Abstract
APETALA2/ethylene response factor (AP2/ERF) is widely found in the plant kingdom and plays crucial roles in transcriptional regulation and defense response of plant growth and development. Based on the research progress related to AP2/ERF genes, this paper focuses on the classification and structural features of AP2/ERF transcription factors, reviews the roles of rice AP2/ERF genes in the regulation of growth, development and stress responses, and discusses rice breeding potential and challenges. Taken together; studies of rice AP2/ERF genes may help to elucidate and enrich the multiple molecular mechanisms of how AP2/ERF genes regulate spikelet determinacy and floral organ development, flowering time, grain size and quality, embryogenesis, root development, hormone balance, nutrient use efficiency, and biotic and abiotic response processes. This will contribute to breeding excellent rice varieties with high yield and high resistance in a green, organic manner.
Keywords: rice, AP2/ERF, classification and feature, gene function, regulatory mechanism, breeding potential
1. Introduction
Transcription factors (TFs) are a group of protein molecules that can specifically bind to cis-acting elements in the promoter region of eukaryotic genes to ensure that target genes are expressed at a specific intensity in a specific time and space, thus playing important roles in plant growth, development and environmental responses. In plants, about 60 kinds of transcription factors have been identified, such as AP2/ERF, bZIP, C2H2, MYB, MADS, NAC, and WRKY [1]. TFs and various DNA binding domains control various regulation processes in higher plants. AP2/ERF is one of the evolutionary oldest and largest transcription factor families in plants. It was first isolated from Arabidopsis thaliana by Jofuku in 1994 and is widely involved in plant growth and development processes, including plant height, root development, flower development, seed germination, organ size, resistance to biotic and abiotic stress, and plant hormone signal transduction pathways [1,2]. As an important food crop, rice (Oryza sativa) encounters pests, diseases, and abiotic stresses such as extreme temperatures, drought and flooding, during the life cycle, which greatly affects the rice production. Studies have shown that rice AP2/ERF transcription factors play crucial roles in growth and development, and also play important roles in improving the resistance of rice to various stresses, pests and diseases.
2. Classification, Features and Binding Elements
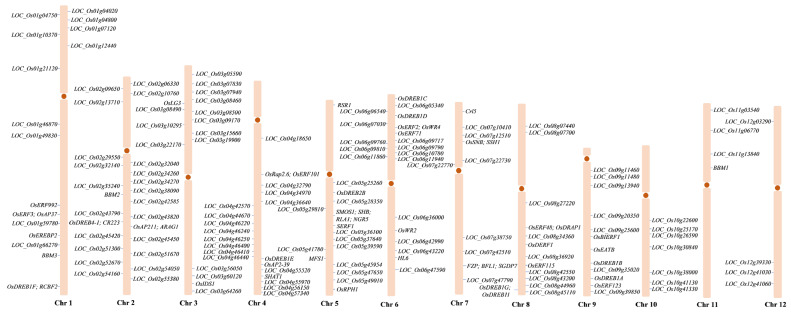
In the plant kingdom, the AP2/ERF family is a large family of transcription factors with conserved AP2/ERF domains composed of about 60–70 amino acid residues [3,4,5,6]. According to the number of AP2/ERF domains and the different functions of genes, the superfamily can be divided into four families: AP2, ERF, RAV, and Soloist [7,8,9,10]. The AP2 family has two duplicated AP2/ERF domains, which can be further subdivided into AP2 and ANT subfamilies [11]. ERF family proteins have only one AP2/ERF domain, which can be subdivided into two subfamilies, ERF and CBF/DREB, according to the sequence of the DNA binding domain. The proteins encoded by ERF subfamily genes bind to AGCCGCC core motifs, while CBF/DREB subfamily members recognize the cis-acting elements A/GCCGAC, named the C-repeat [12,13,14]. ERF and DREB proteins are associated with abiotic stress in plants and are affected by drought, low temperature and high salt. In addition to the AP2/ERF domain, RAV family proteins possess a VP1/ABI3 binding domain. These proteins are negative regulators of growth and development in arabidopsis and play key regulatory roles in plant defense pathways. Phylogenetic analysis of AP2/ERF TFs in rice showed that alanine 14 (Ala-14) and aspartic acid 19 (Asp-19) of ERF subfamily proteins are conserved among the members containing a single AP2/ERF domain, while CBF/DREB subfamily proteins contained valine (Val-14) at the 14th and glutamine (Glu-19) at the 19th positions [9,15]. Compared with the conserved Arg-6 of a single domain protein, Thr-6 was more conserved among the proteins containing two AP2/ERF domains [9,15]. It was also found that there is histidine in each AP2 domain of the AP2 subfamily proteins with two AP2 domains, but not in the other AP2/ERF proteins with one AP2 domain [9,15]. There are 165 AP2/ERF TFs in rice, including 24 AP2 subfamily members, 136 ERF (ERF/DREB) subfamily members, five RAV subfamily members, and zero Soloist subfamily members [1,8] (Figure 1, Table 1).
Figure 1.
The distribution of AP2/ERF genes on chromosomes in rice.
Table 1.
Classification and quantity of AP2/ERF transcription factors in rice.
| Classification | AP2 | ERF | RAV | Soloist |
|---|---|---|---|---|
| Number | 24 | 136 | 5 | 0 |
3. Molecular Roles of AP2/ERF TFs in Rice
Numerous reports have documented that rice AP2/ERF TFs are important regulators involved in plant growth and hormonal regulation, including spikelet development, grain characteristics, root development and formation, drought tolerance, salinity tolerance, temperature tolerance, and nutrient use [1,16,17]. Here, we review the functions and the regulatory networks of AP2/ERF TFs and discuss potential applications in rice breeding.
3.1. Spikelet Determinacy and Organ Development
The spikelet, as a grass-specific basal inflorescence unit, can be divided into two types: determinate spikelet (DS) and indeterminate spikelet. In rice with DS, the spikelet meristem produces two lateral floral meristems and one terminal floral meristem (TFM). Two LFMs form one pair of sterile lemmas, and the TFM develops one terminal floret, which generates one seed [18,19].
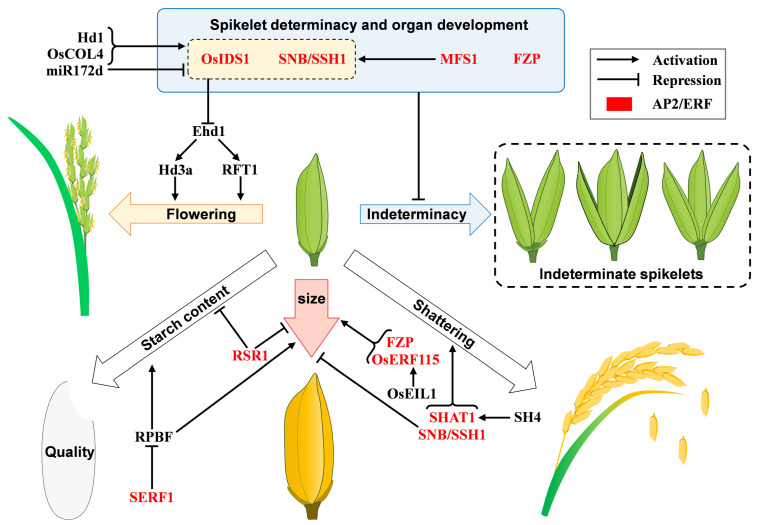
So far, few genes have been reported to maintain spikelet meristem fate, but AP2/ERF genes have been found to be involved in this process [17,18]. AP2/ERF family genes play an important role in the regulation of spikelet meristem determinacy and organ identity (Figure 2) [18,19]. FRIZZY PANICLE (FZP) delays the transition from spikelet meristem to flower meristem, determines the identity of sterile lemma, and is required for the formation of axillary meristem in the spikelet meristem and floral meristem [19,20]. Loss-of-function of FZP results in extra rudimentary glumes and the absence of sterile lemmas and florets on the rachilla position [19]. In weak allelic mutants fzp-12 and fzp-13, the sterile lemmas are converted to rudimentary glumes, while the sterile lemmas are converted to lemmas (degraded florets) in FZP over-expression plants [19]. SUPERNUMERARY BRACT (SNB) is necessary for the correct transition from spikelet meristem to floral meristem and the correct formation of floral organ pattern [21]. In the snb mutant, the rudimentary glume-like and lemma/palea-like organs are generated, and the sterile lemmas fail to develop [21]. Meantime, ectopic lodicules are elongated and occasionally form on the palea side, the stamens were reduced, and additional pistils were formed [21]. In a few cases, extra florets are produced in the axils of sterile lemma [21]. In the Oryza sativa indeterminate spikelet (osids1) mutant, sterile lemmas are replaced by rudimentary glumes [21]. The osids1/snb mutant develops more rudimentary glumes, lemmas, and paleae than the single mutant. Similarly, the osids1/snb mutant shows abnormal floral organs, including lemma/palea-like lodicules, fewer stamens, lodicule-stamen chimera and stamen-pistil chimera organs [22]. Together, SNB and OsIDS1 co-regulate spikelet development and organ identity and play a redundant role in the spikelet meristem transition and determinacy. Moreover, SNB and OsIDS1 cooperatively participate in the determination of floral meristem fate in a dose-dependent manner. MULTI-FLORET SPIKELET 1 (MFS1) determines the spikelet meristem determinacy and floral organ identity, and positively regulates the expressions of spikelet development-related gene G1 and IDS-like genes SNB and OsIDS1 [18]. In the mfs1 mutant, the transition from spikelet meristem to floral meristem is delayed, resulting in extra lemmas and paleae (degraded florets), and degraded sterile lemmas and paleae [18]. These results suggest that AP2/ERF genes inhibit spikelet indeterminacy, and determine organ fate, and it is possible to breed rice cultivars with multi-floret spikelets by altering meristem determinacy and/or inducing lateral florets, thereby increasing the grain number per panicle (Figure 2, Table 2).
Figure 2.
Schematic representation of spikelet development, flowering time and grain properties mediated by AP2/ERF transcription factors in rice. OsIDS1, SNB/SSH1, MFS1 and FZP, which are mentioned in Section 3.1, inhibit spikelet indeterminacy. Of these, OsIDS1 and SNB/SSH1 are mentioned in Section 3.2 to inhibit flowering. In Section 3.3, SHAT1 and SNB/SSH1 are mentioned to promote grain shattering. FZP and OsERF115 promote grain size, while SNB/SSH1, RSR1 and SERF1 inhibit grain size. SERF1 inhibits starch content, and RSR1 affect amylose content and amylopectin structure. The AP2/ERF transcription factors and other regulators are shown in red and black, respectively.
Table 2.
The genes associated with spikelet determinacy and organ development in rice.
3.2. Flowering Regulation
Flowering time is a key agronomic trait affecting rice yield and quality. OsIDS1 and SNB are involved in the regulation of flowering in rice (Figure 2, Table 3). Over-expression of OsIDS1 or SNB delays rice flowering. In over-expression plants, two florigen genes Heading date-3a (Hd3a) and Rice Flowering Lous T1 (RFT1), and their direct upstream regulator Early heading date 1 (Ehd1), were inhibited [22]. MiR172d promoted rice flowering by reducing the expression of SNB and OsIDS1. The expression levels of SNB and OsIDS1 were positively regulated by CONSTANS-LIKE 4 (OsCOL4) and Heading date 1 (Hd1), and they inhibited floral transition by repressing Ehd1 expression [23]. This showed that the AP2 subfamily IDS-like genes OsIDS1 and SNB delay rice flowering (Figure 2), and suggest creation of rice new germplasms with early flowering and early maturing can be achieved by editing IDS-like genes.
Table 3.
Genes associated with flowering in rice.
3.3. Role in Grain Size, Quality and Shattering
Grain properties such as size, quality and shattering determine final edible production and the degree of mechanized harvesting. SNB influences grain size and weight by regulating cell division and elongation and participating in both brassinosteroid signaling and auxin signaling pathways [24]. CRISPR/Cas9 knockdown of SNB plants showed an increase in grain length, width and 1000-grain weight, while SNB over-expression decreased grain length, width and 1000-grain weight. SNB is also involved in regulating grain shattering. The allele SUPPRESSION OF SHATTERING 1 (SSH1) of SNB affects the deposition of lignin and the development of abscission zone (AZ) by positively regulating the expression of SEED SHATTERING 1 (qSH1) and SEED SHATTERING 5 (SH5) genes and regulates rice grain shattering. The ssh1 gene has the genetic effect of increasing grain length and grain weight, and the introduction of ssh1 into the excellent indica rice variety 93–11 further increased grain length and weight, indicating that the ssh1 gene has the potential to improve rice yield [25]. SEED SHATTERING ABORTION 1 (SHAT1), a key gene for rice grain shattering, is continuously expressed in AZ at early spikelet development stages by participating in AZ development. SEED SHATTERING 4 (SH4) and qSH1 are also necessary to reduce grain shattering during rice domestication. Genetic analysis showed that SHAT1 expression in AZ was positively regulated by SH4. qSH1 acts downstream of SHAT1 and SH4, and promotes AZ differentiation by maintaining the expression of SHAT1 and SH4 in AZ, thereby controlling grain shattering [26]. As a transcriptional repressor, OsERF115 interacts with ETHYLENE-INSENSITIVE 3-LIKE 1 (OsEIL1) to regulate grain size [27]. Haplotype analysis showed that the SNP variation of the OsERF115 promoter that can be bound by ETHYLENE INSENSITIVE 3 (EIN3), was significantly correlated with OsERF115 expression level and grain weight, suggesting that natural variation of the OsERF115 promoter contribute to grain size diversity. Over-expression of OsERF115 promotes longitudinal cell elongation and lateral cell division of the hull, enhances grain filling, and increases grain length, width, thickness, and weight, whereas knockdown of OsERF115 does the opposite, indicating that OsERF115 positively regulates grain size and weight [27]. FZP was also reported to positively regulate grain size. The strong allele mutant of fzp could not form complete spikelets or floral organs, while the weak fzp-12 mutant produced smaller grains [19]. Another allelic mutant small grain and dense panicle 7 (sgdp7) has an inserted 18-bp repeat in the upstream promoter of FZP gene, which inhibits the expression of FZP, prolongs spikelet branching, increases spikelet number per panicle, and decreases grain weight [28]. SALT-RESPONSIVE ERF 1 (SERF1) negatively regulates grain size and filling by affecting RICE PROLAMIN-BOX BINDING FACTOR (RPBF) expression. Loss of SERF1 function increases the expression of RPBF, leading to an increase in grain size and starch content, while SERF1 over-expression decreases the expression of RPBF, leading to a decrease in grain size [29].
Starch is the main component of endosperm, and its content and structure directly affect grain quality. RICE STARCH REGULATOR 1 (RSR1) encodes a AP2/ERF transcription factor, the loss function of rsr1 increases amylose content and changes amylopectin structure [30]. The changed starch grains have reduced gelatinization temperature. Moreover, the rsr1 mutant grains become larger and yield increases. In RSR1 over-expression plants, the structure of amylopectin and starch gelatinization characteristics both change in the opposite trend, indicating that RSR1 regulates starch synthesis in rice grains [31].
Taken together, AP2/ERF genes play key roles in determining grain size, quality and shattering, thereby influencing grain yield and quality (Table 4, Figure 2). Exploring AP2/ERF genes and excellent alleles will further reveal genetic mechanism and creating a series of new germplasms through gene pyramiding and transgenic methods will provide a blueprint for breeding new rice varieties with appropriate grain size and good quality, thereby achieving the goal of high yield and quality.
Table 4.
Genes associated with grain properties in rice.
| Gene Name | Gene Family | Gene Locus | Gene Function | Reference(s) |
|---|---|---|---|---|
| OsSNB; SSH1 | AP2 | LOC_Os07g13170 | grain size; grain shattering | [24,25] |
| SHAT1 | AP2 | LOC_Os04g55560 | grain shattering | [26] |
| OsERF115 | ERF | LOC_Os08g41030 | grain size | [27] |
| FZP; BFL1; SGDP7 | ERF | LOC_Os07g47330 | grain size, grain number | [19,28] |
| SERF1 | ERF | LOC_Os05g34730 | grain size; grain quality | [29] |
| RSR1 | AP2 | LOC_Os05g03040 | grain quality | [30] |
3.4. Role in Embryogenesis
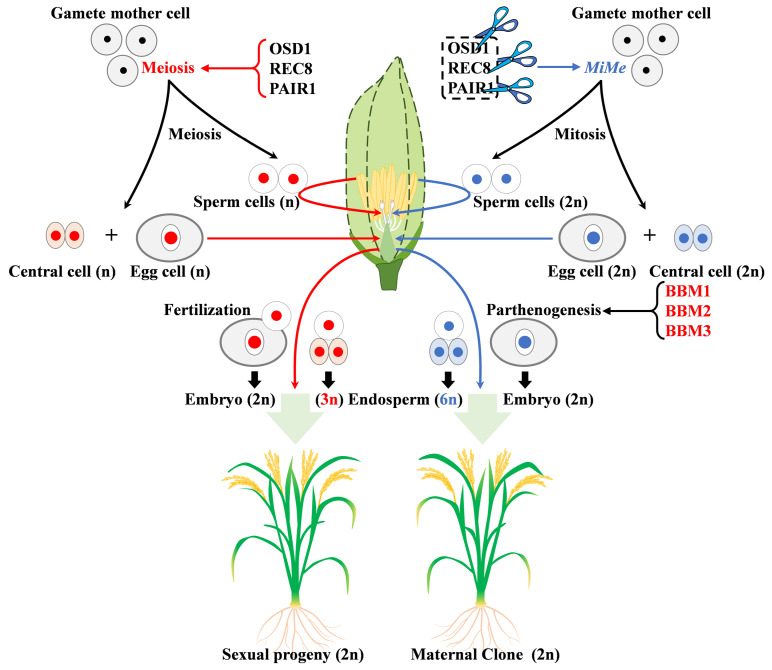
Apomixis includes parthenogenesis, apogamety and apospory. In apomictic plants, seed formation does not undergo exchange during meiosis, and the offspring retain the mother genotype, which plays an important role in heterosis fixation. The BABY BOOM (BBM) genes of the AP2/ERF family are involved in the initiation of rice embryogenesis (Figure 3, Table 5). Ectopic expression of BBM1 in fertilized eggs can induce the production of somatic embryos, and ectopic expression in egg cells can induce parthenogenesis [31]. In the transgenic plant BBM1-ee with specific expression of BBM1 in egg cells, the embryo structure could be observed, but the endosperm was lacking, and the seeds were aborted, suggesting that BBM1 could still induce embryo formation without spermatogenesis. Synthetic apomictic (S-APO) plants were further generated by using a mitosis instead of a meiosis technique (MiMe, knockdown of OSD1, PAIR1 and REC8) in BBM1-ee transgenic plants. It was found that S-APO plants could successfully induce parthenogenesis through seeds [31]. The bbm1/bbm2/bbm3 mutant had no obvious vegetative growth and flower defects and could eventually form normal seeds. These results indicate that BBM2 and BBM3 have redundant functions compared to BBM1. These data show that AP2/ERF genes can induce parthenogenesis, making it possible to propagate rice seeds asexually (Figure 3) [31].
Figure 3.
Schematic representation of sexual reproduction and synthetic apomixis regulated by MiMe and BBMs in rice. The genes in this figure are described in Section 3.4. Left is the sexual reproduction process in which the germ cells form haploid gametes through processes such as meiosis and double fertilization that produce a diploid (2n) embryo and a triploid (3n) endosperm. Apomixis on the right consists of two main modifications: one is the knockdown of OSD1, REC8 and PAIR1, which converts meiosis into mitosis (a technique called MiMe), resulting in 2n egg, sperm cells and central cells. The second is parthenogenesis induced by BBMs (AP2/ERF transcription factors), which converts (2n) egg cells into embryos and produces (6n) endosperm.
Table 5.
Genes associated with embryogenesis in rice.
3.5. Root Initiation and Formation
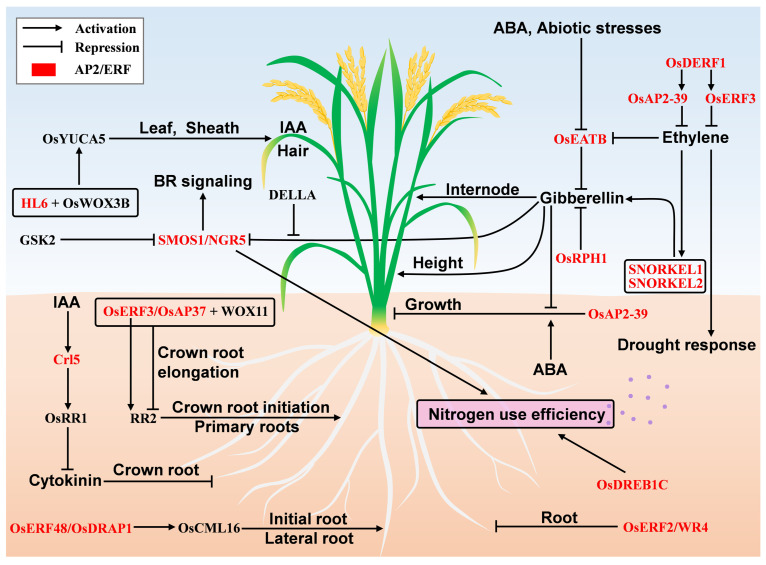
Plant roots mainly absorb water and nutrients, metabolize, are involved in nitrogen fixation, and reproduce. The growth has a complex regulatory system [32]. AP2/ERF family genes, such as Crown rootless 5 (Crl5), OsERF48 and ERF3, are involved in root formation and development. Crl5 encoded as a nitrpoERF transcription factor promotes crown root development, and its expression is induced by auxin, thereby positively regulating the expression of RESPONSE REGULATOR 1 (OsRR1) that inhibits the cytokinin signal [33]. The crl5 mutant produces a small number of crown roots, and the initiation of crown root primordia is destroyed. Crl5 over-expression plants do not induce ectopic roots, but their callus forms adventitious roots. The promoter of Crl5 has an auxin responsive element, and driving the over-expression of OsRR1 by the promoter restores the defect of adventitious roots formation in the crl5 mutant. CROWN ROOTLESS 1 (CRL1) is the essential factor in regulating the crown root. The crl1/crl5 mutant shows additive effects, indicating that Crl1 and Crl5 regulate the occurrence of adventitious roots through two different genetic pathways. The radicleless 1 (ral1) mutant cannot produce seed roots but can form crown roots. The crl1/ral1 mutant has an additive phenotype, indicating that the occurrence of seed roots and crown roots is regulated by different genetic mechanisms in rice [34]. In the process of root development, ETHYLENE-RESPONSE AP2/ERF FACTOR (OsERF48)/DROUGHT RESPONSIVE AP2/EREBP 1 (OsDRAP1) enhance root growth by regulating the expression of the calmodulin gene CALMODULIN 16 (OsCML16). OsERF48 over-expression plants have longer initial roots, more lateral roots and increased dry weight of roots, and the lateral roots of RNAi plants are reduced [35]. OsERF3/OsAP37 regulates crown roots development and the expression of auxin and cytokinin response genes [36]. ERF3 directly binds to the promoter of the cytokinin response gene RR2, thereby positively regulating the expression of RR2 and controlling the initiation of crown roots. In contrast, ERF3 interacts with the WUSCHEL-related homeobox 11 (WOX11) to inhibit the expression of RR2, thereby promoting crown root elongation [36]. The number of crown roots is decreased, and the primary roots become shorter in ERF3 knockout plants, while the opposite is true for over-expression lines [36]. These AP2/ERF genes ae involved in root development and formation by complex regulatory pathways (Figure 4, Table 6).
Figure 4.
Schematic representation of development, phytohormones and nutrient use efficiency regulation mediated by AP2/ERF transcription factors in rice. In Section 3.5, OsERF3/OsAP37, Crl5 and OsERF48/OsDRAP1 are described as affecting root initiation and formation. In Section 3.6, HL6, OsEATB, OsERF2/WR4, OsRPH1, SMOS1/NGR5, SNORKEL1, SNORKEL2, OsDERF1, OsAP2-39 and OsERF3 are described to regulate various growth processes of plants by participating in phytohormone pathways. SMOS1/NGR5 and OsDREB1C promote rice nitrogen use efficiency, as described in Section 3.7. The AP2/ERF transcription factors and other regulators are shown in red and black, respectively. Abbreviations: indole-3-acetic acid, IAA; abscisic acid, ABA; gibberellin, GA; brassinosteroid, BR.
Table 6.
Genes associated with root development in rice.
3.6. AP2/ERF Regulatory Roles Mediated by Hormones
Plant hormones, including auxin, gibberellin, cytokinin, abscisic acid, ethylene and brassinosteroids, play important roles in regulating cell growth and differentiation, organogenesis and abscission, flowering, senescence and maturation.
Ethylene is an important phytohormone for plant growth, development and stress tolerance, and its biosynthesis is regulated by the AP2/ERF transcription factor. The drought-responsive ERF gene, OsDERF1, negatively regulates ethylene synthesis by activating the transcription of OsERF3 and OsAP2-39, and plays a negative role in drought stress [37]. SNORKEL1 and SNORKEL2 are strongly induced by ethylene and derived internode elongation through the GA pathway [38]. Under deep water conditions, ethylene is accumulated in both deep rice and non-deep rice; ethylene accumulated in deep rice can induce the expression of SNORKEL1 and SNORKEL2 and promote internode elongation. However, non-deep rice cannot elongate internodes due to the deletion of these two genes. OsERF2/WR4 is a downstream component of the ethylene signaling pathway and affects the expression of auxin and cytokinin signaling pathway-related genes, ABA accumulation and response, and plays a negative regulatory role in rice root growth [39]. Ethylene and gibberellin synergistically regulate internode development, which determines plant architecture. The ERF transcription factor OsEATB, cloned from indica 93–11, mediates crosstalk between ethylene and gibberellin to repress internode elongation [40]. OsEATB expression is negatively regulated by ethylene, ABA and abiotic stresses. Over-expression of OsEATB decreases the endogenous GA level, and OsEATB limits the ethylene-induced GA response by down-regulating the expression of GA biosynthesis gene ent-kaurene synthase A during internode elongation [40].
GA is the main phytohormone for regulating plant height and its biosynthesis can be regulated by the AP2/ERF family gene. REDUCED PLANT HEIGHT1 (OsRPH1) negatively regulates plant height, internode length, and leaf sheath length by controlling the expression of GA-related genes. Exogenous GA3 treatment restores the defect of plant growth in over-expression lines [41]. OsAP2-39 controls ABA/GA balance, which regulates plant growth and seed production [42]. Over-expression of OsAP2-39 causes a decrease of plant height, tillering number, leaf number and grain number per panicle, and delays heading. Exogenous GA can restore the phenotype of seedling height, germination rate and other defects at heading stage in over-expression lines. Exogenous ABA can aggravate the defects of delayed germination and growth in the over-expression lines. A recent study showed that a AP2 transcription factor, SMALL ORGAN SIZE1 (SMOS1)/NITROGEN-MEDIATED TILLER GROWTH RESPONSE 5 (NGR5) was a key element of gibberellin signaling pathway and interacted with gibberellin receptor GA-INSENSTIVE DWARF1 (GID1) protein. NGR5 can also interact with the protein complex of Polycomb Repressive Complex 2 (PRC2) to regulate the expression of target genes by mediating the methylation level of histone H3K27me3. Gibberellin can reduce epigenetic modifications by promoting the degradation of the NGR5 protein, thereby enhancing the transcriptional activity of target genes to achieve gibberellin inhibition of rice growth and development [43]. RLA1/SMOS1 interacts with and is phosphorylated by GSK2, an importance regulating factor in BR signaling pathway, thereby reducing its stability. RLA1/SMOS1 is also involved in the regulation of OsBZR1, a positive regulator of BR signaling pathway [44]. SMOS1 interacts with a member of the GRAS family gene SMOS2/DWARF AND LOW-TILLERING (DLT), to form a key complex in auxin-brassinosteroids signaling crosstalk, and synergistically enhances transcriptional trans-activation activity [45].
AP2/ERF transcription factor HAIRY LEAF 6 (HL6) regulates auxin biosynthesis. HL6 controls the elongation of epidermis hair, and its regulatory role in the elongation of epidermis hair is mainly dependent on the function of OsWOX3B [46]. The protein complex of HL6 and OsWOX3B enhances the binding ability of HL6 to the auxin-related gene OsYUCCA5. The surfaces of leaves, leaf sheaths and hulls of HL6 over-expression plants ae covered with trichomes/hairs, and the content of indole-3-acetic acid (IAA) in leaves is increased, whereas, the number of long hairs is decreased in the hl6 mutant. In addition, HL6 regulates hair elongation in a dose-dependent manner, and a higher HL6 expression leads to longer hairs. These studies show that AP2/ERF genes induce hormone responses by activating target genes or regulating various growth processes of rice as response factors (Figure 4, Table 7).
Table 7.
Genes associated with hormone regulation in rice.
| Gene Name | Gene Family | Gene Locus | Gene Function | Reference(s) |
|---|---|---|---|---|
| OsDERF1 | ERF | LOC_Os08g35240 | drought tolerance | [37] |
| OsEATB | ERF | LOC_Os09g28440 | internode development | [40] |
| HL6 | ERF | LOC_Os06g44750 | epidermis hair development | [46] |
| OsERF2; OsWR4 | ERF | LOC_Os06g08340 | root development and formation | [39] |
| OsRPH1 | ERF | LOC_Os05g49700 | internode development | [41] |
| SMOS1; SHB; RLA1; NGR5 | ERF | LOC_Os05g32270 | signal transduction | [43,44,45] |
| OsAP2-39 | ERF | LOC_Os04g52090 | signal transduction | [42] |
| SNORKEL1 | ERF | AB510478 | internode development | [38] |
| SNORKEL2 | ERF | AB510479 | internode development | [38] |
3.7. Regulation of Nutrient Use Efficiency
If rice is short of nitrogen, it grows slowly, with short plants and yellow leaves, while excessive nitrogen fertilizer induces more leaves, easy lodging, decreased yield and quality, and serious environmental pollution. Improving the efficiency of nitrogen fertilizer utilization, reducing the input of chemical fertilizer and environmental pollution while ensuring the continuous increase of grain yield is a new breeding strategy. As AP2/ERF transcription factors, OsDREB1C and NGR5 increased rice nitrogen use efficiency. OsDREB1C can simultaneously improve the photosynthetic efficiency and nitrogen use efficiency of rice, leading to early heading, high yield and early maturity, thereby increasing rice yield [47]. Over-expression of OsDREB1C enhances the ability of nitrogen uptake and transport, which allocates more nitrogen to grains, thereby increasing the nitrogen utilization efficiency by at least 25%. Over-expression of OsDREB1C in rice varieties Nipponbare and Xiushui 134 increases yield by at least 30%. NGR5 is a positive regulator of rice growth and development in response to nitrogen. The expression level and protein accumulation of NGR5 increases with the increase of fertilizer application [43]. NGR5 interacts with DELLA protein that competitively binds to the GA receptor GID1 protein, which inhibits GA-mediated degradation of the NGR5 protein, thereby increasing its stability. The accumulation of the DELLA protein led to the first “Green Revolution”, achieving high-yield goals, high fertilizer tolerance, and lodging resistance of semi-dwarf plants, but was accompanied by a reduction of nitrogen utilization efficiency. By contrast, the high accumulation of NGR5 and GRF4 proteins did not change the semi-dwarf trait of the “Green Revolution”, but could increase the number of tillers, and promote the absorption and utilization of nitrogen fertilizer, so as to improve the yield and nitrogen utilization efficiency of the existing main cultivars under low nitrogen fertilizer conditions [44]. Therefore, AP2/ERF genes can improve nitrogen utilization efficiency (Figure 4, Table 8), and create excellent germplasms that not only retain the high-yield characteristics of the “Green Revolution” varieties, but also reduce the amount of exogenous nitrogen, which is conducive to cultivate the “less input, more yield”, high nitrogen utilization efficiency and early-maturing rice varieties.
Table 8.
Genes associated with nutrient use in rice.
3.8. Role of AP2/ERF TFs in Stress Response
Plants can resist biotic and abiotic stresses from different sources by activating various defense mechanisms. AP2/ERF family transcription factors have been found to regulate diverse stress response processes in higher plants, such as abiotic stress (cold, heat, drought, salinity) and biotic stress (insects and pathogens). The mining of AP2/ERF genes and the analysis of regulatory network provides a theoretical basis for breeding rice varieties resistant to biotic and abiotic stresses.
3.8.1. Abiotic Stress Response and Tolerance
Abiotic stresses such as drought, cold damage, high salinity and high temperature seriously affect plant growth and development. AP2/ERF TFs, especially the ERF subfamily members, play a more obvious role in abiotic stress response. OsDREB1A, OsDREB1BI, OsDREB1D and OsDREB1E are positive regulators that resist abnormal temperature. OsDREB1A activates the expression of the ion channel gene CYCLIC NUCLEOTIDE-GATED ION CHANNEL 9 (OsCNGC9), which promotes the expressions of genes related to extracellular calcium influx, intracellular calcium concentration and cold stress, and improves the cold tolerance of rice [48]. OsDREB1BI can be induced by high and low temperatures and plays a role in cold and heat tolerance of plants [49]. OsDREB1BI was transferred into Arabidopsis thaliana and the transgenic plants showed tolerance to low and high temperatures. Over-expression of OsDREB1BI in tobacco resulted in tolerance to biotic and abiotic stresses [50,51,52]. OsDREB1D and OsDREB1E were involved in cold and salt stress response. Over-expression of OsDREB1D and OsDREB1E enhanced cold and high salt resistance in transgenic Arabidopsis thaliana [52].
Moreover, many AP2/ERF TFs are involved in drought tolerance in rice, such as OsDREBs, Wax Synthesis Regulatory 2 (OsWR2), OsERF71 and OsLG3. OsDREB1E, OsDREB1G and OsDREB2B specifically bound to the C-repeat/DRE elements to regulate drought tolerance. Over-expression of OsDREB1G and OsDREB2B significantly improves rice drought tolerance, while over-expression of OsDREB1E slightly improves rice drought tolerance [53]. OsDREB1F is induced by high salt, drought, cold stress and ABA, and specifically binds to DRE/CRT elements. Transgenic rice and Arabidopsis thaliana carrying the OsDREB1F gene have enhanced resistance to high salt, drought and low temperature [54]. Tian et al. cloned three DREB homologs (OsDREB1-1, OsDREB4-1 and OsDREB4-2). In rice seedlings, OsDREB4-1 was induced by drought and high salt stress, while OsDREB1-1 and OsDREB4-2 were constitutively expressed [55]. OsWR2 positively regulated wax and keratin synthesis and enhanced the tolerance to water scarcity and high temperature [56]. Sensing Ca2+ transcription factor 1/2 (SCT1 and SCT2) directly binds to the promoter of OsWR2 to inhibit its expression, thereby negatively regulating the heat tolerance of rice. SCT1/SCT2 decodes intracellular calcium signals by interacting with calmodulin (CaM). High temperature induces the generation of calcium signal by G protein γ subunit, and high concentration of calcium ions is sensed by CaM and promotes the interaction between CaM and SCT1/SCT2, thereby strengthening the transcriptional inhibitory activity of SCT1/SCT2, which ultimately leads to the rapid down-regulation of OsWR2 expression at high temperature [57]. Low expression of OsWR2 reduces wax accumulation, resulting in a heat sensitive phenotype that was unable to resist high temperature [57]. OsERF71 positively regulates drought tolerance by promoting the formation and expansion of lignification and aerenchyma [58]. Over-expression of OsERF71 enhances the expression of OsCCR1, a key gene for lignin synthesis, thus ensuring the adaptability of root morphology and enhancing drought resistance. OsLG3 positively regulates rice drought tolerance by inducing the removal of reactive oxygen species [59]. Over-expression of OsLG3 significantly improves drought tolerance, while inhibition of OsLG3 expression results in increased sensitivity to drought. As a transcription factor that binds to DRE elements, OsAP211 plays a role in stress signaling [60]. Transgenic seedlings with OsAP211 inhibition show decreased tolerance to drought stress, while OsAP211 over-expression plants show slightly increased resistance to drought stress. GUDK mediates drought stress signal transduction through the phosphorylation of OsAP37, leading to the activation of transcription of stress-related genes, which improved stress tolerance, thus increasing yield under drought conditions [61]. Over-expression of AP37 significantly enhances tolerance to drought, high salt and low temperature stress during vegetative and reproductive growth periods. Under severe drought conditions in the field, transgenic plants significantly improved drought tolerance, and did not affect growth [62,63].
OsAP23, OsEREBP2 and SERF1 are involved in the salinity tolerance. OsAP23 is a negative regulator of salt stress response. When exposed to high salt stress, some stress-responsive regulatory genes are significantly induced in the wild type compared with the OsAP23 over-expression lines [64]. OsEREBP2 participates in the salt stress response and plays a central role in regulating different abiotic stress responses [64]. Cold stress, ABA, drought and high salt increase the transcription level of the OsEREBP2 gene [65]. SERF1 is a salt stress response factor, which amplifies the MAPK cascade signaling pathway activated by reactive oxygen species in the initial response stage of salt stress, and then converts salt-induced signals into an appropriate expression response, thus generating salt tolerance [29]. MAPK5 phosphorylated SERF1 and enhanced the transcriptional activity of SERF1. SERF1 RNAi plants are more sensitive to salt stress, while SERF1 over-expression plants show increased salt tolerance. In addition to regulating spikelet determinacy, IDS1 also plays an important role in regulating salt tolerance. IDS1 directly binds to the GCC-box in the promoter of the salt stress response genes LEA1 and SOS, and recruits the transcription co-repressor topless related protein TPR1 and histone deacetylase HDA1 to form the IDS1-TPR1-HDA1 module, which inhibits the expression of LEA1 and SOS, thereby negatively regulating the salt tolerance of rice [66]. OsERF922 negatively regulates salt tolerance in rice [67]. The aboveground parts of OsERF922 over-expression plants show decreased tolerance to salt stress with a high Na+/K+ ratio. Genomic association and ecotilling method analysis showed that OsAE115 and OsAE128 participated in drought stress response [68]. The above information suggests that ERF subfamily genes are involved in high/low temperature, salinity, and drought responses in rice (Figure 5, Table 9).
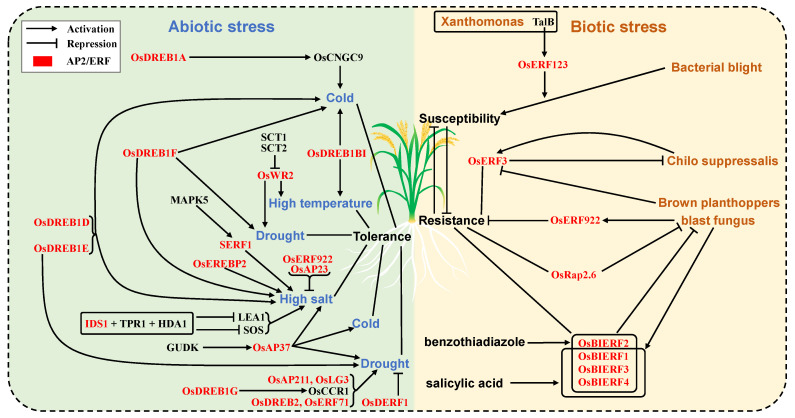
Figure 5.
Schematic representation of AP2/ERF transcription factors involved in abiotic and biotic stress regulation in rice. Left are the AP2/ERF regulatory networks of cold, high temperature, high salt and drought tolerance in rice; these factors are discussed in Section 3.8.1. OsDREB1A/D/E/F, OsDREB1BI and OsAP37 promote rice cold tolerance. OsDREB1BI and OsWR2 promote rice high temperature tolerance. OsDREB1E/F/G, OsDREB2, OsWR2, OsAP37, OsAP211, OsLG3 and OsERF71 promote rice drought tolerance, while OsDERF1 inhibits drought tolerance. OsDREB1D/E/F, SERF1, OsEREBP2, OsAP37 and IDS1 promote rice high salt tolerance, while OsERF922 and OsAP23 inhibit high salt tolerance. On the right, AP2/ERF genes participate in the regulation of disease and insect resistance in rice; these factors are discussed in Section 3.8.2. TalB, which is a transcriptional activator effector in Xanthomonas, promotes rice susceptibility to bacterial blight fungus X11-5A by promoting OsERF123. OsERF3 is activated by Chilo suppressalis attack, which improves rice resistance against chewing herbivores but is slightly suppressed by brown planthoppers attack. OsRap2.6 promotes rice blast fungus resistance, while OsERF922 inhibits rice blast fungus resistance. OsBIERF1/2/3/4 is involved in the rice blast defense response, and among them OsBIERF1/2/3/4 can be induced by benzothiadiazole, and OsBIERF1/3/4 can be induced by salicylic acid and blast fungus. The AP2/ERF transcription factors and other regulators are shown in red and black, respectively.
Table 9.
Genes associated with abiotic stress in rice.
| Gene Name | Gene Family | Gene Locus | Gene Function | Reference(s) |
|---|---|---|---|---|
| OsDREB1A | ERF | LOC_Os09g35030 | cold tolerance | [48] |
| OsDREB1B | ERF | LOC_Os09g35010 | cold tolerance; heat tolerance; | [49,50,51,52] |
| OsDREB1D | ERF | LOC_Os06g06970 | cold tolerance; salinity tolerance | [52] |
| OsDREB1E | ERF | LOC_Os04g48350 | drought tolerance | [52] |
| OsDREB1G; OsDREB1I | ERF | LOC_Os08g43210 | drought tolerance | [53] |
| OsDREB2B | ERF | LOC_Os05g27930 | drought tolerance | [53] |
| OsDREB1E | ERF | LOC_Os04g48350 | drought tolerance | [53] |
| OsDREB1F; RCBF2 | ERF | LOC_Os01g73770 | salinity tolerance; drought tolerance; temperature tolerance | [54] |
| OsDREB1-1; CR350 | ERF | LOC_Os04g48350 | salinity tolerance; drought tolerance | [55] |
| OsDREB4-1; CR223 | ERF | LOC_Os02g43940 | salinity tolerance; drought tolerance | [55] |
| OsWR2 | ERF | LOC_Os06g40150 | temperature tolerance | [56,57] |
| OsERF71 | ERF | LOC_Os06g09390 | drought tolerance | [58] |
| OsLG3; OsERF62; OsRAF | ERF | LOC_Os03g08470 | drought tolerance | [59] |
| OsAP211; ARAG1 | ERF | LOC_Os02g43970 | drought tolerance | [60] |
| OsAP37 | ERF | LOC_Os01g58420 | drought tolerance | [61,62,63] |
| OsAP23 | ERF | LOC_Os03g05590 | salinity tolerance | [64] |
| OsEREBP2 | ERF | LOC_Os01g64790 | salinity tolerance; drought tolerance; temperature tolerance | [65] |
| SERF1 | ERF | LOC_Os05g34730 | salinity tolerance | [29] |
| OsIDS1 | AP2 | LOC_Os03g60430 | salinity tolerance | [66] |
| OsERF922 | ERF | LOC_Os01g54890 | salinity tolerance; drought tolerance | [67,68] |
3.8.2. Biotic Stress Response and Tolerance
ERF subfamily transcription factors play an important role in resistance to blast fungus, Chilo suppressalis, rice planthopper and other biological stresses in rice. OsERF123 is a bacterial blight susceptibility gene. TalB, a transcriptional activator effector from bacterial pathogen Xanthomonas, can act on OsERF123 and OsTFX1, leading to higher host sensitivity to bacterial blight fungus X11-5A [69]. OsERF922 is induced by blast fungus and negatively regulates resistance to blast fungus [67]. Knockout of OsERF922 can enhance resistance to blast fungus, while over-expression of OsERF922 down regulates the expression of related defense genes and makes plants susceptible to blast fungus. OsRap2.6 participates in the rice innate immune response through interaction with RECEPTOR FOR ACTIVATED C KINASE 1 (RACK1), which is a positive regulator of innate immunity in rice [70]. OsRap2.6-RNAi plants are highly susceptible to blast fungus, whereas OsRap2.6 over-expression plants show increased resistance to blast fungus [70]. Four benzothiadiazole (BTH)-induced ERF genes, including OsBIERF1, OsBIERF2, OsBIERF3 and OsBIERF4 are found in rice. The expressions of OsBIERF1, OsBIERF3 and OsBIERF4 are induced by BTH and salicylic acid, which results in a resistance response in rice. Different blast fungi have different abilities to induce the expressions of OsBIERF1 and OsBIERF3, indicating that OsBIERF protein is involved in different signaling pathways of disease resistance responses [71]. OsERF3 regulates rice resistance to Chilo suppressalis. The expression of OsERF3 is rapidly upregulated by Chilo suppressalis feeding, and acts as a central switch in the regulation of plant metabolism to adapt to chewing or sucking insects. OsERF3 affects the early components of herbivore-induced defense response by inhibiting MAPK inhibitors, modulating plant resistance, and JA, SA, ethylene, H2O2-mediated signaling pathways [72]. In addition, OsERF3 is slightly inhibited by brown planthoppers and becomes more sensitive after feeding by brown planthoppers, which might be caused by the inhibition of H2O2 biosynthesis [72]. These results suggest that ERF subfamily genes play key roles in resistance to rice diseases and insect pests (Figure 5, Table 10).
Table 10.
Genes associated with biotic stress in rice.
| Gene Name | Gene Family | Gene Locus | Gene Function | Reference |
|---|---|---|---|---|
| OsERF123 | ERF | LOC_Os09g39810 | bacterial blight | [69] |
| OsERF922 | ERF | LOC_Os01g54890 | blast fungus | [67] |
| OsRap2.6; OsERF101 | ERF | LOC_Os04g32620 | blast fungus | [70] |
| OsBIERF1 | ERF | LOC_Os09g26420 | blast fungus | [71] |
| OsERF3; OsAP37 | ERF | LOC_Os01g58420 | chilo suppressalis; brown planthoppers | [72] |
4. Discussion and Prospects
As an important food crop, rice provides the staple food source for half of the world’s population and is one of the key crops studied [73,74]. High yield, high resistance, high quality and green rice have always been the goal of researchers and production development. While the demand for food is increasing, problems such as water shortage or surplus, cold, drought, pests and diseases limit the improvement of rice yield per unit area. As a large plant-specific transcription factor superfamily, AP2/ERF participates in several plant biological and physiological processes and plays a variety of regulatory roles, such as plant reproductive growth, vegetative growth and various stress-induced stress responses, and plays important roles in determining crop yield and adapting to stress. In this review, we summarize the classification, features, function, regulatory mechanisms, and potential applications of AP2/ERF transcription factors in rice. Although some AP2/ERF regulators and their molecular roles have been identified, their regulatory networks remain fragmented. In fact, the research on AP2/ERF transcription factors in rice is still at the stage of single gene function analysis, and the research on its underlying mechanisms is carried out in the laboratory rather than in the field. One future challenge is to explore the upstream and downstream components of AP2/ERF transcription factors and the possible connections between regulatory pathways under field conditions. The other challenge is to solve the trade-off effect among high yield, high resistance and green, organic production. With our greatly increased knowledge of the molecular mechanisms that determine these traits, the combination of beneficial alleles controlling these characters will help breeders to develop desired rice varieties with high yield, high resistance, and green, organic production.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grants from the National Natural Science Foundation of China (32071993, 91735304).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feng K., Hou X.L., Xing G.M., Liu J.X., Duan A.Q., Xu Z.S., Li M.Y., Zhuang J., Xiong A.S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020;40:750–776. doi: 10.1080/07388551.2020.1768509. [DOI] [PubMed] [Google Scholar]
- 2.Jofuku K.D., den Boer B.G., Van Montagu M., Okamuro J.K. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamuro J.K., Caster B., Villarroel R., Van Montagu M., Jofuku K.D. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1997;94:7076–7081. doi: 10.1073/pnas.94.13.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riechmann J.L., Meyerowitz E.M. The AP2/EREBP family of plant transcription factors. Biol. Chem. 1998;379:633–646. doi: 10.1515/bchm.1998.379.6.633. [DOI] [PubMed] [Google Scholar]
- 5.Magnani E., Sjölander K., Hake S. From endonucleases to transcription factors: Evolution of the AP2 DNA binding domain in plants. Plant Cell. 2004;16:2265–2277. doi: 10.1105/tpc.104.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano T., Suzuki K., Fujimura T., Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakuma Y., Liu Q., Dubouzet J.G., Abe H., Shinozaki K., Yamaguchi-Shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- 8.Sharoni A.M., Nuruzzaman M., Satoh K., Shimizu T., Kondoh H., Sasaya T., Choi I.R., Omura T., Kikuchi S. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 2011;52:344–360. doi: 10.1093/pcp/pcq196. [DOI] [PubMed] [Google Scholar]
- 9.Rashid M., Guangyuan H., Guangxiao Y., Hussain J., Xu Y. AP2/ERF Transcription Factor in Rice: Genome-Wide Canvas and Syntenic Relationships between Monocots and Eudicots. Evol. Bioinform. 2012;8:321–355. doi: 10.4137/EBO.S9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licausi F., Ohme-Takagi M., Perata P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013;199:639–649. doi: 10.1111/nph.12291. [DOI] [PubMed] [Google Scholar]
- 11.Shigyo M., Ito M. Analysis of gymnosperm two-AP2-domain-containing genes. Dev. Genes Evol. 2004;214:105–114. doi: 10.1007/s00427-004-0385-5. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi-Shinozaki K., Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohme-Takagi M., Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7:173–182. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal P.K., Agarwal P., Reddy M.K., Sopory S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006;25:1263–1274. doi: 10.1007/s00299-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 15.Sharma M.K., Kumar R., Solanke A.U., Sharma R., Tyagi A.K., Sharma A.K. Identification, phylogeny, and transcript profiling of ERF family genes during development and abiotic stress treatments in tomato. Mol. Genet. Genomics. 2010;284:455–475. doi: 10.1007/s00438-010-0580-1. [DOI] [PubMed] [Google Scholar]
- 16.Gu C., Guo Z.H., Hao P.P., Wang G.M., Jin Z.M., Zhang S.L. Multiple regulatory roles of AP2/ERF transcription factor in angiosperm. Bot. Stud. 2017;58:6. doi: 10.1186/s40529-016-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren D.Y., Li Y.F., He G.H., Qian Q. Multifloret spikelet improves rice yield. New Phytol. 2020;225:2301–2306. doi: 10.1111/nph.16303. [DOI] [PubMed] [Google Scholar]
- 18.Ren D.Y., Li Y.F., Zhao F.M., Sang X.C., Shi J.Q., Wang N., Guo S., Ling Y.H., Zhang C., Yang Z., et al. MULTI-FLORET SPIKELET1, which encodes an AP2/ERF protein, determines spikelet meristem fate and sterile lemma identity in rice. Plant Physiol. 2013;162:872–884. doi: 10.1104/pp.113.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren D.Y., Hu J., Xu Q.K., Cui Y.J., Zhang Y., Zhou T.T., Rao Y.C., Xue D.W., Zeng D.L., Zhang G.H., et al. FZP determines grain size and sterile lemma fate in rice. J. Exp. Bot. 2018;69:4853–4866. doi: 10.1093/jxb/ery264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsu M., Chujo A., Nagato Y., Shimamoto K., Kyozuka J. FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development. 2003;130:3841–3850. doi: 10.1242/dev.00564. [DOI] [PubMed] [Google Scholar]
- 21.Lee D.Y., Lee J., Moon S., Park S.Y., An G. The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem. Plant J. 2007;49:64–78. doi: 10.1111/j.1365-313X.2006.02941.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee D.Y., An G. Two AP2 family genes, SUPERNUMERARY BRACT (SNB) and OsINDETERMINATE SPIKELET 1 (OsIDS1), synergistically control inflorescence architecture and floral meristem establishment in rice. Plant J. 2012;69:445–461. doi: 10.1111/j.1365-313X.2011.04804.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y.S., Lee D.Y., Cho L.H., An G. Rice miR172 induces flowering by suppressing OsIDS1 and SNB, two AP2 genes that negatively regulate expression of Ehd1 and florigens. Rice. 2014;7:31. doi: 10.1186/s12284-014-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X.S., Feng F.J., Zhang Y., Elesawi I.E., Xu K., Li T.F., Mei H.W., Liu H.Y., Gao N.N., Chen C.L., et al. A novel rice grain size gene OsSNB was identified by genome-wide association study in natural population. PLoS Genet. 2019;15:e1008191. doi: 10.1371/journal.pgen.1008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L.Y., Ma X., Zhao S.S., Tang Y.Y., Liu F.X., Gu P., Fu Y.C., Zhu Z.F., Cai H.W., Sun C.Q., et al. The APETALA2-Like Transcription Factor SUPERNUMERARY BRACT Controls Rice Seed Shattering and Seed Size. Plant Cell. 2019;31:17–36. doi: 10.1105/tpc.18.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y., Lu D.F., Li C.Y., Luo J.H., Zhu B.F., Zhu J.J., Shangguan Y.Y., Wang Z.X., Sang T., Zhou B., et al. Genetic control of seed shattering in rice by the APETALA2 transcription factor shattering abortion1. Plant Cell. 2012;24:1034–1048. doi: 10.1105/tpc.111.094383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Ma T., Yuan D.Y., Zhou Y., Long Y., Li Z.W., Dong Z.Y., Duan M.J., Yu D., Jing Y.Z., et al. The OsEIL1-OsERF115-target gene regulatory module controls grain size and weight in rice. Plant Biotechnol. J. 2022;20:1470–1486. doi: 10.1111/pbi.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai X.F., Huang Y., Hu Y., Liu H.Y., Zhang B., Smaczniak C., Hu G., Han Z.M., Xing Y. Duplication of an upstream silencer of FZP increases grain yield in rice. Nat. Plants. 2017;3:885–893. doi: 10.1038/s41477-017-0042-4. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt R., Schippers J.H., Mieulet D., Watanabe M., Hoefgen R., Guiderdoni E., Mueller-Roeber B. SALT-RESPONSIVE ERF1 is a negative regulator of grain filling and gibberellin-mediated seedling establishment in rice. Mol. Plant. 2014;7:404–421. doi: 10.1093/mp/sst131. [DOI] [PubMed] [Google Scholar]
- 30.Fu F.F., Xue H.W. Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 2010;154:927–938. doi: 10.1104/pp.110.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanday I., Skinner D., Yang B., Mercier R., Sundaresan V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature. 2019;565:91–95. doi: 10.1038/s41586-018-0785-8. [DOI] [PubMed] [Google Scholar]
- 32.Xing Y.D., Wang N., Zhang T.Q., Zhang Q.L., Du D., Chen X.L., Lu X., Zhang Y.Y., Zhu M.D., Liu M.M., et al. SHORT-ROOT 1 is critical to cell division and tracheary element development in rice roots. Plant J. 2021;105:1179–1191. doi: 10.1111/tpj.15095. [DOI] [PubMed] [Google Scholar]
- 33.Kitomi Y., Kitano H., Inukai Y. Molecular mechanism of crown root initiation and the different mechanisms between crown root and radicle in rice. Plant Signal. Behav. 2011;6:1270–1278. doi: 10.4161/psb.6.9.16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitomi Y., Ito H., Hobo T., Aya K., Kitano H., Inukai Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011;67:472–484. doi: 10.1111/j.1365-313X.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 35.Jung H., Chung P.J., Park S.H., Redillas M., Kim Y.S., Suh J.W., Kim J.K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant Biotechnol. J. 2017;15:1295–1308. doi: 10.1111/pbi.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y., Cheng S.F., Song Y.L., Huang Y.L., Zhou S.L., Liu X.Y., Zhou D.X. The Interaction between Rice ERF3 and WOX11 Promotes Crown Root Development by Regulating Gene Expression Involved in Cytokinin Signaling. Plant Cell. 2015;27:2469–2483. doi: 10.1105/tpc.15.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan L.Y., Zhang J.F., Zhang H.W., Zhang Z.J., Quan R.D., Zhou S.R., Huang R.F. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE. 2011;6:e25216. doi: 10.1371/journal.pone.0025216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hattori Y., Nagai K., Furukawa S., Song X.J., Kawano R., Sakakibara H., Wu J., Matsumoto T., Yoshimura A., Kitano H., et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- 39.Xiao G.Q., Qin H., Zhou J.H., Quan R.D., Lu X.Y., Huang R.F., Zhang H.W. OsERF2 controls rice root growth and hormone responses through tuning expression of key genes involved in hormone signaling and sucrose metabolism. Plant Mol. Biol. 2016;90:293–302. doi: 10.1007/s11103-015-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi W.W., Sun F., Wang Q.J., Chen M.L., Huang Y.Q., Feng Y.Q., Luo X.J., Yang J.S. Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiol. 2011;157:216–228. doi: 10.1104/pp.111.179945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Z.M., Wu T., Huang K., Jin Y.M., Li Z., Chen M.J., Yun S., Zhang H.J., Yang X., Chen H.Y., et al. A Novel AP2/ERF Transcription Factor, OsRPH1, Negatively Regulates Plant Height in Rice. Front. Plant Sci. 2020;11:709. doi: 10.3389/fpls.2020.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaish M.W., El-Kereamy A., Zhu T., Beatty P.H., Good A.G., Bi Y.M., Rothstein S.J. The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 2010;6:e1001098. doi: 10.1371/journal.pgen.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu K., Wang S.S., Song W.Z., Zhang J.Q., Wang Y., Liu Q., Yu J.P., Ye Y.F., Li S., Chen J.F., et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science. 2020;367:eaaz2046. doi: 10.1126/science.aaz2046. [DOI] [PubMed] [Google Scholar]
- 44.Qiao S.L., Sun S.Y., Wang L.L., Wu Z.H., Li C.X., Li X.M., Wang T., Leng L.N., Tian W.S., Lu T.G., et al. The RLA1/SMOS1 Transcription Factor Functions with OsBZR1 to Regulate Brassinosteroid Signaling and Rice Architecture. Plant Cell. 2017;29:292–309. doi: 10.1105/tpc.16.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirano K., Yoshida H., Aya K., Kawamura M., Hayashi M., Hobo T., Sato-Izawa K., Kitano H., Ueguchi-Tanaka M., Matsuoka M. SMALL ORGAN SIZE 1 and SMALL ORGAN SIZE 2/DWARF AND LOW-TILLERING Form a Complex to Integrate Auxin and Brassinosteroid Signaling in Rice. Mol. Plant. 2017;10:590–604. doi: 10.1016/j.molp.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Sun W.Q., Gao D.W., Xiong Y., Tang X.X., Xiao X.F., Wang C.R., Yu S.B. Hairy Leaf 6, an AP2/ERF Transcription Factor, Interacts with OsWOX3B and Regulates Trichome Formation in Rice. Mol. Plant. 2017;10:1417–1433. doi: 10.1016/j.molp.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Wei S.B., Li X., Lu Z.F., Zhang H., Ye X.Y., Zhou Y.J., Li J., Yan Y.Y., Pei H.C., Duan F.Y., et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science. 2022;377:eabi8455. doi: 10.1126/science.abi8455. [DOI] [PubMed] [Google Scholar]
- 48.Wang J.C., Ren Y.L., Liu X., Luo S., Zhang X., Liu X., Lin Q.B., Zhu S.S., Wan H., Yang Y., et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant. 2021;14:315–329. doi: 10.1016/j.molp.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Qin Q.L., Liu J.G., Zhang Z., Peng R.H., Xiong A.S., Yao Q.H., Chen J.M. Isolation, optimization, and functional analysis of the cDNA encoding transcription factor OsDREB1B in Oryza Sativa L. Mol. Breed. 2007;19:329–340. doi: 10.1007/s11032-006-9065-7. [DOI] [Google Scholar]
- 50.Gutha L.R., Reddy A.R. Rice DREB1B promoter shows distinct stress-specific responses, and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. Plant Mol. Biol. 2008;68:533–555. doi: 10.1007/s11103-008-9391-8. [DOI] [PubMed] [Google Scholar]
- 51.Figueiredo D.D., Barros P.M., Cordeiro A.M., Serra T.S., Lourenço T., Chander S., Oliveira M.M., Saibo N.J. Seven zinc-finger transcription factors are novel regulators of the stress responsive gene OsDREB1B. J. Exp. Bot. 2012;63:3643–3656. doi: 10.1093/jxb/ers035. [DOI] [PubMed] [Google Scholar]
- 52.Mao D., Chen C. Colinearity and similar expression pattern of rice DREB1s reveal their functional conservation in the cold-responsive pathway. PLoS ONE. 2012;7:e47275. doi: 10.1371/journal.pone.0047275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J.Q., Meng X.P., Zhang Y., Xia M., Wang X.P. Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol. Lett. 2008;30:2191–2198. doi: 10.1007/s10529-008-9811-5. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q.Y., Guan Y.C., Wu Y.R., Chen H.L., Chen F., Chu C.C. Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 2008;67:589–602. doi: 10.1007/s11103-008-9340-6. [DOI] [PubMed] [Google Scholar]
- 55.Tian X.H., Li X.P., Zhou H.L., Zhang J.S., Gong Z.Z., Chen S.Y. OsDREB4 Genes in Rice Encode AP2-Containing Proteins that Bind Specifically to the Dehydration-Responsive Element. J. Integr. Plant Biol. 2005;47:467–476. doi: 10.1111/j.1744-7909.2005.00028.x. [DOI] [Google Scholar]
- 56.Zhou X.Y., Jenks M.A., Liu J., Liu A.L., Zhang X.W., Xiang J.H., Zou J., Peng Y., Chen X.B. Overexpression of Transcription Factor OsWR2 Regulates Wax and Cutin Biosynthesis in Rice and Enhances its Tolerance to Water Deficit. Plant Mol. Biol. Rep. 2014;32:719–731. doi: 10.1007/s11105-013-0687-8. [DOI] [Google Scholar]
- 57.Kan Y., Mu X.R., Zhang H., Gao J., Shan J.X., Ye W.W., Lin H.X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants. 2022;8:53–67. doi: 10.1038/s41477-021-01039-0. [DOI] [PubMed] [Google Scholar]
- 58.Lee D.K., Jung H., Jang G., Jeong J.S., Kim Y.S., Ha S.H., Do Choi Y., Kim J.K. Overexpression of the OsERF71 Transcription Factor Alters Rice Root Structure and Drought Resistance. Plant Physiol. 2016;172:575–588. doi: 10.1104/pp.16.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong H.Y., Yu J.P., Miao J.L., Li J.J., Zhang H.L., Wang X., Liu P.L., Zhao Y., Jiang C.H., Yin Z.G., et al. Natural Variation in OsLG3 Increases Drought Tolerance in Rice by Inducing ROS Scavenging. Plant Physiol. 2018;178:451–467. doi: 10.1104/pp.17.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao F., Chen J.M., Xiong A.S., Peng R.H., Liu J.G., Cai B., Yao Q.H. Isolation and characterization of a novel AP2/EREBP-type transcription factor OsAP211 in Oryza sativa. Biol. Plantarum. 2009;53:643–649. doi: 10.1007/s10535-009-0117-9. [DOI] [Google Scholar]
- 61.Ramegowda V., Basu S., Krishnan A., Pereira A. Rice GROWTH UNDER DROUGHT KINASE is required for drought tolerance and grain yield under normal and drought stress conditions. Plant Physiol. 2014;166:1634–1645. doi: 10.1104/pp.114.248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim Y.S., Kim J.K. Rice transcription factor AP37 involved in grain yield increase under drought stress. Plant Signal. Behav. 2009;4:735–736. doi: 10.4161/psb.4.8.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh S.J., Kim Y.S., Kwon C.W., Park H.K., Jeong J.S., Kim J.K. Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol. 2009;150:1368–1379. doi: 10.1104/pp.109.137554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang Y.H., Liu K., Zhang J., Li X., Xu K.D., Zhang Y., Qi J., Yu D.S., Wang J., Li C.W. JcDREB2, a Physic Nut AP2/ERF Gene, Alters Plant Growth and Salinity Stress Responses in Transgenic Rice. Front. Plant Sci. 2017;8:306. doi: 10.3389/fpls.2017.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serra T.S., Figueiredo D.D., Cordeiro A.M., Almeida D.M., Lourenço T., Abreu I.A., Sebastián A., Fernandes L., Contreras-Moreira B., Oliveira M.M., et al. OsRMC, a negative regulator of salt stress response in rice, is regulated by two AP2/ERF transcription factors. Plant Mol. Biol. 2013;82:439–455. doi: 10.1007/s11103-013-0073-9. [DOI] [PubMed] [Google Scholar]
- 66.Cheng X.L., Zhang S.X., Tao W.C., Zhang X.X., Liu J., Sun J.Q., Zhang H.W., Pu L., Huang R.F., Chen T. INDETERMINATE SPIKELET1 Recruits Histone Deacetylase and a Transcriptional Repression Complex to Regulate Rice Salt Tolerance. Plant Physiol. 2018;178:824–837. doi: 10.1104/pp.18.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu D.F., Chen X.J., Liu J.Q., Ye J.C., Guo Z.J. The rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae and salt tolerance. J. Exp. Bot. 2012;63:3899–3911. doi: 10.1093/jxb/ers079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu S.W., Liao F.X., Wang F.M., Wen W.W., Li J.J., Mei H.W., Luo L.J. Identification of rice transcription factors associated with drought tolerance using the Ecotilling method. PLoS ONE. 2012;7:e30765. doi: 10.1371/journal.pone.0030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran T.T., Pérez-Quintero A.L., Wonni I., Carpenter S., Yu Y.H., Wang L., Leach J.E., Verdier V., Cunnac S., Bogdanove A.J., et al. Functional analysis of African Xanthomonas oryzae pv. oryzae TALomes reveals a new susceptibility gene in bacterial leaf blight of rice. PLoS Pathog. 2018;14:e1007092. doi: 10.1371/journal.ppat.1007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wamaitha M.J., Yamamoto R., Wong H.L., Kawasaki T., Kawano Y., Shimamoto K. OsRap2.6 transcription factor contributes to rice innate immunity through its interaction with Receptor for Activated Kinase-C 1 (RACK1) Rice. 2012;5:35. doi: 10.1186/1939-8433-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Y.F., Song F.M., Goodman R.M., Zheng Z. Molecular characterization of four rice genes encoding ethylene-responsive transcriptional factors and their expressions in response to biotic and abiotic stress. J. Plant Physiol. 2006;163:1167–1178. doi: 10.1016/j.jplph.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Lu J., Ju H.P., Zhou G.X., Zhu C.S., Erb M., Wang X.P., Wang P., Lou Y.G. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011;68:583–596. doi: 10.1111/j.1365-313X.2011.04709.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang L., Wang D.D., Yang Z.H., Jiang S., Qu J.N., He W., Liu Z.M., Xing J.J., Ma Y.C., Lin Q.L., et al. Roles of FERONIA-like receptor genes in regulating grain size and quality in rice. Sci. China Life Sci. 2021;64:294–310. doi: 10.1007/s11427-020-1780-x. [DOI] [PubMed] [Google Scholar]
- 74.Xu Q.K., Yu X.Q., Cui Y.J., Xia S.S., Zeng D.L., Qian Q., Ren D.Y. LRG1 maintains sterile lemma identity by regulating OsMADS6 expression in rice. Sci. China Life Sci. 2021;64:1190–1192. doi: 10.1007/s11427-020-1816-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.