Abstract
Several different families of vacuolating toxin (vacA) alleles are present in Helicobacter pylori, and they encode products with differing functional activities. H. pylori strains containing certain types of vacA alleles have been associated with an increased risk for peptic ulcer disease. In this study, we tested serum samples and gastric juice from 19 H. pylori-negative and 39 H. pylori-positive patients for enzyme-linked immunosorbent assay reactivity with two different types of VacA antigens (types s1/m1 and s2/m2), which were purified from H. pylori 60190 and 86-338, respectively. Both antigens were recognized better by serum immunoglobulin G (IgG) from H. pylori-positive persons than by serum IgG from H. pylori-negative persons (P < 0.01). The s1/m1 VacA antigen was better recognized by sera from patients carrying vacA type s1/m1 strains than by sera from patients carrying vacA type s2/m2 or s1/m2 strains (P < 0.01). Conversely, the s2/m2 VacA antigen was better recognized by sera from patients carrying type s2/m2 or s1/m2 strains (P = 0.03). Serum IgG anti-VacA antibodies were present more frequently in patients carrying type s1/m1 strains than in other H. pylori-positive patients (P = 0.0002). In addition, the highest levels of IgA anti-VacA antibodies were detected in the gastric juice of patients carrying type s1/m1 strains. These data indicate that different VacA isoforms have distinct antigenic properties and that multiple forms of VacA elicit antibody responses in H. pylori-positive humans.
More than half of the world’s human population is colonized with the gram-negative gastric bacterium Helicobacter pylori. Colonization with this organism consistently gives rise to infiltration of the gastric mucosa by immune and phagocytic cells and is associated with an increased risk for development of peptic ulceration, distal gastric adenocarcinoma, and gastric lymphoma (1, 2, 14).
Many H. pylori strains secrete a toxin (VacA) that induces cytoplasmic vacuolation in mammalian cell lines in vitro (6–8, 17). In addition to this action, VacA interferes with normal maturation and trafficking of lysosomal enzymes (27), disrupts antigen presentation by T cells (20), and increases the permeability of polarized epithelial cell monolayers (23). VacA is initially translated as a 140-kDa protoxin, which then undergoes proteolytic processing to yield a mature ∼90-kDa secreted toxin (12, 29). These VacA monomers assemble into complex six- or seven-sided oligomeric structures (11, 18).
Sequence analysis has revealed that there is considerable allelic variation among vacA genes from different H. pylori strains. Diversity is particularly striking in the region of vacA that encodes the N-terminal signal sequence and in the midregion of vacA. Two different families of vacA alleles (s1 and s2) have been recognized based on analysis of the signal sequence region, and two families of vacA alleles (m1 and m2), which are about 70% identical in nucleotide sequences within a 0.7-kb region, have been recognized based on analysis of the midregion (3, 12). H. pylori strains that produce vacuolation of HeLa cells in vitro usually contain type s1/m1 or occasionally type s1/m2 vacA alleles, whereas strains with type s2/m2 vacA alleles lack detectable cytotoxin activity for HeLa cells (3). Some strains with type s1/m2 vacA alleles produce vacuolation of RK-13 cells but not HeLa cells, which suggests that VacA sequence differences confer cell type-specific activities (21). Several studies performed in the United States and Europe have shown that the risk of peptic ulcer disease is higher among persons colonized with H. pylori strains containing type s1 vacA alleles than among those with strains containing type s2 vacA alleles (3, 28, 30).
The considerable diversity in the deduced amino acid sequences of different vacA products suggests that there might be marked antigenic diversity among VacA proteins from different strains. In previous studies (9, 10), it has been shown that about 50% of H. pylori-positive persons in the United States produce serum immunoglobulin G (IgG) antibodies that react with a purified VacA antigen from H. pylori 60190 (a type s1/m1 VacA antigen). However, other types of VacA proteins have not yet been tested for possible utility as antigens in serodiagnostic assays. In addition, it is not known whether the vacA genotypes of H. pylori strains influence anti-VacA immunoglobulin responses. Therefore, in this study we used two different purified VacA antigens in enzyme-linked immunosorbent assays (ELISAs) to analyze specific serum and gastric juice anti-VacA responses in patients who were colonized with H. pylori strains of known vacA genotype. We report here that both type s1/m1 and type s2/m2 forms of VacA are useful antigens in serologic assays and that these two isoforms have distinguishable antigenic properties.
MATERIALS AND METHODS
Purification of H. pylori VacA antigens.
Two different H. pylori strains (60190 and 86-338) were selected as the sources for VacA antigens used in this study. H. pylori 86-338 was isolated from a patient with nonulcer dyspepsia in the United States, and H. pylori 60190 (ATCC 49503) was isolated from a patient in the United Kingdom. The vacA sequence of strain 60190 (GenBank U05676) is the prototype for the s1/m1 family of vacA alleles (12). PCR-based typing and partial sequence analysis of vacA from strain 86-338 indicate that this strain contains a type s2/m2 vacA allele (3, 15). Both strains produce high-molecular-mass VacA oligomers with similar ultrastructural morphologies (11). Both strains were cultured in sulfite-free brucella broth containing 0.5% charcoal, and proteins in the broth culture supernatants were concentrated by precipitation with a 50% saturated solution of ammonium sulfate. VacA oligomers then were purified by gel filtration chromatography with a Superose 6 HR 16/50 column, as described previously (8, 11). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining demonstrated isolation of ∼90-kDa VacA bands from both strains (Fig. 1), and both VacA bands reacted with polyclonal rabbit anti-VacA serum (8) (data not shown). The VacA band from strain 86-338 was slightly larger than the VacA band from strain 60190, which is consistent with previous analysis of a type s2/m2 VacA protein from a different H. pylori strain (3). Supernatant from H. pylori 60190 yielded about 10-fold-higher quantities of purified VacA than supernatant from strain 86-338 (15), which accounts for the increased representation of trace contaminant bands in preparations of the latter VacA protein (Fig. 1, lane b).
FIG. 1.
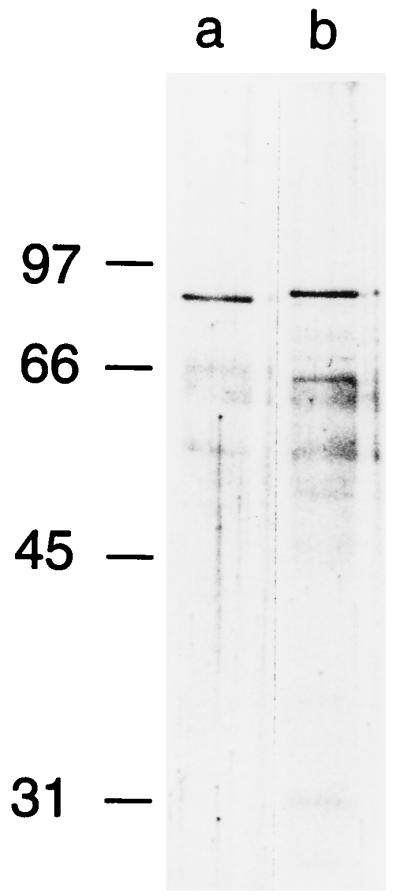
Purification of type s1/m1 and s2/m2 VacA antigens. VacA was purified from broth culture supernatants of H. pylori 60190 and 86-338 (lanes a and b, respectively), and the two preparations (standardized by protein concentration) were analyzed by SDS-PAGE and silver staining. Yields of VacA from strain 60190 were about 10-fold higher than those from strain 86-338; therefore, the VacA preparation from the latter strain was concentrated 10-fold by ultrafiltration with a Centricon-30 device (Amicon) prior to SDS-PAGE.
Collection of H. pylori isolates, sera, and gastric juice specimens.
We studied 58 human patients (mean age, 55.9 ± 12.6 years) who underwent routine upper gastrointestinal endoscopy at the VA Medical Center in Nashville, Tenn., between February 1993 and May 1995 (Table 1). This group of patients has been described in several previous studies (3, 4, 24). Two biopsy specimens from each patient, taken from the gastric antrum and gastric corpus, were cultured under microaerobic conditions at 37°C for isolation of H. pylori. Unstimulated gastric juice aspirates, obtained after an overnight fast, were collected from each patient and immediately separated into aliquots, which were stored at −20°C. Gastric biopsy specimens from each patient were examined and histologic parameters were graded, as described previously (4). All patients classified as colonized by H. pylori (n = 39) had a positive gastric mucosal culture for H. pylori, and all were seropositive by an ELISA that tests for the presence of IgG antibodies to a pool of antigens from several different sonicated H. pylori strains (25). IgG antibody results in this ELISA are reported as optical density (OD) ratios, based on comparison with a pool of sera from H. pylori-negative children (13). The vacA genotypes of H. pylori isolates were determined by a PCR-based typing method, as described previously (3). All patients classified as not colonized (n = 19) had a negative H. pylori culture, a negative rapid urease test, and no evidence of H. pylori on histologic examination and were seronegative by an ELISA that tested for antibodies to sonicated H. pylori. In addition to being tested for reactivity with sonicated H. pylori, sera were tested for ELISA reactivity with purified recombinant CagA, as described previously (5). A total of 21 of the 39 patients colonized with H. pylori, and 3 of the noncolonized patients were reported to have past or present peptic ulcer disease.
TABLE 1.
Characteristics of the study population of 58 dyspeptic patients from Tennessee
| Characteristic | Value for group
|
|||
|---|---|---|---|---|
| H. pylori negative |
H. pylori positive, by
vacA genotype
|
|||
| s2/m2 | s1/m2 | s1/m1 | ||
| Total subjects | 19 | 8 | 12 | 19 |
| Mean age ± SEM (yr) | 52.3 ± 3.0 | 52.9 ± 3.9 | 61.5 ± 3.3 | 57.8 ± 3.2 |
| Seropositive for CagA antibodies (%) | 0 | 0 | 75.0 | 77.8 |
| With peptic ulcer (%) | 15.8 | 25.0 | 75.0 | 52.6 |
ELISA for detection of antibodies to VacA in human sera.
Serum samples were tested for reactivity with purified VacA proteins in ELISAs. Plates (96-well, Immulon 2; Dynatech, Alexandria, Va.) were coated with VacA antigens (approximately 25 ng/well) in carbonate buffer and stored overnight at room temperature. Sera were diluted in phosphate-buffered saline and were incubated with the bound antigens for 1 h at 37°C. After the plates were washed, peroxidase-conjugated anti-human IgG or anti-human IgA (Biosource International, Camarillo, Calif.) was added and the conjugate dilutions were incubated for 1 h. Antigen-antibody complexes were resolved with 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (1 mg/ml) in McIlvain’s buffer (consisting of titrations of 0.2 M Na2HPO4 plus 0.1 M citric acid, pH 4.6) (25). The optimal concentrations of patient sera and peroxidase-conjugated anti-human antibodies, determined by checkerboard titrations (25), were a serum dilution of 1:100, an IgG conjugate dilution of 1:4,000, and an IgA conjugate dilution of 1:2,000. OD values were determined with an MRX ELISA reader (Dynatech) at dual wavelengths (405 and 490 nm). OD values were linear in a range from 0 to 2.5. Each serum sample was tested in duplicate.
ELISA for detection of antibodies to VacA in human gastric juice.
Gastric juice samples were first mixed (1:1 [vol/vol]) with a buffer containing 100 mM Tris (pH 7.6), 60 mM sodium chloride, 0.2% sodium azide, 0.05 U of aprotinin protease inhibitor (Sigma, St. Louis, Mo.) per ml, and 2 mM tetrasodium EDTA (33). This procedure consistently resulted in neutralization of the gastric juice samples; i.e., pH >6.8. Neutralized gastric juice samples were diluted 1:4 with Dulbecco’s phosphate-buffered saline (Gibco-BRL) containing 2.5% bovine serum albumin, and 100-μl aliquots were added to wells of ELISA plates that had been coated with purified VacA antigens (25 ng/well). After the plates were incubated at 37°C for 1 h, biotin-conjugated goat anti-human IgA (Sigma), diluted 1:1,000, was added to the wells for 1 h at 37°C. Avidin-conjugated alkaline phosphatase (Sigma), diluted 1:1,000, was added for 1 h, followed by substrate solution (1 mg of P-nitrophenyl phosphate per ml in 0.1 M diethylanolamine-HCl, pH 9.8) for 15 min. OD values were determined as described above.
Statistical analysis.
Distributions of ELISA OD values (mean ± standard error of the mean) were compared by using Student’s t test for independent variables.
RESULTS
Serum IgG and IgA responses to VacA.
Serum and gastric juice specimens were collected from 58 patients, of whom 39 (67.1%) were classified as H. pylori positive and 19 were classified as H. pylori negative (Table 1). The vacA genotypes of the 39 H. pylori isolates were classified as type s1/m1 (n = 19), s1/m2 (n = 12), or s2/m2 (n = 8). Sera from the 58 patients were tested for the presence of IgG and IgA anti-VacA antibodies in two different ELISAs, which used either type s1/m1 or s2/m2 VacA antigens. Sera from the 39 persons colonized with H. pylori demonstrated significantly greater IgG and IgA reactivity with the type s1/m1 VacA antigen from strain 60190 than did sera from the 19 noncolonized persons (Table 2). Among the 39 patients carrying H. pylori, there was no significant association between levels of anti-VacA IgG antibodies and levels of IgG antibodies to H. pylori whole-cell sonicates (not shown). Sera from H. pylori-colonized patients demonstrated significantly greater IgG reactivity with the type s2/m2 VacA antigen from strain 86-338 than did sera from noncolonized patients (Table 2). However, there were no significant differences in IgA reactivity to the s2/m2 VacA antigen.
TABLE 2.
Serum antibody reactivity to two different VacA antigens
| Patient classification | No. of patients | ELISA
values by antibody class and type of VacA
antigena
|
||||
|---|---|---|---|---|---|---|
| IgG
|
IgA
|
|||||
| H. pylori sonicate | s1/m1 | s2/m2 | s1/m1 | s2/m2 | ||
| H. pylori negative | 19 | 0.41 ± 0.05 | 0.07 ± 0.02 | 0.16 ± 0.05 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| H. pylori positive | 39 | 6.02 ± 0.30 | 0.37 ± 0.04c | 0.33 ± 0.04b | 0.39 ± 0.03c | 0.11 ± 0.02 |
| s1/m1 | 19 | 5.54 ± 0.46 | 0.56 ± 0.09e | 0.25 ± 0.04d | 0.56 ± 0.08e | 0.07 ± 0.01 |
| s1/m2 | 12 | 6.17 ± 0.56 | 0.20 ± 0.04 | 0.42 ± 0.07 | 0.26 ± 0.08 | 0.15 ± 0.08 |
| s2/m2 | 8 | 6.90 ± 0.49 | 0.18 ± 0.07 | 0.38 ± 0.09 | 0.17 ± 0.08 | 0.14 ± 0.08 |
Values for ELISA reactivity of sera with H. pylori sonicate are reported as OD ratios. All other values are ODs.
P = 0.007 compared with values obtained for the H. pylori-negative group.
P < 0.0005 compared with values obtained for the H. pylori-negative group.
P = 0.03 compared with values obtained for the other two groups of H. pylori-positive patients.
P < 0.01 compared with values obtained for each of the three other groups (H. pylori negative, s2/m2, s1/m2).
In the next analysis, H. pylori-positive patients were divided into three groups, based on the vacA genotypes of the H. pylori strains that they carried (s1/m1, s1/m2, or s2/m2). Sera from patients carrying s1/m1 strains demonstrated significantly greater IgG and IgA reactivity with the s1/m1 antigen than did those from patients carrying s2/m2 or s1/m2 strains (P < 0.01) (Table 2). Conversely, the s2/m2 antigen was recognized more strongly by serum IgG from patients carrying type s2/m2 or s1/m2 strains than by serum IgG from patients carrying type s1/m1 strains (P = 0.03).
Based on cutoffs for seropositivity defined as 2 standard deviations above the mean OD value for sera from H. pylori-negative persons, 21 of the 39 H. pylori-positive patients had serum IgG antibodies that reacted with the type s1/m1 VacA antigen, and 16 of these 21 were colonized with type s1/m1 strains. Only 6 of the 39 patients had IgG antibodies that reacted with the type s2/m2 VacA antigen. Of these 6 patients, 5 were colonized with strains containing type m2 vacA alleles (two s2/m2 and three s1/m2 alleles) and 1 was colonized with a type s1/m1 strain. Thus, 16 (84.2%) of the 19 patients colonized with type s1/m1 strains had detectable serum anti-VacA IgG antibodies in an assay that used a type s1/m1 antigen. In comparison, 5 of 20 patients colonized with type m2 strains had detectable serum IgG anti-VacA antibodies (P = 0.0002), and sera from these 5 patients yielded positive results in ELISAs with either type s1/m1 or type s2/m2 antigens. Based on the combined results of both assays, we conclude that anti-VacA IgG antibodies are detectable in sera from patients colonized with type s1/m1 strains significantly more frequently than in sera from patients colonized with type m2 strains.
Gastric juice IgA responses to VacA.
In the next series of experiments, we sought to determine whether anti-VacA antibodies could be detected in human gastric juice. Since preliminary studies indicated that gastric juice samples from H. pylori-positive patients contain IgA but minimal IgG antibodies to H. pylori sonicates (26), only gastric juice anti-VacA IgA responses were analyzed. The type s1/m1 VacA antigen was recognized significantly more strongly by gastric juice IgA from H. pylori-colonized persons than by gastric juice IgA from H. pylori-negative persons (P = 0.002), and as expected, gastric juice from patients carrying vacA type s1/m1 strains yielded the highest reactivity (Table 3). Reactivity to the s2/m2 antigen was higher for gastric juice from H. pylori-positive patients colonized with vacA type s1/m1 strains than for gastric juice from H. pylori-negative patients (P = 0.02); the differences in reactivity of gastric juice from s1/m2, s2/m2, and H. pylori-negative patient groups were not statistically significant. Thus, there are differences in the patterns of serum and gastric juice reactivity to s1/m1 and s2/m2 VacA antigens (compare Tables 2 and 3). Interestingly, among H. pylori-positive patients, levels of gastric juice IgA antibodies to H. pylori sonicate antigens and to purified VacA were significantly associated, and this relationship was detected with both VacA antigens (r = 0.52 and 0.61 for assays using type s1/m1 and s2/m2 VacA antigens, respectively; P ≤ 0.001). In summary, these data are consistent with the production of secretory IgA antibodies to VacA in the gastric mucosa of H. pylori-colonized persons.
TABLE 3.
Gastric juice IgA antibody reactivities to two different VacA antigens
| Patient classification | No. of patients | ELISA
OD values by type of VacA antigen
|
|
|---|---|---|---|
| s1/m1 | s2/m2 | ||
| H. pylori negative | 19 | −0.04 ± 0.01 | −0.03 ± 0.01 |
| H. pylori positive | 39 | 0.45 ± 0.01a | 0.14 ± 0.06 |
| s1m1 | 19 | 0.73 ± 0.26b | 0.20 ± 0.10c |
| s1m2 | 12 | 0.35 ± 0.22 | 0.15 ± 0.014 |
| s2m2 | 8 | −0.004 ± 0.04 | −0.006 ± 0.02 |
P = 0.002 compared with values obtained for the H. pylori-negative group.
P = 0.004 compared with values obtained for the H. pylori-negative group.
P = 0.02 compared with values obtained for the H. pylori-negative group.
Relationship between vacA genotype, gastric histology, and gastric juice pH.
As reported previously (4), patients colonized with type s1/m1 vacA-containing strains of H. pylori had higher scores for epithelial degeneration and gastric mucosal inflammation than did patients carrying H. pylori with type s2/m2 strains. However, there was no evidence that the magnitude of the humoral immune response to specific VacA antigens was a determinant of tissue injury or inflammation (data not shown). In addition, the occurrence of peptic ulceration was unrelated to the presence or absence of an anti-VacA antibody response.
DISCUSSION
The initial classification of vacA alleles into several major families was based on analysis of H. pylori strains isolated from patients in the United States (3). Subsequent analysis has confirmed the existence of these major families of vacA alleles in H. pylori strains isolated from patients throughout the world and has led to the identification of vacA subfamilies that predominate in certain geographic regions (16, 22, 28, 31, 32). Interestingly, nearly all H. pylori strains isolated from patients in Asia or developing countries contain type s1 vacA alleles (16, 22, 31, 32). The deduced amino acid sequences of type s1/m1 and s2/m2 VacA proteins are quite different from each other (3), and the results of this study suggest that these isoforms also differ in antigenic properties. Thus, it seems likely that regions of maximum VacA sequence diversity may correspond to surface-exposed epitopes.
Previous studies (9, 10) have demonstrated that about 50% of H. pylori-positive persons in the United States possess serum antibodies that react in an ELISA with purified VacA from H. pylori 60190 (a type s1/m1 antigen). One interpretation of these results is that these patients were colonized with strains that produce VacA antigens closely related to the type s1/m1 VacA antigen used in the ELISA. In agreement with this explanation, the current study indicates that 84% of patients colonized with type s1/m1 strains possess serum IgG anti-VacA antibodies detectable in an ELISA that uses the type s1/m1 VacA antigen from H. pylori 60190. Of the 20 patients in this study who were colonized with strains containing either s2/m2 or s1/m2 vacA alleles, only 5 (25%) had detectable serum IgG anti-VacA antibodies. Anti-VacA antibodies in the latter 5 patients reacted relatively more strongly in an ELISA with a type s2/m2 VacA antigen than in one with a type s1/m1 antigen, but there was clearly cross-reactivity.
The results of this study indicate that strains producing type s1/m1 forms of VacA are more likely to elicit production of serum anti-VacA antibodies than are H. pylori strains producing alternate forms. Similarly, gastric juice anti-VacA IgA responses are detected most commonly in patients harboring H. pylori strains producing type s1/m1 VacA proteins. There are several possible explanations for these differences. First, strains of H. pylori containing type s1/m1 vacA alleles typically produce and secrete higher levels of VacA in vitro than do strains containing alternate alleles (3, 8, 15), and if similar differences exist in vivo, this could account for the observed pattern of serologic responses. Alternatively, type s1/m1 VacA may be more highly immunogenic than other forms. Finally, s1/m1 strains have been associated with higher levels of gastric epithelial injury than other forms (4), and this may be associated with increased entry of VacA into the gastric mucosa, where antigen recognition probably occurs.
Since in this study, humoral immune responses to VacA were best detected in assays using homologous VacA antigens, the use of multiple VacA antigens may be desirable if VacA is to be used as a component of diagnostic assays for the detection of H. pylori infection. If only one antigen is to be used, assays that use a type s1/m1 antigen rather than a type s2/m2 antigen are recommended, since they seem to have greater sensitivity. Immunization of mice with a type s1/m1 VacA antigen has been reported to confer protective immunity only against H. pylori strains expressing homologous types of VacA (19). Thus, if VacA is to be used as a component of an H. pylori vaccine, the use of both type s1/m1 and s2/m2 isoforms may yield an increased level of protection.
ACKNOWLEDGMENTS
We thank Beverly Hosse for technical assistance and Kyi Tham for providing histologic data.
This study was supported in part by NIH grant AI-39657 and by the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Anonymous. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 2.Anonymous. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton J C, Cao P, Peek R M, Jr, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of H. pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 4.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 5.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with H. pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 6.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 7.Cover T L, Berg D E, Blaser M J. VacA and the cag pathogenicity island of H. pylori. In: Ernst P B, Michetti P, Smith P D, editors. “The immunobiology of H. pylori: from pathogenesis to prevention.”. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 75–90. [Google Scholar]
- 8.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 9.Cover T L, Cao P, Lind C D, Tham K T, Blaser M J. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect Immun. 1993;61:5008–5012. doi: 10.1128/iai.61.12.5008-5012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cover T L, Cao P, Murthy U K, Sipple M S, Blaser M J. Serum neutralizing antibody response to the vacuolating cytotoxin of Helicobacter pylori. J Clin Investig. 1992;90:913–918. doi: 10.1172/JCI115967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover T L, Hanson P I, Heuser J E. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J Cell Biol. 1997;138:759–769. doi: 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cover T L, Tummuru M K R, Cao P, Thompson S A, Blaser M J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 13.Drumm B, Perez-Perez G I, Blaser M J, Sherman P M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990;322:359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- 14.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsyth M H, Atherton J C, Blaser M J, Cover T L. Heterogeneity in levels of vacuolating cytotoxin gene (vacA) transcription among Helicobacter pylori strains. Infect Immun. 1998;66:3088–3094. doi: 10.1128/iai.66.7.3088-3094.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 18.Lupetti P, Heuser J E, Manetti R, Massari P, Lanzavecchia S, Bellon P L, Dallai R, Rappuoli R, Telford J L. Oligomeric and subunit structure of the Helicobacter pylori vacuolating cytotoxin. J Cell Biol. 1996;133:801–807. doi: 10.1083/jcb.133.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 20.Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;87:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagliaccia C, de Bernard M, Lupetti P, Xuhuai J, Burroni D, Cover T L, Papini E, Rappuoli R, Telford J L, Reyrat J-M. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc Natl Acad Sci USA. 1998;95:10212–10217. doi: 10.1073/pnas.95.17.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan Z-J, Berg D E, van der Hulst R W M, Su W-W, Raudonikiene A, Xiao S-D, Dankert J, Tytgat G N J, van der Ende A. Prevalence of vacuolating cytotoxin production and distribution of distinct vacA alleles in Helicobacter pylori from China. J Infect Dis. 1998;178:220–226. doi: 10.1086/515601. [DOI] [PubMed] [Google Scholar]
- 23.Papini E, Satin B, Norais N, de Bernard M, Telford J L, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Investig. 1998;102:813–820. doi: 10.1172/JCI2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peek R M, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA+Helicobacter pylori strains. Lab Investig. 1995;71:760–770. [PubMed] [Google Scholar]
- 25.Perez-Perez G I, Dworkin B M, Chodos J E, Blaser M J. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Perez G I, Tham K T, Peek R M, Atherton J C, Blaser M J. Not all Helicobacter pylori-infected persons produce specific IgA in gastric juice. Gastroenterology. 1994;112:A1061. [Google Scholar]
- 27.Satin B, Norais N, Telford J, Rappuoli R, Murgia M, Montecucco C, Papini E. Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J Biol Chem. 1997;272:25022–25028. doi: 10.1074/jbc.272.40.25022. [DOI] [PubMed] [Google Scholar]
- 28.Strobel S, Bereswill S, Balig P, Allgaier P, Sonntag H-G, Kist M. Identification and analysis of a new vacA genotype variant of Helicobacter pylori in different patient groups in Germany. J Clin Microbiol. 1998;36:1285–1289. doi: 10.1128/jcm.36.5.1285-1289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telford J L, Ghiara P, Dell-Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Doorn L J, Figueiredo C, Rossau R, Jannes G, van Asbroeck M, Sousa J C, Carneiro F, Quint W G V. Typing of Helicobacter pylori vacA gene and detection of cagA gene by PCR and reverse hybridization. J Clin Microbiol. 1998;36:1271–1276. doi: 10.1128/jcm.36.5.1271-1276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Doorn L-J, Figueiredo C, Sanna R, Pena S, Midolo P, Ng E K W, Atherton J C, Blaser M J, Quint W G V. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol. 1998;36:2597–2603. doi: 10.1128/jcm.36.9.2597-2603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H-J, Kuo C-H, Yeh A A M, Chang P C L, Wang W-C. Vacuolating toxin production in clinical isolates of Helicobacter pylori with different vacA genotypes. J Infect Dis. 1998;178:207–212. doi: 10.1086/515600. [DOI] [PubMed] [Google Scholar]
- 33.Wirth H P, Vogt P, Ammann R, Altorfer J. IgA antibodies against Helicobacter pylori in gastric secretions: gastric secretory immune response or salivary contamination? Schweiz Med Wochenschr. 1993;123:1106–1110. [PubMed] [Google Scholar]


