Abstract
Breast cancers have been shown to elicit tumor-specific immune responses. As in other types of cancer, the antitumor immune response fails to contain breast tumor growth, and a reduction in both the quantity and cytotoxic effectiveness of tumor-infiltrating lymphocytes (TILs) is associated with a poorer prognosis. Fas ligand (FasL) induces apoptotic death of activated lymphocytes that express its cell surface receptor, FasR (CD95/APO-1). FasL-mediated apoptosis of activated lymphocytes contributes to normal immune downregulation through its roles in tolerance acquisition, immune response termination, and maintenance of immune privilege in the eye, testis, and fetus. In this report, we demonstrate that breast carcinomas express FasL. Using in situ hybridization and immunohistochemistry, we show that breast tumors constitutively express FasL at both the mRNA and protein levels, respectively. FasL expression is prevalent in breast cancer: 100% of breast tumors (17 of 17) were found to express FasL, and expression occurred over more than 50% of the tumor area in all cases. By immunohistochemistry, FasR was found to be coexpressed with FasL throughout large areas of all the breast tumors. This suggests that the tumor cells had acquired intracellular defects in FasL-mediated apoptotic signaling. FasL and FasR expression were independent of tumor type or infiltrative capacity. FasL expressed by tumor cells has previously been shown to kill Fas-sensitive lymphoid cells in vitro and has been associated with apoptosis of TILs in vivo. We conclude that mammary carcinomas express FasL in vivo as a potential inhibitor of the antitumor immune response.
Despite expression of tumor-associated antigens such as MAGE 1-3, HER-2/neu (9), and DF3/MUC-1 (11) and the presence of tumor-specific cytotoxic T lymphocytes (12), the immune system fails to contain breast carcinoma. Evidence suggests that a poor local immune response contributes to a poor prognosis in patients with breast cancer. As with other cancers (30), a reduction in the level of tumor-infiltrating lymphocytes (TILs) correlates with a poorer prognosis in patients with breast cancer (22). Also in common with other cancers (24), TILs residing in breast cancers exhibit decreased cytotoxic effectiveness relative to that of peripheral blood lymphocytes (32). The mechanisms by which breast cancers inhibit and evade antitumor immune responses are poorly understood.
Fas ligand (FasL) induces apoptotic death of sensitive lymphoid cells expressing its cell surface receptor, FasR (CD95/APO-1) (25). FasL-mediated apoptosis of activated lymphocytes contributes to immune downregulation through its roles in tolerance acquisition (23), T-cell activation-induced cell death (1), and immune response termination (8). FasL is expressed as a mediator of immune privilege in the eye (13), the testis (6), and the placenta (15). By inducing apoptosis of infiltrating proinflammatory immunocytes, the FasL expressed in these organs may help to prevent potential inflammatory damage to vision and reproduction. In rodent transplantation experiments, prolonged allograft survival has been obtained for FasL-expressing tissues (6, 36) or for FasL-negative pancreatic islets coengrafted with FasL-expressing cells (18, 20). Transplantation of murine tumor cell allografts stably transfected with the FasL gene showed that FasL can cause local suppression of both humoral and cellular allograft-specific immune responses (4).
Recent evidence has shown that tumors can also express FasL as a possible mediator of tumor immune privilege (29). Cancer cell lines that express FasL have been shown to kill lymphoid cells by Fas-mediated apoptosis in vitro (28). This suggests a Fas counterattack mechanism of tumor immune escape, by which a cancer cell, by expressing FasL, can delete Fas-sensitive antitumor immune effector cells by apoptosis. Melanoma (14), hepatocellular carcinoma (35), lung cancer (27), astrocytoma (31), and liver metastases of colon adenocarcinomas (34) have been shown to express FasL in vivo. FasL expression by esophageal carcinoma cells was found to be associated with apoptotic depletion of tumor-infiltrating lymphocytes in vivo (7).
The aim of this study was to establish if mammary carcinomas expressed FasL as a possible mediator of tumor immune privilege in breast cancer. Immunohistochemistry and in situ hybridization were used to localize both FasL protein and mRNA within neoplastic breast tissue in vivo.
MATERIALS AND METHODS
Specimens.
Human mammary carcinomas (n = 17) were collected following surgical resections performed at the Mercy Hospital, Cork, Ireland, by a protocol approved by the University Teaching Hospitals Ethics Committee. Specimens were from patients with newly diagnosed breast carcinoma, and the clinicopathological characteristics of the tumors are shown in Table 1. Sections of normal breast tissue, distal to the tumors, were used as controls (n = 10). None of the patients had received chemo-, radio-, or immunotherapy prior to resection.
TABLE 1.
Clinicopathological characteristics and extent of expression of FasL and FasR in breast tumors
| Patient | Tumor type | Gradea | Extent of
expressionb
|
|
|---|---|---|---|---|
| FasL | FasR | |||
| 1 | Intraduct and infiltrating carcinoma | 3 | +++ | +++ |
| 2 | Intraduct and infiltrating carcinoma | 3 | +++ | ++++ |
| 3 | Intraduct and infiltrating carcinoma | 2 | ++++ | ++++ |
| 4 | Intraduct and infiltrating carcinoma | 3 | +++ | +++ |
| 5 | Intraduct and infiltrating carcinoma | 2 | ++++ | ++++ |
| 6 | Intraduct and infiltrating carcinoma | 2 | ++++ | ++++ |
| 7 | Infiltrative ductal carcinoma | 2 | +++ | +++ |
| 8 | Infiltrative ductal carcinoma | 1 | +++ | ++++ |
| 9 | Infiltrative ductal carcinoma | 2 | +++ | +++ |
| 10 | Ductal carcinoma in situ | NAc | +++ | ++++ |
| 11 | Ductal carcinoma in situ | NA | ++++ | ++++ |
| 12 | Ductal carcinoma in situ | NA | +++ | ++++ |
| 13 | Infiltrative mucinous carcinoma | Lowd | +++ | +++ |
| 14 | Mucinous adenocarcinoma | NDe | +++ | ++++ |
| 15 | Infiltrative lobular carcinoma | 2 | +++ | +++ |
| 16 | Infiltrative lobular carcinoma | 2 | +++ | +++ |
| 17 | Tubular carcinoma | 1 | +++ | +++ |
Graded by the Nottingham modification of Scarrf-Bloom-Richardson.
Extent of expression was graded on the basis of the percentage of positive cells, as follows: +, 0 to 25%; ++, 25 to 50%; +++, 50 to 75%; ++++, 75 to 100%.
NA, not applicable.
Graded by conventional histopathological classification.
ND, not determined.
Immunohistochemical detection of FasL and FasR protein.
Formalin-fixed, paraffin-embedded, surgically resected tumor sections were deparaffinized in xylene followed by rehydration in a graded series of alcohol. Sections were postfixed in 4% paraformaldehyde for 1 h and were washed twice for 5 min each time in a wash buffer containing 50 mM Tris-HCl (pH 7.6), 50 mM NaCl, and 0.001% saponin. Endogenous peroxidase activity was quenched by incubation with 3.0% hydrogen peroxide in methanol for 5 min. Sections were then washed as described above except that the wash buffer for this and all subsequent steps included 1% normal goat serum. The sections were blocked for 1 h in wash buffer containing 5% normal goat serum. Sections were washed and incubated overnight at 4°C with affinity-purified, rabbit polyclonal anti-human FasL-specific immunoglobulin G (IgG; Santa Cruz Biotechnology, Santa Cruz, Calif.) at 0.1 μg ml−1 in wash buffer. Antibody binding was localized with a biotinylated secondary antibody, avidin-conjugated horseradish peroxidase, and diaminobenzidine chromogenic substrate, contained within the Vectastain ABC detection kit (Vector Laboratories, Burlingame, Calif.). In control sections, the peptide immunogen to which the antibody was raised (FasL; N-terminal amino acids 260 to 279; Santa Cruz Biotechnology) was included at 1 μg ml−1 during primary antibody incubation as a direct, internal competitive control for antibody specificity. The peptide was preincubated with the antibody at room temperature for 2 h prior to incubation with the sections. The FasL peptide abolished staining in the tumors. Addition of an irrelevant peptide (FasR; C-terminal amino acids 316 to 335; Santa Cruz Biotechnology) did not affect the FasL staining, further confirming that staining was specific for FasL. The FasL specificity of the Santa Cruz Biotechnology antibody had previously been verified by us (28) and others (27, 34). FasL protein detection in the breast tumors was confirmed by immunohistochemistry with another, FasL-specific monoclonal antibody (clone G247-4; Pharmingen, San Diego, Calif.) as described above. The monoclonal antibody was used at a concentration of 5 μg ml−1, and an isotype-matched control antibody was also used. Slides were counterstained with hematoxylin. FasL expression was also confirmed at the mRNA level by in situ hybridization as described below. FasR was detected in the tumors as described above with an affinity-purified, rabbit polyclonal anti-human FasR-specific IgG (Santa Cruz Biotechnology). The peptide immunogen to which the antibody was raised (FasR; C-terminal amino acids 316 to 335) was used in control stainings as described above, and the FasL peptide did not affect FasR staining.
Localization of FasL mRNA expression by in situ hybridization.
A biotinylated, FasL-specific RNA hybridization probe (riboprobe) was generated as follows. A 344-bp fragment of the human FasL cDNA sequence corresponding to codons 96 to 210 was amplified by PCR with a proofreading thermostable polymerase (UlTma DNA polymerase; Perkin-Elmer, Norwalk, Conn.). The fragment was cloned into the EcoRV site of pBluescript (Stratagene, La Jolla, Calif.), which is flanked by the T3 and T7 RNA promoters in opposite orientations. The orientation of the cloned insert relative to those of these promoters was ascertained by restriction mapping. By using the recombinant plasmid as a template, the FasL-specific antisense riboprobe was synthesized by in vitro transcription of the cloned insert with biotin-16-UTP and T3 RNA polymerase (Boehringer Mannheim GmbH, Mannheim, Germany). A sense control riboprobe was synthesized from the same template in the opposite direction by using T7 RNA polymerase (Boehringer Mannheim GmbH). The nucleotide sequence of the FasL-specific riboprobe showed no significant homology to any other sequence in the EMBL DNA sequence database.
In situ hybridization was performed with paraffin-embedded human breast tumor sections (4-μm thick) mounted on aminopropylethoxysilane-treated slides. In situ hybridization was performed with the GenPoint in situ hybridization kit (DAKO Corp., Glostrup, Denmark), according to the manufacturer’s instructions. Briefly, this involved microwave treatment followed by limited proteinase K digestion to enable probe access to tissue mRNA. Hybridization was performed at 42°C for 16 h with the biotinylated FasL-specific riboprobe at a final concentration of 0.5 ng/μl. Following posthybridization washes at 42°C in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), the sections were incubated with streptavidin-conjugated horseradish peroxidase at room temperature for 15 min. Following washes in TBST (50 mM Tris-HCl [pH 7.6], 300 mM NaCl, 0.1% Tween 20), a signal amplification step was performed by incubating the sections with biotinyl-tyramide at room temperature for 5 min. Horseradish peroxidase catalyzes oxidation of biotinyl-tyramide, which rapidly forms covalent bonds with adjacent aromatic groups in the tissue. This results in additional biotin deposition at sites of riboprobe binding. Sections were washed in TBST, and a second incubation with streptavidin-conjugated horseradish peroxidase was performed. Following final TBST washes, color development was performed with the diaminobenzidine chromogenic substrate, which generates a brown color. A control hybridization was performed with a consecutive section from each specimen by using conditions identical to those described above, except that a biotinylated FasL sense riboprobe was used.
RESULTS
Mammary carcinomas express FasL protein.
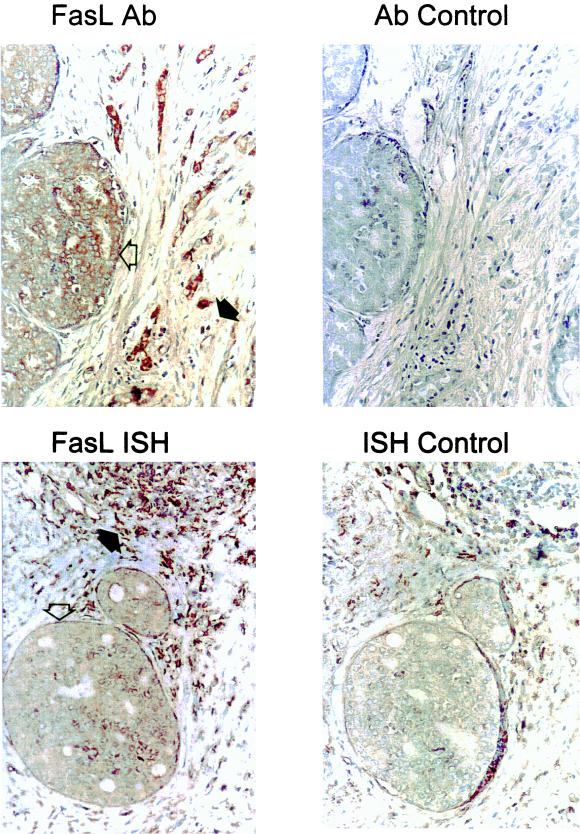
FasL expression by tumor cells was immunohistochemically detected in all (n = 17) surgically resected breast carcinomas examined (Fig. 1). Immunohistochemistry was performed with a FasL-specific polyclonal IgG (Santa Cruz Biotechnology) raised against a synthetic FasL peptide. FasL specificity was confirmed in consecutive control sections by using the FasL peptide immunogen (FasL amino acids 260 to 279) as an internal competitive control. Inclusion of the soluble peptide immunogen during primary antibody incubation resulted in direct, competitive displacement of positive staining (Fig. 1). Inclusion of an irrelevant peptide had no effect on FasL staining. Detection of the FasL protein in the breast tumors was confirmed by immunohistochemistry with a FasL-specific monoclonal antibody (FasL clone G247-4; Pharmingen). With consecutive tumor sections, the Pharmingen monoclonal antibody resulted in a pattern of staining identical to that obtained with the Santa Cruz Biotechnology polyclonal antibody. An isotype-matched monoclonal antibody did not stain control sections.
FIG. 1.
Human breast carcinomas express FasL. Immunoperoxidase staining with a FasL-specific rabbit polyclonal IgG antibody (FasL Ab) was performed with paraffin-embedded breast carcinoma sections. Slides were counterstained with hematoxylin. FasL-positive immunohistochemical staining (brown) is shown in a representative breast carcinoma (magnification, ×80). In addition to positive staining of the tumor island (open arrow), positive staining is also observed among isolated cells of lymphoid morphology (solid arrow), possibly representing FasL-expressing, activated T and NK cells. As a control for specificity of antibody detection, the FasL-immunizing peptide was included during primary antibody incubation (Ab control). Competitive displacement of staining by the soluble peptide immunogen confirms FasL specificity. Breast tumor expression of FasL mRNA was detected by in situ hybridization with a biotinylated FasL-specific riboprobe (FasL ISH). A positive brown hybridization signal is seen within a representative tumor island (open arrow) (magnification, ×80). FasL mRNA was also detected in cells within a lymphoid aggregate (solid arrow). In control sections for in situ hybridization (ISH control), the biotinylated sense control probe failed to hybridize, confirming the specificity of the FasL hybridization. These results are representative of 17 breast carcinomas.
The magnitude and extent of FasL protein expression detected immunohistochemically were variable both within individual tumors and between tumors. FasL staining varied from weakly positive neoplastic areas to intensely staining regions of tumors, where the intensity of staining was stronger than that observed in local FasL-positive TILs. However, FasL staining was of locally uniform intensity within nests of tumor cells. FasL-positive and -negative tumor islands were frequently found to occur within the same tumor, although all tumors expressed FasL throughout more than 50% of the tumor area (Table 1). Expression of FasL in mammary tumors occurred in ductal carcinomas (12 of 12), lobular carcinomas (2 of 2), mucinous carcinomas (2 of 2), and a tubular carcinoma (1 of 1). There was no apparent difference in FasL expression between infiltrative and in situ carcinomas.
Localization of FasL mRNA to breast carcinoma cells.
Nontumor cells, including lymphocytes and neurons, are known to express FasL. Detection of FasL mRNA in whole tumor tissue by Northern blotting or reverse transcription-PCR would not necessarily confirm that the mRNA detected was expressed by tumor cells. In order to confirm that the FasL protein detected in the breast tumors was expressed by tumor cells, we performed in situ hybridization to detect and localize FasL mRNA within the tumor tissue.
A biotinylated, FasL-specific RNA probe (riboprobe) was synthesized by in vitro transcription of a 344-bp fragment of FasL cDNA (codons 96 to 210) cloned into pBluescript. The nucleotide sequence of the FasL riboprobe showed no significant homology to any other sequence within the EMBL DNA sequence database. Using in situ hybridization with this probe, FasL mRNA expression was detected in tumor cells in the resected mammary carcinomas. Positive hybridization occurred within neoplastic cells throughout extensive areas of the tumors (Fig. 1). FasL mRNA detection colocalized with the FasL protein detected immunohistochemically with serial tumor sections. Colocalization of FasL mRNA and protein confirmed that breast carcinoma cells expressed FasL. Cells of lymphoid morphology were also positive by hybridization with the FasL-specific riboprobe, possibly representing activated, FasL-expressing cytotoxic T lymphocytes and natural killer (NK) cells. The specificity of hybridization was confirmed with a biotinylated control riboprobe with a sequence complementary to that of the FasL-specific probe (sense control probe). This sense control probe failed to generate positive signals in control hybridizations with consecutive tumor sections, thus confirming the specificity of FasL mRNA detection (Fig. 1).
Coexpression of FasR and FasL in breast cancers.
FasR expression was immunohistochemically detected in all (n = 17) surgically resected breast carcinomas examined (Fig. 2). Immunohistochemistry was performed with a FasR-specific polyclonal IgG (Santa Cruz Biotechnology) raised against a synthetic FasR peptide. FasR specificity was confirmed in consecutive control sections with the FasR peptide immunogen (FasR amino acids 316 to 335) as an internal competitive control. Inclusion of the soluble peptide immunogen during primary antibody incubation resulted in direct, competitive displacement of positive staining. Inclusion of an irrelevant peptide had no effect on FasR staining.
FIG. 2.
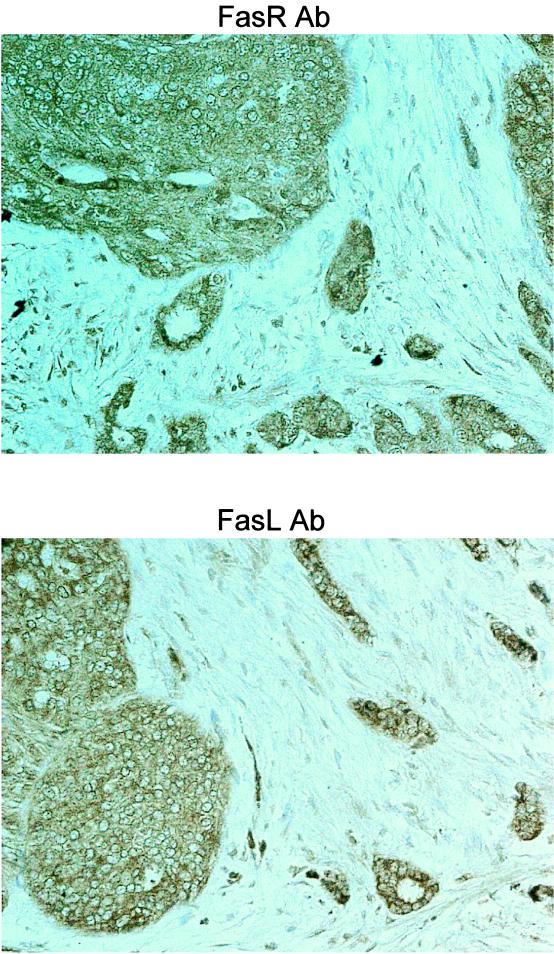
Human breast carcinomas coexpress FasR and FasL. Immunoperoxidase staining with a FasR-specific rabbit polyclonal IgG antibody (FasR Ab) was performed with paraffin-embedded breast carcinoma sections. A consecutive section from each tumor was used for immunohistochemical detection of FasL with a FasL-specific rabbit polyclonal IgG antibody (FasL Ab). Slides were counterstained with hematoxylin. FasR-positive immunohistochemical staining (brown) is shown in a representative breast carcinoma (magnification, ×100). The same tumor region is also positive for FasL expression, indicating coexpression of FasR and FasL by breast tumor cells in vivo. As a control for specificity of antibody detection, the appropriate immunizing peptide (FasR or FasL) was included during primary antibody incubation. Competitive displacement of staining by the soluble peptide immunogen confirmed the specificity of FasL detection (Fig. 1) and FasR detection (data not shown). These results are representative of 17 breast carcinomas.
The magnitude and extent of FasR expression were variable both within individual tumors and between tumors (Table 1). FasR was expressed over more than 50% of the tumor area in all specimens. By using consecutive immunohistochemically stained tumor sections, FasL and FasR were found to be coexpressed by tumor cells throughout large areas of all tumors (Fig. 2). This suggests that the tumor cells had acquired intracellular defects in FasL-mediated apoptotic signaling.
DISCUSSION
In this report, we demonstrate that breast cancers express FasL, an inducer of immunocyte cell death, via the FasR-mediated pathway of apoptosis. Because activated leukocytes express abundant cell-surface FasR, expression of FasL potentially enables breast tumors to counterattack and kill Fas-sensitive, antitumor immune effector cells. We and others have previously demonstrated that FasL expressed by diverse tumor cells in vitro is biologically active: FasL-expressing tumor cells can induce Fas-mediated apoptosis of cocultured FasR-bearing lymphoid cells. The fact that mammary tumors express FasL in vivo suggests that FasL contributes to the immune evasion of breast cancer.
FasL expression by human breast cancers in vivo was prevalent: 100% of carcinomas were found to express FasL mRNA and protein, irrespective of tumor type or infiltrative capacity. Colocalization of FasL mRNA and protein confirmed that FasL was expressed by tumor cells. Since activated lymphocytes are known to shed FasL (37), confirmation of tumor cell expression of FasL mRNA precludes the possibility that the detected FasL protein was derived from TILs. While the magnitude and extent of FasL expression were variable, extensive expression (>50% of the tumor area) occurred in all tumors. Although we noted that myoepithelial cells were immunohistochemically positive, FasL expression was otherwise absent in control normal breast tissue (n = 10) (data not shown), suggesting that FasL expression is upregulated during the transformation process. These results are consistent with those from a previous study that demonstrated FasL expression by estrogen receptor-negative breast carcinoma cell lines in vitro (38). Using reverse transcription-PCR, we have also found FasL expression in the estrogen receptor-positive breast carcinoma cell line MCF-7 (data not shown).
FasL expression within ocular tissues has been shown to trigger FasR-mediated apoptosis of eye-infiltrating, activated leukocytes. This limits accumulation of potentially hazardous proinflammatory cells and is critical to maintenance of immune privilege in the eye, where inflammatory damage could permanently impair vision (13). Endogenous expression of FasL by Sertoli cells (6) and cells within the placenta (15) may contribute to the immune privilege enjoyed by the testis and the fetus, respectively, preventing inflammatory damage to sensitive reproductive tissues. FasL-mediated apoptosis of antiallograft lymphocytes has been implicated as a reason for the remarkable success of human corneal transplantation (36), as well as the prolonged survival of FasL-expressing allografts in animal transplantation experiments (4, 18, 20). FasL has broad immunosuppressive effects: activated T (1), B (8), and NK (10) cells, neutrophils (21), and monocytes (4) have all been shown to be sensitive to FasL-mediated apoptosis. These findings support the view that FasL expressed by breast tumors may help to limit antitumor immune responses, maintaining breast cancers in a state of immune privilege. Although in some animal transplantations (2, 3, 16, 33) allografts of cells transfected with the FasL gene were rapidly rejected due to proinflammatory neutrophil infiltration, factors relating to the experimental setting may account for the paradoxical effect of recombinant FasL in these instances (19). In contrast, adenovirus-mediated expression of FasL suppressed inflammation within experimentally induced arthritic ankle joints in mice (39). In the present study, significant neutrophil infiltration was absent from all FasL-expressing areas of the mammary tumors. All available evidence indicates that in its native context of expression, FasL mediates immunological downregulation, tolerance, and privilege, and its absence, through mutation, leads to autoimmune disease in mice (26).
Evidence which directly implicates FasL as an inhibitor of immunological responses to tumors in vivo has accumulated. When a murine FasL-expressing melanoma cell line was injected into syngeneic host mice, this cell line quickly developed tumors. In syngeneic hosts that express a defective, mutant FasR (lpr [lymphoproliferation]), tumor formation was impaired (14). The greater efficiency of tumor containment by these syngeneic lpr mice may have been due to their lymphocytes’ insensitivity to tumor-expressed FasL. Although other mechanisms of immune evasion enabled the eventual establishment of tumors in FasL-insensitive lpr mice, these experiments showed that FasL contributed to the immune privilege of the tumor, expediting tumor formation in wild-type mice. A recent experiment involving allograft transplantation of murine tumor cells stably transfected with the FasL gene showed that FasL caused profound local suppression of both humoral and cellular allograft-specific immune responses (4). FasL expression by human esophageal carcinoma cells was found to be associated with apoptotic depletion of tumor-infiltrating lymphocytes in vivo (7).
In order to express FasL, tumor cells must be insusceptible to FasL-mediated apoptosis. In the present study, FasR and FasL were found to be coexpressed throughout large areas of all the breast tumors (n = 17). This suggests that complete loss of FasR cannot account for the resistance of breast cancer cells to FasL-mediated apoptosis. Breast cancer cells have been shown to have defective Fas signal transduction in vitro (17). Resistance to Fas-mediated apoptosis is a common feature of cancers, irrespective of cell surface expression of FasR (17, 28, 29). Fas resistance of breast cancer cells has been overcome in vitro by transfection of cDNAs encoding the intracellular proapoptotic proteins caspase 1 (17) or bax (5). The Fas sensitivity of breast cancer cell lines has also been restored by gamma interferon pretreatment (17) or treatment with vitamin E succinate (38). Fas sensitization in response to gamma interferon was associated with upregulation of caspase 1, while vitamin E succinate upregulated expression of FasR, FasL, and bax. These results indicate that Fas resistance in breast cancer may be due to a combination of low-level FasR expression and intracellular defects in Fas signal transduction. The ability of agents such as vitamin E succinate to sensitize breast cancer cells to Fas-mediated apoptosis suggests exciting therapeutic potential in promoting autocrine suicide of FasL-expressing breast tumor cells in vivo. Indeed, this approach may represent a potential alternative to antiestrogen therapy in estrogen-receptor negative tumors (38).
Our results conclusively demonstrate, at both the mRNA and protein levels, that human mammary tumors express FasL, an established mediator of immunological tolerance and privilege. FasL may therefore contribute to the immunologically privileged status of breast cancer. The importance of FasL to the immune evasion of breast cancer is suggested by the high prevalence of its expression among all examined mammary tumors.
ACKNOWLEDGMENTS
This study was supported by the Health Research Board of Ireland, the Cancer Research Appeal at the Mercy Hospital, Cork, Ireland, and the Irish Government Science and Technology Board (Forbairt).
We are grateful to Gary Lee, Pathology Department, Mercy Hospital, Cork, for access to surgically resected tissues and for the use of Pathology Laboratory facilities and Regina Limmer for tissue sectioning.
REFERENCES
- 1.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D H. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison J, Georgiou H M, Strasser A, Vaux D L. Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allograft. Proc Natl Acad Sci USA. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai H, Gordon D, Nabel E G, Nabel G J. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci USA. 1997;94:13862–13867. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai H, Chan S Y, Bishop D K, Nabel G J. Inhibition of the alloantibody response by CD95 ligand. Nat Med. 1997;3:843–848. doi: 10.1038/nm0897-843. [DOI] [PubMed] [Google Scholar]
- 5.Bargou R C, Wagener C, Bommert K, Mapara M Y, Daniel P T, Arnold W, Dietel M, Guski H, Feller A, Royer H D, Dorken B. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97:2651–2659. doi: 10.1172/JCI118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 7.Bennett M W, O’Connell J, O’Sullivan G C, Brady C, Roche D, Collins J K, Shanahan F. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998;160:5669–5675. [PubMed] [Google Scholar]
- 8.Daniel P T, Krammer P H. Activation induces sensitivity toward APO-1 (CD95)-mediated apoptosis in human B cells. J Immunol. 1994;152:5624–5632. [PubMed] [Google Scholar]
- 9.Disis M L, Smith J W, Murphy A E, Chen W, Cheever M A. In vitro generation of human cytolytic T-cells specific for peptides derived from the HER-2/neu protooncogene protein. Cancer Res. 1994;54:1071–1076. [PubMed] [Google Scholar]
- 10.Eischen C M, Schilling J D, Lynch D H, Krammer P H, Leibson P J. Fc receptor-induced expression of Fas ligand on activated NK cells facilitates cell-mediated cytotoxicity and subsequent autocrine NK cell apoptosis. J Immunol. 1996;156:2693–2699. [PubMed] [Google Scholar]
- 11.Gimmi C D, Morrison B W, Mainprice B A, Gribben J G, Boussiotis V A, Freeman G J, Lee Park S Y, Watanabe M, Gong J, Hayes D F, Kufe D W, Nadler L M. Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated T cells. Nat Med. 1996;2:1367–1370. doi: 10.1038/nm1296-1367. [DOI] [PubMed] [Google Scholar]
- 12.Goedegebuure P S, Douville C C, Doherty J M, Linehan D C, Lee K Y, Ganguly E K, Eberlein T J. Simultaneous production of T helper-1-like cytokines and cytolytic activity by tumor-specific T cells in ovarian and breast cancer. Cell Immunol. 1997;175:150–156. doi: 10.1006/cimm.1996.1055. [DOI] [PubMed] [Google Scholar]
- 13.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 14.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J. Melanoma cell expression of Fas (APO-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 15.Hunt J S, Vassmer D, Ferguson T A, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158:4122–4128. [PubMed] [Google Scholar]
- 16.Kang S-M, Schneider D B, Lin Z, Hanahan D, Dichek D A, Stock P G, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 17.Keane M M, Ettenberg S A, Lowrey G A, Russell E K, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res. 1996;56:4791–4798. [PubMed] [Google Scholar]
- 18.Korbutt G S, Elliott J F, Rajotte R V. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317–322. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- 19.Lau H T, Stoeckert C J. FasL—too much of a good thing? Nat Med. 1997;3:727–728. doi: 10.1038/nm0797-727. [DOI] [PubMed] [Google Scholar]
- 20.Lau H T, Yu M, Fontana A, Stoeckert C J., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;270:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 21.Liles W C, Kiener P A, Ledbetter J A, Aruffo A, Klebanoff S J. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrogi A J, Munshi A, Merogi A J, Ohadike Y, El-Habashi A, Marrogi O L, Freeman S M. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74:492–501. doi: 10.1002/(sici)1097-0215(19971021)74:5<492::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Mountz J D, Zhou T, Bluethmann H, Wu J, Edwards C K. Apoptosis defects analyzed in TcR transgenic and Fas transgenic lpr mice. Int Rev Immunol. 1994;11:321–342. doi: 10.3109/08830189409051178. [DOI] [PubMed] [Google Scholar]
- 24.Mulder W M C, Bloemena E, Stukart M J, Kummer J A, Wagstaff J, Scheper R J. T cell receptor-ζ and granzyme B expression in mononuclear cell infiltrates in normal colon mucosa and colon carcinoma. Gut. 1997;40:113–119. doi: 10.1136/gut.40.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 26.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 27.Niehans G A, Brunner T, Frizelle S P, Liston J C, Salerno C T, Knapp D J, Green D R, Kratzke R A. Human lung carcinomas express Fas ligand. Cancer Res. 1997;57:1007–1012. [PubMed] [Google Scholar]
- 28.O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connell J, Bennett M W, O’Sullivan G C, Collins J K, Shanahan F. The Fas counterattack: a molecular mechanism of tumor immune privilege. Mol Med. 1997;3:294–300. [PMC free article] [PubMed] [Google Scholar]
- 30.Ropponen K M, Eskelinen M J, Lipponen P K, Alhava E, Kosma V-M. Prognostic value of tumor-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Saas P, Walker P R, Hahne M, Quiquerez A-L, Schnuriger V, Perrin G, French L, Van Meir E G, de Tribolet N, Tschopp J, Dietrich P-Y. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest. 1997;99:1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schondorf T, Engel H, Lindemann C, Kolhagen H, von Rucker A A, Mallmann P. Cellular characteristics of peripheral blood lymphocytes and tumor-infiltrating lymphocytes in patients with gynaecological tumors. Cancer Immunol Immunother. 1997;44:88–96. doi: 10.1007/s002620050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seino K, Kayagaki N, Okumura K, Yagita H. Antitumor effect of locally produced CD95 ligand. Nat Med. 1997;3:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 34.Shiraki K, Tsuji N, Shioda T, Isselbacher K J, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997;94:6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strand S, Hofmann W J, Hug H, Muller M, Otto G, Strand D, Mariani S M, Stremmel W, Krammer P H, Galle P R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasion? Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 36.Stuart P M, Griffith T S, Usui N, Pepose J, Yu X, Ferguson T A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turley J M, Fu T, Ruscetti F W, Mikovits J A, Bertolette III D C, Birchenall-Roberts M C. Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer Res. 1997;57:881–890. [PubMed] [Google Scholar]
- 39.Zhang H, Yang Y, Horton J L, Samoilova E B, Judge T A, Turka L A, Wilson J M, Chen Y. Amelioration of collagen-induced arthritis by CD95 (APO-1/Fas)-ligand gene transfer. J Clin Invest. 1997;100:1951–1957. doi: 10.1172/JCI119726. [DOI] [PMC free article] [PubMed] [Google Scholar]