. 2009). After observing the data and consequences of the health of Tobacco on people, World Health Organization (WHO) provided the Framework Convention on Tobacco Control (FCTC) in 2003. This convention aimed to reduce the use of cigarettes globally, and this agreement was signed by 168 countries with control strategies on tobacco (Boutayeb and Boutayeb 2005). The first country was the USA which executed a harsh control campaign on tobacco in the 1960s, resulting in a reduction in the number of smokers (Siegel et al. 2020).
Alcohol
Chronic alcohol consumption leads to causes of certain cancer, such as aerodigestive tract, also enhanced risk of breast, hepatocellular, and colorectal cancer (Boutayeb and Boutayeb 2005). Ethanol acts as a carcinogen, and when it is metabolized, it leads to the generation of acetaldehyde and free radicals which primarily result in alcohol-associated carcinogenesis (Anand et al. 2008). The International Agency for Research on Cancer (IARC) categorized alcoholic drinks as cancer-causing agents for humans (Katzke et al. 2015). Alcohol intake is highly associated with cancer development, including mouth (Jemal et al. 2011), pharynx (Sung et al. 2021), larynx (Boutayeb and Boutayeb 2005), and esophagus (Jemal et al. 2011). Some other cancers associated with alcohol consumption are cancer of stomach, gall bladder (Katzke et al. 2015), pancreas, and lung (Gupta et al. 2016).
Diet
Carcinogens, such as nitrates, nitrosamines, pesticides, and dioxins, come from food, food additives, or cooking, those can be the causes of cancer (Boutayeb and Boutayeb 2005). Excessive intake of red meat may be correlated to colorectal, prostate, bladder, breast, gastric, pancreatic, and oral cancer (Jemal et al. 2011; Katzke et al. 2015). Nitrates and nitrites are potent carcinogens employed in meat processing as they bind to myoglobin and prevent the generation of botulinum exotoxin (Anand et al. 2008). Long-term exposure to food additives, including azo dyes and nitrite preservatives, have been linked to the development of cancer. Bisphenol from plastic food containers can also contaminate food, raising the risk of breast and prostate cancer (Srikrishna and Sachsenmeier 2021). Most trans fatty acids, processed sugars, refined wheat, saturated and unsaturated fatty acids foods have been linked to cancer (Katzke et al. 2015; Anand et al. 2008).
Obesity
Overweight and obesity are considered as more common cause over the world than ever before-in adults (by about 27%, and in children by about 47%). When caloric intake exceeds and energy spent decreases through metabolism and physical exercise, obesity sets in. Fat is deposited and accumulated as ectopic fat in tissues as a result of excessive or aberrant fat tissue formation that surpasses the genetically and epigenetically specified adipose tissue reserves, increasing the risk for numerous diseases, including cancer (Avgerinos et al. 2019). According to American Society study, obesity is linked to enhanced mortality cases among various cancer types, such as colon (Jemal et al. 2011), breast, endometrium (Boutayeb and Boutayeb 2005), kidney (Jemal et al. 2011), esophagus (Jemal et al. 2011), gastric, pancreas (Boutayeb and Boutayeb 2005), prostate (Boutayeb and Boutayeb 2005), gallbladder (Anand et al. 2008), and liver cancer (Katzke et al. 2015). A relative risk of more than 1.5/5 kg/m2 enhances the BMI and was reported in esophageal cancer for both males and females (Katzke et al. 2015).
Infectious agents
Viruses are one of the main source of cancer caused by infection. The infection caused by Helicobacter pylori is a relevant illustration of the inflammatory process of carcinogenesis (Gurunathan et al. 2015). About two-thirds of people on the planet have the stomach infection caused by H. pylori bacteria. Fusobacterium nucleatum influences the tumor immune microenvironment and encourages the development of intestinal and colorectal tumors (Gurunathan et al. 2015). Other viruses which are associated with various types of cancer are the Human T-lymphotropic virus, Herpes, heaptitis B and HIV virus (Jemal et al. 2011), and Hepatitis C virus (Anand et al. 2008). These agents cause more harm to the people who are deficient of antioxidants and antioxidant nutrients taken from fresh fruits and vegetables (Gupta et al. 2016). HBV and HCV viruses generate reactive oxygen species by chronic inflammation as well as activate NF-kB (Nuclear Factor Kappa B) an inflammatory marker and lead to mutagenesis (Anand et al. 2008).
Environmental pollution
This aspect includes both outdoor as well as indoor pollution (Sung et al. 2021). Outdoor pollution may be caused by various pollutants including Polycyclic Aromatic Hydrocarbons (PAHs), nitric oxide, and motor vehicle exhaust, while indoor pollution might be caused by tobacco smoke formaldehyde, volatile organic compounds, food additives, nitrates, pesticides, dioxins, metals, metalloids, pharmaceuticals medicines, and cosmetics etc (Sung et al. 2021). All of these pollutants are reported to enhance the risk of leukemia, lymphoma, testicular, colorectal (Anand et al. 2008), bladder, brain tumor (Firdhouse and Lalitha 2015).
Radiation
Cancer has been reported to be caused by both ionizing (X-ray, gamma rays) and non-ionizing radiations (UV, IR, microwaves). These might be involved in enhanced cases of leukemia, lymphoma, thyroid (Sung et al. 2021), skin, sarcoma, lung, and breast cancers (Anand et al. 2008). UV radiations have also been reported to be the more common cause of lip cancer specifically for lower lips (Gupta et al. 2016).
Abruption of cell signaling pathways
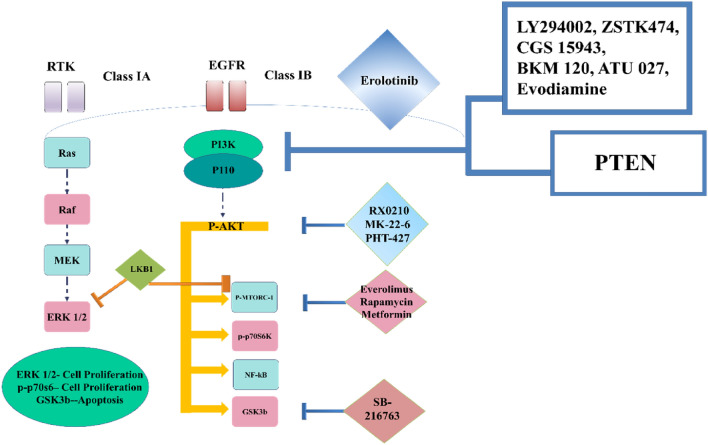
The cell signaling process is the way by which a cell responds to external signaling molecules that bind to receptors on the cell membrane or in the cytoplasm of cells (Nair et al. 2019). The binding of receptors sends signals to the nucleus, where they are translated into gene expression, resulting in biological effects and cellular responses. Signaling pathways are less tightly controlled during carcinogenesis. Cancer is characterized by abnormal regulation and cross-talk of cell signal transduction pathways (Khan et al. 2019a), and obstruction of or anomalies in signaling pathways can lead to excessive cell proliferation (Huynh et al. 2017), apoptotic resistance, angiogenesis, invasion, and metastasis (Huynh et al. 2017), all of which contribute to cancer development and progression (Xia et al. 2018). Aberrant Rat Sarcoma Virus “RAS” protein activation (family of related proteins that shows expression in all the animal cells lineage and organs), the principal oncogenic operator that controls the functioning of important signaling pathways involved in the onset (Khan et al. 2019a) and development of human malignancies is abnormally activated RAS proteins (Nair et al. 2019). RAS gene mutation, which are most common in human malignancies, are the primary cause of persistent RAS activation and associated pathological diseases, such as cancer (Huynh et al. 2017). RAS is the key upstream regulator of several highly conserved signaling systems linked to a variety of important cellular processes that are required for appropriate homeostasis (Khan et al. 2019a) (Fig. 2). Mutated or oncogenic RAS activates a complexly interlinked signaling pathway, such as Mitogen-Activated Protein Kinases (MAPK) (Murthy et al. 2018), Phosphatidylinositol-3-Kinase (PI3K)/Akt pathways (Chamcheu et al. 2019), Protein Kinase C (PKC), and Ral Guanine Nucleotide Dissociation Stimulator (RalGDS), among others, resulting in uncontrolled transcriptional expression and reprogramming in the functioning of a range of nuclear and cytosolic effectors critically associated with the hallmarks of carcinogenesis (Khan et al. 2019a). The PI3K/Akt is a survival pathway and deregulation in this signaling pathway leads to malignancy. This pathway is a hub involved in a variety of physiologic functions linking growth factors nutrients, energy availability to lipid and protein synthesis, metabolism, cell growth, survival, apoptosis, angiogenesis, and tissue development (Nair et al. 2019). Disruption of these pathways leads to variety of malignancies, such as melanoma and non-melanoma skin tumors, and is emerging as clinically important therapeutic targets (Chamcheu et al. 2019). Signal Transducer and Activator of Transcription 3 (STAT3) signaling in tumor cells are important for cell proliferation and survival, and it also regulates many activities in the non-transformed cells that make up the tumor microenvironment. As a result, increased STAT3 activation is a characteristic of many cancers, and it happens frequently in response to cytokines from the Interleukin (IL-6 and IL-10) families (these are proinflammatory cytokines) (Haura et al. 2005), on the other hand, regulates the effector's function of tumor-associated immune and stromal cells, which help tumors grow by inhibiting the host’s antitumor immune response (Wang et al. 2018). The molecular actors of STAT3 signaling and its upstream JAK kinases represent feasible therapeutic targets for the treatment of cancer since STAT3 mediates tumorigenic effects in a variety of cell types (Huynh et al. 2017).
Fig. 2.
The PI3K/Akt survival pathway is a critical downstream target of the rat sarcoma virus (Ras) family of proteins, largely involved in cell proliferation. De-regulation of the PI3K/Akt signaling pathway is thought to affect at least 50% of all cancer patients and 60% of all PDAC patients (Murthy et al. 2018)
Cancer management
There have been several treatment plans and medications for the management of cancer. However, none of them are delivering results that are adequate to full satisfaction (Jubeen et al. 2019). Chemoprevention is a method to prevent or avoid chronic degenerative diseases by using natural or artificial agents. Chemopreventive components are those components that can cease cancer furtherance and progression or perhaps also target at the stage of initiation of carcinogenesis itself (Fimognari et al. 2012). Chemically synthesized drugs have not significantly improved the overall survival rate over the past decades (Choudhari et al. 2020). These target a particular pathway through a specific mechanism. These medications may adversely damage the body’s organs and may cause renotoxicity, hepatotoxicity, and other undesirable consequences (Ashrafizadeh et al. 2020). The main drawback of chemotherapy includes cancer recurrence, drug resistance, and very harmful effects on tissues that are not being treated easily which might limit the use of anticancer medications and reduce the quality of life of patients (Choudhari et al. 2020). The naturally occurring bioactive compounds are of more interest to researchers because they can affect a wide range of molecular signaling pathways and reduce associated side effects (Yadav and Sangwan 2022; Diwan et al. 2021; Ashrafizadeh et al. 2020). The multi-targeting properties of herbal substances have sparked a lot of interest (Yadav and Sangwan 2022; Sharma and Sangwan 2022). A large amount of structural diversity in natural compounds exists because of molecular modifications which provide added advantage of an improvement in therapeutics (Srivastava and Sangwan 2020; Karikas 2010).
Cancer management using plant products
Compounds’ various actions are caused by certain structural characteristics. Numerous naturally occurring chemicals, including antibiotics, vitamins, plant products, and nucleic acids, include heterocyclic compositions with nitrogen as essential building blocks have been used for various purposes. Since its discovery in 1957, 5-fluorouracil (5-FU), an antimetabolite of pyrimidine and a commonly used anticancer medication, has been the subject of much research. The derivatives of 5-FU molecules of the enone functional group are related to many other naturally occurring molecules, which are known for their significant antiproliferative and anticancer properties (Jubeen et al. 2019). Non-targeted cytotoxicity and other health-related problems, such as alopecia, vomiting, and diarrhea, are linked to downside with 5-FU treatment, whether intravenously, orally, or topically (Jubeen et al. 2019). There is a requirement for novel therapeutic agents to reduce these challenges (Maqsood et al. 2021). Phytochemical have received a lot of attention for their possible role in cancer prevention (Elangovan et al. 2015). Selected phytochemicals have exhibited anticancer properties in a variety of studies and some have in clinical trials. Several chemical entities of secondary plant metabolites, microbial metabolites, peptides plant alkaloids, flavonoids, terpenoids, steroids, flavonolignans, and organosulfur compounds (Diwan et al. 2021; Greenwell and Rahman 2015), have been discovered, isolated, described, and clinically tested for their potential to treat cancer (Dhupal and Chowdhury 2020). Herbal chemicals have been shown to have low or even no toxicity to normal cells in addition to having beneficial properties, like enhancing antioxidant status, inactivation of carcinogens, halting growth, as well as induction of cell cycle arrest and programmed cell death (Choudhari et al. 2020). All of these observations support the idea that the herbal chemicals are relative safe and more effective disease-treating agents and that they may be used to target different biochemical pathways (Ashrafizadeh et al. 2020).
Cruciferous vegetables, i.e., Brassicaceae families of plants, have abundant economic significance and have around 340 genera, 3700 species. Valuable metabolites present in vegetable Brassica were found to be very effective in cancer chemoprevention and reduce various types of cancers, such as prostate, renal, colorectal, lung, breast, and oral cancer because they contain vitamins C and E, carotenoids (Avato and Argentieri 2015), and antioxidant enzymes, like Superoxide Dismutase (SOD), catalase (Gaziano et al. 2009), polyphenols (Sharma et al. 2018b), peroxidase, and sulfur organic compounds (Mandrich and Caputo 2020). Moreover, these greens are enriched with unstable compound glucosinolates, which convert into biologically active compounds indoles and isothiocyanates under the effect of the myrosinase enzyme, present in plant tissues (Mandrich and Caputo 2020). These components via xenobiotic metabolism instruction may result in neutralization or removal of carcinogenic as well as mutagenic factors which further inhibit the process of DNA methylation and progression of tumor (Kapusta-Duch 2012).
Dietary supplements which are herbal plant products under Dietary Supplement Health and Education Act (DSHEA) are utilized being part of their supportive health practices (Clarke et al. 2015). Dietary supplements are mostly used by cancer patients in comparison with healthy individuals.
Plant products are reported to inhibit various cancers (Table 1). A phytochemical-rich diet decreases the risk of cancer substantially depending upon the enriched diet. Phytochemicals play a significant role in the formulation of drugs; the use of these phytochemicals can be an effective strategy as it conquers mortality with a frightening rate of 1.6 million deaths every year. Early reports have proved that various phytochemicals possess powerful reactive oxygen species scavenging vehicles for lung cancer. Plant secondary metabolites in vitro have been demonstrated to have powerful cytotoxic effects against malignant cells of liver (Wang et al. 2013), colon (Baskar et al. 2010), lung (Li et al. 2013), ovarian (Moghadamtousi et al. 2014), cervical, and neuroblastoma (Sharma et al. 2018b). Various types of phytochemicals, their occurrence and effectiveness are as follows:
Table 1.
In vivo and in vitro anticancer activities of medicinal plants and their active constituents
| Plant’s name | Common name | Parts utilized | Tested on cancer cell lines | Animal Models | References |
|---|---|---|---|---|---|
| Allium sativum | Garlic | Leaves | WEHI 164 cells | Balb/c mice | Shirzad et al. (2011) |
| Alpinia galanga | Lengkuas, blue ginger | Rhizomes | DLA and A549 cells | Balb/c mice | Lakshmi et al. (2019) |
| Alstonia scholaris | Blackboard | Stem bark | HeLa cells | Mice | Mondal et al. (2012) |
| Andrographis paniculata | Green chiretta | Aerial parts | SW620 and A-498 cells | Swiss Albino mice | Khan et al. (2020) |
| Angelica archangelica | Wild celery | Root and rhizome | MCF-7 and 4T1 cells |
Balb/c Mice Female |
Oliveira et al. (2019) |
| Aralia elata | Japanese angelica tree | Leaves | MCF-7 cells |
Tumor Bearing-nude Mice |
Li et al. (2019) |
| Asclepias curassavica | Tropical milkweed | Shade dried leaves | COLO320 DM cells | Wistar male rats | Baskar et al. (2010) |
| Brassica napus | Mustard | Roots and leaves | Renal, prostate, oral, colorectal lung, and breast cancer | – | Thomson et al. (2010) |
| Copaifera multijuga | Hayne oil | Tree trunk | B16-F10 cells | MaleC57/black Mice | Carneiro et al. (2020) |
| Coptidis rhizoma | Huanglian, Copaiba | Root | HepG2 and MHC97-l cells | – | Wang et al. (2010) |
| Curcuma longa | Turmeric | Rhizome | Ht-29 cells | – | Khan et al. (2020) |
| Elephantopus scaber | Elephants Foot | Whole plant | EAC cells | Swiss male albino mice | Kabeer et al. (2019) |
| Garcinia indica | Kokum | Fruits | HCT-116 andHT -29 cells | – | Khan et al. (2020) |
| Garcinia oblongifolia | Lingnan garcinia | Branch | MCF -7 cells | – | Li et al. (2016) |
| Hedyotis diffusa | White flower Snake-tongue grass | HeLa cells | Nude xenograft mice | Li et al. (2012) | |
| Kaempferia parviflora | Black ginger | Rhizomes | SKOV3 cells | – | Paramee et al. (2018) |
| Loranthus parasiticus | Mulberry Mistletoe | Leaves | SKOV-3, CAOV-3, OVCAR-3 cells | Rat | Moghadamtousi et al. (2014) |
| Menyanthes trifoliata | Bogbean, Buckbean | Aerial part and root | Grade IV glioma cells | – | Kowalczyk et al. (2019) |
| Morus alba | white mulberry | Root | HL-60 and CRL-1579 cells | – | Kikuchi et al. (2010) |
| Morus nigra | Blackberry | Airy portion | PC-3 cells | – | Turan et al. (2017) |
| Nitraria retusa | Salt tree | Leaves | B16-F10 cells | Balb/c mice | Boubaker et al. (2018) |
| Paris polyphylla | Herb Paris | Rhizomes | A549 cells | c57bl/6mice | Li et al. (2013) |
| Perilla frutescens | Beefsteak plant | Leaves | Huh-7 cells | Tumor nude xenograft mice | Wang et al. (2013 |
| Prunus armeniaca | Apricot | Fruit, seeds | HCT116, MCF-7 andHep G2 cells | Rat | Gomaa (2013) |
| Platycodon grandiflorus | Balloon flower | Root | MCF-7 cells | – | Yu et al. (2010) |
| Rabdosia rubescens | Bing Ling | Whole plant | SGC-996 andNOZ cells |
Nude athymic Mice |
Bao et al. (2014) |
| Rhizoma curcumae | Turmeric | Rhizome | JF- 305 andMCF-7cells | Tropical fish | Zhu et al. (2019) |
| Scutellaria barbata | Barbed Skullcap | Whole plant | 95-D cells | Xenograft | Yang et al. (2014) |
| Scutellaria baicalensis | Baikal skullcap | Root | OSCC cells | – | Sato et al. (2013) |
| Tussilago farfara | Coltsfoot | Flower buds | HT-29 cells | – | Lee et al. (2014) |
| Withania somnifera | Ashwagandha | Leaves, roots, stem and flower | MCF-7 (breast), WRL-68 (liver), PC-3 (prostate) and CACO-2 | – | Siddique et al. (2014) Srivastava and Sangwan (2020) |
| Wedelia chinensis |
Chinese Wedelia |
Leaves | B16-F10 cells | C57bl/6 mice | Manjamalai and Grace (2012) |
Polyphenols
Polyphenols, which are natural antioxidants, are considered to boost health and lower the cancer risk when included in diet (Greenwell and Rahman 2015). They are secondary metabolites generated by plants that have a large number of phenolic rings in them. Berries, grapes, olive oil, chocolate, almonds, peanuts, and other fruits and vegetables are high in polyphenols, including up to 300 mg/100 g fresh weight (Sharma et al. 2018b). Polyphenols are also found in substantial levels of goods made from these fruits, such as tea, wine, berries, and beer (Turan et al. 2017). The amount and features of phenolic groups determine the specific qualities of each polyphenol class. They have a variety of roles in plants, including several that are required for plant physiological processes (Khan et al. 2020). These assist in the defense of the body against various kinds of stress and other environmental stimuli (Montane et al. 2020). Azadirachta indica is a medicinal tree studied for its various phytochemicals including gallic acid, caffeic acid, tannic acid, chlorogenic acid, ferulic acid, quinones, kaempferol, epicatechin, quercetin, hydroquinone, 4-caffeoryl quinic acid, and protocatechuic acid having anticarcinogenic properties (Narnoliya et al. 2021; Sharma et al. 2018b).
Stilbenes
These are hydroxylated derivative compounds produced from several plants, including strawberries, grapes, peanuts, and cannabis (Amarowicz and Pegg 2019). Resveratrol (3,5,4ʹ-trihydroxy-trans-stilbene) a natural polyphenol, present in a variety of foods, such as peanuts, grapes, and red wines, has been identified as a possible anticancer treatment in recent studies (Ren et al. 2021). It inhibits tumor growth and reduces metastatic potential by preventing tumor initiation, and acts as antimutagen to reduce tumor promotion. Stilbenes affect several signaling pathways, including the insulin-like growth factor system (Ko et al. 2017), (Wingless-Related Integration Site) Wnt signaling (Lohse et al. 2018), Notch-1 signaling, STAT3 (Signal Transducers and Activators of Transcription 3), the Akt/mTOR (Mammalian Target of Rapamycin Pathway), and Sirt1/MAPK (Mitogen-Activated Protein Kinase Pathway) (Lohse et al. 2018). They suppressed the expression of β-catenin, an important component of Wnt signaling. The expression of β-catenin, a protein required for the canonical Wnt signaling pathway, was down-regulated by resveratrol. Another research discovered that the STAT-1, Notch-1, and Wnt signaling pathways in cervical cancer cells are found to be suppressed by resveratrol (Zhang et al. 2014). Resveratrol was also discovered to promote apoptosis via blocking the Akt/mTOR pathways and up-regulating the p38-MAPK in a different investigation employing on T-cell leukemia cells (Ge et al. 2013). Yet another study pointed toward the Sirt/AMPK pathway as a potential target of resveratrol (Ko et al. 2017). Because of its therapeutic effects resveratrol affects so many various pathways and have so many possible interactions (Lohse et al. 2018).
Curcuminoids
Curcumin is a natural polyphenol curcuminoid, extracted from Curcuma longa rhizomes, having an antineoplastic activity (Lohse et al. 2018). Mainly, dietary foods, i.e., curries made with ginger and turmeric, are rich in curcumin. Curcumin is referred in studies to treat various types cancer, including pancreatic, breast, bone, liver, lung, cervix, and prostate (Dhupal and Chowdhary 2020). Curcumin affects a variety of cellular signaling pathways and has a wide range of targets. WNT/β-catenin (Ashrafizadeh et al. 2020), NOTCH, (Transforming Growth Factor) TGF/Smads (Ashrafizadeh et al. 2020), SHH, STAT3 (Huyanh et al. 2017), PI3K/AKT (Ashrafizadeh et al. 2020), and NF-κB/COX-2 signaling pathways are among them, and they play essential roles in cancer formation and progression (Sultana et al. 2021). Curcumin in vitro causes apoptosis and inhibits cell growth and invasion in pancreatic cancer cells in vivo by decreasing tumor development and angiogenesis. Curcumin therapy also reduces NF-κB binding and IkappaB kinase activity. This change was linked to a time-dependent decrease in cancer cell proliferation and an increase in apoptosis by increase in FOXO1 (Forkhead BoxProtein O 1) expression (Sultana et al. 2021). Dimethoxy curcumin is a curcumin derivative utilized in the treatment of breast and kidney cancer (Yallapu et al. 2013). Other studies also demonstrated that curcumin derivatives can be effective in the treatment of colon cancer cells by lowering surviving expression and increasing E-cadherin, a cell adhesion protein whose loss contributes to the development of epithelial tumors (Montane et al. 2020).
Flavonoids
A group of polyphenols consists of more than 6000 molecules. They are present in green vegetables and other colored fruits, including apples, strawberries, blueberries, plums (Turan et al. 2017), grapes (Sharma et al. 2018a, b), and oranges (Montane et al. 2020). Camellia sinensis isolated polyphenol, Epigallocatechin Gallate (EGCG), and Vinca rosea isolate vinorelbine tartrate were found to have formidable antineoplastic activity as compared to various cancers thus exhibited therapeutics potential (Dhupal and Chowdhury 2020). Acacetin a flavonoid of the Fabaceae family possibly inhibits the proliferation of A549 cell lines as well as enhances protein p53 and p21, which leads to apoptosis activation and arrest of the cell cycle. Glycine max soy isoflavones was reported to possess antineoplastic activity to cure cancer cases (Dhupal and Chowdhury 2020). Dysoxylum binectariferum extract alvocidib which is flavopiridol was found to activate apoptosis through inhibiting CDK9 with CDKs (Cyclin-Dependent Kinases), stopping phosphorylation, and cause cell cycle arrest at the G1 phase (Zocchi et al. 2018). In MDA-MB-231 cancer cells, kaempferol a natural flavonol cause apoptosis and DNA damage by upregulating the phosphorylated version of the H2A Histone Family Member X (H2AX), caspase 3, caspase 9, and the protein serine/threonine kinase (p-ATM) (Baharara et al. 2015).
Alkaloids
These natural products are present in almost all plants specifically in the specific blooming plants family.
Camptothecin analog Topotecan drug (topoisomerase-1 inhibitor) extracted from Camptotheca acuminate tree was approved by FDA for nursing cervical, small cell lung, and ovarian cancer. Vinca rosea (Dhupal and Chowdhury 2020) alkaloid extract vincristine sulfate arrests tumor cell in S phase and M phase of cell cycle, which is named as Oncovin approved by FDA, given to leukemias patient, lymphoblastic lymphoma, Philadelphia Chromosome-Negative (Ph-) Acute Lymphoblastic Leukemia (ALL), Burkitt lymphoma, and solid tumors of all groups. Vinorelbine is also accepted by FDA as the brand name Navelbine for treating advanced gastroesophageal adenocarcinoma, small lung cancer (Zhang et al. 2020), breast, human hepatocarcinoma (Huyanh et al. 2017), and human colon cancer when used in combinational therapy (Dhupal and Chowdhury 2020).
Arctigenin is isolated from seeds of Arctium lappa causing senescence in gallbladder cancer tissue by the down-regulating expression level of Epidermal Growth Factor Receptor (EGFR) by enhancing Fibrosarcoma (RAF)–Mitogen-Activated Protein Kinase (MEK)–Extracellular-Regulated Kinase (ERK) signaling pathway. It leads to cell cycle arrest at G1/G0 phase via decreasing expression of EGFR and causes apoptosis (Mondal et al. 2019).
Vincristine, vinblastine, and vindesine compounds were isolated from the Catharanthus roseus plant belonging to the Apocynaceae family and have anticancer effects used to treat various types of cancer, including lymphomas, leukemias (Choudhari et al. 2020), breast, lung cancer (Devaraj et al. 2014), advanced testicular, and Kaposi’s sarcoma (Mondal et al. 2019).
Glycine max isolates phytoestrogen, isoflavone, and genistein have antineoplastic properties. It has topoisomerase-II and protein-tyrosine kinase inhibition activity, which leads to cell cycle arrest at the G2/M phase along with activation of apoptosis. It is also used for treating early-stage prostate cancer (phase II), metastatic colorectal cancer (phase I/II), and stage IV breast cancer (phase II) under different clinical trials (Dhupal and Chowdhury 2020).
Steroids
Withania somnifera is a rich source of steroids withanolide having unique pharmacological properties, including anticancer (Sangwan et al. 2017). Several withanolidal moieties have proven to be useful candidate in prostate cancer via activating cell cycle arrest, apoptosis, and autophagy along with inhibition of different cancer-causing pathways (Ramakanth et al. 2016; Sangwan and Sangwan 2014; Srivastava and Sangwan 2020).
Cancer management using biological macromolecules
Tetrapyrrolic macrocycles, including porphyrins, chlorins, bacteriochlorins, and phthalocyanines are among the photosensitizers (PS) being studied for cancer therapy. Porphyrin compounds with pharmaceutical activity imitate photosynthetic centers, P-450, and vitamin B12. Porphyrins are a new chemotype that might be used to treat cancer. Porfimer sodium was the first PS to be approved by the FDA in clinical trials for bladder cancer, while verteporfin and phthalocyanines are now being tested. It does, however, have significant drawbacks, such as hydrophobicity, target specificity, long-term skin photosensitivity, and a lack of absorption at longer wavelengths (Diwan et al. 2021; Kirar et al. 2021).
Multidrug-Resistant (MDR) infections have been successfully treated with synthetic macromolecular antimicrobials. Antimicrobial Peptides (AMPs) are synthetic macromolecules that damage microbial cell membranes or target several intracellular proteins or genes to reduce resistance (Tan et al. 2020). AMPs can be utilized as anticancer treatments, according to recent research. The use of AMPs as anticancer medicines has been motivated by the never-ending search for new anticancer drugs known as Anticancer Peptides (ACPs) (Mader et al. 2006).
ACPs, which typically include 5–50 amino acid residues, frequently acquire secondary structures, like α-helical or β-sheets. They also have various distinct physical characteristics, such as strong cationic charges and hydrophobicity, that aid in the development of amphiphilic complexes and improve their interactions with cancer cells. ACPs killed cancer cells by including apoptosis, necrosis, or a combination of two (Tan et al. 2020).
A series of research look into the possibility of using polymer–drug conjugates to improve conventional chemotherapy. Anticancer therapies, such as paclitaxel, doxorubicin, camptothecin, and peptides, were delivered to solid tumors using polymers, such as styrene–maleic acid, Polyethylene Glycol (PEG), poly(l-glutamic acid), and poly[N-(2-hydroxypyropyl)methacrylamide] (MacEwan et al. 2010). When doxorubicin was conjugated to poly[N-(2-hydroxypropyl)methacrylamide], it increased circulation and tumor accumulation, resulting in greater anticancer activity relative to the free drug (Yallapu et al. 2013). In melanoma, colon, and lung tumors, camptothecin conjugated to poly(l-glutamic acid) had higher anticancer activity than the free drug, with increased drug loading and a higher polymer molecular weight boosting this activity. In comparison to free medication, dendrimers conjugated with doxorubicin and camptothecin showed better pharmacokinetics, biodistribution, and antitumor effectiveness (MacEwan et al. 2010). Herbal drugs and phytomolecules-based drugs have low solubility, which results in less systemic absorption, which has hampered their development as possible therapeutic candidates. Nanoformulation allows hydrophobic medicines to enter and remain in the tumor region due to the EPR effects (Dutta et al. 2019).
Nanoparticles
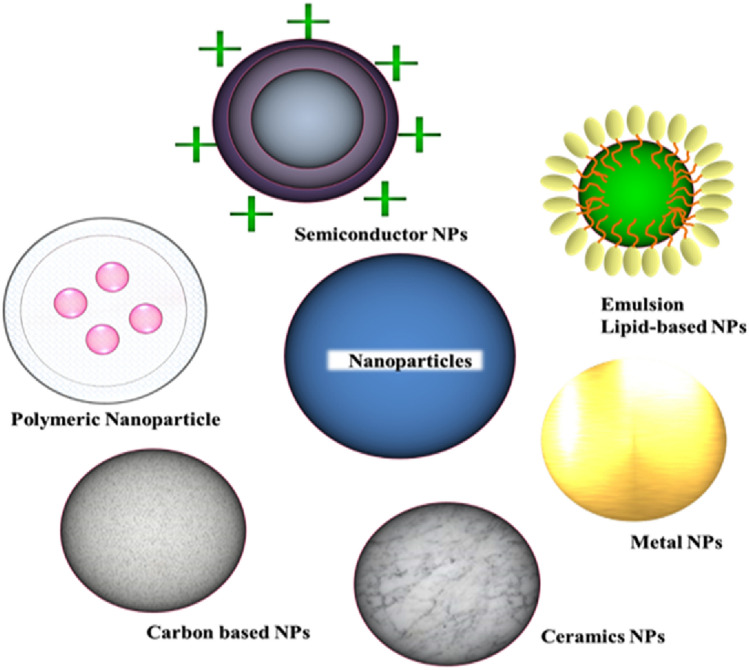
Nanotechnology is a multidisciplinary research whose goal is to build more functional atomic, molecular, and supramolecular materials for their effective utilization in health. NPs are divided according to their shapes, sizes, and properties into various classes. NPs have distinctive physical and chemical properties because of their large surface area and nanoscale size. NPs are ideal in catalysis, imaging, medicinal applications, energy-based research, and environmental applications due to these properties they are used not only in domestic but also in commercial applications (Khan et al. 2019b). They are separated into many categories according to their characteristics. Carbon-based nanoparticles, ceramic NPs, metal NPs, lipid-based NPs, semiconductor NPs, and polymeric NPs (Fig. 3) (Aghebati‐Maleki et al. 2020). Metallic nanoparticles are widely used in biological sciences for a variety of applications, including drug administration, bioimaging (Gomes et al. 2021), biosensing (Conde et al. 2012a), and catalysis (Tripathi et al. 2019a, b). Ceramic NPs are inorganic non-metallic solids, which are incorporated by heating and successive cooling. Researchers are particularly interested in these NPs as they have possible applications in catalysis (Tripathi et al. 2019a, b), dye photodegradation (Conde et al. 2012b), and imaging (Wang et al. 2021a, b). Semiconductor NPs have properties that are intermediate to metals and non-metals and since they have large bandgaps, they show considerable changes in their properties when the bandgap is tuned (Conde et al. 2012b). As a result, they are key materials in photo optics, photocatalysis, and electronic devices (Saleem et al. 2018).
Fig. 3.
Different types of nanocarriers
However, for above all the applications a cost-effective, commercially viable, and environmentally acceptable synthesis approach is required (Gomes et al. 2021). In recent years, Silver Nanoparticles (AgNPs) for having a broad spectrum of biological activities are recommended for new anticancer drug development (Valsalam et al. 2019). NPs were shown to be highly toxic to cancerous cells, so there is a great chance to be used in the diagnosis and therapy of cancer cells (Bhattacharyya et al. 2011). AgNPs reduce tumor cell development, so they can be used as antitumor agents (Gomes et al. 2021), which might be because they could inhibit various signaling cascades which are responsible for cancer development and its pathogenesis. By observing all these it was suggested that AgNPs could be cytotoxic on tumor cells, and also inhibit their development by not affecting non-tumor cells (Priyadarshni and Mahalingam 2017). CeO2 NPs, like AgNPs, play a potential role in therapeutics due to their unique properties. They show antioxidant and antibacterial properties in a dose-dependent manner. This antioxidant behavior can play a major role in their anticancer preparation. Studies showed that CeO2 shows no toxicity for aquatic life up to 100 mg/ml and can be a potential candidate for making phyto-nanocarriers (Marghoob et al. 2021).
Role of nanoparticles in cancer treatment
Different processes have been utilized for the prevention of cancer, such as screening, vaccination, surgery, radiation, cryotherapy, and chemotherapy. Chemotherapeutic drug targets cancerous cells exposing healthy cells followed by side effects with the reduction in the immune response and increased vulnerability to host diseases (Peppercorn et al. 2005).
Despite various traditional methods available for the treatment of cancer, a complete cure is still difficult, which has been proven by the fact that in recent years no appreciable reduction was seen in the mortality of cancer patients with more than 50% of deaths (Bray et al. 2018). For these reasons, nanomedicine came into the role intending to find economical components with higher specificity and sensitivity toward cells (Acebes-Fernandez et al. 2020). NPs offer many advantages as they have a significant impact on a variety of cancer cell lines (He et al. 2016a, b), with higher perforation (Bhattacharyya et al. 2011), as well as they can trail NPs inside the body which makes them a better systematic tool with less undesired effects in cancer treatment compared to standard therapeutic procedures (Gomes et al. 2021). The microenvironment in tumor cells and normal healthy cells vary in terms of an acidic environment, a different expression of enzymes and receptors, etc. The main reason for this is difference in pH due to high rate of glycolysis in cancer cells during anaerobic and aerobic conditions. Tumor cells over-express specific receptors compared to normal cells. These specific features affect the function of NPs, so following proper care during nanocarriers design will be an advantage in targeted cancer treatment (Akhtar et al. 2014). Nanoparticles work as drug carrier it changes the pharmacokinetic property of a drug and increases its efficiency with minimum side effects, regulating the drug release process inside the body (Patra et al. 2018), enhancing encapsulation, solubilization, protecting pharmaceutical molecules, as it is smaller in size than the cell, so they can cross-biological barriers and deliver the drug to the specific place, enhances the longevity of drug inside the blood flow, specific target delivery and biocompatibility (Aghebati‐Maleki et al. 2020). Some of the most studied NPs for cancer therapeutics are detailed in the following sections.
Carbon-based NPs
Carbon Nanomaterials (CNMs) have piqued the scientific community’s interest due to their electronic, optical, thermal, and mechanical properties, as well as their versatile functionalization chemistry and the fact that they appear to be more biocompatible and safer than metal-based nanomaterials for cancer therapeutics. Carbonaceous nanoparticles can load the drug of interest by hydrophobic interactions or stacking and can be employed as effective drug delivery platforms because of their inherent hydrophobic character. Carbon nanomaterials, such as graphene, fullerenes, Carbon Nanotubes (CNTs), and Carbon Quantum Dots (CQTs) are the most commonly employed for cancer treatment (Saleem et al. 2018). These exhibit various properties of commercial interests, like high strength, electrical conductivity, electron affinity, structure, and versatility (Astefanei et al. 2015). CNMs possess various sizes in Acidic tumor Microenvironment (TME); they can penetrate deep into tumors, which is aided by the EPR effect. Furthermore, CNMs are biodegradable, bolstering their potential as cancer treatment agents in the future. Cancer stem cells, tumor migration and invasion, tumor-associated inflammation, hypoxia, metabolism, and angiogenesis are among the pathways that CNMs can regulate (Cheng et al. 2021); thus, they are attractive nanocarriers and nanomedicines for TME-targeted cancer treatment due to their physiochemical features (Saleem et al. 2018).
Metal NPs
These are purely made from metal precursors having the characteristic of Localized Surface Plasmon Resonance (LSPR) and distinctive optoelectrical properties. These are used in cutting-edge materials because of their precise and shape-controlled synthesis (Dreaden et al. 2012). Nobel metal NPs have distinctive qualities, such as high surface-to-volume ratio, wide optical properties, simplicity of production, simple surface chemistry, and functionalization, which make them promising candidates for cancer therapies in clinics (Table 2) (Conde et al. 2012a). Metal NPs use two ways to target tumor cells: passive targeting and active targeting. The EPR effect is a passive phenomenon in which NPs of specific sizes aggregate in tumor tissue at a greater rate than in normal tissues, and due to their leaky vasculature, arrangement, and inadequate lymphatic drainage, tumor tissues are more permeable to macromolecules than healthy tissues, allowing nanoparticles to rapidly infiltrate into malignant cells and destroy them. As NPs have distinctive shapes and enhanced vascular permeability, metal NPs can be used to target cancer cells (Table 2). When compared to passive targeting, active targeting has greater specificity and selectivity toward cancerous cells while causing minimal or no harm to healthy cells. The use of functionalized nanoparticles to deliver chemotherapeutic agents not only improves therapeutic efficacy but also helps prevent drug resistance (Sharma et al. 2018a).
Table 2.
Plants studied for the synthesis of nanoparticles (NPs) in cancer therapeutics
| Plant’s name | Part utilized | Nanoparticles | Cancer cell line | References |
|---|---|---|---|---|
| Abelmoschus esculentus | Pulp | AgNPs, AuNPs | Lymphoma cancer | Korani et al. (2021) |
| Abutilon indicum | Leaf | AgNPs, CuO NPs | COLO205 cells, A549, MDA-MB-231 | Sathiyavimal et al. (2022) |
| Achillea biebersteinii | Flowers | AgNPs, AuNPs | MCF-7 cells, NTERA-2 | Baharara et al. (2015) and Mobaraki et al. (2021) |
| Acorus calamus | Rhizomes | ZnONPs | SK-MEL-3 cells | Vakayil et al. (2021) |
| Allium sativum | Whole plant | AuNPs | HT-29, HCT116 | Liu et al. (2021) |
| Alternanthera sessilis | Airy potion | AgNPs, AuNPs | MCF-7 cells | Firdhouse and Lalitha (2015, 2020) |
| Andrographis echioides | Leaf | Solid lipid NPs, PLGA NPs, AgNPs | MCF-7 cells, HepG2, HeLa cells | Elangovan et al. (2015) and Parveen et al. (2019) |
| Artemisia marschalliana | Airy Portion | AgNPS, AuNPs, ZnNPs, CuNPs | AGS cells, HCT-116, HT29 | Andleeb et al. (2021) |
| Artemisia princeps | Leaves | AgNPs | A549 cells | Gurunathan et al. (2015) |
| Azadirachta indica | Leaves | AuNPs, ZnONPs | SiHa, HeLa, Hek-293 cells | Agarwal et al. (2018) |
| Butea monosperma | Leaves | Fe3O4 | A549 and B16F10 cells | Behera et al. (2020) |
| Calotropis gigantea | Latex | ZnONPs | MCF-7, HeLa, HEK-293 | Gobinath et al. (2022) |
| Curcuma longa | Rhizome | Bipolymer NPs | Hep3B, hBMSCs | Montalban et al. (2018) |
| Alstonia scholaris | Bark | Glycol-chitosan NPs, PLGA NPs | HeLa, Vero cells | Tripathi et al. (2019a, b) and Zheng et al. (2019) |
| Terminalia arjuna | Fruit | MnO2 | MCF-7 | Reddy et al. (2022) |
| Ziziphus mauritiana | Fruit | Ag/AgClNPs | MCF-7 | Kabir et al. (2020) |
| Manilkara zapota | Leaf | AgNPs | MCF-7 | Madakka et al. (2021) |
| Ficus benghalensis | Dry powder | CuNPs | Oral cancer | Imtiaz et al. (2022) |
| Betula pubescens | Whole plant | PLGANPs, lipid NPs | HCC, MCF | Kim et al. (2021) |
| Withania somnifera | Root | TiO2 NPs | HepG2, HEK-293 | Al-Shabib et al. (2020) |
| Glycyrrhiza glabra | Root | SeNPs | PC12 | Maheswari et al. (2020) |
| Cymodocea serrulata | Whole plant, leaves | AgNPs | HeLa, A549 cells | Chanthini et al. (2015) and Palaniappan et al. (2015) |
| Dimocarpus longan | Peel | AgNPs | H1299, BxPc3, VCaP cells | He et al. (2016a, b) |
| Erythrina indica | Root | AgNPs | Hep-G2 cells | Sre et al. (2015) |
| Excoecaria agallocha L | Leaf | AgNPs, AgONPs | B16F10 cells | Banerjee et al. (2017) |
| Gymnema sylvestre | Leaf | AgNPs | HT29 cells | Arunachalam et al. (2015) |
| Moringa olifera | leaf | AgNPs, AuNPs, Ag/AuNPs | HepG2, MDA-MB-231, MCF-7 | Gupta et al. (2020) |
| Piper longum | Fruit | AgNPs | MCF-7 cells | Krishanan et al. (2016) |
| Syzygium cumini | Flower | AgNPs | MCF-7, HeLaandHek-293 cells | Mittal et al. (2016) and Ramar et al. (2015) |
| Tabernaemontana divaricata | Leaves | AgNPs | MCF-7 cells | Devaraj et al. (2014) |
| Taxus yunnanensis | Callus | AgNPs | SMMC-7721 cells | Xia et al. (2016) |
| Vitex negundo | Leaf | AgNPs, ZnONPs | HCT15 cells | Agarwal et al. (2018) |
Polymeric NPs
Polymeric NPs are normally organic-based NPs and mostly their shape is nanosphere or nanocapsules (Mansha et al. 2017). Curcumin-loaded polymeric nanoparticles, PLGA, a biodegradable, biocompatible synthetic polymer of 100–200 nm in size, are broadly studied in cell culture and animal models (Yallapu et al. 2013). Advantages of using polymeric nanoparticles in therapies include refined physiochemical properties, the ability to easily play with several types of molecules, biodegradable, non-toxic, overcome limitations, like poor solubility, and can be used as an alternative therapeutic agent (Indoria et al. 2020). Poly(lactic-co-glycolic acid) and poly(lactic acid) are synthetic polymers that have been approved by the FDA for human use. Multifunctional NPs can facilitate visualization of malignant cells, target them, and destroy cancer cells without damaging healthy cells in the process (Ahlawat et al.2018).
Lipid-based NPs
These NPs have lipid moieties and can be used in a variety of biomedical applications. A lipid NP is typically spherical, with a diameter ranging from 10 to 1000 nm. Lipid nanotechnology (Mashaghi et al. 2013) is a specialized topic that focuses on the design and synthesis of lipid NPs, which can be utilized for a variety of applications, such as medication administration and RNA release in cancer therapy. Liposomes, Solid Lipid Nanoparticles (SLNs), and Nanostructured Lipid Carriers (NLCs) are Lipid-Based Nanoparticles (LBNPs) that generate a lot of attention in drug development and cancer treatment (Wang et al. 2021a, b). These NPs can transport both hydrophobic and hydrophilic molecules, have very low or no toxicity, and extend the duration of pharmacological activity by having a longer half-life and regulated drug release. Chemical changes to lipid nanosystems can be used to evade immune system detection or to improve medication solubility. They can also be made in pH-sensitive formulations to increase drug release in an acidic environment, and they can be linked to antibodies that target tumor cells or their receptors (such as folic acid); because of their biocompatibility and adaptability, liposomes are the most extensively utilized LBNPs (Garcia-Pinel et al. 2019), with the help of their lipophilic tails, amphiphilic phospholipids self-assemble into a spherical lipid bilayer structure, providing a framework for the encapsulation of hydrophobic medicines (Mashaghi et al. 2013). As opposed to this, the hydrophilic heads of phospholipids form an aqueous core and external surface that can contain hydrophilic chemicals. Both charge–charge interactions and interactions with chemical linkers on the liposomal surface can result in the encapsulation of different medicinal compounds into liposomes. It is significant to note that the encapsulation of therapeutics within various liposomal compartments enables safe and precise drug delivery because liposomes can shield enclosed cargos from immune system degradation while transporting them across biological membranes where the free drugs are frequently incompatible (Wang et al. 2021a); however, SLNs and NLCs have recently gained a lot of attention (Garcia-Pinel et al. 2019).
Phyto-nanocarriers and their role in cancer therapy
Nanoparticles synthesized through the conventional methods cause toxicity therefore using green synthesis, i.e., reduction by application of antioxidant plant material results in not only less toxicity (Chanthini et al. 2015) but also more stability. The manufacture of nanoparticles using plant extracts is a straightforward one-step reduction technique with large-scale production (Table 2) (Agarwal et al. 2018). Plant-based methods may also help in achieving desired NPs size (Banerjee et al. 2017; Agarwal et al. 2018) as well as also offer eco-friendliness, economic effectiveness (Baharara et al. 2015), and compatibility for pharmaceutical and biomedical applications (Baharara et al. 2015; Arunachalam et al. 2015). Plant extracts/ molecules act as both capping and stabilizing agents for nanoparticle synthesis (Banerjee et al. 2017) (Fig. 4). Phytomolecule and nanoparticles can help achieve this goal by passively accommodating many solid tumors due to the Enhanced Permeability and Retention (EPR) effect. This effect is due to the abnormalities in tumor vasculature as well as the poorly developed lymphatic system. This limits the drainage of tumor tissue molecules, following the discovery of the EPR effect. In visualizing anticancer new products, which are available commercially, were obtained from natural sources either by structural natural compounds modification or by new compounds synthesis (Table 2). Phytonaocarriers has enhanced effect due to their biocompatibility (Yadav and Sangwan 2022), less cytotoxicity, less resistance, and dynamic physiochemical properties used to treat different cancers (Dhupal and Chowdhary 2020). These well-known plant extracts and phytochemicals (the plant secondary metabolites) with promising anticancer properties have been encapsulated in biocompatible and biodegradable polymeric nanoparticles (Dutta et al. 2019; Indoria et al. 2020), solid lipid nanocarriers (Garcia-Pinel et al. 2019), carbon-based NPs (Saleem et al. 2018), nanocapsules, liposomes, metallic nanoparticles (Valsalam et al. 2019), and non-metallic NPs (Dhupal and Chowdhary 2020). Green synthesized metallic nanoparticles using Achillea biebersteinii plant flower extract showed a significant anti-apoptosis against human breast cancer cell line (MCF-7) (Baharara et al. 2015); NPs synthesized using Excoecaria agallocha L., leaf extracts showed potential anticancer properties against several cell lines, such as Murine Lung Adenocarcinoma (3LL), Murine Ehrlich Ascites Carcinoma (EAC), Murine Colon Cancer (CT26), and Murine Melanoma (B16F10) cells with no side effects. In the case of B16F10 cells, cell death was caused by nuclear translocation which was observed with the help of fluorescence microscopy and flow cytometry (Benerjee et al. 2017). Cytotoxicity studies of NPs synthesized using Nelumbo nucifera and Cymodocea serrulata found that these plant extracts have a significant role in inhibiting lung cancer (HeLa cells) (Chanthini et al. 2015). Gold NPs synthesized using Antigonon leptopus powdered extract and copper oxide NPs synthesized using Acalypha indica have cytotoxic properties against MCF-7 breast cancer cell lines (Greenwell and Rahman 2015). When berberine was encapsulated in chitosan NPs, its bioavailability was improved without changing its structure or performance. By specifically targeting cancer cells, it also improved cytotoxic behavior against them. Oral administration increased bioavailability according to in vivo tests, while berberine capsules were allowed for up to three times lower doses (Chou et al. 2013). Camptothecin is a strong anticancer compound, but its unstable lactone ring and limited solubility made it unsuitable for clinical use. More than 80% of the hydrophobically modified glycol chitosan nanoparticle could be loaded with it. It aids in defending the lactone ring from physiological circumstances. It was also shown to have excellent antitumoral efficacy and to penetrate tumors more deeply, likewise when curcumin was encapsulated with PLGA, it showed enhancement in biological activity against prostate cancer (Dhupal and Chowdhury 2020).
Fig. 4.
Schematic presentation of phyto-nanocarriers used as anticancer
Conclusion
The widespread occurrence of cancer worldwide is a matter of great concern. The existing therapies available, such as radiation, chemotherapy, and other medications are widely used but they have adverse effects associated with them; this review mainly highlights various alternative methods that have been explored and might have minimal or negligible side effects. Various metabolites present in fruits and vegetables are effective in the prevention or inhibition of cancer. Studies have revealed that plant-based molecules possess potent anticancerous properties. The drug formulations of these molecules are restricted due to their limited occurrence, aqueous solubility, and bioavailability. Nanotechnology has arisen as a modern way of therapeutics to overcome some of these issues and treat various stages of cancer by targeted drug delivery of anticancerous agents. Nanoparticles synthesized using plant parts have shown to be effective in in vitro cell lines. Several specific metabolites, such as resveratrol, acacetin, curcumin, epigallocatechin, and gallate, act as a potent scavenger of free radicals and hence protect from oxidative stress. These molecules serve to exhibit properties to be used as phyto-nanocarriers. A few studies have been reported for the toxicity and allergic reactions in people treated with nanodrugs with varying compositions. Therefore, an extensive study is further required to validate the observations for exposure as well as the level of chemical composition used for the study. The dose, shape, surface chemistry, purity, and exposure route also need to be further warranted.
Acknowledgements
NY is thankful to UGC (536 CSIR-UGC NET DEC. 2018) for fellowship. NSS is thankful to DST for financial grant. The funding agency had no role in the interpretation or writing the manuscript.
Declarations
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
5. References
- Acebes-Fernández V, Landeira-Viñuela A, Juanes-Velasco P, Hernández AP, Otazo-Perez A, Manzano-Román R, Gongora R, Fuentes M. Nanomedicine and onco-immunotherapy: from the bench to bedside to biomarkers. Nanomaterials. 2020;10:1274. doi: 10.3390/nano10071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal H, Menon S, Kumar SV, Rajeshkumar S. Mechanistic study on antibacterial action of zincoxide nanoparticles synthesized using green route. Chemico-Biol Interact. 2018;286:60–70. doi: 10.1016/j.cbi.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Aghebati-Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, Yousefi M, Aghebati-Maleki L. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235:1962–1972. doi: 10.1002/jcp.29126. [DOI] [PubMed] [Google Scholar]
- Ahlawat J, Henriquez G, Narayan M. Enhancing the delivery of chemotherapeutics: role of biodegradable polymeric nanoparticles. Molecules. 2018 doi: 10.3390/molecules23092157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar MJ, Ahamed M, Alhadlaq HA, Alrokayan SA, Kumar S. Targeted anticancer therapy: overexpressed receptors and nanotechnology. Clin Chim Acta. 2014;436:78–92. doi: 10.1016/j.cca.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Almatroodi SA, Alsahli MA, Almatroudi A, Verma AK, Aloliqi A, Allemailem KS, Rahmani AH. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules. 2021;26(5):1315. doi: 10.3390/molecules26051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabib NA, Husain FM, Qais FA, Ahmad N, Khan A, Alyousef AA, Shahzad SA. Phyto-mediated synthesis of porous titanium dioxide nanoparticles from Withania somnifera root extract: broad-spectrum attenuation of biofilm and cytotoxic properties against HepG2 cell lines. Front Microbiol. 2020;11:1680. doi: 10.3389/fmicb.2020.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarowicz R, Pegg RB. Advances in food and nutrition research. New York: Academic Press; 2019. Natural antioxidants of plant origin; pp. 1–81. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andleeb A, Andleeb A, Asghar S, Zaman G, Tariq M, Mehmood A, Abbasi BH. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers. 2021;13(11):2818. doi: 10.3390/cancers13112818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam KD, Arun LB, Annamalai SK, Arunachalam AM. Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. Int J Nanomed. 2015;10:31. doi: 10.2147/IJN.S71182.eCollection2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh M, Zarrabi A, Hushmandi K, Zarrin V, Moghadam ER, Hashemi F, Mirzaei H. Toward regulatory effects of curcumin on transforming growth factor-beta across different diseases: a review. Front Pharmacol. 2020 doi: 10.3389/fphar.2020.585413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astefanei A, Núñez O, Galceran MT. Characterisation and determination of fullerenes: a critical review. Anal Chim Acta. 2015;882:1–21. doi: 10.1016/j.aca.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Avato A, Argentieri MP. Brassicaceae: a rich source of health improving phytochemicals. Phytochem Rev. 2015;14:1019–1033. doi: 10.1007/s11101-015-9414-4. [DOI] [Google Scholar]
- Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Baharara J, Namvar F, Ramezani T, Mousavi M, Mohamad R. Silver nanoparticles biosynthesized using Achillea biebersteinii flower extract: apoptosis induction in MCF-7 cells via caspase activation and regulation of Bax and Bcl-2 gene expression. Molecules. 2015;20:2693–2706. doi: 10.3390/molecules20022693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee K, Das S, Choudhury P, Ghosh S, Baral R, Choudhuri SK. A novel approach of synthesizing and evaluating the anticancer potential of silver oxide nanoparticles in vitro. Chemotherapy. 2017;62:279–289. doi: 10.1159/000453446. [DOI] [PubMed] [Google Scholar]
- Bao R, Shu Y, Wu X, Weng H, Ding Q, Cao Y, Li M, Mu J, Wu W, Ding Q. Oridonin induces apoptosis and cell cycle arrest of gallbladder cancer cells via the mitochondrial pathway. BMC Cancer. 2014;14:1–13. doi: 10.1186/1471-2407-14-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar AA, Ignacimuthu S, Paulraj GM, Al Numair KS. Chemopreventive potential of β-sitosterol in experimental colon cancer model-an in vitro and in vivo study. BMC Complement Altern Med. 2010;10:1–10. doi: 10.1186/1472-6882-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera B, Pradhan S, Samantaray A, Pradhan D. Antiproliferative and cytotoxic activity of Hematite (-Fe2O3) nanoparticles from Butea monosperma on MCF-7 Cells. Afr J Pharm Pharmacol. 2020;14(2):29–40. doi: 10.5897/AJPP2019.5111. [DOI] [Google Scholar]
- Bhattacharyya S, Kudgus RA, Bhattacharya R, Mukherjee P. Inorganic nanoparticles in cancer therapy. Pharm Res. 2011;28:237–259. doi: 10.1007/s11095-010-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubaker J, Ben Toumia I, Sassi A, Bzouich-Mokded I, Ghoul Mazgar S, Sioud F, Bedoui A, Safta Skhiri S, Ghedira K, Chekir-Ghedira L. Antitumoral potency by immunomodulation of chloroform extract from leaves of Nitraria retusa, Tunisian medicinal plant, via its major compounds -sitosterol and palmitic acid in BALB/c mice bearing induced tumor. Nutr Cancer. 2018;70:650–662. doi: 10.1080/01635581.2018.1460683. [DOI] [PubMed] [Google Scholar]
- Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4:1–8. doi: 10.1186/1475-9276-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Carneiro LJ, Tasso TO, Santos MF, Goulart MO, Santos RAD, Bastos JK, da Silva JJ, Crotti AE, Parreira RL, Orenha RP, Veneziani R. Copaifera multijuga, Copaifera pubiflora and Copaifera trapezifolia oleoresins: chemical characterization and in vitro cytotoxic potential against tumoral cell lines. J Braz Chem Soc. 2020;3:1679–1689. doi: 10.2157/0103-5053.20200054. [DOI] [Google Scholar]
- Chamcheu JC, Roy T, Uddin MB, Banang-Mbeumi S, Chamcheu RN, Walker AL, Liu YY, Huang S. Role and therapeutic targeting of the PI3K/Akt/mTOR signaling pathway in skin cancer: a review of current status and future trends on natural and synthetic agents therapy. Cells. 2019;8(8):803. doi: 10.3390/cells8080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanthini AB, Balasubramani G, Ramkumar R, Sowmiya R, Balakumaran MD, Kalaichelvan PT, Perumal P. Structural characterization, antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R.Br.) Asch. and magnus mediated silver nanoparticles. J Photochem Photobiol B. 2015;153:145–152. doi: 10.1016/j.jphotobiol.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CW, Batnyam O, Hung HS, Harn HJ, Lee WF, Lin HR. Highly bioavailable anticancer herbal-loaded nanocarriers for use against breast and colon cancer in vitro and in vivo systems. Polym Chem. 2013;4(6):2040–2052. doi: 10.1039/c2py20972a. [DOI] [Google Scholar]
- Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol. 2020 doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christina F, Christopher JL, Murray TA. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability and disability-adjusted life-years for 29 cancer groups, 1990 to 2017. JAMA Oncol. 2019;5:1–21. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015;79:1. [PMC free article] [PubMed] [Google Scholar]
- Conde J, Doria G, Baptista P. Noble metal nanoparticles applications in cancer. J Drug Deliv. 2012 doi: 10.1155/2012/751075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J, Rosa J, Lima JC, Baptista PV. Nanophotonics for molecular diagnostics and therapy applications. Int J Photoenergy. 2012 doi: 10.1155/2012/619530b. [DOI] [Google Scholar]
- Devaraj P, Aarti C, Kumari P. Synthesis and characterization of silver nanoparticles using Tabernaemontana divaricata and its cytotoxic activity against MCF7 cell line. Int J Pharm Sci. 2014;6:86–90. [Google Scholar]
- Dhupal M, Chowdhury D. Phytochemical-based nanomedicine for advanced cancer theranostics: perspectives on clinical trials to clinical use. Int J Nanomed. 2020;15:9125. doi: 10.2147/IJN.S259628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan D, Cheng L, Usmani Z, Sharma M, Holden N, Willoughby N, Sangwan N, Raju R, Liu C, Gupta VK (2021) Microbial cancer therapeutics: a promising approach. Semin Cancer Biol S1044-579X(21) 00131-0 [DOI] [PubMed]
- Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Paul B, Mukherjee B, Mondal L, Sen S, Chowdhury C, Debnath MC. Nanoencapsulated betulinic acid analogue distinctively improves colorectal carcinoma in vitro and in vivo. Sci Rep. 2019;9:1–20. doi: 10.1038/s41598-019-47743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan K, Elumalai D, Anupriya S, Shenbhagaraman R, Kaleena P, Murugesan K. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographis echioides and its bioefficacy on anticancer and antibacterial activities. J Photochem Photobiol B Biol. 2015;151:118–124. doi: 10.1016/j.jphotobiol.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Elisabete W. Lifestyle and cancer risk. J Prevent Med Public Health. 2010;43(6):459–471. doi: 10.3961/jpmph.2010.43.6.459. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Turrini E, Ferruzzi L, Lenzi M, Hrelia P. Natural isothiocyanates: genotoxic potential versus chemoprevention. Mutat Res. 2012;750:107–131. doi: 10.1016/j.mrrev.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Firdhouse J, Lalitha P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog Biomater. 2015;4:113–121. doi: 10.1007/s40204-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdhouse MJ, Lalitha P. Facile synthesis of anisotropic gold nanoparticles and its synergistic effect on breast cancer cell lines. IET Nanobiotechnol. 2020;14(3):224–229. doi: 10.1049/iet-nbt.2019.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, Sarabia F, Prados J, Melguizo C, López-Romero JM. Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomaterials. 2019;9:638. doi: 10.3390/nano9040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen BJE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Liu Y, LiQ GX, Gu L, Ma ZG, Zhu YP. Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38-MAPK. Biomed Environ Sci. 2013;26:902–911. doi: 10.3967/bes2013.019. [DOI] [PubMed] [Google Scholar]
- Gobinath P, Packialakshmi P, Hatamleh AA, Al-Dosary MA, Al-Wasel YA, Balasubramani R, Idhayadhulla A. Calotropis gigantea assisted synthesis of zinc oxide nanoparticle catalysis: synthesis of novel 3-amino thymoquinone connected 1, 4-dihyropyridine derivatives and their cytotoxic activity. J Nanomater. 2022 doi: 10.1155/2022/9697057. [DOI] [Google Scholar]
- Gomaa EZ. In vitro antioxidant, antimicrobial, and antitumor activities of bitter almond and sweet apricot (Prunus armeniaca L.) kernels. Food Sci Biotechnol. 2013;22:455–463. doi: 10.1007/s10068-013-0101-1. [DOI] [Google Scholar]
- Gomes HIO, Martins CSM, Prior JAV. Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials. 2021;11:964. doi: 10.1007/s10068-013-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell M, Rahman PKSM. Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res. 2015;6:4103. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Johnson NW, Kumar N. Global epidemiology of head and neck cancers: a continuing challenge. Oncology. 2016;91(1):13–23. doi: 10.1159/000446117. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hemlata H, Tejavath K. Synthesis, characterization and comparative anticancer potential of phytosynthesized mono and bimetallic nanoparticles using Moringa oleifera aqueous leaf extract. Beilstein Arch. 2020;1:95. doi: 10.3762/bxiv.2020.95.v1. [DOI] [Google Scholar]
- Gurunathan S, Jeong JK, Han JW, Zhang XF, Park JH, Kim JH. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res Lett. 2015;10:1–17. doi: 10.1186/s11671-015-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- He Y, Du Z, Ma S, Cheng S, Jiang S, Liu Y, Li D, Huang H, Zhang K, Zheng X. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus longan Lour. Nanoscale Res Lett. 2016;11:1–10. doi: 10.1186/s11671-016-1511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Du Z, Ma S, Liu Y, Li D, Huang H, Jiang S, Cheng S, Wu W, Zhang K. Effects of green synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int J Nanomed. 2016;11:1879a. doi: 10.2147/IJN.S103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J, Etemad N, Hollande F, Ernst M, Buchert M. Seminars in cancer biology. New York: Academic Press; 2017. The JAK/STAT3 axis: a comprehensive drug target for solid malignancies; pp. 13–22. [DOI] [PubMed] [Google Scholar]
- Imtiaz T, Rajeshkumar S, Ezhilarasan D, Lakshmi T. Preparation of Ficus benghalensis mediated copper nanoparticles and its based mouthwash and to check its cytotoxic activity. Int J Early Childh. 2022;14(03):2022. [Google Scholar]
- Indoria S, Singh V, Hsieh MF. Recent advances in theranostic polymeric nanoparticles for cancer treatment: a review. Int J Pharm. 2020;582:119314. doi: 10.1016/j.ijpharm.2020.119314. [DOI] [PubMed] [Google Scholar]
- Iqbal SZ, Jubeen F, Sher F. Future of 5-fluorouracil in cancer therapeutics, current pharmacokinetics issues and a way forward. J Cancer Res Pract. 2019;6(4):155. doi: 10.4103/JCRP.JCRP_10_19. [DOI] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jubeen F, Liaqat A, Sultan M, Zafar Iqbal S, Sajid I, Sher F. Green synthesis and biological evaluation of novel 5-fluorouracil derivatives as potent anticancer agents. Saudi Pharm J. 2019;27(8):1164–1173. doi: 10.1016/j.jsps.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubeen F, Liaqat A, Amjad F, Sultan M, Iqbal SZ, Sajid I, Sher F. Synthesis of 5-fluorouracil cocrystals with novel organic acids as coformers and anticancer evaluation against HCT-116 colorectal cell lines. Cryst Growth Des. 2020;20(4):2406–2414. doi: 10.1021/acs.cgd.9b01570. [DOI] [Google Scholar]
- Kabeer FA, Rajalekshmi DS, Nair MS, Prathapan R. In vitro and in vivo antitumor activity of deoxyelephantopin from a potential medicinal plant Elephantopus scaber against Ehrlich ascites carcinoma. Biocatal Agric Biotechnol. 2019;19:101106. doi: 10.1016/j.bcab.2019.101106. [DOI] [Google Scholar]
- Kabir SR, Asaduzzaman AKM, Amin R, Haque AT, Ghose R, Rahman MM, Alam MT. Zizyphus mauritiana fruit extract-mediated synthesized silver/silver chloride nanoparticles retain antimicrobial activity and induce apoptosis in MCF-7 cells through the Fas pathway. ACS Omega. 2020;5(32):20599–20608. doi: 10.1021/acsomega.0c02878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta-Duch J, Kopec A, Piatkowska E, Borczak B, Leszczynska T (2012) The beneficial effects of Brassica vegetables on human health. Roczniki Państwowego Zakładu Higieny 63 [PubMed]
- Karikas GA. Anticancer and chemopreventing natural products: some biochemical and therapeutic aspects. J BUON. 2010;15:627–638. [PubMed] [Google Scholar]
- Katzke VA, Kaaks R, Kühn T. Lifestyle and cancer risk. Cancer J. 2015;21(2):104–110. doi: 10.1097/PPO.0000000000000101. [DOI] [PubMed] [Google Scholar]
- Khan AQ, Kuttikrishnan S, Siveen KS, Prabhu KS, Shanmugakonar M, Al-Naemi HA, Haris M, Dermime S, Uddi S. Seminars in cancer biology. New York: Academic Pressa; 2019. RAS-mediated oncogenic signaling pathways in human malignancies; pp. 1–13. [DOI] [PubMed] [Google Scholar]
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–931b. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Khan T, Ali M, Khan A, Nisar P, Jan SA, Afridi S, Shinwari ZK. Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2020;10:47. doi: 10.3390/biom10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, NiheiM NH, Fukushi H, Tabata K, Suzuki T, Akihisa T. Albanol A from the root bark of Morus alba L. induces apoptotic cell death in HL60 human leukemia cell line. Chem Pharm Bull. 2010;58:568–571. doi: 10.1248/cpb.58.568. [DOI] [PubMed] [Google Scholar]
- Kim B, Park JE, Im E, Cho Y, Lee J, Lee HJ, Kim SH. Recent advances in nanotechnology with nano-phytochemicals: molecular mechanisms and clinical implications in cancer progression. Int J Mol Sci. 2021;22(7):3571. doi: 10.3390/ijms22073571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirar S, Chaudhari D, Thakur NS, Jain S, Bhaumik J, Laha JK, Banerjee UC. Light-assisted anticancer photodynamic therapy using porphyrin-doped nanoencapsulates. J Photochem Photobiol B. 2021;220:112209. doi: 10.1016/j.jphotobiol.2021.112209. [DOI] [PubMed] [Google Scholar]
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Ahn KS. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korani S, Rashidi K, Hamelian M, Jalalvand AR, Tajehmiri A, Korani M, Sahebkar A. Evaluation of antimicrobial and wound healing effects of gold nanoparticles containing Abelmoschus esculentus (L.) aqueous extract. Bioinorgan Chem Appl. 2021 doi: 10.1155/2021/7019130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kowalczyk T, Sitarek P, Skała E, Toma M, Wielanek M, PytelD WJ, Szemraj J, Śliwiński T. Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells. Cytotechnology. 2019;71:165–180. doi: 10.1007/s10616-018-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Bupesh G, Manikandan E, Thanigai AK, Magesh S, Kalyanaraman R, Maaza M. Green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell lines. J Antimicro. 2016;2(3):2472–1212. doi: 10.4172/2472-1212.1000123. [DOI] [Google Scholar]
- Lakshmi S, Suresh S, Rahul BS, SaikantR MV, Gopi M, Padmaja G, Remani P. In vitro and in vivo studies of 5, 7-dihydroxy flavones isolated from Alpinia galanga (L.) against human lung cancer and ascetic lymphoma. Med Chem Res. 2019;28:39–51. doi: 10.1007/s00044-018-2260-3. [DOI] [Google Scholar]
- Lee HJ, Cho HS, Jun S, Lee JJ, Yoon J, Lee JH, Song H, Choi S, Kim S, Saloura V, et al. Tussilago farfara L. augments TRAIL-induced apoptosis through MKK7/JNK activation by inhibition of MKK7TIPRL in human hepatocellular carcinoma cells. Oncol Rep. 2014;32:1117–1123. doi: 10.3892/or.2014.3279. [DOI] [PubMed] [Google Scholar]
- Li J, Sun J, Song J. Experimental research on effect of Hedyotis diffusa Willd on blood metastasis in H22 mice. Lishizhen Med Mater Med Res. 2012;23:2434–2435. doi: 10.3390/molecules21060710. [DOI] [Google Scholar]
- Li Y, Gu JF, Zou X, Wu J, Zhang MH, Jiang J, Qin D, Zhou JY, Liu BXZ, Zhu YT. The anti-lung cancer activities of steroidal saponins of P. polyphylla Smith var. chinensis (Franch.) Hara through enhanced immunostimulation in experimental Lewis tumor-bearing C57BL/6 mice and induction of apoptosis in the A549 cell line. Molecules. 2013;18:12916–12936. doi: 10.3390/molecules181012916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Kumar AS, Wu SB, Liu B, Guo ZY, FataJE KEJ, Long CL. Comparative UPLC-QTOF-MS-based metabolomics and bioactivities analyses of Garcinia oblongifolia. J Chromatogr B. 2016;1011:179–195. doi: 10.1016/j.jchromb.2015.12.061. [DOI] [PubMed] [Google Scholar]
- Li F, Wang W, Xiao H. The evaluation of anti-breast cancer activity and safety pharmacology of the ethanol extract of Aralia elata Seem. leaves. Drug Chem Toxicol. 2019 doi: 10.1080/01480545.2019.1601211. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wu F, Chen Y, Alrashood ST, Alharbi SA. Anti-human colon cancer properties of a novel chemotherapeutic supplement formulated by gold nanoparticles containing Allium sativum L. leaf aqueous extract and investigation of its cytotoxicity and antioxidant activities. Arab J Chem. 2021;14(4):103039. doi: 10.1016/j.arabjc.2021.103039. [DOI] [Google Scholar]
- Lohse I, Wildermuth E, Brothers SP. Naturally occurring compounds as pancreatic cancer therapeutics. Oncotarget. 2018;9:35448. doi: 10.18632/oncotarget.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan SR, Callahan DJ, Chilkoti A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery. Nanomedicine. 2010;5:793–806. doi: 10.2217/nnm.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madakka M, Jayaraju N, Rajesh N. Evaluating the antimicrobial activity and antitumor screening of green synthesized silver nanoparticles compounds, using Syzygium jambolanum, towards MCF7 cell line (Breast cancer cell line) J Photochem Photobiol. 2021;6:100028. doi: 10.1016/j.jpap.2021.100028. [DOI] [Google Scholar]
- Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs. 2006;15:933–946. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- Maheswari P, Harish S, Navaneethan M, Muthamizhchelvan C, PonnusamyS HY. Bio-modified TiO2 nanoparticles with Withania somnifera, Eclipta prostrata and Glycyrrhiza glabra for anticancer and antibacterial applications. Mater Sci Eng C. 2020;108:110457. doi: 10.1016/j.msec.2019.110457. [DOI] [PubMed] [Google Scholar]
- Mandrich L, Caputo E. Brassicaceae-derived anticancer agents: towards a green approach to beat cancer. Nutrients. 2020;12:868. doi: 10.3390/nu12030868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjamalai A, Grace V. Antioxidant activity of essential oils from Wedelia chinensis (Osbeck) in vitro and in vivo lung cancer bearing C57BL/6 mice. Asian Pac J Cancer Prev. 2012;13:3065–3071. doi: 10.7314/apjcp.2012.13.7.3065. [DOI] [PubMed] [Google Scholar]
- Mansha M, Khan I, Ullah N, Qurashi A. Synthesis, characterization and visible-light-driven photoelectrochemical hydrogen evolution reaction of carbazole-containing conjugated polymers. Int J Hydrogen Energy. 2017;42:10961. doi: 10.1016/j.dyepig.2017.04.028. [DOI] [Google Scholar]
- Maqsood M, Mushtaq Z, Rasheed T, Nisa ZU, Sher F. Thrombolytic and cytotoxic activity of different bioactive extracts of E. coli case studies in chemical and environmental. Engineering. 2021;3:100080. doi: 10.1016/j.cscee.2021.100080. [DOI] [Google Scholar]
- Marghoob MU, Noureen A, Raza A, Khan WS, Iftikhar M, Sher F. Synthesis and toxicity assessment of environment friendly high yield ceria nanoparticles for biosafety. J Environ Chem Eng. 2021;10(1):107029. doi: 10.1016/j.jece.2021.107029. [DOI] [Google Scholar]
- Mashaghi S, Jadidi T, Koenderink G, Mashaghi A. Lipid nanotechnology. Int J Mol Sci. 2013 doi: 10.3390/ijms14024242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal AK, Thanki K, Jain S, Banerjee UC. Comparative studies of anticancer and antimicrobial potential of bioinspired silver and silver-selenium nanoparticles. Appl Nanomed. 2016;1:1–6. [Google Scholar]
- Mobaraki F, MomeniM YMET, Meshka Z, ToosiM S, Hosseini SM. Plant-derived synthesis and characterization of gold nanoparticles: Investigation of its antioxidant and anticancer activity against human testicular embryonic carcinoma stem cells. Process Biochem. 2021;111:167–177. doi: 10.1016/j.procbio.2021.09.010. [DOI] [Google Scholar]
- Moghadamtousi SZ, Kamarudin MNA, Chan CK, Goh BH, Kadir HA. Phytochemistry and biology of Loranthus parasiticus Merr, a commonly used herbal medicine. Am J Chin Med. 2014;42:23–35. doi: 10.1142/S0192415X14500025. [DOI] [PubMed] [Google Scholar]
- Mondal S, Bandyopadhyay S, Ghosh KM, Mukhopadhyay S, Roy S, Mandal C. Natural products: promising resources for cancer drug discovery. Anti-Cancer Agents Med Chem (formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2012;12:49–75. doi: 10.2174/187152012798764697. [DOI] [PubMed] [Google Scholar]
- Mondal A, Gandhi A, Fimognari C, AtanasovAG BA. Alkaloids for cancer prevention and therapy: current progress and future perspectives. Eur J Pharmacol. 2019;858:172472. doi: 10.1016/j.ejphar.2019.172472. [DOI] [PubMed] [Google Scholar]
- Montalbán MG, Coburn JM, Lozano-Pérez AA, CenisJL VG, Kaplan DL. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials. 2018;8(2):126. doi: 10.3390/nano8020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montané X, Kowalczyk O, Reig-Vano B, Bajek A, Roszkowski K, Tomczyk R, Tylkowski B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecule. 2020;25:3342. doi: 10.3390/molecules25153342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy D, Attri KS, Singh PK. Phosphoinositide 3-kinase signaling pathway in pancreatic ductal adenocarcinoma progression, pathogenesis, and therapeutics. Front Physiol. 2018;9:335. doi: 10.3389/fphys.2018.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Chauhan P, Saha B, Kubatzky KF. Conceptual evolution of cell signaling. Int J Mol Sci. 2019;20:3292. doi: 10.3390/ijms20133292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narnoliya LK, Sangwan N, Jadaun JS, Bansal S, Sangwan RS. Defining the role of a caffeic acid 3-O-methyltransferase from Azadirachta indica fruits in the biosynthesis of ferulic acid through heterologous over-expression in Ocimum species and Withania somnifera. Planta. 2021;253:20. doi: 10.1007/s00425-020-03514-y. [DOI] [PubMed] [Google Scholar]
- Oliveira CR, Spindola DG, Garcia DM, ErustesA BA, Palmeira-dos-Santos C, Smaili SS, Pereira GJ, Hinsberger A, Viriato EP. Medicinal properties of Angelica archangelica root extract: cytotoxicity in breast cancer cells and its protective effects against in vivo tumor development. J Integr Med. 2019;17:132–140. doi: 10.1016/j.joim.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Palaniappan P, Sathishkumar G, Sankar R. Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;138:885–890. doi: 10.1016/j.saa.2014.10.072. [DOI] [PubMed] [Google Scholar]
- Paramee S, Sookkhee S, Sakonwasun C, Takuathung MN, Mungkornasawakul P, Nimlamool W, Potikanond S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement Altern Med. 2018;18:178. doi: 10.1186/s12906-018-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen R, Parveen B, Parveen A, Ahmad S. Andrographis paniculata: from traditional to nano drug for cancer therapy. Nanomater Plant Potential. 2019 doi: 10.1007/978-3-030-05569-1_13. [DOI] [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn J, Herndon J, Kornblith AB, Peters W, Ahles T, Vredenburgh J, Cancer and Leukemia Group B (CALGB), The Southwestern Oncology Group (SWOG) Group Quality of life among patients with Stage II and III breast carcinoma randomized to receive high-dose chemotherapy with autologous bone marrow support or intermediate-dose chemotherapy: results from Cancer and Leukemia Group B 9066. Cancer. 2005;104:1580–1589. doi: 10.1002/cncr.21363. [DOI] [PubMed] [Google Scholar]
- Priyadarshni and Mahalingam Antimicrobial and anticancer activity of silver nanoparticles from Edible Mushroom: a review. Asian J Pharmaceut Clin Res. 2017;10:37–40. doi: 10.22159/ajpcr.2017.v10i3.16027. [DOI] [Google Scholar]
- Rai M, Kon K, Ingle A, DuranN GS, Galdiero M. Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol. 2014;98:1951–1961. doi: 10.1007/s00253-013-5473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakanth GSH, Kumar CU, Kishan PV, Usharani P. A randomized, double blind placebo controlled study of efficacy and tolerability of Withaina somnifera extracts in knee joint pain. J Ayurveda Integr Med. 2016;7(3):151–157. doi: 10.1016/j.jaim.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramar M, Manikandan B, Marimuthu PN, Raman T, Mahalingam A, Subramanian P, KarthickS MA. Synthesis of silver nanoparticles using Solanum trilobatumfruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;140:223–228. doi: 10.1016/j.saa.2014.12.060. [DOI] [PubMed] [Google Scholar]
- Reddy P, Pradeep S, GopinathSM RR, Ramu R, Kollur SP, Shivamallu C. Anti-breast cancer potential of MnO2 nanoparticles using Terminalia chebula fruit extract against MCF-7 cell line through in vitro cell cycle and apoptotic studies. Mater Today Proc. 2022;62:5526. doi: 10.1016/j.matpr.2022.04.330. [DOI] [Google Scholar]
- Ren B, Kwah MXY, Liu C, Ma Z, Shanmugam MK, Ding L, Goh BC. Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett. 2021;515:63–72. doi: 10.1016/j.canlet.2021.05.001. [DOI] [PubMed] [Google Scholar]
- Saleem J, Wang L, Chen C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv Healthc Mater. 2018;7:1800525. doi: 10.1002/adhm.201800525. [DOI] [PubMed] [Google Scholar]
- Sangwan NS, Sangwan RS. Secondary metabolites of traditional medical plants – a case study Ashwaganda. In: Opatrný Z, Nick P, editors. Applied cell technology. Germany: Springer; 2014. pp. 325–367. [Google Scholar]
- Sangwan NS, Tripathi S, Srivastava Y, Mishra B, Pandey N. Science of Ashwagandha: preventive and therapeutic potentials. Cham: Springer; 2017. Phytochemical genomics of Ashwagandha; pp. 3–36. [Google Scholar]
- Sathiyavimal S, Durán-Lara EF, Vasantharaj S, Saravanan M, Sabour A, Alshiekheid M, Pugazhendhi A. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaves extract and their evaluation of antibacterial, anticancer in human A549 lung and MDA-MB-231 breast cancer cells. Food Chem Toxicol. 2022 doi: 10.1016/j.fct.2022.113330. [DOI] [PubMed] [Google Scholar]
- Sato D, Kondo S, Yazawa K, Mukudai Y, Li C, Kamatani T, Katsuta H, Yoshihama Y, Shirota T, Shintani S. The potential anticancer activity of extracts derived from the roots of Scutellaria baicalensis on human oral squamous cell carcinoma cells. Mol Clin Oncol. 2013;1:105–111. doi: 10.3892/mco.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Sangwan NS (2022) Biotechnology applications in neurodegenerative diseases in biotechnology in healthcare: technologies and innovations. In: Barh D (ed) Biotechnology approaches in developing novel drug delivery systems, Biotechnology in Healthcare: Technologies and Innovations, vol 2
- Sharma A, Goyal AK, Rath G. Recent advances in metal nanoparticles in cancer therapy. J Drug Targ. 2018;26:617–632a. doi: 10.1080/1061186X.2017.1400553. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kaur M, Katnoria JK, Nagpal AK. Polyphenols in food: cancer prevention and apoptosis induction. Curr Med Chem. 2018;25:4740–4757b. doi: 10.2174/0929867324666171006144208. [DOI] [PubMed] [Google Scholar]
- Shirzad H, Taji F, Rafieian-Kopaei M. Correlation between antioxidant activity of garlic extracts and WEHI-164 fibrosarcoma tumor growth in BALB/c mice. J Med Food. 2011;14:969–974. doi: 10.1089/jmf.2011.1594. [DOI] [PubMed] [Google Scholar]
- Siddique AA, Joshi P, Misra LN, Sangwan NS, Darokar MP. 5,6-De-epoxy-5-en-7-one-17-hydroxy withaferin A, a new cytotoxic steroid from Withania somnifera L. Dunal leaves. Nat Product Res. 2014;28(6):392–398. doi: 10.1080/14786419.2013.871545. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020 doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Sre PR, Reka M, Poovazhagi R, Kumar MA, Murugesan K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indicam. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;135:1137–1144. doi: 10.1016/j.saa.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Srikrishna D, Sachsenmeier K (2021) We need to bring R0 < 1 to treat cancer too. Genome Med 13(1):120. 10.1186/s13073-021-00940-9 [DOI] [PMC free article] [PubMed]
- Srivastava Y, Sangwan NS. Improving medicinal crops through phytochemical perspective: Withania somnifera (Ashwagandha) In: Tuteja N, editor. Advancement in crop improvement techniques. Sawston: WoodHead Publishing; 2020. pp. 275–295. [Google Scholar]
- Sultana S, Munir N, Mahmood Z, Riaz M, Akram M, Rebezov M, Rengasamy KR. Molecular targets for the management of cancer using Curcuma longa Linn. phytoconstituents: a review. Biomed Pharmacother. 2021;135:111078. doi: 10.1016/j.biopha.2020.111078. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tan J, Tay J, Hedrick J, Yang YY. Synthetic macromolecules as therapeutics that overcome resistance in cancer and microbial infection. Biomaterials. 2020;252:120078. doi: 10.1016/j.biomaterials.2020.120078. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Dickinson S, Bowden T. Cruciferous vegetables, isothiocyanates, indoles, and cancer prevention. Nutrition and health: bioactive compounds and cancer. Berlin: Springer; 2010. pp. 535–566. [Google Scholar]
- Tripathi D, Modi A, Narayan G, Rai SP. Green and cost effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater Sci Eng C. 2019;100:152–164. doi: 10.1016/j.msec.2019.02.113. [DOI] [PubMed] [Google Scholar]
- Tripathi SK, Mahakud AK, Biswa BK. A green approach towards formulation, characterization, and antimicrobial activity of poly (Lactic-co-Glycolic) acid-Alstonia scholaris based nanoparticle. Mater Res Express. 2019;6(9):095325. doi: 10.1088/2053-1591/ab30d1. [DOI] [Google Scholar]
- Turan I, Demir S, Kilinc K, Burnaz NA, Yaman SO, Akbulut K, Mentese A, Aliyazicioglu Y, Deger O. Antiproliferative and apoptotic effect of Morus nigra extract on human prostate cancer cells. Saudi Pharm J. 2017;25:241–248. doi: 10.1016/j.jsps.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakayil R, Muruganantham S, Kabeerdass N, Rajendran M, Ramasamy S, Alahmadi TA, Mathanmohun M. Acorus calamus-zinc oxide nanoparticle coated cotton fabrics shows antimicrobial and cytotoxic activities against skin cancer cells. Process Biochem. 2021;111:1–8. doi: 10.15666/aeer/2002_919929. [DOI] [Google Scholar]
- Valsalam ASP, Arasu MV, Al-Dhabi NA, Ghilan AM, Kaviyaras K, Ravindran B, Chang SW, Arokiyaraj S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J Photochem Photobiol B Biol. 2019;191:65–74. doi: 10.1016/j.jphotobiol.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, Tsao SW. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem. 2010;111:1426–1436. doi: 10.1002/jcb.22869. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen M, Wang Y. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med. 2013;41:177–196. doi: 10.1142/S0192415X13500134. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shen Y, Wang S, Shen Q, Zhou X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018;415:117–128. doi: 10.1016/j.canlet.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Dheen ST, Fuh JYH, Kumar AS. A review of multi-functional ceramic nanoparticles in 3D printed bone tissue engineering. Bioprinting. 2021;23:e00146. doi: 10.1016/j.bprint.2021.e00146. [DOI] [Google Scholar]
- Wang T, SuitaY MS, Dean J, Tapinos N, Shen J. Advances in lipid-based nanoparticles for cancer chemoimmunotherapy. Pharmaceutics. 2021;13(4):520a. doi: 10.3390/pharmaceutics13040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia QH, Ma YJ, Wang JW. Biosynthesis of silver nanoparticles using Taxus yunnanensis callus and their antibacterial activity and cytotoxicity in human cancer cells. Nanomaterials. 2016;6:160. doi: 10.3390/nano6090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Tan S, Zhou Y, Lin J, Wang H, Oyang L, Tian Y, Liu L, Su M, Wang H, Cao D. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther. 2018;11:2063. doi: 10.2147/OTT.S161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav N, Sangwan NS (2022) Biotechnology approaches in developing novel drug delivery systems. In: Barh D (ed) Elsevier Chapter 13: Biotechnology approaches in developing novel drug delivery systems. Biotechnology in Healthcare: Technologies and Innovations, vol 1
- Yallapu MM, Jaggi M, Chauhan CS. Curcumin nanomedicine: a road to cancer therapeutics. Curr Pharm Des. 2013;19:1994–2010. doi: 10.2174/138161213805289219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang Y, Tang S, Tang H, Yang G, Xu Q, Wu J. Anti-tumor effect of polysaccharides from Scutellaria barbata D. Don on the 95-D xenograft model via inhibition of the C-met pathway. J Pharmacol Sci. 2014;125:255–263. doi: 10.1254/jphs.13276fp. [DOI] [PubMed] [Google Scholar]
- Yao Y, Dai W. Genomic instability and cancer. J Carcinogenes Mutagenis. 2014 doi: 10.4172/2157-2518.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Kim AK. Platycodin D induces apoptosis in MCF-7 human breast cancer cells. J Med Food. 2010;13:298–305. doi: 10.1089/jmf.2009.1226. [DOI] [PubMed] [Google Scholar]
- Zargar T, Kumar D, Sahni B, Shoket N, Bala K, Angurana S. Dietary risk factors for colorectal cancer: A hospital-based case–control study. Cancer Res Stat Treatment. 2021;4(3):479. doi: 10.4103/crst.crst_116_21. [DOI] [Google Scholar]
- Zhang P, Li H, Yang B, Yang F, Zhan LL, Kong QY, Liu J. Biological significance and therapeutic implication of resveratrol-inhibited Wnt, Notch and STAT3 signaling in cervical cancer cells. Genes Cancer. 2014;5(5–6):154. doi: 10.18632/genesandcancer.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xiong Y, Xia Q, Wu F, Liu L, Zhou Y, Zen L, Zhou C, Xia C, Jiang W, Liao XL, Liu L, Yang H, Guan R, Li K, Wang J, Lei G, Zhang Y, Yang N. Efficacy and safety of apatinib plus vinorelbine in patients with wild-type advanced non-small cell lung cancer after second-line treatment failure: a nonrandomized clinical trial. JAMA Netw Open. 2020;3(3):e201226. doi: 10.1001/jamanetworkopen.2020.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Li H, He Y, Yuan M, Shen M, Yang R, Yang C. Preparation and in vitro release of total alkaloids from alstonia scholaris leaves loaded mPEG-PLA microspheres. Materials. 2019;12(9):1457. doi: 10.3390/ma12091457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Guo DW, Lao QC, Xu YQ, Meng ZK, Xia B, Yang H, Li CQ, Li P. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci Rep. 2019;9:4541. doi: 10.1038/s41598-019-40866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi L, Wu SC, Wu J, Hayama KL, Benavente CA. The cyclin-dependent kinase inhibitor flavopiridol (alvocidib) inhibits metastasis of human osteosarcoma cells. Oncotarget. 2018;9:23505. doi: 10.18632/oncotarget.25239. [DOI] [PMC free article] [PubMed] [Google Scholar]