Abstract
Among all the NAD+ precursors, nicotinamide riboside (NR) has gained the most attention as a potent NAD+-enhancement agent. This recently discovered vitamin, B3, has demonstrated excellent safety and efficacy profiles and is orally bioavailable in humans. Boosting intracellular NAD+ concentrations using NR has been shown to provide protective effects against a broad spectrum of pathological conditions, such as neurodegenerative diseases, diabetes, and hearing loss. In this review, an integrated overview of NR research will be presented. The role NR plays in the NAD+ biosynthetic pathway will be introduced, followed by a discussion on the synthesis of NR using chemical and enzymatic approaches. NR’s effects on regulating normal physiology and pathophysiology will also be presented, focusing on the studies published in the last five years.
Keywords: vitamin B3, nicotinamide riboside, health, COVID-19, dietary supplements
1. Introduction
Vitamins are a group of structurally diversified, small organic molecules that are essential for almost all forms of life. Although often required in small amounts and designated as “micronutrients”, vitamins have proven critical for maintaining normal physiology. Their absence or deficiency is known to cause disorders or diseases such as anemia [1], beriberi [2], pellagra [3], scurvy [4], night blindness [5], and blood coagulation disorders [6]. The study of vitamins has advanced significantly during the last century, being recognized by the Nobel Prize Committee in the form of numerous awards for vitamin-related research since 1928 [7]. All the research efforts have also resulted in the development of dietary recommendations and vitamin supplementation for disease prevention and treatment [8,9,10]. The human nutrition market was worth 252.38 billion USD in 2020 and is expected to expand during the next decade [11]. This growth can be attributed to increased health awareness, as well as surging demand for additional protection from the devastating COVID-19 pandemic [12,13].
This review focuses on a newly discovered form of vitamin B3, nicotinamide riboside (NR). Initially, NR was shown to increase intracellular NAD+ concentrations and to extend the life span without calorie restriction (CR) in yeast [14]. Subsequent studies established that NR is a potent NAD+ booster [15,16,17]. Together with two other NAD+ precursors, nicotinamide (NAM) and nicotinic acid (NA), NR belongs to the vitamin B3 family [14]. The literature is replete with the beneficial effects of NR-mediated NAD+ elevation in a broad spectrum of diseases, including neurodegenerative diseases [18,19], metabolic disorders [20,21], and cardiac fibrosis [22]. Notably, SARS-CoV-2 infection disrupts NAD+ homeostasis by depleting cellular NAD+ contents and upregulating poly(ADP-ribose) polymerases (PARPs), the NAD+-utilizing enzymes [23,24]. Reduced NAD+ level has been shown to promote inflammation and increase cellular injury [25,26]. Consequently, NAD+-depletion may be held partially responsible for the higher mortality rate in patients with pre-existing medical conditions, such as respiratory diseases, cardiovascular diseases, and diabetes. PARP inhibitors have been suggested as potential therapeutics for COVID-19 by blocking virus proliferation, preventing immune cell hyperactivation, and reducing the levels of circulating cytokines [27]. Interestingly, restoring NAD+ concentration using precursors such as NR is also under intense investigation to attenuate COVID-19-induced complications [28].
In this review, our effort is directed at discussing the distinct NAD+ biosynthetic pathways; the synthesis of NR; the role NR plays in health promotion; and disease prevention. The therapeutic potential of NR in treating COVID-19 is discussed separately towards the end. Several databases, including SciFinder, PubMed, Google Scholar, and Researchgate, were researched for peer-reviewed articles on the subject matter. Special attention is given to the publications after 2018 for NR in health and diseases. Publications prior to that have also been included to establish the foundation knowledge of NR.
2. NR and NAD+ Biosynthesis
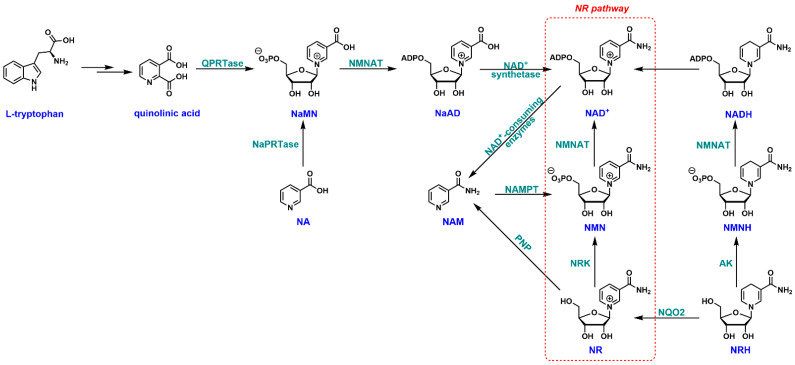
In mammals, an intricate NAD+ biosynthetic network has been established, including the de novo, salvage, NR, and dihydronicotinamide riboside (NRH) pathways (Figure 1) [29,30]. L-Tryptophan is the starting point of the de novo pathway [31]. It is enzymatically transformed through several steps to quinolinic acid, an immediate precursor of nicotinic acid mononucleotide (NaMN). Nicotinamide mononucleotide adenylyltransferase (NMNAT)-catalyzed adenylation converts NaMN to nicotinic acid adenine dinucleotide (NaAD), which can be further amidated to NAD+ by NAD+ synthetase. Intracellular NAD+ can be degraded to NAM by a class of enzymes called “NAD+-consuming enzymes”, such as sirtuins, poly(ADP-ribose) polymerase (PARP), and CD38 [32,33]. The salvage pathway is known to recycle NAM by nicotinamide phosphoribosyltransferase (NAMPT) to nicotinamide mononucleotide (NMN) [34]. NMN can be fully incorporated into NAD+ by the action of NMNAT. Another branch of the salvage pathway starts with NA. It is recycled by nicotinate phosphoribosyltransferase (NaPRTase) to NaMN through the Preiss–Handler pathway [35]. NaMN thus serves as a common intermediate for NA salvage and de novo NAD+ biosynthesis. NR is a naturally occurring metabolite initially found in milk [36]. It can be directly phosphorylated by NR kinases (NRK1/NRK2) to NMN [15,37,38], and ultimately to NAD+. This pathway has been extensively studied because NR is considered a potent NAD+ precursor [38,39,40], and boosting intracellular NAD+ content has been suggested as a potential anti-aging strategy [41,42,43]. Additionally, gut microbiota can also convert the dietary NR into various NAD+ precursors such as NAM, NA, and nicotinic acid riboside (NAR) in the colonic lumen and boost NAD+ biosynthesis [44]. NR is water-soluble and cell-permeable with no apparent toxicity [16]. Unlike other NAD+ precursors, such as NAM or NA, NR is not associated with any severe side effects [15,45]. All of these features render NR an ideal candidate as a therapeutic agent for NAD+ restoration. NRH is the “new kid on the block” as an NAD+ precursor. It has been suggested that NRH is metabolically handled by adenosine kinase (AK) and NMNAT in a sequential order to generate NADH, which can then be equilibrated to NAD+ through redox reactions [30,46]. NRH is also used by NRH:quinone oxidoreductase 2 (NQO2) as the electron donor to detoxify quinones, leading to the formation of endogenous NR [47].
Figure 1.
NAD+ biosynthetic pathways in mammalian cells. NA: nicotinic acid; NAM: nicotinamide; NR: nicotinamide riboside; NMN: nicotinamide mononucleotide; NaMN: nicotinic acid mononucleotide; NaAD: nicotinic acid adenine dinucleotide; NRH: reduced nicotinamide riboside; NMNH: reduced nicotinamide mononucleotide; QPRTase: quinolinate phosphoribosyltransferase; NMNAT: nicotinamide mononucelotide adenylyltransferase; NaPRTase: nicotinic acid phosphoribosyl transferase; NAMPT: nicotinamide phosphoribosyl transferase; NRK: nicotinamide riboside kinase; PNP: purine nucleoside phosphorylase; AK: adenosine kinase; NQO2: NRH: quinone oxidoreductase 2.
3. Synthesis of NR
3.1. Biosynthesis of NR
The study of the biosynthesis of NR has been rather scarce. In yeast, NR can be produced via phosphatase-mediated NMN dephosphorylation [48]. The deletion of nrt1, an NR transporter, in a genetically altered yeast strain has resulted in increased secretion of NR [49], suggesting a possible biosynthetic approach for this vitamin. Endogenous NR biosynthesis has also been observed in mammalian cells [50]. It was further demonstrated that the dephosphorylation of NMN and NaMN by cytosolic 5′-nucleotidases (5′-NTs) led to the formation of NR and NAR in vitro.
3.2. Chemical Synthesis of NR
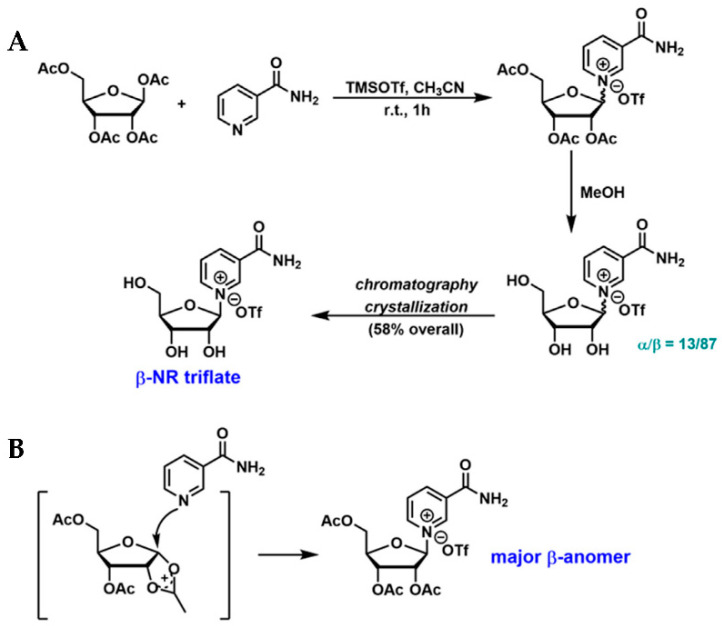
The N-glycosidic bond in NR is considered the “weakest link” of the molecule. The presence of this labile chemical moiety poses a significant challenge in the chemical synthesis and modification of NR. The initial synthetic effort was focused on the coupling of NAM with peracylated-D-ribose, leading to the formation of a mixture of both α- and β-isomers with variable ratios [51,52]. A TMSOTf-mediated coupling reaction between NAM and commercially available tetra-O-acetyl-β-D-ribofuranose was later reported (Figure 2A) [53]. The glycosylation reaction was conducted in acetonitrile at room temperature, resulting in the formation of triacetylated NR. The subsequent methanolysis led to a mixture of both anomers in a 13:87 ratio (α:β), which was further purified by chromatography on activated charcoal and crystallization to afford the desired β-NR triflate in 58% overall yield [53]. The stereoselectivity of the glycosylation can be explained by “neighboring group participation”, as illustrated in Figure 2B [54].
Figure 2.
NR synthesis: glycosylation of NAM and acetylated-D-ribofuranose. (A) Synthetic scheme of β-NR triflate developed by Kirihata et al. [53]; (B) Neighboring group participation leads to the formation of β-NR as the major anomer.
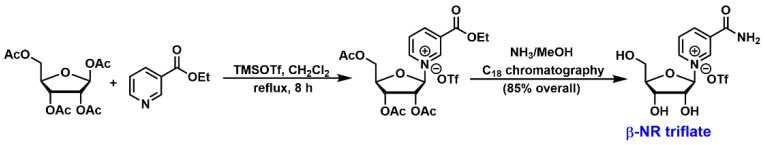
A simple two-step procedure was also developed to synthesize NR in a stereoselective manner [39,55]. Tetra-O-acetyl-β-D-ribofuranose and ethyl nicotinate in the presence of a stoichiometric amount of TMSOTf were refluxed in CH2Cl2 for 8 h (Figure 3). NMR results indicated the formation of only the β-anomer, suggesting that the coupling reaction went through an acyloxonium ion intermediate similar to the one shown in Figure 2B. The resulting ethyl nicotinate 2′,3′,5′-tri-O-acetylriboside triflate was then treated with ammonia in methanol for the simultaneous deprotection of acetyl groups and the conversion of ester to amide. The crude product was purified by C18 column chromatography to afford β-NR triflate in 85% overall yield. It is important to note that NR triflate is not a pharmaceutically acceptable form. Ion exchange with saturated sodium chloride solution provided NR chloride salt [56], which is commonly used as a dietary supplement. Other chemical syntheses of NR and its analogs have also been reported. Please refer to a wonderful review article on this topic if interested [57].
Figure 3.
Synthetic scheme of β-NR triflate developed by Sauve et al. [39,55].
3.3. Chemo-Enzymatic Synthesis of NR
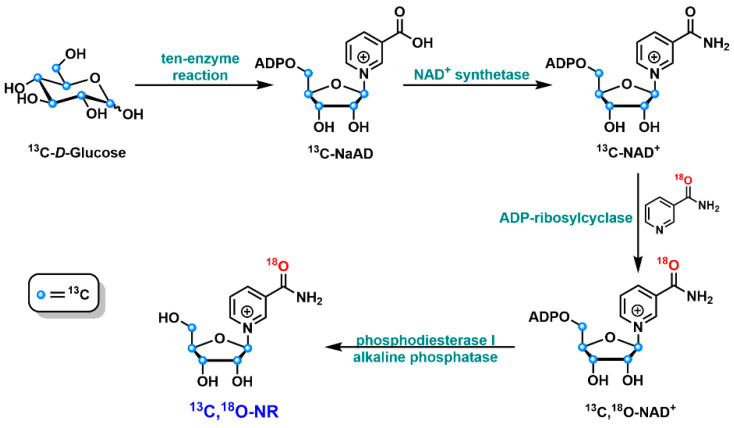
Accessing NR and its derivatives has also been explored using a chemo-enzymatic approach [58]. It started with a one-pot ten-enzyme coupled reaction to convert 13C-labeled glucose to 13C-labeled NaAD, which can then be transformed to NAD+ via NAD+ synthetase-catalyzed amidation reaction (Figure 4). The 13C-labeled NAD+ was then treated with chemically synthesized 18O-NAM in the presence of ADP-ribosylcyclase. This enzyme-mediated “base exchange” reaction allowed the formation of NAD+ with 13C labels in the ribose moiety and 18O label in NAM. The subsequent degradations of this NAD+ isotopomer by phosphodiesterase and alkaline phosphatase generated 13C, 18O-labeled NR in good yield. A similar method was applied to the formation of 14C-labeled NR [58]. These NR isotopomers have been used for the investigation of NR metabolism in the cellular setting.
Figure 4.
Schematic representation of the chemo-enzymatic synthesis of 13C, 18O-NR.
4. NR in Health and Diseases
As a potent NAD+ precursor, NR has profound implications for human health and diseases [59,60]. Many inflammation-related conditions—such as Alzheimer’s disease (AD), sclerosis, and fibrosis—are known to deplete NAD+ contents, aggravate cellular injury, and upregulate proinflammatory cytokines [19,61]. NR-mediated restoration of intracellular NAD+ pool has been shown to stimulate sirtuin activity; improve mitochondrial biogenesis and function; and provide benefits in health span and life span extension [17,62,63,64]. The role NR plays in neuroinflammation, fibrosis, and aging is discussed below and summarized in Table 1. It should be noted that despite the promising results in animal models, the therapeutic benefits of NR in human trials have been modest. This is partially due to the poor metabolic stability of this molecule. NR can be degraded to NAM in circulation, presumably by purine nucleoside phosphorylase (PNP) [14,65]. This degradation may compromise the clinical efficacy of NR.
Table 1.
Role of NR in health and different disease conditions.
| Condition | Route of Administration | Mechanism of Action | Ref. |
|---|---|---|---|
| Neuroinflammation | Intracerebro ventricular | suppresses CD38-mediated neuroinflammation by increasing NAD+ levels and suppressing NF-κB in mice | [19] |
| Oral (supplemented with drinking water) (12 mM) for 5 months | reduces NLRP3 inflammasome expression and proinflammatory cytokines in AD mouse model | [66] | |
| Oral (supplemented with drinking water) (12 mM) for 6 months | suppresses neuroinflammation in AD/Polβ mice by reducing the levels of proinflammatory cytokines IL-α, TNFα, MCP-1, IL-1β, MIP-1α and increasing the levels of anti-inflammatory cytokine IL-10 | [67] | |
| Oral (supplemented with diet; 100 µg/kg daily) for 2 months | reduces inflammation in Gulf War Illness mice by increasing the deacetylation of NF-κB p65 subunit and PGC-1α | [68] | |
| Oral (supplemented with diet at 400 mg/kg); Oral (185 mg/kg) |
decreases neuroinflammatory markers in amyotrophic lateral sclerosis (ALS) mice models | [69,70] | |
| Oral, via stomach gavage (400 mg/kg) for 6 weeks | reduces the level of amyloid-β precursor protein and inflammatory markers NLRP3, ASC, and caspase-1 in AD mice models | [71] | |
| Oral (400 mg/kg) for 4 weeks; Oral (supplemented with food 300 mg/kg) for 28 days | reversed the increased levels of TNFα in the hypothalamus of obese rats and cerebral small vessel disease mice | [72,73] | |
| 100 µM for 24 h | suppressed endothelial inflammation by reducing ICAM1 and von Willebrand factor expression in IL-1β and TNFα-stimulated human aortic endothelial cells | [74] | |
| Liver Fibrosis | Oral, via stomach gavage (400 mg/kg) for 8 weeks | reversed the development of CCl4-induced liver fibrosis in C57BL/6 mice by reducing TGF-β and serum ALT levels | [75] |
| 100 µM to 10 mM for 24 h | reduced the levels of proinflammatory cytokines TNFα and IL-6, and upregulated the levels of the anti-inflammatory molecule, adiponectin, in AML12 mouse hepatocytes | [76] | |
| Oral (400 mg/kg daily) for 20 weeks | Inhibits activation of HSCs by reducing the levels of fibrotic markers α-smooth muscle actin, collagen 1α1, and collagen 6α1 | [77] | |
| Heart failure and cardiac fibrosis | Oral (2 × 250–1500 mg daily) for 9 days | reduced the expression of proinflammatory IL-6 in PBMCs of individuals with Stage D heart failure | [78] |
| Oral (400 mg/kg) for 6–8 weeks | improves the expression of prohibitin to suppress the progression of TGF-1β-induced endothelial-to-mesenchymal transition in cardiac fibrosis | [79] | |
| Oral (supplemented with diet at 400 mg/kg) for 4 weeks | improved mitochondrial function in heart failure with preserved ejection fraction mice by repleting NAD+ levels | [22] | |
| Aging | Oral (1 g daily) for 21 days | reduces circulatory levels of inflammatory cytokines IL-2, IL-5, IL-6, TNFα and augments skeletal muscle NAD+ without altering its mitochondrial bioenergetics in humans | [80] |
| Oral (400 mg/kg) for 8 weeks | reduces amyloid aggregation, improves mitochondrial membrane potential and function in mammalian cells | [81] | |
| Oral (supplemented with drinking water at 50 mg/kg) for 6 weeks | rejuvenates intestinal stem cells in aged mice by activating SIRT1 and mTORC1 | [82] | |
| Oral (supplemented with drinking water at 12 mM) for 2 months | restores mitochondrial function and homeostasis in ataxia telangiectasia mice models | [83] | |
| Oral (500 mg) | improved physical performance and decreased oxidative stress in old individuals | [84] | |
| Oral (400 mg/kg) for 8 weeks | induces change in hematopoietic stem cells composition of aged mice towards a more youthful state by regulating the levels of mitophagy-promoting genes’ transcription | [85] |
4.1. Neuroinflammation
Neuroinflammation is considered one of the common pathophysiological mechanisms of neurodegeneration [86]. Cytokine activation, pathogen-associated molecular patterns (PAMPs), or damage-associated molecular patterns (DAMPs) can lead to the formation and activation of NOD-like receptor protein 3 (NLRP3) inflammasome [87]. NLRP3 inflammasome activation subsequently upregulates caspase-1-mediated release of proinflammatory cytokines and promotes pyroptosis [87,88]. A declined NAD+ level has been identified as a distinct feature of neuroinflammation [66,67,89]. Therefore, the repletion of cellular NAD+ contents using NR may ameliorate neuroinflammation through the downregulation of inflammation-related pathways. Administration of NR in DNA repair-deficient AD mice improved cognitive functions and reduced neuropathological hallmarks of AD, presumably through the elevation of neuronal NAD+ levels and the subsequent stimulation of SIRT3 and SIRT6 activity [67]. In another transgenic AD mouse model, the increase in brain NAD+ via NR treatment downregulated NLRP3 inflammasome and proinflammatory cytokines and decreased the activation of neuronal immune cells, partially in a cGAS-STING-dependent manner [66]. AD-like alterations—such as an accumulation of Aβ aggregates and phosphorylated tau—can be triggered by high-fat-diet-induced brain insulin resistance [90]. In a type 2 diabetic mouse model, NR supplementation decreased neuroinflammation and amyloidogenesis with improved cognitive function [90].
Gulf War Illness (GWI), which occurs predominantly in veterans of the Gulf War [91], is characterized by impaired cognitive function, difficulties with memory, chronic fatigue, and pain. In a GWI mouse model, increased expression of proinflammatory cytokines IL-1β, IL-6, and interferon-gamma (IFN-γ) have been detected in the brains [68], along with decreased brain NAD+ and Sirt1. NR treatment not only restored NAD+ levels but also stimulated Sirt1 and Sirt3 activity for improved mitochondrial biogenesis and reduced neuroinflammation [68].
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by progressive loss of motor neurons [92]. NAD+ has been shown to exhibit remarkable neuroprotective properties in cultured neurons [93]. Indeed, the repletion of NAD+ using NR was shown to delay neurodegeneration, reduce neuroinflammation markers, and alter muscle metabolism in an ALS mouse model [69]. Furthermore, NAMPT, one of the NAD+ biosynthetic enzymes, was upregulated in ALS patients, suggesting an inherent regulation mechanism for the neurons [69].
4.2. Fibrosis
Liver fibrosis is a condition defined by the activation of hepatic stellar cells (HSCs) in response to DAMPs and the over-deposition of extracellular matrix proteins [94]. It leads to chronic inflammation and hepatocellular dysfunction [95]. An elevated serum level of alanine transaminase (ALT) is an indicator of hepatocellular injury [96]. In a CCl4-induced liver fibrosis mouse model, the oral administration of NR at 400 mg/kg significantly reduced serum ALT level and hepatocyte collagen deposition [75]. NR ameliorated liver fibrosis by restoring NAD+ contents, activating NAD+-dependent SIRT1 activity, and downregulating transcription coactivator p300. Subsequently, the TGF-β/Smads pathway-mediated HSC activation was inhibited, leading to reduced severity of liver fibrosis [75]. In other studies, the administration of NR did not reduce serum ALT levels significantly but decreased the levels of accumulated collagen and fibrotic markers [77,97]. In female C57BL/6J mice fed a high-fat diet (HF), NR supplementation at 400 mg/kg daily for 20 weeks did not improve live fibrosis remarkably. Rather, it improved the fibrosis in white adipose tissue in old (16 weeks) female mice [98].
In peripheral blood mononuclear cells (PBMCs), NAD+ augmentation by NR reduced the secretion of IL-6, a cytokine that is upregulated in patients with heart failure (HF) [78]. Additionally, the expression levels of IL-1β, IL-18, and NLRP3 inflammasome were also suppressed. Similar results were obtained in HF patients after oral NR administration [78]. Transforming growth factor-β1 (TGF-β1)-induced endothelial–mesenchymal transition (EndMT) contributes to the progression of cardiac fibrosis [99]. It has been suggested that TGF-β1-induced EndMT may regulate mitochondrial unfolded protein response (mtUPR) in endothelial cells [79]. NR treatment increased the expression of mtUPR, which was suppressed upon TGF-β1 exposure. Additionally, NR supplementation elevated the levels of prohibitin proteins, PHB and PHB2, the overexpression of which upregulated endothelial cell markers and the mtUPR marker and downregulated the fibroblast marker [79]. Moreover, transverse aortic constriction (TAC)-induced EndMT was also inhibited by NR in vivo, suggesting NR as a potential therapeutic for the treatment of cardiac fibrosis [79].
4.3. Aging
Aging is characterized by chronic inflammation and increased cell senescence. Together, these factors cause age-related disorders, such as cardiovascular diseases, osteoporosis, and diabetes mellitus [100,101]. Oral NR administration in aged participants has been shown to increase the levels of NAD+ and related metabolites in skeletal muscle and significantly reduce the levels of circulating inflammatory cytokines, such as IL-6, IL-5, IL-2, and tumor necrosis factor alpha (TNF-α) [80]. Amyloidosis, together with the loss of mitochondrial function, contributes to muscle aging in multiple species, such as C. elegans, mouse skeletal muscle, and human primary myotubes [81]. More importantly, NR-mediated NAD+ elevation restored muscle homeostasis and mitochondrial function and decreased muscle amyloid-like deposition in the same species [81]. In aged mice, elongation of villi and reduction in intestinal stem cell (ISC) number and function have been observed [82]. NR administration led to the inhibition of villi elongation and an increase in ISC population and function. Activation of the SIRT1/mTORC1 pathway upon NAD+ boosting has been suggested as the molecular mechanism of NR-mediated ISC rejuvenation [82].
Senescence is increasingly recognized as a key contributor to the aging process [102]. Ataxia telangiectasia (A-T), a rare premature aging disease, was characterized by senescence phenotypes [83]. At the cellular level, mitochondrial dysfunction and compromised mitophagy have been detected in A-T fibroblasts, and increased cytoplasmic dsDNA resulting from impaired DNA damage repair was observed in ataxia-telangiectasia-mutated (ATM)-deficient cells. NR treatment alleviated senescence phenotypes in cells via the inhibition of the stimulator of the interferon genes (STING) pathway, featuring enhanced mitophagy, restored mitochondrial function, and reduced cytoplasmic dsDNA [83].
Most of the studies on NR focus on its NAD+-increasing capability. A recent study documented its effect on NADH and NADPH levels in humans [84]. Acute NR administration increased erythrocytic NAD(P)H levels in young and old individuals. However, this treatment improved redox homeostasis and physical performance only in old individuals [84], highlighting the importance of further investigation on NR as an ergogenic supplement.
5. NR and COVID-19
The novel coronavirus SARS-CoV-2, the infective agent causing COVID-19, has caused a global pandemic and had a significant socio-economic impact. Novel targets and therapeutic interventions are highly sought after to combat deadly viruses. Multiple independent lines of evidence point to NAD+ metabolism as a potential target of intervention. Viral infections are known to cause cellular NAD+ depletion [103,104]. Indeed, declined levels of NMN, an NAD+ precursor, have been detected in the blood of COVID-19 patients [105]. Furthermore, the upregulations of PARP genes and NAD+ biosynthetic gene nampt were observed in SARS-CoV-2 infected individuals [23,106]. PARPs play key roles in antiviral immune response [107]. The induction of PARPs that are known to use NAD+ as the co-substrate to catalyze mono-ADP-ribosylation (MARylation) further decreased the cellular NAD+ contents. Moreover, the upregulation of NAMPT, the rate-limiting enzyme of the salvage pathway, can be viewed as a compensative mechanism in response to the increased demand for NAD+ [23]. Boosting intracellular NAD+ pool using precursors such as NR has been shown to block the replication of murine hepatitis virus (MHV) sensitive to MARylation PARP activity [23], lending support to the idea that restoration of NAD+ homeostasis may mitigate COVID-19 severity.
NR is studied clinically in COVID-19 patients (Table 2). In one trial, the metabolic condition was investigated using a combination of NR and other metabolic cofactors, including N-acetylcysteine, L-carnitine tartrate, and serine together with hydroxychloroquine treatment (NCT04573153) [108]. The metabolic cofactors treated group demonstrated a significantly shortened recovery time with improved metabolic profiles [108]. In another trial, the dietary supplement of NR, Niagen, is evaluated for the improvement of recovery in patients suffering from Long-COVID (NCT04809974).
Table 2.
Clinical trials of NR in COVID-19.
| Treatment Regimen | Description | Type | Status | Clinical Trial |
|---|---|---|---|---|
| 1 g of NR or placebo orally every morning for 14 days | to investigate whether NR supplementation can attenuate the severity of SARS-CoV-2 infections in elderly patients | randomized double-blinded case–control trial | Unknown | NCT04407390 |
| 250 mg NR capsules administered twice daily for 10 days | treatment with NR in COVID-19 patients for renal protection | prospective, double-blind, placebo-controlled clinical interventional trial | Active, not recruiting | NCT04818216 |
| 2000 mg NR in the form of capsules daily | to examine recovery in people with persistent cognitive and physical symptoms after COVID-19 illness | Double-blinded, randomized, parallel-group, placebo-controlled design | Recruiting | NCT04809974 |
| hydroxychloroquine (standard therapy) + dietary supplement consisting of serine, L-carnitine tartrate, N-acetylcysteine, and NR | metabolic cofactor supplementation and hydroxychloroquine combination in COVID-19 patients | parallel-group, randomized, and open-label study | Recruiting | NCT04573153 |
In addition to serving as an NAD+ booster, NR is also predicted to be a direct inhibitor of viral enzymes. SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) regulates viral genome replication and gene transcription [109,110,111] and has been suggested as a potential therapeutic target for COVID-19. Nucleoside inhibitors (NIs)—such as Rivabirin [112] and Favipiravir—exhibited clinical efficacy against COVID-19 [113,114], presumably through the inhibition of RdRp activity [115]. NR, a structural mimic of the aforementioned NIs, has been proposed to have antiviral activity [116]. Molecular docking analysis and dynamic simulation studies suggested that NR may serve as a competitive inhibitor of SARS-CoV-2 RdRp, independent of its NAD+-elevating capability [116]. A recent docking study—together with target prediction, toxicity prediction, and ADME prediction—also hypothesized the clinical efficacy of NR in combating COVID-19 [117].
6. Conclusions
The initial discovery of NR-mediated lifespan extension in yeast without CR has ignited an intense interest in vitamin B3 for age-related studies [14]. NR supplementation has been increasingly recognized as an effective strategy to augment intracellular NAD+ concentrations to benefit human health. Chemical and enzymatic approaches have been developed to produce this rather labile molecule with good stereoselectivity, yield, and synthetic easiness. The biological function and therapeutic potential of NR have been heavily pursued in the last few years. This NAD+ precursor has been shown to prevent or alleviate multiple pathophysiological conditions in diverse model organisms. The clinical significance of NR is also investigated in several trials for metabolic disorders, aging, neurodegenerative diseases, and, most recently, COVID-19. Accumulating evidence unequivocally establish NR far ahead of other NAD+ precursors in improving human wellness. The future will reveal whether ample preclinical investigations can be translated into clinical applications.
Author Contributions
Conceptualization, C.S. and Y.C.; writing—original draft preparation, C.S., D.D. and Y.C.; writing—review and editing, C.S., D.D. and Y.C.; supervision, Y.C.; funding acquisition, Y.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported in part by 1R01GM143176-01A1 from NIH/NIGMS (to Y.C.) and VCU CCTR Endowment Fund (sub-award of UL1TR002649 from the National Center for Advancing Translational Sciences to VCU) (to Y.C.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azzini E., Raguzzini A., Polito A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. Int. J. Mol. Sci. 2021;22:9694. doi: 10.3390/ijms22189694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith T.J., Johnson C.R., Koshy R., Hess S.Y., Qureshi U.A., Mynak M.L., Fischer P.R. Thiamine deficiency disorders: A clinical perspective. Ann. N. Y. Acad. Sci. 2021;1498:9–28. doi: 10.1111/nyas.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holubiec P., Leonczyk M., Staszewski F., Lazarczyk A., Jaworek A.K., Rojas-Pelc A. Pathophysiology and clinical management of pellagra—A review. Folia Med. Cracov. 2021;61:125–137. doi: 10.24425/fmc.2021.138956. [DOI] [PubMed] [Google Scholar]
- 4.Trapani S., Rubino C., Indolfi G., Lionetti P. A Narrative Review on Pediatric Scurvy: The Last Twenty Years. Nutrients. 2022;14:684. doi: 10.3390/nu14030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewett D., Lam-Kamath K., Poupault C., Khurana H., Rister J. Mechanisms of vitamin A metabolism and deficiency in the mammalian and fly visual system. Dev. Biol. 2021;476:68–78. doi: 10.1016/j.ydbio.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mladenka P., Macakova K., Kujovska Krcmova L., Javorska L., Mrstna K., Carazo A., Protti M., Remiao F., Novakova L. OEMONOM Researchers and Collaborators, Vitamin K—Sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022;80:677–698. doi: 10.1093/nutrit/nuab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souganidis E. Nobel laureates in the history of the vitamins. Ann. Nutr. Metab. 2012;61:265–269. doi: 10.1159/000343122. [DOI] [PubMed] [Google Scholar]
- 8.US Preventive Services Task Force. Mangione C.M., Barry M.J., Nicholson W.K., Cabana M., Chelmow D., Coker T.R., Davis E.M., Donahue K.E., Doubeni C.A., et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327:2326–2333. doi: 10.1001/jama.2022.8970. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan M., Treiman K.A., Kish-Doto J., Middleton J.C., Coker-Schwimmer E.J., Nicholson W.K. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:190–203. doi: 10.1001/jama.2016.19193. [DOI] [PubMed] [Google Scholar]
- 10.Annweiler C., Dursun E., Feron F., Gezen-Ak D., Kalueff A.V., Littlejohns T., Llewellyn D.J., Millet P., Scott T., Tucker K.L., et al. ‘Vitamin D and cognition in older adults’: Updated international recommendations. J. Intern. Med. 2015;277:45–57. doi: 10.1111/joim.12279. [DOI] [PubMed] [Google Scholar]
- 11.Granato D., Carocho M., Barros L., Zabetakis I., Mocan A., Tsoupras A., Cruz A.G., Pimentel T.C. Implementation of Sustainable Development Goals in the dairy sector: Perspectives on the use of agro-industrial side-streams to design functional foods. Trends Food Sci. Technol. 2022;124:128–139. doi: 10.1016/j.tifs.2022.04.009. [DOI] [Google Scholar]
- 12.Lordan R., Rando H.M., Consortium C.-R., Greene C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. Msystems. 2021;6:e00122-21. doi: 10.1128/mSystems.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lordan R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic—Implications for consumers and regulatory oversight. PharmaNutrition. 2021;18:100282. doi: 10.1016/j.phanu.2021.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belenky P., Racette F.G., Bogan K.L., McClure J.M., Smith J.S., Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Bogan K.L., Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 16.Trammell S.A., Schmidt M.S., Weidemann B.J., Redpath P., Jaksch F., Dellinger R.W., Li Z., Abel E.D., Migaud M.E., Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canto C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaur P., Brugg B., Mericskay M., Li Z., Schmidt M.S., Vivien D., Orset C., Jacotot E., Brenner C., Duplus E. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. 2017;31:5440–5452. doi: 10.1096/fj.201700221RR. [DOI] [PubMed] [Google Scholar]
- 19.Roboon J., Hattori T., Ishii H., Takarada-Iemata M., Nguyen D.T., Heer C.D., O’Meally D., Brenner C., Yamamoto Y., Okamoto H., et al. Inhibition of CD38 and supplementation of nicotinamide riboside ameliorate lipopolysaccharide-induced microglial and astrocytic neuroinflammation by increasing NAD. J. Neurochem. 2021;158:311–327. doi: 10.1111/jnc.15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trammell S.A., Weidemann B.J., Chadda A., Yorek M.S., Holmes A., Coppey L.J., Obrosov A., Kardon R.H., Yorek M.A., Brenner C. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci. Rep. 2016;6:26933. doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dollerup O.L., Christensen B., Svart M., Schmidt M.S., Sulek K., Ringgaard S., Stodkilde-Jorgensen H., Moller N., Brenner C., Treebak J.T., et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018;108:343–353. doi: 10.1093/ajcn/nqy132. [DOI] [PubMed] [Google Scholar]
- 22.Tong D., Schiattarella G.G., Jiang N., Altamirano F., Szweda P.A., Elnwasany A., Lee D.I., Yoo H., Kass D.A., Szweda L.I., et al. NAD(+) Repletion Reverses Heart Failure with Preserved Ejection Fraction. Circ. Res. 2021;128:1629–1641. doi: 10.1161/CIRCRESAHA.120.317046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heer C.D., Sanderson D.J., Voth L.S., Alhammad Y.M.O., Schmidt M.S., Trammell S.A.J., Perlman S., Cohen M.S., Fehr A.R., Brenner C. Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity. J. Biol. Chem. 2020;295:17986–17996. doi: 10.1074/jbc.RA120.015138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunewald M.E., Chen Y., Kuny C., Maejima T., Lease R., Ferraris D., Aikawa M., Sullivan C.S., Perlman S., Fehr A.R. The coronavirus macrodomain is required to prevent PARP-mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathog. 2019;15:e1007756. doi: 10.1371/journal.ppat.1007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryan S., Baregzay B., Spicer D., Singal P.K., Khaper N. Redox-inflammatory synergy in the metabolic syndrome. Can. J. Physiol. Pharmacol. 2013;91:22–30. doi: 10.1139/cjpp-2012-0295. [DOI] [PubMed] [Google Scholar]
- 26.Amjad S., Nisar S., Bhat A.A., Shah A.R., Frenneaux M.P., Fakhro K., Haris M., Reddy R., Patay Z., Baur J., et al. Role of NAD(+) in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021;49:101195. doi: 10.1016/j.molmet.2021.101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtin N., Banyai K., Thaventhiran J., Le Quesne J., Helyes Z., Bai P. Repositioning PARP inhibitors for SARS-CoV-2 infection(COVID-19); a new multi-pronged therapy for acute respiratory distress syndrome? Br. J. Pharmacol. 2020;177:3635–3645. doi: 10.1111/bph.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M., Schultz M.B., Sinclair D.A. NAD(+) in COVID-19 and viral infections. Trends Immunol. 2022;43:283–295. doi: 10.1016/j.it.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curry A., White D., Cen Y. Small Molecule Regulators Targeting NAD(+) Biosynthetic Enzymes. Curr. Med. Chem. 2022;29:1718–1738. doi: 10.2174/0929867328666210531144629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Zhang N., Zhang G., Sauve A.A. NRH salvage and conversion to NAD(+) requires NRH kinase activity by adenosine kinase. Nat. Metab. 2020;2:364–379. doi: 10.1038/s42255-020-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurnasov O., Goral V., Colabroy K., Gerdes S., Anantha S., Osterman A., Begley T.P. NAD biosynthesis: Identification of the tryptophan to quinolinate pathway in bacteria. Chem. Biol. 2003;10:1195–1204. doi: 10.1016/j.chembiol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Lin H. Nicotinamide adenine dinucleotide: Beyond a redox coenzyme. Org. Biomol. Chem. 2007;5:2541–2554. doi: 10.1039/b706887e. [DOI] [PubMed] [Google Scholar]
- 33.Camacho-Pereira J., Tarrago M.G., Chini C.C.S., Nin V., Escande C., Warner G.M., Puranik A.S., Schoon R.A., Reid J.M., Galina A., et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietrich L.S., Fuller L., Yero I.L., Martinez L. Nicotinamide mononucleotide pyrophosphorylase activity in animal tissues. J. Biol. Chem. 1966;241:188–191. doi: 10.1016/S0021-9258(18)96977-2. [DOI] [PubMed] [Google Scholar]
- 35.Preiss J., Handler P. Biosynthesis of diphosphopyridine nucleotide. I. Identification of intermediates. J. Biol. Chem. 1958;233:488–492. doi: 10.1016/S0021-9258(18)64789-1. [DOI] [PubMed] [Google Scholar]
- 36.Bieganowski P., Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/S0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 37.Tempel W., Rabeh W.M., Bogan K.L., Belenky P., Wojcik M., Seidle H.F., Nedyalkova L., Yang T., Sauve A.A., Park H.W., et al. Nicotinamide riboside kinase structures reveal new pathways to NAD+ PLoS Biol. 2007;5:e263. doi: 10.1371/journal.pbio.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fletcher R.S., Ratajczak J., Doig C.L., Oakey L.A., Callingham R., Da Silva Xavier G., Garten A., Elhassan Y.S., Redpath P., Migaud M.E., et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab. 2017;6:819–832. doi: 10.1016/j.molmet.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang T., Chan N.Y., Sauve A.A. Syntheses of nicotinamide riboside and derivatives: Effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. J. Med. Chem. 2007;50:6458–6461. doi: 10.1021/jm701001c. [DOI] [PubMed] [Google Scholar]
- 40.Ryu D., Zhang H., Ropelle E.R., Sorrentino V., Mazala D.A., Mouchiroud L., Marshall P.L., Campbell M.D., Ali A.S., Knowels G.M., et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl. Med. 2016;8:361ra139. doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonkowski M.S., Sinclair D.A. Slowing ageing by design: The rise of NAD(+) and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu L.E., Sinclair D.A. Restoring stem cells—All you need is NAD(.) Cell Res. 2016;26:971–972. doi: 10.1038/cr.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canto C., Auwerx J. Targeting sirtuin 1 to improve metabolism: All you need is NAD(+)? Pharmacol. Rev. 2012;64:166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shats I., Williams J.G., Liu J., Makarov M.V., Wu X., Lih F.B., Deterding L.J., Lim C., Xu X., Randall T.A., et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020;31:564–579.e7. doi: 10.1016/j.cmet.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehmel M., Jovanović N., Spitz U. Nicotinamide Riboside—The Current State of Research and Therapeutic Uses. Nutrients. 2020;12:1616. doi: 10.3390/nu12061616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giroud-Gerbetant J., Joffraud M., Giner M.P., Cercillieux A., Bartova S., Makarov M.V., Zapata-Perez R., Sanchez-Garcia J.L., Houtkooper R.H., Migaud M.E., et al. A reduced form of nicotinamide riboside defines a new path for NAD(+) biosynthesis and acts as an orally bioavailable NAD(+) precursor. Mol. Metab. 2019;30:192–202. doi: 10.1016/j.molmet.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long D.J., 2nd, Jaiswal A.K. NRH: Quinone oxidoreductase2 (NQO2) Chem. Biol. Interact. 2000;129:99–112. doi: 10.1016/S0009-2797(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 48.Lu S.P., Lin S.J. Phosphate-responsive signaling pathway is a novel component of NAD+ metabolism in Saccharomyces cerevisiae. J. Biol. Chem. 2011;286:14271–14281. doi: 10.1074/jbc.M110.217885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belenky P., Stebbins R., Bogan K.L., Evans C.R., Brenner C. Nrt1 and Tna1-independent export of NAD+ precursor vitamins promotes NAD+ homeostasis and allows engineering of vitamin production. PLoS ONE. 2011;6:e19710. doi: 10.1371/journal.pone.0019710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulikova V., Shabalin K., Nerinovski K., Dolle C., Niere M., Yakimov A., Redpath P., Khodorkovskiy M., Migaud M.E., Ziegler M., et al. Generation, Release, and Uptake of the NAD Precursor Nicotinic Acid Riboside by Human Cells. J. Biol. Chem. 2015;290:27124–27137. doi: 10.1074/jbc.M115.664458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes L.J., Hughes N.A., Kenner G.W., Todd A. Codehydrogenases. 2. A Synthesis of Nicotinamide Nucleotide. J. Chem. Soc. 1957:3727–3732. doi: 10.1039/JR9570003727. [DOI] [Google Scholar]
- 52.Jeck R., Woenckhaus C. Simple methods for preparing nicotinamide mononucleotide and related analogs. Methods Enzymol. 1980;66:62–70. doi: 10.1016/0076-6879(80)66439-8. [DOI] [PubMed] [Google Scholar]
- 53.Tanimori S., Ohta T., Kirihata M. An efficient chemical synthesis of nicotinamide riboside (NAR) and analogues. Bioorg. Med. Chem. Lett. 2002;12:1135–1137. doi: 10.1016/S0960-894X(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 54.Franchetti P., Pasqualini M., Petrelli R., Ricciutelli M., Vita P., Cappellacci L. Stereoselective synthesis of nicotinamide beta-riboside and nucleoside analogs. Bioorg. Med. Chem. Lett. 2004;14:4655–4658. doi: 10.1016/j.bmcl.2004.06.093. [DOI] [PubMed] [Google Scholar]
- 55.Zhang N., Sauve A.A. Synthesis of beta-Nicotinamide Riboside Using an Efficient Two-Step Methodology. Curr. Protoc. Nucleic Acid Chem. 2017;71:14.14.1–14.14.9. doi: 10.1002/cpnc.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szczepankiewicz B., KOPPETSCH K., Perni R.B. Preparation and Use of Crystalline Beta-d-Nicotinamide Riboside. PCT/IB2015/054181. Google Patents. 2015 December 10;
- 57.Makarov M.V., Migaud M.E. Syntheses and chemical properties of beta-nicotinamide riboside and its analogues and derivatives. Beilstein J. Org. Chem. 2019;15:401–430. doi: 10.3762/bjoc.15.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran A., Yokose R., Cen Y. Chemo-enzymatic synthesis of isotopically labeled nicotinamide riboside. Org. Biomol. Chem. 2018;16:3662–3671. doi: 10.1039/C8OB00552D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cercillieux A., Ciarlo E., Canto C. Balancing NAD(+) deficits with nicotinamide riboside: Therapeutic possibilities and limitations. Cell Mol. Life Sci. 2022;79:463. doi: 10.1007/s00018-022-04499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiten O.K., Wilvang M.A., Mitchell S.J., Hu Z., Fang E.F. Preclinical and clinical evidence of NAD(+) precursors in health, disease, and ageing. Mech. Ageing Dev. 2021;199:111567. doi: 10.1016/j.mad.2021.111567. [DOI] [PubMed] [Google Scholar]
- 61.Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H., Ryu D., Wu Y., Gariani K., Wang X., Luan P., D’Amico D., Ropelle E.R., Lutolf M.P., Aebersold R., et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 63.Gong B., Pan Y., Vempati P., Zhao W., Knable L., Ho L., Wang J., Sastre M., Ono K., Sauve A.A., et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang E.F., Kassahun H., Croteau D.L., Scheibye-Knudsen M., Marosi K., Lu H., Shamanna R.A., Kalyanasundaram S., Bollineni R.C., Wilson M.A., et al. NAD(+) Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts E.L., Newton R.P., Axford A.T. Plasma purine nucleoside phosphorylase in cancer patients. Clin. Chim. Acta. 2004;344:109–114. doi: 10.1016/j.cccn.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Hou Y., Wei Y., Lautrup S., Yang B., Wang Y., Cordonnier S., Mattson M.P., Croteau D.L., Bohr V.A. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc. Natl. Acad. Sci. USA. 2021;118:e2011226118. doi: 10.1073/pnas.2011226118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou Y., Lautrup S., Cordonnier S., Wang Y., Croteau D.L., Zavala E., Zhang Y., Moritoh K., O’Connell J.F., Baptiste B.A., et al. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. USA. 2018;115:E1876–E1885. doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joshi U., Evans J.E., Pearson A., Saltiel N., Cseresznye A., Darcey T., Ojo J., Keegan A.P., Oberlin S., Mouzon B., et al. Targeting sirtuin activity with nicotinamide riboside reduces neuroinflammation in a GWI mouse model. Neurotoxicology. 2020;79:84–94. doi: 10.1016/j.neuro.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Harlan B.A., Killoy K.M., Pehar M., Liu L., Auwerx J., Vargas M.R. Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp. Neurol. 2020;327:113219. doi: 10.1016/j.expneurol.2020.113219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Obrador E., Salvador R., Marchio P., Lopez-Blanch R., Jihad-Jebbar A., Rivera P., Valles S.L., Banacloche S., Alcacer J., Colomer N., et al. Nicotinamide Riboside and Pterostilbene Cooperatively Delay Motor Neuron Failure in ALS SOD1(G93A) Mice. Mol. Neurobiol. 2021;58:1345–1371. doi: 10.1007/s12035-020-02188-7. [DOI] [PubMed] [Google Scholar]
- 71.Lee H.J., Yang S.J. Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice. Int. J. Mol. Sci. 2019;20:4196. doi: 10.3390/ijms20174196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Castro J.M., Stein D.J., Medeiros H.R., de Oliveira C., Torres I.L.S. Nicotinamide Riboside Neutralizes Hypothalamic Inflammation and Increases Weight Loss without Altering Muscle Mass in Obese Rats under Calorie Restriction: A Preliminary Investigation. Front. Nutr. 2021;8:648893. doi: 10.3389/fnut.2021.648893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li C.C., Chen W.X., Wang J., Xia M., Jia Z.C., Guo C., Tang X.Q., Li M.X., Yin Y., Liu X., et al. Nicotinamide riboside rescues angiotensin II-induced cerebral small vessel disease in mice. CNS Neurosci. Ther. 2020;26:438–447. doi: 10.1111/cns.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mateuszuk L., Campagna R., Kutryb-Zajac B., Kus K., Slominska E.M., Smolenski R.T., Chlopicki S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem. Pharmacol. 2020;178:114019. doi: 10.1016/j.bcp.2020.114019. [DOI] [PubMed] [Google Scholar]
- 75.Jiang R., Zhou Y., Wang S., Pang N., Huang Y., Ye M., Wan T., Qiu Y., Pei L., Jiang X., et al. Nicotinamide riboside protects against liver fibrosis induced by CCl4 via regulating the acetylation of Smads signaling pathway. Life Sci. 2019;225:20–28. doi: 10.1016/j.lfs.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 76.Lee H.J., Yang S.J. Nicotinamide riboside regulates inflammation and mitochondrial markers in AML12 hepatocytes. Nutr. Res. Pract. 2019;13:3–10. doi: 10.4162/nrp.2019.13.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pham T.X., Bae M., Kim M.B., Lee Y., Hu S., Kang H., Park Y.K., Lee J.Y. Nicotinamide riboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:2451–2463. doi: 10.1016/j.bbadis.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou B., Wang D.D., Qiu Y., Airhart S., Liu Y., Stempien-Otero A., O’Brien K.D., Tian R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020;130:6054–6063. doi: 10.1172/JCI138538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang M., Weng H., Zheng J. NAD(+) repletion inhibits the endothelial-to-mesenchymal transition induced by TGF-beta in endothelial cells through improving mitochondrial unfolded protein response. Int. J. Biochem. Cell Biol. 2019;117:105635. doi: 10.1016/j.biocel.2019.105635. [DOI] [PubMed] [Google Scholar]
- 80.Elhassan Y.S., Kluckova K., Fletcher R.S., Schmidt M.S., Garten A., Doig C.L., Cartwright D.M., Oakey L., Burley C.V., Jenkinson N., et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD(+) Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019;28:1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romani M., Sorrentino V., Oh C.M., Li H., de Lima T.I., Zhang H., Shong M., Auwerx J. NAD(+) boosting reduces age-associated amyloidosis and restores mitochondrial homeostasis in muscle. Cell Rep. 2021;34:108660. doi: 10.1016/j.celrep.2020.108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Igarashi M., Miura M., Williams E., Jaksch F., Kadowaki T., Yamauchi T., Guarente L. NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell. 2019;18:e12935. doi: 10.1111/acel.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang B., Dan X., Hou Y., Lee J.H., Wechter N., Krishnamurthy S., Kimura R., Babbar M., Demarest T., McDevitt R., et al. NAD(+) supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell. 2021;20:e13329. doi: 10.1111/acel.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dolopikou C.F., Kourtzidis I.A., Margaritelis N.V., Vrabas I.S., Koidou I., Kyparos A., Theodorou A.A., Paschalis V., Nikolaidis M.G. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: A double-blind cross-over study. Eur. J. Nutr. 2020;59:505–515. doi: 10.1007/s00394-019-01919-4. [DOI] [PubMed] [Google Scholar]
- 85.Sun X., Cao B., Naval-Sanchez M., Pham T., Sun Y.B.Y., Williams B., Heazlewood S.Y., Deshpande N., Li J., Kraus F., et al. Nicotinamide riboside attenuates age-associated metabolic and functional changes in hematopoietic stem cells. Nat. Commun. 2021;12:2665. doi: 10.1038/s41467-021-22863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jellinger K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell Mol. Med. 2010;14:457–487. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao C., Zhao W. NLRP3 Inflammasome—A Key Player in Antiviral Responses. Front. Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang E.F., Lautrup S., Hou Y., Demarest T.G., Croteau D.L., Mattson M.P., Bohr V.A. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017;23:899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang S., Kim C.H., Jung H., Kim E., Song H.T., Lee J.E. Agmatine ameliorates type 2 diabetes induced-Alzheimer’s disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Pt ANeuropharmacology. 2017;113:467–479. doi: 10.1016/j.neuropharm.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 91.Mawson A.R., Croft A.M. Gulf War Illness: Unifying Hypothesis for a Continuing Health Problem. Int. J. Environ. Res. Public Health. 2019;16:111. doi: 10.3390/ijerph16010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 93.Lautrup S., Sinclair D.A., Mattson M.P., Fang E.F. NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019;30:630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan Z., Sun H., Xue T., Gan C., Liu H., Xie Y., Yao Y., Ye T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021;9:730176. doi: 10.3389/fcell.2021.730176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim W.R., Flamm S.L., Di Bisceglie A.M., Bodenheimer H.C., Public Policy Committee of the American Association for the Study of Liver Disease Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 97.Han X., Bao X., Lou Q., Xie X., Zhang M., Zhou S., Guo H., Jiang G., Shi Q. Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ. 2019;7:e7568. doi: 10.7717/peerj.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim M.B., Pham T.X., vanLuling M., Kostour V., Kang H., Corvino O., Jang H., Odell W., Bae M., Park Y.K., et al. Nicotinamide riboside supplementation exerts an anti-obesity effect and prevents inflammation and fibrosis in white adipose tissue of female diet-induced obesity mice. J. Nutr. Biochem. 2022;107:109058. doi: 10.1016/j.jnutbio.2022.109058. [DOI] [PubMed] [Google Scholar]
- 99.Zeisberg E.M., Tarnavski O., Zeisberg M., Dorfman A.L., McMullen J.R., Gustafsson E., Chandraker A., Yuan X., Pu W.T., Roberts A.B., et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 100.Jaul E., Barron J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and over Population. Front. Public Health. 2017;5:335. doi: 10.3389/fpubh.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrucci L., Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Korolchuk V.I., Miwa S., Carroll B., von Zglinicki T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine. 2017;21:7–13. doi: 10.1016/j.ebiom.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murray M.F., Nghiem M., Srinivasan A. HIV infection decreases intracellular nicotinamide adenine dinucleotide [NAD] Biochem. Biophys. Res. Commun. 1995;212:126–131. doi: 10.1006/bbrc.1995.1945. [DOI] [PubMed] [Google Scholar]
- 104.Tran T., Pencina K.M., Schultz M.B., Li Z., Ghattas C., Lau J., Sinclair D.A., Montano M. Reduced Levels of NAD in Skeletal Muscle and Increased Physiologic Frailty Are Associated with Viral Coinfection in Asymptomatic Middle-Aged Adults. J. Acquir. Immune Defic. Syndr. 2022;89((Suppl. S1)):S15–S22. doi: 10.1097/QAI.0000000000002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiao N., Nie M., Pang H., Wang B., Hu J., Meng X., Li K., Ran X., Long Q., Deng H., et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021;12:1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luscher B., Verheirstraeten M., Krieg S., Korn P. Intracellular mono-ADP-ribosyltransferases at the host-virus interphase. Cell Mol. Life Sci. 2022;79:288. doi: 10.1007/s00018-022-04290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Altay O., Arif M., Li X., Yang H., Aydin M., Alkurt G., Kim W., Akyol D., Zhang C., Dinler-Doganay G., et al. Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. Adv. Sci. 2021;8:e2101222. doi: 10.1002/advs.202101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hillen H.S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584:154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- 112.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joshi S., Parkar J., Ansari A., Vora A., Talwar D., Tiwaskar M., Patil S., Barkate H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed. Forces India. 2020;76:370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang W.F., Stephen P., Theriault J.F., Wang R., Lin S.X. Novel Coronavirus Polymerase and Nucleotidyl-Transferase Structures: Potential to Target New Outbreaks. J. Phys. Chem. Lett. 2020;11:4430–4435. doi: 10.1021/acs.jpclett.0c00571. [DOI] [PubMed] [Google Scholar]
- 116.Esam Z., Akhavan M., Lotfi M., Bekhradnia A. Molecular docking and dynamics studies of Nicotinamide Riboside as a potential multi-target nutraceutical against SARS-CoV-2 entry, replication, and transcription: A new insight. J. Mol. Struct. 2022;1247:131394. doi: 10.1016/j.molstruc.2021.131394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arora M.K., Grover P., Asdaq S.M.B., Mehta L., Tomar R., Imran M., Pathak A., Jangra A., Sahoo J., Alamri A.S., et al. Potential role of nicotinamide analogues against SARS-COV-2 target proteins. Saudi J. Biol. Sci. 2021;28:7567–7574. doi: 10.1016/j.sjbs.2021.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.